Abstract
In Ontario, within the past few years, there has been a marked increase in the number of non-commercial poultry flocks (referred to as “small flocks”). Small poultry flocks may act as a reservoir of avian and zoonotic pathogens, given the flocks’ limited access to veterinary services, inadequate biosecurity practices, and increased risk of contact with wild birds. Despite these potential risks, there is a scarcity of data concerning the prevalence of poultry and zoonotic pathogens among these flocks. To assess the baseline prevalence of bacterial and viral infectious pathogens, prospective surveillance of small flock postmortem submissions to the Animal Health Laboratory was conducted over a 2-y period. With the owner’s consent, a postmortem examination and pre-set tests for infectious agents were conducted. A total of 160 submissions, mainly chickens (84%), were received. Among bacterial pathogens, Brachyspira spp., Mycoplasma synoviae, Campylobacter spp., Mycoplasma gallisepticum, and Salmonella spp. were detected in 37%, 36%, 35%, 23%, and 3% of tested submissions, respectively. Among viral pathogens, infectious bronchitis virus, fowl adenovirus, infectious laryngotracheitis virus, avian reovirus, and infectious bursal disease virus were detected in 39%, 35%, 15%, 4%, and 1% of submissions, respectively. We detected non-virulent avian avulavirus 1 from two chickens in a single submission, and low-pathogenic H10N8 influenza A virus from a single turkey submission. Our study provides baseline prevalence of viral and bacterial pathogens circulating in Ontario small flocks and may help animal and human health professionals to educate small flock owners about disease prevention.
Keywords: avian avulavirus 1, avian influenza virus, backyard flocks, disease surveillance, epidemiology, infectious laryngotracheitis virus, mixed respiratory infection, poultry pathogens
Introduction
In countries with an industrial poultry sector, such as Canada, there has been a substantial increase in the number of non-commercial poultry flocks (i.e., small flocks) raised in urban and peri-urban areas (Canadian Broadcasting Corporation, http://www.cbc.ca/news/the-backyard-chicken-debate-1.916153). In Canada, poultry production (chicken and turkey industries) is regulated by a quota system. In the province of Ontario, non-commercial poultry operations may have up to 299 broilers, 99 layers, and 49 turkeys. Non-commercial poultry owners can register their flock with a provincial marketing board (such as the Chicken Farmers of Ontario [CFO]) at the time of purchase of birds from broker dealers or hatcheries; in 2016, >16,000 non-commercial poultry growers registered their flocks (CFO, https://www.familyfoodgrower.ca/). However, a definitive number of non-commercial flocks within the province is not available and is likely higher than this, given that registration is voluntary and not done by owners who keep birds in urban areas where the practice is illegal (Yonge Street, www.yongestreetmedia.ca/features/backyardchickens05082013.aspx; Global News Radio 640 Toronto, https://globalnews.ca/news/3782510/toronto-city-council-approves-pilot-project-allowing-backyard-chickens).
In Canada and the United States, surveys have shown that relaxed biosecurity practices, insufficient veterinary supervision, and lack of prophylactic measures (e.g., vaccination) are common in non-commercial poultry flocks.4,5,12,20,23,32 A survey directed to Ontario’s small flock owners registered with the CFO in 2010–2011, demonstrated that nearly half of evaluated flocks had an increased risk of contact with wild birds.4 Wild birds are considered potential reservoirs of avian pathogens, and increased likelihood of contact could facilitate spillover of several infectious agents, including avian influenza virus (AIV; species Influenza A virus) and avian avulavirus 1 (AAvV-1; species Avian orthoavulavirus 1; previously Newcastle disease virus). In Ontario, a spatial analysis4 estimated the average distance between commercial and non-commercial flocks to be 1.6 km, which is lower than the culling radius considered effective in containing the spread of AIV during an outbreak.2 This density can also increase the risk of transmission of other avian pathogens, such as infectious laryngotracheitis virus (ILTV; species Gallid alphaherpesvirus 1), for which wind-borne transmission has been demonstrated.18 During the Newcastle disease outbreaks in California in 1999–2000 and 2018, exotic (or “virulent”) AAvV-1 was isolated in small chicken flocks,8,9 stressing the importance of strict biosecurity to avoid disease transmission between commercial and non-commercial flocks.9
Despite these concerns, the contribution of small flocks to the spread of high-consequence infectious agents, such as AIV and AAvV-1, has been small when compared to commercial flocks.1,33 For instance, during the H5N2 highly pathogenic AIV outbreak in Ontario in 2015, no small flocks tested positive for the virus (Canadian Food Inspection Agency [CFIA], http://www.inspection.gc.ca/animals/terrestrial-animals/diseases/reportable/ai/eng/1323990856863/1323991018946), and during the H7N3 highly pathogenic AIV outbreaks in British Columbia in 2004, only 2 small flocks tested positive, in contrast to 28 positive commercial premises.29
Non-commercial flocks also represent a concern for public health by increasing the risk of human exposure to zoonotic and foodborne pathogens, such as Salmonella spp. and Campylobacter spp.14 In Ontario, poultry meat and eggs that are not sold to third parties can be consumed by the owner and their family without veterinary inspection, a practice that could increase the risk of foodborne diseases in households that raise poultry for domestic consumption (Ontario Ministry of Agriculture, Food and Rural Affairs, http://www.omafra.gov.on.ca/english/livestock/urbanagbib/sellingyourproducts.htm). In addition, keeping poultry as pets can increase the risk of pathogen transmission through direct contact with birds that might be actively shedding pathogens. A statement from the Centers for Disease Control and Prevention (CDC) was issued encouraging parents to avoid contact between poultry species (including ducklings) and children <5 y old because of possible exposure to salmonellae (CDC, https://www.cdc.gov/features/salmonellapoultry/index.html).
Despite the ever-increasing popularity of small poultry flocks, to our knowledge, there is limited published data documenting the baseline prevalence of poultry and zoonotic pathogens among these flocks in Canada, and more specifically, Ontario.19 To establish the baseline prevalence of poultry pathogens and diseases among these flocks in Ontario, we conducted a prospective surveillance study of small flock postmortem submissions to the Animal Health Laboratory (AHL) over a 2-y period (October 2015–September 2017). An extensive array of pre-set microbiology tests and postmortem examinations were carried out for each submission. Results of our study are presented in 2 companion papers. In this first paper in the series, we report the results derived from the pre-set microbiology tests (pathogen prevalence). In the second paper of the series, we report detailed results of the postmortem investigation (disease prevalence).3
Materials and methods
Advertisement and inclusion criteria
From October 1, 2015 to September 29, 2017, Ontario small poultry flock owners were encouraged to submit their birds for postmortem examination through a licensed veterinarian to the Guelph or Kemptville AHL (University of Guelph) locations. Submissions could be dropped off directly or delivered to these laboratories through a prepaid courier service. Owners were charged a nominal fee to include all testing.
The study was advertised through professional veterinary organizations (Ontario Association of Poultry Veterinarians, Ontario Veterinary Medical Association), industry groups (CFO, Poultry Industry Council, Ontario Broiler Hatching Egg and Chick Commission), poultry research networks (Poultry Health Research Network at the University of Guelph), blogs, social media, and periodicals specifically addressing poultry fanciers (The Exhibitor). The study was also advertised at cooperatives, hatcheries, and poultry fairs because these are common sites for the purchase of new birds.
To be included in our study, both the flock and flock owner must reside within the province of Ontario, the owner was required to complete a husbandry and biosecurity questionnaire, and the flock must be within the numerical limits imposed by the quota system and not have a primarily commercial aim (i.e., non-quota, non-commercial). In addition, each owner was required to sign a consent form as per University of Guelph requirements (Research Ethics Board REB-16-12-657). Included in the study were broilers, layers, and turkeys from non-quota flocks (i.e., flocks composed of a maximum of 299 broiler chickens, 99 layer chickens, or 49 turkeys). For other poultry species not regulated by the quota system (game birds and waterfowl), a maximum flock size of 300 birds was adopted, because it was considered representative of flocks not raised for commercial purposes. Birds in the Columbiformes order were excluded from the study. A submission was defined as having a maximum of 5 sick and/or dead birds (fresh dead or frozen) of 1 species from the same flock (i.e., different species were divided into different submissions, even if from the same owner). As per AHL policy, each submission was made by a referring veterinarian.
Postmortem examination and pre-set microbiology tests
Two sets of data were derived from each submission; one focusing on pathogen prevalence at the submission level, and the other on diseases and lesions at the individual bird level (Supplementary Fig. 1). The pre-set array of microbiology tests for infectious agents was conducted on pooled samples from all of the birds in each submission, regardless of history or pathology findings (Table 1). Therefore, negative or positive test results for a pathogen are reported on a submission basis, rather than on a bird basis. This was done to detect infection of the flock, rather than of single birds. A postmortem examination was also performed on each bird; the methodology and results for that part of the study are reported in the second paper of the series.3 All tests were conducted in accordance with the standard operating procedures adopted by the AHL, an American Association of Veterinary Laboratory Diagnosticians–accredited diagnostic facility that functions as the provincial animal health laboratory for Ontario.
Table 1.
Pathogen prevalence at the submission level, and details of pre-set microbiology tests employed in a small poultry flock surveillance study in Ontario, over a 2-y period.
| Pathogen | Type of test | Pooled samples for each submission | Species tested | Submissions tested (N) | Submissions positive (n) | Prevalence (%) |
|---|---|---|---|---|---|---|
| Avian bornavirus10 | RT-rtPCR | Brains | All species | 160 | 0 | 0 |
| Influenza A virus17 | RT-rtPCR | Tracheal and cloacal swabs | All species | 160 | 1 | 0.6 (0.02–3) |
| Avian avulavirus 134 | RT-rtPCR | Tracheal and cloacal swabs | All species | 160 | 1 | 0.6 (0.02–3) |
| Fowl adenovirus (A/C, D, E)‡ | RT-rtPCR | Cloacal swabs | Chickens | 134 | 47 | 35 (27–44) |
| Infectious bronchitis virus7 | RT-rtPCR | Tracheal and cloacal swabs | Chickens | 134 | 52 | 39 (31–48) |
| Infectious laryngotracheitis virus6 | RT-rtPCR | Tracheal swabs | Chickens and game birds | 142 | 22 | 15 (10–23) |
| Infectious bursal disease virus | RT-rtPCR | Cloacal swabs | Chickens | 134 | 2 | 1 (0.2–5) |
| Avian reovirus | RT-rtPCR | Cloacal swabs | Chickens and turkeys | 144 | 6 | 4 (2–9) |
| Mycoplasma gallisepticum | rtPCR | Tracheal swabs | All species except ducks | 151* | 35 | 23 (17–31) |
| Mycoplasma synoviae | Standard PCR | Tracheal swabs | All species except ducks | 151* | 54 | 36 (28–44) |
| Mycoplasma meleagridis | rtPCR | Tracheal swabs | Turkeys | 10 | 0 | 0 |
| Mycoplasma iowae | rtPCR | Tracheal swabs | Turkeys and game birds | 18 | 0 | 0 |
| Mycoplasma spp. | Culture | Airsac swabs | Ducks | 8 | 1 | 13 (0.3–53) |
| Campylobacter total‡ | Enrichment and isolation | Cecal tissue | All species | 158† | 56 | 35 (28–43) |
| C. jejuni | 29 | 18 (13–25) | ||||
| C. coli | 31 | 20 (14–27) | ||||
| Salmonella spp. | Enrichment and isolation | Cecal tissue | All species | 159* | 5 | 3 (1–7) |
| Brachyspira total‡ | rtPCR | Cecal tissue | All species | 159* | 59 | 37 (30–45) |
| Brachyspira spp. | 58 | 36 (29–44) | ||||
| B. innocens | 26 | 16 (11–23) | ||||
| B. intermedia | 16 | 10 (6–16) | ||||
| B. murdochii | 9 | 6 (3–10) | ||||
| B. pilosicoli | 1 | 0.6 (0.02–3) |
Prevalence estimates were calculated by dividing the number of submissions that tested positive for the pathogen by the total number of submissions tested for the pathogen (n/N). Exact binomial 95% confidence intervals (CI) for the non-zero prevalence estimates were computed, in parentheses. Superscripts by the name of the pathogen indicate references. All species = chickens, turkeys, ducks, pheasants, peafowl, and quail; game birds = pheasants, peafowl, and quail; rtPCR = real-time PCR; RT-rtPCR = reverse-transcription rtPCR.
Sample from one chicken submission not available.
Sample from 1 chicken and 1 peafowl submission not available.
At least one submission positive for species- or genotype-specific test.
All Salmonella spp. isolates were submitted for serotyping to the Laboratory for Foodborne Zoonoses, Public Health Agency of Canada (Guelph, ON). For samples positive for infectious bronchitis virus (IBV; species Avian coronavirus) samples, sequencing was attempted on a 621-bp fragment of the S gene, as described previously.24 Nucleotide identity was compared to IBV sequences present on a publicly available database (NCBI GenBank, https://www.ncbi.nlm.nih.gov/genbank/). Genetic sequencing of ILTV-positive samples was performed to differentiate vaccine and wild-type strains based on nucleotide polymorphism of the partial sequence of the UL47 and glycoprotein G genes, as described previously.27 For fowl adenovirus (FAdV), genotypes A/C, D, and E were differentiated based on multiplex assays designed to detect the FAdV genotypes found most commonly in Canadian chickens.13,28 All samples positive for AAvV-1 and AIV were submitted to the National Microbiology Laboratory (NML) in the Canadian Science Centre for Human and Animal Health (Winnipeg, MB) for virus isolation and serotyping.
Database formatting and data analysis
Data derived from the pre-set microbiology tests were entered into a spreadsheet (Excel 2016; Microsoft, Redmond, WA) formatted by submissions, and results were entered as 0 (negative) or 1 (positive). If a specific test was not performed on a submission (e.g., reverse-transcription, real-time PCR [RT-rtPCR] for IBV was not performed for waterfowl, game birds, or turkeys), results were entered as not applicable. Inconclusive RT-rtPCR results, as determined in the final report, were considered as negative.
For each pathogen, prevalence estimates were calculated by dividing the number of submissions that tested positive for the pathogen by the total number of submissions tested for the pathogen (Excel 2016; Microsoft). Exact binomial 95% confidence intervals for the non-zero prevalence estimates were computed (Stata v.14.1; StataCorp, College Station, TX). Venn diagrams (jvenn, http://jvenn.toulouse.inra.fr/app/index.html) were used to illustrate relations among sets (e.g., co-detection of respiratory pathogens) and subsets (e.g., co-detection of different species of Brachyspira) of pathogens. A choropleth map (ArcGIS 10.2.1; Environmental Systems Research Institute, Redlands, CA) was used to illustrate the distribution and density of submissions by forward sortation area (first 3 characters of the postal code) across Ontario.
Results
Types of submissions received
Over the 2-y study period, we received 160 submissions of 1–5 birds (median: 1; mean: 1.3), for a total of 245 individual birds. Chickens accounted for 84% of submissions (134 submissions), followed by turkeys (10 submissions), game birds (8 submissions: 4 peafowl, 2 quail, and 2 pheasants), and ducks (8 submissions). Submissions originated from flocks of 1–299 birds (median: 25; mean: 26), mainly from southern and eastern Ontario (Fig. 1).
Figure 1.
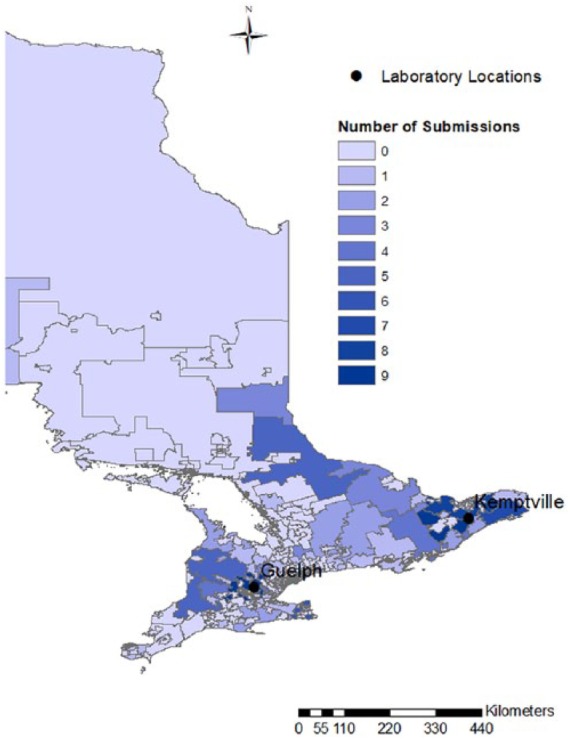
Choropleth map of Ontario, Canada, subdivided by forward sortation areas (first 3 characters of the postal code). The number of submissions from each area is represented using a heat map, with lighter colors denoting low numbers and darker colors denoting high numbers. The locations of the 2 Animal Health Laboratories are identified.
Pathogen detection by submissions
Salmonella spp., Campylobacter spp., and Brachyspira spp
Salmonella spp., Campylobacter spp., and Brachyspira spp were isolated from 3% of 159 tested submissions (Tables 1, 2). Isolates were identified to be S. Anatum, S. Indiana, and S. Ouakam (3 chicken submissions), S. Uganda (1 turkey submission), and S. Montevideo (1 duck submission).
Table 2.
Number of positive submissions and total number of submissions tested (in parentheses), by species, in a small poultry flock surveillance study in Ontario, over a 2-y period.
| AAvV-1 | AIV | IBV | ILTV | FAdV total† | FAdV-E | FAdV-D | FAdV-A/C | ABV | |
|---|---|---|---|---|---|---|---|---|---|
| Chicken | 1 (134) | 0 (134) | 52 (134) | 21 (134) | 47 (134) | 30 (134) | 29 (134) | 27 (134) | 0 (134) |
| Turkey | 0 (10) | 1 (10) | NA | NA | NA | NA | NA | NA | 0 (10) |
| Duck | 0 (8) | 0 (8) | NA | NA | NA | NA | NA | NA | 0 (8) |
| Pheasant | 0 (2) | 0 (2) | NA | 0 (2) | NA | NA | NA | NA | 0 (2) |
| Peafowl | 0 (4) | 0 (4) | NA | 1 (4) | NA | NA | NA | NA | 0 (4) |
| Quail | 0 (2) | 0 (2) | NA | 0 (2) | NA | NA | NA | NA | 0 (2) |
| Avian reovirus | IBDV | Brachyspira total† | Brachyspira spp. | B. innocens | B. intermedia | B. murdochii | B. pilosicoli | ||
| Chicken | 6 (134) | 2 (134) | 49 (133*) | 48 (133*) | 25 (133*) | 12 (133*) | 9 (133*) | 1 (133*) | |
| Turkey | 0 (10) | NA | 4 (10) | 4 (10) | 0 (10) | 1 (10) | 0 (10) | 0 (10) | |
| Duck | NA | NA | 5 (8) | 5 (8) | 0 (8) | 2 (8) | 0 (8) | 0 (8) | |
| Pheasant | NA | NA | 1 (2) | 1 (2) | 1 (2) | 1 (2) | 0 (2) | 0 (2) | |
| Peafowl | NA | NA | 0 (4) | 0 (4) | 0 (4) | 0 (4) | 0 (4) | 0 (4) | |
| Quail | NA | NA | 0 (2) | 0 (2) | 0 (2) | 0 (2) | 0 (2) | 0 (2) | |
| MG | MS | MM | MI | Mycoplasma culture | Campylobacter total† | C. jejuni | C. coli | Salmonella spp. | |
| Chicken | 30 (133*) | 52 (133*) | NA | NA | NA | 44 (133*) | 25 (133*) | 23 (133*) | 3 (133*) |
| Turkey | 3 (10) | 1 (10) | 0 (10) | 0 (10) | NA | 7 (10) | 1 (10) | 6 (10) | 1 (10) |
| Duck | NA | NA | NA | NA | 1 (8) | 2 (8) | 2 (8) | 0 (8) | 1 (8) |
| Pheasant | 1 (2) | 0 (2) | NA | 0 (2) | NA | 1 (2) | 0 (2) | 1 (2) | 0 (2) |
| Peafowl | 1 (4) | 1 (4) | NA | 0 (4) | NA | 0 (3*) | 0 (3*) | 0 (3*) | 0 (4) |
| Quail | 0 (2) | 0 (2) | NA | 0 (2) | NA | 2 (2) | 1 (2) | 1 (2) | 0 (2) |
AAvV-1 = avian avulavirus 1; ABV = avian bornavirus; AIV = avian influenza virus; FAdV = fowl adenovirus; IBDV = infectious bursal disease virus; IBV = infectious bronchitis virus; ILTV = infectious laryngotracheitis virus; MG = Mycoplasma gallisepticum; MI = Mycoplasma iowae; MM = Mycoplasma meleagridis; MS = Mycoplasma synoviae; NA = not applicable.
Sample from one submission not available.
At least one submission positive for species- or genotype-specific test.
Campylobacter spp. (C. jejuni and C. coli only) were isolated from 35% of 158 tested submissions (Tables 1, 2). Among chicken submissions, C. jejuni and C. coli were both common, whereas among turkey submissions, C. coli was the most common.
Real-time PCR for Brachyspira was conducted on 159 submissions, of which 37% were positive for at least 1 set of primers, with multiple species of Brachyspira often detected from the same submission (Tables 1, 2). All submissions that tested positive for at least 1 of the 4 species evaluated were also positive for the general Brachyspira spp. PCR, with the exception of 1 chicken submission that tested positive for B. innocens and yet tested negative for Brachyspira spp. (Fig. 2).
Figure 2.

Venn diagram illustrating relations among species in Brachyspira-positive chicken submissions in a small poultry flock surveillance study in Ontario. The pathogen was detected using real-time PCR on pooled ceca. This PCR included a primer set for broad detection of the Brachyspira genus (B. spp.), and specific primer sets for detection of single species, including B. innocens, B. intermedia, B. murdochii, and B. pilosicoli.
Fowl adenovirus, avian reovirus, infectious bursal disease virus, and avian bornavirus
FAdV was detected in 35% of 134 chicken submissions (Tables 1, 2). A total of 30, 29, and 27 submissions were positive for FAdV genotype E, D, and A/C, respectively; many were positive for more than 1 genotype (Fig. 3). Avian reovirus was detected in 4% of 144 chicken and turkey submissions; all of the positives were chicken submissions. Infectious bursal disease virus (IBDV) was detected in 1% of 134 chicken submissions. Avian bornavirus was not detected in any submissions.
Figure 3.
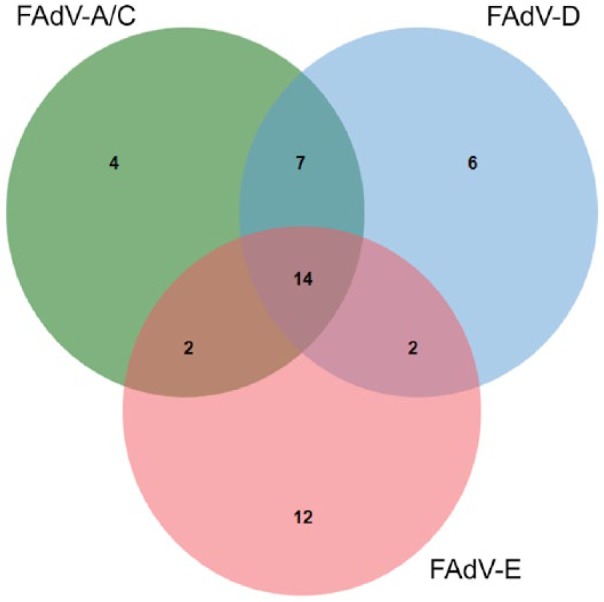
Venn diagram illustrating relations among genotypes in fowl adenovirus (FAdV)-positive chicken submissions in a small poultry flock surveillance study in Ontario. The presence of FAdV was detected using reverse-transcription, real-time PCR on pooled cloacal swabs. Genotypes A/C, D, and E were differentiated based on multiplex assays designed to detect the FAdV genotypes found most commonly in Canadian chickens.13,28
Mycoplasma spp
Mycoplasma gallisepticum (MG) and Mycoplasma synoviae (MS) were detected in 23% and 36% of 151 tested submissions, respectively (Tables 1, 2). Of 133 chicken submissions tested, both MG and MS were detected in 23; MG and MS alone were detected in 7 and 29 submissions, respectively. Of the 3 positive turkey submissions, 2 were positive for MG alone, and the other was positive for both MG and MS. Of the 2 positive peafowl submissions, 1 tested positive for MG and 1 for MS; MG was detected in the only positive pheasant submission. All submissions tested for Mycoplasma meleagridis (MM; turkeys) and Mycoplasma iowae (MI; turkeys and game birds) were negative. Of the 8 duck submissions, 1 was positive for Mycoplasma spp. on culture, further identified as M. verecundum.
Infectious bronchitis virus
IBV was detected in 39% of 134 chicken submissions (Tables 1, 2). Specifically, in 26 submissions, IBV was detected in both tracheal and cloacal swabs, in 1 submission it was detected in the tracheal swab only, and in 25 submissions it was detected in the cloacal swab only.
Of the 52 positive submissions, partial sequencing of the S gene was successful in 46 submissions, with 2 different partial sequences detected in 1 submission, for a total of 47 unique partial sequences. Overall, 15 samples had the closest identity with strain CA/1737/04 (GenBank EU925393), 8 with strain QU/mv (AF349621), 5 with strain GA (KJ538776), 4 with strain 4/91/ON (KJ196215), 4 with strain PA/Wolg/98 (AF305595), 3 with strain DMV (EU694402), 2 with strain 4/91/UK (JN192154), 2 with Mass/A vaccine strain (EU283073), 1 with strain SH1 (DQ075323), and 1 each of Mass/B, MassD/C, and 4/91 vaccine strains (EU283076, EU283085, and KF377577, respectively).
Infectious laryngotracheitis virus
ILTV was detected in 15% of 142 chicken and game bird submissions, of which 21 were from chickens and 1 from a peafowl (Tables 1, 2). Partial sequencing detected a vaccine strain in 12 of 22 positive submissions (including the peafowl submission) and a wild-type strain with high similarity to the Niagara strain reported in the 2004 Ontario outbreak27 in 9 submissions. The ILTV strain of one submission differed from both the vaccine strain and the Niagara strain, and did not match strains in GenBank.
Avian avulavirus and avian influenza virus
Non-virulent AAvV-1 was detected from a 2.5-wk-old chicken submission (2 birds); AIV was detected from one 2-wk-old turkey poult (Tables 1, 2). Both samples were sent to the NML for further characterization and were determined to be a lentogenic vaccine strain (AAvV-1) and a wild-bird origin low pathogenicity AIV (H10N8).
Co-detection of respiratory pathogens
The frequency of co-detection of MG, MS, IBV, and ILTV was evaluated for chicken submissions (Fig. 4). A total of 44 (33%) of 133 tested chicken submissions were positive for more than one of these respiratory pathogens. The most common combinations included MG, MS, and IBV (9%); MS and IBV (8%); and MG and MS (4%). For other species, 1 peafowl submission was positive for both MS and ILTV, and 1 turkey submission was positive for both MG and MS.
Figure 4.
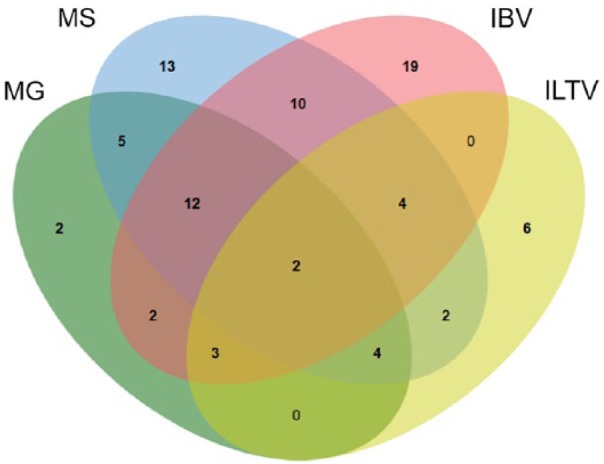
Venn diagram illustrating relations among 4 respiratory pathogens (MG = Mycoplasma gallisepticum; MS = Mycoplasma synoviae; IBV = infectious bronchitis virus; ILTV = infectious laryngotracheitis virus) in chicken submissions in a small poultry flock surveillance study in Ontario.
Prevalence of pathogens by poultry species
Among chicken submissions, the most commonly detected pathogens were MS and IBV (39% each), Brachyspira (37%), FAdV (35%), Campylobacter spp. (33%), MG (23%), and ILTV (16%). Among turkey and game bird submissions, the most commonly detected pathogen was Campylobacter spp., and among duck submissions, the most commonly detected pathogen was Brachyspira (Table 2).
Discussion
A 2014–2015 survey of healthy birds sampled at Ontario provincial abattoirs reported a significantly lower prevalence of Salmonella spp. in small flocks (1%) compared to commercial flocks (15%) processed during the same period.19 Similarly, we found a 3% overall prevalence of Salmonella spp., despite the inclusion of only sick or dead birds that might already have had a compromised immune system. The source of the isolated salmonellae for the positive flocks in our study is unclear. In the submission questionnaire, owners reported that they obtained birds from hatcheries, friends, or other (nonspecified) sources (data not shown). Regardless of the origin, with only 3% of tested submissions positive for Salmonella spp., it appears that Ontario small poultry flocks pose a limited threat with regard to human salmonellosis.
The 35% overall prevalence of Campylobacter spp. in our submissions is higher than the reported 20% prevalence in commercial broiler chickens submitted to federal slaughter plants in Ontario during the 2012–2013 national microbiologic baseline study in broiler chickens (CFIA, http://www.inspection.gc.ca/food/chemical-residues-microbiology/food-safety-testing-bulletins/2016-08-17/december-2012-december-2013/eng/1471358115567/1471358175297). Fecal shedding can lead to the spread of the pathogen within the environment, leading to contamination of food, tools, and materials coming into contact with people, flies, and other flocks. Given that small flock poultry are often kept as pets, close contact (e.g., kissing, petting) could increase the risk of exposure further, especially for people at higher risk (e.g., children). These results underscore the importance of proper sanitation and hand hygiene measures for flock owners to protect both flocks and the public from these pathogens.
Certain Brachyspira spp. are potential zoonotic agents, particularly in immunocompromised individuals.16 Although individual species tend to be host specific, B. pilosicoli has been associated with intestinal disease in numerous mammalian and avian species, including humans.16 In poultry, B. pilosicoli and B. intermedia have been associated with intermittent chronic diarrhea and decreased egg production.31 The detection of at least one Brachyspira species in 37% of our submissions further emphasizes the need for proper sanitation measures to avoid spread to humans and other flocks.
Estimated prevalence of FAdV, IBDV, and avian reovirus in Ontario’s commercial broiler flocks, based on slaughter samples, has been reported as 96%, 49%, and 91%, respectively.13,26 The prevalence of the same pathogens for chicken submissions in our survey was markedly lower, with only 35% positive for at least one genotype of FAdV, 1% positive for IBDV, and 4% positive for avian reovirus. However, it should be noted that both serology and pathogen detection methods were used in the estimation of prevalence in the studies involving commercial flocks. Further, the chickens in our study were primarily layers or dual-purpose birds. These methodologic and population differences may explain some of the discrepancy between studies. Overall, none of the tested enteric pathogens (Salmonella, Campylobacter, Brachyspira, FAdV, IBDV, and reovirus) were associated with clinical signs or lesions in our submissions, suggesting that positive birds were subclinical carriers.3
The strains of IBV that we detected in Ontario small flocks between 2015 and 2017 are similar to those reported within Canada between 2000 and 2013.24 Concurrent with the sampling period of our small flock study, the AHL detected an increase in both submissions and positive test results for IBV in all commercial chicken commodities in Ontario (Ontario Animal Health Network Poultry Expert Network, https://oahn.ca/wp-content/uploads/2016/12/Dec-2016-OAHN-special-report-on-IBV-FINAL.pdf). During this time, the Delmarva (DMV) strain emerged as the predominant strain in commercial poultry, whereas only 3 of the small flocks in our study tested positive for the DMV strain. These 3 positive flocks were sampled during October 2016 and July 2017, after the DMV strain was identified in the commercial industry in September 2016, suggesting spread from the commercial sector to small flocks.
Strains of ILTV from our study included both wild-type and Niagara outbreak–like strains, both of which have been described in Ontario’s commercial poultry industries.27 The presence of a vaccine-like strain in 12 submissions suggests that vaccine strains might be circulating among small flocks, possibly as a direct consequence of ILTV vaccination of these flocks (likely a rare event), or routine vaccination in commercial layers and breeders.27 The detection of the Niagara-like strain in 9 submissions suggests that the strain from the 2004 commercial outbreak has now become endemic in the province.27
The seroprevalence of various pathogens, such as those detected in our samples, has also been reported in various studies of small poultry flocks from the United States, Belgium, and Finland conducted between 1988 and 2016.11,15,21,22,25,30 In those studies, the prevalence of AAvV-1, AIV, IBV, IBDV, ILTV, MG, and MS was 0–78%, 0–23%, 47–91%, 20–74%, 12–77%, 13–73%, and 56–96%, respectively. One of the studies from California11 found that birds purchased from hatcheries certified under the National Poultry Improvement Plan, a U.S. program similar to the Ontario Hatchery and Supply Flock Policy to ensure pathogen-free replacement chicks, had a lower antibody prevalence for AAvV-1, MG, and MS compared to flocks with birds from other sources. The Belgian study15 also reported a 100% flock seroprevalence for Ornithobacterium rhinotracheale and 96% for avian metapneumovirus, which were not part of our testing protocol. Overall, 48% of the Belgian small flocks tested positive for 6 different respiratory pathogens. Although percentages are generally higher than those found in our study, this variability is likely a reflection of the inherent difference between pathogen detection and serologic reactivity, which can also be affected by vaccination, and indicates simple contact, rather than infection. Overall, our study and these reports agree that multiple respiratory pathogens can affect a flock at the same time, contributing to multifactorial respiratory infections. Indeed, lesions of variable severity (often associated with secondary gram-negative bacterial infection) were observed in birds that tested positive for mycoplasma, IBV, and ILTV in our cohort, as shown by the postmortem data.3
The potential for pathogen exposure and spread from small flocks to commercial poultry flocks has long been a point of contention.11,15,23,25 An outbreak of any of the 4 federally reportable poultry diseases in Canada (Newcastle disease, notifiable avian influenza, pullorum disease, fowl typhoid; CFIA, http://www.inspection.gc.ca/animals/terrestrial-animals/diseases/reportable/eng/1303768471142/1303768544412) could have a dramatic impact on the commercial poultry industry. With only one positive test result for non-notifiable AIV and one for vaccine strain AAvV-1, and no Salmonella Pullorum or S. Gallinarum detected, our survey shows no significant indication that small flocks are presently acting as reservoirs for these diseases in Ontario.
Given that this was a passive surveillance project based on voluntary submissions, the 160 flocks tested in our study may not be representative of all small flocks within the province. On the other hand, an active surveillance program was not logistically or financially feasible in a province with a population of >13 million people spread over a million square kilometers (Statistics Canada, http://www12.statcan.gc.ca/census-recensement/2016/as-sa/fogs-spg/Facts-pr-eng.cfm?Lang=Eng&GK=PR&GC=35&TOPIC=1), and where a complete registry of small flocks is not available. The majority of submissions originated from areas relatively close to the AHL laboratories, possibly indicating a higher population density in metropolitan areas near the AHL facilities, or a lower willingness of submitters to ship postmortem samples over long distances. The $25 processing fee required for each submission, albeit heavily subsidized, could have decreased the number of submissions depending on an individual owner’s perceived value of the bird(s). In addition, small flock owners who did not have a veterinarian–client–patient relationship already in place might not have been able to submit their birds. Finally, some small flock owners might have been reticent to submit birds, either because of the ambiguity of municipal regulations or from fear of investigation by government authorities if a reportable disease was detected.
Supplemental Material
Supplemental material, DS1_JVDI_10.1177_1040638719843577 for A two-year prospective study of small poultry flocks in Ontario, Canada, part 1: prevalence of viral and bacterial pathogens by Nancy M. Brochu, Michele T. Guerin, Csaba Varga, Brandon N. Lillie, Marina L. Brash and Leonardo Susta in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank the Animal Health Laboratory for the invaluable work in coordinating and processing all submissions and testing performed in this study, as well as Al Dam and Dr. Melanie Barham for their help in advertising the study. Summer students from the Ontario Veterinary College, Thisuri Eagalle, Kai Moore, and Elysha Smith, were invaluable in helping to organize the data. We also thank all of the veterinarians and small flock owners who submitted birds and participated in this study.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This project was funded by the Ontario Ministry of Agriculture, Food and Rural Affairs–University of Guelph Strategic Partnership (grant UofG 2015-2282), under the Disease Surveillance Plan, which was a joint federal-provincial Growing Forward 2 project. Financial support for Dr. Brochu’s stipend derived from the Ontario Veterinary College (OVC) DVSc fellowship fund. Summer students were supported through the OVC Andrea Leger Dunbar summer assistantship fund.
Supplementary material: Supplementary material for this article is available online.
ORCID iDs: Csaba Varga  https://orcid.org/0000-0003-2751-3677
https://orcid.org/0000-0003-2751-3677
Leonardo Susta  https://orcid.org/0000-0002-4578-6145
https://orcid.org/0000-0002-4578-6145
References
- 1. Bavinck V, et al. The role of backyard poultry flocks in the epidemic of highly pathogenic avian influenza virus (H7N7) in the Netherlands in 2003. Prev Vet Med 2009;88:247–254. [DOI] [PubMed] [Google Scholar]
- 2. Boender GJ, et al. Risk maps for the spread of highly pathogenic avian influenza in poultry. PLoS Comput Biol 2007;3:704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brochu NM, et al. A two-year prospective study of small poultry flocks in Ontario, Canada, part 2: causes of morbidity and mortality. J Vet Diagn Invest 2019;31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buregeya J, et al. Biosecurity practices and geospatial map of Ontario backyard poultry flocks. J Environ Sci Eng 2013;2:694–701. [Google Scholar]
- 5. Burns TE, et al. Preliminary investigation of bird and human movements and disease-management practices in noncommercial poultry flocks in southwestern British Columbia. Avian Dis 2011;55:350–357. [DOI] [PubMed] [Google Scholar]
- 6. Callison SA, et al. Development and validation of a real-time Taqman PCR assay for the detection and quantitation of infectious laryngotracheitis virus in poultry. J Virol Methods 2007;139:31–38. [DOI] [PubMed] [Google Scholar]
- 7. Callison SA, et al. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J Virol Methods 2006;138:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carvallo FR, et al. Diagnosis of virulent Newcastle disease in southern California, May 2018. J Vet Diagn Invest 2018;30:493–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crespo R, et al. Exotic Newcastle disease in a game chicken flock. Avian Dis 1999;43:349–355. [PubMed] [Google Scholar]
- 10. Delnatte P, et al. Avian bornavirus in free-ranging waterfowl: prevalence of antibodies and cloacal shedding of viral RNA. J Wildl Dis 2014;50:512–523. [DOI] [PubMed] [Google Scholar]
- 11. Derksen T, et al. Biosecurity assessment and seroprevalence of respiratory diseases in backyard poultry flocks located close to and far from commercial premises. Avian Dis 2018;62:1–5. [DOI] [PubMed] [Google Scholar]
- 12. Elkhoraibi C, et al. Backyard chickens in the United States: a survey of flock owners. Poult Sci 2014;93:2920–2931. [DOI] [PubMed] [Google Scholar]
- 13. Eregae ME, et al. Flock prevalence of exposure to avian adeno-associated virus, chicken anemia virus, fowl adenovirus, and infectious bursal disease virus among Ontario broiler chicken flocks. Avian Dis 2014;58:71–77. [DOI] [PubMed] [Google Scholar]
- 14. Grunkemeyer VL. Zoonoses, public health, and the backyard poultry flock. Vet Clin Exot Anim 2011;14:477–490. [DOI] [PubMed] [Google Scholar]
- 15. Haesendonck R, et al. High seroprevalence of respiratory pathogens in hobby poultry. Avian Dis 2014;58:623–627. [DOI] [PubMed] [Google Scholar]
- 16. Hampson DJ, et al. Potential for zoonotic transmission of Brachyspira pilosicoli. Emerg Infect Dis 2006;12:869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Howden KJ, et al. An investigation into human pandemic influenza virus (H1N1) 2009 on an Alberta swine farm. Can Vet J 2009;50:1153–1161. [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson YJ, et al. Wind-borne transmission of infectious laryngotracheitis between commercial poultry operations. Int J Poult Sci 2005;4:263–267. [Google Scholar]
- 19. Lebert L, et al. Prevalence and antimicrobial resistance among Escherichia coli and Salmonella in Ontario smallholder chicken flocks. Zoonoses Public Health 2018;65:134–141. [DOI] [PubMed] [Google Scholar]
- 20. Madsen JM, et al. Evaluation of Maryland backyard flocks and biosecurity practices. Avian Dis 2013;57:233–237. [DOI] [PubMed] [Google Scholar]
- 21. Madsen JM, et al. Prevalence and differentiation of diseases in Maryland backyard flocks. Avian Dis 2013;57:587–594. [DOI] [PubMed] [Google Scholar]
- 22. Madsen JM, et al. Avian influenza seroprevalence and biosecurity risk factors in Maryland backyard poultry: a cross-sectional study. PLoS One 2013;8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mainali C, Houston I. Small poultry flocks in Alberta: demographics and practices. Avian Dis 2017;61:46–54. [DOI] [PubMed] [Google Scholar]
- 24. Martin EA, et al. Genotyping of infectious bronchitis viruses identified in Canada between 2000 and 2013. Avian Pathol 2014;43:264–268. [DOI] [PubMed] [Google Scholar]
- 25. McBride MD, et al. Health survey of backyard poultry and other avian species located within one mile of commercial California meat-turkey flocks. Avian Dis 1991;35:403–407. [PubMed] [Google Scholar]
- 26. Nham EG, et al. Flock-level prevalence, geographical distribution, and seasonal variation of avian reovirus among broiler flocks in Ontario. Can Vet J 2017;58:828–834. [PMC free article] [PubMed] [Google Scholar]
- 27. Ojkic D, et al. Characterization of field isolates of infectious laryngotracheitis virus from Ontario. Avian Pathol 2006;35:286–292. [DOI] [PubMed] [Google Scholar]
- 28. Ojkic D, et al. Genotyping of Canadian isolates of fowl adenoviruses. Avian Pathol 2008;37:95–100. [DOI] [PubMed] [Google Scholar]
- 29. Pasick J, et al. The roles of national and provincial diagnostic laboratories in the eradication of highly pathogenic H7N3 avian influenza virus from the Fraser Valley of British Columbia, Canada. Avian Dis 2007;51:309–312. [DOI] [PubMed] [Google Scholar]
- 30. Pohjola L, et al. A survey for selected avian viral pathogens in backyard chicken farms in Finland. Avian Pathol 2017;46:166–172. [DOI] [PubMed] [Google Scholar]
- 31. Robineau B. Brachyspira spp (avian intestinal spirochetosis). In: Brugere-Picoux J, Vaillancourt JP, eds. Manual of Poultry Diseases. 1st ed Paris, France: AFAS, 2015:376–379. [Google Scholar]
- 32. Smith EI, et al. Epidemiologic characterization of Colorado backyard bird flocks. Avian Dis 2016;56:263–271. [DOI] [PubMed] [Google Scholar]
- 33. Smith G, Dunipace S. How backyard poultry flocks influence the effort required to curtail avian influenza epidemics in commercial poultry flocks. Epidemics 2011;3:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wise MG, et al. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J Clin Microbiol 2004;42:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_JVDI_10.1177_1040638719843577 for A two-year prospective study of small poultry flocks in Ontario, Canada, part 1: prevalence of viral and bacterial pathogens by Nancy M. Brochu, Michele T. Guerin, Csaba Varga, Brandon N. Lillie, Marina L. Brash and Leonardo Susta in Journal of Veterinary Diagnostic Investigation


