Summary
Enhancers can regulate the promoters of their target genes over very large genomic distances. It is widely assumed that mechanisms of enhancer action involve the reorganization of three-dimensional chromatin architecture, but this is poorly understood. The predominant model involves physical enhancer-promoter interaction by looping out the intervening chromatin. However, studying the enhancer-driven activation of the Sonic hedgehog gene (Shh), we have identified a change in chromosome conformation that is incompatible with this simple looping model. Using super-resolution 3D-FISH and chromosome conformation capture, we observe a decreased spatial proximity between Shh and its enhancers during the differentiation of embryonic stem cells to neural progenitors. We show that this can be recapitulated by synthetic enhancer activation, is impeded by chromatin-bound proteins located between the enhancer and the promoter, and appears to involve the catalytic activity of poly (ADP-ribose) polymerase. Our data suggest that models of enhancer-promoter communication need to encompass chromatin conformations other than looping.
Keywords: chromatin looping, enhancer, PARP, poly(ADP-ribosyl)ation, Sonic hedgehog, TAL-effector
Graphical Abstract
Highlights
-
•
Super-resolution microscopy reveals increased enhancer-promoter separation upon activation
-
•
Synthetic enhancer activation supports decreased enhancer-promoter proximity
-
•
Enhancer-promoter separation can be driven by poly(ADP-ribose) polymerase 1
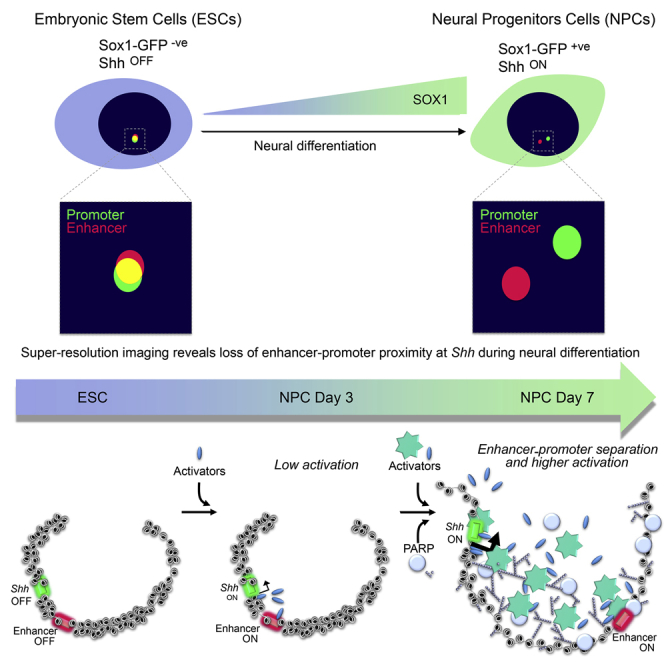
Combining 3D-FISH and chromosome conformation capture, this study monitors long-range chromatin reorganization upon enhancer-driven activation of the Sonic hedgehog gene (Shh) during neural differentiation. The increased separation between Shh and Shh brain enhancers, which is recapitulated in embryonic stem cells using synthetic activators, seems incompatible with looping models of enhancer function and suggests that other models for enhancer action at a distance need to be considered.
Introduction
Enhancers are cis-regulatory sequences, often located within the non-coding portion of the genome, which regulate spatial and temporal gene expression in development and physiology. Enhancers can operate when located proximal to or very distant (100s–1000s of kb) from their target gene (Vernimmen and Bickmore, 2015). Well-established molecular signatures of active enhancers include clustered sequence-specific transcription factor (TF) binding sites and DNase I hypersensitive (DHS) sites, specific histone modifications (e.g., H3K4me1, acetylation of specific lysine residues on histone H3 [H3K27ac, H3K64ac, H3K122ac] and H4 [H4K16ac]), and, in some cases, enhancer RNA (eRNA) transcription (Kim et al., 2010, Pradeepa et al., 2016, Shlyueva et al., 2014, Taylor et al., 2013). Less is known about the mechanisms by which enhancers communicate with and control the expression of their target gene promoter(s).
For proximal enhancers, it has been proposed that activation signals nucleated by bound TFs can move toward the target gene by facilitated diffusion or by tracking along the intervening chromatin, even modifying the intervening chromatin along the way (Bulger and Groudine, 2011, Benabdallah and Bickmore, 2015). These models have been considered unlikely as mechanisms for more distal enhancers.
For very-long-range regulation, communication between the enhancer and the promoter is generally thought to occur through the interaction of protein complexes bound at both sites, with looping out of the intervening chromatin. Chromatin “looping” has been best illustrated for interactions between the β-globin gene and its locus control region (LCR); enhancer-promoter interactions have been detected by chromosome conformation capture (3C) methods (Carter et al., 2002, Tolhuis et al., 2002), and experimentally forced enhancer-promoter chromatin looping can contribute to transcriptional activation (Bartman et al., 2016, Deng et al., 2012, Deng et al., 2014, Morgan et al., 2017). Using fluorescence in situ hybridization (FISH), we have visualized the spatial juxtaposition of a target gene (Shh) with its distant (1 Mb) limb enhancer (ZRS), with a looping out of the intervening chromatin, specifically in Shh-expressing tissue of the developing limb bud (Williamson et al., 2016). However, the generality of enhancer-promoter looping remains unclear, and other chromatin conformations may contribute to long-range gene regulation from enhancers (Benabdallah and Bickmore, 2015, Brown et al., 2018). Live cell imaging also fails to provide evidence for enhancer-promoter spatial proximity driving transcription in mouse embryonic stem cells (mESCs) (Alexander et al., 2019).
The Sonic hedgehog morphogen (Shh) governs the growth and patterning of many tissues during development. Precise spatial and temporal control of Shh expression is regulated by tissue-specific enhancers located within the introns of the gene, upstream of the Shh transcription start site (TSS) in a large (750 kb) gene desert and within genes at the far end of the gene desert (Anderson and Hill, 2014). Shh expression is important for several aspects of brain development. Shh-Brain-Enhancers-6 (SBE6), SBE2/3, SBE4, and SBE5, located ≥100 kb upstream of the Shh TSS, drive expression in the midbrain and anterior domains of the developing brain (Benabdallah et al., 2016, Jeong et al., 2006, Yao et al., 2016). Here, we observe that during the induction of Shh expression in neural progenitor cells (NPCs) or in vivo in the Shh-expressing cells of the embryonic neural tube, there is a decrease in enhancer-promoter proximity that is not compatible with an enhancer-promoter looping model. We show that synthetic enhancer activation in mESCs also leads to enhanced separation between Shh and SBE6, SBE4, or SBE2/3 enhancers. Our data suggest a role for polyADP-ribosylation in this increased spatial separation of enhancers and promoters, and we discuss these findings in the context of new biophysical models of enhancer function.
Results
Decreased Shh and Shh-Brain-Enhancers Co-localization upon Neural Differentiation
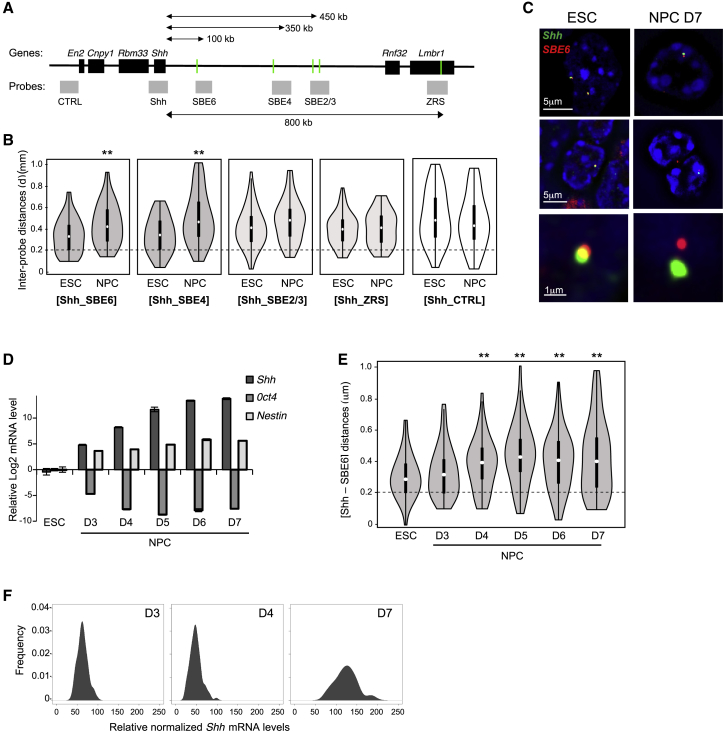
As the known Shh-Brain-Enhancers SBE5, SBE2/3, SBE4, and SBE6 are located 780, 450, 350, and 100 kb upstream of Shh, respectively (Figure 1A), the present models assume that these enhancers would physically loop to the Shh promoter in neural cells and tissues. We have previously used super-resolution microscopy, in conjunction with three-dimensional (3D)-FISH, to demonstrate the spatial juxtaposition of Shh and its limb enhancer (ZRS), with displacement of an intervening genomic region, restricted to the time and place of Shh expression in the developing limb (Williamson et al., 2016).
Figure 1.
Loss of Shh-Brain-Enhancer Proximity during Neuronal Differentiation
(A) Map of the Shh regulatory domain showing the genes (black boxes), enhancers (green bars), and fosmid FISH probes (gray boxes). Probe and enhancer coordinates are listed in Table S8.
(B) Violin plots showing the distribution of inter-probe distances (μm) between Shh and SBE6, SBE4, SBE2/3, ZRS, and CTRL probes in the nuclei of ESCs and D7 NPCs. Distances below the dotted horizontal line at 0.2 μm are considered co-localized. The asterisks on the FISH data represent Mann-Whitney U test significance between ESC and NPC populations. ∗∗p < 0.01. Each violin plot represents one biological replicate; for other replicates and statistics, see Figure S1 and Table S1.
(C) 3D-SIM images illustrating Shh-SBE6 separation in ESCs or in D7 NPCs. Scales bars are 5 μm (top two rows) and 1 μm (bottom, inset from center row).
(D) Shh, Oct4, and Nestin expression assayed by qRT-PCR during a time course of NPC differentiation. The graph shows mean (±SEM) log2 mRNA levels relative to Gapdh and normalized to the level in ESC (three technical replicates).
(E) Violin plots showing Shh-SBE6 inter-probe distances in cell populations corresponding to the expression data in (D).
(F) Kernel density plots showing Shh mRNA expression in single NPCs relative to Gapdh and normalized to the expression in ESC. Density is an arbitrary unit based on the frequency of the occurrence and the total counts and the size of the population (i.e., the binning of the data).
Data from a biological replicate are shown in Figure S1B.
To analyze the spatial relation of Shh to its known brain enhancers, we differentiated 46c mESCs (Ying et al., 2003) into NPCs, monitoring differentiation by Sox1-GFP fluorescence (Benabdallah et al., 2016). We performed 3D-FISH on mESCs that do not express Shh and on NPCs obtained after 7 days of differentiation, when Shh is expressed (Benabdallah et al., 2016), and we imaged the slides by 3D-structured illumination microscopy (3D-SIM). Within the Shh domain, the most prominent peaks of H3K4me1 and H3K27ac gained during differentiation occur at SBE6, an enhancer required for the full induction of Shh during this NPC differentiation program (Benabdallah et al., 2016). Upon Shh activation, there was a significant increase in inter-probe distances between Shh and both SBE6 and SBE4, located 100 and 350 kb 5′ of Shh, respectively, and a decrease in the proportion of co-localized alleles (enhancer-promoter inter-probe distances ≤0.2 μm) (Figures 1B and 1C; Table S1). For example, for SBE6-Shh, the proportion of alleles with inter-probe distances ≤0.2 μm ranged between 7.8% and 26.3% in mESCs between replicate experiments and fell to 3.6%–19.2% in NPCs (Table S1). For SBE4-Shh, the ranges were 10.6%–23.8% in ESCs and 5.4%–6.6% in NPCs. Distances between Shh and the more distant ZRS or a control probe (CTRL) located outside the Shh regulatory domain were not significantly changed (Figures 1A and 1B; Table S1).
The decreased proportion of alleles with enhancer-promoter juxtaposition upon Shh induction does not seem compatible with the formation of a chromatin loop between Shh and the SBE6/SBE4/SBE2 neural enhancers. To assess whether looping occurs at an earlier time point, we analyzed Shh expression during the NPC differentiation time course (days 3–7; D3–D7). Some Shh expression initiated on D3, increasing steadily until D6 or D7 (Figure 1D). In all of the replicate experiments, Shh-SBE6 separation increased significantly from D4 onward. Shh-SBE6 distances were somewhat increased at D3, but only reached statistical significance for two of the three biological replicates (Figures 1E and S1A). These data support the notion that no stable chromatin loops are formed between SBE6 and Shh at an earlier time point during this neural differentiation program (Table S1).
Single-cell qRT-PCR showed that there is increased Shh expression in most cells of the population at D3 and D4 of NPC differentiation (Figures 1F and S1B), and even higher levels by D7, consistent with the cell population averaged expression data (Figure 1D). This excludes that there is a small subpopulation of NPCs that express Shh at high levels, possibly with a looped chromatin conformation. For the same cell populations, Shh-SBE6 inter-probe distances start to increase at D3–D4 and shift homogenously toward greater distances at D5–D7 (Figures 1E and S1A). There is no statistical evidence for bimodality in the data distribution.
No Increased Enhancer-Promoter Co-localization In Vivo
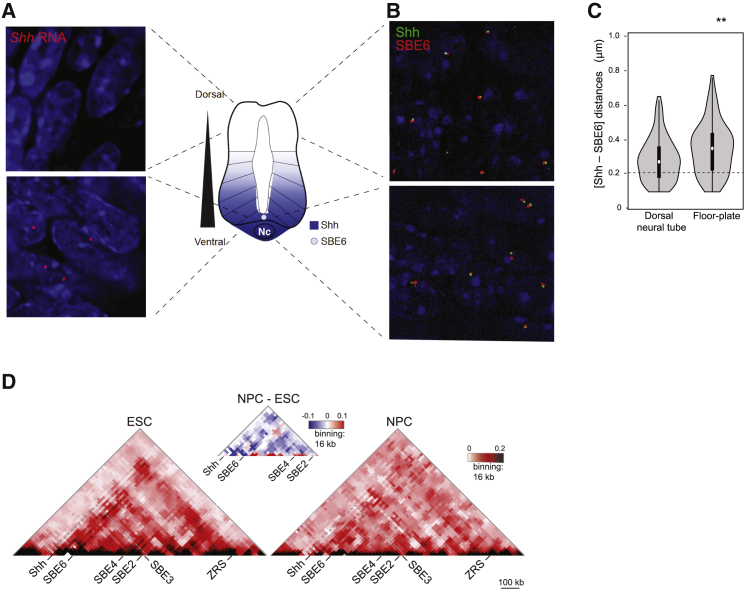
Shh is expressed in ventral regions of the neural tube—in the floor plate and notochord (Jeong et al., 2006) (Figure 2A)—and transgene assays indicate that SBE6 is also active in the floor plate (Benabdallah et al., 2016). To assess SBE6-Shh proximity in vivo, we used FISH to examine Shh-SBE6 inter-probe distances in sections through the neural tube of an E10.5 embryo. RNA FISH indicated that 49% of Shh alleles are expressed in this region of the neural tube (Figure 2A). DNA FISH showed that Shh-SBE6 inter-probe distances were greater, and the proportion of alleles with enhancer-promoter co-localization lower (9.9%), in nuclei from the floor plate region (9.9%) compared to dorsal neural tube cells (21.9%) (Figures 2B and 2C). This suggests that there is no elevated enhancer-promoter proximity in Shh-expressing cells in vivo during neurogenesis.
Figure 2.
Enhancer-Promoter Separation Occurs In Vivo
(A) Schematic of a transverse section through the neural tube with a gradient of Shh emanating from ventral Shh-expressing cells in the notochord (Nc) and the floor plate, where SBE6 activity is also detected (Benabdallah et al., 2016). Panels at left show RNA FISH signal (red) for Shh in sections from the dorsal neural tube (top panel) or the floor plate (bottom panel) of E10.5 mouse embryos.
(B) 3D-FISH SIM images illustrating Shh-SBE6 separation in nuclei from the dorsal neural tube (top) or ventral floor plate (bottom) of E10.5 mouse embryos.
(C) Violin plots showing Shh-SBE6 inter-probe distances for FISH data from E10.5 dorsal neural tube and ventral floor plate cells. ∗∗p < 0.01 for this biological replicate. For two other biological replicates, p = 0.002 and p < 0.001.
(D) 5C heatmaps of the Shh regulatory region (mm9, chr5:28604000-29780000) from ESCs and D7 NPCs with 16 kb binning and smoothing. A difference plot for the 5C heatmaps between NPCs and ESCs is shown above.
Data from a biological replicate are shown in Figure S2C.
To determine whether other as yet unidentified cis-regulatory elements gain interactions with the Shh promoter during the differentiation of ESCs to NPCs, we used chromosome conformation capture carbon copy (5C) to assay cross-linked ligation frequencies across the entire Shh regulatory domain. Consistent with Hi-C data from ESCs (Smallwood and Ren, 2013) and 5C data from E11.5 embryos (Williamson et al., 2016), in both ESCs and NPCs, all of the SBEs are contained in a topologically associated domain (TAD) that extends from downstream of Shh (before Rbm33) to just beyond Lmbr1, the gene where the ZRS is located. Comparison of 5C data from ESCs and D7 NPCs revealed no evidence for a gain of specific interactions in NPCs that may indicate the formation of a loop between Shh and its neural enhancers (Figures 2D and S2A).
SBE Activation Increases Enhancer-Promoter Separation
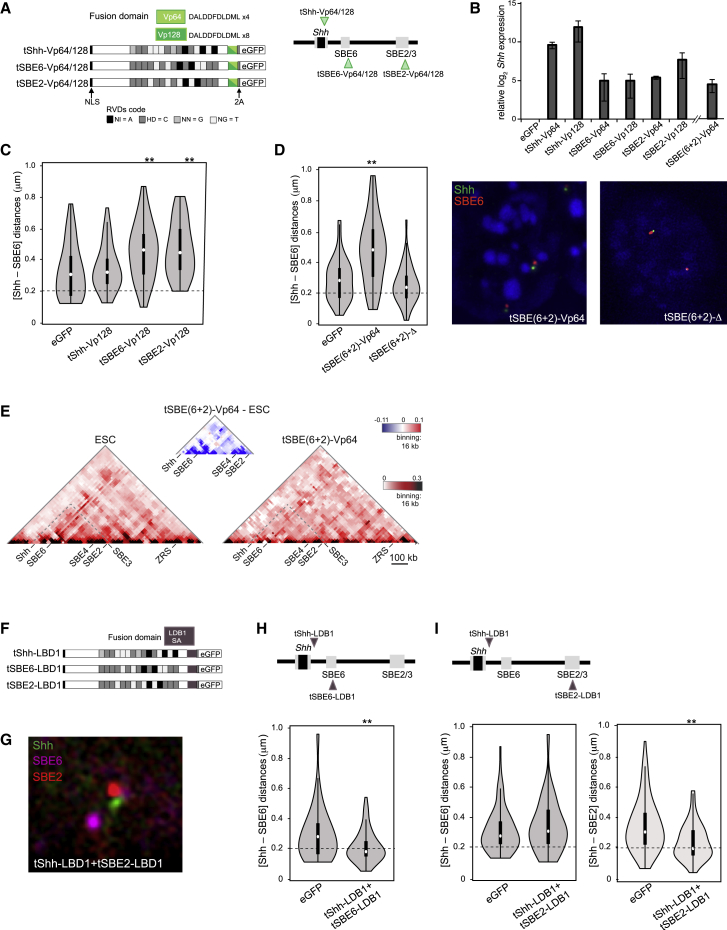
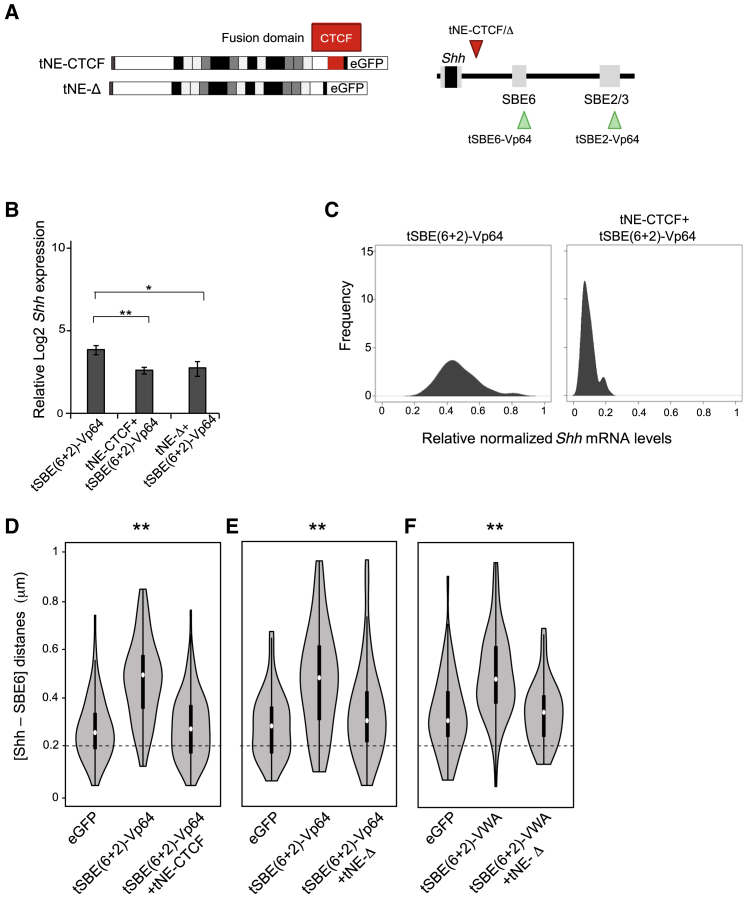
Supercoiling associated with transcription decondenses large chromatin domains (Naughton et al., 2013); therefore, the altered chromatin conformations we observe during NPC differentiation could occur as a passive consequence of Shh transcription. To test this, we bypassed the need for Shh enhancers, fusing a transcription activator-like effector (TALE) targeted to the Shh promoter (tShh) to repeats of the small viral acidic protein VP16 (VP64/VP128) that can strongly activate gene expression (Zhang et al., 2011) (Figure 3A), including in ESCs (Therizols et al., 2014). The expression of tShh-VP64/128 in mESCs led to the activation of Shh expression to levels similar to those seen in NPCs (Figure 3B), but without perturbing markers of pluripotency or neuronal differentiation (Figure S3A). Synthetic activation was restricted to Shh and the long non-coding RNA 9530036O11Rik that is transcribed in the opposite direction from the Shh promoter (Figures S3B and S4C). Like Shh, 9530036O11Rik expression is also induced during NPC differentiation. The two other genes in the Shh TAD (Rnf32 and Lmbr1) are not activated during NPC differentiation or by TALE-VP16 targeting to the Shh promoter or SBE6. The same is true of two genes flanking the Shh TAD (Rbm33 and Nom1) (Figure S3B).
Figure 3.
Synthetic Activation of Shh and Increased Enhancer-Promoter Separation Using TALE-VP16
(A) Schematic of TALE-VP64 and TALE-VP128 constructs targeting the Shh promoter (tShh), SBE6, or SBE2. Repeat variable diresidue (RVD) code is displayed with one-letter abbreviations for amino acids. Self-cleaving (2A) peptide allows the expression of eGFP and cell isolation by fluorescence-activated cell sorting (FACS). A map of the targeting sites is shown at right.
(B) Log2 mRNA levels of Shh, relative to Gapdh, assayed by qRT-PCR after TALE-VP64/128 expression in ESCs. Data show means (±SEMs) of three biological replicates normalized to ESCs expressing a control eGFP.
(C) Violin plots representing one biological replicate of Shh-SBE6 inter-probe distances (μm) in ESCs expressing control eGFP, TALE-VP128 fusions targeting Shh promoter (tShh), SBE6, or SBE2. ∗∗p < 0.01. Statistical data and replicate experiments are in Table S2. Distances below the dotted horizontal line at 0.2 μm are considered co-localized.
(D) As in (C), but for VP64 recruitment to both SBE6 and SBE2 simultaneously (tSBE6+2), or a TALE with no fusion protein (tSBE6+2)-Δ. ∗∗p < 0.01. Representative FISH images with probes for Shh (green) and SBE6 (red) in mESCs expressing tSBE(6+2)-VP64 and tSBE(6+2)-Δ are shown at right.
(E) 5C heatmaps of the Shh regulatory region (chr5:28604000-29780000) with 16 kb binning and smoothing for ESCs and for ESCs expressing TALE-VP64 fusions targeting both SBE6 and SBE2. Difference 5C plots are shown above the main 5C plots.
(F) Schematic representing TALE-LDB1 targeting sequences.
(G) Three-color FISH with probes for Shh (green), SBE6 (magenta), and SBE2 (red) in mESCs expressing tShh-LDB1+tSBE2-LDB1.
(H) Violin plots displaying Shh and SBE6 inter-probe distances (μm) in ESCs expressing eGFP or tShh-LDB1+tSBE6-LDB1. ∗∗p < 0.01.
(I) As in (H), but in cells expressing tShh-LDB1+tSBE2-LDB1. Shh-SBE6 distances are shown in the left-hand panel, and Shh-SBE2 distances are in the right-hand panel. ∗∗p < 0.01.
Statistical data relating to this figure are included in Table S3.
Synthetic activation of Shh using promoter-targeted VP16 did not alter Shh-SBE6 inter-probe distances (Figure 3C; Table S2). Therefore, the increased enhancer-Shh promoter separation observed during NPC differentiation is not simply a consequence of activating Shh or 9530036O11Rik expression.
Recruiting VP64/128 to SBE2 or SBE6 also induced Shh expression, albeit less markedly compared to direct recruitment to the Shh promoter (Figure 3B), and without detectable effects on the expression of the pluripotency or neuronal markers tested (Figure S3A). However, VP128 recruitment to either enhancer resulted in an increase in enhancer-promoter separation (Figure 3C) and decreased enhancer-promoter proximity—the percentage of alleles with inter-probe distances <200 nm decreasing from 22%–32% in eGFP controls to <7.5% in SBE-VP128-expressing transfectants (Table S2). A similar result was achieved by recruiting VP64 to SBE6 and SBE2 simultaneously: tSBE(6+2)-VP64 (Figures 3B and 3D; Table S2). Decreased enhancer-promoter proximity was specific to VP64 activity as recruiting TALEs without a fusion domain (tSBE(6+2)-Δ) had no effect (Figure 3D; Table S2). 5C analysis (Figure 3E) and virtual 4C analysis of that data (Figure S3C) also revealed a loss of interactions between the activated enhancers and the Shh promoter and with other sequences in the Shh-SBE2 interval.
To show that FISH is capable of detecting enhancer-promoter proximity as a result of chromosome looping, we created artificial Shh-SBE interactions. Targeted tethering (using zinc fingers) of the self-association (SA) domain of LIM domain-binding protein 1 (LDB1) has been used previously to force a chromatin loop at the β-globin locus (Bartman et al., 2016, Deng et al., 2012, Deng et al., 2014). Using a similar approach, but with TALE proteins, we tethered the LBD1 SA to the Shh promoter (tShh-LDB1) and to either SBE6 or SBE2 (tSBE6-LDB1 and tSBE2-LDB1) in mESCs (Figure 3F). The configurations of FISH probe signals were consistent with the predicted loop (Figure 3G). Quantification revealed dramatically increased co-localization (35%–50% of alleles at ≤200 nm) between the tethering sites upon tShh-LDB1 and tSBE6/tSBE2-LDB1 co-transfection (Figures 3H and 3I; Table S3). In cells transfected with tShh-LDB1 and tSBE2-LDB1, SBE6 was further from Shh than was the genomically more distant SBE2/3 (Figure 3I), which is consistent with a chromatin loop anchored by LDB1 interactions. We conclude that 3D FISH is able to detect a chromatin loop in ESCs, albeit an artificially constructed one.
Endogenous Activators and Co-activators Also Reduce Enhancer-Promoter Proximity
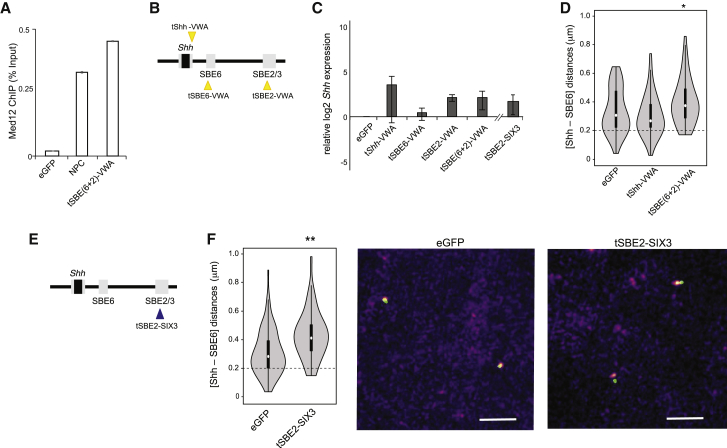
VP16 is a very effective transcriptional activator, but of viral origin. We therefore wished to analyze whether mammalian endogenous activators and co-activators could induce similar alterations in enhancer-promoter proximity. The Mediator complex is recruited to active enhancers and promoters (Soutourina, 2018) and can work alongside cohesin to alter 3D chromosome conformation upon enhancer-driven gene activation (Kagey et al., 2010, Phillips-Cremins et al., 2013). Chromatin immunoprecipitation (ChIP) showed that Mediator is recruited to the Shh promoter during the differentiation of 46c ESCs to NPCs (Figure 4A).
Figure 4.
Enhancer-Promoter Separation Induced by Endogenous Activators and Co-activators
(A) Med12 ChIP (percentage of input, normalized to β-actin promoter) at Shh promoter measured by qPCR in NPC or in ESCs expressing eGFP or tSBE(6+2)-vWA.
(B) Schematic showing the targeting of TALE-vWA constructs.
(C) Log2 mRNA levels of Shh relative to Gapdh in ESCs expressing TALE-vWA constructs targeting the Shh promoter (tShh), SBE6, SBE2, or both SBE6 and SBE2. Data for a TALE targeting SIX3 to SBE2 are also shown. Data show means (±SEMs) of three biological replicates normalized to ESCs expressing eGFP.
(D) Violin plots of Shh-SBE6 inter-probe distances (μm) in cells expressing eGFP or TALE-vWA targeting the Shh promoter (tShh), or both SBE6 and SBE2. ∗p = 0.018. Statistical data and replicate experiments are shown in Table S4.
(E) Schematic showing the targeting of TALE-SIX3 construct to SBE2.
(F) As in (D), but for ESCs expressing tSBE2-SIX3. ∗∗p < 0.01. Example FISH images are shown at right. Bar, 2 μm.
Mediator interacts with RNA polymerase II and many transcription factors and is believed to bridge between them. VP16 interacts with the Med25 subunit located in the tail domain of the complex (Milbradt et al., 2011, Vojnic et al., 2011), and Med25 is recruited into the Mediator complex through its N-terminal von Willebrand factor A (vWA) domain (Mittler et al., 2003). We therefore fused TALEs to the Med25 vWA domain (Figure 4B). Compared to TALE-VP16, Med25-vWA recruitment induced only low-level Shh expression, even for the Shh promoter-targeted TALE (tShh-vWA) (Figure 4C). Recruitment of vWA to the Shh promoter did not alter chromatin conformation upstream of Shh (Table S4). Targeting to both SBE6 and SBE2 (tSBE(6+2)-vWA) resulted in the recruitment of the Med12 subunit of the Mediator kinase module to the Shh promoter, compatible with a long-range effect (Figure 4A); as for VP64 recruitment, it led to increased promoter-enhancer separation, and the frequency of co-localized (≤200 nm) SBE6-Shh alleles decreased from 17.4%–18.6% in eGFP controls to 2.6%–4.3% (Figure 4D; Table S4).
The endogenous TFs that bind and activate SBEs in neural tissues are largely unknown. However, Six homeobox 3 (SIX3) is known to bind to SBE2, and mutation of its binding site, or of SIX3 itself, affects Shh expression in the developing brain, leading to holoprosencephaly (HPE) (Geng et al., 2008, Jeong et al., 2008). Six3 is also upregulated during the ex vivo differentiation of ESCs to NPCs (Benabdallah et al., 2016). This prompted us to investigate whether tethering SIX3 was sufficient to recapitulate chromatin conformation changes in the region 5′ of Shh. TALE-directed recruitment of SIX3 to SBE2 (Figure 4E) induced only very low-level and variable Shh expression (Figure 4C), but nevertheless led to increased inter-probe distances 5′ of Shh (Figure 4F; Table S4). Therefore, recruitment of either an endogenous activator (SIX3) or a co-activator (Mediator) to enhancers 5′ of Shh leads to increased enhancer-promoter separation.
Intervening Proteins Abrogate the Loss of Enhancer-Promoter Proximity
The absence of detectable enhancer-promoter juxtaposition upon Shh activation in vitro and in vivo and evidence of increased enhancer-promoter separation in these conditions seem incompatible with chromatin-looping mechanisms for enhancer action, but they may be more consistent with spreading and/or linking or tracking-like models (Bulger and Groudine, 2011, Engel et al., 2008, Vernimmen and Bickmore, 2015). To investigate this further, we attempted to insert obstacles between SBE6 and Shh. We chose a site 65 kb upstream of the Shh TSS (chr5:28859721; mm9) that lacks evidence both of enhancer activity (H3K4me1/H3K27ac marks) during ESC-NPC differentiation (Benabdallah et al., 2016) and evolutionary conservation. We fused a TALE construct specific to this site (NE [Non-Enhancer]) to CTCF (tNE-CTCF) (Figure 5A), as CTCF has been proposed to have general enhancer-blocking functions (Burgess-Beusse et al., 2002) and to block enhancer-promoter tracking (Engel et al., 2008). Transfection of tNE-CTCF, in conjunction with TALE-VP64 co-activation of SBE6 and SBE2, reduced Shh activation in the cell population (Figure 5B), and single-cell qRT-PCR confirmed that the majority of the cells transfected with tNE-CTCF+tSBE(6+2)-VP64 had low levels of Shh expression (Figure 5C). tNE-CTCF also prevented the increase in enhancer-promoter separation induced by the TALE-VP64 co-activation of SBE6 and SBE2 (Figure 5D; Table S5), which is consistent with the intervening CTCF molecule interrupting a mechanism initiated at SBE6/2. As a control, we also recruited a TALE without any fused protein (tNE-Δ) (Figure 5A). The introduction of tNE-Δ also abrogated Shh induction (Figure 5B) and the increased enhancer-promoter separation induced by tSBE(6+2)-VP64 or tSBE(6+2)-vWA (Figures 5E and 5F; Table S5).
Figure 5.
Enhancer-Promoter Spatial Separation Is Blocked by Intervening Chromatin-Bound Proteins
(A) Schematic showing TALEs targeting the NE site with either no fusion protein (tNE-Δ) or fused to CTCF (tNE-CTCF).
(B) Log2 mean (±SEM) Shh mRNA levels relative to Gapdh in ESCs expressing TALE-VP64 fusions targeting SBE6 and SBE2 and in cells that also express either tNE-CTCF (three biological replicates) or tNE-Δ (four biological replicates). Data are normalized to those from ESCs expressing control eGFP. The asterisks represent p values for a one-tailed Student’s t test between conditions. ∗p < 0.05 and ∗∗p < 0.01.
(C) Kernel density plots showing Shh expression in single ESCs expressing TALE-VP64 fusions targeting SBE6+SBE2 and in these cells when tNE-CTCF is also expressed. Expression is normalized to that in ESCs.
(D and E) Violin plots representing one biological replicate of Shh-SBE6 inter-probe distances (μm) in ESCs expressing eGFP, TALE-VP64 fusions targeting SBE6+SBE2, and these cells when either tNE-CTCF (D) or tNE-Δ (E) is also expressed.
(F) As in (E), but using a TALE-vWA fusion targeting SBE6 and SBE2. ∗p < 0.05 and ∗∗p < 0.01.
Statistical data for FISH data from this figure are shown in Table S5.
H3K27me3, H3K27ac, and Transcription Are Not Mediating Decreased Enhancer-Promoter Proximity at Shh
A class of poised enhancers in mESCs are marked by H3K27me3 (Rada-Iglesias et al., 2011), and our data may therefore result from a loss of long-range interactions between such poised enhancers and Shh upon differentiation or synthetic gene activation. However, H3K27me3 ChIP profiling in mESCs (Illingworth et al., 2015) showed that whereas the Shh gene itself is a target of polycomb, there are no other blocks of H3K27me3 in the Shh regulatory region that could indicate the presence of additional repressors or poised enhancers (Figure S4A).
There are examples in which histone acetylation spreads between an enhancer and a target gene and is blocked by CTCF (Zhao and Dean, 2004). ChIP showed that Shh activation using TALE-VP64 constructs targeted to SBE6 or SBE2 induced histone acetylation (H3K27ac), but this was limited precisely to the TALE binding site, with no indication of spreading along the intervening chromatin to Shh (Figure S4B). Similarly, RNA polymerase II has been demonstrated to track between some enhancer and promoter regions, synthesizing short poly-adenylated RNAs (Zhu et al., 2007). The expression of the long non-coding RNA 9530036O11Rik on the opposite strand from Shh is also upregulated during neural differentiation (Figures S3B and S4C). However, as 9530036O11Rik transcription is also induced by targeting VP16 to the Shh promoter (Figure S3B), this does not account for the increase in inter-probe separation between Shh and SBE6 (Figure 3C).
Assaying nascent transcription in mESCs and during NPC differentiation using 4-thiouridine (4sU) sequencing also provided no evidence for any additional transcription, apart from Shh and 9530036O11Rik, induced across the Shh intergenic region on either strand during NPC differentiation (Figure S4C).
Increased Enhancer-Promoter Separation Appears to Involve Poly(ADP-Ribosyl)ation
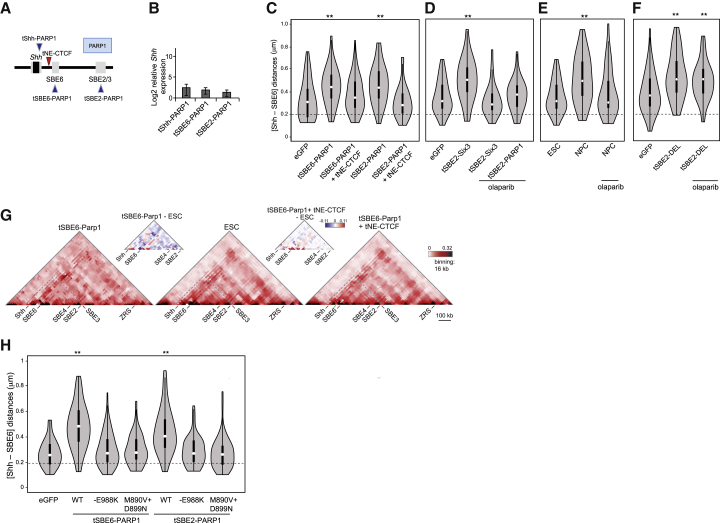
Because poly(ADP-ribosyl)ation (PARylation) catalyzed by poly(ADP-ribose) polymerases has been linked to large-scale changes in chromatin structure such as decompaction (Huletsky et al., 1989, Poirier et al., 1982), with chromatin remodeling (Singh et al., 2017) and gene activation at ecdysone and heat shock-induced puffs on Drosophila polytene chromosomes (Sawatsubashi et al., 2004, Tulin and Spradling, 2003, Tulin et al., 2003), we wanted to determine whether it could be involved in the localized changes in chromosome conformation at Shh. We therefore used TALEs to investigate whether targeting PAR polymerase 1 (PARP1) to Shh, SBE6, or SBE2 altered chromatin conformation at the Shh region (Figure 6A). PARP1 recruitment had a minimal effect on Shh expression (Figure 6B), but it led to an increased Shh and SBE6 separation when targeted to SBE6 or SBE2 (Figure 6C; Table S6). This could be blocked by co-transfection with tNE-CTCF (Figure 6C), as was observed with TALE-VP16 constructs (Figure 5D). Similarly, 5C analysis showed a relative loss of interactions in the region 5′ of Shh when Parp1 was recruited to SBE6 (Figure 6G), and these interactions were restored when CTCF was tethered to NE between SBE6—the site of PARP1 recruitment—and Shh (Figures 6G and S5).
Figure 6.
PARP1 Catalytic Activity Decreases Enhancer-Promoter Proximity
(A) Schematic of TALEs that target PARP1 to the Shh promoter (tShh), SBE6, or SBE2.
(B) Log2 mRNA levels of Shh relative to Gapdh in ESCs expressing the TALE-PARP1 constructs shown in (A). Data show means (±SEMs) of five biological replicates normalized to ESCs expressing eGFP.
(C) Violin plots representing one biological replicate of Shh-SBE6 inter-probe distances (μm) in ESCs expressing eGFP, tSBE6-PARP1, tSBE6-PARP1+tNE-CTCF, tSBE2-PARP1, and tSBE2-PARP1+tNE-CTCF.∗∗p < 0.01.
(D) As in (C) but for ESCs expressing eGFP and tSBE2-Six3, and then in tSBE2-Six3 or tSBE2-PARP1-expressing cells treated with olaparib.
(E) Violin plots representing one biological replicate of Shh-SBE6 inter-probe distances in ESCs or NPCs and in NPCs treated with olaparib.
(F) As in (D), but for ESCs expressing eGFP or tShh-DEL with and without olaparib treatment. ∗∗p < 0.01. Statistical data and replicate experiments are shown in Table S6.
(G) 5C heatmaps of the Shh regulatory region (chr5:28604000-29780000) with 16 kb binning and smoothing for ESCs and for ESCs expressing TALE-PARP1 fusions targeting SBE6 or ESCs co-expressing tSBE6-PARP1 and TALE-CTCF targeting NE. Difference 5C plots are shown above the main 5C plots. Data for a replicate experiment are shown in Figure S5.
(H) Violin plots representing one biological replicate of Shh-SBE6 inter-probe distances (μm) in ESCs expressing tSBE6-PARP1 or tSBE2-PARP1, PARP1 catalytic mutant E988K, or PARP1 catalytic double mutant M890V+D899N. ∗∗p < 0.01.
Statistical data relating to FISH data are shown in Table S7.
To assess whether the increased enhancer-promoter spatial separation seen by targeted recruitment of other activators such as SIX3 (Figures 4G and 5H) could also involve PARP1 catalytic activity, we used the PARP inhibitor olaparib. Olaparib treatment prevented the Shh-SBE6 distance increases mediated by either SIX3 or PARP1 recruitment to SBE2 (Figure 6D) and those seen upon the differentiation of mESCs to NPCs (Figure 6E; Table S6). The effect of olaparib is not a generic effect on chromatin conformation. Visible chromatin decompaction could also be induced by recruiting the small acidic peptide DELQPASIDP (DEL) to SBE2. This peptide decompacts chromatin without leading to gene activation (Carpenter et al., 2005, Therizols et al., 2014). Olaparib had no effect on the increased enhancer-promoter distances induced by DEL recruitment (Figure 6F).
We further tested a requirement for PARP1 catalytic activity using TALE-mediated recruitment of PARP1 E988K and M890V+D899N, which are mutations in the catalytic domain of PARP1 (Rolli et al., 1997). In contrast to wild-type PARP1, recruitment of mutant PARP1 to SBE2 or SBE6 did not alter Shh-SBE6 distances (Figure 6H; Table S7). We conclude that most of the changes in chromatin structure that we have detected as a result of recruitment of activators or PARP1 to the regulatory region 5′ of Shh rely on catalytically active PARP1.
Discussion
A popular model of enhancer-promoter communication has involved chromatin looping to juxtapose the two elements in 3D nuclear space (Vernimmen and Bickmore, 2015), and we have provided visual evidence that supports this model in the context of long-range gene activation of Shh by the ZRS enhancer during limb development (Williamson et al., 2016). However, recent live-cell imaging approaches challenge the idea of stable enhancer-promoter loops as the basis for all enhancer-promoter communication (Fukaya et al., 2016, Alexander et al., 2019).
Here, we have analyzed the spatial relation between Shh and its neural enhancers SBE6, SBE4, and SBE2/3 using FISH and 5C during neural differentiation and in vivo. We found evidence for a decreased rather than an increased frequency of enhancer-promoter juxtaposition in Shh-expressing cells and enhancer-promoter distances in the regulatory domain upstream of Shh increase (Figures 1 and 2). We recapitulated this using synthetic activators (based upon TALE-mediated recruitment of VP16) to either induce Shh expression directly through activator recruitment to the promoter or induce expression from a distance through recruitment to distal enhancers up to 400 kb away in the Shh regulatory domain. Activation from a distance recapitulated the increased enhancer-promoter separation and the decreased enhancer-promoter co-localization that we saw during NPC differentiation (Figure 3). Similar results were seen by the distal recruitment of an endogenous activator (Six3) or a co-activator (Mediator) (Figure 4). There is no evidence for bimodality in our datasets that may suggest a small proportion of looped alleles, although we cannot exclude that such structures are extremely transient.
CTCF bound at cognate binding sites is known to alter enhancer and promoter communication (Ali et al., 2016) and could be consistent with our observation that TALE-mediated CTCF recruitment blocks the increase in enhancer-Shh separation induced by activator and co-activator recruitment to enhancers (Figure 5). Overall, our data do not seem compatible with enhancer-promoter loops being essential for enhancer-driven gene transcription.
PARP and Enhancer Activation
We could also induce enhancer-promoter separation at Shh by recruiting catalytically active PARP1 and could block it with a PARP inhibitor (olaparib) (Figure 6). Although PARP1 is usually studied in the context of DNA damage sensing and repair, it has also been associated with the regulation of gene expression and with regulatory regions (Nalabothula et al., 2015). PARP and Parp1-dependent PARylation have been implicated in gene regulation from distal enhancer elements that are controlled by nuclear hormone receptors (Sawatsubashi et al., 2004, Schiewer et al., 2012, Wright et al., 2012, Zhang et al., 2013). PARylation is also known to modify the insulating and enhancer blocking activity of CTCF (Yu et al., 2004).
PARylation of histones leads to the decompaction of nucleosome arrays in vitro (Huletsky et al., 1989, Poirier et al., 1982) and in vivo (Petesch and Lis, 2012, Tulin and Spradling, 2003) and may facilitate chromatin access for factors involved in transcriptional activation, or it may increase the mobility and nuclear search space for enhancers (Gu et al., 2018). The nucleic acid-like PAR chains can also seed phase separation through liquid de-mixing (Altmeyer et al., 2015), and PARylation has been proposed to be involved in the formation of dynamic non-membrane-bound subnuclear and subcellular compartments involved in a variety of processes, including DNA damage and post-transcriptional regulation (Leung et al., 2011).
Enhancer Condensates and Chromatin Organization
Enhancer function has recently been reconsidered from the perspective of liquid-liquid phase separation (LLPS). It has been proposed that TFs bound at enhancers drive LLPS, nucleating a high concentration of activators, co-activators, and the transcriptional machinery into dynamic condensates to drive transcription (Hnisz et al., 2017). Target genes of super-enhancers can be shown to co-localize at some frequency with condensates that appear to be on the order of several hundreds of nanometers in diameter (Cho et al., 2018, Chong et al., 2018, Sabari et al., 2018, Boija et al., 2018). However, the spatial relation between enhancers and their target genes has not been studied in this context. A recent study indicates that such condensates can physically affect chromatin structure, pushing chromatin out, and in turn, a more open flexible chromatin can facilitate LLPS and condensate formation (Shin et al., 2018). This could be consistent with the increased intra-nuclear distances we have seen between enhancers and their target promoters. Future work should explore the topology of the chromatin linking distal enhancers to their target gene promoters in relation to condensates of the activators and co-activators that are nucleated by these enhancers.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| MED12 | Bethyl laboratories | Cat# A300-774A; RRID:AB_669756 |
| H3K27ac | Millipore | Cat# 07-360; RRID:AB_310550 |
| Bacterial and Virus Strains | ||
| Modified TALEN backbone Vp64 | Therizols et al. 2014 | N/A |
| Fosmid FISH probe | BACPAC resource | See Table S8 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Protease Inhibitor Cocktail Set III, EDTA-Free | Calbiochem, Merck | 539134-1SET |
| PhosSTOP | Roche, Merck | 4906837001 |
| GMEM | Gibco, ThermoFisher | 11710-035 |
| DMEM/F-12, HEPES | Gibco, ThermoFisher | 11330-032 |
| Neurobasal Medium | Gibco, ThermoFisher | 21103-049 |
| B-27 Supplement (50X), serum free | Gibco, ThermoFisher | 17504044 |
| N-2 Supplement (100X) | Gibco, ThermoFisher | 17502048 |
| 2-Mercaptoethanol | ThermoFisher | 31350-010 |
| Lipofectamine 2000 Transfection Reagent | Invitrogen, ThermoFisher | 11668027 |
| Olaparib (AZD2281, Ku-0059436) | Selleck Chemicals | S1060 |
| Green dUTP | Abbott Molecular | 02N32-050, 00884999002913 |
| ChromaTide Alexa Fluor 594-5-dUTP | Invitrogen, ThermoFisher | C11400 |
| Mouse Cot-1 DNA | Invitrogen, ThermoFisher | 18440-016 |
| EGS (ethylene glycol bis(succinimidyl succinate) | Thermo Scientific, ThermoFisher | 21565 |
| Pierce 16% Formaldehyde (w/v), Methanol-free | Thermo Scientific, ThermoFisher | 28908 |
| Proteinase K | Genaxxon bioscience | M3037.0001 |
| 4-Thiouridine | Sigma, Merck | T4509 |
| TRIzol Reagent | Invitrogen, ThermoFisher | 15596026 |
| EZ-Link HPDP-Biotin | Thermo Scientific, ThermoFisher | 21341 |
| SYBR Green I nucleic acid gel stain | Sigma, Merck | S9430 |
| Critical Commercial Assays | ||
| Wizard SV Gel and PCR Clean-Up | Promega | A9282 |
| RNeasy Mini Kit | Qiagen | 74106 |
| SuperScript II Reverse Transcriptase | Invitrogen, ThermoFisher | 18064014 |
| LightCycler 480 SYBR Green I Master | Roche Diagnostics | 507203180 |
| CellsDirect One-Step qRT-PCR Kit | Invitrogen, ThermoFisher | 11753500 |
| Musunuru/Cowan Lab TALEN Kit | Addgene | 1000000034 |
| Protein G Sepharose, GE Healthcare | Sigma, Merck | GE17-0618-01 |
| GenomePlex Complete Whole Genome Amplification (WGA) Kit | Sigma, Merck | WGA2-50RXN |
| MinElute PCR Purification Kit | Qiagen | 28004 |
| μMACS Streptavidin Kit | Miltenyi | 130-074-101 |
| TURBO DNA-free Kit | Invitrogen, ThermoFisher | AM1907M |
| RNeasy MinElute Cleanup Kit | Qiagen | 74204 |
| Low Input RiboMinus Eukaryote System v2 | Invitrogen, ThermoFisher | A15027 |
| NEBNext Ultra II Directional RNA Library Prep Kit for Illumina | NEB | E7760 |
| Deposited Data | ||
| 5C sequencing | This paper | GEO: GSE89388 |
| Chip-chip Data Nimblegen 720K, H3K27ac | This paper | GEO: GSE115642 |
| 4SU-seq | This paper | GEO: GSE115774 |
| Experimental Models: Cell Lines | ||
| 46c mESCs derived from E14tg2A containing Sox1-GFP | Ying et al., 2003 | N/A |
| Experimental Models: Organisms/Strains | ||
| CD1 mice | Williamson et al., 2016 | N/A |
| Oligonucleotides | ||
| RT-PCR Primers | This paper | See Table S9 |
| ChIP-qPCR Primers | This paper | See Table S9 |
| SA domain of LDB1, gBlocks Gene Fragments | IDT | N/A |
| VWA domain of Med25, gBlocks Gene Fragments | IDT | N/A |
| CTCF, Invitrogen GeneArt Gene Synthesis | ThermoFisher | N/A |
| SIX3, Invitrogen GeneArt Gene Synthesis | ThermoFisher | N/A |
| PARP1, Invitrogen GeneArt Gene Synthesis | ThermoFisher | N/A |
| PARP1 E988K, Invitrogen GeneArt Gene Synthesis | ThermoFisher | N/A |
| PARP1 M890V+D899N, Invitrogen GeneArt Gene Synthesis | ThermoFisher | N/A |
| DELQPASIDP peptide | Carpenter et al., 2005, Therizols et al., 2014 | N/A |
| A-key and P1-key primers (5C library) | Fraser et al., 2012 | N/A |
| 5C primers covering USP22 and Shh region | Williamson et al. 2016 | N/A |
| NEBNext Multiplex Oligos for Illumina (Index Primers Set 1) | NEB | E7335 |
| Shh Custom Stellaris RNA FISH Probes | Stellaris RNA FISH | This study |
| Software and Algorithms | ||
| Nikon Nis-Elements Imaging software | Nikon UK Ltd | https://www.microscope.healthcare.nikon.com/en_EU/products/software/nis-elements |
| Volocity 3D Image Analysis Softwar | PerkinElmer Inc | https://www.perkinelmer.co.uk/lab-products-and-services/cellular-imaging/volocity-3d-visualization.html |
| TAL Effector Nucleotide Targeter 2.0 (TALE design) | Doyle et al., 2012 | https://tale-nt.cac.cornell.edu/ |
| LightCycler 480 Software | Roche | 04898915001 |
| Linear Models for Microarray Data (Limma) Bioconductor package (R) |
Bioconductor | http://www.bioconductor.org/packages/devel/bioc//manuals/limma/man/limma.pdf |
| Torrent 5C data transformation pipeline on Galaxy | Galaxy | https://main.g2.bx.psu.edu |
| HOMER v4.8 (RNA seq) | http://homer.ucsd.edu/homer/ | |
| Other | ||
| H3K27me3 Chip-Seq data | Illingworth et al., 2015 | GEO: GSE69955 |
Lead Contact and Materials Availability
Materials Availability Statement: Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Wendy Bickmore (Wendy.Bickmore@igmm.ed.ac.uk).
Experimental Model and Subject Details
46c male mouse embryonic stem cells (mESCs), derived from E14tg2A, contain a GFP insertion into the Sox1 locus (Ying et al., 2003) were used for this study. mESCs were cultured in GMEM supplemented with 10% fetal bovine serum (FBS), 1000 units/ml LIF, nonessential amino acids, sodium pyruvate, 2-β-mercaptoethanol, L-glutamine and penicillin/streptomycin.
Method Details
Cell Culture, differentiation and transfection
ESCs were differentiated into NPCs essentially as described previously (Pollard et al., 2006, Ying et al., 2003).To ensure a homogeneous starting population of ESCs, cells were harvested using trypsin 24 h prior to initiating differentiation and seeded at 3x106 on 0.1% gelatin coated Corning flasks. Differentiation media (1:1 DMEM/F12:Neurobasal medium (GIBCO, #31330-032 and 21103-049 respectively) supplemented with 0.5x B27 (Invitrogen, #17504044), 0.5x N2 (Invitrogen, # 17502048), L-Glutamine and 50 μM 2-β-mercaptoethanol (GIBCO, #31350-010)) was prepared fresh on the day of differentiation. On D0, ESCs were harvested, washed twice with PBS and twice with differentiation media then seeded at a density of 1x106 ESCs on 0.1% gelatin coated T75 Corning flasks (gelatin coated at RT for 3-6 h and washed twice with PBS prior to seeding). Cells were then cultured for 7 days with daily media changes after day 2. NPC differentiation was monitored visually through expression of the GFP reporter.
ESCs were transfected with TALE plasmids using Lipofectamine® 2000 Reagent (Invitrogen, #11668) and FAC-sorted for GFP as previously described (Therizols et al., 2014). Briefly, 1x106 ESCs were transfected in a 6-well plate with 2.5μg of plasmid and 7μl of Lipofectamine. The culture medium was changed 6h after transfection. Transfected cells were FACS sorted based on eGFP expression 24h after transfection and re-seeded on slides or 6-well-plates. Flow cytometric analysis was performed using the 488nm laser of a BD FACSAriaII SORP (Becton Dickinson) with 525/50 nm bandpass filters. BD FACSDiva software (Becton Dickinson, Version 6.1.2) was used for instrument control and data analysis.
For PARP inhibition, 24h after transfection or 5h after FACs olaparib was added to media to a concentration of 10μM for 1.5 h before cells were fixed for FISH.
3D-FISH
1x106 ESCs or NPCs were seeded on Sigma Poly-Prep glass slides for 5h. Cells were fixed in 4% paraformaldehyde (pFA) for 10 mins at room temperature (r.t.) and then permeabilized using 0.5% TritonX for 10 mins (Eskeland et al., 2010). Fosmid clones were labeled with green-dUTP (Abbott Molecular 02N32-050, 00884999002913) or red-dUTP (ChromaTide Alexa Fluor 594-5-dUTP C11400). Approximately 150 ng of labeled fosmid probes were used per slide, together with 15 μg of mouse Cot1 DNA (GIBCO BRL) and 10 μg salmon sperm DNA. Probes were denatured at 70°C for 5 min, reannealed with CotI DNA for 15 min at 37°C and hybridized to the denatured slides overnight. Slides were incubated in 2XSSC with RNase for 1h at 37°C, dried in a series of EtOH washes and pre-warmed in an oven at 75°C for 15 mins before being denatured at 80°C for 20 mins in 70% formamide/2 × SSC pH 7.5 followed by 3 minutes in ice-cold 70% ethanol, dried in EtOH gradient and hybridized at 37°C overnight. Slides were then washed in 2XSSC at 37°C then in 0.5XSSC at 65°C and finally in 4XSSC-Tween. FISH probes are described in Table S8.
For tissues sections 3D-FISH mouse embryos were collected, fixed, embedded, sectioned and processed as previously described (Morey et al., 2007). Briefly, embryos from CD1 mice were collected at E10.5, fixed in 4% pFa overnight at 4°C, dehydrated through an ethanol series, cleared in xylene and embedded in paraffin blocks. Sections laid on Superfrost slides were heated to 60°C for 20 mins and washed in xylene before rehydration through an ethanol series. Slides were microwaved for 20 mins in 0.1 M citrate pH 6.0 buffer, washed in water and rinsed once in 2 × SSC before use. FISH was performed as described above, but with a denaturation step of 3 minutes at 75°C.
RNA FISH
Custom Stellaris® RNA FISH Probes were designed against Shh nascent mRNA (pool of 48 unique 22-mer probes) by utilizing the Stellaris® RNA FISH Probe Designer (Biosearch Technologies, Inc., Petaluma, CA) (https://www.biosearchtech.com/support/tools/design-software/stellaris-probe-designer, version 4.2). Slides were hybridized with the Shh Stellaris FISH Probe set labeled with Quasar 570 (Biosearch Technologies, Inc.), following the manufacturer’s instructions (https://www.biosearchtech.com/support/resources/stellaris-protocols). Briefly, FFPE tissue sections from E10.5 embryos were deparaffinised in xylene, hydrated in ethanol and permeabilised in 70% ethanol overnight at 4°C. Slides were incubated in 10 μg/mL proteinase K in 1X PBS for 20 minutes at 37°C followed washes in 1X PBS and Stellaris Wash Buffer A (#SMF-WA1-60). Shh RNA FISH probes were diluted in Stellaris RNA FISH hybridization buffer (#SMF-HB1-10) to 125 nM and hybridized to slides overnight in humidified chamber at 37°C. Slides were washed 2 × 30 minutes in Wash Buffer A at 37°C, counterstained with 5 ng/mL DAPI, washed in Stellaris Wash Buffer B (#SMF-WB1-20) and mounted in Vectashield.
Image Capture
Super-resolution images from 3D FISH were acquired using structured illumination microscopy (SIM). 3D-SIM images were acquired on a N-SIM (Nikon Instruments, UK) using a 100x Nikon Plan Apo TIRF objective (NA 1.49, oil immersion) and refractive index matched immersion oil (Nikon Instruments). Images were captured using an Andor DU-897X-5254 EMCCD camera using 405, 488, 561 and 640nm laser lines. Step size for z stacks was set to 0.12 μm as required by the manufacturer’s software. For each focal plane, 15 images (5 phases, 3 angles) were captured with the NIS-Elements software.
RNA FISH slides were imaged using a Photometrics Coolsnap HQ2 CCD camera and a Zeiss AxioImager A1 fluorescence microscope with a Plan Apochromat 100x 1.4NA objective, a Nikon Intensilight Mercury based light source (Nikon UK Ltd, Kingston-on-Thames, UK) and Chroma #89014ET (3 color) single excitation and emission filters (Chroma Technology Corp., Rockingham, VT) with the excitation and emission filters installed in Prior motorised filter wheels. A piezoelectrically driven objective mount (PIFOC model P-721, Physik Instrumente GmbH & Co, Karlsruhe) was used to control movement in the z dimension. Step size for z stacks was set to 0.2 μm. Hardware control and image capture were performed using Nikon Nis-Elements software (Nikon UK Ltd, Kingston-on-Thames, UK). Images were deconvolved using a calculated PSF in Volocity (PerkinElmer Inc, Waltham MA). Image analysis was carried out using the Quantitation module of Volocity (PerkinElmer Inc, Waltham MA).
RNA extraction and Real Time quantitative Polymerase Chain Reaction (qRT-PCR)
RNA was prepared using RNeasy mini kit (QIAGEN) according to the manufacturer’s protocol, including a DNaseI (QIAGEN) treatment for 15 mins at r.t. cDNA was synthesized from 2 μg purified RNA with Superscript II reverse transcriptase (Invitrogen) primed with random hexamers (Promega).
Real-time PCR was carried on the Roche LightCycler 480 Real-Time PCR System using a Lightcycler 480 Sybr Green detection kit (Roche). The real-time thermal cycler was programmed as follows: 15 min Hotstart; 44 PCR cycles (95°C for 15 s, 55°C for 30 s, 72°C for 30 s). A standard curve for each primer set was obtained using a mix of each of the cDNAs. Primers are listed in Table S9. Ptn and Nrp1 primers were taken from (Therizols et al., 2014).
Single Cell RT-qPCR
RNA reverse transcription and cDNA pre-amplification from single cells were performed as previously described (Dalerba et al., 2011) with some modifications. Each well of a 96-well PCR plate was loaded with 5 μl 2x Reaction Mix, 0.2 μl Superscript III RT/Platinum Taq Mix with RNaseOUT Ribonuclease Inhibitor (Invitrogen Cells Direct One-Step qRT-PCR kit, Life Technologies), 2.5 μl primer mix (containing 200 nM of each gene-specific primer), 1.3 μl H2O. Single-cell suspensions were sorted on their GFP reporter into separate wells of the 96-well PCR plate. 32 cells were sorted into one well, to be used for serial dilution for generation of qRT-PCR standard curves. RNA reverse transcription and 22 cycles of cDNA pre-amplification were performed as previously described (Dalerba et al., 2011). The cDNA was diluted 1:5 in H2O. For the qRT-PCR, 9 μl of cDNA was loaded into a 96-well plate with one 96-well plate loaded with one primer pair i.e Gapdh or Shh.
TALE Design & Assembly
TALEs were designed using TAL Effector Nucleotide Targeter 2.0 software (Doyle et al., 2012) and the assembly was performed following the protocol described in (Therizols et al., 2014). TALE DNA binding domains specific for 16 base pairs of the Shh promoter, SBE6, SBE2, and NE were assembled following the methods described in (Ding et al., 2013). Four pre-assembled multimeric TALE repeat modules (three 4-mer and one 3-mer) were assembled into a modified TALEN backbone in which the BamHI-BsrGI fragment containing hFokI2-2A-eGFP was replaced by a gBlocks® (IDT) fragment encoding Vp64-2A-eGFP (Therizols et al., 2014). The BamHI-BglII fragment containing Vp64 of the TALE-Vp64 plasmid was deleted to generate Tale-Δ.
To generate other TALE-fusions, the BamHI-NheI cloning site was further used to fuse the TALE with; the SA domain of LDB1 designed and ordered from gBlocks® (IDT), with CTCF, PARP1 and PARP1 mutants (PARP1 E988K and M890V+D899N) designed and ordered from GeneArt® (Life Technologies). To generate Tale-BP, the BamHI-NheI fragment containing Vp64 was replaced by a double strand oligonucleotide encoding the DELQPASIDP peptide (Carpenter et al., 2005).
Chromatin Immunoprecipitation and Microarrays
For cross-link ChIP, 0.5-1x107 ESC were first cross-linked with 2 mM EGS (Pearce, Thermo Scientific, #21565) in PBS for 60 min at r.t. Methanol-free formaldehyde was then added at a final concentration of 1% (Thermo Scientific Pierce, PN28906) for 10 min at r.t. and quenched for 5 min with 125mM glycine and then washing in PBS. All buffers were supplemented with the following additives just prior to use: 0.2 mM PMSF, 1 mM DTT, 1x Protease inhibitors (Calbiochem, 539134-1SET) and 1x phosphatase inhibitors (Roche, PhosSTOP, 04906837001). Purified DNA was isolated using a QIAquick PCR Purification Kit (QIAGEN). Med12 ChIP was performed as previously described (Vernimmen et al., 2007) using Med12 antibody (Bethyl laboratories, A300-774A). Quantitative (q)PCR was performed on a LightCycler480 (Roche) using the same guideline as for qRT-PCR. ChIP qPCR primers are displayed in Table S9.
For examination of histone H3 K27 acetylation (H3K27ac) by native ChIP, nuclei were prepared and resuspended in NB-R as previously described (Gilbert et al., 2003). Nuclei corresponding to 0.5-1x107 ESCs were digested with 50-80 Boehringer units of MNase (Sigma) for 10 min at r.t. in the presence of 20 μg RNase A to obtain a chromatin ladder enriched in tri-, tetra-, and some pentanucleosomes. The reaction was stopped by adding equal volume of Stop Buffer (215 mM NaCl, 10 mM TrisHCl pH 8, 20 mM EDTA, 5.5% Sucrose, 2% Triton X-100, 0.2 mM PMSF 1 mM DTT and complete protease inhibitor cocktail) and incubated on ice overnight. Between 50-150 μg released chromatin were pre-cleared with Protein G Sepharose (GE Healthcare) for 2 hr and mixed with 10 μg prebound H3K27ac antibody (Millipore 07-360) in the presence of 100 μg BSA and incubated for 3 hr at 4°C. Beads were then washed 3x with Wash Buffer (150 mM NaCl, 10 mM TrisHCl pH 8, 2 mM EDTA, 1% NP40, 1% Sodium deoxycholate, 0.2mM PMSF, 1mM DTT and protease inhibitor cocktail) and once in TE. Bound complexes were eluted with 0.1 M NaHCO3, 1% SDS at r.t. Immunoprecipitated and input DNA were purified with Proteinase K (Genaxxon) and QIAGEN PCR purification kit.
For Nimblegen Arrays (H3K27ac), 10ng of input (MNase digested) or ChIP DNA were amplified using the WGA2 whole genome amplification kit (Sigma). Amplified material was labeled with Cy3 or Cy5 by random priming according to the NimbleGen ChIP-chip protocol (Roche). Samples were hybridized for 20 h and washed according to manufacturer's protocol. A custom 3x720K mouse tiling array (NimbleGen, Roche) containing 179,493 unique probes from different genomic regions, with each probe represented by 4 replicates was used. Arrays were scanned on a NimbleGen MS 200 Microarray scanner (Roche) using 100% laser power and 2 μm resolution.
ChIP-seq data for H3K27me3 in mESCs were taken from (Illingworth et al., 2015) GEO: GSE69955.
5C primer, 5C library design and preparation
3C library preparation was performed as previously described (Williamson et al., 2014). 1 × 107 ESCs or NPCs were fixed with 1% formaldehyde for 10 min at r.t. Cross-linking was stopped with 125 mM glycine for 5 min at r.t. followed by 15 min on ice. Cells were centrifuged at 400 g for 10 min at 4°C, supernatants were removed, and cell pellets were flash-frozen on dry ice. Cell pellets were then treated as previously described (Williamson et al., 2016).
Briefly, 1-2 × 107 fixed cells were incubated for 15 min on ice in 200 μL of lysis buffer (10 mM Tris pH 8.0, 10 mM NaCl, 0.2% NP40, supplemented with fresh protease inhibitor cocktail). Cells were then disrupted on ice with a Dounce homogenizer (pestle B; 2 × 20 strokes) and cell suspensions centrifuged at 2000 g for 5 min. Supernatants were removed, the cell pellets were washed twice with 100 μL of 1 × CutSmart buffer (New England Biolabs), and resuspended in 100 μL of 1 × CutSmart buffer and divided into two Eppendorf tubes. 1 × CutSmart buffer (337 μL) was added to each tube, and the mixture was incubated for 10 min at 65°C with 0.1% SDS. Forty-four microliters of 10% Triton X-100 were added before overnight digestion with 400 U of HindIII. The restriction enzyme was inactivated by adding 86 μL 10% SDS and incubation for 30 min at 65°C. Samples were then individually diluted into 7.62 mL of ligation mix (750 μL 10% Triton X-100, 750 μL 10 × ligation buffer, 80 μL 10 mg/ml of BSA, 80 μL 100 mM ATP, 3000 cohesive end units of T4 DNA ligase) and incubated for 2 h at 16°C.
3C libraries were incubated overnight at 65°C with 50 μL of Proteinase K (10 mg/ml) and an additional 50 μL of Proteinase K the following day for 2 h. The DNA was purified by one phenol and one phenol–chloroform extraction and precipitated with 0.1 vol (800 μl) of 3 M NaOAc (pH 5.2) and 2.5 vol of cold EtOH (20 ml). After at least 1 h at −80°C, the DNA was centrifuged at 20,000 g for 25 min at 4°C, and the pellets washed with cold 70% EtOH. DNA was resuspended in 400 μL of TE (pH 8.0) and transferred to Eppendorf tubes for another phenol–chloroform extraction and precipitation with 40 μL of 3 M NaOAc (pH 5.2) and 1.1 mL of cold EtOH. DNA was recovered by centrifugation and washed eight times with cold 70% EtOH. Pellets were then dissolved in 100 μL of TE pH 8.0 and incubated with1 μL of 10 mg/ml RNase A for 15 min at 37°C.
For PARP1 experiments a modified 3C library protocol for low cell numbers (∼2 million) was used as described (Berlivet et al., 2013). Essentially this is the same as the standard protocol, the modifications being mainly in the buffer volumes for DNA ligation and purification and the number of alcohol washes.
5C primers covering the USP22 (mm9, chr11: 60,917,307–61,017,307) and Shh (mm9, chr5: 28317087-30005000) regions, library design, and preparation, were performed as described (Williamson et al., 2016). 5C libraries were prepared and amplified with the A-key and P1-key primers (Fraser et al., 2012). 3C libraries were first titrated by PCR for quality control (single band, absence of primer dimers, etc.) and to verify that contacts were amplified at frequencies similar to that usually obtained from comparable libraries. We used 1–11 μg of 3C library per 5C ligation reaction.
5C primer stocks (20 μM) were diluted individually in water on ice and mixed to a final concentration of 0.002 μM. Mixed diluted primers (1.7 μl) were combined with 1 μL of annealing buffer (10 × NEBuffer 4, New England Biolabs) on ice in reaction tubes. Salmon testis DNA (1.5 μg) was added to each tube, followed by the 3C libraries and water to a final volume of 10 μl. Samples were denatured for 5 min at 95°C and annealed for 16 h at 48°C. Ligation with 10 U of Taq DNA ligase was performed for 1 h at 48°C. One-tenth (3 μl) of each ligation was then PCR-amplified individually with primers against the A-key and P1-key primer tails. We used 26 or 28 cycles based on dilution series showing linear PCR amplification within that cycle range. The products from two to four PCR reactions were pooled before purifying the DNA on MinElute columns (QIAGEN).
5C libraries were quantified on agarose gels and diluted to 0.0534 ng/μL (for Xpress template kit version 2.0) or 12 pmol (for Ion Proton). One microliter of diluted 5C library was used for sequencing with an Ion Proton sequencer. Samples were sequenced as recommended by the manufacturer (Life Technologies).
4SU-seq
4SUseq was performed essentially as described previously (Rabani et al., 2011). Briefly, 4-thiouridine (4SU; Sigma, #T4509) was added to ESCs/NPCs in culture to a final concentration of 500 μM and incubated at 37°C for 20 min. Cells were harvested by trypsinisation and washed twice with PBS at r.t. Total RNA was isolated from 5x106 cells using Trizol according to the manufacturer’s instructions (Invitrogen, #15596026). Following precipitation, purified RNA was resuspended in 100 μL RNase-free water and DNase treated using the TURBO DNA-free kit according to the manufacturer’s instructions (Invitrogen, #AM1907M). Residual inactivation beads were removed by spinning the RNA sample through a QIAshredder column at 1000 g for 1 min (QIAGEN, #79654). Following quantification, 50 μg of RNA were incubated for 1.5 h at r.t. with 100 μg of Biotin-HPDP (Pierce, #21341; reconstituted in dimethylformamide at 1 mg/ml) in 1x Biotinylation Buffer (10 mM Tris pH 7.4 and 1 mM EDTA) to a total volume of 500 μl. Uncoupled biotin was removed through two consecutive rounds of 1:1 v/v chloroform extraction followed by isopropanol/NaCl precipitation. RNA was resuspended in 100 μL of RNase free water and mixed 1:1 w/w with μMacs Streptavidin beads (Miltenyi, #130-074-101) and incubated for 15 min at RT with rotation. The RNA / bead mixture was applied to a μMacs column following pre-equilibration with wash buffer (100 mM Tris pH 7.5, 10 mM EDTA, 1 M NaCl and 0.1% Tween20). The captured beads were then washed with 3 × 900 μl of 65°C wash buffer and 3 × 900 μl RT wash buffer. RNA was then eluted from the column by adding two consecutive rounds of 100 mM DTT. The eluate was added to 700 μl Buffer RLT (RNeasy MinElute Cleanup Kit; QIAGEN, #74204) and then purified according to the manufacturer’s instructions. Prior to library preparation, ribosomal RNA was depleted from the purified 4SU incorporate RNA using the low Input RiboMinus Eukaryote System v2 kit according to the manufacturer’s instructions (Ambion, # A15027).
Sequencing libraries were constructed using the NEBNext® Ultra II Directional RNA Library Prep Kit for Illumina according to the protocol for ribosome depleted RNA and with a 10 min RNA fragmentation step (NEB, #E7760). Library PCRs were supplemented with 2x SYBR dye (Sigma, #S9430) so that amplification could be monitored by quantitative PCR on a Roche lightcycler 480. To allow for sample multiplexing, PCRs were performed using index primers (NEBNext Multiplex Oligos for Illumina - Set 1. #E7335) and amplified to linear phase. Libraries were purified and then combined into 4 sample equimolar pools containing the indexes 1, 3, 6 and 8.
Quantification and Statistical Analysis
FISH image analysis
SIM image processing and reconstruction were carried out using the N-SIM module of NIS-Element Advanced Research software. Images were reconstructed using NiS Elements software (Nikon Instruments) from a z-stack comprising of no less than 1 μm of optical sections. In all SIM image reconstructions the Wiener and Apodization filter parameters were kept constant.
Image analysis was carried out using the Quantitation module of Volocity (PerkinElmer). Reconstructed SIM data was directly uploaded and analyzed on Volocity. The statistical significance of differences in inter-probe distances was assessed using the nonparametric Mann-Whitney U test to examine the null hypothesis. Bimodality analysis was done using Hartigan’s dip test. Each dataset consisted of 20 to 50 nuclei (40 to 100 loci). Biological replicates are shown under their p values in Supplemental figures and Tables.
qRT-PCR
The relative expression of each sample was measured by the Lightcycler software and normalized to the mean for Gapdh from replicates. The log2 of the ratio relative to eGFP transfected ESCs was calculated.
For single-cell qRT-PCR, 3 plates were used for technical replicates. Data analysis was performed as for regular qRT-PCR using the first well (32 cells) of ESCs and NPCs plates as a mean for Gapdh and Shh. For data analysis, wells without data for Gapdh or Shh were removed. Values normalized to Gapdh were then plotted in to R using a density plot.
Microarray analysis
Raw signal intensities were quantified from TIFF images using MS 200 Data Collection software. ChIP microarray data were analyzed in R using the bioconductor package Limma. Raw signal intensity values were averaged across replicate probes (4 replicated per unique probe sequence). Averaged signal intensities were subsequently corrected for dye bias between each pair of co-hybridized ChIP and input samples using loess normalization. Corrected values were then transformed into log2 ChIP/Input ratios and scale normalized. For visualization, normalized log2 intensity ratios were assigned to the mm9 location of the probe sequence.
5C analysis
Analysis of 5C sequencing data was performed as described previously (Berlivet et al., 2013). Sequencing data were processed through a Torrent 5C data transformation pipeline on Galaxy (https://usegalaxy.org). NPC-enriched and TALE activated-enriched 5C interactions were obtained by subtracting wild-type ESC 5C-seq data (Williamson et al., 2014). Before normalizing, interactions between adjacent fragments were removed due to the high noise: signal ratio likely to occur here. Data were normalized by dividing the number of reads of each 5C contact by the total number of reads from the corresponding sequence run. All scales shown correspond to this ratio multiplied by 103. Sequencing technical and biological replicates reads are displayed in Table S10.
4SU-seq analysis
Pooled 4SU libraries were sequenced on an Ilumina NextSeq 550 and the resulting fastq files were aligned to the mouse genome (mm9) using bowtie2 v2.2.6 with standard parameters for paired end sequence data to generate. SAM files. Mapped data was processed using HOMER v4.8. SAM files were converted into tag directories using ‘makeTagDirectory’ with the following parameters: -format sam -flip –sspe. Genomic intervals which extended beyond the end of the chromosomes were removed using ‘removeOutOfBoundsReads.pl’. Strand specific browser track files (.bigWig) were generated by combining replicate tag directories with the ‘makeTagDirectory’ function and then running ‘makeUCSCfile’ with the following parameters: -fsize 1e20 -strand + (or -) -norm 1e8 -color 25,50,200.
Data and Code Availability
Data from this paper are available at NCBI GEO under the series GSE89557 which includes sub-series for the 5C (GSE89388), ChIP-chip (GSE115642) and 4SU-seq data (GSE115774).
Acknowledgments
We thank the staff of the Institute of Genetics and Molecular Medicine (IGMM) fluorescence-activated cell sorting (FACS) and imaging facilities for their invaluable technical help. This work was supported by a Ph.D. studentship to N.S.B., funded through a grant from the UK Medical Research Council to the Edinburgh Super Resolution Imaging Consortium (ESRIC). Work in the group of W.A.B. is supported by MRC University Unit grant MC_UU_00007/2.
Author Contributions
N.S.B. conducted most of the experiments and data analysis, prepared the figures, and wrote the manuscript. I.W. assisted with the 5C experiments and manuscript editing. L.K. performed the RNA FISH. R.S.I. assisted with 4sU sequencing (4sU-seq), ChIP, and microarray analyses and manuscript editing. S.B. assisted with tissue section FISH. D.S. helped with ESC to NPC differentiation. G.R.G. assisted with the microarray data processing and statistical analyses. P.T. assisted with TALE design and building. W.A.B. conceived of the study, designed the study, coordinated the study, and wrote the manuscript. All of the authors gave final approval for publication.
Declaration of Interests
The authors declare no competing interests.
Published: September 4, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.molcel.2019.07.038.
Supplemental Information
References
- Alexander J.M., Guan J., Li B., Maliskova L., Song M., Shen Y., Huang B., Lomvardas S., Weiner O.D. Live-cell imaging reveals enhancer-dependent Sox2 transcription in the absence of enhancer proximity. eLife. 2019;8:e41769. doi: 10.7554/eLife.41769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali T., Renkawitz R., Bartkuhn M. Insulators and domains of gene expression. Curr. Opin. Genet. Dev. 2016;37:17–26. doi: 10.1016/j.gde.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Altmeyer M., Neelsen K.J., Teloni F., Pozdnyakova I., Pellegrino S., Grøfte M., Rask M.D., Streicher W., Jungmichel S., Nielsen M.L., Lukas J. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose) Nat. Commun. 2015;6:8088. doi: 10.1038/ncomms9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E., Hill R.E. Long range regulation of the sonic hedgehog gene. Curr. Opin. Genet. Dev. 2014;27:54–59. doi: 10.1016/j.gde.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Bartman C.R., Hsu S.C., Hsiung C.C.-S., Raj A., Blobel G.A. Enhancer Regulation of Transcriptional Bursting Parameters Revealed by Forced Chromatin Looping. Mol. Cell. 2016;62:237–247. doi: 10.1016/j.molcel.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabdallah N.S., Bickmore W.A. Regulatory Domains and Their Mechanisms. Cold Spring Harb. Symp. Quant. Biol. 2015;80:45–51. doi: 10.1101/sqb.2015.80.027268. [DOI] [PubMed] [Google Scholar]
- Benabdallah N.S., Gautier P., Hekimoglu-Balkan B., Lettice L.A., Bhatia S., Bickmore W.A. SBE6: a novel long-range enhancer involved in driving sonic hedgehog expression in neural progenitor cells. Open Biol. 2016;6:160197. doi: 10.1098/rsob.160197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlivet S., Paquette D., Dumouchel A., Langlais D., Dostie J., Kmita M. Clustering of tissue-specific sub-TADs accompanies the regulation of HoxA genes in developing limbs. PLoS Genet. 2013;9:e1004018. doi: 10.1371/journal.pgen.1004018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boija A., Klein I.A., Sabari B.R., Dall’Agnese A., Coffey E.L., Zamudio A.V., Li C.H., Shrinivas K., Manteiga J.C., Hannett N.M. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell. 2018;175:1842–1855.e16. doi: 10.1016/j.cell.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.M., Roberts N.A., Graham B., Waithe D., Lagerholm C., Telenius J.M., De Ornellas S., Oudelaar A.M., Scott C., Szczerbal I. A tissue-specific self-interacting chromatin domain forms independently of enhancer-promoter interactions. Nat. Commun. 2018;9:3849. doi: 10.1038/s41467-018-06248-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger M., Groudine M. Functional and mechanistic diversity of distal transcription enhancers. Cell. 2011;144:327–339. doi: 10.1016/j.cell.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess-Beusse B., Farrell C., Gaszner M., Litt M., Mutskov V., Recillas-Targa F., Simpson M., West A., Felsenfeld G. The insulation of genes from external enhancers and silencing chromatin. Proc. Natl. Acad. Sci. USA. 2002;99(Suppl 4):16433–16437. doi: 10.1073/pnas.162342499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A.E., Memedula S., Plutz M.J., Belmont A.S. Common effects of acidic activators on large-scale chromatin structure and transcription. Mol. Cell. Biol. 2005;25:958–968. doi: 10.1128/MCB.25.3.958-968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D., Chakalova L., Osborne C.S., Dai Y.F., Fraser P. Long-range chromatin regulatory interactions in vivo. Nat. Genet. 2002;32:623–626. doi: 10.1038/ng1051. [DOI] [PubMed] [Google Scholar]
- Cho W.K., Spille J.H., Hecht M., Lee C., Li C., Grube V., Cisse I.I. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science. 2018;361:412–415. doi: 10.1126/science.aar4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S., Dugast-Darzacq C., Liu Z., Dong P., Dailey G.M., Cattoglio C., Heckert A., Banala S., Lavis L., Darzacq X., Tjian R. Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science. 2018;361:eaar2555. doi: 10.1126/science.aar2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalerba P., Kalisky T., Sahoo D., Rajendran P.S., Rothenberg M.E., Leyrat A.A., Sim S., Okamoto J., Johnston D.M., Qian D. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat. Biotechnol. 2011;29:1120–1127. doi: 10.1038/nbt.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Lee J., Wang H., Miller J., Reik A., Gregory P.D., Dean A., Blobel G.A. Controlling long-range genomic interactions at a native locus by targeted tethering of a looping factor. Cell. 2012;149:1233–1244. doi: 10.1016/j.cell.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Rupon J.W., Krivega I., Breda L., Motta I., Jahn K.S., Reik A., Gregory P.D., Rivella S., Dean A., Blobel G.A. Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell. 2014;158:849–860. doi: 10.1016/j.cell.2014.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q., Lee Y.-K., Schaefer E.A.K., Peters D.T., Veres A., Kim K., Kuperwasser N., Motola D.L., Meissner T.B., Hendriks W.T. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013;12:238–251. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle E.L., Booher N.J., Standage D.S., Voytas D.F., Brendel V.P., Vandyk J.K., Bogdanove A.J. TAL Effector-Nucleotide Targeter (TALE-NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 2012;40:W117–W122. doi: 10.1093/nar/gks608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel N., Raval A.K., Thorvaldsen J.L., Bartolomei S.M. Three-dimensional conformation at the H19/Igf2 locus supports a model of enhancer tracking. Hum. Mol. Genet. 2008;17:3021–3029. doi: 10.1093/hmg/ddn200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskeland R., Leeb M., Grimes G.R., Kress C., Boyle S., Sproul D., Gilbert N., Fan Y., Skoultchi A.I., Wutz A., Bickmore W.A. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol. Cell. 2010;38:452–464. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J., Ethier S.D., Miura H., Dostie J. A Torrent of data: mapping chromatin organization using 5C and high-throughput sequencing. Methods Enzymol. 2012;513:113–141. doi: 10.1016/B978-0-12-391938-0.00005-7. [DOI] [PubMed] [Google Scholar]
- Fukaya T., Lim B., Levine M. Enhancer Control of Transcriptional Bursting. Cell. 2016;166:358–368. doi: 10.1016/j.cell.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X., Speirs C., Lagutin O., Inbal A., Liu W., Solnica-Krezel L., Jeong Y., Epstein D.J., Oliver G. Haploinsufficiency of Six3 fails to activate Sonic hedgehog expression in the ventral forebrain and causes holoprosencephaly. Dev. Cell. 2008;15:236–247. doi: 10.1016/j.devcel.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert N., Boyle S., Sutherland H., de Las Heras J., Allan J., Jenuwein T., Bickmore W.A. Formation of facultative heterochromatin in the absence of HP1. EMBO J. 2003;22:5540–5550. doi: 10.1093/emboj/cdg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B., Swigut T., Spencley A., Bauer M.R., Chung M., Meyer T., Wysocka J. Transcription-coupled changes in nuclear mobility of mammalian cis-regulatory elements. Science. 2018;359:1050–1055. doi: 10.1126/science.aao3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D., Shrinivas K., Young R.A., Chakraborty A.K., Sharp P.A. A Phase Separation Model for Transcriptional Control. Cell. 2017;169:13–23. doi: 10.1016/j.cell.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huletsky A., de Murcia G., Muller S., Hengartner M., Ménard L., Lamarre D., Poirier G.G. The effect of poly(ADP-ribosyl)ation on native and H1-depleted chromatin. A role of poly(ADP-ribosyl)ation on core nucleosome structure. J. Biol. Chem. 1989;264:8878–8886. [PubMed] [Google Scholar]
- Illingworth R.S., Moffat M., Mann A.R., Read D., Hunter C.J., Pradeepa M.M., Adams I.R., Bickmore W.A. The E3 ubiquitin ligase activity of RING1B is not essential for early mouse development. Genes Dev. 2015;29:1897–1902. doi: 10.1101/gad.268151.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y., El-Jaick K., Roessler E., Muenke M., Epstein D.J. A functional screen for sonic hedgehog regulatory elements across a 1 Mb interval identifies long-range ventral forebrain enhancers. Development. 2006;133:761–772. doi: 10.1242/dev.02239. [DOI] [PubMed] [Google Scholar]
- Jeong Y., Leskow F.C., El-Jaick K., Roessler E., Muenke M., Yocum A., Dubourg C., Li X., Geng X., Oliver G., Epstein D.J. Regulation of a remote Shh forebrain enhancer by the Six3 homeoprotein. Nat. Genet. 2008;40:1348–1353. doi: 10.1038/ng.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey M.H., Newman J.J., Bilodeau S., Zhan Y., Orlando D.A., van Berkum N.L., Ebmeier C.C., Goossens J., Rahl P.B., Levine S.S. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–435. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.-K., Hemberg M., Gray J.M., Costa A.M., Bear D.M., Wu J., Harmin D.A., Laptewicz M., Barbara-Haley K., Kuersten S. Widespread transcription at neuronal activity-regulated enhancers. Nature. 2010;465:182–187. doi: 10.1038/nature09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A.K., Vyas S., Rood J.E., Bhutkar A., Sharp P.A., Chang P. Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol. Cell. 2011;42:489–499. doi: 10.1016/j.molcel.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbradt A.G., Kulkarni M., Yi T., Takeuchi K., Sun Z.-Y.J., Luna R.E., Selenko P., Näär A.M., Wagner G. Structure of the VP16 transactivator target in the Mediator. Nat. Struct. Mol. Biol. 2011;18:410–415. doi: 10.1038/nsmb.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler G., Stühler T., Santolin L., Uhlmann T., Kremmer E., Lottspeich F., Berti L., Meisterernst M. A novel docking site on Mediator is critical for activation by VP16 in mammalian cells. EMBO J. 2003;22:6494–6504. doi: 10.1093/emboj/cdg619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey C., Da Silva N.R., Perry P., Bickmore W.A. Nuclear reorganisation and chromatin decondensation are conserved, but distinct, mechanisms linked to Hox gene activation. Development. 2007;134:909–919. doi: 10.1242/dev.02779. [DOI] [PubMed] [Google Scholar]
- Morgan S.L., Mariano N.C., Bermudez A., Arruda N.L., Wu F., Luo Y., Shankar G., Jia L., Chen H., Hu J.F. Manipulation of nuclear architecture through CRISPR-mediated chromosomal looping. Nat. Commun. 2017;8:15993. doi: 10.1038/ncomms15993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalabothula N., Al-jumaily T., Eteleeb A.M., Flight R.M., Xiaorong S., Moseley H., Rouchka E.C., Fondufe-Mittendorf Y.N. Genome-Wide Profiling of PARP1 Reveals an Interplay with Gene Regulatory Regions and DNA Methylation. PLoS One. 2015;10:e0135410. doi: 10.1371/journal.pone.0135410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton C., Avlonitis N., Corless S., Prendergast J.G., Mati I.K., Eijk P.P., Cockroft S.L., Bradley M., Ylstra B., Gilbert N. Transcription forms and remodels supercoiling domains unfolding large-scale chromatin structures. Nat. Struct. Mol. Biol. 2013;20:387–395. doi: 10.1038/nsmb.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petesch S.J., Lis J.T. Activator-induced spread of poly(ADP-ribose) polymerase promotes nucleosome loss at Hsp70. Mol. Cell. 2012;45:64–74. doi: 10.1016/j.molcel.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Cremins J.E., Sauria M.E., Sanyal A., Gerasimova T.I., Lajoie B.R., Bell J.S., Ong C.T., Hookway T.A., Guo C., Sun Y. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell. 2013;153:1281–1295. doi: 10.1016/j.cell.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier G.G., de Murcia G., Jongstra-Bilen J., Niedergang C., Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc. Natl. Acad. Sci. USA. 1982;79:3423–3427. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard S.M., Benchoua A., Lowell S. Neural stem cells, neurons, and glia. Methods Enzymol. 2006;418:151–169. doi: 10.1016/S0076-6879(06)18010-6. [DOI] [PubMed] [Google Scholar]
- Pradeepa M.M., Grimes G.R., Kumar Y., Olley G., Taylor G.C.A., Schneider R., Bickmore W.A. Histone H3 globular domain acetylation identifies a new class of enhancers. Nat. Genet. 2016;48:681–686. doi: 10.1038/ng.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabani M., Levin J.Z., Fan L., Adiconis X., Raychowdhury R., Garber M., Gnirke A., Nusbaum C., Hacohen N., Friedman N. Metabolic labeling of RNA uncovers principles of RNA production and degradation dynamics in mammalian cells. Nat. Biotechnol. 2011;29:436–442. doi: 10.1038/nbt.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A., Bajpai R., Swigut T., Brugmann S.A., Flynn R.A., Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolli V., O’Farrell M., Ménissier-de Murcia J., de Murcia G. Random mutagenesis of the poly(ADP-ribose) polymerase catalytic domain reveals amino acids involved in polymer branching. Biochemistry. 1997;36:12147–12154. doi: 10.1021/bi971055p. [DOI] [PubMed] [Google Scholar]
- Sabari B.R., Dall’Agnese A., Boija A., Klein I.A., Coffey E.L., Shrinivas K., Abraham B.J., Hannett N.M., Zamudio A.V., Manteiga J.C. Coactivator condensation at super-enhancers links phase separation and gene control. Science. 2018;361:eaar3958. doi: 10.1126/science.aar3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatsubashi S., Maki A., Ito S., Shirode Y., Suzuki E., Zhao Y., Yamagata K., Kouzmenko A., Takeyama K., Kato S. Ecdysone receptor-dependent gene regulation mediates histone poly(ADP-ribosyl)ation. Biochem. Biophys. Res. Commun. 2004;320:268–272. doi: 10.1016/j.bbrc.2004.05.157. [DOI] [PubMed] [Google Scholar]
- Schiewer M.J., Goodwin J.F., Han S., Brenner J.C., Augello M.A., Dean J.L., Liu F., Planck J.L., Ravindranathan P., Chinnaiyan A.M. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2:1134–1149. doi: 10.1158/2159-8290.CD-12-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y., Chang Y.C., Lee D.S.W., Berry J., Sanders D.W., Ronceray P., Wingreen N.S., Haataja M., Brangwynne C.P. Liquid Nuclear Condensates Mechanically Sense and Restructure the Genome. Cell. 2018;175:1481–1491.e13. doi: 10.1016/j.cell.2018.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlyueva D., Stampfel G., Stark A. Transcriptional enhancers: from properties to genome-wide predictions. Nat. Rev. Genet. 2014;15:272–286. doi: 10.1038/nrg3682. [DOI] [PubMed] [Google Scholar]
- Singh H.R., Nardozza A.P., Möller I.R., Knobloch G., Kistemaker H.A.V., Hassler M., Harrer N., Blessing C., Eustermann S., Kotthoff C. A Poly-ADP-Ribose Trigger Releases the Auto-Inhibition of a Chromatin Remodeling Oncogene. Mol. Cell. 2017;68:860–871.e7. doi: 10.1016/j.molcel.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Smallwood A., Ren B. Genome organization and long-range regulation of gene expression by enhancers. Curr. Opin. Cell Biol. 2013;25:387–394. doi: 10.1016/j.ceb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutourina J. Transcription regulation by the Mediator complex. Nat. Rev. Mol. Cell Biol. 2018;19:262–274. doi: 10.1038/nrm.2017.115. [DOI] [PubMed] [Google Scholar]
- Taylor G.C.A., Eskeland R., Hekimoglu-Balkan B., Pradeepa M.M., Bickmore W.A. H4K16 acetylation marks active genes and enhancers of embryonic stem cells, but does not alter chromatin compaction. Genome Res. 2013;23:2053–2065. doi: 10.1101/gr.155028.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therizols P., Illingworth R.S., Courilleau C., Boyle S., Wood A.J., Bickmore W.A. Chromatin decondensation is sufficient to alter nuclear organization in embryonic stem cells. Science. 2014;346:1238–1242. doi: 10.1126/science.1259587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhuis B., Palstra R.J., Splinter E., Grosveld F., de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol. Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Tulin A., Spradling A. Chromatin loosening by poly(ADP)-ribose polymerase (PARP) at Drosophila puff loci. Science. 2003;299:560–562. doi: 10.1126/science.1078764. [DOI] [PubMed] [Google Scholar]
- Tulin A., Chinenov Y., Spradling A. Regulation of chromatin structure and gene activity by poly(ADP-ribose) polymerases. Curr. Top. Dev. Biol. 2003;56:55–83. doi: 10.1016/s0070-2153(03)01007-x. [DOI] [PubMed] [Google Scholar]
- Vernimmen D., De Gobbi M., Sloane-Stanley J.A., Wood W.G., Higgs D.R. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007;26:2041–2051. doi: 10.1038/sj.emboj.7601654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernimmen D., Bickmore W.A. The Hierarchy of Transcriptional Activation: From Enhancer to Promoter. Trends Genet. 2015;31:696–708. doi: 10.1016/j.tig.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Vojnic E., Mourão A., Seizl M., Simon B., Wenzeck L., Larivière L., Baumli S., Baumgart K., Meisterernst M., Sattler M., Cramer P. Structure and VP16 binding of the Mediator Med25 activator interaction domain. Nat. Struct. Mol. Biol. 2011;18:404–409. doi: 10.1038/nsmb.1997. [DOI] [PubMed] [Google Scholar]
- Williamson I., Berlivet S., Eskeland R., Boyle S., Illingworth R.S., Paquette D., Dostie J., Bickmore W.A. Spatial genome organization: contrasting views from chromosome conformation capture and fluorescence in situ hybridization. Genes Dev. 2014;28:2778–2791. doi: 10.1101/gad.251694.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson I., Lettice L.A., Hill R.E., Bickmore W.A. Shh and ZRS enhancer colocalisation is specific to the zone of polarising activity. Development. 2016;143:2994–3001. doi: 10.1242/dev.139188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R.H.G., Castellano G., Bonet J., Le Dily F., Font-Mateu J., Ballaré C., Nacht A.S., Soronellas D., Oliva B., Beato M. CDK2-dependent activation of PARP-1 is required for hormonal gene regulation in breast cancer cells. Genes Dev. 2012;26:1972–1983. doi: 10.1101/gad.193193.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Minor P.J., Zhao Y.T., Jeong Y., Pani A.M., King A.N., Symmons O., Gan L., Cardoso W.V., Spitz F. Cis-regulatory architecture of a brain signaling center predates the origin of chordates. Nat. Genet. 2016;48:575–580. doi: 10.1038/ng.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Q.-L., Stavridis M., Griffiths D., Li M., Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Yu W., Ginjala V., Pant V., Chernukhin I., Whitehead J., Docquier F., Farrar D., Tavoosidana G., Mukhopadhyay R., Kanduri C. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat. Genet. 2004;36:1105–1110. doi: 10.1038/ng1426. [DOI] [PubMed] [Google Scholar]
- Zhang F., Cong L., Lodato S., Kosuri S., Church G.M., Arlotta P. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat. Biotechnol. 2011;29:149–153. doi: 10.1038/nbt.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Wang Y., Wang L., Luo X., Huang K., Wang C., Du M., Liu F., Luo T., Huang D., Huang K. Poly(ADP-ribose) polymerase 1 is a key regulator of estrogen receptor α-dependent gene transcription. J. Biol. Chem. 2013;288:11348–11357. doi: 10.1074/jbc.M112.429134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Dean A. An insulator blocks spreading of histone acetylation and interferes with RNA polymerase II transfer between an enhancer and gene. Nucleic Acids Res. 2004;32:4903–4919. doi: 10.1093/nar/gkh832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Ling J., Zhang L., Pi W., Wu M., Tuan D. A facilitated tracking and transcription mechanism of long-range enhancer function. Nucleic Acids Res. 2007;35:5532–5544. doi: 10.1093/nar/gkm595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this paper are available at NCBI GEO under the series GSE89557 which includes sub-series for the 5C (GSE89388), ChIP-chip (GSE115642) and 4SU-seq data (GSE115774).