Abstract
The apelinergic pathway has been generating increasing interest in the past few years for its potential as a therapeutic target in several conditions associated with the cardiovascular and metabolic systems. Indeed, preclinical and, more recently, clinical evidence both point to this G protein–coupled receptor as a target of interest in the treatment of not only cardiovascular disorders such as heart failure, pulmonary arterial hypertension, atherosclerosis, or septic shock, but also of additional conditions such as water retention/hyponatremic disorders, type 2 diabetes, and preeclampsia. While it is a peculiar system with its two classes of endogenous ligand, the apelins and Elabela, its intricacies are a matter of continuing investigation to finely pinpoint its potential and how it enables crosstalk between the vasculature and organ systems of interest. In this perspective article, we first review current knowledge on the role of the apelinergic pathway in the above systems, as well as the associated therapeutic indications and existing pharmacological tools. We also offer a perspective on the challenges and potential ahead to advance the apelinergic system as a target for therapeutic intervention in several key areas.
Keywords: apelin, Apela/Toddler/Elabela, apelin receptor/APLNR/APJ, cardiovascular protection, heart failure, pulmonary arterial hypertension, water retention/hyponatremic disorders, hypertension, septic shock, type 2 diabetes, preeclampsia
Graphical abstract
This perspective article reviews current knowledge on the role of the apelinergic pathway in the cardiovascular and metabolic systems and discusses recent evidence pointing to this G protein–coupled receptor as a target of interest in the treatment of not only cardiovascular disorders, but also of conditions such as water retention/hyponatremic disorders, type 2 diabetes, and preeclampsia.
Introduction
Cardiovascular and metabolic diseases are prevalent in most societies and continue to rise, despite knowledge of important and preventable risk factors such as unhealthy diet, insufficient physical activity, and smoking.1 These risk factors and their determinants are major contributors to vascular endothelial and multi-organ dysfunctions. The development of novel and more effective therapies is thus urgently needed to curb the incidence, morbidity, and mortality associated with cardiovascular and metabolic diseases.
Among systems contributing to the maintenance of vascular homeostasis, the apelinergic system stands out for its pleiotropic beneficial impacts on cardiovascular and metabolic insults. In this perspective article, we first review current knowledge of the apelinergic system as it pertains broadly to cardiovascular, body fluid, and metabolic homeostasis, then synthesize recent developments and identify questions to address in order to advance this target toward therapeutic applications. This manuscript is a joint effort resulting from the RegPep2018 symposium on the apelinergic system organized by the International Regulatory Peptide Society in October 2018.
Discovery of the apelinergic system
In 1993, O’Dowd et al. cloned from a human genomic library the cDNA of an orphan receptor that was then named APJ (putative receptor protein related to the type 1 angiotensin receptor).2 Subsequently, the APJ receptor (also known as the apelin receptor (APLNR)) was isolated in amphibians3 and rodents.4, 5 The human receptor is 380 amino acids (aa) long and belongs to the seven transmembrane domain G protein–coupled receptors (GPCRs) superfamily. It shares 31% aa sequence identity with the human angiotensin type 1 (AT1) receptor,2 yet does not bind radiolabeled angiotensin II (Ang II). Moreover, treatment of the rat apelin receptor with Ang II does not affect cyclic adenosine monophosphate (cAMP) production, confirming that it is not an angiotensin receptor subtype.4
As an example of reverse pharmacology, the apelin receptor was deorphanized in 1998 when apelin (APJ Endogenous LIgaNd) was isolated from bovine stomach tissue extracts as apelin-36, its longest endogenous ligand with sub-nanomolar affinity, by Tatemoto et al.6 Apelin is generated from a 77-aa precursor, preproapelin. Alignment of preproapelin sequences from cattle, humans, rats, and mice shows strict conservation of the C-terminal portion (aa 61–77), known as apelin-17 (also known as K17F).6 In vivo, apelin circulates as several isoforms differing in length (corresponding to the 36-, 17-, or 13-aa of the C-terminal part of preproapelin), commonly called apelin-36, apelin-17, and apelin-13 (Fig.1).7–9 Apelin-13 is naturally pyroglutamylated at its N-terminus (Fig.1). Apelin-36 predominates in rat lung, testis, uterus, and bovine colostrum, while it is detected together with Pyr1-apelin-13 in the rat mammary gland.8 The most abundant forms of apelin in rat brain as well as rat and human plasma are apelin-17 and Pyr1-apelin-13, whereas the concentration of apelin-36 is much lower.7, 10 In the heart, the most abundant form is Pyr1-apelin-13.11 Due to the presence of pairs of basic residues in preproapelin, prohormone convertases are presumed to be responsible for the processing of its precursor to generate apelin-17 and apelin-13 (Fig.1). The ability of the proprotein convertase subtilisin/kexin 3 (also known as furin) to cleave proapelin directly into apelin-13 without generating longer isoforms was shown in vitro.12
Figure 1.
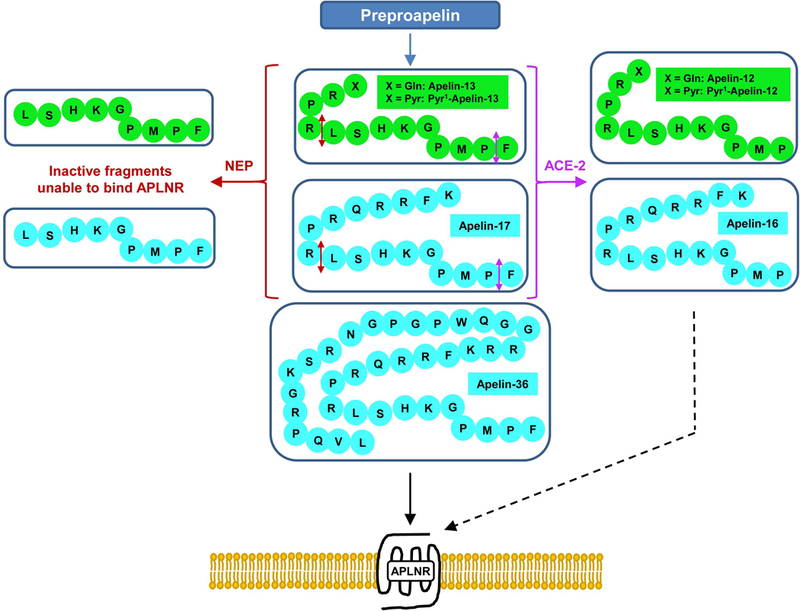
The different forms of apelin. Proteolysis of preproapelin at putative cleavage sites leads to three fragments of 36, 17, and 13 amino acids named apelin-36, apelin-17, and apelin-13, respectively. Pyroglutamylated apelin-13 is formed by spontaneous cyclization of apelin-13. The cleavage sites for neprilysin and angiotensin-converting enzyme 2 on apelin-13 and apelin-17 are indicated by red and purple double-headed arrows, respectively. The straight and dashed black arrows represent full and biased agonism of the different apelin forms, respectively.
A recent peculiar discovery came from the cloning by two independent groups of Apela (also known as Elabela or Toddler), a distinct gene encoding a second endogenous ligand of the apelin receptor with sub-nanomolar affinity.13, 14 Mature Elabela is a 32-aa peptide relatively well conserved in vertebrates and secreted following the removal of its N-terminal 22 aa-long signal peptide. Although putative furin cleavage sites were predicted to generate shorter Elabela peptides,13, 15 their in vivo detection still needs to be demonstrated. While Elabela is broadly expressed during development, its mRNA transcript remains highly expressed during adulthood in the prostate and kidney.15 Yet other sources of Elabela secretion, such as the endothelial cells (ECs) of adult human arterial vessels,16 may also significantly contribute to its presence in the circulation.
Proteolytic inactivation of apelins
Angiotensin-converting enzyme 2 (ACE2, EC 3.4.17.23), the first protease implicated in the physiological regulation of apelins, removes the C-terminal phenylalanine residue of apelin-36, apelin-17, apelin-13, and Pyr1-apelin-13 (Fig.1).17, 18 Cleavage of apelin-17 by ACE2 generates apelin-16, which retains sub-nanomolar affinity for the apelin receptor and similar efficacy of Gαi/o protein coupling. However, apelin-16 exhibits an opposite profile of ligand-induced receptor internalization and blood pressure regulation,19, 20 making apelin-16 a bona fide biased agonist of the receptor. Interestingly, the apelin–ACE2 axis forms a feedback loop as demonstrated by the necessity of apelin-13 to maintain and upregulate ACE2 expression in vivo (Fig.2).21 Using a peptide-based fluorescent probe, the membrane-bound, zinc-dependent metalloprotease neprilysin (NEP, EC 3.4.24.11) was recently identified as another enzyme that cleaves apelin isoforms (Fig.1).22 In vitro, proteolysis by NEP generated fragments lacking affinity for the apelin receptor, making NEP the first protease to fully inactivate apelin (Fig. 2).23 Synthetic analogs of apelin modified within the NEP degradation site (“RPRL” motif, Fig.1) show improved in vitro proteolytic stability while maintaining receptor affinities. Many such analogs proved physiologically inactive even with relatively conservative modifications, highlighting the importance of this region for full agonist activity. The recognition that NEP is a major enzyme that completely inactivates apelin peptides provides a novel mode of action of NEP inhibitors as potential therapeutic agents in cardiovascular diseases.22, 24, 25 More recently, human plasma kallikrein (KLKB1) has been identified as a protease that cleaves the first three N-terminal amino acids (KFR) of apelin-17.26
Figure 2.
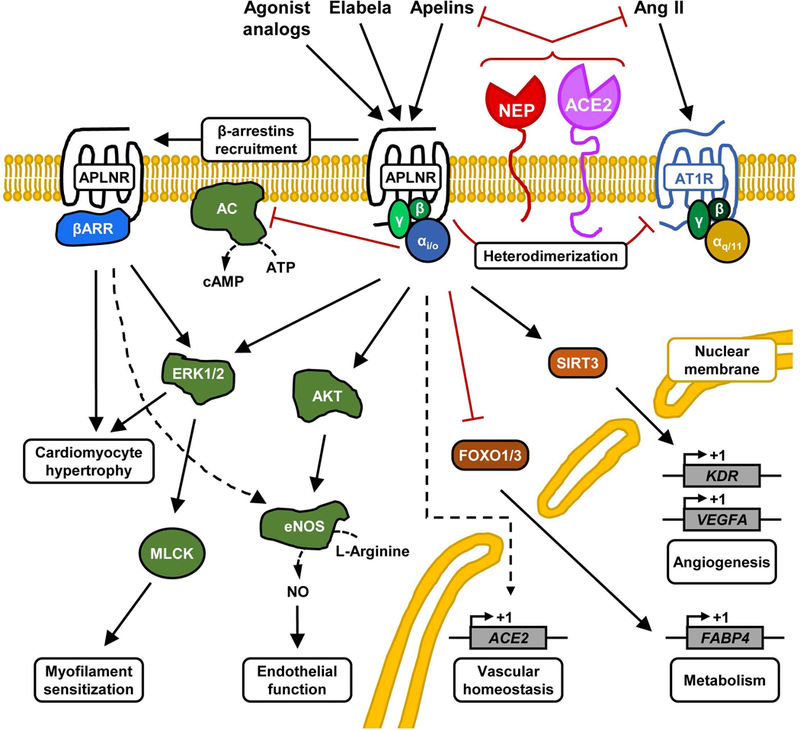
Schematic of the different apelinergic signaling pathways. The activation of apelin receptor by its endogenous ligands, apelin or Elabela, triggers various signaling pathways that exert protective cardiovascular and metabolic effects in the organism. Some of the transcriptional gene regulation effects following the activation of apelinergic pathway are depicted in the nucleus. Abbreviations: AC, adenylate cyclase; ACE2, angiotensin-converting enzyme 2; Ang II, angiotensin II; APLNR, apelin receptor; AT1R, angiotensin type 1 receptor; βARR, β-arrestins; eNOS, endothelial nitric oxide synthase; ERK1/2, extracellular-regulated kinases 1/2; FABP4, fatty acid binding protein 4; FOXO1/3, forkhead box protein O1/3; KDR, kinase insert domain receptor (also known as VEGFR2); MLCK, myosin light chain kinase; NEP, neprilysin; SIRT3, sirtuin 3; VEGFA, vascular endothelial growth factor A.
Signaling
Evidence for the coupling of apelin receptor with Gαi/o protein was revealed by the team of Fujino and other laboratories (Fig.2).4, 6, 8, 9, 27 Stable overexpression in CHO cells of the rat apelin receptor fused at its C-terminal portion with enhanced green fluorescent protein showed the sub-nanomolar efficacy of apelin isoforms (i.e., apelin-17 and Pyr1-apelin-13) to inhibit forskolin-induced cAMP production.4, 28 Pyr1-apelin-13, apelin-13, and apelin-17 stimulation of ERK1/2 MAPK phosphorylation depends on a pertussis toxin–sensitive G protein in CHO cells overexpressing the receptor (Fig.2).19, 28, 29 While the apelin receptor preferentially couples with protein Gαi1 and Gαi2, but not Gαi3,30 the identity of the subtypes of the Gβγ protein subunits released upon activation remains largely unknown. Additional coupling to the Gαq protein upon ligand binding was implied in studies of cultured rat basophil–derived cells, adipocytes, and neuronal cells.31–33 However, the lack of Ca2+ mobilization and arachidonic acid release from CHO cells stably overexpressing the apelin receptor,27 and the decreased amplitude of Ca2+ release upon apelin receptor–dependent signaling in healthy adult cardiomyocytes,34 collectively suggest that significant Gαq protein signaling following apelin receptor activation, if any, may depend on cellular context.
As expected for a GPCR, agonist-induced apelin receptor activation also elicits recruitment of β-arrestins (Fig. 2) and subsequent internalization through a clathrin-dependent mechanism, while removal of apelin’s C-terminal phenylalanine abrogates this process.20, 35 Accordingly, apelin analogs biased toward Gαi/o protein signaling and inefficiently recruiting β-arrestins lose the ability to induce vasorelaxation and reduce blood pressure.19, 20, 36 This functional dissociation between Gαi/o proteins and β-arrestin signaling has been shown to lead to the phosphorylation of MAPK ERK1/2 by two different pathways, one dependent on Gαi/o protein coupling and the other dependent on β-arrestins (Fig. 2).19, 37 Interestingly, apelin-13 and apelin-36 determine different fates of internalized apelin receptor by modulating its interaction with β-arrestin1.38 Based on accumulating evidence about β-arrestin–dependent signaling of internalized GPCRs,39 it is likely that the endocytosed apelin receptor exerts important biological functions of the apelinergic system that have yet to be uncovered.
Distribution
Analysis of the apelin receptor transcript expression indicates its presence in rat and human brain (e.g., hypothalamus and medulla),2, 5, 4, 40 anterior pituitary,4, 5 and highly vascularized organs of the body (e.g., lungs, heart, spleen, and kidney).8, 33, 40, 41 Important interspecies differences for the expression profile of transcripts of apelin and its receptor in tissues were noted.33 However, expression of the receptor, as revealed by immunohistochemistry and binding assays performed on ECs of both murine42 and human vessels,43, 44 is in accordance with its potent cardiovascular and metabolic effects. Similarly, the endothelial production of apelin was demonstrated by in situ hybridization,45 immunohistochemistry,46 and reporter gene expression assays,47 while the mRNA encoding Elabela was found to be highly expressed during adulthood mainly in the kidney.15 Based on the distribution of the receptor and its endogenous ligands, apelin and Elabela, the therapeutic potential of the apelinergic system has been predominantly explored based on its physiological roles in cardiovascular, respiratory, and metabolic functions (Fig. 3), as well as body fluid homeostasis (Fig. 4). As a result, the apelinergic system has emerged as a potential target for diseases associated with the vascular system, heart failure (HF), pulmonary arterial hypertension (PAH), water retention/hyponatremic disorders, and type 2 diabetes, but also more recently in conditions bearing a strong cardiac and/or vascular component, such as septic shock and preeclampsia. These are further elaborated below.
Figure 3.
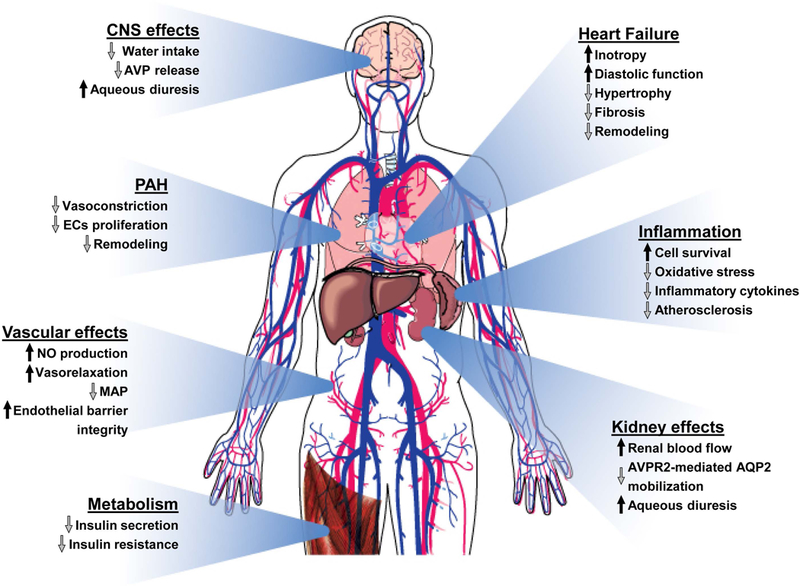
The apelinergic system counteracts cardiovascular and metabolic failures. The activation of the apelinergic pathway results in multiple physiological effects that can alleviate an array of cardiovascular and metabolic disorders. Abbreviations: AVP, vasopressin; ECs, endothelial cells; MAP, mean arterial pressure; NO, nitric oxide; PAH, pulmonary arterial hypertension.
Figure 4.
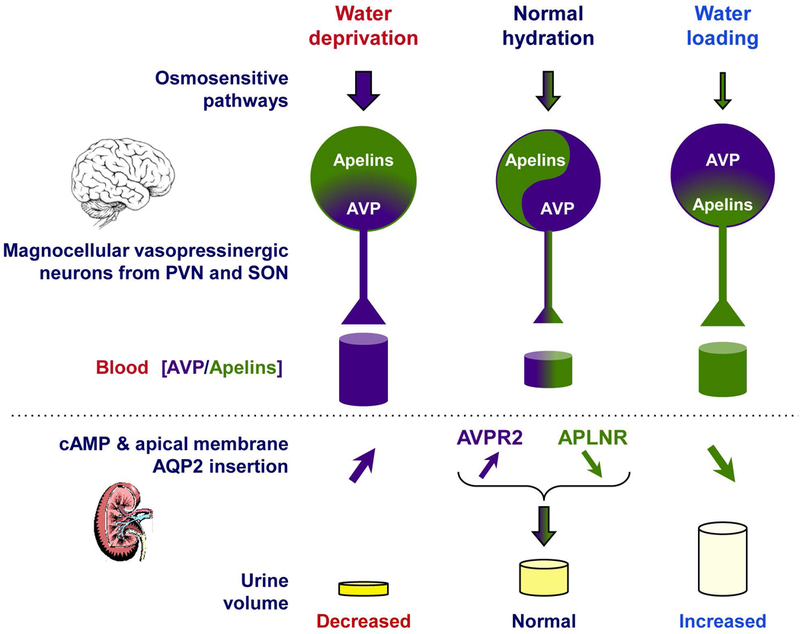
Vasopressin and apelins—the yin and yang of water balance. The interrelated actions of apelins and vasopressin (AVP) are required to ensure and finely tune water balance in the entire organism. The hydration state determines the overall release of apelins and AVP from the hypothalamic magnocellular neurons into the bloodstream. Their opposing receptor-mediated functions in the brain and kidney results in the regulation of urine osmolality and volume.
Vascular physiological effects of apelinergic signaling
Numerous studies have provided insight into the vascular effects of apelinergic signaling.48, 49 For example, apelin-13 produced relaxation in normal human splanchnic arteries via NO release after activation of apelin receptors located in the endothelium (Fig.2).50 While administration of apelin-36 and Pyr1-apelin-13 to humans caused NO-dependent arterial vasodilation,51 apelin-17 provoked NO-dependent vasorelaxation of rat renal glomerular arterioles or rat aorta that were precontracted with Ang II or norepinephrine, respectively.41, 52 Establishing the expression profile of apelin and its receptor relied on several immunohistochemistry, in situ hybridization, and reporter gene expression studies investigating the exact cell types that express the ligand and/or its receptor (see above). The recent identification of Elabela as another endogenous ligand of the apelin receptor added another dimension of complexity to this signaling paradigm.13, 14 In the developing vasculature of the mouse retina, apelin expression is highly restricted to tip cells that demarcate the leading edge of the developing vasculature, while apelin receptor expression is restricted to the endothelium of the stalk cells that form developing luminal structures.53 Evaluation of ligand and receptor expression in adult mice demonstrated the predominantly endothelial expression of the ligand/receptor combination.54, 55 An important caveat to these findings is that the expression profiling discussed above are limited to evaluation in normal homeostatic or developmental context. While other studies have described the expression of apelin or its receptor in disease models, the use of newly developed and highly specific knock-out validated antibodies56, 57 and reporter gene expression models is expected to provide more rigorous, reproducible, and reliable findings in future studies.
Role of apelin and its receptor in vascular disease models
Multiple studies have focused on apelinergic signaling in vascular disease models.49 Key vascular pathologies found to be impacted by the apelinergic system include atherosclerosis,58 arterial hypertension,45 and PAH (Fig. 3).16, 59–61 In the context of atherosclerosis, loss of apelin resulted in exacerbation of atherosclerosis in Apoe−/− mice subjected to a high fat diet, and supplemental apelin administration led to significant improvement in atherosclerosis, which was due, at least in part, to its activation of the nitric oxide pathway and inhibition of Ang II signaling.58 These findings contrast with the pro-atherosclerotic effect of targeting the apelin receptor in Apoe−/− mice fed with high fat diet,62 but the experimental design of these two studies differ in several aspects. Indeed, the distinct mouse genetic backgrounds, the moderate vs. high cholesterol–containing diets, and the potential activation of compensatory loci alleviating the profound disruptive effect of targeting the apelin receptor gene on embryonic vascular development might explain these confounding results, as previously discussed.58 Considering the increased expression of apelin in human atherosclerotic plaques63 and stenotic aortic valves,64 the anti- or pro-atherosclerotic roles of the apelinergic system in this context still have to be clarified.
In diabetic (Leprdb/db) mice, apelin-36 restores the altered aortic vascular responsiveness to acetylcholine and Ang II by potentiating phosphorylation of Akt and eNOS (Fig. 2).65 In the context of blood pressure regulation, acute intravenous (i.v.) administration of various forms of apelin (i.e., apelin-36, apelin-17, Pyr1-apelin-13, and apelin-12) in anesthetized rodents consistently leads to short-term lowering of blood pressure.20, 28, 45, 66 Consistent with these data, Aplnr−/− mice display an exaggerated pressor response to systemic Ang II, suggesting a counterregulatory effect of apelin on Ang II.67 Interestingly, Apln-/y mice do not have significant differences in systemic blood pressure, suggesting that compensatory mechanisms may be activated or downregulated to maintain blood pressure homeostasis.68 This is discussed in more detail in the section on body fluid homeostasis.
With respect to PAH, a number of approved classes of drugs have led to improvements in clinical outcomes,69 yet mortality remains exceedingly high.70 PAH is a progressive pulmonary vasculature disease that results from an intricate combination of vasoconstriction, thrombosis, inflammation, and proliferative-obstructive remodeling of the pulmonary arterial vasculature, which subsequently leads to right ventricular failure.71 In fact, impaired pulmonary arterial endothelial cell (PAEC) function is a key feature of PAH. Apelin has been identified among the principal genes affected by right HF and PAH.72 Although acute hypoxia or hypoxia-inducible factor exposure induce apelin cellular expression/secretion,73–75 low circulating levels and cardiopulmonary expression of apelin-13 and Elabela are ultimately observed in patients with established PAH.16 Accordingly, PAECs from PAH patients display decreased expression of apelin, higher apoptotic index, and the promotion of pulmonary arterial smooth muscle cells proliferation that can be rescued by the administration of apelin or bone morphogenetic protein receptor 2 ligands.76 However, contradictory data have been published on apelins and the proliferation of smooth muscle cells,76, 77 which needs to be further investigated. Importantly, acute apelin infusion in patients with primary PAH produces beneficial hemodynamic effects without associated systemic hypotension and adverse effects,78 suggesting a potential therapeutic role for apelinergic signaling in this devastating disease.
Apln−/− mice exhibit worsened hypoxia-induced PAH, and multiple downstream targets and upstream regulators of apelin signaling have been identified to have key roles in pulmonary vascular homeostasis.16, 59, 61 For instance, significant disruption of an endothelial miRNA-dependent apelin–FGF2 link was demonstrated to trigger endothelial dysfunction and PAH.61 Additional evidence of the deleterious impact of apelin disruption and/or antagonism of its receptor is exemplified by the serious adverse reactions sometimes observed using protamine (a sub-micromolar affinity competitive antagonist of the apelin receptor that reverses heparin anticoagulation), and by the occurrence of sudden rises in pulmonary arterial pressure, sustained hypotension, and impaired cardiac fractional shortening.59, 79–82 Apelins can also potentially impact long-term vascular remodeling by reducing inflammation83–87 and fibrosis.88–92
Cardiac effects of the apelinergic system and its potential for heart failure treatment
While HF is common and characterized by high morbidity and mortality,24 better understanding and treatment of HF has significantly improved outcomes over the past two decades.93 Despite better medical and surgical therapies, ischemic, hypertensive, and obesity-related heart diseases remain leading causes of HF.94, 95 Acute and chronic HF are characterized by activation of several signaling pathways associated with pathological hypertrophy and maladaptive ventricular remodeling. HF is caused by damage to or loss of cardiomyocytes, and contributes to diminished systolic performance and diastolic dysfunction in the failing heart.94, 96 HF involves changes in cardiac structure, myocardial composition, myocyte deformation, and multiple biochemical and molecular alterations, collectively referred to as adverse myocardial remodeling.97, 98 The patient profile of HF has evolved significantly recently; while HF with reduced ejection fraction (HFrEF) is declining due to effective revascularization of patients with acute coronary syndromes, the prevalence and incidence of HF with preserved ejection fraction (HFpEF), mainly characterized by diastolic dysfunction, is still increasing.99 Myocardial hypertrophy, systemic inflammation, and endothelial dysfunction are the major pathophysiological events in HFpEF.100–102
Lessons from loss-of-function experiments
Important observations on the potential impact of the apelinergic system in HF were obtained from loss-of-function approaches. Indeed, aged Apln-/y mice develop progressive impairment of myocardial contractility along with systolic dysfunction, and loss of apelin contributes to HF in response to pressure overload.88 Deletion of apelin also leads to aggravated left ventricular injury following MI, while a synthetic apelin receptor agonist markedly protects from myocardial ischemia/reperfusion (I/R) injury.103 This is accompanied by greater activation of survival pathways and promotion of angiogenesis. Stretch-dependent apelin receptor signaling, rather than ligand-induced apelin receptor signaling, contributes to increased cardiomyocyte cell size and myocardial hypertrophy (Fig. 2).104 Since the apelin receptor responds to both mechanical stretch and endogenous apelin in cardiac hypertrophy (Fig. 2), interventions aiming to balance these stimuli may determine the resulting adaptive physiology of the apelinergic system.104 In the two-kidney, one-clip hypertensive rats, reduction in apelin and its receptor in the heart and aorta aggravates pathophysiological responses to hypertension and HF.25 Reduced plasma and myocardial apelin levels in patients with advanced HF, with concomitant reduction in expression of its receptor, clearly suggest that the apelinergic system is compromised in HF.105, 106 However, discrepant findings have been reported, with no difference in plasma apelin levels noted in patients with dilated cardiomyopathy. Discrepancies among these studies may be related to the quality of the assays, often immunoassays with different qualities of antibodies. In this context, the development of antibody-free quantification methods based, for example, on tandem mass spectrometry, would be a great boost to the quality of future studies.107–109
Several mechanistic studies provide insight into the role of apelinergic signaling in cardiac function and disease. Inactivation of Forkhead box protein O1 (FOXO1) and inhibition of endothelial expression of fatty acid (FA) binding protein 4 (FABP4) were identified as key downstream signaling targets of apelin/receptor signaling (Fig. 2). Both Apln−/− and apelin receptor constitutive and EC-specific knock-out (KO) mice demonstrate increased endothelial FABP4 expression and excess tissue FA accumulation.55 In obese patients, higher left ventricular ejection fraction is associated with higher levels of plasma apelin, and in an obese murine model of I/R injury, apelin prevents nuclear translocation of FOXO3 in response to oxygen deprivation, providing insights into its potential clinical relevance in obese patients with HF.110 Ang II infusion potentiated oxidative stress, pathological hypertrophy, and myocardial fibrosis in young Apln-/y mice, resulting in exacerbation of cardiac dysfunction in association with reduced ACE2 levels.111 Downregulation of the apelin pathway action lowers ACE2 levels, leading to activation of the renin-angiotensin system (Fig. 2).21, 88, 112 Indeed, deletion of apelin in mice leads to cardiac dysfunction, which is abrogated by blockade of the AT1 receptor or using AT1 receptor KO mice.21 The intricate interplay between the apelin and angiotensin pathways is also demonstrated by the heterodimerization of their receptors induced by apelin-13, which negatively regulates the allosteric binding site and function of the AT1 receptor (Fig. 2).113 These findings provide mechanistic insights that could greatly expand the therapeutic applications for the apelinergic system in cardiomyopathic, hypertensive, and metabolic disorders.55, 88
Gain-of-function approaches
Systolic and diastolic function, as well as adverse cardiovascular remodeling, were drastically improved in response to infusion of Pyr1-apelin-13 in hypertensive rats, and this was accompanied by decreased expression of inflammatory cytokines.114, 115 Apelin-mediated activation of PKCε and ERK1/2 was responsible for enhancement of cardiac contractility, myosin light chain kinase being the downstream target (Fig.2).25, 116 Circulating apelin levels are reduced in patients with essential hypertension, and lower plasma apelin levels are independently associated with greater left ventricular systolic and diastolic dysfunction, indicating that apelin may serve as a biomarker for increased risk of chronic HF.108, 117 However, the measurement of blood apelins, unlike N-terminal pro B-type natriuretic peptide and galectin-3, is reported not to be useful to fine tune the diagnosis of acute HF,118 whereas the blood levels of cardiac troponin are more likely prognostic.119 A bolus of exogenous apelin lowers blood pressure, an effect abrogated in Aplnr−/− mice.67 These effects are paralleled in humans where apelin peptides induce peripheral and coronary vasodilatation while increasing cardiac output and contractility.120 Importantly, both local vascular and systemic hemodynamic responses to apelin are preserved in patients with stable symptomatic chronic HF treated by current medical therapy.120, 121 Expression of apelin and its receptor in human ECs is associated with shear stress, in which expression of the receptor is induced independently of its ligand.122
Plasma apelin levels predict the major cardiovascular event after percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction, and adverse events are higher in patients with lower plasma apelin levels.123 Apelin and its receptor are markedly upregulated in the heart and skeletal muscle following myocardial injury or systemic hypoxic exposure through a hypoxia-inducible factor-mediated pathway.54 In mice fed with a high-fat diet subjected to myocardial I/R injury, apelin-13 alleviated infarct size, myocardial apoptosis, and mitochondrial damage.25, 124 Apelin gene therapy increases vascular density and alleviates diabetic cardiomyopathy via a mechanism involving activation of Sirt3 and upregulation of VEGF/VEGFR2 expression (Fig. 2).25, 125 Intracellular Ca2+ abnormality and endoplasmic reticulum stress mediates cardiac dysfunction induced by myocardial I/R injury, and apelin-13 suppresses these pathogenic pathways.25, 126 In cultured primary cardiomyocytes under hypoxia/re-oxygenation, apelin administration suppresses apoptosis and generation of reactive oxygen species. In addition, apelin improves cardiac dysfunction after myocardial I/R injury by inhibiting myocardial apoptosis and oxidative stress, along with upregulation of eNOS levels and activation of PI3K/Akt and ERK1/2 phosphorylation signaling.25, 127 Apelin-mediated protection against cardiac fibrosis results primarily from direct modulation of plasminogen activator inhibitor-1 gene expression, associated with synergistic inhibition of Ang II signaling and increased production of NO.25 Since apelin-13 upregulates ACE2 levels (Fig. 2),21 it is also likely to decrease superoxide production and expression of hypertrophy- and fibrosis-related genes, as seen with daily recombinant human ACE2 treatment that significantly attenuated myocardial hypertrophy, fibrosis, and dysfunction in the Ang II-infused Ace2-/y mice.128 Based on the above, the apelin receptor represents a promising target in the treatment of HF. As a result, a first clinical trial with the orally administered small-molecule apelin receptor agonist AMG986 represents an important milestone toward the validation of the target in this indication.129
Central and renal effects of apelin on body fluid homeostasis
The colocalization of apelin and arginine-vasopressin (AVP; also know as antidiuretic hormone), together with the apelin and vasopressin type-1 receptors in hypothalamic magnocellular neurons, suggested an interaction between these two hormones (Fig. 4).10, 130 Early experiments were performed in lactating rats, which exhibit increased activity in magnocellular AVP neurons, leading to increased AVP synthesis and release in order to preserve water for maximal milk production. In this model, intracerebroventricular (i.c.v.) administration of apelin-17 triggered inhibition of the phasic electrical activity of AVP neurons, reduced AVP release in the bloodstream, and increased diuresis, with no change in sodium and potassium excretion.10 Together, these suggest that apelin is likely released from AVP cell bodies to inhibit both AVP neuron activity and release by autocrine action on the apelin receptor expressed by AVP/apelin-containing neurons.
Moreover, following water deprivation in rodents, increased plasma AVP levels are associated with depletion of AVP neuronal contents in the paraventricular nucleus and supraoptic nucleus magnocellular AVP/apelin neurons (Fig. 4). Conversely, water deprivation decreases plasma apelin levels and increases apelin neuronal contents in the same neurons (Fig. 4).10, 131 Interestingly, i.c.v. administration of a selective V1 receptor antagonist markedly reduces the increase in neuronal apelin contents induced by water deprivation, whereas i.c.v. infusion of AVP has similar effects to dehydration on neuronal apelin concentration.131 Together, these imply that AVP and apelin are released separately by AVP/apelin magnocellular neurons in which they are produced, and that they reciprocally control their release into the bloodstream upon osmotic stimuli. Physiologically, this cross-regulation of apelin and AVP, which follows osmotic stimuli, maintains body fluid homeostasis by controlling water excretion at the kidney level, depending on the hydration state (Fig. 4). This makes apelin a natural physiological inhibitor of the antidiuretic effect of AVP. Such cross-regulation of apelin and AVP following osmotic stimuli has also been observed in humans.7 Indeed, increased plasma osmolality simultaneously raised plasma AVP levels and decreased plasma apelin levels. Conversely, decreased plasma osmolality simultaneously reduced plasma AVP levels and rapidly increased plasma apelin levels (Fig. 4).7 These observations are consistent with the fact that plasma osmolality is a major physiologic regulator of plasma apelin in humans and rodents, strongly suggesting that apelin and AVP contribute to maintain body fluid homeostasis in both species.
In addition to its central action, the aquaretic effect of apelin probably involves a renal component because of the renal expression of the apelin receptor, Elabela, and preproapelin, as well as apelin immunoreactivity.5, 13, 15, 33, 44, 132, 133 A high level of apelin receptor mRNA expression was also detected in glomeruli, and a moderate expression was observed in all nephron segments, especially in collecting ducts (CDs) that express AVP type-2 receptors (V2R).41 Application of apelin-17 on glomerular arterioles precontracted by Ang II induced NO-dependent vasorelaxation by inhibiting Ang II-induced intracellular calcium mobilization.41 This vasorelaxation induced by apelin in glomerular efferent arterioles, which gives rise to the vasa recta, is in agreement with a key role of apelin in the control of renal medullary circulation.41 In addition, the expression of the apelin receptor mRNA in the CDs strongly suggests that apelin could act as an aquaretic peptide through direct action on this nephron segment. As confirmation, treatment of microdissected medullary CDs with apelin-17 inhibits cAMP production induced by dDAVP, an AVP analog agonist of the V2R expressed in CD cells.134 Intravenous injection of increasing doses of apelin-17 in lactating rats dose-dependently increases diuresis, concomitantly with a decrease in aquaporin-2 insertion at the apical membrane of CD cells and a significant decrease in urine osmolality (Fig. 4).134 Thus, the diuresis induced by i.v. injection of apelin-17 seems linked to a direct action of apelin on CDs. Similarly, in other studies, acute or chronic i.v. treatment with apelin-13 potently increased diuresis in male Sprague-Dawley rats (Fig.4).133, 135
In contrast with these studies demonstrating the aquaretic role of apelin in rodents,136 water-deprived Aplnr−/− mice were unable to concentrate their urine to the same extent as wild-type mice.137, 138 A possible explanation for this discrepancy is that in whole-body KO mouse model, the total absence of apelin receptor during fetal and adult life elicits compensatory mechanisms, leading to opposite effects on urine output observed following exogenous apelin administration. Hopefully, the study of an apelin receptor conditional KO mouse model55, 68 will circumvent the limitations of those prior studies and provide insight. Altogether, these studies demonstrate that the aquaretic effect of apelin is elicited by its central effect that inhibits AVP release in the blood circulation, as well as its direct renal action that increases renal blood flow and counteracts the V2R-dependent antidiuretic effect of AVP at the CD (Fig. 4). In this context, the development of apelin receptor agonists or apelin analogs will represent potential therapeutic tools for the treatment of water retention/hyponatremic disorders.
Integrating cardiac and renal aspects and the impact on cardio-renal syndrome
Disturbance of the cardio-renal axis is common in acute and chronic HF (see above), septic shock, and severe forms of preeclampsia (see below).139 Indeed, the bidirectional crosstalk between the heart and kidneys can induce acute or chronic dysfunction in other organs with parallel and/or consecutive occurrences, leading to complex overlapping syndromic conditions difficult to treat and with poor outcomes.140 Apelin circulating levels are strongly associated with and inversely proportional to cardiovascular risk and the progression of renal dysfunction in patients with type 2 diabetes mellitus.141 Elabela and apelin-13 differentially affect the cardio-renal axis, mainly through opposing effects on counter-regulation in the vasopressinergic system, and possibly additional unknown mechanisms. Elabela is abundantly expressed in the kidney142 and is more effective than apelin-13 in reducing renal dysfunction/injury.15, 85, 143 Elabela and apelin-13 also significantly improve fluid homeostasis in experimental endotoxemia and septic shock, thus limiting hypovolemia.85, 144 An anti-inflammatory activity of apelin-13 and Elabela may contribute to reduce systemic vascular permeability, thus preserving plasma volume and hemodynamics.85, 144 The latter effects are closely related to a specific ligand-dependent interplay between the apelinergic and vasopressinergic systems (see above). In fact, i.v. injection of Elabela does not impact the increase of sepsis-induced AVP circulating levels, as opposed to apelin-13 which lowers AVP release in the blood circulation and rather tilts the balance toward undesired aquaresis and subsequent plasma volume loss.85 Indeed, a dynamic reduction of AVP bloodstream release is observed in severe sepsis in humans, and AVP supply offers a non-catecholaminergic pathway that helps to limit both the extent and duration of low blood pressure in patients with septic shock.145, 146
Potential of the apelinergic system for the treatment of septic shock
Septic shock is essentially initiated by microbial infections growing systemically, and sepsis-induced myocardial dysfunction is a special condition related to acute HF, with its own specific molecular mechanisms, treatment, and outcome (Fig.3).147, 148 The heart, along with the brain and lungs, is one of the three organs most strongly affected and associated with septic shock mortality,149 often with a combination of systolic and diastolic dysfunctions.147, 148, 150 Systemic vascular resistance (afterload) time-dependently and dynamically declines in septic shock, whereas it remains high to very high in non-septic acute HF.151 However, as a member of the vasoplegia syndrome family, this shock is complex, and absolute-to-relative preload deficiency (fluid deficit and leakage) is almost always associated with this condition.152–154 Vascular permeability during septic shock is an early concern, along with related organ dysfunctions and differential leaking intensity.155, 156
During experimental sepsis, circulating and myocardial apelin-13 and Elabela contents are low85, 157 and apelin receptor expression is rather downregulated.85 Receptor blockade further exacerbates myocardial dysfunction and mortality,144 and apelin gene expression is down-regulated in the hearts of non-survivors.158 On the other hand, circulating apelin-13 is marginally increased in early human sepsis, suggesting an inappropriately low reactivity of the system.159
Exogenous delivery of apelin-13 is beneficial in several experimental models of sepsis (e.g., endotoxemia, peritonitis), reducing multiple organ inflammation/injury and improving outcome.144, 157, 160–162 More specifically and remarkably, Elabela is superior to apelin-13 in enhancing cardiac performance and hemodynamics in rats with peritonitis,85 optimizing both inotropy and pressure-volume relationships on the Frank-Starling curve, and reducing myocardial inflammation, oxidation, and stress.144 Interestingly, the potency of cardiac response with apelinergic agonists is enhanced under systemic inflammatory conditions or polymicrobial infection, in spite of myocardial AR downregulation.85, 144 In comparison, while adrenoceptor β1 agonists are actually recommended for supporting septic shock–related hemodynamic failure, sepsis dampens myocardial responsiveness,163–165 with possible decreased expression of adrenoceptor β1,166 and impairs GPCR signaling (e.g., enhanced Gαi/o and reduced Gαs activities).167, 168
Metabolic implications of apelin signaling and potential links with vascular effects.
Although most studies have addressed the vascular49 and metabolic48 effects of the apelinergic system independently, recent results suggest the two may be highly integrated, whereby the vasculature may be a critical driving force behind several beneficial metabolic effects of apelin. Indeed, preceded by a number of studies suggesting differential regulation of apelin expression in metabolically challenged states, including diabetes169 and obesity,170 Dray and colleagues reported that acute administration of apelin-13 led to robust reduction of blood glucose, both at baseline as well as in the setting of an oral glucose tolerance test,171 without affecting circulating insulin levels. Subsequent studies corroborated these findings, including the demonstration of impaired insulin sensitivity in Apln-/y mice.172 Recent human studies demonstrated that Pyr1-apelin-13 injection in a group of obese but otherwise healthy humans led to improved insulin sensitivity.173 These findings provide clinical evidence for yet another potential indication for the apelinergic system.
In addition to regulating glucose utilization and insulin sensitivity, the apelinergic pathway was recently found to have a key role in skeletal muscle function as related to aging. Indeed, apelin expression is decreased in aged skeletal muscles, and Apln-/y and Aplnr−/− mice were found to have impaired muscle function with age.174 Apelin administration led to increased mitochondriogenesis, as well as increased proliferation and myogenic differentiation of muscle stem cells. Lastly, a strong correlation was found between activity/exercise levels and circulating apelin levels in humans, characterizing apelin as an exerkine, a recently coined term for peptides and nucleic acids released from skeletal muscle and other organs upon exercise and mediating systemic adaptations to exercise.175
While the profound metabolic effects of apelinergic signaling, including human disease implications, provide a potential therapeutic role for targeting this pathway, the significantly greater expression of apelin and its receptor in ECs over non-endothelial cell types provides a mechanistic enigma. A number of key questions emerge regarding how this signaling mechanism truly impacts the metabolic phenotypes observed in different KO strains: (1) Is the primary source of apelin the ECs, and is endothelially-derived apelin responsible for the bulk of metabolic effects in a paracrine manner? (2) Is the lower-level expression of the apelin receptor in the non-endothelial cell types, such as adipocytes and skeletal myocytes, sufficient to drive the paracrine effects of circulating apelin? And (3) can autocrine or paracrine endothelial apelin/receptor signaling be responsible for the metabolic effects attributed to this signaling pathway? These important questions are beginning to be addressed by the generation of conditional, cell type–specific deletions of Aplnr. Mice with an endothelial-specific deletion of Aplnr were found to exhibit insulin resistance and significantly impaired glucose utilization, and failed to improve glucose utilization in glucose tolerance tests after exogenous apelin administration.55 Moreover, these mice were found to have augmented trans-endothelial fatty acid transport and accumulation of fatty acids in the skeletal muscle, which at least partly explains their impaired insulin resistance. While these studies suggest that endothelial-specific apelin/receptor signaling may be a critical component of how apelin regulates metabolic balance, further studies involving selective deletion of Aplnr in other non-endothelial cell types, including skeletal myocytes, adipocytes, hepatocytes, and others, will provide further insights into both the metabolic and the myogenic effects of apelinergic signaling as we continue to develop this pathway into a therapeutic modality.
Emerging potential of the apelinergic system for treating preeclampsia
Preeclampsia, a clinical condition observed during pregnancy, is characterized by high blood pressure and damage to vital organs, most often liver and kidneys. Placental impaired trophoblast invasion, spiral artery transformation, and endothelial dysfunction are commonly observed in preeclampsia, with mostly unknown mechanisms.176, 177 Several miRNAs, peptides, and proteins derived from the placenta were shown as potential biomarkers of preeclampsia,178, 179 but valuable and safe therapeutic targets are still urgently needed. Interestingly, the apelin/Elabela/APLNR axis is a major player in the development, growth, and function of the vascular system, including the extensive angiogenesis necessary for placental growth as well as normal embryonic and fetal development.180, 181 More specifically, Elabela is known to be a hormonal guide for angioblasts during angiogenesis.182 Both apelin receptor and its ligands are strongly present in placental (including cytotrophoblast, syncytiotrophoblast, and stromal cells) and uterine tissues,183 with possible overexpression in preeclampsia.184 On the other hand, knockdown of LIN28B (an RNA-binding protein that influences stem cell maintenance and metabolism) suppresses Elabela expression185 and Elabela deficiency is a promoter of preeclampsia and cardiovascular defects (including in angiogenesis) in mice.186 This experimental link has to be further investigated, since two recent clinical studies did not confirm the lack of Elabela in the blood and placenta of women with early and late preeclampsia.187, 188 This topic is a still emerging and relevant one with regards to both pathophysiological events and the potential function of the apelinergic system. There is no intervention along this axis currently in progress to our knowledge.
Toward pharmacological probes and novel drugs
In parallel to the above investigations on the role of the apelinergic axis in the physiology and pathology associated with multiple systems and diseases, efforts have been dedicated to understanding the structure–function of the endogenous ligands of the apelin receptor. Indeed, the apelinergic system possesses the rare characteristic of being associated with two classes of ligands detected in vivo: apelins (apelin-36, apelin-17, apelin-13, and Pyr1-apelin-13) and Elabela. Not surprisingly, based on the nature of its endogenous ligands, peptide derivatives of apelin and, more recently, Elabela have dominated chemistry efforts along several lines aimed at (1) better characterizing the structure–signaling and structure–activity relationship (SAR) of apelin and Elabela; (2) providing biologically more stable ligands; and (3) generating pharmacological probes to validate the potential of the apelin receptor as a target in the above indications. More recently, nonpeptide small molecules have also been reported, some of which have been clinically tested.189, 190
Peptides and mimetics
Initial efforts to understand the SAR of peptide molecules classically involve truncation, deletion, alanine scanning, as well as aspartate and N- methylation scanning. Together, these efforts usually lead to the identification of critical pharmacophoric elements, as well as preliminary information on the structure of the ligand. When applied to apelin fragments, N-terminal truncation was found to decrease forskolin-induced cAMP production, receptor internalization, AVP release, and the effect on blood pressure.20, 28 Subsequently, single replacements with alanine (alanine scanning) and stereoinversion collectively pointed to Arg2, Arg4, Leu5, and Phe13 as important pharmacophoric elements.22, 33, 191, 192 This is congruent with NMR structures of apelin-17, which identified these regions as structurally less flexible.193, 194
Important insights into the role of the C-terminal Phe residue in signaling came early on with the demonstration that its deletion in apelin-17 (i.e., generating apelin-16)20 abrogated the hypotensive effect of the parent peptide, and its substitution by Ala in apelin-13 inhibited the apelin-13-induced transient blood pressure decrease.195 Surprisingly, apelin-16 possesses similar affinity for the apelin receptor and efficacy to activate Gαi/o signaling as apelin-17.196 The profound decrease in the hypotensive effect of apelin-16 was due to its reduced ability to promote β-arrestin recruitment and apelin receptor internalization. This demonstrated that β-arrestin signaling may account for the hypotensive activity of apelin-17.19, 20 Recently, a broader analysis of signaling bias in a collection of compounds confirmed the correlation between the hypotensive effect of apelin and the activation of the β-arrestin pathway subsequent to binding to the receptor.36 Knowing that the C-terminal Phe residue of apelin-17 or apelin-13 is removed by ACE2 (Fig.1), this suggests that ACE2 might be an important regulator of the activity of apelins in the control of blood pressure.18
Substitution of the C-terminal residue with unnatural aromatic amino acids led to compounds possessing mid-pM affinity.191, 197, 198 This class of modifications also increased plasma stability. Furthermore, aromatic amino acid substitutions of the C-terminal residue also identified a determinant of signaling, likely associated with the presence of multiple aromatic residues on several transmembrane domains in that area of the binding pocket (i.e. residues Y35, W85, Y88, Y93 and Y299), as observed in the recently reported X-ray structure of the stabilized apelin receptor (modifications include N- and C-terminus truncations as well as the mutations V117A and W126K) complexed with a macrocyclic apelin-17 derivative.17, 23, 33, 189, 196, 199 Additional work in this area relied on the introduction of Pro12Aib and Phe13(4-Br)Phe, which were beneficial for plasma stability of apelin-13 and apelin-17.17, 23, 33, 192 Further stabilizing modifications involved N-Me, α-Me, aza-Arg, and aza-Leu in replacement of Arg4 and Leu5, combined with the above Pro12 and Phe13 replacements, which led to improved plasma stability while keeping low nanomolar binding affinity. N-PEGylation and N-palmitoylation of the native peptides also increased plasma stability by several orders of magnitude while maintaining good Ca2+ mobilization.200, 201 Interestingly, preserved agonist activity with prolonged in vivo half-life was also achieved by fusing apelin-13 to IgG Fc fragment or by encapsulating Pyr1-apelin-13 in PEGylated liposomal nanocarriers.202, 203 More recently, the addition of a fluoroalkyl chain to the N-terminal part of apelin-17 was shown to strongly increase plasma stability while maintaining its in vitro pharmacological properties and significantly improving the in vivo activity of the peptide on diuresis and arterial blood pressure.192
Finally, macrocyclization was applied to apelin-13 in different ways.204 Among several attempts, double cyclization of apelin-13 turned produced a competitive antagonist with a Ki of 82 nM that demonstrated efficacy in reducing tumor growth in a mouse model of glioblastoma.205, 206 MM07 was also reported as a G protein–biased agonist with potency comparable to Pyr1-apelin-13 in the saphenous vein contraction assay, yet with lower potency on β-arrestin recruitment and receptor internalization.207 MM07 significantly increased cardiac output in anesthesized rats, and dose-dependently increased forearm blood flow in human volunteers with higher efficacy than apelin. A further systematic study of macrocyclization in Pyr1-apelin-13 identified the N- and C-termini as preferable sites for cyclization to retain good binding and signaling properties, and to significantly increase plasma stability. In this series, low nM, Gαi-biased agonists were identified, and they demonstrated, as suggested above, very low hypotensive properties in rats in vivo, as well as strong inotropic properties in the Langendorff isolated perfused heart model.135, 208
Beyond apelin-13, a few studies have dissected the SARs of apelin-17, apelin-36, and Elabela. Essentially, conclusions drawn from SAR on apelin-13 have held true for the other apelins.23, 33, 192 Importantly, the combined SARs of the three apelins point to the presence of two distant pharmacophoric elements, one corresponding to the N-terminus of Pyr1-apelin-13, with cationic elements found in Arg2 and Arg4 interacting with anionic residues in the extracellular domain of the receptor, and the second involving a C-terminal aromatic moiety found in Phe13, buried deeper in the transmembrane domain.196 This view is consistent with the X-ray structure of the human apelin receptor complexed with a macrocyclic derivative of apelin-17, 198 and site-directed mutagenesis studies on the rat apelin receptor.196, 209 This is also consistent with a semi-peptide derivative reported by Iturrioz et al., which links a cationic peptide portion with a polyaromatic fluorophore via a flexible linker.52, 210 Interestingly, preliminary SAR of Elabela allowed the determination that the shortest significantly active fragment corresponds to its 14 C-terminal amino acids.211 Despite large differences in primary sequence between apelins and Elabela, distant epitopes seem necessary, although their chemical nature varies somewhat.
Receptor structure
The X-ray structure of the apelin receptor complexed with a macrocyclic apelin-17 analog (AMG3054) was reported recently.199 This follows several studies of either the ligand in solution, individual transmembrane domains by solution NMR, or site-directed mutagenesis, as summarized recently.189 Altogether, these studies provide invaluable structural information on the binding and dynamics of the receptor. The C-terminal binding pocket described in the X-ray structure and 3D model of the apelin receptor, which is very rich in aromatic residues located on several transmembrane domains, helps to explain why and how modifications to the C-terminal Phe residue of apelins is such an important determinant of β-arrestin signaling.19, 196, 199 Accordingly, this region also emerges as a region of interest for virtual screening for the identification of small molecules. Future efforts combining 3D solid structure with solution dynamics and computational modeling will be critical for fully comprehending the ligand-receptor complex structure and dynamics, and how these relate to signaling. This knowledge will serve the dual purpose of identifying novel small molecule ligands and how to bias their signaling toward beneficial pathways at the expense of undesired ones. Indeed, as described in the previous sections, different signaling pathways may be necessary to optimize efficacious and safe drugs for different therapeutic indications.
Small molecules
Few small molecules have been reported to date as apelin receptor ligands. The presence of two distant pharmacophoric elements (see above) may contribute to the difficulty of identifying small enough molecules to bind and activate the receptor. As far as antagonists are concerned, two small molecule antagonists with low/sub-micromolar efficacy, ML221 and ML223, were identified from a high-throughput screen. Yet, although functional efficacy data were reported, these compounds have not been shown to actually bind the orthosteric apelin receptor site.212, 213 Protamine was also identified as an apelin receptor antagonist with high nanomolar efficacy.79 More recently, CMF-019 was identified as an agonist with EC50 on 47 nM on calcium mobilization. CMF-019 binds to the human left ventricle (pKi = 8.6), and potently inhibits cAMP production, with a low impact on β-arrestin recruitment and receptor internalization. As such, CMF-019 emerges as a Gαi-biased apelin receptor agonist, and it showed increased cardiac contractility upon i.v. administration to rats. Several series of low/sub-nanomolar apelin receptor agonists were reported in the patent literature based on various heterocyclic scaffolds.189, 190 Finally, the oral administration of the small-molecule apelin agonist AMG986 is currently in phase I clinical trials for the treatment of HF.129
Challenges and perspectives
Several key points remain to further establish the druggability of the apelinergic pathway. First, a clear understanding of an optimal candidate signaling profile for the therapeutic indications under scrutiny is required. At the moment, it is not clear, for each indication, whether a compound acting on cAMP signaling via the Gαi/o pathway, or a compound that mediates receptor internalization, or even a G protein–independent but β-arrestin–dependent signaling, is preferred. In order to validate this, compounds possessing strongly biased signaling profiles on these diverse pathways are necessary, as well as the validation of these compounds in relevant animal models. This will be invaluable to orient drug discovery on the right pathways. Second, and in the same vein, like many peptidergic GPCRs it has been difficult to identify small molecule agonists of the apelin receptor. Although peptide and semi-peptide compounds provide high levels of affinity and represent good probes to decipher the above structure-signaling relationship or potential drug candidates for indications compatible with parenteral administration, there is only a handful of orally bioavailable small molecules that would be compatible with indications such as heart failure, PAH, or diabetes. One of the difficulties in identifying such small molecules may be the necessity of two topologically distant epitopes for binding, as revealed by studies based on peptide molecules. Third, translational models, particularly those relying on genetic interference, all possess their drawbacks such as, for example, heavy compensation. This makes translation more difficult. Their predictive value will be enhanced by the validation of the observed effects with emerging molecules, and the availability of tissue-specific genetic interference approaches. Finally, although several investigator trials have provided encouraging results on the role of apelins in several conditions, much remains to be done to demonstrate the clinical efficacy of apelin or Elabela in pathological conditions. Some differences are observed between the two peptide classes in animal models; however, these need to be clinically validated.
Conclusion
The apelinergic system is an emerging target with broad potential therapeutic impact in cardiovascular, renal, and metabolic disorders, as well as other indications with a strong vascular component. Animal models of diseases provide convincing evidence that it should be pursued in several indications. Among the most advanced indications in terms of preclinical and clinical validation are HF and PAH. However, type 2 diabetes, water retention/hyponatremic disorders, kidney diseases, septic shock, and preeclampsia are also among therapeutic indications that would benefit from intervention via the apelinergic system. Moreover, the interplay between the involved systems and the particular role of the vasculature emphasizes the need to better understand the impact of vascular dysfunctions in these diseases, as well as how intervention via the apelinergic system contributes to the positive effects observed.
Several classes of molecules are now available, some with biased signaling, to decipher which signaling pathway to favor for therapeutic intervention in the above areas. With these tools in hand, the coming years will certainly be very stimulating for answering some of the questions associated with the apelinergic system.
Acknowledgments
The authors acknowledge their respective research teams and network of collaborators. Funding from the following agencies is gratefully acknowledged: the Canadian Institutes for Health Research, the Heart and Stroke Foundation of Canada, Fonds de Recherche du Québec - Santé, the French National Institute of Health and Medical Research (INSERM), College de France, the French National Agency for Research, the National Institutes of Health (US), and the American Heart Association.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. 2017. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 135: e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Dowd BF, Heiber M, Chan A, et al. 1993. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 136: 355–360. [DOI] [PubMed] [Google Scholar]
- 3.Devic E, Paquereau L, Vernier P, et al. 1996. Expression of a new G protein-coupled receptor X-msr is associated with an endothelial lineage in Xenopus laevis. Mech Dev. 59: 129–140. [DOI] [PubMed] [Google Scholar]
- 4.De Mota N, Lenkei Z & Llorens-Cortes C. 2000. Cloning, pharmacological characterization and brain distribution of the rat apelin receptor. Neuroendocrinology. 72: 400–407. [DOI] [PubMed] [Google Scholar]
- 5.O’Carroll AM, Selby TL, Palkovits M, et al. 2000. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim Biophys Acta. 1492: 72–80. [DOI] [PubMed] [Google Scholar]
- 6.Tatemoto K, Hosoya M, Habata Y, et al. 1998. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 251: 471–476. [DOI] [PubMed] [Google Scholar]
- 7.Azizi M, Iturrioz X, Blanchard A, et al. 2008. Reciprocal regulation of plasma apelin and vasopressin by osmotic stimuli. J Am Soc Nephrol. 19: 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosoya M, Kawamata Y, Fukusumi S, et al. 2000. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem. 275: 21061–21067. [DOI] [PubMed] [Google Scholar]
- 9.Kawamata Y, Habata Y, Fukusumi S, et al. 2001. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 1538: 162–171. [DOI] [PubMed] [Google Scholar]
- 10.De Mota N, Reaux-Le Goazigo A, El Messari S, et al. 2004. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc Natl Acad Sci U S A. 101: 10464–10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maguire JJ, Kleinz MJ, Pitkin SL, et al. 2009. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension. 54: 598–604. [DOI] [PubMed] [Google Scholar]
- 12.Shin K, Pandey A, Liu XQ, et al. 2013. Preferential apelin-13 production by the proprotein convertase PCSK3 is implicated in obesity. FEBS Open Bio. 3: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chng SC, Ho L, Tian J, et al. 2013. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev Cell. 27: 672–680. [DOI] [PubMed] [Google Scholar]
- 14.Pauli A, Norris ML, Valen E, et al. 2014. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 343: 1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen H, Wang L, Wang W, et al. 2017. ELABELA and an ELABELA Fragment Protect against AKI. J Am Soc Nephrol. 28: 2694–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang P, Read C, Kuc RE, et al. 2017. Elabela/Toddler Is an Endogenous Agonist of the Apelin APJ Receptor in the Adult Cardiovascular System, and Exogenous Administration of the Peptide Compensates for the Downregulation of Its Expression in Pulmonary Arterial Hypertension. Circulation. 135: 1160–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W, McKinnie SM, Farhan M, et al. 2016. Angiotensin-Converting Enzyme 2 Metabolizes and Partially Inactivates Pyr-Apelin-13 and Apelin-17: Physiological Effects in the Cardiovascular System. Hypertension. 68: 365–377. [DOI] [PubMed] [Google Scholar]
- 18.Vickers C, Hales P, Kaushik V, et al. 2002. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 277: 14838–14843. [DOI] [PubMed] [Google Scholar]
- 19.Ceraudo E, Galanth C, Carpentier E, et al. 2014. Biased signaling favoring gi over beta-arrestin promoted by an apelin fragment lacking the C-terminal phenylalanine. J Biol Chem. 289: 24599–24610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Messari S, Iturrioz X, Fassot C, et al. 2004. Functional dissociation of apelin receptor signaling and endocytosis: implications for the effects of apelin on arterial blood pressure. J Neurochem. 90: 1290–1301. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, Suzuki T, Watanabe H, et al. 2013. Apelin is a positive regulator of ACE2 in failing hearts. The Journal of clinical investigation. 123: 5203–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinnie SMK, Wang W, Fischer C, et al. 2017. Synthetic Modification within the “RPRL” Region of Apelin Peptides: Impact on Cardiovascular Activity and Stability to Neprilysin and Plasma Degradation. J Med Chem. 60: 6408–6427. [DOI] [PubMed] [Google Scholar]
- 23.McKinnie SM, Fischer C, Tran KM, et al. 2016. The Metalloprotease Neprilysin Degrades and Inactivates Apelin Peptides. Chembiochem. 17: 1495–1498. [DOI] [PubMed] [Google Scholar]
- 24.McMurray JJ, Packer M, Desai AS, et al. 2014. Angiotensin-neprilysin inhibition versus enalapril in heart failure. The New England journal of medicine. 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 25.Zhong JC, Zhang ZZ, Wang W, et al. 2017. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim Biophys Acta. 1863: 1942–1950. [DOI] [PubMed] [Google Scholar]
- 26.Fischer C, Lamer T, Wang W, et al. 2019. Plasma kallikrein cleaves and inactivates apelin-17: Palmitoyl- and PEG-extended apelin-17 analogs as metabolically stable blood pressure-lowering agents. Eur J Med Chem. 166: 119–124. [DOI] [PubMed] [Google Scholar]
- 27.Habata Y, Fujii R, Hosoya M, et al. 1999. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta. 1452: 25–35. [DOI] [PubMed] [Google Scholar]
- 28.Reaux A, De Mota N, Skultetyova I, et al. 2001. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 77: 1085–1096. [DOI] [PubMed] [Google Scholar]
- 29.Masri B, Lahlou H, Mazarguil H, et al. 2002. Apelin (65–77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem Biophys Res Commun. 290: 539–545. [DOI] [PubMed] [Google Scholar]
- 30.Masri B, Morin N, Pedebernade L, et al. 2006. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J Biol Chem. 281: 18317–18326. [DOI] [PubMed] [Google Scholar]
- 31.Yue P, Jin H, Xu S, et al. 2011. Apelin decreases lipolysis via G(q), G(i), and AMPK-Dependent Mechanisms. Endocrinology. 152: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choe W, Albright A, Sulcove J, et al. 2000. Functional expression of the seven-transmembrane HIV-1 co-receptor APJ in neural cells. J Neurovirol. 6 Suppl 1: S61–69. [PubMed] [Google Scholar]
- 33.Medhurst AD, Jennings CA, Robbins MJ, et al. 2003. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem. 84: 1162–1172. [DOI] [PubMed] [Google Scholar]
- 34.Parikh VN, Liu J, Shang C, et al. 2018. Apelin and APJ orchestrate complex tissue-specific control of cardiomyocyte hypertrophy and contractility in the hypertrophy-heart failure transition. Am J Physiol Heart Circ Physiol. 315: H348–H356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans NA, Groarke DA, Warrack J, et al. 2001. Visualizing differences in ligand-induced beta-arrestin-GFP interactions and trafficking between three recently characterized G protein-coupled receptors. J Neurochem. 77: 476–485. [DOI] [PubMed] [Google Scholar]
- 36.Besserer-Offroy E, Berube P, Cote J, et al. 2018. The hypotensive effect of activated apelin receptor is correlated with beta-arrestin recruitment. Pharmacol Res. 131: 7–16. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Bai B, Tian Y, et al. 2014. Identification of serine 348 on the apelin receptor as a novel regulatory phosphorylation site in apelin-13-induced G protein-independent biased signaling. J Biol Chem. 289: 31173–31187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee DK, Ferguson SS, George SR, et al. 2010. The fate of the internalized apelin receptor is determined by different isoforms of apelin mediating differential interaction with beta-arrestin. Biochem Biophys Res Commun. 395: 185–189. [DOI] [PubMed] [Google Scholar]
- 39.Peterson YK & Luttrell LM. 2017. The Diverse Roles of Arrestin Scaffolds in G Protein-Coupled Receptor Signaling. Pharmacol Rev. 69: 256–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edinger AL, Hoffman TL, Sharron M, et al. 1998. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 72: 7934–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hus-Citharel A, Bouby N, Frugiere A, et al. 2008. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int. 74: 486–494. [DOI] [PubMed] [Google Scholar]
- 42.Kidoya H, Ueno M, Yamada Y, et al. 2008. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 27: 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Falco M, De Luca L, Onori N, et al. 2002. Apelin expression in normal human tissues. In Vivo. 16: 333–336. [PubMed] [Google Scholar]
- 44.Kleinz MJ, Skepper JN & Davenport AP. 2005. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept. 126: 233–240. [DOI] [PubMed] [Google Scholar]
- 45.Tatemoto K, Takayama K, Zou MX, et al. 2001. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 99: 87–92. [DOI] [PubMed] [Google Scholar]
- 46.Chen MM, Ashley EA, Deng DX, et al. 2003. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation. 108: 1432–1439. [DOI] [PubMed] [Google Scholar]
- 47.Liu Q, Hu T, He L, et al. 2015. Genetic targeting of sprouting angiogenesis using Apln-CreER. Nat Commun. 6: 6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castan-Laurell I, Masri B & Valet P. 2019. The apelin/APJ system as a therapeutic target in metabolic diseases. Expert Opin Ther Targets. 23: 215–225. [DOI] [PubMed] [Google Scholar]
- 49.Mughal A & O’Rourke ST. 2018. Vascular effects of apelin: Mechanisms and therapeutic potential. Pharmacol Ther. 190: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salcedo A, Garijo J, Monge L, et al. 2007. Apelin effects in human splanchnic arteries. Role of nitric oxide and prostanoids. Regul Pept. 144: 50–55. [DOI] [PubMed] [Google Scholar]
- 51.Japp AG & Newby DE. 2008. The apelin-APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem Pharmacol. 75: 1882–1892. [DOI] [PubMed] [Google Scholar]
- 52.Iturrioz X, Alvear-Perez R, De Mota N, et al. 2010. Identification and pharmacological properties of E339–3D6, the first nonpeptidic apelin receptor agonist. FASEB J. 24: 1506–1517. [DOI] [PubMed] [Google Scholar]
- 53.del Toro R, Prahst C, Mathivet T, et al. 2010. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 116: 4025–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheikh AY, Chun HJ, Glassford AJ, et al. 2008. In vivo genetic profiling and cellular localization of apelin reveals a hypoxia-sensitive, endothelial-centered pathway activated in ischemic heart failure. Am J Physiol Heart Circ Physiol. 294: H88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwangbo C, Wu J, Papangeli I, et al. 2017. Endothelial APLNR regulates tissue fatty acid uptake and is essential for apelin’s glucose-lowering effects. Sci Transl Med. 9: eaad4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradbury A & Pluckthun A. 2015. Reproducibility: Standardize antibodies used in research. Nature. 518: 27–29. [DOI] [PubMed] [Google Scholar]
- 57.Baker M 2015. Reproducibility crisis: Blame it on the antibodies. Nature. 521: 274–276. [DOI] [PubMed] [Google Scholar]
- 58.Chun HJ, Ali ZA, Kojima Y, et al. 2008. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. The Journal of clinical investigation. 118: 3343–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandra SM, Razavi H, Kim J, et al. 2011. Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol. 31: 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Falcao-Pires I, Goncalves N, Henriques-Coelho T, et al. 2009. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 296: H2007–2014. [DOI] [PubMed] [Google Scholar]
- 61.Kim J, Kang Y, Kojima Y, et al. 2013. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 19: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hashimoto T, Kihara M, Imai N, et al. 2007. Requirement of apelin-apelin receptor system for oxidative stress-linked atherosclerosis. Am J Pathol. 171: 1705–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pitkin SL, Maguire JJ, Kuc RE, et al. 2010. Modulation of the apelin/APJ system in heart failure and atherosclerosis in man. Br J Pharmacol. 160: 1785–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peltonen T, Napankangas J, Vuolteenaho O, et al. 2009. Apelin and its receptor APJ in human aortic valve stenosis. J Heart Valve Dis. 18: 644–652. [PubMed] [Google Scholar]
- 65.Zhong JC, Yu XY, Huang Y, et al. 2007. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc Res. 74: 388–395. [DOI] [PubMed] [Google Scholar]
- 66.Galanth C, Hus-Citharel A, Li B, et al. 2012. Apelin in the control of body fluid homeostasis and cardiovascular functions. Curr Pharm Des. 18: 789–798. [DOI] [PubMed] [Google Scholar]
- 67.Ishida J, Hashimoto T, Hashimoto Y, et al. 2004. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 279: 26274–26279. [DOI] [PubMed] [Google Scholar]
- 68.Charo DN, Ho M, Fajardo G, et al. 2009. Endogenous regulation of cardiovascular function by apelin-APJ. Am J Physiol Heart Circ Physiol. 297: H1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J 2014. Apelin-APJ signaling: a potential therapeutic target for pulmonary arterial hypertension. Mol Cells. 37: 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Humbert M, Sitbon O, Chaouat A, et al. 2010. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 122: 156–163. [DOI] [PubMed] [Google Scholar]
- 71.Galie N, Palazzini M & Manes A. 2010. Pulmonary arterial hypertension: from the kingdom of the near-dead to multiple clinical trial meta-analyses. Eur Heart J. 31: 2080–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Drake JI, Bogaard HJ, Mizuno S, et al. 2011. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol. 45: 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glassford AJ, Yue P, Sheikh AY, et al. 2007. HIF-1 regulates hypoxia- and insulin-induced expression of apelin in adipocytes. Am J Physiol Endocrinol Metab. 293: E1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang J, Liu Q, Hu X, et al. 2015. Apelin/APJ signaling promotes hypoxia-induced proliferation of endothelial progenitor cells via phosphoinositide-3 kinase/Akt signaling. Mol Med Rep. 12: 3829–3834. [DOI] [PubMed] [Google Scholar]
- 75.Ronkainen VP, Ronkainen JJ, Hanninen SL, et al. 2007. Hypoxia inducible factor regulates the cardiac expression and secretion of apelin. FASEB J. 21: 1821–1830. [DOI] [PubMed] [Google Scholar]
- 76.Alastalo TP, Li M, Perez Vde J, et al. 2011. Disruption of PPARgamma/beta-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. The Journal of clinical investigation. 121: 3735–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He L, Zhou Q, Huang Z, et al. 2019. PINK1/Parkin-mediated mitophagy promotes apelin-13-induced vascular smooth muscle cell proliferation by AMPKalpha and exacerbates atherosclerotic lesions. J Cell Physiol. 234: 8668–8682. [DOI] [PubMed] [Google Scholar]
- 78.Brash L, Barnes GD, Brewis MJ, et al. 2018. Short-Term Hemodynamic Effects of Apelin in Patients With Pulmonary Arterial Hypertension. JACC Basic Transl Sci. 3: 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le Gonidec S, Chaves-Almagro C, Bai Y, et al. 2017. Protamine is an antagonist of apelin receptor, and its activity is reversed by heparin. FASEB J. 31: 2507–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wakefield TW, Bies LE, Wrobleski SK, et al. 1990. Impaired myocardial function and oxygen utilization due to protamine sulfate in an isolated rabbit heart preparation. Ann Surg. 212: 387–393; discussion 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Del Re MR, Ayd JD, Schultheis LW, et al. 1993. Protamine and left ventricular function: a transesophageal echocardiography study. Anesth Analg. 77: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 82.Park KW 2004. Protamine and protamine reactions. Int Anesthesiol Clin. 42: 135–145. [DOI] [PubMed] [Google Scholar]
- 83.Zhou H, Yang R, Wang W, et al. 2018. Fc-apelin fusion protein attenuates lipopolysaccharide-induced liver injury in mice. Sci Rep. 8: 11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coquerel D, Sainsily X, Dumont L, et al. 2018. The apelinergic system as an alternative to catecholamines in low-output septic shock. Crit Care. 22: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coquerel D, Chagnon F, Sainsily X, et al. 2017. ELABELA Improves Cardio-Renal Outcome in Fatal Experimental Septic Shock. Crit Care Med. 45: e1139–e1148. [DOI] [PubMed] [Google Scholar]
- 86.Han S, Englander EW, Gomez GA, et al. 2017. Apelin Regulates Nuclear Factor-kappaB’s Involvement in the Inflammatory Response of Pancreatitis. Pancreas. 46: 64–70. [DOI] [PubMed] [Google Scholar]
- 87.Leeper NJ, Tedesco MM, Kojima Y, et al. 2009. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 296: H1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang ZZ, Wang W, Jin HY, et al. 2017. Apelin Is a Negative Regulator of Angiotensin II-Mediated Adverse Myocardial Remodeling and Dysfunction. Hypertension. 70: 1165–1175. [DOI] [PubMed] [Google Scholar]
- 89.Huang Z, Wu L & Chen L. 2018. Apelin/APJ system: A novel potential therapy target for kidney disease. J Cell Physiol. 233: 3892–3900. [DOI] [PubMed] [Google Scholar]
- 90.Xu R, Zhang ZZ, Chen LJ, et al. 2016. Ascending aortic adventitial remodeling and fibrosis are ameliorated with Apelin-13 in rats after TAC via suppression of the miRNA-122 and LGR4-beta-catenin signaling. Peptides. 86: 85–94. [DOI] [PubMed] [Google Scholar]
- 91.Lv SY, Cui B, Chen WD, et al. 2017. Apelin/APJ system: A key therapeutic target for liver disease. Oncotarget. 8: 112145–112151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X, Hu W, Feng F, et al. 2016. Apelin-13 protects against myocardial infarction-induced myocardial fibrosis. Mol Med Rep. 13: 5262–5268. [DOI] [PubMed] [Google Scholar]
- 93.McMurray JJ 2011. CONSENSUS to EMPHASIS: the overwhelming evidence which makes blockade of the renin-angiotensin-aldosterone system the cornerstone of therapy for systolic heart failure. Eur J Heart Fail. 13: 929–936. [DOI] [PubMed] [Google Scholar]
- 94.Yancy CW, Jessup M, Bozkurt B, et al. 2013. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 95.Mozaffarian D, Benjamin EJ, Go AS, et al. 2015. Heart disease and stroke statistics−−2015 update: a report from the American Heart Association. Circulation. 131: e29–322. [DOI] [PubMed] [Google Scholar]
- 96.Braunwald E & Bristow MR. 2000. Congestive heart failure: fifty years of progress. Circulation. 102: IV14–23. [DOI] [PubMed] [Google Scholar]
- 97.Bhatt AS, Ambrosy AP & Velazquez EJ. 2017. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Curr Cardiol Rep. 19: 71. [DOI] [PubMed] [Google Scholar]
- 98.Peterzan MA, Lygate CA, Neubauer S, et al. 2017. Metabolic remodeling in hypertrophied and failing myocardium: a review. Am J Physiol Heart Circ Physiol. 313: H597–H616. [DOI] [PubMed] [Google Scholar]
- 99.Katz AM & Rolett EL. 2016. Heart failure: when form fails to follow function. Eur Heart J. 37: 449–454. [DOI] [PubMed] [Google Scholar]
- 100.Putko BN, Wang Z, Lo J, et al. 2014. Circulating levels of tumor necrosis factor-alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: evidence for a divergence in pathophysiology. PloS one. 9: e99495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Senni M, Paulus WJ, Gavazzi A, et al. 2014. New strategies for heart failure with preserved ejection fraction: the importance of targeted therapies for heart failure phenotypes. Eur Heart J. 35: 2797–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paulus WJ & Tschope C. 2013. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology. 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 103.Wang W, McKinnie SM, Patel VB, et al. 2013. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia-reperfusion injury: therapeutic potential of synthetic Apelin analogues. Journal of the American Heart Association. 2: e000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scimia MC, Hurtado C, Ray S, et al. 2012. APJ acts as a dual receptor in cardiac hypertrophy. Nature. 488: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Foldes G, Horkay F, Szokodi I, et al. 2003. Circulating and cardiac levels of apelin, the novel ligand of the orphan receptor APJ, in patients with heart failure. Biochem Biophys Res Commun. 308: 480–485. [DOI] [PubMed] [Google Scholar]
- 106.Iwanaga Y, Kihara Y, Takenaka H, et al. 2006. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: Possible role of angiotensin II-angiotensin type 1 receptor system. J Mol Cell Cardiol. 41: 798–806. [DOI] [PubMed] [Google Scholar]
- 107.Basu R, Poglitsch M, Yogasundaram H, et al. 2017. Roles of Angiotensin Peptides and Recombinant Human ACE2 in Heart Failure. J Am Coll Cardiol. . 69 805–819. [DOI] [PubMed] [Google Scholar]
- 108.Dalzell JR, Rocchiccioli JP, Weir RA, et al. 2015. The Emerging Potential of the Apelin-APJ System in Heart Failure. Journal of cardiac failure. 21: 489–498. [DOI] [PubMed] [Google Scholar]
- 109.Onorato JM, Xu C, Chen XQ, et al. 2019. Linking (Pyr)(1)apelin-13 pharmacokinetics to efficacy: Stabilization and measurement of a high clearance peptide in rodents. Anal Biochem. 568: 41–50. [DOI] [PubMed] [Google Scholar]
- 110.Boal F, Roumegoux J, Alfarano C, et al. 2015. Apelin regulates FoxO3 translocation to mediate cardioprotective responses to myocardial injury and obesity. Sci Rep. 5: 16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhong J, Basu R, Guo D, et al. 2010. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 122: 717–728. [DOI] [PubMed] [Google Scholar]
- 112.Sato T, Kadowaki A, Suzuki T, et al. 2019. Loss of Apelin Augments Angiotensin II-Induced Cardiac Dysfunction and Pathological Remodeling. Int J Mol Sci. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siddiquee K, Hampton J, McAnally D, et al. 2013. The apelin receptor inhibits the angiotensin II type 1 receptor via allosteric trans-inhibition. Br J Pharmacol. 168: 1104–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pang H, Han B, Yu T, et al. 2014. Effect of apelin on the cardiac hemodynamics in hypertensive rats with heart failure. Int J Mol Med. 34: 756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koguchi W, Kobayashi N, Takeshima H, et al. 2012. Cardioprotective effect of apelin-13 on cardiac performance and remodeling in end-stage heart failure. Circ J. 76: 137–144. [DOI] [PubMed] [Google Scholar]
- 116.Perjes A, Skoumal R, Tenhunen O, et al. 2014. Apelin increases cardiac contractility via protein kinase Cepsilon- and extracellular signal-regulated kinase-dependent mechanisms. PloS one. 9: e93473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Przewlocka-Kosmala M, Kotwica T, Mysiak A, et al. 2011. Reduced circulating apelin in essential hypertension and its association with cardiac dysfunction. J Hypertens. 29: 971–979. [DOI] [PubMed] [Google Scholar]
- 118.van Kimmenade RR, Januzzi JL Jr., Ellinor PT, et al. 2006. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. Journal of the American College of Cardiology. 48: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 119.Wettersten N & Maisel A. 2015. Role of Cardiac Troponin Levels in Acute Heart Failure. Card Fail Rev. 1: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Japp AG, Cruden NL, Barnes G, et al. 2010. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 121: 1818–1827. [DOI] [PubMed] [Google Scholar]
- 121.Barnes GD, Alam S, Carter G, et al. 2013. Sustained cardiovascular actions of APJ agonism during renin-angiotensin system activation and in patients with heart failure. Circulation. Heart failure. 6: 482–491. [DOI] [PubMed] [Google Scholar]
- 122.Busch R, Strohbach A, Pennewitz M, et al. 2015. Regulation of the endothelial apelin/APJ system by hemodynamic fluid flow. Cellular signalling. 27: 1286–1296. [DOI] [PubMed] [Google Scholar]
- 123.Liu HT, Chen M, Yu J, et al. 2015. Serum apelin level predicts the major adverse cardiac events in patients with ST elevation myocardial infarction receiving percutaneous coronary intervention. Medicine (Baltimore). 94: e449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boal F, Timotin A, Roumegoux J, et al. 2016. Apelin-13 administration protects against ischaemia/reperfusion-mediated apoptosis through the FoxO1 pathway in high-fat diet-induced obesity. Br J Pharmacol. 173: 1850–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li L, Zeng H & Chen JX. 2012. Apelin-13 increases myocardial progenitor cells and improves repair postmyocardial infarction. Am J Physiol Heart Circ Physiol. 303: H605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tao J, Zhu W, Li Y, et al. 2011. Apelin-13 protects the heart against ischemia-reperfusion injury through inhibition of ER-dependent apoptotic pathways in a time-dependent fashion. Am J Physiol Heart Circ Physiol. 301: H1471–1486. [DOI] [PubMed] [Google Scholar]
- 127.Zeng XJ, Zhang LK, Wang HX, et al. 2009. Apelin protects heart against ischemia/reperfusion injury in rat. Peptides. 30: 1144–1152. [DOI] [PubMed] [Google Scholar]
- 128.Zhong J, Basu R, Guo D, et al. 201o. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 122: 717–728. [DOI] [PubMed] [Google Scholar]
- 129. https://clinicaltrials.gov/ct2/show/NCT03276728.
- 130.Reaux A, Gallatz K, Palkovits M, et al. 2002. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience. 113: 653–662. [DOI] [PubMed] [Google Scholar]
- 131.Reaux-Le Goazigo A, Morinville A, Burlet A, et al. 2004. Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology. 145: 4392–4400. [DOI] [PubMed] [Google Scholar]
- 132.Wang Z, Yu D, Wang M, et al. 2015. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci Rep. 5: 8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deng C, Chen H, Yang N, et al. 2015. Apela Regulates Fluid Homeostasis by Binding to the APJ Receptor to Activate Gi Signaling. J Biol Chem. 290: 18261–18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hus-Citharel A, Bodineau L, Frugiere A, et al. 2014. Apelin counteracts vasopressin-induced water reabsorption via cross talk between apelin and vasopressin receptor signaling pathways in the rat collecting duct. Endocrinology. 155: 4483–4493. [DOI] [PubMed] [Google Scholar]
- 135.Murza A, Sainsily X, Cote J, et al. 2017. Structure-activity relationship of novel macrocyclic biased apelin receptor agonists. Org Biomol Chem. 15: 449–458. [DOI] [PubMed] [Google Scholar]
- 136.Flahault A, Couvineau P, Alvear-Perez R, et al. 2017. Role of the Vasopressin/Apelin Balance and Potential Use of Metabolically Stable Apelin Analogs in Water Metabolism Disorders. Front Endocrinol (Lausanne). 8: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roberts EM, Pope GR, Newson MJ, et al. 2010. Stimulus-specific neuroendocrine responses to osmotic challenges in apelin receptor knockout mice. J Neuroendocrinol. 22: 301–308. [DOI] [PubMed] [Google Scholar]
- 138.Roberts EM, Newson MJ, Pope GR, et al. 2009. Abnormal fluid homeostasis in apelin receptor knockout mice. J Endocrinol. 202: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smyth A, Ronco C & Garovic VD. 2017. Preeclampsia: a Cardiorenal Syndrome in Pregnancy. Curr Hypertens Rep. 19: 15. [DOI] [PubMed] [Google Scholar]
- 140.Di Lullo L, Bellasi A, Barbera V, et al. 2017. Pathophysiology of the cardio-renal syndromes types 1–5: An uptodate. Indian Heart J. 69: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Silva AP, Fragoso A, Silva C, et al. 2013. What is the role of apelin regarding cardiovascular risk and progression of renal disease in type 2 diabetic patients with diabetic nephropathy? Biomed Res Int. 2013: 247649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O’Carroll AM, Salih S, Griffiths PR, et al. 2017. Expression and functional implications of the renal apelinergic system in rodents. PloS one. 12: e0183094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schreiber CA, Holditch SJ, Generous A, et al. 2017. Sustained ELABELA Gene Therapy in High-salt Diet-induced Hypertensive Rats. Curr Gene Ther. 16: 349–360. [DOI] [PubMed] [Google Scholar]
- 144.Chagnon F, Coquerel D, Salvail D, et al. 2017. Apelin Compared With Dobutamine Exerts Cardioprotection and Extends Survival in a Rat Model of Endotoxin-Induced Myocardial Dysfunction. Crit Care Med. 45: e391–e398. [DOI] [PubMed] [Google Scholar]
- 145.Russell JA, Walley KR, Singer J, et al. 2008. Vasopressin versus norepinephrine infusion in patients with septic shock. The New England journal of medicine. 358: 877–887. [DOI] [PubMed] [Google Scholar]
- 146.Russell JA, Fjell C, Hsu JL, et al. 2013. Vasopressin compared with norepinephrine augments the decline of plasma cytokine levels in septic shock. Am J Respir Crit Care Med. 188: 356–364. [DOI] [PubMed] [Google Scholar]
- 147.Walley KR 2018. Sepsis-induced myocardial dysfunction. Curr Opin Crit Care. 24: 292–299. [DOI] [PubMed] [Google Scholar]
- 148.Martin L, Derwall M, Al Zoubi S, et al. 2019. The Septic Heart: Current Understanding of Molecular Mechanisms and Clinical Implications. Chest. 155: 427–437. [DOI] [PubMed] [Google Scholar]
- 149.Schuler A, Wulf DA, Lu Y, et al. 2018. The Impact of Acute Organ Dysfunction on Long-Term Survival in Sepsis. Crit Care Med. 46: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ehrman RR, Sullivan AN, Favot MJ, et al. 2018. Pathophysiology, echocardiographic evaluation, biomarker findings, and prognostic implications of septic cardiomyopathy: a review of the literature. Crit Care. 22: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cotter G, Moshkovitz Y, Kaluski E, et al. 2003. The role of cardiac power and systemic vascular resistance in the pathophysiology and diagnosis of patients with acute congestive heart failure. Eur J Heart Fail. 5: 443–451. [DOI] [PubMed] [Google Scholar]
- 152.Lesur O, Delile E, Asfar P, et al. 2018. Hemodynamic support in the early phase of septic shock: a review of challenges and unanswered questions. Ann Intensive Care. 8: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lambden S, Creagh-Brown BC, Hunt J, et al. 2018. Definitions and pathophysiology of vasoplegic shock. Crit Care. 22: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cecconi M, Evans L, Levy M, et al. 2018. Sepsis and septic shock. Lancet. 392: 75–87. [DOI] [PubMed] [Google Scholar]
- 155.Aslan A, van Meurs M, Moser J, et al. 2017. Organ-Specific Differences in Endothelial Permeability-Regulating Molecular Responses in Mouse and Human Sepsis. Shock. 48: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jaffee W, Hodgins S & McGee WT. 2018. Tissue Edema, Fluid Balance, and Patient Outcomes in Severe Sepsis: An Organ Systems Review. J Intensive Care Med. 33: 502–509. [DOI] [PubMed] [Google Scholar]
- 157.Pan CS, Teng X, Zhang J, et al. 2010. Apelin antagonizes myocardial impairment in sepsis. Journal of cardiac failure. 16: 609–617. [DOI] [PubMed] [Google Scholar]
- 158.Rudiger A, Dyson A, Felsmann K, et al. 2013. Early functional and transcriptomic changes in the myocardium predict outcome in a long-term rat model of sepsis. Clin Sci (Lond). 124: 391–401. [DOI] [PubMed] [Google Scholar]
- 159.Lesur O, Roussy JF, Chagnon F, et al. 2010. Proven infection-related sepsis induces a differential stress response early after ICU admission. Crit Care. 14: R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Luo K, Long H, Xu B, et al. 2015. Apelin attenuates postburn sepsis via a phosphatidylinositol 3-kinase/protein kinase B dependent mechanism: A randomized animal study. Int J Surg. 21: 22–27. [DOI] [PubMed] [Google Scholar]
- 161.Fan XF, Xue F, Zhang YQ, et al. 2015. The Apelin-APJ axis is an endogenous counterinjury mechanism in experimental acute lung injury. Chest. 147: 969–978. [DOI] [PubMed] [Google Scholar]
- 162.Soliman M & Arafah M. 2015. Apelin protect against multiple organ injury following hemorrhagic shock and decrease the inflammatory response. Int J Appl Basic Med Res. 5: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kumar A, Schupp E, Bunnell E, et al. 2008. Cardiovascular response to dobutamine stress predicts outcome in severe sepsis and septic shock. Crit Care. 12: R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cariou A, Pinsky MR, Monchi M, et al. 2008. Is myocardial adrenergic responsiveness depressed in human septic shock? Intensive Care Med. 34: 917–922. [DOI] [PubMed] [Google Scholar]
- 165.Vallet B, Chopin C, Curtis SE, et al. 1993. Prognostic value of the dobutamine test in patients with sepsis syndrome and normal lactate values: a prospective, multicenter study. Crit Care Med. 21: 1868–1875. [DOI] [PubMed] [Google Scholar]
- 166.Shepherd RE, Lang CH & McDonough KH. 1987. Myocardial adrenergic responsiveness after lethal and nonlethal doses of endotoxin. Am J Physiol. 252: H410–416. [DOI] [PubMed] [Google Scholar]
- 167.Bohm M, Kirchmayr R, Gierschik P, et al. 1995. Increase of myocardial inhibitory G-proteins in catecholamine-refractory septic shock or in septic multiorgan failure. Am J Med. 98: 183–186. [DOI] [PubMed] [Google Scholar]
- 168.Matsuda N, Hattori Y, Akaishi Y, et al. 2000. Impairment of cardiac beta-adrenoceptor cellular signaling by decreased expression of G(s alpha) in septic rabbits. Anesthesiology. 93: 1465–1473. [DOI] [PubMed] [Google Scholar]
- 169.Li L, Yang G, Li Q, et al. 2006. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 114: 544–548. [DOI] [PubMed] [Google Scholar]
- 170.Heinonen MV, Purhonen AK, Miettinen P, et al. 2005. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 130: 7–13. [DOI] [PubMed] [Google Scholar]
- 171.Dray C, Knauf C, Daviaud D, et al. 2008. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 8: 437–445. [DOI] [PubMed] [Google Scholar]
- 172.Yue P, Jin H, Aillaud M, et al. 2010. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 298: E59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gourdy P, Cazals L, Thalamas C, et al. 2018. Apelin administration improves insulin sensitivity in overweight men during hyperinsulinaemic-euglycaemic clamp. Diabetes Obes Metab. 20: 157–164. [DOI] [PubMed] [Google Scholar]
- 174.Vinel C, Lukjanenko L, Batut A, et al. 2018. The exerkine apelin reverses age-associated sarcopenia. Nat Med. 24: 1360–1371. [DOI] [PubMed] [Google Scholar]
- 175.Safdar A, Saleem A & Tarnopolsky MA. 2016. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 12: 504–517. [DOI] [PubMed] [Google Scholar]
- 176.Lyall F, Bulmer JN, Kelly H, et al. 1999. Human trophoblast invasion and spiral artery transformation: the role of nitric oxide. Am J Pathol. 154: 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lyall F, Robson SC & Bulmer JN. 2013. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: relationship to clinical outcome. Hypertension. 62: 1046–1054. [DOI] [PubMed] [Google Scholar]
- 178.Whigham CA, MacDonald TM, Walker SP, et al. 2019. The untapped potential of placenta-enriched molecules for diagnostic and therapeutic development. Placenta. [DOI] [PubMed] [Google Scholar]
- 179.Phipps EA, Thadhani R, Benzing T, et al. 2019. Pre-eclampsia: pathogenesis, novel diagnostics and therapies. Nat Rev Nephrol. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kidoya H & Takakura N. 2012. Biology of the apelin-APJ axis in vascular formation. J Biochem. 152: 125–131. [DOI] [PubMed] [Google Scholar]
- 181.Freyer L, Hsu CW, Nowotschin S, et al. 2017. Loss of Apela Peptide in Mice Causes Low Penetrance Embryonic Lethality and Defects in Early Mesodermal Derivatives. Cell Rep. 20: 2116–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Helker CS, Schuermann A, Pollmann C, et al. 2015. The hormonal peptide Elabela guides angioblasts to the midline during vasculogenesis. Elife. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Greenlee MW, Gerling J & Waltenspiel S. 1990. Spatial-frequency discrimination of drifting gratings. Vision Res. 30: 1331–1339. [DOI] [PubMed] [Google Scholar]
- 184.Cobellis L, De Falco M, Mastrogiacomo A, et al. 2007. Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol Histopathol. 22: 1–8. [DOI] [PubMed] [Google Scholar]
- 185.Canfield J, Arlier S, Mong EF, et al. 2019. Decreased LIN28B in preeclampsia impairs human trophoblast differentiation and migration. FASEB J. 33: 2759–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ho L, van Dijk M, Chye STJ, et al. 2017. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science. 357: 707–713. [DOI] [PubMed] [Google Scholar]
- 187.Panaitescu B, Romero R, Gomez-Lopez N, et al. 2018. ELABELA plasma concentrations are increased in women with late-onset preeclampsia. J Matern Fetal Neonatal Med. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pritchard N, Kaitu’u-Lino TJ, Gong S, et al. 2018. ELABELA/APELA Levels Are Not Decreased in the Maternal Circulation or Placenta among Women with Preeclampsia. Am J Pathol. 188: 1749–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Murza ATK; Bru neau-Cossette L; Lesur O; Auger-Messier M; Lavigne P; Sarret P; Marsault E 2018. Apelins, ELABELA and their derivatives: peptidic regulators of the cardiovascular system and beyond. Peptide Science. e24064. [Google Scholar]
- 190.Narayanan S, Harris DL, Maitra R, et al. 2015. Regulation of the Apelinergic System and Its Potential in Cardiovascular Disease: Peptides and Small Molecules as Tools for Discovery. J Med Chem. 58: 7913–7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Murza A, Parent A, Besserer-Offroy E, et al. 2012. Elucidation of the structure-activity relationships of apelin: influence of unnatural amino acids on binding, signaling, and plasma stability. ChemMedChem. 7: 318–325. [DOI] [PubMed] [Google Scholar]
- 192.Gerbier R, Alvear-Perez R, Margathe JF, et al. 2017. Development of original metabolically stable apelin-17 analogs with diuretic and cardiovascular effects. FASEB J. 31: 687–700. [DOI] [PubMed] [Google Scholar]
- 193.Langelaan DN & Rainey JK. 2009. Headgroup-dependent membrane catalysis of apelin-receptor interactions is likely. J Phys Chem B. 113: 10465–10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Langelaan DN, Reddy T, Banks AW, et al. 2013. Structural features of the apelin receptor N-terminal tail and first transmembrane segment implicated in ligand binding and receptor trafficking. Biochim Biophys Acta. 1828: 1471–1483. [DOI] [PubMed] [Google Scholar]
- 195.Lee DK, Saldivia VR, Nguyen T, et al. 2005. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 146: 231–236. [DOI] [PubMed] [Google Scholar]
- 196.Iturrioz X, Gerbier R, Leroux V, et al. 2010. By interacting with the C-terminal Phe of apelin, Phe255 and Trp259 in helix VI of the apelin receptor are critical for internalization. J Biol Chem. 285: 32627–32637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Murza A, Belleville K, Longpre JM, et al. 2014. Stability and degradation patterns of chemically modified analogs of apelin-13 in plasma and cerebrospinal fluid. Biopolymers. 102: 297–303. [DOI] [PubMed] [Google Scholar]
- 198.Murza A, Besserer-Offroy E, Cote J, et al. 2015. C-Terminal modifications of apelin-13 significantly change ligand binding, receptor signaling, and hypotensive action. J Med Chem. 58: 2431–2440. [DOI] [PubMed] [Google Scholar]
- 199.Ma Y, Yue Y, Ma Y, et al. 2017. Structural Basis for Apelin Control of the Human Apelin Receptor. Structure. 25: 858–866 e854. [DOI] [PubMed] [Google Scholar]
- 200.Jia ZQ, Hou L, Leger A, et al. 2012. Cardiovascular effects of a PEGylated apelin. Peptides. 38: 181–188. [DOI] [PubMed] [Google Scholar]
- 201.Juhl C, Els-Heindl S, Schonauer R, et al. 2016. Development of Potent and Metabolically Stable APJ Ligands with High Therapeutic Potential. ChemMedChem. 11: 2378–2384. [DOI] [PubMed] [Google Scholar]
- 202.Wang W, Zhang D, Yang R, et al. 2018. Hepatic and cardiac beneficial effects of a long-acting Fc-apelin fusion protein in diet-induced obese mice. Diabetes Metab Res Rev. 34: e2997. [DOI] [PubMed] [Google Scholar]
- 203.Serpooshan V, Sivanesan S, Huang X, et al. 2015. [Pyr1]-Apelin-13 delivery via nano-liposomal encapsulation attenuates pressure overload-induced cardiac dysfunction. Biomaterials. 37: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Marsault E, Peterson ML 2017. Practical medicinal chemistry with macrocycles; Wiley, Hoboken. [Google Scholar]
- 205.Macaluso NJ & Glen RC. 2010. Exploring the ‘RPRL’ motif of apelin-13 through molecular simulation and biological evaluation of cyclic peptide analogues. ChemMedChem. 5: 1247–1253. [DOI] [PubMed] [Google Scholar]
- 206.Macaluso NJ, Pitkin SL, Maguire JJ, et al. 2011. Discovery of a competitive apelin receptor (APJ) antagonist. ChemMedChem. 6: 1017–1023. [DOI] [PubMed] [Google Scholar]
- 207.Brame AL, Maguire JJ, Yang P, et al. 2015. Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. Hypertension. 65: 834–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tran K, Murza A, Sainsily X, et al. 2018. A Systematic Exploration of Macrocyclization in Apelin-13: Impact on Binding, Signaling, Stability, and Cardiovascular Effects. J Med Chem. 61: 2266–2277. [DOI] [PubMed] [Google Scholar]
- 209.Gerbier R, Leroux V, Couvineau P, et al. 2015. New structural insights into the apelin receptor: identification of key residues for apelin binding. FASEB J. 29: 314–322. [DOI] [PubMed] [Google Scholar]
- 210.Margathe JF, Iturrioz X, Alvear-Perez R, et al. 2014. Structure-activity relationship studies toward the discovery of selective apelin receptor agonists. J Med Chem. 57: 2908–2919. [DOI] [PubMed] [Google Scholar]
- 211.Murza A, Sainsily X, Coquerel D, et al. 2016. Discovery and Structure-Activity Relationship of a Bioactive Fragment of ELABELA that Modulates Vascular and Cardiac Functions. J Med Chem. 59: 2962–2972. [DOI] [PubMed] [Google Scholar]
- 212.Khan P, Maloney PR, Hedrick M, et al. 2010. “Functional Agonists of the Apelin (APJ) Receptor”. In Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD). [Google Scholar]
- 213.Maloney PR, Khan P, Hedrick M, et al. 2012. Discovery of 4-oxo-6-((pyrimidin-2-ylthio)methyl)-4H-pyran-3-yl 4-nitrobenzoate (ML221) as a functional antagonist of the apelin (APJ) receptor. Bioorg Med Chem Lett. 22: 6656–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]


