Abstract
Endocytosis is a prominent clathrin-mediated mechanism for concentrated uptake and internalization of ligand-receptor complexes, also known as cargo. Internalization of cargo is the fundamental mechanism for receptor-dependent regulation of cell membrane function, intracellular signal transduction, and neurotransmission, as well as other biological and physiological activities. However, the intrinsic mechanisms of receptor endocytosis and contemporaneous intracellular signaling are not well understood. We review emerging concepts of receptor endocytosis with concurrent intracellular signaling, using a typical example of guanylyl cyclase/natriuretic peptide receptor-A (NPRA) internalization, subcellular trafficking, and simultaneous generation of second-messenger cGMP and signaling in intact cells. We highlight the role of short-signal motifs located in the carboxyl-terminal regions of membrane receptors during their internalization and subsequent receptor trafficking in organelles that are not traditionally studied in this context, including nuclei and mitochondria. This review sheds light on the importance of future investigations of receptor endocytosis and trafficking in live cells and intact animals in vivo in physiological context.
Keywords: Membrane receptors, internalization, intracellular signaling, subcellular trafficking, short-signal motifs, guanylyl cyclase/natriuretic peptide receptor
1. Introduction
There are at least two types of cardiac-derived peptide hormones, namely atrial and brain natriuretic factor or peptides (ANF/ANP, BNP), which mediate natriuretic, diuretic, vasorelaxant, and antimitogenic responses directed to reduce blood pressure and maintain fluid volume homeostasis [1–7]. Both ANP and BNP are primarily synthesized in the heart atrial myocytes and to a lesser extent in the ventricular cells [8–10]. Pro-ANP, but not ANP, is stored in the atrial dense granules [11–13]. ANP is largely released as a 28-amino acid residues biologically active mature hormone, whereas BNP is released as a prohormone (Pro-BNP). Upon secretion, Pro-BNP is enzymatically cleaved to produce biologically active 32-residues BNP [11–13]. It is unclear whether BNP or Pro-BNP is stored in those granules, which contain ANP. A third peptide in the natriuretic peptide hormone family, differs from ANP and BNP in the tissue expression and biological function, is known as C-Type natriuretic peptide (CNP), which is highly conserved among species, is basically present in endothelial cells and the central nervous system [14, 15]. The receptors of these peptide hormones have been classified as natriuretic peptide receptor-A (NPRA), -B (NPRB), -C (NPRC), and ANF-RGC [16–24]. NPRA and NPRB show similar homology and contain an extracellular ligand-binding domain, a single transmembrane region, and an intracellular region containing both a protein kinase-like homology domain (protein-KHD) and guanylyl cyclase (GC) catalytic domain [16, 17, 20, 23–25]. As shown in Figure 1, both ANP and BNP specifically bind and activate NPRA that produces intracellular second-messenger cGMP in response to hormone binding in various cells and tissues [2, 26–29]. BNP is also considered as a neuropeptide hormone, which is expressed in a subset of transient receptor potential vanilloid-1 (TRPV1) neurons. A study showed that Nppb−/− mice selectively lost almost all behavioral responses to itch-inducing agents [30]. CNP activates NPRB that also produces intracellular cGMP [17, 24, 28, 29]. Moreover, all three natriuretic peptides arbitrarily bind to NPRC, which lacks a GC domain and does not produce cGMP [5, 22, 28, 31]. GC-A/NPRA is considered to be the biologically active cognate receptor of both ANP and BNP. Because most of the physiological effects of GC-A/NPRA are triggered by generating second-messenger cGMP, this receptor is generally considered the biologically active cognate receptors of ANP and BNP [5, 19, 29, 32–35].
Figure 1. Diagrammatic representation of ligand specificity, transmembrane assembly, and the intracellular signaling system of natriuretic peptide receptors, namely NPRA, NPRB, and NPRC:
The extracellular and intracellular domains of these receptors are shown in different colors. Extracellular LBD and intracellular protein-KHD and GCD domains of NPRA and NPRB are indicated with receptor topology. The extracellular domain and short intracellular tail of NPRC are differentially shown. NPRA and NPRB are represented to bind ATP in the intracellular KHD region of the receptors and to generate second-messenger cGMP from the hydrolytic conversion of GTP to cGMP. The increased level of cGMP activates three known effector molecules: cGMP-dependent PKG, cGMP-gated CNGs, and cGMP-activated PDEs. The activation of PKGs, CNGs, and PDEs elicits natriuretic peptide-dependent physiological responses, including natriuresis/diuresis, vasorelaxation, anti-inflammation, antifibrosis, anti-mitogenesis, and anti-hypertrophy. ANP/BNP binding to NPRC has been shown to decrease cAMP and to increase IP3 in target cells. ANP, atrial natriuretic peptide; ATP, adenosine triphosphate; BNP, brain natriuretic peptide; CNG, cyclic nucleotide-gated ion channel; CNP, C-type natriuretic peptide; GCB, guanylyl cyclase catalytic domain; GTP, guanosine trisphosphate; IP3, inositol trisphosphate; KHD, kinase homology domain; LBD, ligand-binding domain; NP, natriuretic peptide; PDE, phosphodiesterase; PKG, protein kinase G; TM, transmembrane domain; NPRA, natriuretic peptide receptor-A; NPRB, natriuretic peptide receptor B; NPRC, natriuretic peptide receptor C.
The biological function of NPRA is demonstrated primarily through the ANP/BNP-dependent GC catalytic activity of the receptor and the production of cGMP, which is regulated by several factors, including hormones, growth factors, physiological milieu, and the ligand itself [26, 34, 36–42]. Several of our studies have established that the gene targeting (gene-disruption and gene-duplication) of Npr1 (encoding NPRA) in mouse models shows the hallmark significance of NPRA signaling in lowering arterial pressure and protecting against renal, cardiac, and vascular diseases [43–48]. More recent evidences suggest that reduced levels of ANP seem to be associated with insulin resistance, obesity, lifestyle-associated metabolic syndrome, and essential hypertension [10, 49–51]. In addition, ANP/NPRA system has been suggested to play role in vascular remodeling of uterine spiral artery [52]. Previous studies have suggested that endothelial action of ANP enhances the myocardial inflammatory infiltration in early phase after acute infarction [53]. Further, ANP has been shown to exhibit anti-inflammatory properties and tumor-associated immune response [54, 55]. Moreover, the synthetic novel designer natriuretic peptides have provided the renal enhancing actions of these hormones over the naturally occurring molecules [56–59].
NPRA is designated as the cognate receptor for both ANP and BNP; nevertheless, ANP binds to the receptor with a higher affinity than BNP [60]. However, it is not yet clear whether ANP and BNP complete each other for the receptor binding. The ligand binding of both NPRA and NPRB has demonstrated that bound ligand-receptor complexes of NPRA are promptly internalized into cells, redistributed into intracellular compartments, and ultimately degraded in lysosomes [35, 40, 61–64]. Ligand-receptor complexes of ANP-NPRA are distributed, possibly through the endosomes, to lysosomal compartments, where they are largely metabolized; however, a population of ligand-receptor complexes escapes the lysosomal compartments, allowing these receptors to recycle back to the plasma membrane [26, 34, 42, 62]. Moreover, internalization of ANP, BNP, and CNP also occurs through NPRC [65, 66]. Virtually, the ligand-mediated receptor endocytosis is a vital mechanism for physiological responses. Clathrin-mediated endocytosis is a well-established mechanism for numerous membrane-bound hormone receptors containing glucagon, insulin, platelet-derived growth factor (PDGF), and epidermal growth factor (EGF), which are also internalized by ligand-mediated endocytosis [35, 67, 68]. Our recent studies have demonstrated that NPRA is dynamin-dependently endocytosed in clathrin-coated vesicles [69]. Here, we provide a comprehensive summary of past and present findings on the internalization, intracellular trafficking, and concurrent signaling mechanisms of NPRA focused on cell-based studies as well as in intact live cells and animals in vivo to establish the actions of NPRA in the dynamic physiological context.
2. Historical background
Several studies have clearly established that ANP-BNP/NPRA ligand-receptor complexes are internalized in various cell types upon stimulation by ligand. However, the question of whether NPRA is internalized remained open for a long time. For example, earlier it has been stated that neither NPRA nor NPRB ligand-receptor complexes are internalized and that only NPRC receptor is internalized to downregulate the cGMP signaling process [70]. Although remarkable progress has been made toward determining the structure-function relationship of NPRA and NPRB, the issues of internalization and the trafficking itinerary of these GC receptors remained controversial until recently. In the past, debate focused on whether ANP/NPRA complexes were internalized at all or whether cells used some other mechanisms to cell-specifically release ANP from its receptor. Indeed, it was reported by default that among the three natriuretic peptide receptors, only NPRC was internalized with bound ligand [71]. It was suggested that endogenous NPRA was not internalized in cultured renal medullary interstitial cells and that rapid dissociation of ligand-receptor complexes occurred after ANP binding to NPRA at 370C. However, it was difficult to interpret the results of such findings because the dissociation of ligand was carried out in a medium containing high concentrations of unlabeled ANP to preclude the rebinding of dissociated hormone to receptors [71]. One study using this ligand-binding protocol indicated that intact 125I-ANP was released into the culture medium of 293 T cells expressing recombinant NPRA [72]. However, in a later study, the authors reconciled the absence of ANP/NPRA internalization in 293 T cells, stating that it might have resulted from the slow rate of cell-specific ANP degradation in these cells [73].
Multiple studies from our laboratory and others, which have used stoichiometric kinetic analyses of ANP/NPRA, have provided strong evidence that bound ligand-receptor complexes are internalized and processed intracellularly, and that degraded products are released into the culture medium of various cell types harboring endogenous receptors [34, 61, 63, 64, 74], The same events have been demonstrated in transfected COS-7 and HEK-293 cells expressing recombinant NPRA [62, 75, 76]. Moreover, in response to CNP-binding, NPRB has been shown to be internalized and recycled back to the cell surface in cultured glioma cells and hippocampus neurons [77]. The prevailing consensus now is that, in response to ligand binding, both NPRA and NPRB are internalized into the cell interior and redistributed in subcellular compartments. To obtain unequivocal evidence, it became important to visualize cell trafficking and redistribution of ANP/NPRA complexes in intact cells. Recent findings from our laboratory have resolved this important issue, unequivocally demonstrating ligand-dependent endocytosis and intracellular trafficking of NPRA by confocal immunofluorescence (IF) and co-immunoprecipitation (Co-IP) analyses with organelle-specific marker proteins in eGFP/NPRA-transfected HEK-293 cells and primary MMCs [26, 42]. Our findings have facilitated direct visualization of ligand-dependent endocytosis, trafficking, and redistribution of eGFP-NPRA into subcellular compartments with concurrent production of intracellular second-messenger cGMP. Earlier we have also demonstrated the kinetics of 125I-ANP-NPRA internalization in recombinant Cos-7 and HEK-293 cells [34, 75]. However, in recent studies, we examined immunofluorescence-based NPRA internalization in MMCs, a cell system with physiological relevance [26]. We used eGFP-NPRA to capture immunofluorescence images generated by this receptor protein only during the processes of endocytosis and trafficking, without any interference from either NPRB or NPRC. Also, since ANP binds to both NPRA and NPRC, we have used c-ANF to block ANP binding to NPRC in the 125I-ANP binding assay. The peptide c-ANF binds only to NPRC, not to either NPRA nor NPRB. Thus, all the binding parameter for ligand-dependent internalization of ligand-receptor complexes were accounted due to NPRA as previously reported [78].
The mechanisms of NPRA signaling during the glycosylation of NPRA is not well understood. Previously, it was suggested that glycosylation sites in guanylyl cyclase-coupled receptors might be important for proper folding and stability of the receptor protein, as well as essential to facilitate ligand binding of NPRA and NPRB [18, 79, 80]. However, one study has suggested that glycosylation is not essential for ligand-binding of NPRA [81]. More experimentations are needed to confirm the functional role of glycosylation in the ligand binding and trafficking of both NPRA and NPRB GC-coupled receptors.
3. Ligand-stimulated internalization of NPRA
After ANP binding, NPRA dimerizes, while the GC catalytic domain of the receptor becomes activated [82]. Studies have shown that after ANP binding, ligand-receptor complexes are internalized, redistributed in the cell interior, and metabolized in lysosomes [40, 61, 63, 64, 74]. It has been suggested that internalized receptor traffic into intracellular compartments, as well as a population of receptor, escapes the lysosomal compartments and recycles back to plasma membranes [34, 35, 42, 61, 75, 83]. Our classical studies using Leydig tumor (MA-10) cells containing a high density of endogenous NPRA and recombinant human embryonic kidney-293 (HEK-293), as well as COS-7 cells transfected with NPRA cDNA construct, firmly established that ligand-receptor complexes of ANP-NPRA are rapidly and ligand-dependently internalized and redistributed intracellularly [61, 84, 85]. Those findings demonstrated that after ligand-binding NPRA is internalized with bound hormone by an energy- and temperature-dependent mechanism, after which degraded ligand is released into the culture medium [34, 42, 61, 62, 86].
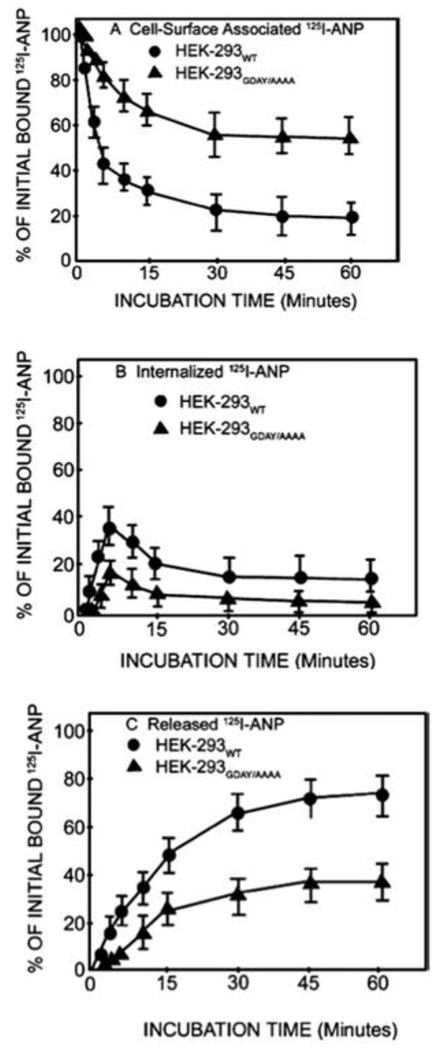
Distribution of 125Iodine-labeled-ANP (125I-ANP) radioactivity on the cell surface, in the intracellular compartments, and in culture medium established a dynamic equilibrium of receptor-mediated 125I-ANP uptake, degradation, and release outside of cells (Fig. 2). A major proportion of internalized 125I-ANP was released into the culture medium, which consisted of approximately 75%-80% degraded products and about 20%-30% intact ligand [26, 34, 42, 61, 62]. The release of both degraded and intact ligands was prevented by introducing the lysosomotropic agents such as ammonium chloride (NH4Cl2), chloroquine, or nigericin in the culture media [40, 42, 61]. Early work suggested that most of the internalized 125I-ANP was metabolized through the pathways of lysosomal degradative compartments in intact cells; however, a population of internalized ligand-receptor complexes recycled back to the plasma membrane and ligand was extruded outside the cell (Fig. 3). It is believed that the released intact ligand could rebind to the receptor and re-enter the cell by the pathway of the retro-endocytosis mechanism [35, 61, 84].
Figure 2. Quantitative analyses of cell-surface-associated, internalized, and released 125I-ANP radioactivity in recombinant HEK-293 cells expressing wild-type or GDAY/AAAA mutant receptors:
Confluent HEK-293 cells expressing wild-type or mutant receptors were allowed to bind 125I-ANP at 4 0C for 1 h. Cells were washed four times with 2 ml of assay medium to remove unbound ANP, then reincubated in fresh medium at 37 °C. At the indicated time points, cells were placed on ice at 4 0C and media were collected. Cell-surface-associated (acid-sensitive) radioactivity was eluted with glycine acidic buffer (pH 3.8) and cells were dissolved in 1 M NaOH to determine the internalized (acid-resistant) radioactivity. Cell-surface-associated (A), internalized (B), and released (C) 125I-ANP radioactivity was determined in the acid eluate, cell extract, and culture medium. Figure adapted from Pandey et. al., 2005 with permission from Biochemical Journal.
Figure 3. Immunofluorescence localization of cGMP and schematic representation of internalization, intracellular trafficking, and signaling pathway of NPRA in primary MMCs:
Immunofluorescence localization of cGMP was done at 48 h after transfection of cells with eGFP-NPRA-WT and mutated motif FQQI/AAAA. To inhibit the endogenous expression of NPRA, cells had been transiently transfected with pcDNA-6.2- GW/GFP-miR expression vector containing Npr1-miRNA insert pCMVNpr1miRNA-1595 and pCMV-Npr1miRNA-2931. The cells were treated with 100 nM ANP for 10 min at 37°C in the presence of IBMX. A: After treatment with ANP for 10 min, eGFP-NPRA-WT showed significantly more diffused fluorescence intensity in the cytoplasm than did mutated motif FQQI/AAAA. B: To assay the stimulation of intracellular accumulation of cGMP, cells were transiently transfected with eGFP-NPRA-WT and mutated motif FQQI/AAAA. Intracellular accumulation of cGMP through eGFP-NPRA-WT and mutated motif FQQI/AAAA was quantitated. cGMP assay was performed to determine the concurrent generation of cGMP during receptor internalization and trafficking. Cells were treated with 100 nM ANP for 5, 10, 15 or 30 min in the presence of 0.2 mM IBMX. Cells were washed three times with PBS and scraped in 0.1 M HCl. Cell suspensions were subjected to five cycles of freeze and thaw and then centrifuged at 10 000 x g for 15 min at 4 ◦ C. The supernatant was collected for cGMP assay using direct cGMP complete ELISA kit (Arbor Assay, Ann Arbor, MI) according to the manufacturer’s protocol. C: Scheme depicting the sequential events of internalization, trafficking, recycling, and degradation of ligand-receptor complexes in intracellular compartments. After ANP-binding to NPRA, ligand-receptor complexes entered cells via clathrin-coated pits. Ligand-bound receptor complex trafficked intracellularly through endosomes, lysosomes, and a population of receptor recycled back to the plasma membrane through the recycling endosomes, with concurrent generation of intracellular cGMP. Sorting of bound ANP-NPRA complex occurs by endosomal dissociation metabolic and lysosomal degradative pathways. On the other hand, mutation of FQQI residues to AAAA (FQQI/AAAA) significantly inhibited receptor internalization and intracellular trafficking. Note that the multivesicular body formation likely places the receptor in the lumen of the lysosomes. IBMX, 3-isobutyl-1-methylxanthine; ECD, extracellular domain; KHD, kinase homology domain; GCD, guanylyl cyclase domain. Figure adapted from Mani et. al., 2016 with permission from American Journal of Physiology-Renal Physiol.
Although, after internalization, most of the endocytosed ligand was degraded in the lysosomes and then released into the culture medium, a population of ligand-receptor complexes escaped the lysosomal degradative pathway and extruded intact outside of cells [34, 62, 87]. By using an antibody-tracking method, one study indicated that, in a cell-specific manner, both NPRA and NPRB are ligand-independently internalized [70]. However, antibody-tracking could determine the internalization kinetics of ligand-receptor complexes only qualitatively. Our recent studies using confocal immunofluorescence microscopy clearly visualized and unequivocally established that ligand-dependent internalization and subcellular trafficking of eGFP-tagged-NPRA (eGFP-NPRA) occurs in stably transfected recombinant HEK-293 cells and transiently NPRA-transfected primary murine mesangial cells (MMCs) [26, 42]. Furthermore, NPRB has been shown, in response to CNP binding, to be internalized and recycled back to the cell surface in cultured hippocampal neurons and C6 glioma cells [77].
4. Role of clathrin adaptor proteins in the endocytosis of NPRA
It is thought that the tyrosine-based Yxxphi sorting signals located in the carboxyl-terminus domain direct the internalization and trafficking of various membrane receptors by interacting with mu 1, mu 2, mu 3, and mu 4 subunits of adaptor proteins (APs), including, respectively, AP-1, AP-2, AP-3, and AP-4 [26, 88, 89]. Recent studies have demonstrated the role of adaptor protein 2 in endocytosis of insulin-like growth factor-I receptor (IGF-IR), which interacts with insulin-receptor substrate (IRS)-1 with the clathrin adaptor complex AP2 [90]. However, IRS-1 inhibits recruitment of IGF-IR into clathrin-coated structures; for this reason, IGF-IR avoids rapid endocytosis and prolongs its activity on the cell surface. Beta-arrestins are known to act as endocytic adaptors by recruiting the AP-2 complex to G-protein-coupled receptors (GPCRs), linking them to clathrin-coated pits (CCPs) for internalization [91]. Upon activation by Wnt, the Frizzled receptor is internalized in a process that requires the recruitment of Dishevelled, a family of proteins involved in the Wnt signaling pathways [92]. Interaction between Dishevelled2 (Dvl2) and μ2-adaptin, a subunit of the clathrin adaptor AP-2, is required to engage activated Frizzled4 with the endocytic machinery for its internalization.
Our recent studies have shown that the μ1B subunit of AP-1 directly binds to a phenylalanine-based FQQI motif in the cytoplasmic tail of NPRA receptor protein [26]. However, more studies are needed to establish the exact role of adapter proteins in the endocytotic pathways of NPRA. To delineate the critical role of endocytic signals in the intracellular sorting and signaling of NPRA, immunofluorescence staining and Co-IP of eGFP-NPRA with the AP-1 marker have been used to follow the intracellular trafficking and concurrent signaling of mutated and WT receptors by confocal immunofluorescence microscopy and immunoblotting in MMCs (Fig. 3). Subcellular trafficking of receptor has shown that the immunofluorescence colocalization of mutated receptors with μ1B markers was decreased by 50% for the subunit of AP-1 as compared with the WT receptor in intact MMCs [26]. It has been shown that the autosomal recessive hypercholesterolemia protein (ARH) is important in clathrin-mediated endocytosis of low-density lipoprotein receptors (LDLRs) [93]. In addition to its role in endocytosis, ARH unites with AP-1B in basolateral exocytosis of LDLR from recycling endosomes (REs) to plasma membrane.
5. Role of dynamin in NPRA endocytosis
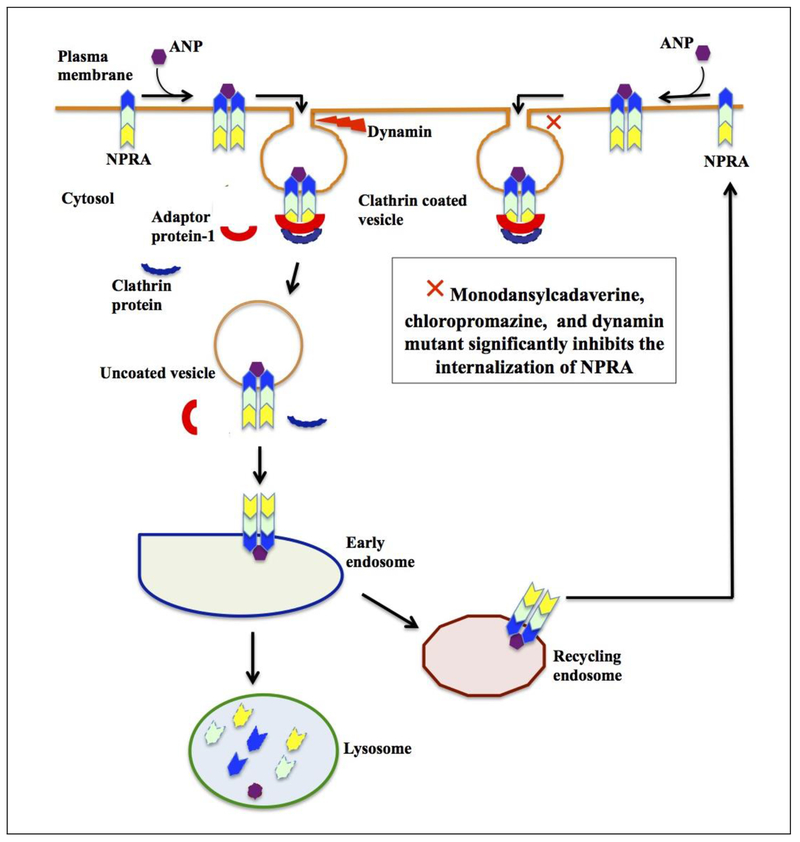
In recent studies using 125I-ANP binding assay and confocal microscopy, we have examined the role of dynamin in the internalization and trafficking of NPRA in a stably transfected HEK-293 cells [69]. Our findings indicated that ANP treatment of high-density NPRA expressing recombinant HEK-293 cells time-dependently accelerates internalization of receptor from the plasma membrane to the cell interior. However, the internalization of ligand-receptor complexes of NPRA was significantly decreased by specific inhibitors of clathrin- and dynamin-dependent receptor internalization. These decreases were approximately 80%-90% using monodansylcadaverine (MDC), 75%-80% using chlorpromazine (CPZ), and 85%-90% using mutant dynamin, which are specific blockers of endocytic vesicle formation and internalization pathways (Fig. 4). Moreover, IF visualization of the internalization of NPRA and enhanced GFP-tagged NPRA in HEK-293 cells by confocal microscopy have demonstrated the formation of endocytic vesicles after 5 min of ANP treatment; this effect was blocked by clathrin inhibitors (MDC and CPZ) and mutant dynamin construct [69]. These studies showed that NPRA undergoes internalization via clathrin-mediated endocytosis as part of its normal cellular itinerary, including receptor trafficking, signaling, and metabolic degradation.
Figure 4. Schematic representation of clathrin-dependent endocytosis of NPRA in recombinant HEK-293 cells:
This scheme describes the sequential events of internalization, trafficking, recycling, and degradation of ligand-receptor complexes in intracellular compartments. After binding of ANP to NPRA, ligand–receptor complexes enter the cell via clathrin-coated pits with the help of dynamin GTPase. The ligand-bound receptor complex is endocytosed and traffics intracellularly through the endosomes and lysosomes. A population of receptor recycles back to the plasma membrane through the recycling endosomes. On the other hand, with the expression of mutant dynamin and the blockers of dynamin GTPase (monodansylcadaverine, chlorpromazine) inhibiting clathrin-coated vesicle formation, the internalization of NPRA was significantly restricted. Figure adapted from Somanna et. al., 2018 with permission from Molecular and Cellular Biochemistry.
Our earlier findings suggested that ANP-mediated internalization of NPRA in the recombinant HEK-293 cells occurred via the clathrin-dependent pathway and involved initial clustering of receptor-ligand cargo in clathrin-coated pits, with plasma membrane invagination forming clathrin-coated vesicles. Blockade of ligand-induced endocytosis of the receptor by MDC and CPZ is the most effective means of the mechanistic action that inhibits the receptor internalization. Confocal microscopy clearly showed the formation of endocytic vesicles, which is a characteristic feature of internalized receptor within the cytoplasm. Several previous studies have shown that both CPZ and MDC, as well as mutant dynamin, have effectively blocked endocytosis of the membrane receptors and affected both the assembly of clathrin components and the formation of clathrin-coated pits in cells of various types [94–98]. The classical clathrin-mediated endocytic pathway clearly seems to be a major route for the endocytosis of NPRA. Demonstration of this provides a significant advance in our understanding of the role of this receptor in regulating hypertension and cardiovascular homeostasis.
In recent studies, we have found that MDC and CPZ effectively blocked the internalization of ANP-NPRA complexes in recombinant HEK-293 cells by approximately 80%-90% [69]. MDC acts as a competitive blocker of transglutaminase by creating an isopeptide bond between the glutamine and lysine amino-acid residues of two proteins, thus inhibiting the clathrin-coated vesicle-dependent endocytic process [96, 99, 100]. Earlier findings suggested that MDC blocks the internalization and trafficking of various types of ligand-receptor complexes [101–103]. Similarly, CPZ has been shown to block the assembly of clathrin-coated pits and to inhibit coated-vesicle-mediated internalization of membrane proteins [104–106]. We demonstrated that CPZ inhibits endocytosis of ANP-NPRA complexes by 80%-85%, which is consistent with the previous finding that CPZ inhibits the endocytosis of several different types of hormone receptors [106–109]. CPZ, MDC, and dynamin-mutant have also been shown to play inhibitory roles for endocytosis in Wnt signaling [110].
The expression of a dominant-negative mutant of dynamin (K44A/dynamin) has been shown to block the formation of coated vesicles during the internalization of various ligand-receptor complexes [67, 111–113]. Previous studies have also indicated that dynamin, through its interaction with AP2, has a critical role in the localization of clathrin-coated pits and clathrin-mediated endocytosis [114, 115]. After recruitment to coated pits, dynamin forms a ring structure, and then pinches off coated vesicles into the cytoplasm. This process, known as pinchase, through its intrinsic GTPase activity, generates clathrin-coated vesicles in the clathrin-mediated endocytosis [105, 116–118]. It is thought that the GTPase-defective dominant mutant interferes with wild-type dynamin and blocks the formation of endocytic coated-vesicles and internalization of membrane receptors [119–123].
The endocytic inhibitors MDC and CPZ and dynamin GTPase blocker (dynamin mutant) have been shown to significantly decrease the intracellular accumulation of cGMP. Moreover, earlier reports suggested that G-protein-coupled receptor (GPCRs) continue to signal by generating cAMP throughout internalization processes with their bound ligands [124]. Signaling from inside the cell is persistent and appears to trigger specific downstream effects. We have recently demonstrated that intracellular accumulation of cGMP significantly decreases after treatment with endocytic inhibitors MDC and CPZ and transfection with dynamin mutant GTPase blocker [69]. Diminution in the intracellular accumulation of cGMP indicates that these same endocytic inhibitors and dynamin GTPase blocker (dynamin mutant) have a concerted regulatory role in NPRA trafficking and signaling process.
6. Post-endocytic sorting of NPRA
Recently we visualized the internalization and subcellular trafficking of enhanced GFP (eGFP)-tagged NPRA (eGFP–NPRA) in intact recombinant HEK-293 cells and primary MMCs, using immunofluorescence and co-IP analyses of eGFP–NPRA [26, 42]. These studies showed that treatment of cells with ANP initiated rapid internalization and co-localization of the receptor with early endosome antigen-1 (EEA-1), which was increased in a time-dependent manner. The highest visualization, which occurred at 5 min, gradually decreased within 30 min. Similarly, co-localization of the receptor was time-dependently accomplished with lysosome-associated membrane protein-1 (LAMP-1) in MMCs [26]. These immunofluorescence and co-IP studies demonstrated that after trafficking in the endosomes, eGFP-NPRA routed intracellularly within the late endosomes and/or lysosomes. The accumulation of eGFP–NPRA in intracellular compartments was observed after treatment of MMCs with lysosomotropic agents such as chloroquine and NH4Cl2. In ANP-treated cells, eGFP–NPRA was time-dependently co-localized with LAMP-1; however, after treatment with lysosomotropic agents, intracellular accumulation of the receptor gradually increased within 30 min. It is known that both chloroquine and NH4Cl2 inhibit lysosomal degradation of intracellular trafficking of the various ligand-receptor complexes [34, 35, 40, 125]. Moreover, lysosomotropic agents did not completely block the receptor degradation of ANP/NPRA complexes in lysosomes, suggesting that the trafficking of intact eGFP–NPRA also occurred through a lysosome-independent pathway. In our studies, co-IP assays confirmed that the localization of internalized NPRA occurred within subcellular organelles during endocytosis of eGFP-NPRA [42]. The endocytic recycling marker Rab 11, which was used as a recycling endosome, showed that ∼20 % of receptors recycled back to the plasma membrane [26, 42]. Our results support the notion that ANP-treated cells exhibit a marked increase in the IF of cGMP, whereas receptor still trafficked into intracellular compartments. This suggests that after ligand binding, NPRA is rapidly internalized and trafficked from the cell surface into endosomes, including recycling endosomes, late endosomes, and lysosomes, with concurrent generation of intracellular second-messenger cGMP.
On the other hand, subcellular trafficking of NPRA indicated that IF of the mutated receptor with EEA-1, LAMP-1, and Rab 11 markers was decreased by almost 50%-58% in early endosomes, 45%-50% in lysosomes, and 35%-45% in recycling endosomes, respectively, compared with WT NPRA in MMCs and HEK-293 cells [26, 42]. To support the findings of immunofluorescence-based internalization kinetics and cell trafficking of WT and mutated eGFP-NPRA in intact MMCs, we have used the 125I-ANP binding assay to examine cell-surface and intracellular ligand-receptor complexes [26]. These studies demonstrated that 125I-ANP binds to cell-surface eGFP-NPRA, enters through the process of receptor-mediated endocytosis, and then is delivered to intracellular compartments until it reaches a steady-state level, which occurs after 15-30 min. The kinetic rates of internalization, degradation, and release of 125I-ANP were markedly decreased in the mutant receptor, suggesting that the FQQI motif is important for the internalization and subcellular trafficking itinerary of eGFP-NPRA in intact cells [26]. Thus, it seems that homeostatic regulation of native NPRA and the cell sensitivity of ANP depends on a dynamic equilibrium and reuse of ligand-receptor complexes from the cell surface to intracellular compartments.
Moreover, we have demonstrated that internalization and subcellular trafficking of NPRA, using IF staining (IFS) and co-IP of plasma membrane, endosomal, lysosomal, and recycling endosome markers to follow intracellular trafficking and signaling by confocal IF microscopy (CIF) and immunoblotting (IB) analyses in recombinant HEK-293 cells [42]. These functional subcellular compartments provide the physical basis for the assembly and turnover of intracellular kinetics of ligand-receptor complexes, which in turn define the specialized endosomal–lysosomal signaling platforms for hormone-dependent physiological functions of membrane receptors and other membrane proteins.
7. Role of short-signal motif in the internalization and trafficking of NPRA:
Co-immunoprecipitation assays confirmed that colocalization of the mutant receptor (FQQI/AAAA) was significantly decreased in subcellular compartments during endocytosis and trafficking processes [26]. These earlier studies showed that the FQQI signal sequence motif is critically essential for the internalization and subcellular trafficking of NPRA during the hormone signaling process in intact primary MMCs [26]. The confocal immunofluorescence studies also showed that, after ligand binding, WT receptor eGFP-NPRA is rapidly internalized and trafficked from the cell surface to subcellular compartments. However, the mutated motif FQQI/AAAA significantly inhibited internalization, subcellular trafficking, and signaling processes of NPRA receptor protein [26]. These studies also demonstrated that FQQI has a significant role in sustaining receptor signaling in intact MMCs and thus advanced our understanding of the molecular mechanisms of vesicular transport, subcellular trafficking, and concurrent NPRA signaling. Similarly, another mutation in the GDAY motif (GDAY/AAAA) in the NPRA carboxyl-terminal domain has been shown to reduce internalization of mutant receptors by almost 40%-45% as compared with the WT receptor [62].
Small signal-sequence motifs have been shown to play pivotal roles in endocytosis and intracellular trafficking of membrane receptor proteins to direct cargo into trafficking vesicles [126, 127]. Short signal sequence motifs constitute the short sequence of amino acids in a linear array that consists of two-to-six amino-acid residues. Nevertheless, only two or three residues have critical roles in receptor endocytosis and trafficking pathways [126, 128, 129]. The most common small-signal sequence motifs found in various membrane receptors are shown in Table 1. Tetrameric sequence Gly920-Asp921-Ala922-Tyr923 (GDAY) motif in the carboxyl terminal-region of NPRA serves as a signal for endocytosis [62]. The amino acid residues Gly920 and Tyr923 constitute the critical elements for internalization of NPRA in the GDAY signal motif, however, Asp921 exhibits an acidic environment for efficient signaling of GDAY during the subcellular trafficking process. The mutation of Gly920 and Tyr923 to Ala prevented the internalization of NPRA by almost 50%, but had no effect on the recycling process. Although, the mutation of Asp921 to Ala did not exhibit a major effect on receptor endocytosis, but critically attenuated the recycling of internalized receptors to the plasma membrane.
Table 1.
A list of important short-sequence signal motifs for the internalization and trafficking of membrane receptors and proteins.
| Membrane Receptor/Protein | Signal Motifs | Targeted Pathways | Reference |
|---|---|---|---|
| Membrane Receptors: | |||
| CD-Mannose-6-phosphate receptor | YKYSKV | Endocytosis/lysosomal targeting | [185] |
| CI-Mannose-6-phosphate receptor | YSKV | Endocytosis/lysosomal targeting | [136] |
| Glutamate receptor | YWL | Endocytosis | [146] |
| GC-A/NPRA | GDAY | Endocytosis | [62] |
| GC-ANPRA | FQQI | Endocytosis | [26] |
| Insulin-like growth factor receptor | YxxPhi | Endocytosis | [136, 185] |
| LDL receptor | FDNPVY | Endocytosis | [145] |
| LH receptor | GTALL | Endocytosis | [186] |
| LDL-related receptor | YATL | Endocytosis | [187] |
| Mannose phosphate receptor | FENTLY | Lysosomal targeting | [136, 185] |
| Platelet activating factor receptor | [YF]xNPx[YF] | Endocytosis | [188] |
| Protease-activated receptor-1 | YKKL | Endocytosis | [140] |
| P2X receptor | YEQGL | Endocytosis | [189] |
| Transferrin receptor | YTRF/Q | Endocytosis | [136, 185] |
| T-cell receptor | YQPL | Endocytosis | [136] |
| Membrane Proteins: | |||
| Acetylcholine transporter | DSLL | Endosomal targeting | [147] |
| Beta-amyloid precursor protein | YENPTY | Endocytosis | [190] |
| CD3 Chains | [DE]xxxL[LI] | Endocytosis | [191] |
| Integrin | NPxY | Endocytosis | [192] |
| GLUT4 | FQQI | Endocytosis/lysosomal targeting | [193] |
The evidence suggest that various short sequence signal motifs usually contain tyrosine or phenylalanine residues, followed by hydrophobic and/or aromatic residues. Certain sequence motifs also contain acidic residues with required amino acid residue tyrosine. Membrane receptors and proteins also make the use of dileucine short signal motifs. GC-A, guanylyl cyclase-A; NPRA, natriuretic peptide receptor-A; LDL, low density lipoprotein; LH, leutinizing hormone; x, refers to any amino acid residue; P2X receptor, purinergic P2Xreceptor; Phi, refers to hydrophobic amino acid residue; GLUT4, glucose transporter 4.
The NPxY signal sequence is known to facilitate the internalization of low density lipoprotein (LDL) receptor [130]. Endocytosis of platelet-activating factor is governed by a putative DPxxY signal motif and type-2 vasopressin receptor by NPxxY motif [131, 132]. Similarly, YxxL signal motif also functions in endocytosis of LDL receptor-related protein [133]. A common feature of these internalization signal motifs, including DPxxy, NPxY, and GDAY, is the presence of an amino acid tyrosine at the end of the tetrapeptide short sequence motif [62, 131]. Tyrosine residues are also involved in endocytosis of mannose-6-phosphate receptor and in the influenza virus hemagglutinin, even though they are not present in the context of NPxY or YxRF consensus sequence suggesting that if a universal internalization signal exists, it may not contain a universal peptide sequence [131, 134]. The critical characteristics of all these sequences might be their specification of a particular conformation, such as a tight beta-turn in protein structure [135]. Tyr recognition short sequence signal motifs present a small surface loop but they differ in primary structure in the context of the positioning of Tyr residue in the loop structure [128, 136, 137]. The substitution of Tyr with amino acid known to be inert in endocytosis resulted in disruption of the beta-turn structure. The tyrosine-based motifs such as NPxY and YxxL recruit clathrin and adaptor protein molecules and act as a ligand-receptor cargo recognition sequence for their delivery to endosomes and lysosomes [134, 138]. These motifs initially recruit AP-2 at the plasma membrane and activate mu2 subunit that facilitates the binding of beta-2 subunit of AP-2 to clathrin at the cell surface, leading to clathrin-mediated internalization of receptor molecules [128, 134, 139, 140].
NPxY was recognized as the first short signal sequence motif in the cytoplasmic domain of membrane receptors and proteins with critical roles in internalization and trafficking of receptor molecules [130]. Including LDL receptor, beta-1 integrin, megalin, beta-amyloid precursor protein, EGF receptor, and neurotrophin receptor [93, 128, 130, 141–144]. The early studies demonstrated that substitution of a cysteine residue for a tyrosine residue in NPxY (Asn-Pro-x-Tyr) motif of LDL receptor rapidly abolished its endocytosis [145]. Several dileucine-based motifs, including YGLL, SLL, and YWLL are also located in the protein-KHD and GC regions of NPRA, however, their roles in the internalization and trafficking of NPRA is not yet known. Dileucine (LL) motifs regulate internalization and trafficking of several membrane receptors and proteins through the endocytic and secretory pathways [128, 146–150]. LL motifs contain 3-7 amino acid residues in which LL amino acid residues are preceded by a polar amino acids and a negatively charged residue that may be aspartic acid, glutamic acid, or phosphoserine. Dileucine motifs with acidic amino acids are constitutively active and regulate both endocytosis and secretory pathways of membrane receptors and proteins [148].
A short sequence motif Yxxphi is located in the carboxyl-terminal domain of NPRA at residues Y988-x-x-F991; however, its role in the internalization of NPRA remains be determined. These signal motifs with internalization specificity are located within 10-30 amino acid residues from the transmembrane domain of membrane receptors [151–153]. The tyrosine-based Yxxphi sorting signals direct the internalization by interacting with mu1, mu2, mu3, and mu4 subunits of adaptor proteins, including AP-1, AP-2, AP-3, and AP-4, respectively [88, 89, 139]. The tetrapeptide sequence Yxxphi is usually located in the cytoplasmic domains of the receptors including transferrin and asialoglycoprotein receptors and exhibit critical roles in the endocytosis. In Yxxphi signal motifs, Y represents a tyrosine residue, x is any amino acid residue, and phi represents a residue with large bulky hydrophobic side-chain. Yxxphi signal motifs contain dual specificity with endocytotic functional motif and a trafficking signal within the endosomal and/or secretory pathways [128, 134, 153, 154]. The adaptor protein complexes capture the cargo in the coated vesicles for trafficking of receptors in the subcellular compartments.
8. Receptor internalization in non-traditional sub-cellular compartments:
Here, we predict that the alternative fates of activated NPRA are emerging, including trafficking to two other subcellular compartments such as the nucleus and mitochondria (Fig. 5). Comprehensive investigations of receptor trafficking have demonstrated that growth factors induce the internalization of receptor tyrosine kinases (RTKs) and then are either sorted to lysosomes or recycled back on plasma membranes [155–157]. In addition, RTKs traffic through different subcellular compartments such as the nucleus and mitochondria. It has been recognized that trafficking to these novel destinations involves newly identified biochemical and cellular mechanisms and that these trafficking events function in the signal transduction pathways, implicating the receptor itself as a signaling element between the cell surface and the subcellular organelles [158, 159]. Similarly, after binding of ligand, cell-surface epidermal growth factor receptor (EGFR) is internalized and trafficked through the endo-lysosomal subcellular compartments or returned to the plasma membrane by recycling mechanisms. Other than these subcellular compartments, EGFR also traffics from the cell surface to the nucleus, Golgi apparatus, endoplasmic reticulum, and mitochondria after endocytosis, where it seems to be involved in multiple biological and physiological functions [160].
Figure 5. Schematic representation of the proposed intracellular trafficking pathways of NPRA through the multi-organelle systems in cell compartments:
Activated cell-surface NPRA is internalized and sorted at the early endosome. The fate of the receptor, with the recycling endosome, has important consequences for biological responses, although the degradative pathway is via multivesicular bodies (MVBs)/lysosome. Typical pathways to the nucleus and mitochondria have been proposed to favor survival, but the transport mechanisms have not yet been established. Conversely, ligand-activated ANP-NPRA complexes recycle back on plasma membranes for re-endocytosis.
Evidence suggest that angiotensin (Ang II) type 1 receptor (AT1R) levels are greatly increased in the mitochondria of aged mice, however, AT2R was localized in mitochondria at a greatest density in young animals and decreased with age [161, 162]. Those previous studies indicated that Ang II and AT2R constitute essential components of the mitochondrial inner membrane of various cell types and regulates nitric oxide synthesis to enhance mitochondrial function. These observations also support the hypothesis of intracrine Ang II as previously suggested to illustrate the well –established physiological system [163]. The mechanisms by which these receptors are translocated into the inner membrane of the mitochondria remain unknown. Usually several receptors and proteins localized in mitochondria, are synthesized in the cytoplasm and contain mitochondrial localization sequence at the amino-terminal, which are transported with the help of cytosolic chaperons and delivered to the inner and outer membranes of mitochondria [164, 165]. Interestingly, alternative pathways have been suggested for internalization of Ang II ligand-receptor complexes into the subcellular compartments, including microtubule-dependent endocytic pathway [166]. Recent studies have indicated that luminal receptor-bound Ang II is endocytosed in a complex of both AT1R and AT2R as a heterodimer in the endoplasmic reticulum in cultured LLC-PK1 cells [167]. More studies are needed to determine the diversified pathways of the internalization and trafficking of various ligand-receptor complexes in diverse subcellular compartments in both physiological and pathophysiological contexts.
9. Intracellular trafficking with concurrent signaling of NPRA
Our recent studies have shown internalization and concurrent signaling of NPRA in subcellular compartments; this had not been previously demonstrated [26, 42]. Preparation of the enhanced GFP (eGFP)-tagged NPRA (eGFP-NPRA) construct has greatly helped to visualize the internalization, intracellular trafficking, and subsequent signaling of receptor in the subcellular compartments. These studies delineated the molecular mechanisms of NPRA trafficking with ANP/NPRA/cGMP signaling in recombinant HEK-293 cells and primary MMCs in the physiological context [26, 42]. Earlier studies showed that cGMP production is not exclusively activated at the cell surface, but also occurs after receptors have been internalized and continued trafficking in subcellular locations [26, 42]. However, endocytic inhibitors MDC and CPZ and dynamin GTPase blocker (dynamin mutant) significantly decrease the intracellular accumulation of cGMP [69]. Moreover, the receptor containing the mutated short signal motif (FQQI/AAAA) also produced a significantly lower level of intracellular cGMP during subcellular trafficking than do the WT receptor without this mutation [26].
Interestingly, early studies reported that G-protein-coupled receptor (GPCR) continued, using their bound ligands, to produce signals by generating intracellular second-messenger cAMP throughout the internalization and trafficking itinerary [124]. Moreover, cGMP regulates various processes, including hypertension, cardiovascular homoeostasis, antimitogenic effects vascular reactivity, sensory transduction, anti-inflammation responses, neuronal plasticity, and learning [124, 168]. Generally, cGMP interacts with three distinct kinds of intracellular effector protein molecules, including cGMP-dependent protein kinases (PKG), cGMP-activated phosphodiesterases (PDEs), and cGMP-regulated ion channels (CNGs) [169]. It is possible that cGMP-binding proteins transduce the cGMP signal to alter cell function through different mechanisms, comprising an inhibitory effect and/or by stimulating protein phosphorylation [170, 171]. Using immunofluorescence microscopy of cGMP, it was possible to clearly visualize that ligand-receptor complexes of ANP/NPRA continuously produce intracellular cGMP at various time points after treatment with ANP during the internalization and trafficking of this receptor protein [26, 42, 69]. Based on our previous studies, it became evident that signaling from inside the cell into subcellular compartments is persistent. This signaling appears to trigger specific downstream effects of cGMP [26, 42]. Attenuation of the intracellular accumulation of cGMP suggested that endocytic inhibitors and dynamin GTPase blocker may have a concerted regulatory role in NPRA trafficking and signaling in intracellular compartments [69].
10. Live-cell and intact-animal in vivo approaches to internalization and trafficking of membrane receptors
Internalization and subcellular trafficking are critical for most membrane receptors with approaches in different subcellular compartments and some receptors that are recycled back to the plasma membrane during the signaling process. Live-cell imaging eliminates the possible effects of fixation on cell volume, as well as the loss reduction, or redistribution of GFP-chimeric receptors in physiological and pathophysiological contexts. Live-cell imaging permits investigation of receptor trafficking while avoiding confounding variables associated with fixation, particularly the loss or artifactual movement of eGFP-tagged membrane receptors. As individual cells are tracked in real time, the subcellular localization of receptors can be imaged and measured. Live-cell imaging allows the study of protein trafficking, migration, proliferation, apoptosis, cell-cycle pathway, autophagy, and protein-protein interactions and dynamics [172]. To understand the mechanistic pathway of receptor endocytosis, trafficking, and signaling of live cells and animal in vivo have been used to determine the roles of various receptors in their physiological milieu.
Interestingly, in vivo infusion and uptake of Ang II in intact mice indicated that multiligand endocytic receptor megalin has at least some role in the uptake of Ang II and the downstream signaling process in proximal tubule cells (PTCs) in vivo [173]. Earlier in vivo studies showed that PTCs of the kidney also accumulate both systematically infused 125I-labeled and unlabeled Ang II [174–176]. Live-cell imaging has also simultaneously captured time-lapse images of Ang II type 1a receptors (AT1aR) and intracellular compartments in transfected HEK-293 cells following stimulation with Ang II [172]. It has been reported that a proton-sensing GPCR, ovarian cancer G protein-coupled receptor 1 (OGR1), is related to acidosis and diseases that cause tissue acidification. pH-dependent intracellular trafficking of OGR1 has been visualized in living leukocytes by a real-time fluorescence microscopic method based on sortase A-mediated pulse labeling of OGR1. Quantitative single-cell image analysis and live-cell monitoring demonstrated that the signal transduction activity of OGR1 is regulated by pH-dependent receptor internalization and recycling to the plasma membrane [177]. Recently, internalization of G protein-coupled receptors (GPCRs) was visualized with high spatial resolution near the plasma membrane by means of total internal reflection fluorescence (TIRF) microscopy at a level of discrete single events. Methods have also been developed to determine the relative extent of internalized fluorescent receptor-ligand complexes by comparative fluorescence quantification in living cells of Chinese hamster ovaries (CHO) [178]. Live-cell imaging of other GPCRs using confocal microscopes enabled investigators to observe the internalization and trafficking of receptors in individual cells [179, 180].
Confocal imaging revealed detailed receptor neuroanatomy throughout the nervous system of knock-in mice, where δ-opioid receptor (DOR) was replaced by an active DOR-EGFP fusion protein [181]. Real-time imaging in primary neurons allowed dynamic visualization of drug-induced receptor trafficking. In DOR-EGFP animals, drug treatment triggered receptor endocytosis that correlated with the behavioral response. It also has been shown that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors play an important role in brain functions such as learning and memory process. Modulation of synaptic strength through trafficking of AMPA receptors is an essential mechanism underlying synaptic plasticity. Several studies have used live time-lapse imaging of fluorescence-tagged AMPA receptors to directly monitor membrane trafficking in these receptors in their basal state, as well as during synaptic plasticity in vivo using confocal microscopy [182]. Overexpression of lung 5-hydroxytryptamine7 (5-HT7) receptor is an important protective mechanism during lipopolysaccharide (LPS)-induced sepsis-related cell damage. 5-HT7 receptor trafficking has been evaluated using in-vivo and in-vitro models of LPS-induced inflammatory cell injury in rats and LPS-treated A549 cells, which suggested that lung 5-HT7 receptor expression is increased in LPS-induced endotoxemia [183]. Tumor hypoxia is linked with progression of malignant state and resistance to cancer treatments and therapies. It has been indicated that the ligand-bound EGF receptor (EGFR) trafficking is prolonged due to hypoxia-inducible factor (HIF)-medicated delays of endocytic cycle [184]. These authors suggested a role for oxygen-sensing pathway of tumor activation of HIF-associated EGFR-mediated signaling by delayed enocytic trafficking and deactivation of receptor protein. To understand endocytosis, trafficking, and signaling of receptors, more studies with live-cell and animal in-vivo approaches will allow greater detail that should illustrate the mechanistic pathways of NPRA in both physiological and pathophysiological contexts of hormone signaling. The gene-knockout studies of Npr1 in mice have demonstrated that ANP resistance is associated with sodium and volume retention and kidney dysfunction in the mutant animals [45]. Nevertheless, the role of NPRA trafficking in the recycling endosomes and the desensitization of this receptor under pathological conditions is not well understood.
11. Conclusions and future perspectives
Upon ligand-induced activation, NPRA is endocytosed and intracellularly sorted to endosomes, recycling endosomes, late endosomes, and finally to lysosomes. ANP binding to the receptor accelerates internalization and trafficking of ligand-receptor complexes of ANP/NPRA into intracellular compartments. Ligand is subsequently delivered through the endosomes to lysosomal compartments, where, in large measure, it is degraded. However, a population of ligand–receptor complexes escape and are diverted into recycling endosomes, allowing these receptors to recycle back to the plasma membrane. After exposure to ligand, the receptors, at various times during the subsequent internalization and subcellular trafficking processes, continuously produce the intracellular second messenger cGMP. Evidence suggests that intracellular accumulation of cGMP is significantly decreased after treatment with endocytic inhibitors MDC and CPZ and introduction of dynamin GTPase blocker (dynamin mutant), suggesting that dynamin has an intrinsic role in the endocytic process.
The review provides compelling evidence of the specificity of ligand-induced endocytosis of membrane receptors through clathrin-coated vesicle formation, as well as the role of dynamin GTPase in this process. Detailed investigation of endocytic proteins will be necessary to elucidate the precise functions of these proteins in receptor internalization and trafficking in intracellular compartments, as well as their subsequent signaling in physiological and pathophysiological conditions. Immunofluorescence studies have provided critical information regarding mechanisms of the trafficking itinerary of ANP-NPRA, during which it undergoes internalization via clathrin-mediated endocytosis and potentiates the development of pathophysiological conditions such as hypertension and cardiovascular disease. Exploitation of molecular and pharmacologic perturbants has facilitated delineation of the pathways of ligand-bound receptor endocytosis, trafficking, and redistribution in subcellular compartments from the plasma membrane into the cell interior. The demonstration of receptor trafficking in organelle that have not traditionally been studied, including nuclei and mitochondria, has opened a new line of investigation of receptor trafficking into subcellular compartments. Further concerted studies are needed to delineate receptor endocytosis and trafficking in their physiological contest in live cells and whole-animal models. Our understanding of the molecular determinants mediating the association of membrane receptors with subcellular organelles during intracellular trafficking with concurrent signaling should provide a basis for new molecular studies and important targets for therapeutic developments in translational health and disease.
Highlights.
Internalization is a prominent clathrin-mediated mechanism for concentrated uptake of ligand-receptor complexes for receptor-dependent regulation of cell membrane function, intracellular signal transduction, and physiological activities.
The receptor endocytosis and contemporaneous intracellular signaling occur concurrently during internalization and subcellular trafficking with simultaneous generation of second-messenger signals in intact cells.
The short-signal motifs located in the carboxyl-terminal regions of membrane receptors play pivotal roles during the internalization and subsequent receptor trafficking in subcellular organelles.
Emphasis is placed on future investigations of receptor endocytosis and trafficking in live cells and intact animals in vivo in physiological context.
Acknowledgements
The authors thank Mrs. Kamala Pandey for the help in the preparation of this manuscript. This work was supported by NIH grants R01HL057531 and R01HL062147.
Abbreviations
- APs
adaptor proteins
- NH4Cl2
ammonium chloride
- Ang II
angiotensin II
- AT1R
angiotensin type 1 receptor
- ANP
atrial natriuretic peptide
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ARH
autosomal recessive hypercholesterolemia
- CNGs
cGMP-regulated gated ion channels
- CPZ
chlorpromazine
- CHO
Chinese hamster ovaries
- CCPs
clathrin-coated pits
- CIF
confocal IF microscopy
- CNP
C-type natriuretic peptide
- DOR
δ-opioid receptor
- eGFP
enhanced green fluorescent protein
- EGFR
epidermal growth factor receptor
- GPCRs
G-protein-coupled receptors
- OGR1
ovarian cancer G protein-coupled receptor 1
- GC-A/NPRA
Guanylyl cyclase/natriuretic peptide receptor-A
- 5-HT7
5-hydroxytryptamine7
- HEK-293
Human embryonic kidney-293
- IB
immunoblotting
- IF
immunofluorescence
- Co-IP
co-immunoprecipitation
- IGF-IR
insulin-like growth factor-I receptor
- IRS
insulin-receptor substrate
- protein-KHD
protein Kinase-like homology domain
- LDLR
Low-density lipoprotein receptor
- MDC
monodansylcadaverine
- MMCs
murine mesangial cells
- NPRA
natriuretic peptide receptor-A
- NPRB
natriuretic peptide receptor-B
- NPRC
natriuretic peptide receptor-C
- PDEs
phosphodiesterases
- PDGF
platelet-derived growth factor
- PKG
protein kinases G
- PTCs
proximal tubule cells
- REs
recycling endosomes
- TIRF
total internal reflection fluorescence
- TRPV1
transient receptor potential vanilloid-1
Footnotes
Declarations of interest: None
References
- [1].Anand-Srivastava MB, Trachte GJ, Atrial natriuretic factor receptors and signal transduction mechanisms, Pharmacol Rev 45(4) (1993) 455–97. [PubMed] [Google Scholar]
- [2].Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML, Diverse biological actions of atrial natriuretic peptide, Physiol Rev 70(3) (1990) 665–99. [DOI] [PubMed] [Google Scholar]
- [3].de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H, A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats, Life Sci 28(1) (1981) 89–94. [DOI] [PubMed] [Google Scholar]
- [4].Kishimoto I, Tokudome T, Nakao K, Kangawa K, Natriuretic peptide system: an overview of studies using genetically engineered animal models, The FEBS journal 278(11) (2011) 1830–41. [DOI] [PubMed] [Google Scholar]
- [5].Pandey KN, Biology of natriuretic peptides and their receptors, Peptides 26(6) (2005) 901–32. [DOI] [PubMed] [Google Scholar]
- [6].Pandey KN, The functional genomics of guanylyl cyclase/natriuretic peptide receptor-A: perspectives and paradigms, The FEBS journal 278(11) (2011) 1792–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Delporte C, Poloczek P, Tastenoy M, Winand J, Christophe J, Atrial natriuretic peptide binds to ANP-R1 receptors in neuroblastoma cells or is degraded extracellularly at the Ser-Phe bond, Eur J Pharmacol 227(3) (1992) 247–56. [DOI] [PubMed] [Google Scholar]
- [8].Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, et al. , Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide, J Clin Invest 87(4) (1991) 1402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hasegawa K, Fujiwara H, Itoh H, Nakao K, Fujiwara T, Imura H, Kawai C, Light and electron microscopic localization of brain natriuretic peptide in relation to atrial natriuretic peptide in porcine atrium. Immunohistocytochemical study using specific monoclonal antibodies, Circulation 84(3) (1991) 1203–9. [DOI] [PubMed] [Google Scholar]
- [10].Zois NE, Bartels ED, Hunter I, Kousholt BS, Olsen LH, Goetze JP, Natriuretic peptides in cardiometabolic regulation and disease, Nat Rev Cardiol 11(7) (2014) 403–12. [DOI] [PubMed] [Google Scholar]
- [11].Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, Jougasaki M, Burnett JC Jr., Corin is present in the normal human heart, kidney, and blood, with pro-B-type natriuretic peptide processing in the circulation, Clin Chem 57(1) (2011) 40–7. [DOI] [PubMed] [Google Scholar]
- [12].Sawada Y, Suda M, Yokoyama H, Kanda T, Sakamaki T, Tanaka S, Nagai R, Abe S, Takeuchi T, Stretch-induced hypertrophic growth of cardiocytes and processing of brain-type natriuretic peptide are controlled by proprotein-processing endoprotease furin, J Biol Chem 272(33) (1997) 20545–54. [DOI] [PubMed] [Google Scholar]
- [13].Yan W, Wu F, Morser J, Wu Q, Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme, Proc Natl Acad Sci U S A 97(15) (2000) 8525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sudoh T, Minamino N, Kangawa K, Matsuo H, C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain, Biochemical and biophysical research communications 168(2) (1990) 863–70. [DOI] [PubMed] [Google Scholar]
- [15].Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H, Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”, J Clin Invest 90(3) (1992) 1145–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garbers DL, Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands, Cell 71(1) (1992) 1–4. [DOI] [PubMed] [Google Scholar]
- [17].Khurana ML, Pandey KN, Receptor-mediated stimulatory effect of atrial natriuretic factor, brain natriuretic peptide, and C-type natriuretic peptide on testosterone production in purified mouse Leydig cells: activation of cholesterol side-chain cleavage enzyme, Endocrinology 133(5) (1993) 2141–9. [DOI] [PubMed] [Google Scholar]
- [18].Lowe DG, Fendly BM, Human natriuretic peptide receptor-A guanylyl cyclase. Hormone cross-linking and antibody reactivity distinguish receptor glycoforms, J Biol Chem 267(30) (1992) 21691–7. [PubMed] [Google Scholar]
- [19].Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA, Guanylyl cyclases and signaling by cyclic GMP, Pharmacol Rev 52(3) (2000) 375–414. [PubMed] [Google Scholar]
- [20].Duda T, Goraczniak RM, Sharma RK, Site-directed mutational analysis of a membrane guanylate cyclase cDNA reveals the atrial natriuretic factor signaling site, Proc Natl Acad Sci U S A 88(17) (1991) 7882–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, Goeddel DV, Schulz S, A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor, Nature 338(6210) (1989) 78–83. [DOI] [PubMed] [Google Scholar]
- [22].Fuller F, Porter JG, Arfsten AE, Miller J, Schilling JW, Scarborough RM, Lewicki JA, Schenk DB, Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones, J Biol Chem 263(19) (1988) 9395–401. [PubMed] [Google Scholar]
- [23].Pandey KN, Singh S, Molecular cloning and expression of murine guanylate cyclase/atrial natriuretic factor receptor cDNA, J Biol Chem 265(21) (1990) 12342–8. [PubMed] [Google Scholar]
- [24].Schulz S, Singh S, Bellet RA, Singh G, Tubb DJ, Chin H, Garbers DL, The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family, Cell 58(6) (1989) 1155–62. [DOI] [PubMed] [Google Scholar]
- [25].Sharma RK, Evolution of the membrane guanylate cyclase transduction system, Molecular and cellular biochemistry 230(1–2) (2002) 3–30. [PubMed] [Google Scholar]
- [26].Mani I, Garg R, Pandey KN, Role of FQQI motif in the internalization, trafficking, and signaling of guanylyl-cyclase/natriuretic peptide receptor-A in cultured murine mesangial cells, American journal of physiology. Renal physiology 310(1) (2016) F68–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pandey KN, Pavlou SN, Inagami T, Identification and characterization of three distinct atrial natriuretic factor receptors. Evidence for tissue-specific heterogeneity of receptor subtypes in vascular smooth muscle, kidney tubular epithelium, and Leydig tumor cells by ligand binding, photoaffinity labeling, and tryptic proteolysis, J Biol Chem 263(26) (1988) 13406–13. [PubMed] [Google Scholar]
- [28].Drewett JG, Garbers DL, The family of guanylyl cyclase receptors and their ligands, Endocr Rev 15(2) (1994) 135–62. [DOI] [PubMed] [Google Scholar]
- [29].Levin ER, Gardner DG, Samson WK, Natriuretic peptides, N Engl J Med 339(5) (1998) 321–8. [DOI] [PubMed] [Google Scholar]
- [30].Mishra SK, Hoon MA, The cells and circuitry for itch responses in mice, Science 340(6135) (2013) 968–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Anand-Srivastava MB, Natriuretic peptide receptor-C signaling and regulation, Peptides 26(6) (2005) 1044–59. [DOI] [PubMed] [Google Scholar]
- [32].Duda T, Pertzev A, Sharma RK, The ANF-RGC gene motif (669)WTAPELL(675) is vital for blood pressure regulation: biochemical mechanism, Biochemistry 52(13) (2013) 2337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Duda T, Pertzev A, Sharma RK, Atrial natriuretic factor receptor guanylate cyclase, ANF-RGC, transduces two independent signals, ANF and Ca(2+), Front Mol Neurosci 7 (2014) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pandey KN, Nguyen HT, Sharma GD, Shi SJ, Kriegel AM, Ligand-regulated internalization, trafficking, and down-regulation of guanylyl cyclase/atrial natriuretic peptide receptor-A in human embryonic kidney 293 cells, J Biol Chem 277(7) (2002) 4618–27. [DOI] [PubMed] [Google Scholar]
- [35].Pandey KN, Endocytosis and Trafficking of Natriuretic Peptide Receptor-A: Potential Role of Short Sequence Motifs, Membranes (Basel) 5(3) (2015) 253–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cao L, Gardner DG, Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts, Hypertension 25(2) (1995) 227–34. [DOI] [PubMed] [Google Scholar]
- [37].Garg R, Pandey KN, Regulation of guanylyl cyclase/natriuretic peptide receptor-A gene expression, Peptides 26(6) (2005) 1009–23. [DOI] [PubMed] [Google Scholar]
- [38].Kumar P, Garg R, Bolden G, Pandey KN, Interactive roles of Ets-1, Sp1, and acetylated histones in the retinoic acid-dependent activation of guanylyl cyclase/atrial natriuretic peptide receptor-A gene transcription, J Biol Chem 285(48) (2010) 37521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tripathi S, Pandey KN, Guanylyl cyclase/natriuretic peptide receptor-A signaling antagonizes the vascular endothelial growth factor-stimulated MAPKs and downstream effectors AP-1 and CREB in mouse mesangial cells, Molecular and cellular biochemistry 368(1–2) (2012) 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pandey KN, Kinetic analysis of internalization, recycling and redistribution of atrial natriuretic factor-receptor complex in cultured vascular smooth-muscle cells. Ligand-dependent receptor down-regulation, Biochem J 288 ( Pt 1) (1992) 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kumar P, Periyasamy R, Das S, Neerukonda S, Mani I, Pandey KN, All-trans retinoic acid and sodium butyrate enhance natriuretic peptide receptor a gene transcription: role of histone modification, Molecular pharmacology 85(6) (2014) 946–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mani I, Garg R, Tripathi S, Pandey KN, Subcellular trafficking of guanylyl cyclase/natriuretic peptide receptor-A with concurrent generation of intracellular cGMP, Bioscience reports 35(5) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Das S, Periyasamy R, Pandey KN, Activation of IKK/NF-kappaB provokes renal inflammatory responses in guanylyl cyclase/natriuretic peptide receptor-A gene-knockout mice, Physiological genomics 44(7) (2012) 430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N, Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A, Proc Natl Acad Sci U S A 94(26) (1997) 14730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shi SJ, Vellaichamy E, Chin SY, Smithies O, Navar LG, Pandey KN, Natriuretic peptide receptor A mediates renal sodium excretory responses to blood volume expansion, American journal of physiology. Renal physiology 285(4) (2003) F694–702. [DOI] [PubMed] [Google Scholar]
- [46].Subramanian U, Kumar P, Mani I, Chen D, Kessler I, Periyasamy R, Raghavaraju G, Pandey KN, Retinoic acid and sodium butyrate suppress the cardiac expression of hypertrophic markers and proinflammatory mediators in Npr1 gene-disrupted haplotype mice, Physiological genomics 48(7) (2016) 477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Vellaichamy E, Khurana ML, Fink J, Pandey KN, Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A, J Biol Chem 280(19) (2005) 19230–42. [DOI] [PubMed] [Google Scholar]
- [48].Kumar P, Gogulamudi VR, Periasamy R, Raghavaraju G, Subramanian U, Pandey KN, Inhibition of HDAC enhances STAT acetylation, blocks NF-kappaB, and suppresses the renal inflammation and fibrosis in Npr1 haplotype male mice, American journal of physiology. Renal physiology 313(3) (2017) F781–F795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS, Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals, Circulation 115(11) (2007) 1345–53. [DOI] [PubMed] [Google Scholar]
- [50].Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW, Vasan RS, Impact of obesity on plasma natriuretic peptide levels, Circulation 109(5) (2004) 594–600. [DOI] [PubMed] [Google Scholar]
- [51].Pandey KN, Molecular and genetic aspects of guanylyl cyclase natriuretic peptide receptor-A in regulation of blood pressure and renal function, Physiological genomics 50(11) (2018) 913–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, Huang X, Liu M, Fang C, Peng J, Chen S, Wu S, Liu Z, Dong L, Zhou Y, Wu Q, Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy, Nature 484(7393) (2012) 246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chen W, Spitzl A, Mathes D, Nikolaev VO, Werner F, Weirather J, Spiranec K, Rock K, Fischer JW, Kammerer U, Stegner D, Baba HA, Hofmann U, Frantz S, Kuhn M, Endothelial Actions of ANP Enhance Myocardial Inflammatory Infiltration in the Early Phase After Acute Infarction, Circ Res 119(2) (2016) 237–48. [DOI] [PubMed] [Google Scholar]
- [54].Staedtke V, Bai RY, Kim K, Darvas M, Davila ML, Riggins GJ, Rothman PB, Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S, Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome, Nature 564(7735) (2018) 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Vollmar AM, The role of atrial natriuretic peptide in the immune system, Peptides 26(6) (2005) 1086–94. [DOI] [PubMed] [Google Scholar]
- [56].Chen Y, Harty GJ, Huntley BK, Iyer SR, Heublein DM, Harders GE, Meems L, Pan S, Sangaralingham SJ, Ichiki T, Burnett JC Jr., CRRL269: a novel designer and renal-enhancing pGC-A peptide activator, Am J Physiol Regul Integr Comp Physiol 314(3) (2018) R407–R414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lee CY, Huntley BK, McCormick DJ, Ichiki T, Sangaralingham SJ, Lisy O, Burnett JC Jr., Cenderitide: structural requirements for the creation of a novel dual particulate guanylyl cyclase receptor agonist with renal-enhancing in vivo and ex vivo actions, Eur Heart J Cardiovasc Pharmacother 2(2) (2016) 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC Jr., Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP, J Am Coll Cardiol 52(1) (2008) 60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Meems LMG, Andersen IA, Pan S, Harty G, Chen Y, Zheng Y, Harders GE, Ichiki T, Heublein DM, Iyer SR, Sangaralingham SJ, McCormick DJ, Burnett JC Jr., Design, Synthesis, and Actions of an Innovative Bispecific Designer Peptide, Hypertension 73(4) (2019) 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Koller KJ, Goeddel DV, Molecular biology of the natriuretic peptides and their receptors, Circulation 86(4) (1992) 1081–8. [DOI] [PubMed] [Google Scholar]
- [61].Pandey KN, Stoichiometric analysis of internalization, recycling, and redistribution of photoaffinity-labeled guanylate cyclase/atrial natriuretic factor receptors in cultured murine Leydig tumor cells, J Biol Chem 268(6) (1993) 4382–90. [PubMed] [Google Scholar]
- [62].Pandey KN, Nguyen HT, Garg R, Khurana ML, Fink J, Internalization and trafficking of guanylyl (guanylate) cyclase/natriuretic peptide receptor A is regulated by an acidic tyrosine-based cytoplasmic motif GDAY, Biochem J 388(Pt 1) (2005) 103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Rathinavelu A, Isom GE, Differential internalization and processing of atrial-natriuretic-factor B and C receptor in PC12 cells, Biochem J 276 ( Pt 2) (1991) 493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rathinavelu A, Isom GE, Lysosomal delivery of ANP receptors following internalization in PC12 cell, Life Sci 53(12) (1993) 1007–14. [DOI] [PubMed] [Google Scholar]
- [65].Nussenzveig DR, Lewicki JA, Maack T, Cellular mechanisms of the clearance function of type C receptors of atrial natriuretic factor, J Biol Chem 265(34) (1990) 20952–8. [PubMed] [Google Scholar]
- [66].Cohen D, Koh GY, Nikonova LN, Porter JG, Maack T, Molecular determinants of the clearance function of type C receptors of natriuretic peptides, J Biol Chem 271(16) (1996) 9863–9. [DOI] [PubMed] [Google Scholar]
- [67].Ceresa BP, Kao AW, Santeler SR, Pessin JE, Inhibition of clathrin-mediated endocytosis selectively attenuates specific insulin receptor signal transduction pathways, Mol Cell Biol 18(7) (1998) 3862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Huang F, Khvorova A, Marshall W, Sorkin A, Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference, J Biol Chem 279(16) (2004) 16657–61. [DOI] [PubMed] [Google Scholar]
- [69].Somanna NK, Mani I, Tripathi S, Pandey KN, Clathrin-dependent internalization, signaling, and metabolic processing of guanylyl cyclase/natriuretic peptide receptor-A, Molecular and cellular biochemistry 441(1–2) (2018) 135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Dickey DM, Flora DR, Potter LR, Antibody tracking demonstrates cell type-specific and ligand-independent internalization of guanylyl cyclase a and natriuretic peptide receptor C, Molecular pharmacology 80(1) (2011) 155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Koh GY, Nussenzveig DR, Okolicany J, Price DA, Maack T, Dynamics of atrial natriuretic factor-guanylate cyclase receptors and receptor-ligand complexes in cultured glomerular mesangial and renomedullary interstitial cells, J Biol Chem 267(17) (1992) 11987–94. [PubMed] [Google Scholar]
- [72].Fan D, Bryan PM, Antos LK, Potthast RJ, Potter LR, Down-regulation does not mediate natriuretic peptide-dependent desensitization of natriuretic peptide receptor (NPR)-A or NPR-B: guanylyl cyclase-linked natriuretic peptide receptors do not internalize, Molecular pharmacology 67(1) (2005) 174–83. [DOI] [PubMed] [Google Scholar]
- [73].Flora DR, Potter LR, Prolonged atrial natriuretic peptide exposure stimulates guanylyl cyclase-a degradation, Endocrinology 151(6) (2010) 2769–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pandey KN, Inagami T, Misono KS, Atrial natriuretic factor receptor on cultured Leydig tumor cells: ligand binding and photoaffinity labeling, Biochemistry 25(26) (1986) 8467–72. [DOI] [PubMed] [Google Scholar]
- [75].Pandey KN, Kumar R, Li M, Nguyen H, Functional domains and expression of truncated atrial natriuretic peptide receptor-A: the carboxyl-terminal regions direct the receptor internalization and sequestration in COS-7 cells, Molecular pharmacology 57(2) (2000) 259–67. [PubMed] [Google Scholar]
- [76].Somanna NK, Pandey AC, Arise KK, Nguyen V, Pandey KN, Functional silencing of guanylyl cyclase/natriuretic peptide receptor-A by microRNA interference: analysis of receptor endocytosis, International journal of biochemistry and molecular biology 4(1) (2013) 41–53. [PMC free article] [PubMed] [Google Scholar]
- [77].Brackmann M, Schuchmann S, Anand R, Braunewell KH, Neuronal Ca2+ sensor protein VILIP-1 affects cGMP signalling of guanylyl cyclase B by regulating clathrin-dependent receptor recycling in hippocampal neurons, J Cell Sci 118(Pt 11) (2005) 2495–505. [DOI] [PubMed] [Google Scholar]
- [78].Li Y, Madiraju P, Anand-Srivastava MB, Knockdown of natriuretic peptide receptor-A enhances receptor C expression and signalling in vascular smooth muscle cells, Cardiovascular research 93(2) (2012) 350–9. [DOI] [PubMed] [Google Scholar]
- [79].Heim JM, Singh S, Gerzer R, Effect of glycosylation on cloned ANF-sensitive guanylyl cyclase, Life Sci 59(4) (1996) PL61–8. [DOI] [PubMed] [Google Scholar]
- [80].Koller KJ, Lipari MT, Goeddel DV, Proper glycosylation and phosphorylation of the type A natriuretic peptide receptor are required for hormone-stimulated guanylyl cyclase activity, J Biol Chem 268(8) (1993) 5997–6003. [PubMed] [Google Scholar]
- [81].Miyagi M, Zhang X, Misono KS, Glycosylation sites in the atrial natriuretic peptide receptor: oligosaccharide structures are not required for hormone binding, European journal of biochemistry / FEBS 267(18) (2000) 5758–68. [DOI] [PubMed] [Google Scholar]
- [82].van den Akker F, Zhang X, Miyagi M, Huo X, Misono KS, Yee VC, Structure of the dimerized hormone-binding domain of a guanylyl-cyclase-coupled receptor, Nature 406(6791) (2000) 101–4. [DOI] [PubMed] [Google Scholar]
- [83].Pandey KN, Ligand-mediated endocytosis and intracellular sequestration of guanylyl cyclase/natriuretic peptide receptors: role of GDAY motif, Molecular and cellular biochemistry 334(1–2) (2010) 81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Pandey KN, Intracellular trafficking and metabolic turnover of ligand-bound guanylyl cyclase/atrial natriuretic peptide receptor-A into subcellular compartments, Molecular and cellular biochemistry 230(1–2) (2002) 61–72. [PubMed] [Google Scholar]
- [85].Pandey KN, Nguyen HT, Li M, Boyle JW, Natriuretic peptide receptor-A negatively regulates mitogen-activated protein kinase and proliferation of mesangial cells: role of cGMP-dependent protein kinase, Biochemical and biophysical research communications 271(2) (2000) 374–9. [DOI] [PubMed] [Google Scholar]
- [86].Pandey KN, Functional roles of short sequence motifs in the endocytosis of membrane receptors, Front Biosci (Landmark Ed) 14 (2009) 5339–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Saftig P, Klumperman J, Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function, Nat Rev Mol Cell Biol 10(9) (2009) 623–35. [DOI] [PubMed] [Google Scholar]
- [88].Hirst J, Lindsay MR, Robinson MS, GGAs: roles of the different domains and comparison with AP-1 and clathrin, Mol Biol Cell 12(11) (2001) 3573–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Parent JL, Labrecque P, Driss Rochdi M, Benovic JL, Role of the differentially spliced carboxyl terminus in thromboxane A2 receptor trafficking: identification of a distinct motif for tonic internalization, J Biol Chem 276(10) (2001) 7079–85. [DOI] [PubMed] [Google Scholar]
- [90].Yoneyama Y, Lanzerstorfer P, Niwa H, Umehara T, Shibano T, Yokoyama S, Chida K, Weghuber J, Hakuno F, Takahashi SI, IRS-1 acts as an endocytic regulator of IGF-I receptor to facilitate sustained IGF signaling, Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Fessart D, Simaan M, Zimmerman B, Comeau J, Hamdan FF, Wiseman PW, Bouvier M, Laporte SA, Src-dependent phosphorylation of beta2-adaptin dissociates the beta-arrestin-AP-2 complex, J Cell Sci 120(Pt 10) (2007) 1723–32. [DOI] [PubMed] [Google Scholar]
- [92].Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, Kirchhausen T, Association of Dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling, Dev Cell 12(1) (2007) 129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kang RS, Folsch H, ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells, J Cell Biol 193(1) (2011) 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Le Roy C, Wrana JL, Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling, Nat Rev Mol Cell Biol 6(2) (2005) 112–26. [DOI] [PubMed] [Google Scholar]
- [95].Law HT, Lin AE, Kim Y, Quach B, Nano FE, Guttman JA, Francisella tularensis uses cholesterol and clathrin-based endocytic mechanisms to invade hepatocytes, Sci Rep 1 (2011) 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Schutze S, Machleidt T, Adam D, Schwandner R, Wiegmann K, Kruse ML, Heinrich M, Wickel M, Kronke M, Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling, J Biol Chem 274(15) (1999) 10203–12. [DOI] [PubMed] [Google Scholar]
- [97].Smani Y, Docobo-Perez F, Lopez-Rojas R, Dominguez-Herrera J, Ibanez-Martinez J, Pachon J, Platelet-activating factor receptor initiates contact of Acinetobacter baumannii expressing phosphorylcholine with host cells, J Biol Chem 287(32) (2012) 26901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Schwartz V, Kruttgen A, Weis J, Weber C, Ostendorf T, Lue H, Bernhagen J, Role for CD74 and CXCR4 in clathrin-dependent endocytosis of the cytokine MIF, Eur J Cell Biol 91(6–7) (2012) 435–49. [DOI] [PubMed] [Google Scholar]
- [99].Schlegel R, Dickson RB, Willingham MC, Pastan IH, Amantadine and dansylcadaverine inhibit vesicular stomatitis virus uptake and receptor-mediated endocytosis of alpha 2-macroglobulin, Proc Natl Acad Sci U S A 79(7) (1982) 2291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ivanov AI, Pharmacological inhibition of endocytic pathways: is it specific enough to be useful?, Methods Mol Biol 440 (2008) 15–33. [DOI] [PubMed] [Google Scholar]
- [101].Chow JC, Condorelli G, Smith RJ, Insulin-like growth factor-I receptor internalization regulates signaling via the Shc/mitogen-activated protein kinase pathway, but not the insulin receptor substrate-1 pathway, J Biol Chem 273(8) (1998) 4672–80. [DOI] [PubMed] [Google Scholar]
- [102].Ray E, Samanta AK, Dansyl cadaverine regulates ligand induced endocytosis of interleukin-8 receptor in human polymorphonuclear neutrophils, FEBS Lett 378(3) (1996) 235–9. [DOI] [PubMed] [Google Scholar]
- [103].Ray E, Samanta AK, Receptor-mediated endocytosis of IL-8: a fluorescent microscopic evidence and implication of the process in ligand-induced biological response in human neutrophils, Cytokine 9(8) (1997) 587–96. [DOI] [PubMed] [Google Scholar]
- [104].Donaldson JG, McPherson PS, Membrane trafficking heats up in Pavia. Golgi Meeting on Membrane Trafficking in Global Cellular Responses, EMBO Rep 10(2) (2009) 132–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].McMahon HT, Boucrot E, Molecular mechanism and physiological functions of clathrin-mediated endocytosis, Nat Rev Mol Cell Biol 12(8) (2011) 517–33. [DOI] [PubMed] [Google Scholar]
- [106].Wang LH, Rothberg KG, Anderson RG, Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation, J Cell Biol 123(5) (1993) 1107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Gotte M, Sofeu Feugaing DD, Kresse H, Biglycan is internalized via a chlorpromazine-sensitive route, Cell Mol Biol Lett 9(3) (2004) 475–81. [PubMed] [Google Scholar]
- [108].Nawa M, Takasaki T, Yamada K, Kurane I, Akatsuka T, Interference in Japanese encephalitis virus infection of Vero cells by a cationic amphiphilic drug, chlorpromazine, J Gen Virol 84(Pt 7) (2003) 1737–41. [DOI] [PubMed] [Google Scholar]
- [109].Kuhn DA, Vanhecke D, Michen B, Blank F, Gehr P, Petri-Fink A, Rothen-Rutishauser B, Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages, Beilstein J Nanotechnol 5 (2014) 1625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Blitzer JT, Nusse R, A critical role for endocytosis in Wnt signaling, BMC Cell Biol 7 (2006) 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Johannessen LE, Pedersen NM, Pedersen KW, Madshus IH, Stang E, Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and Grb2-containing clathrin-coated pits, Mol Cell Biol 26(2) (2006) 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Vieira AV, Lamaze C, Schmid SL, Control of EGF receptor signaling by clathrin-mediated endocytosis, Science 274(5295) (1996) 2086–9. [DOI] [PubMed] [Google Scholar]
- [113].Zhang J, Ferguson SS, Barak LS, Menard L, Caron MG, Dynamin and beta-arrestin reveal distinct mechanisms for G protein-coupled receptor internalization, J Biol Chem 271(31) (1996) 18302–5. [DOI] [PubMed] [Google Scholar]
- [114].Wang LH, Sudhof TC, Anderson RG, The appendage domain of alpha-adaptin is a high affinity binding site for dynamin, J Biol Chem 270(17) (1995) 10079–83. [DOI] [PubMed] [Google Scholar]
- [115].Praefcke GJ, Ford MG, Schmid EM, Olesen LE, Gallop JL, Peak-Chew SY, Vallis Y, Babu MM, Mills IG, McMahon HT, Evolving nature of the AP2 alpha-appendage hub during clathrin-coated vesicle endocytosis, EMBO J 23(22) (2004) 4371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Marks B, Stowell MH, Vallis Y, Mills IG, Gibson A, Hopkins CR, McMahon HT, GTPase activity of dynamin and resulting conformation change are essential for endocytosis, Nature 410(6825) (2001) 231–5. [DOI] [PubMed] [Google Scholar]
- [117].Rappoport JZ, Benmerah A, Simon SM, Analysis of the AP-2 adaptor complex and cargo during clathrin-mediated endocytosis, Traffic 6(7) (2005) 539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Warnock DE, Schmid SL, Dynamin GTPase, a force-generating molecular switch, Bioessays 18(11) (1996) 885–93. [DOI] [PubMed] [Google Scholar]
- [119].Miwako I, Schmid SL, Clathrin-coated vesicle formation from isolated plasma membranes, Methods Enzymol 404 (2005) 503–11. [DOI] [PubMed] [Google Scholar]
- [120].Sever S, Damke H, Schmid SL, Dynamin:GTP controls the formation of constricted coated pits, the rate limiting step in clathrin-mediated endocytosis, J Cell Biol 150(5) (2000) 1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Song BD, Leonard M, Schmid SL, Dynamin GTPase domain mutants that differentially affect GTP binding, GTP hydrolysis, and clathrin-mediated endocytosis, J Biol Chem 279(39) (2004) 40431–6. [DOI] [PubMed] [Google Scholar]
- [122].Song BD, Yarar D, Schmid SL, An assembly-incompetent mutant establishes a requirement for dynamin self-assembly in clathrin-mediated endocytosis in vivo, Mol Biol Cell 15(5) (2004) 2243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Tremblay CS, Brown FC, Collett M, Saw J, Chiu SK, Sonderegger SE, Lucas SE, Alserihi R, Chau N, Toribio ML, McCormack MP, Chircop M, Robinson PJ, Jane SM, Curtis DJ, Loss-of-function mutations of Dynamin 2 promote T-ALL by enhancing IL-7 signalling, Leukemia 30(10) (2016) 1993–2001. [DOI] [PubMed] [Google Scholar]
- [124].Calebiro D, Nikolaev VO, Persani L, Lohse MJ, Signaling by internalized G-protein-coupled receptors, Trends Pharmacol Sci 31(5) (2010) 221–8. [DOI] [PubMed] [Google Scholar]
- [125].Garofalo C, Mancarella C, Grilli A, Manara MC, Astolfi A, Marino MT, Conte A, Sigismund S, Care A, Belfiore A, Picci P, Scotlandi K, Identification of common and distinctive mechanisms of resistance to different anti-IGF-IR agents in Ewing’s sarcoma, Mol Endocrinol 26(9) (2012) 1603–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Davey NE, Van Roey K, Weatheritt RJ, Toedt G, Uyar B, Altenberg B, Budd A, Diella F, Dinkel H, Gibson TJ, Attributes of short linear motifs, Mol Biosyst 8(1) (2012) 268–81. [DOI] [PubMed] [Google Scholar]
- [127].Kozik P, Francis RW, Seaman MN, Robinson MS, A screen for endocytic motifs, Traffic 11(6) (2010) 843–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Bonifacino JS, Traub LM, Signals for sorting of transmembrane proteins to endosomes and lysosomes, Annu Rev Biochem 72 (2003) 395–447. [DOI] [PubMed] [Google Scholar]
- [129].Mardones GA, Burgos PV, Lin Y, Kloer DP, Magadan JG, Hurley JH, Bonifacino JS, Structural basis for the recognition of tyrosine-based sorting signals by the mu3A subunit of the AP-3 adaptor complex, J Biol Chem 288(13) (2013) 9563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Chen WJ, Goldstein JL, Brown MS, NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor, The Journal of biological chemistry 265(6) (1990) 3116–23. [PubMed] [Google Scholar]
- [131].Chen Z, Dupre DJ, Le Gouill C, Rola-Pleszczynski M, Stankova J, Agonist-induced internalization of the platelet-activating factor receptor is dependent on arrestins but independent of G-protein activation. Role of the C terminus and the (D/N)PXXY motif, J Biol Chem 277(9) (2002) 7356–62. [DOI] [PubMed] [Google Scholar]
- [132].Bouley R, Sun TX, Chenard M, McLaughlin M, McKee M, Lin HY, Brown D, Ausiello DA, Functional role of the NPxxY motif in internalization of the type 2 vasopressin receptor in LLC-PK1 cells, Am J Physiol Cell Physiol 285(4) (2003) C750–62. [DOI] [PubMed] [Google Scholar]
- [133].Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G, The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein, J Biol Chem 275(22) (2000) 17187–94. [DOI] [PubMed] [Google Scholar]
- [134].Stolt PC, Bock HH, Modulation of lipoprotein receptor functions by intracellular adaptor proteins, Cell Signal 18(10) (2006) 1560–71. [DOI] [PubMed] [Google Scholar]
- [135].Collawn JF, Stangel M, Kuhn LA, Esekogwu V, Jing SQ, Trowbridge IS, Tainer JA, Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis, Cell 63(5) (1990) 1061–72. [DOI] [PubMed] [Google Scholar]
- [136].Ktistakis NT, Thomas D, Roth MG, Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins, The Journal of cell biology 111(4) (1990) 1393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Collawn JF, Kuhn LA, Liu LF, Tainer JA, Trowbridge IS, Transplanted LDL and mannose-6-phosphate receptor internalization signals promote high-efficiency endocytosis of the transferrin receptor, Embo J 10(11) (1991) 3247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM, Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor, Embo J 21(18) (2002) 4915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Paing MM, Johnston CA, Siderovski DP, Trejo J, Clathrin adaptor AP2 regulates thrombin receptor constitutive internalization and endothelial cell resensitization, Molecular and cellular biology 26(8) (2006) 3231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Traub LM, Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection, The Journal of cell biology 163(2) (2003) 203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Singh S, D’Mello V, van Bergen en Henegouwen P, Birge RB, A NPxY-independent beta5 integrin activation signal regulates phagocytosis of apoptotic cells, Biochemical and biophysical research communications 364(3) (2007) 540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Maginnis MS, Mainou BA, Derdowski A, Johnson EM, Zent R, Dermody TS, NPXY motifs in the beta1 integrin cytoplasmic tail are required for functional reovirus entry, J Virol 82(7) (2008) 3181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, Johnson GL, Structural and evolutionary division of phosphotyrosine binding (PTB) domains, J Mol Biol 345(1) (2005) 1–20. [DOI] [PubMed] [Google Scholar]
- [144].Madshus IH, Stang E, Internalization and intracellular sorting of the EGF receptor: a model for understanding the mechanisms of receptor trafficking, Journal of cell science 122(Pt 19) (2009) 3433–9. [DOI] [PubMed] [Google Scholar]
- [145].Davis CG, Lehrman MA, Russell DW, Anderson RG, Brown MS, Goldstein JL, The JD mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors, Cell 45(1) (1986) 15–24. [DOI] [PubMed] [Google Scholar]
- [146].Colgan L, Liu H, Huang SY, Liu YJ, Dileucine motif is sufficient for internalization and synaptic vesicle targeting of vesicular acetylcholine transporter, Traffic 8(5) (2007) 512–22. [DOI] [PubMed] [Google Scholar]
- [147].Letourneur F, Klausner RD, A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains, Cell 69(7) (1992) 1143–57. [DOI] [PubMed] [Google Scholar]
- [148].Geisler C, Dietrich J, Nielsen BL, Kastrup J, Lauritsen JP, Odum N, Christensen MD, Leucine-based receptor sorting motifs are dependent on the spacing relative to the plasma membrane, J Biol Chem 273(33) (1998) 21316–23. [DOI] [PubMed] [Google Scholar]
- [149].Johnson KF, Kornfeld S, A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function, J Biol Chem 267(24) (1992) 17110–5. [PubMed] [Google Scholar]
- [150].Mattera R, Boehm M, Chaudhuri R, Prabhu Y, Bonifacino JS, Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants, J Biol Chem 286(3) (2011) 2022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Rohrer J, Schweizer A, Russell D, Kornfeld S, The targeting of Lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane, The Journal of cell biology 132(4) (1996) 565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Carvajal-Gonzalez JM, Gravotta D, Mattera R, Diaz F, Perez Bay A, Roman AC, Schreiner RP, Thuenauer R, Bonifacino JS, Rodriguez-Boulan E, Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B, Proc Natl Acad Sci U S A 109(10) (2012) 3820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Ortega B, Mason AK, Welling PA, A tandem Di-hydrophobic motif mediates clathrin-dependent endocytosis via direct binding to the AP-2 alphasigma2 subunits, J Biol Chem 287(32) (2012) 26867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Kirchhausen T, Adaptors for clathrin-mediated traffic, Annu Rev Cell Dev Biol 15 (1999) 705–32. [DOI] [PubMed] [Google Scholar]
- [155].Crosetto N, Tikkanen R, Dikic I, Oncogenic breakdowns in endocytic adaptor proteins, FEBS Lett 579(15) (2005) 3231–8. [DOI] [PubMed] [Google Scholar]
- [156].Sorkin A, Goh LK, Endocytosis and intracellular trafficking of ErbBs, Exp Cell Res 314(17) (2008) 3093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].von Zastrow M, Sorkin A, Signaling on the endocytic pathway, Curr Opin Cell Biol 19(4) (2007) 436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Carpenter G, Liao HJ, Trafficking of receptor tyrosine kinases to the nucleus, Exp Cell Res 315(9) (2009) 1556–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Grewal T, Enrich C, Annexins--modulators of EGF receptor signalling and trafficking, Cell Signal 21(6) (2009) 847–58. [DOI] [PubMed] [Google Scholar]
- [160].Wang YN, Hung MC, Nuclear functions and subcellular trafficking mechanisms of the epidermal growth factor receptor family, Cell Biosci 2(1) (2012) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD, Identification and characterization of a functional mitochondrial angiotensin system, Proc Natl Acad Sci U S A 108(36) (2011) 14849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Inagami T, Mitochondrial angiotensin receptors and aging, Circ Res 109(12) (2011) 1323–4. [DOI] [PubMed] [Google Scholar]
- [163].Re RN, The intracrine hypothesis and intracellular peptide hormone action, Bioessays 25(4) (2003) 401–9. [DOI] [PubMed] [Google Scholar]
- [164].Jensen RE, Dunn CD, Protein import into and across the mitochondrial inner membrane: role of the TIM23 and TIM22 translocons, Biochim Biophys Acta 1592(1) (2002) 25–34. [DOI] [PubMed] [Google Scholar]
- [165].Pfanner N, Hoeben P, Tropschug M, Neupert W, The carboxyl-terminal two-thirds of the ADP/ATP carrier polypeptide contains sufficient information to direct translocation into mitochondria, J Biol Chem 262(31) (1987) 14851–4. [PubMed] [Google Scholar]
- [166].Li XC, Hopfer U, Zhuo JL, AT1 receptor-mediated uptake of angiotensin II and NHE-3 expression in proximal tubule cells through a microtubule-dependent endocytic pathway, American journal of physiology. Renal physiology 297(5) (2009) F1342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ferrao FM, Cardoso LHD, Drummond HA, Li XC, Zhuo JL, Gomes DS, Lara LS, Vieyra A, Lowe J, Luminal ANG II is internalized as a complex with AT1R/AT2R heterodimers to target endoplasmic reticulum in LLC-PK1 cells, American journal of physiology. Renal physiology 313(2) (2017) F440–F449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Feil R, Kemp-Harper B, cGMP signalling: from bench to bedside. Conference on cGMP generators, effectors and therapeutic implications, EMBO Rep 7(2) (2006) 149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Lincoln TM, Cornwell TL, Intracellular cyclic GMP receptor proteins, Faseb J 7(2) (1993) 328–38. [DOI] [PubMed] [Google Scholar]
- [170].Kumar R, Cartledge WA, Lincoln TM, Pandey KN, Expression of guanylyl cyclase-A/atrial natriuretic peptide receptor blocks the activation of protein kinase C in vascular smooth muscle cells. Role of cGMP and cGMP-dependent protein kinase, Hypertension 29(1 Pt 2) (1997) 414–21. [DOI] [PubMed] [Google Scholar]
- [171].Sauro MD, Fitzpatrick DF, Atrial natriuretic peptides inhibit protein kinase C activation in rat aortic smooth muscle, Pept Res 3(3) (1990) 138–41. [PubMed] [Google Scholar]
- [172].Kadam P, McAllister R, Urbach JS, Sandberg K, Mueller SC, Live Cell Imaging and 3D Analysis of Angiotensin Receptor Type 1a Trafficking in Transfected Human Embryonic Kidney Cells Using Confocal Microscopy, J Vis Exp (121) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Li XC, Zhuo JL, Mechanisms of AT1a receptor-mediated uptake of angiotensin II by proximal tubule cells: a novel role of the multiligand endocytic receptor megalin, American journal of physiology. Renal physiology 307(2) (2014) F222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Li XC, Navar LG, Shao Y, Zhuo JL, Genetic deletion of AT1a receptors attenuates intracellular accumulation of ANG II in the kidney of AT1a receptor-deficient mice, American journal of physiology. Renal physiology 293(2) (2007) F586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Li XC, Zhuo JL, Selective knockdown of AT1 receptors by RNA interference inhibits Val5-ANG II endocytosis and NHE-3 expression in immortalized rabbit proximal tubule cells, Am J Physiol Cell Physiol 293(1) (2007) C367–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG, Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor, Hypertension 39(1) (2002) 116–21. [DOI] [PubMed] [Google Scholar]
- [177].Tan M, Yamaguchi S, Nakamura M, Nagamune T, Real-time monitoring of pH-dependent intracellular trafficking of ovarian cancer G protein-coupled receptor 1 in living leukocytes, J Biosci Bioeng 126(3) (2018) 363–370. [DOI] [PubMed] [Google Scholar]
- [178].Tabor A, Moller D, Hubner H, Kornhuber J, Gmeiner P, Visualization of ligand-induced dopamine D2S and D2L receptor internalization by TIRF microscopy, Sci Rep 7(1) (2017) 10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Aymerich MS, Lopez-Azcarate J, Bonaventura J, Navarro G, Fernandez-Suarez D, Casado V, Mayor F, Lluis C, Valencia M, Artieda J, Franco R, Real-time G-protein-coupled receptor imaging to understand and quantify receptor dynamics, ScientificWorldJournal 11 (2011) 1995–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Fortian A, Sorkin A, Live-cell fluorescence imaging reveals high stoichiometry of Grb2 binding to the EGF receptor sustained during endocytosis, J Cell Sci 127(Pt 2) (2014) 432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, Basbaum AI, Dierich A, Vonesh JL, Gaveriaux-Ruff C, Kieffer BL, Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo, Proc Natl Acad Sci U S A 103(25) (2006) 9691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Roth RH, Zhang Y, Huganir RL, Dynamic imaging of AMPA receptor trafficking in vitro and in vivo, Curr Opin Neurobiol 45 (2017) 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Ayaz G, Halici Z, Albayrak A, Karakus E, Cadirci E, Evaluation of 5-HT7 Receptor Trafficking on In Vivo and In Vitro Model of Lipopolysaccharide (LPS)-Induced Inflammatory Cell Injury in Rats and LPS-Treated A549 Cells, Biochem Genet 55(1) (2017) 34–47. [DOI] [PubMed] [Google Scholar]
- [184].Wang Y, Roche O, Yan MS, Finak G, Evans AJ, Metcalf JL, Hast BE, Hanna SC, Wondergem B, Furge KA, Irwin MS, Kim WY, Teh BT, Grinstein S, Park M, Marsden PA, Ohh M, Regulation of endocytosis via the oxygen-sensing pathway, Nat Med 15(3) (2009) 319–24. [DOI] [PubMed] [Google Scholar]
- [185].Bansal A, Gierasch LM, The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation, Cell 67(6) (1991) 1195–201. [DOI] [PubMed] [Google Scholar]
- [186].Kishi M, Liu X, Hirakawa T, Reczek D, Bretscher A, Ascoli M, Identification of two distinct structural motifs that, when added to the C-terminal tail of the rat LH receptor, redirect the internalized hormone-receptor complex from a degradation to a recycling pathway, Mol Endocrinol 15(9) (2001) 1624–35. [DOI] [PubMed] [Google Scholar]
- [187].Thies RS, Webster NJ, McClain DA, A domain of the insulin receptor required for endocytosis in rat fibroblasts, J Biol Chem 265(17) (1990) 10132–7. [PubMed] [Google Scholar]
- [188].Mulkearns EE, Cooper JA, FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis, Mol Biol Cell 23(7) (2012) 1330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Levitan ES, Takimoto K, Surface expression of Kv1 voltage-gated K+ channels is governed by a C-terminal motif, Trends Cardiovasc Med 10(7) (2000) 317–20. [DOI] [PubMed] [Google Scholar]
- [190].Herz J, Bock HH, Lipoprotein receptors in the nervous system, Annu Rev Biochem 71 (2002) 405–34. [DOI] [PubMed] [Google Scholar]
- [191].Doray B, Bruns K, Ghosh P, Kornfeld SA, Autoinhibition of the ligand-binding site of GGA1/3 VHS domains by an internal acidic cluster-dileucine motif, Proceedings of the National Academy of Sciences of the United States of America 99(12) (2002) 8072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Bogdanovic O, Delfino-Machin M, Nicolas-Perez M, Gavilan MP, Gago-Rodrigues I, Fernandez-Minan A, Lillo C, Rios RM, Wittbrodt J, Martinez-Morales JR, Numb/Numbl-Opo antagonism controls retinal epithelium morphogenesis by regulating integrin endocytosis, Developmental cell 23(4) (2012) 782–95. [DOI] [PubMed] [Google Scholar]
- [193].Capilla E, Suzuki N, Pessin JE, Hou JC, The glucose transporter 4 FQQI motif is necessary for Akt substrate of 160-kilodalton-dependent plasma membrane translocation but not Golgi-localized (gamma)-ear-containing Arf-binding protein-dependent entry into the insulin-responsive storage compartment, Mol Endocrinol 21(12) (2007) 3087–99. [DOI] [PubMed] [Google Scholar]