Abstract
Pituitary adenylate cyclase activating polypeptide acting through its cognate receptors, PAC1, VPAC1, and VPAC2, is a pleiotropic signaling neuropeptide of the vasoactive intestinal peptide/secretin/glucagon family. PACAP has known functions in neuronal growth, development, repair, and central PACAP signaling has acute behavioral consequences. One of the ways in which PACAP may affect neuronal function is through the modulation of intrinsic membrane currents to control neuronal excitability. Here we review evidence of PACAP-dependent modulation of voltage-gated potassium currents, hyperpolarization activated cation currents, calcium currents, and voltage-gated sodium currents. Interestingly, PACAP signaling pathways diverge into parallel pathways to target different ionic currents for modulation, though single pathways are not limited to modulating just one target ionic current. Despite the various targets of modulation, the weight of the evidence suggests that PACAP signaling most commonly leads to a net-increase in neuronal excitability. We discuss possible mechanisms by which PACAP signaling leads to the modulation of intrinsic membrane currents to change behavior.
Keywords: Currents, PAC1, ERK, HCN, Kv, Endosome
Introduction
Pituitary adenylate cyclase activating polypeptide (PACAP, ADCYAP1), a member of the vasoactive intestinal peptide (VIP)/secretin/glucagon family of related peptides, has diverse functions in development, homeostatic signaling in many physiological systems, and repair/regeneration responses to neural injury or related challenges.1 The expression and function of PACAP are tightly regulated, but notably maladaptive PACAP signaling has been implicated in many psychiatric disorders including post-traumatic stress disorder,2 schizophrenia,3 and major depressive disorder.4 The behavioral effects of PACAP have both acute neurotransmitter and long-term neuroplasticity components to mediate the rapid and sustaining consequences of stress, respectively. The targeted infusion of PACAP into specific regions in the CNS, for example, can produce rapid behavioral changes, suggesting an important role for direct effects of PACAP on neuronal excitability.5 Moreover, a single infusion can also produce behavioral changes that can persist for hours and days to suggest more long-term plasticity changes resulting in altered neuronal function,6 and PACAP signaling can be sensitized by prior chronic stress.7 The long-term effects of PACAP have been well-examined, especially in a neurotrophic context for cell proliferation, survival and repair after injury.1 By contrast, the PACAP mechanisms underlying the regulation of ionic conductances mediating acute responses have not been fully elucidated. PACAP binds to three different heptahelical G protein-coupled receptors with relatively equal high affinity, including the PAC1 (ADCYAP1R1), VPAC1 (VIPR1), and VPAC2 (VIPR2) receptors. The VPAC1 and VPAC2 receptors also bind VIP with similar affinities as PACAP. Whereas the many PAC1 receptor isoforms can be coupled to Gαs and Gαq to engage multiple intracellular signaling pathways, VPAC receptors principally couple Gαs to activate adenylyl cyclase (AC) to increase intracellular cAMP levels.1 More recently, PAC1 receptor activation has also been shown to lead to β-arrestin-mediated receptor internalization and endosomal signaling leading to sustained MEK/ERK signaling.8, 9
From the activation of diverse signaling pathways, PACAP/PAC1 receptor signaling has the potential of coordinating the function of several ionic channels to regulate neuronal excitability. Changes in neuronal excitability can be differentiated broadly into synaptic and intrinsic plasticity. Synaptic plasticity is the modification of synaptic strength or sensitivity and can be modified either presynaptically, via changes in the probability of transmitter release or readily releasable pool of synaptic vesicles, or post-synaptically, such as in AMPA receptor trafficking in long term potentiation (LTP). There is evidence of PACAPergic regulation of synaptic strength in addition to the PACAP modulation of intrinsic currents discussed below 10, 11 though a discussion of synaptic actions is beyond the scope of this review. Beyond synaptic strength, neuronal excitability may also be altered through changes in intrinsic neuronal excitability, due to changes in ionic currents through voltage-gated channels, caused by changes in cell-surface channel expression or alteration in the voltage-dependence of channel activation and/or inactivation. These modifications can alter the basic properties of neuronal electrical activity, such as resting membrane potential, spike threshold, or local excitability in neuronal processes which can produce extensive changes in brain regions that impact behavior (see ref. 5 for review). The functional changes in the intrinsic excitability of neurons can be regulated by canonical signaling pathways that include AC/cAMP/PKA, PLC/DAG/IP3/PKC and MEK/ERK, which is activated by either β-arrestin and endocytosis, or neuritogenic cAMP sensor (NCS) rapgef2; we review how some of the intrinsic membrane currents can be regulated by PACAP/PAC1 receptor activation (figure 1).
Figure 1:
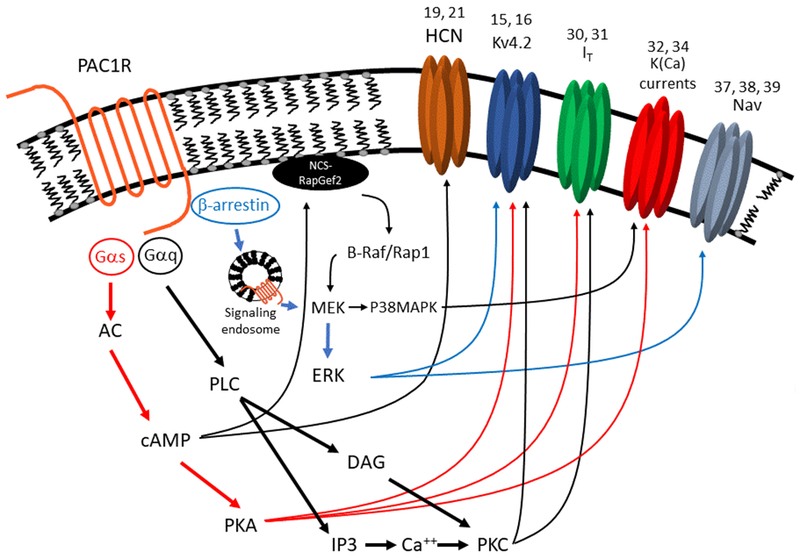
PACAP activates multiple signaling pathways to regulate several ionic currents controlling intrinsic neuronal excitability. The PAC1 receptor is dually-coupled to Gαs and Gαq subunits, which activate adenylyl cyclase and phospholipase c respectively. cAMP and PKA are both capable of modulating ionic currents, as well as stimulating further signaling factors, such as RapGef. Downstream of PLC, PKC has been shown to modulate multiple ionic currents. PAC1 is also capable of stimulating phosphorylation of ERK via β-arrestin and receptor endocytosis, and downstream of cAMP via RapGef and Rap1. Phosphorylated ERK is then capable of modulating both potassium and sodium conductances. Numbers indicate references demonstrating PACAP effect on specified current.
Rapidly inactivating potassium currents or A-Currents (IA)
Canonical voltage-gated potassium channels (Kv) have principal roles in action potential (AP) repolarization but can also regulate neuronal excitability by other means such as the modulation of an A-current (IA). IA is a rapidly inactivating, voltage-dependent, and 4-aminopyridine-sensitive outward potassium current that upon activation, causes rapid repolarization following depolarization. A-type channels are often found on dendritic processes to regulate local excitability; IA suppresses incoming dendritic postsynaptic potentials, thereby diminishing the temporal and spatial summation of signals at the soma to decrease AP generation probability. The assembly of Kv tetramer subunits mediating IA conduction can include either Kv1.4, Kv3.4, or Kv4.1 - 4.3 subunits.13 Kv1.4 and Kv3.4 are found on axons while Kv4.1 - Kv4.3 are localized to dendrites and soma.13, 14 The cellular distribution of these channels implicates their function; the dendritic localization of these channels suggests roles in regulating the summation of incoming postsynaptic potentials and the back-propagation of APs, somatic channels might regulate overall excitability, and axonal channels may regulate AP transmission. Hence, the differential distribution of Kv channels suggests that their modulation by peptides could impact several different factors controlling neuronal excitability.
Direct assessment via voltage clamp by Gupte et al.15 found that PACAP/PAC1 receptor signaling phosphorylated and diminished the surface expression of Kv4.2 in cultured hippocampal neurons, resulting in a reduction of an outwardly rectifying current consistent with IA.15 These effects could be partially recapitulated with forskolin to increase endogenous cAMP production, suggesting a role for PACAP/PAC1 receptor Gαs/adenylyl cyclase/cAMP signaling on IA. However, the PACAP-mediated decrease in IA was completely blocked by co-treatments with the MEK1/2 inhibitor U0126, which also implicated MEK/ERK activation as a critical component of PACAP regulation of IA in these cells.15 As PKA and pERK phosphorylation sites have been identified on the regulatory domains of Kv4.2,14 these mechanisms in aggregate could directly regulate Kv4.2 cell surface expression to reduce IA. Similar effects have been observed in olfactory epithelial tissue where PACAP was shown to down regulate the surface expression of Kv4.2 and Kv1.4; however, unlike the PKA- and MEK/ERK-dependent mechanisms in hippocampal neurons, the downregulatory events in the olfactory epithelium were dependent on PLC activation and intracellular calcium to suggest PKC-dependent processes.16 The reduction/inhibition of IA channels by PACAP would functionally facilitate neural excitation. With the localization of these channels in dendritic arbors, the results in aggregate suggest PACAP roles in modulating the strength of postsynaptic potentials and AP back-propagation, both of which are known to participate in synaptic potentiation, especially important in learning and memory processes.
Hyperpolarization-activated cyclic nucleotide gated cation currents or H-Currents (IH)
Hyperpolarization-activated cyclic nucleotide gated channels (HCN) are voltage-gated cationic channels that upon activation allow inward currents (IH) to counter membrane hyperpolarization, such as the after-hyperpolarization that can follow an AP, and can produce a rebound depolarization following the termination of that hyperpolarization.17 Accordingly, IH can play a critical role in regulating spike frequency and pacemaking in rhythmic firing neurons. A number of regulatory sites have been identified within HCN channels capable of modulating its activity, most notably a cyclic nucleotide binding site that when bound affects the voltage-sensing domain of the channel, to allow the channel to open at a less polarized voltage. Enhancement of IH can effectively decrease the amplitude and duration of the neuronal spike afterhyperpolarization and thereby promote increase spike frequency.18
An example of PACAP modulation of IH contributions to enhanced neuronal excitability occurs in guinea pig cardiac neurons. PACAP/PAC1 receptor signaling enhances IH in these postganglionic parasympathetic neurons due to a positive shift in voltage-dependence of channel activation.19 In current clamp recordings, PACAP enhanced both the rectification in the hyperpolarization elicited by current injection and the rebound depolarization following the termination of the hyperpolarization. A PACAP-induced enhancement of IH also contributed to the peptide-induced increase in AP generation by depolarizing steps. The effects of PACAP on cardiac neuron IH were recapitulated by forskolin, indicating that a PACAP-stimulated increase in cAMP signaling is responsible for the enhancement of IH.19
HCN channels can be found in many cellular compartments including soma, axons, and dendrites, but within a given neuron HCN channel expression is often restricted to specific sites.20 As PACAP enhances IH function, PACAP may regulate rhythmic firing and oscillatory activities in diverse regions of the nervous system, such as in the thalamus.21 For example, the medial septum is a central generator of theta rhythms and heavily expresses both the PAC1 receptor22 and HCN channels.23, 24 Accordingly, a positive shift in HCN channel activation voltage downstream of PACAP signaling could facilitate spike bursts in rhythmically firing neurons to impact many limbic circuit functions.
Transient low voltage-activated calcium currents (IT)
Transient, low voltage-activated calcium currents (IT) are inward currents that flow through T-type calcium channels.25, 26 Their voltage-dependence of activation, transient nature and sensitivity to low concentrations of nickel distinguishes T-type calcium currents from longer-lasting calcium currents carried by other voltage-dependent calcium channels (Cav), such as L-type, N-type and P-type channels.27, 28 The principal pore-forming α-subunit of voltage-gated calcium channels Cav3.1, Cav3.2 and Cav3.3, composed of a tandem of four homologous domains containing 6 transmembrane α-helices each, carry IT. Among these, Cav3.2 is the best studied with the intracellular loop between the second and third domain having known regulation sites by PKC, PKA, and Gβγ.29 IT has an activation voltage more negative than spike-threshold and functionally supports both burst firing and pacemaker activity. Often, T-type calcium currents work in concert with IH to maintain net depolarizing inward current following deactivation of IH.27 Given these complementary roles, T-type channels are often found to be expressed in some of the same regions as HCN channels, including the sinoatrial node, and thalamic regions.27
Guinea pig cardiac neurons express T-type calcium channel transcripts and low concentrations of nickel can suppress the PACAP-induced increase in excitability of guinea pig cardiac neurons suggesting that the PACAP-induced changes in these neurons are due in part to an enhancement of IT.30 PACAP also increases IT in adrenal chromaffin cells, an effect reversed by treatment with PKC inhibitors.31 PACAP modulation of IT is likely a mechanism contributing to the regulation of neuronal excitability by PACAP in CNS neurons and as PACAP can engage multiple signaling pathways, the mechanisms underlying its regulation of IT may be neuronal specific.
Calcium-activated potassium currents
PACAP, like many other neurotransmitters/neuropeptides, depresses a calcium-dependent late slow outward potassium current in CA1 pyramidal cells.32 This slow outward current underlies the late slow component of the three component afterhyperpolarization (AHP) that follows an AP in these neurons. The AHP is markedly enhanced by repetitive AP generation.33 Inhibition of this late slow outward current decreases AP frequency adaptation so that the number of APs generated by depolarizing current injection increases. Part of this PACAP effect is mediated through PAC1 receptor activation, the remainder through activation of VPAC1 receptors. Activation of AC/cAMP/PKA and p38 MAPK signaling cascades mediate the PACAP modulation of the slow calcium-dependent potassium current.32
In cerebellar neurons PACAP enhances calcium-sensitive, voltage-dependent big conductance (BK) channel activity, an action mediated by activation of a cAMP/Epac/p38 MAPK signaling.34 Similarly, PACAP activation of BK channels in smooth muscle myocytes can contribute to the regulation of cerebral artery tone.35 Thus, it is quite likely that PACAP modulation of BK channels in multiple neuronal types regulates resting membrane potentials. PACAP may also alter neuronal calcium-dependent small-conductance (SK) channels, although direct evidence for this has not been demonstrated. SK channels typically regulate a slow-afterhyperpolarization event in some neurons following significant firing events such as bursts, where intracellular calcium rises to activate the channel.17 Tonic levels of PKA-activation regulate SK channel clustering and activity,36 so PACAP activation of AC/cAMP/PKA signaling could potentially modulate SK channel activity.
Overall, these observations demonstrate that PACAP can potentially modulate different types of calcium-dependent potassium-channels; whether enhancing or depressing their activity being dependent on the specific channel and neuronal expression.
Voltage-gated sodium channels
Given the diversity of PACAP-stimulated intracellular signaling cascades, PACAP likely activates and/or inhibits other ionic conductances that regulate excitability in addition to those above. For instance, modulation of different voltage-gated sodium channels (Nav) potentially could mediate some effects of PACAP on neuronal excitability. In early studies examining the effect of PACAP on neuronal activity, Shibuya and colleagues (Ref 37) demonstrated that PACAP induced a sodium-dependent depolarization that increased spiking behavior. More recent studies in guinea pig cardiac ganglion cells showed that the PACAP-induced increase in excitability is sensitive to treatment with putative Nav1.7 channel inhibitors.38 The enhancement of Nav1.7 was mediated through a PACAP/PAC1 activation of MEK/ERK signaling. Consistent with this conclusion, earlier studies reported that Nav1.7 channel α subunits were phosphorylated by pERK; the phosphorylation initiating a hyperpolarizing shift in the voltage dependence of channel activation.39
The results above suggest a role of sodium channel modulation by PACAP as a potential mechanism contributing to neuronal excitability in CNS neurons. However, direct assessment of PACAP modulation of neuronal sodium currents in intact CNS neurons is difficult because of their complicated geometry and because the gating kinetics of neuronal voltage-gated sodium channels are commonly too fast to accurately measure under voltage clamp conditions. While this shortcoming potentially might be addressed with experiments conducted at lower temperatures, PACAP likely does not initiate some of its signaling cascades below physiological temperatures.8 Using pharmacological treatments with sodium channel inhibitors, combined with AP measurements under current clamp recording conditions, also is likely not to be as useful an approach with CNS neurons as with peripheral neurons, in part because of limited selectivity of most inhibitors between Nav channel types in CNS neurons. Thus, although modulation of Nav channels is potentially a component of the PACAP-mediated change in excitability, the role and mechanisms remain to be elucidated.
Channel regulation may lead to behavioral outcomes
The observations that PACAP can cause acute behavioral changes suggest that PACAP may rapidly alter neuronal activity in limbic circuits by regulating in part ion channel conductances. This has yet to be demonstrated, but there are behavioral phenotypes that are clearly regulated by PACAP where the PACAP-modulated downstream ionic conductances could be identified through rigorous study. For example, our laboratory and others have demonstrated that PACAP actions in the central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST) participate in chronic stress and pain mechanisms. Chronic stress and pain paradigms can upregulate PACAP/PAC1 receptor expression.5 PACAP infusions into these regions can alter pain- and stress-coping behaviors. For example, PACAP infusions into the CeA caused pain hypersensitivity and increased avoidance in an elevated plus maze 40; BNST PACAP infusions heightened acoustic startle,6 increased peripheral corticosterone release,41 caused an anorexic phenotype,42 and caused reinstatement of drug seeking.43 The ion channels that participate in the CeA- and BNST-mediated behavioral changes can be variable and notably, the CeA and BNST express channels that can carry IA, IH, and IT described above.44, 45 As PACAP/PAC1 receptor signaling can engage diverse intracellular signaling pathways, PACAP can affect cAMP-dependent enhancement of IH, PKA-dependent enhancement of IT, and MEK/ERK-dependent regulation of IA or gating of a NaV channel. The changes in channel activity would likely lead to changes in CeA or BNST activities to impact hypothalamic functions and other limbic regions that mediate fear and anxiety-like behaviors. There are multiple neurotransmitter and neuropeptidergic systems regulating behavior and an understanding of the mechanisms by which PACAP/PAC1 receptors contribute to the integration of these neurocircuit signaling events has the potential of generating new insights to maladaptive behavioral states.
Conclusions
PACAP binding to PAC1 receptors can potently and efficaciously generate multiple intracellular second messengers. PACAP/PAC1 receptor through its abilities to engage Gαs, Gαq and β-arrestin can activate downstream AC/cAMP/PKA, PLC/DAG/IP3/PKC, cAMP/NCS-RapGef2/Rap1/MEK, and endosomal MEK/ERK pathways to regulate diverse cellular functions that may be acute for rapid neuronal signaling or long term for adaptive neuroplasticity that may accompany development or responses to physiological challenges. Among these functions, PACAP/PAC1 receptor-mediated increase in neuronal excitability can involve the integrated contributions from multiple channel types modulated through the activation of different intracellular signaling pathways. PACAP/PAC1 receptors are interesting representatives of how a single ligand and receptor system can generate multiple signals to modulate diverse function including ionic conductances in the regulation of neuronal excitability (see figure 1) further studies of these mechanisms can lead to important insights into neurocircuits and the cell signaling events in health and disease.
Acknowledgments
Funding Sources: MH097988; UVM Vice President for Research Office; UVM College of Arts and Sciences
Footnotes
Disclosures: None of the authors have any competing interests to disclose.
References
- 1.Vaudry D, Falluel-Morel A, Bourgault S, et al. 2009. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacological reviews. 61: 283–357. [DOI] [PubMed] [Google Scholar]
- 2.Ressler KJ, Mercer KB, Bradley B, et al. 2011. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 470: 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashimoto R, Hashimoto H, Shintani N, et al. 2007. Pituitary adenylate cyclase-activating polypeptide is associated with schizophrenia. Molecular psychiatry. 12: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto R, Hashimoto H, Shintani N, et al. 2010. Possible association between the pituitary adenylate cyclase-activating polypeptide (PACAP) gene and major depressive disorder. Neuroscience letters. 468: 300–302. [DOI] [PubMed] [Google Scholar]
- 5.Hammack SE & May V. 2015. Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biological psychiatry. 78: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammack SE, Cheung J, Rhodes KM, et al. 2009. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 34: 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King SB, Lezak KR, O’Reilly M, et al. 2017. The Effects of Prior Stress on Anxiety-Like Responding to Intra-BNST Pituitary Adenylate Cyclase Activating Polypeptide in Male and Female Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 42: 1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merriam LA, Baran CN, Girard BM, et al. 2013. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. The Journal of neuroscience : the official journal of the Society for Neuroscience. 33: 4614–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May V, Buttolph TR, Girard BM, et al. 2014. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. American journal of physiology. Cell physiology. 306: C1068–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho JH, Zushida K, Shumyatsky GP, et al. 2012. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. The Journal of neuroscience : the official journal of the Society for Neuroscience. 32: 14165–14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa L, Santangelo F, Li Volsi G, et al. 2009. Modulation of AMPA receptor-mediated ion current by pituitary adenylate cyclase-activating polypeptide (PACAP) in CA1 pyramidal neurons from rat hippocampus. Hippocampus. 19: 99–109. [DOI] [PubMed] [Google Scholar]
- 12.Debanne D, Inglebert Y & Russier M. 2018. Plasticity of intrinsic neuronal excitability. Current opinion in neurobiology. 54: 73–82. [DOI] [PubMed] [Google Scholar]
- 13.Kim J & Hoffman DA. 2008. Potassium channels: newly found players in synaptic plasticity. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 14: 276–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrasquillo Y & Nerbonne JM. 2014. IA channels: diverse regulatory mechanisms. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 20: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupte RP, Kadunganattil S, Shepherd AJ, et al. 2016. Convergent phosphomodulation of the major neuronal dendritic potassium channel Kv4.2 by pituitary adenylate cyclase-activating polypeptide. Neuropharmacology. 101: 291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han P & Lucero MT. 2006. Pituitary adenylate cyclase activating polypeptide reduces expression of Kv1.4 and Kv4.2 subunits underlying A-type K(+) current in adult mouse olfactory neuroepithelia. Neuroscience. 138: 411–419. [DOI] [PubMed] [Google Scholar]
- 17.Hille B 2001. Ion channels of excitable membranes. Sinauer; Sunderland, MA. [Google Scholar]
- 18.Chen S, Wang J & Siegelbaum SA. 2001. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. The Journal of general physiology. 117: 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merriam LA, Barstow KL & Parsons RL. 2004. Pituitary adenylate cyclase-activating polypeptide enhances the hyperpolarization-activated nonselective cationic conductance, Ih, in dissociated guinea pig intracardiac neurons. Regulatory peptides. 123: 123–133. [DOI] [PubMed] [Google Scholar]
- 20.He C, Chen F, Li B, et al. 2014. Neurophysiology of HCN channels: from cellular functions to multiple regulations. Progress in neurobiology. 112: 1–23. [DOI] [PubMed] [Google Scholar]
- 21.Sun QQ, Prince DA & Huguenard JR. 2003. Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide activate hyperpolarization-activated cationic current and depolarize thalamocortical neurons in vitro. The Journal of neuroscience : the official journal of the Society for Neuroscience. 23: 2751–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takei N, Torres E, Yuhara A, et al. 2000. Pituitary adenylate cyclase-activating polypeptide promotes the survival of basal forebrain cholinergic neurons in vitro and in vivo: comparison with effects of nerve growth factor. The European journal of neuroscience. 12: 2273–2280. [DOI] [PubMed] [Google Scholar]
- 23.Hangya B, Borhegyi Z, Szilagyi N, et al. 2009. GABAergic neurons of the medial septum lead the hippocampal network during theta activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 29: 8094–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris NP, Fyffe RE & Robertson B. 2004. Characterisation of hyperpolarization-activated currents (I(h)) in the medial septum/diagonal band complex in the mouse. Brain research. 1006: 74–86. [DOI] [PubMed] [Google Scholar]
- 25.Talavera K & Nilius B. 2006. Biophysics and structure-function relationship of T-type Ca2+ channels. Cell calcium. 40: 97–114. [DOI] [PubMed] [Google Scholar]
- 26.Iftinca MC & Zamponi GW. 2009. Regulation of neuronal T-type calcium channels. Trends in pharmacological sciences. 30: 32–40. [DOI] [PubMed] [Google Scholar]
- 27.Catterall WA 2011. Voltage-gated calcium channels. Cold Spring Harbor perspectives in biology. 3: a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simms BA & Zamponi GW. 2014. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron. 82: 24–45. [DOI] [PubMed] [Google Scholar]
- 29.Sekiguchi F & Kawabata A. 2013. T-type calcium channels: functional regulation and implication in pain signaling. Journal of pharmacological sciences. 122: 244–250. [DOI] [PubMed] [Google Scholar]
- 30.Tompkins JD, Merriam LA, Girard BM, et al. 2015. Nickel suppresses the PACAP-induced increase in guinea pig cardiac neuron excitability. American journal of physiology. Cell physiology. 308: C857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill J, Chan SA, Kuri B, et al. 2011. Pituitary adenylate cyclase-activating peptide (PACAP) recruits low voltage-activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells. The Journal of biological chemistry. 286: 42459–42469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor RD, Madsen MG, Krause M, et al. 2014. Pituitary adenylate cyclase-activating polypeptide (PACAP) inhibits the slow afterhyperpolarizing current sIAHP in CA1 pyramidal neurons by activating multiple signaling pathways. Hippocampus. 24: 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sah P & Faber ES. 2002. Channels underlying neuronal calcium-activated potassium currents. Progress in neurobiology. 66: 345–353. [DOI] [PubMed] [Google Scholar]
- 34.Ster J, De Bock F, Guerineau NC, et al. 2007. Exchange protein activated by cAMP (Epac) mediates cAMP activation of p38 MAPK and modulation of Ca2+-dependent K+ channels in cerebellar neurons. Proceedings of the National Academy of Sciences of the United States of America. 104: 2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koide M, Syed AU, Braas KM, et al. 2014. Pituitary adenylate cyclase activating polypeptide (PACAP) dilates cerebellar arteries through activation of large-conductance Ca(2+)-activated (BK) and ATP-sensitive (K ATP) K (+) channels. Journal of molecular neuroscience : MN. 54: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abiraman K, Sah M, Walikonis RS, et al. 2016. Tonic PKA Activity Regulates SK Channel Nanoclustering and Somatodendritic Distribution. Journal of molecular biology. 428: 2521–2537. [DOI] [PubMed] [Google Scholar]
- 37.Shibuya I, Kabashima N, Tanaka K, et al. 1998. Patch-clamp analysis of the mechanism of PACAP-induced excitation in rat supraoptic neurones. Journal of neuroendocrinology. 10: 759–768. [DOI] [PubMed] [Google Scholar]
- 38.Tompkins JD, Clason TA, Hardwick JC, et al. 2016. Activation of MEK/ERK signaling contributes to the PACAP-induced increase in guinea pig cardiac neuron excitability. American journal of physiology. Cell physiology. 311: C643–c651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamboulian S, Choi J-S, Ahn H-S, et al. 2010. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Nav1. 7 and alters its gating properties. 30: 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Missig G, Roman CW, Vizzard MA, et al. 2014. Parabrachial nucleus (PBn) pituitary adenylate cyclase activating polypeptide (PACAP) signaling in the amygdala: implication for the sensory and behavioral effects of pain. Neuropharmacology. 86: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lezak KR, Roelke E, Harris OM, et al. 2014. Pituitary adenylate cyclase-activating polypeptide (PACAP) in the bed nucleus of the stria terminalis (BNST) increases corticosterone in male and female rats. Psychoneuroendocrinology. 45: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kocho-Schellenberg M, Lezak KR, Harris OM, et al. 2014. PACAP in the BNST produces anorexia and weight loss in male and female rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 39: 1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miles OW, Thrailkill EA, Linden AK, et al. 2018. Pituitary Adenylate Cyclase-Activating Peptide in the Bed Nucleus of the Stria Terminalis Mediates Stress-Induced Reinstatement of Cocaine Seeking in Rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 43: 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammack SE, Mania I & Rainnie DG. 2007. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. Journal of neurophysiology. 98: 638–656. [DOI] [PubMed] [Google Scholar]
- 45.Martina M, Royer S & Pare D. 1999. Physiological properties of central medial and central lateral amygdala neurons. Journal of neurophysiology. 82: 1843–1854. [DOI] [PubMed] [Google Scholar]


