Abstract
Objective:
We explored underlying metabolism-related dysfunction by examining metabolomic profiles in adults categorized as lean, having normal weight obesity (NWO), or overweight-obesity.
Methods:
Subjects (n=179) had fasting plasma analyzed using liquid chromatography and high-resolution mass spectrometry for high-resolution metabolomics (HRM). Body composition was assessed by dual energy x-ray absorptiometry. NWO was defined as a body mass index (BMI) <25 and body fat >30% for females and >23% for males. Differentiating metabolomic features were determined using linear regression models and likelihood ratio tests, with false discovery rate (FDR) correction. Mummichog was utilized for pathway and network analyses.
Results:
A total of 222 metabolites significantly differed between the groups at FDR q=0.2. Linoleic acid, beta-alanine, histidine, and aspartate/asparagine metabolism pathways were significantly enriched (all, p<0.01) by metabolites that were similarly upregulated in the NWO and overweight-obesity groups compared to the lean group. Network analysis linked branched chain amino acids and amino acid metabolites as elevated in the NWO and overweight-obesity groups compared to the lean group (all, p<0.05).
Conclusions:
Metabolomic profiles of individuals with NWO reflected similar metabolic disruption as individuals with overweight-obesity. HRM may help identify people at risk for developing obesity-related disease, despite normal BMI.
Keywords: Amino acids, body composition, body mass index, diet, fat distribution, fatty acids, nutrition, overweight, linoleic acid, lipid metabolism
Introduction
Obesity is a leading risk factor for major diseases including cardiovascular disease, type 2 diabetes, and cancer and health conditions such as depression, obstructive sleep apnea, and decreased physical functioning(1). Individuals with obesity have excess fat mass and metabolic dysregulation resulting in increased all-cause mortality risk(1). Body mass index (BMI), calculated using simple anthropometric measures of height and weight, is used clinically to define obesity as a BMI above 30 kg/m2. While BMI is useful for identifying individuals at extreme levels with very high or low adiposity, BMI values in more moderate ranges are not well correlated with body fatness(2, 3). This is because BMI utilizes total body weight and does not account for body composition components such as lean mass and fat mass, which independently influence disease risk.
Within the range of intermediate BMI values is a group of individuals with a body composition phenotype termed normal weight obesity (NWO)(4). These individuals have a BMI within the normal weight range (18.5–24.9 kg/m2) but exhibit excess fat mass. The current reported estimates for NWO are as high as 30%(4, 5). Individuals with NWO have an increased risk of cardiometabolic disease and mortality compared to individuals who are normal weight and lean and individuals who are metabolically healthy with obesity(6). Previous studies show that individuals with NWO have elevated cardiometabolic disease risk factors demonstrated by hyperlipidemia, hypertension, glucose intolerance, insulin resistance, increased inflammation, increased oxidative stress, and decreased physical functioning(7). Although there is a growing literature of metabolic dysregulation in NWO, there is a need to define the nutrition and metabolism-related pathophysiology of NWO.
High-resolution metabolomics (HRM) is an innovative platform that is useful for exploring obesity-related disease from a systems-biology approach(8). HRM is a powerful tool for nutrition research because it enables the profiling of thousands of small-molecular weight metabolites in human biosamples and allows the investigation of important questions regarding the complex metabolite interactions which derive from diet, endogenous nutrient metabolism, the microbiome, and exogenous chemicals(8). Metabolomics has been used to identify specific metabolic signatures related to BMI and obesity(9, 10). However, there is little known regarding the metabolomic profiles of individuals with NWO in comparison to other body composition subtypes utilizing HRM. In this study, we used HRM to investigate differences in the plasma metabolome between three body composition subtypes: lean, NWO, and overweight-obesity. We hypothesized that individuals with NWO would have metabolomic profiles that are similar to subjects who are overweight-obese and distinct from subjects who are lean.
Methods
Subjects and Study Design
Emory University and Emory Healthcare employees were randomly invited to join the Emory-Georgia Tech Center for Health Discovery and Well Being Predictive Health Institute (http://predictivehealth.emory.edu) cohort study between December 2007 and December 2010. Participants underwent extensive dietary, metabolic, and other phenotypic assessments, as described in detail elsewhere (11). All subjects provided written informed consent and the study was approved by the Emory University Institutional Review Board. Exclusion criteria were the addition of a new prescription medication for chronic disease treatment within the previous year (other than anti-hypertensive or anti-diabetic agents), acute illness within 12 weeks of the study visit, hospitalization for an acute or chronic disease within the previous year, history of substance/drug or alcohol abuse, current active malignant neoplasm, women who were pregnant or breastfeeding, or having an uncontrolled (non-medicated) or poorly controlled autoimmune, cardiovascular, endocrine, gastrointestinal, hematologic, infectious, inflammatory, musculoskeletal, neurologic, psychiatric, or respiratory disease (11). All data included in this analysis were collected at baseline visits. The current study included a subset of individuals with available baseline plasma HRM data. Demographic, education, and income information were self-reported. Subjects were classified as having a history of chronic disease (yes/no) if they reported a current diagnosis of diabetes, hypertension, or hyperlipidemia, or if subjects were currently taking anti-hypertensive, anti-diabetic, or lipid-lowering medications.
Clinical Markers, Physical Fitness, and Diet Quality Scores
Fasting concentrations of glucose, insulin, and lipids were measured by Quest Diagnostics (Valencia, CA). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated according to Matthews et al.(12). Systolic and diastolic blood pressure were measured using an automated machine (Omron, Kyoto, Japan). Physical fitness (VO2 maximum) was assessed using a GE T2100 Treadmill (GE Healthcare, Waukesha, WI) following a modified Bruce protocol. Subjects completed the Cross-Cultural Activity Participation Study (CAPS) (13) to determine if individuals met the 2007 American College of Sports Medicine/American Heart Association physical activity and strength guidelines. Dietary intake was assessed with the 2005 Block food frequency questionnaire (NutritionQuest, Berkeley, CA). Any reported intakes below 500 calories or above 5,000 calories were considered implausible and excluded. Three validated diet quality scores, Alternate Healthy Eating Index (aHEI)(14), Dietary Approach to Stop Hypertension (DASH)(15), and Alternate Mediterranean Diet Score (MDS)(16), were calculated from FFQ output, as previously described(17).
Body Composition Analysis and Body Composition Subgroups
Whole and regional body composition were assessed by dual energy x-ray absorptiometry (DXA) using a Lunar iDXA densitometer and enCORE (v.12.2) with CoreScan® software (GE Healthcare, Madison, WI, USA). BMI was calculated from height and weight measured using an electronic scale and stadiometer (Tanita TBF-25, Tanita Health Management, Arlington Heights, IL). Participants were then classified into one of three body composition subtypes (lean, NWO, or overweight-obesity) based on sex-specific body fat percent values and BMI. For males, a body fat percent above 23 was considered elevated, and for females, a body fat percent above 30 was considered elevated based on published literature(18). Participants were categorized as having a lean body composition subtype if BMI was between 18.5 and 24.9 kg/m2 and body fat percent was below the sex-specific cut-off values. NWO was defined as a BMI between 18.5 and 24.9 kg/m2 and a body fat percent above the sex-specific cut-off values. Lastly, overweight-obesity was categorized as a BMI ≥25 kg/m2 and a body fat percent above the sex-specific cut-off values. Waist circumference was measured three times by a health professional trained in anthropometry using a tape measure, and the average value is reported.
Plasma High-Resolution Metabolomics (HRM)
Plasma HRM was performed on 179 fasted individuals using published methods(19) in the Emory University Clinical Biomarkers Laboratory. In brief, fasting plasma previously stored at −80°C was treated with acetonitrile and an internal standard mixture using an established protocol(19). Following protein precipitation, fasting plasma samples were analyzed in triplicate with a Fourier transform mass spectrometer (MS, Dionex Ultimate 3000, Q-Exactive HF, Thermo Fisher, Waltham, MA) using C18 liquid chromatography (LC) and positive electrospray ionization (ESI) to maximize the detection of low-molecular weight chemicals. After analysis of all participant samples and quality control samples, LC/MS data was extracted using the R-based packages apLCMS(20) and xMSanalyzer(21) to provide a mass to charge (m/z) feature table of detected ions denoted by relative retention time and accurate mass. Batch correction was completed by ComBat(22). Data pre-processing included: 1) filtering of features based on coefficient of variation (CV); 2) filtering of samples based on Pearson correlation between averaged technical replicates and percent missing values (features were retained only if there was a signal in at least 50% of samples); and 3) log10 transformation, quantile normalized, and mean centering. A total of 9,967 metabolomic features were included in this analysis following data filtering.
Metabolite Identification
The R package xMSannotator was used for metabolite annotation, which uses multiple criteria to provide a score-based annotation(23). Identities of multiple endogenous metabolites, including the amino acids, have been confirmed by comparing coelution with an authentic standard(24) in the Emory Clinical Biomarkers Laboratory and are equivalent to a Level 1 identification according to the Schymanski et al. criteria(25). Additional annotations were made with a high or medium confidence (≥ Level 2) with M+H adducts. When identity confirmation was not available, metabolites were annotated by searching metabolite databases such as Human Metabolome Database (http://www.hmdb.ca) and Metlin (https://metlin.scripps.edu) for metabolite m/z matches. For selected features which could not be annotated based on MS1 data only, ion dissociation spectra (MS/MS) were collected on a Thermo Scientific Fusion Mass Spectrometer for MS/MS spectral library matching using the mzCloud database (https://www.mzcloud.org).
Statistical Analyses and Bioinformatics
Descriptive statistics (mean ± SD) were performed for clinical variables. Distributions were assessed for normality, and any non-normally distributed clinical variables were natural log-transformed for use in parametric statistics and back-transformed for data presentation. Analysis of covariance (ANCOVA) tests, adjusting for age, race, sex, and history of chronic disease (yes or no), were used to test for overall group differences in clinical, body composition, and lifestyle factors. Post-hoc comparisons between specific groups were assessed with Tukey’s honestly significant different tests. Fisher’s exact tests were used for comparison of categorical variables due to small numbers in the variable levels. HRM bioinformatics analyses were performed using R. HRM analyses used multiple linear regression analyses with likelihood ratio tests, adjusting for age, sex, race, and history of chronic disease to determine differences between the three body composition groups (lean, NWO, and overweight/obesity). False discovery rate (FDR) was controlled for with the Benjamini-Hochberg procedure (q= 0.2). Metabolites that significantly differed between the groups were analyzed by the Mummichog pathway enrichment and modular analysis program(26). Significantly enriched metabolic pathways that included less than four metabolites were excluded from findings. Modular analyses are also produced from Mummichog, which are unbiased from established biological pathways and construct independent networks of highly correlated metabolites(26). To test for differences in significantly enriched pathways and network metabolites between body composition subtypes, intensity values for individual metabolites within each pathway and network were compared, adjusting for age, race, sex, and history of disease. Post-hoc analyses of differing metabolites also controlled for group differences in VO2 maximum. In a subset of the cohort (n=86), sensitivity analyses were performed on metabolites of interest between individuals classified as having NWO and overweight-obesity using student’s T tests. Individuals were matched by age (within two years), race, and sex. Out of 43 individuals classified as having NWO, 32 were matched to individuals classified as having overweight-obesity on all three criteria and 11 were matched on two of the three criteria. Statistics comparing clinical variables and individual metabolite intensity values were performed in JMP Pro (version 13, SAS Institute Inc., Cary, NC).
Results
Demographic and clinical characteristics for all subjects are shown in Table 1. Distributions of age and race did not significantly differ between the three groups (p=0.07 and p=0.3, respectively). There were significantly more females in the NWO group (p<0.05) compared to the lean and overweight-obesity groups. The overweight-obesity group had a significantly higher proportion of individuals with a history a chronic disease compared to the lean or NWO groups (p=0.01). In general, the population was highly educated and reported a high annual household income, which were similar between all groups (p>0.05 for both). Fasting plasma glucose, total cholesterol, LDL cholesterol, and diastolic blood pressure did not significantly differ between the groups (p>0.05). Fasting insulin, HOMA-IR, and triglycerides, were similar between the lean and NWO groups (p>0.05) but were significantly higher in the overweight-obesity group (p<0.05). Systolic blood pressure differed only between the NWO and overweight-obesity groups (p<0.05). HDL cholesterol levels did not differ between lean and NWO groups but were significantly lower in the overweight-obesity group (p<0.05). The proportion of subjects in each group with adverse clinical biomarkers is shown in Supplemental Table 1 (Table S1).
Table 1.
Demographic and clinical characteristics
| Lean (n=26) | NWO (n=43) | Overweight/Obesity (n=110) | |
|---|---|---|---|
| Age (y) | 47.3 ± 2.0 | 47.8 ± 1.6 | 50.6 ± 1.0 |
| Female [n (%)] | 15 (58) | 35 (81)a | 66 (60) |
| White [n (%)] | 23 (88) | 35 (81) | 79 (72) |
| Education | |||
| Less than high school | 1 (4) | - | - |
| Completed high school | 1 (4) | - | 4 (4) |
| Some college | 1 (4) | 5 (12) | 21 (19) |
| Four years of college | 6 (23) | 12 (28) | 25 (23) |
| Any graduate school | 17 (65) | 26 (60) | 60 (55) |
| Annual household income | |||
| ≤ $50,000/year | 1 (4) | 1 (3) | 14 (14) |
| > $50,000–$100,000/year | 6 (24) | 6 (15) | 33 (32) |
| > $100,000–$200,000/year | 10 (40) | 21 (53) | 31 (30) |
| > $200,000/year | 8 (32) | 12 (30) | 25 (24) |
| Chronic disease [n (%)] | 5 (19) | 6 (14) | 38 (35)a |
| Plasma glucose (mg/dL) | 95.7 ± 4.8 | 93.1 ± 4.0 | 94.9 ± 3.1 |
| Plasma insulin (μIU/mL)* | 2.7 ± 0.5a | 3.6 ± 0.5a | 5.5 ± 0.6b |
| HOMA-IR* | 0.6 ± 0.1a | 0.8 ± 0.1a | 1.3 ± 0.1b |
| Total cholesterol (mg/dL) | 193.9 ± 9.4 | 201.4 ± 8.0 | 197.8 ± 6.1 |
| LDL-C (mg/dL) | 105.3 ± 8.2 | 117.8 ± 7.0 | 118.4 ± 5.3 |
| HDL-C (mg/dL) | 72.7 ± 3.8a | 63.5 ± 3.3a | 55.5 ± 2.5b |
| Triglycerides (mg/dL) | 81.1 ± 11.4a | 101.2 ± 9.7a,b | 117.2 ± 7.4b |
| Systolic Blood Pressure (mmHg) | 119.4 ± 3.6a,b | 118.1 ± 3.1a | 126.1 ± 2.3b |
| Diastolic Blood Pressure (mmHg) | 74.7 ± 2.5 | 75.5 ± 2.1 | 79.9 ± 1.6 |
Values are mean ± SE or n (%). Values not connected by the same letter are significantly different at p< 0.05. Plasma variables adjusted for age, sex, race, and history of chronic disease. Abbreviations: NWO, group with normal weight obesity; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol.
Variables were natural log-transformed for analyses and back transformed for data presentation and reported as geometric mean ± SE.
Body Composition, Diet Quality, and Physical Fitness
Body composition and lifestyle variables are presented in Table 2. Per body composition subtype classification, BMI was similar between the lean and NWO groups (p>0.05) but was significantly higher in the overweight-obesity group (p<0.05). Body fat percent increased significantly from subjects classified as lean to NWO to overweight-obesity (p<0.05). Although lean body mass did not differ between the lean and overweight-obesity groups, it was significantly lower in the NWO group (p<0.05). Visceral adipose tissue (VAT) increased significantly with each group, while waist circumference was only significantly higher in the overweight-obesity group (p<0.05). VO2 maximum was highest in the lean group and significantly lower in the NWO and overweight-obesity groups (p<0.05). Based on self-reported data, there were no differences between groups for aerobic physical activity or strength training (p>0.05). MDS and aHEI diet quality scores were similar across all groups (p>0.05). DASH diet quality score was significantly higher in the lean group compared to the overweight-obesity group (p<0.05).
Table 2.
Body Composition Variables, Physical Fitness, and Diet Quality Scores
| Lean (n=26) | NOW (n=43) | Overweight-Obesity (n=110) | |
|---|---|---|---|
| BMI (kg/m2) | 23.9 ± 0.9a | 24.3 ± 0.7a | 30.8 ± 0.6b |
| Total body mass (kg) | 69.7 ± 2.9a | 68.5 ± 2.5a | 87.6 ± 1.9b |
| Lean body mass (kg) | 50.3 ± 1.5a | 44.4 ± 1.2b | 51.1 ± 1.0a |
| Fat mass (kg) | 16.5 ± 1.8a | 21.5 ± 1.5b | 33.6 ± 1.1c |
| Total body fat (%) | 23.1 ± 1.0a | 31.3 ± 0.8b | 37.7 ± 0.6c |
| Visceral adipose tissue (kg)* | 0.22 ± 0.04a | 0.5 ± 0.1b | 1.3 ± 0.2c |
| Waist circumference (cm) | |||
| Males | 82.3 ± 3.2a | 85.8 ± 3.2a | 97.6 ± 2.6b |
| Females | 77.0 ± 2.9a | 77.5 ± 2.1a | 91.7 ± 1.7b |
| VO2 Maximum (mL/min/kg) | 42.9 ± 2.3a | 34.6 ± 2.0b | 34.2 ± 1.5b |
| Met MVPA Guidelines‡ | 6 (23) | 10 (23) | 28 (25) |
| Met Strength Guidelines‡ | 10 (38) | 8 (19) | 23 (21) |
| Mediterranean Diet Score | 4.7 ± 0.4 | 4.1 ± 0.4 | 3.9 ± 0.3 |
| DASH Diet Score | 5.5 ± 0.3a | 5.0 ± 0.2a,b | 4.9 ± 0.2b |
| Alternative Healthy Eating Index | 48.6 ± 2.6 | 46.8 ± 2.2 | 45.0 ± 1.7 |
Values are mean ± SE or n (%). Values not connected by the same letter are significantly different at p< 0.05. Variables adjusted for age, sex, race, and history of chronic disease. Abbreviations: NWO, group with normal weight obesity; BMI, body mass index; MVPA, moderate to vigorous physical activity; DASH, dietary approaches to stop hypertension.
Variable was natural log-transformed for analyses and back transformed for data presentation and reported as geometric mean ± SE.
Met the 2007 guidelines of 30 minutes of moderate activity exercises at least 5 times per week, 20 minutes of vigorous activity three times per week, or a combination of both. Subjects reported meeting the strength guidelines by performing muscular strengthening exercises at least twice per week.
High-Resolution Metabolomics
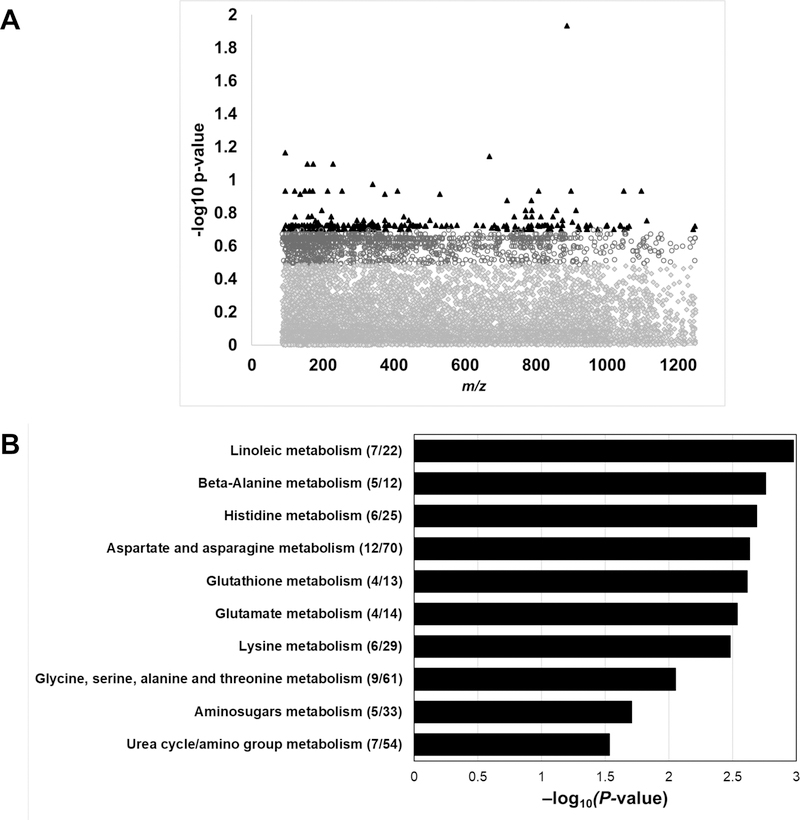
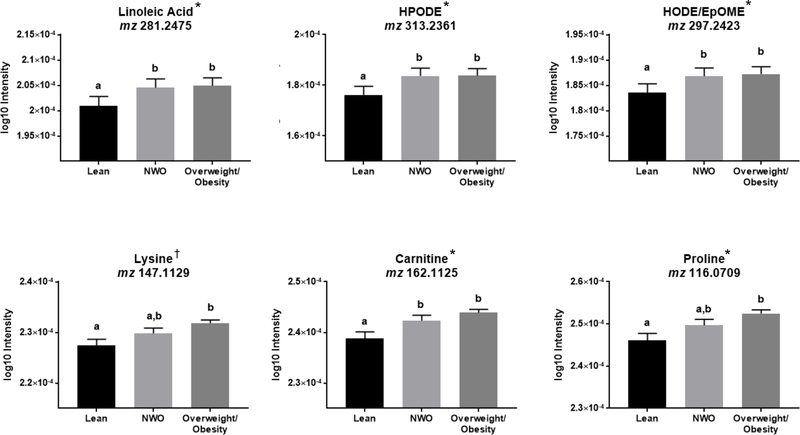
Of the 9,967 filtered metabolomic features, 1,533 features were significantly associated with the body composition subtypes at p<0.05 (Figure 1A). Following FDR correction, there were 222 significantly associated metabolites (q=0.20), which were used as input for Mummichog (26) pathway enrichment and modular analyses. Significantly enriched pathways are shown in Figure 1B. There were ten significantly enriched pathways predominantly related to lipid and amino acid metabolism. Representative metabolites within the significantly enriched pathways are shown in Figure 2. All metabolites included in Figure 2 were matched by an M+H adduct and have a Level 1 or Level 2 annotation with high or medium confidence (23, 27). Metabolites within the linoleic acid metabolism pathway, such as linoleic acid and oxidized linoleic related metabolites, were higher in the NWO and overweight-obesity groups compared to the lean group (p<0.05 for all). Metabolites within beta-alanine, histidine, and aspartate/asparagine metabolism were significantly elevated in the NWO and overweight-obesity groups compared to the lean group. Glutathione and glutamate metabolism contained metabolic features that were similarly elevated in the NWO and overweight-obesity groups compared to the lean group, and metabolites that were elevated in only the overweight-obesity group compared to the lean group. The significantly enriched pathways lysine metabolism, glycine and serine metabolism, and urea cycle contained metabolites that were higher in the overweight-obesity group compared to the lean group. Following further adjustment with VO2 maximum, lysine levels were similar between all three groups. No other findings in pathway analyses changed after adjusting for VO2 maximum, as shown in Figure 2. Significantly enriched pathways with all tentatively annotated metabolic features are shown in Supplemental Table 2 (Table S2).
Figure 1.
Panel A, Manhattan plot of metabolites significantly different between body composition subtypes. There were 1,533 metabolic features that were significant at a p<0.05 (grey open circles) and 222 metabolic features that were significant at an FDR q<0.2 (black triangles). Panel B, Pathway enrichment analysis of the 222 metabolites significantly associated with the body composition subtypes at a q<0.2.
Figure 2.
Representative metabolites within significantly enriched metabolic pathways. All metabolites have been matched by an M+H adduct in positive electrospray ionization mode. Abbreviations: NWO, normal weight obesity; HPODE, hydroperoxy-octadecadienoic acid, an intermediate of linoleic acid metabolism and precursor for the oxidized metabolite octadecadienoic acid; HODE, hydroxyoctadecadienoic acid, a derivative of linoleic acid; EpOME, Epoxyoctadecenoic acid, a peroxidation product of linoleic acid.
*Indicates that findings were confirmed in post-hoc analyses with further adjustment for VO2 maximum, and in a subset of the cohort (n=86) with subjects categorized as having normal weight obesity or overweight-obesity matched by age, race/ethnicity, and sex.
† Following further adjustment with VO2 maximum, metabolite was similar between all three groups.
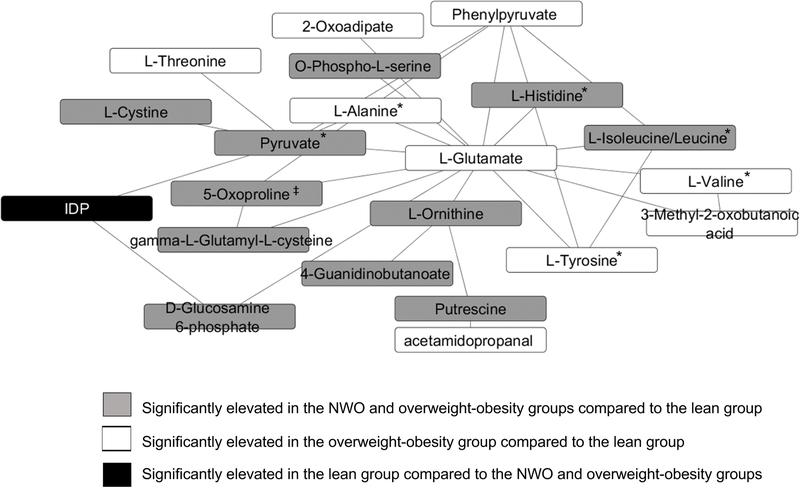
Figure 3 depicts a module analysis of metabolites significantly differing between the body composition subtypes (p<0.05 for all metabolites). The network was predominantly comprised of amino acids and amino acid-related metabolites (17 out of 21 metabolites) including the branched chain amino acids (BCAA) leucine/isoleucine, cystine, pyruvate, histidine, 5-oxoproline, ornithine, and putrescine, which were significantly elevated in the NWO and overweight-obesity groups compared to the lean group. Additional amino acid metabolites such as the BCAA valine, 3-methyl-2-oxobutanoic acid (a valine-related metabolite), the aromatic amino acids (AAA) tyrosine and threonine, glutamate, and phenylpyruvate (a phenylalanine-related metabolite), were higher in the overweight-obesity subtype compared to the lean subtype, but these metabolite intensities in the NWO group did not differ from either of the other groups. Following additional adjustment for VO2 maximum, 5-oxoproline levels were significantly elevated in the overweight-obese group compared to the lean group but did not differ significantly in the NWO group. All of the metabolic features tested followed the same pattern after adjusting for VO2 maximum, as noted in Figure 3.
Figure 3.
Modular analysis of correlated metabolic features that were significantly associated with the three body composition subtypes. Abbreviations: IDP, Inosine diphosphate
*Indicates that findings were confirmed in post-hoc analyses with further adjustment for VO2 maximum, and in a subset of the cohort (n=86) with subjects categorized as having normal weight obesity or overweight-obesity matched by age, race/ethnicity, and sex.
‡ Following further adjustment with VO2 maximum, metabolite was significantly elevated in the overweight-obesity group compared to the lean group.
In sensitivity analyses of matched subjects classified as NWO and overweight-obesity, there were no changes in statistical findings from the results reported above and shown in Figure 2 and Figure 3; all metabolite intensities remained similar between the NWO and overweight-obese groups.
Discussion
In this Atlanta-based cohort, we found that adults with an NWO phenotype had metabolomic profiles that were similar to individuals who have overweight-obesity and distinct from individuals who are lean. In particular, linoleic acid, beta-alanine, histidine, and aspartate/asparagine metabolism, and some BCAA, were upregulated in the NWO and overweight-obesity subtypes compared to the lean subtype. We also found dysregulation of amino acid metabolism related to valine, tyrosine, and phenylalanine in the overweight-obesity group compared to the lean group.
Analysis of classic clinical measures showed similar profiles between individuals with NWO and individuals who are lean for lipid levels, insulin resistance (via HOMA-IR), and blood pressure. While other studies have shown elevated clinical measures in individuals with NWO compared to lean individuals(7, 28), we did not find those distinctions in this cohort. Only triglyceride concentrations were similar between individuals with NWO and overweight-obesity. All other clinical variables were comparable between NWO and lean groups. While the lean group exhibited higher physical fitness, further adjustment of VO2 maximum did not alter our main findings, therefore, we conclude the differences in metabolite intensities is more likely due to differences in the body composition subtypes rather than differences in fitness levels. The combined results of the clinical measures and HRM in individuals with NWO shows that HRM may be a more sensitive measure to detect metabolism-related dysfunction prior to altered clinical measures in middle-aged adults.
Linoleic acid is an essential omega-6 poly-unsaturated fatty acid (n-6, PUFA) whose effects on cardiometabolic health have been debated(29, 30). In our study, linoleic acid metabolism was significantly upregulated in the NWO and overweight-obesity groups compared to the lean group, indicating disruption of this metabolic pathway with elevated adiposity. Additional studies have shown increased total linoleic acid in subjects with a BMI >30 kg/m2 (31), while others reported decreased levels of linoleic acid but increased levels of linoleic acid-related metabolites(32, 33). As it can be converted to arachidonic acid, linoleic acid has been suggested to promote pro-inflammatory pathways(34, 35). Evidence suggests that obese individuals may have a greater pro-inflammatory response to linoleic acid consumption compared to lean individuals(34, 36). Previous studies have shown that individuals with NWO have increased circulating pro-inflammatory biomarkers(7). Through their actions on PPARϒ (peroxisome proliferator-activated receptor gamma) activation(37), the oxidized linoleic acid metabolites, 9-HODE and 13-HODE, may promote both inflammation and adipocyte differentiation(34). Further, through competition with the shared Δ6 desaturase enzyme, a high intake of linoleic acid may blunt the anti-inflammatory effects of alpha-linolenic acid (ALA, a precursor to docosahexanoic acid and eicosapentanoic acid)(34, 38). In aggregate, upregulated linoleic acid metabolism may be indicative of increased inflammation in settings of excess adiposity.
Previous studies have reported elevated amino acid concentrations in individuals with obesity. Our findings show similarly increased levels of amino acids and related metabolites, including histidine, in individuals with NWO compared to individuals classified as lean. Studies utilizing principal components analyses to investigate relationships between cardiometabolic health and the plasma metabolome have identified histidine as a significantly associated metabolite (39, 40), although others have found a negative association or no relationship between histidine with BMI and obesity(9, 31, 41). Metabolites enriched within histidine and beta-alanine overlapped with glutamate metabolism and may represent anaplerotic substrates(31). Lysine metabolism was upregulated in the overweight-obesity group. Lysine is an essential amino acid that is needed to synthesize carnitine for fatty acid transport into the mitochondria for oxidation. Both carnitine and lysine have been shown to be elevated in obesity(41), and here we report higher levels of carnitine in subjects who have NWO and overweight-obesity compared to subjects who are lean. Acylcarnitines, especially C3 and C5 acylcarnitines(42), have been found to be elevated in obesity, perhaps as a result of incompletely oxidized BCAAs. Finally, pathways related to nitrogenous waste excretion, aspartate and asparagine metabolism, were dysregulated in the NWO and overweight-obesity groups compared to the lean group, in line with other obesity and cardiometabolic disease research(43). Our findings of altered amino acid metabolism are in line with published reports regarding obesity pathophysiology and represent new findings for individuals with NWO.
In line with previous obesity-related research(42, 44), we found dysregulation of BCAAs, AAAs, and related metabolites associated with greater adiposity in the modular analysis. There is now a well-established metabolic signature of obesity including elevated concentrations of BCAAs and AAAs (particularly tyrosine and phenylalanine) related to insulin resistance, mitochondrial oxidative capacity overload(42) and, ultimately, increased risk of developing type 2 diabetes(44). The altered flux of BCAA catabolism exceeds mitochondrial oxidative capacity and ultimately leads to release of BCAAs into the blood(42). The increase in AAA may be due to competition for the same cellular transport protein used by large neutral amino acids. Elevated levels of glutamate, alanine, and pyruvate in obese individuals, which we also show in subjects with NWO, may also be linked to altered BCAA metabolism and overload of the Krebs cycle(42). Glutamate is produced in the first step of BCAA catabolism and increased concentrations of glutamate may shift pyruvate towards conversion to alanine(42). In summary, we found altered BCAA and AAA metabolism in subjects with NWO and overweight-obesity, which may reflect the underlying pathophysiology of insulin resistance and mitochondrial energy metabolism overload.
In this study, individuals with NWO had significantly lower lean body mass compared to the lean and overweight-obesity groups, and individuals with NWO had significantly higher VAT compared to lean individuals. Furthermore, individuals with NWO and overweight-obesity had significantly lower fitness levels compared to lean individuals. Relevant to our metabolomics findings, resistance and aerobic training in overweight, insulin resistant adults showed reductions in whole plasma molar sum of the BCAAs and improved clearance of acyl groups(45). Thus, the plasma metabolomic differences observed between individuals with NWO and those who are lean may reflect a combination of differences in body composition and fitness, or other variables that were not assessed.
To our knowledge, this is the first study to examine plasma metabolomic profiles of individuals with NWO and fills an important gap in knowledge about this population. This novel approach allowed for the comparison of detailed health profiles between groups beyond classic clinical laboratory assessments. Furthermore, the use of pathway enrichment analysis provides context to associations of disease with metabolic pathways instead of single metabolites. Pathway analysis also provides the advantage of being downstream from genetic changes and allows insight into products of genetic or epigenetic alterations. A limitation of the study was its cross-sectional nature, which impedes our ability to infer causality in the results. Health status, education, and income were collected by self-report, and therefore may be subject to recall bias. This cohort is predominantly composed of individuals who reported a high education and income, which may not be reflective of the general United States population. Our power to determine differences in outcomes between groups may have been limited by small numbers. For example, several metabolites in the NWO group had intermediate values that were between lean and overweight-obese subjects but were not statistically significantly different. This may be due to small numbers between groups or heterogeneity in the metabolic health of individuals with NWO. Finally, there are no established cut points to define obesity based on body fat percent and applying another threshold to define obesity in this population may have yielded different results.
This study reports novel findings in this adult population that individuals with NWO have altered metabolomic profiles, denoting underlying metabolic dysfunction similar to individuals with overweight-obesity, despite having a normal BMI and generally normal clinical biomarkers. Specifically, linoleic acid and amino acid pathways were dysregulated in the NWO and overweight-obesity subtypes compared to the lean subtype. Thus, the plasma metabolome may be a useful measure of health status to detect perturbations that predict early metabolic changes. Larger, prospective studies are needed to determine if HRM can identify normal weight individuals at risk for obesity-related diseases and if targeted interventions in individuals with NWO can reduce such risk.
Supplementary Material
What is already known about this subject?
Obesity increases a person’s disease and mortality risk.
Body mass index (BMI) is typically used to assess obesity and categorize disease risk.
Individuals with a normal weight but high body fat (normal weight obesity, NWO) have increased disease and mortality risk but may be overlooked when screening for obesity by BMI.
What does this study add?
This study characterizes the plasma metabolome of individuals with NWO compared to individuals classified as lean or having overweight-obesity using high-resolution metabolomics.
Individuals with NWO exhibited altered plasma metabolomic profiles similar to individuals with overweight-obesity, including oxidized linoleic acid and related metabolites and dysregulated amino acid metabolism.
High-resolution metabolomics may be a helpful tool to identify individuals who have increased disease risk despite having a normal BMI.
Acknowledgments
Funding support:
This work is based on information from the Emory Predictive Health Institute and Center for Health Discovery and Well Being Database supported by the National Center for Advancing Translational Sciences of the NIH under award number UL1 TR002378. Additional grant support NIH K01 DK102851 (JAA), R03 DK117246 (JAA), K24 DK096574 (TRZ), and P30 ES019776 (Health and Exposome Research Center at Emory; DPJ, TRZ).
Footnotes
Disclosure: The authors declare no conflict of interest
REFERENCES
- 1.Blüher M Obesity: global epidemiology and pathogenesis. Nature Reviews Endocrinology 2019;15: 288–298. [DOI] [PubMed] [Google Scholar]
- 2.Romero-Corral A, Somers VK, Sierra-Johnson J, Thomas RJ, Collazo-Clavell ML, Korinek J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. International journal of obesity (2005) 2008;32: 959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. International journal of obesity (2005) 2010;34: 791–799. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J, et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. European heart journal 2010;31: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MK, Han K, Kwon HS, Song KH, Yim HW, Lee WC, et al. Normal weight obesity in Korean adults. Clinical endocrinology 2014;80: 214–220. [DOI] [PubMed] [Google Scholar]
- 6.Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, et al. Normal-Weight Central Obesity: Implications for Total and Cardiovascular Mortality. Ann Intern Med 2015;163: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jean N, Somers VK, Sochor O, Medina-Inojosa J, Llano EM, Lopez-Jimenez F. Normal-weight obesity: implications for cardiovascular health. Current atherosclerosis reports 2014;16: 464. [DOI] [PubMed] [Google Scholar]
- 8.Jones DP, Park Y, Ziegler TR. Nutritional metabolomics: progress in addressing complexity in diet and health. Annu Rev Nutr 2012;32: 183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore SC, Matthews CE, Sampson JN, Stolzenberg-Solomon RZ, Zheng W, Cai Q, et al. Human metabolic correlates of body mass index. Metabolomics : Official journal of the Metabolomic Society 2014;10: 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rauschert S, Uhl O, Koletzko B, Hellmuth C. Metabolomic biomarkers for obesity in humans: a short review. Annals of nutrition & metabolism 2014;64: 314–324. [DOI] [PubMed] [Google Scholar]
- 11.Brigham KL. Predictive health: the imminent revolution in health care. Journal of the American Geriatrics Society 2010;58 Suppl 2: S298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28: 412–419. [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. Journal of women’s health & gender-based medicine 1999;8: 805–813. [DOI] [PubMed] [Google Scholar]
- 14.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, et al. Alternative dietary indices both strongly predict risk of chronic disease. The Journal of nutrition 2012;142: 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. The New England journal of medicine 1997;336: 1117–1124. [DOI] [PubMed] [Google Scholar]
- 16.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. The New England journal of medicine 2003;348: 2599–2608. [DOI] [PubMed] [Google Scholar]
- 17.Bettermann EL, Hartman TJ, Easley KA, Ferranti EP, Jones DP, Quyyumi AA, et al. Higher Mediterranean Diet Quality Scores and Lower Body Mass Index Are Associated with a Less-Oxidized Plasma Glutathione and Cysteine Redox Status in Adults. The Journal of nutrition 2018;148: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madeira FB, Silva AA, Veloso HF, Goldani MZ, Kac G, Cardoso VC, et al. Normal weight obesity is associated with metabolic syndrome and insulin resistance in young adults from a middle-income country. PloS one 2013;8: e60673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soltow QA, Strobel FH, Mansfield KG, Wachtman L, Park Y, Jones DP. High-performance metabolic profiling with dual chromatography-Fourier-transform mass spectrometry (DC-FTMS) for study of the exposome. Metabolomics : Official journal of the Metabolomic Society 2013;9: S132–s143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu T, Park Y, Johnson JM, Jones DP. apLCMS--adaptive processing of high-resolution LC/MS data. Bioinformatics (Oxford, England) 2009;25: 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, et al. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC bioinformatics 2013;14: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics (Oxford, England) 2007;8: 118–127. [DOI] [PubMed] [Google Scholar]
- 23.Uppal K, Walker DI, Jones DP. xMSannotator: An R Package for Network-Based Annotation of High-Resolution Metabolomics Data. Analytical chemistry 2017;89: 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Go YM, Walker DI, Liang Y, Uppal K, Soltow QA, Tran V, et al. Reference Standardization for Mass Spectrometry and High-resolution Metabolomics Applications to Exposome Research. Toxicological sciences : an official journal of the Society of Toxicology 2015;148: 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP, et al. Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environmental science & technology 2014;48: 2097–2098. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, et al. Predicting network activity from high throughput metabolomics. PLoS computational biology 2013;9: e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, et al. Proposed minimum reporting standards for chemical analysis. Metabolomics : Official journal of the Metabolomic Society 2007;3: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Progress in cardiovascular diseases 2014;56: 426–433. [DOI] [PubMed] [Google Scholar]
- 29.Wu JHY, Marklund M, Imamura F, Tintle N, Ardisson Korat AV, De Goede J, et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. The Lancet Diabetes & Endocrinology 2017;5: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah SH, Kraus WE, Newgard CB. Metabolomic Profiling for the Identification of Novel Biomarkers and Mechanisms Related to Common Cardiovascular Diseases: Form and Function 2012;126: 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell metabolism 2009;9: 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JY, Park JY, Kim OY, Ham BM, Kim H-J, Kwon DY, et al. Metabolic Profiling of Plasma in Overweight/Obese and Lean Men using Ultra Performance Liquid Chromatography and Q-TOF Mass Spectrometry (UPLC-Q-TOF MS) 2010;9: 4368–4375. [DOI] [PubMed] [Google Scholar]
- 33.Warensjö E, Öhrvall M, Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women 2006;16: 128–136. [DOI] [PubMed] [Google Scholar]
- 34.Naughton SS, Mathai ML, Hryciw DH, McAinch AJ. Linoleic acid and the pathogenesis of obesity. Prostaglandins & other lipid mediators 2016;125: 90–99. [DOI] [PubMed] [Google Scholar]
- 35.Teng KT, Chang CY, Chang LF, Nesaretnam K. Modulation of obesity-induced inflammation by dietary fats: mechanisms and clinical evidence. Nutrition journal 2014;13: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel C, Ghanim H, Ravishankar S, Sia CL, Viswanathan P, Mohanty P, et al. Prolonged Reactive Oxygen Species Generation and Nuclear Factor-κB Activation after a High-Fat, High-Carbohydrate Meal in the Obese. The Journal of Clinical Endocrinology & Metabolism 2007;92: 4476–4479. [DOI] [PubMed] [Google Scholar]
- 37.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 1998;93: 229–240. [DOI] [PubMed] [Google Scholar]
- 38.de Antueno RJ, Knickle LC, Smith H, Elliot ML, Allen SJ, Nwaka S, et al. Activity of human Delta5 and Delta6 desaturases on multiple n-3 and n-6 polyunsaturated fatty acids. FEBS letters 2001;509: 77–80. [DOI] [PubMed] [Google Scholar]
- 39.Batch BC, Shah SH, Newgard CB, Turer CB, Haynes C, Bain JR, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism: clinical and experimental 2013;62: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ntzouvani A, Nomikos T, Panagiotakos D, Fragopoulou E, Pitsavos C, McCann A, et al. Amino acid profile and metabolic syndrome in a male Mediterranean population: A cross-sectional study. Nutrition, Metabolism and Cardiovascular Diseases 2017;27: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 41.Boulet MM, Chevrier G, Grenier-Larouche T, Pelletier M, Nadeau M, Scarpa J, et al. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. American journal of physiology Endocrinology and metabolism 2015;309: E736–746. [DOI] [PubMed] [Google Scholar]
- 42.Newgard CB. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell metabolism 2017;25: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cirulli ET, Guo L, Leon Swisher C, Shah N, Huang L, Napier LA, et al. Profound Perturbation of the Metabolome in Obesity Is Associated with Health Risk. Cell metabolism 2018. [DOI] [PMC free article] [PubMed]
- 44.Zhao X, Han Q, Liu Y, Sun C, Gang X, Wang G. The Relationship between Branched-Chain Amino Acid Related Metabolomic Signature and Insulin Resistance: A Systematic Review. Journal of diabetes research 2016;2016: 2794591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glynn EL, Piner LW, Huffman KM, Slentz CA, Elliot-Penry L, AbouAssi H, et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia 2015;58: 2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.