Abstract
Introduction
Previous studies have shown attenuated cortisol awakening response in patients with posttraumatic stress disorder (PTSD).
Case Presentation
A 15-year-old girl, a survivor of acute sexual violence, received a 7-day oral treatment with cannabidiol. She was followed-up from the first 24 hours after the event for 6 months, for assessment of the effects of this treatment on the reconsolidation of memories related to the traumatic event.
Discussion
Cannabidiol treatment did not prevent the onset of PTSD. Cortisol awakening responses after the onset of the disorder were attenuated compared with those observed in the same individual before the onset of PTSD, in line with previous evidence from studies comparing groups with and without PTSD.
Keywords: cannabidiol, cortisol awakening response, memory reconsolidation, posttraumatic stress disorder, PTSD
INTRODUCTION
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), posttraumatic stress disorder (PTSD) is characterized by abnormal behavioral responses resulting from exposure to a major traumatic event.1 The traumatic event is persistently revived in 1 or more ways, for example, in the form of nightmares or feelings like those experienced during the traumatic event. Also, there is persistent avoidance of stimuli associated with the trauma and persistent symptoms of excitability.
Compared with its previous versions, the DSM-V added a fourth group of symptoms related to negative changes in cognition and mood. These symptoms should last for more than 1 month and be accompanied by considerable distress to the individual.2
Results of a recent meta-analysis showed that among people who have been sexually abused, depressive disorders and PTSD were the most prevalent consequences, with 36% of sexually abused people having PTSD throughout their lives and 26% in the last year.3 Sexual abuse, both in childhood and in adulthood, can have serious consequences for the affected individual, including substance abuse and/or dependence, eating disorders, anxiety, depression, and cognitive consequences.4,5
PTSD could change the activity of the hypothalamic-pituitary-adrenal axis.6 The cortisol awakening response (CAR) is a way to measure hypothalamic-pituitary-adrenal axis function because cortisol levels are expected to increase after awakening and to reach a peak within 1 hour.7,8
We found 7 previous studies that analyzed the CAR in patients with PTSD.6,9–14 Except for the study by Lindley et al,14 the other 4 studies that compared CAR levels in patients with PTSD and healthy controls found decreased CAR in patients with PTSD.6,9,10,13 Also, 2 studies investigated the levels of PTSD symptoms and CAR in patients, and both described an inverse relationship between the severity of PTSD symptoms and CAR.11,12 Reduced CAR in patients with PTSD may be related to increased suppression of the hypothalamic-pituitary-adrenal axis, as suggested by evidence showing that the administration of dexamethasone was associated with increased cortisol suppression in patients exposed to trauma (with or without PTSD) compared with healthy controls.10
The studies cited above compared the CAR in groups with and without PTSD or correlated levels of PTSD symptoms with CAR measures. In the present case report, we describe the evolution of the CAR before and after the diagnosis of PTSD in the same patient.
We have systematically followed-up a survivor of sexual violence since the first 24 hours after the event for a 6-month period. The objective of this follow-up was to assess whether the administration of cannabidiol (CBD) daily for 1 week beginning 72 hours after the traumatic event, interfered with the consolidation/reconsolidation of memories related to the traumatic event.
CBD is a compound of the plant Cannabis sativa, responsible for 40% of the plant composition. This compound is free from the psychoactive effects caused by tetrahydrocannabinol. A series of evidence suggests that CBD, in addition to its anxiolytic effect,15 was able to interfere in aversive memories.16,17 During the processes of consolidation or reconsolidation, memory recall makes stored material labile and susceptible to pharmacologic interference.18,19 Evidence in rodents suggests that CBD blocks reconsolidation of an aversive memory in a context-based fear paradigm.17,20–22 Recently, the effect of CBD on extinction learning has been studied in humans, using a Pavlovian fear-conditioning paradigm in healthy volunteers.23 The results of this study indicate that CBD increased the consolidation of extinction learning, suggesting its potential effect on the extinction of fears learned in anxiety disorders, particularly PTSD.
CASE PRESENTATION
Presenting Concerns
A 15-year-old adolescent girl was referred to an Emergency Department (ED) for survivors of sexual abuse at the University Hospital of the Ribeirão Preto Medical School-University of São Paulo (HCFMRP-USP) in Ribeirão Preto, Brazil, on the same day that she experienced sexual violence by an unknown man. The episode of sexual violence was the only one the patient experienced. The girl was a student, unmarried, and a nonsmoker and belonged to socioeconomic stratum B2 (for example, high school student, middle class, unmarried, and a nonsmoker) according to Brazil’s socioeconomic classification criteria. The presence of organic brain syndromes, psychoactive drug abuse, and other psychiatric disorders was excluded. The recruitment interview for the study occurred while the patient was at the ED, and the procedures ended with an invitation for a more detailed conversation the next day.
The procedures of the present study were developed in parallel to the usual care that female survivors of violence receive in the HCFMRP-USP. This protocol consists of ED care for prophylaxis with contraceptive and antiretroviral methods. After this first approach, the patients are referred to a service of care for survivors of domestic and sexual violence that relies on professionals in the fields of psychiatry, psychology, and social work. In this service, medical and psychosocial treatments are offered for possible consequences of abuse. The Research Ethics Committee of the HCFMRP-USP approved the study (process no. 1398873).
Therapeutic Intervention and Treatment
In the ED, prophylaxis was implemented within 24 hours of the event such as pregnancy prevention and medications for sexually transmitted diseases. In the interview conducted 1 day after the visit to the ED (day 1), the patient signed a consent form to participate in the study and completed instruments to assess eligibility and collect baseline data. The instruments were the following: Questionnaire for clinical and demographic evaluation; Mini-Screening of Mental Disorders (Mini-SMD), a screening tool for specific mental disorders in the general population that was developed and validated in Brazil24; Structured Clinical Interview for DSM-V (SCID-5-Clinical Version)2 if results of the Mini-SMD were positive; Posttraumatic Stress Disorder Checklist-5 (PCL-5)25; State-Trait Anxiety Inventory (STAI-T [Trait] and STAI-S [State])26,27; and Patient Health Questionnaire-9 (PHQ-9).27,28
After the instruments were completed, the patient received instructions and material for the CAR test to be performed the following night and subsequent morning. For the CAR response test, the participant collected 4 saliva samples. The first sample was collected 48 hours after the traumatic event, at bedtime. On the following day, 3 saliva samples were obtained immediately after spontaneous or alarm-induced awakening, considered as T0 (while still in bed), and 30 and 60 minutes after awakening. Specifically, the patient collected saliva samples at 10 pm and at 6, 6:30, and 7 am on the next day. Material for the CAR test was also collected on day 16 (at 11 pm and 7, 7:30, and 8 am), day 60 (at midnight and 10, 10:30, and 11 am), and day 180 (at 10 pm and 7, 7:30, and 8 am) after the traumatic event.
A behavioral test was performed on day 2 (before completing 72 hours of the traumatic event) and repeated 16 days later. The test was an adaptation of the procedure proposed by Pitman et al,29 which was shown to generate subjective symptoms and physiologic responses in patients with PTSD. It consisted of reporting trauma information, which is recorded in the form of digital audio. The recording lasted approximately 1.5 minutes and, after this, the patient was instructed to close her eyes and to imagine the traumatic event as vividly as possible for 30 seconds. Before and after the behavioral test, subjective measures of anxiety (anxiety factor of the Visual Analogue Mood Scales [VAMS]30 and STAI-S26) and data about the physiologic correlates of anxiety (heart rate and systolic and diastolic blood pressure) were recorded. The behavioral testing, which includes rememorizing the traumatic event under controlled conditions, is not expected to be detrimental to the patient because a similar procedure is used in the therapeutic technique of extinction.23
The administration of CBD, a single dose of 300 mg, started on day 2 before the behavioral test and continued daily for 6 additional days. (Treatment was started in the laboratory on day 2 of the study. After that day, the patient took medication for another 6 days of treatment, totaling 7 days and ending on day 8 of the study.) The patient took the drug orally in the form of capsules containing CBD powder with 99.9% purity dissolved in corn oil (BioSynthesis Pharma Group, Sandwich, UK).
A summary of the experimental protocol and a timeline of the case are presented in Tables 1 and 2, respectively.
Table 1.
Experimental protocol
| Day | Phase | Procedure |
|---|---|---|
| 0 | Recruitment | Assistance at the Emergency Department and invitation to participate in study |
| 1 | Selection | Eligibility assessment and signature of the consent form |
| Data collection (demographics, STAI-T, STAI-S, PHQ-9, Mini-SMD, SCID-5 [if Mini-SMD results were abnormal]) | ||
| Instructions and delivery of material for CAR test | ||
| 2 | Pharmacologic intervention (CBD, 300 mg) Behavioral test | Collection of samples for the CAR test by the investigator |
| Oral administration of CBD, 300 mg, and 2.5 h after performing the behavioral test (described in the text) | ||
| 3–8 | Daily treatment with CBD, 300 mg | Daily oral dose of CBD, 300 mg |
| 16 | Assessment of the pharmacologic intervention on the reconsolidation of traumatic memories | Collection of samples for the CAR test by the investigator |
| Measurement of physiologic and psychological (VAMS and STAI-S) correlates of anxiety before and after the behavioral test | ||
| 30 | Stress disorder evaluation | SCID-5 and PCL-5 |
| 60 | Psychiatric evaluation | Collection of samples for the CAR test by the investigator |
| First evaluation for the diagnosis of PTSD (SCID-5) | ||
| 180 | Psychiatric evaluation | Collection of samples for the CAR test by the investigator |
| Second evaluation for the diagnosis of PTSD (SCID-5) |
CAR = cortisol awakening response; CBD = cannabidiol; Mini-SMD = Mini-Screening of Mental Disorders; PCL-5 = Posttraumatic Stress Disorder Checklist for DSM-V; PHQ-9 = Patient Health Questionnaire-9; PTSD = posttraumatic stress disorder; SCID-5 = Structured Clinical Interview for the DSM-V; STAI-S = State-Trait Anxiety Inventory-State; STAI-T = State-Trait Anxiety Inventory-Trait; VAMS = Visual Analogue Mood Scales.
Table 2.
Timeline of the case
| Time, d | Event |
|---|---|
| 0 | Recruitment for study |
| 1 | Selection for study |
| 2 | CAR 1 tested; CBD administered; BT 1 performed |
| 3 | CBD administered |
| 4 | CBD administered |
| 5 | CBD administered |
| 6 | CBD administered |
| 7 | CBD administered |
| 8 | CBD administered |
| 16 | CAR2 tested; BT 2 performed |
| 30 | Stress disorder evaluation |
| 60 | CAR 3 tested; PTSD evaluation |
| 180 | CAR 4 tested; PTSD evaluation |
BT 1 = behavior test 1 (reporting trauma information); BT 2 = behavior test 2 (assessment of intervention on the reconsolidation of memories related to the traumatic event); CAR = cortisol awakening response; CBD = cannabidiol, 300 mg; PTSD = posttraumatic stress disorder.
Follow-up and Outcomes
The patient completed the study protocol until day 180. At baseline (day 1), she had scores of 30 in the STAI-T and 63 in the STAI-S, but the PHQ-9 showed an absence of depressive symptoms.
The results of the anxiety measures before and after the behavioral test are shown in Table 3. After the first CBD administration, the scores of the STAI-S and the anxiety factor of the VAMS increased after the patient recollected the traumatic event, but this increase did not happen 1 week after the end of the 7-day treatment with CBD. The physiologic correlates of anxiety (blood pressure and heart rate) showed discrete and inconsistent changes.
Table 3.
Psychological and physiologic correlates of anxiety before and after recollection of aversive event under acute effect of CBD and after daily treatment with CBD for 7 days
| Measure | Day 2 (acute CBD effect) | Day 16 (7 days after CBD treatment) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| STAI-Sa | 54 | 59 | 56 | 53 |
| VAMS (anxiety factor)a | 52.0 | 64.0 | 48.5 | 47.4 |
| Heart rate, beats/min | 90 | 93 | 75 | 74 |
| BP-systolic, mmHg | 100 | 100 | 98 | 88 |
| BP-diastolic, mmHg | 73 | 66 | 68 | 55 |
Higher number score indicates worse anxiety symptoms.
BP = blood pressure; CBD = cannabidiol; STAI-S = State-Trait Anxiety Inventory-State; VAMS = Visual Analogue Mood Scales.
In the first 2 weeks of the study, the score of depressive symptoms assessed with the PHQ-9 increased from 0 to 20. Because of the depressive symptoms, the participant was medicated with sertraline.
In the evaluation performed on day 30, the participant fulfilled DSM-V criteria for acute stress disorder, and on day 60 the criteria were met for PTSD. The score on the PTSD Checklist-5 (intensity of PTSD symptoms) 30 days after the diagnosis of PTSD (180 days of the traumatic event) was 50.
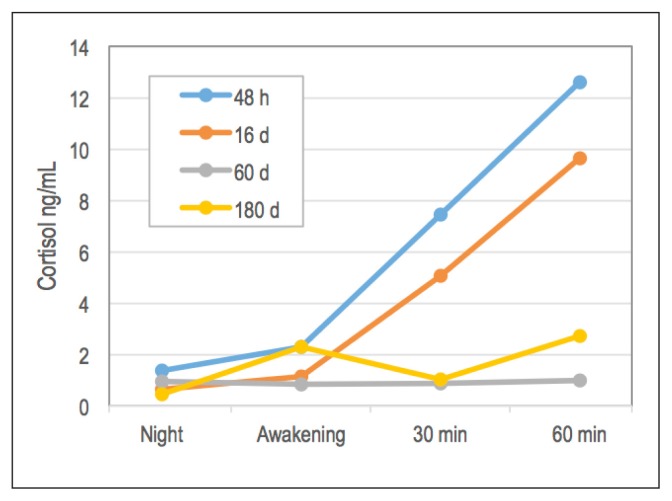
We found an increase in CAR before the pharmacologic intervention and 1 week after the introduction of CBD treatment (day 16; Figure 1). However, this increase in cortisol levels on awakening was attenuated when PTSD developed in the patient (assessments on days 60 and 180).
Figure 1.
Cortisol awakening response before (48 hours and 16 days after traumatic event) and after (60 and 180 days) diagnosis of posttraumatic stress disorder.
DISCUSSION
In this report, an initial single dose of CBD, 300 mg, failed to prevent an increase in anxiety after recollection of the traumatic event (day 2). However, 1 week after daily administration of CBD for 7 days, reexposure to the account of the traumatic event in the absence of the drug did not increase anxiety scores, suggesting a possible interference of CBD in the reconsolidation of traumatic memories. This suggestion should be considered with caution because this is a single case, even though it is in line with preclinical findings in laboratory animals.17,20–22 However, this possible interference in the reconsolidation of aversive memories was not enough to prevent the development of PTSD.
It is possible that a single association of CBD with recollection may not be enough in the case of severe trauma, as suggested by a study with caffeine in the reconsolidation of fear memories in rats.31 In another case report about a 10-year-old patient with PTSD, 12 mg of CBD used as a sublingual spray was able to reduce anxiety and improve sleep quality.32 A recent retrospective case series, which evaluated the clinical benefit of CBD administration in 11 adults with PTSD, concluded that administration of CBD in oral and spray formulations decreased the symptoms of PTSD.33 A relevant finding from this study is that CBD substantially decreased nightmare symptoms when assessed by the PTSD Checklist-5 scale after follow-up sessions at 4 and 8 weeks.33
The interference of other drugs in the consolidation or reconsolidation of aversive memories has been tested, with propranolol being the most studied. A meta-analysis of these studies with propranolol suggests that its administration before the consolidation or reconsolidation of emotional memories reduces memories with negative valences or the expression of cue-elicited fear.34
The main outcome in this case report is related to CAR. Immediately before and 1 week after the pharmacologic intervention with CBD, the usual CAR was observed, with its typical increase on awakening. However, this response was attenuated after PTSD developed (evaluations at 60 and 180 days after the traumatic experience), which corroborates earlier evidence showing that patients with PTSD have an attenuation in CAR compared with healthy controls.6,9–13 Similarly, a systematic review of the literature and meta-analysis examined the psychosocial factors that interfere with CAR in 147 studies and concluded that the presence of PTSD is one of the factors that attenuates CAR.35 Although this report is based on a single case, it was able to show changes in CAR before and after the onset of PTSD in the same individual. Future studies with larger prospective samples examining different doses of CBD in preventing and treating PTSD are necessary and opportune.
CONCLUSION
Although this case suggested an interference of CBD in reconsolidation, it was not sufficient to prevent the development of PTSD. However, the main observation was a change in CAR in the same patient before and after the start of PTSD towards attenuation.
Acknowledgments
José Alexandre Crippa, MD, PhD, received a research grant from University Global Partnership Network, Surrey, UK. Antonio Waldo Zuardi, MD, PhD, and José Alexandre Crippa are recipients of a fellowship award from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Lago Sul, Brazil. The present study was supported by 2 CNPq grants (CNPq/MS/SCTIE/DECIT N_ 26/2014-466805/2014-4 and INCT-TM). Lívia Maria Bolsoni, MS, is the recipient of a doctoral research grant from the São Paulo Research Foundation in São Paulo, Brazil (FAPESP; grant 2016/01801-5).
Kathleen Louden, ELS, of Louden Health Communications performed a primary copy edit.
Footnotes
Disclosure Statement
Antonio Waldo Zuardi, MD, PhD, and José Alexandre Crippa, MD, PhD, are co-inventors of the patent “Fluorinated CBD Compounds, Compositions and Uses thereof” (Publication no. WO/2014/108899; International Application No. PCT/IL2014/050023); Def. US no. Reg. 62193296 on July 29 2015; and INPI (National Institute of Industrial Property, Brazil) on August 8, 2015 (BR1120150164927). The University of São Paulo has licensed the patent to Phytecs Inc, Los Angeles, CA (USP [US Pharmacopeia] Resolution no. 15.1.130002.1.1). The University of São Paulo has an agreement with Prati-Donaduzzi, Toledo, Brazil, to “develop a pharmaceutical product containing synthetic cannabidiol and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson’s disease, and anxiety disorders.” José Alexandre Crippa received travel support from Biosynthesis Pharma Group, Sandwich, UK, and is an advisory consultant to the SCBD Center, Sandwich, UK.
The other authors have no conflicts of interest to disclose.
References
- 1.Araujo AC, Lotufo FN. A nova classificação Americana para os Transtornos Mentais: o DSM-5. Rev Bras Ter Comport Cogn. 2014 Apr;16(1):67–82. doi: 10.31505/rbtcc.v16i1.659. [DOI] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 3.Dworkin ER. Risk for mental disorders associated with sexual assault: A meta-analysis. Trauma Violence Abuse. 2018 Dec;25:1524838018813198. doi: 10.1177/1524838018813198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gros DF, Price M, Magruder KM, Frueh BC. Symptom overlap in posttraumatic stress disorder and major depression. Psychiatry Res. 2012 Apr 30;196(2–3):267–70. doi: 10.1016/j.psychres.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rojas SM, Bujarski S, Babson KA, Dutton CE, Feldner MT. Understanding PTSD comorbidity and suicidal behavior: Associations among histories of alcohol dependence, major depressive disorder, and suicidal ideation and attempts. J Anxiety Disord. 2014 Apr;28(3):318–25. doi: 10.1016/j.janxdis.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31(2):209–15. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Wahbeh H, Oken BS. Salivary cortisol lower in posttraumatic stress disorder. J Trauma Stress. 2013 Apr;26(2):241–8. doi: 10.1002/jts.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto RJ, Correia-Santos P, Costa-Leite J, Levendosky AA, Jongenelen I. Cortisol awakening response among women exposed to intimate partner violence. Psychoneuroendocrinology. 2016 Dec;74:57–64. doi: 10.1016/j.psyneuen.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Yehuda R, Golier JA, Kaufman S. Circadian rhythm of salivary cortisol in Holocaust survivors with and without PTSD. Am J Psychiatry. 2005 May;162(5):998–1000. doi: 10.1176/appi.ajp.162.5.998. [DOI] [PubMed] [Google Scholar]
- 10.de Kloet CS, Vermetten E, Heijnen CJ, Geuze E, Lentjes EG, Westenberg HG. Enhanced cortisol suppression in response to dexamethasone administration in traumatized veterans with and without posttraumatic stress disorder. Psychoneuroendocrinology. 2007 Apr;32(3):215–26. doi: 10.1016/j.psyneuen.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Neylan TC, Brunet A, Pole N, et al. PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology. 2005 May;30(4):373–81. doi: 10.1016/j.psyneuen.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Rauch SA, King AP, Abelson J, et al. Biological and symptom changes in posttraumatic stress disorder treatment: A randomized clinical trial. Depress Anxiety. 2015 Mar;32(3):204–12. doi: 10.1002/da.22331. [DOI] [PubMed] [Google Scholar]
- 13.Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry. 2004 Apr 1;55(7):745–51. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Lindley SE, Carlson EB, Benoit M. Basal and dexamethasone suppressed salivary cortisol concentrations in a community sample of patients with posttraumatic stress disorder. Biol Psychiatry. 2004 May 1;55(9):940–5. doi: 10.1016/j.biopsych.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Zuardi AW, Cosme RA, Graeff FG, Guimarães FS. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993 Jan;7(1 Suppl):82–8. doi: 10.1177/026988119300700112. [DOI] [PubMed] [Google Scholar]
- 16.Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008 Dec;18(12):849–59. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Stern CA, Gazarini L, Takahashi RN, Guimarães FS, Bertoglio LJ. On disruption of fear memory by reconsolidation blockade: Evidence from cannabidiol treatment. Neuropsychopharmacology. 2012 Aug;37(9):2132–42. doi: 10.1038/npp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudai Y. Reconsolidation: The advantage of being refocused. Curr Opin Neurobiol. 2006 Apr;16(2):174–8. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Sara SJ, Hars B. In memory of consolidation. Learn Mem. 2006 Sep-Oct;13(5):515–21. doi: 10.1101/lm.338406. [DOI] [PubMed] [Google Scholar]
- 20.Stern CA, Gazarini L, Vanvossen AC, et al. Involvement of the prelimbic cortex in the disruptive effect of cannabidiol on fear memory reconsolidation. Paper presented at: 27th ECNP (European College of Neuropsychopharmacology) Congress; 2014 Oct 18–21; Berlin, Germany. Eur Neuropsychopharmacol. 2014;24(Suppl 2):S322. [Google Scholar]
- 21.Stern CA, Gazarini L, Vanvossen AC, et al. Δ9-Tetrahydrocannabinol alone and combined with cannabidiol mitigate fear memory through reconsolidation disruption. Eur Neuropsychopharmacol. 2015 Jun;25(6):958–65. doi: 10.1016/j.euroneuro.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Gazarini L, Stern CA, Piornedo RR, Takahashi RN, Bertoglio LJ. PTSD-like memory generated through enhanced noradrenergic activity is mitigated by a dual step pharmacological intervention targeting its reconsolidation. Int J Neuropsychopharmacol. 2014 Oct 31;18(1) doi: 10.1093/ijnp/pyu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das RK, Kamboj SK, Ramadas M, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl) 2013 Apr;226(4):781–92. doi: 10.1007/s00213-012-2955-y. [DOI] [PubMed] [Google Scholar]
- 24.Bolsoni L, Moscovici L, de Azevedo Marques JM, Zuardi AW. Specific mental disorder screening compilation may detect general mental disorders. Rev Bras Med Fam Comunidade. 2018 Apr;13(40):1–13. doi: 10.5712/rbmfc13(40)1685. [DOI] [Google Scholar]
- 25.Osório FL, Silva TD, Santos RG, et al. Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Transcultural adaptation of the Brazilian version. Arch Clin Psychiatry. 2017 Jan-Feb;44(1):10–19. doi: 10.1590/0101-60830000000107. [DOI] [Google Scholar]
- 26.Andrade L, Gorenstein C, Vieira Filho AH, Tung TC, Artes R. Psychometric properties of the Portuguese version of the State-Trait Anxiety Inventory applied to college students: Factor analysis and relation to the Beck Depression Inventory. Braz J Med Biol Res. 2001 Mar;34(3):367–74. doi: 10.1590/S0100-879X2001000300011. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osorio FL, Carvalho AC, Fracalossi TA, Crippa JA, Loureiro ES. Are two items sufficient to screen for depression within the hospital context? Int J Psychiatry Med. 2012 Dec;44(2):141–8. doi: 10.2190/PM.44.2.e. [DOI] [PubMed] [Google Scholar]
- 29.Pitman R, Sanders K, Zusman RM. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002 Jan;51(2):189–92. doi: 10.1016/S0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 30.Zuardi AW, Karniol IG. Transcultural study of a self-rating scale for subjective states. J Bras Psiquiatri. 1981;30(5):403–6. [Google Scholar]
- 31.Pedraza LK, Sierra RO, Lotz FN, Alvares LO. Periodical reactivation under the effect of caffeine attenuates fear memory expression in rats [published correction appears at Sci Rep 2018 Aug 6;8(1):11944] Sci Rep. 2018 May 8;8(1):7260. doi: 10.1038/s41598-018-25648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shannon S, Opila-Lehman J. Effectiveness of cannabidiol oil for pediatric anxiety and insomnia as part of posttraumatic stress disorder: A case report. Perm J. 2016 Fall;20(4):108–11. doi: 10.7812/TPP/16-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elms L, Shannon S, Hughes S, Lewis N. Cannabidiol in the treatment of post-traumatic stress disorder: A case series. J Altern Complement Med. 2018 Dec;:1–6. doi: 10.1089/acm.2018.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lonergan MH, Olivera-Figueroa LA, Pitman RK, Brunet A. Propranolol’s effects on the consolidation and reconsolidation of long-term emotional memory in healthy participants: a meta-analysis. J Psychiatry Neurosci. 2013 Jul;38(4):222–31. doi: 10.1503/jpn.120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol Psychol. 2009 Mar;80(3):265–78. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]