Abstract
Six different xanthophyll cycles have been described in photosynthetic organisms. All of them protect the photosynthetic apparatus from photodamage caused by light-induced oxidative stress. Overexcitation conditions lead, in the chloroplast, to the over-reduction of the NADP pool and production of superoxide, which can subsequently be metabolized to hydrogen peroxide or a hydroxyl radical, other reactive oxygen species (ROS). On the other hand, overexcitation of photosystems leads to an increased lifetime of the chlorophyll excited state, increasing the probability of chlorophyll triplet formation which reacts with triplet oxygen forming single oxygen, another ROS. The products of the light-dependent phase of xanthophyll cycles play an important role in the protection against oxidative stress generated not only by an excess of light but also by other ROS-generating factors such as drought, chilling, heat, senescence, or salinity stress. Four, mainly hypothetical, mechanisms explaining the protective role of xanthophyll cycles in oxidative stress are presented. One of them is the direct quenching of overexcitation by products of the light phase of xanthophyll cycles and three others are based on the indirect participation of xanthophyll cycle carotenoids in the process of photoprotection. They include: (1) indirect quenching of overexcitation by aggregation-dependent light-harvesting complexes (LHCII) quenching; (2) light-driven mechanisms in LHCII; and (3) a model based on charge transfer quenching between Chl a and Zx. Moreover, results of the studies on the antioxidant properties of xanthophyll cycle pigments in model systems are also presented.
Keywords: De-epoxidation, Non-photochemical quenching, Oxidative stress, Xanthophyll cycles
Introduction
An imbalance between the generation of different reactive oxygen forms and a biological system's ability to detoxify reactive intermediates or to repair the resulting damage is commonly known as oxidative stress. Chemically, oxidative stress is associated with the increased production of oxidizing species or a significant decrease in the capability of antioxidant defenses and it may occur in all organisms.1
Most of these oxygen-derived species are produced at a low level by normal aerobic metabolism and the damage they cause to cells is constantly repaired. Reactive oxygen species (ROS) can even be beneficial, as they are used by the immune system as a way to attack and kill pathogens and play important roles in cell signalling through a process called redox signalling.
However, when the production and consumption of ROS becomes unbalanced, proper cellular redox homeostasis is threatened, which can cause cell death. It is known that even moderate oxidation can trigger apoptosis.2 A particular destructive aspect of oxidative stress is the production of ROS, which include free radicals and peroxides. Moreover, some of the less reactive of these species can be converted by oxidation–reduction reactions into more aggressive radical species.3 Minor amounts of ROS are generated by some enzymes such as oxidases through the autooxidation of different molecules. The oxidases known to produce ROS are NADPH oxidase, lipoxygenases, cyclooxygenases, and xanthine oxidase,4 while molecules entering autooxidation may be of exogenous and endogenous origin like neurotoxin 5-hydroxydecanoate5 or catecholamines,6 and many other compounds.
Furthermore, the ability to accept and donate a single electron by metals such as iron, copper, chromium, vanadium, and cobalt is the basis of reactions that produce reactive radicals and ROS can be generated. The most important reactions are probably Fenton's reaction and the Haber–Weiss reaction, in which a hydroxyl radical (OH) is produced from reduced iron and hydrogen peroxide (H2O2). However, the major fraction of ROS in living organisms is produced by electron-transport chains, endoplasmic reticulum, plasmatic, and nuclear membranes.7 In plants ROS are additionally generated during photosynthesis as well as photorespiration8 and by cell wall–bound NAD(P)H oxidases–peroxidases.9
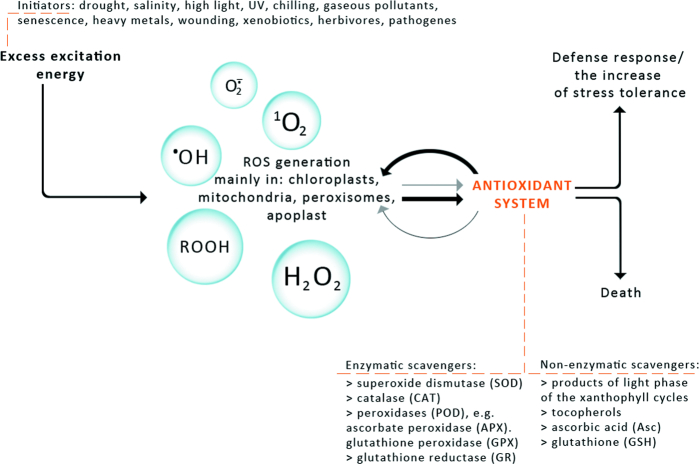
The exposure of plants to stresses such as high-light stress, ultraviolet radiation, drought stress and desiccation, salt stress, chilling, heat shock, heavy metals, air pollutants, mechanical stress, nutrient deprivation, and pathogen attack can give rise to an excess accumulation of ROS at the cellular level.10 Since ROS can be viewed as cellular indicators of stress and as secondary messengers involved in the stress-response signal transduction pathway, their level has to be kept under tight control by ROS scavenging mechanisms, including enzymatic and non-enzymatic antioxidants (Fig. 1).11
Figure 1.
The response of plants to different abiotic and biotic stresses. The antioxidant system (enzymatic and non-enzymatic scavengers) enables plants to regulate ROS level and influence on ROS-dependent signal induction. High ROS generation and weak interaction of the antioxidant system cause cell death. Domination of antioxidant system over ROS generation allows defence response or increase in stress tolerance.
One group of non-enzymatic antioxidants that exists in plants is the products of de-epoxidation occurring in the processes generally called the xanthophyll cycle.
Xanthophyll cycle protects plants against oxidative stress generated by high-light intensity
The regulation of excitation density in the photosynthetic apparatus is particularly important under high-light conditions, owing to the risk of the light-induced generation of ROS, leading to the photo-degradation of the photosynthetic apparatus. Overexcitation conditions on the one hand lead to the over-reduction of the NADP pool and to superoxide (O2−.) production in the chloroplast by Mehler's reaction.12 O2−. can subsequently be converted to H2O2 or a •OH.13 On the other hand, overexcitation of photosystems leads to an increased lifetime of an excited state of chlorophyll (Chl), chlorophyll single formation (1Chl*), increasing the probability of a Chl a triplet formation (3Chl*) which reacts with oxygen (3O2) forming singlet oxygen (1O2), ROS (Fig. 2(A)). Thus, photosystem II (PSII) and light-harvesting complexes (LHCII) localized at the periphery of each PS become an important source of 1O2.14 LHCII comprises Chl a and b (eight and six molecules per protein monomer, respectively), four molecules of xanthophylls (two molecules of lutein (L), one neoxanthin (Nx), and one violaxanthin (Vx))15 appear in a trimeric form in its native state.15
Figure 2.
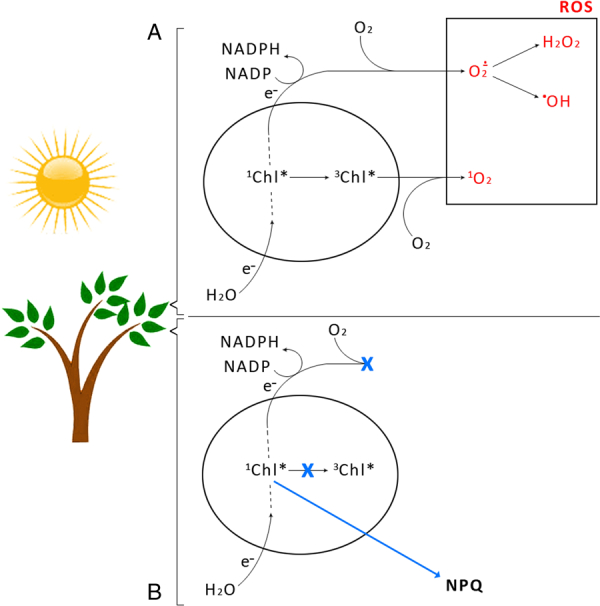
Routes of generation of ROS during the light phase of photosynthesis. 3Chl* on account of increasing chlorophyll excited state (1Chl*) in incomplete photochemical quenching, reacts with oxygen (3O2) to form 1O2. Over-reduction of the NADP pool causes O2− production by Mehler's reaction (A). The photoprotective mechanism of excess energy dissipation through NPQ, blocking generation of ROS (B).
One of the most efficient mechanisms protecting plants and other photosynthesizing organisms under overexcitation conditions is the xanthophyll cycle. At the molecular level some carotenoids of this cycle act as quenchers of 1Chl*, thus preventing the formation of ROS (Fig. 2(B)).16,17
Six types of xanthophyll cycle have been described. Four of them are based on beta-xanthophylls and two on alpha-xanthophylls (Table 1).18 All xanthophyll cycles have in common the light-dependent transformation of epoxidized xanthophylls to de-epoxidized ones in high light, which facilitates the dissipation of excitation energy, and their reversion to epoxidized xanthophylls in low light.16,19 This dissipation is obtained by mechanisms collectively referred to as non-photochemical quenching (NPQ). The predominant NPQ component is induced as a result of the acidification of the thylakoid lumen associated with the formation of the chloroplast proton motive force and defined as energy quenching (qE).20 In addition to qE, relaxing within 2–5 minutes, a slowly relaxing component of the NPQ process is known as qI (photoinhibitory quenching), with a half-time of approximately 30 minutes and longer (depending on the degree of photoinhibition).21 A third quenching component (qT), relaxing within 15–20 minutes, also has been reported.21,22
Table 1.
Types of xanthophyll cycles
| Type of xanthophyll cycle | Products of light phase of the xanthophyll cycles | Description | |
|---|---|---|---|
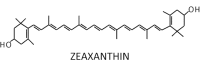
| Violaxanthin cycle (xanthophyll cycle) | 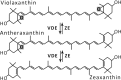 |
 |
Enzymes • Violaxanthin de-epoxidase (VDE) • Zeaxanthin epoxidase (ZE) Occurrence • Higher plants • Mosses • Lichens • Algae (phaeophyta, chlorophyta, rhodophyta) |
| Diadinoxanthin cycle | 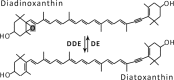 |
 |
Enzymes • Diadinoxanthin de-epoxidase (DDE) • Diatoxanthin epoxidase (DE) Occurrence • Algae (diatoms, phaeophytes, dinophytes, haptophytes) |
| Antheraxanthin cycle | 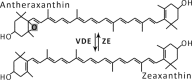 |
 |
Enzymes • Violaxanthin de-epoxidase (VDE) • Zeaxanthin epoxidase (ZE) Occurrence • Gracilaria gracilis • Gracilaria multipartite |
| Xanthophyll cycle in Mantoniella squamata | 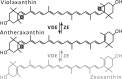 |
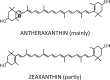 |
Enzymes • Violaxanthin de-epoxidase (VDE) • Zeaxanthin epoxidase (ZE) Occurrence • Mantoniella squamata |
| Lutein epoxide cycle | 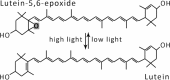 |
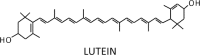 |
Enzymes • Violaxanthin de-epoxidase (VDE) • Zeaxanthin epoxidase (ZE) Occurrence • Leaves of 62% of the 188 tested species |
| Siphonaxanthin cycle | 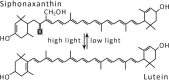 |
 |
Enzymes: no data available Occurrence • Caulerpa racemosa |
Note: Non-specific Xanthophyll cycle – siphonaxanthin is not epoxy xanthophyll.
The most commonly occurring type of the six xanthophyll cycles and most intensively studied is the Vx-cycle, also called the xanthophyll cycle, where the main product of strong light-stimulated de-epoxidation is zeaxanthin (Zx). Thorough and detailed studies by different research groups have shown a dependence between the content of Zx and NPQ in chloroplasts.23 An even better correlation was found between NPQ and the total amount of Zx and antheraxanthin (Ax).24 An increase in NPQ after high-light treatment and its correlation to Vx de-epoxidation in spinach (Spinacia oleracea) leaves, isolated chloroplasts, and purified LHC complexes have also been observed.25 Similarly, in diatoms, the NPQ level was well correlated with the diatoxanthin (Dtx) amount, which was created during the de-epoxidation of diadinoxanthin (Ddx) (Table 1).25,26 In other experiments, a photoprotective action of Dtx during prolonged UV-A and UV-B illumination of diatoms (Thalassiosira weissflogii) has been demonstrated.27 These UV-insensitive diatoms shown increased activity of the Dtx cycle as a response to light stress.
Studies of mutants of the green algae Chlamydomonas have also shown that L, like the de-epoxidation products of Vx or Dtx, has a significant role in energy dissipation.26
It also has been observed that the de-epoxidation of lutein-epoxide (Lx) to L (Table 1) facilitated the rapid engagement of NPQ, and that this process may be fine-tuned by concurrent Zx accumulation inducing strong energy dissipation in plants having both an Lx-cycle and Vx-cycle.18
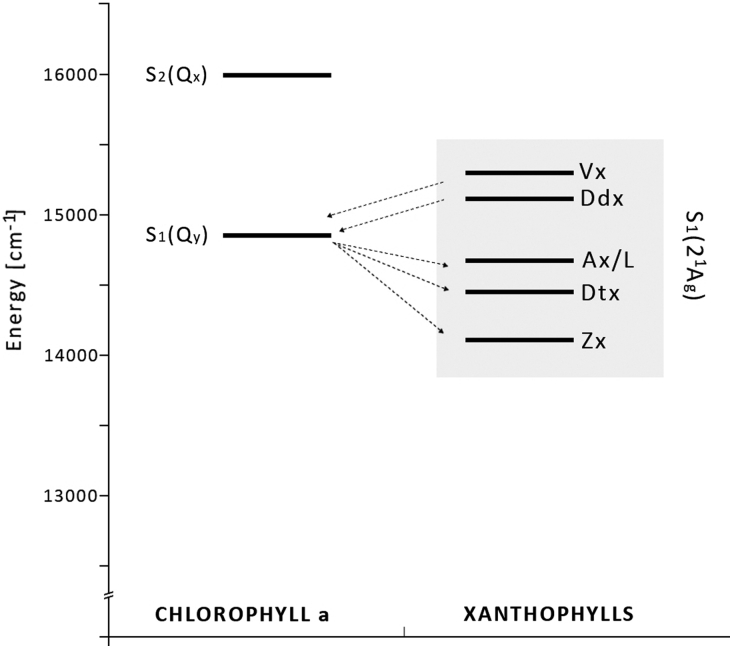
On the other hand, photoconversion of siphonaxanthin (Sx) to L (Table 1) detected in the green alga Caulerpa racemosa, showing significant relationships to Vx–Ax interconversion, suggests a similar activation signal for these two mechanisms. In particular, both Ax and L reached their highest values not only under high light, but also at sunrise when light intensity was lower.28 This last feature suggests a similar and very high sensitivity of the two cycles to light and a photoprotective role for the interconversion between Sx and L may be hypothesized, even if this hypothesis needs to be tested through adequate studies. Also, in favour of this hypothesis are the biochemical similarities between L and Ax reported in the literature, the energetic state analysis revealing similar S1 values for Ax and L (Fig. 3).28
Figure 3.
Energy-level diagram. The localization of the S1 and S2 energy levels of chlorophyll a (Chl a) and S1 energy levels of carotenoids. The energies of Chl a: S2(Qx) 16 000/cm and S1(Qy) 14 700/cm. The energies of the S1 state of xanthophylls: violaxanthin (Vx, 15 290/cm), diadinoxanthin (Ddx, 15 130/cm), antheraxanthin and lutein (Ax/L, 14 720/cm), diatoxanthin (Dtx, 14 485/cm), and zeaxanthin (Zx, 14 170/cm).28 Arrows from left to right represent forward energy transfer (light-harvesting); arrows from right to left – reverse energy transfer (NPQ).
All these observations show that all the products' xanthophyll cycles that are created under light conditions are effective quenchers of ROS.
It is known that carotenoids possess at least two spectroscopically important low-lying excited states denoted S1 and S2,29 with S0 indicating the ground state. Electronic transitions between S0 and S1 are forbidden because these two states have the same (Ag) symmetry in the idealized C2h point group. Electronic transitions to and from S1 and S0 are forbidden by symmetry, but such a transition is allowed between S0 and S2 because S2 possesses Bu symmetry. Vx, Zx, and all xanthophylls display very strong absorption in the visible region. This absorption is associated with an electronic transition between S0 and S2. This higher energy state is denoted 11Bu. Although there is some evidence that additional Ag states lie near the S1 (21Ag) and the 11Bu states in long polyenes and carotenoids,30 the latter is usually referred to as S2 because it is the most easily observed and spectroscopically accessible state above S1.
Direct quenching of overexcitation by products of the light phase of xanthophyll cycles
A key factor in evaluating the efficiency of the mechanisms that were proposed to explain NPQ was the energy of the S1 states of the epoxy- and de-epoxy-xanthophylls.
It was postulated that the xanthophylls created as an effect of light-induced enzymatic conversion (Table 1; Ax, Zx, L, and Dtx) are the pigments that possess a longer conjugated double-bond system, as compared to their oxidized derivatives (11 conjugated double bonds for Zx versus 9 for Vx). This fact implies that the lowest-excited singlet state (S1) of these pigments is located at a lower level on the energy scale with respect to the Qy level of Chl a and suggests a possible quenching of Chl a excessive singlet excitation by these xanthophylls but not by their oxidized derivatives (Fig. 3).31
One of the hypotheses explaining the role of xanthophyll cycles in NPQ assumes a direct quenching of overexcitation by the de-epoxyxanthophylls created under high-light conditions. This model proposes that a downhill energy transfer from Chl a to Zx, Ax, Ddx, or L occurring after a pH-activated structural change in the pigment–protein complex has facilitated the energy exchange.32,33 This idea has gained support from estimates of the energies of the lowest-lying singlet (S1) states of the xanthophylls from either the dynamics of these states or the fluorescence of a series of shorter carotenoids (less than 10 carbon–carbon double bonds), and extrapolation of the energies to the longer molecules including the pigments involved in the xanthophyll cycles.33 Spectroscopic and kinetic investigations have revealed that the energies of the S1 states of the xanthophyll pigments are low enough to quench Chl excited states.
It was previously published that a clear relationship exists between the carotenoid S1 energy level and its ability to quench Chl fluorescence.28 The use of carotenoids with S1 energies above that of Chla had little, if any, effect on NPQ. A carotenoid molecule may be considered as a ‘quencher’ or ‘non-quencher’ depending whether the S1 energy is below or above that of Chl a, respectively.
Model system studies using liposomes with embedded LHCII and xanthophyll cycle pigments revealed that the xanthophylls decrease the relative quantum yield of Chl fluorescence, quenching the Chl via singlet excitation transfer, Zx being a better quencher than Vx.34
The ability of five carotenoids (Zx, Vx, L, Nx, and beta-carotene) to quench Chl a fluorescence was also tested by trapping both types of pigments in micelles of triton X-100. Among the xanthophyll cycle pigments studied, only Zx was a good quencher of Chl a fluorescence, comparable in its efficiency to that of beta-carotene. Vx was a much weaker quencher than Zx. L and Nx actually enhanced the fluorescence. Moreover, it was also demonstrated that the Zx quenching ability was related to Zx dimer formation immediately on addition of this pigment to the Chl-containing micelles. It has been postulated that this dimerization may play a role in Zx functioning in the photosynthetic apparatus.35
It also should be mentioned that some of the recent determinations of the energy levels of the xanthophyll cycle pigments show that the difference between the energies of the S1 state of Vx and Zx is very small, and therefore essentially different Chl excitation quenching efficiencies for these two compounds may not be expected.36 Accordingly, no exceptional Chl singlet excitation quenching has been observed in the antenna complexes isolated from the L mutants of Arabidopsis thaliana, in which L was replaced with Zx.17,37
Moreover, it was also found that the S1 level of not only Zx but also Vx lies below the Qy level of Chl.38 This has significant implications for the mechanism of xanthophyll cycle photoregulation in plants. A mechanism involving direct quenching via singlet–singlet energy transfer seems to be invalid. According to these data, Vx could even be a more efficient quencher than Zx, because its S1 level lies below but closer to the Chl Qy transition than the S1 level of Zx. In this respect, an aggregation model based on the indirect participation of the xanthophyll cycle carotenoids in the process of photoprotection is a more promising candidate to explain the function of the xanthophyll cycle in the quenching of excess energy in the antenna complexes of higher plants.38
Three main models for excess energy dissipation by xanthophyll cycle products created under light conditions have been proposed.
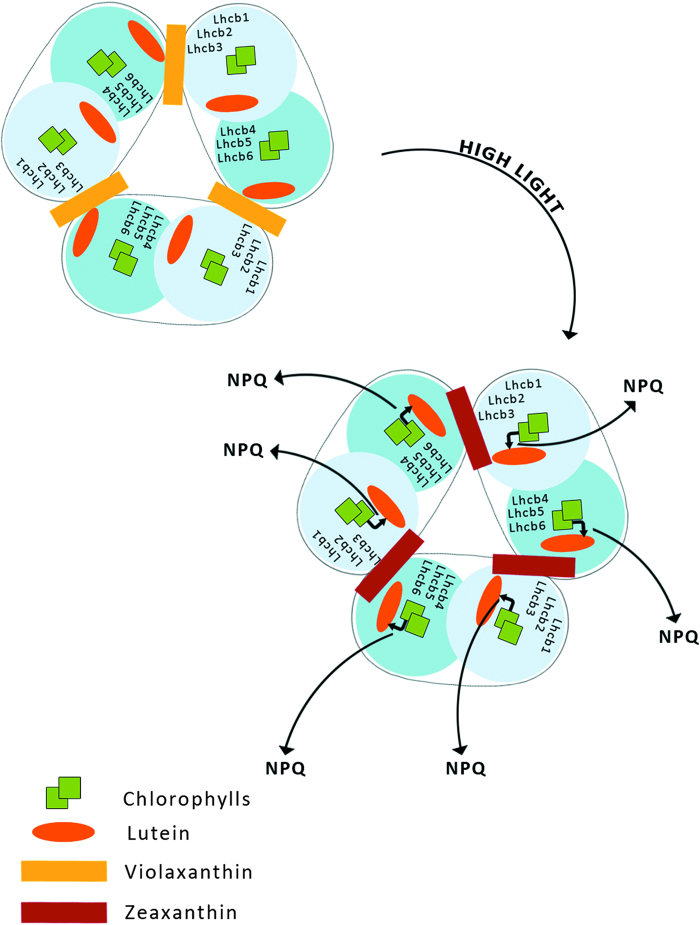
I. LHCII aggregation-dependent indirect quenching of overexcitation
According to this model, qE occurs upon aggregation of the major, trimeric LHCII complex of PSII. This produces a conformational change within the protein and promotes energy transfer from Chl a to S1 excited state of L bound to the LHCII (Fig. 4).39,40
Figure 4.
Aggregation-dependent indirect quenching of overexcitation by LHCII. The conformation change produces energy transfer from Chl a to a lutein.
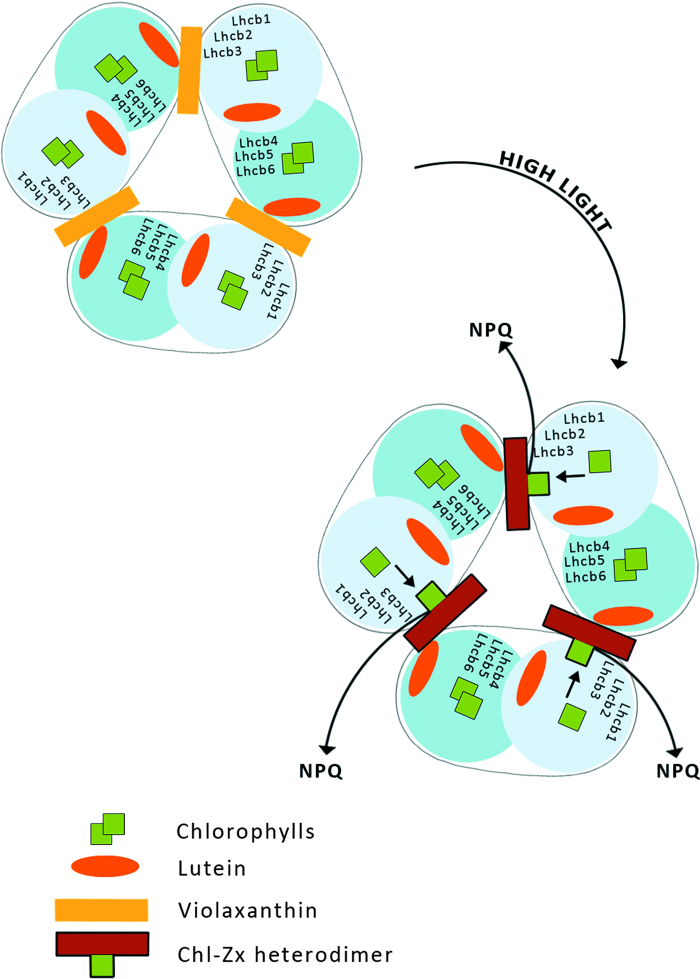
LHCII consists of six types of monomeric proteins called as Lhcb which fall into two groups with respect to the sites where they bind L and Vx or Zx. The first group includes Lhcb1, Lhcb2, and Lhcb3, the components of the major trimeric LHCII, binding L at sites L1 and L2, while Vx or Zx bind at site V1.15,41 The second group of Lhcb proteins includes monomeric Lhcb4, Lhcb5, and Lhcb6, which bind L at site L1 and Vx or Zx at site L2.42,43 These proteins do not have a V1 site and thus exchange Vx with Zx in site L2,44 while Lhcb1-3 do not.
Recently, an aggregation-dependent LHCII quenching model was supported by the observation of a red-shifted fluorescence lifetime component both in aggregated LHCII trimers binding Zx and in quenched leaves.45 Zx bound at site V1 of LHCII acts as an allosteric modulator of L-dependent quenching,45 whereas aggregation in vitro has been proposed to entrain an intrinsic conformational transition in the LHCII complex, responsible for the establishment of the quenching reaction (Fig. 4).46 Carotenoid S1-Chl excited state coupling was recently measured in isolated LHCII complexes and correlated with the NPQ amplitude in vivo in different mutants such as npq1, npq2, lut2, and PsbS over-accumulating lines.47
Criticisms of this model have been raised on the basis that:
-
1.
the effect of down regulating the components of LHCII in vivo, namely Lhcb1+248 or Lhcb3,49 is, at best, very small;
-
2.
quenching and other spectral changes attributed to LHCII occur in Lhcb4 and Lhcb5 as well, even more promptly than in LHCII,50
-
3.
L cannot be the only quencher during NPQ since the lut2npq2 mutant having Zx as the only xanthophyll is active in NPQ as well as the L-less mutant lut2.
II. Light-driven reactions in LHCII as a mechanism of the indirect quenching of overexcitation by products of the light phase of xanthophyll cycles
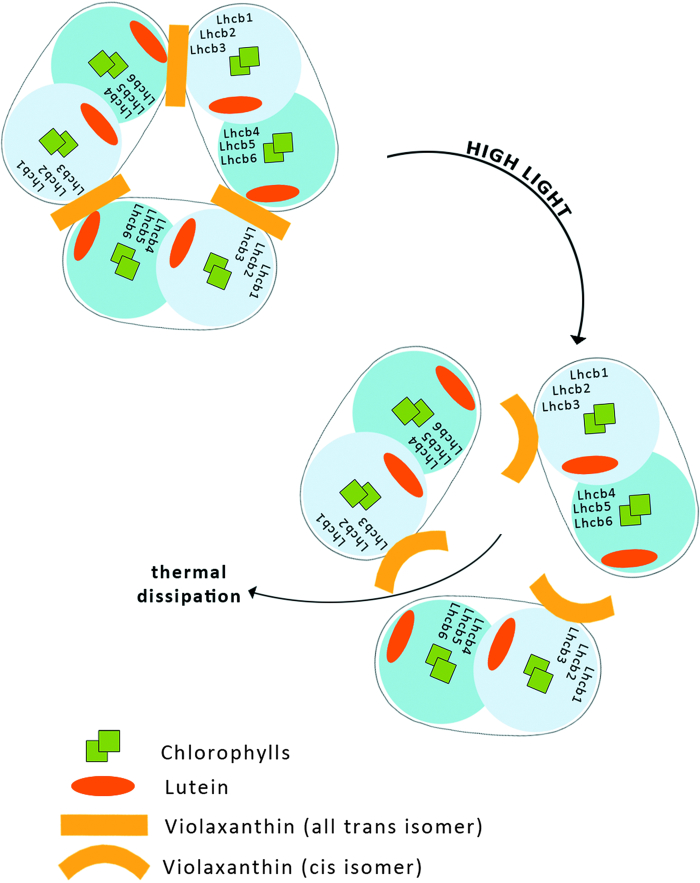
This model is postulated because under strong light illumination both photo-isomerization of all-trans Vx bound to LHCII to the cis isomer and a light-induced trimer to monomer transition in LHCII have been observed (Fig. 5).51,52 A very recent examination of the molecular organization of LHCII, based on fluorescence lifetime imaging microscopy, revealed that all-trans Vx stabilizes the trimeric organization of the complex, in contrast to Zx which promotes a monomeric state of LHCII. The rate of excitation energy transfer from Vx to Chl s in LHCII is extremely low41 and therefore the light energy absorbed by Vx may be utilized to drive the isomerization of the pigment to the cis isomer and to cause a trimer to monomer transition in LHCII, which then leads to a reduction in the Chl fluorescence lifetime (Fig. 5). The shortening of the Chl fluorescence lifetime reflects a more efficient singlet excitation thermal dissipation and therefore the light-dependent process is discussed in terms of photoprotective activity within LHCII. Moreover, the operation of the xanthophyll cycle in the photosynthetic apparatus requires Vx to be freely available within the lipid phase of the thylakoid membrane for de-epoxidation to Zx.43 Vx is a xanthophyll relatively weakly bound to the protein environment of LHCII, and the process of the light-driven change of this pigment's molecular configuration can result in its uncoupling from the protein and its transfer to the lipid environment of the membrane. Certainly, light-dependent LHCII monomerization makes it easier, or even possible, for Vx to migrate from the protein to the lipid environment. Vx in an all-trans, fully relaxed configuration is a specific substrate of the de-epoxidase enzyme53 and the pigment tends to adopt such a configuration after light-driven transformation, due to the energy minimization process.54
Figure 5.
Model of light-induced transformation of the antenna complex LHCII. Illumination in physiological conditions results in Vx all-trans to Vx cis isomerisation, which causes dissociation of LHCII trimers to monomers, resulting in increased thermal energy dissipation.
III. Model of charge transfer quenching between Chl a and Zx
The assumption that NPQ is connected with the formation of a charge-transfer (CT) state between Chl a and Zx has been proposed on the basis of quantum chemical calculations55 and ultra-fast pump–probe experiments on isolated thylakoid membranes.56 The CT mechanism involves energy transfer from bulk Chl molecules to a Chl–Zx heterodimer that undergoes charge separation followed by recombination, thereby transiently producing a Zx radical cation (Zx+.) with a very short relaxation time (50–200 ps), as expected of an efficient quencher (Fig. 6). The formation of Zx+. in thylakoids depends on the three components needed for qE in vivo: lumen acidification, PsbS activation, and Zx production.56,57
Figure 6.
Model of charge transfer quenching between Chl a and Zx. This mechanism involves energy transfer from chl to Chl–Zx heterodimer.
Signals from Zx+. formation have been found in isolated monomeric Lhcbs, but not in LHCII.57–59
The mutation analysis of Chl binding sites in Lhcb458 showed that for CT quenching a Chl pair called Chl A5 and Chl B5 is critical rather than a single Chl a chromophore. The involvement of a Chl pair is reasonable since charge delocalization over the Chl pair would stabilize the CT state. Chl A5–B5 are located in the proximity of the L2 domain while Zx binding to this site induces a conformational change bringing Chl A5 into excitonic interaction with Chl B5, switching the protein to a dissipative state by Zx+. formation. Also, Lhcb6 antenna complexes show a Zx+. formation, while in the Lhcb5 complex two distinct CT quenching sites were detected involving, respectively, Zx and L radical cation species, depending on Zx binding to the L2 binding site. Thus, Zx in site L2 acts both as a quencher and as an allosteric modulator of L CT efficiency into site L1.59 L radical cation was also recently detected in Lhcb6 and Lhcb4 complexes binding L as the only xanthophyll.60
Criticism of this model is connected with:
-
1.
a double mutant lacking both Lhcb5 and Lhcb6 and reduced in Lhcb4 retains most NPQ activity;
-
2.
the low level of minor complexes undergoing CT quenching in vitro (∼1%) versus in vivo (30%), implies the presence of factors, possibly PsbS, ΔpH or interactions with protein partners, which stabilize the dissipative conformation59 in vivo;
-
3.
LHC protein conformational change induced by interaction with PsbS has not been reproduced in vitro, so far;
-
4.
the relation between CT quenching and the S1 population is seen as a consequence of charge recombination on carotenoid radical cation formation.
The interaction of Zx with LHCII via PsbS was also postulated as an explanation of the fact that LHCII is involved in the catalysis of NPQ, despite the fact that LHCII trimers containing Zx+. could not be found in solution.57,60 Moreover, PsbS was not found to bind Zx with LHCII either in in vivo or in vitro studies.61
The xanthophyll cycle is a mechanism protecting plants against oxidative stress not only in the pigment–protein complexes
A very important aspect of the operation of the xanthophyll cycle in the thylakoids as a mechanism protecting plants against oxidative stress is the direct presence of carotenoid pigments in the lipid phase of the thylakoid membrane and not assembled into pigment–protein complexes.17,62,63
The effect of Zx on lipid degradation under strong light conditions was observed in pea leaves.64 The content of lipids in leaf cells decreased and the saturated/unsaturated lipid ratio increased. Lipid degradation was more significant when Zx formation was inhibited by dithiothreitol (DTT).65 Similar results came from experiments in which lipid content was measured in response to high illumination in the npq1 mutant, deficient in the production of Zx.65 In comparison with the wild Arabidopsis form, the npq1 mutant had a significantly higher level of lipid photooxidation. Interestingly, in tomato leaves, the Zx level and lipid degradation (measured as ethylene formation) were also correlated. At 3°C and under high light (low level of created Zx), ethylene production was intensive. But at 23°C and in high light, ethylene secretion was lower and the Zx content increased.66
The antioxidant properties of Zx were also tested in model systems. It was observed that Zx was the most effective against oxidation initiated both in the aqueous and lipid phases of all tested carotenoids such as beta-cryptoxanthin, beta,beta-carotene, astaxanthin, canthaxanthin, and lycopene. In a homogeneous organic solution, all tested carotenoids ameliorated lipid peroxidation. Zx, as well as beta, beta-carotene, reacted with ROS at similar rates, giving a similar degree of protection in an organic solution. The reactivity and protective efficiency of the astaxanthin and canthaxanthin were lower.67 Also L in model systems reacted rapidly with oxidizing agents and was recognized as an important antioxidant factor.68
These results point to the significance of the xanthophyll cycle pigments in direct protection of the photosynthetic apparatus against ROS. Although Zx and Vx are normally bound to the antenna proteins, they must be liberated from their binding sites to the lipid domains surrounding the antenna complexes so as to be accessible as substrates for the xanthophyll cycle enzymes.69 Thus, significant proportions of Zx and Vx are transiently present in the lipid phase, where they may directly quench ROS.
It was also postulated that Vx-cycle and particularly Zx play a role in senescence, as a photoprotectant against lipid photooxidation.70 It was also observed that the level of L increased gradually during the aging of primary cabbage leaves while the level of Lx was decreased, although the correlation of this phenomenon with lipid peroxidation was not tested.71
Notwithstanding differences between authors, the Vx-cycle is recognized as one of the main adaptation mechanisms responsible for a fast response to peroxidation and for the creation of antioxidant substances in thylakoid membranes that can quench singlet oxygen72 and other free radicals.73
It was also observed that some stress factors like drought,74 salt stress75, or chilling stimulate the production of Zx.76 It is commonly suggested that this is due to xanthophyll cycle activity which plays an important role in protecting the photosynthetic apparatus from photoinhibitory damage under a variety of stressors.
Another aspect of the xanthophyll cycle in protection against oxidative stress was postulated when the effect on the xanthophyll cycle of short-term ozone pollution at high doses under photoinhibitory conditions was studied. The plants were also subjected to direct treatment with H2O2, O2−., and to paraquat as a herbicide. Although a degradation of Vx was observed in these experiments, it was not compensated for by the sum of Ax + Zx. It was hypothesized that, under photoinhibitory conditions combined with strong oxidative stress, Vx is used in large part not for the xanthophyll cycle reaction but for the synthesis of growth inhibitory substances such as abscisic acid (ABA).77,78
It was documented79 that Vx is one of the intermediate products in ABA synthesis. One may suppose that conditions causing an increase in Vx de-epoxidase activity would cause a decrease in ABA production. Exogenously added ABA, which inhibits its synthesis, resulted in a higher concentration of Zx and greater photoprotection of PSII.80
Conclusion
All of the six types of the xanthophyll cycle are engaged in antioxidant defence in plant cells. Products of the light-dependent phase of these cycles play an important role in the protection against oxidative stress generated not only by excess of light but also by other ROS-generating factors like drought, chilling, heat, senescence, or salinity stress. It was demonstrated that these products are effective quenchers of ROS. Several molecular mechanisms are presented to explain the protective role of the xanthophyll cycle pigments. Some of them refer to direct quenching of ROS and others are based on facilitation of the energy dissipation in the photosynthetic apparatus, which results in decrease in production of singlet oxygen and other free radicals under overexcitation conditions. The mechanisms explaining the protective role of xanthophyll cycles in oxidative stress based on the indirect participation of the de-epoxidized pigments include: (1) quenching of overexcitation by aggregation-dependent LHCII quenching; (2) light-driven mechanisms in LHCII; and (3) charge transfer quenching between Chl a and Zx.
Acknowledgments
This paper was prepared in the frames of the European Regional Development Fund: the Polish Innovation Economy Operational Program (contract No. POIG.02.01.00-12-167/08, Project Małopolska Centre of Biotechnology) and was financially supported by the Statutory Funds of the Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University.
References
- 1.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 2001;30(11):1191–212. doi: 10.1016/S0891-5849(01)00480-4. PMID 11368918. [DOI] [PubMed] [Google Scholar]
- 2.Lennon SV, Martin SJ, Cotter TG. Dose-dependent induction of apoptosis in human tumour cell lines by widely diverging stimuli. Cell Prolif 1991;24(2):203–14. doi: 10.1111/j.1365-2184.1991.tb01150.x. PMID 2009322. [DOI] [PubMed] [Google Scholar]
- 3.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem 2005;12(10):1161–208. doi: 10.2174/0929867053764635. PMID 15892631. [DOI] [PubMed] [Google Scholar]
- 4.Puddu P, Puddu GM, Cravero E, Rosati M, Muscari A. The molecular sources of reactive oxygen species in hypertension. Blood Press 2008;17(2):70–7. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez-Pallares J, Parga JA, Joglar B, Guerra MJ, Labandeira-Garcia JL. The mitochondrial ATP-sensitive potassium channel blocker 5-hydroxydecanoate inhibits toxicity of 6-hydroxydopamine on dopaminergic neurons. Neurotox Res 2009;15(1):82–95. Epub 2009 Feb 24. [DOI] [PubMed] [Google Scholar]
- 6.Callaway JK, Lawrence AJ, Jarrott B. AM-36, a novel neuroprotective agent, profoundly reduces reactive oxygen species formation and dopamine release in the striatum of conscious rats after endothelin-1-induced middle cerebral artery occlusion. Neuropharmacology 2003;44:787–800. [DOI] [PubMed] [Google Scholar]
- 7.Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci 2008;1147:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parvaiz A, Maryam S, Satyawati S. Reactive oxygen species, antioxidants and signaling in plants. J Plant Biol 2008;51(3):167–73. [Google Scholar]
- 9.Slesak I, Libik M, Karpinska B, Karpinski S, Miszalski Z. The role of hydrogen peroxide in regulation of plant metabolism and cellular signalling in response to environmental stresses. Acta Biochim Pol 2007;54:39–50. [PubMed] [Google Scholar]
- 10.Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 2002;53:1331–41. [PubMed] [Google Scholar]
- 11.Asada K, Takahashi M. Production and scavenging of active oxygen in chloroplasts. In: , Kyle DJ, Osmond CB, Arntzen CJ (eds) Photoinhibition. Amsterdam: Elsevier; 1987. pp.227–87. [Google Scholar]
- 12.Wise RR, Naylor AW. Chilling-enhanced photooxidation: evidence for the role of singlet oxygen and superoxide in the breakdown of pigments and endogenous antioxidants. Plant Physiol 1987;83:278–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osmond CB. What is photoinhibition? Some insights from comparisons of shade and sun plants. , Baker NR, Bowyer JR (eds) Photoinhibition of photosynthesis: from molecular mechanisms to the field. Oxford: Bios. Sci. Pub.; 1994. pp. 1–24. [Google Scholar]
- 14.Dall'Osto L, Fiore A, Cazzaniga S, Giuliano G, Bassi R. Different roles of alpha- and beta-branch xanthophylls in photosystem assembly and photoprotection. J Biol Chem 2007;282:35056–68. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Yan H, Wang K, Kuang T, Zhang J, Gui L, et al.. Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature 2004;428:287–92. [DOI] [PubMed] [Google Scholar]
- 16.Latowski D, Grzyb J, Strzalka K. The xanthophyll cycle – molecular mechanism and physiological significance. Acta Physiol Plant 2004;26:197–212. doi: 10.1007/s11738-004-0009-8. [Google Scholar]
- 17.Gruszecki WI, Grudzinski W, Gospodarek M, Patyra M, Maksymiec W. Xanthophyll-induced aggregation of LHCII as a switch between light-harvesting and energy dissipation systems. Biochim Biophys Acta 2006;1757:1504–11. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Plazaola JI, Matsubara S, Osmond CB. The lutein epoxide cycle in higher plants: its relationships to other xanthophyll cycles and possible functions. Funct Plant Biol 2007;34:759–73. [DOI] [PubMed] [Google Scholar]
- 19.Muller P, Li XP, Niyogi KK. Non-photochemical quenching. A response to excess light energy. Plant Physiol 2001;125:1558–66. doi: 10.1104/pp. 125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niyogi KK. Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 1999;50:333–59. [DOI] [PubMed] [Google Scholar]
- 21.Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol 1996;47:655–84. [DOI] [PubMed] [Google Scholar]
- 22.de Bianchia S, Ballottaria M, Dall'Ostoa L, Bassi R. Regulation of plant light harvesting by thermal dissipation of excess energy. Biochem Soc Trans 2010;38:651–60. [DOI] [PubMed] [Google Scholar]
- 23.Ma YZ, Holt NE, Li XP, Niyogi KK, Fleming GR. Proc Natl Acad Sci USA 2003;100(8):4377–82. Epub 2003 Apr 03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams WW, Demmig-Adams B, Verhoeven AS, Baker DH, Aust J. Photoinhibition during winter stress: involvement of sustained xanthophyll cycle-dependent energy dissipation. Plant Physiol 1995;22:261–76. [Google Scholar]
- 25.Ruban AV, Horton P. The xanthophyll cycle modulates the kinetics of non-photochemical energy dissipation in isolated light-harvesting complexes, intact chloroplasts, and leaves of spinach. Plant Physiol 1999;119:531–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavaud J, Rosseau B, Etienne AL. In diatoms, a transthylakoid proton gradient alone is not sufficient to induce a non-photochemical fluorescence quenching. FEBS Lett 2002;523:163–6. [DOI] [PubMed] [Google Scholar]
- 27.Lavaud J, Rosseau B, van Gorkom HJ, Etienne AL. Influence of the diadinoxanthin pool size on photoprotection in the marine planktonic diatom Phaeodactylum tricornutum. Plant Physiol 2002;129:1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young AJ, Frank HA. Energy transfer reactions involving carotenoids: quenching of chlorophyll fluorescence. J Photochem Photobiol B: Biol 1996;36:3–15. [DOI] [PubMed] [Google Scholar]
- 29.Hudson B, Kohler BE. Electronic structure and spectra of finite linear polyenes. Synth Metals 1984;9:241–53. [Google Scholar]
- 30.Shrevea AP, Trautmana JK, Owensb TG, Albrechta AC. Two-photon excitation spectroscopy of thylakoid membranes from Phaeodactylum tricornutum: Evidence for an in vivo two-photon-allowed carotenoid state. Chem Phys Lett 1990;170(1):51–6. [Google Scholar]
- 31.Frank HA, Bautista JA, Josue SJ, Young AJ. Mechanism of nonphotochemical quenching in green plants: energies of the lowest excited singlet states of violaxanthin and zeaxanthin. Biochemistry 2000;39:2831–7. [DOI] [PubMed] [Google Scholar]
- 32.Demmig-Adamsa B. Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochimic Biophys Acta (BBA) – Bioenerg 1990;1020(1):1–24. [Google Scholar]
- 33.Frank HA, Cua A, Chynwat V, Young A, Gosztola D, Wasielewski MR. Photophysics of the carotenoids associated with the xanthophyll cycle in photosynthesis. Photosynth Res 1994;41:389–95. [DOI] [PubMed] [Google Scholar]
- 34.Gruszecki WI, Matuła M, Myśliwa-Kurdziel B, Kernem P, Krupa Z, Strzałka K. Effect of xanthophyll pigments on fluorescencje of chlorophyll a in LHC II embedded to liposomes. J Photochem Photobiol, B: Biol 1997;37:84–90. [Google Scholar]
- 35.Avital S, Brumfeld V, Malkin S. A micellar model system for the role of zeaxanthin in the non-photochemical quenching process of photosynthesis – chlorophyll fluorescence quenching by the xanthophylls. Biochim Biophys Acta 2006;1757:798–810. [DOI] [PubMed] [Google Scholar]
- 36.Polivka T, Sundstrom V. Ultrafast dynamics of carotenoid excited states – from solution to natural and artificial systems. Chem Rev 2004;104:2021–71. [DOI] [PubMed] [Google Scholar]
- 37.Lokstein H, Tian L, Polle JEW, DellaPenna D. Xanthophyll biosynthetic mutants of Arabidopsis thaliana: altered nonphotochemical quenching of chlorophyll fluorescence is due to changes in Photosystem II antenna size and stability. Biochim Biophys Acta 2002;1553:309–19. [DOI] [PubMed] [Google Scholar]
- 38.Polivka T, Herek JL, Zigmantas D, Akerlund H, Sundstrom V. Direct observation of the (forbidden) S1 state in carotenoids. Proc Natl Acad Sci USA 1999;96:4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berera R, van Stokkum IH, Kodis G, Keirstead AE, Pillai S, Herrero C, et al.. Energy transfer, excited-state deactivation, and exciplex formation in artificial caroteno-phthalocyanine light-harvesting antennas. J Phys Chem B 2007;111:6868–77. [DOI] [PubMed] [Google Scholar]
- 40.Ruban AV, Berera R, Ilioaia C, van Stokkum IH, Kennis JT, Pascal AA, et al.. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 2007;450:575–8. [DOI] [PubMed] [Google Scholar]
- 41.Caffarri S, Croce R, Breton J, Bassi R. The major antenna complex of photosystem II has a xanthophyll binding site not involved in light harvesting. J Biol Chem 2001;276:35924–33. [DOI] [PubMed] [Google Scholar]
- 42.Croce R, Cinque G, Holzwarth AR, Bassi R. The soret absorption properties of carotenoids and chlorophylls in antenna complexes of higher plants. Photosynth Res 2000;64:221–31. [DOI] [PubMed] [Google Scholar]
- 43.Jahns P, Latowski D, Strzalka K. Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochim Biophys Acta 2009;1787:3–14. [DOI] [PubMed] [Google Scholar]
- 44.Wehner A, Storf S, Jahns P, Schmid VH. De-epoxidation of violaxanthin in light-harvesting complex I proteins. J Biol Chem 2004;279:26823–9. [DOI] [PubMed] [Google Scholar]
- 45.Miloslavina Y, Wehner A, Lambrev PH, Wientjes E, Reus M, Garab G, et al.. Far-red fluorescence: a direct spectroscopic marker for LHCII oligomer formation in nonphotochemical quenching. FEBS Lett 2008;582:3625–31. [DOI] [PubMed] [Google Scholar]
- 46.Ilioaia C, Johnson MP, Horton P, Ruban AV. Induction of efficient energy dissipation in the isolated light-harvesting complex of photosystem II in the absence of protein aggregation. J Biol Chem 2008;283:29505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bode S, Quentmeier CC, Liao PN, Hafi N, Barros T, Wilk L, et al.. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc Nat Acad Sci USA 2009;106:12311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersson J, Wentworth M, Walters RG, Howard CA, Ruban AV, Horton P, et al.. Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of photosystem II – effects on photosynthesis, grana stacking and fitness. Plant J 2003;35:350–61. [DOI] [PubMed] [Google Scholar]
- 49.Damkjaer JT, Kereiche S, Johnson MP, Kovacs L, Kiss AZ, Boekema EJ, et al.. The photosystem II light-harvesting protein Lhcb3 affects the macrostructure of photosystem II and the rate of state transitions in Arabidopsis. Plant Cell 2009;21(10):3245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mozzo M, Passarini F, Bassi R, van Amerongen H, Croce R. Photoprotection in higher plants: the putative quenching site is conserved in all outer light-harvesting complexes of photosystem II. Biochim Biophys Acta 2008;1777:1263–7. [DOI] [PubMed] [Google Scholar]
- 51.Garab G, Cseh Z, Kovacs L, Rajagopal S, Varkonyi Z, Wentworth M, et al.. Light-induced trimer to monomer transition in the main light- harvesting antenna complex of plants: thermo-optic mechanism. Biochemistry 2002;41:15121–9. [DOI] [PubMed] [Google Scholar]
- 52.Grudziński W, Matula M, Sielewiesiuk J, Kernem P, Krupa Z, Gruszecki WI. Effect of 13-cis violaxanthin on organization of light harvesting complex II in monomolecular layers. Biochim Biophys Acta 2001;1503:291–302. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto HY, Higashi RM. Violaxanthin de-epoxidase. Lipid composition and substrate specificity. Arch Biochem Biophys 1978;190:514–22. [DOI] [PubMed] [Google Scholar]
- 54.Niedzwiedzki D, Krupa Z, Gruszecki WI. Temperature-induced isomerization of violaxanthin in organic solvents and in light-harvesting complex II. J Photochem Photobiol B, Biol 2005;78:109–14. [DOI] [PubMed] [Google Scholar]
- 55.Schiffer R, Neis M, Holler D, Rodriguez F, Geier A, Gartung C, et al.. Active influx transport is mediated by members of the organic anion transporting polypeptide family in human epidermal keratinocytes. J Invest Dermatol 2003;120:285–91. [DOI] [PubMed] [Google Scholar]
- 56.Holt NE, Zigmantas D, Valkunas L, Li XP, Niyogi KK, Fleming GR. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 2005;307:433–6. [DOI] [PubMed] [Google Scholar]
- 57.Avenson TJ, Ahn TK, Zigmantas D, Niyogi KK, Li Z, Ballottari M, et al.. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J Biol Chem 2008;283:3550–8. [DOI] [PubMed] [Google Scholar]
- 58.Ahn TK, Avenson TJ, Ballottari M, Cheng YC, Niyogi KK, Bassi R, et al.. Architecture of a charge transfer state regulating light harvesting in a plant antenna protein. Science 2008;320:794–7. [DOI] [PubMed] [Google Scholar]
- 59.Avenson TJ, Ahn TK, Niyogi KK, Ballottari M, Bassi R, Fleming GR. Lutein can act as a switchable charge transfer quencher in the CP26 light-harvesting complex. J Biol Chem 2009;284:2830–5. [DOI] [PubMed] [Google Scholar]
- 60.Li Z, Ahn TK, Avenson TJ, Ballottari M, Cruz JA, Kramer DM, et al.. Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. Plant Cell 2009;21:1798–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonente G, Howes BD, Caffarri S, Smulevich G, Bassi R. Interactions between the photosystem II subunit PsbS and xanthophylls studied in vivo and in vitro. J Biol Chem 2008;283:8434–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gruszecki WI, Strzalka K. Does the xanthophyll cycle take part in the regulation of fluidity of the thylakoid membrane? Biochim Biophys Acta 1991;1060:310–14. [Google Scholar]
- 63.Latowski D, Akerlund HE, Strzalka K. Violaxanthin de-epoxidase, the xanthophyll cycle enzyme, requires lipid inverted hexagonal structures for its activity. Biochemistry 2004;43:4417–20. [DOI] [PubMed] [Google Scholar]
- 64.Havaux M, Gruszecki WI, Dupont I, Leblanc RM. Increased heat emission and its relationship to the xanthophyll cycle in pea leaves exposed to strong light stress. J. Photochem Photobiol B Biol 1991;8:361–70. [Google Scholar]
- 65.Havaux M, Niyogi KK. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Nat Acad Sci USA 1999;96:8762–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarry JE, Montillet JL, Sauvaire Y, Havaux M. The protective function of the xanthophyll cycle in photosynthesis. FEBS Lett 1994;353:147–50. [DOI] [PubMed] [Google Scholar]
- 67.Woodall AA, Britton G, Jackson MJ. Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: Relationship between carotenoid structure and protective ability. Biochim Biophys Acta 1997;1336:575–86. [DOI] [PubMed] [Google Scholar]
- 68.Woodall AA, Lee SW-M, Weesie RJ, Jackson MJ, Britton G. Oxidation of carotenoids by free radicals: relationship between structure and reactivity Biochim. Biophys Acta 1997;1336:33–42. [DOI] [PubMed] [Google Scholar]
- 69.Schaller S, Latowski D, Jemioła-Rzemińska M, Wilhelm C, Strzałka K, Goss R. The main thylakoid membrane lipid monogalactosyldiacylglycerol (MGDG) promotes the de-epoxidation of violaxanthin associated with the light-harvesting complex of photosystem II (LHCII). Biochim Biophys Acta-Bioenerg 2010;1797(3):414–24. [DOI] [PubMed] [Google Scholar]
- 70.Munne-Bosch S, Alegre L. Plant aging increases oxidative stress in chloroplasts. Planta 2002;214:608–15. [DOI] [PubMed] [Google Scholar]
- 71.Misra AN, Latowski D, Strzałka K. Photosynthetic excitation pressure causes violaxanthin de-epoxidation in aging cabbage (Brassica oleracea L.) leaves. J Life Sci 2011;5:182–91. [Google Scholar]
- 72.Krinsky NI. Carotenoid protection against oxidation. Pure Appl Chem 1979;51:649–60. [Google Scholar]
- 73.Lim BP, Nagao A, Terao J, Tanaka T, Takama K. Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipid membrane. Biochem Biophys Acta 1992;1126:178–84. [DOI] [PubMed] [Google Scholar]
- 74.Peguero-Pina JJ, Morales F, Flexas J, Gil-Pelegrín E, Moya I. Photochemistry, remotely sensed physiological reflectance index and de-epoxidation state of the xanthophyll cycle in Quercus coccifera under intense drought. Conference Information: 3rd International Workshop on Remote Sensing of Vegetation Fluorescence; 2007; Florence, Italy. Oecologia 2008;156(1):1–11. [DOI] [PubMed] [Google Scholar]
- 75.Lu CM, Qiu NW, Lu QT. Photoinhibition and the xanthophyll cycle are not enhanced in the salt-acclimated halophyte Artimisia anethifolia. Physiol Planta 2003;118(4):532–7. [Google Scholar]
- 76.Sui N, Li M, Liu XY, Wang N, Fang W, Meng QW. Response of xanthophyll cycle and chloroplastic antioxidant enzymes to chilling stress in tomato over-expressing glycerol-3-phosphate acyltransferase gene. Photosynthetica 2007;45(3):447–54. [Google Scholar]
- 77.Pasqualini S, Batini P, Ederli L, Antonielli M. Responses of the xanthophyll cycle pool and ascorbate-glutathione cycle to ozone stress in two tobacco cultivars. Free Rad Res 1999;31(Suppl):67–73. [DOI] [PubMed] [Google Scholar]
- 78.Ederli L, Pasqualini S, Batini P, Antonielli M. Photoinhibition and oxidative stress: effects on xanthophyll cycle, scavenger enzymes and ABA content in tobacco plants. J Plant Physiol 1997;151(4):422–8. [Google Scholar]
- 79.Audran C, Borel C, Frey A, Sotta B, Meyer C, Simonneau T, et al.. Expression studies of the zeaxanthin epoxidase gene in Nicotiana plumbaginifolia. Plant Physiol 1998;118:1021–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ivanov AG, Krol M, Maxwell D, Huner NP. Abscisic acid induced protection against photoinhibition of PSII correlates with enhanced activity of the xanthophyll cycle. FEBS Lett 1995;371:61–4. [DOI] [PubMed] [Google Scholar]