Summary
Background
Population-based cancer survival estimates provide valuable insights into the effectiveness of cancer services and can reflect the prospects of cure. As part of the second phase of the International Cancer Benchmarking Partnership (ICBP), the Cancer Survival in High-Income Countries (SURVMARK-2) project aims to provide a comprehensive overview of cancer survival across seven high-income countries and a comparative assessment of corresponding incidence and mortality trends.
Methods
In this longitudinal, population-based study, we collected patient-level data on 3·9 million patients with cancer from population-based cancer registries in 21 jurisdictions in seven countries (Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the UK) for seven sites of cancer (oesophagus, stomach, colon, rectum, pancreas, lung, and ovary) diagnosed between 1995 and 2014, and followed up until Dec 31, 2015. We calculated age-standardised net survival at 1 year and 5 years after diagnosis by site, age group, and period of diagnosis. We mapped changes in incidence and mortality to changes in survival to assess progress in cancer control.
Findings
In 19 eligible jurisdictions, 3 764 543 cases of cancer were eligible for inclusion in the study. In the 19 included jurisdictions, over 1995–2014, 1-year and 5-year net survival increased in each country across almost all cancer types, with, for example, 5-year rectal cancer survival increasing more than 13 percentage points in Denmark, Ireland, and the UK. For 2010–14, survival was generally higher in Australia, Canada, and Norway than in New Zealand, Denmark, Ireland, and the UK. Over the study period, larger survival improvements were observed for patients younger than 75 years at diagnosis than those aged 75 years and older, and notably for cancers with a poor prognosis (ie, oesophagus, stomach, pancreas, and lung). Progress in cancer control (ie, increased survival, decreased mortality and incidence) over the study period was evident for stomach, colon, lung (in males), and ovarian cancer.
Interpretation
The joint evaluation of trends in incidence, mortality, and survival indicated progress in four of the seven studied cancers. Cancer survival continues to increase across high-income countries; however, international disparities persist. While truly valid comparisons require differences in registration practice, classification, and coding to be minimal, stage of disease at diagnosis, timely access to effective treatment, and the extent of comorbidity are likely the main determinants of patient outcomes. Future studies are needed to assess the impact of these factors to further our understanding of international disparities in cancer survival.
Funding
Canadian Partnership Against Cancer; Cancer Council Victoria; Cancer Institute New South Wales; Cancer Research UK; Danish Cancer Society; National Cancer Registry Ireland; The Cancer Society of New Zealand; National Health Service England; Norwegian Cancer Society; Public Health Agency Northern Ireland, on behalf of the Northern Ireland Cancer Registry; The Scottish Government; Western Australia Department of Health; and Wales Cancer Network.
Introduction
The International Cancer Benchmarking Partnership (ICBP) is a global, multidisciplinary partnership of clinicians, academics, and policy makers seeking to understand how and why cancer survival differs across countries that have high-quality cancer registries and universal access to, and comparable expenditure on, health care.1 The first phase of ICBP resulted in a series of studies that have sought to comprehensively understand and address international cancer survival variations, including an assessment of four cancers over the period 1995–2007 in six countries.2 During the second phase of ICBP, the Cancer Survival in High-Income Countries (SURVMARK-2) project3 was established to further explore the underlying reasons for observed survival differences across an expanded set of cancer types, with a focus on indicators that inform clinical practice and public health, and engagement with a multidisciplinary group of collaborators and stakeholders.
Research in context.
Evidence before this study
We searched PubMed, with no language restrictions, on Jan 15, 2019, for studies containing one or more of the terms “survival”, “cancer”, “incidence”, “mortality”, and “trends” to identify relevant population-based studies assessing survival alongside incidence or mortality, or both, for one or more of the following cancer types: oesophagus, stomach, colon, rectum, pancreas, lung, and ovary. Benchmarking international population-based cancer survival differences has proven to be a highly complex but crucial way to develop and assess early-detection strategies, the quality of clinical care, and the management of cancer patients. Although consistent improvements in cancer survival have been reported in the past two decades, survival differences appear to persist for most cancer sites, motivating policy reforms in specific countries.
Added value of this study
As part of the second phase of the International Cancer Benchmarking Partnership (ICBP), the Cancer Survival in High-Income Countries (SURVMARK-2) benchmarks 1-year and 5-year net survival by age and period of diagnosis for seven cancers (oesophagus, stomach, colon, rectum, pancreas, lung, and ovary) in seven countries (Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the UK), using data from 21 high-quality population-based cancer registries for the period 1995–2014, with follow-up until Dec 31, 2015. High levels of data scrutiny, close interactions with a multidisciplinary group of data providers, epidemiologists, and clinicians, and efforts to extend the set of relevant indicators make this study unique. This report builds on previous studies by providing a combined assessment of trends in incidence, mortality, and survival to measure successes in cancer control.
Implications of all the available evidence
We found that cancer survival continues to improve across high-income countries, although international disparities persist even for cancers with poor prognoses. Progress likely stems from earlier diagnosis and improved treatment, alongside policy reforms that have ensured improved pathways to diagnosis and treatment. The favourable 20-year stomach, colon, lung (males), and ovarian cancer incidence and mortality trends are probably attributable to the delivery of interventions across the spectrum of cancer control, including effective cancer prevention and treatment. Although differences in registration practice, classification, and coding are unlikely to explain the variations reported here, ICBP SURVMARK-2 seeks to quantify the effect of specific registration-related factors on country-specific cancer survival, including definitions of date of diagnosis, and how death certificates (as the initial source of registration), are dealt with, building on previous assessments. Innovative measures of cancer survival will be included in forthcoming papers and made publicly available via an online tool to facilitate the next generation of benchmarking studies.
Several studies in high-income countries have shown consistent improvements in cancer survival over the past two decades, yet survival differences between countries have been a permanent feature for most cancer sites and continue to persist, albeit to varying extents.2, 4, 5 These differences have been interpreted, and in some cases validated, as partly reflecting variations in progress in advancing cancer services, motivating policy reforms across countries.1 However, evolving cancer control plans need to be assessed not only in light of improvements in survival, but also taking into account trends in incidence and mortality to better understand the underlying biological, epidemiological, and clinical processes involved.
Benchmarking international variations in cancer survival remains a highly complex exercise given the numerous potential factors that might explain observed variations. In addition to factors that directly affect patient outcomes, such as stage at diagnosis and treatment,6, 7, 8, 9 survival can also be influenced by differences in how cancer cases are registered and classified.10 Additionally, national and subnational variability in factors linked to the underlying health systems, cancer policy, and clinical practice could all be important drivers. As such, public awareness and beliefs about cancer are considered to have less of an influence on international differences in survival.11, 12
This initial paper from ICBP SURVMARK-2 aims to describe current patterns and recent trends in population-based survival, incidence, and mortality for seven cancers in seven countries using data from high-quality, population-based cancer registries, and to generate hypotheses with regards to the drivers of these trends.
Methods
Data collection
In this longitudinal, population-based study, we obtained patient-level data on primary cancers of the oesophagus, stomach, colon, rectum, liver, pancreas, lung, and ovary from 21 population-based cancer registries covering 21 jurisdictions in seven countries: Australia (New South Wales, Victoria, and Western Australia), Canada (Alberta, British Columbia, Manitoba, New Brunswick, Newfoundland and Labrador, Nova Scotia, Ontario, Prince Edward Island, Quebec, and Saskatchewan), Denmark, Ireland, New Zealand, Norway, and the UK (England, Scotland, Wales, and Northern Ireland). Cancers diagnosed in the period 1995–2014 with follow-up until Dec 31, 2015, were selected and coded according to the following rubrics of the tenth revision of the International Classification of Diseases (ICD-10): oesophagus (C15), stomach (C16), colon (C18–19), rectum (C20), liver (C22), pancreas (C25), lung (C34), and ovary including peritoneum and fallopian tube in females only (C48.1–2, C56, C57.0). Only invasive tumours (behaviour code 3) were included, and all borderline ovarian cancers were excluded (third revision of the ICD for Oncology codes 8442, 8451, 8462, 8472, and 8473).
We collected patient-level information on the following variables: histology, morphology, basis of diagnosis, stage at diagnosis, and treatment. While 18 registries provided full dates of diagnosis and vital status, three provided survival time in days (British Columbia, Norway, and Scotland). Three registries could not provide incidence data for the full 20-year period (data were available for New South Wales for 1995–2012, Ireland for 1995–2013, and Quebec for 2000–2011). Given our focus on temporal comparisons of patients diagnosed during 1995–2014, data from two Canadian jurisdictions were excluded: Quebec, because they only had data available from 2000, and Newfoundland and Labrador, because linkage of registered cases to vital status information was not systematically carried out before 2005. Hence, 19 jurisdictions were included in our analysis. Given the complexities in registration, classification, and differentiation of primary and secondary tumours of the liver, survival comparisons from this cancer site were not included here and will be presented in a separate paper.
Of all cases eligible for analysis in the 19 jurisdictions, we excluded cases diagnosed on the basis of death certificate only or at autopsy; those with invalid or missing dates, age, or non-malignant behaviour; those in patients aged younger than 15 years or older than 99 years at diagnosis; and second or higher order cancers at the same site (ie, if a patient had two colon cancers during the study period, only the first would be included).
National estimates of cancer mortality for the period 1995–2014 were obtained from the following sources: Australian Bureau of Statistics (all Australian jurisdictions), Statistics Canada (all Canadian jurisdictions), the Danish Health Data Authority, Central Statistics Office Ireland, Norwegian Institute of Public Health, New Zealand Ministry of Health, Office for National Statistics (Wales and England, UK), National Records of Scotland, and the General Registrar's Office for Northern Ireland. All data were submitted through a secured portal at the International Agency for Research on Cancer (IARC) and the data were handled according to applicable data protection and privacy laws, rules, and regulations. Ethical approval was obtained from each participating registry and from the IARC Ethics Committee.
Quality control
We subjected each registry dataset to several data quality assessments, which included scrutinising data for specific anomalies, such as instances of negative survival duration (date of death occurring before date of diagnosis); out-of-range dates of diagnosis or dates of death, or both; and invalid vital status codes. Additionally, every case was checked for consistency in terms of patient sex and sex-specific cancer site; site and morphology; age and site; age and morphology; and age, site, and morphology. We constructed tables including data quality indicators and communicated these to the registries and any issues identified were followed up and discussed in detail via one-to-one interviews between the investigators and registry staff. Interviews with key staff at the registries were carried out at an early phase of the study (in parallel with the call for data) to inform local case finding and ascertainment mechanisms, linkage to vital statistics, and coding and classification practices. Revised datasets were submitted by the registries when necessary. Data from all jurisdictions were subsequently harmonised according to international standards. The overall flow of data, including checks and exclusions, are in the appendix (p 25).
Statistical analysis
We calculated net survival to estimate population-based cancer survival, which was taken as the survival of patients in a defined resident population if cancer was the only possible cause of death. We present net survival with accompanying 95% CIs and used this metric to ensure fair comparisons across groups in which the chance of dying of other diseases varies. Net survival was estimated for each primary site in each jurisdiction, and for the Canadian, Australian, and UK jurisdictions combined. Follow-up was available until Dec 31, 2015, for all patients except for those in Ontario, where follow-up was limited to Dec 31, 2014.
We obtained background mortality in the general population of each jurisdiction from lifetables of all-cause mortality by sex, single year of age, and calendar year of death during 1995–2014. Complete lifetables were available for all but one jurisdiction (Prince Edward Island, Canada), for which we used a Poisson model to interpolate 1-year intervals from an abridged (5-year) lifetable. We imputed missing adjacent years (a maximum of 2 years) using the last observation carried forward method. We supplemented missing years or ages, or both, in the lifetables for New Zealand with data from the Human Mortality Database.
We calculated survival estimates at 1 year and 5 years after diagnosis by age group (<75 years vs ≥75 years), time period of diagnosis (ie 1995–99, 2000–04, 2005–09, and 2010–14), and cancer site for each jurisdiction using Pohar Perme estimators and the user-written Stata command stnet.13, 14 We did age-standardised survival estimates using international cancer survival standard weights.15 We used the cohort approach for 1995–99, 2000–04, and 2005–09, and we used the period approach for patients diagnosed in 2010–14 (period window: 2012–14). In secondary analyses, we included cases that were diagnosed on the basis of death certificate or autopsy under the assumption that survival equalled 1 day. This assumption represents the most extreme scenario to assess the potential maximum (unrealistic) effect of inclusion of these cases.
We calculated trends in age-standardised cancer incidence and mortality for patients aged 25 years and older using the age-truncated World Standard Population data16 and expressed per 100 000 person-years. For incidence and mortality estimates, slightly different codes were used for ovarian (includes only C56) and lung cancer (includes C33–34 for mortality). Because previous analyses have highlighted the importance of survival differences by age, we also presented the results by broad age groups at diagnosis (<75 years vs ≥75 years). Additionally, we did sex-specific analyses for lung cancer because of its distinct patterns and trends in incidence and mortality reflecting differences in smoking prevalence in males and females.
All analyses were carried out using Stata version 14.2.
Role of the funding source
The funders had a role in writing of the report and had no role in study design, data collection, data analysis, or data interpretation. MA, MJR, AB, and IS had access to the raw data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Of the 3 890 934 cancer cases that were eligible for analysis in the 19 jurisdictions, 126 391 (3·2%) were excluded due to the cancer diagnosis only being based on a death certificate or at autopsy (77 578 [2·0%]); invalid or missing dates, age, or non-malignant behaviour of the cancer (4471 [0·1%]); the patient being younger than 15 years or older than 99 years at diagnosis (2438 [<0·1%]); and second or higher order cancers being present at the same site as the primary cancer (41 904 [1·1%]). Hence, 3 764 543 (96·8%) of eligible cancer cases were included in the analyses (table 1; appendix p 25).
Table 1.
Number of cancer cases identified and included in analysis 1995–2014 by cancer site and country
| Cases identified |
Exclusion criteria |
Cases included in analyses | ||||
|---|---|---|---|---|---|---|
| Diagnosed on the basis of death certificate or autopsy | Quality control* | Age <15 years or >99 years | Multiple cancers | |||
| Oesophagus | ||||||
| Australia | 15 313 | 193 (1·25%) | 77 (0·50%) | 3 (0·02%) | 9 (0·06%) | 15 032 (98·16%) |
| Canada | 23 560 | 309 (1·31%) | 12 (0·05%) | 7 (0·03%) | 43 (0·18%) | 23 189 (98·43%) |
| Denmark | 8296 | 94 (1·13%) | 0 | 2 (0·02%) | 4 (0·05%) | 8196 (98·79%) |
| Ireland | 6471 | 107 (1·65%) | 0 | 1 (0·02%) | 7 (0·11%) | 6356 (98·22%) |
| New Zealand | 4943 | 33 (0·67%) | 1 (0·02%) | 2 (0·04%) | 1 (0·02%) | 4906 (99·25%) |
| Norway | 3988 | 47 (1·18%) | 0 | 1 (0·03%) | 4 (0·10%) | 3936 (98·70%) |
| UK | 156 125 | 2137 (1·32%) | 5 (<0·01%) | 72 (0·05%) | 115 (0·07%) | 153 877 (98·56%) |
| Stomach | ||||||
| Australia | 26 282 | 294 (1·12%) | 124 (0·47%) | 12 (0·05%) | 51 (0·19%) | 25 801 (98·17%) |
| Canada | 43 370 | 607 (1·40%) | 17 (0·04%) | 10 (0·02%) | 93 (0·21%) | 42 643 (98·32%) |
| Denmark | 10 745 | 159 (1·48%) | 0 | 4 (0·04%) | 3 (0·03%) | 10 579 (98·46%) |
| Ireland | 9413 | 276 (2·93%) | 0 | 1 (0·01%) | 18 (0·19%) | 9118 (96·87%) |
| New Zealand | 7635 | 69 (0·90%) | 2 (0·03%) | 7 (0·09%) | 1 (0·01%) | 7556 (98·97%) |
| Norway | 11 255 | 134 (1·19%) | 0 | 4 (0·04%) | 18 (0·16%) | 11 099 (98·61%) |
| UK | 170 448 | 3454 (1·94%) | 16 (0·01%) | 94 (0·06%) | 216 (0·13%) | 166 812 (97·87%) |
| Colon | ||||||
| Australia | 126 281 | 1291 (1·02%) | 584 (0·46%) | 95 (0·08%) | 4244 (3·36%) | 120 067 (95·08%) |
| Canada | 210 013 | 2321 (1·11%) | 93 (0·04%) | 85 (0·04%) | 3433 (1·63%) | 204 081 (97·18%) |
| Denmark | 51 821 | 444 (0·86%) | 0 | 24 (0·05%) | 447 (0·86%) | 50 906 (98·23%) |
| Ireland | 29 026 | 618 (2·13%) | 1 (<0·01%) | 11 (0·04%) | 852 (2·94%) | 27 544 (94·89%) |
| New Zealand | 40 408 | 245 (0·61%) | 1 (<0·01%) | 13 (0·03%) | 58 (0·14%) | 40 091 (99·22%) |
| Norway | 48 748 | 540 (1·11%) | 116 (0·24%) | 23 (0·05%) | 824 (1·69%) | 47 245 (96·92%) |
| UK | 540 800 | 8955 (1·60%) | 45 (0·01%) | 638 (0·12%) | 14 249 (2·64%) | 517 231 (95·64%) |
| Rectum | ||||||
| Australia | 45 562 | 213 (0·47%) | 102 (0·22%) | 8 (0·02%) | 1195 (2·62%) | 44 044 (96·67%) |
| Canada | 66 449 | 416 (0·63%) | 22 (0·03%) | 20 (0·03%) | 1075 (1·62%) | 64 916 (97·69%) |
| Denmark | 26 173 | 172 (0·66%) | 0 | 7 (0·03%) | 290 (1·11%) | 25 704 (98·21%) |
| Ireland | 11 235 | 121 (1·08%) | 0 | 2 (0·02%) | 202 (1·80%) | 10 910 (97·11%) |
| New Zealand | 13 369 | 65 (0·49%) | 3 (0·02%) | 8 (0·06%) | 243 (1·82%) | 13 050 (97·61%) |
| Norway | 20 557 | 98 (0·48%) | 0 | 7 (0·03%) | 486 (2·36%) | 19 966 (97·13%) |
| UK | 210 073 | 2214 (1·02%) | 14 (0·01%) | 89 (0·04%) | 3232 (1·54%) | 204 586 (97·39%) |
| Pancreas | ||||||
| Australia | 29 636 | 1054 (3·56%) | 599 (2·02%) | 19 (0·06%) | 5 (0·02%) | 27 959 (94·34%) |
| Canada | 53 922 | 2116 (3·92%) | 42 (0·08%) | 34 (0·06%) | 12 (0·02%) | 51 718 (95·91%) |
| Denmark | 16 676 | 411 (2·46%) | 0 | 3 (0·02%) | 1 (0·01%) | 16 261 (97·51%) |
| Ireland | 7922 | 382 (4·82%) | 0 | 2 (0·03%) | 9 (0·11%) | 7529 (95·04%) |
| New Zealand | 8011 | 187 (2·33%) | 2 (0·02%) | 12 (0·15%) | 0 | 7810 (97·49%) |
| Norway | 13 188 | 487 (3·69%) | 0 | 4 (0·03%) | 3 (0·02%) | 12 694 (96·25%) |
| UK | 157 641 | 6623 (4·05%) | 15 (0·01%) | 110 (0·07%) | 70 (0·04%) | 151 064 (95·83%) |
| Lung | ||||||
| Australia | 119 915 | 2826 (2·36%) | 1234 (1·03%) | 36 (0·03%) | 727 (0·61%) | 115 092 (95·98%) |
| Canada | 304 284 | 6818 (2·24%) | 175 (0·06%) | 55 (0·02%) | 2445 (0·80%) | 294 791 (96·88%) |
| Denmark | 79 731 | 1591 (2·00%) | 0 | 7 (0·01%) | 140 (0·18%) | 77 993 (97·82%) |
| Ireland | 35 659 | 1200 (3·37%) | 4 (0·01%) | 7 (0·02%) | 306 (0·86%) | 34 142 (95·75%) |
| New Zealand | 35 666 | 720 (2·02%) | 2 (0·01%) | 8 (0·02%) | 14 (0·04%) | 34 922 (97·91%) |
| Norway | 47 872 | 938 (1·96%) | 0 | 8 (0·02%) | 321 (0·67%) | 46 605 (97·35%) |
| UK | 821 586 | 22 709 (2·63%) | 667 (0·08%) | 209 (0·03%) | 5567 (0·68%) | 793 535 (96·59%) |
| Ovary | ||||||
| Australia | 18 004 | 275 (1·53%) | 119 (0·66%) | 72 (0·40%) | 56 (0·31%) | 17 482 (97·10%) |
| Canada | 39 030 | 546 (1·40%) | 11 (0·03%) | 153 (0·39%) | 107 (0·27%) | 38 213 (97·91%) |
| Denmark | 11 810 | 105 (0·89%) | 0 | 17 (0·14%) | 64 (0·54%) | 11 624 (98·43%) |
| Ireland | 6517 | 147 (2·26%) | 1 (0·02%) | 15 (0·23%) | 22 (0·34%) | 6332 (97·16%) |
| New Zealand | 5796 | 47 (0·81%) | 1 (0·02%) | 29 (0·50%) | 12 (0·21%) | 5707 (98·46%) |
| Norway | 9960 | 116 (1·16%) | 16 (0·16%) | 25 (0·25%) | 36 (0·36%) | 9767 (98·06%) |
| UK | 129 748 | 2655 (1·98%) | 348 (0·27%) | 363 (0·28%) | 574 (0·44%) | 125 892 (97·03%) |
Data are n or n (%). Proportions are calculated using the number of cases identified as the denominator.
Data inconsistencies included invalid age, missing or incomplete dates, tumours with non-malignant behaviour, and tumours with invalid morphological or topographical codes.
In the most recent 5-year period of diagnosis (2010–14), the highest 1-year survival for most cancer sites was in Australia, followed by Canada and Norway (appendix pp 11–12). The lowest 1-year survival was observed for stomach, colon, rectal, and lung cancer in the UK; and for oesophageal cancer in Canada, pancreatic cancer in New Zealand, and ovarian cancer in Ireland. Similar patterns were observed for 5-year survival (table 2), with consistently higher survival in Australia than in the other countries, except for lung (Canada) and ovarian cancer (Norway), and lower survival in the UK, except for oesophageal (Denmark) and ovarian cancer (Ireland). Inclusion of cases that were diagnosed on the basis of death certificate or autopsy in secondary analyses did not substantially change the results (appendix pp 23–24). Using this scenario, 5-year survival estimates were reduced by a maximum of 0·6 percentage points across all countries in 2010–14.
Table 2.
Age-standardised 5-year net survival by cancer site, country, and period of diagnosis
| Australia* | Canada† | Denmark | Ireland‡ | New Zealand | Norway | UK§ | |
|---|---|---|---|---|---|---|---|
| Oesophagus | |||||||
| 1995–99 | 18·3% (16·9–19·9) | 13·5% (12·4–14·6) | 5·1% (3·9–6·6) | 10·9% (9·1–12·9) | 13·6% (11·2–16·1) | 8·7% (6·7–11·0) | 8·6% (8·2–9·0) |
| 2000–04 | 17·8% (16·5–19·2) | 14·2% (13·2–15·3) | 8·6% (7·2–10·1) | 12·8% (11·0–14·7) | 11·6% (9·6–13·9) | 9·0% (6·8–11·6) | 11·4% (11·0–11·8) |
| 2005–09 | 18·4% (17·1–19·8) | 14·8% (13·8–15·8) | 10·5% (9·0–12·0) | 17·2% (15·3–19·2) | 14·3% (12·1–16·6) | 13·2% (10·8–15·7) | 13·8% (13·4–14·2) |
| 2010–14 | 23·5% (21·6–25·4) | 16·3% (15·1–17·5) | 14·7% (12·1–17·5) | 21·9% (19·3–24·5) | 16·9% (14·3–19·7) | 19·4% (16·5–22·5) | 16·2% (15·7–16·7) |
| Absolute change | 5·2 | 2·8 | 9·6 | 11·0 | 3·3 | 10·7 | 7·6 |
| Stomach | |||||||
| 1995–99 | 25·7% (24·6–26·9) | 21·5% (20·6–22·4) | 14·0% (12·5–15·7) | 17·3% (15·5–19·1) | 21·3% (19·2–23·4) | 21·3% (19·5–23·1) | 14·1% (13·7–14·4) |
| 2000–04 | 28·1% (26·9–29·3) | 24·1% (23·2–25·1) | 15·2% (13·7–16·9) | 18·5% (16·7–20·4) | 23·7% (21·6–25·9) | 22·0% (20·2–23·9) | 16·0% (15·6–16·5) |
| 2005–09 | 29·2% (28·0–30·4) | 26·2% (25·3–27·1) | 15·7% (14·2–17·3) | 22·0% (20·2–23·9) | 24·0% (21·9–26·2) | 25·1% (23·1–27·2) | 19·0% (18·5–19·5) |
| 2010–14 | 32·8% (31·3–34·3) | 29·8% (28·6–30·9) | 22·6% (20·6–24·8) | 28·4% (26·1–30·9) | 24·5% (22·1–27·0) | 26·9% (24·6–29·3) | 20·8% (20·2–21·4) |
| Absolute change | 7·1 | 8·3 | 8·6 | 11·1 | 3·2 | 5·6 | 6·7 |
| Colon | |||||||
| 1995–99 | 59·6% (58·9–60·3) | 57·9% (57·3–58·4) | 49·1% (47·9–50·3) | 50·0% (48·5–51·6) | 59·3% (58·0–60·6) | 55·7% (54·4–57·0) | 47·0% (46·7–47·4) |
| 2000–04 | 63·5% (62·8–64·1) | 61·3% (60·7–61·8) | 53·2% (52·0–54·3) | 52·7% (51·3–54·2) | 60·3% (59·1–61·5) | 58·0% (56·8–59·1) | 51·0% (50·6–51·3) |
| 2005–09 | 67·1% (66·5–67·8) | 65·4% (64·9–65·9) | 57·5% (56·4–58·6) | 58·0% (56·7–59·3) | 61·6% (60·4–62·8) | 62·2% (61·1–63·3) | 55·3% (55·0–55·7) |
| 2010–14 | 70·8% (70·0–71·5) | 66·8% (66·2–67·4) | 65·7% (64·5–66·9) | 61·8% (60·2–63·3) | 62·1% (60·8–63·4) | 65·4% (64·2–66·5) | 58·9% (58·6–59·3) |
| Absolute change | 11·2 | 8·9 | 16·6 | 11·8 | 2·8 | 9·7 | 11·9 |
| Rectum | |||||||
| 1995–99 | 59·1% (57·9–60·3) | 57·5% (56·5–58·6) | 48·1% (46·4–49·8) | 47·6% (45·1–50·0) | 56·3% (54·0–58·6) | 57·3% (55·5–59·1) | 47·8% (47·3–48·4) |
| 2000–04 | 64·1% (63·0–65·1) | 61·6% (60·6–62·5) | 54·0% (52·3–55·7) | 51·0% (48·8–53·2) | 59·7% (57·5–61·8) | 61·5% (59·8–63·2) | 53·0% (52·4–53·5) |
| 2005–09 | 68·5% (67·5–69·5) | 65·4% (64·5–66·2) | 60·7% (59·2–62·1) | 57·1% (54·9–59·2) | 62·4% (60·4–64·4) | 65·4% (63·7–67·0) | 57·0% (56·5–57·5) |
| 2010–14 | 70·8% (69·6–72·0) | 67·0% (66·0–68·1) | 69·1% (67·4–70·8) | 62·4% (59·9–64·9) | 65·4% (63·1–67·5) | 68·8% (66·9–70·6) | 62·1% (61·5–62·6) |
| Absolute change | 11·7 | 9·5 | 21·0 | 14·8 | 9·1 | 11·5 | 14·3 |
| Pancreas | |||||||
| 1995–99 | 6·4% (5·7–7·2) | 7·0% (6·4–7·5) | 3·2% (2·5–4·0) | 5·6% (4·4–7·0) | 8·8% (7·3–10·6) | 4·4% (3·5–5·5) | 3·3% (3·0–3·5) |
| 2000–04 | 7·8% (7·1–8·6) | 7·8% (7·2–8·4) | 3·8% (3·1–4·6) | 5·9% (4·7–7·3) | 6·9% (5·6–8·5) | 4·7% (3·8–5·7) | 4·0% (3·7–4·3) |
| 2005–09 | 9·2% (8·5–10·0) | 9·6% (9·1–10·2) | 5·7% (4·9–6·5) | 7·0% (5·8–8·4) | 7·3% (6·1–8·7) | 5·9% (5·0–7·0) | 5·5% (5·2–5·8) |
| 2010–14 | 14·6% (13·5–15·7) | 11·1% (10·4–11·8) | 9·6% (8·5–10·9) | 9·6% (7·8–11·6) | 8·2% (6·8–9·7) | 9·9% (8·5–11·4) | 7·9% (7·6–8·3) |
| Absolute change | 8·2 | 4·1 | 6·4 | 4·0 | −0·6 | 5·5 | 4·6 |
| Lung | |||||||
| 1995–99 | 13·3% (12·9–13·8) | 15·4% (15·1–15·7) | 8·2% (7·7–8·7) | 9·3% (8·5–10·1) | 11·5% (10·7–12·4) | 10·8% (10·1–11·6) | 7·2% (7·0–7·3) |
| 2000–04 | 14·9% (14·5–15·4) | 16·0% (15·7–16·4) | 9·7% (9·2–10·2) | 10·1% (9·3–10·9) | 10·9% (10·2–11·7) | 11·8% (11·1–12·5) | 8·3% (8·2–8·5) |
| 2005–09 | 17·3% (16·8–17·8) | 18·5% (18·2–18·8) | 12·4% (11·9–13·0) | 13·4% (12·7–14·2) | 11·9% (11·1–12·7) | 15·4% (14·7–16·2) | 10·0% (9·8–10·2) |
| 2010–14 | 21·4% (20·8–22·0) | 21·7% (21·3–22·2) | 18·9% (18·1–19·6) | 19·8% (18·7–20·9) | 15·5% (14·5–16·5) | 20·4% (19·4–21·4) | 14·7% (14·5–15·0) |
| Absolute change | 8·1 | 6·3 | 10·7 | 10·5 | 4·0 | 9·6 | 7·5 |
| Ovary | |||||||
| 1995–99 | 35·5% (33·9–37·1) | 35·5% (34·4–36·7) | 32·0% (30·0–33·9) | 27·8% (25·2–30·5) | 31·9% (29·0–34·8) | 37·0% (34·8–39·3) | 27·3% (26·8–27·9) |
| 2000–04 | 36·2% (34·6–37·8) | 36·4% (35·3–37·5) | 34·2% (32·3–36·1) | 29·6% (27·2–32·1) | 33·5% (30·7–36·4) | 40·6% (38·4–42·8) | 29·0% (28·4–29·5) |
| 2005–09 | 40·1% (38·6–41·7) | 39·8% (38·7–40·9) | 38·3% (36·3–40·2) | 31·1% (28·7–33·5) | 32·7% (30·0–35·4) | 41·3% (39·1–43·5) | 32·1% (31·6–32·7) |
| 2010–14 | 43·2% (41·3–45·1) | 40·3% (39·0–41·6) | 42·1% (39·8–44·5) | 36·0% (33·0–38·9) | 36·3% (33·3–39·4) | 46·2% (43·6–48·8) | 37·1% (36·5–37·8) |
| Absolute change | 7·7 | 4·8 | 10·1 | 8·2 | 4·4 | 9·2 | 9·8 |
Data are 5-year age-standardised net survival estimate (95% CI) and absolute change in percentage points. Absolute change is between 1995–99 and 2010–14.
Australia includes New South Wales (1995–2012), Victoria, and Western Australia.
Canada includes Alberta, British Columbia, Manitoba, New Brunswick, Nova Scotia, Ontario, Prince Edward Island, and Saskatchewan.
Ireland (1995–2013).
The UK includes its four consituents countries: England, Scotland, Wales, and Northern Ireland.
Differences in 1-year and 5-year survival across jurisdictions within countries were observed over time, with large variations for 2010–14 found in 5-year survival for oesophageal cancer in Australia (26% in Victoria vs 19% in New South Wales), pancreatic cancer in Canada (13% in Manitoba vs 7% in Nova Scotia), and colon cancer in the UK (63% in Northern Ireland vs 57% in Wales; appendix pp 17–22).
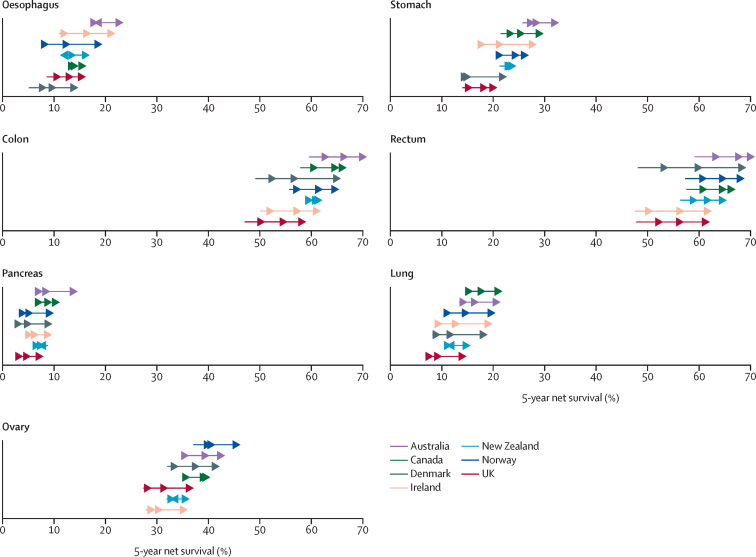
Cancer survival increased in all seven countries over the 20-year study period, and improvements, at least in relative terms, were most substantial for the cancer sites associated with a generally poor prognosis (oesophagus, stomach, pancreas, and lung; figure 1), and for patients younger than 75 years at diagnosis (table 3; appendix pp 13–14). The most pronounced increases in 5-year survival were observed in Denmark for cancers of the colon, with a 16·6 percentage point absolute increase, and rectum, with a 21 percentage point absolute increase, between the 1995–99 and 2010–14 time periods (table 2, figure 1). The survival gap between countries narrowed over time for 1-year survival for all cancers except pancreatic cancer (because of substantial increases in survival in all countries), whereas for 5-year survival the survival gap only narrowed for oesophageal and rectal cancer (table 2). Differences in 5-year survival across countries were greater among patients aged 75 years and older than those younger than 75 years, and the survival gap widened for ovarian cancer in those older than 75 years over the 20-year period but remained fairly stable for other cancer sites (Table 3, Table 4).
Figure 1.
Age-standardised 5-year net survival by site, country, and period of diagnosis, 1995–2014
Age-standardised net survival is for patients aged 15–99 years at diagnosis. Beginning of arrow denotes estimates for 1995–99 and arrow heads from left to right refer to 2000–04, 2005–09, and 2010–14 estimates. Australia includes New South Wales (1995–2012), Victoria, and Western Australia; Canada includes Alberta, British Columbia, Manitoba, New Brunswick, Nova Scotia, Ontario, Prince Edward Island, and Saskatchewan; Ireland (1995–2013); the UK includes its four constituent countries: England, Scotland, Wales, and Northern Ireland; all other countries with national data (1995–2014).
Table 3.
Age-standardised 5-year net survival by cancer site, country and period of diagnosis in patients aged younger than 75 years
| Australia* | Canada† | Denmark | Ireland‡ | New Zealand | Norway | UK§ | |
|---|---|---|---|---|---|---|---|
| Oesophagus | |||||||
| 1995–99 | 21·4% (19·5–23·4) | 15·4% (14·0–16·8) | 6·6% (5·1–8·3) | 12·9% (10·6–15·4) | 15·6% (12·6–19·0) | 10·0% (7·4–13·0) | 10·7% (10·2–11·2) |
| 2000–04 | 21·1% (19·3–22·9) | 16·5% (15·2–17·9) | 10·4% (8·6–12·4) | 15·7% (13·3–18·2) | 15·0% (12·1–18·1) | 11·3% (8·3–14·9) | 14·2% (13·7–14·8) |
| 2005–09 | 21·5% (19·7–23·3) | 16·9% (15·6–18·2) | 13·2% (11·3–15·2) | 20·8% (18·3–23·4) | 17·8% (14·8–21·0) | 17·0% (13·9–20·4) | 17·1% (16·6–17·7) |
| 2010–14 | 26·9% (24·5–29·4) | 18·4% (17·0–20·0) | 17·3% (14·0–20·9) | 27·0% (23·7–30·5) | 20·9% (17·4–24·7) | 24·4% (20·6–28·3) | 19·6% (19·0–20·3) |
| Absolute change | 5·5 | 3·0 | 10·7 | 14·1 | 5·3 | 14·4 | 8·9 |
| Stomach | |||||||
| 1995–99 | 29·1% (27·6–30·6) | 23·3% (22·1–24·4) | 16·3% (14·4–18·3) | 19·7% (17·5–22·0) | 23·4% (20·8–26·0) | 23·5% (21·3–25·8) | 16·5% (16·0–17·0) |
| 2000–04 | 31·7% (30·2–33·2) | 26·8% (25·7–28·0) | 17·7% (15·7–19·7) | 21·0% (18·7–23·3) | 27·2% (24·6–30·0) | 25·2% (22·8–27·6) | 18·9% (18·3–19·5) |
| 2005–09 | 32·9% (31·3–34·4) | 28·6% (27·4–29·7) | 18·7% (16·8–20·6) | 26·4% (24·0–28·8) | 27·0% (24·4–29·7) | 28·3% (25·7–30·9) | 22·7% (22·0–23·3) |
| 2010–14 | 37·4% (35·5–39·3) | 32·9% (31·5–34·3) | 25·9% (23·3–28·5) | 32·8% (29·8–35·8) | 29·1% (26·1–32·3) | 30·5% (27·6–33·5) | 24·0% (23·3–24·8) |
| Absolute change | 8·3 | 9·6 | 9·6 | 13·1 | 5·7 | 7·0 | 7·5 |
| Colon | |||||||
| 1995–99 | 62·1% (61·3–62·9) | 59·0% (58·3–59·7) | 50·2% (48·8–51·6) | 52·4% (50·6–54·2) | 59·4% (58·0–60·8) | 57·7% (56·2–59·2) | 50·6% (50·1–51·0) |
| 2000–04 | 66·3% (65·5–67·0) | 63·2% (62·6–63·8) | 54·0% (52·6–55·3) | 55·7% (54·0–57·3) | 61·0% (59·6–62·4) | 60·3% (58·8–61·7) | 54·7% (54·3–55·2) |
| 2005–09 | 69·8% (69·1–70·5) | 67·9% (67·4–68·5) | 60·2% (58·9–61·4) | 62·0% (60·5–63·5) | 62·9% (61·5–64·3) | 64·1% (62·8–65·4) | 59·6% (59·2–60·0) |
| 2010–14 | 73·3% (72·5–74·1) | 69·6% (69·0–70·3) | 66·4% (65·0–67·8) | 66·5% (64·7–68·2) | 64·5% (62·9–66·0) | 67·5% (66·1–68·8) | 64·2% (63·7–64·6) |
| Absolute change | 11·2 | 10·6 | 16·2 | 14·1 | 5·1 | 9·8 | 13·6 |
| Rectum | |||||||
| 1995–99 | 62·6% (61·3–63·8) | 60·8% (59·6–61·9) | 51·3% (49·4–53·2) | 49·6% (47·0–52·2) | 58·5% (56·0–61·0) | 61·4% (59·3–63·5) | 53·0% (52·4–53·7) |
| 2000–04 | 68·3% (67·1–69·4) | 65·1% (64·1–66·1) | 57·9% (56·1–59·7) | 56·6% (54·1–59·0) | 62·9% (60·5–65·1) | 64·9% (63·0–66·8) | 58·5% (57·9–59·1) |
| 2005–09 | 72·6% (71·5–73·6) | 69·8% (68·9–70·7) | 65·4% (63·8–67·0) | 63·4% (61·1–65·7) | 66·6% (64·4–68·7) | 69·7% (67·9–71·5) | 63·3% (62·7–63·9) |
| 2010–14 | 75·0% (73·7–76·2) | 72·9% (71·9–73·9) | 72·6% (70·8–74·2) | 67·9% (65·3–70·3) | 69·3% (67·0–71·5) | 72·1% (70·1–73·9) | 68·5% (67·9–69·1) |
| Absolute change | 12·4 | 12·1 | 21·3 | 18·3 | 10·8 | 10·7 | 15·5 |
| Pancreas | |||||||
| 1995–99 | 7·6% (6·7–8·7) | 8·1% (7·4–8·9) | 3·5% (2·7–4·5) | 6·3% (4·8–8·2) | 10·1% (8·1–12·4) | 5·4% (4·2–6·8) | 4·1% (3·7–4·4) |
| 2000–04 | 9·4% (8·5–10·5) | 9·4% (8·7–10·1) | 4·9% (4·0–6·0) | 6·3% (4·8–8·1) | 8·2% (6·4–10·2) | 5·7% (4·6–7·1) | 5·1% (4·7–5·4) |
| 2005–09 | 11·5% (10·5–12·5) | 11·5% (10·7–12·2) | 7·4% (6·4–8·6) | 8·0% (6·5–9·8) | 9·4% (7·7–11·3) | 7·2% (5·8–8·6) | 7·0% (6·6–7·4) |
| 2010–14 | 18·1% (16·7–19·6) | 14·1% (13·2–15·1) | 12·2% (10·7–13·8) | 11·7% (9·3–14·5) | 11·2% (9·2–13·4) | 12·3% (10·4–14·3) | 10·0% (9·5–10·5) |
| Absolute change | 10·5 | 6·0 | 8·7 | 5·4 | 1·1 | 6·9 | 5·9 |
| Lung | |||||||
| 1995–99 | 15·8% (15·2–16·4) | 17·6% (17·2–18·0) | 10·1% (9·5–10·7) | 11·1% (10·1–12·1) | 13·0% (11·9–14·0) | 13·3% (12·4–14·2) | 8·9% (8·7–9·1) |
| 2000–04 | 17·3% (16·7–17·9) | 18·3% (17·9–18·7) | 11·7% (11·1–12·3) | 12·2% (11·2–13·2) | 12·9% (11·9–13·9) | 14·3% (13·5–15·3) | 10·2% (9·9–10·4) |
| 2005–09 | 20·2% (19·6–20·8) | 21·2% (20·8–21·6) | 14·9% (14·2–15·6) | 16·3% (15·3–17·4) | 14·3% (13·3–15·3) | 18·8% (17·8–19·8) | 12·1% (11·9–12·3) |
| 2010–14 | 24·6% (23·8–25·4) | 24·8% (24·2–25·4) | 21·3% (20·3–22·2) | 23·5% (22·1–25·0) | 18·8% (17·5–20·2) | 23·9% (22·6–25·1) | 17·4% (17·1–17·8) |
| Absolute change | 8·8 | 7·2 | 11·2 | 12·4 | 5·8 | 10·6 | 8·5 |
| Ovary | |||||||
| 1995–99 | 43·3% (41·4–45·2) | 41·2% (39·9–42·5) | 37·3% (35·1–39·4) | 33·6% (30·6–36·6) | 37·4% (34·0–40·7) | 44·5% (41·9–47·0) | 32·9% (32·3–33·5) |
| 2000–04 | 43·5% (41·6–45·4) | 43·2% (42·0–44·5) | 41·0% (38·8–43·1) | 35·7% (32·8–38·5) | 40·7% (37·3–44·0) | 48·1% (45·6–50·7) | 35·5% (34·9–36·2) |
| 2005–09 | 48·0% (46·2–49·7) | 47·7% (46·4–48·9) | 45·6% (43·3–47·8) | 38·9% (36·1–41·7) | 40·8% (37·6–43·9) | 49·2% (46·6–51·7) | 39·2% (38·5–39·9) |
| 2010–14 | 52·1% (50·0–54·1) | 49·3% (47·8–50·8) | 48·8% (46·2–51·3) | 44·8% (41·4–48·2) | 43·3% (39·8–46·8) | 54·5% (51·7–57·3) | 44·9% (44·1–45·7) |
| Absolute change | 8·8 | 8·1 | 11·5 | 11·2 | 5·9 | 10·0 | 12·0 |
Data are 5-year age-standardised net survival estimate (95% CI) and absolute change in percentage points. Absolute change is between 1995–99 and 2010–14.
Australia includes New South Wales (1995–2012), Victoria, and Western Australia.
Canada includes Alberta, British Columbia, Manitoba, New Brunswick, Nova Scotia, Ontario, Prince Edward Island and Saskatchewan.
Ireland (1995–2013).
The UK includes its four consituents countries: England, Scotland, Wales, and Northern Ireland.
Table 4.
Age-standardised 5-year net survival by cancer site, country and period of diagnosis in patients aged 75 years and older
| Australia* | Canada† | Denmark | Ireland‡ | New Zealand | Norway | UK§ | |
|---|---|---|---|---|---|---|---|
| Oesophagus | |||||||
| 1995–99 | 10·9% (8·9–13·1) | 8·8% (7·0–10·8) | 1·5% (0·4–4·6) | 6·0% (3·6–9·2) | 8·5% (5·2–12·6) | 5·4% (2·8–9·4) | 3·4% (3·0–3·8) |
| 2000–04 | 9·8% (8·1–11·8) | 8·6% (7·0–10·3) | 4·1% (2·4–6·5) | 5·7% (3·7–8·3) | 3·4% (1·8–5·7) | 3·4% (1·6–6·3) | 4·4% (4·0–4·8) |
| 2005–09 | 11·1% (9·4–13·0) | 9·6% (8·1–11·3) | 3·8% (2·2–6·1) | 8·4% (6·0–11·3) | 5·7% (3·7–8·3) | 3·7% (1·8–6·6) | 5·6% (5·1–6·0) |
| 2010–14 | 14·8% (12·3–17·5) | 10·9% (9·0–13·0) | 8·4% (4·9–13·0) | 9·4% (6·4–13·1) | 6·7% (4·2–10·0) | 6·0% (3·0–10·3) | 7·5% (7·0–8·1) |
| Absolute change | 3·9 | 2·1 | 6·9 | 3·4 | −1·8 | 0·6 | 4·1 |
| Stomach | |||||||
| 1995–99 | 17·5% (15·6–19·5) | 17·1% (15·5–18·8) | 8·6% (6·2–11·4) | 11·3% (8·4–14·6) | 16·1% (12·8–19·8) | 15·9% (13·5–18·4) | 8·1% (7·6–8·6) |
| 2000–04 | 19·3% (17·4–21·3) | 17·5% (16·0–19·1) | 9·3% (6·9–12·1) | 12·5% (9·7–15·7) | 15·2% (11·9–18·9) | 14·2% (11·9–16·7) | 9·0% (8·5–9·5) |
| 2005–09 | 20·2% (18·2–22·1) | 20·4% (18·8–22·0) | 8·5% (6·4–11·0) | 11·4% (9·0–14·2) | 16·8% (13·4–20·6) | 17·4% (14·6–20·5) | 9·9% (9·4–10·5) |
| 2010–14 | 21·5% (19·1–24·0) | 22·1% (20·2–24·0) | 14·7% (11·5–18·3) | 17·8% (14·2–21·7) | 13·1% (9·8–16·8) | 18·1% (14·7–21·9) | 12·6% (11·9–13·3) |
| Absolute change | 4·0 | 5·0 | 6·1 | 6·5 | −3·0 | 2·2 | 4·5 |
| Colon | |||||||
| 1995–99 | 53·4% (51·9–54·9) | 55·1% (53·9–56·3) | 46·4% (44·1–48·7) | 44·3% (40·9–47·5) | 59·0% (56·1–61·8) | 50·8% (48·4–53·1) | 38·4% (37·8–39·0) |
| 2000–04 | 56·6% (55·2–57·9) | 56·6% (55·5–57·6) | 51·1% (48·8–53·4) | 45·6% (42·7–48·5) | 58·5% (56·0–61·0) | 52·4% (50·2–54·5) | 41·8% (41·2–42·4) |
| 2005–09 | 60·6% (59·3–61·9) | 59·2% (58·2–60·2) | 50·9% (48·7–53·1) | 48·2% (45·5–50·8) | 58·4% (56·0–60·7) | 57·7% (55·6–59·7) | 45·0% (44·4–45·6) |
| 2010–14 | 64·5% (63·0–66·1) | 59·9% (58·7–61·1) | 64·3% (61·6–66·8) | 50·5% (47·4–53·6) | 56·3% (53·8–58·7) | 60·1% (57·7–62·4) | 46·1% (45·5–46·8) |
| Absolute change | 11·1 | 4·8 | 17·9 | 6·2 | −2·7 | 9·3 | 7·7 |
| Rectum | |||||||
| 1995–99 | 50·8% (48·0–53·4) | 49·7% (47·3–52·0) | 40·3% (36·8–43·8) | 42·6% (37·0–48·1) | 51·0% (45·6–56·2) | 47·2% (43·7–50·6) | 35·1% (34·0–36·1) |
| 2000–04 | 53·8% (51·4–56·2) | 53·0% (50·8–55·1) | 44·5% (40·8–48·0) | 37·4% (32·6–42·2) | 52·0% (47·0–56·8) | 53·2% (49·7–56·6) | 39·5% (38·5–40·5) |
| 2005–09 | 58·6% (56·1–60·9) | 54·6% (52·6–56·6) | 49·1% (45·9–52·3) | 41·5% (37·0–46·0) | 52·2% (47·6–56·6) | 54·9% (51·2–58·4) | 41·7% (40·7–42·7) |
| 2010–14 | 60·7% (57·7–63·5) | 53·4% (51·1–55·6) | 60·7% (56·7–64·5) | 49·4% (43·7–54·9) | 55·7% (50·3–60·7) | 60·9% (56·5–65·0) | 46·2% (45·0–47·3) |
| Absolute change | 9·9 | 3·7 | 20·4 | 6·8 | 4·7 | 13·7 | 11·1 |
| Pancreas | |||||||
| 1995–99 | 3·3% (2·5–4·2) | 4·0% (3·3–4·9) | 2·3% (1·3–3·8) | 3·8% (2·1–6·1) | 5·6% (3·5–8·5) | 2·1% (1·2–3·5) | 1·3% (1·1–1·6) |
| 2000–04 | 3·7% (2·9–4·7) | 3·9% (3·2–4·6) | 1·1% (0·6–2·0) | 4·8% (3·1–7·1) | 4·0% (2·3–6·3) | 2·0% (1·2–3·1) | 1·3% (1·1–1·5) |
| 2005–09 | 3·8% (3·1–4·6) | 5·1% (4·4–5·9) | 1·4% (0·8–2·3) | 4·5% (3·0–6·5) | 2·2% (1·2–3·7) | 3·0% (2·0–4·2) | 1·9% (1·6–2·1) |
| 2010–14 | 6·0% (4·9–7·2) | 3·9% (3·2–4·6) | 3·2% (2·0–4·8) | 4·3% (2·6–6·5) | 1·3% (0·6–2·4) | 4·1% (2·7–5·8) | 2·7% (2·4–3·1) |
| Absolute change | 2·7 | −0·1 | 0·9 | 0·5 | −4·3 | 2·0 | 1·4 |
| Lung | |||||||
| 1995–99 | 7·3% (6·6–8·0) | 10·0% (9·5–10·6) | 3·4% (2·7–4·2) | 5·0% (3·9–6·3) | 7·9% (6·6–9·4) | 4·8% (3·9–5·9) | 3·0% (2·8–3·1) |
| 2000–04 | 9·1% (8·5–9·8) | 10·6% (10·1–11·1) | 4·9% (4·1–5·8) | 4·9% (3·9–5·9) | 6·1% (5·1–7·3) | 5·6% (4·7–6·6) | 3·8% (3·6–4·0) |
| 2005–09 | 10·3% (9·6–10·9) | 11·9% (11·4–12·3) | 6·5% (5·7–7·3) | 6·3% (5·3–7·4) | 6·1% (5·1–7·2) | 7·1% (6·2–8·1) | 4·9% (4·7–5·1) |
| 2010–14 | 13·4% (12·5–14·3) | 14·1% (13·5–14·7) | 12·6% (11·3–13·8) | 10·4% (8·9–12·0) | 7·4% (6·2–8·8) | 11·7% (10·4–13·1) | 7·8% (7·5–8·1) |
| Absolute change | 6·1 | 4·1 | 9·2 | 5·4 | −0·5 | 6·9 | 4·8 |
| Ovary | |||||||
| 1995–99 | 16·4% (13·8–19·2) | 21·6% (19·4–23·9) | 19·0% (15·1–23·3) | 13·7% (9·3–19·0) | 18·4% (13·1–24·3) | 18·8% (14·8–23·2) | 13·7% (12·7–14·6) |
| 2000–04 | 18·2% (15·7–20·9) | 19·6% (17·8–21·6) | 17·5% (14·1–21·2) | 14·9% (10·7–19·7) | 16·0% (11·7–21·1) | 22·1% (18·4–26·0) | 12·9% (12·1–13·8) |
| 2005–09 | 20·9% (18·3–23·7) | 20·6% (18·7–22·5) | 20·4% (16·8–24·2) | 12·0% (8·2–16·5) | 12·8% (8·8–17·6) | 22·0% (18·3–25·9) | 14·8% (14·0–15·7) |
| 2010–14 | 21·8% (18·5–25·2) | 18·9% (16·7–21·1) | 26·2% (21·7–30·9) | 14·3% (10·2–19·1) | 17·5% (12·2–23·5) | 26·5% (21·7–31·6) | 17·7% (16·7–18·9) |
| Absolute change | 5·4 | −2·7 | 7·2 | 0·6 | −0·9 | 7·7 | 4·0 |
Data are 5-year age-standardised net survival estimate (95% CI) and absolute change in percentage points. Absolute change is between 1995–99 and 2010–14.
Australia includes New South Wales (1995–2012), Victoria, and Western Australia.
Canada includes Alberta, British Columbia, Manitoba, New Brunswick, Nova Scotia, Ontario, Prince Edward Island and Saskatchewan.
Ireland (1995–2013).
The UK includes its four consituents countries: England, Scotland, Wales, and Northern Ireland.
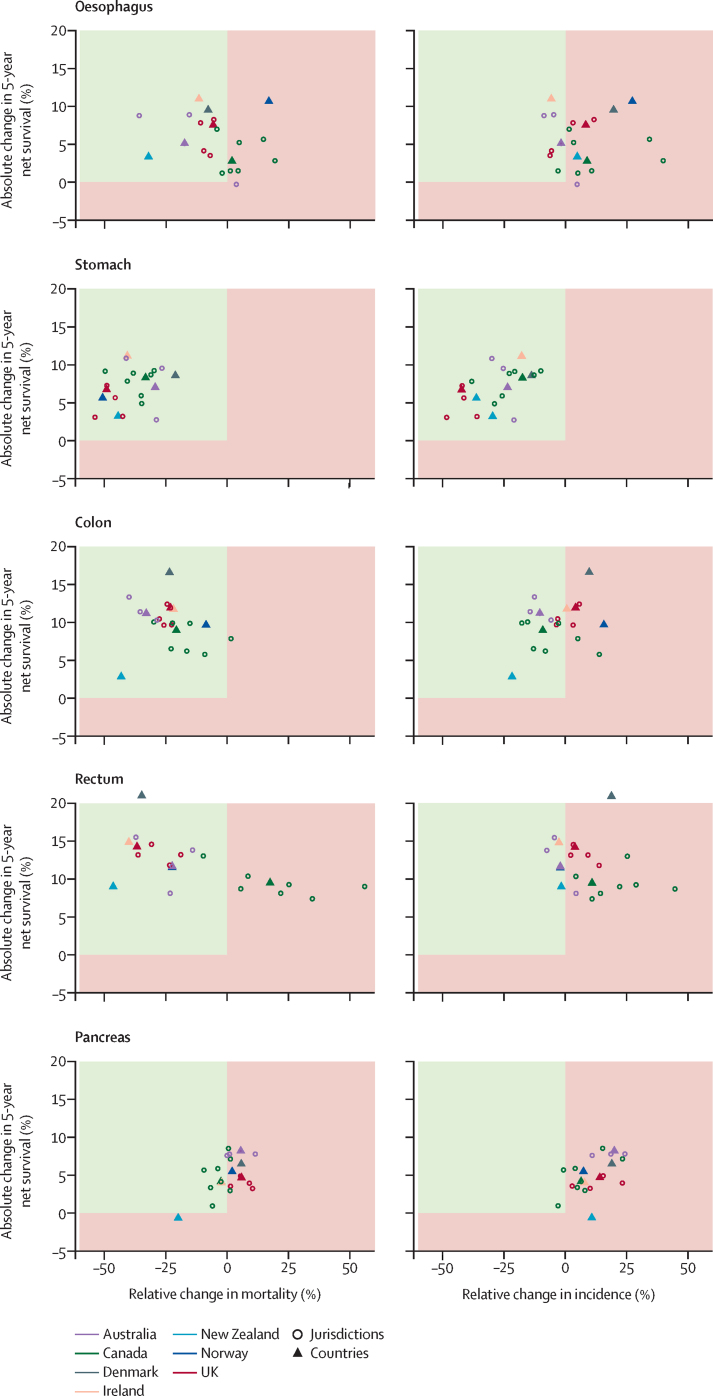
Both 1-year and 5-year survival from oesophageal cancer increased substantially in all countries among patients diagnosed in the period 2010–14 compared with those diagnosed in 1995–99 (table 2; appendix pp 11–12). Comparing the periods 1995–99 and 2010–14, 5-year survival from oesophageal cancer increased by up to 11 percentage points (in Ireland and Norway), which was more pronounced in those younger than 75 years at diagnosis (up to 14 percentage points in Ireland and Norway) than in those aged 75 years and older (up to 7 percentage points in Denmark; Table 3, Table 4). Although mortality has been stable or decreasing across countries over time (except in Norway), incidence has increased (except for in Ireland and Australia; figure 2; appendix p 30).
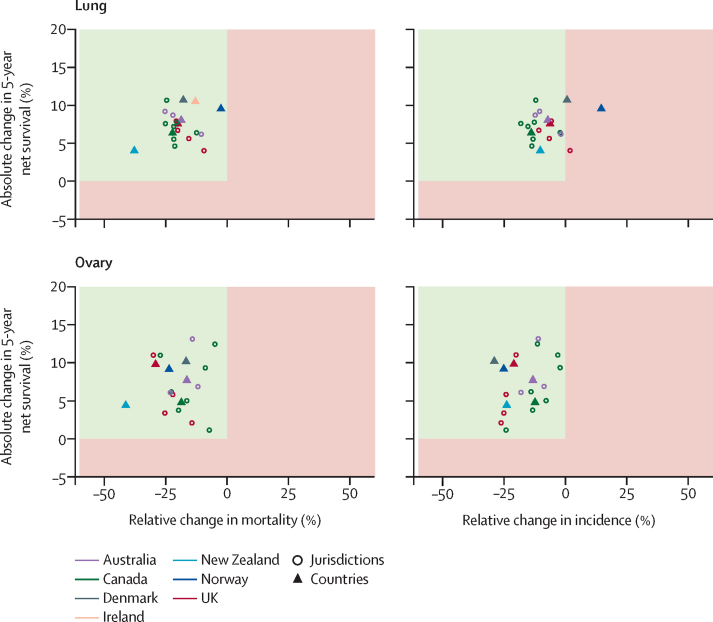
Figure 2.
Changes in age-standardised 5-year net survival against changes in mortality and incidence, 2010–14 compared with 1995–99
Age-standardised net survival is for patients aged 15–99 years at diagnosis, and age-standardised incidence and mortality are for patients aged 25 years and older at diagnosis. Increases in survival paralleled by decreases in incidence and mortality are the optimum scenario and show true progress (green upper left quadrant). Other scenarios, such as increasing survival and decreasing mortality, accompanied by increasing incidence, point towards partial progress (red quadrants). Australia includes New South Wales (1995–2012), Victoria and Western Australia; Canada includes Alberta, British Columbia, Manitoba, New Brunswick, Nova Scotia, Ontario, and Saskatchewan; Ireland (1995–2013); the UK includes its four constituent countries: England, Scotland, Wales, and Northern Ireland; and all other countries with national data (1995–2014). Estimates for Prince Edward Island (Canada) are not shown because of large fluctuations in rates compared with the small number of cases.
Improvements in survival from stomach cancer were equally uniform across countries (table 2; appendix pp 11–12). Increases were more pronounced among patients younger than 75 years, and most notably in Ireland, where 5-year survival increased from 20% to 33% over the 20-year period (table 3). All countries saw concurrent decreases in both incidence and mortality since 1995 (figure 2; appendix p 31).
5-year survival from colon cancer increased in all countries over 1995–2014 (table 2). The increases were greater in patients younger than 75 years than among those older than 75 years, with an at least 13 percentage point increase observed in Ireland, Denmark, and the UK (table 3). Although colon cancer mortality decreased across the study period in all countries, decreases in incidence were less pronounced and varied substantially by country (figure 2). Slight increases in incidence were observed in Norway, Denmark, and the UK (figure 2; appendix p 32).
Cancers of the rectum had the highest survival among the cancers studied, up to 90% for 1-year survival and 71% for 5-year survival in 2010–14, both in Australia (appendix pp 11–12), with the most substantial increases in survival seen among patients younger than 75 years (table 3; appendix pp 13–14). Overall, 5-year rectal cancer survival increased substantially over the 1995–2014 period, by more than 14 percentage points in Denmark, Ireland, and the UK. Considerable variations in incidence and mortality were seen across countries; although mortality decreased in all countries except Canada, incidence remained largely stable, except in Canada and Denmark, where incidence increased slightly (figure 2; appendix p 33).
Pancreatic cancer had the lowest survival among the studied cancer sites, although survival increases were observed over time in most countries except New Zealand (table 2; appendix pp 11–12). Slight increases in pancreatic cancer incidence were seen in all countries, although no appreciable changes were observed in mortality over time (figure 2; appendix p 34).
For lung cancer, although survival was highest in Canada, with a 5-year survival of 22% in 2010–14, survival increased most in Denmark, Ireland, and Norway, with a 10–11 percentage point increase when comparing the periods 1995–99 with 2010–14 (table 2; appendix p 35). Survival was highest and showed the largest absolute increase in patients younger than 75 years, particularly in Denmark, Ireland, and Norway. Although survival improved for both sexes, incidence, and mortality varied by sex (appendix p 29); both incidence and mortality decreased in males, whereas in females incidence increased and mortality remained stable.
The highest 5-year survival for ovarian cancer was observed in Norway, followed by Australia and Denmark, with survival ranging from 36% to 46% across countries for diagnoses in 2010–14 (table 2). Following considerable absolute improvements in survival over 1995–2014 across all countries, including Ireland and the UK, the most recent survival estimates in these countries, and in New Zealand, remained relatively low at around 36–37%. Decreases in both incidence and mortality were observed over the same time period in all countries (figure 2; appendix p 36).
All results are also available through the SURVMARK-2 interactive online tool.
Discussion
This first ICBP SURVMARK-2 study has shown that cancer survival has continued to improve across seven high-income countries over the period 1995–2014. Compared with the first phase of ICBP, the number of cancer sites analysed has been expanded, including lung, colon, rectal, ovarian, oesophageal, stomach, and pancreatic cancer, and survival improvements have been notable for cancers associated with worse prognoses and in patients diagnosed when younger than 75 years. Cancer survival was generally higher in Australia, Canada, and Norway. International differences narrowed across the study period for 1-year survival after diagnosis for all cancers except pancreatic cancer, whereas at 5 years after diagnosis differences across countries only narrowed for oesophageal and rectal cancer. Our combined description of trends in incidence, mortality, and survival suggests substantial progress has been made in cancer control across these seven countries for stomach, colon, lung (in males), and ovarian cancer.
The uniform improvements in cancer survival in this study are probably the direct consequence of major health care reforms and technological advances that have enabled earlier diagnosis, more effective and tailored treatment, and better patient management than in previous periods. For example, rectal cancer had one of the most substantial increases in 5-year net survival over time, increasing between 9 and 21 percentage points in all seven countries. Improvements in surgical techniques, including the implementation of total mesorectal excision and new guidelines that include preoperative radiotherapy are among the key changes that have improved patient outcomes.17 Improvements in survival were largely seen among younger patients (aged <75 years) and might relate to the relatively wider access to adjuvant chemotherapy and ability of these patients to tolerate more aggressive treatments than older age groups. Additionally, better diagnosis and staging with new technologies such as PET-CT imaging, alongside greater precision in the selection of patients for targeted therapies on the basis of molecular markers, have probably contributed. In Denmark, the substantial increases in survival seen across all cancers, particularly colorectal cancer, might have partly resulted from national health-care reforms in 2007 when cancer became regarded as an acute life-threatening disease, after which cancer-specific pathways for diagnosis and treatment were accelerated, including reduced waiting times in hospitals and large investments in radiotherapy.18, 19 International differences in access to diagnostic services, screening practices, treatments, patient pathways, and health-care systems, and their effect on survival are being further explored in ICBP studies.
Differences in coding and classification systems and cancer registration practices in different jurisdictions and countries are potential challenges to international population-based comparisons of cancer survival. Taking the example of staging—a principle determinant of cancer survival—the implementation of common recording systems has been hindered by variations in the availability of clinical information to determine the stage of cancer, the application of different staging guidelines, and differences in the pace of change in diagnostic and treatment practice.20 The validity of the definition of date of diagnosis, accuracy of ascertainment of death, and extent of trace-back of cases first notified by death certificates—eg. tracing notifications back to the source capable of confirming (or contradicting) the death certificate statement—are all possible source of artifacts that might vary by registry and differentially affect survival estimates.10, 21, 22 However, previous studies have shown that only an implausibly high level of missed cases or delayed (later or incorrectly assigned) dates of diagnosis would lead to a substantial effect on cancer survival estimates.21, 23
Cancer registries included in ICBP SURVMARK-2 are covered by reasonably complete and efficient mortality measurement systems. Still, an earlier ICBP study10 has shown that local registration practices, including the prioritisation of earlier incidence dates and the exclusion of second or higher order tumours, might inflate survival and partly account for survival gaps between jurisdictions. However, specific aspects of registration that might influence survival are uncollectable or beyond the control of the registry, such as the completeness of registration, characteristics of patients with cancer who are not included in the registries, and incomplete ascertainment of deaths. The individual and cumulative effect of these factors is extremely difficult, if not impossible, to measure. Insights into data collection processes and an in-depth assessment of coding, classification, and registration in collaboration with registry staff, biostatisticians, and clinicians are key to informing future strategies to improve international survival benchmarking.
The datasets assembled in this study are unique in several ways. ICBP SURVMARK-2 is based on a large international partnership that has facilitated the collation of 20 years of data from 21 longstanding cancer registries of internationally high quality. Stringent protocols for data processing and harmonisation were put in place and extensive exchanges with both registry staff and clinical advisors in each jurisdiction ensured optimal comparability in the final datasets. The resulting survival estimates are analogous to those from previous international studies,2, 4 although slight deviations might have occurred through differences in exclusion criteria and methodological approaches (eg, differences in the diagnostic window for period analyses and cancer definitions).
This study has several limitations. Although net survival is not subject to potential errors in cause of death certification, it remains a conceptual metric that is of little use to express the survival of an individual patient with cancer. Net survival is used at the population level to allow fair comparisons between countries and groups. Although we made every effort to ensure data comparability, differences in registration practices, and sources of information available to the individual registries might account for some of the variation in cancer survival seen across countries. Furthermore, the proportion of cases eligible for analysis varied by country and depended heavily on the proportion of cases of cancer determined by death certificate (or autopsy) only (varying between 2·3% and 4·8% for pancreatic cancer, and from 0·5% to 3·4% for the remaining cancer sites), and other quality control measures such as inconsistencies between dates. In New Zealand, originally higher proportions of cases diagnosed by death certificate only can be linked to the 1994 Cancer Registry Act that mandated all laboratories to submit new diagnoses of cancer to the national registry. Because registrations were thus obtained from pathology, hospital discharge, or mortality sources, some clinically diagnosed cases not admitted to a hospital were notified to the registry via death certificates only after the patients had died. In this study, an additional assessment of inclusion of cases determined by death certificate only—under the extreme assumption that survival equalled 1 day—reduced the 5-year survival estimates by a maximum of 0·6 percentage points in the most recent period. However, including cases diagnosed on the basis of death certificate or autopsy did not alter the overall patterns across countries and jurisdictions. Moreover, data from Canada and Australia were not national but covered most of their populations (76% of Canada's population and 70% of Australia's population), which is unlikely to restrict their representativeness. Different methods were applied to estimate survival in different time periods (cohort approach for patients diagnosed during 1995–2009, and period approach for diagnoses after 2010), which might affect the interpretation of trends.24 Finally, this study was not designed to assess the drivers of survival differences in-depth across countries or over time, but rather generate hypotheses that will be explored in future studies.
In summary, this first ICBP SURVMARK-2 study clearly shows that although cancer survival continues to improve across high-income countries, international disparities persist even for cancers with the worst prognoses. Earlier diagnosis, improved treatment, and other policy reforms have ensured improved pathways for patients to diagnosis and treatment and have all likely contributed to improved outcomes. Such progress in the control of the cancer types studied here, particularly cancers of the stomach, colon, lung (in males), and ovary, can be attributed to the delivery of multiple evidence-based and effective interventions that span the spectrum of cancer control, during the past two decades.
Acknowledgments
Acknowledgments
This study was funded by Canadian Partnership Against Cancer; Cancer Council Victoria; Cancer Institute New South Wales; Cancer Research UK; Danish Cancer Society; National Cancer Registry Ireland; The Cancer Society of New Zealand; National Health Service England; Norwegian Cancer Society; Public Health Agency Northern Ireland, on behalf of the Northern Ireland Cancer Registry; The Scottish Government; Western Australia Department of Health; and Wales Cancer Network. The findings and interpretations in this Article are those of the authors and do not necessarily represent the views of any government agency or funder. The authors alone are responsible for the views expressed in this Article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated. We thank Lucie Hooper, Samantha Harrison, Charles Norell, Shanta Keshwala, Charlotte Lynch, Deborah Robinson, and Irene Reguilon of Cancer Research UK for managing the programme. We also thank the International Cancer Benchmarking Partnership (ICBP) Cancer Survival in High-Income Countries (SURVMARK-2) Local Leads for their advice to understand the data, for the study protocol, and interpretation of the results; the ICBP Clinical Committees for their advice; and the ICBP SURVMARK-2 Academic Reference Group for providing independent peer review and advice for the study protocol and analysis plan development. A full list of all investigators can be found in the appendix (pp 3–10).
Contributors
MA, MJR, IS, and FB conceived the study, contributed to study design and analysis, and wrote the first draft of the manuscript. HT, VT, DR, LS, RRW, DT, SL, SR, NS-J, PD, CM, AVR, HS-P, GE, PMW, CJ, SV, EM, AG, DSM, and DWH contributed to data collection, interpretation of the results, and critically reviewed the manuscript. AB and JF contributed to data preparation and analysis. TM-LA, TÅM, GP, JB, HB, DCC, SH, DMP, PS, PCL, and BM contributed to the study design, interpretation of the results, and finalising the report. All authors agreed with the decision to submit for publication.
Declaration of interests
TM-LA reports taking part in a private–public collaboration between Karolinska Institutet and Janssen Pharmaceuticals. All other authors declare no competing interests.
Supplementary Material
References
- 1.Butler J, Foot C, Bomb M. The International Cancer Benchmarking Partnership: an international collaboration to inform cancer policy in Australia, Canada, Denmark, Norway, Sweden and the United Kingdom. Health Policy. 2013;112:148–155. doi: 10.1016/j.healthpol.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Coleman MP, Forman D, Bryant H. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377:127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SURVMARK: cancer survival in high-income countries. International Agency for Research on Cancer, World Health Oragniaztion. http://survival.iarc.fr/Survmark/en/
- 4.Allemani C, Matsuda T, Di Carlo V. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Angelis R, Sant M, Coleman MP. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5— a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 6.Maringe C, Walters S, Butler J. Stage at diagnosis and ovarian cancer survival: evidence from the International Cancer Benchmarking Partnership. Gynecol Oncol. 2012;127:75–82. doi: 10.1016/j.ygyno.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Maringe C, Walters S, Rachet B. Stage at diagnosis and colorectal cancer survival in six high-income countries: a population-based study of patients diagnosed during 2000–07. Acta Oncol. 2013;52:919–932. doi: 10.3109/0284186X.2013.764008. [DOI] [PubMed] [Google Scholar]
- 8.Walters S, Maringe C, Butler J. Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000–2007: a population-based study. Br J Cancer. 2013;108:1195–1208. doi: 10.1038/bjc.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walters S, Maringe C, Coleman MP. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–07. Thorax. 2013;68:551–564. doi: 10.1136/thoraxjnl-2012-202297. [DOI] [PubMed] [Google Scholar]
- 10.Eden M, Harrison S, Griffin M. Impact of variation in cancer registration practice on observed international cancer survival differences between International Cancer Benchmarking Partnership (ICBP) jurisdictions. Cancer Epidemiol. 2019;58:184–192. doi: 10.1016/j.canep.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Forbes LJ, Simon AE, Warburton F. Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): do they contribute to differences in cancer survival? Br J Cancer. 2013;108:292–300. doi: 10.1038/bjc.2012.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simon AE, Forbes LJ, Boniface D. An international measure of awareness and beliefs about cancer: development and testing of the ABC. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perme MP, Stare J, Estève J. On estimation in relative survival. Biometrics. 2012;68:113–120. doi: 10.1111/j.1541-0420.2011.01640.x. [DOI] [PubMed] [Google Scholar]
- 14.Coviello E, Seppä K, Dickman PW, Pokhrel A. Estimating net survival using a life-table approach. Stata J. 2015;15:173–185. [Google Scholar]
- 15.Corazziari I, Quinn M, Capocaccia R. Standard cancer patient population for age standardising survival ratios. Eur J Cancer. 2004;40:2307–2316. doi: 10.1016/j.ejca.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Segi M, Fujisaku S, Kurihara M. Geographical observation on cancer mortality by selected sites on the basis of standardised death rate. Gan. 1957;48:219–225. [PubMed] [Google Scholar]
- 17.Bertelsen CA, Neuenschwander AU, Jansen JE. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16:161–168. doi: 10.1016/S1470-2045(14)71168-4. [DOI] [PubMed] [Google Scholar]
- 18.Probst HB, Hussain ZB, Andersen O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians—a national Danish project. Health Policy. 2012;105:65–70. doi: 10.1016/j.healthpol.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Moseholm E, Lindhardt BO. Patient characteristics and cancer prevalence in the Danish cancer patient pathway for patients with serious non-specific symptoms and signs of cancer-a nationwide, population-based cohort study. Cancer Epidemiol. 2017;50:166–172. doi: 10.1016/j.canep.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Walters S, Maringe C, Butler J, Brierley JD, Rachet B, Coleman MP. Comparability of stage data in cancer registries in six countries: lessons from the International Cancer Benchmarking Partnership. Int J Cancer. 2013;132:676–685. doi: 10.1002/ijc.27651. [DOI] [PubMed] [Google Scholar]
- 21.Rutherford MJ, Møller H, Lambert PC. A comprehensive assessment of the impact of errors in the cancer registration process on 1- and 5-year relative survival estimates. Br J Cancer. 2013;108:691–698. doi: 10.1038/bjc.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner H, Hakulinen T. Implications of incomplete registration of deaths on long-term survival estimates from population-based cancer registries. Int J Cancer. 2009;125:432–437. doi: 10.1002/ijc.24344. [DOI] [PubMed] [Google Scholar]
- 23.Woods LM, Coleman MP, Lawrence G, Rashbass J, Berrino F, Rachet B. Evidence against the proposition that “UK cancer survival statistics are misleading”: simulation study with National Cancer Registry data. BMJ. 2011;342 doi: 10.1136/bmj.d3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner H, Hakulinen T. Period versus cohort modeling of up-to-date cancer survival. Int J Cancer. 2008;122:898–904. doi: 10.1002/ijc.23087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.