Abstract.
Antimicrobial resistance (AMR) is a growing threat to global health. Although AMR endangers continued effectiveness of antibiotics, the impact of AMR has been poorly estimated in low-income countries. This study sought to quantify the effect of AMR on treatments for pediatric pneumococcal disease in Ethiopia. We developed the DREAMR (Dynamic Representation of the Economics of AMR) model that simulate children younger than 5 years who acquire pneumococcal disease (pneumonia, meningitis, and acute otitis media) and seek treatment from various health facilities in Ethiopia over a year. We examined the AMR levels of three antibiotics (penicillin, amoxicillin, and ceftriaxone), treatment failures, and attributable deaths. We used the cost-of-illness method to assess the resulting economic impact of AMR from a societal perspective by estimating the direct and indirect treatment costs and productivity losses. Findings showed that AMR against antibiotics that were used to treat pneumococcal disease led to 195,763 treatment failures per year, which contributed to 2,925 child deaths annually in Ethiopia. Antimicrobial resistance resulted in a first-line treatment failure rate of 29.4%. In 1 year, the proportion of nonsusceptible Streptococcus pneumoniae bacteria increased by 2.1% and 0.5% for amoxicillin and penicillin, and reduced by 0.3% for less commonly used ceftriaxone. Annual costs of AMR to treat pneumococcal disease were around US$15.8 million, including US$3.3 million for ineffective first-line treatments, US$3.7 million for second-line treatments, and US$8.9 million for long-term productivity losses. Antibiotic stewardship to reduce misuse and overuse of antibiotics is essential to maintain the effectiveness of antibiotics, and lessen the health and economic burden of AMR.
INTRODUCTION
Antimicrobial resistance (AMR) poses a global public health threat by diminishing the effectiveness of existing antimicrobials, leaving individuals prone to prolonged hospitalization and mortality from simple bacterial infections.1,2 Antimicrobial resistance is the mechanism by which microbes such as bacteria deactivate the efficacy of antimicrobials from destroying them and stopping their growth.3 Bacteria become resistant through various mechanisms, including producing destructive enzymes that neutralize antimicrobials, modifying antimicrobial targets by mutation so that drugs cannot recognize them, or removing antimicrobial agents by pumping them out of cells.4 Resistant bacteria can also prevent antimicrobials from entering bacterial cells by modifying the outer cell membrane or creating bypasses that allow bacteria to function without the enzymes targeted by antimicrobials.4 Resistance to all first-line and last-resort antimicrobials is increasing globally, and only a few antimicrobials have been developed in recent decades, resulting in an overall decline in the total portfolio of antimicrobial effectiveness.5–7
The global costs of AMR are estimated to increase to US$100 trillion annually by 2050.8 Antimicrobial resistance results in longer treatment duration, greater side effects from second- or third-line treatment, higher mortality and morbidity, more treatment costs, as well as income losses.9 In the United States, antimicrobial-resistant bacterial pathogens are responsible for more than two million infections and 23,000 deaths each year at a direct cost of US$20 billion, and additional productivity losses of US$35 billion.10 In Europe, in 2007, 25,000 deaths were attributable to resistant infections, costing €1.5 billion annually in direct and indirect costs, according to an estimate from the European Medicines Agency and European Center for Disease Prevention and Control.11 Although efforts have been made to estimate the impact of AMR, most studies have focused on high-income countries.12 Similar estimates of the impact of AMR are not currently available in low- and middle-income countries, although these countries are experiencing the greatest increase in antimicrobial use.5
The World Health Organization (WHO) lists Streptococcus pneumoniae as a community-acquired infection of high global concern for resistance.13 Streptococcus pneumoniae is the pathogen that causes pneumococcal infections resulting in several diseases, such as acute otitis media, pneumonia, and meningitis. Streptococcus pneumoniae is known to cause at least 18% of severe pneumonia episodes and 33% of pneumonia deaths worldwide.14 Understanding the influence of AMR on pneumococcal infections is particularly important in countries with high rates of pneumonia and child deaths. Ethiopia is among the top five countries globally with the highest number of child deaths. Estimates suggest that between 33,000 and 37,000 Ethiopian children younger than age 5 die annually from pneumonia.14 We examined the impact of AMR on treatments for pediatric pneumococcal infections in Ethiopia.
Agent-based modeling (ABM), a type of individual-based model, can aid in simulating complex interactions among agents and assess their effects on the system as a whole.15,16 In an ABM, agents such as bacteria and humans follow predetermined rules based on their heterogenous characteristics and the environment. Some characteristics change over time, whereas others do not, resulting in dynamic interactions between agents and the environment to produce aggregate results over a period.15 ABM can provide better insights into the dynamics of infectious diseases compared to previous studies which primarily used compartmental approaches to model AMR.12,16 In addition, most models that have examined the impact of AMR were deterministic and did not incorporate an economic perspective. We aimed to develop a stochastic ABM with humans and bacterial agents to examine the broader health and economic effect of AMR on treatment for pneumococcal disease.12
MATERIALS AND METHODS
We developed the DREAMR (Dynamic Representation of the Economics of AMR) model, an ABM with two interactive submodels for bacteria and humans. Creating two submodels facilitated dynamic interactions between humans and bacteria. In the human submodel, children become infected by S. pneumoniae, seek care at varying health facilities, and use antibiotic treatment or do not seek care. When antibiotics are used, bacterial agents survive or die, and replicate based on the magnitude of antibiotic exposure. This results in a change in the proportion of resistant strains in the bacterial population also known as the AMR pattern. The AMR pattern subsequently affects treatment outcomes of pneumococcal diseases among human agents, where treatment failures increase as the proportion of resistant strains increase. As a consequence, adverse health outcomes and costs of treatment increase because of prolonged treatment duration and use of second-line antibiotics. We used NetLogo 6.0.2 for our simulation, a freely available and widely used educational software with a multi-agent programmable modeling environment.17 We estimated the annual health and economic impact of AMR on treatment of pediatric pneumococcal infections in Ethiopia.
Bacteria submodel.
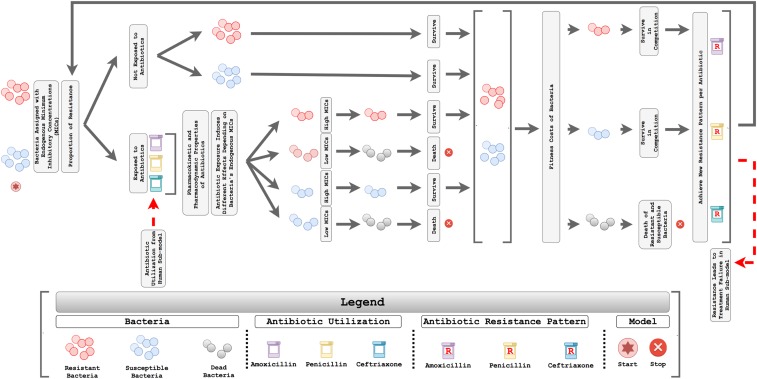
The DREAMR bacteria submodel included two types of bacteria: resistant strains and susceptible strains (Figure 1). The initial proportions of each type of bacteria were set based on resistance patterns for three antimicrobials (amoxicillin, penicillin, and ceftriaxone) that are commonly used to treat pneumococcal diseases in Ethiopia.18 The model simulated 5,000 bacterial agents, including both resistant and susceptible strains for each antibiotic. Each agent represented 0.02% of bacteria in the entire bacterial reservoir. The bacterial reservoir consisted of S. pneumoniae that colonized on either symptomatic or asymptomatic individuals, where bacterial agents could move randomly over the entire bacteria space. This represented disease transmission, where bacteria can move from one individual to another.
Figure 1.
Conceptual framework of the bacteria submodel to estimate the accumulation of antimicrobial resistance. This figure appears in color at www.ajtmh.org.
Every bacterial agent was categorized as either resistant or susceptible against each antibiotic. Individual bacterium was also assigned a minimum inhibitory concentration (MIC) value based on its resistant/susceptible characteristic, where resistant bacteria were more likely to be assigned higher MIC values. The MIC values were obtained from the gamma distribution to represent the highly right-skewed nature of MIC values, with variance obtained from the literature.19,20 The method of moments approach was applied to derive parameters needed for gamma distributions.21 The 90th percentile of MIC values in the gamma distribution for susceptible strains was set to be equal to the breakpoint for susceptible strains determined by the Clinical Laboratory Standards Institute (CLSI). The 90th percentile of MIC values for resistant strains was set to the breakpoint of intermediate strains by combining intermediate and resistant strains from CLSI as a resistant category.20
We simulated pharmacokinetics based on currently approved dosages for the three antibiotics and evaluated the resulting pharmacodynamics to determine whether each bacterium will die under antibiotic exposure (see Supplemental Appendix). Pharmacokinetic parameters, including the volume of distribution, total body clearance, and elimination rate constant were retrieved from the literature and product information for each antibiotic.22–25 The probability that adequate antibiotic exposure was achieved depended on defined daily doses (DDDs) and the proportion of children colonized with S. pneumoniae.26 DDDs were derived from the human submodel, which represented the proportion of children using antibiotics. Because not every individual carries S. pneumoniae, the model divided DDDs by the proportion of humans colonized to estimate the probability that bacterial agents would be exposed to antibiotics. As a result, larger DDDs led to a greater propensity for bacteria to be exposed to antibiotics.27 Antibiotic utilization was simulated based on common recommended dosages and intervals.28,29
Because the three beta-lactam antibiotics are time dependent, we simulated the effectiveness of antibiotics based on the percentage of time the exposed antibiotic concentration is above the MIC, where time is highly correlated with the overall dose, dosing frequency, and other pharmacokinetic characteristics.30,31 Bacteria would die if the percentage of time the antibiotic concentration is above its MIC value became larger than the threshold, under the assumption that all bacteria are susceptible to the antibiotic if adequate exposure is achieved.30,32,33 Susceptible strains of bacteria were more likely to be killed compared with resistant strains because of a lower MIC distribution.
The model also included fitness costs of resistant bacteria to counter the AMR growth.12,34 Fitness of resistant bacteria depended on several factors, such as host immune status, the environment in which the bacteria are growing, and prior drug pressure.35 As the literature suggests that resistant strains are often less fit than susceptible strains when not facing antibiotic pressure, resistant bacteria in the model faced a lower probability of survival when competing with susceptible strains.34 Survived bacteria, either resistant or susceptible strains, subsequently had an identical chance to reproduce regardless of their MIC values, until the total number of bacteria in the population reached 5,000 (100%) again to obtain a new resistant pattern. The rate of change in the proportion of resistant bacteria was associated with antibiotic pressure. We examined the AMR patterns for each antibiotic, which were dynamically updated over time in accordance with antibiotic exposure.
Human submodel.
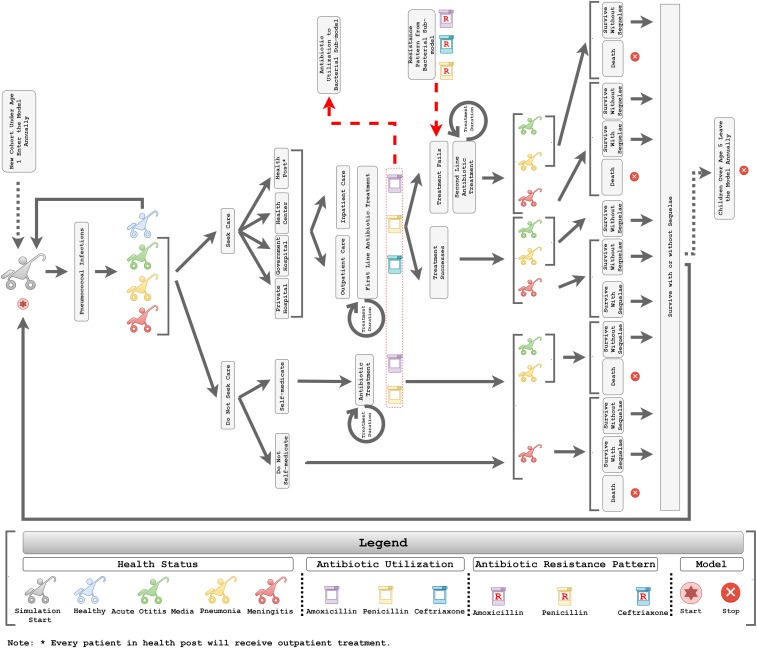
We also developed the DREAMR human submodel, where we simulated 8,000 child agents between 0 and 5 years of age with incidence of pneumococcal infections resulting in pneumonia, meningitis, and acute otitis media (Figure 2). Each child agent in the model represented 10 children, where the study modeled a total of 80,000 children over a 1-year horizon. A similar method was also used in other ABMs to overcome the computational burden.36 Every child agent in the model was assigned an immunization status for receiving the pneumococcal conjugate vaccine (PCV) based on the WHO and United Nations Children’s Fund (UNICEF) immunization coverage estimates in 2017 for Ethiopia.37 Daily pneumococcal disease incidence rates were applied based on the child agent’s PCV immunization status and vaccine efficacy.26,38–40 Disease incidence also took into consideration the effects of herd immunity, where immunization can protect unvaccinated populations by preventing disease transmission when immunization coverage reaches a high threshold.41,42 This study simulated herd immunity at the vaccine coverage rate of 2017 (i.e., 68%), where lower disease incidence rates were applied to unvaccinated populations to simulate the indirect effect of immunization.43
Figure 2.
Conceptual framework of the human submodel to estimate the health and economic impact of antimicrobial resistance. This figure appears in color at www.ajtmh.org.
Care-seeking and treatment of pneumococcal disease in children was modeled over 1 year. Specifically, caregivers of children with pneumococcal infections chose whether or not to seek care from healthcare facilities based on care-seeking rates from the Ethiopian Demographic and Health Survey.44 Children who did not receive treatment from healthcare facilities would either receive self-medication or remain untreated. Untreated children faced a greater propensity to develop adverse health outcomes (i.e., disability and death).45 Children who sought health care received treatment from one of the following facilities: 1) health post, 2) health center, 3) government hospital, or 4) private hospital/clinic.46 All children with acute otitis media were treated as outpatients, whereas all meningitis cases were treated as inpatients.47,48 Hospitalization rates from the literature were applied to children with pneumococcal pneumonia who sought care at the health center, government hospital, or private hospital/clinic.46 At each health facility, we simulated child agents receiving first-line antibiotic treatment in an outpatient or inpatient setting. Three antibiotics were commonly used as first-line treatment for acute otitis media, pneumonia, and meningitis in Ethiopia: 1) amoxicillin, 2) penicillin, and 3) ceftriaxone.49 In outpatient/self-medication scenarios, the model assumed that patients received antibiotics that could be given through oral route (i.e., amoxicillin and penicillin). Antibiotic regimens and treatment durations were extracted from the literature.28,29,50,51 The study also included the effects of noncompliance, where noncompliant individuals who received antibiotics from a healthcare facility or self-medication faced a 2-fold higher risk of adverse health outcomes.52
The proportion of child agents using antibiotics was derived on a daily basis to estimate the magnitude of antibiotic utilization in the bacteria submodel. When exposed to antibiotics, the chance of bacterial survival depended on the antibiotic dose and dosing frequency, pharmacokinetic characteristics, the MIC values of bacteria, and fitness costs. In addition, the model assumed a 75% effective dose in the bacteria submodel when bacteria encountered antibiotic exposure caused by noncompliant patients. The proportion of antibiotic resistance in the bacteria submodel subsequently affected the rates of treatment failures observed among child agents receiving the third day of first-line treatment in the human submodel.53
Child agents encountered first-line treatment failure if they used an antibiotic and had a susceptibility value (between 0 and 1 from a uniform distribution) lower than that of the same antibiotic’s AMR pattern. Child agents with treatment failure would then be switched to second-line therapy, and the overall treatment duration was prolonged. Child agents with illness either died or recovered from the disease episode based on overall case fatality rates.39 Child agents who survived meningitis faced a chance of developing neurological sequelae.50
Outcomes and uncertainty analyses.
We estimated the annual change in the AMR pattern, measuring the proportion of bacterial strains for each antibiotic. We simulated the number and percentage of treatment failures for pneumonia, meningitis, and acute otitis media for children under 5 in Ethiopia.54 Other predicted health outcomes included average DDDs, cumulative incidence of disease episodes, and annual numbers of death and disability due to pneumococcal disease in Ethiopia. We also assessed the number of deaths due to treatment failures. All outcomes are based on an average of 10,000 simulation runs in the model.
In addition to health effects, we also assessed the economic impact using the cost-of-illness method taking a societal perspective, where we estimated direct medical costs, direct nonmedical costs, productivity losses for caregivers, and productivity losses due to death/disability.55,56 Direct medical costs combined costs of 1) registration/consultation, 2) laboratory diagnosis, 3) medicines and supplies, 4) hospital bed, and 5) traditional healer visits.46 Direct nonmedical costs included costs of transportation and others such as food and lodging. Unit costs for each health facility were obtained from the literature.46 We also estimated the productivity losses for caregivers and productivity losses due to death and disability based on Ethiopia’s gross domestic product per capita using a human capital approach.57,58 A disability-adjusted life year weight was applied to productivity losses for children living with disability due to meningitis.59 All costs are expressed in U.S. dollars (2018), and future costs such as productivity losses due to premature death and disability were discounted back to 2018 using a 3% discount rate.
We performed sensitivity analyses to capture uncertainties resulting from 1) the stochastic nature of ABM, 2) model inputs, and 3) model assumptions. Some uncertainty is inherent in the ABM, as the modeling approach uses random processes to capture heterogeneities across individual agents and environments. For instance, MIC values were randomly assigned to bacterial agents, and bacterial agents stochastically encountered antibiotic exposure resulting in different AMR patterns across simulations. This uncertainty from ABM was minimized by running the base case simulation 10,000 times and taking an average across iterations (see Supplemental Appendix). In addition, we conducted a multivariate probabilistic sensitivity analysis (PSA) to incorporate the additional uncertainty due to model inputs. By using Monte Carlo simulations, the PSA randomly drew parameter values from underlying distributions 10,000 times and estimated variances.21 Parameters that were varied during the PSA along with their underlying distributions and uncertainty ranges are listed in Table 1. The 95% confidence intervals were derived from taking the 2.5th and 97.5th percentiles of PSA results. We observed that the variance largely came from the stochastic nature of ABM rather than from key input parameters. Lastly, uncertainties from key model assumptions were also examined. Specific sensitivity analyses were carried out to assess the effect of the number of child agents in the model (i.e., simulating 2,500, 5,000, and 8,000 child agents) and to examine the outcome without herd immunity (see Supplemental Appendix). Estimated outcomes were comparable between 5,000 and 8,000 child agents, confirming that the model simulated adequate numbers of agents. Sensitivity analyses were also conducted to examine the impact of the multiplication approach, where one child agent represented 10 children. Similar results were obtained between simulating 800 child agents of 10 children compared with 8,000 child agents of one child, showing that the underlying model was robust.
Table 1.
DREAMR model inputs
| Parameter variable | Unit | Value | Standard error or uncertainty range | Underlying distribution | Source |
|---|---|---|---|---|---|
| Demographics | |||||
| Total population | Thousands | 99,873 | – | – | UN DESA54 |
| Population, age 0–4 years | Thousands | 14,901 | – | – | UN DESA54 |
| Population growth rate | % | 2.60 | – | – | UN DESA54 |
| Gross domestic product per capita | USD | 706.76 | – | – | World Bank58 |
| Life expectancy at birth | Years | 65.97 | – | – | UNDESA54 |
| Vaccine characteristics | |||||
| Vaccine effectiveness | % | 60.20 | – | – | Moore et al.38 |
| Pneumococcal conjugate vaccine 13 coverage rate | % | 68 | – | – | WHO/UNICEF37 |
| Pneumococcal disease incidence | |||||
| Streptococcus pneumoniae colonization prevalence | % | 43.78 | 2.61 | Beta | Gabre et al.26 |
| Pneumonia | |||||
| Incidence | Per 100,000 | 3,397 | – | – | O’Brien et al.39 |
| Case fatality rate | % | 11 | 7–18 | Beta | O’Brien et al.39 |
| Meningitis | |||||
| Incidence | Per 100,000 | 38 | – | – | O’Brien et al.39 |
| Case fatality rate | % | 73 | 18–94 | Beta | O’Brien et al.39 |
| Neurological sequelae | % | 32 | 3.80 | Beta | Arditi et al.50 |
| Acute otitis media | |||||
| Incidence | Per 100 | 60.99 | – | – | Monasta et al.40 |
| AOM caused by S. pneumoniae | % | 40.00 | – | – | Gebre et al.26 |
| Clinical resolution rate, amoxicillin | % | 92.80 | – | – | Le Saux et al.47 |
| Clinical resolution rate, no treatment | % | 84.17 | – | – | Le Saux et al.47 |
| Probability of hearing loss | Per 10,000 | 22.84 | – | – | Monasta et al.40 |
| Herd immunity (indirect effect) | |||||
| Pneumonia (in months) | |||||
| < 12 | % | 33 | – | – | Blank et al.43 |
| 12–23 | % | 42 | – | – | Blank et al.43 |
| 24–35 | % | 37 | – | – | Blank et al.43 |
| 36–47 | % | 37 | – | – | Blank et al.43 |
| 48–59 | % | 54 | – | – | Blank et al.43 |
| Meningitis (months) | |||||
| < 12 | % | 48 | – | – | Blank et al.43 |
| 12–23 | % | 56 | – | – | Blank et al.43 |
| 24–35 | % | 43 | – | – | Blank et al.43 |
| 36–47 | % | 43 | – | – | Blank et al.43 |
| 48–59 | % | 41 | – | – | Blank et al.43 |
| Acute otitis media (months) | |||||
| < 12 | % | 22 | – | – | Blank et al.43 |
| 12–23 | % | 27 | – | – | Blank et al.43 |
| 24–35 | % | 0 | – | – | Blank et al.43 |
| 36–47 | % | 0 | – | – | Blank et al.43 |
| 48–59 | % | 0 | – | – | Blank et al.43 |
| Care-seeking behaviors | |||||
| Facility types | |||||
| Health post | % | 12.17 | 1.76 | Dirichlet | Memirie et al.46 |
| Health center | % | 53.33 | 2.69 | Dirichlet | Memirie et al.46 |
| Government hospital | % | 24.06 | 2.30 | Dirichlet | Memirie et al.46 |
| Private clinic/hospital | % | 10.43 | 1.65 | Dirichlet | Memirie et al.46 |
| Hospitalization rate | |||||
| Pneumonia | |||||
| Health post | % | 0 | 0 | Beta | Memirie et al.46 |
| Health center | % | 1.63 | 0.93 | Beta | Memirie et al.46 |
| Government hospital | % | 31.33 | 5.09 | Beta | Memirie et al.46 |
| Private clinic/hospital | % | 36.11 | 8.01 | Beta | Memirie et al.46 |
| Meningitis | % | 100 | – | – | Assumption |
| Acute otitis media | % | 0 | – | – | Assumption |
| Sought care at formal healthcare facilities | |||||
| Pneumonia | % | 27.00 | 0.43 | Beta | EDHS44 |
| Meningitis | % | 35.30 | 0.47 | Beta | EDHS44 |
| Acute otitis media | % | 27.00 | 0.43 | Beta | EDHS44 |
| Mortality rate of nonseeking vs. seeking care | Odds ratio | 7.56 | 3.77–15.10 | Normal | Reyes et al.45 |
| Proportion of self-medication | % | 30.93 | 2.34 | Beta | Gebeyehu et al.73 |
| Noncompliance | |||||
| Formal healthcare facility | % | 17.11 | 3.05 | Beta | Yadesa et al.74 |
| Self-medication | % | 27.31 | 2.26 | Beta | Gebeyehu et al.73 |
| Antibiotic’ effective dose | % | 75.00 | – | – | Assumption |
| Risk of adverse health outcomes | Fold | 2 | – | – | WHO52 |
| Pneumonia | |||||
| Inpatient | Days | 10 | – | – | Harris et al.53 |
| Outpatient | Days | 7 | – | – | Assumption |
| Meningitis | Days | 15 | – | – | Arditi et al.50 |
| Acute otitis media | Days | 7 | – | – | Klein et al.48 |
| Follow-up since onset of therapy | Days | 3 | – | – | Harris et al.53 |
| Initial antibiotics resistance rate | |||||
| Amoxicillin | % | 29.00 | – | – | Anagaw et al.18 |
| Penicillin | % | 31.30 | – | – | Anagaw et al.18 |
| Ceftriaxone | % | 9.80 | – | – | Anagaw et al.18 |
| Antibiotic utilization | |||||
| Pneumonia | |||||
| Amoxicillin | % | 20.80 | 3.63 | Dirichlet | Achalu et al.49 |
| Penicillin | % | 25.60 | 3.90 | Dirichlet | Achalu et al.49 |
| Ceftriaxone | % | 53.60 | 4.46 | Dirichlet | Achalu et al.49 |
| Meningitis | |||||
| Ceftriaxone | % | 100.00 | – | – | Achalu et al.49 |
| Acute otitis media | |||||
| Amoxicillin | % | 57.14 | 18.70 | Beta | Achalu et al.49 |
| Penicillin | % | 42.86 | 18.70 | Beta | Achalu et al.49 |
| Costs | |||||
| Direct medical costs | |||||
| Outpatient | % | 78.95 | – | – | Memirie et al.46 |
| Inpatient | % | 79.52 | – | – | Memirie et al.46 |
| Mean medical expenditure | |||||
| Outpatient | |||||
| Health post | USD | 1.61 | 2.71 | Gamma | Memirie et al.46 |
| Health center | USD | 4.06 | 5.91 | Gamma | Memirie et al.46 |
| Government hospital | USD | 12.08 | 12.05 | Gamma | Memirie et al.46 |
| Private clinic/hospital | USD | 28.12 | 8.85 | Gamma | Memirie et al.46 |
| Inpatient | |||||
| Health post | USD | – | – | – | Memirie et al.46 |
| Health center | USD | 12.13 | 8.80 | Gamma | Memirie et al.46 |
| Government hospital | USD | 47.89 | 28.81 | Gamma | Memirie et al.46 |
| Private clinic/hospital | USD | 139.66 | 71.97 | Gamma | Memirie et al.46 |
| DALY weight for hearing loss | DALYs | 0.158 | – | – | Salomon et al.59 |
| Pharmacokinetics and pharmacodynamics characteristics | |||||
| Penicillin G | |||||
| Dose | IU/kg/day | 50,000 | – | – | AAP29 |
| Dosing interval | Hours | 6 | – | – | AAP29 |
| mg to IU conversion | IU/mg | 1,670 | – | – | Humphrey et al.22 |
| Volume of distribution | L/kg | 1.39 | – | – | Bolme et al.23 |
| Clearance | mL/min/kg | 22.2 | – | – | Bolme et al.23 |
| Ceftriaxone | |||||
| Dose | mg/kg/day | 50–100 | – | – | Bradley et al.28 |
| Dosing interval | Hours | 12 | – | – | Bradley et al.28 |
| Vd | mL/kg | 387 | – | – | Steele et al.25 |
| Elimination half-life (t1/2) | Hours | 5.4 | – | – | Steele et al.25 |
| Amoxicillin | |||||
| Dose | mg/kg/dose | 30 | – | – | Bradley et al.28 |
| Dosing interval | Hours | 8 | – | – | Bradley et al.28 |
| Vd | mL/kg | 764 | – | – | Schaad et al.24 |
| Elimination half-life (t1/2) | Hours | 1.17 | – | – | Schaad et al.24 |
| Fitness cost (resistant vs. susceptible) | Relative fitness | 0.86 | – | – | Maher et al.34 |
AAP = American Academy of Pediatrics; AOM = acute otitis media; DALY = disability-adjusted life year; EDHS = Ethiopia Demographic and Health Survey; IU = international units; UN DESA = United Nations Department of Economic and Social Affairs; UNICEF = United Nations Children’s Fund; USD = United States dollars; Vd = volume of distribution; WHO = World Health Organization.
Model validation.
We assessed the model by examining its face and empirical validities. Face validity ensured that the model contained important elements, while empirical validity examined whether generated data are in line with other existing data. We validated the model at different levels, including at the micro-level representing fundamental rules the agents must follow, and at the macro-level reflecting the outcomes produced by complex interactions between agents and environments. Because this study included two submodels that had within- and between-model interactions, we looked into the validity of each submodel and the composite outcomes that resulted from their interactions. For face validity, we consulted with several experts in microbiology and pharmacokinetics to ensure that the bacteria submodel included important components of AMR, such as antibiotic selection pressure and fitness costs. For the human submodel, we included factors that impacted disease incidence and treatment patterns, including direct and indirect effects of immunization and care-seeking behaviors. The aggregate results met face validity where the magnitude of emerging resistance against different antibiotics was correlated with antibiotic utilization, where antibiotics prescribed frequently were more likely to gain resistance, and those used less could reverse the trend of accumulating resistance due to fitness costs. For empirical validation, we compared our results with several outcomes of interest, such as disease incidence and burden of malaria. We calibrated the model so that the study outputs aligned with available data from Ethiopia.
RESULTS
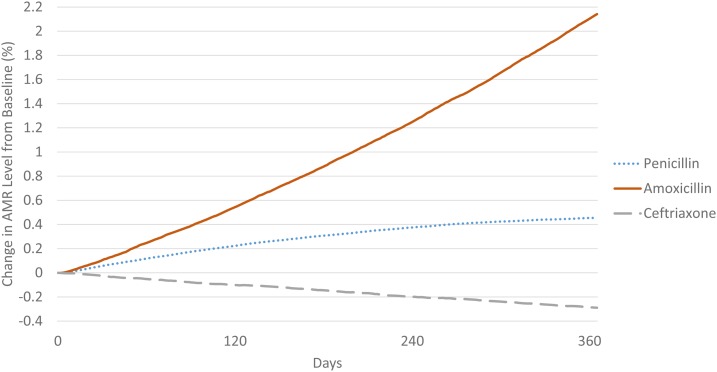
Over the 1-year simulation, the proportion of nonsusceptible S. pneumoniae increased by 2.14% (95% CI: 0.11–4.99%) and 0.47% (95% CI: −0.23–1.08%) for amoxicillin and penicillin, respectively, and reduced by 0.30% (95% CI: −1.22–0.67%) for less commonly used ceftriaxone (Figure 3). Increase in resistance was greater among antibiotics prescribed frequently such as amoxicillin. For antibiotics not commonly prescribed for pneumococcal diseases such as ceftriaxone, our simulation showed that resistance in the bacteria population could be reduced because of the effect of fitness costs.
Figure 3.
Changes in proportions of antimicrobial resistance over time. This figure appears in color at www.ajtmh.org.
We observed a large disease burden from S. pneumoniae infections among children under age 5 in Ethiopia (Table 2). We estimated that pneumococcal infections were annually associated with 227,834 (95% CI: 215,319–240,656) cases of pneumonia, 2,405 (95% CI: 1,118–3,725) cases of meningitis, and 2,230,302 (95% CI: 2,191,006–2,269,795) cases of acute otitis media. Pneumococcal infections in children under 5 were responsible for 26,979 (95% CI: 17,695–36,698) deaths per year, with many individuals not seeking care. Meningitis and acute otitis media were simulated to result in 1,310 (95% CI: 373–2,235) disabilities annually. We projected that pediatric pneumococcal infections resulted in 666,370 (95% CI: 636,459–696,435) first-line antibiotic treatments per year in Ethiopia. On average, 1.43 (95% CI: 1.33–1.53) in every 1,000 children under 5 used antibiotics per day to treat pneumococcal diseases.
Table 2.
Annual impact of antibiotic resistance on health outcomes due to pneumococcal infections in Ethiopia
| Outcomes | Value* | 95% Probabilistic sensitivity analysis CI† | |
|---|---|---|---|
| Incremental change in resistance | |||
| Amoxicillin, % | 2.14 | 0.11 | 4.99 |
| Penicillin, % | 0.47 | −0.23 | 1.08 |
| Ceftriaxone, % | −0.30 | −1.22 | 0.67 |
| Average defined daily dose, per 1,000 patient days | 1.43 | 1.33 | 1.53 |
| Disease cases | |||
| Pneumococcal pneumonia, n | 227,834 | 215,319 | 240,656 |
| Pneumococcal meningitis, n | 2,405 | 1,118 | 3,725 |
| Pneumococcal AOM, n | 2,230,302 | 2,191,006 | 2,269,795 |
| Adverse health outcomes | |||
| Overall death, n | 26,979 | 17,695 | 36,698 |
| Death during formal treatment, n | 2,979 | 1,118 | 5,774 |
| Death due to resistance in formal treatment, n | 519 | 0 | 1,490 |
| Death due to resistance in self-medication, n | 2,406 | 745 | 4,843 |
| Death due to not seeking care, n | 21,075 | 15,832 | 24,591 |
| Disability, n | 1,310 | 373 | 2,235 |
| Treatment behaviors | |||
| Overall treatments, n | 666,370 | 636,459 | 696,435 |
| Overall treatment failures , n | 195,763 | 180,856 | 215,506 |
| Treatment failures (pneumonia), n | 11,931 | 9,872 | 16,950 |
| Treatment failures (meningitis), n | 228 | 0 | 745 |
| Treatment failures (AOM) , n | 183,604 | 170,984 | 197,811 |
| Proportion of treatment failures, % | 29.38 | 28.06 | 31.28 |
AOM = acute otitis media; CI = confidence interval.
* Point estimates were derived by taking average values across 10,000 base case simulations.
† The 2.5th and 97.5th percentiles across 10,000 probabilistic sensitivity analyses were used to derive 95% CIs.
Antimicrobial resistance resulted in an overall first-line treatment failure rate of 29.38% (95% CI: 28.06–31.28%), where patients needed to switch to second-line therapy, endured a longer duration of illness, and incurred greater costs. Antimicrobial resistance against antibiotics led to 195,763 (95% CI: 180,856–215,506) treatment failures annually, where the majority of treatment failures in the model came from AMR against amoxicillin and penicillin to treat acute otitis media (183,604, 95% CI: 170,984–197,811). Resistance-related treatment failures contributed to 519 (95% CI: 0–1,490) and 2,406 (95% CI: 745–4,843) deaths through treatment at healthcare facilities and from self-medication, respectively.
On average, costs for each successful first-line treatment and treatment failure due to resistance were US$16.69 (95% CI: US$12.05–$25.89) and US$35.39 (95% CI: US$25.90–$52.97), respectively (Table 3). Annual overall costs related to AMR were around 15.8 million, including US$3,267,389 (95% CI: US$2,149,468–US$5,658,071) for ineffective first-line treatments, US$3,661,208 (95% CI: US$2,547,098–$5,741,984) for second-line treatments, and $8,872,107 (95% CI: US$2,158,860–US$20,017,281) for long-term productivity losses due to premature deaths and disabilities. Costs of ineffective first-line treatments included US$1,432,290 (95% CI: US$478,281–US$3,609,864) in direct costs and US$1,835,099 (95% CI: $1,671,186–$2,048,206) in productivity losses for the caregiver. Second-line treatments, which could have been prevented by effective first-line treatments, added US$1,391,019 (95% CI: US$416,648–US$3,275,032) in direct costs and US$2,270,190 (95% CI: US$2,130,451–US$2,466,952) in productivity losses to the caregiver.
Table 3.
Annual impact of antibiotic resistance on costs of pneumococcal infections in Ethiopia
| Outcomes | Value* | 95% Probabilistic sensitivity analysis CI† | |
|---|---|---|---|
| Average costs per successful first-line treatment | |||
| Overall costs, USD | 16.69 | 12.05 | 25.89 |
| Direct medical costs, USD | 5.78 | 2.13 | 13.03 |
| Direct nonmedical costs, USD | 1.54 | 0.56 | 3.46 |
| Productivity losses for caregiver, USD | 9.37 | 9.36 | 9.40 |
| Average costs per treatment failure due to resistance | |||
| Overall costs, USD | 35.39 | 25.90 | 52.97 |
| Direct medical costs, USD | 11.39 | 3.91 | 25.23 |
| Direct nonmedical costs, USD | 3.03 | 1.03 | 6.72 |
| Short-term productivity losses (caregiver), USD | 20.97 | 20.95 | 21.02 |
| Long-term productivity losses (death/disability), USD | 45.32 | 11.94 | 92.89 |
| Annual costs incurred by first-line treatments due to resistance | |||
| Overall costs, USD | 3,267,389 | 2,149,468 | 5,658,071 |
| Direct medical costs, USD | 1,131,602 | 378,609 | 2,851,699 |
| Direct nonmedical costs, USD | 300,688 | 99,672 | 758,165 |
| Productivity losses for caregiver, USD | 1,835,099 | 1,671,186 | 2,048,206 |
| Annual costs incurred by second-line treatments due to resistance | |||
| Overall costs, USD | 3,661,208 | 2,547,098 | 5,741,984 |
| Direct medical costs, USD | 1,098,473 | 329,218 | 2,585,820 |
| Direct nonmedical costs, USD | 292,546 | 87,430 | 689,212 |
| Short-term productivity losses (caregiver), USD | 2,270,190 | 2,130,451 | 2,466,952 |
| Long-term productivity losses (death/disability), USD | 8,872,107 | 2,158,860 | 20,017,281 |
CI = confidence interval; USD = United States dollars.
* Point estimates were derived by taking average values across 10,000 base case simulations.
† The 2.5th and 97.5th percentiles across 10,000 probabilistic sensitivity analyses were used to derive 95% CIs.
DISCUSSION
This is the first study to estimate the health and economic impact of AMR on treatment of pediatric pneumococcal infections in Ethiopia by developing an agent-based model. Our simulation estimated that among all first-line antibiotics used to treat pneumococcal disease, around 30% resulted in ineffective treatments because of AMR, necessitating the need to switch to second-line antibiotic therapy. Treatment failures led to significant child deaths, higher costs due to prolonged treatment duration, greater loss in productivity, and societal costs associated with increasing AMR. Treatment failures and resulting impact can be averted by controlling the development of AMR associated with antibiotics.
Our results demonstrate the substantial impact of AMR in low-income countries such as Ethiopia, with a large infectious disease burden and high rates of antibiotic utilization. Although this study focused specifically on antibiotic utilization for treatment of pediatric pneumococcal diseases over a year, we still observed sizable treatment failures and avertable costs to patients and healthcare systems. These results are important because the impact of AMR is often not measured and underappreciated in developing countries, although antibiotics are commonly available without prescriptions, and widespread antibiotic misuse is prevalent in these settings.
Our results revealed that resistance tended to increase for amoxicillin and penicillin, whereas it tended to decrease for ceftriaxone to treat pediatric pneumococcal infections in Ethiopia over a year. Differences in these estimates can be explained by two influential factors: the magnitude of antibiotic utilization and fitness costs. Whereas higher antibiotic exposure increased AMR, fitness costs provided negative feedback as resistant bacteria often have reduced fitness to compete with susceptible strains.34 In most cases, the effects of antibiotic exposure overrode the opposite influence of bacteria fitness, resulting in an increase in resistance.60 However, when antibiotics were less frequently used, the effect of fitness costs was observed where the proportion of resistant strains reduced.60 By incorporating antibiotic exposure and fitness costs of bacteria in the DREAMR model, we demonstrated the dynamic relationship between antibiotic use and AMR.
Because AMR growth is directly attributable to antibiotic use, antibiotic stewardship to improve appropriate use of antibiotics is essential. Antimicrobial stewardship can be defined as “a coherent set of actions which promote using antimicrobials in ways that ensure sustainable access to effective therapy for all who need them.”61 On the supply side, this may entail making antibiotics obtainable by prescription, making accurate diagnosis, following antimicrobial guidelines, monitoring antimicrobial use and resistance, and investing in a clinical decision support system to improve prescribing and responsible use. On the demand side, interventions may involve education and community engagement programs to ensure that patients understand issues surrounding rational medication use, including how and when to take antimicrobials, and ensuring that patients do not store and use leftover antimicrobials. Improved antimicrobial stewardship involving both demand- and supply-side initiatives are critical to mitigating the global impact of AMR. Increased PCV immunization coverage could also reduce pneumococcal disease incidence, thereby reducing antibiotic utilization and curbing AMR. Future research should demonstrate the impact of antibiotic stewardship and vaccination on controlling the development of AMR.
Our findings are consistent with previous studies on the impact of pneumococcal disease, antibiotic utilization, and proportion of resistance. Global estimate of the burden of S. pneumoniae in children under 5 has projected approximately 57,000 pneumococcal deaths in Ethiopia in 2000, before the country introduced PCV.39 In our model, overall deaths due to pneumococcal diseases reflect current vaccine coverage and vaccine efficacy, resulting in fewer deaths. As for the rate of antibiotic utilization, previous studies report average overall antibiotic use at around 10 to 35 DDDs across countries.13,62 We believe our lower antibiotic utilization rate is reasonable, given that our model only focused on utilization of three antibiotics for pediatric pneumococcal infections, and because Ethiopia has a relatively low proportion of individuals seeking care. Although data about antibiotic resistance in Africa are scarce due to the lack of susceptibility testing and weak surveillance systems, previous studies have reported the proportion of nonsusceptible S. pneumoniae to be between 9% and 69%, which aligns with our analysis.13,63 Our results on the positive correlation between antibiotic use and AMR are consistent with previous findings in other countries.64,65
There are a number of potential limitations to our study. First, our model relies on the quality of data reported in the existing literature. Although we conducted an extensive literature search to incorporate the most recent and best quality published data, results of the study are subject to the quality of data inputs. Data availability also limits the model’s ability to capture heterogeneity across the population. For example, data on antibiotic utilization for people who do not seek care at formal health facilities were limited, including those who obtain antibiotics from pharmacies or from other individuals. Real-world antibiotic utilization may also differ from recommended treatment guidelines, and antibiotic prescription behaviors may change in accordance with resistance patterns. While previous studies have suggested the examination of S. pneumoniae serotypes, this study was not able to incorporate the impact of different serotypes, assuming no difference in bacterial virulence and response to PCV13. This limited our ability to examine how heterogeneous serotypes of infected patients may affect vaccine effectiveness and disease severity. Further studies should be conducted to examine the heterogeneity of several crucial data, including bacterial susceptibility, effect of serotypes, antibiotic quality, antibiotic utilization, impact of vaccination, bacterial fitness, and treatment costs, in order to provide a more comprehensive insight on the impact.
Secondly, the bacteria submodel does not incorporate the effects of genetic mutation and substandard and falsified antibiotics. Although bacteria can develop resistance under strong selection pressure due to antibiotic exposure, they may also become resistant as a result of genetic mutation under subinhibitory concentrations or acquire resistant genes from other strains.66–68 Mutation-related resistance may play an important role among countries with high prevalence of substandard and falsified medicines, where bacteria are more likely to be exposed to subinhibitory antibiotic concentrations.69 Future studies should examine these impacts.
Third, our model examined the AMR impact across three antibiotics commonly used in Ethiopia to treat pediatric pneumococcal disease. These results may not be easily generalizable to other antibiotics and contexts. In addition, antibiotic treatment might not be optimized, where the route of administration and studies of combination therapies that can result in more realistic exposures among patients were not available for the study. Finally, our model results focused solely on the use of antibiotics for human health. Further studies should estimate the impact of AMR from a broader scope under a One Health approach, taking into account antibiotic use across animal and environmental health sectors and additional stakeholders influenced by the accumulation of AMR.70
The DREAMR model is the first to estimate the annual impact of AMR in a low-income country by estimating the impact of antibiotic resistance on treatment of pediatric pneumococcal disease in Ethiopia. The results can inform in-country stakeholders, international donors, and national and regional child health experts to recognize the burden of AMR and examine interventions to improve appropriate antibiotic use. Reducing the impact of AMR is essential to achieve the Sustainable Development Goals (SDGs)—to achieve access to safe, effective, quality, and affordable essential medicines for all.71 Maintaining the effectiveness of current antibiotics is also vital to meet the Global Health Security Agenda (GHSA) to help create a world safe and secure from infectious disease threats.72
Supplemental appendix
Acknowledgments:
We thank Jim Herrington, Brian Conlon, and Ashley Marx for their advice and insights. We also thank the Global Health Economics for Pharmacy (GHEP) team at the University of North Carolina at Chapel Hill (Daniel Evans, Colleen Higgins, Sarah Laing, and Tatenda Yemeke) for their feedback and support.
Note: Supplemental appendix appear at www.ajtmh.org.
REFERENCES
- 1.Laxminarayan R, Sridhar D, Blaser M, Wang M, Woolhouse M, 2016. Achieving global targets for antimicrobial resistance. Science 353: 874–875. [DOI] [PubMed] [Google Scholar]
- 2.Woolhouse M, Waugh C, Perry MR, Nair H, 2016. Global disease burden due to antibiotic resistance–state of the evidence. J Glob Health 6: 010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention , 2017. About Antimicrobial Resistance. Available at: https://www.cdc.gov/drugresistance/about.html. Accessed February 16, 2018. [Google Scholar]
- 4.Penesyan A, Gillings M, Paulsen IT, 2015. Antibiotic discovery: combatting bacterial resistance in cells and in biofilm communities. Molecules 20: 5286–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelband H, Molly Miller P, Pant S, Gandra S, Levinson J, Barter D, White A, Laxminarayan R, 2015. The state of the world’s antibiotics 2015. Wound Healing South Afr 8: 30–34. [Google Scholar]
- 6.Ventola CL, 2015. The antibiotic resistance crisis: part 1: causes and threats. P T 40: 277–283. [PMC free article] [PubMed] [Google Scholar]
- 7.Spellberg B, Gilbert DN, 2014. The future of antibiotics and resistance: a tribute to a career of leadership by John Bartlett. Clin Infect Dis 59 (Suppl 2): S71–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Neill J, 2016. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. London, United Kingdom: Review on Antimicrobial Resistance. [Google Scholar]
- 9.Gandra S, Barter DM, Laxminarayan R, 2014. Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect 20: 973–980. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention , 2013. Antibiotic Resistance Threats in the United States, 2013. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed February 16, 2018. [Google Scholar]
- 11.European Medicines Agency (EMA) and European Center for Disease Prevention and Control (ECDC) , 2009. The Bacterial Challenge: Time to React a Call to Narrow the Gap Between Multidrug-Resistant Bacteria in the EU and Development of New Antibacterial Agents. Available at: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf. Accessed February 16, 2018. [Google Scholar]
- 12.Niewiadomska AM, Jayabalasingham B, Seidman JC, Willem L, Grenfell B, Spiro D, Viboud C, 2019. Population-level mathematical modeling of antimicrobial resistance: a systematic review. BMC Med 17: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO , 2014. Antimicrobial Resistance: Global Report on Surveillance. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 14.Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE, 2013. Global burden of childhood pneumonia and diarrhoea. Lancet 381: 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilensky U, Rand W, 2015. An Introduction to Agent-Based Modeling: Modeling Natural, Social, and Engineered Complex Systems with NetLogo. Cambridge, MA: MIT Press. [Google Scholar]
- 16.Bershteyn A, et al. 2018. Implementation and applications of EMOD, an individual-based multi-disease modeling platform. Pathog Dis 76: fty059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilensky U, 1999. NetLogo: Center for Connected Learning and Computer-Based Modeling. Evanston, IL: Northwestern University. [Google Scholar]
- 18.Anagaw B, Gezachew M, Biadgelgene F, Anagaw B, Geleshe T, Taddese B, Getie B, Endris M, Mulu A, Unakal C, 2013. Antimicrobial susceptibility patterns of Streptococcus pneumoniae over 6 years at Gondar University Hospital, northwest Ethiopia. Asian Pac J Trop Biomed 3: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical Laboratory Standards Institute , Patel JB, 2015. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Fifth Informational Supplement. Wayne, PA: Committee for Clinical Laboratory Standards. [Google Scholar]
- 20.Turnidge J, Kahlmeter G, Kronvall G, 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin Microbiol Infect 12: 418–425. [DOI] [PubMed] [Google Scholar]
- 21.Briggs AH, Sculpher MJ, Claxton K, 2006. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press. [Google Scholar]
- 22.Humphrey JH, Musset MV, Perry WL, 1953. The second international standard for penicillin. Bull World Health Organ 9: 15–28. [PMC free article] [PubMed] [Google Scholar]
- 23.Bolme P, Eriksson M, Paalzow L, Stintzing G, Zerihun G, Woldemariam T, 1995. Malnutrition and pharmacokinetics of penicillin in Ethiopian children. Pharmacol Toxicol 76: 259–262. [DOI] [PubMed] [Google Scholar]
- 24.Schaad UB, Casey PA, Cooper DL, 1983. Single-dose pharmacokinetics of intravenous clavulanic acid with amoxicillin in pediatric patients. Antimicrob Agents Chemother 23: 252–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steele RW, Eyre LB, Bradsher RW, Weinfeld RE, Patel IH, Spicehandler J, 1983. Pharmacokinetics of ceftriaxone in pediatric patients with meningitis. Antimicrob Agents Chemother 23: 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gebre T, Tadesse M, Aragaw D, Feye D, Beyene HB, Seyoum D, Mekonnen M, 2017. Nasopharyngeal carriage and antimicrobial susceptibility patterns of Streptococcus pneumoniae among children under five in southwest Ethiopia. Children (Basel) 4: E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO, International Working Group for Drug Statistics Methodology, Collaborating Centre for Drug Statistics Methodology, Collaborating Centre for Drug Utilization Research and Clinical Pharmacological Services , 2003. Introduction to Drug Utilization Research. Oslo, Norway: World Health Organization. [Google Scholar]
- 28.Bradley JS, et al. 2011. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 53: e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Academy of Pediatrics , 2015. Pneumococcal infections. Red Book: 2015 Report of the Committee on Infectious Diseases, 30th edition Elk Grove Village, IL: American Academy of Pediatrics. [Google Scholar]
- 30.Kuti JL, 2016. Optimizing antimicrobial phamacodynamics: a guide for your stewardship program. Revista Médica Clínica Las Condes 27: 615–624. [Google Scholar]
- 31.Quintiliani R, 2004. Using pharmacodynamic and pharmacokinetic concepts to optimize treatment of infectious diseases. Infect Med 21: 219–232. [Google Scholar]
- 32.Craig WA, 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis 22: 89–96. [DOI] [PubMed] [Google Scholar]
- 33.Levison ME, Levison JH, 2009. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect Dis Clin North Am 23: 791–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maher MC, Alemayehu W, Lakew T, Gaynor BD, Haug S, Cevallos V, Keenan JD, Lietman TM, Porco TC, 2012. The fitness cost of antibiotic resistance in Streptococcus pneumoniae: insight from the field. PLoS One 7: e29407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geisinger E, Isberg RR, 2017. Interplay between antibiotic resistance and virulence during disease promoted by multidrug-resistant bacteria. J Infect Dis 215: S9–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hackl J, Dubernet T, 2019. Epidemic spreading in urban areas using agent-based transportation models. Future Internet 11: 92. [Google Scholar]
- 37.WHO, UNICEF , 2018. WHO/UNICEF Coverage Estimates for 1980–2017. Available at: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tswucoveragepcv3.html. Accessed October 23, 2018. [Google Scholar]
- 38.Moore MR, et al. 2016. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med 4: 399–406. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374: 893–902. [DOI] [PubMed] [Google Scholar]
- 40.Monasta L, Ronfani L, Marchetti F, Montico M, Vecchi Brumatti L, Bavcar A, Grasso D, Barbiero C, Tamburlini G, 2012. Burden of disease caused by otitis media: systematic review and global estimates. PLoS One 7: e36226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fine PE, 1993. Herd immunity: history, theory, practice. Epidemiol Rev 15: 265–302. [DOI] [PubMed] [Google Scholar]
- 42.Greenwood B, 2014. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci 369: 20130433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blank PR, Szucs TD, 2012. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine in Switzerland. Vaccine 30: 4267–4275. [DOI] [PubMed] [Google Scholar]
- 44.Central statistical agency (CSA) [Ethiopia], ICF , 2016. Ethiopia Demographic and Health Survey 2016. Addis Ababa, Ethiopia, and Rockville, MA: CSA and ICF. [Google Scholar]
- 45.Reyes H, Perez-Cuevas R, Salmeron J, Tome P, Guiscafre H, Gutierrez G, 1997. Infant mortality due to acute respiratory infections: the influence of primary care processes. Health Policy Plan 12: 214–223. [DOI] [PubMed] [Google Scholar]
- 46.Memirie ST, Metaferia ZS, Norheim OF, Levin CE, Verguet S, Johansson KA, 2017. Household expenditures on pneumonia and diarrhoea treatment in Ethiopia: a facility-based study. BMJ Glob Health 2: e000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Saux N, Gaboury I, Baird M, Klassen TP, MacCormick J, Blanchard C, Pitters C, Sampson M, Moher D, 2005. A randomized, double-blind, placebo-controlled noninferiority trial of amoxicillin for clinically diagnosed acute otitis media in children 6 months to 5 years of age. CMAJ 172: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein J, Pelton S, 2018. Acute Otitis Media in Children: Treatment. Available at: https://www.uptodate.com/contents/acute-otitis-media-in-children-treatment. Accessed April 10, 2018. [Google Scholar]
- 49.Achalu T, Mensa M, 2017. Retrospective drug use pattern of antibiotics in pediatric ward of Shenan Gibe Hospital, Oromia Region, Ethiopia. J Antibiotics Res 1: 106. [Google Scholar]
- 50.Arditi M, et al. 1998. Three-year multicenter surveillance of pneumococcal meningitis in children: clinical characteristics, and outcome related to penicillin susceptibility and dexamethasone use. Pediatrics 102: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 51.Baxter Healthcare Corporation , 2016. Penicillin G Potassium–Penicillin G Injection, Solution. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9e58122f-5c75-4905-a774-d3a4dae4ff8c. Accessed February 16, 2018. [Google Scholar]
- 52.WHO , 2017. A Study on the Public Health and Socioeconomic Impact of Substandard and Falsified Medical Products. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 53.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, Thomson A, 2011. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 66 (Suppl 2): ii1–23. [DOI] [PubMed] [Google Scholar]
- 54.United Nations Department of Economic and Social Affairs Population Division , 2017. World Population Prospects: the 2017 Revision. Available at: https://population.un.org/wpp/. Accessed October 20, 2018. [Google Scholar]
- 55.Drummond M, 1992. Cost-of-illness studies. Pharmacoeconomics 2: 1–4. [DOI] [PubMed] [Google Scholar]
- 56.Hodgson TA, Meiners MR, 1982. Cost-of-illness methodology: a guide to current practices and procedures. Milbank Mem Fund Q Health Soc 60: 429–462. [PubMed] [Google Scholar]
- 57.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW, 2015. Methods for the Economic Evaluation of Health Care Programmes. Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- 58.The World Bank , 2017. GDP Per Capita (Current US$) 2016. Available at: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD. Accessed Febuary 15, 2018. [Google Scholar]
- 59.Salomon JA, et al. 2015. Disability weights for the global burden of disease 2013 study. Lancet Glob Health 3: e712–e723. [DOI] [PubMed] [Google Scholar]
- 60.Whittles LK, White PJ, Didelot X, 2017. Estimating the fitness cost and benefit of cefixime resistance in Neisseria gonorrhoeae to inform prescription policy: a modelling study. PLoS Med 14: e1002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dyar OJ, Huttner B, Schouten J, Pulcini C, 2017. What is antimicrobial stewardship? Clin Microbiol Infect 23: 793–798. [DOI] [PubMed] [Google Scholar]
- 62.Goossens H, Ferech M, Coenen S, Stephens P, 2007. Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries. Clin Infect Dis 44: 1091–1095. [DOI] [PubMed] [Google Scholar]
- 63.Frean J, et al. 2012. External quality assessment of national public health laboratories in Africa, 2002–2009. Bull World Health Organ 90: 191–199A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melander E, Ekdahl K, Jonsson G, Molstad S, 2000. Frequency of penicillin-resistant pneumococci in children is correlated to community utilization of antibiotics. Pediatr Infect Dis J 19: 1172–1177. [DOI] [PubMed] [Google Scholar]
- 65.Australian Commission on Safety and Quality in Health Care , 2014. Preliminary Report on Antimicrobial Use and Resistance in Australia (AURA). Sydney, Australia: ACSQHC. [Google Scholar]
- 66.Henderson-Begg SK, Livermore DM, Hall LM, 2006. Effect of subinhibitory concentrations of antibiotics on mutation frequency in Streptococcus pneumoniae. J Antimicrob Chemother 57: 849–854. [DOI] [PubMed] [Google Scholar]
- 67.Croucher NJ, et al. 2011. Rapid pneumococcal evolution in response to clinical interventions. Science 331: 430–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zinner SH, Lubenko IY, Gilbert D, Simmons K, Zhao X, Drlica K, Firsov AA, 2003. Emergence of resistant Streptococcus pneumoniae in an in vitro dynamic model that simulates moxifloxacin concentrations inside and outside the mutant selection window: related changes in susceptibility, resistance frequency and bacterial killing. J Antimicrob Chemother 52: 616–622. [DOI] [PubMed] [Google Scholar]
- 69.Bate R, Jensen P, Hess K, Mooney L, Milligan J, 2013. Substandard and falsified anti-tuberculosis drugs: a preliminary field analysis. Int J Tuberc Lung Dis 17: 308–311. [DOI] [PubMed] [Google Scholar]
- 70.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, 2016. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press. [Google Scholar]
- 71.United Nations , 2015. Sustainable Development Goals. Available at: https://sustainabledevelopment.un.org/sdgs. Accessed October 30, 2018. [Google Scholar]
- 72.Global Health Security Agenda , 2018. Available at: https://www.ghsagenda.org/. Accessed October 30, 2018.
- 73.Gebeyehu E, Bantie L, Azage M, 2015. Inappropriate use of antibiotics and its associated factors among urban and rural communities of Bahir Dar city administration, northwest Ethiopia. PLoS One 10: e0138179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yadesa TM, Gudina EK, Angamo MT, 2015. Antimicrobial use-related problems and predictors among hospitalized medical in-patients in southwest Ethiopia: prospective observational study. PLoS One 10: e0138385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.