Abstract
Although the robust antidepressant effects of the N-methyl-d-aspartate receptor (NMDAR) antagonist ketamine in patients with treatment-resistant depression are beyond doubt, the precise molecular and cellular mechanisms underlying its antidepressant effects remain unknown. NMDAR inhibition and the subsequent α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) activation are suggested to play a role in the antidepressant effects of ketamine. Although (R)-ketamine is a less potent NMDAR antagonist than (S)-ketamine, (R)-ketamine has shown more marked and longer-lasting antidepressant-like effects than (S)-ketamine in several animal models of depression. Furthermore, non-ketamine NMDAR antagonists do not exhibit robust ketamine-like antidepressant effects in patients with depression. These findings suggest that mechanisms other than NMDAR inhibition play a key role in the antidepressant effects of ketamine. Duman’s group demonstrated that the activation of mammalian target of rapamycin complex 1 (mTORC1) in the medial prefrontal cortex is reportedly involved in the antidepressant effects of ketamine. However, we reported that mTORC1 serves a role in the antidepressant effects of (S)-ketamine, but not of (R)-ketamine, and that extracellular signal-regulated kinase possibly underlie the antidepressant effects of (R)-ketamine. Several lines of evidence have demonstrated that brain-derived neurotrophic factor (BDNF) and its receptor, tyrosine kinase receptor B (TrkB), are crucial in the antidepressant effects of ketamine and its two enantiomers, (R)-ketamine and (S)-ketamine, in rodents. In addition, (2R,6R)-hydroxynormetamine [a metabolite of (R)-ketamine] and (S)-norketamine [a metabolite of (S)-ketamine] have been shown to exhibit antidepressant-like effects on rodents through the BDNF–TrkB cascade. In this review, we discuss recent findings on the molecular and cellular mechanisms underlying the antidepressant effects of enantiomers of ketamine and its metabolites. It may be time to reconsider the hypothesis of NMDAR inhibition and the subsequent AMPAR activation in the antidepressant effects of ketamine.
Subject terms: Depression, Clinical pharmacology
Introduction
Antidepressants, including selective serotonin reuptake inhibitors (SSRIs) and selective noradrenaline reuptake inhibitors (SNRIs), are widely prescribed for the treatment of depression in patients with major depressive disorder (MDD). However, there is a significant time lag of weeks to months for the antidepressant effects of these drugs to be achieved in patients with MDD1. In addition, approximately one-third of patients with MDD do not experience satisfactory therapeutic benefits following treatment with SSRIs or SNRIs1. Importantly, the delayed onset of these antidepressants is extremely harmful to patients with depression who experience suicidal ideation2,3. Therefore, the development of rapid-acting and robust antidepressants is imperative to relieve the symptoms of severe depression and suicidal ideation in patients with MDD or bipolar disorder (BD)4–12.
In 2000, Berman et al.13 demonstrated that a sub-anesthetic dose (0.5 mg/kg) of ketamine, an N-methyl-d-aspartate receptor (NMDAR) antagonist, produced rapid-acting and sustained antidepressant effects in patients with MDD. This is a first double-blind, placebo-controlled study of ketamine in depressed patients13. Subsequently, Zarate et al.14 replicated the rapid-acting and sustained antidepressant effects of ketamine for patients with treatment-resistant MDD. In addition, ketamine possesses robust antidepressant effects in patients with bipolar depression15–18. Ketamine has been shown to alleviate suicidal ideation in patients with treatment-resistant MDD19–21. Several meta-analyses revealed that ketamine has robust antidepressant and anti-suicidal ideation effects in depressed patients with treatment-resistant MDD or BD2,3,22,23.
The antidepressant effects of ketamine have attracted increasing academic attention due to its effects being rapid-acting and long-lasting effects in treatment-resistant depression8,12,24. Although ketamine has a robust antidepressant effect, its side effects may limit its widespread use for the treatment of depression12,25–31. Ketamine has detrimental side effects, which include psychotomimetic effects, dissociative effects, and abuse liability; which may be associated with the blockade of NMDAR25,26,32. It is known that dissociative symptoms following ketamine infusion are not associated with its clinical benefits24, suggesting that NMDAR inhibition may not serve a key role in the antidepressant effects of ketamine. Fava et al.33 also reported that there were no statistically significant correlations between Clinician Administered Dissociative States Scale (CADSS) scores 40 min after the ketamine infusion and Hamilton Depression Rating Scale-6 (HAMD-6) scores at day 1 and day 3 in treatment-resistant patients with depression, in contrast to the hypothesis by Luckenbaugh et al.34. In addition, brain-imaging findings suggest that reduced subgenual anterior cingulate cortex is implicated in the antidepressant effects of ketamine in humans35,36. However, the precise molecular and cellular mechanisms underlying its antidepressant effects remain unclear. In this review article, recent findings on the molecular and cellular mechanisms underlying the antidepressant effects of enantiomers of ketamine and its metabolites are summarized.
Enantiomers of ketamine
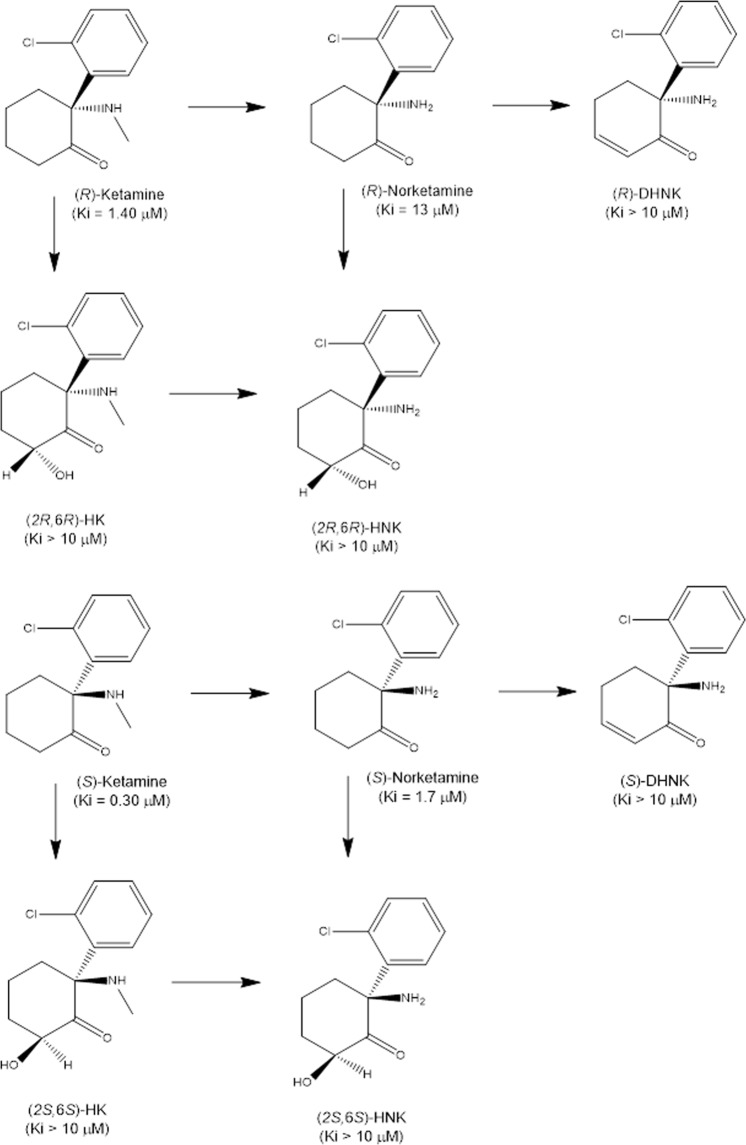
Ketamine (Ki = 0.53 μM for NMDAR) (Fig. 1) is a racemic mixture consisting of equal parts of (R)-ketamine (or arketamine) and (S)-ketamine (or esketamine). The binding affinity of (S)-ketamine (Ki = 0.30 μM) for NMDAR is ~4-fold greater than that of (R)-ketamine (Ki = 1.4 μM) (Fig. 1)37. Furthermore, the anesthetic potency of (S)-ketamine is ~3–4-fold greater and the undesirable psychotomimetic side effects are greater than those of (R)-ketamine38. We reported that (R)-ketamine has more potent and longer-lasting antidepressant-like effects than (S)-ketamine in neonatal dexamethasone-treated, chronic social defeat stress (CSDS), and learned helplessness (LH) models of depression39,40. Subsequent studies have also shown that (R)-ketamine has more potent antidepressant-like effects than (S)-ketamine in rodents41,42. A recent study showed that the order of antidepressant-like effects in a CSDS model following the intranasal administration is (R)-ketamine > (R,S)-ketamine > (S)-ketamine43, and that the order of side effects in rodents is (S)-ketamine > (R,S)-ketamine > (R)-ketamine43. The side effects of (R)-ketamine in rodents were lower than those of (S)-ketamine40,43–45. A positron emission tomography study showed a marked reduction in dopamine D2/3 receptor binding in the conscious monkey striatum following a single intravenous infusion of (S)-ketamine but not that of (R)-ketamine, suggesting that the (S)-ketamine-induced dopamine release may be associated with acute psychotomimetic and dissociative side effects in humans46.
Fig. 1. Chemical structure of enantiomers of ketamine and its metabolites.
(R)-ketamine [or (S)-ketamine] is initially metabolized to (R)-norketamine [or (S)-norketamine] by either CYP2B6 or CYP3A4, and then metabolized to (R)-dehydronorketamine (DHNK) [or (S)-DHNK]. Hydroxylation of (R)-norketamine [or (S)-norketamine] at the sixth position by CYP2A6 results in (2R,6R)-hydroxynorketamine (HNK) [or (2S,6S)-HNK]. (R)-ketamine [or (S)-ketamine] is also metabolized to (2R,6R)-hydroxyketamine (HK) [or (2S,6S)-HK], then to (2R,6R)-HNK [or (2S,6S)-HNK]49. The values in the parenthesis are the Ki value for the NMDAR37,41
In 1995, Mathisen et al.47 reported that the incidence of psychotomimetic side effects of (S)-ketamine in patients with orofacial pain was higher than that of (R)-ketamine, despite the dose of (S)-ketamine (0.45 mg/kg) being lower than that of (R)-ketamine (1.8 mg/kg). In addition, Vollenweider et al.48 reported that (R)-ketamine did not produce psychotic symptoms in healthy subjects and that the majority experienced a state of relaxation, whereas the same dose of (S)-ketamine caused psychotic reactions including depersonalization and hallucinations. These findings suggest that (S)-ketamine contributes to the acute side effects of ketamine, whereas (R)-ketamine may not be associated with these side effects49. Importantly, non-ketamine NMDAR antagonists (i.e., memantine, traxoprodil, lanicemine, rapastinel, and AV-101) did not exhibit robust ketamine-like antidepressant effects in patients with MDD12,22,23. These clinical findings suggest that NMDAR may not be the primary target for the antidepressant effects of ketamine.
Taken together, (R)-ketamine is considered to be a safer antidepressant than (R,S)-ketamine and (S)-ketamine in humans12,50–52. On March 5, 2019, the US Food Drug Administration (FDA) approved (S)-ketamine nasal spray for treatment-resistant depression. However, it is only available through a restricted distribution system, under a Risk Evaluation and Mitigation Strategy due to the risk of serious side adverse outcomes. A clinical trial of (R)-ketamine in humans is currently underway by Perception Neuroscience, Inc.12.
Mechanisms of action of ketamine’s antidepressant action
NMDAR inhibition and subsequent AMPAR activation
In 1990, Skolnick’ group reported antidepressant-like effects of NMDAR antagonists in rodents53,54. Although the precise mechanisms underlying the antidepressant effects of ketamine and its metabolites remain unclear, their rapid antidepressant effects are considered to occur via the blockade of NMDARs located on inhibitory interneurons (Fig. 2). This blockage leads to the disinhibition of pyramidal cells, resulting in a burst of glutamatergic transmission. In 2008, Maeng et al.55 reported that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) antagonists blocked the antidepressant-like effects of ketamine in rodents, suggesting a role of AMPAR activation in the antidepressant-like effects of ketamine. It has been suggested that increased glutamate release activates AMPARs, as AMPAR antagonists inhibit the antidepressant-like effects of ketamine and its two enantiomers40–42,56–58. Collectively, it appears that AMPAR activation serves an important role in the antidepressant-like effects of ketamine and its enantiomers5–10,40,58.
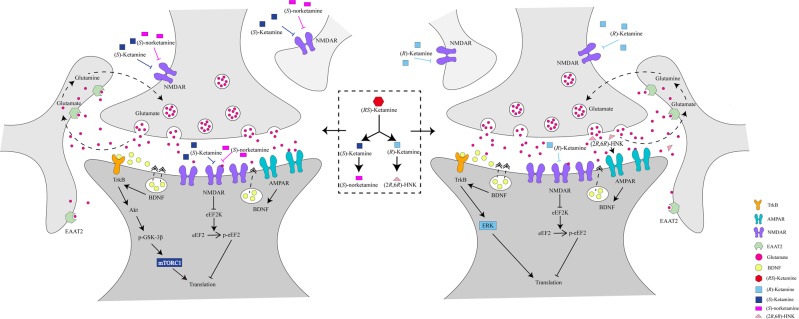
Fig. 2. Proposed cellular mechanisms of antidepressant actions of enantiomers of ketamine, and its metabolites.
Left: (S)-Ketamine is metabolized to (S)-norketamine. (S)-Ketamine activate AMPAR, subsequently, (S)-ketamine activates mTORC1 signaling, resulting in activation of BDNF–TrkB signaling40,72. Although (S)-norketamine does not activate AMPAR, (S)-norketamine activates mTORC1 signaling, resulting in activation of BDNF–TrkB signaling119. Right: (R)-Ketamine is metabolized to (2R,6R)-HNK. Antidepressant-like effects of (R)-ketamine in rodents are more potent than (S)-ketamine, and antidepressant-like effects of (2R,6R)-HNK are inconsistent. (R)-Ketamine activates AMPAR, subsequently, (R)-ketamine might activate MEK–ERK signaling, resulting in activation of BDNF–TrkB signaling40,72. AMPAR activation may be necessary for antidepressant-like actions of (2R,6R)-HNK41. The mTORC1 signaling and BDNF-TrkB signaling may play a role in the antidepressant effects of (R)-ketamine40,72
In contrast, non-ketamine NMDAR antagonists did not produce robust ketamine-like antidepressant effects in depressed patients12,22,23. In addition, (R)-ketamine has more potent antidepressant-like effects in rodents than (S)-ketamine, despite (R)-ketamine being less potent at NMDAR inhibition than (S)-ketamine. A recent functional MRI (fMRI) study in conscious rats demonstrated that, similar to the potent and selective NMDAR antagonist (+)-MK-801 (0.1 mg/kg), (R,S)-ketamine (10 mg/kg), and (S)-ketamine (10 mg/kg) produced a significant positive response in the cortex, nucleus accumbens, and striatum. In contrast, (R)-ketamine (10 mg/kg) produced negative response in the several regions59. This study suggests that (R)-ketamine and (S)-ketamine induce completely different fMRI response patterns in rat brain, and that (S)-ketamine-induced pattern is similar to (+)-MK-801. Collectively, it is likely that at the antidepressant-like dose (10 mg/kg), (R)-ketamine does not produce NMDAR antagonist-like brain activation in the brain.
Therefore, it may be time to reconsider the hypothesis of NMDAR inhibition and the subsequent AMPAR activation in the antidepressant effects of ketamine and its two enantiomers. In addition to NMDA inhibition and AMPAR activation, other important pathways, including mechanistic target of rapamycin (mTOR), the brain-derived neurotrophic factor (BDNF)-tyrosine kinase receptor B (TrkB) pathway, may be involved in the antidepressant-like effects of ketamine, as discussed below.
Monoaminergic systems
A recent study using in vivo microdialysis showed that (R)-ketamine and (S)-ketamine acutely increased serotonin (5-HT: 5-hydroxytryptamine) release in the PFC in a dose-dependent manner, and the effect of (R)-ketamine was greater than that of (S)-ketamine60. In contrast, (S)-ketamine caused a robust increase in dopamine release compared with (R)-ketamine. Differential effects between (R)-ketamine and (S)-ketamine were also observed in a LPS-induced model of depression. An AMPAR antagonist NBQX attenuated (S)-ketamine-induced, but not (R)-ketamine-induced 5-HT release, whereas NBQX blocked DA release induced by both enantiomers. This paper suggests differences between (R)-ketamine and (S)-ketamine in their abilities to induce prefrontal 5-HT and dopamine60. Furthermore, Zhang et al.61 reported that 5-HT depletion did not affect the antidepressant-like effects of (R)-ketamine in a CSDS model, suggesting that 5-HT does not play a major role in the antidepressant-like effects of (R)-ketamine.
A recent study showed that dopamine D1 receptor activation in the medial PFC may play a role in the antidepressant-like effects of ketamine62. However, Chang et al.63 reported that the pretreatment with dopamine D1 receptor antagonist did not block the antidepressant-like effects of (R)-ketamine in a CSDS model, suggesting that dopamine D1 receptors may not play a major role in the antidepressant-like actions of (R)-ketamine, consistent with the previous report64.
Collectively, it is unlikely that monoamines such as 5-HT and dopamine do not play a key role in the antidepressant-like effects of ketamine and its enantiomers although the monoaminergic system may play a role in their other pharmacological effects. A further detailed study is needed.
Mechanistic target of rapamycin complex 1 (mTORC1)
mTOR is an atypical serine/threonine protein kinase consisting of 2549 amino acids belonging to the phosphatidylinositol 3-kinase-related kinase family, which combines with several proteins to form two different complexes, mTORC1 and mTORC265. In addition, the signaling pathway controlled by mTOR can regulate physiological function in the central nervous system, such as neuronal development, synaptic plasticity, memory storage, and cognitive function66.
In 2010, Li et al. demonstrated that rapamycin, an mTOR inhibitor, inhibited the antidepressant-like effects of ketamine in rodents, which acted by increasing the number of synaptic proteins and synaptic spine density by rapidly activating the mTORC1-signaling pathway in the medial prefrontal cortex (PFC)56. In a forced swimming test, ketamine decreased the immobility time and increased the levels of hippocampal mTOR and BDNF, suggesting that the antidepressant-like effects of ketamine may be associated with increased hippocampal levels of mTOR and BDNF67. Furthermore, tramadol, an analgesic agent, enhanced the antidepressant-like effects of ketamine by increasing mTOR levels in the rat hippocampus and mPFC68. In addition, ketamine and its metabolites [i.e., norketamine and (2S,6S)-hydroxynorketamine (HNK)] may produce an antidepressant-like effect by increasing the phosphorylation level of mTOR and its downstream targets69. By contrast, rapamycin can cause neurobehavioral changes, including anxiety-like behavior, in rats and can impede the antidepressant-like effects of ketamine70. In addition, neuropeptide VGF (non-acronymic) knockdown attenuated the rapid antidepressant-like effects of ketamine by reducing mTOR phosphorylation71. In dorsal raphe neurons, ketamine transiently increased the neurotransmission mediated by spontaneous AMPAR via mTOR signaling72. Furthermore, activation of mTOR in the PFC was involved in the antidepressant-like effects of ketamine, whereas inhibition of this pathway may protect the brain from oxidative stress or endoplasmic reticulum stress73,74. The mood stabilizer lithium, a GSK-3 inhibitor, can indirectly activate mTORC1 signaling, thereby enhancing the antidepressant-like effects of ketamine75. In addition, we previously reported that ketamine-induced antidepressant-like effects are associated with the AMPAR-mediated upregulation of mTOR and BDNF in the hippocampus and PFC76. Collectively, it is likely that mTORC1 signaling serves an important role in the mechanism underlying the antidepressant-like effects of ketamine.
Although these aforementioned studies support the role of mTORC1 in the antidepressant-like effect of ketamine, inconsistent results have emerged in subsequent studies. Autry et al.57 showed that the level of phosphorylated mTOR was not altered in the hippocampus of control and Bdnf-knockout mice following acute administration of ketamine, and that the antidepressant-like effects of ketamine in wild-type mice were not affected by rapamycin. In addition, another study showed no significant changes in the levels of phosphorylated mTOR in the hippocampus and prefrontal cortex of mice following administration of ketamine or (2R,6R)-HNK, whereas the levels of phosphorylated eEF2 and BDNF were significantly increased in the hippocampus following administration of ketamine or (2R,6R)-HNK41. This increase may partially explain the mechanisms underlying the sustainable antidepressant-like effects of ketamine41. Of note, we reported that mTORC1 serves a major role in the antidepressant effect of (S)-ketamine, but not (R)-ketamine, in a CSDS model77. The antidepressant effects of (R)-ketamine may be mediated by the activation of ERK as pretreatment with SL327 (an ERK inhibitor) inhibited the antidepressant effects of (R)-ketamine77.
There are few clinical studies reporting the role of mTORC1 in the antidepressant effects of ketamine in depressed patients. Denk et al.78 reported the first evidence of increased phosphorylated mTOR protein in the blood from a patient with MDD following a single injection of (S)-ketamine. Furthermore, we reported that the plasma levels of phosphorylated mTOR, GSK-3β, and eEF2 were significantly increased following a single injection of ketamine79. It is, therefore, of interest to investigate whether (R)-ketamine can influence ERK and its phosphorylation in the blood from patients with MDD or BD.
A recent randomized, placebo-controlled clinical study demonstrated that pretreatment with rapamycin did not alter the acute effects of ketamine in patients with treatment-resistant MDD, whereas its combination with ketamine prolonged the antidepressant effect of ketamine and the response rate 2 weeks following treatment80. At present, there is no evidence that a low dose of rapamycin can achieve sufficient brain levels to inhibit mTOR. It is also suggested that rapamycin may produce beneficial effects through the inflammatory system in the periphery, although further investigation is required. Taken together, the role of mTORC1 in the antidepressant effect of ketamine in patients with MDD remains contradictory. Further investigation using a larger sample size is required to determine the role of mTORC1 in the antidepressant effects of ketamine and its metabolites in patients with MDD.
BDNF
Multiple lines of evidence show that BDNF and its receptor TrkB serve a critical role in the pathogenesis of depression and therapeutic mechanisms of antidepressants81–87. In 2011, Autry et al.57 reported that the rapid-acting antidepressant effects of ketamine depend on the rapid synthesis of BDNF, as ketamine did not elicit antidepressant-like effects in inducible Bdnf-knockout mice, indicating a key role of the BDNF–TrkB cascade in the antidepressant effects of ketamine. Subsequent studies have supported the role of the BDNF–TrkB cascade in the antidepressant effects of ketamine67,76. In addition, the TrkB inhibitor ANA-12 significantly inhibited the rapid and long-lasting antidepressant effects of (R)-ketamine, and (S)-ketamine in a CSDS model40. Furthermore, (R)-ketamine produced more marked beneficial effects on reduced synaptogenesis and the BDNF–TrkB cascade in the PFC and hippocampus (i.e., CA3 and DG) of CSDS-susceptible mice than (S)-ketamine40. It has also been reported that the regulation of glutamate transporter 1 on astrocytes through the activation of TrkB is involved in the beneficial effects of ketamine on behavioral abnormalities and morphological changes in the hippocampus of chronic unpredictable mild stress (CUMS)-exposed rats88. A recent study showed that ketamine restores depression-like phenotypes in CUMS-exposed vulnerable rats by rescuing the dendritic trafficking of Bdnf mRNA89. In addition, the ketamine-induced regulation of TrkB is independent of HNK90. Collectively, it is likely that long-lasting activation of the BDNF–TrkB cascade in the PFC and hippocampus may be implicated in the long-lasting antidepressant effects of ketamine and its enantiomers.
Synaptogenesis
Preclinical studies have shown that ketamine rapidly induces synaptogenesis and reverses the synaptic deficits caused by chronic stress, resulting in its antidepressant-like effects56,80–94. We reported that ketamine and its two enantiomers, improved decreased spine density in the mPFC of CSDS-susceptible mice 7 or 8 days following a single dose40,95, suggesting long-lasting effects on synaptogenesis. A recent study using single-cell two-photon calcium imaging in awake mice showed that the effects of ketamine on spine formation in the PFC were slower: spine formation rates were not significantly altered at 3–6 h following a single injection of ketamine, but were markedly altered at 12–24 h96. This suggests that dendritic spine formation in the PFC was required for the sustained antidepressant effects of ketamine but not for its acute antidepressant effects. By contrast, Zhang et al.97 reported that (R)-ketamine rapidly (<3 h) ameliorated the decreased spine density in the medial PFC and hippocampus of CSDS susceptible mice, resulting in its rapid acting antidepressant-like effects in rodents. In addition, a recent study showed that (S)-ketamine rapidly (<1 h) reversed dendritic spine deficits in CA1 pyramidal neurons of Flinders Sensitive rats with a depression-like phenotype98. Therefore, further investigation of the acute effects of ketamine and its enantiomers in the dendritic spine deficits of rodents with depression-like phenotype is required.
Opioid system
It is well known that ketamine can interact with opioid receptors. The order of affinity for opioid receptor subtypes is mu > kappa > delta. The binding of (S)-ketamine is also known to be ~2–4-fold stronger to mu and kappa receptors than that of (R)-ketamine38,99. In addition, ketamine has been reported to exert antagonistic effects at both mu and kappa opioid receptors, suggesting that ketamine use does not lead to opioid addiction99. Recently, pretreatment with an opiate receptor antagonist naltrexone (50 mg) significantly inhibited the antidepressant and anti-suicidal effects of ketamine, but not its dissociative effects, in patients with treatment-resistant MDD, suggesting that activation of the opioid system is necessary to produce the rapid-acting antidepressant effects of ketamine100,101. By contrast, Yoon et al.102 demonstrated that pretreatment with naltrexone did not affect the antidepressant effects of ketamine in depressed patients with alcohol use disorder. Furthermore, ketamine had antidepressant efficacy in patients concurrently on high-affinity mu opioid receptor agonists (i.e., buprenorphine, methadone, or naltrexone), suggesting that the chronic use of opioid receptor agonists is not a contraindication for ketamine treatment for depression103. Therefore, the role of the opioid system in the antidepressant effects of ketamine is controversial.
Recently, we reported that pretreatment with naltrexone did not inhibit the antidepressant-like effects of ketamine in a CSDS model and inflammation-induced model of depression, suggesting that the opioid system may not serve a role in the antidepressant-like effects of ketamine104. However, further clinical trials with a large sample size are required to better understand whether opioid receptor activation is necessary for the antidepressant and anti-suicidal effects of ketamine in patients with MDD and BD.
(2R,6R)-hydroxynorketamine (HNK)
Ketamine is metabolized to norketamine via N-demethylation by cytochrome P450 (CYP) enzymes in the liver (Fig. 1). Following N-demethylation, norketamine is further metabolized to HNKs and dehydronorketamine (DHNK) (Fig. 1)49. Several metabolites of HNKs, including (2R,6R;2S,6S)-HNK and (2S,6R; 2R,6S)-HNK, were detected in human plasma following ketamine infusion105.
In 2016, Zanos et al. demonstrated that the generation of (2R,6R)-HNK (Ki > 10 μM for NMDAR) (Fig. 1) in the body was essential for the antidepressant-like effects of (R,S)-ketamine in rodents, and that NMDAR may not be involved in the antidepressant-like effects of (2R,6R)-HNK41. Of note, (2R,6R)-HNK did not produce detrimental side effects (i.e., hyperlocomotion, pre-pulse inhibition deficits, motor incoordination, and abuse liability) of ketamine in rodents at a high dose37. Subsequently, several groups have replicated the antidepressant-like effects of (2R,6R)-HNK in rodents106,107. Furthermore, Lumsden et al.108 demonstrated that antidepressant-relevant concentrations of (2R,6R)-HNK did not inhibit NMDAR function, whereas a high concentration (50 μM) of (2R,6R)-HNK inhibited NMDAR synaptic function109. It is also suggested that the metabotropic glutamate mGlu2 receptors are involved in the antidepressant-like effects of (2R,6R)-HNK as the antidepressant-like effects of (2R,6R)-HNK were absent in mice lacking the Grm2 gene, but not the Grm3 gene110. It is currently unknown whether mGlu2 receptors play a role in the antidepressant-like effects of (R)-ketamine in rodents.
By contrast, our group found that (2R,6R)-HNK did not exhibit antidepressant-like effects in rodent models of depression, however, its parent compound (R)-ketamine exhibited robust antidepressant-like effects in the same models111–116. Pretreatment with two CYP inhibitors (ticlopidine hydrochloride and 1-aminobenzotriazole) prior to (R)-ketamine (3 mg/kg) injection increased the levels of (R)-ketamine in the blood, whereas (2R,6R)-HNK was not detected in the blood. In the presence of these CYP inhibitors, (R)-ketamine (3 mg/kg) exhibited antidepressant-like effects, although the same dose did not exhibit antidepressant-like effects in the absence of CYP inhibitors117. In addition, we reported that the direct infusion of (R)-ketamine in brain regions produced antidepressant-like effects in a rat LH model, suggesting that (R)-ketamine itself, but not its metabolite, produced antidepressant-like effects118. These data suggest that the metabolism of (2R,6R)-HNK from (R)-ketamine is not essential for the antidepressant-like effects of (R)-ketamine119,120. The US FDA approved (S)-ketamine, however, (2R,6R)-HNK is not prepared from (S)-ketamine, indicating that (2R,6R)-HNK is not essential for the antidepressant effects of ketamine12. A recent study from Zanos et al.121 showed that (R)-ketamine may exert antidepressant-like effects partly via conversion to (2R,6R)-HNK. The conclusion was toned down 3 years after the first publication of (2R,6R)-HNK41.
It has also been demonstrated that (2R,6R)-HNK exerts antidepressant effects through AMPAR activation as AMPAR antagonist inhibited the antidepressant effects of (2R,6R)-HNK41. By contrast, at a clinically relevant unbound brain concentration (0.01–10 μM), (2R,6R)-HNK did not bind orthosterically or directly to functionally activated AMPARs122. Furthermore, (2R,6R)-HNK failed to evoke AMPAR-centric changes in any electrophysiological endpoint from adult rodent hippocampal sections122. Unfortunately, the AMPAR potentiator Org 26576 did not have antidepressant effects in depressed patients123. At present, a clinical trial of TAK-653, an AMPAR potentiator with minimal agonistic effects, is underway in patients with treatment-resistant depression (NCT03312894). Further investigation on the role of AMPAR in the action of enantiomers of ketamine and its metabolites (norketamine and HNK) is required.
A recent study demonstrated that a single injection of (2R,6R)-HNK (1–10 mg/kg), but not (2S,6S)-HNK, increased aggressive behaviors through AMPAR-dependent mechanisms in the ventrolateral periaqueductal gray matter124. A clinical trial of (2R,6R)-HNK in humans is currently underway at the National Institute of Mental Health, USA12. The aggressive effects of (2R,6R)-HNK in humans warrant investigation. In addition, it is of interest to compare the antidepressant effects of (R)-ketamine and its final metabolite (2R,6R)-HNK in patients with MDD.
(S)-Norketamine
(S)-Ketamine is metabolized to (S)-norketamine [Ki = 1.70 μM for NMDAR] by CYP enzymes (Fig. 1). We reported that (S)-norketamine, but not (R)-norketamine, exhibits rapid and sustained antidepressant-like effects in CSDS and inflammation models of depression. The potency of the antidepressant-like effects of (S)-norketamine is similar to that of its parent compound (S)-ketamine, although the antidepressant-like effects of (S)-norketamine are less potent than those of (R)-ketamine125. Unlike (R,S)-ketamine and its enantiomers, AMPAR antagonists do not inhibit the antidepressant effects of (S)-norketamine, suggesting that AMPAR activation appears to be unnecessary for the antidepressant-like effects of (S)-norketamine125. Therefore, it is unlikely that a rapid increase in glutamate due to the direct inhibition of NMDARs localized to interneurons is involved in the antidepressant-like effects of (S)-norketamine (Fig. 2)125. Furthermore, we reported that, similar to (S)-ketamine, BDNF-TrkB and mTOR signaling might play a role in the antidepressant-like effects of (S)-norketamine in rodents125. Interestingly, the side effects of (S)-norketamine in rodents are significantly lower than those of (S)-ketamine; ketamine-induced side effects may be associated with NMDAR inhibition. Taken together, (S)-norketamine appears to be a safer alternative antidepressant without the side effects of (S)-ketamine in humans12,125,126. Of note, unlike (S)-ketamine, (S)-norketamine is not a schedule compound.
Conclusions
The discovery of the antidepressant effects of ketamine in depressed patients was serendipitous24. The mechanisms underlying the antidepressant effects of ketamine have been investigated for almost 20 years, however, its precise molecular and cellular mechanisms remain to be fully elucidated. Although NMDAR inhibition is considered to serve a key role in the antidepressant effects of ketamine, clinical data of non-ketamine NMDAR antagonists (i.e., memantine, traxoprodil, lanicemine, rapastinel, and AV-101)12 and preclinical data using two ketamine enantiomers suggest that mechanisms other than NMDAR inhibition may be involved in the antidepressant effects of ketamine. For example, a randomized, placebo-controlled study using a large sample demonstrated that lanicemine did not exert antidepressant effects in patients with MDD with a history of inadequate treatment response127, supporting a lack of antidepressant-like effects of lanicemine in a CSDS model128. On March 6, 2019, Allergan announced phase three results of rapastinel as an adjunctive treatment of MDD. In three acute trials, rapastinel treatment did not produce primary and key secondary endpoints compared with the placebo group. By contrast, rapastinel exerted rapid-acting antidepressant-like effects in a CSDS model although, unlike (R)-ketamine, rapastinel did not exhibit long-lasting antidepressant effects129. Collectively, non-ketamine NMDAR antagonists did not produce robust ketamine-like antidepressant effects in patients with MDD, although certain NMDAR antagonists may exhibit rapid ketamine-like antidepressant-like effects in rodents. There is no guarantee that preclinical data will translate to humans130. At present, the general consensus is that NMDAR inhibition and the subsequent AMPAR activation serve a role in the antidepressant-like effects of ketamine and two enantiomers. However, the precise molecular and cellular mechanisms underlying ketamine’s antidepressant actions are complex5–10,94,131. Considering the clinical data and new preclinical data using ketamine enantiomers, it is the time to reconsider the current hypothesis for the antidepressant effects of ketamine. Recently, Heifets and Malenka130 suggested a need to conceptualize molecular mechanisms with more nuance than action at a single, broadly distributed glutamate receptor.
A number of researchers have used control stress-naive rodents to investigate the antidepressant-like effects of ketamine and its metabolites. Healthy control subjects showed significant increases in depressive symptoms for up to 1 day following a single ketamine infusion132, suggesting that ketamine does not produce antidepressant effects in healthy control subjects. It is also well known that ketamine can produce schizophrenia-like symptoms (i.e., positive symptoms, negative symptoms, cognitive impairment) in healthy control subjects32,38,133. Therefore, the use of control naive rodents may contribute to discrepancies in the antidepressant-like effects of ketamine and its metabolite HNK12,134. Collectively, rodents with depression-like phenotypes should be used to investigate the antidepressant effects of ketamine and its metabolites, although it is clear that animal models of depression cannot fully represent the complexity of human depression134,135.
On March 5, 2019, the US FDA approved (S)-ketamine nasal spray (Spravato™) for treatment-resistant depression. The clinical study of (R)-ketamine and (2R,6R)-HNK in humans is currently underway12. Therefore, it is of interest to compare the antidepressant effects of (R)-ketamine and (S)-ketamine [or (2R,6R)-HNK] in patients with MDD or BD. Finally, the identification of novel molecular and cellular targets responsible for the rapid and sustained antidepressant effects of enantiomers of ketamine and its metabolites is useful for the development of novel antidepressants without the detrimental side effects of ketamine.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (to C.Y., 81703482 and 81974171; to J.Y., 81771156; to A.L., 81771159 and 81571047), the AMED, Japan (to K.H., JP19dm0107119). C.Y. received the research support from B. Braun Medical Inc. K.H. is the inventor of filed patent applications on “The use of R-ketamine in the treatment of psychiatric diseases” and “(S)-norketamine and salt thereof as pharmaceutical” by the Chiba University. K.H. has received research support and consultant from Dainippon Sumitomo, Otsuka, and Taisho. J.Y. and A.L. report no biochemical financial interests or potential conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Trivedi MH, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 2.Bartoli F, et al. Ketamine as a rapid-acting agent for suicidal ideation: a meta-analysis. Neurosci. Biobehav. Rev. 2017;77:232–236. doi: 10.1016/j.neubiorev.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson ST, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am. J. Psychiatry. 2018;175:150–158. doi: 10.1176/appi.ajp.2017.17040472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res. Rev. 2009;61:105–123. doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat. Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nat. Rev. Drug Discov. 2017;16:472–486. doi: 10.1038/nrd.2017.16. [DOI] [PubMed] [Google Scholar]
- 7.Abdallah CG, Sanacora G, Duman RS, Krystal JH. The neurobiology of depression, ketamine and rapid-acting antidepressants: Is it glutamate inhibition or activation? Pharm. Ther. 2018;190:148–158. doi: 10.1016/j.pharmthera.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duman RS. Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Res. 2018;7:659. doi: 10.12688/f1000research.14344.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkin JM, Knutson DE, Rodriguez GJ, Shi S. Rapid-acting antidepressants. Curr. Pharm. Des. 2018;24:2556–2563. doi: 10.2174/1381612824666180730104707. [DOI] [PubMed] [Google Scholar]
- 10.Gould TD, Zarate CA, Jr, Thompson SM. Molecular pharmacology and neurobiology of rapid-acting antidepressants. Annu. Rev. Pharm. Toxicol. 2019;59:213–236. doi: 10.1146/annurev-pharmtox-010617-052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang K, Hashimoto K. An update on ketamine and its two enantiomers as rapid-acting antidepressants. Expert Rev. Neurother. 2019;19:83–92. doi: 10.1080/14737175.2019.1554434. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto Kenji. Rapid‐acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective. Psychiatry and Clinical Neurosciences. 2019;73(10):613–627. doi: 10.1111/pcn.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berman RM, et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 14.Zarate CA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 15.Diazgranados N, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarate CA, Jr., et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraus C, et al. Administration of ketamine for unipolar and bipolar depression. Int. J. Psychiatry Clin. Pract. 2017;21:2–12. doi: 10.1080/13651501.2016.1254802. [DOI] [PubMed] [Google Scholar]
- 18.Zheng W, et al. Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. Psychiatry Res. 2018;106:61–68. doi: 10.1016/j.jpsychires.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murrough JW, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol. Med. 2015;45:3571–3580. doi: 10.1017/S0033291715001506. [DOI] [PubMed] [Google Scholar]
- 21.Grunebaum MF, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am. J. Psychiatry. 2018;175:327–335. doi: 10.1176/appi.ajp.2017.17060647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newport DJ, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am. J. Psychiatry. 2015;172:950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto T, et al. Single-dose infusion ketamine and non-ketamine N-methyl-D-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol. Med. 2016;46:1459–1472. doi: 10.1017/S0033291716000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krystal JH, et al. Ketamine: a paradigm shift for depression research and treatment. Neuron. 2019;101:774–778. doi: 10.1016/j.neuron.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C, Hashimoto K. Rapid antidepressant effects and abuse liability of ketamine. Psychopharmacology. 2014;231:2041–2042. doi: 10.1007/s00213-014-3543-0. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y, Lin D, Wu B, Zhou W. Ketamine abuse potential and use disorder. Neurosci. Bull. 2016;126:68–73. doi: 10.1016/j.brainresbull.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W, et al. Risks associated with misuse of ketamine as a rapid-acting antidepressant. Neurosci. Bull. 2016;32:557–564. doi: 10.1007/s12264-016-0081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh I, et al. Ketamine treatment for depression: opportunities for clinical innovation and ethical foresight. Lancet Psychiatry. 2017;4:419–426. doi: 10.1016/S2215-0366(17)30102-5. [DOI] [PubMed] [Google Scholar]
- 29.Sanacora G, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry. 2017;74:399–405. doi: 10.1001/jamapsychiatry.2017.0080. [DOI] [PubMed] [Google Scholar]
- 30.Wilkinson ST, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am. J. Psychiatry. 2017;174:695–696. doi: 10.1176/appi.ajp.2017.17020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Short B, et al. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78. doi: 10.1016/S2215-0366(17)30272-9. [DOI] [PubMed] [Google Scholar]
- 32.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 33.Fava, M. et al. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol. Psychiatry10.1038/s41380-018-0256-5 (2018). [DOI] [PMC free article] [PubMed]
- 34.Luckenbaugh DA, et al. Do the dissociative side effects of ketamine mediate its antidepressant effects? J. Affect. Disord. 2014;159:56–61. doi: 10.1016/j.jad.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deakin JF, et al. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch. Gen. Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- 36.Wong JJ, et al. Ketamine modulates subgenual cingulate connectivity with the memory-related neural circuit—a mechanism of relevance to resistant depression? PeerJ. 2016;4:e1710. doi: 10.7717/peerj.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur. J. Pharmacol. 1997;333:99–104. doi: 10.1016/S0014-2999(97)01116-3. [DOI] [PubMed] [Google Scholar]
- 38.Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113:678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JC, Li SX, Hashimoto K. R(-)-ketamine shows greater potency and longer lasting antidepressant effects than S(+)-ketamine. Pharm. Biochem. Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 40.Yang C, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl. Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zanos P, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukumoto K, et al. Antidepressant potential of (R)-Ketamine in rodent models: comparison with (S)-ketamine. J. Pharm. Exp. Ther. 2017;361:9–16. doi: 10.1124/jpet.116.239228. [DOI] [PubMed] [Google Scholar]
- 43.Chang L, et al. Comparison of antidepressant and side effects in mice after intranasal administration of (R,S)-ketamine, (R)-ketamine, and (S)-ketamine. Pharm. Biochem. Behav. 2019;181:53–59. doi: 10.1016/j.pbb.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Yang C, et al. Loss of parvalbumin-immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R-ketamine. Psychiatry Res. 2016;239:281–283. doi: 10.1016/j.psychres.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 45.Tian Z, et al. Expression of heat shock protein HSP-70 in the retrosplenial cortex of rat brain after administration of (R,S)-ketamine and (S)-ketamine, but not (R)-ketamine. Pharm. Biochem. Behav. 2018;172:17–21. doi: 10.1016/j.pbb.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto K, et al. Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R-ketamine: a PET study in conscious monkeys. Eur. Arch. Psychiatry Clin. Neurosci. 2017;267:173–176. doi: 10.1007/s00406-016-0692-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathisen LC, Skjelbred P, Skoglund LA, Oye I. Effect of ketamine, an NMDA receptor inhibitor, in acute and chronic orofacial pain. Pain. 1992;61:215–220. doi: 10.1016/0304-3959(94)00170-J. [DOI] [PubMed] [Google Scholar]
- 48.Vollenweider FX, et al. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur. Neuropsychopharmacol. 1997;7:25–38. doi: 10.1016/S0924-977X(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 49.Zanos P, et al. Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharm. Rev. 2018;70:621–630.. doi: 10.1124/pr.117.015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without risk of brain toxicity. Psychol. Med. 2016;46:2449–2451. doi: 10.1017/S0033291716000969. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto K. Ketamine’s antidepressant action: beyond NMDA receptor inhibition. Expert Opin. Ther. Targets. 2016;20:1389–1392. doi: 10.1080/14728222.2016.1238899. [DOI] [PubMed] [Google Scholar]
- 52.Hashimoto K. Detrimental side effects of repeated ketamine infusions in the brain. Am. J. Psychiatry. 2016;173:1044–1045. doi: 10.1176/appi.ajp.2016.16040411. [DOI] [PubMed] [Google Scholar]
- 53.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur. J. Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-J. [DOI] [PubMed] [Google Scholar]
- 54.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharm. Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Maeng S, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 56.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monteggia LM, Zarate CA., Jr. Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr. Opin. Neurobiol. 2015;30:139–143. doi: 10.1016/j.conb.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Masaki, Y., Kashiwagi, Y., Watabe, H. & Abe, K. (R)- and (S)-ketamine induce differential fMRI responses in conscious rats. Synapse73, e22126 (2019). [DOI] [PubMed]
- 60.Ago Yukio, Tanabe Wataru, Higuchi Momoko, Tsukada Shinji, Tanaka Tatsunori, Yamaguchi Takumi, Igarashi Hisato, Yokoyama Rei, Seiriki Kaoru, Kasai Atsushi, Nakazawa Takanobu, Nakagawa Shinsaku, Hashimoto Kenji, Hashimoto Hitoshi. (R)-Ketamine Induces a Greater Increase in Prefrontal 5-HT Release Than (S)-Ketamine and Ketamine Metabolites via an AMPA Receptor-Independent Mechanism. International Journal of Neuropsychopharmacology. 2019;22(10):665–674. doi: 10.1093/ijnp/pyz041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang K, et al. 5-Hydroxytryptamine-independent antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Int. J. Neuropsychopharmacol. 2018;21:157–163. doi: 10.1093/ijnp/pyx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hare BD, et al. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat. Commun. 2019;10:223. doi: 10.1038/s41467-018-08168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang L, et al. Lack of dopamine D1 receptors in the antidepressant actions of (R)-ketamine in a chronic social defeat stress model. Eur. Arch. Psychiatry Clin. Neurosci. 2019 doi: 10.1007/s00406-019-01012-1. [DOI] [PubMed] [Google Scholar]
- 64.Li Y, et al. Dopamine D2/D3 but not dopamine D1 receptors are involved in the rapid antidepressant-like effects of ketamine in the forced swim test. Behav. Brain Res. 2015;279:100–105. doi: 10.1016/j.bbr.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 65.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Switon K, et al. Molecular neurobiology of mTOR. Neuroscience. 2017;341:112–153. doi: 10.1016/j.neuroscience.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 67.Yang C, et al. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups. J. Med. Sci. 2013;118:3–8. doi: 10.3109/03009734.2012.724118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang C, et al. Tramadol pretreatment enhances ketamine-induced antidepressant effects and increases mammalian target of rapamycin in rat hippocampus and prefrontal cortex. J. Biomed. Biotechnol. 2012;2012:175619. doi: 10.1155/2012/175619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paul RK, et al. (R,S)-Ketamine metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine increase the mammalian target of rapamycin function. Anesthesiology. 2014;121:149–159. doi: 10.1097/ALN.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hadamitzky M, et al. Acute systemic rapamycin induces neurobehavioral alterations in rats. Behav. Brain Res. 2014;273:16–22. doi: 10.1016/j.bbr.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 71.Shen M, et al. Essential roles of neuropeptide VGF regulated TrkB/mTOR/BICC1 signaling and phosphorylation of AMPA receptor subunit GluA1 in the rapid antidepressant-like actions of ketamine in mice. Brain Res. Bull. 2018;143:58–65. doi: 10.1016/j.brainresbull.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 72.Llamosas N, et al. Ketamine promotes rapid and transient activation of AMPA receptor-mediated synaptic transmission in the dorsal raphe nucleus. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;88:243–252. doi: 10.1016/j.pnpbp.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 73.Abelaira HM, et al. Ketamine exhibits different neuroanatomical profile after mammalian target of rapamycin inhibition in the prefrontal cortex: the role of inflammation and oxidative stress. Mol. Neurobiol. 2017;54:5335–5346. doi: 10.1007/s12035-016-0071-4. [DOI] [PubMed] [Google Scholar]
- 74.Abelaira HM, et al. Effects of ketamine administration on mTOR and reticulum stress signaling pathways in the brain after the infusion of rapamycin into prefrontal cortex. J. Psychiatr. Res. 2017;87:81–87. doi: 10.1016/j.jpsychires.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Chiu CT, et al. The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int. J. Neuropsychopharmacol. 2014;18:pyu102. doi: 10.1093/ijnp/pyu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou W, et al. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur. Psychiatry. 2014;29:419–423. doi: 10.1016/j.eurpsy.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 77.Yang C, et al. Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol. Psychiatry. 2018;83:18–28. doi: 10.1016/j.biopsych.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 78.Denk MC, et al. Monitoring ketamine treatment response in a depressed patient via peripheral mammalian target of rapamycin activation. Am. J. Psychiatry. 2011;168:751–752. doi: 10.1176/appi.ajp.2011.11010128. [DOI] [PubMed] [Google Scholar]
- 79.Yang C, et al. Acute increases in plasma mammalian target of rapamycin, glycogen synthase kinase-3beta, and eukaryotic elongation factor 2 phosphorylation after ketamine treatment in three depressed patients. Biol. Psychiatry. 2013;73:e35–e36. doi: 10.1016/j.biopsych.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 80.Abdallah, C. G., et al. Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment. Preprint at 10.1101/500959 (2018).
- 81.Nestler EJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 82.Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Brain Res. Rev. 2004;45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 83.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 84.Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin. Neurosci. 2010;64:341–357. doi: 10.1111/j.1440-1819.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- 85.Björkholm C, Monteggia LM. BDNF—a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang JC, Yao W, Hashimoto K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr. Neuropharmacol. 2016;14:721–731. doi: 10.2174/1570159X14666160119094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhan G, et al. PGC-1α-FNDC5-BDNF signaling pathway in skeletal muscle confers resilience to stress in mice subjected to chronic social defeat. Psychopharmacology. 2018;235:3351–3358. doi: 10.1007/s00213-018-5041-2. [DOI] [PubMed] [Google Scholar]
- 88.Liu WX, et al. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology. 2016;233:405–415. doi: 10.1007/s00213-015-4128-2. [DOI] [PubMed] [Google Scholar]
- 89.Tornese P, et al. Chronic mild stress induces anhedonic behavior and changes in glutamate release, BDNF trafficking and dendrite morphology only in stress vulnerable rats. The rapid restorative action of ketamine. Neurobiol. Stress. 2019;10:100160. doi: 10.1016/j.ynstr.2019.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kohtala S, et al. Ketamine-induced regulation of TrkB-GSK3β signaling is accompanied by slow EEG oscillations and sedation but is independent of hydroxynorketamine metabolites. Neuropharmacology. 2019;157:107684. doi: 10.1016/j.neuropharm.2019.107684. [DOI] [PubMed] [Google Scholar]
- 91.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duman CH, Duman RS. Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci. Lett. 2015;601:20–29. doi: 10.1016/j.neulet.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ohgi Y, Futamura T, Hashimoto K. Glutamate signaling in synaptogenesis and NMDA receptors as potential therapeutic targets for psychiatric disorders. Curr. Mol. Med. 2015;15:206–221. doi: 10.2174/1566524015666150330143008. [DOI] [PubMed] [Google Scholar]
- 94.Duman, R. S., Shinohara, R., Fogaça, M. V. & Hare, B. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol. Psychiatry10.1038/s41380-019-0400-x (2019). [DOI] [PMC free article] [PubMed]
- 95.Dong C, et al. Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: comparison with ketamine. Int. J. Neuropsychopharmacol. 2017;20:228–236. doi: 10.1093/ijnp/pyw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moda-Sava, R. N. et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science364, pii: eaat8078 (2019). [DOI] [PMC free article] [PubMed]
- 97.Zhang Jiancheng, Qu Youge, Chang Lijia, Pu Yaoyu, Hashimoto Kenji. (R)-Ketamine Rapidly Ameliorates the Decreased Spine Density in the Medial Prefrontal Cortex and Hippocampus of Susceptible Mice After Chronic Social Defeat Stress. International Journal of Neuropsychopharmacology. 2019;22(10):675–679. doi: 10.1093/ijnp/pyz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Treccani Giulia, Ardalan Maryam, Chen Fenghua, Musazzi Laura, Popoli Maurizio, Wegener Gregers, Nyengaard Jens Randel, Müller Heidi Kaastrup. S-Ketamine Reverses Hippocampal Dendritic Spine Deficits in Flinders Sensitive Line Rats Within 1 h of Administration. Molecular Neurobiology. 2019;56(11):7368–7379. doi: 10.1007/s12035-019-1613-3. [DOI] [PubMed] [Google Scholar]
- 99.Hirota K, Lambert DG. Ketamine: its mechanism(s) of action and unusual clinical uses. Br. J. Anaesth. 1996;77:441–444. doi: 10.1093/bja/77.4.441. [DOI] [PubMed] [Google Scholar]
- 100.Williams NR, et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonisms. Am. J. Psychiatry. 2018;175:1205–1215. doi: 10.1176/appi.ajp.2018.18020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williams, N. R. et al. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonisms. Mol. Psychiatry10.1038/s41380-019-0503-4 (2019). [DOI] [PubMed]
- 102.Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry. 2019;176:337–338. doi: 10.1001/jamapsychiatry.2018.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marton T, Barnes DE, Wallace A, Woolley JD. Concurrent use of buprenorphine, methadone, or naltrexone does not inhibit ketamine’s antidepressant activity. Biol. Psychiatry. 2019;85:e76–e76. doi: 10.1016/j.biopsych.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 104.Zhang K, Hashimoto K. Lack of opioid system in the antidepressant actions of ketamine. Biol. Psychiatry. 2019;85:e25–e27. doi: 10.1016/j.biopsych.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 105.Zarate CA, Jr, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol. Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pham TH, et al. Common neurotransmission recruited in (R,S)-ketamine and (2R,6R)-hydroxynorketamine-induced sustained antidepressant-like effects. Biol. Psychiatry. 2018;84:e3–e6. doi: 10.1016/j.biopsych.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 107.Fukumoto K, et al. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc. Natl Acad. Sci. USA. 2019;116:97–302. doi: 10.1073/pnas.1814709116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lumsden EW, et al. Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function. Proc. Natl Acad. Sci. USA. 2019;116:5160–5169. doi: 10.1073/pnas.1816071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suzuki K, et al. Effects of a ketamine metabolite on synaptic NMDAR function. Nature. 2017;546:E1–E3. doi: 10.1038/nature22084. [DOI] [PubMed] [Google Scholar]
- 110.Zanos Panos, Highland Jaclyn N., Stewart Brent W., Georgiou Polymnia, Jenne Carleigh E., Lovett Jacqueline, Morris Patrick J., Thomas Craig J., Moaddel Ruin, Zarate Carlos A., Gould Todd D. (2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions. Proceedings of the National Academy of Sciences. 2019;116(13):6441–6450. doi: 10.1073/pnas.1819540116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang, C. et al. (R)-ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)-hydroxynorketamine. Biol. Psychiatry82, e43–e44 (2017). [DOI] [PubMed]
- 112.Shirayama Y, Hashimoto K. Lack of antidepressant effects of (2R,6R)-hydroxynorketamine in a rat learned helplessness model: comparison with (R)-ketamine. Int. J. Neuropsychopharmacol. 2018;21:84–88. doi: 10.1093/ijnp/pyx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang K, Fujita Y, Hashimoto K. Lack of metabolism in (R)-ketamine’s antidepressant actions in a chronic social defeat stress model. Sci. Rep. 2018;8:4007. doi: 10.1038/s41598-018-22449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang K, et al. Lack of deuterium isotope effects in the antidepressant effects of (R)-ketamine in a chronic social defeat stress model. Psychopharmacology. 2018;235:3177–3185. doi: 10.1007/s00213-018-5017-2. [DOI] [PubMed] [Google Scholar]
- 115.Chang L, et al. No sex-specific differences in the acute antidepressant actions of (R)-ketamine in an inflammation model. Int. J. Neuropsychopharmacol. 2018;21:932–937. doi: 10.1093/ijnp/pyy053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiong Zhongwei, Fujita Yuko, Zhang Kai, Pu Yaoyu, Chang Lijia, Ma Min, Chen Jincao, Hashimoto Kenji. Beneficial effects of (R)-ketamine, but not its metabolite (2R,6R)-hydroxynorketamine, in the depression-like phenotype, inflammatory bone markers, and bone mineral density in a chronic social defeat stress model. Behavioural Brain Research. 2019;368:111904. doi: 10.1016/j.bbr.2019.111904. [DOI] [PubMed] [Google Scholar]
- 117.Yamaguchi Jun-ichi, Toki Hidetoh, Qu Youge, Yang Chun, Koike Hiroyuki, Hashimoto Kenji, Mizuno-Yasuhira Akiko, Chaki Shigeyuki. (2R,6R)-Hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacology. 2018;43(9):1900–1907. doi: 10.1038/s41386-018-0084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shirayama Y, Hashimoto K. Effects of a single bilateral infusion of R-ketamine in the rat brain regions of a learned helplessness model of depression. Eur. Arch. Psychiatry Clin. Neurosci. 2017;267:177–182. doi: 10.1007/s00406-016-0718-1. [DOI] [PubMed] [Google Scholar]
- 119.Chaki S. Is metabolism of (R)-ketamine essential for the antidepressant effects? Int. J. Neuropharmacol. 2018;21:154–156. doi: 10.1093/ijnp/pyx120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chaki S, Yamaguchi JI. Now is the time for (2R,6R)-hydroxynorketamine to be viewed independently from its parent drug. Pharm. Biochem. Behav. 2018;175:24–26. doi: 10.1016/j.pbb.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 121.Zanos, P. et al. (R)-ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Bri. J.Pharmacol.176, 2573–2592 (2019). [DOI] [PMC free article] [PubMed]
- 122.Shaffer CL, et al. Pharmacological evaluation of clinically relevant concentrations of (2R,6R)-hydroxynorketamine. Neuropharmacology. 2019;153:73–81. doi: 10.1016/j.neuropharm.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 123.Nations KR, et al. Examination of Org 26576, an AMPA receptor positive allosteric modulator, in patients diagnosed with major depressive disorder: an exploratory, randomized, double-blind, placebo-controlled trial. J. Psychopharmacol. 2012;26:1525–1539. doi: 10.1177/0269881112458728. [DOI] [PubMed] [Google Scholar]
- 124.Ye L, et al. Ketamine metabolite (2R,6R)-hydroxynorketamine enhances aggression via periaqueductal gray glutamatergic transmission. Neuropharmacology. 2019;157:107667. doi: 10.1016/j.neuropharm.2019.107667. [DOI] [PubMed] [Google Scholar]
- 125.Yang C, et al. AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-norketamine. Biol. Psychiatry. 2018;84:591–600. doi: 10.1016/j.biopsych.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 126.Hashimoto, K. & Yang, C. Is (S)-norketamine an alternative antidepressant for esketamine? Eur. Arch. Psychiatry Clin. Neurosci.269, 867–868 (2019). [DOI] [PMC free article] [PubMed]
- 127.Sanacora G, et al. Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: a randomized, placebo-controlled study. Neuropsychopharmacology. 2017;42:844–853. doi: 10.1038/npp.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qu, Y. et al. Comparison of (R)-ketamine and lanicemine on depression-like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci. Rep.7, 15725 (2017). [DOI] [PMC free article] [PubMed]
- 129.Yang B, et al. Comparison of R-ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology. 2016;233:3647–3657. doi: 10.1007/s00213-016-4399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heifets Boris D., Malenka Robert C. Disruptive Psychopharmacology. JAMA Psychiatry. 2019;76(8):775. doi: 10.1001/jamapsychiatry.2019.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kadriu B, et al. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int. J. Neuropsychopharmacol. 2019;22:119–135. doi: 10.1093/ijnp/pyy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nugent AC, et al. Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol. Psychiatry. 2019;24:1040–1052. doi: 10.1038/s41380-018-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 134.Hashimoto K, Shirayama Y. What are the causes for discrepancies of antidepressant actions of (2R,6R)-hydroxynorketamine? Biol. Psychiatry. 2018;84:e7–e8. doi: 10.1016/j.biopsych.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 135.Bale TL, et al. The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology. 2019;44:1349–1353. doi: 10.1038/s41386-019-0405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]