Abstract
Background
Gaucher disease is a rare multi-systemic metabolic disorder resulting from the deficiency of acid β-glucosidase activity, with consequent accumulation of glucocerebroside. Less than 15% of mean normal acid β-glucosidase activity in leukocytes is the gold standard for the diagnosis of Gaucher disease, and is generally supplemented by a massive elevation in chitotriosidase activity. We report here our experience in the biochemical diagnosis of Gaucher disease by showing the heterogeneity of the activity of enzymes over 25 years from 1993-2017, through the analysis of 5128 clinically suspected Gaucher disease cases referred to the Biochemical Genetics Department, National Research Centre, as the main reference lab in Egypt for the diagnosis of Inherited Metabolic Disorders.
Methods
Acid β-glucosidase and chitotriosidase activities were measured in all referred cases. Sphinogmylinase activity was estimated for all cases with normal β-glucosidase activity and moderate elevation of chitotriosidase.
Results
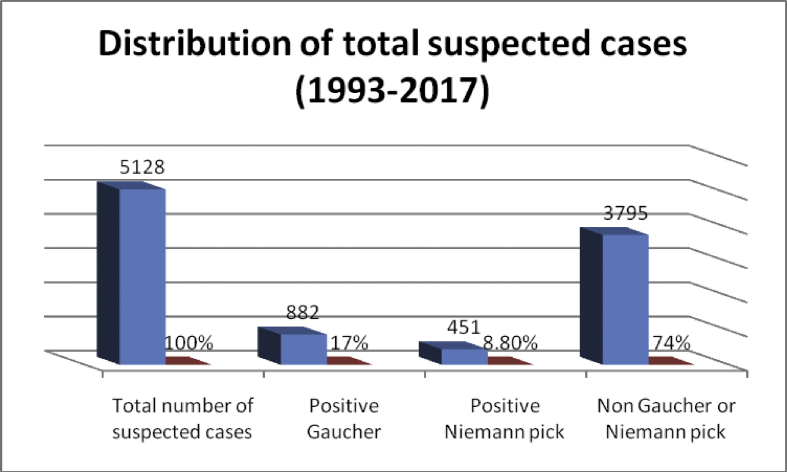
Out of the 5128 suspected cases, 882 (17%) showed a deficiency in acid β-glucosidase activity, accompanied by a raised chitotriosidase activity, ranges (213-66700 umol/l/h) and mean (7255 umol/l/h). Deficient chitotriosidase activity was found in 9 patients (1%) with low β-glucosidase. 451 cases were diagnosed with acid sphingomyelinase deficiency patients (8.8%).
Conclusion
Other biochemical markers are needed in addition to chitotriosidase for the diagnosis and follow up. Molecular testing was done to a relatively small number but needs to be done to all diagnosed patients as many mutations are known to predict the course of the disease.
Keywords: Metabolism, Biochemistry, Chitotriosidase, Experience., Gaucher disease, Acid β-glucosidase, Egypt
1. Introduction
Gaucher disease (GD; MIM #230800) is an autosomal recessive lysosomal storage disorder. Primarily resulting from the deficiency of beta-glucocerebrosidase activity (acid β-glucosidase; GBA; EC 3.2.1.45) which, leads to accumulation of undegraded glucocerebroside in several tissues, mainly in monocyte-macrophage origin of cells [1] or secondarily due to mutations in the sphingolipid activator protein SAP C gene [2] The disease prevalence in general population is between 1:20,000 to 1:200,000, with a high prevalence in the Ashkenazi Jewish population (1:450) [3].
GD presents as three variants according to the clinical signs and the onset of disease: GD type 1 Non-neuronopathic (OMIM# 230800) [4, 5], GD type 2 acute neuronopathic [6] and GD type 3 subacute neuronopathic [7, 8] GD type 3 is mainly seen in Northern Europe, Egypt and East Asia [9].
Acid β-glucosidase, cleaves the β -glucosyl linkage of glucosylceramide (GC) and glucosylsphingosine (GS); deficiency leads to accumulations of GC and GS in visceral organs and CNS regions. GC displays the greatest accumulation by mass, which is formed of β-D-glucose and ceramide. The latter consists of sphingosyl as well as fatty acid acyl chains (FAAC) of varying chain length from 16 to >26 carbons [10].
Glucosylsphingosine is the deacylated form of GC and belongs to the lyso-glycosphingolipid family, glucosylsphingosine is almost undetectable in tissues in healthy individuals, but is elevated in neuronopathic disease and correlates more with phenotype severity compared to glucosylceramide [11].
The location of acid β-glucosidase gene is on chromosome 1q21. More than 350 different GBA mutations have been reported [12].
Point mutations, deletions, splice-site mutations, and recombinant alleles are included in the identified mutant alleles. The most common known reported mutations are L444P, N370S, IVS2+1G4A, and 84GG, which account for 90% of the mutant alleles in the Ashkenazi Jewish populations and about 50–60% of alleles in non-Jewish individuals [13].
Some mutations have been associated with specific phenotypes. For example, the N370S mutation has been linked to GD type 1 [14].
The L444P mutation can occur as either a single base substitution or a part of a complex allele and can be related to all three types of GD [13]. Homozygotes for the L444P mutation typically develop GD type 3, whereas heterozygotes are more likely to develop GD type 1 or GD type 2 [15].
The standard biochemical diagnosis of GD is by demonstrating acid β-glucosidase deficiency in blood leukocytes or cultured skin fibroblasts and recently, in dried blood spot using synthetic substrates and tandem mass spectrometry [16, 17].
In affected cases the activity of acid β-glucosidase is about 0%–15% of normal activity; however, enzyme activity cannot detect the disease phenotype or heterozygote carriers of GD nor of saposin C deficiency [16].
Increased chitotriosidase (CT) activity is an epiphenomenon resulting from activation of macrophages upon up take of glucosylceramide; it is used as biomarker for GD since 1994 and to reflect the total storage of Gaucher cells and response to therapy [18].
Although GD patients usually display a massive (10–100fold) elevation in chitotriosidase, other lysosomal storage diseases (LSDs) may show elevated levels too but to lesser extent [19].
However, some individuals including GD patients (4–5%) are homozygous for 24 bp duplication in exon 10 chitotriosidase gene which renders the enzyme inactive. So, other biochemical markers will have to be tested [20].
Gaucher cells, such as activated macrophages, are responsible for several cellular responses manifested in GD, like cytokines (interleukin-1b, interleukin-1 receptor antagonist, interleukin-2 receptor, CD14 and M-CSF): [21, 22]. In 2013, Rolfs et al have proposed that, glucosylsphingosine is a more specific and sensitive biomarker than CT and CCL18/PARC in normal individuals, GD cases, GD carrier and other LSD patients [23].
The aim of the treatment is to reduce the excessive amount of glucocerebroside and other glycolipids. There are four routes for treatment: enzyme replacement therapy [24], substrate synthesis inhibition therapy (SRT) [25], pharmacological chaperone (PC) therapy [26, 27, 28], and gene therapy [29].
The aim of this study focuses on five different points: 1 – to describe our biochemical experience and recommendations for the diagnosis of Gaucher disease through (1993–2017) as a guide for an effective diagnosis, 2- to enroll all previously diagnosed cases and their total number over 25 years from 1993 to 2017,3 – to give an overview of the annually diagnosed cases, 4- to estimate approximately the incidence of the diagnosed cases among our population and 5- to compare the number of diagnosed cases versus the cases under treatment.
2. Material and methods
2.1. Materials
From March 1993 till December 2017, the Biochemical Genetics laboratory; the reference laboratory for inborn errors of metabolism in Egypt at National Research Centre, received blood samples from 5128 patients with suspected Gaucher signs and symptoms. These samples came from various areas throughout Egypt.
Ethical approval following the regulations of Helsinky-Ethical principles for medical research involving human subjects through the institutional review board at the NRC was obtained (n# 19004).
2.2. Methods
5 ml of whole blood were collected by venous puncture in EDTA tube. Plasma was obtained for CT assay and leukocytes were separated to determine the activity of acid β-glucosidase using synthetic substrate.
Leukocytes were isolated from peripheral blood according to Skoog and Beck (1956) [30]. Plasma was obtained through centrifugation (2,500 rpm for 5 min). Before initiating enzymatic analysis, protein content was estimated according to Lowry et al. (1951) [31].
For all cases, measurement of acid β-glucosidase activity was done with the assessment of chitotriosidase and finally sphinogmylinase activity in peripheral leukocytes.
Normal enzyme activities in healthy controls, for chitotriosidase was 4–80 umol/l/h, for acid β-glucosidase was 1-5umol/g prot./h. and for sphingomylinase was 9–47 umol/g prot./h. All the chemicals were purchased from Sigma- Aldrich, St. Louis, MO. USA.
Assay of acid β-glucosidase activity was done by using fluorogenic substrate 4-methyl-umbelliferyl-β-D-glucopyranoside in the presence of pure sodium taurocholate and Triton X-100.Activity was expressed in umol/g prot./h [32].
Chitotriosidase activity was measured by incubating plasma with 4-methylumbelliferyl-β-D-NN,N'triacetylchitotriose (4 MU-chitotrioside) as substrate in citrate/phosphate buffer pH 5.2, at 37 °C. The result was expressed in umol/l/hr [18].
3. Results
The present study included a total number of 5128 patients suspected to have Gaucher disease referred to the Biochemical Genetics Department from 1993 to 2017. From the patients reporting, age range was (3 months to adulthood) and the positive parental consanguinity was present in 3897 cases (76%) of the studied group.
Acid β-glucosidase and chitotriosidase activities were determined for all suspected cases to diagnose Gaucher patients.
Sphingomylinase activity was determined in all non Gaucher patients, with elevated chitotriosidase activity.
Out of 5128 suspected cases, 882 were diagnosed as Gaucher patients (17 %) and 451 cases were diagnosed as acid sphingomyelinase deficiency patients (8.8%), Fig. 1.
Fig. 1.
Total number of referred cases, Gaucher and Acid Sphingomyelinase deficiency patients during the period (1993–2017).
In 719 of 882 GD patients (81.5%) showed positive parental consanguinity, with male to female ratio (1.6:1). The age range of diagnosed Gaucher patients was (3 months - 45 years), the age of majority of patients (859, 97.5%) ranged (1.7:8 years), followed by 16 patients (1.8%) with a range (25 : 45 years) and six patients (0.7%) aged below 2 years (3 months: 1.4 years).
Most of the patients 873 (99%) revealed a decrease in acid β-glucosidase activity with a mean of (0.3 umol/g prot./h, 30% of low normal value) and showed dramatically increased in chitotriosidase activity with range (213-66700 umol/l/h) and mean value (6243 umol/l/h, ±20211 umol/l/h) Table 1.
Table 1.
Activities of acid β-glucosidase and chitotriosidase in diagnosed Gaucher patients.
| Number of cases | Normal level of acid β-glucosidase (1-5 umol/g prot./h) (Mean) | Normal level of chitotriosidase (4-80 umol/l/h) (Mean) | The Percent out of 882 cases |
|---|---|---|---|
| 9 | 0.45 | Zero | 1% |
| 873 | 0.3 | 6243 | 99% |
Out of 882 cases 9 patients (1%) showed a zero chitotriosidase activity, accompanied with low activity of acid β-glucosidase as well, Table 1.
A group of suspected cases 103 (2%) showed an elevation in chitotriosidase level with mean (131.8 umol/l/h, ±24 umol/l/h), normal activity of acid β-glucosidase with mean (3.2 umol/g prot./h) and normal sphingomylinase activity.
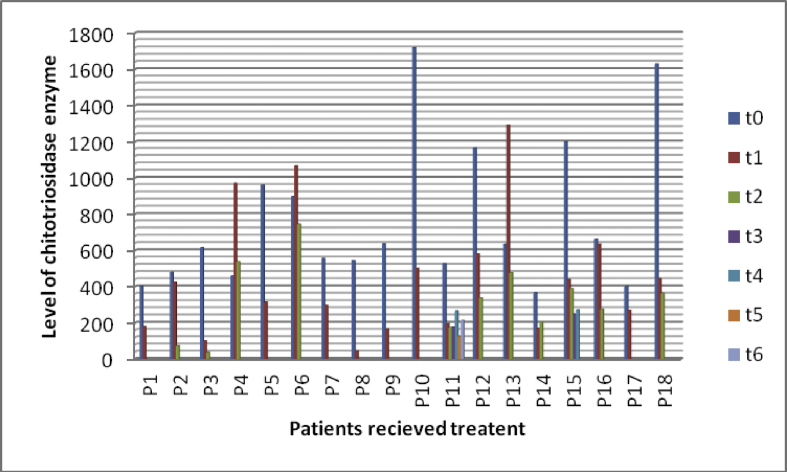
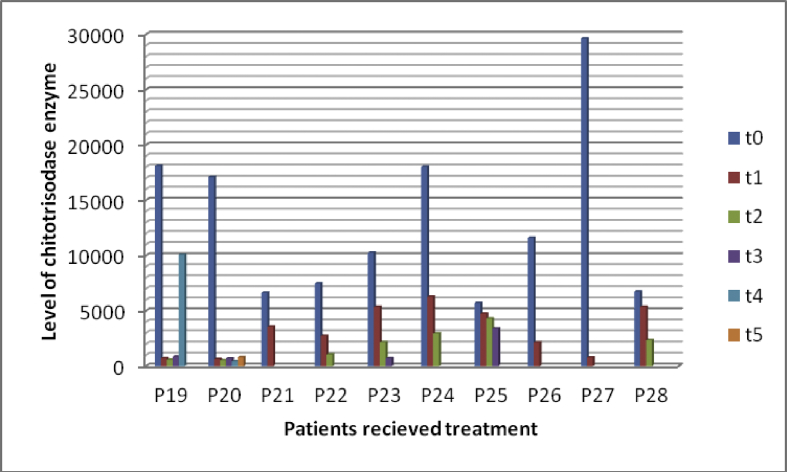
Figs. 2 and 3 showed reduction in chitotriosidase activity in 28 patients under treatment.
Fig. 2.
Reduction of chitotriosidase activity in some cases under treatment. t0: time at diagnosis, t1 to t6: times of follow-up at different intervals. P1: patient 1, P2: patient 2, P3: patient 3……………etc.
Fig. 3.
Reduction of chitotriosidase activity in some cases under treatment. t0: time at diagnosis, t1 to t6: times of follow-up at different intervals. P19: patient 19, P20: patient 20, P21: patient 21……………etc.
Out of 882 diagnosed cases, only 282 cases received the treatment (32%) and out of this 28 patients (10%) followed up their treatment by measuring CT enzyme activity.
The range of chitotriosidase activity at time of diagnosis was (367-29636 umol/l/h), with mean (5185 umol/l/h, ±7461 umol/l/h) while after the last follow-up the range was (38-3572 umol/l/h), and mean was (904 umol/l/h, ±1112 umol/l/h).
Large number of suspected cases were referred in the years 2014, 2015, 2016 and 2017 while the percent of diagnosed cases was not equally high. In the years 1999, 2000 and 2001 the number of referred cases was not as high but on the other hand the number of diagnosed cases was high, Table 2.
Table 2.
Number of referred cases, diagnosed GD patients and their percent in certain years.
| Years | Referred cases | Diagnosed GD cases | Precent of diagnosed cases |
|---|---|---|---|
| 1999 | 37 | 13 | 35% |
| 2000 | 100 | 25 | 25% |
| 2001 | 179 | 43 | 24% |
| 2014 | 424 | 59 | 14% |
| 2015 | 430 | 61 | 14% |
| 2016 | 551 | 68 | 12.3% |
| `2017 | 710 | 136 | 19.2% |
4. Discussion
Biochemical Genetics Department, National Research Centre, the main reference lab in Egypt for the diagnosis of Inherited Metabolic Disorders received 5128 patients suspected to have Gaucher disease from different hospitals and diagnostic centers throughout Egypt.
The consanguinity rate among Egyptians was reported in many articles, Temtamy and Loutife in 1970 [33], stated that the consanguinity rate was 30%, in 1983 Hafze et al. [34], reported that it was 28.9%. While in 2010, Temtamy et al. [35], stated that the consanguinity rate was 40%.
In the present study, the parental consanguinity was found in 3897 (76%) of the referred cases. While among the diagnosed GD patients, it was 719 (81.5%) and supported by previous studies among Egyptians GD patients, Ragab et al., 2000 (100%) [36], El-Beshlawy et al., 2006 (68%) [37], Khalifa et al., 2011 (88.8%) [38] and Fateen et al., 2017 (45.5%) [39].
Among 19 Syrian GD patients, the consanguinity rate was 82%, which is very similar to our results [40], while among 425 Tunisian GD patients the consanguinity was (64.94%) [41].
Among 412 Brazilian GD patients, the consanguinity rate reached to 58% [42]. All these high consanguinity rates among GD patients match its autosomal recessive mode of inheritance.
Although GD is an autosomal recessive disorder affecting both sexes equally [43] our results showed a difference between male patients (541) and female patients number (341) with a ratio (1.6: 1) this reflects traditions in Arab and Eastern countries; who give more care to males than females especially in rural areas where the consanguinity rate and metabolic disorders are high.
El-Beshlawy et al., 2006 showed that, the male to female ratio among 21 GD patients was (1.2:1) [37], Khalifa et al., 2011, found the ratio among 48 patients was (3.5:1) [38]. El-Morsy et al., 2011 [44] found that, the ratio among17 GD patients was (3.25:1), Fateen et al., 2017 [39] showed the ratio in 34 GD patients was (1.6:1) However Ragab et al., 2000 showed that, the ratio in 13 GD patients was (1:1.16) [36], and Tahia et al, 2017 [45] revealed that the ratio was (1.2:1) in 29 patients from Upper Egypt. Our result agrees with all previous reported studies.
Similarly among 30 Tunisian GD patients the ratio was (1.5:1) [46]. A study in India showed that among all the confirmed lysosomal storage disorders, the incidence in males was observed to be more common than in females, with male to female ratio (3.5:1) except for metachromatic leukodystrophy and mucopolysaccharidosis type IVA [47]. This study has no scientific explanation except the small number of the studied groups, which cannot give accurate estimate of the real ratios.
While among 412 Brazilian Gaucher patients, the ratio was (1: 1.35) [42] which is different from our findings and the findings in other Arab countries.
About 14% of GD patients were diagnosed between the age of 31 and 50 years [46]. In this study, only16 patients (1.8%) were diagnosed between the ages 25–45 years, with mild type of GD pointing to type 1, our finding does not reflect the real number of adult Gaucher patients in Egypt, it only reflects that adult GD patients are under diagnosed either because of the mild course of the disease or low awareness by the physician in a country like Egypt with so many other causes of hepatosplenomegaly. But from our experience it is mostly due to the mild form of disease in the adult diagnosed cases.
Six cases (0.7%) aged below 2 years were diagnosed as GD type 2, which also do not reflect the real number of type 2 patients, who die very early due to severe neurologic manifestations without being diagnosed [48]. These two types need more awareness among the physicians to estimate the real number of these patients among our population.
Most of the diagnosed patients 859 (97.4%) were around the age of two years reflect severe disease with performed anemia, thrombocytopenia, organomegaly and early onset of mild neurological signs, pointing to GD type 3.
Our results agree with previous studies on Egyptian Gaucher patients; El-Beshlawy et al., 2006 [37] found that, out of 22 patients, 13 (59.1%) patients were diagnosed as GD type 3, Khalifa et al., 2011 [38] detected among 48 GD patients, 32 (66.7%) exhibited type 3 followed by type 1 10 (20.8%) while type 2 had the lowest frequency 6 (12.5%). Tylki-Szymanska et al., 2010 [9] mentioned that GD type 3 is mainly seen in Northern Europe, Egypt and East Asia.
Fateen et al., 2017 carried out a study on 34 Gaucher patients. This study showed that, GD 3 was the most common form followed by GD 1 and one patient with type 2 [39].
Out of 30 Tunisian Gauher patients, 28 were classified type 1 GD and 2 patients were diagnosed types 2 and 3 [49].
In conclusion the prevalence of type GD 3 among Egyptian patients correlates well with the age of diagnosis of 97.4% of the patients which are diagnosed at around the age of 2 years and the severity of the disease.
The protocol for the diagnosis of GD starts with measurement of acid β-glucosidase activity in peripheral blood leukocytes, Baris et al., 2014 [11] pointed that acid β-glucosidase activity in Gaucher patient was 0–15% and Cabrera-Ortiz et al., 2016 [50] stated that cut off value of acid β-glucosidase was 30% in the studied cases confirmed by molecular study. The cut off value in the present study was 30% of low normal value accompanied with a massive elevation of chitotriosidase, on average 78 times of high normal value of CT activity, Table 1.
Molecular study for diagnosed cases is an important step for further confirmation and prediction of the disease type, but unfortunately, it has not been done on all 882 diagnosed Gaucher patients.
Molecular analysis has been done in some of these patients in several previous studies (El-Morsy et al., 2011 [44], Khalifa et al., 2011 [38] and Fateen et al., 2017 [39], who stated that, the most common mutation was L444P/L444P.
The second step in the protocol is assessment of CT activity. As mentioned previously, 873 GD cases (99%) showed a massive elevation in CT level over other LSDs [51]. The level of CT in this study was higher than the high normal value (80umol/L) with range (213-66700umol/l/h) and mean (7254.8umol/L, ±20211.38 umol/l/h).
One hundred and three patients out of 5128 (2%) showed increased CT with normal acid β-glucosidase activity. This led us to search for another LSD and allowed us to establish other diagnoses, especially acid sphingomyelinase deficiency types among the referred cases.
Lo et al., 2010 reported a misdiagnosed acid sphingomyelinase deficiency case, who was diagnosed first as GD patient, then after a negative response to the therapy the case was re-evaluated and diagnosed as acid Sphingomyelinase deficiency type C patient [52].
According to our similar experience through the years, we always assess sphingomylinase activity in all the referred GD patients. Still, some cases are diagnosed with GD. We attributed this to the poor quality of blood of these patients; which to unknown reason improves after ERT and by reassessment after few months of treatment a secured diagnosis of acid sphingomyelinase deficiency was obvious.
Although, chitotriosidase activity is a reliable biomarker for the diagnosis and follow up of GD patients, there were 9 cases (1%) with zero CT activity. Boot et al., 1998 [20] stated that about 4–5% of the population showed CT deficiency due to the presence of 24 bp duplication homozygous in exon 10 of the CHIT1 gene.
This raises the demands for new biomarkers for different LSDs, Lobato et al., 2016 [53] mentioned different biomarkers for different LSDs; glucosylsphingosine as biomarker for GD, proposed by Rolfs et al. [23] has emerged as a more effective alternative biomarker. Their results showed that the concentration of glucosylsphingosine in Gaucher samples was about 100 times higher than in healthy individuals and in patients with other LSDs. In the same direction, Murugesan et al., 2016 stated that, the utility of glucosylsphingosine as a biomarker of GD is underscored by its biological role and impressive associations with indicators of disease severity as well as treatment status [54].
Misdiagnosis of GD and resulting diagnostic delays can occur in all types of GD patients, due to the vast heterogeneity in overall disease severity as well as differing patterns of organ involvement [55].
As a result, the parents of the affected child lose out on timely genetic counseling, and the advantage of prenatal diagnosis. This puts the couple on the risk of having another affected child. In the present study, 33 families (0.77 %) had history of an affected sibling notably in the first years of study. In the recent years all diagnosed families sought prenatal diagnosis.
In 1999, Project HOPE began to implement the Gaucher Disease Initiative in Egypt in partnership with Genzyme Corporation, through donation of more than 30 million units of Cerezyme (imiglucerase for injection).
A total of 282 cases that received therapy in Egypt till now, represented 32% of the number of GD patients diagnosed in this study (882), therefore large numbers of patients did not receive any therapy.
According to the data we had in this study, 28 patients (10%) are regularly having follow up by measuring CT enzyme activity. Their CT activity has decreased from mean 5185umol/l/h to mean 904umol/l/h. By this regular follow up the ERT can be tuned; which is very important for such very an expensive medication.
The time of follow up was variable between patients and there were massive differences in CT activity between patients and between the same patient (Figs. 2, 3). Due to financial reasons and we are a developmental country, there is no guarantee to receive the therapy in regular way or receive a suitable dose. Moreover, the transportation is expensive for families as they live in rural areas and there is no specific centre near them. Besides, the awareness of the importance of follow up is not equal between patients and must be increased among them and treating physicians.
The increased annual number of referred cases in the last four years of this study and the lower percent of positive GD cases compared with the first years of study reflect the increased awareness among the pediatricians about the diverse clinical features of GD and the availability of the specific investigations and treatment.
In the general population the prevalence of GD type 3 is 1:100.000 [56,57] so the total number of diagnosed Gaucher patients in Egypt should be 1000 cases according to the calculation using the prevalence and the total number of the population in Egypt (100.000.000), that means the difference between what we diagnosed over 25 years and what we predict is small 1000-882 = 118 cases around Egypt. Which lead us to think that the prevalence in Egypt may be higher than 1:100.000.
Lysosomal disorders are rare, inherited disorders that impact metabolic processes resulting from dysfunction or loss of function of lysosomal enzymes. Early diagnosis is critical in determining treatment options for patients, and there is evidence that starting treatment early mostly results in a better outcome. There is a need to review the molecular and biologic principles behind early treatment of lysosomal disorders with a focus on Fabry and Pompe diseases. The panel of experts in lysosomal diseases will explore the opportunities and challenges of starting treatment early before irreversible organ damage occurs.
Declarations
Author contribution statement
Ekram M. Fateen: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Zeinab Y. Abdallah: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work is supported financially through National Research Centre (10010602).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Jmoudiak M., Futerman A.H. Gaucher disease: pathological mechanisms and modern management. Br. J. Haematol. 2005;129:178–188. doi: 10.1111/j.1365-2141.2004.05351.x. [DOI] [PubMed] [Google Scholar]
- 2.Tylki-Szymanska A., Czartoryska B., Vanier M.T. Non-neuronopathic Gaucher disease due to saposin C deficiency. Clin. Genet. 2007;72:538–542. doi: 10.1111/j.1399-0004.2007.00899.x. [DOI] [PubMed] [Google Scholar]
- 3.Martins A.M., Valadares E.R., Porta G., Coelho J., Filho S.J., Pianovski M.A., Kerstenetzky M.S., Mde M.F., Aranda P.C., Pires R.F., Mota R.M., Bortolheir T.C. Brazilian study group on Gaucher disease and other lysosomal storage diesases recommendations on diagnosis, treatment, and monitoring for Gaucher disease. J. Pediatr. 2009;155:S10–18. doi: 10.1016/j.jpeds.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Grabowski G.A. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372:1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 5.Walton-Bowen K., Mantick N. 2000. Gaucher Registry Annual Aggregate Data Report. [Google Scholar]
- 6.Grabowski G.A., Andria G., Baldellou A. Pediatric non-neuronopathic Gaucher disease: presentation, diagnosis and assessment: consensus statements. Eur. J. Pediatr. 2004;163:58–66. doi: 10.1007/s00431-003-1362-0. [DOI] [PubMed] [Google Scholar]
- 7.Mehta A. Epidemiology and natural history of Gaucher’s disease. Eur. J. Intern. Med. 2006;17:S2–S5. doi: 10.1016/j.ejim.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Pastores G.M. Neuropathic Gaucher disease. Wien. Med. Wochenschr. 2010;160:605–608. doi: 10.1007/s10354-010-0850-x. [DOI] [PubMed] [Google Scholar]
- 9.Tylki-Szymanska A., Vellodi A., El-Beshlawy A., Cole J.A., Kolodny E. Neuronopathic Gaucher disease: demographic and clinical features of 131 patients enrolled in the International Collaborative Gaucher group neurological outcomes subregistry. J. Inherit. Metab. Dis. 2010;33:339–346. doi: 10.1007/s10545-009-9009-6. [DOI] [PubMed] [Google Scholar]
- 10.Grabowski G.A., Petsko G.A., Kolodny E.H. Gaucher disease. In: Valle D., Beaudet A., Vogelstein B., Kinzler K.W., Antonarakis S.E., editors. The Online Metabolic and Molecular Bases of Inherited Diseases. The McGraw-Hill Companies, Inc.; New York: 2010. [Google Scholar]
- 11.Orvisky E., Park J.K., LaMarca M.E. Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: correlation with phenotype and genotype. Mol. Genet. Metab. 2002;76:262–270. doi: 10.1016/s1096-7192(02)00117-8. [DOI] [PubMed] [Google Scholar]
- 12.Siebert M., Bock H., Michelin-Tirelli K., Coelho J.C., Giugliani R., Saraiva-Pereira M. Novel mutations in the glucocerebrosidase gene of Brazilian patients with Gaucher disease. JIMD Rep. 2013;9:7–16. doi: 10.1007/8904_2012_174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastores G.M., Barnett N.L., Kolodny E.H. An open-label, noncomparative study of miglustat in type I Gaucher disease: efficacy and tolerability over 24 months of treatment. Clin. Ther. 2005;27:1215–1227. doi: 10.1016/j.clinthera.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Goker-Alpan O., Hruska K.S., Orvisky E. Divergent phenotypes in Gaucher disease implicate the role of modifiers. J. Med. Genet. 2005;42:e37. doi: 10.1136/jmg.2004.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ron I., Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum. Mol. Genet. 2005;14:2387–2398. doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 16.Baris H.N., Cohen I.J., Mistry P.K. Gaucher Disease: the metabolic defect, pathophysiology, phenotypes and natural history. Pediatr. Endocrinol. Rev. 2014;12(0 1):72–81. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X.K., Elbin C.S., Chuang W.L. Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin. Chem. 2008;54:1725–1728. doi: 10.1373/clinchem.2008.104711. [DOI] [PubMed] [Google Scholar]
- 18.Hollak C.E., Van W.S., Van Oers M.H. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J. Clin. Investig. 1994;93:1288–1292. doi: 10.1172/JCI117084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodamer O.A., Hung C. Laboratory and genetic evaluation of Gaucher disease. Wien. Med. Wochenschr. 2010;160:600–604. doi: 10.1007/s10354-010-0814-1. [DOI] [PubMed] [Google Scholar]
- 20.Boot R.G., Renkema G.H., Verhoek M. The human chitotriosidase gene. Nature of inherited enzyme deficiency. J. Biol. Chem. 1998;273:25680–25685. doi: 10.1074/jbc.273.40.25680. [DOI] [PubMed] [Google Scholar]
- 21.Barak V., Acker M., Nisman B. Cytokines in Gaucher’s disease. Eur. Cytokine Netw. 1999;10:205–210. [PubMed] [Google Scholar]
- 22.Hollak C.E., Evers L., Aerts J.M. Elevated levels of M-CSF, sCD14 and IL8 in type 1 Gaucher disease. Blood Cells Mol. Dis. 1997;23:201–212. doi: 10.1006/bcmd.1997.0137. [DOI] [PubMed] [Google Scholar]
- 23.Rolfs A., Giese A.K., Grittner U. Glucosylsphingosine is highly sensitive and specific biomarker for primary diagnostic and follow-up monitoring in Gaucher disease in a non-Jewish, Caucasian cohort of Gaucher disease patients. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson H.C., Charrow J., Kaplan P. Individualization of long-time enzyme replacement therapy for Gaucher disease. Genet. Med. 2005;7(2):105–110. doi: 10.1097/01.gim.0000153660.88672.3c. [DOI] [PubMed] [Google Scholar]
- 25.Mc Eachern K.A., Fung J., Komarnitsky S. A specific and potent inhibitor of glucosylceramide synthase for substrate reduction therapy of Gaucher disease. Mol. Genet. Metab. 2007;91:259–267. doi: 10.1016/j.ymgme.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Ron I., Horowitz M. ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum. Mol. Genet. 2005 doi: 10.1093/hmg/ddi240. [DOI] [PubMed] [Google Scholar]
- 27.Kornhaber G.J., Tropak M.B., Maegawa G.H. Isofagomine induced stabilization of glucocerebrosidase. Chem. Biochem. 2008;9:2643–2649. doi: 10.1002/cbic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maegawa G.H., Tropak M.B., Buttner J.D. Identification and characterization of ambroxol as an enzyme enhancement agent for Gaucher disease. J. Biol. Chem. 2009;284:23502–23516. doi: 10.1074/jbc.M109.012393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters C., Krivit W. Hematopoietic stem cell therapy, stem cells and gene therapy. In: Futerman A.H., Zimran A., editors. Gaucher Disease. CRC Press; Boca Raton: 2007. pp. 423–443. [Google Scholar]
- 30.Skoog W., Beck W.S. Studies on the fibrinogen, dextran and phytohemagylutinin methods of isolation leukocytes. Blood. 1956;11:436. [PubMed] [Google Scholar]
- 31.Lowry O.H., Rosenbrough N.J., Farr A., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 32.Wenger D.A., Clark C., Sattler M., Wharton C. Synthetic substrate β-glucoside activity in leucocyte: a reproducible method for the identification of the patients and carriers of Gaucher disease. Clin. Genet. 1978;13:145–153. doi: 10.1111/j.1399-0004.1978.tb04242.x. [DOI] [PubMed] [Google Scholar]
- 33.Temtamy S.A., Loutife A. CL Palate J; 1970. Surgical aspects of cleft lip-cleft palate problems in Egypt; pp. 578–581. [PubMed] [Google Scholar]
- 34.Hafez M.M., El-Tahan H., Awad Allah M.O. Consanguineous meeting in Egyptian population. J. Med. Genet. 1983;20:53–60. doi: 10.1136/jmg.20.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temtamy S.A., Aglan M.S., Meguid N.A. Genetic disorders in the Egyptians. In: Teebi A.S., editor. Genetic Disorders Among Arab Population. Springer; 2010. [Google Scholar]
- 36.Ragab L., Mostafa N., Fateen E., Kamal A. Estimation of β-glucocerebrosidase and plasma chitotriosidase in Gaucher diseases. Gaz. Egypt. Paed. 2000;48:73. [Google Scholar]
- 37.El-Beshlawy A., Ragab L., Youssry I., Yakout K., El-Kiki H., Eid K., Mansour I.M., Abd El-Hamid S., Yang M., Mistry P.K. Enzyme replacement therapy and bony changes in Egyptian paediatric Gaucher disease patients. J. Inherit. Metab. Dis. 2006;29:92–98. doi: 10.1007/s10545-006-0121-6. [DOI] [PubMed] [Google Scholar]
- 38.Khalifa A.S., Tantawy A.A., Shawky R.M., Monir E., Elsayed S.M., Fateen E., Cooper A. Outcome of enzyme replacement therapy in children with Gaucher disease: the Egyptian experience. Egypt. J. Med. Hum. Genet. 2011;12:9–14. [Google Scholar]
- 39.Ekram F., Heba F., Dina A.M., Noha K.M., Alice A.A. Mutational analysis of a cohort of Egyptian patients with Gaucher disease. Middle East J. Med. Genet. 2017;6:61–69. [Google Scholar]
- 40.Alasmar D. Gaucher disease in Syrian children: common mutations identification, and clinical futures. Ann. Saudi Med. 2015;35(2):127–132. doi: 10.5144/0256-4947.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ben Halim N., Hsouna S., Lasram K., Rejeb I., Walha A., Talmoudi F. Differential impact of consanguineous marriages on autosomal recessive diseases in Tunisia. Am. J. Hum. Biol. 2016;28(2):171–180. doi: 10.1002/ajhb.22764. [DOI] [PubMed] [Google Scholar]
- 42.Michelin K. Application of a comprehensive protocol for the identification of Gaucher disease in Brazil. Am. J. Med. Genet. 2005;136A:58–62. doi: 10.1002/ajmg.a.30787. [DOI] [PubMed] [Google Scholar]
- 43.Griffiths, Anthony J.F. W.H. Freeman; New York: 1999. An Introduction to Genetic Analysis. [Google Scholar]
- 44.El-Morsy Z., Khashaba M.T., Soliman O.E.S. Glucosidase acid beta gene mutations in Egyptian children with Gaucher disease and relation to disease phenotypes. World J. Pediatr. 2011;7:326. doi: 10.1007/s12519-011-0309-1. [DOI] [PubMed] [Google Scholar]
- 45.Tahia H.S., Mohammed H.H., Ahmed E.A., Ayat A.S., Nahed A.M., Khalid I.E., Abdallah M.A.A.E., Norhan B.B.M. Clinical and genetic assessment of pediatric patients with Gaucher’s disease in Upper Egypt. Egypt. J. Med. Hum. Genet. 2017;18:249–255. [Google Scholar]
- 46.Dandana A., Ben Khelifa S., Chahed H., Miled A., Ferchichi S. Gaucher disease: clinical, biological and therapeutic aspects pathobiology. 2016;83:13–23. doi: 10.1159/000440865. [DOI] [PubMed] [Google Scholar]
- 47.Kadali S. The relative frequency of lysosomal storage disorders: a medical genetics referral laboratory’s experience from India. J. Child Neurol. 2014;29(10):1377–1382. doi: 10.1177/0883073813515075. [DOI] [PubMed] [Google Scholar]
- 48.Weiss K., Gonzalez A., Lopez G., Pedoeim L., Groden C.M.S., CRNP, Sidransky E. The clinical management of type 2 Gaucher disease. Mol. Genet. Metab. 2015;114(2):110–122. doi: 10.1016/j.ymgme.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cherif W., Ben Turkia H., Ben Rhouma F. Gaucher disease in Tunisia: high frequency of the most common mutations. Blood Cells Mol. Dis. 2009;43:161–162. doi: 10.1016/j.bcmd.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Ortiz-Cabrera N.V., Gallego-Merlo J., Vélez-Monsalve C., de Nicolas R., Mas S.F., Ayuso C., Trujillo-Tiebas M.J. Nine-year experience in Gaucher disease diagnosis at the Spanish reference center Fundación Jiménez Díaz. Mol. Genet. Metab. Rep. 2016;13(9):79–85. doi: 10.1016/j.ymgmr.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo Y., He W., Boer A.M., Wevers R.A., de Bruijn A.M., Groener J.E., Hollak C.E., Aerts J.M., Galjaard H., van Diggelen O.P. Elevated plasma chitotriosidase activity in various lysosomal storage disorders. J. Inherit. Metab. Dis. 1995;18(6):717–722. doi: 10.1007/BF02436762. [DOI] [PubMed] [Google Scholar]
- 52.Lo S.M., McNamara J., Seashore M.R., Mistry P.K. Misdiagnosis of Niemann-pick disease type C as Gaucher disease. J. Inherit. Metab. Dis. 2010;33:S429–S433. doi: 10.1007/s10545-010-9214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lobato J.B., Hidalgo M.J., Jiménez L.M. Review biomarkers in lysosomal storage diseases. Diseases. 2016;(4):40. doi: 10.3390/diseases4040040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murugesan V., Chuang W.L., Liu J., Lischuk A., Kacena K., Lin H., Pastores G.M., Yang R., Keutzer J., Zhang K., Mistry P.K. Glucosylsphingosine is a key biomarker of Gaucher disease. Am. J. Hematol. 2016 Nov;91(11):1082–1089. doi: 10.1002/ajh.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox T.M., Cachón-González M.B. The cellular pathology of lysosomal diseases. J. Pathol. 2012;226:241–254. doi: 10.1002/path.3021. [DOI] [PubMed] [Google Scholar]
- 56.Abdelwahab M., Blankenship D., Schiffmann R. Long-term follow-up and sudden unexpected death in Gaucher disease type 3 in Egypt. Neurol. Genet. 2016;2(2):e55. doi: 10.1212/NXG.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shawky R.M., Elsayed S.M. Review.Treatment options for patients with Gaucher disease. Egypt. J. Med. Hum. Genet. 2016;17(3):281–285. [Google Scholar]