Abstract
Background:
The striatum is abnormal in schizophrenia, and possibly represents a common neurobiological mechanism underlying psychotic disorders. Resting-state fMRI (RS-fMRI) studies have not reached a consensus regarding striatal dysconnectivity in schizophrenia, although these studies generally find impaired fronto-parietal and salience network connectivity. The goal of the current study was to clarify the pattern of cortico-striatal connectivity, including whether cortico-striatal dysconnectivity is transdiagnostic and extends into psychotic bipolar disorder.
Methods:
We examined cortico-striatal functional connectivity in 60 healthy subjects and 117 individuals with psychosis, including 77 with a schizophrenia spectrum illness and 40 with psychotic bipolar disorder. We conducted both a cortical seed-based region-of-interest (ROI) analysis with follow-up voxel-wise analysis for any significant results. Further, a striatum seed-based analysis was conducted to examine group differences in connectivity between the striatum and the whole cortex.
Results:
Cortical ROI analysis indicated that overall connectivity of the salience network with the striatum was reduced in psychotic disorders, which follow-up voxel-wise analysis localized to the left putamen. Striatum seed-based analyses showed reduced ventral rostral putamen connectivity with the salience network portion of the medial PFC in both schizophrenia and psychotic bipolar disorders.
Conclusions:
The current study found evidence of transdiagnostic cortico-striatal dysconnectivity in both schizophrenia and psychotic bipolar disorder, including reduced salience network connectivity, as well as reduced connectivity between the putamen and the medial PFC. Overall, the current study points to the relative importance of salience network hypo-connectivity in psychotic disorders.
Keywords: Psychosis, Resting-state fMRI, Striatum, Cortex, Schizophrenia, Psychotic bipolar disorder
INTRODUCTION
Multiple lines of evidence implicate the striatum in the pathophysiology of schizophrenia (1–3). The discovery that antipsychotics’ efficacy relates directly to their ability to block dopamine receptors led to the dopamine hypothesis of schizophrenia, which hypothesizes that psychotic symptoms result from exaggerated dopamine signaling (4,5) in the striatum (6). The dopamine hypothesis was directly supported with the advent of positron emission tomography (PET) imaging (3). Specifically, PET studies in schizophrenia have consistently found elevated presynaptic synthesis capacity (2,7), exaggerated dopamine release following amphetamine challenge (8), and increased postsynaptic dopamine D2 receptors (9,10).
Over the past several years, resting-state fMRI (RS-fMRI) has joined the armamentarium of neuroimaging methods for investigating psychiatric disorders (11–13). A key advantage of RS-fMRI is that it can be used to assess distributed neural networks rather than brain regions in isolation. The striatum is a key node within cortico-striatal loops, or functional networks composed of cortex, basal ganglia, and thalamus (14–16). RS-fMRI studies have reliably identified five cortico-striatal networks: the fronto-parietal (FPN), default mode (DMN), limbic (LN), salience (SAL), and motor networks (15). RS-fMRI studies of cortico-striatal networks in psychotic disorders have not reached a consensus regarding the nature of dysconnectivity in these five cortico-striatal networks. Research consistently finds decreased FPN connectivity [e.g., involved in goal representation (17)] in psychotic disorders (18–21). Research also generally implicates decreased salience network connectivity [e.g., information integration, salience attribution (22,23)] in psychotic disorders (24,25). Research also finds hyper-connectivity in motor networks [e.g., execution of motor plans (26)] (25,27), although the direction of connectivity in the DMN [e.g., attention to internal states, self-referential thinking (28)] (29–32) and limbic (e.g., motivation) networks (33,34) is mixed. Furthermore, there is a lack of consistency regarding the associations between cortico-striatal connectivity, clinical symptoms, and impaired cognition. In terms of associations between cortico-striatal connectivity and symptoms, recent research indicates that salience network hypo-connectivity is correlated with impaired cognition (35) and positive psychotic symptoms (24). However, other networks, such as the FPN and DMN, have also been linked to positive symptoms (18,19,36). Most previous studies involving psychotic disorders involved limited sample sizes (e.g., <30 individuals in the patient groups) (19,20,24,25,29,32,33), raising the possibility that these studies were underpowered to examine cortico-striatal connectivity differences and identify reliable clinical-connectivity associations.
Psychiatry is increasingly focusing on transdiagnostic phenomena, as exemplified by the National Institute of Mental Health (NIMH) RDoC initiative which posits common neurobiological mechanisms underlie transdiagnostic symptom domains (37). Evidence that psychotic bipolar disorder is also associated with exaggerated dopamine signaling in the striatum (38,39), consistent with schizophrenia, suggests striatal dysfunction may be a common neurobiology underlying psychosis. Research examining connectivity in schizophrenia and bipolar groups have found mixed results. This evidence includes intact connectivity in a group at risk for bipolar disorder (40) and disorder-specific connectivity impairments in schizophrenia spectrum groups (40,41). In contrast, there is some evidence for cortico-striatal impairments in bipolar groups (42) as well as trans-diagnostically across psychotic disorders (43).
The current study investigated cortico-striatal connectivity in schizophrenia and psychotic bipolar disorder in order to: 1) Further clarify cortico-striatal network dysconnectivity in schizophrenia; 2) Determine if schizophrenia and psychotic bipolar disorder demonstrate similar or different patterns of cortico-striatal dysconnectivity; and 3) Replicate prior findings linking cortico-striatal network dysconnectivity to cognitive impairment (35) and positive psychotic symptoms (24).
METHODS
Study Participants
193 individuals (61 healthy subjects, 132 individuals with a psychotic disorder) that participated in an on-going NIMH-funded study on brain connectivity in psychotic disorders were initially screened for inclusion in this investigation. 16 participants did not meet our neuroimaging quality assurance (QA) procedures described below. Thus, the final cohort consisted of 177 study participants: 60 healthy individuals, 77 individuals with a schizophrenia spectrum illness (i.e. schizophrenia, schizoaffective, and schizophreniform disorder), and 40 individuals with bipolar disorder with psychotic features (i.e. psychotic bipolar disorder). Demographic data are presented in Table 1. Patients were recruited through the Psychotic Disorders Program within the Department of Psychiatry and Behavioral Sciences at Vanderbilt University Medical Center (VUMC). Healthy individuals were recruited from Nashville and surrounding area via advertisement and word-of-mouth. This study was approved by the Vanderbilt University Institutional Review Board. All study participants provided written informed consent prior to participating.
Table 1.
Sample Demographics
| Healthy Subjects |
Psychosis | Statistics | Contrasts | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SCZ | BPP | ||||||||
| N=60 | N=77 | N=40 | F/t/x2 | p | |||||
| Gender (male:female) | 38:22 | 53:24 | 23:17 | 1.52 | .47 | -- | |||
| Race (White:AA:Other) | 39:15:6 | 54:21:2 | 32:6:2 | 5.81 | .21 | -- | |||
| Clinical Status (% first episode) | -- | 44.2 | 40.0 | 0.19 | .67 | -- | |||
| Mood Stabilizer Use (%) | -- | 28.6 | 70.0 | 18.46 | <.001a | -- | |||
| Mean | SD | Mean | SD | Mean | SD | ||||
| Age | 29.27 | 9.45 | 27.43 | 9.71 | 29.20 | 11.19 | 0.72 | .49 | -- |
| Premorbid IQ | 109.90 | 6.73 | 102.01 | 10.08 | 105.53 | 9.93 | 12.66 | <.001 | HS>SCZ |
| SCIP Global Cognition z-score | 0.29 | 0.59 | −0.80 | 0.82 | −0.29 | 0.90 | 33.83 | <.001 | HS>BP>SCZ |
| Median Signal-to-Noise Ratio | 58.87 | 14.15 | 61.31 | 13.85 | 60.49 | 14.33 | 0.51 | .60 | -- |
| Median Voxel Displacement | 0.05 | 0.02 | 0.05 | 0.02 | 0.05 | 0.02 | 0.33 | .72 | -- |
| PANSS Positive | -- | -- | 14.43 | 8.27 | 10.83 | 6.26 | 2.42 | .02 | -- |
| PANSS Negative | -- | -- | 13.62 | 5.41 | 9.80 | 2.73 | 4.20 | <.001 | -- |
| PANSS General | -- | -- | 27.53 | 7.83 | 23.75 | 6.48 | 2.62 | .01 | -- |
| CPZ Equivalents | -- | -- | 354.64 | 199.53 | 231.84 | 160.81 | 2.65 | .01 | -- |
| Illness Duration (Months)b | -- | -- | 75.00 | 95.75 | 72.51 | 88.44 | 0.14 | .89 | -- |
Abbreviations: SCZ=Schizophrenia; BPP=Bipolar with Psychotic Features; HS=Healthy Subjects; SCIP=Screen for Cognitive Impairment in Psychiatry; PANSS=Positive and Negative Syndrome Scale; CPZ=Chlorpromazine
The groups did not differ in mood stabilizer dose: t(44)=−0.27, p=.79.
Average duration of illness was not significantly associated with any of the significant corticostriatal connectivity estimates (ps> .45).
Psychiatric diagnoses were confirmed in patients and ruled out in healthy subjects using the Structured Clinical Interview for Diagnosing DSM-IV Disorders (SCID) (44). Patients were further assessed with the Positive and Negative Syndrome Scale (PANSS) (45), Young Mania Rating Scale (YMRS) (46), and Hamilton Depression Scale (HAM-D) (47) to quantify severity of psychotic, mania, and depression symptoms, respectively. The Wechsler Test of Adult Reading (WTAR) (48) was administered to all subjects to provide an estimate of pre-morbid intellect. Study participants also completed the Screen for Cognitive Impairment in Psychiatry (SCIP) (49), a brief neuropsychological battery that includes a word list learning test of verbal memory, a version of the Auditory Consonant Trigrams test of working memory, phonemic verbal fluency, and a coding test of processing speed. SCIP sub-test raw scores were converted to z-scores using previously published normative data and averaged to create a “composite” z-score of overall cognitive functioning (49). Exclusion criteria included age less than 16 or greater than 55; estimated pre-morbid IQ less than 70; presence of a medical illness or central nervous system disorder (e.g. multiple sclerosis, epilepsy) that would affect study results; reported pregnancy or lactation; history of significant head trauma; psychotropic drug use (healthy subjects only); substance abuse within last three months (patients) or lifetime history of substance abuse/dependence (healthy subjects); and MRI contra-indicators (e.g. metal implants, claustrophobia).
Neuroimaging Data Acquisition, Preprocessing, and Quality Assurance
A 10-minute resting-state (eyes open, fixation) echo planar imaging functional scan and T1-weigthed anatomical scan were collected on each subject during a single scanning session on a 3T Philips Intera Achieva MRI scanner located at Vanderbilt University Institute of Imaging Sciences (VUIIS). The resting-state fMRI scan had the following parameters: 38 axial slices (slice thickness=3.0 mm; gap=0.3 mm), field of view (FOV)=80×80 matrix (3.0 mm×3.0 mm in-plane resolution), 90-degree flip angle, 300 volumes, TR/TE=2000/25 ms. A high resolution T1-weighted turbo field echo (TFE) structural scan (170 sagital slices, FOV=256×256 matrix, 1.0 mm isovoxel resolution, TR/TE=8.9/4.6 ms) was also acquired.
T1-weighted anatomical images were segmented into grey matter, white matter, and CSF using the Voxel-based Morphometry, version 8 toolbox (VBM8; http://dbm.neuro.uni-jena.de/vbm/) for Statistical Parametric Mapping version 12 (SPM12: https://www.fil.ion.ucl.ac.uk/spm/). Following segmentation, each tissue class was spatially warped to an MNI-space template image comprised of 550 subjects included with the VBM8 toolbox (http://dbm.neuro.uni-jena.de.libproxy.wustl.edu/vbm/) using the high dimensional DARTEL normalization method. Functional resting-state scans were slice-time corrected, motion corrected, co-registered to native space structural data, and normalized to MNI space using DARTEL deformation fields obtained from spatially normalizing the T1-weighted anatomical images.
All resting state data underwent quality assurance (QA) using an automated QA pipeline that calculated median voxel displacement, median temporal signal-to-noise ratio (SNR), and 95th percentile signal change. Scans with log SNR and log median voxel displacement above the 95th percentile of the distribution of the entire dataset were excluded from the analysis, leaving (as previously mentioned) n=177 subjects passing these criteria. Diagnostic groups did not differ on median signal-to-noise ratio (F(2,174)=0.51, p=.60), median voxel displacement (F(2,174)=0.33, p=.72), and 95th percentile signal change (F(2,174)=1.24, p=.29).
Functional Connectivity Analysis
Prior striatal functional connectivity studies in psychosis used either cortical seeds to examine connectivity of cortical areas with the striatum (i.e. cortex seed-based analysis) (27,50–52), or striatal seeds to examine connectivity of striatal sub-regions with the rest of the brain (i.e. striatum seed-based analysis) (18,19,53). We used both approaches to comprehensively map functional connectivity of the striatum and facilitate comparison of our results to prior studies. Each approach is described in detail below.
Functional connectivity maps of cortex and striatum seeds were generated using the CONN-fMRI toolbox v.17.f (http://www.nitrc.org/projects/conn). The mean BOLD time series was extracted from seeds and entered as a predictor in a multiple regression general linear model (GLM). Regressors corresponding to the 6 motion correction parameters and their first temporal derivatives, along with grey matter, white matter, and CSF were included to remove variance related to head motion, the global grey matter signal, white matter, and CSF, respectively. In addition to 12 nuisance regressors related to motion (6 translations/rotations and their temporal first derivatives), the first 6 principal components were extracted from each subject’s white matter and CSF segmentations (i.e. anatomical CompCor) (54). Anatomical CompCor has been shown to be at least as effective as another commonly used approach, the “scrubbing” procedure described by Power et al. (55), at mitigating the residual effects of head motion on functional connectivity (56). Motion correction parameters were regressed out prior to temporal band-pass filtering. Performing these steps in reverse order (i.e. band-pass filtering before nuisance regression) re-introduces nuisance related variation thereby overestimating connectivity estimates and exacerbating the effects of head motion (57).
In addition, a mask was created for each subject’s mean fMRI image to identify in-brain voxels (i.e., an in-brain mask) and exclude voxels with low SNR. Briefly, after rescaling and standardizing each subject’s voxel intensity, the mean voxel intensity for all 177 subjects was calculated, and a threshold was identified using the SPM12’s antimode function to estimate a brain-nonbrain threshold (58). Any voxels that did not meet this in-brain threshold were removed, after applying the mask to MNI space. This mask was used in all cortical analyses in to remove non-brain and low SNR voxels from the analyses.
Cortex Seed-based Analysis
The 7-network cortical parcellation identified by Yeo et al. (59) was used to define seeds for the cortical seed-based analysis (see Figure 1a). Briefly, the Yeo 7-network cortical parcellation is comprised of the default mode (DMN), fronto-parietal (FPN), salience (SAL), limbic (LN), sensorimotor (SMN), dorsal attention (DAN), and visual (VN) networks. Functional connectivity maps were generated for each of the Yeo networks, except the DAN and VN which have minimal functional connectivity with the striatum (15). The functional connectivity maps were masked to only include voxels in the striatum. To maximize statistical power, we first performed a region-of-interest (ROI) analysis that generated a single value approximating overall functional connectivity of each cortical seed with the striatum. Specifically, the unsmoothed cortical seed-based connectivity maps were entered into separate one-sample voxel-wise t-tests to identify voxels in the striatum exhibiting positive functional connectivity with each cortical seed. This analysis included the entire cohort of study participants, including healthy subjects and individuals with a psychotic disorder. The resultant statistical parametric maps (SPM) were thresholded at voxel-wise family-wise error (FWE) corrected p=.05, minimum cluster size=10 voxels, and the ‘eigenvariate’ function in SPM12 was used to extract the first principal component from the voxels that survived thresholding. This yielded a single measure of functional connectivity with the striatum for each cortical seed for each subject. These measures served as dependent variables in a repeated measures ANCOVA with network entered as a repeating variable and diagnostic group (healthy controls, schizophrenia, psychotic bipolar disorder) entered as a between-subjects factor along with age and sex as covariates. Significant main effects and interactions were followed-up with post-hoc t-tests.
Figure 1.
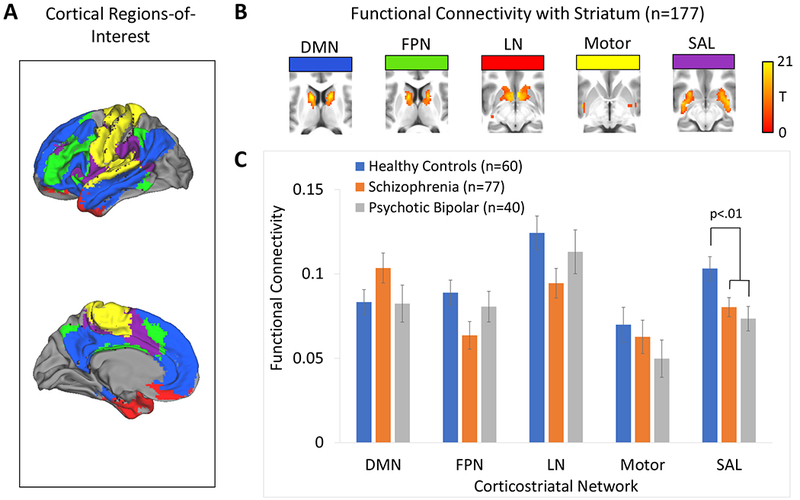
Cortical seed-based analysis of cortico-striatal functional connectivity in healthy subjects, schizophrenia, and psychotic bipolar disorder. Panel A: The cortex was partitioned into 5 non-overlapping regions-of-interest (ROIs) that were used as seeds in a seed-based functional connectivity analysis. Panel B: Average cortico-striatal functional connectivity within the striatum for each of the five cortical ROIs. Panel C: Average cortico-striatal functional connectivity within the striatum for each of the five cortical ROIs for each of the three diagnostic groups (i.e., controls, schizophrenia, psychotic bipolar disorder). Error bars indicate standard error of the mean.
The well-powered ROI approach described above was followed-up with voxel-wise analyses comparing functional connectivity of each cortical seed within the striatum between groups, in order to examine the specific locations of connectivity differences between the groups. Briefly, the unsmoothed cortical seed-based connectivity maps were analyzed separately using one-way ANOVAs with group (healthy controls, schizophrenia, psychotic bipolar disorder) entered as a between-subjects variable along with age and sex as covariates. Voxel-wise ANOVAs were masked with the one-sample t-test results described above such that only voxels that demonstrated positive functional connectivity with the cortex-based seeds were included in the analysis. Results were thresholded at cluster-level pFWE=.05 for voxel-wise p=.001.
Striatum Seed-based Analysis
We used the striatum seeds from previous striatal seed-based connectivity research (18,19,60). These striatum seeds are comprised of six 3.5-mm-radius spherical ROIs, totaling three bilateral caudate and three bilateral putamen regions corresponding to: dorsal caudate (DC; x=±13, y=15, z=9), superior ventral caudate (sVC; x=±10, y=15, z=0), inferior ventral caudate (iVC; x=±9, y=9, z=−8), dorsocaudal putamen (dcPT; x=±28, y=1, z=3), dorsorostral putamen (drPT; x=±25, y=8, z=6), and the ventrorostral putamen (vrPT; x=±20, y=12, z=−3). Functional connectivity maps were generated for each of these striatum seeds. Functional connectivity maps were masked to only include voxels in the cortex (using the in-brain mask detailed in the Supplement) and smoothed (6 mm). A one-sample voxel-wise t-test was performed for each striatum seed to identify voxels in the cortex exhibiting positive functional connectivity with each striatum seed. The resultant statistical parametric maps (SPM) were thresholded at voxel-wise p=.001. Group differences in functional connectivity of each striatum seed were then analyzed using a one-way ANOVA with group (healthy controls, schizophrenia, psychotic bipolar disorder) entered as a between-subjects variable along with age and sex as covariates. These analyses were masked with the one-sample t-test results described above such that only voxels that demonstrated positive functional connectivity with striatum-based seeds were included in the analysis. Results were thresholded at cluster-level pFWE=.05 for voxel-wise p=.001.
Testing Associations Between Striatal Connectivity and Clinical Variables
Next, we examined whether there were associations between connectivity impairments and both cognitive impairment and symptoms (and specifically positive psychotic symptoms), as has been found in previous research (24,35). To test these hypotheses, we examined whether there were significant associations between each significant cortico-striatal connectivity finding and both cognitive impairment on the SCIP and PANSS symptoms. These regressions included the following predictors: sex, age, diagnostic group (i.e., healthy subjects, schizophrenia, psychotic bipolar disorder), the variable of interest (e.g., SCIP “composite” z-score), and an interaction between diagnostic group and the variable of interest. For the cortex seed-based ROI analysis described above, overall connectivity between each cortical network and the striatum served as the dependent variable in regression analyses. For the voxel-wise analyses, mean functional connectivity was extracted from the clusters that survived thresholding using the eigenvariate function in SPM12 and this served as the dependent variable in regression analyses. Regression analyses were corrected for multiple comparisons (using False Discovery Rate (FDR) correction) based on the number of associations examined (i.e., FDR-corrected for every cognitive impairment and symptom variable of interest for a total of four FDR-corrected comparisons) for each significant cortico-striatal connectivity finding.
RESULTS
Striatum Functional Connectivity: Cortical Seed-based Analysis
ROI analysis:
Results of the ROI analysis are shown in Figure 1b. Repeated measures ANCOVA revealed a main effect of diagnostic group (F(2,172)=3.82, p=.02) and a network by diagnosis interaction (F(8,340)=2.06, p=.04). Bonferroni-corrected post-hoc comparison of estimated marginal means indicated that the main effect of diagnostic group was due to the schizophrenia group overall showing reduced cortico-striatal connectivity with the striatum compared to healthy subjects (mean difference=0.14, SE=.005, p=.04). Connectivity was not significantly different between psychotic bipolar disorder and healthy subjects: mean difference=0.14, SE=.006, p=.09. Connectivity was also similar between schizophrenia and psychotic bipolar: mean difference=0.001, SE=.006, p>.99 (see Figure 1c). Post-hoc univariate analysis indicated that the network by diagnosis interaction effect was due to group differences in SAL network connectivity with striatum (F(2,172)=5.24, p=.006, see Figure 2). SAL connectivity with striatum was greater in healthy subjects compared to schizophrenia (p=.007) and psychotic bipolar disorder (p=.005), which did not differ from one another (p=.58; see Figure 2b). Although not significant, there were also trends towards diagnostic effects for the FPN and LN (F(2,172)=2.87, p=.06 and F(2,172)=2.41, p=.09, respectively). Post-hoc contrasts indicated that FPN and LN connectivity with striatum was significantly lower in schizophrenia compared to healthy subjects (p=.02 and p=.03, respectively), whereas connectivity in psychotic bipolar disorder was similar to healthy subjects (p=.53 and p=.41, respectively).
Figure 2.
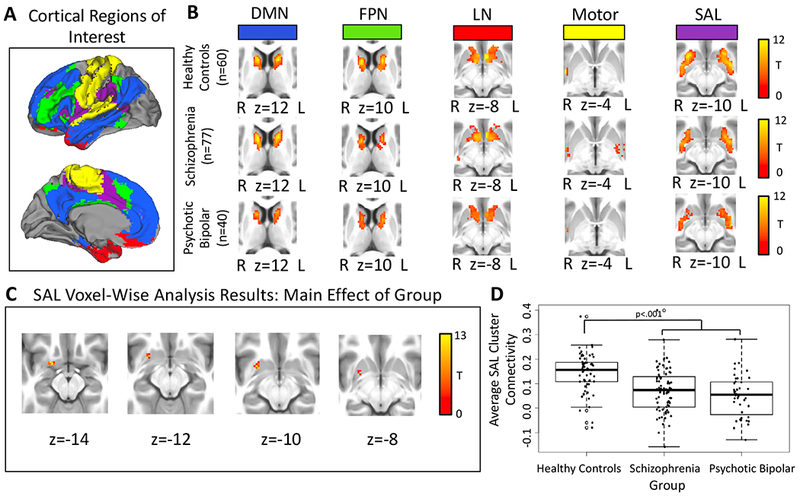
Panel A: Cortical parcellation used to define the five cortical regions-of-interest (ROIs). Panel B: The pattern of functional connectivity within the striatum for each cortical ROI in healthy controls, schizophrenia, and psychotic bipolar disorder. Results were thresholded at cluster-level Family-wise error-corrected p(FWE)=.05 for voxel-wise p(uncorrected)=.001. Panel C: Voxel-wise results for the salience network (SAL) cortical seed within the striatum revealed a main effect of group for the SAL cortical seed within the striatum, thresholded at whole-brain voxel-wise p(uncorrected)=.001. Panel D: Direct comparison between groups revealed decreased SAL network connectivity in the striatum in schizophrenia and psychotic bipolar disorder.
Median voxel displacement was not significantly associated with SAL connectivity (β=.30, p=.20). In a regression which included the combined patient sample examining the association between connectivity and antipsychotic dose in chlorpromazine equivalents (61), SAL connectivity did not significantly correlate with antipsychotic dose (β=−.04, p=.74). With respect to associations with cognitive functioning and symptoms, SAL connectivity was not significantly related to cognitive functioning (i.e., SCIP composite z-score; β=.03, p=.89; there was also no evidence that the relation between connectivity and cognitive functioning varied by group, β=.08, p=.75). Nor was SAL connectivity significantly related to PANSS positive, negative, or general symptoms (βs<−.67, FDR-corrected ps>.45; there was no evidence that the relation between connectivity and PANSS symptoms varied by group, βs<.58, FDR-corrected ps>.47).
Voxel-wise Analysis:
Consistent with the ROI analysis presented above, connectivity between the SAL cortical seed and striatum was decreased in schizophrenia and psychotic bipolar disorder in a cluster located in the putamen (see Table 2 and Figure 2). No additional cortical seeds demonstrated altered connectivity with the striatum. Post-hoc univariate analysis revealed that the significant effect of diagnostic group for this cluster was due to greater SAL cluster connectivity in healthy subjects compared to both schizophrenia (p<.001) and psychotic bipolar disorder (p<.001). Connectivity was similar in schizophrenia and psychotic bipolar disorder (p=.53; see Figure 2). Median voxel displacement was not significantly associated with SAL cluster connectivity (β=−.18, p=.42). In a regression which included the combined patient sample examining the association between connectivity and antipsychotic dose in chlorpromazine equivalents, SAL cluster connectivity did not significantly correlate with antipsychotic dose (β=−.11, p=.33). SAL cluster connectivity was unrelated to cognitive functioning (β=.24, p=.32; there was also no evidence that the relation between connectivity in this cluster and cognitive functioning varied by group, β=−.12, p=.60). SAL cluster connectivity was not significantly related to PANSS positive, negative, or general symptoms (βs<−.80, FDR-corrected ps>.39; nor was there any evidence that PANSS symptoms varied by patient group, βs<.69, FDR-corrected ps>.54).
Table 2.
Voxel-wise Analysis Results for the Cortical and Striatum Regions of Interest.a
| Cortical Targ | Brain Region | MNI Coordinates |
Peak F-value | Cluster P(FWE-corr) | Cluster Size (voxels) | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Cortical Regions of Interest | |||||||
| SAL | L. Putamen/L. Olfactory cortex | −20 | 6 | −14 | 12.37 | <.001 | 28 |
| L. Putamen | −24 | 6 | −6 | 9.50 | |||
| Striatum Regions of Interest | |||||||
| vrPT | R Medial Prefrontal Cortex | 4 | 14 | 48 | 11.62 | 0.003 | 208 |
| L Medial Prefrontal Cortex | −2 | 8 | 46 | 8.47 | |||
| R. Anterior Cingulate Cortex | 8 | 20 | 28 | 8.35 | |||
Abbreviations: FWE-corr=Cluster-level Family-wise error corrected p=.05 for voxel-wise p(uncorrected)=.001;SAL=Salience network; vrPT=ventral rostral putamen; sVC=superior ventral caudate; L=Left; R=Right.
Regions of Interest not presented had no clusters survived the cluster-level threshold.
Striatum Functional Connectivity: Striatum Seed-based Analysis
The between-group analyses (see Figure 3) revealed a main effect of diagnostic group between the vrPT striatal seed and cortical regions in a cluster located in the medial prefrontal cortex (see Figure 3c; Table 2). Bonferroni-corrected post-hoc univariate analysis revealed that the significant effect of diagnostic group for this cluster was due to greater vrPT cluster connectivity in healthy subjects compared to both schizophrenia (p<.005) and psychotic bipolar disorder (p<.001), whereas cluster connectivity was similar in schizophrenia compared to psychotic bipolar disorder (p=.89; see Figure 3c). Median voxel displacement was not significantly associated with vrPT cluster connectivity (β=−.13, p=.57). In a regression which included the combined patient sample examining the association between connectivity and antipsychotic dose in chlorpromazine equivalents, vrPT cluster connectivity did not significantly correlate with antipsychotic dose (β=1.05, p=.24). In terms of cognitive functioning, greater vrPT cluster connectivity was unrelated to SCIP composite z-scores, β=.24, p=.33 (there was also no evidence that the relation between connectivity and SCIP scores varied by group, β=−.18, p=.46). In terms of PANSS symptoms, vrPT cluster connectivity was unrelated related to PANSS positive, negative, or general symptoms (βs<|.83|, FDR-corrected ps>.21; nor was there any evidence that PANSS symptoms varied by patient group, βs<|.81|, FDR-corrected ps>.20).
Figure 3.
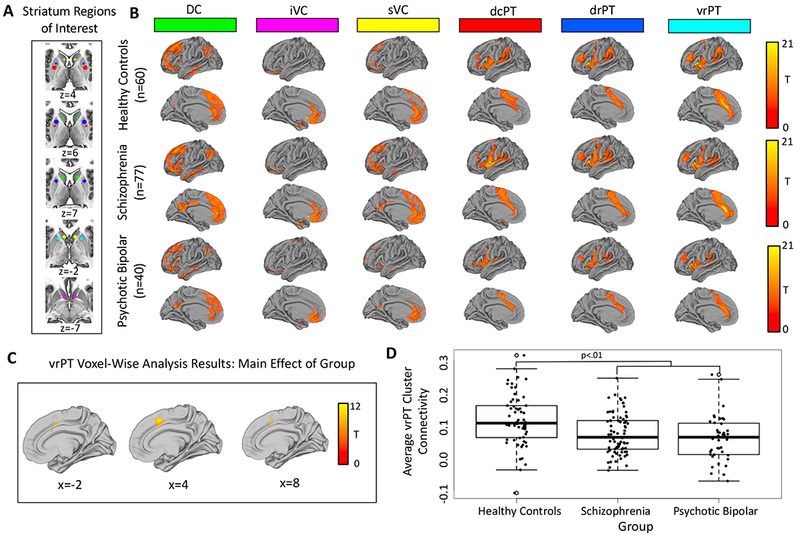
Panel A: The six striatum seeds used as regions-of-interest (ROIs) in seed-based functional connectivity analyses. Panel B: The pattern of functional connectivity within the cortex for each striatum ROI in healthy controls, schizophrenia, and psychotic bipolar disorder, thresholded at whole-brain voxel-wise p(uncorrected)=.001. Panel C: Voxel-wise results for the main effect of group for the ventral rostral putamen (vrPT) striatum seed, thresholded at whole-brain voxel-wise p(uncorrected)=.001. Panel D: Direct comparison between groups revealed decreased vrPT connectivity in schizophrenia and psychotic bipolar disorder.
DISCUSSION
We examined both cortical connectivity between the striatum and pre-defined cortical seeds and connectivity of striatal seeds with the rest of the brain in a large sample. We found evidence for reduced SAL network connectivity observed in several prior investigations of patients with schizophrenia (22,33,35) is also present in psychotic bipolar disorder. We also replicated evidence for hypo-connectivity between the ventrorostral putamen (vrPT) and the SAL network portion of the medial PFC in both schizophrenia and psychotic bipolar disorder. This research supports evidence for a transdiagnostic neurobiology underlying psychosis (39), providing evidence that the SAL network may be specifically associated with hypo-connectivity across psychotic disorders. Thus, the current study helps clarify cortico-striatal dysconnectivity in psychotic disorders.
With regard to cortical connectivity to striatal regions, first of all, there was a main effect of group, whereby the schizophrenia group overall showed reduced connectivity compared to healthy subjects. Furthermore, we replicated previous findings of SAL network hypo-connectivity (22,33,35), as the voxel-wise analysis helped localize the SAL network connectivity differences to the putamen. The SAL network is consistently linked to functions associated with impairment in psychotic disorders, including directing attention towards important stimuli (23), making the SAL network a likely suspect for impaired connectivity across psychotic disorders. SAL-related dysfunction in psychotic disorders has been found in neuroimaging studies, including reductions in gray-matter volume in SAL regions (62), impairments in task-related activation (63,64), as well as altered structural connectivity in key SAL regions (65,66). Likewise, and consistent with this study, reduced SAL connectivity in psychotic disorders has been found in a number of studies, including reduced connectivity within the insula (24,52,67), dorsal ACC (34,51), as well as overall reduced SAL within-network connectivity (29,66,68). Lastly, post-hoc analyses indicated that FPN and LN networks showed reduced connectivity specifically for the schizophrenia group, replicating findings from previous cortico-striatal connectivity work (18,19,21,69). Thus, while the SAL network exhibited transdiagnostic connectivity impairments across psychotic disorders, the FPN and LN connectivity impairments were unique to schizophrenia. The post-hoc findings of cortical seed-based impairments in the FPN and LN networks in this group indicate that there are connectivity impairments that perhaps represent more proximal risk factors unique to the diagnosis of schizophrenia (70).
With regard to striatal connectivity to cortical regions, the vrPT seeds showed reduced connectivity to the medial prefrontal cortex. This hypo-connectivity between the ventrorostal putamen and a salience network portion of the medial PFC is consistent with the current study’s cortical seed-based findings of salience network hypo-connectivity and provides further evidence that salience network dysfunction is specifically associated with transdiagnostic impairment across psychotic disorders. This medial PFC hypo-connectivity is also consistent with several studies finding that hypo-connectivity of this region linked to self-referential thinking is associated with schizophrenia spectrum symptoms (25,33,34). Furthermore, the vrPT hypo-connectivity extended into the anterior cingulate cortex, a region that has been heavily implicated in psychotic disorders (32,71,72), including recent research indicating that increased connectivity between the ACC and putamen is associated with more favorable treatment response (73).
Hypo-connectivity in SAL network regions in both the cortical and striatum seed-based analyses is also consistent with the dopamine hypothesis of psychotic disorders (6). As noted in the Introduction, hyper-dopaminergic functioning is heavily implicated in psychosis (40–42) and the dopaminergic system is also broadly implicated in cortico-striatal circuits (43). The dopaminergic system likely has multiple influences on the development and expression of psychotic symptoms, including influencing salience misattribution and failures in information integration, functions associations with reduced SAL connectivity (44,45). Such a model could, if supported by future studies designed to test it explicitly, unify behavioral, functional, and neural correlates of psychotic symptoms. However, inconsistent with previous research and perhaps the dopamine hypothesis of psychosis, we did not replicate previous correlations between SAL connectivity (or vrPT-mPFC connectivity) and cognition (35) and positive symptoms (24).
Our investigation has several limitations. First of all, it is unclear if the functional dysconnectivity found in the current study is a consequence of compromised structural connectivity, as has been reported in previous studies (22,65,66). Multi-modal investigations will be helpful in clarifying the nature of cortico-striatal dysconnectivity. Second, the relatively small number of psychotic bipolar patients (n=40) included in our sample is another limitation. Third, the schizophrenia sample was quite heterogeneous (i.e., 42.7% first episode, 6.2% schizoaffective). Future research should replicate these transdiagnostic effects in other homogeneous psychotic samples (e.g., first-episode, medication naïve (32)) to examine the generalizability of results. Lastly, somewhat surprising, the cortical pattern of connectivity for each of the putamen seeds (i.e., dcPT, drPT, and vrPT) were very similar (see Figure 3), and therefore it will be important for future research to replicate these patterns of cortico-striatal connectivity using other striatum parcellations.
In conclusion, using a combination of cortical and striatal seed-based approaches, we confirmed that SAL network hypo-connectivity is present in both schizophrenia and psychotic bipolar disorder, perhaps representing a transdiagnostic biomarker. According to striatum seed-based approach, there is evidence for transdiagnostic hypo-connectivity between the ventrorostral putamen and salience network portion of the medial PFC. Thus, overall, the current study points to the relative importance of salience network hypo-connectivity in psychotic disorders.
Acknowledgements
This work was supported by NIMH grant R01 MH102266 (awarded to NDW) and T32 MH014677 (awarded to NRK), the Charlotte and Donald Test Fund, and the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-TR000445 from the National Center for Research Resources/NIH). This work was conducted in part using the resources of the Advanced Computing Center for Research and Education at Vanderbilt University, Nashville, TN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kesby JP, Eyles DW, McGrath JJ, Scott JG (2018): Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Translational psychiatry. 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howes OD, Fusar-Poli P, Bloomfield M, Selvaraj S, McGuire P (2012): From the prodrome to chronic schizophrenia: the neurobiology underlying psychotic symptoms and cognitive impairments. Curr Pharm Des. 18:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fusar-Poli P, Meyer-Lindenberg A (2013): Striatal presynaptic dopamine in schizophrenia, part II: meta-analysis of [(18)F/(11)C]-DOPA PET studies. Schizophr Bull. 39:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapur S, Mizrahi R, Li M (2005): From dopamine to salience to psychosis--linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 79:59–68. [DOI] [PubMed] [Google Scholar]
- 5.Seeman P, Kapur S (2000): Schizophrenia: more dopamine, more D2 receptors. Proceedings of the National Academy of Sciences. 97:7673–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howes OD, Kapur S (2009): The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MA, Bhattacharyya S, Allen P, et al. (2013): Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 74:106–112. [DOI] [PubMed] [Google Scholar]
- 8.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, et al. (1996): Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America. 93:9235–9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. (2000): Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 97:8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seeman P (2013): Schizophrenia and dopamine receptors. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 23:999–1009. [DOI] [PubMed] [Google Scholar]
- 11.Du Y, Fryer SL, Fu Z, Lin D, Sui J, Chen J, et al. (2017): Dynamic functional connectivity impairments in early schizophrenia and clinical high-risk for psychosis. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Heuvel MP, Hulshoff Pol HE (2010): Exploring the brain network: a review on resting-state fMRI functional connectivity. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 20:519–534. [DOI] [PubMed] [Google Scholar]
- 13.Woodward ND, Heckers S (2016): Mapping Thalamocortical Functional Connectivity in Chronic and Early Stages of Psychotic Disorders. Biol Psychiatry. 79:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander GE, Crutcher MD, DeLong MR (1990): Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 85:119–146. [PubMed] [Google Scholar]
- 15.Choi EY, Yeo BT, Buckner RL (2012): The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 108:2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, et al. (2008): Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 28:7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE (2008): A dual-networks architecture of top-down control. Trends Cogn Sci. 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dandash O, Fornito A, Lee J, Keefe RS, Chee MW, Adcock RA, et al. (2014): Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 40:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, et al. (2013): Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 70:1143–1151. [DOI] [PubMed] [Google Scholar]
- 20.Horga G, Cassidy CM, Xu X, Moore H, Slifstein M, Van Snellenberg JX, et al. (2016): Dopamine-Related Disruption of Functional Topography of Striatal Connections in Unmedicated Patients With Schizophrenia. JAMA Psychiatry. 73:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon JH, Westphal AJ, Minzenberg MJ, Niendam T, Ragland JD, Lesh T, et al. (2014): Task-evoked substantia nigra hyperactivity associated with prefrontal hypofunction, prefrontonigral disconnectivity and nigrostriatal connectivity predicting psychosis severity in medication naive first episode schizophrenia. Schizophr Res. 159:521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palaniyappan L, Liddle PF (2012): Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. Journal of psychiatry & neuroscience : JPN. 37:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uddin LQ (2015): Salience processing and insular cortical function and dysfunction. Nature reviews Neuroscience. 16:55–61. [DOI] [PubMed] [Google Scholar]
- 24.Peters H, Riedl V, Manoliu A, Scherr M, Schwerthoffer D, Zimmer C, et al. (2017): Changes in extra-striatal functional connectivity in patients with schizophrenia in a psychotic episode. Br J Psychiatry. 210:75–82. [DOI] [PubMed] [Google Scholar]
- 25.Raij TT, Mantyla T, Kieseppa T, Suvisaari J (2015): Aberrant functioning of the putamen links delusions, antipsychotic drug dose, and compromised connectivity in first episode psychosis--Preliminary fMRI findings. Psychiatry research. 233:201–211. [DOI] [PubMed] [Google Scholar]
- 26.Haber SN (2016): Corticostriatal circuitry. Dialogues in clinical neuroscience. 18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernard JA, Orr JM, Mittal VA (2017): Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. NeuroImage Clinical. 14:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raichle ME (2015): The brain’s default mode network. Annu Rev Neurosci. 38:433–447. [DOI] [PubMed] [Google Scholar]
- 29.Koch K, Rus OG, Reess TJ, Schachtzabel C, Wagner G, Schultz CC, et al. (2014): Functional connectivity and grey matter volume of the striatum in schizophrenia. Br J Psychiatry. 205:204–213. [DOI] [PubMed] [Google Scholar]
- 30.Peeters SC, Gronenschild EH, van de Ven V, Habets P, Goebel R, van Os J, et al. (2015): Altered mesocorticolimbic functional connectivity in psychotic disorder: an analysis of proxy genetic and environmental effects. Psychol Med. 45:2157–2169. [DOI] [PubMed] [Google Scholar]
- 31.Rolland B, Amad A, Poulet E, Bordet R, Vignaud A, Bation R, et al. (2015): Resting-state functional connectivity of the nucleus accumbens in auditory and visual hallucinations in schizophrenia. Schizophr Bull. 41:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt K, et al. (2015): Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 72:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orliac F, Naveau M, Joliot M, Delcroix N, Razafimandimby A, Brazo P, et al. (2013): Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 148:74–80. [DOI] [PubMed] [Google Scholar]
- 34.Orr JM, Turner JA, Mittal VA (2014): Widespread brain dysconnectivity associated with psychotic-like experiences in the general population. NeuroImage Clinical. 4:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avram M, Brandl F, Bauml J, Sorg C (2018): Cortico-thalamic hypo- and hyperconnectivity extend consistently to basal ganglia in schizophrenia. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotarska-Jagiela A, van de Ven V, Oertel-Knochel V, Uhlhaas PJ, Vogeley K, Linden DE (2010): Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 117:21–30. [DOI] [PubMed] [Google Scholar]
- 37.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. (2010): Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 167:748–751. [DOI] [PubMed] [Google Scholar]
- 38.Ashok AH, Marques TR, Jauhar S, Nour MM, Goodwin GM, Young AH, et al. (2017): The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol Psychiatry. 22:666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jauhar S, Nour MM, Veronese M, Rogdaki M, Bonoldi I, Azis M, et al. (2017): A Test of the Transdiagnostic Dopamine Hypothesis of Psychosis Using Positron Emission Tomographic Imaging in Bipolar Affective Disorder and Schizophrenia. JAMA Psychiatry. 74:1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sole-Padulles C, Castro-Fornieles J, de la Serna E, Romero S, Calvo A, Sanchez-Gistau V, et al. (2016): Altered Cortico-Striatal Connectivity in Offspring of Schizophrenia Patients Relative to Offspring of Bipolar Patients and Controls. PLoS One. 11:e0148045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandl F, Avram M, Weise B, Shang J, Simoes B, Bertram T, et al. (2018): Specific Substantial Dysconnectivity in Schizophrenia: A Transdiagnostic Multimodal Meta-analysis of Resting-State Functional and Structural Magnetic Resonance Imaging Studies. Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 42.Dandash O, Yucel M, Daglas R, Pantelis C, McGorry P, Berk M, et al. (2018): Differential effect of quetiapine and lithium on functional connectivity of the striatum in first episode mania. Translational psychiatry. 8:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma A, Wolf DH, Ciric R, Kable JW, Moore TM, Vandekar SN, et al. (2017): Common Dimensional Reward Deficits Across Mood and Psychotic Disorders: A Connectome-Wide Association Study. Am J Psychiatry. 174:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.First M, Spitzer R, Gibbon M, Williams JJBW (1996). Structured clinical interview for DSM-IV Axis I disorders, Clinician Version (SCID-CV). New York Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- 45.Kay SR, Fiszbein A, Opler LA (1987): The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 13:261–276. [DOI] [PubMed] [Google Scholar]
- 46.Young R, Biggs J, Ziegler V, Meyer D (1978): A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 133:429–435. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton M (1960): A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 23:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weschler D (2001): Weschler Test of Adult Reading (WTAR). The Psychological Corporation, San Antonio, TX. [Google Scholar]
- 49.Purdon S (2005): The screen for cognitive impairment in psychiatry (SCIP): administration manual and normative data. Edmonton, Alberta: PNL Inc. [Google Scholar]
- 50.Hoffman RE, Fernandez T, Pittman B, Hampson M (2011): Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 69:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobson McEwen SC, Connolly CG, Kelly AM, Kelleher I, O’Hanlon E, Clarke M, et al. (2014): Resting-state connectivity deficits associated with impaired inhibitory control in nontreatment-seeking adolescents with psychotic symptoms. Acta psychiatrica Scandinavica. 129:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, et al. (2007): Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neuroscience letters. 417:297–302. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Liu WH, Li Z, Wei XH, Jiang XQ, Geng FL, et al. (2016): Altered corticostriatal functional connectivity in individuals with high social anhedonia. Psychol Med. 46:125–135. [DOI] [PubMed] [Google Scholar]
- 54.Behzadi Y, Restom K, Liau J, Liu TT (2007): A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 37:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014): Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH (2014): Reduction of motion-related artifacts in resting state fMRI using aCompCor. NeuroImage. 96:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hallquist MN, Hwang K, Luna B (2013): The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage. 82:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo WL, Nichols TE (2003): Diagnosis and exploration of massively univariate neuroimaging models. Neuroimage. 19:1014–1032. [DOI] [PubMed] [Google Scholar]
- 59.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (2011): The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, et al. (2008): Functional connectivity of human striatum: a resting state FMRI study. Cerebral cortex (New York, NY: 1991). 18:2735–2747. [DOI] [PubMed] [Google Scholar]
- 61.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ (2010): International consensus study of antipsychotic dosing. American Journal of Psychiatry. 167:686–693. [DOI] [PubMed] [Google Scholar]
- 62.Shao J, Meng C, Tahmasian M, Brandl F, Yang Q, Luo G, et al. (2018): Common and distinct changes of default mode and salience network in schizophrenia and major depression. Brain imaging and behavior. [DOI] [PubMed] [Google Scholar]
- 63.Sepede G, Spano MC, Lorusso M, De Berardis D, Salerno RM, Di Giannantonio M, et al. (2014): Sustained attention in psychosis: Neuroimaging findings. World journal of radiology. 6:261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smieskova R, Roiser JP, Chaddock CA, Schmidt A, Harrisberger F, Bendfeldt K, et al. (2015): Modulation of motivational salience processing during the early stages of psychosis. Schizophr Res. 166:17–23. [DOI] [PubMed] [Google Scholar]
- 65.Iwabuchi SJ, Liddle PF, Palaniyappan L (2015): Structural connectivity of the salience-executive loop in schizophrenia. European archives of psychiatry and clinical neuroscience. 265:163–166. [DOI] [PubMed] [Google Scholar]
- 66.Wang C, Ji F, Hong Z, Poh JS, Krishnan R, Lee J, et al. (2016): Disrupted salience network functional connectivity and white-matter microstructure in persons at risk for psychosis: findings from the LYRIKS study. Psychol Med. 46:2771–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, et al. (2014): Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 40:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt A, Palaniyappan L, Smieskova R, Simon A, Riecher-Rossler A, Lang UE, et al. (2016): Dysfunctional insular connectivity during reward prediction in patients with first-episode psychosis. Journal of psychiatry & neuroscience : JPN. 41:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang GJ, Murray JD, Wang XJ, Glahn DC, Pearlson GD, Repovs G, et al. (2016): Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 113:E219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nolen-Hoeksema S, Watkins ER (2011): A Heuristic for Developing Transdiagnostic Models of Psychopathology: Explaining Multifinality and Divergent Trajectories. Perspectives on psychological science : a journal of the Association for Psychological Science. 6:589–609. [DOI] [PubMed] [Google Scholar]
- 71.Knolle F, Ermakova AO, Justicia A, Fletcher PC, Bunzeck N, Duzel E, et al. (2018): Brain responses to different types of salience in antipsychotic naive first episode psychosis: An fMRI study. Translational psychiatry. 8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyata J (2019): Toward integrated understanding of salience in psychosis. Neurobiology of disease. [DOI] [PubMed] [Google Scholar]
- 73.Cadena EJ, White DM, Kraguljac NV, Reid MA, Jindal R, Pixley RM, et al. (2019): Cognitive control network dysconnectivity and response to antipsychotic treatment in schizophrenia. Schizophr Res. 204:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]


