Abstract
Introduction
N-Acetylcysteine (NAC) may have efficacy in treating tobacco use disorder (TUD) by reducing craving and smoking reward. This study examines whether treatment with NAC may have a clinical efficacy in the treatment of TUD.
Methods
A 12-week double blind randomized controlled trial was conducted to compare the clinical efficacy of NAC 3 g/day versus placebo. We recruited 34 outpatients with therapy resistant TUD concurrently treated with smoking-focused group behavioral therapy. Participants had assessments of daily cigarette use (primary outcome), exhaled carbon monoxide (COEXH) (secondary outcome), and quit rates as defined by COEXH<6 ppm. Depression was measured with the Hamilton Depression Rating Scale (HDRS). Data were analyzed using conventional and modified intention-to-treat endpoint analyses.
Results
NAC treatment significantly reduced the daily number of cigarettes used (Δ mean±SD = −10.9 ± 7.9 in the NAC-treated versus −3.2 ± 6.1 in the placebo group) and COEXH (Δ mean± SD = −10.4 ± 8.6 ppm in the NAC-treated versus −1.5 ± 4.5 ppm in the placebo group); 47.1% of those treated with NAC versus 21.4% of placebo-treated patients were able to quit smoking as defined by COEXH<6 ppm. NAC treatment significantly reduced the HDRS score in patients with tobacco use disorder.
Conclusions
These data show that treatment with NAC may have a clinical efficacy in TUD. NAC combined with appropriate psychotherapy appears to be an efficient treatment option for TUD.
Keywords: N-Acetylcysteine, Smoking cessation, Depression, Oxidative stress, Inflammation, Glutathione.
Introduction
N-Acetylcysteine (NAC) is a widely available, tolerable, and affordable nutraceutical supplement that increases the intracellular levels of glutathione, a major antioxidant, and modulates oxidative, immune-inflammatory, glutamatergic, and neurotrophic pathways.1,2 NAC is well tolerated, with a side-effect profile that does not differ significantly from placebo when administered orally at doses up to 3 g/day.3 NAC reduces cue extinction and craving in models of opiate and cocaine dependence.4–6
There is evidence that glutamate, including the cystine–glutamate exchange system, mediate behavioral sensitization, craving and drug intake in preclinical models of addiction.7 In addictions, glutamate, which is restored by NAC, is a core determinant of relapses.8 NAC may reduce craving and reward behaviors in nicotine dependence, which are both modulated by glutamate.9 NAC by restoring glutamate levels at the inhibitory GluR2/3 pre-synaptic receptor may reduce the reinstatement of drug seeking.10 Treatment with NAC by activating cystine–glutamate exchange may prevent withdrawal and craving in addiction.11 Moreover, NAC can improve some of the damage caused by tobacco smoke exposure, such as oxidative damage to the lung and other tissues.12 Finally, animal models of addiction show that glutamate uptake is involved in the effects of NAC.13
There are only two studies that examined treatment with NAC for smoking cessation. A first small placebo-controlled study with 29 nicotine-dependent patients used 2.4 g/day of NAC as a treatment for tobacco cessation. There were no significant differences in exhaled carbon monoxide (COEXH) levels between patients treated with NAC and placebo, and no significant difference between NAC and placebo treatment in the daily use of cigarettes.14 A second pilot double-blind-controlled study examined the effects of NAC 3.6 g/day (n = 10) versus placebo (n = 12) on smoking reward.8 These authors found that smokers treated with NAC reported that the first cigarette after an abstinence period of 3.5 days was significantly less rewarding than reported by subjects who were treated with placebo. The authors also reported a non-significant trend toward fewer withdrawal symptoms in the patients treated with NAC.8 All in all, these two studies provide inconsistent evidence that NAC may have some efficacy in the treatment of tobacco use disorder.
There is a strong comorbidity between mood disorders, including depression, and tobacco use disorder.15,16 Tobacco use disorder is two to four times more likely to be diagnosed in subjects with psychiatric disorders. In major depressive disorder, the prevalence of tobacco use disorder is as high as 40–60%.17 There is some evidence that NAC has clinical efficacy in unipolar depression and bipolar disorder.18,19 Trials in bipolar disorder showed large effect sizes for the treatment of depression and quality of life.18,20 These findings suggest that, in addition to the previously highlighted mechanisms of reducing craving and drug-seeking behavior, NAC may potentially be of benefit in alleviating depressive symptoms that are common in nicotine withdrawal states.
The aim of this study was to delineate whether NAC (3 g/day) may have a clinical efficacy in treating tobacco use disorder by reducing both daily cigarette use and COEXH, and whether these effects are associated with reductions in severity of depression.
Experimental procedures
Study participants
The study was conducted at the Center of Smoking Cessation, at Londrina State University (UEL), Brazil. We included 34 outpatients with tobacco use disorder. They received monthly group behavioral therapy treatments before and during the course of the study. All were current smokers and were refractory to first-line smoking cessation treatments, which included nicotine replacement therapy, bupropion or varenicline.17 Subjects were men and women aged 18–65 years of all ethnicities.
The diagnosis of tobacco use disorder was made by a research psychiatrist using a Portuguese translation of the semi-structured DSM-IV interview (SCID).21 We diagnosed ‘current smokers’ according to the US Centers for Disease Control and Prevention (CDC) criteria, i.e. individuals who and at the time of interview reported smoking every day or some days and had during their lifetime smoked at least 100 cigarettes.22 We included individuals with and without mood disorders. Females of childbearing potential who were sexually active were only included if they were using effective contraception. Exclusion criteria were: unstable systemic disease that requires medical treatment, active gastrointestinal ulcers, pregnancy or breast-feeding and a history of anaphylactic reaction to NAC or any other component of the preparation. All participants had results in the normal range on routine laboratory tests, such as hemogram, aspartate transaminase (AST), alanine transaminase (ALT), and creatinine. The study period extended from January 2013 to March 2014. The study was evaluated and approved by the Ethics Research Committee of UEL. All individuals gave written informed consent. The study was registered on clinicaltrials.gov number NCT02124525.
Measurements
Prior to randomization into the clinical trial, all individuals underwent a semi-structured interview and provided information on socio-demographic and clinical data and current and lifetime smoking history. We also scored the Fagerstrom Test for Nicotine Dependence (FTND) and used this scale to assess the severity of tobacco by dependence.23 The test was translated and adapted to Portuguese by Carmo and Pueyo.24 We used a cut-off point for FTND nicotine dependence >5.25,26 The number of pack-years was calculated as the number of cigarettes smoked per day multiplied by number of years smoked and divided by 20 (1 pack has 20 cigarettes).
The primary outcome measurement (number of cigarettes per day) was collected by face-to-face interviews by a trained, senior psychiatrist (blinded to treatment conditions) using a semi-structured interview at baseline and 4, 8, and 12 weeks later. The secondary outcome measure of smoking reduction was evaluated using exhaled carbon monoxide (COEXH). The latter was measured at baseline and 4, 8, and 12 weeks later using a Micro CO Meter with an electrochemical sensor (Micro CO – Micro Medical Ltd, Rochester, Kent, UK). All participants took a deep breath, held their breath for 20 seconds and exhaled completely through a mouthpiece. A cut-off point for COEXH levels ≤6 ppm was used as a criterion for smoking cessation.27 The raters of the primary and secondary outcome measures were blind to treatment assignment. The third outcome measure was severity of depression as measured with the Hamilton Depression Rating Scale – 17 items (HDRS) at baseline and endpoint. The HDRS was translated and adapted for the Brazilian population.28
The Sheehan Disability Scale, a self-rated scale, was used to assess the disability in three areas: (1) occupational, (2) social life and leisure, (3) family life, activities and household activities. Items are scored from 0 to 10: 0–3 indicates mild, 4–6 moderate and 7–10 severe disability.29 We computed the body mass index as the body weight (kg)/height (m)2 ratio. We measured the systolic and diastolic blood pressure using a mercury sphygmomanometer (after 10-minute rest; right arm; sitting position) and computed the mean value of two consecutive measurements (5 minutes apart). The primary and secondary outcome measures, the Sheehan Disability Scale, and BMI were measured at baseline and 4, 8, and 12 weeks later. The HDRS and blood pressure were assessed at baseline and endpoint.
Study design and procedures
This randomized clinical trial was designed to examine the clinical efficacy of NAC for tobacco use disorder. We randomized the patients (n = 34) into two groups (17 patients in each group) in a double-blind manner to receive NAC or indistinguishable placebo. The dose of NAC was 3 g/day administered in 500 mg capsules in two daily doses, three capsules in the morning and three in the evening. This dosage was based on previous studies in which similar dosages had shown to be effective and well tolerated.
Statistical analyses
We checked baseline data, including clinical and socio-demographic data for balance between the NAC and placebo group using analysis of variance (ANOVA) and analyses of contingence tables (χ2-tests). The primary outcome measure was the daily number of cigarettes, the secondary outcome measure was the COEXH, and the tertiary outcome measure was the HDRS. Systolic and diastolic blood pressure, creatinine, AST, ALT, and quality of life measurements were other outcome measurements. We performed a conventional intention-to-treat (ITT) analysis on the basis of an as-randomized approach with inclusion of all patients treated or not; and a modified ITT (mITT) with an as-treated (AT) protocol design, i.e. patients who started treatment and who had at least one rating 1 month after starting the treatment. These analyses were performed using the last observation carried forward method. We also performed a per protocol (PP) approach whereby only patients who completed all measurements were included (treatment received). The recommendations of the FDA and the Committee for Proprietary Medicinal Products are that an RCT should be assessed through both PP and ITT analyses and that when these approaches reach the same conclusion confidence in the results increases. The primary analyses were generalized linear model (GLM) analyses with the endpoint measurements as dependent variables and the baseline levels and treatment modality as predictor variables. We also performed RM design ANOVAs, which considered baseline and endpoint measurement as time effect and treatment (NAC versus placebo) as factor and the interaction time × treatment as the primary outcome. Side effects were analyzed by using Fisher's exact probability test and analysis of contingence tables (χ2 = tests). Repeated measurements of binary data over time were assessed using the non-parametric McNemar test. We analyzed the data using SPSS (version 19). Statistical significance was set at α = 0.05 (two tailed).
Results
Socio-demographic and clinical data
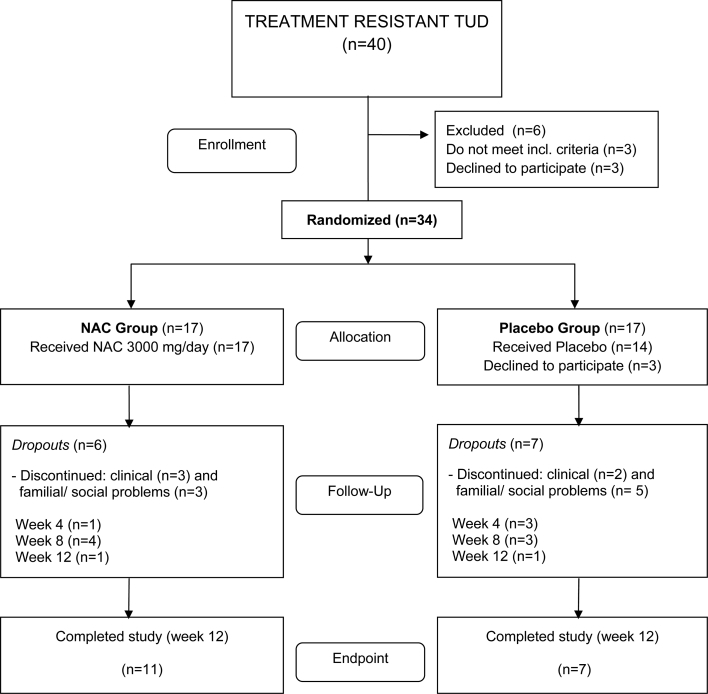
Figure 1 shows the CONSORT flow diagram illustrating the progress of the patients through the trial. Of the 40 individuals screened for inclusion and exclusion criteria, 34 were randomized to enter the study. Three randomized patients declined to participate (all three were randomized to the placebo group). Therefore, 17 patients allocated to the NAC treatment arm and 14 patients allocated to the placebo treatment arm started the study. One month later, 27 patients continued to participate in the study, i.e. 16 in the NAC group and 11 in the placebo group. Two months after starting treatment 20 patients participated, i.e. 12 in the NAC group and 8 in the placebo group. Eighteen patients completed the treatment protocol without violations of the protocol and thus participated in the study for the full 3 months. Eleven patients were in the NAC and seven in the placebo treatment groups. Thus, at endpoint there were 6 dropouts in the NAC group and 10 in the placebo group. The causes for dropouts in the study were refusal to take medication (three patients), having family and/or social matters (eight patients), and referral for clinical problems (five patients). Adverse effects reported as causes of discontinuation were nausea in two patients allocated to the NAC group, and general clinical problems not related to the intervention in the other cases.
Figure 1.
CONSORT flow chart.
Table 1 compares the clinical and socio-demographic data between both the NAC and placebo treatment groups. There were no significant differences at baseline in clinical and socio-demographic data, including age, years of education, onset of tobacco use disorder, duration of tobacco use, Sheehan disability scale, cigarettes smoked per day, FTND score, lifetime consumption, HDRS score, BMI, and BP, between the two treatment groups.
Table 1.
Clinical and socio-demographic data of the NAC and placebo treatment groups
| Characteristics | NAC group (n = 17) | Placebo group (n = 14) | F | df | P | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Current age | 51.93 | 7.022 | 50.76 | 11.819 | 0.105 | 1/29 | 0.748 |
| Years of education | 10.86 | 5.318 | 9.18 | 5.040 | 0.812 | 1/29 | 0.375 |
| Smoking onset age | 16.86 | 2.507 | 16.18 | 3.340 | 0.396 | 1/29 | 0.534 |
| Years of smoking | 35.00 | 7.766 | 33.29 | 11.889 | 0.213 | 1/29 | 0.648 |
| Pack-years | 32.64 | 18.519 | 31.43 | 18.369 | 0.038 | 1/29 | 0.846 |
| FTND | 4.50 | 1.743 | 4.82 | 2.186 | 0.201 | 1/29 | 0.657 |
| Gender | M = 7 F = 10 | M = 2 F = 12 | NA | NA | 0.132* | ||
Effects of treatment on the primary outcome
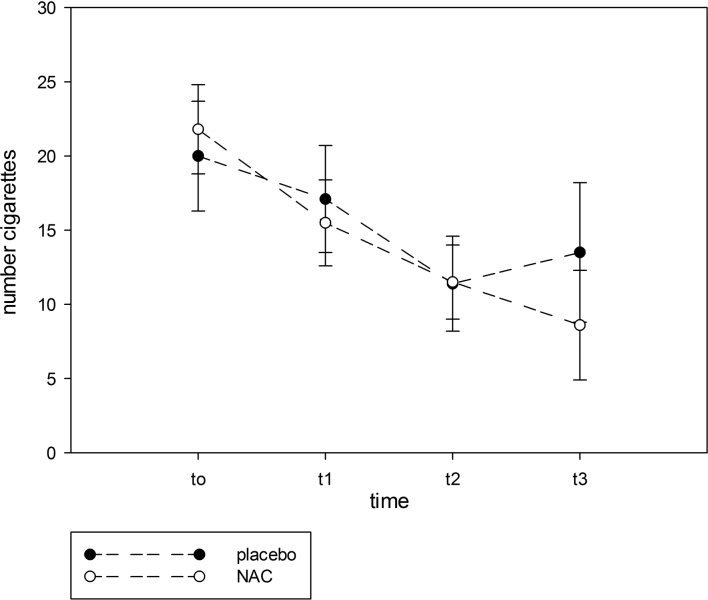
Table 2 shows the effects of treatment with NAC or placebo on the primary and secondary outcome measurements. In the ITT GLM analysis, we found that the number of cigarettes smoked at endpoint was significantly lower in the NAC group compared to the placebo group and that there were significant effects of baseline daily cigarette number. The Δ (mean± SD) daily number of cigarettes from baseline to three months later was −10.9 ± 7.9 in NAC-treated versus −3.2 ± 6.1 in placebo-treated patients (F = 8.77, df = 1/29, P = 0.006). RM design ANOVA performed on the time series of cigarette smoking showed a significant interaction time × treatment (F = 8.77, df = 1/29, P = 0.006), a significant effect of time (F = 29.65, df = 1/29, P < 0.001), but no overall difference between NAC and placebo (F = 0.83, df = 1/29, P = 0.364). The mITT GLM analysis showed that the endpoint daily cigarette usage was significantly lowered by treatment with NAC compared to the placebo group (Wald = 7.38, df = 1, P = 0.007; there were significant effects of baseline number of cigarettes/day: Wald = 39.15, df = 1, P < 0.001). In the PP GLM analysis, we found that there was a trend toward a significant effect of NAC reducing daily cigarette use as compared with placebo (Wald = 3.23, df = 1, P = 0.072; there were significant effects of baseline cigarette use: Wald = 24.05, df = 1, P < 0.001). Figure 2 shows the time course data for the effects of NAC versus placebo at the 4 time points. It can be seen that NAC began to diverge from placebo 8–12 weeks after starting treatment.
Table 2.
Effects of treatments on outcome measurements
| Variable | Mean (±SD) | Treatment Effect | Effect of Baseline | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NAC/ Placebo | Time 0 (Baseline) | Week 12 (Endpoint) | Wald | df | P | Wald | df | P | |
| Cigarettes | Placebo | 19.6 (9.7) | 16.4 (11.7) | 9.52 | 1 | 0.002 | 43.03 | 1 | <0.001 |
| NAC | 20.3 (9.8) | 9.4 (10.1) | |||||||
| COexh | Placebo | 16.6 (5.8) | 15.1 (7.6) | 9.92 | 1 | 13.96 | 1 | ||
| NAC | 20.8 (7.6) | 10.4 (8.3) | 0.002 | <0.001 | |||||
| HAM-D | Placebo | 13.6 (4.4) | 12.1 (4.6) | 5.62 | 1 | 17.75 | 1 | ||
| NAC | 12.7 (7.1) | 7.2 (6.3) | 0.018 | <0.001 | |||||
| BMI | Placebo | 26.2 (6.6) | 26.4 (6.9) | 3.98 | 1 | 0.046 | 916.52 | 1 | <0.001 |
| NAC | 27.1 (5.0) | 26.5 (5.3) | |||||||
| SBP | Placebo | 121.4 (23.4) | 118.9 (15.3) | 0.18 | 1 | 0.893 | 4.42 | 1 | 0.035 |
| NAC | 128.2 (16.7) | 121.8 (13.3) | |||||||
| DBP | Placebo | 75.7 (12.7) | 77.1 (11.1) | 0.35 | 1 | 0.554 | 2.67 | 1 | 0.102 |
| NAC | 75.5 (8.2) | 79.1 (5.4) | |||||||
| Sheehan (Social) | Placebo | 3.7 (3.1) | 2.6 (3.6) | 0.28 | 1 | 0.594 | 9.62 | 1 | 0.002 |
| NAC | 1.6 (2.5) | 1.0 (2.0) | |||||||
| Sheehan (Work) | Placebo | 2.6 (3.2) | 2.7 (3.9) | 2.85 | 1 | 0.092 | 88.97 | 1 | <0.001 |
| NAC | 1.7 (2.5) | 0.9 (2.1) | |||||||
| Sheehan (Family) | Placebo | 4.0 (3.7) | 2.9 (3.0 | 2.66 | 1 | 0.103 | 56.21 | 1 | <0.001 |
| NAC | 1.7 (2.5) | 0.6 (1.5) | |||||||
Figure 2.
The time course data for the effects of NAC versus placebo at the 4 time points (shown are the estimated marginal means with standard error). t0 = baseline, t1 = 4 weeks, t2 = 8 weeks, t3 = 12 weeks.
Effects of treatment on secondary outcomes
Table 2 shows the effect of NAC versus placebo on COEXH. In the ITT GLM analysis, we found that endpoint COEXH was significantly lower in the NAC treatment group than in the placebo group and that there was a significant effect of baseline COEXH. RM design ANOVA performed on the time series of COEXH showed a significant interaction time X treatment (F = 12.20, df = 1/29, P = 0.002), a significant effect of time (F = 21.79, df = 1/29, P < 0.001) but no overall difference between NAC and placebo (F = 0.00, df = 1/29, P = 0.932). In the mITT GLM analysis, we found that endpoint COEXH was significantly lowered by treatment with NAC compared to the placebo group (Wald = 5.60, df = 1, P = 0.018; there were significant effects of baseline COEXH: Wald = 6.22, df = 1, P = 0.013). In the PP GLM analysis, we found that there was a trend toward a significant effect of NAC reducing COEXH (Wald = 2.55, df = 1, P = 0.11; there were also no significant effects of baseline number of COEXH: Wald = 1.91, df = 1, P = 0.167).
The Δ (mean ± SD) COEXH from baseline to 3 months later was −10.4 ± 8.6 in NAC-treated patients versus −1.5 ± 4.5 in placebo-treated patients (F = 12.20, df = 1/29, P = 0.002). There was a significant correlation between the Δ COEXH and Δ daily cigarette usage (r = 0.705, P < 0.001, n = 31). In NAC-treated subjects there was a significantly greater quit rate (as defined by COEXH <6 ppm), i.e. 8/17 (McNemar test: P = 0.008), whereas in placebo-treated patients no such effect was found, i.e. 3/14 (P = 0.250).
Effects of treatment on other outcome measurements and side effects
Table 2 shows the effects of treatment on other outcome measurements according to PP GLM analyses. We found a significant effect of NAC reducing the HDRS score as compared with placebo. There were no significant correlations between the Δ HDRS (from baseline to endpoint) and Δ number of daily cigarettes (r = 0.316, P = 0.201, n = 18) or Δ COEXH (r = 0.14, P = 0.577 n = 18). There was also a marginally significant effect of NAC reducing the BMI. There were no significant effects of NAC versus placebo treatment on the other measurements (see Table 2).
Adverse events were monitored at each contact and they are summarized in Table 3. No serious treatment emerging adverse events were reported during the course of the study. The most common adverse effect was nausea and some patients reported treatment emergent diarrhea, skin allergy and respiratory allergies. There were no significant differences in any of these adverse events between patients treated with NAC or placebo.
Table 3.
Side effects reported during the course of the study
| Side effects | Week 4 | Week 8 | Week 12 | P | |||
|---|---|---|---|---|---|---|---|
| NAC | Placebo | NAC | Placebo | NAC | Placebo | ||
| Nausea | 6 | 0 | 4 | 1 | 6 | 0 | 0.596 |
| Diarrhea | 1 | 0 | 0 | 1 | 0 | 0 | 0.389 |
| Skin allergy | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Respiratory allergy | 1 | 0 | 2 | 0 | 1 | 0 | 0.497 |
Discussion
In this study, we found that treatment with NAC in combination with smoking-focused psychotherapy during 3 months significantly impacted the primary and secondary outcome measures, i.e. reducing the daily number of cigarettes used (Δ mean ± SD = −10.9 ± 7.9 in the NAC versus −3.2 ± 6.1 in the placebo group) and COEXH levels (Δ mean ± SD = −10.4 ± 8.6 ppm versus −1.5 ± 4.5 ppm in the placebo group) in patients with therapy-resistant tobacco use disorder. 47.1% of those treated with NAC versus 21.4% of placebo-treated patients were able to quit smoking (as defined by COEXH <6 ppm). The results of our study extend those of previous papers that treatment with NAC may reduce logged daily use of cigarettes.14 The latter study, however, was unable to find effects of NAC on COEXH. Overall, NAC treatment (3 g/day) was well tolerated and only few participants reported adverse effects, nausea being the most frequent, not exceeding that of placebo. NAC is a well-tolerated treatment across a number of different conditions and illnesses.1,2
Another important finding is that treatment with NAC significantly reduced the HDRS score from baseline to 3 months later. It has been shown that NAC has a clinical efficacy in the depressed phase of bipolar disorder reducing the severity of depressive symptoms.9,18 In addition, this study replicates the efficacy of NAC in the management of unipolar depression, showing arguably more robust effects.19 While this may be due to participant selection or methodological issues, dose may be a critical factor. This study used a 3 g daily dose, compared to 2 g in the original study. There is some albeit weak dose finding data suggesting that the higher dose may be of greater efficacy.30 Mood effects of NAC may be important as tobacco use is associated with mood changes, including depressive symptoms, and functional impairment.31 Depressive smokers more strongly endorse beliefs that smoking reduces negative affect and craving.32 In the present study; however, we found that the reduction in cigarette use and COEXH levels were not related the NAC-induced changes in the HDRS score. Thus, the effects of NAC on tobacco use disorder occur independently from the antidepressant effects of NAC. However, the most robust meta-analytic evidence shows that smoking cessation is associated with long-term improvement in mental health.33
Another finding of our study is that treatment with NAC may reduce BMI although there is no significant difference in BMI between placebo and NAC in the post-treatment condition. During tobacco withdrawal, body weight increases on average 2–3 kg (American psychiatric Association, 2013).34 This is important as worry about weight gain may be a disincentive to quitting especially in women.35 Non-significant decreases in body weight with NAC have been seen in other human trials and in preclinical models.20,36 Our findings thus suggest that treatment with NAC may have another advantage since it does not induce weight gain.
Quality of life is another important issue as many patients with tobacco use disorder show a decreased quality of life. This is important as the diagnosis of tobacco use disorder is more associated with work disability.35,37 There are now many placebo-controlled trials using treatment with NAC in different conditions and illnesses showing significant benefits in quality of life parameters.1 In the present study; however, we were unable to detect a significant effect of NAC treatment on quality of life measurements. This may be explained by a relative shorter duration of treatment (3 months) and the smaller number of patients included.
The findings should be interpreted in the context of a number of limitations. Firstly, the present randomized controlled trial was a pilot study. Nevertheless, we recruited patients with therapy-resistant tobacco use disorder who additionally showed a high lifetime cigarette consumption. Secondly, the generalizability of the results of this study to the broad population of the tobacco users is limited as we only included patients between 18 and 65 years old and we did not examine the comorbidity of tobacco use disorders with several medical and psychiatric illnesses. Thirdly, follow-up studies are needed to investigate the maintenance treatment effects of NAC.
The clinical efficacy of NAC in tobacco use disorder may be explained by its effects on craving and reward, which are in part modulated by glutamate metabolism.9 Another theory revolves around the effects of NAC on immune-inflammatory and oxidative and nitrosative (IO&NS) pathways in relation to serotonin metabolism. Recently, we found that both tobacco use disorder and mood disorders are associated with the STin2.12 (a 17-bn variable number of tandem repeats in the functional 5-HTT intron) allele, leading to a lowered availability of serotonin.38 Both tobacco use disorder and depression are also accompanied by activation of IO&NS pathways, which in turn through induction of indoleamine 2,3-dioxygenase cause a depletion of tryptophan thereby driving further decreases in serotonin.35,39 There is now evidence that nicotine has short-term antidepressant effects by increasing the metabolism of serotonin.40–43 Nicotine dependence may therefore be regarded as an operational conditioned response aiming to compensate the depleted serotonergic metabolism in specific brain areas. NAC treatment by attenuating IO&NS pathways1 may therefore dampen the IO&NS forces that drive serotonin depletion. Such an effect would attenuate the use of nicotine and thus lead to an improvement in tobacco use disorder. NAC has robust effects on glutathione, while smoking is associated with reduced peripheral as well as brain glutathione levels.44 The extent to which glutathione changes are related to the efficacy of NAC in addiction is however unknown.20
All in all, our findings provide some evidence that treatment with NAC 3 g/day may significantly augment the efficacy of behavioral therapy in the treatment of tobacco use disorder. Moreover, our results show an effect of NAC reducing depressive symptoms during smoking cessation. Another factor regarding NAC treatment is that it does not increase body weight.
Acknowledgements
The authors wish to thank the Centre of Approach and Treatment for Smokers at Londrina State University, Paraná, Brazil (UEL). M.B. is supported by a NHMRC Senior Principal Research Fellowship 1059660. M.M. is supported by a CNPq (Conselho Nacional de Desenvolvimento Cientifico e Technologia) P.V.E. fellowship and the Health Sciences Graduate Program fellowship, Londrina State University (UEL).
Disclaimer statements
Contributors All authors contributed equally to the writing up of this paper.
Funding This study was supported by Health Sciences Postgraduate Program at Londrina State University, Paraná, Brazil (UEL), and Phloracea Pharmaceutics, Londrina, Paraná, Brazil.
Conflict of interest The authors declare that they have no competing interests.
Ethics approval The study was evaluated and approved by the Ethics Research Committee of UEL. All individuals gave written informed consent. The study was registered on clinicaltrials.gov number NCT02124525.
References
- 1.Morris G, Anderson G, Dean O, Berk M, Galecki P, Martin-Subero M, Maes M. The glutathione system: a new drug target in neuroimmune disorders. Mol Neurobiol. Epub ahead of print 18 April. 2014 DOI: 10.1007/s12035-014-8705-x. [DOI] [PubMed] [Google Scholar]
- 2.Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci 2013;34:167–77. [DOI] [PubMed] [Google Scholar]
- 3.Dodd S, Dean O, Copolov DL, Malhi GS, Berk M. N-Acetylcysteine for antioxidant therapy: pharmacology and clinical utility. Exp Opin Biol Ther 2008;8:1955–62. [DOI] [PubMed] [Google Scholar]
- 4.Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm J. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatr 2007;31:389–94. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatr 2008;63:338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azevedo E, Mendes AC, Berk M, Brietzke E. Systematic review of N-acetylcysteine in the treatment of addictions. Rev Bras Psiquiatr Epub ahead of print 18 may 2014 DOI: 10.1590/1516-4446-2013-1244. [DOI] [PubMed] [Google Scholar]
- 7.Bossert JM, Liu SY, Lu L, Shaham A. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci 2004;24:10726–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmaal L, Berk L, Hulstijn KP, Cousijn J, Wiers RW, Van den Brink W. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double blind placebo-controlled pilot study. Eur Addict Res 2011;17:211–16. [DOI] [PubMed] [Google Scholar]
- 9.Dean O, Giorland F, Berk M. N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatr Neurosci 2011;36:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalivas P, Volkow N. The neural of addiction: a pathology of motivation and choice. Am J Psychiatr 2005;162:1403–13. [DOI] [PubMed] [Google Scholar]
- 11.Koob G. Neurobiology of addiction. Focus. 2011;9:55–65. [Google Scholar]
- 12.Gould NS, Min E, Gauthier S, Martin RJ, Day BJ. Lung glutathione adaptive responses to cigarette smoke exposure. Respiratory Res 2011;12:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci 2007;27:13968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knackstedt LA, LaRowe S, Mardikian P, Malcom R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatr 2009;65:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bortolasci CC, Vargas HO, Souza-Nogueira A, Barbosa DS, Moreira EG, Nunes SO, Berk M, Dodd S, Maes M. Lowered plasma paraoxonase (PON)1 activity is a trait marker of major depression and PON1 Q192R gene polymorphism-smoking interactions differentially predict the odds of major depression and bipolar disorder. J Affect Disord 2014;159:23–30. [DOI] [PubMed] [Google Scholar]
- 16.Nunes SOV, De Castro MRP, Watanabe MAW, Guembarovski RL, Vargas HO, Reiche EM, Kaminami HM, Dodd S, Berk M. Genetic polymorphisms in glutathione-S-transferases are associated with anxiety and mood disorders in nicotine dependence. Psychiatr Genet 2014;24:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonelli RC. Nicotine-related disorders. In: Gabbard GO. Gabbard's treatment of Psychiatric disorders- DSM-5 Edition. (5th edn) Washington: American Psychiatric Publishing; 2014; pp. 871–83. [Google Scholar]
- 18.Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol Psychiatr 2008;64:361–58. [DOI] [PubMed] [Google Scholar]
- 19.Berk M, Dean OM, Cotton SM, Jeavons S, Tanious M, Kohlmann K, Hewitt K, Moss K, Allwang C, Schapkaitz I, Robbins J, Cobb H, Ng F, Dodd S, Bush AI, Malhi GS. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatr 2014;75:628–36. [DOI] [PubMed] [Google Scholar]
- 20.Berk M, Ng F, Dean O, Dodd S, Bush AI. Glutathione: a novel treatment target in psychiatry. Trends Pharmacol Sci 2008;29:346–51. [DOI] [PubMed] [Google Scholar]
- 21.Del Ben CM, Vilela JAA, Crippa JAS, Hallak JEC, Labate CM, Zuardi AW. Reliability of the ‘Structured Clinical Interview for DSM – IV’ - clinical version translated into Portuguese. Rev Bras Psychiatr 2001;23:156–9. [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) Quitting smoking among adults: United States, 2001–2011. MMWR Morb Mortal Weekly Rep 2011;60:1513–9. [PubMed] [Google Scholar]
- 23.Fagerström KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerström Tolerance Questionnaire. J Behav Med 1989;12:159–82. [DOI] [PubMed] [Google Scholar]
- 24.Carmo JT, Pueyo AA. Adaptation to Portuguese of the Fagerstrom Test for Nicotine Dependence (FTND) to assess dependence and tolerance to nicotine in smokers. Rev Bras Med 2002;59:73–80. [Google Scholar]
- 25.Storr CL, Reboussin BA, Anthony JC. The Fagerstrom test for nicotine dependence : a comparison of standard scoring and latent class analysis approaches. Drug Alcohol Depend 2005;80:241–250. [DOI] [PubMed] [Google Scholar]
- 26.Reichert J, Araújo AJ, Gonçalves CMC, Godoy I, Chatkin JM, Sales MPU, Santos SRRA. BTA guidelines: guidelines for smoking cessation - 2008. J Bras Pneumol 2008;34:845–80. [DOI] [PubMed] [Google Scholar]
- 27.Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest 2000;117:758–63. [DOI] [PubMed] [Google Scholar]
- 28.Moreno RA, Moreno DH. Hamilton and Montgomery & Asberg depression rating scales. Rev Psychiatr Clin 1998;25:262–72. [Google Scholar]
- 29.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurements of disability. Int Clin Psychopharmacol 1996;3(III Suppl):89–95. [DOI] [PubMed] [Google Scholar]
- 30.Garcia RJ, Francis L, Dawood M, Lai ZW, Faraone SV, Perl A. Attention deficit and hyperactivity disorder scores are elevated and respond to N-acetylcysteine treatment in patients with systemic lupus erythematous. Arthr Rheumat 2013;65:1313–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasco JA, Williams LJ, Jacka FN, Ng F, Henry MJ, Nicholson GC, Kotowicz MA. Tobacco smoking as a risk factor for major depressive disorder: population-based study. Brit J Psychiatr 2008;193:322–6. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg A, Georg T, Mc Knee S. Differences in smoking expectancies in smokers with and without a history of major depression. Addict Beh 2011;36:434–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 2014;13:348, g1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Psychiatric Association Tobacco-related disorders of DSM-5. In: American Psychiatric Association, Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: APA; 2013 p. 571–7. [Google Scholar]
- 35.Nunes SOV, De Castro MRP, Vargas HO, Vargas MM, Machado RCBR, Fonseca ICB, Dodd S, Berk M. Clinical characteristics and smoking cessation: an analysis of sex and depressive disorders differences. Addict Disord Treatm 2013;12:158–65. [Google Scholar]
- 36.Souza GA, Ebaid GX, Seiva FR, Rocha KH, Galhardi CM, Mani F, Novelli EL. N-acetylcysteine an allium plant compound improves high-sucrose diet-induced obesity and related effects. Evid Based Compl Altern Med 2011;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nunes SO, Vargas HO, Brum J, Prado E, Vargas MM, Castro MR, Berk M. A comparison of inflammatory markers in depressed and nondepressed smokers. Nicotine Tob Res 2012;14:540–6. [DOI] [PubMed] [Google Scholar]
- 38.Pizzo de Castro MR, Maes M, Guembarovski RL, Ariza CB, Reiche EM, Vargas HO, Vargas MM, de Melo LG, Dodd S, Berk M, Watanabe MA, Nunes SO. SLC6A4 STin2 VNTR genetic polymorphism is associated with tobacco use disorder, but not with successful smoking cessation or smoking characteristics: a case control study. BMC Genet 2014;15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 2012;36:764–85. [DOI] [PubMed] [Google Scholar]
- 40.O'Loughlin J, Lambert M, Karp I, McGrath J, Gray-Donald K, Barnett TA, Delvin EE, Levy E, Paradis G. Association between cigarette smoking and C-reactive protein in a representative, population-based sample of adolescents. Nicotine Tob Res 2008;10:525–32. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez-Lopes S, Gardunõ J, Mihailescu S. Nicotinic modulation of serotonergic activity in the dorsal raphe nucleus. Rev Neurosci 2013;24:455–69. [DOI] [PubMed] [Google Scholar]
- 42.Khadrawy YA, El-Shamy KAI, Mohamed SI. Nicotine restores monoamine neurotransmitter changes in the cortex and hippocampus of reserpinized rats as a model of depression. Eur Rev Med Pharmacol Sci 2011;15:863–70. [PubMed] [Google Scholar]
- 43.Vieyra-Reyes P, Venebra-Muñoz A, Rivas-Santiago B, García-García F. Acción de la nicotina como antidepresivo y regulador del sueño en sujetos deprimidos. Rev Neurol 2009;49:661–7. [PubMed] [Google Scholar]
- 44.Chitty KM, Lagopoulos J, Hickie IB, Hermens DF. The impact of alcohol and tobacco use on in vivo glutathione in youth with bipolar disorder: an exploratory study. J Psychiatr Res 2014; 55:59–67. [DOI] [PubMed] [Google Scholar]