Abstract
We report the case of a girl with Asparagine synthetase deficiency, an autosomal recessive metabolic disorder characterized by severe microcephaly and epileptic encephalopathy secondary to pathogenic variants in the ASNS gene. Genetic explorations found a deletion of ASNS and a missense variant on the other allele detected respectively by array comparative genomic hybridization (CGH) and Sanger sequencing. Amino acid analysis provided a biochemical confirmation. Previous cases of Asparagine synthetase deficiency were diagnosed though exome Sequencing. The combination of several techniques (array CGH, sequencing, and biochemical analysis) improves the opportunity to provide accurate diagnosis.
Keyswords: Asparagine synthetase deficiency, Microcephaly, Epileptic encephalopathy, Array CGH, Intragenic deletion, ASNS
1. Introduction
Asparagine synthetase deficiency (ASNSD) (OMIM#615574) is a newly described rare autosomal recessive neurodevelopmental disease. To date, all cases of ASNSD have been identified through exome sequencing. Asparagine is a nonessential amino acid synthetized by asparagine synthetase (AS) via the transfer of ammonia from glutamine to aspartic acid [9]. ASNSD is due to homozygous or compound heterozygous pathogenic variants in the ASNS gene, located on chromosome 7q21. ASNS is a 20.4 Kb gene comprising 13 exons, presenting 17 transcripts, and playing an important role in cortical development and brain size [12]. ASNSD is characterized by severe progressive congenital microcephaly and epileptic encephalopathy associated with severe development delay, axial hypotonia, severe spasticity, brain atrophy, and mild nonspecific dysmorphic [11]. Moreover, patients with ASNSD also present feeding and respiratory disorders that can lead to premature death in infancy (Alfadhel and El-Hattab, 1993; [5]). Low asparagine level in cerebro spinal fluid (CSF) and/or plasma might be helpful for the diagnosis of ASNSD. However, normal CSF and blood level of asparagine do not exclude this disease [16]. Brain magnetic resonance imaging (MRI) usually shows decreased cerebral volume, decreased pons size and gyral simplification [2,14,15].
We report here a new case of ASNSD for which array comparative genomic hybridization (array-CGH) enabled the identification of a causative, compound heterozygote genotype with a deletion of ASNS, and a novel missense variant.
2. Materials and methods
2.1. Ethics statement
Informed written consent for genetic explorations and the authorization of publication were obtained from the parents.
2.2. Array CGH
DNA was isolated from peripheral blood using the QIAamp DNA blood Midi Kit (Qiagen, Venlo, The Netherlands). An Array CGH was performed with an 180,000 oligonucleotides microarray (SurePrint G3 Human CGH Microarray Kit, 4*180 K, Agilent Technologies, Santa Clara, CA, USA). Array CGH procedures were performed according to the manufacturer's instructions. The 180 K slides were scanned on Agilent's DNA Microarray Scanner system and read with its Feature Extraction software. Data analysis was carried out with Agilent's CytoGenomics software. A dye-swap method was used to analyze copy number variations (CNVs). The array CGH results were analyzed with Cartagenia software and UCSC hg19 assembly. CNVs were confirmed thereafter by using quantitative polymerase chain reaction (PCR).
2.3. Sanger DNA sequencing
PCR primers were designed using Primer3Plus software. MP Biomedicals' Taq DNA Polymerase (Applied Biosystems, Foster City, CA, USA) was used to amplify the target regions. Sanger sequencing was performed with the BigDye Terminator v3.1 Cycle Sequencing Kit using an ABI 3130 genetic analyzer (Applied Biosystems). DNA chromatograms were analyzed using SeqScape v2.5.0 and Sequence Scanner v1.0 (Applied Biosystems) software and compared to a reference sequence (NM_133436.3).
2.4. Quantitative PCR (qPCR)
Results from array CGH were confirmed with qPCR. It was performed on a genomic DNA with two primer pairs amplifying unique sequence in intron 9 in ASNS within ADORA2B as the reference gene using the QuantiTect SYBR Green PCR Kit (Qiagen, Courtaboeuf, France). Experiments were conducted on a light Cycler 2000 (Roche Applied Science, Indianipolis, USA) according to the manufacturer's recommendations.
3. Results
The patient was a French 4-year-old girl presenting with an intrauterine growth retardation and then severe progressive microcephaly with epileptic encephalopathy. Her parents were unrelated. The patient was born at 39 weeks of gestation with weight, length and head circumference measurements of respectively 2.730 kg (-2SD), 45 cm (-3SD), and 30 cm (-4SDSD). She developed severe progressive post-natal microcephaly (head circumference at 5 months: -6SD) and psychomotor delay. Neurological examination found axial hypotonia, spastic quadriplegia, and hyperreflexia. She had minor facial morphologic variations, including large ears and micro-retrognathism. At the age of eight months, she experienced a first tonic-clonic seizure. Subsequently, she developed generalized tonic-clonic seizures, myoclonic seizures and absences. Electroencephalogram-recording at one year showed symmetric low-intensity background activity with multifocal spikes with predominant centrotemporal location. Multiple antiepileptic drugs were required to control seizures. Brain MRI, at two years, showed global brain atrophy, a thin corpus callosum, and delayed myelination. The results of an initial metabolic screening at one year of age were considered normal particularly with regard to amino acid plasma analysis with an asparagine level at the lower limit of the control range, i.e., 33 μmol/L (28–65 μmol/L). She subsequently evolved to severe psychomotor development delay: at the age of five years and half she could only hold her head up, roll from back to side, hold herself in the chest-up position, sit with support and use a palmar grasp. She had no language development. Clinical examination reveals spasticity particularly in lower limbs. Seizures remain weekly despite antiepileptic drugs.
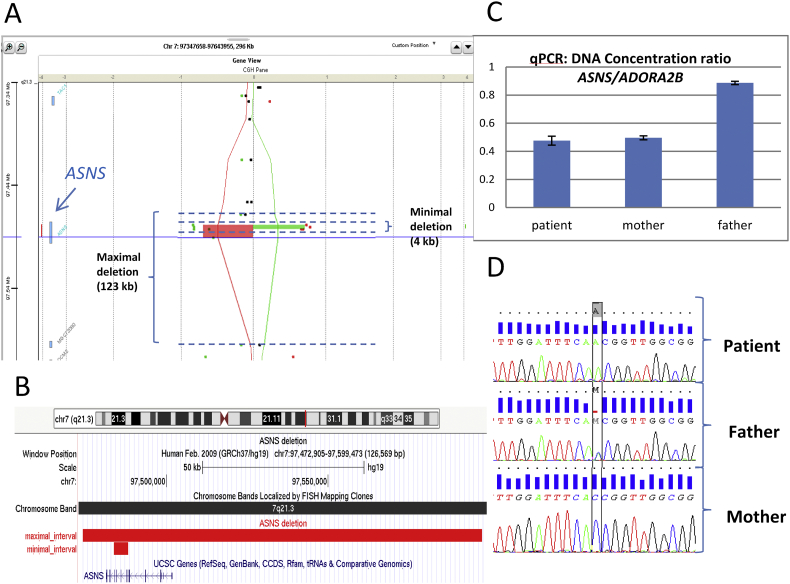
Array CGH, which was performed as a first step of genetic testing, showed a 123 Kb heterozygote deletion of ASNS. The boundaries of the deletion were not precisely defined because of the poor density of probes within this region. It was considered a large 123 kb deletion with maximal bounds deletion from 97,474,170 to 97,597,701 removing the entire ASNS gene and a small one of 4.3 Kb with minimal bound deletion from 97,483,869 to 97,488,230, removing 5 exons from exon 6 to exon 10 of ASNS (Fig. 1A and B). This deletion is probably responsible for an absence of production of the AS from the deleted allele or the production of a nonfunctional truncated protein. The deletion was confirmed in the patient using qPCR with a ratio between ASNS and ADORA2B (reference gene) of 0.5 (Fig. 1C). Since the phenotype of the patient was consistent with ASNSD, we sequenced the exons and intronic margins of ASNS using the Sanger method. We found a hemizygous transversion in exon 3, c.144C>A, chr7:g.97498325G>T (NM_133436.3) (hg19) (Fig. 1D). This variant was absent from the gnomAD database of control individuals. It was predicted to replace a highly-conserved histidine, located in the glutamine transferase domain, by a glutamine, p.(His48Gln). This domain is an important and conserved functional domain of AS. Accordingly, in silico prediction programs (Mutation Taster, SIFT, Align GVGD) were in favor of deleterious effects. The hemizygous nature of the transversion informed us that exon 3 was actually deleted in the other allele. Quantitative PCR showed that the deletion was inherited from the mother (Fig. 1C). The mutation was inherited from the father (Fig. 1D). Both parents were healthy. Therefore, the association of a deletion and a missense variant, each inherited from one parent, leads to a haploinsufficiency and was in favor of the diagnosis of ASNSD. Following the results of the genetic analysis, we repeated amino acid analyses in fasted plasma and CSF. In the former, asparagine levels had fallen to 11 μmol/L (28–65 μmol/L) and in the latter, they were at 4 μmol/L (6–14 μmol/L). Glutamine and the other amino acids were in the control range in both plasma and CSF. These metabolic results enabled a definite confirmation of the diagnosis.
Fig. 1.
Deletion in array-CGH analysis confirmed by qPCR and pathogenic variant in Sanger sequencing in ASNS in our patient and her parents.
A: A view of the heterozygote deletion involving the ASNS gene detected by array-CGH using a dye-swap method. Patient and control are compared twice with the dye assignment reversed in the second hybridization. In the first assignment, patient is compared to the control (red line and rectangle) and in the second assignment control is compared to the patient (green line and rectangle). A maximal 123 kilobases (kb) deletion with boundaries from 97,474,170 to 97,597,701 and a minimal 4 kb deletion from 97,483,869 to 97,488,230 were considered because of the poor density of probes within the region. B: A UCSC view with the interval of the maximal and the minimal deletion, both concerning ASNS coding sequences. C: Quantitative PCR of DNA concentration ratio between ASNS and a reference gene (ADORA2B) confirming the presence of the deletion in our patient inherited from her mother. D: Genetic chromatograms showing the hemizygous mutation in exon 3 c.144C > A; p.His48Gln in our patient inherited from her father. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This observation confirms the description of ASNSD as a severe neurodevelopmental disease associating: clinical findings of severe microcephaly, global development delay, spastic quadriplegia and seizures; radiological findings of brain atrophy and delayed myelination; and laboratory findings of low asparagine levels in fasting plasma or CSF, this latter being inconstant and fluctuating, but nonetheless useful for confirming the pathogenicity of the genetic variants identified in ASNS [3]. Half of the patients reported so far in the literature died during their first years and the others had poor neurological outcomes as our patient [11].
Concerning the management of this disease, only supportive therapy can be offered as there is no effective treatment to date. Alrifai and Alfadhel tested asparagine supplementation (500 mg, once daily) in a child with ASNSD [4]. Unfortunately, they reported worsening seizures leading to the discontinuation of the supplementation. They speculated that the asparagine supplementation, through the activity of asparaginase, increased the levels of aspartate and glutamate, both being excitatory neurotransmitters, or alternatively, that transport across the blood brain barrier may not have been effective. They finally proposed high doses of valproic acid to control seizures. Asparagine oral supplementation was tested on 2 others patients with no worsening of the disease and improvement of attention and nonverbal communication in one of them [14]. More studies are needed to understand better the effectiveness of this treatment.
We report here an unusual molecular mechanism for ASNSD comprising a missense mutation in one allele and a deletion in the other highly likely to be responsible for loss of function. All previously published cases of ASNSD were diagnosed via exome sequencing considered to be the most efficient technique for the diagnosis of rare autosomal recessive disorders [1,[6], [7], [8],10,13]. However, because the detection of CNVs remains a challenge in routine exome sequencing, array CGH continues to be pertinent for initial genetic explorations to detect (homozygous or heterozygous) deletions in genes causing autosomal recessive disorders. Moreover, our case underlines the difficulty of the diagnosis for some rare hereditary metabolic diseases via metabolic screening, which is burdened by the variability of its results. In contrast, amino acid analyses, particularly in CSF, provide the geneticist with valuable confirmatory information when candidate gene variations are suspected in rare amino acid deficiencies.
Acknowledgments
The proofreading of this article was supported by the “Bibliothèque Scientifique de l'Internat de Lyon and the Hospices Civils de Lyon”.
References
- 1.Abhyankar A., Lamendola-Essel M., Brennan K., Giordano J.L., Esteves C., Felice V., Wapner R., Jobanputra V. Clinical whole exome sequencing from dried blood spot identifies novel genetic defect underlying asparagine synthetase deficiency. Clin. Case Rep. 2018;6:200–205. doi: 10.1002/ccr3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfadhel M., Alrifai M.T., Trujillano D., Alshaalan H., Al Othaim A., Al Rasheed S., Assiri H., Alqahtani A.A., Alaamery M., Rolfs A., Eyaid W. Asparagine synthetase deficiency: new inborn errors of metabolism. JIMD Rep. 2015;22:11–16. doi: 10.1007/8904_2014_405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfadhel M., El-Hattab A.W. Asparagine synthetase deficiency. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J., Stephens K., Amemiya A., editors. GeneReviews®. University of Washington; Seattle, Seattle (WA): 2018. [PubMed] [Google Scholar]
- 4.Alrifai M.T., Alfadhel M. Worsening of seizures after asparagine supplementation in a child with asparagine synthetase deficiency. Pediatr. Neurol. 2016;58:98–100. doi: 10.1016/j.pediatrneurol.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Salem S., Gleeson J.G., Al-Shamsi A.M., Islam B., Hertecant J., Ali B.R., Al-Gazali L. Asparagine synthetase deficiency detected by whole exome sequencing causes congenital microcephaly, epileptic encephalopathy and psychomotor delay. Metab. Brain Dis. 2015;30:687–694. doi: 10.1007/s11011-014-9618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galada C., Hebbar M., Lewis L., Soans S., Kadavigere R., Srivastava A., Bielas S., Girisha K.M., Shukla A. Report of four novel variants in ASNS causing asparagine synthetase deficiency and review of literature. Congenit. Anom. 2018 doi: 10.1111/cga.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gataullina S., Lauer-Zillhardt J., Kaminska A., Galmiche-Rolland L., Bahi-Buisson N., Pontoizeau C., Ottolenghi C., Dulac O., Fallet-Bianco C. Epileptic phenotype of two siblings with asparagine synthesis deficiency mimics neonatal pyridoxine-dependent epilepsy. Neuropediatrics. 2016;47:399–403. doi: 10.1055/s-0036-1586222. [DOI] [PubMed] [Google Scholar]
- 8.Gupta N., Tewari V.V., Kumar M., Langeh N., Gupta A., Mishra P., Kaur P., Ramprasad V., Murugan S., Kumar R., Jana M., Kabra M. Asparagine Synthetase deficiency-report of a novel mutation and review of literature. Metab. Brain Dis. 2017;32:1889–1900. doi: 10.1007/s11011-017-0073-6. [DOI] [PubMed] [Google Scholar]
- 9.Lomelino C.L., Andring J.T., McKenna R., Kilberg M.S. Asparagine synthetase: function, structure, and role in disease. J. Biol. Chem. 2017;292:19952–19958. doi: 10.1074/jbc.R117.819060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monies D., Abouelhoda M., Assoum M., Moghrabi N., Rafiullah R., Almontashiri N., Alowain M., Alzaidan H., Alsayed M., Subhani S., Cupler E., Faden M., Alhashem A., Qari A., Chedrawi A., Aldhalaan H., Kurdi W., Khan S., Rahbeeni Z., Alotaibi M., Goljan E., Elbardisy H., ElKalioby M., Shah Z., Alruwaili H., Jaafar A., Albar R., Akilan A., Tayeb H., Tahir A., Fawzy M., Nasr M., Makki S., Alfaifi A., Akleh H., Yamani S., Bubshait D., Mahnashi M., Basha T., Alsagheir A., Abu Khaled M., Alsaleem K., Almugbel M., Badawi M., Bashiri F., Bohlega S., Sulaiman R., Tous E., Ahmed S., Algoufi T., Al-Mousa H., Alaki E., Alhumaidi S., Althagafi M., Alghamdi H., Alghamdi M., Sahly A., Nahrir S., Al-Ahmari A., Alkuraya H., Almehaidib A., Abanemai M., Alsohaibaini F., Alsaud B., Arnaout R., Abdel-Salam G.M.H., Aldhekri H., AlKhater S., Alqadi K., Alsabban E., Alshareef T., Awartani K., Banjar H., Alsahan N., Abosoudah I., Alashwal A., Aldekhail W., Alhajjar S., Al-Mayouf S., Alsemari A., Alshuaibi W., Altala S., Altalhi A., Baz S., Hamad M., Abalkhail T., Alenazi B., Alkaff A., Almohareb F., Al Mutairi F., Alsaleh M., Alsonbul A., Alzelaye S., Bahzad S., Manee A.B., Jarrad O., Meriki N., Albeirouti B., Alqasmi A., AlBalwi M., Makhseed N., Hassan S., Salih I., Salih M.A., Shaheen M., Sermin S., Shahrukh S., Hashmi S., Shawli A., Tajuddin A., Tamim A., Alnahari A., Ghemlas I., Hussein M., Wali S., Murad H., Meyer B.F., Alkuraya F.S. Lessons learned from large-scale, first-tier clinical exome sequencing in a highly consanguineous population. Am. J. Hum. Genet. 2019;104:1182–1201. doi: 10.1016/j.ajhg.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radha Rama Devi A., Naushad S.M. Molecular diagnosis of asparagine synthetase (ASNS) deficiency in two Indian families and literature review of 29 ASNS deficient cases. Gene. 2019;704:97–102. doi: 10.1016/j.gene.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 12.Ruzzo E.K., Capo-Chichi J.-M., Ben-Zeev B., Chitayat D., Mao H., Pappas A.L., Hitomi Y., Lu Y.-F., Yao X., Hamdan F.F., Pelak K., Reznik-Wolf H., Bar-Joseph I., Oz-Levi D., Lev D., Lerman-Sagie T., Leshinsky-Silver E., Anikster Y., Ben-Asher E., Olender T., Colleaux L., Décarie J.-C., Blaser S., Banwell B., Joshi R.B., He X.-P., Patry L., Silver R.J., Dobrzeniecka S., Islam M.S., Hasnat A., Samuels M.E., Aryal D.K., Rodriguiz R.M., Jiang Y., Wetsel W.C., McNamara J.O., Rouleau G.A., Silver D.L., Lancet D., Pras E., Mitchell G.A., Michaud J.L., Goldstein D.B. Deficiency of asparagine synthetase causes congenital microcephaly and a progressive form of encephalopathy. Neuron. 2013;80 doi: 10.1016/j.neuron.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacharow S.J., Dudenhausen E.E., Lomelino C.L., Rodan L., El Achkar C.M., Olson H.E., Genetti C.A., Agrawal P.B., McKenna R., Kilberg M.S. Characterization of a novel variant in siblings with asparagine synthetase deficiency. Mol. Genet. Metab. 2018;123:317–325. doi: 10.1016/j.ymgme.2017.12.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprute R., Ardicli D., Oguz K.K., Malenica-Mandel A., Daimagüler H.-S., Koy A., Coskun T., Wang H., Topcu M., Cirak S. Clinical outcomes of two patients with a novel pathogenic variant in ASNS: response to asparagine supplementation and review of the literature. Hum. Genome Var. 2019;6:24. doi: 10.1038/s41439-019-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J., McGillivray A.J., Pinner J., Yan Z., Liu F., Bratkovic D., Thompson E., Wei X., Jiang H., Asan null, Chopra M. Diaphragmatic Eventration in sisters with asparagine synthetase deficiency: a novel homozygous ASNS mutation and expanded phenotype. JIMD Rep. 2017;34:1–9. doi: 10.1007/8904_2016_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto T., Endo W., Ohnishi H., Kubota K., Kawamoto N., Inui T., Imamura A., Takanashi J.-I., Shiina M., Saitsu H., Ogata K., Matsumoto N., Haginoya K., Fukao T. The first report of Japanese patients with asparagine synthetase deficiency. Brain and Development. 2017;39:236–242. doi: 10.1016/j.braindev.2016.09.010. [DOI] [PubMed] [Google Scholar]