Abstract
Objectives. To evaluate the effectiveness of point-of-care informational interventions in general practitioner clinics to improve influenza and pneumococcal vaccination uptake among elderly patients.
Methods. We conducted a pragmatic, cluster-randomized crossover trial in 22 private general practitioner clinics in Singapore, from November 2017 to July 2018. We included all patients aged 65 years or older. Clinics were assigned to a 3-month intervention (flyers and posters encouraging vaccination) plus 1-month washout period, and a 4-month control period (usual care). Primary outcomes were differences in vaccination uptake rates between periods. Secondary outcomes were identification of other factors associated with vaccination uptake.
Results. A total of 4378 and 4459 patients were included in the intervention and control periods, respectively. Both influenza (5.9% vs 4.8%; P = .047) and pneumococcal (5.7% vs 3.7%; P = .001) vaccination uptake rates were higher during the intervention period compared with the control period. On multilevel logistic regression analysis, follow-up for hypertension, diabetes mellitus, hyperlipidemia, or any combination of the 3 was associated with uptake of both vaccines.
Conclusions. Point-of-care informational interventions likely contributed to increased influenza and pneumococcal vaccination uptake. Patients on follow-up for hypertension, diabetes mellitus, hyperlipidemia, or any combination of the 3 were more likely to receive influenza and pneumococcal vaccination and should be actively engaged by physicians.
Trial Registration. ClinicalTrials.gov Identifier: NCT03445117.
Influenza and pneumococcal vaccines have been shown to be effective in reducing the risk of influenza virus and Streptococcus pneumoniae bacterial infections, respectively, in elderly persons.1–3 Current international guidelines recommend that all persons aged 65 years or older receive annual influenza vaccination4 and pneumococcal vaccination with single doses of PCV13 and PPSV23.5
However, vaccination uptake rates among the elderly vary substantially across countries.6 Barriers to vaccination include a lack of awareness, vaccine misconceptions, doubts about necessity of vaccines, and cost issues.7–9 Failure of health care workers to provide recommendations also results in missed opportunities to vaccinate eligible patients.10 Conversely, effective measures to increase vaccination uptake include invitational brochures, brief messages with cues to action, improving accessibility, clinician reminders, and providing information on available financial schemes.11–13
Singapore is a tropical country that experiences year-round circulation of influenza viruses. Typically, there are bimodal peaks in annual influenza activity,14 and an estimated 1 in 5 adults are infected over a 1-year period.15 Both influenza and pneumococcal disease are important causes of mortality and morbidity among the elderly.16,17 However, despite national recommendations18 and the widespread availability of vaccines, vaccination rates in the elderly are low, estimated at 17.0% for influenza and 6.1% for at least 1 pneumococcal vaccination.19,20
Private general practitioner (GP) clinics provide 80% of primary care services in Singapore, including 55% of chronic disease care.21 Each clinic is staffed by 1 or more regular GPs and clinic assistants (CAs) who assist with patient registration, dispensing of medication, and billing of patients. Vaccination services are available on site, and many clinics offer the use of Medisave (a compulsory medical savings scheme for all Singapore residents),22 which can be used to pay for vaccinations, thereby reducing out-of-pocket costs. These clinics are hence well-suited for opportunistic vaccination of patients.
However, current evidence on increasing influenza and pneumococcal vaccination uptake is largely from Western temperate countries, which differ from settings such as Singapore in terms of seasonal patterns, cultural norms, primary care infrastructure, and health care financing. Studies in additional settings are hence needed to verify the effectiveness of specific interventions in different cultures and health systems.
We evaluated the effectiveness of an intervention utilizing informational materials, sited at the point of care in private GP clinics, to improve influenza and pneumococcal vaccination uptake among elderly patients.
METHODS
We conducted a pragmatic, cluster-randomized crossover trial in private GP clinics in Singapore, from November 2017 through July 2018.
Setting
We engaged the senior management of 3 private GP clinic chains (comprising 30 clinics in total) to participate in the study. The senior management subsequently shared the study details (as provided by the study team) with the lead GPs in each clinic during their regular business meetings as well as by e-mail dissemination and sought their agreement to participate.
Of the 3 chains, 1 (comprising 7 clinics) declined participation because of concerns about additional administrative workload. Within the other 2 chains (comprising 23 clinics), 1 clinic was excluded because of differences in clinic software and operational challenges with data extraction. The remaining 22 clinics were included in the study (Figure A, available as a supplement to the online version of this article at http://www.ajph.org). The participating clinics were well-distributed across urban areas and housing estates in the country, providing primary care services to community-dwelling elderly patients with wide demographic variation.
Participants
We included all patients aged 65 years or older, with or without chronic disease, who visited and were registered as clinic patients during the study period.
Randomization and Allocation
We conducted randomization at the clinic level, with each clinic comprising 1 cluster. The study team used a computerized random number generator to allocate clinics to start with either the intervention or control period.
The study comprised 2 phases: a 4-month initial phase followed by a 4-month crossover phase. During the initial phase, half of the clinics underwent a 3-month intervention period (during which patients received the informational intervention), followed by a 1-month washout period. The other half of the clinics underwent the control period (during which patients received usual care) for 4 months. The clinics subsequently switched over in the crossover phase (Figure A). Because of the nature of the intervention, blinding of clinic staff and patients was not possible.
Intervention
The intervention materials comprised informational flyers and posters carrying uncomplicated messages encouraging patients to get vaccinated against influenza and pneumococcal disease (Figure B). These messages stated key benefits identified to be important to seniors from previous qualitative studies.7 The option to make payment by using Medisave (available in all clinics in our study) was also highlighted. The design and message content of the materials were developed by an external commercial designer and discussed with a health communications expert (M. O. L.), with revisions made to ensure realism and GP clinic context appropriateness before dissemination. Materials were first developed in English, and then translated to Chinese to cater to the large proportion of mainly Chinese-literate elderly patients who were expected to visit the clinics (70% of Singaporeans are Chinese).
Before each study phase, the study team briefed all GPs and CAs from clinics undergoing the intervention period on the workflow (Figure C, available as a supplement to the online version of this article at http://www.ajph.org). In each clinic, CAs managed the distribution of the flyers, and 1 or 2 posters were put up in prominent areas. At the point of registration, CAs identified patients aged 65 years or older and handed each patient a flyer to read while awaiting their turn for medical consultation. Patients were instructed to show the doctor the flyer during consultation, and the doctor would counsel and vaccinate patients who were agreeable and fulfilled eligibility criteria (e.g., no recent similar vaccine given, no previous allergic reactions).
Data Collection
All study data were obtained from the clinics’ electronic medical records, extracted with the help of information technology vendors for the clinic management software. All key patient identifiers were anonymized before use by the study team. Within each study phase, each patient was identified by a unique study identity number to match repeat visits. We collected data on age, gender, ethnicity, postal codes (to match housing type, commonly used as a surrogate measure for income status in Singapore as it correlates with household income),23 and all dispensed medications and vaccines over each study phase for each patient. We considered patients to be on chronic disease follow-up with the clinic if they had been dispensed medications identified to treat hypertension, diabetes mellitus, hyperlipidemia, asthma, or chronic obstructive pulmonary disease at any point over the study period.
Outcomes
Primary outcomes were differences in uptake rates for influenza and pneumococcal vaccinations between the intervention period and the control period. For pneumococcal vaccination, patients could have received either PCV13 or PPSV23 vaccines, based on the clinical management of the GPs. Vaccinations given during the postintervention washout period were considered to be part of the intervention period (to include patients who had received the intervention and were only vaccinated slightly later, for reasons such as needing more time to consider or having acute illness and needing to recover first).
Secondary outcomes were identification of other factors at the individual and cluster levels associated with vaccination uptake.
Statistical Analysis
We originally hypothesized that the intervention would be less effective for pneumococcal vaccination, given its much higher overall cost, and, hence, we based power calculation on estimated pneumococcal vaccination uptake rates. To detect an absolute difference of 5% between the intervention and control periods (10% vs 5%, respectively), at 80% study power, an α level of 5%, an assumed within-cluster, within-period intracluster correlation (ICC) of 0.04 and a within-cluster, between-period ICC of 0, we would require data from a minimum of 200 patients per clinic in each phase, across a total of 22 clinics.
We performed descriptive analysis of participant characteristics, with categorical variables presented as proportions. We described age by using ordered categories or as a continuous variable with nonparametric properties and summarized by using the median value with interquartile ranges. To evaluate primary outcomes, we constructed separate multilevel logistic regression models for influenza and pneumococcal vaccination uptake. In both models, we used vaccination uptake within the period (intervention or control) as the outcome and included as covariates a fixed intervention effect, a fixed study phase effect (to control for changes occurring over time that were unrelated to the intervention), a random cluster effect, and a random cluster-by-study phase effect.24,25 The latter 2 variables were included to adjust for similarities likely present among patients within clusters, both within the same study phase and across study phases.
To assess secondary outcomes, we added to these models other independent individual-level variables, including age, gender, ethnicity, housing type, and follow-up for various chronic diseases. We also added in 1 cluster-level variable (i.e., number of unique elderly patients seen by each clinic over each 4-month phase [as a measure of clinic workload]).
We present measures of association as adjusted odds ratios (AORs) with 95% confidence intervals (CIs). We performed statistical analysis by using Stata version 13 (StataCorp LP, College Station, TX) with P values of less than .05 regarded as statistically significant.
We calculated the actual within-cluster, within-period ICC and the within-cluster, between-period ICC by using the linear regression approach outlined by Morgan et al.24 These approaches used an analysis of variance and pairwise estimating approach, respectively, and were coded in R (2018; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
The study had 1 major deviation. Originally, the initial phase was to run from November 2017 to February 2018, and the crossover phase from March to June 2018. However, because of logistics issues faced by all clinics with obtaining seasonal influenza vaccine supplies ahead of the midyear season, commencement of the crossover phase was delayed by 1 month. We retained the study design of two 4-month phases and ran the initial and crossover phases from November 2017 to February 2018 and from April to July 2018, respectively.
A total of 4378 and 4459 patients visited the clinics during the intervention and control periods, respectively. Distributions of age, gender, housing type, and follow-up for chronic diseases were generally comparable between intervention and control periods, as well as initial and crossover phases (Table 1). There were slightly more persons of Chinese origin in control period clinics and a higher percentage of persons of Malay origin in intervention period clinics during the initial phase, with the reverse observed during the crossover phase. This reflected variations in the ethnic composition of patients across different clinics.
TABLE 1—
Baseline Characteristics of Patients in Intervention- and Control-Period Clinics, by Study Phases: Singapore, November 2017–July 2018
| Initial Phase |
Crossover Phase |
|||||
| Patient Characteristic | Intervention-Period Clinics (n = 2267), Median (IQR) or No. (%) | Control-Period Clinics (n = 2277), Median (IQR) or No. (%) | Pa | Intervention-Period Clinics (n = 2111), Median (IQR) or No. (%) | Control-Period Clinics (n = 2182), Median (IQR) or No. (%) | Pa |
| Age, y | 70 (67–76) | 71 (68–77) | .41 | 71 (68–77) | 71 (68–76) | .41 |
| Age group, y | .7 | .58 | ||||
| 65–69 | 944 (41.6) | 925 (40.6) | 849 (40.2) | 879 (40.3) | ||
| 70–74 | 621 (27.4) | 602 (26.4) | 565 (26.8) | 623 (28.6) | ||
| 75–79 | 355 (15.7) | 372 (16.3) | 333 (15.8) | 334 (15.3) | ||
| 80–84 | 195 (8.6) | 214 (9.4) | 197 (9.3) | 181 (8.3) | ||
| ≥ 85 | 152 (6.7) | 164 (7.2) | 167 (7.9) | 165 (7.6) | ||
| Gender = male | 1021 (45.0) | 1021 (44.8) | .89 | 950 (45.0) | 996 (45.6) | .68 |
| Ethnic group | <.01 | <.01 | ||||
| Chinese | 1779 (78.5) | 1864 (81.9) | 1770 (83.8) | 1670 (76.5) | ||
| Malay | 217 (9.6) | 116 (5.1) | 100 (4.7) | 211 (9.7) | ||
| Indian | 93 (4.1) | 83 (3.6) | 94 (4.5) | 84 (3.8) | ||
| Others | 52 (2.3) | 57 (2.5) | 58 (2.7) | 59 (2.7) | ||
| Not stated | 126 (5.6) | 157 (6.9) | 89 (4.2) | 158 (7.2) | ||
| Rental or smaller housing flats | 143 (6.3) | 141 (6.2) | .46 | 111 (5.3) | 134 (6.1) | .15 |
| On follow-up with clinic for | ||||||
| Diabetes | 120 (5.3) | 111 (4.9) | .52 | 114 (5.4) | 113 (5.2) | .75 |
| Hypertension | 435 (19.2) | 400 (17.6) | .16 | 390 (18.5) | 441 (20.2) | .15 |
| Hyperlipidemia | 311 (13.7) | 300 (13.2) | .59 | 300 (14.2) | 319 (14.6) | .7 |
| Asthma or COPD or both | 24 (1.1) | 22 (1.0) | .76 | 18 (0.9) | 27 (1.2) | .22 |
Note. COPD = chronic obstructive pulmonary disease; IQR = interquartile range.
P values compare differences in characteristics of patients from clinics undergoing intervention versus control periods during the initial phase and crossover phase, respectively.
Primary Outcomes
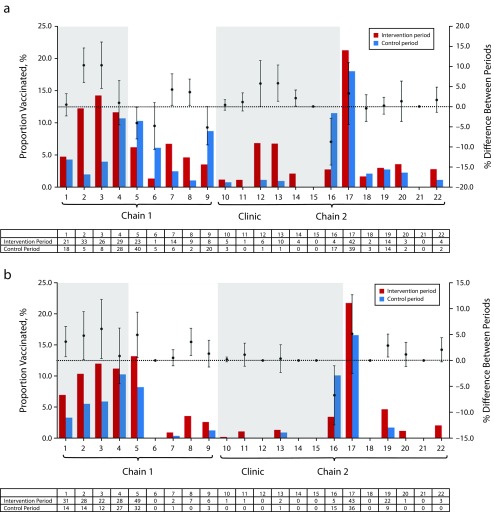
Figure 1 shows the influenza and pneumococcal vaccination uptake rates, respectively. Overall uptake rates were significantly higher in clinics during the intervention period compared with the control period for both influenza (5.9% vs 4.8%; P = .047) and pneumococcal (5.7% vs 3.7%; P = .001) vaccines. A large proportion of patients had concurrent receipt of vaccines: of 602 patients receiving any vaccination, 286 (47.5%) received both vaccinations within the same period, 187 (31.1%) received influenza vaccination only, and 129 (21.4%) received pneumococcal vaccination only.
FIGURE 1—
Rates Across Clinics, by Period, of (a) Influenza Vaccination and (b) Pneumococcal Vaccination: Singapore, November 2017–July 2018
Note. Shaded background indicates clinics that underwent the intervention period during the initial phase (Nov 2017–Feb 2018). White backgrounds indicate clinics that underwent the intervention period during the crossover phase (Apr–Jul 2018). Error bars indicate 95% confidence intervals for differences in vaccination rates between periods. Tables show total vaccines given in each clinic during intervention and control periods.
On multivariable analysis (Tables 2 and 3), patients who visited the clinic during the intervention period were more likely to receive influenza vaccination (AOR = 1.43; 95% CI = 0.99, 2.07; P = .06) than were those who visited during the control period. They were also more likely to receive pneumococcal vaccination (AOR = 1.78; 95% CI = 1.28, 2.48; P < .01).
TABLE 2—
Factors Associated With Influenza Vaccination Uptake: Singapore, November 2017–July 2018
| Variable | Vaccinated (n = 473), No. (%) | Not Vaccinated (n = 8364), No. (%) | AOR (95% CI) |
| Clinic undergoing intervention period (vs control period) | 259 (54.8) | 4119 (49.2) | 1.43 (0.99, 2.07) |
| Study phase Nov–Feb (vs Apr–Jul) | 272 (57.5) | 4272 (51.1) | 1.27 (0.86, 1.87) |
| Age group, y | |||
| 65–69 | 211 (44.6) | 3386 (40.5) | 1 (Ref) |
| 70–74 | 128 (27.1) | 2283 (27.3) | 0.98 (0.77, 1.23) |
| 75–79 | 70 (14.8) | 1324 (15.8) | 0.99 (0.74, 1.32) |
| 80–84 | 44 (9.3) | 743 (8.9) | 1.00 (0.70, 1.42) |
| ≥ 85 | 20 (4.2) | 628 (7.5) | 0.58 (0.35, 0.94) |
| Male gender | 221 (46.7) | 3767 (45.0) | 1.08 (0.89, 1.31) |
| Ethnic group | |||
| Chinese | 413 (87.3) | 6670 (79.7) | 1 (Ref) |
| Malay | 20 (4.2) | 624 (7.5) | 0.63 (0.39, 1.00) |
| Indian | 13 (2.8) | 341 (4.1) | 0.70 (0.39, 1.25) |
| Others | 9 (1.9) | 217 (2.6) | 0.67 (0.33, 1.33) |
| Not stated | 18 (3.8) | 512 (6.1) | 0.85 (0.51, 1.41) |
| Rental or smaller housing flats | 19 (4.0) | 510 (6.1) | 0.63 (0.39, 1.03) |
| On follow-up for diabetes, hypertension, or hyperlipidemia | 170 (35.9) | 1868 (22.3) | 1.61 (1.29, 2.00) |
| On follow-up for COPD or asthma | 8 (1.7) | 83 (1.0) | 1.27 (0.60, 2.72) |
| No. of elderly patients (≥ 65 y) seen by clinic over 4 mo | |||
| 0–100 (5 clinics) | 18 (3.8) | 573 (6.9) | 0.28 (0.09, 0.88) |
| 101–200 (8 clinics) | 93 (19.7) | 2344 (28.0) | 0.39 (0.16, 0.94) |
| 201–300 (5 clinics) | 224 (47.4) | 2135 (25.5) | 1 (Ref) |
| > 300 (4 clinics) | 138 (29.2) | 3312 (39.6) | 0.41 (0.16, 1.10) |
Note. AOR = adjusted odds ratio; CI = confidence interval; COPD = chronic obstructive pulmonary disease. Multilevel logistic regression model with fixed intervention effect, fixed period effect, random cluster effect (by clinic), and random cluster-by-period effect.
TABLE 3—
Factors Associated With Pneumococcal Vaccination Uptake: Singapore, November 2017–July 2018
| Variable | Vaccinated (n = 415), No. (%) | Not Vaccinated (n = 8422), No. (%) | AOR (95% CI) |
| Clinic undergoing intervention period (vs control period) | 251 (60.5) | 4127 (49.0) | 1.78 (1.28, 2.48) |
| Study phase Nov–Feb (vs Apr–Jul) | 199 (48.0) | 4345 (51.6) | 0.75 (0.51, 1.11) |
| Age group, y | |||
| 65–69 | 176 (42.4) | 3421 (40.6) | 1 (Ref) |
| 70–74 | 112 (27.0) | 2299 (27.3) | 1.04 (0.80, 1.35) |
| 75–79 | 66 (15.9) | 1328 (15.8) | 1.13 (0.83, 1.54) |
| 80–84 | 42 (10.1) | 745 (8.8) | 1.25 (0.87, 1.82) |
| ≥ 85 | 19 (4.6) | 629 (7.5) | 0.72 (0.44, 1.20) |
| Gender = male | 201 (48.4) | 3787 (45.0) | 1.25 (1.01, 1.55) |
| Ethnic group | |||
| Chinese | 373 (89.9) | 6710 (79.7) | 1 (Ref) |
| Malay | 26 (6.3) | 618 (7.3) | 0.90 (0.59, 1.39) |
| Indian | 8 (1.9) | 346 (4.1) | 0.47 (0.23, 0.99) |
| Others | 2 (0.5) | 224 (2.7) | 0.16 (0.04, 0.67) |
| Not stated | 6 (1.4) | 524 (6.2) | 0.38 (0.16, 0.89) |
| Rental or smaller housing flats | 32 (7.7) | 497 (5.9) | 1.32 (0.88, 1.99) |
| On follow-up for diabetes, hypertension, or hyperlipidemia | 199 (48.0) | 1839 (21.8) | 2.64 (2.10, 3.31) |
| On follow-up for COPD or asthma | 13 (3.1) | 78 (0.9) | 2.81 (1.47, 5.37) |
| No. of elderly patients (≥ 65 y) seen by clinic over 4 mo | |||
| 0–100 (5 clinics) | 1 (0.2) | 590 (7.0) | 0.02 (0.00, 0.40) |
| 101–200 (8 clinics) | 68 (16.4) | 2369 (28.1) | 0.28 (0.05, 1.56) |
| 201–300 (5 clinics) | 188 (45.3) | 2171 (25.8) | 1 (Ref) |
| > 300 (4 clinics) | 158 (38.1) | 3292 (39.1) | 0.51 (0.07, 3.51) |
Note. AOR = adjusted odds ratio; CI = confidence interval; COPD = chronic obstructive pulmonary disease. Multilevel logistic regression model with fixed intervention effect, fixed period effect, random cluster effect (by clinic), and random cluster-by-period effect.
Secondary Outcomes
Being on follow up for hypertension, diabetes mellitus, hyperlipidemia, or any combination of the 3 was significantly associated with both influenza and pneumococcal vaccinations. As compared with the persons of Chinese origin, persons of Malay origin were less likely to receive influenza vaccination, whereas Indian and other ethnic groups were less likely to receive pneumococcal vaccination. Pneumococcal vaccination was also positively associated with male gender and follow up for asthma or COPD or both. By contrast, influenza vaccination was negatively associated with being aged 85 years or older (vs being aged 65–69 years).
In addition, patients in clinics that saw 201 to 300 elderly patients over a 4-month study phase were more likely to receive influenza and pneumococcal vaccination, compared with those in clinics that saw fewer elderly patients (0–100 or 101–200 over the same duration), or clinics that saw more (although results were not significantly different for both types of vaccination).
For influenza vaccination, the estimated within-cluster, within-period ICC was 0.044, and the within-cluster, between-period ICC was 0.024. For pneumococcal vaccination, the corresponding ICCs were 0.057 and 0.040.
DISCUSSION
Our point-of-care study intervention appeared to contribute to modest but significantly increased vaccination rates among elderly primary care patients. While there were variations in both influenza and pneumococcal vaccination uptake rates across clinics and study phases, our analysis took into account key variables to be included when evaluating a cluster randomized crossover trial to ensure that we accurately assessed the overall effect of our intervention.
The effect size of our intervention was greater for pneumococcal vaccination compared with influenza vaccination, possibly because low awareness was a more important barrier toward pneumococcal vaccination,7 and this was easily addressed by our intervention. In contrast, while patients were more likely to know about influenza vaccination, they might not have viewed it as a necessity because of low perceived susceptibility to infection or perceived severity of health complications.26,27
Among vaccinated patients, a high proportion received both influenza and pneumococcal vaccines, which has been similarly observed in previous studies.28 Concurrent recommendations of influenza and pneumococcal vaccinations to elderly patients would help reduce missed opportunities for vaccination. The safety profile of concurrent vaccination has been established.29
Vaccination uptake varied widely across clinics, likely reflective of differing practices among GPs. Absolute differences in vaccination rates between intervention and control periods were higher by up to 10% for influenza vaccination and 6% for pneumococcal vaccination. At the cluster level, influenza and pneumococcal vaccination uptake appeared to be most strongly associated with clinics that saw a moderate number of elderly patients (201–300 unique patients over a 4-month period, or about 3 per work day), compared with those that saw greater or lesser numbers of elderly. This may reflect a balance between clinics’ experience in elderly management and proactiveness in preventive care, and operational constraints limiting consult time and quality of counseling for each patient.
Multivariable analysis showed that patients on follow up for hypertension, diabetes mellitus, hyperlipidemia, or any combination of the 3 conditions were more likely to receive influenza and pneumococcal vaccination. Those on follow-up for asthma or COPD or both were also more likely to receive pneumococcal vaccine. Vaccination is associated with having a regular family doctor and receiving recommendations by health care professionals19,20,26 and is also more likely in adult patients with comorbidities.26,30 These suggest that the therapeutic relationship can influence patients’ decisions to receive vaccination. Hence, primary care physicians should actively engage elderly patients on their regular follow up on the topic of vaccination.
However, the oldest patients were less likely to take influenza vaccine, probably because of financial constraints (including less Medisave to utilize) and low perceived benefits of the vaccine. There were also differences among the ethnic groups. Persons of Malay origin were less likely to receive influenza vaccine, and persons of Indian and other ethnic origins were less likely to receive pneumococcal vaccine as compared with persons of Chinese origin. The effects of ethnicity may be mediated through language barriers and cultural receptiveness to vaccines. It could also have reflected confounding by socioeconomic factors such as educational level and household income. These were not collected as part of this study but have been shown to be positively associated with influenza and pneumococcal vaccine uptake rates.31,32
Interestingly, some clinics assigned to the control period during the initial phase (November–February) had higher influenza vaccination uptake during the control compared with the intervention period. Although there is year-round risk of influenza in tropical Singapore, some patients might have associated influenza vaccination with pretravel preparations and, hence, timed their vaccination around the year-end holiday season, independent of the intervention.
Current recommendations in Singapore are that the elderly (aged ≥ 65 years) and those with key medical conditions should receive annual influenza vaccination.18 There is currently debate on whether vaccinating the elderly twice a year in the tropics may be necessary33 to counteract observed waning of antibody titers and effectiveness in older individuals.34,35 Overall, public education should target the oldest elderly and lower income subgroups and aim to change perceptions regarding the benefit of repeat influenza vaccination, as well as highlight the risk of severe influenza-associated outcomes in vulnerable persons who do not travel.
Strengths and Limitations
Our study had a number of strengths. We systematically collected routine data from clinic electronic medical records (a robust resource for patients’ clinical and demographic data) to conduct and evaluate our study. This method may also be more acceptable to GPs who are considering participating in research, as it reduces additional administrative workload of clinic staff.36 We recruited patients with wide demographic variation, from clinics sited across different localities, which increases the generalizability of our findings. We relied on nonphysician staff (CAs) to drive our intervention by activating patients through personal contact, which has been shown to be effective and more likely to be sustainable.11,12 Our interventions were brief and low-cost, and we believe them to be practically implementable in the GP clinic setting.
Our study also had some limitations. We were unable to determine the baseline vaccination rates of the clinics (including records from other health care institutions) because of absence of a comprehensive national adult vaccination database. High baseline rates would have placed a ceiling effect on the effectiveness of our intervention. However, the clinic chains had never participated in any adult vaccination-related programs before this study, and we believe that baseline vaccination rates were low (similar to national estimates around the time of the study), with a large proportion of patients still requiring vaccination. By including the postintervention washout period as part of the intervention period, we might have captured patients who did not receive the intervention at all in our intervention period group; this would have caused a bias toward the null in terms of estimating the effect of the intervention on our study outcomes.
We were unable to control for other external factors that may have acted as possible confounders toward vaccination uptake, such as the content and quality of any health counseling by GPs or the CAs. However, we verified that, over the study period, there were no other major factors—such as use of other educational materials on vaccination, changes in the lead GPs for each clinic, widespread campaigns on vaccination, or changes in funding mechanisms—which could have affected the way health counseling was conducted.
We were also unable to measure the true compliance of each clinic to the intervention because of limitations in staff capacity (both for the study team and the clinics) for collecting these data. Similar pragmatic trials have demonstrated study compliance to be as low as 21.0%.13 Nevertheless, the study intent was to evaluate the real-world impact of such an intervention, which likely played a contributory role in modestly increasing vaccination uptake.
Public Health Implications
Point-of-care informational interventions delivered in private GP clinics likely contributed to modest increases in influenza and pneumococcal vaccination uptake. Concurrent administration of both vaccinations should be recommended to reduce missed opportunities. Clinics seeing moderate elderly patient loads were most likely to have high vaccination rates. Health promotion efforts should also target the oldest elderly subgroup and emphasize the importance of annual influenza vaccination. Patients on follow-up for hypertension, diabetes mellitus, hyperlipidemia, or any combination of the 3 conditions were more likely to receive influenza and pneumococcal vaccination and should be actively engaged by physicians.
ACKNOWLEDGMENTS
This study was funded by the Health Services Research New Investigator Grant administered by the National Medical Research Council under the Ministry of Health, Singapore (grant HNIG16Jul007).
Note. The funder had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
CONFLICTS OF INTEREST
All authors declare no conflict of interest.
HUMAN PARTICIPANT PROTECTION
The National Healthcare Group Domain Specific Review Board approved the study, with waiver of informed consent from patients (DSRB number: 2017/00441). This study was registered at https://www.clinicaltrials.gov (trial registry number: NCT03445117).
REFERENCES
- 1.Falkenhorst G, Remschmidt C, Harder T, Hummers-Pradier E, Wichmann O, Bogdan C. Effectiveness of the 23-Valent Pneumococcal Polysaccharide Vaccine (PPV23) against pneumococcal disease in the elderly: systematic review and meta-analysis. PLoS One. 2017;12(1):e0169368. doi: 10.1371/journal.pone.0169368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonten MJ, Huijts SM, Bolkenbaas M et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 3.Demicheli V, Jefferson T, Di Pietrantonj C et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2018;2:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Vaccines against influenza WHO position paper—November 2012. Wkly Epidemiol Rec. 2012;87(47):461–476. [PubMed] [Google Scholar]
- 5.Tomczyk S, Bennett NM, Stoecker C et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2014;63(37):822–825. [PMC free article] [PubMed] [Google Scholar]
- 6.de Gomensoro E, Del Giudice G, Doherty TM. Challenges in adult vaccination. Ann Med. 2018;50(3):181–192. doi: 10.1080/07853890.2017.1417632. [DOI] [PubMed] [Google Scholar]
- 7.Ho HJ, Chan YY, Ibrahim MAB, Wagle AA, Wong CM, Chow A. A formative research-guided educational intervention to improve the knowledge and attitudes of seniors towards influenza and pneumococcal vaccinations. Vaccine. 2017;35(47):6367–6374. doi: 10.1016/j.vaccine.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Kwon DS, Kim K, Park SM. Factors associated with influenza vaccination coverage among the elderly in South Korea: the Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV) BMJ Open. 2016;6(12):e012618. doi: 10.1136/bmjopen-2016-012618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bödeker B, Remschmidt C, Schmich P, Wichmann O. Why are older adults and individuals with underlying chronic diseases in Germany not vaccinated against flu? A population-based study. BMC Public Health. 2015;15(1):618. doi: 10.1186/s12889-015-1970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casalino E, Ghazali A, Bouzid D et al. Patient’s behaviors and missed opportunities for vaccination against seasonal epidemic influenza and evaluation of their impact on patient’s influenza vaccine uptake. PLoS One. 2018;13(3):e0193029. doi: 10.1371/journal.pone.0193029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas RE, Lorenzetti DL. Interventions to increase influenza vaccination rates of those 60 years and older in the community. Cochrane Database Syst Rev. 2018;5:CD005188. doi: 10.1002/14651858.CD005188.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau D, Hu J, Majumdar SR, Storie DA, Rees SE, Johnson JA. Interventions to improve influenza and pneumococcal vaccination rates among community-dwelling adults: a systematic review and meta-analysis. Ann Fam Med. 2012;10(6):538–546. doi: 10.1370/afm.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrett E, Williamson E, van Staa T et al. Text messaging reminders for influenza vaccine in primary care: a cluster randomised controlled trial (TXT4FLUJAB) BMJ Open. 2016;6(2):e010069. doi: 10.1136/bmjopen-2015-010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee VJ, Yap J, Ong JB et al. Influenza excess mortality from 1950–2000 in tropical Singapore. PLoS One. 2009;4(12):e8096. doi: 10.1371/journal.pone.0008096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh EH, Jiang L, Hsu JP et al. Epidemiology and relative severity of influenza subtypes in Singapore in the post-pandemic period from 2009 to 2010. Clin Infect Dis. 2017;65(11):1905–1913. doi: 10.1093/cid/cix694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow A, Ma S, Ling AE, Chew SK. Influenza-associated deaths in tropical Singapore. Emerg Infect Dis. 2006;12(1):114–121. doi: 10.3201/eid1201.050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low S, Chan FL, Cutter J, Ma S, Goh KT, Chew SK. A national study of the epidemiology of pneumococcal disease among hospitalised patients in Singapore: 1995 to 2004. Singapore Med J. 2007;48(9):824–829. [PubMed] [Google Scholar]
- 18.Society of Infectious Diseases Singapore. Clinical practice guidelines on adult vaccination in Singapore. Available at: http://www.sids.org.sg/publications. Accessed March 10, 2019.
- 19.Ang LW, Cutter J, James L, Goh KT. Factors associated with influenza vaccine uptake in older adults living in the community in Singapore. Epidemiol Infect. 2017;145(4):775–786. doi: 10.1017/S0950268816002491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ang LW, Cutter J, James L, Goh KT. Epidemiological characteristics associated with uptake of pneumococcal vaccine among older adults living in the community in Singapore: results from the National Health Surveillance Survey 2013. Scand J Public Health. 2018;46(2):175–181. doi: 10.1177/1403494817720105. [DOI] [PubMed] [Google Scholar]
- 21.Khoo HS, Lim YW, Vrijhoef HJ. Primary healthcare system and practice characteristics in Singapore. Asia Pac Fam Med. 2014;13(1):8. doi: 10.1186/s12930-014-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Central Provident Fund Board Singapore. Medisave 2019. Available at: https://www.cpf.gov.sg/Members/Schemes/schemes/healthcare/medisave. Accessed March 10, 2019.
- 23.Department of Statistics Singapore. Key household income trends, 2017–2018. Available at: https://www.singstat.gov.sg/-/media/files/publications/households/pp-s24.pdf. Accessed March 10, 2019.
- 24.Morgan KE, Forbes AB, Keogh RH, Jairath V, Kahan BC. Choosing appropriate analysis methods for cluster randomised cross-over trials with a binary outcome. Stat Med. 2017;36(2):318–333. doi: 10.1002/sim.7137. [DOI] [PubMed] [Google Scholar]
- 25.Turner RM, White IR, Croudace T. Analysis of cluster randomized cross-over trial data: a comparison of methods. Stat Med. 2007;26(2):274–289. doi: 10.1002/sim.2537. [DOI] [PubMed] [Google Scholar]
- 26.Nagata JM, Hernandez-Ramos I, Kurup AS, Albrecht D, Vivas-Torrealba C, Franco-Paredes C. Social determinants of health and seasonal influenza vaccination in adults ≥ 65 years: a systematic review of qualitative and quantitative data. BMC Public Health. 2013;13(1):388. doi: 10.1186/1471-2458-13-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans MR, Prout H, Prior L, Tapper-Jones LM, Butler CC. A qualitative study of lay beliefs about influenza immunisation in older people. Br J Gen Pract. 2007;57(538):352–358. [PMC free article] [PubMed] [Google Scholar]
- 28.Domínguez A, Soldevila N, Toledo D et al. Factors associated with pneumococcal polysaccharide vaccination of the elderly in Spain: a cross-sectional study. Hum Vaccin Immunother. 2016;12(7):1891–1899. doi: 10.1080/21645515.2016.1149661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo YB, Choi WS, Lee J, Song JY, Cheong HJ, Kim WJ. Comparison of immunogenicity and safety of an influenza vaccine administered concomitantly with a 13-valent pneumococcal conjugate vaccine or 23-valent polysaccharide pneumococcal vaccine in the elderly. Clin Exp Vaccine Res. 2017;6(1):38–44. doi: 10.7774/cevr.2017.6.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganczak M, Gil K, Korzeń M, Bażydło M. Coverage and influencing determinants of influenza vaccination in elderly patients in a country with a poor vaccination Implementation. Int J Environ Res Public Health. 2017;14(6):665. doi: 10.3390/ijerph14060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La EM, Trantham L, Kurosky SK, Odom D, Aris E, Hogea C. An analysis of factors associated with influenza, pneumoccocal, Tdap, and herpes zoster vaccine uptake in the US adult population and corresponding inter-state variability. Hum Vaccin Immunother. 2018;14(2):430–441. doi: 10.1080/21645515.2017.1403697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen M, Lindegaard H, Hendricks O, Friis-Møller N. Factors associated with influenza and pneumococcal vaccine uptake among rheumatoid arthritis patients in Denmark invited to participate in a pneumococcal vaccine trial (Immunovax_RA) Scand J Rheumatol. 2017;46(6):446–453. doi: 10.1080/03009742.2016.1242774. [DOI] [PubMed] [Google Scholar]
- 33.Haur SY, Sadarangani S, Young B et al. Six-monthly versus annual influenza vaccination in older adults in the tropics: an observer-blind, active-comparator controlled, randomised superiority trial. Clin Infect Dis. 2018;69(1):121–129. doi: 10.1093/cid/ciy836. [DOI] [PubMed] [Google Scholar]
- 34.Young B, Zhao X, Cook AR, Parry CM, Wilder-Smith A, Chen MI. Do antibody responses to the influenza vaccine persist year-round in the elderly? A systematic review and meta-analysis. Vaccine. 2017;35(2):212–221. doi: 10.1016/j.vaccine.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Young B, Sadarangani S, Jiang L, Wilder-Smith A, Chen MI. Duration of influenza vaccine effectiveness: a systematic review, meta-analysis, and meta-regression of test-negative design case–control studies. J Infect Dis. 2018;217(5):731–741. doi: 10.1093/infdis/jix632. [DOI] [PubMed] [Google Scholar]
- 36.van Staa TP, Dyson L, McCann G et al. The opportunities and challenges of pragmatic point-of-care randomised trials using routinely collected electronic records: evaluations of two exemplar trials. Health Technol Assess. 2014;18(43):1–146. doi: 10.3310/hta18430. [DOI] [PMC free article] [PubMed] [Google Scholar]