Abstract
Psoriasis is a common inflammatory skin disease involving a cross‐talk between epidermal and immune cells. The role of specific epidermal stem cell populations, including hair follicle stem cells (HF‐SCs) in psoriasis is not well defined. Here, we show reduced expression of c‐JUN and JUNB in bulge HF‐SCs in patients with scalp psoriasis. Using lineage tracing in mouse models of skin inflammation with inducible deletion of c‐Jun and JunB, we found that mutant bulge HF‐SCs initiate epidermal hyperplasia and skin inflammation. Mechanistically, thymic stromal lymphopoietin (TSLP) was identified in mutant cells as a paracrine factor stimulating proliferation of neighboring non‐mutant epidermal cells, while mutant inter‐follicular epidermal (IFE) cells are lost over time. Blocking TSLP in psoriasis‐like mice reduced skin inflammation and decreased epidermal proliferation, VEGFα expression, and STAT5 activation. These findings unravel distinct roles of HF‐SCs and IFE cells in inflammatory skin disease and provide novel mechanistic insights into epidermal cell interactions in inflammation.
Keywords: epidermal hyper‐proliferation, hair follicle stem cells, lineage tracing, psoriasis, thymic stromal lymphopoietin
Subject Categories: Regenerative Medicine, Skin
Introduction
The epidermis is a stratified epithelium undergoing constant renewal due to the presence of stem cells (Alberts et al, 2002). It is composed of the inter‐follicular epidermis (IFE), which consists of layers of keratinocytes, hair follicles, and associated sebaceous and sweat glands (Fuchs, 2008). Within the IFE, epidermal stem cells are localized in the basal cell layer. Upon commitment to terminal differentiation, basal stem cells asymmetrically divide giving rise to post‐mitotic suprabasal layers establishing an epidermal barrier (Fuchs, 2008). In addition to the IFE, distinct regions in the hair follicle contain hair follicle stem cells (HF‐SCs), such as the bulge, isthmus and junctional zone of hair follicle, and sebaceous glands (Liu et al, 2003; Horsley et al, 2006; Jensen et al, 2009). Differential expression of specific markers characterizes the different stem cell subpopulations, allowing the study of their specific contributions to different aspects of cell behavior. For instance, in mice, HF‐SCs in the bulge region express CD34, while both HF‐SCs and IFE basal keratinocytes express integrin‐α6 (CD49f; Liu et al, 2003; Trempus et al, 2003; Horsley et al, 2006, 2008; Jaks et al, 2008; Jensen et al, 2009; Snippert et al, 2010). These different stem cell populations from IFE and hair follicles also have distinct roles during skin remodeling, wound healing, and homeostasis. For example, during homeostasis, committed progenitor cells (transit‐amplifying keratinocytes) of the IFE maintain the tissue renewal, while HF‐SCs remain largely quiescent until the onset of HF growth (Ito et al, 2005; Blanpain & Simons, 2013). During wound healing and acute inflammation, both IFE epidermal stem cells and K15+ bulge HF‐SCs contribute to early wound re‐epithelialization by increasing their proliferation rates, thereby expanding the pool of progenitor cells that migrate out of the follicles toward the wound (Ito et al, 2005; Levy et al, 2005; Arwert et al, 2012; Mascre et al, 2012; Blanpain & Simons, 2013). Furthermore, it is reported that basal and squamous skin cell carcinomas arise from K15+ bulge HF‐SCs (Trempus et al, 2007; Lapouge et al, 2011; Schober & Fuchs, 2011; Wang et al, 2011). However, little is known about the specific contribution of K15+ bulge HF‐SCs and IFE stem cells to the development of chronic inflammatory skin diseases such as psoriasis.
Psoriasis is a common heterogeneous inflammatory disease of unknown etiology, with an estimated worldwide prevalence of 3% in the population (Wagner et al, 2010). The vast majority of patients develop a chronic “plaque‐type” psoriasis referred to as psoriasis vulgaris. The most common sites of psoriasis are the elbows, knees, forearms, shins, retro‐auricular regions, and scalp (Wolf‐Henning Boehncke, 2015). Scalp psoriasis is the most common type of psoriasis, representing 50% of the cases of psoriatic patients (Icen et al, 2009). Approximately 75–90% of patients with plaque‐type psoriasis in other areas also develop psoriasis in the scalp (Ortonne et al, 2009). Psoriatic plaques are characterized and perpetuated by hyper‐proliferation of keratinocytes, impaired differentiation and permanent infiltration of neutrophils and T cells (Wagner et al, 2010). Many studies have focused on immunological aspects of the disease; however, treatments against immunological targets are not completely effective, and recurrence and chronicity prevail. In an epidermal context, it has been demonstrated that human transit‐amplifying keratinocytes in psoriatic plaques exhibit increased proliferation and a reduced apoptotic rate, fostering epidermal hyper‐proliferation and altered differentiation in situ and in vitro (McKay & Leigh, 1995; Truzzi et al, 2011). However, it remains unclear which epidermal lineages give rise to psoriasis, since a longitudinal analysis of the behavior of psoriatic keratinocytes in human patients is not achievable.
Genetically engineered mouse models (GEMMs) of psoriasis have been generated to mimic various aspects of the disease (Wagner et al, 2010). We have previously generated an inducible epidermal‐specific double knockout mouse model by epidermal deletion of c‐Jun and JunB using the K5 promoter (referred as DKO*). This model develops a psoriasis‐like disease within 2 weeks after induction, exhibiting several psoriatic hallmarks including hyper‐ and parakeratosis, inflammatory infiltrate, elevated levels of cytokines/chemokines, increased subepidermal vascularization, and some co‐morbidities like bone loss and arthritic joints (Zenz et al, 2005). Our goal in this study is to elucidate the specific contribution of different epidermal stem cell populations during the development of psoriasis‐like disease, by applying a novel strategy to simultaneously track the IFE and K15+ bulge HF‐SCs stem cell lineages in the DKO*‐psoriasis‐like mouse model. This model allows us to permanently label different cell populations involved in the development of psoriasis‐like disease in vivo, and to investigate the specific role of each population during disease initiation and development. Using this new mouse model, we found that a small fraction of K15+ bulge HF‐SCs can initiate a psoriasis‐like disease. Mutant bulge HF‐SCs are able to survive, whereas IFE cells disappear during disease progression. Furthermore, mutant bulge HF‐SCs and basal keratinocytes secrete thymic stromal lymphopoietin (TSLP), which induces proliferation of neighboring non‐mutant keratinocytes. These results emphasize the heterogeneity of epidermal stem cells and their role in psoriasis‐like development, and define a mechanistic basis for epidermal hyperplasia through TSLP paracrine signaling.
Results
c‐JUN and JUNB levels are reduced in bulge hair follicle stem cells (HF‐SCs) from scalp psoriasis patients
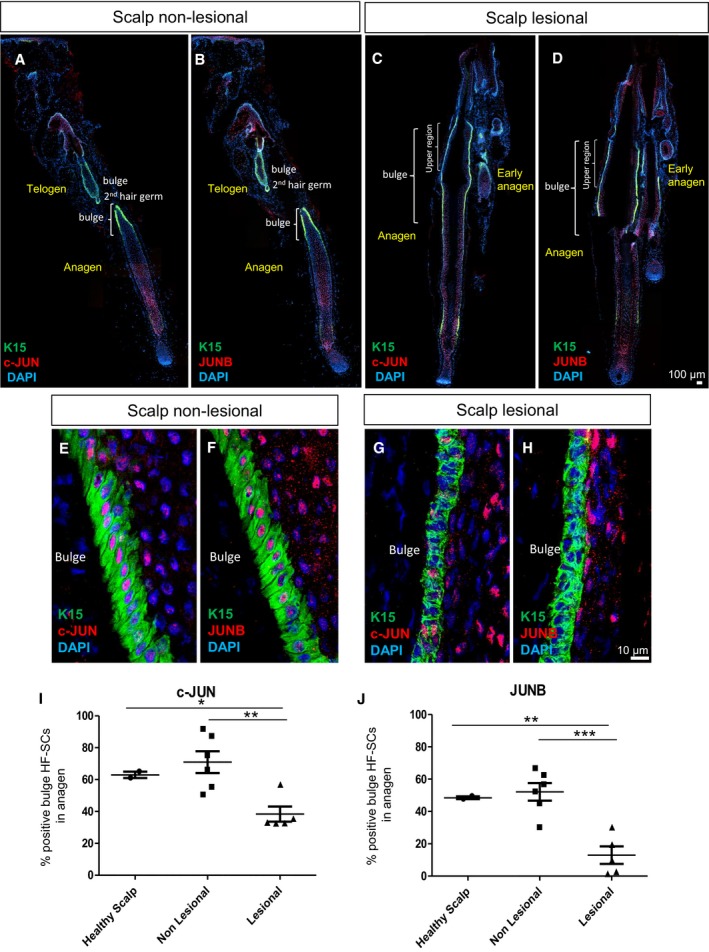
Previous results have reported alterations in the expression levels of c‐JUN and JUNB in the inter‐follicular epidermis (IFE) of psoriatic plaques from human patients (Zenz et al, 2005); however, it is not known whether the expression levels of c‐JUN and JUNB are affected in epidermal stem cells. While there are no specific markers to identify IFE stem cells, keratin 15 (K15)‐positive cells represent the vast majority of HF‐SCs located in the bulge of hair follicles (Liu et al, 2003; Purba et al, 2014). Another marker that identifies putative bulge HF‐SCs in human HFs is CD200 (Purba et al, 2014). We analyzed the expression of c‐JUN and JUNB in the bulge region of HFs from scalp psoriatic patients, a common form of human psoriasis (Figs 1 and EV1A). K15 was highly expressed in hair follicles, and low expression was also found in some basal keratinocytes of the scalp epidermis (Fig 1A and B). K15+ basal cells located in the outer root sheath (ORS) of the bulge region showed significantly reduced expression of c‐JUN and JUNB in lesional hair follicles in anagen phase (38 and 12%, respectively), while c‐JUN and JUNB were expressed in 70 and 50%, respectively, in non‐lesional hair follicles and healthy scalp (Fig 1A–J). The reduction was confirmed in putative bulge HF‐SCs identified by the surface marker CD200 (Fig EV1A).
Figure 1. Scalp psoriasis exhibits reduced expression of c‐JUN and JUNB in hair follicle stem cells (HF‐SCs).
-
A–DRepresentative composite immunofluorescence images of whole hair follicle units from non‐lesional and lesional scalp psoriasis patients. K15 (green), c‐JUN (red in A, C) and JUNB (red in B, D) and DAPI (blue).
-
E–HConfocal images of the bulge region of human psoriatic hair follicles from non‐lesional and lesional regions of the scalp. K15 (green), c‐JUN (red in E, G) and JUNB (red in F, H) and DAPI (blue).
-
I, JPercentage of HF‐SCs (K15+) that express c‐JUN (I) and JUNB (J) in the bulge region of human psoriatic hair follicles from non‐lesional and lesional scalp in comparison with healthy scalp. n = 2–6 hair follicles in anagen per group from five psoriatic patients and two healthy patients. Data represent mean ± SD. Statistical significance *P < 0.05, **P < 0.01, ***P < 0.001 (Student's two‐tailed t‐test relative to controls). See Appendix Table S2 for exact P‐values.
Source data are available online for this figure.
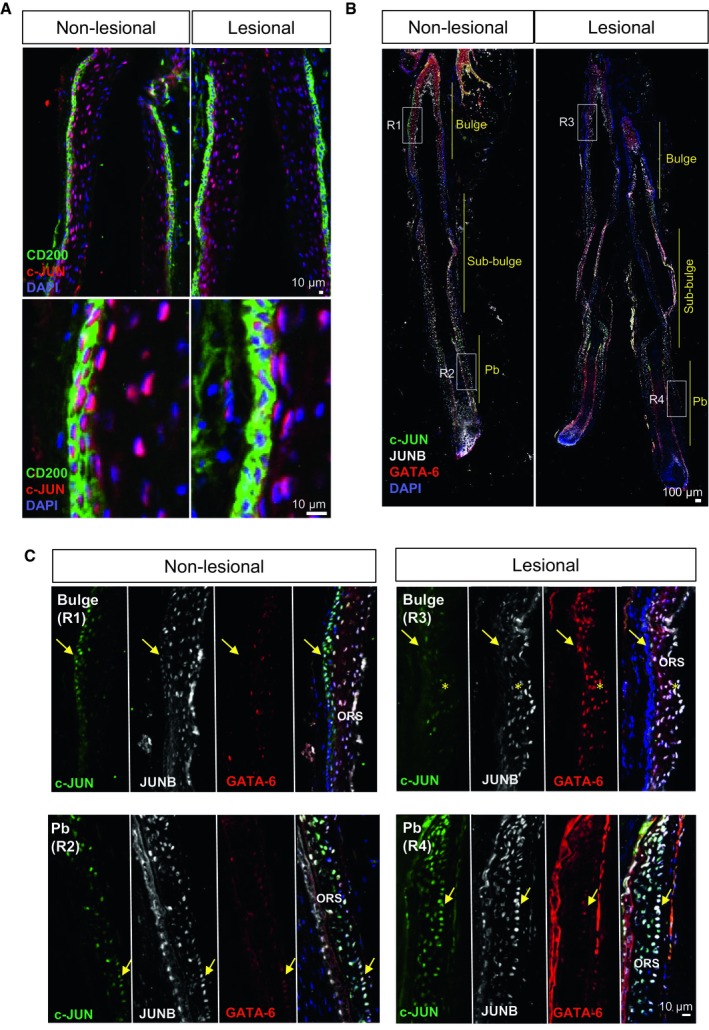
Figure EV1. Characterization of c‐JUN/JUNB in distinct hair follicle stem cell populations from scalp psoriasis patients (related to Fig 1).
-
AConfocal images of the bulge region of human psoriatic hair follicles from non‐lesional and lesional regions of the scalp. CD200 (green), c‐JUN (red), DAPI (blue).
-
B, CRepresentative composite immunofluorescence images of whole hair follicle units by confocal from non‐lesional and lesional scalp psoriasis patients (B) and magnification images of specific regions from the HFs (C). c‐JUN (green), JUNB (gray), GATA‐6 (red), and DAPI (blue). Yellow arrows represent the outer root sheath basal layer in the bulge (R1, R3) and proximal bulb (Pb, R2, R4). Yellow asterisks represent overexpression of GATA‐6 in suprabasal layers of the outer root sheath in lesional bulge region.
Only bulge HF‐SCs exhibited reduced c‐JUN/JUNB expression, while basal and suprabasal ORS layers in the sub‐bulge and proximal bulb regions maintained the expression of c‐JUN/JUNB (Fig EV1B and C). In addition, other regulators of HF‐SC renewal from other regions in the hair follicle, such as GATA‐6, expressed in progenitor matrix cells (bulb) in mice were analyzed (Wang et al, 2017). Interestingly, GATA‐6 was up‐regulated in suprabasal ORS layers in the bulge region from psoriatic lesional scalps, while no differences were observed in GATA‐6‐positive cells from the proximal bulb (Fig EV1C). These findings show that scalp psoriasis is associated with a reduction of c‐JUN and JUNB in bulge HF‐SCs along with GATA‐6 increased expression.
Epidermal stem cell lineage tracing in the DKO* psoriasis‐like mouse model
The differential expression of c‐JUN and JUNB in CD200+/K15+ bulge HF‐SCs in human samples of psoriatic plaques directed us to explore the potential causal contribution of epidermal stem cell populations to the initiation and development of psoriasis. To this end, we applied mouse genetics to ablate the expression of c‐Jun and JunB, first globally in all epidermal stem cell populations present in both the basal layer of the IFE and hair follicle units during homeostasis (Byrne et al, 1994). We crossed the well‐established DKO* psoriasis‐like mouse model (Zenz et al, 2005) with the mT/mG fluorescent reporter mouse. The psoriasis‐like phenotype in DKO*‐mT/mG mice was induced by the genetic deletion of c‐Jun and JunB floxed alleles in K5+ basal keratinocytes and K5+ epidermal stem cells after tamoxifen administration. This treatment also induced an irreversible replacement of the constitutive Tomato expression (red) by the expression of GFP only in K5+ basal keratinocytes and K5+ epidermal stem cells (Fig 2A), allowing tracing of epidermal stem cells and their progeny (transient‐amplifying and differentiated keratinocytes) during psoriasis progression. Mice expressing Cre recombinase under the control of the K5 promoter with wild‐type or heterozygous c‐Jun and JunB were used as controls (Co‐mT/mG).
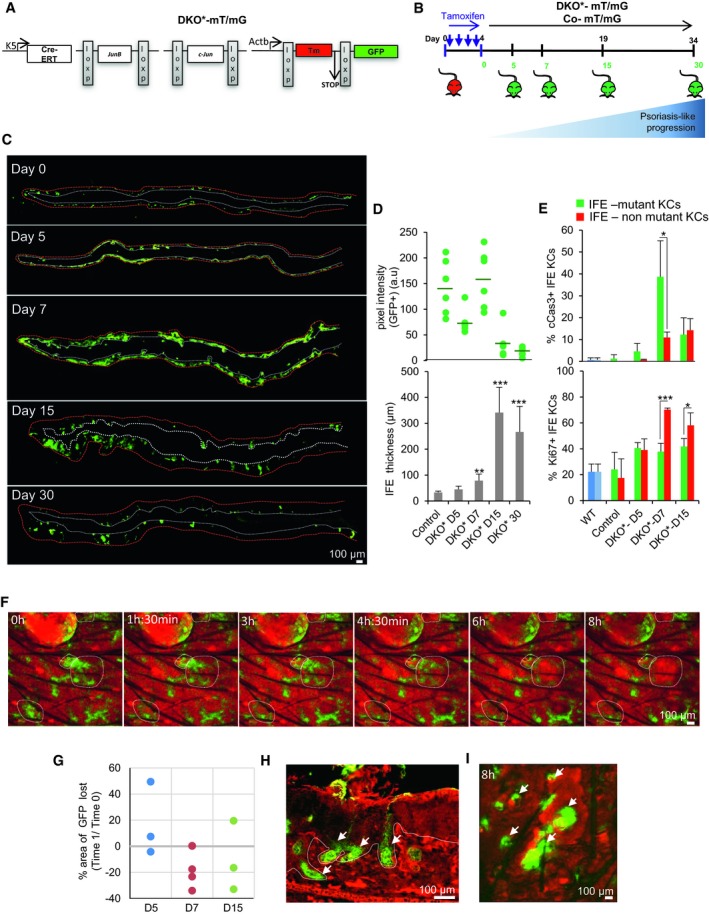
Figure 2. Mutant and non‐mutant inter‐follicular keratinocytes have different proliferation and apoptotic rates during psoriasis‐like disease progression.
-
ASchematic representation of the JunB and c‐Jun double knockout mouse model (DKO*) with lineage tracing used to investigate the psoriasis‐like disease development in mice. Briefly, DKO* psoriasis‐like mouse model was generated by the cross of mice carrying the floxed JunB allele (JunB f/f) and floxed c‐Jun allele (c‐Jun f/f) with the transgenic mice expressing the Cre recombinase–estrogen receptor fusion under the control of the basal keratinocyte‐specific K5 promoter (K5‐Cre‐ERT) to obtain JunB f/f c‐Jun f/f K5‐Cre‐ERT mice. To trace epidermal‐specific deletion of JunB f/f and c‐Jun f/f in DKO* mice, global double‐fluorescent Cre reporter mouse Gt(ROSA)26Sortm4(ACTB‐tdTomato,‐EGFP)Luo/J (referred as mT/mG) was crossed with DKO* mice (referred as DKO*‐mT/mG).
-
BExperimental timeline to induce psoriasis‐like disease in 8‐week‐old mice with four consecutive doses of tamoxifen injections (2 mg). Upon induction, non‐mutant keratinocytes express Tomato, while mutant keratinocytes express GFP. GFP expression was analyzed at 0, 5, 7, 15, and 30 days post‐induction.
-
CComposite immunofluorescence images showing GFP expression of whole ear sections from DKO*‐mT/mG mice at day 5, 7, 15, and 30. Increased GFP expression is shown at day 7 followed by progressive decrease of GFP+ keratinocytes (white dotted line represents basal layer, and red dotted line represents outermost skin layer). n = 3 per time point.
-
DQuantification analysis of GFP expression (top) and epidermal thickness (bottom) of DKO* mice at different time points during psoriasis‐like disease progression. n = 6 per time point. Data represent mean ± SD. Statistical significance **P < 0.01, ***P < 0.001 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐values.
-
EQuantification analysis of cleaved caspase‐3 (cCas3, top) and Ki67 (bottom) in ear skin of DKO* mice at different time points during psoriasis‐like disease progression. n = 3 per time point. Data represent mean ± SD. Statistical significance *P < 0.05, ***P < 0.001 (Student's two‐tailed t‐test). See Appendix Table S2 for exact P‐values.
-
FIntravital confocal time‐lapse imaging of non‐mutant Tomato keratinocytes and mutant GFP keratinocytes of DKO*‐mT/mG at day 7 after first tamoxifen injection. Dotted circles emphasize areas in which mutant GFP+ keratinocytes are replaced by non‐mutant Tomato+ keratinocytes.
-
GPercentage of GFP+ area eliminated during intravital confocal time‐lapse imaging at day 5, 7, and 15 in DKO*‐mT/mG mice (Time‐lapse 1:8 h). Positive value: Area of GFP increased. Negative value: Area of GFP reduced.
-
HFluorescence imaging of DKO*‐mT/mG ear skin at day 30 after first tamoxifen injection shows that remaining mutant GFP+ keratinocytes reside along the hair follicles. White dotted line separates epidermis and dermis. White arrows represent GFP+ hair follicles.
-
IIntravital confocal time‐lapse imaging of DKO*‐mT/mG at day 15 after first tamoxifen injection. Mutant GFP+ keratinocytes (white arrows) are maintained around hair follicles. White arrows represent GFP+ hair follicles.
Source data are available online for this figure.
We first analyzed the labeling efficiency and epidermal specificity of GFP in Co‐mT/mG ear skin 15 days after tamoxifen injection. Without tamoxifen, 95 ± 5% of epidermal cells expressed Tomato and only few keratinocytes expressed GFP (Fig EV2A). Following tamoxifen treatment, Tomato expression decreased and GFP expression increased by ~ 60–70% in K5+ epidermal cells of IFE, hair follicles, and sebaceous glands (Fig EV2B, upper panels). DKO*‐mT/mG mice treated with tamoxifen developed a psoriasis‐like phenotype 2 weeks after induction, mainly in the ears, head, paws, and tail, as previously reported (Zenz et al, 2005). We next analyzed the expression of GFP in this psoriasis‐like DKO*‐mT/mG ear skin. Interestingly, although hyperplasia was present throughout the epidermis, only small patches of hyperplastic keratinocytes expressed GFP in IFE, hair follicles, and sebaceous glands, showing a mosaic pattern of mutantGFP‐positive keratinocytes among a majority of non‐mutantTom‐positive keratinocytes (Fig EV2B, lower panels). We confirmed that the deletion of c‐Jun and JunB occurred only in mutantGFP epidermal cells using fluorescence microscopy (Fig EV2C). Therefore, our tracing system successfully allows studying the dynamics of mutantGFP epidermal stem cells and their progenitor cells, and non‐mutantTom epidermal stem cells and their progenitor cells in this psoriasis‐like mouse model.
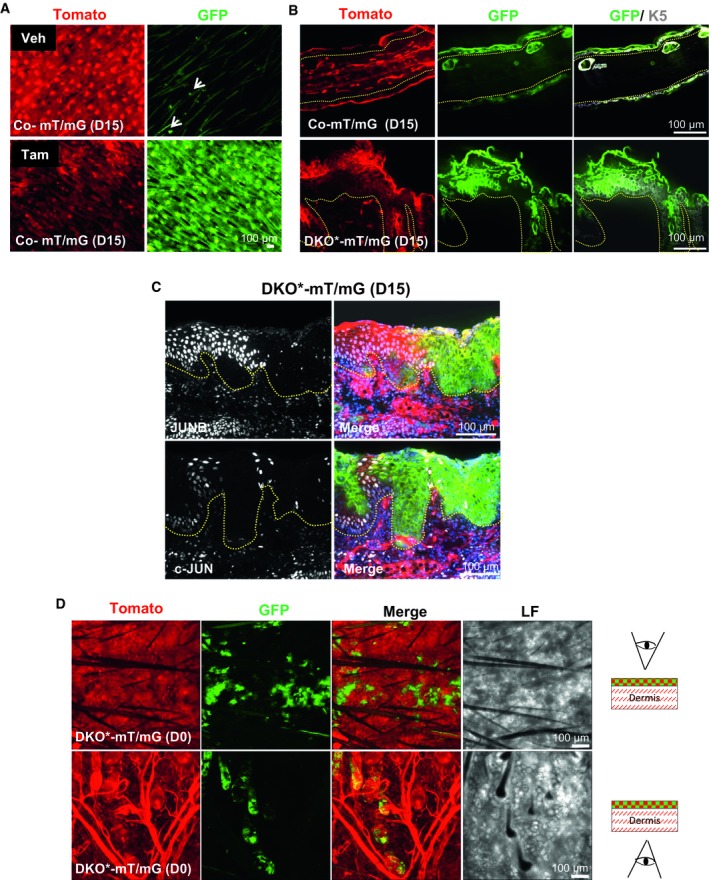
Figure EV2. Validation of the lineage tracing system in Co‐mT/mG and DKO*‐mT/mG mice (related to Fig 2).
-
AWhole mount of ear skin from co‐mT/mG mice before (VEHICLE) and after tamoxifen at day 15. White arrows represent isolated spots of GFP in vehicle skin.
-
BImmunofluorescence images of Co‐mT/mG ear skin sections and DKO*‐mT/mG at day 15 after tamoxifen treatment, co‐stained with K5 (white). Yellow dotted line separates epidermis and dermis.
-
CImmunofluorescence images of c‐Jun and JunB staining in DKO*‐mT/mG at day 15 after tamoxifen treatment showing GFP+ keratinocytes are negative for c‐Jun and JunB expression. GFP (green), Tomato (red), JunB and c‐Jun (white), DAPI (blue). Yellow dotted line separates epidermis and dermis.
-
DRepresentative images of whole mounts of ear skin from DKO*‐mT/mG at day 0 after tamoxifen treatment in two views, from the epidermis and from the dermis, to distinguish the expression of GFP+ epidermal cell into the IFE and HFs.
Mutant and non‐mutant IFE keratinocytes exhibit different proliferation and apoptosis rates during psoriasis‐like development
To further understand the mosaic deletion pattern in the DKO*‐mT/mG psoriasis‐like mouse model, we traced GFP expression after tamoxifen injection during the initiation (day 0–5), mid‐term (day 7–9), and late‐term (day 15–30) stages of the disease (Fig 2B). Initial epidermal cells labeled for GFP+ at day 0 after the last injection with tamoxifen are observed in isolated groups of cells in IFE and hair follicles (Figs 2C and EV2D). Composite images of whole ear sections showed that mutantGFP keratinocytes are increased at 5–7 days followed by a drastic reduction at days 15–30, whereas immune cell infiltration and epidermal thickening sharply increased over time (Fig 2C and D; Appendix Fig S1A–C).
We then analyzed apoptosis and proliferation by cleaved caspase‐3 (cCas3) and Ki67 expression, respectively. Apoptosis was observed in mutantGFP and non‐mutantTom keratinocytes from the IFE of DKO*‐mT/mG mice during psoriasis‐like progression, although at day 7 after induction, the apoptosis rate was higher in mutantGFP keratinocytes (38%), when compared to non‐mutantTom keratinocytes (10%) (Fig 2E, upper panel). Interestingly, 78% of non‐mutantTom keratinocytes expressed Ki67 vs. 40% of mutantGFP keratinocytes, suggesting a proliferative advantage of non‐mutantTom keratinocytes (Fig 2E, lower panel). The rapid loss of mutantGFP keratinocytes in the ear skin of DKO*‐mT/mG mice was also confirmed by intravital confocal imaging analyses at day 7 after induction (Fig 2F and G, and Movie EV1). Overall, these findings suggest that the psoriasis‐like phenotype in the IFE of DKO*‐mT/mG mice is characterized by different proliferation and apoptotic rates of distinct mutant and non‐mutant populations during psoriasis‐like progression.
The global proliferation/apoptosis imbalance led to almost complete depletion of mutantGFP basal and suprabasal keratinocytes in the IFE at the latest stage (day 15–30). This drastic loss could imply that the psoriasis‐like phenotype should also decrease over time. However, the psoriasis‐like phenotype is sustained until the late term of disease progression (D30) (Fig 2D). Interestingly, remaining mutantGFP keratinocytes at the late term of disease progression were located in the outer root sheath (ORS) in both hair follicles and in adjacent IFE keratinocytes (Fig 2H and I). In fact, same dynamic pattern of GFP/Tomato cells was observed in the IFE of tail and back skin during psoriasis development in DKO* mice (Appendix Fig S2). Interestingly, mutant GFP+ hair follicles from tail psoriatic skin underwent anagen induction suggesting the activation of these mutantGFP HF‐SCs in psoriasis‐like disease. Our next goal was to further investigate the contribution of these HF‐associated mutantGFP keratinocytes during psoriasis‐like progression.
Deletion of c‐Jun/JunB in bulge HF‐SCs is sufficient for the development of inflammatory skin disease
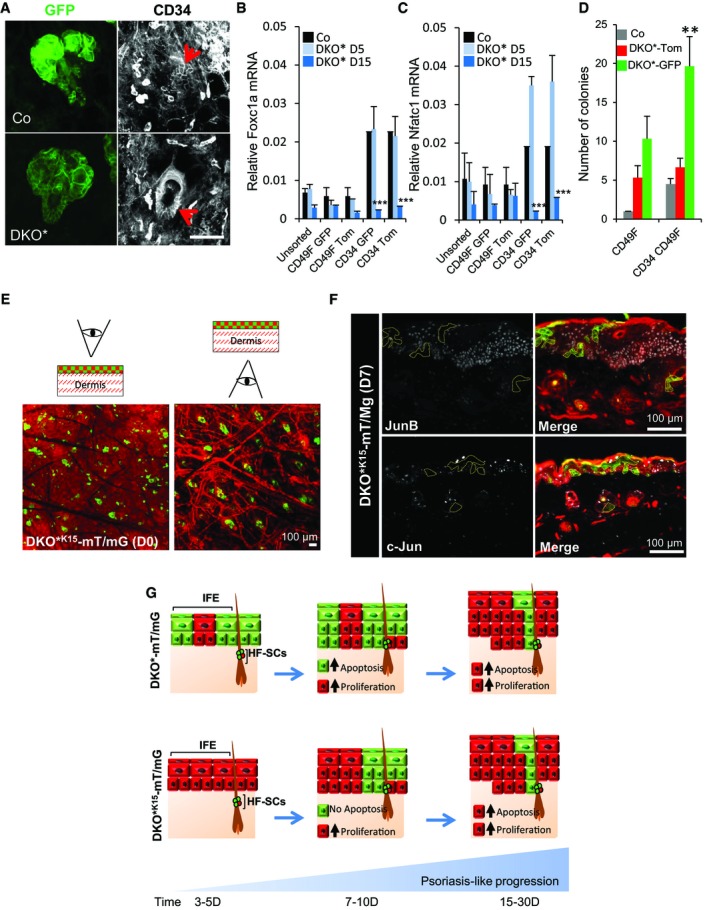
Bulge HF‐SCs are an important stem cell population for wound healing and for the initiation and maintenance of skin carcinomas, and are characterized by the expression of K15 and the surface marker CD34 in mice. We hypothesized that the remaining mutantGFP keratinocytes observed in the ORS of the hair follicles and in adjacent IFE regions are mainly derived from mutant HF‐SCs. To test this hypothesis, we first conducted FACS analyses to determine the proportion of the different HF‐SC subpopulations expressing CD34, CD49f, and Sca‐1 markers (Jensen et al, 2010) in the ear skin of the Co‐mT/mG and DKO*‐mT/mG mice during the psoriasis‐like progression (Appendix Fig S3A). As expected, the number of mutantGFP basal IFE cells decreased during disease progression, whereas non‐mutantTom basal and suprabasal IFE cells increased over time (Appendix Fig S3B and C). With regard to the hair follicles, the number of mutantGFP HF‐SCs from bulge and junctional zone was increased twofold during disease progression, whereas the number of non‐mutantTom HF‐SCs decreased over time (Appendix Fig S3D–F). To confirm that CD34+ mutantGFP cells belong to bulge HF‐SCs and not to the IFE epidermis, we conducted histological section and ear whole‐mount confocal analyses and observed co‐expression of GFP+ and CD34+ cells only in the bulge region of hair follicles (Fig EV3A and Appendix Fig S4A). Interestingly, both bulge HF‐SC populations (mutantGFP and non‐mutantTom) had reduced mRNA levels of the quiescence transcription factors Foxc1 and Nfatc1 (Horsley et al, 2008; Wang et al, 2016; Fig EV3B and C), suggesting an activation of bulge HF‐SCs during psoriasis progression. In fact, FACS‐isolated CD34+ mutantGFP bulge HF‐SCs displayed an increased clonogenic capacity in vitro, compared to non‐mutantTom bulge HF‐SC counterparts and control bulge HF‐SCs (Fig EV3D and Appendix Fig S4B). Overall, all these findings suggest that bulge HF‐SCs are activated and exit quiescence in the psoriasis‐like disease.
Figure EV3. MutantGFP bulge HF‐SCs are active and initiate psoriasis‐like development (related to Fig 3).
-
AImmunofluorescence images of the ears of control (Co) and DKO* mice showing co‐expression of mutantGFP with CD34 (red arrows) in HF‐SCs. n = 3. Scale bar = 50 μm.
-
B, CGene expression of quiescence transcription factors, Foxc1a and Nfatc1 show that HF‐SCs exit the quiescent stage during psoriasis‐like development in DKO* mice. n = 3 mice per group. Data represent mean ± SD. Statistical significance ***P < 0.001 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐values.
-
DColony formation in vitro of bulge hair follicle stem cells (HF‐SCs, CD34+ CD49fhigh) vs. basal keratinocytes (b‐KCs, CD49fhigh) from control and DKO*‐mT/mG ears shows that mutantGFP HF‐SCs have significant increased colony formation when compared to non‐mutantTom HF‐SCs or control HF‐SCs. n = 3 mice per group. Data represent mean ± SD. Statistical significance **P < 0.01 (Student's two‐tailed t‐test relative to control groups). See Appendix Table S2 for exact P‐values.
-
ERepresentative images of whole mount of ear skin from DKO*K15‐mT/mG at day 0 after mifepristone treatment in two views from the epidermis and from the dermis, to distinguish the expression of GFP+ epidermal cell in IFE and HFs.
-
FImmunofluorescence images of c‐Jun and JunB staining in DKO* K15‐mT/mG at days 5–7 after tamoxifen treatment show GFP+ keratinocytes are negative for c‐Jun and JunB expression.
-
GRepresentation of both epidermal lineage‐tracking system, DKO*‐mT/mG and DKO* K15‐mT/mG, for investigating the origin and contribution of epidermal stem cells in the development of psoriasis‐like.
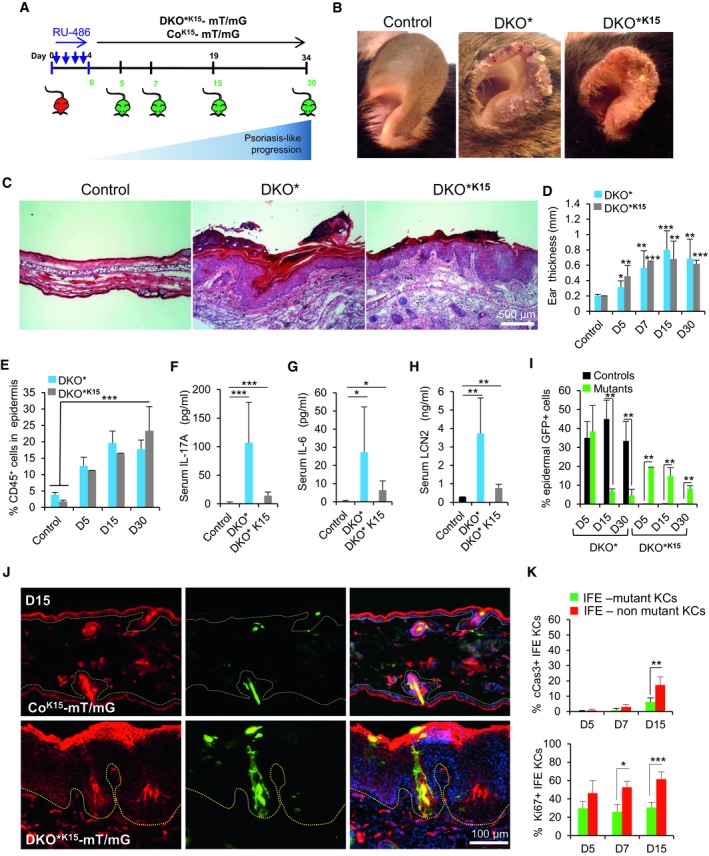
We then investigated whether mutantGFP keratinocytes from bulge HF‐SCs are sufficient to induce and maintain skin inflammation. We generated a new DKO* model to specifically target K15+ HF‐SCs and termed it DKO* K15‐mT/mG. This was achieved by crossing mice carrying the floxed JunB (JunB f/f) and c‐Jun (c‐Jun f/f) alleles with a transgenic mouse line expressing the Cre recombinase–prostaglandin receptor fusion under the control of keratin 15 promoter (K15‐Cre‐PGR; Liu et al, 2003). After mifepristone (RU‐486) treatment, this model allowed the specific targeting of K15+ HF‐SCs in the bulge to delete c‐Jun and JunB and to render targeted cells to become GFP positive (Fig 3A). Interestingly, DKO* K15‐mT/mG mice developed a similar psoriasis‐like phenotype as the DKO* mouse model 2 weeks after induction, displaying similar histological and chronological features in the skin at day 30 of the disease (Fig 3B–E). However, the psoriasis‐like severity was less pronounced in DKO* K15 mice when compared to the DKO* mice. The expression of specific pro‐inflammatory cytokines/alarmins, such as IL‐17A, IL‐6, and lipocalin‐2 (LCN2), was higher in the serum of DKO* mice compared to DKO* K15 mice at day 30 after psoriasis‐like induction (Fig 3F–H). These cytokines and LCN2 were significantly increased in DKO* K15 mice in comparison with control mice, suggesting a mild systemic inflammatory process in the HF‐SC DKO* K15 psoriasis‐like mouse model. We next analyzed the dynamic GFP expression in the DKO* K15‐mT/mG ear skin and compared it to DKO*‐mT/mG mice during psoriasis‐like progression. At day 0 after the last injection with mifepristone, the majority of hair follicles are labeled for GFP including some isolated spots found in the IFE (Fig EV3E). During initiation of the psoriasis‐like disease (day 5), 20% of mutantGFP epidermal cells derived from mutantGFP K15+ bulge HF‐SCs were quantified by FACS analysis (CD45−CD49f+CD34− epidermal total population), whereas mutantGFP epidermal cells derived from mutantGFP K5+ basal keratinocytes/HF‐SCs was increased by 35–50% (Fig 3I). In contrast, during late stages of the psoriasis‐like disease (day 15–30), the percentage of mutantGFP epidermal cells was only twofold reduced in DKO* K15 mice (from 20 to 10%), whereas in DKO* mice, it was drastically reduced fivefold at days 15–30 (from 35–50 to 8%). Immunofluorescence analyses for c‐Jun and JunB expression confirmed that the mutantGFP keratinocytes derived from mutantGFP K15+ bulge HF‐SCs did not express c‐Jun or JunB (Fig EV3F). Interestingly, DKO* K15‐mT/mG psoriatic‐like mice showed the same mosaic pattern of green and Tomato epidermal cells than the one previously described in the DKO* mouse model at the late term of psoriasis‐like development (Fig 3J). In fact, a similar proliferation pattern of mutantGFP and non‐mutantTom keratinocytes was observed in both models, although the apoptosis rate of mutantGFP keratinocytes was reduced in DKO* K15‐mT/mG mice when compared to DKO*‐mT/mG mice (Figs 3K and 2E).
Figure 3. Inactivation of c‐Jun and JunB in bulge HF‐SCs is sufficient to initiate psoriasis‐like development.
-
AExperimental timeline showing the different time points analyzed after mifespristone induction (day 5, 7, 15, and 30, green) to evaluate GFP expression in ear skin from control (CoK15‐mT/mG) and mutant (DKO* K15‐mT/mG) mice.
-
BEar pictures of control, DKO*, and DKO* K15 mice after 15 days of induction show clear signs of inflammation and psoriatic‐like scales in both models.
-
CRepresentative images of hematoxylin and eosin (H&E) staining of ear sections from control, DKO*, and DKO*K15 mice.
-
DEar thickness measurement at different time points during psoriasis‐like development in control, DKO*, and DKO* K15 mice. n > 5 per group. Data represent mean ± SD. Statistical significance *P < 0.05, **P < 0.01, ***P < 0.001 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐values.
-
EFACS quantification of CD45+ cells from ear skin of control, DKO*, and DKO* K15 mice at different time points during psoriasis‐like development. n > 5 per group. Data represent mean ± SD. Statistical significance *P < 0.05, **P < 0.01, ***P < 0.001 (Student's two‐tailed t‐test relative to controls). See Appendix Table S2 for exact P‐values.
-
F–HQuantification of IL‐17A, IL‐6, and LCN2 production in sera of control, DKO*, and DKO* K15 mice at day 30 of psoriasis‐like disease by ELISA. n > 4 per group. Data represent mean ± SD. Statistical significance *P < 0.05, **P < 0.01, ***P < 0.001 (Student's two‐tailed t‐test relative to controls). See Appendix Table S2 for exact P‐values.
-
IQuantification of total number of GFP+ epidermal cells by FACS analysis from DKO*‐mT/mG and DKO* K15‐mT/mG mice at different time points during psoriasis‐like progression compared to co‐mT/mG mice. n = 3–6 per group and time point. Data represent mean ± SD. Statistical significance **P < 0.01 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐values.
-
JRepresentative immunofluorescence images of ear sections from CoK15‐mT/mG and DKO*K15‐mT/mG mouse models after 15 days of induction. Red (Tomato), green (GFP), and blue (DAPI). Hyperplasia of Tomato+ keratinocytes in psoriatic‐like DKO*K15‐mT/mG mouse with clusters of GFP+ keratinocytes is derived from hair follicle K15+ GFP+ stem cells. n = 3 per group. Dotted lines separate epidermis and dermis.
-
KQuantification analysis of cleaved caspase‐3 (cCas3) and Ki67 in ear skin of DKO*K15‐mT/mG mice at different time points during psoriasis‐like disease progression. n = 3–5 per group. Data represent mean ± SD. Statistical significance *P < 0.05, **P < 0.01, ***P < 0.001 (Student's two‐tailed t‐test). See Appendix Table S2 for exact P‐values.
Source data are available online for this figure.
Overall, these data show that the specific inactivation of c‐Jun and JunB in bulge HF‐SCs is sufficient to induce a psoriasis‐like phenotype in the DKO* K15‐mT/mG mice. Importantly, these data uncovered that bulge HF‐SCs may act as a reservoir of new mutant epidermal cells, sustaining epidermal hyperplasia and skin inflammation, thereby recapitulating the observed skin phenotype of DKO* mice at later time points (Fig EV3G).
Keratinocytes derived from mutantGFP HF‐SCs have increased cell survival compared to mutantGFP keratinocytes derived from IFE
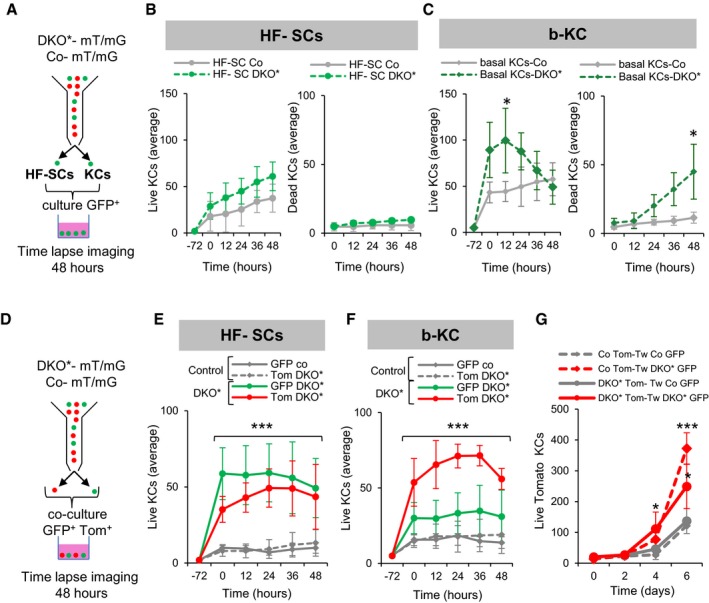
To determine whether keratinocytes derived from mutantGFP bulge HF‐SCs behave differently than IFE mutantGFP basal keratinocytes (b‐KCs), we FACS‐isolated and cultured these two populations at low concentration of 2,000 cells/cm2, as well as GFP+ bulge HF‐SCs and b‐KCs from Co‐mT/mG mice as controls (Fig 4A). Next, we conducted a time‐lapse confocal microscopy analysis of small colonies after 3 days in culture to evaluate the activity of different cell populations. Interestingly, over a 48‐h time course, mutantGFP KCs derived from mutantGFP bulge HF‐SCs showed similar growth compared to controlGFP KCs from bulge HF‐SCs (Fig 4B), while mutantGFP b‐KCs had a rapid growth during the first 24 h followed by a drastic decrease in cell number due to cell death compared to controlGFP b‐KCs (Fig 4C and Movie EV2). These results show that the same mutation in primary keratinocytes from distinct epidermal stem cell populations induces different proliferation and survival phenotypes, and suggest that bulge HF‐SCs play a major role in the maintenance of the psoriasis‐like disease.
Figure 4. Differential behavior of inter‐follicular and follicular stem cells.
-
ASchematic representation of cell sorting and single culture conditions. GFP+ bulge hair follicle stem cells (HF‐SCs, CD34+ CD49fhigh) and GFP+ basal keratinocytes (b‐KC, CD49fhigh) from ear skin of Co‐mT/mG and DKO*‐mT/mG mice were collected at day 10 after tamoxifen induction, sorted by FACS, and cultured at low density (2,000 cells/cm2) during 3–5 days as primary cultures. Time‐lapse imaging of six fields was performed during 48 h, once small colonies of 5–10 cells were observed.
-
BAverage number of live and dead (TOPRO‐3+ DAPI+) primary keratinocytes derived from HF‐SCGFP at different time points of time‐lapse capture during 48 h. There is a slight increase over time of live HF‐SCs from DKO* mice when compared to control cells, and no difference in average of dead cells. n = 4 fields from two independent experiments. Data represent mean ± SD. Non‐statistical significance (two‐way ANOVA and Bonferroni post‐test).
-
CAverage number of live and dead (TOPRO‐3+ DAPI+) primary keratinocytes derived from basal keratinocytesGFP show a significant increase in DKO* basal live keratinocytes at 12 h, followed by a significant increase in cell death at 48 h. n = 4 fields from two independent experiments. Data represent mean ± SD. Statistical significance *P < 0.05 (two‐way ANOVA and Bonferroni post‐test).
-
DSchematic representation of cell sorting and co‐culture conditions. GFP+ and Tom+ bulge HF‐SCs (CD34+ CD49fhigh) and GFP+ and Tom+ basal keratinocytes (CD49fhigh) from ear skin of Co‐mT/mG and DKO*‐mT/mG collected at day 10 after tamoxifen induction were sorted by FACS and co‐cultured (same proportion of GFP and Tom cells) at low density (2,000 cells/cm2) during 3–5 days. When small colonies of 5–10 cells were formed, time‐lapse imaging of six fields was performed during 48 h.
-
EAverage number of live primary keratinocytes from co‐cultures of GFP+ with Tomato+ bulge HF‐SCs at different time points of time‐lapse capture during 48 h. Co‐culture of HF‐SCGFP with HF‐SCTom from DKO*‐mT/mG mice shows a significant increase of HF‐SCTom, while the HF‐SCTom from DKO*‐mT/mG mice show a normal growth when they are co‐cultured with HF‐SCGFP from control mice. n = 4 fields from two independent experiments. Data represent mean ± SD. Statistical significance ***P < 0.001 (two‐way ANOVA and Bonferroni post‐test).
-
FAverage number of live primary keratinocytes from co‐cultures of GFP+ with Tomato+ basal keratinocytes at different time points of time‐lapse capture during 48 h. Co‐culture of b‐KCGFP with b‐KCTom from DKO*‐mT/mG mice shows significant increase of b‐KCTom, while these b‐KCTom from DKO*‐mT/mG mice show a normal growth when they are co‐cultured with b‐KCGFP from control mice. n = 4 fields from two independent experiments. Data represent mean ± SD. Statistical significance ***P < 0.001 (two‐way ANOVA and Bonferroni post‐test).
-
GAverage total number of live Tomato+ basal keratinocytes (from DKO* or control mice) at different time points cultured in the bottom part of trans‐well system cultures with GFP+ basal keratinocytes (from DKO* or control mice) cultured in the upper trans‐well (Tw). n = 5 fields from three independent experiments. Data represent mean ± SD. Tom+ basal keratinocytes significantly increased when these were cultured with mutant GFP+ basal keratinocytes in the trans‐well in comparison with control GFP+ basal keratinocytes. Statistical significance *P < 0.05, ***P < 0.001 (two‐way ANOVA and Bonferroni post‐test).
Source data are available online for this figure.
Secreted factors from mutantGFP keratinocytes induce hyper‐proliferation of neighboring non‐mutantTom keratinocytes
We have shown that in both psoriasis‐like mouse models, DKO*‐mT/mG and DKO* K15‐mT/mG, non‐mutantTom KCs mainly sustain epidermal hyperplasia. We hypothesized that the establishment of cell–cell interactions between mutantGFP KCs and non‐mutantTom KCs primed the non‐mutantTom population to increase their proliferation, leading to chronic hyperplasia in the psoriasis‐like disease. To determine if the hyper‐proliferation of non‐mutantTom KCs is conditioned by the presence of mutantGFP KCs, we co‐cultured sorted mutantGFP bulge HF‐SCs and mutantGFP b‐KCs with their corresponding non‐mutantTom bulge HF‐SC and b‐KC counterparts (Fig 4D). MutantGFP KCs derived from both bulge HF‐SC and b‐KC populations were able to stimulate the proliferation of co‐cultured neighboring non‐mutantTom KCs (Fig 4E and F, and Movie EV3). This increase in the proliferation of Tomato+ KCs was also observed when mutantGFP bulge HF‐SCs and mutantGFP b‐KCs were co‐cultured with controlTom bulge HF‐SC and b‐KC counterparts (Appendix Fig S5A and B). In addition, non‐mutantTom bulge HF‐SCs conditioned the proliferation of mutantGFP bulge HF‐SCs, whereas non‐mutantTom b‐KCs did not affect the proliferation of mutantGFP b‐KCs (Fig 4E and F). Interestingly, mutantGFP KCs from b‐KCs also induced an increased cell death in neighboring non‐mutant/controlTom KCs, while mutantGFP KCs from bulge HF‐SCs did not induce a significant increase in cell death of their non‐mutant/controlTom KC counterparts (Appendix Fig S5C and D). To determine whether the observed increased proliferation and cell death are a result of a direct cell–cell contact rather than stimulation by secreted factors, we cultured b‐KCs in trans‐wells, in which the non‐mutantTom b‐KCs were cultured at the bottom of the plate and the mutantGFP b‐KCs were cultured on the trans‐well inserts. We observed similar cell proliferation and death rates as the ones observed in the co‐cultures, suggesting that cell–cell contact is not required for the hyper‐proliferation of non‐mutantTom KCs (Fig 4G). These observations suggest an induction of a proliferative advantage in non‐mutantTom KCs by paracrine factors secreted from mutantGFP KCs, independently of their origin from the hair follicle or IFE during the psoriasis‐like progression in the DKO* mouse model.
Mutant and non‐mutant epidermal stem cells display different molecular signatures
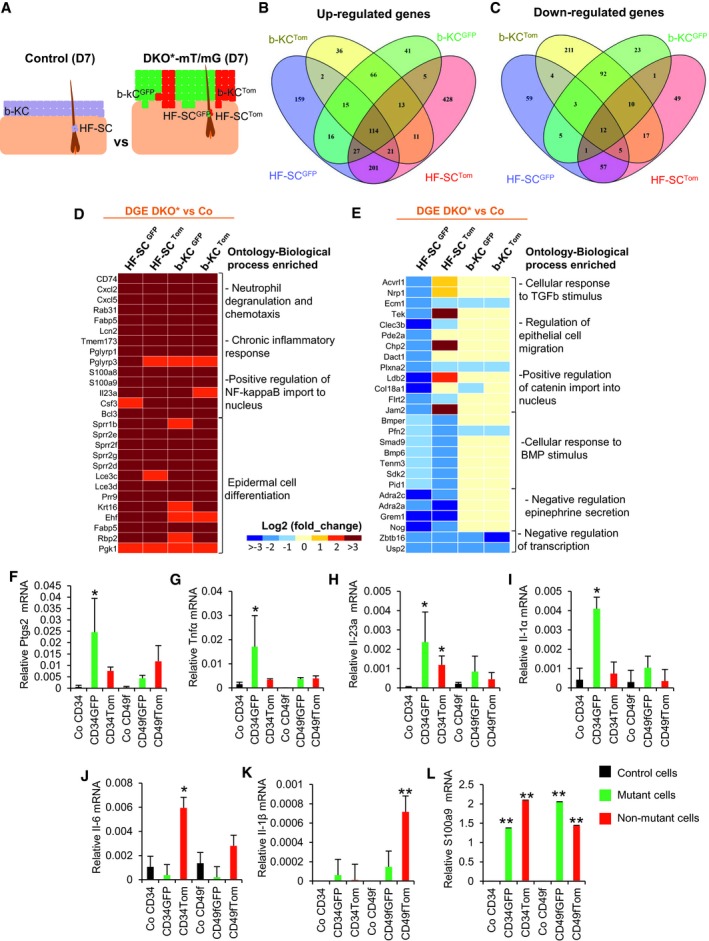
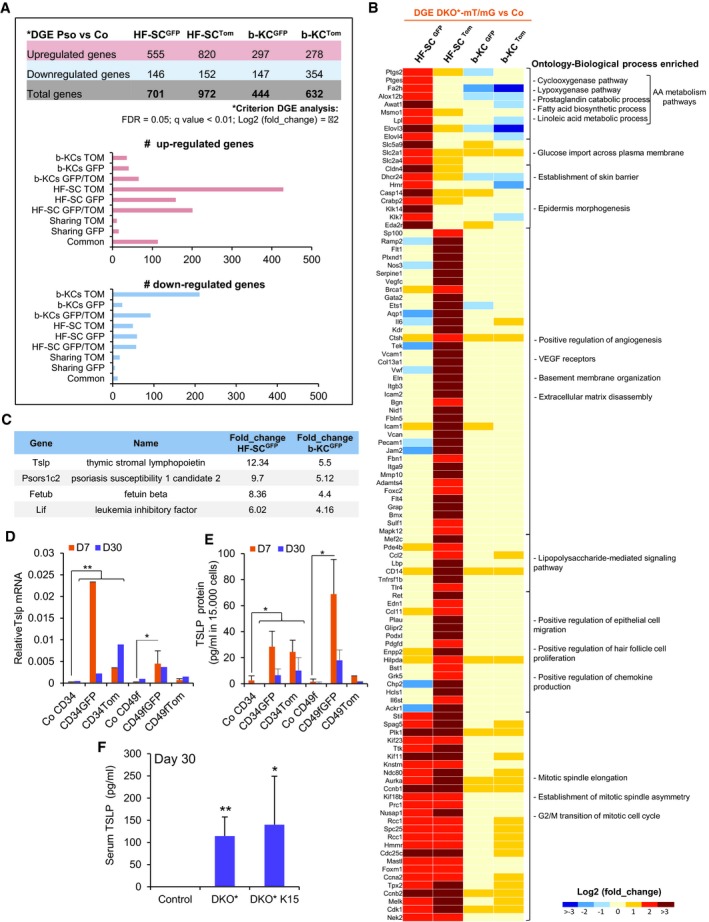
To gain insight into the potential molecular mechanisms that govern the behavior of the distinct mutant and non‐mutant bulge HF‐SCs and IFE cell populations during the progression of the psoriasis‐like disease, we performed mRNA sequencing analyses. We FACS‐isolated GFP+, Tomato+ bulge HF‐SCs and b‐KCs from DKO*‐mT/mG mice, and their counterpart populations from control mice at the mid‐term of the psoriasis‐like progression (Fig EV4A). Interestingly, non‐mutantTom populations exhibited a higher differential gene expression (972 total genes in HF‐SCs and 632 total genes in b‐KCs) than the one observed in mutantGFP populations (701 total genes in HF‐SCs and 444 total genes in b‐KCs) (Fig 5A, upper panel). A Venn diagram including these differentially expressed genes (DEG) was generated to depict the results in each population (Fig EV4B and C). In fact, the bulge HF‐SC population displayed more up‐regulated genes, including genes specifically up‐regulated in non‐mutantTom HF‐SCs (> 400 genes), mutantGFP HF‐SCs (> 150 genes) and genes commonly up‐regulated in both populations of HF‐SCs (GFP/TOM > 200 genes; Fig 5A). Conversely, the mutant b‐KC population presents the majority of down‐regulated genes (> 200 genes; Fig 5A), whereas around 100 common genes were up‐regulated in all cell populations (Fig 5A).
Figure EV4. Isolation of bulge HF‐SCs and basal keratinocytes from Co‐mT/mG and DKO*‐mT/mG ear skin for RNA‐seq and gene expression profiling (related to Fig 5).
-
AHair follicle stem cells (HF‐SC, CD34+ CD49fhigh) and basal keratinocytes (b‐KC, CD49fhigh) from ear skin of DKO*‐mT/mG collected at day 7 after tamoxifen induction were sorted by FACS for GFP+ vs. Tomato+. HF‐SCs (CD34+ CD49fhigh) and b‐KC (CD49fhigh) from control mice were also sorted to sequence the RNA. n = 3 mice per condition. Differentially expressed genes were obtained by the comparison of mutant cell populations with their same cell populations from control mice.
-
B, COverlapping of differentially expressed genes (DEGs) from the four subpopulations (HF‐SCGFP; HF‐SCTom; b‐KCGFP; and b‐KCTom) by Venn diagram.
-
D, EHeat map of up (C)‐ and down (D)‐regulated biological processes based on Gene Ontology IDs for DEGs commonly up‐ and down‐regulated in the four subpopulations (HF‐SCGFP; HF‐SCTom; b‐KCGFP; and b‐KCTom)
-
F–LGene expression for Ptgs2, TNFa, IL‐23a, IL‐1α, IL‐6, IL‐1β, and S100a9 in the different epidermal cell subpopulations sorted at mid‐term of psoriasis‐like development from DKO* mice. n = 3 mice. Data represent mean ± SD. Statistical significance *P < 0.05, **P < 0.01 (Student's two‐tailed t‐test relative to control groups). See Appendix Table S2 for exact P‐values.
Figure 5. Different molecular signatures of mutant and non‐mutant epidermal stem cells (EpSCs).
-
ADifferentially expressed genes (DEGs) from the four subpopulations (bulge HF‐SCGFP; bulge HF‐SCTom; b‐KCGFP; and b‐KCTom) from psoriatic DKO*‐mT/mG mice in comparison with their control counterparts (Co). The graphs represent the number of genes differentially up‐regulated or down‐regulated specifically in each group of interest obtained by Venn diagram analysis (Fig EV4B and C).
-
BHeat map for specific genes relative to biological process enriched based on Gene Ontology IDs for DEGs that are: (i) exclusively up‐regulated in HF‐SCGFP; (ii) exclusively up‐regulated in HF‐SCTom; (iii) commonly up‐regulated in HF‐SCGFP and HF‐SCTom.
-
CPrediction of secreted proteins enriched in GFP+ subpopulations by the analysis of DEG commonly up‐regulated in HF‐SCGFP and b‐KCGFP by ProteINSIDE analysis of RNA‐seq.
-
D, EGene and protein expression of TSLP in different epidermal cell subpopulations sorted at mid‐term (D7) or late term (D30) of psoriasis‐like progression in DKO* mice. n = 2–3. Data represent mean ± SD. Statistical significance *P < 0.05, **P < 0.01 (Student's two‐tailed t‐test relative to control groups). See Appendix Table S2 for exact P‐values.
-
FQuantification of TSLP production in sera of control, DKO*, and DKO* K15 mice at day 30 of psoriasis‐like disease by ELISA. n = 4 per group. Data represent mean ± SD. Statistical significance *P < 0.05, **P < 0.01 (Student's two‐tailed t‐test relative to controls). See Appendix Table S2 for exact P‐values.
Source data are available online for this figure.
Next, we conducted gene ontology (GO) analyses, to identify the specific enrichment of the obtained genetic signatures in different biological processes, focusing attention on the bulge HF‐SCs up‐/down‐regulated genes and on the common genes up‐regulated in all cell populations (Figs 5B, and EV4D and E). The most prominent biological process enriched in the genetic signatures of all cell populations are related to neutrophil degranulation and chemotaxis, chronic inflammatory responses, and epidermal cell differentiation (Fig EV4D). These findings support our prior findings observed in vivo and in vitro, where non‐mutantTom KCs acquire psoriasis‐like hallmarks without having c‐Jun and JunB mutation.
MutantGFP bulge HF‐SCs displayed a specific enrichment of genes encoding pro‐inflammatory mediators in psoriasis, in particular an increase in genes related to arachidonic acid (AA) metabolism, such as Ptgs2 (Figs 5B and EV4F), and specific pro‐inflammatory cytokines, such as Tnf‐α, Il‐23, and Il1‐α (Fig EV4G–I), whereas genes related to epithelial cell migration were down‐regulated (Fig EV4E). In contrast, non‐mutantTom bulge HF‐SCs showed an enrichment of genes related to angiogenesis, VEGF receptors, extracellular matrix disassembly, and epithelial cell migration and proliferation (Fig 5B). Both mutantGFP and non‐mutantTom bulge HF‐SCs shared up‐regulated genes related to mitotic cell cycle and asymmetric cell division (Fig 5B). These results are in agreement with our previous observations that both bulge HF‐SC populations were active during psoriasis progression. In addition, other pro‐inflammatory cytokines important in psoriasis, such as Il‐6, were only up‐regulated in non‐mutantTom cell populations (Fig EV4J), and we also observed that Il‐1β was significantly up‐regulated in non‐mutantTom b‐KCs (Fig EV4K). These data suggest that mutantGFP bulge HF‐SCs have an important pro‐inflammatory role in the initiation of psoriasis‐like disease and unravel the expression of a unique transcriptomic profile of non‐mutantTom bulge HF‐SCs in the development of psoriasis.
We next investigated the differential expression of genes coding for secreted proteins in mutantGFP populations of bulge HF‐SCs and b‐KCs, since our in vitro results showed that mutantGFP cell populations secreted paracrine factors that induced the proliferation of non‐mutantTom KCs. Four secreted proteins were overexpressed Tslp, Psors1c2, Fetub, and Lif (Fig 5C). All four proteins are involved in systemic inflammation and TSLP, PSORS1C2 and LIF in inflammatory skin diseases (Asumalahti et al, 2000; Szepietowski et al, 2001; He et al, 2008). Thymic stromal lymphopoietin (TSLP) is known for its important role in allergies and atopic dermatitis (He et al, 2008), and it is up‐regulated in the epidermis of psoriatic patients (Volpe et al, 2014). We detected a 12‐fold increase in mutantGFP bulge HF‐SCs and a fivefold increase in mutantGFP b‐KCs at the early/mid‐term (D7) of the psoriasis‐like development (Fig 5C). Interestingly, while the expression of TSLP remained increased in mutantGFP bulge HF‐SCs and mutantGFP b‐KCs at the mid‐term of the psoriasis‐like development, its expression was reduced at the latest stage (D30) (Fig 5D and E). In contrast, an increased expression of TSLP in non‐mutantTom bulge HF‐SCs was observed at D30, while non‐mutantTom b‐KCs did not express TSLP at any stage of psoriasis‐like development (Fig 5D and E). Furthermore, TSLP was increased in sera of both psoriasis‐like mouse models, DKO* and DKO*K15, at day 30 after psoriasis‐like induction (Fig 5F). These results suggest that the secretion of TSLP may contribute to psoriasis‐like initiation and maintenance in DKO* and DKO* K15 mice. In summary, the RNA‐seq analyses revealed the heterogeneity and complexity of different epidermal stem cell populations during psoriasis‐like disease initiation and progression.
TSLP secreted by mutantGFP keratinocytes induces hyper‐proliferation of non‐mutantTom keratinocytes leading to epidermal hyperplasia
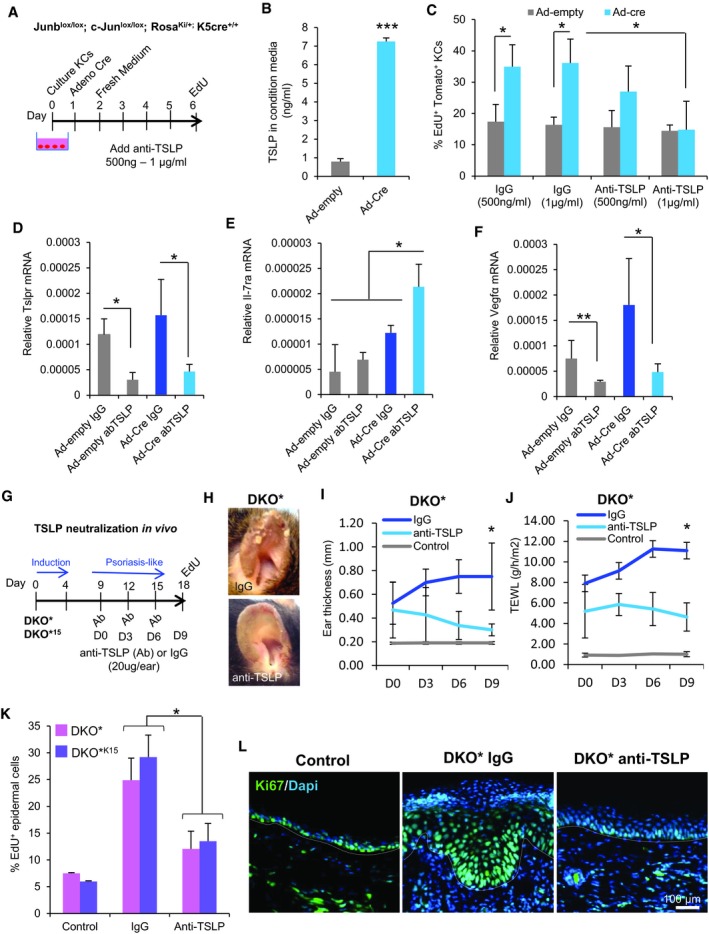
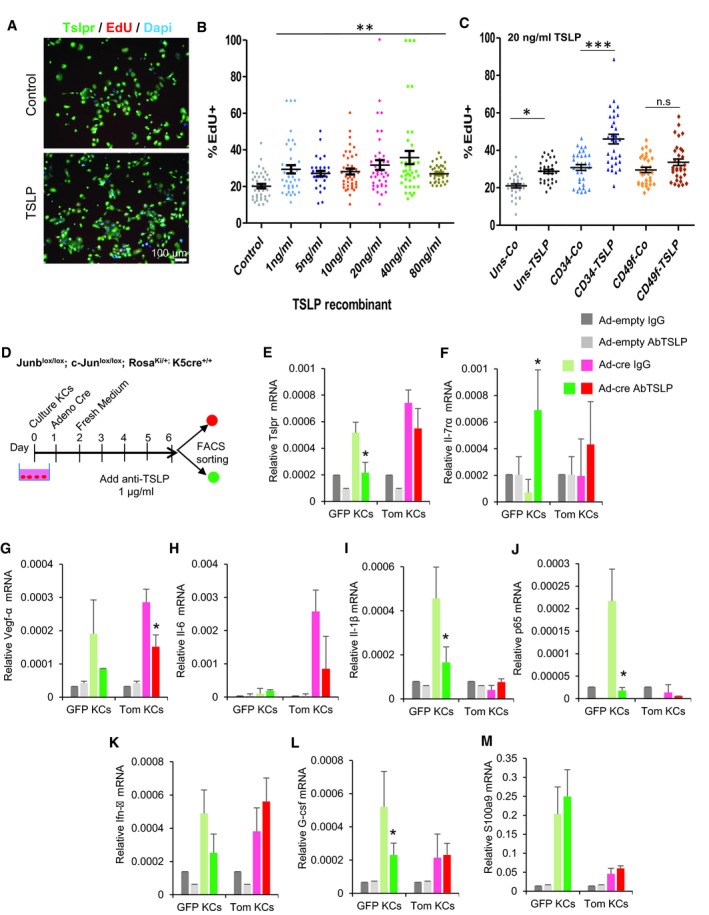
To mechanistically explore the effect of the pro‐inflammatory TSLP protein on non‐mutantTom KC proliferation, we first induced the deletion of c‐Jun and JunB in primary DKO*‐mT/mG KC cultures by infecting cells with Adenovirus‐Cre or empty Adenovirus (control). We observed that 24 h after infection, 50% of KCs were GFP+ and deficient for c‐Jun and JunB expression, while the other 50% were Tomato+ KCs and maintained the expression of c‐Jun and JunB. To assess the causal involvement of TSLP on non‐mutant KCs proliferation, cells were treated with a blocking TSLP antibody for three consecutive days after Adenovirus infection (Fig 6A). The Adeno‐Cre‐infected cells significantly increased the amount of TSLP in conditioned media, when compared to Adeno‐empty‐infected control cells (Fig 6B). This correlated with a significant increase in the proliferation of non‐mutantTom KCs present in the Adeno‐Cre‐infected cell cultures, when compared to Adeno‐empty control cells, while the hyper‐proliferation was blocked by the addition of 1 μg/ml TSLP antibody (Fig 6C). In order to test whether TSLP is sufficient to induce proliferation in KCs, recombinant TSLP was added at different concentrations (Fig EV5A and B) to primary wild‐type (WT) KCs. Primary WT KCs expressed TSLPR in culture and increased proliferation rate after recombinant TSLP treatment, even at low concentrations. Interestingly, primary WT KCs derived from bulge HF‐SCs (CD34+) significantly increased the proliferation rate rather than basal KCs (CD49f+) in comparison with their control cells (Fig EV5C) suggesting that bulge HF‐SCs are more sensitive to TSLP. These data show that the proliferation of non‐mutant KCs is dependent on TSLP secretion from mutant KCs. TSLP exerts its biological effects by binding to a high‐affinity heteromeric complex composed of TSLP receptor chain and IL‐7Rα. Blocking TSLP led to down‐regulation of TSLPR chain in co‐cultures, while the IL‐7rα chain increased possibly as a compensatory mechanism (Fig 6D and E). We also observed that VEGFα expression, a pro‐inflammatory mediator in psoriasis, decreased with anti‐TSLP treatment (Fig 6F). To determine the TSLP autocrine/paracrine regulation in co‐cultures of mutantGFP with non‐mutantTom KCs, we purified by FACS GFP+ and Tomato+ KCs after TSLP neutralization and analyzed gene expression of pro‐inflammatory mediators in psoriasis‐like disease (Fig EV5D–M). We confirmed the reduction of TSLP receptor expression and overexpression of Il‐7rα in both populations, mutantGFP and non‐mutantTom KCs, but statistically significant in mutantGFP KCs (Fig EV5E and F). In addition, VEGFα was increased in mutantGFP and non‐mutantTom KCs after Adeno‐Cre infection, while its expression was down‐regulated after TSLP neutralization in both populations (Fig EV5G). Interestingly, Il‐6 was increased only in non‐mutantTom KCs after the induction with Adeno‐Cre and was reduced after blocking with TSLP antibody (Fig EV5H). Other pro‐inflammatory cytokines and mediators, such as IL‐1β and p65 (NF‐κB), increased only in mutantGFP KCs after c‐Jun/JunB deletion with the consecutive reduction after TSLP neutralization (Fig EV5I and J). Furthermore, IFN‐γ or G‐CSF was up‐regulated in both populations, GFP and Tom KCs; however, only mutantGFP KCs responded to TSLP neutralization (Fig EV5K and L). S100A9 increased in both populations, higher in mutantGFP KCs, but its expression was not reduced after blocking TSLP (Fig EV5M). These data suggest that TSLP acts as autocrine and paracrine factor in the cross‐talk between mutant and non‐mutant KCs and regulates the expression of different pro‐inflammatory mediators.
Figure 6. TSLP secreted by mutantGFP epidermal cells induces hyper‐proliferation of non‐mutantTom keratinocytes.
-
AExperimental design for induction of mutantGFP KCs in vitro and neutralization of secreted TSLP by anti‐TSLP. Primary keratinocytes were cultured from the skin of Junblox/lox; c‐Junlox/lox; RosaKi/+; and K5cre‐ERT+/+ mice. 50% of mutantGFP KCs were induced by infection with Cre Adenovirus, the non‐infected cells were non‐mutantTom KCs. Adeno‐empty was used as control. EdU was added for 5 h to label proliferating Tomato+ cells in 6‐day co‐culture. Quantification was performed by confocal imaging analysis.
-
BTSLP production in conditioned media quantified by ELISA three days after infection with Ad‐empty or Ad‐cre. n = 3 independent experiments. Data represent mean ± SD. Statistical significance ***P < 0.001 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐values.
-
CPercentage of EdU+ Tomato+ keratinocytes after blocking with anti‐TSLP. Anti‐TSLP neutralizes the hyper‐proliferation of non‐mutantTom KCs in vitro after infection with Ad‐cre. n = 3 independent experiments, six fields per experiment. Data represent mean ± SD. Statistical significance *P < 0.05 (two‐way ANOVA and Bonferroni post‐test).
-
DGene expression of Tslpr after TSLP neutralization is reduced. n = 3 independent experiments. Data represent mean ± SD. Statistical significance *P < 0.05 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐value.
-
EGene expression of IL‐7ra after TSLP neutralization is increased. n = 3 independent experiments. Data represent mean ± SD. Statistical significance *P < 0.05 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐value.
-
FGene expression of VEGFα after TSLP neutralization is reduced. n = 3 independent experiments. Data represent mean ± SD. Statistical significance *P < 0.05, **P < 0.01 (Student's two‐tailed t‐test relative to control groups). See Appendix Table S2 for exact P‐value.
-
GExperimental design for TSLP neutralization in vivo. TSLP neutralization was performed by triple intradermal injections of 20 μg of anti‐TSLP or IgG to the right ears of DKO* and DKO*15 mice 5 days after psoriasis‐like induction. EdU was added into mice by IP injection 2 h before euthanasia.
-
HRepresentative images of treated ears after 9 days of anti‐TSLP or IgG treatment.
-
I, JLongitudinal analysis of ear thickness (I) and trans‐epidermal water loss (TEWL) (J) in DKO* mice at different time points during psoriasis‐like disease progression. Data represent mean ± SD. n = 4. Statistical significance *P < 0.05 (two‐way ANOVA and Bonferroni post‐test).
-
KPercentage of EdU+ epidermal cells (CD45−CD49f+) after blocking with anti‐TSLP in DKO* and DKO*15 mice. DKO* n = 4 and DKO*15 n = 3 per group. Data represent mean ± SD. Statistical significance *P < 0.05 (two‐way ANOVA and Bonferroni post‐test).
-
LRepresentative immunofluorescence images of ear sections from control or DKO* mice treated with IgG or anti‐TSLP and stained for Ki67. Ki67 expression was reduced in mice treated with anti‐TSLP (n = 3).
Source data are available online for this figure.
Figure EV5. Regulation of keratinocyte proliferation and pro‐inflammatory mediators by TSLP (related to Fig 6).
-
AImmunofluorescence images of TSLPR (green) and EdU (red) staining in primary keratinocyte cultures from wild‐type (WT) mouse skin treated with recombinant TSLP during 48 h.
-
BPercentage of EdU+ WT keratinocytes after blocking with recombinant TSLP at different concentrations in culture. WT keratinocytes responded to recombinant TSLP treatment and increased their proliferation. n = 3 independent experiments. Data represent mean ± SD. Statistical significance **P < 0.01 (two‐way ANOVA and Bonferroni post‐test). See Appendix Table S2 for exact P‐values.
-
CPercentage of EdU+ WT keratinocytes derived from bulge HF‐SCs (CD34+) and basal keratinocytes (CD49f+) after treating with TSLP recombinant in culture. Bulge HF‐SCs increased the proliferation rate rather than basal keratinocytes. n = 2 independent experiments. Data represent mean ± SD. Statistical significance *P < 0.05, ***P < 0.001 (two‐way ANOVA and Bonferroni post‐test). See Appendix Table S2 for exact P‐values.
-
DExperimental design for induction of mutantGFP KCs in vitro and neutralization of secreted TSLP by anti‐TSLP. Primary keratinocytes were cultured from the skin of Junblox/lox; c‐Junlox/lox; RosaKi/+; and K5cre‐ERT+/+ mice. 50% of mutantGFP KCs were induced by infection with Cre Adenovirus, and the non‐infected cells were non‐mutantTom KCs. Adeno‐empty was used as control. After neutralization, GFP+ and Tomato+ KCs were sorted by FACS and RNA isolation was carried out for further gene expression profiling.
-
E–MGene expression profiling of Tslpr, IL‐7ra, VEGFα, IL‐6, IL‐1β, p65, IFN‐γ, G‐CSF, and S100a9 in sorted GFP vs. Tom KCs after TSLP neutralization. n = 2 independent experiments. Data represent mean ± SD. Statistical significance *P < 0.05 (Student's two‐tailed t‐test relative to control group). See Appendix Table S2 for exact P‐value.
To determine whether local neutralization of TSLP reduces epidermal cell proliferation and psoriasis‐like progression in vivo, intradermal injections of TSLP antibody were applied to the right ear of both mouse models, DKO* and DKO*K15, 5 days after psoriasis‐like induction (Fig 6G). Anti‐TSLP treatment ameliorated psoriasis‐like plaque formation, ear thickness, and trans‐epidermal water loss (TEWL) in both mouse models, significantly in DKO* mice (Fig 6H–J) but not in DKO*K15 mice (Appendix Fig S6A and B). In order to determine whether this disease amelioration was caused by the reduction of epidermal cell proliferation and/or by reduction in the inflammatory response, FACS analyses were performed to quantify proliferative epidermal cells (Fig 6K) and immune cells infiltration (Appendix Fig S6C and D) from the ear of DKO* and DKO*K15 mice. Proliferating epidermal cells were significantly reduced in both mouse models after anti‐TSLP treatment (Fig 6K), confirmed by a reduction of epidermal hyperplasia and proliferation by Ki67 expression in ear sections (Fig 6L). Furthermore, the number of neutrophils on the treated ears was reduced in DKO* mice, but less in DKO*K15 mice (Appendix Fig S6C and D).
The JAK/STAT signaling pathway through TSLP/TSLPR has been widely studied for the activation of dendritic cells in allergies/inflammatory diseases such as atopic dermatitis or airway allergies (Rochman et al, 2010). We next analyzed the expression of STAT3 and STAT5 phosphorylation (p‐STAT3, p‐STAT5) by immunofluorescence in ear sections of psoriasis‐like mice treated with anti‐TSLP or IgG (Appendix Fig S6E). DKO* mice treated with anti‐TSLP had decreased epidermal thickness and STAT5 phosphorylation, while p‐STAT3 remained unaffected in both models, suggesting that TSLP acts through p‐STAT5.
Overall, these data show that TSLP secretion by bulge HF‐SCs and mutant basal keratinocytes stimulates neighboring epidermal cells to hyper‐proliferate, activate p‐STAT5 and induce expression of VEGFα and IL‐6, contributing to the initiation of epidermal hyperplasia and the maintenance of chronic skin inflammation observed in psoriasis lesions. Autocrine regulation by TSLP secretion may activate pro‐inflammatory mediators, such as NF‐κB, IL‐1β, and G‐SCF in mutant bulge HF‐SCs and mutant basal keratinocytes, pushing the inflammatory signaling toward psoriasis progression.
TSLP increases in hair follicles and IFE of scalp psoriasis patients
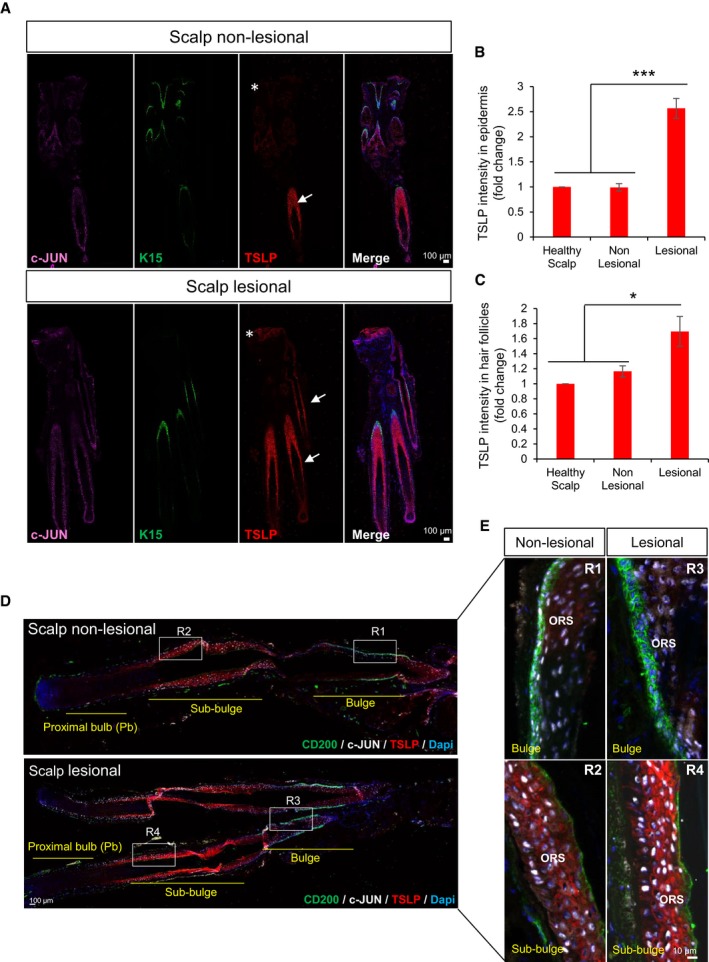
Finally, we analyzed the expression levels of TSLP in human scalp psoriasis patients (Fig 7). TSLP was expressed along the ORS in homeostasis of non‐lesional or healthy scalp, while IFE did not show any expression. In contrast, TSLP expression level was increased in lesional areas with psoriasis in both compartments, IFE and hair follicles (Fig 7A–C). Interestingly, bulge HF‐SCs labeled with CD200 did not express TSLP in psoriasis patients, neither lesional nor non‐lesional scalp, while sub‐bulge ORS increased its expression in lesional HFs (Fig 7D and E).
Figure 7. Scalp psoriasis patient skin shows increased expression of TSLP in hair follicles and epidermis.
-
ARepresentative composite immunofluorescence images of whole hair follicle units by confocal from non‐lesional and lesional scalp psoriasis patients. c‐JUN (purple), K‐15 (green), TSLP (red), and DAPI (blue). Asterisks represent the epidermis and arrows hair follicles.
-
B, CTSLP intensity measurement in hair follicle units and epidermis by ImageJ from healthy scalp, non‐lesional, and lesional scalp psoriasis patients. Representation of fold change relative to control healthy scalp. n = 3 samples. Data represent mean ± SD. Statistical significance *P < 0.05, ***P < 0.001 (Student's two‐tailed t‐test). See Appendix Table S2 for exact P‐values.
-
D, ERepresentative composite immunofluorescence images of whole hair follicle units by confocal from non‐lesional and lesional scalp psoriasis patients (D) and magnification images of specific regions from the HFs (E). CD200 (green), c‐JUN (white), TSLP (red), DAPI (blue).
Discussion
It is well established that Jun/AP‐1 transcription factors are involved in chronic skin inflammation. Reduced expression of JUNB/c‐JUN in lesional regions of the inter‐follicular epidermis (IFE) of psoriatic patients might be critical for the development of the disease (Zenz et al, 2005) despite the high variability in the expression of these factors (Haider et al, 2006; Park et al, 2009; Guinea‐Viniegra et al, 2014). Using an epidermal lineage tracing approach in skin inflammatory mouse models with inducible epidermal deletion of c‐Jun and JunB, we show that mutant bulge hair follicle stem cells (HF‐SCs) are sufficient to initiate and maintain disease development, whereas mutant IFE cells, also initiators of the disease, are lost during psoriasis‐like progression. The lesional psoriasis‐like areas of the mouse IFE displayed a mosaic pattern of cells expressing c‐Jun/JunB, which is highly reminiscent to the observed variability in expression of JUNB/c‐JUN in psoriatic plaques of patients (Haider et al, 2006; Park et al, 2009; Guinea‐Viniegra et al, 2014). Interestingly, the analyses of human scalp psoriatic patients show for the first time a significant reduction in the levels of c‐JUN and JUNB specifically in bulge HF‐SCs, supporting our hypothesis that AP‐1 transcription factors in the bulge may control HF‐SC homeostasis.
The data from the mouse models suggest that the inducible deletion of c‐Jun and JunB in HF‐SCs can initiate and maintain psoriasis‐like plaques in ear skin, the epidermal hyperplasia and inflammatory features of the disease, but it does not fully recapitulate the systemic effects observed in the DKO* psoriasis‐like mice. These differences provide a unique opportunity to study separately the specific systemic features derived from mild/moderate psoriasis in DKO* K15 mice vs. severe psoriasis‐like disease in DKO* mice, including different target treatments in two complementary mouse models. Furthermore, these genetic modifications in IFE or HF‐SCs might also reflect the high variability of the disease severity and psoriasis types in human patients, in which some patients show small psoriatic plaques with mild severity, while others develop multiple psoriatic plaques and co‐morbidities.
An important finding using lineage tracing and cell dynamic studies in these mouse models is the distinct behavior of different epidermal stem cell populations during disease initiation and progression. Interestingly, the inflammatory skin disease is sustained mainly by non‐mutant keratinocytes, while IFE mutant keratinocytes, basal and suprabasal, may contribute to the initiation, but not to the progression of the disease. Recent studies demonstrated that the epidermis is able to correct aberrant growth and inflammation to maintain tissue homeostasis through the elimination of damaged/mutant epidermal cells (Brown et al, 2017; Kashiwagi et al, 2017). However, in both psoriasis‐like mouse models, although mutant IFE cells are eliminated over time, the chronic skin inflammation is perpetuated by the survival of mutant HF‐SCs. We propose that the expression of c‐Jun and JunB is differentially regulated in distinct stem cell populations in the epidermis and that specific disruptions in the levels of these AP‐1 factors are important in HF‐SCs, which enhance their survival and promote the development of psoriasis‐like disease.
Importantly, mutant HF‐SCs have an inflammatory transcriptomic signature distinct from other epidermal populations. For instance, several pro‐inflammatory cytokines and arachidonic acid metabolic pathways described to be critical in psoriasis, such as IL‐23 (Rizzo et al, 2011), IL‐1α (Sanmiguel et al, 2009), TNF‐α (Kock et al, 1990; Kumari et al, 2013), TSLP (Volpe et al, 2014), or PTGS2 (Samuelsson, 1991), were up‐regulated. A recent report showed that during hair follicle regeneration, CD34+ bulge HF‐SCs displayed up‐regulated expression levels of genes involved in the regulation of the cell cycle and inflammatory responses, such as TNF‐α and IL‐23 (Hoeck et al, 2017). The aberrant up‐regulation of a pro‐inflammatory signaling cascade in mutant HF‐SCs may be sufficient to increase cell survival and the acquisition of a psoriasis‐like phenotype, not only in mutant HF‐SCs, but also in neighboring non‐mutant cells. Interestingly, some arachidonic acid metabolites can regulate the survival of different adult stem cell populations (Widera et al, 2006; Porter et al, 2013). Furthermore, these pro‐inflammatory mediators may also be involved in the recruitment of inflammatory cells, such as dendritic cells, neutrophils, or T cells (Albanesi et al, 2018).
Similarly, mutant epidermal cells can also stimulate neighboring non‐mutant epidermal cells to trigger hyperplasia in a paracrine fashion. In both mouse models, we show that non‐mutant keratinocytes acquire a proliferative advantage, which is induced by paracrine factors secreted by mutant keratinocytes such as TSLP. TSLP is a cytokine expressed by epithelial cells, including keratinocytes, and plays an important role in allergic inflammation, such as atopic dermatitis (AD) (He et al, 2008). Overproduction of TSLP was found in keratinocytes of human AD skin lesions (Soumelis et al, 2002) and in psoriasis patients (Volpe et al, 2014). Most studies focus on the role of TSLP in activating inflammatory cells, such as dendritic cells, although its role in epidermal cell–cell communication is not well understood. Our data indicate that TSLP secretion from mutant keratinocytes enhances cell proliferation and VEGFα expression in non‐mutant keratinocytes, an effect that was found reduced by neutralizing TLSP. We have previously reported in the DKO* mouse model that treatment with anti‐VEGF antibody ameliorates the disease (Schonthaler et al, 2009) and epidermal‐specific VEGFα overexpression in transgenic mice leads to spontaneous chronic skin inflammation (Xia et al, 2003). Importantly, during psoriasis‐like disease initiation, TSLP is produced by mutant HF‐SCs and at late stage also by non‐mutant HF‐SCs. These mutant HF‐SCs also up‐regulate several kallikreins (Klk7 and Klk14), and elevated Klk expression in keratinocytes results in significantly enhanced protease activity and augmentation of TSLP levels (Briot et al, 2010; Bin et al, 2011). Local administration of anti‐TSLP in psoriasis‐like mice resulted in the reduction of the phenotype due to epidermal hyperplasia regression and inhibition of STAT‐5 phosphorylation (p‐STAT5) in the epidermis. The activation of STAT‐5 by TSLP has been described to play an important role in survival and proliferation of CD4+ T cells in allergic/inflammatory skin and airway disorders (Rochman et al, 2010). However, phosphorylation of STAT3 in epidermal cells and immune cell infiltration remained equally activated in both mouse models, suggesting that epidermal proliferation induced by TSLP in psoriasis‐like disease maybe acting through activation of STAT5, and not STAT3 phosphorylation. These novel findings extend the role of TSLP to the regulation of keratinocyte proliferation in psoriasis‐like progression, which is an important hallmark in psoriasis. Interestingly, scalp psoriasis patients overexpressed TSLP in the IFE and in the ORS of the sub‐bulge region of HFs; however, bulge HF‐SCs did not express TSLP. CD34 labels sub‐bulge cells in human HFs, but bulge HF‐SCs are negative for this marker and positive for CD200 (Purba et al, 2014). Whether CD34 sub‐bulge populations in human HFs or other stem cell populations respond to TSLP is still unsolved. The specific response to TSLP by different stem cell subpopulations in the hair follicles of psoriatic patients as well as potential cross‐talk between cell populations will require further investigation. In addition, further investigations will be required to determine whether local neutralization of TSLP in combination with specific HF‐SCs pro‐inflammatory mediators such as PGE2 could be an effective treatment for psoriasis.
In conclusion, our study describes a novel contribution of epidermal stem cells in psoriasis‐like development and provides insights into new cellular and molecular mechanisms underlying the disease. These findings open new avenues for further investigations toward discovering novel therapies against psoriatic HF‐SCs, which might be beneficial for treating specifically psoriasis from different skin regions.
Materials and Methods
Human psoriasis biopsies
A total of seven scalp human biopsies were obtained in this study. Five were obtained from psoriatic patients with lesional scalp psoriasis and non‐lesional scalp psoriasis from the same patient, and two healthy scalp samples from occipital scalp region of volunteer hair transplant patient. All samples were obtained through informed consent after protocol approval by the Clinical Research Ethical Committees of Hospital Donostia and the University of Las Palmas de Gran Canaria, and the experiments conformed to the principles set out in the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report. Scalp biopsies were harvested using between 1 and 4 mm diameter punch (at least with a hair follicle unit). Scalp biopsies were directly embedded in optimal cutting temperature (OCT) compound for frozen sections, or fixed 24–48 h with neutral buffered 4% paraformaldehyde (PFA) and directly paraffin‐embedded. Five‐micrometer sections were stained either with hematoxylin and eosin (H&E) or processed for immunofluorescence staining (see below).
Generation of lineage tracing system in psoriasis‐like mouse models
All mouse experiments were performed in accordance with the royal decree D53/2013 on the protection of animals used for scientific purposes (DIRECTIVE 2010/63/EU), and the Ethical Committee of the Health Institute Carlos III, “authorized Body” by the Comunidad de Madrid (CAM), has evaluated and approved all the procedures involving animal experimentation for this project.
The generation of the DKO* psoriasis‐like mouse model has previously been described (Zenz et al, 2005). Briefly, DKO* psoriasis‐like mouse model with mix background was generated by the cross of mice carrying the floxed JunB allele (JunB f/f) and floxed c‐Jun allele (c‐Jun f/f) with the transgenic mice expressing the Cre recombinase–estrogen receptor fusion under the control of the basal keratinocyte‐specific K5 promoter (K5‐Cre‐ERT) to obtain JunB f/f c‐Jun f/f K5‐Cre‐ERT mice. DKO*K15 psoriasis‐like mouse model was generated by the cross of mice carrying the floxed JunB allele (JunB f/f) and floxed c‐Jun allele (c‐Jun f/f) with the transgenic mice expressing the Cre recombinase–prostaglandin receptor fusion under the control of the bulge hair follicle stem cell‐specific keratin 15 promoter (K15‐Cre‐PGR) to obtain JunB f/f c‐Jun f/f K15‐Cre‐PGR mice.
To trace epidermal‐specific deletion of JunB f/f and c‐Jun f/f in DKO* and DKO*K15 mice, global double‐fluorescent Cre reporter mouse Gt(ROSA)26Sortm4(ACTB‐tdTomato,‐EGFP)Luo/J (referred as mT/mG: membrane‐Tomato/membrane‐Green) was crossed with DKO* and DKO*K15 mice (referred as DKO*‐mT/mG and DKO*K15‐mT/mG). Mice expressing Cre recombinase under the control of the K5 promoter or K15 promoter with wild‐type (+/+) or heterozygous (+/f) for JunB and c‐Jun were used as controls (CoK5‐mT/mG and CoK15‐mT/mG, respectively). Eight‐week‐old mutant mice and controls (males and females) were injected daily (intraperitoneal), four times with 2 mg tamoxifen (Sigma) or mifepristone (Cayman Chemical) in DKO*‐mT/mG and DKO*K15‐mT/mG to induce the deletion of floxed JunB and c‐Jun alleles in the DKO*‐mT/mG and DKO*K15‐mT/mG, respectively. In addition, constitutive Tomato expression was irreversibly replaced by the expression of GFP upon the removal of the loxP‐flanked stop sequence only in the basal layer of epidermal cells that delete JunB and c‐Jun in DKO*‐mT/mG, or only in bulge hair follicle stem cells in the case of DKO*K15‐mT/mG animals.
Intravital imaging
Psoriasis‐like DKO*‐mT/mG mice at day 7 after tamoxifen induction were anaesthetized with vaporized isoflurane and placed in a hand‐made platform for the mounting of the ear into a coverslip until it was completely flattened and immobile. Mice were well hydrated by a subcutaneous injection of 1 ml saline, and their temperature was kept at 35°C in a microscope incubator equipped with an air heater and temperature probe (The Cube, Life Imaging Services). Mice were provided with vaporized isofluorane at 1.5% through a nose cone for the course of the live imaging session during 8 h.
All mice were imaged with a TCS‐SP5 confocal microscope (Leica Microsystems) equipped with AOBS and a HCX PLAN APO 20×/0.7 N.A. dry objective. EGFP and TdTomato emission were acquired every 10 min. Image processing, measurements, assembly, and editing of time‐lapse movies were performed using Image J.
Immunofluorescence
Serial sections from human scalp biopsies and mouse ears embedded in OCT or human scalp biopsies embedded in formalin‐fixed paraffin were processed for immunofluorescence. Frozen sections were fixed with neutral buffered 4% paraformaldehyde (PFA) for 15 min at room temperature (RT) and washed three times with phosphate‐buffered saline (PBS). Paraffin sections were processed for their deparaffinization and antigen retrieval. Primary antibodies specific for c‐Jun (Cell Signaling, clone 60A8, ref # 9165, dilution 1:100), JunB (Cell Signaling, clone C37F9, ref # 3753, dilution 1:100), Keratin 15 (Santa Cruz, clone LHK15, dilution 1:400), CD200 (Bio‐Rad, ref # MCA1960GA, dilution 1:100), GATA‐6 (R&D Systems, ref # AF1700, dilution 1:400), TSLP (BioLegend, ref # 515901, dilution 1:200), TSLPR (Invitrogen, ref # Pa5‐20380, dilution 1:200), Keratin 5 (BioLegend, ref # 905901, dilution 1:1,000), cleaved caspase‐3 (Cell Signaling, clone Asp175, ref # 9661S, dilution 1:100), Ki67 (Master Diagnostica, clone SP6, dilution 1:5), pSTAT3 (Cell Signaling ref # 9145, dilution 1:100), and pSTAT5 (Cell Signaling ref # 9351, dilution 1:100) were incubated overnight (O/N) at 4°C. Sections were then washed three times with PBS and incubated with secondary antibodies conjugated to the appropriate fluorophores (Alexa Fluor 647, Life Technologies, dilution 1:200) for 1 h at RT. Sections were then washed three times with PBS and mounted with gel mount to be analyzed by fluorescence microscopy or confocal.
For whole‐mount immunostaining, ear skin was dissected, fixed in 4% paraformaldehyde O/N at 4°C, and washed three times in PBS. The samples were incubated in 1.5% Triton X‐100 and 0.1% Tween in PBS (PBS‐TT) for 3 h at RT. Then, samples were blocked with 10% donkey serum in PBS‐TT (blocking buffer) O/N at RT. Primary antibody for staining bulge hair follicle stem cells, CD34 (BD Pharmingen™, clone RAM34, ref # 553731, dilution 1:100), was diluted in blocking buffer and incubated 24 h at RT. After the staining, samples were washed three times during 6 h in PBS‐TT. For immunofluorescence detection, the samples were incubated in blocking buffer containing Alexa‐647‐conjugated secondary antibodies for 24 h at RT. After three washes as described previously, the samples were stained with Hoechst (1:500) O/N at 4°C. Three washes with PBS‐TT were performed, and samples were kept in glycerol at 80° for confocal microscopy imaging.
Flow cytometry analysis and FACS
To obtain fresh dissociated epidermal cells from ear skin of psoriasis‐like and control mice, the epidermis was separated from the dermis by trypsin digestion (0.75%) for 1 h at 37°C. After mechanical dissociation, the cell suspension was filtered through a 70 μm cell strainer and centrifuged 5 min at 445 g. Pellet was re‐suspended in culture medium for further flow cytometry analysis or FACS depends on experiment.
Analysis of different epidermal subpopulations by flow cytometry was carried out. Epidermal cells were counted, centrifuged and re‐suspended in PBS with 1% chelated FBS and 1% BSA (staining buffer), and stained for 30–45 min at 4°C with the following conjugated surface markers depending on the experiment: CD45‐APC‐Cy7 (BioLegend, clone 30‐F11, dilution 1:400), CD31‐PerCP/Cy5.5 (BioLegend, dilution 1:100), CD49f‐PE‐Cy7 (BioLegend, dilution 1:400), CD34‐Alexa‐647 (BD Pharmingen™, clone RAM34, dilution 1:100), Sca‐1‐PerCP/Cy5.5 (BioLegend, clone D7, dilution 1:400), CD3e‐PE‐Cy7 (BioLegend, dilution 1:100), CD11b‐FITC (BioLegend, dilution 1:100), CD11c‐APC (BioLegend, dilution 1:100), and Ly6G‐PeRP/Cy5.5 (BioLegend, dilution 1:100). EdU staining was carried out following the manufacturing protocol (Click‐iT Plus EdU Alexa Fluor 647 Flow cytometry Assay Kit, Molecular probes, # C10634).
After staining, cells were washed with staining buffer, stained with DAPI to exclude dead cells, and analyzed by FACS CANTO II (BD, San Jose CA). At least 25,000 alive single events were collected. All data are analyzed using FlowJo 7.6.5 (Treestar, Oregon).
Sorting of different epidermal subpopulations (basal keratinocytes and hair follicle stem cells) was performed after carrying out the staining explained above. After staining, cells were filtered through a 40 μm cell strainer onto 5 ml polypropylene sterile tubes. Sorting procedures were done excluding dead cells and doublets. GFP+ and Tomato+ epidermal basal keratinocytes and hair follicle stem cells were purified into TRIzol LS for RNA isolation, or into cell lysis buffer 1× (Cell Signaling, ref # 9803S) for ELISAs, or collected in keratinocyte serum‐free medium with growth factors (KGM, Invitrogen) for primary keratinocyte cultures.
Primary cultures and live‐cell imaging
For keratinocyte colony formation assay, basal keratinocytes and hair follicle stem cells sorted by FACS were cultured in a 12‐well culture plate treated with Coating Matrix Kit (Cascade Biologicals) in KGM (Invitrogen) in a concentration of 500 cells/cm2. After 10 days in cultures, Tomato+ and GFP+ colonies of keratinocytes were quantified by fluorescence microscopy. Isolated keratinocytes and keratinocyte colonies formed by less of 10 cells were discarded in this study. Three independent experiments with technical duplicates were performed for statistical analysis.
For live‐cell time‐lapse imaging of purified single cultures or co‐cultures of Tomato+ and GFP+ keratinocytes, fresh isolated epidermal cells from ear skin of psoriatic‐like DKO* or control mice were cultured in KGM in a 24‐well culture plate treated with Coating Matrix Kit in a concentration of 2,000 cells/cm2. Each type of epidermal subpopulation was cultured alone, or in contact co‐cultures (1:1 proportion) of mutantGFP cell with non‐mutantTom cells or in co‐cultures using 12‐well trans‐well system, in which non‐mutantTom cells were cultured in the bottom and mutantGFP cells were cultured in the insert. Time‐lapse images were captured using CCD confocal incubator microscope at 37°C and 5% CO2. Images of small colonies formed after 3–5 days in culture from alone GFP+ epidermal cells, or co‐cultures of GFP+: Tomato+ keratinocytes were captured with 20× objective as six selected fields where keratinocyte colonies were located in 15‐min intervals for 48 h. Images of Tomato+ keratinocytes from trans‐well experiments were captured each 48 h during 6 days. Cell death was analyzed by TOPRO‐3 and DAPI staining. Image processing, measurements, assembly, and editing of time‐lapse movies were performed using Image J. Three independent experiments were performed for statistical analysis.
To recombinant TSLP treatment, fresh isolated epidermal cells from ear and tail skin or purified bulge HF‐SCs and basal KCs of WT mice were cultured in KGM in a 24‐well culture plate treated with Coating Matrix Kit in a concentration of 10,000 cells/cm2. Next day, mouse recombinant TSLP (eBioscence, ref # 14‐8498‐80) was added at different concentrations during 48 h. 5‐Ethynyl‐2′‐deoxyuridine (EdU) was added to the cultures after 2 days with mouse recombinant TSLP in a concentration of 10 μM during 5 h at 37°C. EdU and anti‐TSLPR staining was performed, and random confocal images were captures. EdU+ keratinocytes were quantified by Image J. Three independent experiments were performed for statistical analysis.
Adenovirus infection and TSLP neutralization
Fresh dissociated epidermal cells from ear and tail epidermis of adult JunB f/f c‐Jun f/f RosamT/mGf/f mice were plated in a 24‐well culture plate treated with Coating Matrix Kit (Cascade Biologicals) in KGM (Invitrogen) in a concentration of 15,000 cells/cm2. Twenty‐four hours later, JunB f/f c‐Jun f/f RosamT/mGf/f keratinocyte cultures were infected with adenoviruses expressing Cre (Ad‐cre) or empty (Ad‐empty) purchased from the University of lowa in a concentration of 3 × 106 particles of virus per ml of KGM. The medium with adenoviruses was changed by fresh KGM 16 h after infection. Two days after infection, around 50–60% of primary keratinocytes became GFP positive and the rest were Tomato positive and anti‐TSLP antibody (PROALT‐Protein Alternatives S.L, clone 28F12) was added to the cells every day during three consecutive days in a concentration of 500 ng/ml or 1 μg/ml. Mouse IgG was added in parallel as control. 5‐Ethynyl‐2′‐deoxyuridine (EdU) was added to the cultures after 3 days with anti‐TSLP antibody in a concentration of 10 μM during 5 h at 37°C. For further analysis, cells were fixed with PFA 4% in PBS for EdU staining (Thermo Fisher, C10086) or they were harvested for RNA isolation from total cultures or from purified GFP+ and Tom+ KCs by FACS. Conditioned medium was also harvested to quantify soluble TSLP by ELISA according to the manufacturer's instructions (Mouse TSLP Quantikine ELISA Kit, Ref # MTLP00, R&D Systems). Conditioned medium was diluted to 1:50.
In vivo neutralization of TSLP was performed by three intradermal injections of 20 μg of ab‐TSLP or IgG each 72 h on the right ear of DKO* and DKO*15 5 days after psoriasis‐like induction. Left ears were injected with PBS. EdU was applied to mice by IP injection 2 h before euthanasia. FACS analyses were performed as described above.
RNA isolation and qPCRs
After FACS, each purified epidermal subpopulation was collected in TRIzol LS (Thermo Fisher Scientific) for further RNA isolation as described by manufacturer's instructions. RNA extraction was followed by reverse transcription using Promega kit (A2801). qPCR analyses were carried out using SYBR Green Master Mix Kit (Qiagen, 204143). Expression levels were compared to housekeeping gene RPL4. The primer sequences are shown in Appendix Table S1.
RNA‐seq
Whole‐genome transcriptomic analysis was performed by Next‐Gen RNA‐sequencing in four groups of psoriatic‐like epidermal subpopulations after FACS and RNA isolation described above: MutantGFP, non‐mutantTom basal keratinocytes, mutantGFP, and non‐mutantTom hair follicle stem cells were purified from the ears of three independent psoriatic‐like DKO* mice after 7 days of tamoxifen induction. RNA‐seq was also performed in control basal keratinocytes and hair follicle stem cells from the ears of three Co‐mT/mG mice for further analysis. Next‐Gen library construction and sequencing procedures were carried out by the Genomic Unit at CNIO, and further analysis was carried out by Bioinformatics Unit at CNIO. After confirmation of RNA quality by Agilent 2100 Bioanalyzer, variable amounts of total RNA samples, between 4 and 10 ng, were processed with the SMART‐Seq v4 Ultra Low input RNA Kit (Clontech) by following manufacturer's instructions. Briefly, oligo(dT) primes preferentially reverse transcription of poly(A) RNA in the presence of a template switching oligonucleotide, and then, limited‐cycle PCR produces double‐stranded cDNA. Resulting cDNA was subsequently processed with the “Nextera XT DNA Library Prep Kit” (Illumina), which fragments and inserts Illumina adapters. Adapter‐ligated non‐directional libraries were completed by 12 cycles of PCR.
The resulting purified cDNA library was applied to an Illumina flow cell for cluster generation and sequenced on an Illumina HiSeq 2500 following manufacturer's protocols. Fifty‐one base single‐end sequencing reads were analyzed with the nextpresso pipeline (Grana et al, 2017), as follows: Sequencing quality was checked with FastQC v0.11.0 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were aligned to the mouse genome (NCBI37/mm9) with TopHat‐2.0.10 (Trapnell et al, 2012) using Bowtie 1.0.0 (Langmead et al, 2009) and SAMtools 0.1.19 (Li et al, 2009), allowing two mismatches and 20 multihits. Differential expression was tested with Cufflinks (Trapnell et al, 2012), using the mouse NCBI37/mm9 transcript annotations from https://ccb.jhu.edu/software/tophat/igenomes.shtml. GSEAPreranked (Subramanian et al, 2005) was used to perform gene set enrichment analysis of the described gene signatures on a pre‐ranked gene list, setting 1,000 gene set permutations. Differentially expressed genes (DEGs) were further analyzed for Venn's diagram (Venny2.1, http://bioinfogp.cnb.csic.es/tools/venny/), Gene Ontology Biological Process (Enrichr, http://amp.pharm.mssm.edu/Enrichr/), and predictable secreted proteins (ProteINSIDE, http://www.proteinside.org/).
Statistical analyses
For studies including animals, n is the number of total animals tested, and for in vitro studies, the number of independent experimental replicates is indicated in Figure legends, with n representing number of repeats with different sets of cells. For RNA‐seq, three control mice and 3 DKO* mice were used. Data in bar graphs represent mean ± standard deviation (SD). Statistical analyses were performed using Prism5 (GraphPad) software. When comparing two groups, a two‐tailed Student's t‐test was used, and to compare three or more groups, a two‐way ANOVA and Bonferroni post‐test was implemented. P‐values were calculated with a confidence interval of 95% to indicate the statistical significance between groups. Statistically significant differences between groups are noted in figures with asterisks (*P < 0.05, **P < 0.01, ***P < 0.001).
Author contributions
NG‐L conceived the project, designed and performed the experiments, and wrote the article. LFM performed experiments and contributed to manuscript writing. DM analyzed confocal microscopy experiments. GM‐S analyzed RNA‐seq. AI and FJ provided the human samples. EFW supervised the project, provided funding, and edited the article.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Movie EV1
Movie EV2
Movie EV3
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Acknowledgements
We thank Drs. M. Serrano and M. Pérez‐Moreno for the Gt(ROSA)26Sortm4(ACTB‐tdTomato,‐EGFP)Luo/J and K15‐Cre‐PGR mouse lines. We are very grateful to Drs. M. Pérez‐Moreno, F. Real, Ö. Uluҫkan, L. Bakiri and the laboratory members of the Sibilia and Wagner groups for critical reading of the manuscript and valuable suggestions. We thank V. Bermeo, G. Medrano, S. Leceta, O. Graña, and M. Pérez for their technical help and IT support. We acknowledge R. Paus laboratory members for the shipment of hair follicle samples. N.G.L. received funding from the People programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007‐2013) under REA grant agreement no 608765. A.I is funded by the Institute of Health Carlos III (PI16/01430). The Wagner laboratory was funded by a grant from the Spanish Ministry of Economy and competitiveness (SAF2015‐70857RE, cofounded by the European Regional Development Fund) and is supported by the ERC (ERC‐AdG 2016 CSI‐Fun).
EMBO Mol Med (2019) 11: e10697
Data availability
All primary data, detailed protocols, and non‐commercially available materials can be requested from the corresponding author. The RNA‐seq datasets have been deposited to GEO under the accession number GSE119762 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119762).
References
- Albanesi C, Madonna S, Gisondi P, Girolomoni G (2018) The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front Immunol 9: 1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002) Epidermis and its renewal by stem cells In Molecular biology of the cell, Anderson MS, Dilernia B, Goatly B. (eds), 4th edn, pp 1616 New York, NY: Garland Science; [Google Scholar]
- Arwert EN, Hoste E, Watt FM (2012) Epithelial stem cells, wound healing and cancer. Nat Rev Cancer 12: 170–180 [DOI] [PubMed] [Google Scholar]
- Asumalahti K, Laitinen T, Itkonen‐Vatjus R, Lokki ML, Suomela S, Snellman E, Saarialho‐Kere U, Kere J (2000) A candidate gene for psoriasis near HLA‐C, HCR (Pg8), is highly polymorphic with a disease‐associated susceptibility allele. Hum Mol Genet 9: 1533–1542 [DOI] [PubMed] [Google Scholar]
- Bin L, Kim BE, Hall CF, Leach SM, Leung DY (2011) Inhibition of transcription factor specificity protein 1 alters the gene expression profile of keratinocytes leading to upregulation of kallikrein‐related peptidases and thymic stromal lymphopoietin. J Invest Dermatol 131: 2213–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Simons BD (2013) Unravelling stem cell dynamics by lineage tracing. Nat Rev Mol Cell Biol 14: 489–502 [DOI] [PubMed] [Google Scholar]
- Briot A, Lacroix M, Robin A, Steinhoff M, Deraison C, Hovnanian A (2010) Par2 inactivation inhibits early production of TSLP, but not cutaneous inflammation, in Netherton syndrome adult mouse model. J Invest Dermatol 130: 2736–2742 [DOI] [PubMed] [Google Scholar]
- Brown S, Pineda CM, Xin T, Boucher J, Suozzi KC, Park S, Matte‐Martone C, Gonzalez DG, Rytlewski J, Beronja S et al (2017) Correction of aberrant growth preserves tissue homeostasis. Nature 548: 334–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C, Tainsky M, Fuchs E (1994) Programming gene expression in developing epidermis. Development 120: 2369–2383 [DOI] [PubMed] [Google Scholar]
- Fuchs E (2008) Skin stem cells: rising to the surface. J Cell Biol 180: 273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grana O, Rubio‐Camarillo M, Fdez‐Riverola F, Pisano DG, Glez‐Pena D (2017) Nextpresso: next generation sequencing expression analysis pipeline. Curr Bioinform 13: 583–591 [Google Scholar]
- Guinea‐Viniegra J, Jimenez M, Schonthaler HB, Navarro R, Delgado Y, Concha‐Garzon MJ, Tschachler E, Obad S, Dauden E, Wagner EF (2014) Targeting miR‐21 to treat psoriasis. Sci Transl Med 6: 225re221 [DOI] [PubMed] [Google Scholar]
- Haider AS, Duculan J, Whynot JA, Krueger JG (2006) Increased JunB mRNA and protein expression in psoriasis vulgaris lesions. J Invest Dermatol 126: 912–914 [DOI] [PubMed] [Google Scholar]
- He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS (2008) TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci USA 105: 11875–11880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeck JD, Biehs B, Kurtova AV, Kljavin NM, de Sousa EMF, Alicke B, Koeppen H, Modrusan Z, Piskol R, de Sauvage FJ (2017) Stem cell plasticity enables hair regeneration following Lgr5(+) cell loss. Nat Cell Biol 19: 666–676 [DOI] [PubMed] [Google Scholar]
- Horsley V, O'Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, Nussenzweig M, Tarakhovsky A, Fuchs E (2006) Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 126: 597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E (2008) NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132: 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Maradit Kremers H (2009) Trends in incidence of adult‐onset psoriasis over three decades: a population‐based study. J Am Acad Dermatol 60: 394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11: 1351–1354 [DOI] [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R (2008) Lgr5 marks cycling, yet long‐lived, hair follicle stem cells. Nat Genet 40: 1291–1299 [DOI] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM (2009) Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4: 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Driskell RR, Watt FM (2010) Assaying proliferation and differentiation capacity of stem cells using disaggregated adult mouse epidermis. Nat Protoc 5: 898–911 [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Hosoi J, Lai JF, Brissette J, Ziegler SF, Morgan BA, Georgopoulos K (2017) Direct control of regulatory T cells by keratinocytes. Nat Immunol 18: 334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel JC, Luger TA (1990) Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med 172: 1609–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Bonnet MC, Ulvmar MH, Wolk K, Karagianni N, Witte E, Uthoff‐Hachenberg C, Renauld JC, Kollias G, Toftgard R et al (2013) Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin‐24‐dependent psoriasis‐like skin inflammation in mice. Immunity 39: 899–911 [DOI] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory‐efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapouge G, Youssef KK, Vokaer B, Achouri Y, Michaux C, Sotiropoulou PA, Blanpain C (2011) Identifying the cellular origin of squamous skin tumors. Proc Natl Acad Sci USA 108: 7431–7436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy V, Lindon C, Harfe BD, Morgan BA (2005) Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell 9: 855–861 [DOI] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The sequence alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang Z, Cotsarelis G (2003) Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J Invest Dermatol 121: 963–968 [DOI] [PubMed] [Google Scholar]
- Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, Simons BD, Blanpain C (2012) Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489: 257–262 [DOI] [PubMed] [Google Scholar]
- McKay IA, Leigh IM (1995) Altered keratinocyte growth and differentiation in psoriasis. Clin Dermatol 13: 105–114 [DOI] [PubMed] [Google Scholar]
- Ortonne J, Chimenti S, Luger T, Puig L, Reid F, Trueb RM (2009) Scalp psoriasis: European consensus on grading and treatment algorithm. J Eur Acad Dermatol Venereol 23: 1435–1444 [DOI] [PubMed] [Google Scholar]
- Park CC, Kim KJ, Woo SY, Chun JH, Lee KH (2009) Comparison of the expression profile of JunB, c‐Jun, and S100A8 (Calgranulin A) in psoriasis vulgaris and guttate psoriasis. Ann Dermatol 21: 35–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RL, Georger MA, Bromberg O, McGrath KE, Frisch BJ, Becker MW, Calvi LM (2013) Prostaglandin E2 increases hematopoietic stem cell survival and accelerates hematopoietic recovery after radiation injury. Stem Cells 31: 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purba TS, Haslam IS, Poblet E, Jimenez F, Gandarillas A, Izeta A, Paus R (2014) Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. BioEssays 36: 513–525 [DOI] [PubMed] [Google Scholar]
- Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A (2011) IL‐23‐mediated psoriasis‐like epidermal hyperplasia is dependent on IL‐17A. J Immunol 186: 1495–1502 [DOI] [PubMed] [Google Scholar]
- Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez‐Rodriguez J, Wagner KU, Leonard WJ (2010) Thymic stromal lymphopoietin‐mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL‐7‐induced signaling. Proc Natl Acad Sci USA 107: 19455–19460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B (1991) Arachidonic acid metabolism: role in inflammation. Z Rheumatol 50(Suppl 1): 3–6 [PubMed] [Google Scholar]
- Sanmiguel JC, Olaru F, Li J, Mohr E, Jensen LE (2009) Interleukin‐1 regulates keratinocyte expression of T cell targeting chemokines through interleukin‐1 receptor associated kinase‐1 (IRAK1) dependent and independent pathways. Cell Signal 21: 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober M, Fuchs E (2011) Tumor‐initiating stem cells of squamous cell carcinomas and their control by TGF‐beta and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci USA 108: 10544–10549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonthaler HB, Huggenberger R, Wculek SK, Detmar M, Wagner EF (2009) Systemic anti‐VEGF treatment strongly reduces skin inflammation in a mouse model of psoriasis. Proc Natl Acad Sci USA 106: 21264–21269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG et al (2010) Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327: 1385–1389 [DOI] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A et al (2002) Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 3: 673–680 [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES et al (2005) Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci USA 102: 15545–15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szepietowski J, Walker C, Hunter JA, McKenzie RC (2001) Elevated leukaemia inhibitory factor (LIF) expression in lesional psoriatic skin: correlation with interleukin (IL)‐8 expression. J Dermatol 28: 115–122 [DOI] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L (2012) Differential gene and transcript expression analysis of RNA‐seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW (2003) Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol 120: 501–511 [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Ehinger M, Elmore A, Bortner CD, Ito M, Cotsarelis G, Nijhof JG, Peckham J, Flagler N et al (2007) CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res 67: 4173–4181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truzzi F, Marconi A, Atzei P, Panza MC, Lotti R, Dallaglio K, Tiberio R, Palazzo E, Vaschieri C, Pincelli C (2011) P75 neurotrophin receptor mediates apoptosis in transit‐amplifying cells and its overexpression restores cell death in psoriatic keratinocytes. Cell Death Differ 18: 948–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe E, Pattarini L, Martinez‐Cingolani C, Meller S, Donnadieu MH, Bogiatzi SI, Fernandez MI, Touzot M, Bichet JC, Reyal F et al (2014) Thymic stromal lymphopoietin links keratinocytes and dendritic cell‐derived IL‐23 in patients with psoriasis. J Allergy Clin Immunol 134: 373–381 [DOI] [PubMed] [Google Scholar]
- Wagner EF, Schonthaler HB, Guinea‐Viniegra J, Tschachler E (2010) Psoriasis: what we have learned from mouse models. Nat Rev Rheumatol 6: 704–714 [DOI] [PubMed] [Google Scholar]
- Wang GY, Wang J, Mancianti ML, Epstein EH Jr (2011) Basal cell carcinomas arise from hair follicle stem cells in Ptch1(+/−) mice. Cancer Cell 19: 114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Siegenthaler JA, Dowell RD, Yi R (2016) Foxc1 reinforces quiescence in self‐renewing hair follicle stem cells. Science 351: 613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AB, Zhang YV, Tumbar T (2017) Gata6 promotes hair follicle progenitor cell renewal by genome maintenance during proliferation. EMBO J 36: 61–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B (2006) Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF‐kappaB signaling. BMC Neurosci 7: 64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf‐Henning Boehncke MPS (2015) Psoriasis. Lancet 386: 983–994 [DOI] [PubMed] [Google Scholar]
- Xia YP, Li B, Hylton D, Detmar M, Yancopoulos GD, Rudge JS (2003) Transgenic delivery of VEGF to mouse skin leads to an inflammatory condition resembling human psoriasis. Blood 102: 161–168 [DOI] [PubMed] [Google Scholar]
- Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, Mehic D, Scheuch H, Angel P, Tschachler E, Wagner EF (2005) Psoriasis‐like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437: 369–375 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Movie EV1
Movie EV2
Movie EV3
Review Process File
Source Data for Figure 1
Source Data for Figure 2
Source Data for Figure 3
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 6
Data Availability Statement
All primary data, detailed protocols, and non‐commercially available materials can be requested from the corresponding author. The RNA‐seq datasets have been deposited to GEO under the accession number GSE119762 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119762).


