Abstract
Background
Tumor mutations and tumor microenvironment are associated with resistance to cancer immunotherapies. However, peripheral T cell in effective anti-programmed death 1 (PD-1) antibody treatment is poorly understood.
Methods
Mass spectrometry and conventional flow cytometry were used to investigate peripheral blood cells isolated from patients. Furthermore, melanoma mouse model was performed to assess the role of CXCR3 signaling in anti-PD-1 antibody treatment.
Findings
We revealed a marked increase in the percentage of CXCR3+ T cells in the blood of cancer patients after the first pembrolizumab infusion. This percentage decreased after the second infusion in responsive patients, whereas a sustained high percentage of CXCR3+ T cells was observed in patients with progressive disease. A low percentage of CXCR3+ T cells presented in patients with stable disease or a partial response was confirmed by conventional flow cytometry. Intriguingly, blockade of CXCR3 signaling exacerbated tumor growth in mice. Intratumoral injection with recombinant CXCL9/10 plus intraperitoneal injection of anti-PD1 antibody inhibited the tumor growth in mice.
Interpretation
The dynamic changes in CXCR3+ T cells in blood may be a prognostic factor in anti-PD-1 immunotherapy, and promotion of CXCR3-mediated signaling may be beneficial to the anti-PD-1 therapy.
Fund
This work was supported by the National Natural Science Foundation of China (Nos. 81722047, 81871944, 81670553, 81874317, 81572389, 81730100) and Jiangsu province key medical talents (Nos. ZDRCA2016026), The “Deng Feng” Distinguished Scholars Program, National Science & Technology Major Project “Key New Drug Creation and Manufacturing Program”, China (Number: 2018ZX09201002), and the Fundamental Research Funds for the Central Universities (020814380117).
Keywords: Anti-PD-1, CXCR3, Biomarker, Immunotherapy
Highlights
-
•
The first infusion of anti-PD-1 antibody rapidly increases CXCR3+ T cells in blood in melanoma and head and neck cancer patients.
-
•
Retention of CXCR3+ T cells in blood is associated with the resistance to anti-PD-1 therapy.
-
•
CXCR3-dependent T cell tumor accumulation is required for effective anti-PD-1 immunotherapy.
-
•
Intratumoural injection with recombinant CXCL9/10 improved the anti-PD-1 antibody treatment
Research in context.
Evidence before this study
Immune checkpoint blockade therapy demonstrates unprecedented clinical efficacy in treating advanced cancers. However, only a subset of patients can greatly benefit from the anti-programmed death 1 (PD-1) antibody treatment. Otherwise, it is still challenging to predict and reinforce the efficacy of immunotherapy.
Added value of this study
Our findings highlight that the retention of CXCR3+ T cells in blood may reflect failure in the infiltration of IFN-γ-producing T cells into tumors, leading to ineffective outcomes for anti-PD-1 therapy. We think that the detection of dynamic changes in CXCR3+ T cells in blood after therapy initiation may provide early guidance for choosing the appropriate therapy and will greatly improve patient response rates to anti-PD-1 therapy.
Implications of all the available evidence
We report that the percentage of CXCR3+ T cells in blood changes in a specific pattern during multiple infusions of anti-PD-1 antibody. Interestingly, a lower percentage of CXCR3+ T cells in blood indicated a better therapeutic effect in patients receiving pembrolizumab. Consistently, CXCR3 blockade led to tumor hyper-progression in mice, suggesting CXCR3-mediated T cell tumor trafficking is required for Anti-PD-1 therapy.
Alt-text: Unlabelled Box
1. Introduction
Immune checkpoint blockade therapy demonstrates unprecedented clinical efficacy in treating advanced cancers, including melanoma, non-small-cell lung cancer, renal cell carcinoma, bladder cancer, head and neck squamous cell carcinoma, microsatellite instability (MSI)-high colorectal carcinoma, Merkel cell carcinoma, and Hodgkin lymphoma, and has changed the practice of medical oncology [[1], [2], [3], [4], [5]]. Keytruda (pembrolizumab) has been approved by the FDA as the first cancer treatment for any solid tumor with a specific genetic feature regardless of the tissue of origin. However, many patients failed to benefit from the pembrolizumab treatment [6]. Clinical data have shown that anti-programmed death 1 (PD-1) therapy induces disease hyperprogression in a subset of patients [7]. Furthermore, both innate and acquired resistance to anti-PD-1 therapy have been observed in patients with melanoma [8]. Studies aiming to reveal the mechanistic basis of resistance focus on the tumor microenvironment and tumor mutations, such as the characterization of genomic and transcriptomic features [9], JAK1/2 mutations [8,10], biallelic PTEN loss [11], the IFN-γ-related mRNA profile [12], the tumor microenvironment [13], and inactivation of antigen presentation [14]. Although tumor mutations and the immune microenvironment have been shown to be associated with resistance to cancer immunotherapy, dynamic immune responses in peripheral blood remains unclear. It is extremely important to identify prognostic factors for this promising therapeutic modality. However, only a limited number of strategies are currently available for predicting drug responses in the clinical practice, including molecular biomarkers, which are complicated and sometimes inapplicable. The application of key predictive markers for anti-PD-1 therapy response in peripheral blood is promising but currently remains undeveloped.
Here, we report that the percentage of CXCR3+ T cells in blood changes in a specific pattern during multiple infusions of anti-PD-1 antibody. Mass cytometry with peripheral blood mononuclear cells (PBMCs) periodically collected from patients before and after receiving a periodic infusion of pembrolizumab revealed significant alterations in the constitution of lymphocytes, especially in CXCR3+ T cells. Interestingly, a lower percentage of CXCR3+ T cells in blood indicated a better therapeutic effect in patients receiving pembrolizumab. Consistently, CXCR3 blockade led to tumor hyperprogression in mice. Thus, the amount of peripheral CXCR3+ T cells may be a novel and potential predictive biomarker for the efficacy of anti-PD-1 therapy.
2. Materials & methods
2.1. Study design
The aim of the study was to detect the changes in the systemic immune landscape after anti-PD-1 immunotherapy and to identify a special cell population that is associated with a curative effect. Whole blood samples from all patients were provided by the Jiangsu Province Hospital, China. In total, forty-three patients were enrolled (Supplementary Table 1). The patients met all four selection criteria. First, these patients must have had locally advanced (unresectable, III phase) or metastatic tumors (IV phase) with histological determination and must have received no local treatment. Second, these patients should have failed to respond to multiline chemotherapy (excluding adjuvant chemotherapy or neoadjuvant chemotherapy) or targeted therapy. Third, the patients were assessed by imaging computed tomography (CT) or magnetic resonance imaging (MRI) before participating in the treatment with pembrolizumab (Merck & Co., Inc., Kenilworth, NJ, USA) and according to the images, the patients must have had at least one measurable lesion identified by the Immune Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST 1.1). Fourth, patients who had previously received anti-PD-1, anti-PDL1 and anti-PDL2 therapy were excluded. PBMCs were isolated from heparin-treated whole blood from patients using ficoll gradient centrifugation. After isolation, the PBMCs were cryopreserved in vapor-phase liquid nitrogen. Sixteen samples of PBMCs isolated from ten patients were assigned to the discovery cohort. Among these sixteen samples, twelve were collected before and after every cycle of anti-PD-1 infusion, and the remaining four were collected after four infusions and used to estimate the curative effect. The patients were treated with 2 mg per kg body weight (mg/kg) of pembrolizumab every three weeks, and after every four infusions, their clinical status was reassessed. The validation cohort was composed of twenty-nine samples from new patients, whose clinical status was evaluated after four or five infusions of anti-PD-1 therapy. Frozen PBMCs were used in this retrospective study to balance the cohorts in terms of response and to reduce batch effects through a unique barcoding strategy. PBMCs cryopreserved in vapor-phase liquid nitrogen were thawed in a 37 °C-thermostat water bath and washed with cell staining buffer (Fluidigm, South San Francisco, CA). All biological samples from humans were collected after written informed consent was obtained from patients and with approval of the local ethics committee.
2.2. Mass cytometry analyses
Approximately eight million PBMCs were stained for mass cytometry analyses, and the data were acquired on a CyTOF 2 Helios system (Fluidigm) in PLTTECH (Zhejiang, China). PBMC cells were added cisplatin with a final concentration of 0.5 μM for 2 min. Wash cells and block cells with Block Mix consist of 5 μL Fc Receptor Blocking Solution and 50 μL Cell Stain Buffer for 10 min. Then add 50 μL of the surface marker antibody cocktail (anti-CD45, CXCR3, CD16, CD27, CCR4, IL-7R) for 30 min. Following the incubation, wash cells twice. Then suspend each sample in 1 mL Fix I Buffer, and incubate for 10 min at room temperature. Then wash twice with 1 mL of Barcode Perm Buffer. Suspend each sample with 800 μL Barcode Perm Buffer with barcodes, and incubate for 30 min. Wash cell twice. Then stain cells with 50 μL of the antibody cocktail (antibodies that are slightly affected by barcoding, the remaining 28 antibodies) for 30 min at room temperature. Wash cells twice. Add 1 mL of the cell intercalation solution to the tube and gently vortex. Incubate for 1 h at room temperature or leave overnight at 4 °C. Wash cells twice. Leave cells pelleted until ready to run on CyTOF. Immediately prior to CyTOF data acquisition, adjust cell concentration to 0.25–0.5 million/mL with water and filter cells into cell strainer cap tubes. Acquire data on CyTOF. Analyse the data by debarcoding software and cytobank.org.
2.3. Response assessment
Patients who were enrolled in this clinical trial received anti-PD-1 therapy. After every four infusions of treatment with anti-PD-1 therapy (pembrolizumab), we evaluated tumor responses using the RECIST v1.1 by CT. The responder group included every patient who showed signs of a clinical benefit during every four treatment infusions, which included partial response, complete response and stable disease. The non-responder group included every patient who discontinued treatment due to disease progression or who showed signs of progression during the four infusions of treatment. Progression was defined as a measurable increase in tumor size, the presence of new metastatic sites or the need to treat the patient with a secondary treatment, such as radiotherapy.
2.4. Validation by conventional flow cytometry
The CyTOF data were validated using a combination of markers with significantly different expression levels from the initial discovery mass cytometry approach and by markers that defined the cellular composition in blood using conventional flow cytometry. A set of twenty-nine samples of PBMCs was analyzed from patients with melanoma, lung cancer, gallbladder cancer and other tumor types. The panel is consisted of antibodies against CD2 (Cat# 561759, BD Biosciences, San Jose, CA), CD3 (Cat# 560275, BD Biosciences), CD16 (Cat# 561248, BD Biosciences), CD4 (Cat# 550631, BD Biosciences), CD8 (Cat# 560917, BD Biosciences), and CD183 (Cat# 550633, BD Biosciences). At least 100,000 live cells were acquired and analyzed using FACSDiva software on an Aria II flow cytometer (BD).
2.5. Mice
Six-to eight-week-old male C57BL/6 mice were supplied by the Model Animal Research Institute of Nanjing University. All of the C57BL/6 mice received humane care according to the criteria outlined in the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985). The mice were housed five per cage under pathogen-free conditions with soft bedding under controlled temperature (22 ± 2 °C) and photoperiods (12:12-h light–dark cycle). They were allowed to acclimate to these conditions for at least two days before inclusion in the experiments. All animal experimental procedures were approved by the Animal Care Committee of Nanjing University (Nanjing, China).
2.6. Animal experiments
The mice were equally assigned into four groups and each group had twelve mice. Each mouse was subcutaneously injected with 106 B16-F10 melanoma cells into the right flank. Tumor masses were measured every two days by assessing the length of the long and short axis of the tumor using a digital caliper. Tumor volume was calculated according to the formula: Volume = (long axis * short axis2)/2. The maximum tumor size was achieved when the tumor mass reached approximately 10% of the body weight. The treatment was initiated three days after the tumor injection when tumors were visible to the naked eyes. For anti-PD-1 experiments, mice were injected intraperitoneal with anti-PD-1 antibody (200 μg per mice; Cat# BE0146, Bio X Cell, West Lebanon, NH) every 6 days. For anti-CXCR3 experiments, mice were injected intraperitoneal with either anti-CXCR3 antibody (200 μg per mice; Cat# 126537, BD Biosciences) or isotype control (200 μg per mice; Cat# BE0260, Bio X Cell) every six days. For combination therapy, treatment with anti-PD-1 and anti-CXCR3 was initiated at the same time. Another set of animal experiments was performed in four groups with seven mice in each group. B16-F10 melanoma cells were injected into each mouse as described above. The anti-PD-1 group was treated as the former experiment at indicated time points. The combination group was treated by intraperitoneal injection of anti-PD-1 antibody and intratumoral injection of 500 ng CXCL9 and 500 ng CXCL10 (mixed and diluted in 100 μl PBS) per tumor. To deplete CD4+ and CD8+ T cells, anti-CD4 mAb (200 μg, Cat# BE0003-1, Bio X Cell) plus anti-CD8 mAb (200 μg, Cat# BE BE0061, Bio X Cell) were injected i.p. on days +6, +10, +14, and +18 with respect to the day of tumor challenge.
2.7. Enzyme-linked immunosorbent assay (ELISA)
The expressions of patient serum CXCL9/10 were assayed by ELISA. Prepare all reagents and working standards as directed in the previous sections. Add 300 μl Wash Buffer (1×) per well, and allow the Wash Buffer to sit in the wells for about 30 s before aspiration. Use the microwell strips immediately after washing. Add 100 μl of 2-fold diluted Standard in duplicate. Add 100 μl of Standard Diluent to Blank well in duplicate. Add 80 μl of Assay Buffer (1×) and 20 μl sample to the sample well. Add 50 μl of diluted Detect Antibody to each well. Cover with an adhesive strip. Incubate at room temperature (18 to 25 °C) for 1.5 h on a microplate shaker set at 300 rpm. Aspirate each well and wash, repeating the process five times for a total six washes. Wash by filling each well with 300 μl Wash Buffer (1×). After the last wash, remove any remaining Wash Buffer by aspirating or decanting. Invert the plate and blot it against clean paper towels. Cover with a new adhesive strip. Incubate at room temperature (18 to 25 °C) for 30 min on a microplate shaker set at 300 rpm. Repeat aspiration/wash. Add 100 μl of Substrate Solution to each well. Incubate for 5–30 min at room temperature. Add 100 μl of Stop Solution to each well. The absorbance at 450 nm was measured using a microplate reader, and data were normalized to total protein content.
2.8. Reverse transcriptase-PCR and quantitative PCR
Total RNA was extracted using Trizol reagent (Roche Diagnostics, Indianapolis, IN) as described by the manufacturer. Single-stranded cDNA was synthesized from 2 μg of total RNA by reverse transcription using 0.5 μg of oligo (dT)18 primer. PCR was performed at 95 °C for 30 s, 60 °C for 1 min and 72 °C for 1 min. The level of actin RNA expression was used to normalize the data.
2.9. Statistical analysis
The results were expressed as the mean ± standard error of the mean (SEM). Statistical evaluations were performed using Student's t-test. P < 0.05 was considered significant.
3. Results
3.1. A marked increase in CXCR3+ T cells in blood is observed during the first infusion of anti-PD-1 antibody in melanoma and head and neck cancer patients
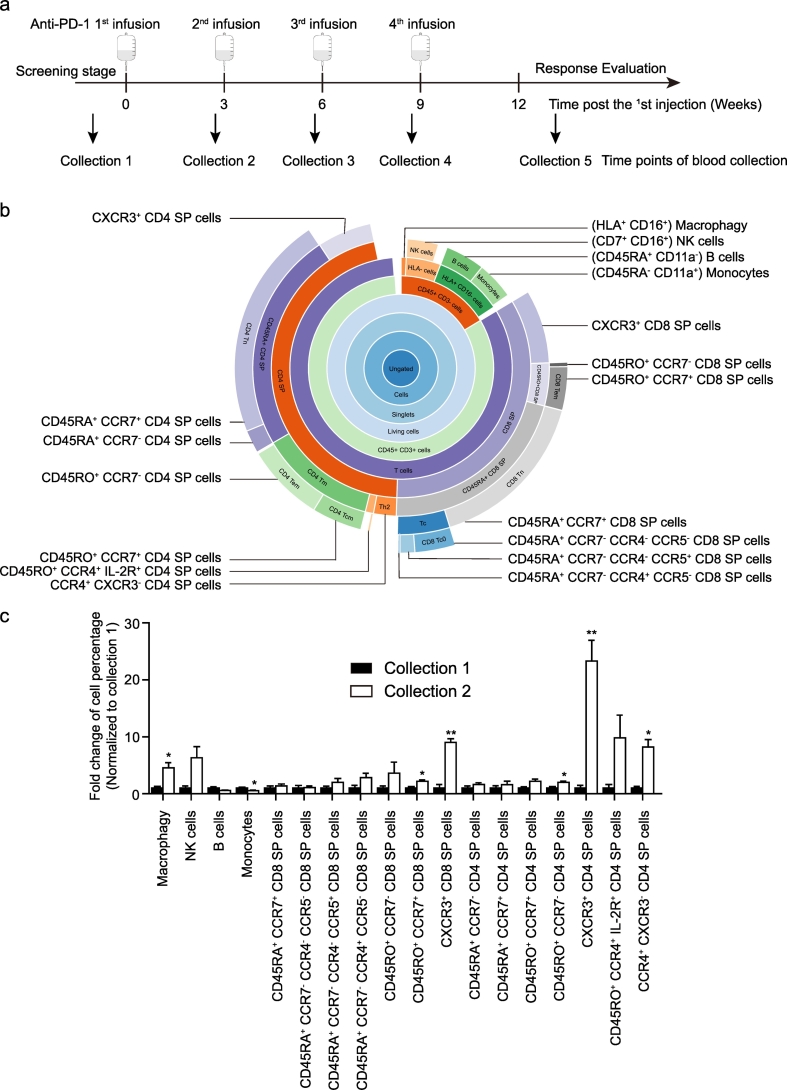
We enrolled forty-three patients (Supplementary Table 1). Patients were treated with pembrolizumab, and blood was collected at the indicated time points (Fig. 1a). PBMCs were isolated from blood samples and analyzed by mass cytometry according to 34 human T cell immune-oncology-related markers (Supplementary Table 2), and 18 populations were gated (Fig. 1b). Compared with that in collection 1, significant increases in the percentages of macrophages, natural killer (NK) cells, CD45RO+ CCR7+ CD8 single-positive (SP) T cells, CD45RO+ CCR7− CD4 SP T cells, CXCR3+ CD4 SP T cells, CXCR3+ CD8 SP T cells, and CCR4+ CXCR3− CD4 SP T cells were observed in collection 2 (Fig. 1c), suggesting a systemic immune signature change. Interestingly, a 20-fold induction was observed in the percentage of CXCR3+ CD4 SP T cells (Fig. 1c).
Fig. 1.
The first infusion of anti-PD-1 antibody induces changes in the systemic immune landscape in melanoma and head and neck cancer patients. (a) Infusions of anti-PD-1 therapy and the time points for collection of the patients' peripheral blood. (b) A classification diagram of 18 cell populations stratified by the expression of cell surface antibodies, and the relationship among them. (c) Fold changes of the percentage of 18 cell populations (mean ± s.e.m.; collection 1 n = 5; collection 2 n = 4). *P < 0.05 and **P < 0.01 by Student's t-tests, collection 2 vs collection 1.
3.2. Anti-PD-1 therapy causes a reverse-U change of CXCR3+ T cell percentage in blood
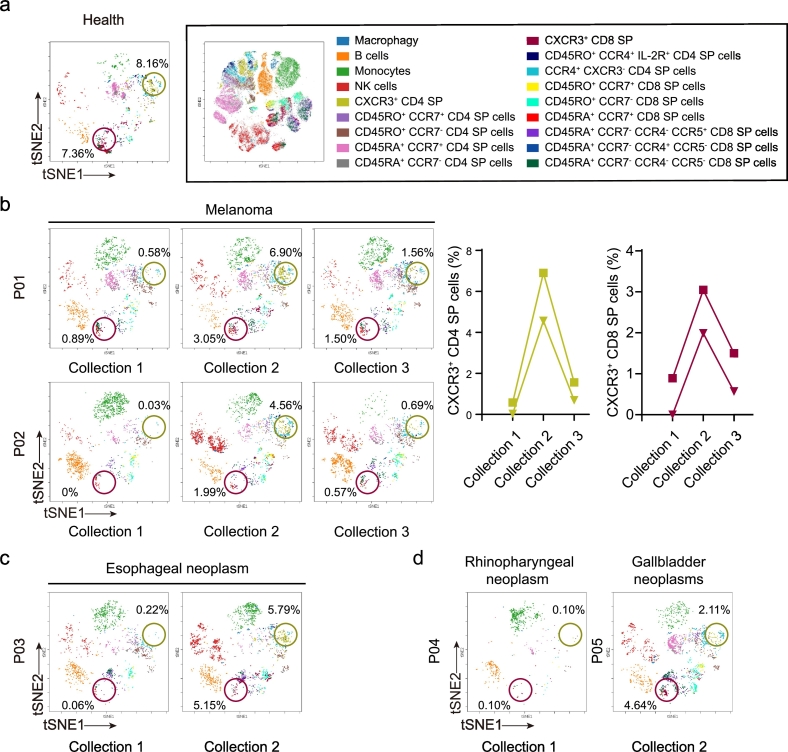
CXCR3 is expressed primarily on activated T cells and NK cells that produce IFN-γ. CXCL9 and CXCL10, which are ligands of CXCR3, are induced by IFN-γ stimulation. In addition, the IFN-γ-CXCL9/10-CXCR3 loop plays an important role in the recruitment of IFN-γ-producing cells [15]. The infiltration of T cells into tumors induced by anti-PD-1 therapy has not been well characterized. Thus, we focused on investigating the strong fold changes in the percentage of CXCR3+ T cells in blood. CXCR3+ CD4+ CD8− and CXCR3+ CD4− CD8+ T cells constitute a clear population in the blood of one healthy donor (Fig. 2a), while the percentages of CXCR3+ CD4+ CD8− and CXCR3+ CD4− CD8+ T cells were very low in patients with melanoma before pembrolizumab treatment (Fig. 2b, collection 1). Interestingly, the percentage of CXCR3+ CD4+ CD8− and CXCR3+ CD4− CD8+ T cells increased in the blood of patients with the first infusion of pembrolizumab (Fig. 2b, collection 2), suggesting an activation of systemic immunity in blood by immune checkpoint blockade. Periodic infusion of pembrolizumab led to a decrease in the percentages of CXCR3+ CD4+ CD8− and CXCR3+ CD4− CD8+ T cells in blood (Fig. 2b, collection 3). In addition, the other sixteen cell populations in PBMC did not show a similar change (Supplementary Fig. 1). The increases in the percentages of CXCR3+ CD4+ CD8− and CXCR3+ CD4− CD8+ T cells were observed in patients with esophageal neoplasms, rhinopharyngeal neoplasms, and gallbladder neoplasms (Fig. 2c and d), indicating that this phenomenon may be common among various cancer types.
Fig. 2.
Anti-PD-1 therapy induces a reverse-U change in CXCR3+ T cells. (a) viSNE analysis of immune cells from a healthy donor labeled by the relative expression of CyTOF markers, with 18 cell populations indicated (right). CXCR3+ CD4+ CD8− and CXCR3+ CD8+ CD4− T cells are separately circled in khaki and kermesinus. (b) Dynamic changes in the percentages of CXCR3+ CD4+ CD8− and CXCR3+ CD8+ CD4− T cells in two melanoma patients are presented by viSNE analysis (left) and a line diagram (right) before and after anti-PD-1 therapy. (c) viSNE analysis of the percentages of CXCR3+ CD4+ CD8− and CXCR3+ CD8+ CD4− T cells in a patient with esophageal neoplasm before and after the 1st infusion of anti-PD-1 therapy. (d) viSNE analysis of the percentages of CXCR3+ CD4+ CD8− and CXCR3+ CD8+ CD4− T cells in a patient with rhinopharyngeal neoplasm before anti-PD-1 therapy initiation and in a patient with a gallbladder neoplasm patient after the 1st infusion of anti-PD-1 therapy.
3.3. An increase of CXCR3+ T cells in blood is associated with resistance to anti-PD-1 therapy
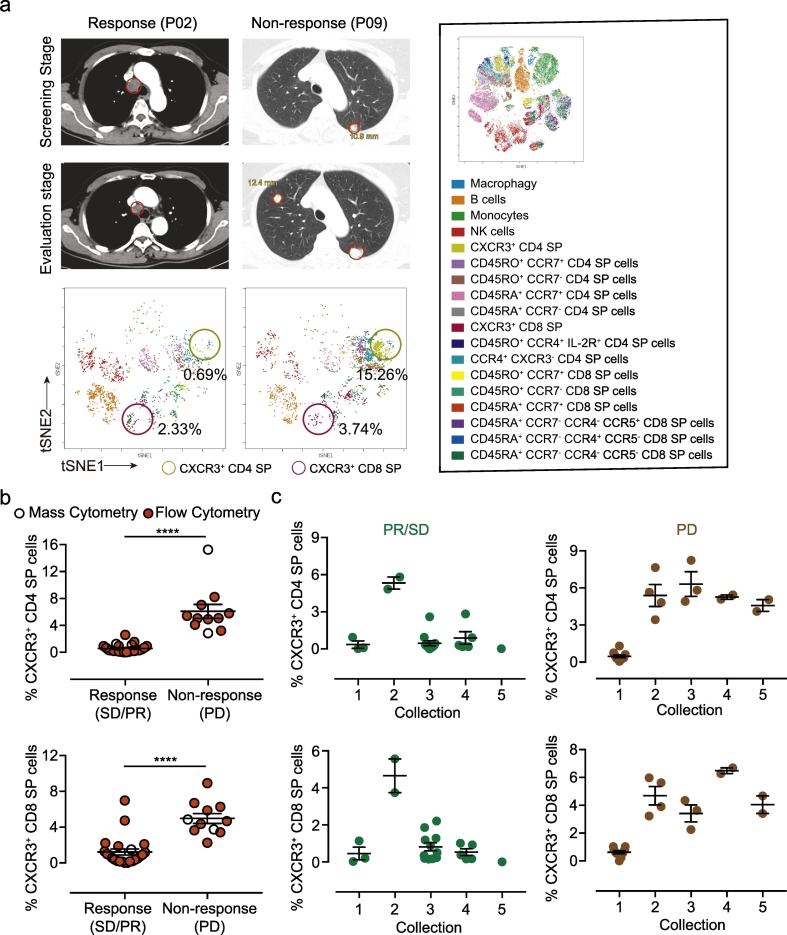
In addition to trafficking, CXCR3 plays an important role in the function of T helper (Th)-1 and cytotoxic T (Tc) cells [16]. More CXCR3+ T cells may correlate with the clinical response. However, an increased percentage of CXCR3+ T cells in blood is in fact an indicator of a poor prognosis according to our study. The experimental information of forty-three patients is described in Supplementary Fig. 2. Patients with a response to pembrolizumab treatment had lower percentages of CXCR3+ CD4+ CD8− and CXCR3+ CD4− CD8+ T cells in blood (Fig. 3a). Using conventional flow cytometry, we also confirmed that the percentages of CXCR3+ CD4+ CD8− and CXCR3+ CD4− CD8+ T cells in blood were positively correlated with tumor progression (Fig. 3b and c). Interferon signaling mutations are associated with acquired resistance to PD-1 blockade in melanoma [17]. It was reported that the expressions of CXCL9 and CXCL10 in tumors from patients who are resistant to PD-1 blockade was reduced post-anti-PD-1 therapy [11]. Consistently, slight decreases of serum CXCL9/10 were also observed in non-response patients (Supplementary Fig. 3a and b).
Fig. 3.
CXCR3+ T cells in PBMCs are associated with the effects of anti-PD-1 therapy in melanoma. (a) CT scans of one responsive patient and one non-responsive patient were contrasted by the screening stage and evaluation stage (upper). viSNE analysis of the percentage of CXCR3+ CD4+ CD8− T cells in the responsive and non-responsive patient in the evaluation stage (below). (b) Statistical chart of the percentages of CXCR3+ T cells in responders (n = 29) and non-responders (n = 11). Blank dots represent data from mass cytometry, and red dots represent data from conventional flow cytometry (mean ± s.e.m. ****P < 0.0001 by Student's t-Tests). (c) Statistical chart of the percentages of CXCR3+ T cells in patients with collections 1–5. Green dots represent data from response patients, and brown dots represent data from non-response patients (mean ± s.e.m). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Role of CXCR3 signaling in B16-F10 mouse model
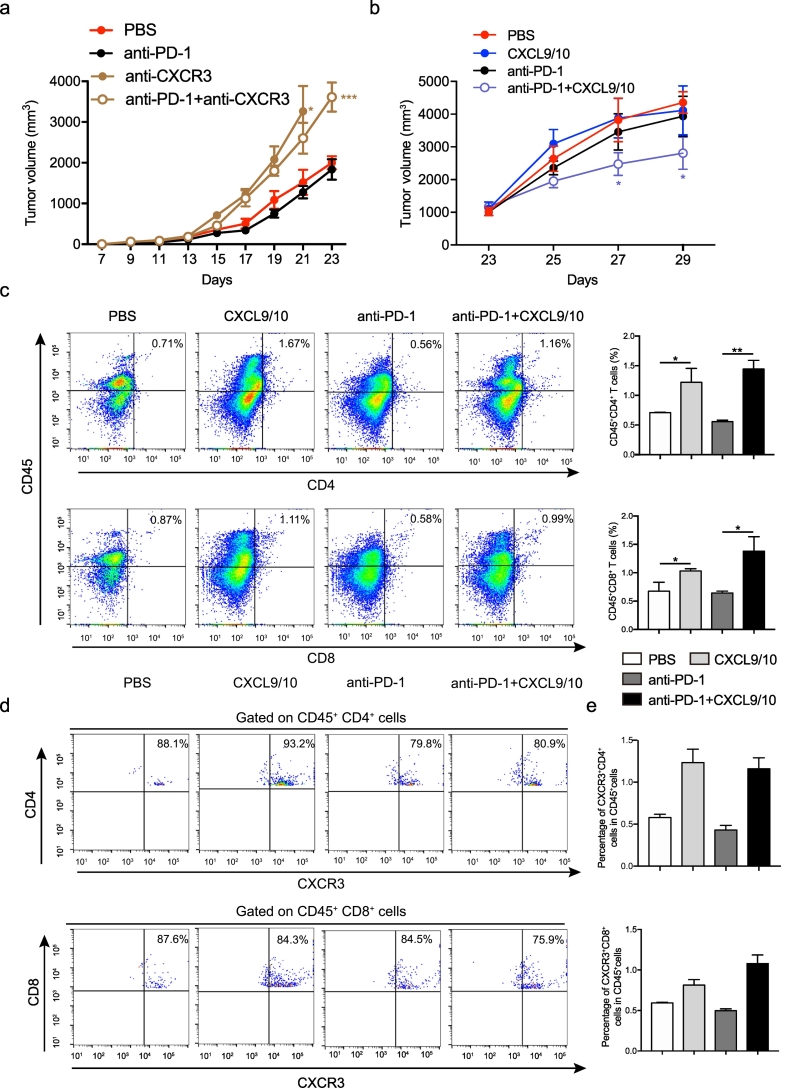
To confirm our discovery, we carried out two different sets of animal experiments. Consistently, the blockade of CXCR3-mediated signaling by using anti-CXCR3 antibody exacerbated tumor growth in mice engrafted with B16-F10 tumor cells (Fig. 4a). Furthermore, intratumoral injection with recombinant CXCL9/10 plus intraperitoneal injection of anti-PD1 inhibited the tumor growth (Fig. 4b), suggesting CXCR3 signaling plays an important role in the tumor growth. The intratumoral injection with recombinant CXCL9/10 increased CD4+ and CD8+ cells, that were mainly expressed CXCR3 (Fig. 4c–e). In addition, the intratumoral injection with recombinant CXCL9/10 promoted mRNA expressions of CXCL9/10 on tumor tissues (Supplementary Fig. 4). In addition, it is possible that tumors in the T cell depleted animals grow so fast that would limit the possibility to change the natural course of these tumors. Consistently, anti-CXCR3 did not exacerbate tumor growth in mice treated with anti-PD-1 plus anti-CD4/CD8 antibodies (Supplementary Fig. 5, green line comparison). Thus, we speculate that the increase of CXCR3+ T cells in the blood of resistant patients is may be caused by a reduced induction of CXCL9 and CXCL10 in tumors and is a reflection of changes in the tumor environment (Fig. 5).
Fig. 4.
CXCR3-mediated signaling is required for anti-PD-1 therapy. (a) Tumor volumes were observed for 23 days and presented in a line chart (n = 12). (b) Tumor volumes were observed for 29 days and presented in a line chart (n = 7). (c–e) The cytometry analysis of CXCR3+ T cells in tumor from different treatment groups. (mean ± s.e.m.; n = 3, *P < 0.05, **P < 0.01 and ***P < 0.001 by Student's t-tests).
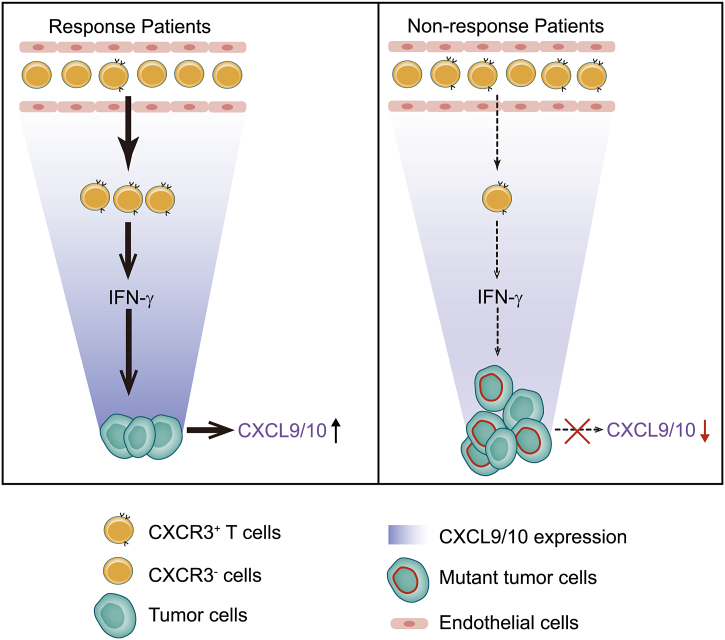
Fig. 5.
CXCR3 mediated T cell tumor accumulation contributes to anti-PD-1 therapy. In response patients, CXCR3+ T cells migrate into the tumor and activate IFN-γ signaling, which induces CXCL9 and CXCL10 expressions for recruiting T cells into the tumor. In contrast, deficient IFN-γ signaling in resistant tumor cells with gene mutations decreases CXCL9/10 expression and inhibits the recruitment of CXCR3+ T cells. Thus, the CXCR3+ T cells will accumulate in the blood in non-response patients.
4. Discussion
Given the broad application of anti-PD-1 therapy, the number of non-responsive patients will increase. Immune checkpoint blockade for anticancer therapy faces challenges due to the lack of prognostic markers. Using mass cytometry, we revealed dynamic changes in the immune signature in blood from cancer patients treated with pembrolizumab. Interestingly, a unique pattern of change was observed in the percentage of CXCR3+ T cells in blood during multiple infusions of anti-PD-1 agents. Increases of CXCR3+ T cells in blood is associated with resistance to anti-PD-1 therapy and may be caused by reduced expression of CXCL9/10 in resistant tumor, which may be a result of JAK1 and JAK2 truncating mutations [8]. Thus, the increases of CXCR3+ T cells may be a new predictive marker reflecting both tumor mutations and systemic immunity. In addition to tumor variation, changes in blood cells play important roles in anti-PD-1 therapy [9,10,18,19]. Exhausted CD8+ T cells are preferentially reinvigorated by anti-PD-1 therapy [18,19]. Here, we found that the IFN-γ-CXCL9/10-CXCR3 loop induced the increases of T cells into tumors by anti-PD-1 therapy in patients, which suggests a crosstalk between local and systemic immunity induced by anti-PD-1 therapy. Importantly, the increases of CXCR3+ T cells in blood could predict responsiveness to anti-PD-1 therapy.
Trafficking of immune cells into tumors induced by immune checkpoint blockade is critical for cancer immunotherapies [20]. Peng et al. identified IFN-γ as a crucial nexus for the tumor infiltration of T cells induced by PD-1 blockade in mice [21]. In addition, mutations in the IFN-γ signaling pathway have been reported to be involved in anti-PD-1 therapy resistance [12]. Thus, analysis of dynamic changes in immune cells in blood during infusions of pembrolizumab may provide prognostic indicators for anti-PD-1 therapy. We think that the first infusion of anti-PD-1 antibody reinvigorates CXCR3+ T cells in blood (Fig. 1c). The CXCR3+ T cells increase in tumors and activate IFN-γ signaling. Subsequently, IFN-γ induces CXCL9 and CXCL10 expressions in tumors, and CXCL9/10 recruit T cells into the tumor. In contrast, in resistant tumors, mutations in tumors interfere with IFN-γ signaling, decrease CXCL9/10 expressions, inhibit the recruitment of CXCR3+ T cells and cause increases of CXCR3+ T cells in blood (Fig. 5).
In conclusion, dynamic changes in CXCR3+ T cells in blood is a potential biomarker for predicting anti-PD-1 therapy response. The increase of CXCR3+ T cells in blood may reflect failure in the infiltration of IFN-γ-producing T cells into tumors, leading to ineffective outcomes for anti-PD-1 therapy. Detection of the dynamic changes in CXCR3+ T cells in blood after therapy initiation may therefore provide early guidance for selecting appropriate therapy and substantially improve patient response rates to anti-PD-1 therapy.
However, because of the limited sample size in our study, to further confirm our conclusion, we need to collect more samples from larger, multicenter cohorts of patients with more cancer types for which anti-PD-1 therapy has been established. In addition, there are limitations in our animal model study. B16-F10 mouse model is an anti-PD-1 resistant model, which is not suitable for making conclusions by using CD4/CD8 depletion and CXCR3 blockade on mice treated with anti-PD-1 antibody. Consistently, Chow et al. reported that CXCR3 chemokine system is required for an effective response to PD-1 blockade therapy in mouse model [22]. In our study, we paid more attention to clinical samples from patients treated with anti-PD-1 antibody. In our future study, we will pay more attention to the role and characteristic of CXCR3+ cells. Recently, Carsten Krieg et al. reported that before commencing therapy, the frequency of classical CD14+ CD16− HLA-DRhi monocytes may be a strong predictor of progression-free and overall survival in response to anti-PD-1 immunotherapy. The authors collected samples from stage IV melanoma patients before and after 12 weeks of anti-PD-1 immunotherapy and observed that the presence of activated classical monocytes may be a prerequisite for a successful response [23]. In contrast to the study by Carsten Krieg et al., we monitored the dynamic changes before and after every infusion of anti-PD-1 therapy. Thus, to better conform with clinical guidelines, the combined detection of activated CD14+ CD16− HLA-DRhi monocytes in PBMCs before therapy initiation and dynamic changes in CXCR3+ T cells after therapy initiation may be a new research direction. In addition, our understanding of the complexity of CXCR3+ T cells remains limited, and thus we need to conduct gene sequencing to characterize this cell population. Uncovering the mechanisms underlying the association between the IFN-γ-CXCR3 loop and responsiveness to anti-PD-1 therapy is an ongoing challenge.
Author contributions
X.H., Y.W., XX.W., YH.G. and Q.X. designed the study. X.H., Y.W., T.T., J.S., XM.C., PN.L., Y.T., B.W., JW.W., LY.X. and ZY.Y. performed experiments and analyzed data. H.X., Y.W., T.T. and XX.W. interpreted data and contributed to the discussion. X.H., Y.W., T.T., XX.W. and YH.G. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Data and materials availability
B16-F10 melanoma cell lines were obtained from the Chinese Academy of Science.
Declaration of Competing Interest
The authors declare no competing financial interests.
Acknowledgments
We thank Dr. Jie Zhu and Xinlei Chen for technical assistance in multiparameter cell flow cytometry.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2019.08.067.
Contributor Information
Qiang Xu, Email: molpharm@163.com.
Xingxin Wu, Email: xingxin.wu@nju.edu.cn.
Yanhong Gu, Email: guyanhong@njmu.edu.cn.
Appendix A. Supplementary data
Supplementary material
References
- 1.Khalil D.N., Smith E.L., Brentjens R.J., Wolchok J.D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13(5):273–290. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X., Song W., Shao C., Shi Y., Han W. Emerging predictors of the response to the blockade of immune checkpoints in cancer therapy. Cell Mol Immunol. 2019;16(1):28–39. doi: 10.1038/s41423-018-0086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascierto P.A., Marincola F.M. 2015: The year of anti-PD-1/PD-L1s against melanoma and beyond. EBioMedicine. 2015;2(2):92–93. doi: 10.1016/j.ebiom.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Donnell J.S., Long G.V., Scolyer R.A., Teng M.W., Smyth M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev. 2017;52:71–81. doi: 10.1016/j.ctrv.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Saada-Bouzid E., Defaucheux C., Karabajakian A., Coloma V.P., Servois V., Paoletti X. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605–1611. doi: 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- 8.Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hugo W., Zaretsky J.M., Sun L., Song C., Moreno B.H., Hu-Lieskovan S. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin D.S., Zaretsky J.M., Escuin-Ordinas H., Garcia-Diaz A., Hu-Lieskovan S., Kalbasi A. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7(2):188–201. doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George S., Miao D., Demetri G.D., Adeegbe D., Rodig S.J., Shukla S. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity. 2017;46(2):197–204. doi: 10.1016/j.immuni.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayers M., Lunceford J., Nebozhyn M., Murphy E., Loboda A., Kaufman D.R. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai J., Gao Z., Li X., Dong L., Han W., Nie J. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget. 2017;8(66):110693–110707. doi: 10.18632/oncotarget.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sade-Feldman M., Jiao Y.J., Chen J.H., Rooney M.S., Barzily-Rokni M., Eliane J.P. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8(1):1136. doi: 10.1038/s41467-017-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groom J.R., Luster A.D. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89(2):207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groom J.R., Luster A.D. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X., Lahiri A., Haines G.K., III, Flavell R.A., Abraham C. NOD2 regulates CXCR3-dependent CD8+ T cell accumulation in intestinal tissues with acute injury. J Immunol. 2014;192(7):3409–3418. doi: 10.4049/jimmunol.1302436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang A.C., Postow M.A., Orlowski R.J., Mick R., Bengsch B., Manne S. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pourchet A., Fuhrmann S.R., Pilones K.A., Demaria S., Frey A.B., Mulvey M. CD8(+) T-cell immune evasion enables oncolytic virus immunotherapy. Ebiomedicine. 2016;5:59–67. doi: 10.1016/j.ebiom.2016.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slaney C.Y., Kershaw M.H., Darcy P.K. Trafficking of T cells into tumors. Cancer Res. 2014;74(24):7168–7174. doi: 10.1158/0008-5472.CAN-14-2458. [DOI] [PubMed] [Google Scholar]
- 21.Peng W., Liu C., Xu C., Lou Y., Chen J., Yang Y. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72(20):5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chow M.T., Ozga A.J., Servis R.L., Frederick D.T., Lo J.A., Fisher D.E. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity. 2019;50(6):1498–512 e5. doi: 10.1016/j.immuni.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieg C., Nowicka M., Guglietta S., Schindler S., Hartmann F.J., Weber L.M. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24(2):144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material