An effervescent microneedle patch has been designed for rapid separation of drug-loaded microneedles and long-term contraception.
Abstract
To increase access to long-acting contraception, we developed a reversible contraceptive microneedle patch that is simple-to-administer, slowly releases contraceptive hormone (levonorgestrel) for >1 month, and generates no biohazardous sharps waste. After manually pressing the patch to skin for 1 min, microneedles rapidly separate from the patch within the skin due to effervescence triggered by contact with skin’s interstitial fluid, as demonstrated in rats and human participants. Long-acting contraception is achieved by formulating microneedles with a biodegradable polymer [poly(lactic-co-glycolic) acid] that slowly releases levonorgestrel for ~1 month in vitro. In rats, the patch maintained levonorgestrel concentration above the human contraceptive threshold level for >1 month, and a placebo microneedle patch was well-tolerated in human participants. Women of reproductive age in three continents demonstrated interest in and preference for long-acting contraception by microneedle patch. These studies indicate that an effervescent microneedle patch could facilitate greater access to long-acting contraception.
INTRODUCTION
Contraceptive methods empower women and couples to make decisions about the timing and spacing of pregnancies. Family planning, in turn, provides important health, social, and economic benefits for individuals, families, and nations (1). With the global population exceeding 7.5 billion people (2), universal access to effective contraception is critical for both personal and societal needs. Although many family planning methods exist, 85 million pregnancies worldwide were unintended in 2012, which represents 40% of all pregnancies (3, 4). In the United States, where 45% of pregnancies were unplanned in 2011 (3, 4), total public expenditures on unintended pregnancies nationwide were estimated to be $21 billion in 2010 (5).
Expanding contraceptive options with new methods that better meet the needs of women throughout their reproductive life spans is critical to reduce the number of unwanted pregnancies. Important characteristics of a contraceptive include (i) high safety and efficacy with minimal side effects; (ii) simple administration, enabling self-administration; (iii) long-acting (≥1 month) to increase user compliance by requiring infrequent dosing; (iv) generation of no biohazardous sharps waste to avoid needle-stick injury; (v) cost-effectiveness; and (vi) high acceptability among women of reproductive age (6). Nonhormonal contraceptive methods such as condoms and diaphragms do not provide long-lasting protection and have limited effectiveness due to poor user acceptance and adherence with correct use (7, 8). Oral contraceptive pills are easy to use but are also not long acting, and their efficacy suffers because of poor compliance (9). Injectable long-acting hormonal contraceptives have reduced acceptability due to their invasive nature and can have poor compliance due to the need for administration by health care professionals, who are in limited supply in developing countries or regions (10, 11). Implantable long-acting hormonal contraceptives, such as intrauterine devices (IUDs) and subcutaneous implants, can last for years but are even more invasive and require administration by health care professionals with even greater training compared to other methods (12, 13).
To address these limitations, we developed a long-acting contraceptive administered by a microneedle (MN) patch containing an effervescent backing to facilitate rapid separation of the MNs from the patch. We chose levonorgestrel (LNG) as the contraceptive hormone because of its long history as a safe and effective contraceptive (14), and we chose poly(lactic-co-glycolic) acid (PLGA) as the biodegradable MN polymer, mediating slow drug release, because of its extensive use in pharmaceutical and medical products with an excellent safety profile (15).
Our design includes an MN patch as the delivery method. MN patches contain arrays of solid needles measuring hundreds of micrometers in length that encapsulate drugs (16, 17). By manually pressing the patch to the skin, the MNs painlessly penetrate into the skin’s upper layers, where they release the encapsulated drug. Most work with MN patches involves water-soluble formulations that quickly release drug and thereby enable a short patch wear time (e.g., minutes) and fabrication using aqueous casting or dipping methods. This type of MN patch has received extensive attention in the literature (18–21) and has been successfully used in a number of clinical trials (22, 23). MN patches have also been shown to be easy to administer, including via self-administration (22, 24); to generate no biohazardous sharps waste, because MNs dissolve in the skin (25); to find high acceptability in human studies (22, 26, 27); and to enable low-cost manufacturing (18).
Using MN patches for slow drug release has two important challenges. First, the MNs must be designed to use a non–water-soluble formulation that slowly releases the drug and contains sufficient drug to last the targeted time period. Second, to enable a short MN patch wear time (i.e., to avoid the need to keep the patch on the skin for the duration of drug release), the MNs must be biodegradable and have a mechanism to rapidly separate from the patch backing to remain embedded in the skin. MN patches with these properties have received limited attention in prior literature (28–32).
Here, we developed a MN patch using biodegradable MNs for slow release of LNG that enables rapid separation in skin by including an effervescent formulation in the patch backing (Fig. 1), which builds on our prior work in this area (32). Upon contacting interstitial fluid (ISF) in the skin, sodium bicarbonate and citric acid in the MN patch backing react to form carbon dioxide bubbles that weaken MN attachment to the patch and enable separation within 1 min of skin insertion. This is faster than what other MN designs have achieved without involving a mechanical separation mechanism. The MN patch design also involves casting nonaqueous solutions onto MN molds by a method that achieves high drug loading and retains MN shape during patch drying. In this study, we first developed the effervescent MN patch and characterized its mechanical, rapid detachment, and controlled release properties in vitro, then determined the pharmacokinetics of long-acting LNG delivery in rats, demonstrated rapid MN separation from effervescent patch backing in human participants, and lastly assessed the acceptability of long-acting contraception by MN patch among women in India, Nigeria, and the United States. In this way, this translational study goes from patch design and fabrication through animal studies and ends with two studies in human participants.
Fig. 1. Design and fabrication of effervescent MN patches.
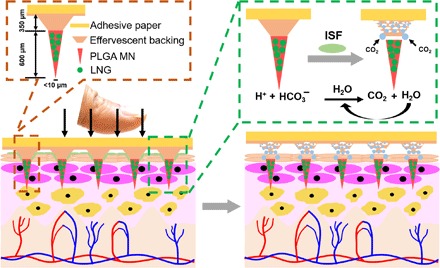
Schematic illustration of the design of an MN patch with effervescent backing and of the process of MN patch application to skin to rapidly deliver MNs into the skin by fast dissolution of the effervescent backing. Photo credit: Wei Li, Georgia Tech.
RESULTS
Design and fabrication of effervescent MN patches
The MN patch was fabricated by casting a PLGA solution in diglyme/water (95%/5%, v/v) with suspended LNG crystals with an average diameter of 17.1 ± 7.6 μm (Fig. 2, D and E) onto a silicone MN mold. Polymer and LNG formulations were filled into mold cavities by centrifugation to form the MNs and enhance MN strength by minimizing void formation. Because we found that organic solvents can enter into the silicone molds and swell them, which can distort MN shape, we used diglyme, which minimally swelled the mold (i.e., pure diglyme swelled the mold by 25.7 ± 1.1%, n = 3 replicate measurements), as the solvent. The addition of a small amount of water swelled the mold even less [diglyme/water (95%/5%, v/v) swelled the mold by 15.8 ± 0.3%, n = 3]. The MN patches were then dried to remove residual solvent. In addition to minimizing mold swelling, this casting formulation was able to produce and encapsulate fine LNG particles at 40% loading in PLGA MNs for slow release of LNG over time as the PLGA biodegrades.
Fig. 2. Characterization of effervescent MN patches.
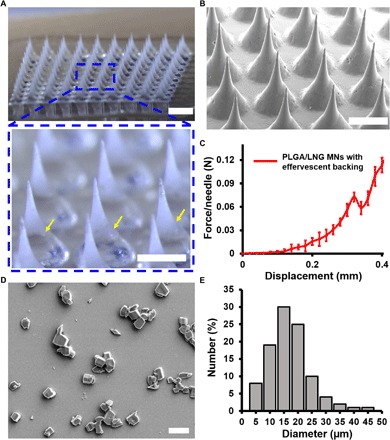
(A) Representative bright-field microscopy images of an MN patch with effervescent backing. The yellow arrows indicate the interface of the MN and backing. Scale bars, 1 mm (top) and 500 μm (bottom). (B) Scanning electron micrograph of MN array. Scale bar, 500 μm. (C) Mechanical behavior of the effervescent MN patch. (D) Scanning electron micrograph of LNG crystals extracted from MNs. Scale bar, 20 μm. (E) Size distribution of LNG crystals.
Next, an effervescent backing solution in ethanol was cast on the mold surface. The backing solution was formulated to facilitate rapid separation of the MNs from the patch backing by containing polyvinylpyrrolidone (PVP), which is very water soluble, as well as citric acid and sodium bicarbonate (Fig. 1) to produce effervescence. Upon insertion into skin, the skin’s ISF penetrates the patch and begins to solubilize the patch backing. Once in contact with ISF, the sodium bicarbonate and citric acid react and form CO2 bubbles and water [C6H8O7(aq) + 3NaHCO3(aq) → Na3C6H5O7(aq) + 3H2O(l) + 3CO2(g)]. The effervescent CO2 bubbles serve to mechanically weaken the interface between the MNs and the patch backing, and the water facilitates further dissolution of the patch backing at this interface. In this way, the effervescence initiated by MN patch application to skin enables rapid separation of the MNs from the patch backing. The patch can then be removed from the skin after a short application time, after which the PLGA MNs remain embedded under the skin surface for long-acting drug release.
The fabricated patch consists of a 10 × 10 array of sharp MNs over an area of ~0.5 cm2, mounted on a slightly larger, rigid tape, containing 0.28 ± 0.04 mg of LNG per patch. Each MN is conical with a base radius of 150 μm, a height of 600 μm, and a tip radius of ~10 μm (Figs. 1 and 2, A and B). Measurement of mechanical strength of the effervescent PLGA/LNG patch using a force gauge showed a failure force of 0.07 N per needle (Fig. 2C), which indicates that the effervescent MN patch should have sufficient strength to penetrate the skin without breaking (33).
Rapid detachment test of effervescent MN patches
To investigate whether effervescent patches can achieve rapid MN detachment, patches were immersed into phosphate-buffered saline (PBS), which was used to mimic contact with the skin’s ISF (Fig. 3A). MNs were loaded with a fluorescent dye, Nile red, for better visualization (Fig. 3B). Upon placing the patch in PBS, the patch backing immediately generated a large field of gas bubbles due to the reaction between citric acid and sodium bicarbonate, and the MNs were rapidly separated from the patch (Fig. 3, C and D). It only took 10.7 ± 1.2 s (mean ± SD) to separate MNs from the effervescent backing (Fig. 3E and movie S1), compared to 53.3 ± 3.1 s for a PVP patch backing without effervescence or 94.0 ± 6.6 s for a patch with backing made of polyvinyl alcohol (PVA) and sucrose, which is a commonly used noneffervescent MN patch formulation (Fig. 3E, fig. S1, and movie S2). This demonstrates the rapid detachment of MNs from a patch enabled by an effervescent backing in vitro.
Fig. 3. Detachment of MNs from effervescent MN patches.
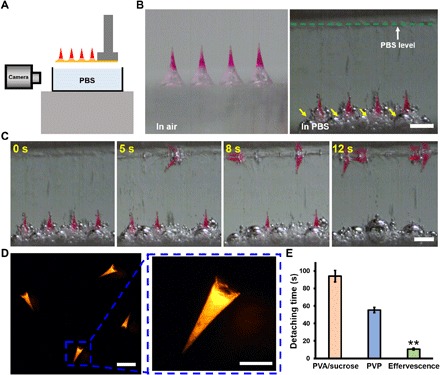
(A) Schematic illustration of the experimental setup for the detachment test of effervescent MN patches. (B) Representative bright-field microscopy images of MN patches before (left) and immediately after (right) placement in PBS solution. The yellow arrows indicate gas bubbles generated in the effervescent backing. Scale bar, 500 μm. (C) Dissolution of the effervescent backing and detachment of MNs in PBS over time. Scale bar, 500 μm. (D) Fluorescent images of MNs separated from the patch with effervescent backing in PBS solution. Scale bars, 500 μm (left) and 200 μm (right). (E) Quantification of detachment time of 100% of MNs from patches with effervescent backing, PVA/sucrose backing, or PVP backing in PBS buffer. Each point represents mean ± SD (n = 3), **P < 0.01.
Application of effervescent MN patches to skin ex vivo
To determine whether effervescent MN patches enable rapid MN separation in the skin (as opposed to PBS in the previous section), patches were applied to porcine skin ex vivo. MNs loaded with Nile red dye were pressed firmly against the skin and then kept in place for 50 s to enable separation of MNs in the skin due to effervescence (Fig. 4, A and B). After patch removal from the skin, there was almost no fluorescent dye left in the residual patch, indicating efficient MN separation (Fig. 4C). Histological sections indicate that the separated MNs were embedded under the skin surface (Fig. 4D). On average, 96 ± 4% of MNs separated from the effervescent patches and 90 ± 4% of encapsulated fluorescent dye (simulating encapsulated hormone) was delivered into the skin (Fig. 4E). In contrast, MN patches with noneffervescent PVP backing or PVA/sucrose backing had <45% MN detachment efficiency and <35% dye delivery efficiency after a 50-s application time on the skin (Fig. 4E). Together, these results demonstrate rapid and efficient MN separation and high delivery efficiency into skin using the effervescent patch ex vivo.
Fig. 4. Application of effervescent MN patches to porcine skin ex vivo.
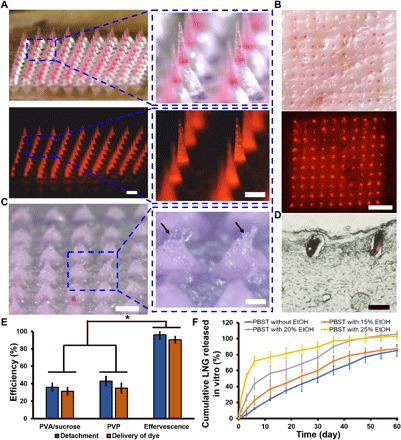
(A) Representative bright-field (above) and fluorescence (below) microscopy images of an effervescent MN patch with MNs containing red fluorescent dye (Nile red). Scale bars, 500 μm. (B) Representative bright-field (above) and fluorescence (below) microscopy images of porcine skin after MN patch insertion and MN detachment in porcine skin ex vivo. Scale bar, 2 mm. (C) Representative bright-field microscopy images of a residual patch after application to porcine skin. Black arrows indicate the dissolved PVP polymer in the backing at the sites of MN separation. Scale bars, 500 μm (left) and 200 μm (right). (D) Representative image of a histological section of porcine skin after MN patch insertion and separation of MNs imaged by bright-field microscopy. Scale bar, 200 μm. (E) Quantification of the efficiency of MN detachment and delivery of Nile red dye from MN patches with effervescent backing, PVA/sucrose backing, and PVP backing. Each point represents mean ± SD (n = 5), *P < 0.05. (F) Cumulative LNG release in vitro from LNG-loaded MN patches in PBST solution containing ethanol at different concentrations at 37°C, shown as a function of time. Each point represents mean ± SD (n = 3).
Sustained release of LNG from effervescent MN patches in vitro
Effervescent MN patches were further tested for the release kinetics of LNG using release media of PBS containing Tween 80 surfactant (PBST) and 0 to 25% ethanol. Ethanol was added to the release media to better simulate in vivo release kinetics (34). LNG release into PBST exhibited essentially no burst release and released LNG at a rate of ~1.4% per day over the course of at least 60 days (Fig. 4F). Inclusion of ethanol in the release medium [to simulate faster release kinetics often seen in vivo (34)] proportionally increased burst release up to 25.7 ± 1.5% after the first day in 25% ethanol, but the presence of ethanol did not significantly affect release rate after that (two-tailed Student’s t test, P = 0.83), such that LNG release persisted in all release media for >1 month, which is the target delivery time frame in this study.
Effervescent MN patch application and LNG pharmacokinetics in vivo
To study effervescent MN patches in vivo, MN patches (containing Nile red dye) were manually pressed against the skin of shaved rats and then left in place for 50 s. As seen ex vivo, the fluorescent MNs were efficiently separated from the patch after application to skin with a detachment efficiency of 91.7 ± 2.4% (Fig. 5A); histological sections showed MNs embedded under the skin surface in rats (Fig. 5B).
Fig. 5. LNG pharmacokinetics from effervescent MN patches in vivo.
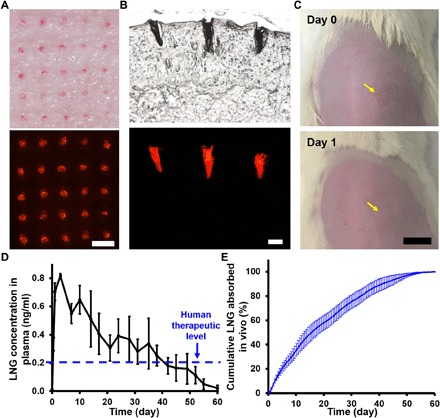
(A) Representative images of rat skin in vivo after MN patch application and removal showing MNs embedded in the skin by bright-field (top) and fluorescence (bottom) microscopy. Scale bar, 500 μm. (B) Representative histological section of rat skin imaged by bright-field (top) and fluorescence (bottom) microscopy, showing MNs embedded in skin after application of effervescent MN patch in vivo. Scale bar, 200 μm. (C) Representative images of rat skin are shown immediately or 1 day after application of an effervescent MN patch in vivo. Yellow arrows indicate the MN patch application site. Scale bar, 1 cm. (D) Rat plasma concentrations of LNG after administration of LNG-loaded effervescent MN patches. The therapeutic LNG level in humans is indicated by the blue dashed line. Each point represents mean ± SD (n = 10). (E) Cumulative LNG absorbed in vivo after administration of LNG-loaded effervescent MN patches as a function of time as determined by pharmacokinetic modeling of the data shown in (D). Each point represents mean ± SD (n = 10). Photo credit: Wei Li, Georgia Tech.
To assess LNG pharmacokinetics from MN patches, rats were each administered an LNG-loaded effervescent MN patch, which was applied to the skin for <1 min. LNG concentrations in rat plasma reached a peak concentration (Cmax) of 0.83 ± 0.03 ng/ml (mean ± SD) at a time (Tmax) of 98 ± 84 hours after MN patch application (Fig. 5D). Thereafter, the LNG plasma concentration gradually decreased, keeping above the human therapeutic threshold level of 0.2 ng/ml (35) for >30 days, and lastly dropped to essentially zero concentration after 60 days (Fig. 5D). The area the under the plasma concentration-time curve (AUC) for LNG delivery from effervescent MN patches was 482 ± 79 ng·hour/ml (table S1).
Pharmacokinetic analysis of the data allowed calculation of the LNG absorption kinetics from the MNs in vivo, which can be compared to the measured LNG release kinetics from MNs in vitro. The estimated absorption curve in vivo shows essentially no burst release and exhibits roughly first-order release kinetics (Fig. 5E). This is in reasonable agreement with the in vitro release profile when ethanol was included in the release medium (Fig. 4F).
Examination of the rats during this study showed that the effervescent MN patches were well tolerated, with no erythema, edema, or other signs of irritation during the 60-day study (Fig. 5C and fig. S2). Histological analysis of the rat skin treated with MN patches with effervescent backing did not show evidence of changes in skin architecture, inflammatory cells, or other signs of tissue damage (fig. S2). Overall, these results demonstrate the following key functions of the effervescent MN patch in vivo: rapid, efficient separation of MNs in skin; sustained release of LNG, maintaining target plasma concentration of LNG for >1 month; and good tolerability of the drug-releasing MNs in the skin.
Painless application and rapid separation of effervescent MN patches in women in the United States
We next determined whether effervescent MN patches could be applied painlessly and then separate rapidly in skin of women of reproductive age. We also studied skin tolerability and acceptability of MN patch administration. To study these effects, placebo MN patches were pressed against the dorsal skin of participants’ hands and then left on the skin for 50 s. These MN patches used the same effervescent patch backing formulation used throughout this study, but the MNs did not contain LNG and were made of water-soluble excipients (i.e., PVA and sucrose) rather than PLGA to avoid the complexity of leaving PLGA MNs in the skin of human participants, which was beyond the scope of this study.
Consistent with findings in pig and rat skin, MNs were able to efficiently penetrate skin and separate from the patch backing (Fig. 6). Microscopic examination of MNs before and after application to skin shows good separation of the MNs from the patch, similar to that seen in vitro and in rats (Fig. 6A). Skin staining shows a 10 × 10 array of micropores created in the skin by penetration of the MNs (Fig. 6C), and quantitative analysis of the remaining patches indicated that 98% of MNs penetrated the skin and 92% of MNs separated from the patch within a wear time of <1 min (Fig. 6D). For comparison, MN patches of similar design, but without effervescent formulation, were left in skin for up to 20 min to enable complete MN separation in prior studies in human participants (36).
Fig. 6. Application of effervescent MN patches to human participants.
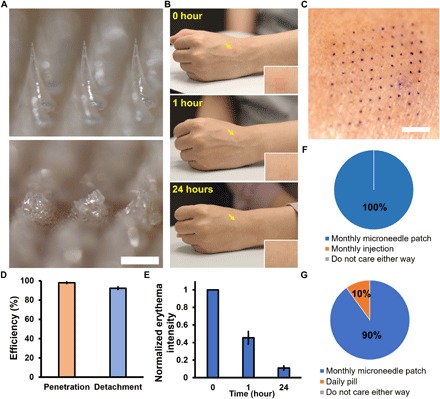
(A) Representative bright-field microscopy images of a section of an effervescent MN patch before (top) and after (bottom) application to human skin. Scale bar, 500 μm. (B) Representative images of the site of effervescent MN patch application (yellow arrows) to the skin of a human participant over time. Inset shows magnified images of the skin application site. These images are all from the same participant. (C) Representative photographic image of skin on a human participant stained to show where a 10 × 10 array of MNs punctured into skin. Scale bar, 2 mm. (D) The efficiency of penetration and detachment of effervescent MN patches in skin of human participants. Each point represents mean ± SD (n = 3). (E) Normalized erythema intensity of human skin over time at the site of effervescent MN patch application. Each point represents mean ± SD (n = 10). (F) Preference of human participants for monthly application of effervescent MN patch compared to monthly hypodermic injection for delivery of contraceptive (n = 10). (G) Preference of human participants for monthly application of effervescent MN patch compared to daily oral administration by pill for delivery of contraceptive (n = 10). Photo credit: Wei Li, Georgia Tech.
Immediately after MN patch application, faint erythema was seen at the site of patch administration, which largely disappeared within 1 hour and almost completely disappeared after 24 hours (Fig. 6, B and E). During MN patch administration, all 10 participants reported either no pain or slight pain that was much less than that caused by a conventional hypodermic injection (fig. S3). No participants reported pain 1 hour or 24 hours after MN patch application. There was no tenderness, swelling, or other notable effect seen at the patch application site at any time (table S2). These findings are consistent with previous observations for MN patches without effervescent formulation (22, 36), indicating that the effervescent MN patches were well tolerated.
Last, in response to a standard set of questions, 100% of participants said they would prefer contraceptive administration by a monthly MN patch compared to monthly hypodermic injection (Fig. 6F), and 90% of participants said they would prefer a monthly MN patch compared to oral administration of daily pills (Fig. 6G). Sixty percent of participants preferred self-administration to physician administration (fig. S4). This indicates that long-acting contraception by an MN patch was well accepted and overwhelmingly preferred over daily pills or a monthly injection in the small population of reproductive-age women studied.
Acceptability of long-acting contraception by MN patch among women in India and Nigeria
To further understand the acceptability of long-acting contraception by an MN patch, we conducted a qualitative study involving 16 focus group discussions and 20 in-depth interviews with women of reproductive age, as well as 20 in-depth interviews with public and private sector providers in India and Nigeria. Participants were provided a brief description of an MN patch, a prototype MN patch, and a magnified image of MNs to inform their opinions. Overall, we found that women and providers were receptive to the idea of a long-acting contraceptive MN patch and perceived it as very easy to use. Relative to other contraceptive methods, perceived advantages of the MN patch included no need to take daily pills, avoidance of invasive placement of implants (Nigeria) and IUDs (India), and discreet use due to short patch wear time.
Women in most interviews liked the idea of self-administration, although many wanted to first receive it from a provider to learn to apply it correctly. Interest in the patch grew with the duration of protection against pregnancy, where 3- or 6-month patches were much preferred, but a 1-month patch was still of interest to some women. Many women and providers were initially concerned about possible pain caused by MNs, but further discussion indicated that a feeling akin to an insect bite or injection would generally be acceptable. There was discussion but ultimately less concern about issues regarding the location of patch application, patch size, and possible erythema, although there was a general preference to hide skin “rashes” to conceal contraceptive use and an interest to better understand possible side effects of the contraceptive. We recently published an expanded analysis of this study that further supports these conclusions (37).
DISCUSSION
MN patch effervescence enabling short patch application time
This study developed an effervescent MN patch that can provide women with better access to contraception. A novel feature of the patch design is that it incorporates an effervescent backing that causes rapid separation of MNs from the patch, enabling separation within 1 min, while providing contraception for >1 month. Upon penetrating the skin and contact with ISF, the effervescent reaction of sodium bicarbonate and citric acid in the patch backing immediately produces CO2 gas bubbles that mechanically weaken the attachment of MNs to the patch and generates water that facilitates dissolution of the MN-patch interface. These effects lead to rapid MN detachment. The detached MNs remain embedded under the skin surface, where they slowly biodegrade and release LNG for long-acting contraception. Effervescence is classically used in oral drug formulation, for example, for taste masking or for improved patient acceptability and/or marketing (38), but we believe that this is the first use of effervescence associated with MNs.
The use of the ISF to initiate effervescence and MN detachment within 1 min provides a trigger that is inherently associated with skin penetration and requires no special intervention by the user. Without effervescence, a water-soluble patch backing formulation can also dissolve in ISF, but such dissolution can be slow, requiring patch application times, for example, of 20 min (22, 39) or 6 hours (39), as shown in recent clinical trials using dissolvable MNs. Coated MNs, which can sometimes dissolve faster but have other disadvantages like generating sharps waste, also required 2 min (40) or 30 min (23) to release drugs (23). A few other studies have developed methods for rapid MN separation, but those designs require complicated experimental setup and operating procedures (e.g., high-voltage supply and micrometer-scale alignment) (41, 42), metal-polymer hybrid systems (17, 43), and/or physical intervention required by the user (e.g., application of shear force) (32). The effervescent MN patch developed here is expected to enable low-cost manufacturing and simple, reliable use.
MN patch acceptability among women of reproductive age and health care providers
In addition to studies in vitro and in rats, this study examined the acceptability of MN patches for long-acting contraception in the United States, India, and Nigeria. When experiencing placebo effervescent MN patches, a small population of women in the United States reported slight or no pain associated with patch application, faint erythema that generally resolved within hours, and a very strong preference for long-acting contraception administered by a monthly MN patch compared to monthly injections or daily pills. Findings from the qualitative study in India and Nigeria also indicated high reported acceptability of the MN patch for contraceptive delivery. Its simple administration and opportunities for self-application were compelling to women, as was the possibility of discreet use. While low levels of pain at the application site may be acceptable, there was concern with the perception that MN patches might hurt (although these study participants never experienced application of MN patches and only saw pictures and prototypes). Acceptability studies like these can guide continued MN patch development, e.g., by helping to optimize trade-offs between patch size, location, and erythema, in support of discreet use or addressing the importance of possible local cosmetic skin effects in ensuring acceptability and uptake of a future product.
Translational potential of long-acting contraception by effervescent MN patch
In this study, the effervescent MN patch delivered LNG, which is a well-established contraceptive hormone, with an extensive record of safety and efficacy. A prior study examined LNG delivery by MN patch, but it was for bolus delivery in the context of emergency contraception (44). The effervescent MN patch was designed for self-administration by women without special training. Previous studies have shown that human participants are able to reliably self-administer MN patches (26), and the fact that the effervescent MN patch application time is so short should increase patient compliance with correct use. Because the MNs separate from the patch backing, the patch does not generate biohazardous sharps waste after use, which should increase safety and reduce disposal costs. The high patch loading with 40% LNG can facilitate minimizing patch size containing the required drug dose. Last, the straightforward fabrication method—sequentially cast two solutions onto a mold, allow them to dry, and place the resulting product in packaging—should lend itself to low-cost, mass manufacturing that can meet health care cost constraints in developing countries.
Study limitations
A limitation of this study is that it was conducted in a relatively small number of animals, which emphasized pharmacokinetic analysis, and in small cohorts of human participants, which addressed the use of effervescent MN patches on human skin and stated preference for contraceptive delivery by MN patches. Future studies should increase the LNG dose to enable contraception in humans for 1-month or possibly 6-month use, further develop MN formulations to achieve an optimal LNG release profile, improve MN patch design based on a more complete understanding of needs and preferences of women in target populations, and translate the work to clinical trials to evaluate safety and efficacy in large populations.
CONCLUSIONS
In this study, we developed an MN patch with a backing that effervesces upon application to skin and contact with the skin’s ISF. This enables the MNs to separate from the patch within 1 min and remain embedded under the skin surface after patch removal. The MNs were made of a biodegradable polymer that released LNG for >1 month in vitro and in vivo in rats, thereby demonstrating potential to serve as a long-acting contraceptive. The effervescent MN patch was well tolerated in the skin of rats and human participants, leaving little visible sign of use. An acceptability study among women of reproductive age in the United States showed overwhelming reported preference for long-acting contraception by MN patch compared with daily pills or monthly injections. Health care providers and women of reproductive age in India and Nigeria also reported interest in MN patches for long-acting contraception, where ease of use, discreetness, and duration of action were identified as important attributes. We conclude that effervescent MN patches can achieve month-long delivery of contraceptive hormone that is simple to administer, minimally invasive, and strongly preferred and can offer women improved access to long-acting contraception.
MATERIALS AND METHODS
Fabrication of effervescent MN patches
Effervescent MN patches were fabricated using polydimethylsiloxane (PDMS) (Dow Corning, Midland, MI) molds. Each MN was conical with a base radius of 150 μm, a height of 600 μm, and a tip radius of ~10 μm. The MNs were arranged in a 10 × 10 array in an area of 7 mm by 7 mm with a center-to-center spacing of 600 μm. To elevate the MNs, the patch backing contained an array of pedestals (base diameter, 600 μm; top diameter, 300 μm; and height, 350 μm) positioned at the base of each MN.
To fabricate MN patches, two solutions were cast sequentially onto the mold. The first casting solution contained 10% (w/w) solids, PLGA/LNG (60/40%, w/w) in diglyme/water (95%/5%, w/w). To prepare the first casting solution, 0.6 g of PLGA (50/50 lactide/glycolide molar ratio; inherent viscosity, 0.59 dl/g; Durect, Birmingham, AL) and 0.4 g of LNG (Chemo Industriale Chimica S.R.L, Saronno, Italy) were dissolved in a mixture of 7 g of diglyme (Sigma-Aldrich, St. Louis, MO), and 2.5 g of tetrahydrofuran (Thermo Fisher Scientific, Waltham, MA), and then LNG was crystalized and precipitated by slow evaporation of the tetrahydrofuran. Last, additional diglyme and deionized (DI) water were added to obtain the final casting solution. To fabricate MN patches containing Nile red (Sigma-Aldrich), 20 mg of Nile red powder was added into the casting solution and mixed to homogeneity.
Seven microliters of casting solution were applied to the top of the MN mold and then centrifuged at 3200g for 20 min to fill the mold. After waiting for 5 min, 20 μl of diglyme/water (95%/5%, w/w) was pipetted on the top of the mold, followed by centrifugation at 3200g for 20 min to wash residual casting solution on the top of the mold into the mold cavities. After that, the mold was placed in a 60°C oven with vacuum for 12 hours for drying.
Next, a second casting solution was prepared, consisting of 13% (w/v) PVP with two molecular weights (360/55 kDa, 50/50%, w/w; Sigma-Aldrich), and 4% (w/v) citric acid (Sigma-Aldrich) in pure ethanol (Koptec, King of Prussia, PA), with 5% (w/v) sodium bicarbonate (Sigma-Aldrich) suspended in the solution. Then, 80 μl of the second casting solution was gently applied to the dried PDMS mold surface to form the effervescent patch backing. For the control groups with noneffervescent backing, the second casting solution contained 13% (w/v) PVA (Sigma-Aldrich) and 13% (w/v) sucrose (Sigma-Aldrich) in DI water or 13% (w/v) PVP (360/55 kDa, 50/50%, w/w) in DI water. After drying in the chemical hood for 1 hour for the effervescent backing or 3 hours for the PVA/sucrose or PVP backing, the mold with effervescent backing or noneffervescent backing was placed in a desiccator overnight or for 2 days, respectively, at room temperature (20° to 25°C) for complete drying, after which a layer of adhesive paper was gently attached onto the top surface of the PDMS mold and used to carefully peel the patch from the mold, which was then stored in a desiccator until use.
To make MN patches for use in human participants, the first cast solution comprised 13% (w/v) PVA (Sigma-Aldrich) and 13% (w/v) sucrose (Sigma-Aldrich) in DI water. The second cast solution was the effervescent patch backing formulation containing PVP, citric acid, and sodium bicarbonate, as described above. The solutions were sterilized by passing through a sterile 0.2-μm filter (PES, VWR International, Radnor, PA). Molds were sterilized by autoclaving. Fabrication was carried out in a biological safety cabinet (Forma Class II, Thermo Fisher Scientific), and sample MN patches were tested to assure adherence to a low-bioburden specification.
Swelling of PDMS mold
Films of the PDMS used to make MN casting molds were cast at a thickness of 2 to 3 mm on a glass dish and cured at 37°C for 2 days and then at 60°C for 4 hours under vacuum. Circular discs with a radius of 1.25 cm were then punched from the silicone film. A minimum of three discs were used for each solvent test. The discs were individually weighed, placed in a vial, and covered with 50 ml of the solvent to be tested. The container was sealed and the discs were allowed to soak for 2 hours. After soaking, each disc was removed from the solvent, lightly blotted onto absorbant paper (Kimwipes, Kimberly Clark, Irving, TX), and then quickly weighed to minimize evaporation of solvent. The percent swelling was determined as the difference between the weight of the disc before and after soaking, and dividing by the initial disc weight.
Effervescent MN patch mechanical properties
Mechanical properties of MN patches with effervescent backing were measured using a displacement force test station (Force Gauge, Mark-10, Copiague, NY). Briefly, a single patch was attached to a rigid stainless steel platform positioned vertically (MNs facing up). Then, the test station sensor probe moved toward the MNs in the vertical direction at a speed of 0.1 mm/s. The distance between the sensor and MN tips was initially 1 cm. Force and displacement measurements began when the sensor first touched the MN tips and continued until the sensor traveled 0.4 mm from the MN tips toward the patch backing.
Detachment test of effervescent MN patches
To study the kinetics of MN detachment from patches with effervescent backing or noneffervescent backing (PVP or PVA/sucrose), a single patch facing up was attached to a holder and then immersed in PBS. A camera (MU300, AmScope, Irvine, CA) was used to image the MN detachment process until all MNs detached from the patch.
Skin insertion of effervescent MN patches ex vivo
Separation, retention, and delivery efficiency of effervescent MN patches were evaluated by inserting MN patches loaded with fluorescent dye (Nile red) into stretched porcine skin ex vivo by pressing with thumb against the skin and then leaving the patches on the skin for 50 s to give time for dissolution of the effervescent backing and separation of MNs. The skin was then examined by optical microscopy (Olympus, Tokyo, Japan) to identify detached MNs embedded in the skin. To remove any detached MNs that were partially protruding above the skin surface, a swab was gently and repeatedly scraped across the site of MN patch treatment for 10 s in some cases. Separation and retention efficiency were calculated by dividing the number of colored spots due to the presence of fluorescent MNs in the skin by the number of MNs in the patch (i.e., 100 MNs).
Delivery efficiency of the MN patch was evaluated by using quantitative fluorescence analysis (Microplate Reader, Bio-Rad, Hercules, CA) to measure fluorescence intensity from the dye in the MN patch before and after skin insertion and fluorescence from the dye on the skin surface. The amounts of dye in the residual backing and on the skin surface were subtracted from that in the MN patch before insertion to determine the amount of dye delivered into the skin. To calculate delivery efficiency, the delivered dye in the skin was divided by the amount of dye in the MN patch before insertion. Last, skin was frozen and then cut into 10-μm sections for histological analysis.
LNG release from effervescent MN patches in vitro
In vitro release of LNG from effervescent MN patches was measured by placing an MN patch into 1 liter of PBST with varying concentrations of ethanol as the release media in a glass vessel incubated in a shaker water bath at 37°C and shaken at 80 rpm. PBST solution contained 137 mM NaCl, 2.68 mM KCl, 10.14 mM Na2HPO4, 1.76 mM KH2PO4, and 0.02% (w/v) Tween 80. Ethanol was added to PBST to final concentrations of 0, 15, 20, or 25% (v/v) ethanol. One milliliter of release medium was collected at predetermined time points (0, 1, 3, 6, 12, 18, 24, 30, 36, 42, 48, 54, and 60 days) and replaced with the same amount of fresh medium.
Sample analysis by ultraperformance liquid chromatography (UPLC)–mass spectrometer (Waters, Milford, MA) was used to quantify LNG concentration. LNG was separated on an Acquity UPLC Ethylene-Bridged Hybrids (BEH) C18 column (100 mm by 2.1 mm; inner diameter, 1.7-μm particle size) at 50°C. A mixture of acetonitrile containing 0.1% formic acid and water containing 0.1% formic acid (8:2 ratio, v/v) comprised the mobile phase. The injection volume was 10 μl, with a flow rate of 0.3 ml/min. LNG detection was performed by electrospray ionization mass spectrometry in the positive ion mode. Quantification was based on the target analyte of LNG [M + H+; m/z (mass/charge ratio) = 313.4].
LNG pharmacokinetics after release from effervescent MN patches in vivo
LNG-loaded effervescent MN patches were applied to adult female Sprague-Dawley rats (~200 g) while under isoflurane anesthesia. The rats’ dorsal skin was shaved before application of one MN patch per rat, taking care not to damage skin during shaving. Animal studies were performed with approval by the Georgia Institute of Technology Institutional Animal Care and Use Committee (IACUC), and IACUC guidelines were followed in all studies with animal subjects.
To investigate detachment of PLGA/LNG MNs in rats, effervescent MN patches containing Nile red were administered to the rats using the methods described above for ex vivo MN patch application to porcine skin. The administration sites on the skin were imaged by fluorescence microscopy (Olympus).
To study pharmacokinetics of LNG release from MNs in skin, a group of 10 rats received LNG-loaded effervescent MN patches. A power analysis indicated that a sample size of 10 rats per group would be sufficient to distinguish pharmacokinetic profiles in animals receiving LNG from those without application of patches with 95% confidence. The primary endpoint of the animal study was LNG plasma concentration above the human therapeutic threshold level (0.2 ng/ml in plasma) for 1 month. The secondary endpoint was irritation at the site of MN patch administration. All data collected in this study were retained; no outliers were excluded.
Blood samples (~500 μl) were drawn from the tail vein 0, 12, and 24 hours and 3, 7, 10, 14, 17, 21, 24, 28, 31, 35, 38, 42, 45, 49, 52, 55, and 60 days after MN patch application. Plasma was separated by centrifuging blood samples at 2000g for 15 min at 4°C, and LNG concentration in the plasma was analyzed by enzyme-linked immunosorbent assay (Thermo Fisher Scientific) following the manufacturer’s instruction. Biocompatibility of LNG delivery from effervescent MN patches was evaluated by euthanizing rats by CO2 asphyxiation at the end of the study (i.e., 60 days after MN patch application) and excising tissue surrounding the patch application site. This tissue was fixed in 10% neutral-buffered formalin for 2 days at 4°C and then embedded in paraffin after complete dehydration, cut into sections of 5-μm thickness, and stained using hematoxylin and eosin for histological analysis.
Pharmacokinetic analysis
Pharmacokinetic parameters were calculated using noncompartmental pharmacokinetic analysis (45) with Phoenix WinNonlin v. 8 (Certara, Princeton, NJ). The following parameters were calculated: Cmax, the observed maximum plasma concentration; Tmax, the time when Cmax was achieved; Ke, the terminal rate constant of LNG, estimated by log-linear regression of the terminal phase of the concentration-time profile after patch administration; AUC0–t, the area under the plasma concentration-time curve from time zero to the time of last quantifiable concentration (Clast) using the linear trapezoidal rule; and AUC0–∞, the area under the curve from time zero to infinity, calculated as AUC0–t + Clast/Ke. The percentage of LNG absorbed in vivo as a function of time was calculated by numerical deconvolution of the patch LNG plasma concentration versus time profiles within Phoenix WinNonlin. Reference intravenous pharmacokinetic data for the calculation of absolute bioavailability (F) and percentage of LNG absorbed as a function of time were obtained from the literature (46).
Application of MN patches with effervescent backing to human participants
To be eligible to participate in this study, participants had to be healthy nonpregnant female adults with normal skin, no known problems with pain perception, and no known allergies to the materials used in this study. Ten participants (21 to 36 years old) were recruited from students, faculty, and staff at the Georgia Institute of Technology. This study was approved by the Georgia Institute of Technology Institutional Review Board (IRB), informed consent was obtained from all participants, and IRB guidelines were followed in all studies with human participants.
Participants received MN patches on the dorsal surface of their hands. Each participant received one patch on their left hand, and three participants received an additional patch on their right hand. The patches were pressed to the skin for 3 s and then left in place on the skin for 50 s. Skin of the left hands was imaged by a camera (EOS 60D, Canon, Japan) using the same setup (e.g., same light condition, same exposure time) 0, 1, and 24 hours after patch application. For those who received two MN patches, the application site on their right hands was stained with Gentian violet solution (Humco, Linden, TX) and then imaged 5 min after staining. All participants were asked a standard set of questions to solicit information about possible pain of the MN patch administration and the acceptability of MN patches for delivery of contraceptives. Pain was scored from 0 (no pain) to 10 (pain of a hypodermic injection).
MN patch acceptability among women in India and Nigeria
A qualitative study in New Delhi, India and Ibadan, Nigeria gathered initial data on the hypothetical acceptability of a contraceptive MN patch. A total of 16 focus group discussions and 20 in-depth interviews were conducted in 2017–2018 with women of reproductive age across the two countries, as well as 20 in-depth interviews with public and private sector health care providers. Women who had previously used modern contraception and women who had not were interviewed; in India, all women were married, whereas in Nigeria, the sample included married and unmarried women. Participants were provided with a short description of the patch and shown a patch prototype (that did not contain MNs or LNG) and a close-up picture of the MN seen under a microscope. The target product profile was not fully specified; rather, interviews examined initial acceptability of the patch and attitudes toward several patch attributes, including pain at application, location of application, patch size, wear time, duration of protection, and skin reaction (erythema) at application, and several possible levels for each of these attributes. All interviews were audio-recorded, transcribed and translated into English, and analyzed using coding and analytic memos. This study was approved by the Sigma IRB in India, the Oyo State Research Ethical Review Committee in Nigeria, and FHI 360’s Protection of Human Subjects Committee in the United States, and informed consent was obtained from all participants.
Statistical analysis
All results presented in this study are means ± SD. Statistical analysis was perform using two-sided Student’s t test or an analysis of variance (ANOVA) test with Origin software (OriginLab, Northampton, MA). Probability values of less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank D. Owen, G. S. Kopf, and J. Ayres of FHI 360 for valuable technical discussions and review of the manuscript and D. Bondy and A. Troxler for administrative support. Funding: This publication is made possible by the support of the American people through the U.S. Agency for International Development (USAID) and was prepared under a subcontract funded by Family Health International under Cooperative Agreement no. AID-OAA-15-00045 funded by USAID. The qualitative acceptability study in India and Nigeria was also cofunded by an Interagency Agreement with the National Institute of Child Health and Human Development (NICHD). The content of this publication does not necessarily reflect the views, analysis, or policies of FHI 360, USAID, NICHD, or the U.S. Government, nor does any mention of trade names, commercial products, or organizations imply endorsement by FHI 360, USAID, NICHD, or the U.S. Government. Author contributions: W.L., J.T., S.P.S., and M.R.P. designed the project, and A.B. and R.L.C. designed the human acceptability study in India and Nigeria. W.L. and M.R.P. wrote the manuscript. J.T., R.N.T., A.B., and S.P.S. also contributed to the writing of the manuscript. R.N.T. developed the polymer-LNG formulation of the first casting solution, and W.L. developed the effervescent formulation of the second casting solution. W.L., J.T., S.L., and R.K.N. performed the experiments, and A.B. and R.L.C. performed the human acceptability study in India and Nigeria. C.A.R. performed pharmacokinetic analysis. All authors analyzed and interpreted the data. Competing interests: W.L., R.N.T., and M.R.P. are inventors on a patent related to this work filed by Georgia Tech Research Corporation. The patent application was filed with the World Intellectual Property Organization (WIPO) (no. WO2019075275A1, 11 October 2018). M.R.P. is also a paid advisor to companies developing MN-based products and is a founder/shareholder of companies developing MN-based products (Micron Biomedical). This potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University. The other authors declare no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/11/eaaw8145/DC1
Fig. S1. Representative images of the slow dissolution of an MN patch with PVA/sucrose backing (i.e., noneffervescent) in PBS over time.
Fig. S2. Local effects of an effervescent MN patch for LNG delivery on rat skin in vivo.
Fig. S3. Assessment of reported pain during MN patch administration scored by participants on a visual analog scale of 0 (no pain) to 10 (pain of a hypodermic injection).
Fig. S4. Percentage of participants showing preference for self-administration or doctor administration for future application of an effervescent MN patch for long-acting contraception.
Table S1. Pharmacokinetic parameters of LNG following administration of effervescent MN patch containing LNG.
Table S2. Summary of the prevalence of skin reactions at different time points after the application of effervescent MN patches to human participants.
Movie S1. Separation of MNs from effervescence backing in PBS solution.
Movie S2. Separation of MNs from noneffervescence backing in PBS solution.
REFERENCES AND NOTES
- 1.Petruney T., Wilson L. C., Stanback J., Cates W., Family planning and the post-2015 development agenda. Bull. World Health Organ. 92, 548–548 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.www.census.gov/popclock/.
- 3.Sedgh G., Singh S., Hussain R., Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud. Fam. Plann. 45, 301–314 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finer L. B., Zolna M. R., Declines in unintended pregnancy in the United States, 2008-2011. N. Engl. J. Med. 374, 843–852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.A. Sonfield, K. Kost, Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: National and state estimates for 2010 (The Guttmacher Institute, 2015)(www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf).
- 6.P. K. Park, in Park K. Park’s Textbook of Preventive and Social Medicine (Banarsidas Bhanot Publishers, ed. 24, 2017), pp. 525–552. [Google Scholar]
- 7.Rose E., DiClemente R. J., Wingood G. M., Sales J. M., Latham T. P., Crosby R. A., Zenilman J., Melendez J., Hardin J., The validity of teens and young adults self-reported condom use. Arch. Pediatr. Adolesc. Med. 163, 61–64 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Macaluso M., Kelaghan J., Artz L., Austin H., Fleenor M., Hook E. W., Valappil T., Mechanical failure of the latex condom in a cohort of women at high STD risk. Sex. Transm. Dis. 26, 450–458 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Molloy G. J., Graham H., McGuinness H., Adherence to the oral contraceptive pill: A cross-sectional survey of modifiable behavioural determinants. BMC Public Health 12, 838 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y., Wang J. C., Zhang X., Zhang Z. J., Zheng Y., Chen D. W., Zhang Q., Synchronic release of two hormonal contraceptives for about one month from the PLGA microspheres: In vitro and in vivo studies. J. Control. Release 129, 192–199 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Kaunitz A. M., Long-acting injectable contraception with depot medroxyprogesterone acetate. Am. J. Obstet. Gynecol. 170, 1543–1549 (1994). [PubMed] [Google Scholar]
- 12.Espey E., Ogburn T., Long-acting reversible contraceptives: Intrauterine devices and the contraceptive implant. Obstet. Gynecol. 117, 705–719 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Hoggart L., Newton V. L., Young women’s experiences of side-effects from contraceptive implants: A challenge to bodily control. Reprod. Health Matters 21, 196–204 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Hapgood J. P., Kaushic C., Hel Z., Hormonal contraception and HIV-1 acquisition: Biological mechanisms. Endocr. Rev. 39, 36–78 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makadia H. K., Siegel S. J., Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3, 1377–1397 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M., Hu L. Z., Xu C. J., Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab Chip 17, 1373–1387 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Larraneta E., Lutton R. E. M., Woolfson A. D., Donnelly R. F., Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mat. Sci. Eng. R. 104, 1–32 (2016). [Google Scholar]
- 18.Prausnitz M. R., Engineering Microneedle patches for vaccination and drug delivery to skin. Annu. Rev. Chem. Biomol. 8, 177–200 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Ye Y. Q., Yu J. C., Wen D., Kahkoska A. R., Gu Z., Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 127, 106–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin X., Zhu D. D., Chen B. Z., Ashfaq M., Guo X. D., Insulin delivery systems combined with microneedle technology. Adv. Drug Deliv. Rev. 127, 119–137 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Cheung K., Das D. B., Microneedles for drug delivery: Trends and progress. Drug Deliv. 23, 2338–2354 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Rouphael N. G., Paine M., Mosley R., Henry S., McAllister D. V., Kalluri H., Pewin W., Frew P. M., Yu T. W., Thornburg N. J., Kabbani S., Lai L. L., Vassilieva E. V., Skountzou I., Compans R. W., Mulligan M. J., Prausnitz M. R., Grp T. M. S., The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 390, 649–658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daddona P. E., Matriano J. A., Mandema J., Maa Y. F., Parathyroid hormone (1-34)-coated microneedle patch system: Clinical pharmacokinetics and pharmacodynamics for treatment of osteoporosis. Pharm. Res. 28, 159–165 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Yu J. C., Zhang Y. Q., Ye Y. Q., DiSanto R., Sun W. J., Ranson D., Ligler F. S., Buse J. B., Gu Z., Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. U.S.A. 112, 8260–8265 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J. W., Park J. H., Prausnitz M. R., Dissolving microneedles for transdermal drug delivery. Biomaterials 29, 2113–2124 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ripolin A., Quinn J., Larrañeta E., Vicente-Perez E. M., Barry J., Donnelly R. F., Successful application of large microneedle patches by human volunteers. Int. J. Pharm. 521, 92–101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen T. T., Park J. H., Human studies with microneedles for evaluation of their efficacy and safety. Expert Opin. Drug Deliv. 15, 235–245 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y. Q., Liu Q. M., Yu J. C., Yu S. J., Wang J. Q., Qiang L., Gu Z., Locally induced adipose tissue browning by microneedle patch for obesity treatment. ACS Nano 11, 9223–9230 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeMuth P. C., Garcia-Beltran W. F., Ai-Ling M. L., Hammond P. T., Irvine D. J., Composite dissolving microneedles for coordinated control of antigen and adjuvant delivery kinetics in transcutaneous vaccination. Adv. Funct. Mater. 23, 161–172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J. H., Allen M. G., Prausnitz M. R., Polymer microneedles for controlled-release drug delivery. Pharm. Res. 23, 1008–1019 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Chen M. C., Lin Z. W., Ling M. H., Near-infrared light-activatable microneedle system for treating superficial tumors by combination of chemotherapy and photothermal therapy. ACS Nano 10, 93–101 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Li W., Terry R. N., Tang J., Feng M. R., Schwendeman S. P., Prausnitz M. R., Rapidly separable microneedle patch for the sustained release of a contraceptive. Nat. Biomed. Eng. 3, 220–229 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Davis S. P., Landis B. J., Adams Z. H., Allen M. G., Prausnitz M. R., Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 37, 1155–1163 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Wang S. H., Zhang L. C., Lin F., Sa X. Y., Zuo J. B., Shao Q. X., Chen G. S., Zeng S., Controlled release of levonorgestrel from biodegradable poly(D,L-lactide-co-glycolide) microspheres: In vitro and in vivo studies. Int. J. Pharm. 301, 217–225 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Fotherby K., Levonorgestrel. Clinical pharmacokinetics. Clin. Pharmacokinet. 28, 203–215 (1995). [DOI] [PubMed] [Google Scholar]
- 36.Arya J., Henry S., Kalluri H., McAllister D. V., Pewin W. P., Prausnitz M. R., Tolerability, usability and acceptability of dissolving microneedle patch administration in human subjects. Biomaterials 128, 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunie A., Callahan R. L., Godwin C. L., Bajpai J., OlaOlorun F. M., User preferences for a contraceptive microarray patch in India and Nigeria: Qualitative research on what women want. PLOS ONE 14, e0216797 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu F., Ranmal S., Batchelor H. K., Orlu-Gul M., Ernest T. B., Thomas I. W., Flanagan T., Tuleu C., Patient-centred pharmaceutical design to improve acceptability of medicines: Similarities and differences in paediatric and geriatric populations. Drugs 74, 1871–1889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirobe S., Azukizawa H., Hanafusa T., Matsuo K., Quan Y. S., Kamiyama F., Katayama I., Okada N., Nakagawa S., Clinical study and stability assessment of a novel transcutaneous influenza vaccination using a dissolving microneedle patch. Biomaterials 57, 50–58 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Fernando G. J. P., Hickling J., Flores C. M. J., Griffin P., Anderson C. D., Skinner S. R., Davies C., Witham K., Pryor M., Bodle J., Rockman S., Frazer I. H., Forster A. H., Safety, tolerability, acceptability and immunogenicity of an influenza vaccine delivered to human skin by a novel high-density microprojection array patch (Nanopatch (TM)). Vaccine 36, 3779–3788 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Chen M. C., Huang S. F., Lai K. Y., Ling M. H., Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials 34, 3077–3086 (2013). [DOI] [PubMed] [Google Scholar]
- 42.Zhu D. D., Wang Q. L., Liu X. B., Guo X. D., Rapidly separating microneedles for transdermal drug delivery. Acta Biomater. 41, 312–319 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Chu L. Y., Prausnitz M. R., Separable arrowhead microneedles. J. Control. Release 149, 242–249 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao G. T., Quan G. L., Lin S. Q., Peng T. T., Wang Q. Q., Ran H., Chen H. P., Zhang Q., Wang L. L., Pan X., Wu C. B., Novel dissolving microneedles for enhanced transdermal delivery of levonorgestrel: In vitro and in vivo characterization. Int. J. Pharm. 534, 378–386 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Gabrielsson J., Weiner D., Non-compartmental analysis. Methods Mol. Biol. 929, 377–389 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Naqvi R. H., Mitra S. B., Lindberg M. C., Effect of dose on the pharmacokinetics of levonorgestrel in the rat during the rapid elimination phase following subcutaneous administration. Contraception 30, 599–605 (1984). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/11/eaaw8145/DC1
Fig. S1. Representative images of the slow dissolution of an MN patch with PVA/sucrose backing (i.e., noneffervescent) in PBS over time.
Fig. S2. Local effects of an effervescent MN patch for LNG delivery on rat skin in vivo.
Fig. S3. Assessment of reported pain during MN patch administration scored by participants on a visual analog scale of 0 (no pain) to 10 (pain of a hypodermic injection).
Fig. S4. Percentage of participants showing preference for self-administration or doctor administration for future application of an effervescent MN patch for long-acting contraception.
Table S1. Pharmacokinetic parameters of LNG following administration of effervescent MN patch containing LNG.
Table S2. Summary of the prevalence of skin reactions at different time points after the application of effervescent MN patches to human participants.
Movie S1. Separation of MNs from effervescence backing in PBS solution.
Movie S2. Separation of MNs from noneffervescence backing in PBS solution.


