Abstract
OBJECTIVES
Evidence suggests use of proton pump inhibitors (PPIs) and H2 blockers may provoke disease flares in individuals with established inflammatory bowel disease (IBD); however, there are no studies investigating the relationship of these medications with risk of developing pediatric IBD. The hypothesis was that use of acid suppression therapy in children might be associated with development of pediatric IBD.
METHODS
This was a nested case-control study of 285 Kaiser Permanente Northern California members, age ≤21 years diagnosed with IBD from 1996 to 2016. Four controls without IBD were matched to each case on age, race, and membership status at the case's index date. Disease risk scores (DRS) were computed for each subject. Odds ratios and 95% confidence intervals were calculated by using conditional logistic regression models adjusted for DRS.
RESULTS
The children's mean age was 15.1 ± 2.6 years and 49.5% were female. Six cases (n = 3 Crohn's disease [CD], n = 3 ulcerative colitis [UC]) and 6 controls were prescribed PPIs and 10 cases (n = 7 CD, n = 3 UC) and 28 controls were prescribed H2 blockers. The OR for the association of at least 1 PPI or H2 blocker prescription with subsequent IBD was 3.6 (95% CI, 1.1–11.7) for PPIs and 1.6 (95% CI, 0.7–3.7) for H2 blockers.
CONCLUSIONS
Early-life PPI use appears to be associated with subsequent IBD risk. These findings have implications for clinical treatment of children with gastrointestinal symptoms and warrant further investigation in a larger cohort.
Keywords: child, epidemiology, inflammatory bowel disease, pharmacoepidemiology
Introduction
From 1990 through the 2000s, the burden of pediatric inflammatory bowel disease (IBD) has increased globally.1 In North America, more than 1.3% of the general population has IBD, amounting to over 3.1 million Americans.2 Pediatric IBD can result in adverse consequences, such as delayed puberty and growth failure.3
Previous longitudinal and cross-sectional studies have shown that IBD is associated with changes in the composition of the gut microbiota,4,5 emphasizing the role of environmental factors in the development and progression of the disease. If microbiome changes influence IBD risk, then it is plausible that drugs that alter the microbiome may also alter the risk of IBD. One drug class that is known to alter the gut microbiome is acid-reducing medications, such as proton pump inhibitors (PPIs) and H2-receptor agonists (H2 blockers);6,7 however, whether these drugs alter the microbiome in a way that increases the risk of IBD is still unclear. There has been a significant upsurge in the use of PPIs and H2 blockers among infants and children in the past decade.8–10 Proton pump inhibitors and H2 blockers may provoke disease flares in individuals with established IBD;11 however, no study has investigated the relationship between the use of PPIs, H2 blockers, and incident IBD. Given the higher severity of IBD in the pediatric population,12,13 and the limited evidence of PPI and H2 blocker safety from clinical trials in younger populations, this gap in knowledge is concerning. The hypothesis was that the use of acid suppression therapy in children might be associated with the development of pediatric IBD.
Materials and Methods
Study Setting. Kaiser Permanente Northern California (KPNC) is a closed, prepaid, integrated health plan that serves 30% of the San Francisco Bay Area population, with over 4 million currently enrolled members.14,15 The membership of KPNC is representative of the underlying population of the San Francisco Bay Area with respect to race and socioeconomic status (SES).14 All patient encounters, prescription fills, and laboratory results have been recorded in a computerized database since 1996.
Study Design, Subjects, and Data Source. This nested case-control study16,17 used data from KPNC electronic health records as described earlier for the Kaiser Permanente Autoimmune Registry.18 Inpatient and outpatient data were used to identify all children diagnosed with IBD at ≤21 years of age between 1996 and 2016. Cases were selected as those 285 children in the health plan with at least 5 years of continuous membership (with no coverage gaps longer than 60 days) prior to the date of IBD diagnosis (i.e., the index date). Four controls drawn from the general KPNC pediatric population were matched to each case on age (within 1 year), race (Asian/Pacific Islander, black, white, Native American, multiracial, or unknown/other), primary clinic location, and membership status at the case's index date. These data were obtained from membership data and the electronic medical record. Controls were required to have been members at least as long as their matched case, and not to have an IBD diagnosis as of the index date for the case to which they were matched.
Classification of Outcome. IBD diagnosis was defined by International Classification of Disease, Clinical Modifications 9 (ICD-9-CM) codes for IBD (555 for ulcerative colitis [UC] and 556 for Crohn's disease [CD]) and by ICD, Clinical Modifications 10 (ICD-10-CM) codes for IBD (K50 for CD and K51 for UC), using diagnostic codes recorded during inpatient and outpatient encounters. Cases were required to have had at least 2 inpatient or outpatient visits with IBD diagnoses recorded. According to previous research, this case definition has 95% positive predictive value (95% CI, 94%–96%).19
Classification of Exposure. Use of PPIs and H2 blockers was assessed from the health plan's outpatient pharmacy database that records all details of prescriptions and dispensing of medications to health plan members. This database was searched for National Drug Codes (NDC) matching PPIs and H2 blockers. Owing to the possibility that these medications were prescribed for treatment of symptoms attributable to undiagnosed IBD, a 2-year “lag period” was implemented when assessing exposure. Thus, a case or control was only considered to be exposed if there was PPI or H2 use between 2 and 5 years before the index date. This reduced the possibility of protopathic bias,20 which occurs when a drug of interest is initiated to treat symptoms of the disease under study before it is diagnosed.
Chart Review. A chart review was conducted for all cases and controls in the study who were prescribed PPIs, and a random sample of subjects who were prescribed H2 blockers. The review covered the period 2 to 5 years prior to index date to determine the medical indication for the prescription and whether there was contemporaneous evidence of IBD symptoms (e.g., diarrhea, bloody stools, abdominal pain). The goal was to assess the indication for which the drug was prescribed to determine if prodromal symptoms were plausible as an explanation. The note from the clinic visit preceding the first prescription during the study period was examined for patient-reported symptoms, concomitant diagnostic codes, additional medications, and the primary clinical reason for the prescription.
Potential Confounders. Covariates that preceded and may have been associated with both IBD and exposure to PPIs were included in our analysis as potential confounding factors. In addition to the matching factors (age at index date, race, and primary clinic location), these candidate confounders included antibiotic medication use, sex, and SES. Data on antibiotic exposure were obtained by using the same pharmacy databases that were used to identify PPI and H2 blocker prescriptions. Sex was obtained from the electronic medical record. Two census tract-level measures21 representing SES were identified by using residential address geocodes: proportion of resident adults who are high-school graduates and proportion of family households with below-poverty level income.
Because very few children were prescribed PPI or H2 blocker medications in this database, traditional statistical adjustment methods were not used. Instead, to control for potential differences in risk of IBD between users of PPI and H2 blockers and non-users of either type of drug, a disease risk score (DRS) was calculated for all subjects. In general, a DRS estimates the probability of disease for each member of a study population in the absence of the exposure, regardless of true exposure status.22 This method allowed us to account for medication exposure adjusted for a single measure of disease risk, which was critical because of the few children exposed to PPI and H2 blockers in this population. In addition, the method is compatible with conducting a nested case-control design.23 Both DRSs and propensity scores address statistical problems that arise when there is a large number of covariates and a rare exposure (DRS) or outcome (propensity scores), but a DRS is preferable to propensity scores when the exposure is rare.22 The DRS was estimated from a logistic regression analysis of sex, year of birth, number of antibiotic prescriptions in the 2 to 5 years prior to index date, and the 2 census-level measures described above on the odds of being an IBD case in this study.
Statistical Analysis. Conditional logistic regression16 was used to compare cases and controls with respect to PPI and H2 blocker prescriptions filled between 2 and 5 years before the diagnosis, adjusting for the DRS as a continuous measure. To consider the possibility that early symptoms might drive an increase in PPI and H2 blocker prescriptions in the 2 years prior to diagnosis, additional analyses considered exposure within 1 year and 1 to 2 years prior to diagnosis. These analyses allowed for comparison with our a priori exposure period of interest to look for an increase in prescriptions in this time frame (Supplementary Table 1 (18.6KB, pdf) ). Odds ratios and 95% CIs were calculated by using conditional logistic regression models that included both PPIs and H2 blockers. All analyses were conducted by using SAS version 9.4 (SAS Institute, Cary, North Carolina) and proc logistic. This study was approved by the Kaiser Foundation Research Institute Institutional Review Board.
Results
Demographic Characteristics. Two hundred eighty-six cases and 1144 controls were identified (Table 1). One case was dropped after it was discovered that his IBD diagnosis preceded his PPI prescription, for a total of 285 cases. Two controls were missing data for SES measures and thus were dropped from the analysis for a total of 1142 controls. The mean age at index date was 15 years for both cases and controls. More than half (54%) of cases and controls were white. Hispanic was the second most common race/ethnicity category for both cases and controls (14%), followed by multiracial (12%), Asian/Pacific Islander (11%), black (7%), and Native American (1%). Controls were more likely to be female than cases (51% versus 43%). Cases and controls received similar numbers of antibiotic prescriptions in the 2 to 5 years prior to index date. Socioeconomic status measures were also similar between cases and controls.
Table 1.
Demographic Characteristics of IBD Cases and Matched Controls
| Cases, n = 285 | Controls,* n = 1142 | |
|---|---|---|
| Index age, yr, mean (range) | 15.1 (10–21) | 15.1 (10–21) |
| Female, % | 43.4 | 51.1 |
| Race/ethnicity, % | ||
| Asian/Pacific Islander | 11.0 | 11.0 |
| Black | 7.4 | 7.4 |
| Hispanic | 14.3 | 14.2 |
| White | 53.7 | 53.6 |
| Native American | 1.4 | 1.4 |
| Multiracial | 12.0 | 12.0 |
| Unknown/other | 0.4 | 0.3 |
| Missing | <1% (n = 3) | <1% (n = 12) |
| No. of antibiotic prescriptions, mean ± SD | 0.9 ± 1.5 | 0.8 ± 1.4 |
| Socioeconomic status†, mean ± SD | ||
| Proportion of family households with below-poverty level income | 0.04 ± 0.05 | 0.04 ± 0.06 |
| Proportion of high-school graduates | 0.2 ± 0.1 | 0.2 ± 0.1 |
| PPI prescriptions, mo supply/patient, mean ± SD | 3.7 ± 43.6 | 0.6 ± 11.7 |
| H2 blocker prescriptions, mo supply/patient, mean ± SD | 2.8 ± 22.8 | 2.6 ± 33.5 |
IBD, inflammatory bowel disease; PPI, proton pump inhibitor
* Matched to cases on age, race, primary location, and duration of membership.
† Based on census tract of residence.
Association With PPIs and H2 Blockers. During the 2 to 5 years before the index date, 2.1% of cases (n = 6) and 0.5% of controls (n = 6) were prescribed PPIs; 3.5% of cases (n = 10) and 1.6% of controls (n = 18) were prescribed H2 blockers (4 children were prescribed both [2 cases and 2 controls]). The OR for the association of receipt of at least 1 prescription with risk of subsequent IBD was 3.6 (95% CI, 1.1–11.7) for PPIs and 1.6 (95% CI, 0.7–3.7) for H2 blockers after accounting for potential confounders and use of the other medication class (Figure).
Figure.
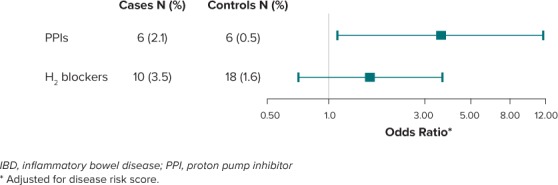
Forest plot of adjusted odds ratios and 95% CI for the association between receipt of 1 or more PPI or H2 blocker prescriptions 2 to 5 years before diagnosis and pediatric-onset IBD.
There was a substantial increase in the OR for the association of receipt of at least 1 PPI prescription with risk of subsequent IBD during the 2 years prior to diagnosis (Supplementary Table 1 (18.6KB, pdf) ).
Chart Review. Among the 12 children prescribed PPIs during the study period, all but 2 (1 case and 1 control) had been diagnosed with gastrointestinal symptoms or conditions at the visit preceding the PPI prescription (Table 2). Two controls and 4 cases were prescribed PPIs for gastroesophageal reflux disease (GERD) or possible GERD. There were no discernible differences in PPI indication between cases and controls. Our analyses of 4 cases and 5 controls with H2 blocker use similarly did not identify differences between cases and controls (Supplementary Table 2 (18.9KB, pdf) ).
Table 2.
Clinical Indications, Symptoms, and Additional Prescriptions Among 6 IBD Cases and 6 Controls Prescribed PPIs, Kaiser Permanente Northern California, 1996–2016
| Case/Control (Index Age) | PPI Use, Age, Duration | H2 Blocker and Antibiotic Use, Age, Drug Type* | Additional Prescriptions† | Diagnosis | Gastrointestinal Symptoms | Non-gastrointestinal Symptoms | Primary Reason for Visit |
|---|---|---|---|---|---|---|---|
| Control (14) | 9, 14 days | 9, penicillin | Metronidazole, amoxicillin, polyethylene glycol 3350, ondansetron | H pylori | Abdominal pain, constipation, vomiting | Decreased appetite | H pylori treatment |
| Control (14) | 11, 30 days 12, 30 days |
9, penicillin 10, penicillin 10, penicillin 11, penicillin 11, H2 blocker |
Amoxicillin, acetaminophen, codeine; acetaminophen, dicyclomine, ranitidine, isometheptene, sertraline, phenobarbital, belladonna alkaloid, dichloralphenazone | Chronic abdominal pain | Nausea, vomiting, abdominal pain | Migraine, weight loss | Abdominal pain, nausea, vomiting |
| Control (14) | 11, 30 days | None | Ondansetron, cyproheptadine, guanfacine, sertraline, mirtazapine | Anxiety disorder, oppositional defiant disorder | Diarrhea, abdominal pain (pain noted as better at visit), vomiting | Migraine | Diarrhea |
| Control (15) | 12, 100 days | 11, penicillin 11, penicillin 11, H2blocker 12, penicillin 13, penicillin |
Amoxicillin, ranitidine | Gastritis, GERD | Upset stomach, diarrhea | Flu-like symptoms (congestion, sore throat, runny nose, fever) | GERD, not taking ranitidine as prescribed |
| Control (16) | 11, 182 days 12, 90 days 12, 90 days |
None | Metoclopramide | GERD | Regurgitation, worsening GERD | None | Worsening GERD |
| Control (19) | 16, 50 days 17, 50 days |
14, tetracycline | Ondansetron | Suspected gastritis | Abdominal pain and nausea, vomiting | Dizziness | Abdominal pain and vomiting |
| Case (12) | 8, 30 days | 7, cephalosporin 8, penicillin 10, penicillin |
None | Sinus infection, possible GERD | Not reported | Not reported | GERD symptoms |
GERD, gastroesophageal reflux disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; PUD, peptic ulcer disease; PPI, proton pump inhibitor
* During the 2 to 5 years before index date.
† Additional prescriptions within ± 6 months of PPI prescription.
Table 2.
Clinical Indications, Symptoms, and Additional Prescriptions Among 6 IBD Cases and 6 Controls Prescribed PPIs, Kaiser Permanente Northern California, 1996–2016 (cont)
| Case/Control (Index Age) | PPI Use, Age, Duration | H2 Blocker and Antibiotic Use, Age, Drug Type* | Additional Prescriptions† | Diagnosis | Gastrointestinal Symptoms | Non-gastrointestinal Symptoms | Primary Reason for Visit |
|---|---|---|---|---|---|---|---|
| Case (13) | 8, 100 days | 9, cephalosporin | Azithromycin, clindamycin, fluticasone proprionate, guaifenesin, sulfamethoxazole | Eosinophilic esophagitis, GERD | Chronic heartburn, cough, reflux | Coughing attacks, dizziness | Eosinophilic esophagitis |
| Case (14) | 10, 30 days 10, 30 days 11, 30 days 11, 50 days 11, 75 days 11, 502 days |
9, sulfonamide 9, cephalosporin 10, cephalosporin 9, H2blocker 10, H2 blocker 10, H2 blocker 10, H2 blocker 10, sulfonamide 11, sulfonamide 11, sulfonamide |
Sulfasalazine, erythromycin, ranitidine, valproate sodium | Diarrhea, acid reflux, GERD | Constipation, diarrhea, emesis | Weight loss, poor growth | Diarrhea, constipation, blood in stool and melena, gagging, vomiting |
| Case (15) | 10, 30 days 10, 30 days 12, 30 days |
13, cephalosporin 13, sulfonamide |
Ondansetron, clindamycin phosphate | Abdominal migraines, differential diagnosis: H pylori, PUD, IBS, GERD | Abdominal pain | None | Continued abdominal pain |
| Case (16) | 12, 10 days 12, 10 days |
12, penicillin | Amoxicillin, clarithromycin, desonide, azithromycin | Pharyngitis | Difficulty swallowing | Sore throat | Sore throat and difficulty swallowing, elevated H pylori |
| Case (17) | 14, 60 days 14, 100 days |
14, penicillin 15, H2 blocker | Venlafaxine, neomycin polymyxin HD, amoxicillin, ondansetron, sodium chloride, hydroxyzine, citalopram, polyethylene glycol 3350 | Major depression, panic disorder with agoraphobia | Constipation (severe), encopresis, abdominal pain, nausea | Weight loss, depression, anxiety | Weight loss (15 lb over 1 year), abdominal pain, constipation, encopresis |
GERD, gastroesophageal reflux disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; PUD, peptic ulcer disease; PPI, proton pump inhibitor
* During the 2 to 5 years before index date.
† Additional prescriptions within ± 6 months of PPI prescription.
Discussion
Cases were 3.6 times more likely than controls to have been prescribed PPIs in the 2 to 5 years prior to diagnosis. The results from a chart review reveal a variety of indications for the prescription of PPIs with similar indications for initiating the medication class in cases and controls. However, owing to the small number of exposed individuals, further research is needed to assess this potential association.
Patients in our study were prescribed PPIs between 25 and 60 months prior to IBD diagnosis, while the average prodromal phase of IBD in pediatric populations is thought to be 7 to 11 months for CD and 5 to 8 months for UC.24 Despite this, there is a chance that these results reflect protopathic bias, due to treatment for early indications of IBD where the diagnosis process is delayed or complex. However, the association between H2 blockers and IBD was weak, even though the indications for H2 blockers were seen to be similar to the indications for PPIs (Supplementary Table 2 (18.9KB, pdf) ). This makes it less likely that the association between PPI use and IBD is solely due to protopathic bias and suggests other possibilities.20 However, the large increase in risk during the 2 years prior to diagnosis (Supplementary Table 1 (18.6KB, pdf) ) suggests that there may be some role of prodromal IBD symptoms in the use of these medications.
Previous literature has implicated PPIs as a risk factor for several health complications, including gastrointestinal conditions such as Clostridium difficile (C difficile) infection25 and small intestinal bacterial overgrowth.26 Although, to date, no studies have investigated the role of PPIs in the development of IBD, there is evidence that PPI exposure increases the severity of disease when prescribed to adults with a history of IBD.11,27 A large cohort study conducted in Canada found that patients with IBD given a new prescription for PPIs were more likely to experience IBD treatment escalation than patients with IBD who were not prescribed PPIs.11 A nested case-control study conducted within the Veterans Health Affairs system found that PPI prescriptions were associated with an increased risk of IBD-related hospitalization in patients with IBD.27
There is a link between PPIs and intestinal dysbiosis.28,29 In an age-sex-matched cohort study, Takagi et al.29 analyzed fecal samples from 36 PPI users and 36 non-users. They found significant differences in the microbial composition of the gut, comparing users and non-users. This dysbiosis might be a mechanism by which PPIs increase risk of C difficile and small intestinal bacterial overgrowth. Given that IBD is also associated with disturbances to the gut microbiome,5,30 and that C difficile is very common among pediatric IBD patients,31 this adds plausibility to a potential association between PPIs and pediatric-onset IBD.
This study has several limitations. First, as mentioned above, the number of exposed individuals in our sample was small. This limits the conclusions that can be drawn from our results, although it is notable that there is a safety signal despite the small number of exposed children, which strongly suggests the importance of replication in additional cohorts. In particular, despite efforts to avoid it, the possibility of lengthy protopathic bias cannot be fully eliminated. This will always be a concern in diseases like IBD with long latency periods, especially when the exposure of interest is a drug that targets symptoms that may, at least in part, be similar to manifestations of early disease. However, if the disease is being misdiagnosed for such long periods in pediatric care, this is (in itself) a call for studies with improved power to document this and to improve the diagnostic process for IBD to allow for early and effective treatment.
Another limitation is the inability to capture PPI and H2 blockers that may have been taken over the counter. Thus, there may be some exposure misclassification in this study, mostly in underascertainment of medication use. However, this bias most likely would be non-differential relative to IBD case status, because it is unlikely that over-the-counter PPI or H2 blocker use differs by future disease status. It also seems unlikely that, in a population with health insurance, there would be significant self-treatment of children with these medications in the absence of medical visits or advice. The net effect of this misclassification is likely to be an attenuated measure of association by including some exposed children in the reference category and making the risk of this group more similar to that of children prescribed medication.
Pediatric IBD is a growing clinical concern that confers a substantial economic burden on families and health care systems.32,33 In the context of increasing numbers of acid-blocker prescriptions among children,8–10 understanding the adverse effects of these drugs is a public health priority. Short-term treatment of eosinophilic esophagitis and of GERD are the only 2 FDA-approved indications for PPI use in children.34 Only one-half of the children in this study who had been prescribed these drugs had symptoms consistent with these indications, highlighting the need for appropriate prescribing patterns. PPI use in childhood is also associated with allergic disease and bone loss.35,36 Our study adds to the growing body of evidence for prudent use of PPIs by providing evidence of an association between childhood use of PPIs and future IBD diagnoses. Overprescription of PPIs is not limited to pediatric populations37; thus, there is a need to investigate this association in adult populations, as well. Our results have implications for clinical treatment of children with gastrointestinal symptoms, suggesting either a safety signal for common drugs or underdiagnosis of IBD in children, and should be investigated further in a larger cohort of children.
Acknowledgment
Poster presentation at Digestive Disease Week 2018 – “Proton Pump Inhibitors (PPI), H2 Blocker Use, and Risk of Inflammatory Bowel Disease (IBD) in Children”
ABBREVIATIONS
- CD
Crohn's disease
- DRS
disease risk score
- GERD
gastroesophageal reflux disease
- H2 blockers
H2-receptor agonists
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- ICD
International Classification of Disease
- KPNC
Kaiser Permanente Northern California
- NDC
National Drug Codes
- PPI
proton pump inhibitors
- PUD
peptic ulcer disease
- SES
socioeconomic status
- UC
ulcerative colitis
Supplemental Material
DOI: 10.5863/1551-6776-24.6.489.S1; DOI: 10.5863/1551-6776-24.6.489.S2
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data and take responsibility for the integrity and accuracy of the data analysis.
REFERENCES
- 1.Benchimol EI, Fortinsky KJ, Gozdyra P et al. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17(1):423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 2.Dahlhamer J, Zammitti E, Ward B et al. Prevalence of inflammatory bowel disease among adults aged =18 years—United States, 2015. Morb Mortal Wkly Rep. 2016;65(42):1166–1169. doi: 10.15585/mmwr.mm6542a3. [DOI] [PubMed] [Google Scholar]
- 3.Heuschkel R, Salvestrini C, Beattie RM et al. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(6):839–849. doi: 10.1002/ibd.20378. [DOI] [PubMed] [Google Scholar]
- 4.Frank DN, St. Amand AL, Feldman RA et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. PNAS. 104(34):13680–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw KA, Bertha M, Hofmekler T et al. Dysbiosis, inflammation, and response to treatment: a longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Med. 2016;8(1):75. doi: 10.1186/s13073-016-0331-y. doi:10.1186/s13073-016-0331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin CM, Kim N, Kim YS et al. Impact of long-term proton pump inhibitor therapy on gut microbiota in f344 rats: pilot study. Gut Liver. 2016;10(6):896–901. doi: 10.5009/gnl15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterbini FP, Palladini A, Masucci L et al. Effects of proton pump inhibitors on the gastric mucosa-associated mi-crobiota in dyspeptic patients. Appl Environ Microbiol. 2016;82(22):6633–6644. doi: 10.1128/AEM.01437-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orenstein SR, Hassal E. Infants and proton pump inhibitors: tribulations, no trials. J Pediatr Gastroenterol Nutr. 2007;45(4):395–398. doi: 10.1097/MPG.0b013e31812e011d. [DOI] [PubMed] [Google Scholar]
- 9.Barron JJ, Tan H, Spalding J et al. Proton pump inhibitor utilization patterns in infants. J Pediatr Gastroenterol Nutr. 2007;45(4):421–427. doi: 10.1097/MPG.0b013e31812e0149. [DOI] [PubMed] [Google Scholar]
- 10.Faden HS, Ma CX. Trends in oral antibiotic, proton pump inhibitor, and histamine 2 receptor blocker prescription patterns for children compared with adults: Implications for clostridium difficile infection in the community. Clin Pediatr (Phila) 2016;55(8):712–716. doi: 10.1177/0009922815604596. [DOI] [PubMed] [Google Scholar]
- 11.Juillerat P, Schneeweiss S, Cook EF et al. Drugs that inhibit gastric acid secretion may alter the course of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36(3):239–247. doi: 10.1111/j.1365-2036.2012.05173.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Limbergen J, Russell RK, Drummond HE et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135(4):1114–1122. doi: 10.1053/j.gastro.2008.06.081. [DOI] [PubMed] [Google Scholar]
- 13.Jakobsen C, Bartek J, Jr, Wewer V et al. Differences in phenotype and disease course in adult and paediatric inflammatory bowel disease—a population-based study. Aliment Pharmacol Ther. 2011;34(10):1217–1224. doi: 10.1111/j.1365-2036.2011.04857.x. [DOI] [PubMed] [Google Scholar]
- 14.Gordon NP. How does the adult Kaiser Permanente membership in Northern California compare with the larger community? https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/comparison_kaiser_vs_non-Kaiser_adults_kpnc.pdf Accessed September 12, 2017.
- 15.Ricciuto A, Fish JR, Tomalty DE et al. Diagnostic delay in Canadian children with inflammatory bowel disease is more common in Crohn's disease and associated with decreased height. Arch Dis Child. 2018;103(4):319–326. doi: 10.1136/archdischild-2017-313060. [DOI] [PubMed] [Google Scholar]
- 16.Suissa S. Novel approaches to pharmacoepidemiology study design and statistical analysis. In: Strom BL, editor. Pharmacoepidemiology. 3rd ed. Chichester, UK: John Wiley & Sons, Ltd; 2000. pp. 383–395. [Google Scholar]
- 17.Ernster VL. Nested case-control studies. Prev Med. 1994;23(5):587–590. doi: 10.1006/pmed.1994.1093. [DOI] [PubMed] [Google Scholar]
- 18.Herrinton LJ, Liu L, Lewis JD et al. Incidence and prevalence of inflammatory bowel disease in a Northern California managed care organization, 1996–2002. Am J Gastroenterol. 2008;103(8):1998–2006. doi: 10.1111/j.1572-0241.2008.01960.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Allison James E, Herrinton Lisa J. Validity of computerized diagnoses, procedures, and drugs for inflammatory bowel disease in a Northern California managed care organization. Pharmacoepidemiol Drug Saf. 2009;18(11):1086–1093. doi: 10.1002/pds.1824. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz RI, Feinstein AR. The problem of “protopathic bias” in case-control studies. Am J Med. 1980;68(2):255–258. doi: 10.1016/0002-9343(80)90363-0. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Census Bureau 2016 American Community Survey 5-Year Estimates. https://www.census.gov/data/developers/data-sets/acs-5year.2016.html Accessed September 28, 2017.
- 22.Arbogast PG, Seeger JD, DEcIDE Methods Center Summary Variable Working Group Summary Variables in Observational Research: Propensity Scores and Disease Risk Scores. Rockville, Maryland: Agency for Healthcare Research and Quality; 2012. [Google Scholar]
- 23.Arbogast PG, Ray WA. Use of disease risk scores in pharmacoepidemiologic studies. Stat Methods Med Res. 2009;18(1):67–80. doi: 10.1177/0962280208092347. [DOI] [PubMed] [Google Scholar]
- 24.Mamula P, Markowitz JE, Baldassano RN. Inflammatory bowel disease in early childhood and adolescence: special considerations. Gastroenterol Clin North Am. 2003;32(3):967–995. viii. doi: 10.1016/s0889-8553(03)00046-3. [DOI] [PubMed] [Google Scholar]
- 25.Dial S, Delaney JaC, Barkun AN et al. Use of gastric acid–suppressive agents and the risk of community-acquired Clostridium difficile–associated disease. JAMA. 2005;294(23):2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 26.Su T, Lai S, Lee A et al. Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol. 2018;53(1):27–36. doi: 10.1007/s00535-017-1371-9. [DOI] [PubMed] [Google Scholar]
- 27.Shah R, Richardson P, Yu H et al. Gastric acid suppression is associated with an increased risk of adverse outcomes in inflammatory bowel disease. Digestion. 2017;95(3):188–193. doi: 10.1159/000455008. [DOI] [PubMed] [Google Scholar]
- 28.Naito Y, Kashiwagi K, Takagi T et al. Intestinal dysbiosis secondary to proton-pump inhibitor use. Digestion. 2018;97(2):195–204. doi: 10.1159/000481813. [DOI] [PubMed] [Google Scholar]
- 29.Takagi T, Naito Y, Inoue R et al. The influence of long-term use of proton pump inhibitors on the gut microbiota: an age-sex-matched case-control study. J Clin Biochem Nutr. 2018;62(1):100–105. doi: 10.3164/jcbn.17-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halfvarson J, Brislawn CJ, Lamendella R et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2 doi: 10.1038/nmicrobiol.2017.4. 17004. doi:10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hourigan SK, Oliva-Hemker M, Hutfless S. The prevalence of clostridium difficile infection in pediatric and adult patients with inflammatory bowel disease. Dig Dis Sci. 2014;59(9):2222–2227. doi: 10.1007/s10620-014-3169-4. [DOI] [PubMed] [Google Scholar]
- 32.Heaton PC, Tundia NL, Schmidt N et al. National burden of pediatric hospitalizations for inflammatory bowel disease: results from the 2006 kids' inpatient database. J Pediatr Gastroenterol Nutr. 2012;54(4):477–485. doi: 10.1097/MPG.0b013e318239bc79. [DOI] [PubMed] [Google Scholar]
- 33.Sin AT, Damman JL, Ziring DA et al. Out-of-pocket cost burden in pediatric inflammatory bowel disease: a cross-sectional cohort analysis. Inflamm Bowel Dis. 2015;21(6):1368–1377. doi: 10.1097/MIB.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proton pump inhibitors: use in pediatric patients. https://www.cms.gov/Medicare-Medicaid-Coordination/Fraud-Prevention/Medicaid-Integrity-Education/Pharmacy-Education-Materials/Downloads/ppi-pediatric-factsheet11-14.pdf Accessed September 12, 2017.
- 35.Freedberg DE, Haynes K, Denburg MR et al. Use of proton pump inhibitors is associated with fractures in young adults: a population-based study. Osteoporos Int. 2015;26(10):2501–2507. doi: 10.1007/s00198-015-3168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitre E, Susi A, Kropp LE et al. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. 2018 doi: 10.1001/jamapediatrics.2018.0315. e180315. doi:10.1001/jamapediatrics.2018.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savarino V, Dulbecco P, De Bortoli N et al. The appropriate use of proton pump inhibitors (ppis): need for a reappraisal. Eur J Intern Med. 2017;37:19–24. doi: 10.1016/j.ejim.2016.10.007. [DOI] [PubMed] [Google Scholar]


