Abstract
Background
X-linked hypophosphatemia (XLH) causes rickets, osteomalacia, skeletal deformities and growth impairment, due to elevated fibroblast growth factor 23 and hypophosphatemia. Conventional therapy requires high doses of phosphate salts combined with active vitamin D analogues. Risks of this regimen include nephrocalcinosis and secondary hyperparathyroidism or progression to tertiary (hypercalcemic) hyperparathyroidism.
Methods
The primary goals were to estimate the prevalence of hyperparathyroidism and to characterize parathyroidectomy outcomes regarding hypercalcemia among XLH patients. XLH patients attending our center from 1/2000 to 12/2017 were included in a retrospective chart review. Prevalence of nephrocalcinosis and eGFR<60 mL/min/1.732 was also assessed.
Results
Of 104 patients with XLH, 84 had concurrent measurements of calcium and PTH (40 adults and 44 children). Of these, 70/84 (83.3%), had secondary or tertiary hyperparathyroidism at any time point. Secondary hyperparathyroidism was persistent in 62.2% of those with data at multiple timepoints. Tertiary hyperparathyroidism had an overall prevalence of 14/84 (16.7%) patients.
Parathyroidectomy was performed in 8/84 (9.5%) of the total population. After parathyroidectomy, persistent or recurrent tertiary hyperparathyroidism was detected in 6/8 (75%) patients at a median of 6 years (from 0 to 29 years). One patient had chronic postoperative hypoparathyroidism and one patient remained normocalcemic 4 years after surgery.
Nephrocalcinosis was more prevalent in patients with tertiary hyperparathyroidism than those without (60.0% vs 18.6%). Chronic kidney disease (eGFR<60 mL/min/1.732) was also more prevalent in patients with tertiary hyperparathyroidism than those without (35.7% vs 1.5%).
Conclusion
The majority of patients with XLH develop secondary hyperparathyroidism during treatment with phosphate and active vitamin D. A significant proportion develops tertiary hyperparathyroidism and most have recurrence or persistence of hypercalcemia after surgery.
1. Introduction
X-linked hypophosphatemic rickets (XLH), an X-linked dominant disorder, is the most prevalent genetic form of hypophosphatemic rickets, with an estimated incidence of approximately one case per 20,000 −25,000 live births [1]. XLH occurs due to loss of function mutations in PHEX, which causes increased bone expression of fibroblast growth factor 23 (FGF23) [2, 3]. FGF23 alters both vitamin D and phosphorus metabolism. FGF23 decreases surface expression of sodium phosphate cotransporters from proximal renal tubule cells resulting in renal phosphorus losses [4]. FGF23 also down regulates 1alpha-hydroxylase and up regulates 24-hydroxylase, thus decreasing levels of 1,25-dihydroxyvitamin D [1,25(OH)2D] by decreasing synthesis and increasing catabolism [3, 5].
Conventional therapy for XLH has for many years been administration of high doses of oral phosphate salts and active vitamin D or analogs [5, 6]. Conventional therapy requires dosing multiple times a day and can cause gastrointestinal symptoms due to the phosphate. However, a number of other clinically important side effects of conventional therapy have been described, including nephrocalcinosis, hypercalciuria, hypertension, and hyperparathyroidism [4, 5, 7]. Hyperparathyroidism may contribute to nephrolithiasis, chronic kidney disease, and hypertension [7, 8].
Patients with XLH are at risk for both secondary and tertiary (hypercalcemic) hyperparathyroidism. However, most descriptions are limited to case reports or series [9–13]. Of note, prior to initiating conventional therapy, about a third of patients may have evidence of secondary hyperparathyroidism [14], which often improves after starting treatment with active vitamin D. In 1995, Knudtzon et al estimated the prevalence of hypercalcemic hyperparathyroidism as 7/319 (2.2%) based on collating data from twenty-one published reports [15]. The current prevalence of hyperparathyroidism in XLH is uncertain. Limited studies have addressed treatment and outcomes of tertiary hyperparathyroidism [13, 15–21].
The goal of this study was to estimate the prevalence of hyperparathyroidism and its association with renal outcomes among XLH patients. We also aimed to estimate the frequency of parathyroidectomy and the recurrence or persistence of hyperparathyroidism after parathyroidectomy in this population.
2. Material and Methods
2.1. Study design and data source
We conducted a retrospective chart review study of patients with XLH at our institution to identify the occurrence of hyperparathyroidism and associated treatments. Clinical data was extracted from a combination of paper and electronic medical records (EMR), including the Indiana Network for Patient Care (INPC), which is a multi-institution health information exchange including data from over 90 hospitals and multiple health systems across Indiana. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Indiana University Institutional Review Board prior to any data collection.
Patients
All patients attending either adult or pediatric endocrinology practices of the Indiana University School of Medicine in Indianapolis, Indiana from 1/1/2000 to 12/31/2017 with a clinical diagnosis of XLH were eligible for inclusion if having at least one concurrent measurement of intact parathyroid hormone (PTH) and serum calcium. Patients were not contacted during this study. Both children (age < 18 years) and adults (age ≥ 18 years) were included. Patients were counted as children or as adults depending on their earliest clinical encounter that classified them as having hyperparathyroidism, even if this clinical encounter occurred before 2000 (for example patients being seen as adults that had parathyroidectomy as children). Those that never had hyperparathyroidism were classified based on their earliest available encounter date.
Standard clinical criteria for XLH diagnosis included hypophosphatemia with hyperphosphaturia, beginning in childhood and clinical history otherwise consistent with XLH, in the absence of other causes of hypophosphatemia. Appropriate family history of X-linked dominant inheritance was supportive, but not necessary, as spontaneous mutations causing XLH are common. PHEX mutation testing also supported the diagnosis of XLH but was not considered necessary as most patients did not have genotyping as part of their standard clinical care. FGF23 concentrations that were elevated or in the upper normal range were also considered when available, but most were not routinely available from their clinical care. Measurement of PHEX mutation or of FGF23 concentrations were not required for diagnosis. However, patients with clear evidence of other FGF23-mediated or of non-FGF23 mediated forms of hypophosphatemia were excluded (such as autosomal dominant or recessive forms of hypophosphatemia, tumor induced osteomalacia, or fibrous dysplasia of bone). Patients were also excluded for lack of available data to assess hyperparathyroidism and its outcomes. Patients with XLH may have been receiving no therapy or may have received calcitriol and phosphate salts. Although some subjects subsequently participated in burosumab clinical trials, no data was available to the investigators for this analysis from subjects during or after burosumab therapy.
Records were initially screened for the following ICD codes for hypophosphatemia (ICD9 code 275.3 or ICD10 codes E83.31 or E83.30). Since these codes are nonspecific (and the ICD9 code 275.3 includes both hyperphosphatemia and hypophosphatemia), records were then reviewed by S.D. to confirm diagnosis.
2.2. Variables collected
Laboratory test values from clinical laboratories included intact PTH, serum creatinine, calcium, phosphorus, and alkaline phosphatase when available. The estimated glomerular filtration rate (eGFR) was calculated from the last creatinine available for each patient, using the CKD-EPI equation for adults and the modified Schwartz equation for children. When children did not have a simultaneous height available at time of last creatinine, the height closest in time was used (within one year); this occurred for 5 children. Medication records were searched for cinacalcet. Compliance with medications was not assessable.
2.3. Classification of Hyperparathyroidism
Patients were categorized according to the following criteria. Secondary hyperparathyroidism was defined as PTH value ≥65 pg/mL with serum calcium <10.2 mg/dl. Tertiary hyperparathyroidism was defined as any one of the following: 1) PTH ≥65 pg/mL plus serum calcium ≥10.2 mg/dL; 2) PTH ≥50 pg/mL plus serum calcium ≥10.5 mg/dL; or 3) history of parathyroidectomy. Some patients fulfilled criteria for multiple categories over time, but for the purposes of this study were classified based on the highest simultaneous PTH and calcium values and on whether hypercalcemia persisted across multiple measurements. If a patient met criteria for tertiary hyperparathyroidism but did not meet such criteria on multiple contiguous encounters they were classified as having secondary hyperparathyroidism. Exceptions were made when the only available laboratory values were during cinacalcet treatment (n=1) or if the patient’s only available laboratory assessment was consistent with tertiary hyperparathyroidism (n=1). However, if patients had a subsequent persistent normocalcemia without having parathyroidectomy or cinacalcet treatment, they were classified as secondary hyperparathyroidism. Based on the presumed pathogenesis of tertiary hyperparathyroidism, patients meeting criteria for tertiary hyperparathyroidism were presumed to have previously had secondary hyperparathyroidism, even if prior laboratory documentation was not available.
Information regarding parathyroidectomy was obtained from physician notes, operative notes or other medical records where available. Some parathyroidectomies were performed prior to establishing care at our institution and thus data was only available via history. If there was a discrepancy between records, the operative report was prioritized, followed by the attending surgeon note, and the attending endocrinologist note. If a patient had parathyroidectomy, but we did not have access to presurgical laboratory testing, we rated these as being tertiary hyperparathyroidism based on recorded endocrinologist notes that tertiary hyperparathyroidism was the indication for surgery, or on evidence that they ever had tertiary hyperparathyroidism post-surgery.
2.4. Statistical Analysis
Categorical variables were summarized by frequencies (percentages, %) and were compared between groups using Fishers exact test. Continuous variables were summarized by mean ± standard deviation (SD), except that years were summarized by median (minimum, maximum). Prevalence of nephrocalcinosis was evaluated and compared as lifetime prevalence, while prevalence of eGFR<60 mL/min/1.732 was evaluated at the last known creatinine measurement.
3. Results
3.1. Hyperparathyroidism
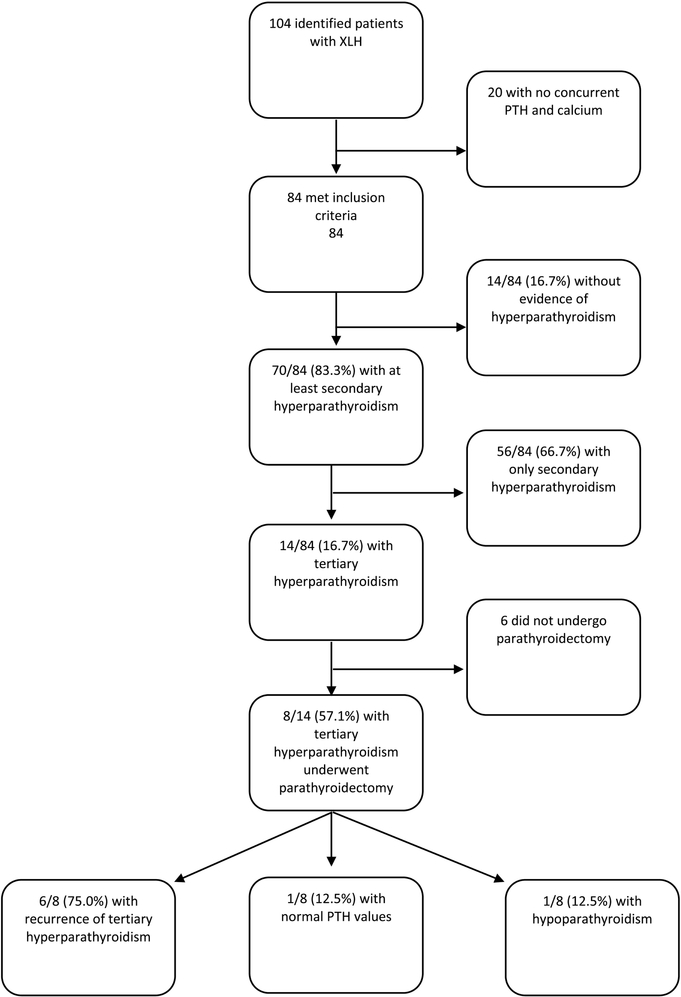
ICD codes supporting hypophosphatemia were detected in 376 patients from this practice group, of which 104 were confirmed on chart review to have a diagnosis of XLH. Of these, 84 had concurrent measurements of calcium and PTH and could be included in this analysis (Figure 1). Data were available from 40 patients as adults and 44 as children. Table 1 shows the characteristics of the studied population.
Fig. 1.
Flowchart of XLH patients and hyperparathyroidism.
Table 1.
Characteristics of XLH patients
| Study Group Characteristics | ||||
|---|---|---|---|---|
| Category | Whole population | Normal | Secondary Hyperparathyroidism | Tertiary Hyperparathyroidism |
| Sex (n=84) | Male: 25 (29.8%) | 4 (28.6%) | 17 (30.4%) | 4 (28.6%) |
| Female: 59 (70.2%) | 10 (71.4%) | 39 (69.6%) | 10 (71.4%) | |
| Race (n=84) | White: 80 (95.2%) | 13 (92.9%) | 53 (94.6%) | 14 (100%) |
| Black: 4 (4.8%) | 1 (7.1%) | 3 (5.4%) | 0 (0%) | |
| Age (n=84) | Adult (≥18): 40 (47.6%) | 6 (42.9%) | 22 (39.3%) | 12 (85.7%) |
| Child (<18): 44 (52.4%) | 8 (57.1%) | 34 (60.7%) | 2 (14.3%) | |
| First Calcium available (n=84) mg/dL | 9.42 (0.58) | 9.62 (0.40) | 9.35 (0.45) | 9.55 (1.11) |
| First PTH available (n=84) pg/mL | 99.57 (100.11) | 47.5 (10.94) | 81.52 (32.68) | 224.54 (196.65) |
| First Phosphorus available (n=84)* mg/dL | 2.41 (0.65) | 2.56 (0.80) | 2.46 (0.61) | 2.09 (0.49) |
| Nephrocalcinosis on renal ultrasound (n=69) | Never noted: 52 (75.4%) | 7 (63.6%) | 41 (85.4%) | 4 (40%) |
| Noted at least once: 17 (24.6%) | 4 (36.4%) | 7 (14.6%) | 6 (60%) | |
| eGFR at last measure (n=83) mL/min/1.73m2 | 111.39 (35.69) | 129.83 (40.68) | 116.03 (29.22) | 68.79 (25.29) |
Data are presented as n (%) or as Mean (standard deviation [SD]).
Some patient’s earliest accessible laboratory values occurred during therapy with calcitriol and phosphate.
Evidence for either secondary or tertiary hyperparathyroidism occurred in 70/84 (83.3%), during the study period. Fourteen patients (16.7%) never had evidence of hyperparathyroidism of any kind, 56 (66.7%) had evidence of secondary hyperparathyroidism (PTH>65) without ever having hypercalcemia, and 14 (16.7%) met criteria for tertiary hyperparathyroidism. Of the 14 patients with tertiary hyperparathyroidism, 10 had available data only during their period of tertiary hyperparathyroidism (or following its treatment) and four had secondary hyperparathyroidism that progressed to tertiary hyperparathyroidism during our study period. Some form of hyperparathyroidism occurred in 36/44 (81.2%) children and 34/40 (85.0%) adults. Resolution of secondary hyperparathyroidism was observed in all patients classified as children during our study period of 2000–2017. However, two adult patients did have a prior history of progression to tertiary hyperparathyroidism during adolescence (before the year 2000). Thus, most patients with secondary hyperparathyroidism did not progress to tertiary hyperparathyroidism [34/36 (94.4%) of children and 22/34 (64.7%) of adults]. Of the patients with only secondary hyperparathyroidism (n=56), 11 had single elevated PTH values without earlier or subsequent values to assess. Of the remaining 45 patients with secondary hyperparathyroidism and multiple datapoints, the most recent PTH value was <65 pg/mL for 17 (37.8%) patients, and persisted at >65 pg/mL for 28 (62.2%) patients. The follow up interval from first elevated to last known PTH value ranged from 0–16 years with a median of five years.
Tertiary hyperparathyroidism had an overall prevalence of 14/84 (16.7%) patients, including 2/44 (4.5%) children and 12/40 (30.0%) adults. According to the highest category achieved based on PTH and calcium concentrations, patients with tertiary hyperparathyroidism included: n=10 with PTH ≥65 pg/mL plus calcium ≥10.5 mg/dL; n=1 with PTH ≥65 pg/mL plus calcium 10.2–10.4 mg/dL; n=2 with PTH ≥50 pg/mL plus calcium ≥10.5 mg/dL; n=1 with parathyroidectomy prior to presentation to our center with resultant chronic post-surgical hypoparathyroidism.
3.2. Surgical Outcomes
Of the 14 patients with tertiary hyperparathyroidism, 8 (57.1%) had at least one parathyroidectomy procedure. This represented in 8/84 (9.5%) of the total population, 2/44 (4.5%) of children and 6/40 (15.0%) of adults. Table 2 describes the clinical features of patients who had parathyroidectomy.
Table 2:
Patients with Tertiary Hyperparathyroidism
| Sex | Calcium (mg/dL) before surgery | PTH (pg/mL) before surgery | Age at First Surgery | Glands Removed | Implantati on | Time after surgery to recurrence | Calcium (mg/dL) at recurrence | PTH (pg/mL) at recurrence | Cinacalc et used |
|---|---|---|---|---|---|---|---|---|---|
| F | Not available | Not available | 15 | Unknown | Unknown | 29 years | 10.7 | 74 | No |
| F | Not available | Not available | 17 | Unknown | Arm | No recurrence (Hypoparathyroidis m) | No | ||
| F | Not available | Not available | 24 | 2 | No | 29 years a | 10.7 | 785 | No |
| M | Not available | Not available | 36 | 3.5 | Neck | 5 years b | 11.4 | 558 | Yes |
| F | 12.5 | 525 | 40 | 3.5 | No | 4 years | 10.7 | 81 | No |
| F | Not available | Not available | 60 | Unknown | Unknown | 7 years | 10.2 | 74 | No |
| F | 10.2 | 286.5 | 60 | 1 | No | 0 years | 10.2 | 287 c | Yes |
| M | 10.2 | 259 | 61 | 3 | No | No recurrence | Yes | ||
| F | 11.6 | 60 | None | Yes | |||||
| F | 10.6 | 54 | None | No | |||||
| M | 10.7 | 63 | None | No | |||||
| M | 10.2 | 178 | None | No | |||||
| F | 11.2 | 161 | None | No | |||||
| F | 10.8 | 106 | None | No |
First labs available after surgery
Per notes patient had recurrence at 5 years, but no labs available for that time. Labs are given 8 years after parathyroidectomy.
This patient had a repeat parathyroidectomy removing 2 glands at age 63, three years after the original surgery.
Four of the parathyroidectomies occurred during our study period. Four surgeries were performed at Indiana University. Operative details were lacking for some of the surgeries performed outside of our institution and/or study period. The number of glands removed varied from 1 to 3.5, but three surgeries had an unreported number of glands removed. Two surgeries included parathyroid autotransplantation in the neck or arm, four surgeries involved no autotransplantation, and for three surgeries gland implantation was unknown as no operative note was available (Table 2).
After parathyroidectomy, persistent or recurrent tertiary hyperparathyroidism was detected in 6/8 patients at a median of six years (ranging from 0 to 29 years). One patient had chronic post-surgical hypoparathyroidism, and one patient remained normocalcemic four years after surgery. One patient with persistent hyperparathyroidism had resolution of hyperparathyroidism after a second surgery.
3.3. Cinacalcet
Five of 14 (35.7%) patients with tertiary hyperparathyroidism attempted management with cinacalcet. The time on the medication, doses used, and response to therapy was variable.
The first patient had a subtotal parathyroidectomy for tertiary hyperparathyroidism with an associated brown tumor in 2003. He had recurrence of tertiary hyperparathyroidism at an uncertain time after surgery and was treated with 30 mg of cinacalcet three times a day with adequate control (patient reported). Due to progressive kidney failure, he underwent kidney transplant in 2009. After transplant, PTH levels rose progressively to 400–600 pg/ml per text notes and calcium to 11.7 mg/dl. Approximately two years (2011) after transplant, he was treated with cinacalcet 60 mg twice a day limited by nausea. One year later (2012) he presented to our institution where PTH was 558 pg/ml and calcium 11.4 mg/dl. He was initially continued on cinacalcet 60 mg twice daily and decreased to 60 mg in the morning and 30 mg in the afternoon 6 months later when PTH was 524 pg/ml and calcium 9.7mg/dl. The patient was subsequently lost to follow up.
A second patient underwent a subtotal parathyroidectomy in 2013 with recurrent tertiary hyperparathyroidism in 2014. In light of the recurrent hyperparathyroidism, oral phosphate was discontinued while continuing calcitriol and vitamin D. However, in 2015 calcium was 10.7 mg/dl and PTH 186 pg/ml therefore the patient started cinacalcet 30 mg daily. After one year on cinacalcet (2016), PTH was 165 pg/ml and calcium 10.2 mg/dl. Calcium continued to be relatively controlled (calcium 10.4 mg/dl, PTH 236 pg/ml) in 2017. However, due to symptoms of osteomalacia, alkaline phosphatase of 212 U/L, and inability to treat with phosphorus and calcitriol because of tertiary hyperparathyroidism, she had repeat parathyroidectomy in 2017. One year after the repeat parathyroidectomy (2018) his calcium and PTH were normal.
A third patient started cinacalcet 30 mg daily when found to have tertiary hyperparathyroidism in 2013 (calcium 11.0 mg/dl, PTH unavailable). Two months later the cinacalcet dose was increased to 60 mg daily (after calcium level of 10.9 mg/dl and PTH 168 pg/ml). Calcium remained mildly elevated despite increases in cinacalcet to 60 mg twice daily (calcium 10.4 mg/dl, PTH 248 pg/ml), and the patient was sent to surgery for a 3.5 gland parathyroidectomy. Four years after parathyroidectomy, the PTH and calcium remained normal.
A fourth patient presented to care in 2015 after already receiving cinacalcet 30 mg twice daily for unknown duration (PTH 119 pg/ml, calcium 9.3 mg/dl). During the subsequent years of treatment (until the end of our study) this patient has been generally normocalcemic except for one occurrence of calcium 11.6 mg/dl and PTH 60 pg/ml.
A fifth patient briefly attempted cinacalcet but could not tolerate it due to nausea and underwent 3.5 gland resection.
3.4. Renal Findings
Renal ultrasound reports were available for 66 patients, of whom 10 also had tertiary hyperparathyroidism. Renal ultrasounds were available for 71.4% of patients with tertiary hyperparathyroidism and for 80% of those without tertiary hyperparathyroidism. The prevalence of nephrocalcinosis among patients with tertiary hyperparathyroidism was 60% (6/10), while the prevalence of nephrocalcinosis among those without tertiary hyperparathyroidism, was 18.6% (11/59, p = 0.01). (See Table 3)
Table 3:
Nephrocalcinosis and Renal Function in relation to Hyperparathyroidism
| Category | Tertiary hyperparathyroidism | Secondary hyperparathyroidism | Patients without any hyperparathyroidism | All patients without tertiary hyperparathyroidism | p value (comparing tertiary hyperparathyroidism to other groups) |
|---|---|---|---|---|---|
| Nephrocalcinosis prevalence | 6/10 (60.0%) | 7/48 (14.6%) | 4/11 (36.4%) | 11/59 (18.6%) | p=0.005 (secondary) p=0.26 (normal) p=0.011 (all) |
| eGFR <60 mL/min/1.732 | 5/14 (35.7%) | 1/56 (1.8%) | 0/14 (0%) | 1/69 (1.5%) | p=0.009 (secondary) p=0.025 (normal) p=0.004 (all) |
The mean (SD) eGFR for patients (n=83) was 111.01 (35.69) mL/min/1.732 (94.48 (28.51) for adults and 132.60 (32.56) for children). Those with tertiary hyperparathyroidism had a mean eGFR of 68.79 (25.30) mL/min/1.732, while those without tertiary hyperparathyroidism had a mean eGFR of 119.58 (31.18) mL/min/1.732. Of patients with tertiary hyperparathyroidism 35.7% had an eGFR<60 mL/min/1.732, compared to 1.5% of those without tertiary hyperparathyroidism (p<0.0001). (Table 3). Patients with nephrocalcinosis were more likely to have eGFR<60 mL/min/1.732 than those without nephrocalcinosis [3/17 (17.6%) versus 1/48 (2.0%), p=0.05].
Discussion
In this retrospective study, we found a high rate of hyperparathyroidism among patients with XLH: 83.3% had evidence for secondary hyperparathyroidism and 16.7% had tertiary hyperparathyroidism. Of all patients with XLH, 9.5% underwent one or more parathyroid operations. However, 75% of patients who underwent parathyroidectomy had persistence or recurrence of hyperparathyroidism.
Our study describes a higher prevalence of secondary and tertiary hyperparathyroidism than described previously [10, 15]. Most reports of secondary and tertiary hyperparathyroidism are limited to case studies and, based on the number of such reports, secondary and tertiary has been characterized as rare. Firth et al, in their review of the literature, found only 12 patients described with coincident hypercalcemic hyperparathyroidism and hypophosphatemic bone disease, eight of which were classified as having familial hypophosphatemic rickets [13]. Rivkees et al in 1992 found that only 20 cases of hypercalcemic hyperparathyroidism had been described in the literature [10]. In 1995 Knudtzon et al reviewed the literature for reports of tertiary hyperparathyroidism concluding that the prevalence of hyperparathyroidism is 2.2–4.1% [15]. Carpenter et al prospectively described elevations of PTH in 7/19 patients during routine daytime sampling, while 15/19 of their patients had nocturnal elevations in PTH [14]. This prevalence of nocturnal secondary hyperparathyroidism of 78.9% is close to our estimate of 83.3%, though we used daytime measurements. Potential reasons for differences include the ages of patients included, and the definitions used for hyperparathyroidism in various studies. Indeed, as the co-occurrence of hyperparathyroidism and XLH has become well known, PTH monitoring has become routine. We assessed both children and adults, and the rate of secondary hyperparathyroidism was high in both, while tertiary hyperparathyroidism was less common in children, but more common in adults. However, some of our patients had only one or a few time points assessed, and some were only assessed as children. It is possible that the lifetime risk of tertiary hyperparathyroidism may be higher than we detected.
Treatment of tertiary hyperparathyroidism in the XLH patient population is challenging. Of five patients who attempted cinacalcet, four still required surgery, though one was retreated for recurrence and controlled with cinacalcet after surgery. While studies have suggested that parathyroidectomy can be an effective treatment for XLH [17, 21], we found a high rate of recurrence or persistence of tertiary hyperparathyroidism after surgery. This may be influenced by the number of glands removed, as some patients only had a single gland removed, while the number of glands removed was not known for some patients who could only provide a history of past surgery. The nature of hyperparathyroidism in XLH typically involves multi-gland hyperplasia. Thus, frequently patients require removal of multiple glands to achieve normocalcemia. However, among our patients with 3 or 3.5 glands removed, we still saw evidence of recurrence. In addition, one patient who underwent parathyroidectomy developed chronic hypoparathyroidism, complicating her management with resultant hypocalcemia. These surgeries were performed by different providers at different centers over many years. It is possible that more patients will undergo either initial or a repeat surgery over their lifetimes.
While in our study we found a recurrence rate of 75% of hyperparathyroidism after parathyroidectomy for XLH patients, previous studies have described a lower rate of recurrence. In a retrospective chart review of six patients who underwent parathyroidectomy Savio found lower rates of recurrence (16.7%) after initial parathyroidectomy than we did in our population [17]. Only one out of six of their patients required reoperation for recurrent tertiary hyperparathyroidism. The differences in the outcome compared to our study may be due to multiple factors, including that patients in their study typically had a higher number of glands removed at the time of first surgery, with fewer gland implantation outside the neck, and differences in follow-up time.
We demonstrated a significantly higher prevalence of nephrocalcinosis among patients with tertiary hyperparathyroidism compared to those without tertiary hyperparathyroidism (60.0% vs 19.6% respectively). Sampling bias for assessment with renal ultrasound is unlikely as 71% of patients with tertiary hyperparathyroidism had at least one renal ultrasound, while 80% of those without tertiary hyperparathyroidism had renal ultrasounds. Furthermore, patients with tertiary hyperparathyroidism or nephrocalcinosis were more likely to develop impaired kidney function. Our 8.4% frequency of eGFR < 60 mL/min/1.732 is similar to that reported by Nakumura et al (9.1%) [22].
Limitations include the retrospective nature of the study, and wide variation in the amount of data available for each patient, even though we included available health records from outside our own institution, and from the Indiana Network for Patient Care. Full details on the parathyroidectomy, such as number of glands removed, were not always available. Some patients had only a single record of PTH and calcium, while others had more than 20 occurrences. To obtain the largest amount of data possible, laboratory tests were included from both our own hospital’s clinical laboratory as well as those from outside institutions. Therefore, laboratory assays may have been run at different places and using different assays, increasing variability. We also used total calcium without adjusting for albumin level, as most patients did not have albumin levels available.
Another limitation is the lack of available data to evaluate the effect of calcitriol and phosphate doses and exposure on complications of hyperparathyroidism and nephrocalcinosis. However, the lifetime duration and doses of therapy, along with its compliance, are likely to be relevant to the patient’s risk, rather than the values solely at the time of testing. However, data was pulled from available clinical notes, which was often not adequate to determine the exposure of the individual patient. In addition, some patients came to our center as new patients after decades of treatment at various healthcare providers for which we did not have access to sufficiently detailed records. Thus, we were not able to characterize the duration or dosage of phosphate and calcitriol in this population, and whether these related to the development of hyperparathyroidism or nephrocalcinosis. In addition, some patients presented with evidence of tertiary hyperparathyroidism years after having stopped conventional therapy.
The etiology of parathyroid hormone elevation in patients with XLH is complex. Elevated parathyroid hormone levels have been reported even in untreated patients with XLH [14, 15, 23]. These typically improve after initiating therapy with active vitamin D. However, the development of secondary and tertiary hyperparathyroidism in patients on conventional therapy appears to be driven primarily by the phosphate doses. After phosphate doses, a transient increase in serum phosphorus is accompanied by slight decreases in serum calcium, which is expected to stimulate PTH production [24]. Indeed prolonged use of high dose phosphate therapy has been identified as a risk factor for PTH elevation [25]. Additionally, treatment with calcitriol and phosphate has independently been shown to increase levels of FGF23 [26, 27]. Elevated levels of FGF23 has been directly linked to elevations in PTH in XLH patients [27]. Similarly, animal models of FGF23 excess have enlarged parathyroid glands and elevated PTH concentrations [28]. In excised tissue from a parathyroidectomy of a patient with XLH, PHEX was highly expressed in parathyroid tissue [23]. Given parathyroid PHEX expression, the study concluded PHEX may play a role in PTH inactivation. Such evidence suggests there may be a mechanism inherent to XLH, aside from phosphate treatment, that increases the tendency to parathyroid autonomy in this population. Given the propensity to hyperparathyroidism, it may be necessary to limit phosphate doses or increase calcitriol doses in the face of rising PTH levels, in an attempt to normalize PTH, and prevent development of hypercalcemic hyperparathyroidism.
The paradigm of treatment of XLH is likely to shift over time with the recent approval of an anti-FGF23 antibody therapy (burosumab) as monotherapy. Notably, none of the data from this study comes from patients receiving burosumab. As patients switch from using phosphate salts with active vitamin D to using burosumab, over time there may be a change in the prevalence of hyperparathyroidism. However, long term studies will be needed to determine whether or not there is any impact of burosumab on the prevalence of hyperparathyroidism in XLH patients.
Conclusion
Patients with XLH have a high risk of developing secondary and tertiary hyperparathyroidism during treatment with conventional therapy which includes phosphate salts. Tertiary hyperparathyroidism is difficult to manage, with frequent recurrence or persistence after surgery, risk of developing post-surgical hypoparathyroidism, and mixed responses to calcimimetics. Hyperparathyroidism and nephrocalcinosis both were associated with impairment in kidney function. Further studies are needed to determine the best approach to preventing hyperparathyroidism, and the effect of novel therapies on its development.
Highlights:
Secondary hyperparathyroidism occurred in 81.8% of children and 85.0% of adults.
Tertiary hyperparathyroidism occurred in 16.7% of all patients, or 30% of adults.
Hypercalcemic hyperparathyroidism persisted or recurred in 6/8 (75%) of patients, while one (12.5%) developed chronic post-surgical hypoparathyroidism.
Tertiary hyperparathyroidism was associated with nephrocalcinosis and with low eGFR though other factors not assessed (including medication doses, duration of disease, duration of treatment) may also have contributed to renal disease.
Acknowledgments
Funding: This work was supported in part by funding from the NIH/NIAMS: 1P30AR072581.
Disclosures:
EAI has received research funding and fees for consulting with Kyowa Hakko Kirin and Ultragenyx, Pharmaceuticals. All other authors have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beck-Nielsen SS, et al. , Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol, 2009. 160(3): p. 491–7. [DOI] [PubMed] [Google Scholar]
- 2.A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet, 1995. 11(2): p. 130–6. [DOI] [PubMed] [Google Scholar]
- 3.Liu S and Quarles LD, How fibroblast growth factor 23 works. J Am Soc Nephrol, 2007. 18(6): p. 1637–47. [DOI] [PubMed] [Google Scholar]
- 4.Alizadeh Naderi AS and Reilly RF, Hereditary disorders of renal phosphate wasting. Nat Rev Nephrol, 2010. 6(11): p. 657–65. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter TO, et al. , A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res, 2011. 26(7): p. 1381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linglart A, et al. , Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect, 2014. 3(1): p. R13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alon US, et al. , Hypertension in hypophosphatemic rickets--role of secondary hyperparathyroidism. Pediatr Nephrol, 2003. 18(2): p. 155–8. [DOI] [PubMed] [Google Scholar]
- 8.Tournis ST, et al. , Co-existence of X-linked hypophosphatemic rickets (XLH) and primary hyperparathyroidism: case report and review of the literature. J Musculoskelet Neuronal Interact, 2005. 5(2): p. 150–4. [PubMed] [Google Scholar]
- 9.Thomas WC Jr. and Fry RM, Parathyroid adenomas in chronic rickets. Am J Med, 1970. 49(3): p. 404–7. [DOI] [PubMed] [Google Scholar]
- 10.Rivkees SA, et al. , Tertiary hyperparathyroidism during high phosphate therapy of familial hypophosphatemic rickets. J Clin Endocrinol Metab, 1992. 75(6): p. 1514–8. [DOI] [PubMed] [Google Scholar]
- 11.Tournis S, et al. , Tertiary hyperparathyroidism in a patient with X-linked hypophosphatemic rickets. J Musculoskelet Neuronal Interact, 2011. 11(3): p. 266–9. [PubMed] [Google Scholar]
- 12.Wu CJ, Song YM, and Sheu WH, Tertiary hyperparathyroidism in X-linked hypophosphatemic rickets. Intern Med, 2000. 39(6): p. 468–71. [DOI] [PubMed] [Google Scholar]
- 13.Firth RG, Grant CS, and Riggs BL, Development of hypercalcemic hyperparathyroidism after long-term phosphate supplementation in hypophosphatemic osteomalacia. Report of two cases. Am J Med, 1985. 78(4): p. 669–73. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter TO, et al. , Nocturnal hyperparathyroidism: a frequent feature of X-linked hypophosphatemia. J Clin Endocrinol Metab, 1994. 78(6): p. 1378–83. [DOI] [PubMed] [Google Scholar]
- 15.Knudtzon J, et al. , Autonomous hyperparathyroidism in X-linked hypophosphataemia. Clin Endocrinol (Oxf), 1995. 42(2): p. 199–203. [DOI] [PubMed] [Google Scholar]
- 16.Yavropoulou MP, et al. , Cinacalcet in hyperparathyroidism secondary to X-linked hypophosphatemic rickets: case report and brief literature review. Hormones (Athens), 2010. 9(3): p. 274–8. [DOI] [PubMed] [Google Scholar]
- 17.Savio RM, et al. , Parathyroidectomy for tertiary hyperparathyroidism associated with X-linked dominant hypophosphatemic rickets. Arch Surg, 2004. 139(2): p. 218–22. [DOI] [PubMed] [Google Scholar]
- 18.Akinci A, Dundar I, and Kivilcim M, The Effectiveness of Cinacalcet as an Adjunctive Therapy for Hereditary 1,25 Dihydroxyvitamin D3-Resistant Rickets. J Clin Res Pediatr Endocrinol, 2017. 9(2): p. 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alon US, et al. , Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol, 2008. 3(3): p. 658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raeder H, et al. , A case of X-linked hypophosphatemic rickets: complications and the therapeutic use of cinacalcet. Eur J Endocrinol, 2008. 159 Suppl 1: p. S101–5. [DOI] [PubMed] [Google Scholar]
- 21.Alon U, Newsome H Jr., and Chan JC, Hyperparathyroidism in patients with X-linked dominant hypophosphatemic rickets--application of the calcium infusion test as an indicator for parathyroidectomy. Int J Pediatr Nephrol, 1984. 5(1): p. 39–43. [PubMed] [Google Scholar]
- 22.Nakamura Y, et al. , Hypertension is a characteristic complication of X-linked hypophosphatemia. Endocr J, 2017. 64(3): p. 283–289. [DOI] [PubMed] [Google Scholar]
- 23.Blydt-Hansen TD, Tenenhouse HS, and Goodyer P, PHEX expression in parathyroid gland and parathyroid hormone dysregulation in X-linked hypophosphatemia. Pediatr Nephrol, 1999. 13(7): p. 607–11. [DOI] [PubMed] [Google Scholar]
- 24.Schmitt CP and Mehls O, The enigma of hyperparathyroidism in hypophosphatemic rickets. Pediatr Nephrol, 2004. 19(5): p. 473–7. [DOI] [PubMed] [Google Scholar]
- 25.Makitie O, Kooh SW, and Sochett E, Prolonged high-dose phosphate treatment: a risk factor for tertiary hyperparathyroidism in X-linked hypophosphatemic rickets. Clin Endocrinol (Oxf), 2003. 58(2): p. 163–8. [DOI] [PubMed] [Google Scholar]
- 26.Imel EA, et al. , Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab, 2010. 95(4): p. 1846–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenter TO, et al. , Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J Clin Endocrinol Metab, 2010. 95(11): p. E352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson T, et al. , Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology, 2004. 145(7): p. 3087–94. [DOI] [PubMed] [Google Scholar]