Abstract
Age-related strength and muscle mass loss is further increased after acute periods of inactivity. To avoid this, resistance training has been proposed as an effective countermeasure, but the additional effect of a protein supplement is not so clear. The aim of this study was to examine the effect of a whey protein supplement enriched with leucine after resistance training on muscle mass and strength gains in a post-hospitalized elderly population. A total of 28 participants were included and allocated to either protein supplementation or placebo supplementation following resistance training for 12 weeks (2 days/week). Physical function (lower and upper body strength, aerobic capacity and the Short Physical Performance Battery (SPPB) test), mini nutritional assessment (MNA) and body composition (Dual X-ray Absorptiometry) were assessed at baseline and after 12 weeks of resistance training. Both groups showed improvements in physical function after the intervention (p < 0.01), but there were no further effects for the protein group (p > 0.05). Muscle mass did not improve after resistance training in either group (p > 0.05). In conclusion, 12 weeks of resistance training are enough to improve physical function in a post-hospitalized elderly population with no further benefits for the protein-supplemented group.
Keywords: elderly, aging, muscle mass, strength, resistance training, leucine, whey protein, protein supplementation
1. Introduction
Aging is characterized by a progressive decline in skeletal muscle mass and function defined as sarcopenia [1]. Sarcopenia is related to an increased risk of falling [1], fractures [2], physical disability [3] and mortality [4]. In healthy aging, muscle mass loss ranges from 3% to 8% per decade [5]. However, this decline is further emphasized by acute or chronic illness [6], inactivity [6] and inadequate protein and/or energy intake [7]. So, physical activity is proposed as an effective countermeasure to delay the age-related muscle mass loss [7]. Indeed, following a healthy lifestyle may help to prevent and reduce the consequences of age-related muscle mass loss [7].
A balanced protein metabolism is important to muscle mass accretion and maintenance [8]. Energy and protein intake are key nutritional factors to achieve protein balance [7]. However, due to several physiological and social factors, elderly people tend to reduce food intake and, in consequence, often fail to meet energy and protein requirements [9]. Likewise, protein-energy malnutrition is frequent in elderly patients [9]. Besides total daily protein intake [10], dietary protein quality and its anabolic potential have also received increased interest with the goal of optimizing skeletal muscle anabolism in the elderly [10,11].
Dietary protein quality depends on its digestibility, amino acid (AA) profile and AA availability [12,13]. Therefore, in studies aiming to optimize muscle mass among elderly people, whey protein (≈20 g/day) is considered superior to other isolated protein sources [14,15]. Whey protein is also characterized for being a high leucine-containing protein, which is the main precursor for activating muscle protein synthesis via mammalian target of rapamycin (mTOR) signaling [8,16]. A protein/AA source containing around 1.8–2.0 g of leucine would be enough to activate post-exercise “leucine trigger”, whereas in rested conditions, a higher dose might be required in young adults [16]. Other authors [15], reported that 20 g of whey protein enriched with 3 g of leucine post-exercise resulted in a greater muscle protein synthesis rate in healthy older people.
Muscle mass accretion and strength gain depend on the synergistic effect of protein consumption and resistance training [8,16]. Protein ingestion close after exercise seems to increase exercise-induced muscle mass sensitivity to anabolism [8]. In a recent systematic review and meta-analysis [17], it was concluded that a combination of protein supplementation and resistance training led to positive effects on body composition, muscle volume and strength, and physical function in elderly people. In contrast, in people aged 70 years or older, it was shown that despite overall improvements from baseline for the majority of outcomes, there were no significant differences between the group receiving protein/AA supplementation along with resistance training and the group with resistance training alone [18].
Acute periods of inactivity, such as a hospital stay, accentuates age-related muscle mass loss [6]. After hospitalization, older individuals are more vulnerable to develop any adverse event [19]. Then, early interventions to accelerate recovery and avoid hospital readmission will be important [20]. For example, implementing interventions that combine nutrition and physical exercise immediately after discharge [20].
In view of this growing interest, the objective of the present study was to examine the effect of a whey protein supplement enriched with leucine after resistance training on muscle mass and strength gains in a post-hospitalized elderly population. We hypothesized that elderly people after hospitalization may benefit most from the synergetic effect of protein supplementation and a resistance training session.
2. Methods
2.1. Study Design
The Sarcopenia and Fragilidad-protein (S and F-PROT) study is a prospective, 24-week, single-blind, randomized, placebo-controlled clinical trial (ClinicalTrials.gov ID: NCT03815201). The study was conducted at the facilities of the Araba University Hospital in Vitoria-Gasteiz (North Spain), from September 2017 to July 2018. The Clinical Research Ethics Committee of the Araba University Hospital (CEIC-HUA: 2017-021) approved the study protocol (S and F-PROT) that complied with the revised ethical guidelines of the Declaration of Helsinki (revision of 2013). All participants were informed about the details of the research and signed an informed consent before their enrolment in the study.
The S and F-PROT project compared relative changes on functional capacity (muscular strength of upper and lower limbs, and aerobic capacity), body composition (lean mass and fat mass at whole body, arms, legs and trunk) and nutritional status between two groups following a resistance training intervention program with post-exercise supplementation (Protein-group) or without supplementation (Placebo-group).
2.2. Participants
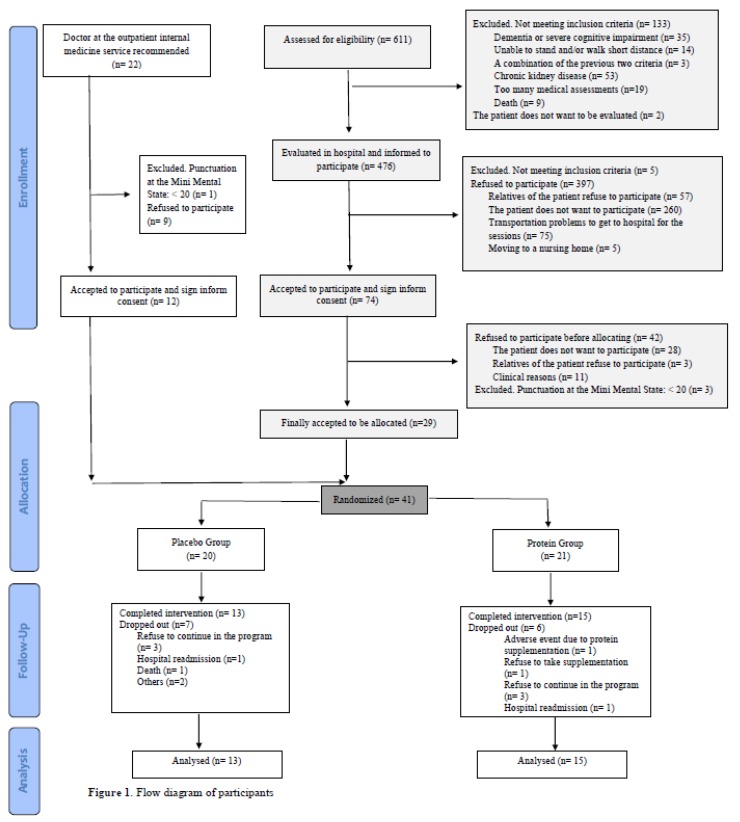
Volunteers accessed the program after hospitalization at the internal medicine service of the Araba University Hospital, or by medical recommendation at the outpatient internal medicine specialty at the Araba University Hospital (Figure 1). Hospitalized patients older than 70 years old were first pre-screened for eligibility. All pre-screened participants met the following criteria: >70 years old, a punctuation of ≥20 at the Mini Mental State Questionnaire (MMSE), fulfilled the criteria for sarcopenia diagnosis of the European Working Group on Sarcopenia in Older People, were able to walk alone or using a walking stick, a walking frame, or parallel walking bars, were able to understand the instructions or what had being said, and signed the informed consent. Patients were excluded for examination if they had any of the following exclusion criteria: history of chronic kidney disease, had suffered a heart attack in the last 3 months, been unable to walk, have suffered any fracture of the upper or lower limbs in the last 3 months, been suffering from severe dementia, a history of autoimmune neuromuscular disorders (for example, myasthenia gravis, Guillain–Barré syndrome, inflammatory myopathies) or amyotrophic lateral sclerosis, or refused to sign the informed consent.
Figure 1.
Flow Diagram of participants.
Patients that were eligible for the intervention program were assessed for nutritional status (Mini Nutritional Assessment-Short Form (MNA-SF; Nestlé Nutrition Institute)) [21], physical function (Short Physical Performance Battery (SPPB) [22] and handgrip strength), frailty (a Spanish language version of the Fried test [23]) and cognitive function (Spanish validated version of the Pfeiffer test, the Short Portable Mental Status Questionnaire (SPMSQ) [24]) during their hospitalization. Patients were informed about the possibility of participating in an exercise training program after hospital discharge and an informed consent was given along with further written information. After a recovery week, patients were cited for baseline physical function assessment before initiating the intervention program.
Many hospitalized patients did not meet inclusion criteria when assessing eligibility (21.8%) or refused to participate (66.6%) because of health issues, lack of interest in the physical exercise program, or had problems to get to hospital for the intervention sessions. As the hospital recruitment proved not to be enough for the intervention aims, the outpatient internal medicine service was chosen as an alternative recruitment source. Those patients at the outpatient internal medicine service potentially meeting inclusion criteria were informed by their doctor about the exercise intervention program. Thereafter, patients were cited for a first eligibility assessment with the investigation team. If participation criteria were met, patients were again cited a week after for baseline physical function assessment (Figure 1).
2.3. Randomization
Following baseline physical function assessment, participants were randomly allocated to one of the two intervention groups: Placebo-group or Protein-group. Participant stratification was based on gender to ensure equal allocation in both groups.
2.4. Supplementation and Blinding
Placebo and protein supplements were delivered by the nutritionist in the first half hour following each training session. The protein supplement contained 20 g of whey protein isolate (Davisco ®: BiPRO all-natural whey protein isolate, Eden Paririe, MN, USA) enriched with 3 g of leucine (Nutricia, Madrid, Spain). The nutritional composition of both the placebo and protein supplement is shown in Table 1. The supplements were energy-matched and were flavored with lemon flavor and solubilized in 150 mL of water. Only participants were blinded for supplementation. Supplements were stored in boxes and only the research team could identify them. All supplements were developed, prepared and stored in boxes by Laboratorium Sanitatis SL (Tecnalia Research and Innovation, Vitoria-Gasteiz, Spain).
Table 1.
Nutritional composition of the protein and placebo supplements.
| Nutritional Composition | Protein Supplement |
|---|---|
| Β-lactoglobulin (g/bottle) | 20 |
| L-Leucine (g/bottle) | 3 |
| Sodium saccharin (g/bottle) | 0.050 |
| Sucralose (g/bottle) | 0.030 |
| Lemon flavor 654500 (g/bottle) | 0.250 |
| Placebo supplement | |
| Maltodextrin (g/bottle) | 23 |
| Hydroxyethylcellulose (g/bottle) | 0.200 |
| Lemon flavor 654500 (g/bottle) | 0.250 |
2.5. Design of the Resistance Training Program
Both groups followed a supervised resistance training program for 12 weeks. The program consisted of 1 h sessions on two non-consecutive days per week. The first week of intervention was used for familiarization, and 1-RM (repetition maximum) estimation by the individual’s functional capacity through Brzycki equation [25]. The load was then gradually increased during a month, and half exercises were performed at 50%–65% of the estimated 1-RM. During the subsequent months, load was increased until 70% of the estimated 1-RM was reached. Two sets were performed per exercise and load and maximum repetition for each exercise was personalized for each participant. All resistance training sessions were designed and supervised by a sport scientist with experience in resistance training for the elderly.
All training sessions started with warm-up exercises (heel stand, calf raises, chair stand exercise and neck movements) and were followed by strengthening exercises of upper and lower limbs (arm-curl exercise with the participant in a seated position and personalized load, knee extension exercise with personalized load in a seated position, standing knee flexion with personalized load, side hip raise, standing hip extension and chair stand exercise). In the same resistance training session, some exercises for dynamic balance improvement were also practiced (side-by-side stand, semi-tandem stand, tandem stand, monopodal stand, timed up and go, stepping around obstacles and step up and down exercises). The session finished with 5 min of cool-down, consisting mainly of stretching exercises.
2.6. Outcome Measures
Primary and secondary outcomes were assessed at baseline and after 12 weeks of intervention by the same trained researchers. Post-intervention measurements were scheduled within one week following the last exercise session.
2.7. Primary Outcome: Physical Function
Physical function was assessed at baseline and at week 13 (once supervised intervention period was finished).
Physical function was assessed using a combination of tests. The tests used to assess lower and upper body strength and aerobic capacity were based on the Senior Fitness Test [26]. For lower and upper body strength, 30-Second Chair Stand Test and 30-Second Arm Curl Test were used, respectively. For upper body strength, isometric handgrip strength was also measured using a handled dynamometer (JAMAR® PLUS + Hand dynamometer). Aerobic capacity was assessed by the 6 min walking test (6MWT). Hence, for physical function assessment, the SPPB test battery was also used [22]. This test includes the 4 m walking speed test, the standing balance test (side-by-side stand, semi-tandem stand and tandem stand) and the time to rise from a chair five times test [22].
2.8. Secondary Outcomes
2.8.1. Nutritional Assessment
A nutritionist completed all nutritional questionnaires along with the participant and/or participant’s relative or caregiver. Participant’s nutritional status was assessed using the MNA questionnaire (Nestlé Nutritional Institute) [27]. This questionnaire contains 18 items divided into 4 categories: anthropometric assessment, general assessment, short dietary assessment and subjective assessment [27]. Each answer has a numerical value contributing to the final punctuation. A maximum of 30 points can be obtained. Punctuation ranging from 24 to 30 reflects normal nutritional status, from 17 to 23.5 risk of malnutrition and a punctuation under 17 reflects malnutrition [27].
2.8.2. Body Composition
Body fat, lean mass, bone mass, bone mineral density (BMD) and bone mineral content (BMC) were assessed by dual-energy X-ray absorptiometry (DXA; HOLOGIC, QDR 4500).
Body mass (OMRON HN-288, Digital Personal Scale, Barcelona, Spain) was measured barefoot following the standard protocols. Height was estimated using knee height determination (SECA 220, Hamburg, Germany) [28]. Body mass index (BMI) was calculated as body weight divided by height squared (kg/m2). Waist circumference, hip circumference, calf circumference and mid-arm circumference were measured with a nonelastic tape (CESCORF, Porto Alegre, Brasil) following the protocol recommended by the International Society for the Advancement of Kinanthropometry (ISAK).
2.8.3. Biochemical Parameters
Biochemical parameters were obtained from fasting venous blood samples in Ethylenediaminetetraacetic acid -containing tubes and in serum tubes. These tubes were immediately carried to the laboratory and EDTA-containing tubes were centrifuged at 1000× g at 4 °C for 10 min, whereas serum tubes were centrifuged 90 min after blood collection at 1000× g at 20 °C for 15 min. Serum albumin, prealbumin and creatinine were measured as protein malnutrition markers.
2.9. Statistical Analysis
Baseline characteristics between groups (i.e., placebo versus protein supplementation) were compared using independent Student’s t-test.
Sample size estimation and power analysis was calculated for muscle mass increase. With a population size of 35 on each group, a significant alpha level of 0.05, and power >80%, the range for a statistically detectable change in muscle mass will be 1.5–2kg with a standard deviation of 1.5–1.7 kg.
Data analysis was performed following the per-protocol principle. Changes in primary and secondary outcomes were calculated as Post-intervention minus Pre-intervention values. Differences between the placebo and the protein groups (fixed factor) in changes on primary outcomes and secondary outcomes were calculated by analyses of covariance adjusting with baseline values.
All statistical analyses were performed using the statistical software SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) with a level of significance of α = 0.05. Data are expressed as means ± standard error of the mean.
3. Results
During the recruitment period, a total of 476 hospitalized patients were evaluated and invited to participate in the study. From them, only 74 (15.5%) accepted to participate, whereas the remaining 402 patients refused to participate (83.4%) or did not meet inclusion criteria (1.15%). Finally, from the 74 patients who accepted to participate, a total of 29 participants (39.2%) were randomized for the study. Overall, 93.9% of the evaluated hospitalized patients did not participate in the study due to inclusion criteria or rejection to participate in the program. Regarding the recruitment from the outpatient internal medicine service, a total of 22 patients were recommended to participate, 40.9% of these patients refused to enter the intervention program or did not meet inclusion criteria (4.5%), whereas 54.54% accepted. In total, 41 patients were randomized for the intervention program, 20 entered the Placebo-group and 21 the Protein-group. From the allocated participants, 13 did not complete the 12 weeks of the intervention program (7 from the Placebo-group and 6 from the Protein-group). The main reason for dropping out from the study in both groups was that participants refused to continue in the program (15.0% of the randomized patients in the Placebo-group and 14.3% in the Protein-group). In the Protein-group, an adverse event was reported with protein supplementation regarding itchy throat and difficulties to inhale, whereas another participant refused to take the protein supplement, so both participants were dropped from the study (Figure 1). Baseline characteristics of the recruited participants can be found in Supplemental Table S1.
Table 2 shows baseline characteristics of participants. There were no statistically significant differences in body composition and nutritional status variables between groups at baseline. However, within the physical function parameters, the protein-group walked significantly more meters in the 6MWT at baseline (p < 0.05). In contrast, the Protein-group showed significantly greater lean mass on the legs (%) than the Placebo-group (p < 0.05).
Table 2.
Characteristics of participants completing the study (intend-to-treat analyses).
| N | Placebo Group | N | Protein Group | p | |
|---|---|---|---|---|---|
| Age (years) | 13 | 81.7 (6.45) | 15 | 82.9 (5.59) | 0.607 |
| Women (N, %) | 13 | 7 (53.8) | 15 | 7 (46.7) | 0.717 |
| Body mass (kg) | 13 | 75.9 (17.95) | 15 | 68.0 (11.43) | 0.188 |
| BMI (Kg/m2) | 13 | 30.8 (6.53) | 15 | 27.4 (3.50) | 0.110 |
| Physical Function | |||||
| Handgrip (kg/body mass) | 13 | 0.3 (0.09) | 15 | 0.4 (0.09) | 0.063 |
| SFT chair stand test 30sec | 13 | 10.6 (4.17) | 15 | 12.3 (2.97) | 0.229 |
| SFT arm curl test 30sec | 13 | 13.5 (5.22) | 15 | 16.3 (3.92) | 0.137 |
| SFT 6MWT (m) | 13 | 314.8 (139.36) | 15 | 411.5 (80.40) | 0.040 |
| SPPB total punctuation | 13 | 8.7 (2.36) | 15 | 10.1 (1.58) | 0.089 |
| SPPB 5Squat | 13 | 14.7 (6.85) | 15 | 12.2 (2.86) | 0.232 |
| Body composition | |||||
| Waist to hip ratio | 13 | 1.00 (0.07) | 15 | 0.98 (0.09) | 0.459 |
| Lean mass arms (kg) | 13 | 2.3 (0.67) | 15 | 2.3 (0.44) | 0.897 |
| Lean mass legs (kg) | 13 | 6.8 (1.70) | 15 | 6.4 (1.08) | 0.441 |
| Lean mass trunk (kg) | 13 | 23.0 (4.83) | 15 | 21.5 (3.89) | 0.380 |
| Total lean mass (kg) | 13 | 45.2 (9.85) | 15 | 42.3 (6.63) | 0.391 |
| Fat mass arms (%) | 13 | 2.6 (0.96) | 15 | 2.4 (0.77) | 0.545 |
| Fat mass legs (%) | 13 | 5.8 (1.85) | 15 | 5.4 (1.84) | 0.603 |
| Fat mass trunk (%) | 13 | 17.1 (3.87) | 15 | 14.9 (3.03) | 0.124 |
| Total fat mass (%) | 13 | 35.4 (8.05) | 15 | 32.1 (6.84) | 0.259 |
| Nutritional Status | |||||
| MNA score | 13 | 23.1 (3.82) | 15 | 24.5 (2.11) | 0.273 |
| Normal nutritional status (N, %) | 13 | 4 (30.8) | 15 | 11 (73.3) | 0.064 |
| At risk of malnutrition (N, %) | 13 | 8 (61.5) | 15 | 4 (26.7) | |
| Malnourished (N, %) | 13 | 1 (7.7) | 15 | 0 (0) | |
| Biomarkers | |||||
| Creatinine (mg/dL) | 10 | 1.1 (0.48) | 15 | 0.9 (0.35) | 0.401 |
| Albumin (g/dL) | 13 | 4.0 (0.39) | 15 | 4.0 (0.31) | 0.994 |
| Prealbumin (mg/dL) | 12 | 22.2 (6.63) | 14 | 23.3 (4.31) | 0.613 |
BMI: body mass index; MNA score: Mini Nutritional Assessment score; SFT chair stand test 30 s: Senior Fitness Test chair stand test 30 s; SFT arm curl test 30 s: Senior Fitness Test arm curl test 30 s; SFT 6MWT (m): Senior Fitness Test 6-min Walking Test (m); SPPB total punctuation: Short Physical Performance Battery total punctuation; SPPB 5Squat: Short Physical Performance Battery 5Squat. Values are means and standard deviations.
3.1. Effects of the Intervention on Primary Outcomes: Physical Function
Both groups showed improvements over time in all the physical function tests (p < 0.01), except for the handgrip strength test (Table 3). However, we did not observe any significant difference between groups in any of the measured physical function tests (Table 3).
Table 3.
Body composition, nutritional status and physical function in elderly patients before (Pre) and after (Post) their participation in the resistance exercise intervention program plus protein supplementation (Protein-group) or placebo (placebo-group) (analyses per protocol).
| Placebo-Group | Protein-Group | Differences between Groups | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Pre | Post | p | N | Pre | Post | p | Δ Placebo | Δ Protein | p | |
| Primary outcome | |||||||||||
| Physical function | |||||||||||
| Handgrip (kg/body mass) | 13 | 0.3 (0.09) | 0.3 (0.09) | 0.775 | 15 | 0.4 (0.09) | 0.4 (0.09) | 0.651 | 0.0 (0.03) | -0.0 (0.06) | 0.971 |
| SFT chair stand test 30sec | 13 | 10.6 (4.17) | 13.5 (4.59) | 0.003 | 15 | 12.3 (2.97) | 14.4 (3.22) | <0.001 | 2.8 (2.79) | 2.1 (1.53) | 0.480 |
| SFT arm curl test 30sec | 13 | 13.5 (5.22) | 21.9 (4.66) | <0.001 | 15 | 16.3 (3.92) | 23.5 (4.53) | <0.001 | 8.4 (5.74) | 7.2 (4.86) | 0.724 |
| SFT 6min WT (m) | 13 | 314.8 (139.36) | 375.0 (128.39) | 0.002 | 15 | 411.5 (80.4) | 455.1 (81.77) | 0.005 | 60.2 (53.67) | 43.6 (51.2) | 0.959 |
| SPPB total score | 13 | 8.7 (2.36) | 10.3 (1.89) | 0.001 | 15 | 10.1 (1.58) | 11.3 (0.96) | 0.002 | 1.6 (1.39) | 1.2 (1.21) | 0.634 |
| SPPB 5Squat | 13 | 14.7 (6.85) | 10.6 (3.67) | 0.005 | 15 | 12.2 (2.86) | 10.0 (2.81) | 0.004 | −4.1 (4.32) | –2.2 (2.4) | 0.491 |
| Secondary outcomes | |||||||||||
| Body composition | |||||||||||
| Body mass (kg) | 13 | 75.9 (17.95) | 75.6 (18.31) | 0.621 | 15 | 68.0 (11.43) | 68.3 11.07) | 0.500 | −0.3 (2.24) | 0.3 (1.60) | 0.471 |
| BMI (kg/m2) | 13 | 30.8 (6.54) | 30.7 (6.64) | 0.575 | 15 | 27.4 (3.5) | 27.5 (3.37) | 0.453 | −0.3 (2.24) | 0.3 (1.60) | 0.493 |
| Waist to hip ratio | 13 | 1.00 (0.07) | 1.00 (0.08) | 0.818 | 15 | 0.98 (0.09) | 0.96 (0.08) | 0.255 | −0.0 (0.06) | −0.0 (0.05) | 0.400 |
| Lean mass arms (kg) | 13 | 2.3 (0.67) | 2.3 (0.41) | 0.937 | 15 | 2.3 (0.44) | 2.2 (0.41) | 0.049 | 0.0 (0.36) | −0.1 (0.24) | 0.088 |
| Lean mass legs (kg) | 13 | 6.8 (1.7) | 6.9 (1.45) | 0.630 | 15 | 6.4 (1.08) | 6.5 (1.04) | 0.260 | 0.1 (0.64) | 0.1 (0.34) | 0.756 |
| Lean mass trunk (kg) | 13 | 23.0 (4.83) | 22.6 (4.47) | 0.212 | 15 | 21.5 (3.88) | 21.7 (3.61) | 0.198 | −0.4 (1.21) | 0.2 (0.67) | 0.128 |
| Total lean mass (kg) | 13 | 45.2 (9.85) | 44.7 (8.54) | 0.545 | 15 | 42.3 (6.63) | 42.5 (6.61) | 0.458 | −0.4 (2.52) | 0.2 (1.02) | 0.611 |
| Fat mass arms (%) | 13 | 2.6 (0.96) | 2.6 (0.85) | 0.808 | 15 | 2.4 (0.77) | 2.3 (0.92) | 0.291 | −0.0 (0.56) | −0.1 (0.41) | 0.575 |
| Fat mass legs (%) | 13 | 5.8 (1.85) | 5.9 (2.07) | 0.165 | 15 | 5.4 (1.84) | 5.5 (1.69) | 0.506 | 0.2 (0.45) | 0.1 (0.46) | 0.549 |
| Fat mass trunk (%) | 13 | 17.1 (3.86) | 16.7 (3.31) | 0.448 | 15 | 14.9 (3.03) | 15.7 (2.61) | 0.061 | −0.4 (1.86) | 0.7 (1.31) | 0.297 |
| Total fat mass (%) | 13 | 35.4 (8.05) | 35.2 (7.53) | 0.728 | 15 | 32.1 (6.84) | 32.7 (6.64) | 0.092 | −0.2 (1.91) | 0.6 (1.31) | 0.357 |
| Nutritional status | |||||||||||
| MNA score | 13 | 23.1 (3.8) | 25.3 (2.2) | 0.010 | 15 | 24.5 (2.1) | 26.2 (1.6) | 0.019 | 2.2 (2.6) | 1.7 (2.5) | 0.512 |
| Normal nutritional status (N. %) | 13 | 4(30.8) | 9(69.3) | 0.123 | 15 | 11(73.3) | 14(93.4) | 0.533 | |||
| At risk of malnutrition (N. %) | 13 | 8(61.6) | 4(30.8) | 15 | 4(26.7) | 1(6.7) | |||||
| Malnourished (N. %) | 13 | 1(7.7) | 0 | 15 | 0 | 0 | |||||
| Biomarkers | |||||||||||
| Creatinine (mg/dL) | 10 | 1.1 (0.48) | 1.1 (0.37) | 0.664 | 15 | 0.9 (0.35) | 0.9 (0.32) | 0.595 | 0.0 (0.21) | 0.0 (0.14) | 0.438 |
| Albumin (g/dL) | 13 | 3.9 (0.39) | 4.1 (0.31) | 0.189 | 15 | 3.9 (0.31) | 4.0 (0.26) | 0.499 | 0.1 (0.22) | 0.0 (0.15) | 0.331 |
| Prealbumin (mg/dL) | 12 | 22.2 (6.63) | 20.5 (4.48) | 0.221 | 14 | 23.3 (4.31) | 21.3 (4.17) | 0.019 | −1.6 (4.36) | −1.9 (2.77) | 0.916 |
SFT chair stand test 30sec: Senior Fitness Test chair stand test 30 sec; SFT arm curl test 30 sec: Senior Fitness Test arm curl test 30sec; SFT 6MWT (m): Senior Fitness Test 6-min Walking Test (m); SPPB total punctuation: Short Physical Performance Battery total punctuation; SPPB 5Squat: Short Physical Performance Battery 5Squat; BMI: body mass index; MNA score: Mini Nutritional Assessment score. Values are means and standard deviations. *P indicates statistical differences between Pre and Post values (paired Student’s t-test). Δ placebo indicates the difference between Pre and Post values in the Placebo-group; Δ Protein indicates the difference between Pre and Post values in the Protein-group. P indicates statistical significance between Δ placebo and Δ Protein (ANOVA).
3.2. Effects of the Intervention on Secondary Outcomes
We did not observe any significant difference on body composition measurements within groups at the end of the intervention, except for lean mass on arms within the protein-group (p < 0.05, Table 3). There were no significant differences in any of the body composition variables between the two groups (Table 3).
The MNA scoring improved significantly within both groups after the intervention program (p < 0.05, Table 3). However, we did not observe any significant difference on changes in MNA score between groups (p < 0.5, Table 3).
Among serum markers of protein malnutrition, creatinine and albumin concentrations did not significantly change over time in either group (Table 3). Prealbumin concentrations significantly decreased in the Protein-group (p < 0.05, Table 3). Nevertheless, there were no significant differences on changes in protein nutritional status serum biomarkers between groups (Table 3).
4. Discussion
The current study aimed to examine the additional effect of a leucine-enriched protein supplementation on physical function, skeletal muscle mass and nutritional status after resistance training in a post-hospitalized elderly population. Results do not show further beneficial effects with protein and leucine-enriched supplementation after 12 weeks of resistance training (2 sessions/week) for any of the measured variables. These findings suggest that protein supplementation might not be determinant to see improvements in muscle mass and strength, and/or the time period of the intervention was not enough to see significant results.
It is well-established that resistance training is an effective countermeasure to combat age-related skeletal muscle mass and strength loss [6,29]. It is proposed as a primary intervention for sarcopenia [30], frailty [31], malnutrition [32] and other geriatric syndromes [7]. Our results are in line with these guidelines, according to physical function measurements as both groups show improvement after resistance training.
Resistance training stimulates muscle protein synthesis [33]. To take advantage of this anabolic stimuli, we considered protein supplementation as a complementary strategy following resistance training. In line with studies supporting this strategy [15,34], the protein-group received 20 g of whey protein enriched with 3 g of leucine after each session twice per week. However, there were no further benefits on physical function for the protein-group in this study. This is in contrast with some [34,35,36], but not all [37,38] previous studies. A recent systematic review [18], concluded that protein/AA supplementation did not further improve muscle strength in older subjects following a resistance training program. Nevertheless, both groups showed significant improvements in physical function parameters, except for handgrip strength. This result was also seen in the study carried out by Leenders et al. [29], where they suggested that handgrip strength is not a clinically relevant and/or valid measure to evaluate changes in muscle function in response to a resistance training program in the elderly.
We observed no changes in body composition after the intervention in either group. Again, we did not see further benefits with protein supplementation. One previous study with participants aged 82 years reported a limited muscle plasticity that further limited strength gains in response to a progressive resistance training program [39]. So, our results in a population with the same average age (82 years) underscore the limited capacity to hypertrophy as we age [40]. Furthermore, when looking for studies regarding muscle mass and strength gains along with protein supplementation, among many of them, the target adult population are younger than age 80 [34,36,41,42,43]. The same issue can be seen in recent systematic reviews and meta-analyses, where most of the included studies are based in younger populations [44,45]. However, this blunted anabolic response might be overcome, or at least minimized, if adequate interventions are designed [46]. It seems that the protein synthesis capacity of the muscle is preserved up to very old age in response to anabolic stimuli [33].
There is still much controversy regarding protein supplementation in the elderly population due to poor compliance, high heterogeneity and underpowered studies evident from meta-analysis and systematic reviews [17,18,45]. Studies underlying protein supplementation as an effective measure to increase resistance training induced adaptations were based on short-term metabolic studies [15,34,47]. Conversely, dietary intervention studies, where long-term protein supplementation have been examined, have failed to observe measurable gains in skeletal muscle mass in the elderly population [35,37,38]. Tieland et al. supplemented the protein-group with 15 g of protein at breakfast and lunch for 24 weeks and they reported that protein intake in this group increased to more than 25 g with each meal (daily protein intake increased from 1.0 ± 0.1 to 1.4 ± 0.1g/kg body mass/day) [35]. However, they did not observe measurable gains in skeletal muscle mass for the protein-group as baseline protein intake was already high [35].
Contrary to Tieland et al. [35], participants in our study entered the interventional program after hospitalization where they had suffered an acute phase of illness and inactivity, and it would be reasonable to think that the protein-group should have benefit more from the resistance training along with protein supplementation. However, during the post-hospitalization period exists an acquired, transient period of vulnerability known as post-hospital syndrome, where the nutritional requirements are higher than normal to reverse this acute situation [19,48]. It has been suggested to increase dietary protein to 1.2–1.5 g/kg body mass/day during acute illness or up to 2.0 g/kg body mass/day in severe situations [48]. Thus, it could be speculated that if the protein supplementation protocol used in the study of Tieland et al. was not enough to see gains in muscle mass [35], and neither was the one applied in our study. It is probably that participants in our study were below the dietary protein recommendation set for acute phases or that the dietary treatment on this study did not increase the daily protein intake to have an effect. However, in contrast to Tieland et al. [35], the protein-group in our study was supplemented immediately after resistance training in order to overcome any daily protein deficiency and take advantage of the increased exercise-induced anabolic stimuli. In a recent study conducted in mobility-limited older adults, the protein-group was supplemented after resistance training with 20 g of whey protein three times per week for six months [42]. Englund et al. concluded that protein supplementation improved body composition [42]. However, the target population in the study conducted by Englund et al. had a total SPPB score of ≤9 [42], whereas the protein group in our study had a baseline total SPPB score of ≥9. This suggests that our participants were in better physical condition and that they had better body composition, although they entered the program after an acute illness phase. So, there might be different hypotheses to assess why further benefits were not seen with protein supplementation in muscle mass accretion. The acute illness phase might have been the limiting factor which had increased the nutritional requirements of our participants, not only for protein needs but also daily energy intake. So, it could be that until all nutritional requirements are met, just twice per week protein supplementation after resistance training is not enough for muscle mass accretion. Conversely, as baseline protein intake was not reported, it could be that it was already within the protein dietary recommendation and that our participants in the protein-group were, after all, within an acceptable range of physical and health condition or that the deviation for protein intake from baseline was not sufficient to induce muscle mass gains [49].
The latter hypothesis might be more probable because muscle mass did not increase with protein supplementation, but even more importantly, it did not decrease after resistance training in either group. This suggests that participants in this study were within the RDA for protein, otherwise negative protein balance would have occurred hampering muscle mass maintenance [50], and it also reinforces the idea that healthy elderly people with an adequate daily protein intake might not benefit from increased protein content [51]. Furthermore, at baseline, participants in the protein-group had an average punctuation of 24.5 in the MNA, which is considered a normal nutritional status. In turn, within the placebo-group, the average punctuation was 23.1, so they were almost at risk of malnutrition according to the MNA. But, following the resistance training program, the mean punctuation in the placebo-group increased to 25.3, achieving a good nutritional status. These results suggest that nutritional requirements were within an acceptable range among both groups leading to improved physical function variables and maintenance of muscle mass in both groups. Nevertheless, it is worth to mention that protein supplementation was not prescribed to participant body weight, which could benefit more muscle mass accretion. It is also important to highlight that the rate of protein turnover in older adults is slower, so improvements in strength and physical performance are often seen before measurable changes in skeletal muscle mass become apparent [48]. Thus, it is unlikely to observe significant changes in muscle mass after only 12 weeks of twice per week supervised intervention. For muscle mass accretion, a positive protein balance must be achieved over time along with resistance exercise [50]. Indeed, it might be that the overall volume employed in our resistance training program was not enough to see gains in muscle mass [52]. In addition, the sample size in this study might not be enough to see beneficial effects in muscle mass accretion following post-training protein supplementation [18,49,52].
Limitations and Strength
The current study has several limitations. Daily protein and energy intake of participants were not controlled, we can merely speculate that probably total daily protein intake, the protein distribution among meals, the protein supplementation and/or the duration of our interventional program were not enough to increase muscle mass in this study. In addition, we assessed changes in body composition by DXA and with this methodology, differences smaller than 1.0 kg are not detectable [35]. The recruitment was lower than what we expected with only 20 and 21 participants in the Placebo-group and in the Protein-group, respectively. However, the dropout rate was high with only 13 and 15 participants completing the intervention study in the Placebo-group and in the Protein-group, respectively. When designing the study, it was proposed that 35 participants should be included to each group to see detectable improvements in muscle mass. So, this might be the main reason for not having seen significant improvements in muscle mass after the intervention. Another limitation is that the study was single-blinded and not double-blinded. One of the strengths of the study is that to the best of our knowledge, this is the first randomized study including post-hospitalized elderly adults in a resistance training program along with protein supplementation.
5. Conclusions
This study reinforces resistance training as a fundamental early intervention strategy to maintain muscle mass and increase gains in physical function parameters in post-hospitalized elderly adults. Thus, 12 weeks of supervised resistance training with a one-hour session over two days/week seems enough to enhance strength and physical function variables in post-hospitalized elderly adults. However, it does not clarify the additional benefits of a protein supplementation.
The elderly population is a very heterogenic group, so future directions should focus on conducting studies among the different subgroups with special needs. There is a need to assess which might be the optimum length of an interventional study including resistance exercise and supplementation to induce gains in muscle mass and strength. Specifically, there is a growing interest in stablishing the characteristics of the best protein supplementation protocol and differences between healthy older adults and older adults with an acute or chronic disease and/or with one or more conditions of the geriatric syndrome. It would also be interesting for future studies to add muscle biopsies as direct measurements for muscle mass hypertrophy.
Acknowledgments
We would like to acknowledge all participants and their families for their participation in the intervention program as well as the professionals and pre/postgraduate students who have been involved in data collection, measurements and intervention. Finally, we would like to thank the Araba University Hospital in Vitoria-Gasteiz for providing their facilities.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/10/2337/s1, Table S1: Characteristics of the recruited participants in the study (intend-to-treat analyses).
Author Contributions
A.B., J.R.R., J.I. and I.L. designed the study, M.A. (Maria Amasene), I.E. and M.U. collected the data, M.A. (Maria Amasene) and I.L. interpreted the data and drafted the manuscript, M.A. (Maria Amasene), I.E., M.U., J.R.R., A.R.-L., M.A. (Mikel Aldamiz), P.A., A.B., J.I., and I.L. have approved the submitted version and agree to be personally accountable for the author’s own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature.
Funding
This study was supported by the Basque Government (2016111138), and the European Regional Development Funds (ERDF), the University of Granada Plan Propio de Investigación 2016 (Excellence Actions: Unit of Excellence on Exercise and Health [UCEES]) and the Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades (ERDF: ref. SOMM17/6107/UGR). MA was supported by a grant from the University of the Basque Country (PIF17/186), IE by a grant from the University of the Basque Country in collaboration with the University of Bordeaux (Université of Bordeaux (UBX)) (PIFBUR16/07) and JRR by grants from the Spanish Ministry of Economy and Competitiveness (RYC 2010-05957; RYC-2011-09011 and BES-2014-068829).This work was also supported by grants from the Public University of Navarra, “Plan de Promoción de Grupos de Investigación (2019)”.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Fielding R.A., Vellas B., Evans W.J., Bhasin S., Morley J.E., Newman A.B., Abellan van Kan G., Andrieu S., Bauer J., Breuille D., et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaap L.A., van Schoor N.M., Lips P., Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: The Longitudinal Aging Study Amsterdam. J. Gerontol. 2018;73:1199–1204. doi: 10.1093/gerona/glx245. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I., Heymsfield S.B., Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 4.Landi F., Liperoti R., Fusco D., Mastropaolo S., Quattrociocchi D., Proia A., Tosato M., Bernabei R., Onder G. Sarcopenia and mortality among older nursing home residents. J. Am. Med. Dir. Assoc. 2012;13:121–126. doi: 10.1016/j.jamda.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell W.K., Williams J., Atherton P., Larvin M., Lund J., Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012;11:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witard O.C., McGlory C., Hamilton D.L., Phillips S.M. Growing older with health and vitality: A nexus of physical activity, exercise and nutrition. Biogerontology. 2016;17:529–546. doi: 10.1007/s10522-016-9637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deutz N.E., Bauer J.M., Barazzoni R., Biolo G., Boirie Y., Bosy-Westphal A., Cederholm T., Cruz-Jentoft A., Krznariç Z., Nair K.S., et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014;33:929–936. doi: 10.1016/j.clnu.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breen L., Phillips S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011;8:68. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung Y., Wijnhoven H.A.H., Visser M., Verbeke W. Appetite and Protein Intake Strata of Older Adults in the European Union: Socio-Demographic and Health Characteristics, Diet-Related and Physical Activity Behaviours. Nutrients. 2019;11:777. doi: 10.3390/nu11040777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonnie M., Hooker E., Brunstrom J.M., Corfe B.M., Green M.A., Watson A.W., Williams E.A., Stevenson E.J., Penson S., Johnstone A.M. Protein for Life: Review of Optimal Protein Intake, Sustainable Dietary Sources and the Effect on Appetite in Ageing Adults. Nutrients. 2018;10:360. doi: 10.3390/nu10030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennings B., Boirie Y., Senden J.M., Gijsen A.P., Kuipers H., van Loon L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011;93:997–1005. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 12.Schaafsma G. Advantages and limitations of the protein digestibility-corrected amino acid score (PDCAAS) as a method for evaluating protein quality in human diets. Br. J. Nutr. 2012;108(Suppl. 2):S333–S336. doi: 10.1017/S0007114512002541. [DOI] [PubMed] [Google Scholar]
- 13.Van Vliet S., Burd N.A., van Loon L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015;145:1981–1991. doi: 10.3945/jn.114.204305. [DOI] [PubMed] [Google Scholar]
- 14.Pennings B., Groen B., de Lange A., Gijsen A.P., Zorenc A.H., Senden J.M., van Loon L.J. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol. Endocrinol. Metab. 2012;302:E992–E999. doi: 10.1152/ajpendo.00517.2011. [DOI] [PubMed] [Google Scholar]
- 15.Luiking Y.C., Deutz N.E., Memelink R.G., Verlaan S., Wolfe R.R. Postprandial muscle protein synthesis is higher after a high whey protein, leucine-enriched supplement than after a dairy-like product in healthy older people: A randomized controlled trial. Nutr. J. 2014;13:9. doi: 10.1186/1475-2891-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reidy P.T., Rasmussen B.B. Role of Ingested Amino Acids and Protein in the Promotion of Resistance Exercise-Induced Muscle Protein Anabolism. J. Nutr. 2016;146:155–183. doi: 10.3945/jn.114.203208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao C.D., Tsauo J.Y., Wu Y.T., Cheng C.P., Chen H.C., Huang Y.C., Chen H.C., Liou T.H. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017;106:1078–1109. doi: 10.3945/ajcn.116.143594. [DOI] [PubMed] [Google Scholar]
- 18.Thomas D.K., Quinn M.A., Saunders D.H., Greig C.A. Protein Supplementation Does Not Significantly Augment the Effects of Resistance Exercise Training in Older Adults: A Systematic Review. J. Am. Med. Dir. Assoc. 2016;17:959.e1–959.e9. doi: 10.1016/j.jamda.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krumholz H.M. Post-hospital syndrome--an acquired, transient condition of generalized risk. N. Engl. J. Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deer R.R., Goodlett S.M., Fisher S.R., Baillargeon J., Dickinson J.M., Raji M., Volpi E. A Randomized Controlled Pilot Trial of Interventions to Improve Functional Recovery After Hospitalization in Older Adults: Feasibility and Adherence. J. Gerontol. 2018;73:187–193. doi: 10.1093/gerona/glx111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaiser M.J., Bauer J.M., Ramsch C., Uter W., Guigoz Y., Cederholm T., Thomas D.R., Anthony P., Charlton K.E., Maggio M., et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging. 2009;13:782–788. doi: 10.1007/s12603-009-0214-7. [DOI] [PubMed] [Google Scholar]
- 22.Soares Menezes K.V.R., Auger C., de Souza Menezes W.R., Guerra R.O. Instruments to evaluate mobility capacity of older adults during hospitalization: A systematic review. Arch. Gerontol. Geriatr. 2017;72:67–79. doi: 10.1016/j.archger.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 24.Martínez de la Iglesia J., Dueñas Herrero R., Onís Vilches M.C., Aguado Taberné C., Albert Colomer C., Luque Luque R. Cross-cultural adaptation and validation of Pfeiffer’s test (Short Portable Mental Status Questionnaire [SPMSQ]) to screen cognitive impairment in general population aged 65 or older. Med. Clín. 2001;117:129–134. doi: 10.1016/S0025-7753(01)72040-4. [DOI] [PubMed] [Google Scholar]
- 25.Brzycki M. Strength testing: Predicting a one-rep max from reps-to-fatigue. J. Phys. Educ. Recreat. Danc. 1993;64:88–90. doi: 10.1080/07303084.1993.10606684. [DOI] [Google Scholar]
- 26.Rikli R.E., Jones C.J. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013;53:255–267. doi: 10.1093/geront/gns071. [DOI] [PubMed] [Google Scholar]
- 27.Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature: What does it tell us? J. Nutr. Health Aging. 2006;10:466–485. [PubMed] [Google Scholar]
- 28.Chumlea W.C., Roche A.F., Steinbaugh M.L. Estimating stature from knee height for persons 60 to 90 years of age. J. Am. Geriatr. Soc. 1985;33:116–120. doi: 10.1111/j.1532-5415.1985.tb02276.x. [DOI] [PubMed] [Google Scholar]
- 29.Leenders M., Verdijk L.B., van der Hoeven L., van Kranenburg J., Nilwik R., van Loon L.J. Elderly men and women benefit equally from prolonged resistance-type exercise training. J. Gerontol. 2013;68:769–779. doi: 10.1093/gerona/gls241. [DOI] [PubMed] [Google Scholar]
- 30.Law T.D., Clark L.A., Clark B.C. Resistance Exercise to Prevent and Manage Sarcopenia and Dynapenia. Annu. Rev. Gerontol. Geriatr. 2016;36:205–228. doi: 10.1891/0198-8794.36.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao C.D., Lee P.H., Hsiao D.J., Huang S.W., Tsauo J.Y., Chen H.C., Liou T.H. Effects of Protein Supplementation Combined with Exercise Intervention on Frailty Indices, Body Composition, and Physical Function in Frail Older Adults. Nutrients. 2018;10:1916. doi: 10.3390/nu10121916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deutz N.E.P., Ashurst I., Ballesteros M.D., Bear D.E., Cruz-Jentoft A.J., Genton L., Landi F., Laviano A., Norman K., Prado C.M. The Underappreciated Role of Low Muscle Mass in the Management of Malnutrition. J. Am. Med. Dir. Assoc. 2019;20:22–27. doi: 10.1016/j.jamda.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Koopman R., van Loon L.J. Aging, exercise, and muscle protein metabolism. J. Appl. Physiol. 2009;106:2040–2048. doi: 10.1152/japplphysiol.91551.2008. [DOI] [PubMed] [Google Scholar]
- 34.Pennings B., Koopman R., Beelen M., Senden J.M., Saris W.H., van Loon L.J. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am. J. Clin. Nutr. 2011;93:322–331. doi: 10.3945/ajcn.2010.29649. [DOI] [PubMed] [Google Scholar]
- 35.Tieland M., van de Rest O., Dirks M.L., van der Zwaluw N., Mensink M., van Loon L.J., de Groot L.C. Protein supplementation improves physical performance in frail elderly people: A randomized, double-blind, placebo-controlled trial. J. Am. Med. Dir. Assoc. 2012;13:720–726. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Mori H., Tokuda Y. Effect of whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: A randomized controlled trial. Geriatr. Gerontol. Int. 2018;18:1398–1404. doi: 10.1111/ggi.13499. [DOI] [PubMed] [Google Scholar]
- 37.Verdijk L.B., Jonkers R.A., Gleeson B.G., Beelen M., Meijer K., Savelberg H.H., Wodzig W.K., Dendale P., van Loon L.J. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am. J. Clin. Nutr. 2009;89:608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- 38.Verhoeven S., Vanschoonbeek K., Verdijk L.B., Koopman R., Wodzig W.K., Dendale P., van Loon L.J. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am. J. Clin. Nutr. 2009;89:1468–1475. doi: 10.3945/ajcn.2008.26668. [DOI] [PubMed] [Google Scholar]
- 39.Slivka D., Raue U., Hollon C., Minchev K., Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: Evidence for limited skeletal muscle plasticity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R273–R280. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cartee G.D., Hepple R.T., Bamman M.M., Zierath J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016;23:1034–1047. doi: 10.1016/j.cmet.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atherton P.J., Kumar V., Selby A.L., Rankin D., Hildebrandt W., Phillips B.E., Williams J.P., Hiscock N., Smith K. Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clin. Nutr. 2017;36:888–895. doi: 10.1016/j.clnu.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 42.Englund D.A., Kirn D.R., Koochek A., Zhu H., Travison T.G., Reid K.F., von Berens Å., Melin M., Cederholm T., Gustafsson T., et al. Nutritional Supplementation with Physical Activity Improves Muscle Composition in Mobility-Limited Older Adults, The VIVE2 Study: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Gerontol. 2017;73:95–101. doi: 10.1093/gerona/glx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkinson D.J., Bukhari S.S.I., Phillips B.E., Limb M.C., Cegielski J., Brook M.S., Rankin D., Mitchell W.K., Kobayashi H., Williams J.P., et al. Effects of leucine-enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clin. Nutr. 2018;37:2011–2021. doi: 10.1016/j.clnu.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morton R.W., Murphy K.T., McKellar S.R., Schoenfeld B.J., Henselmans M., Helms E., Aragon A.A., Devries M.C., Banfield L., Krieger J.W., et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018;52:376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou L., Lei Y., Li X., Huo C., Jia X., Yang J., Xu R., Wang X. Effect of Protein Supplementation Combined with Resistance Training on Muscle Mass, Strength and Function in the Elderly: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging. 2019;23:451–458. doi: 10.1007/s12603-019-1181-2. [DOI] [PubMed] [Google Scholar]
- 46.Wall B.T., Gorissen S.H., Pennings B., Koopman R., Groen B.B., Verdijk L.B., van Loon L.J. Aging Is Accompanied by a Blunted Muscle Protein Synthetic Response to Protein Ingestion. PLoS ONE. 2015;10:e0140903. doi: 10.1371/journal.pone.0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macnaughton L.S., Wardle S.L., Witard O.C., McGlory C., Hamilton D.L., Jeromson S., Lawrence C.E., Wallis G.A., Tipton K.D. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiol. Rep. 2016;4:e12893. doi: 10.14814/phy2.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer J., Biolo G., Cederholm T., Cesari M., Cruz-Jentoft A.J., Morley J.E., Phillips S., Sieber C., Stehle P., Teta D., et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Park Y., Choi J.E., Hwang H.S. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2018;108:1026–1033. doi: 10.1093/ajcn/nqy214. [DOI] [PubMed] [Google Scholar]
- 50.Stokes T., Hector A.J., Morton R.W., McGlory C., Phillips S.M. Recent Perspectives Regarding the Role of Dietary Protein for the Promotion of Muscle Hypertrophy with Resistance Exercise Training. Nutrients. 2018;10:180. doi: 10.3390/nu10020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell W.W., Leidy H.J. Dietary protein and resistance training effects on muscle and body composition in older persons. J. Am. Coll. Nutr. 2007;26:696S–703S. doi: 10.1080/07315724.2007.10719650. [DOI] [PubMed] [Google Scholar]
- 52.Peterson M.D., Sen A., Gordon P.M. Influence of resistance exercise on lean body mass in aging adults: A meta-analysis. Med. Sci. Sports Exerc. 2011;43:249–258. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.