Abstract
Summary
Studying dietary patterns is often more informative than individual nutrients or foods. We found that a Prudent dietary pattern (rich in vegetables and fish) was associated with reduced loss of total hip BMD in older men. A Prudent dietary pattern may be a potential lifestyle strategy for minimizing bone loss.
Introduction
This study aimed to identify baseline dietary patterns using factor analysis in a cohort of older men and to evaluate whether the dietary patterns were associated with bone mineral density change (%ΔBMD) at the total hip and femoral neck over time.
Methods
Participants (n = 4379; mean age 72.9 ±5.5 years) were from the Osteoporotic Fractures in Men (MrOS) prospective cohort study and had dietary data collected at baseline (March 2000-April 2002) and BMD measured at baseline and Visit 2 (March 2005-May 2006). Dietary intake was assessed with a brief Block food frequency questionnaire (FFQ); factor analysis was used to derive dietary patterns. BMD was measured by dual-energy x-ray absorptiometry (DXA); %ΔBMD was calculated from baseline to Visit 2. We used generalized linear regression to estimate least square (LS) means of %ΔBMD in quartiles of the dietary pattern scores adjusted for potential confounding factors.
Results
Two major dietary patterns were derived: Prudent (abundant in vegetables, salad, and non-fried fish) and Western (rich in hamburger, fries, processed meats, cheese, and sweets/desserts). There was an inverse association between adherence to the Prudent pattern and total hip %ΔBMD (p-trend = 0.028 after adjusting for age and clinical site; p-trend = 0.033 after further adjustment for smoking, calcium supplement use, diabetes, hypertension, and total energy intake). No other consistent associations between dietary patterns and %ΔBMD were observed.
Conclusions
Greater adherence to a Prudent dietary pattern may attenuate total hip BMD loss (%ΔBMD) in older men.
Keywords: BMD change, Dietary pattern, Factor analysis, Older men, Prudent, Western
Introduction
Many nutrients work synergistically and are present together in foods, and free-living people generally eat a variety of foods in combination. Moreover, it is challenging to isolate the effects of a single nutrient or food on a health outcome such as bone mineral density (BMD), which is an established risk factor for fracture [1–3]. Therefore, an approach that examines diet as a whole may be more informative than studying single nutrients or individual foods alone [4]. Additionally, studies of dietary patterns, rather than individual nutrients, are more easily translated into public health messages [5].
Dietary patterns can be described in a variety of terms, though exact definitions may vary by study. In general, Prudent (or “nutrient dense”) and Western (or “energy-dense”) are the most reproducible dietary patterns across studies [6]. Prudent dietary patterns are typically characterized by intakes of fruit, vegetables, whole grains, poultry, fish, nuts, legumes, and low-fat dairy. In contrast, Western dietary patterns are usually characterized by a higher proportion of foods of poor nutritional value, such as sugar-sweetened beverages, high-fat processed meats, and sweets [6, 7]. Additionally, different statistical methods can be used to examine relationships between dietary patterns and health outcomes. The most common approaches include a priori methods, which assess how closely participants follow predefined dietary patterns such as the Mediterranean or Baltic Sea diets [8], and a posteriori or data-driven approaches, which derive patterns empirically from dietary intake data using post hoc techniques [6, 9, 10]. Our group has expertise in using an a posteriori approach with factor analysis to derive dietary patterns [10–13]. In contrast to a priori methods, which are based on preconceived ideas of established dietary patterns, factor analysis allows for exploration and quantification of real-world dietary data in a scientific manner. When factor analysis is used to derive dietary patterns, the dietary patterns are named based on factor loadings that contribute the most to each pattern [10, 13].
The current literature predominately supports a beneficial effect of Prudent dietary patterns on bone-related outcomes [6, 11], but some studies have found no consistent associations between dietary patterns and risk of fracture [14] or BMD loss [12]. Additionally, since most studies have been conducted in female populations [6], further work is needed to elucidate associations between dietary patterns and bone outcomes in men.
The Osteoporotic Fractures in Men (MrOS) is an ongoing prospective cohort study that has followed ambulatory community-dwelling US men ≥ 65 years old since 2000 and has generated a rich dataset with which to examine multiple health outcomes in older men [15]. Dietary data at baseline (MrOS Visit 1) have been used to examine demographic factors and dietary quality [16] and macronutrients, diet quality, and frailty status [17], but associations between dietary patterns and BMD have not been investigated in this cohort.
The aims of the present study were to derive baseline dietary patterns among MrOS participants using factor analysis and to evaluate associations of dietary pattern scores with percent change in total hip and femoral neck BMD (%ΔBMD) from baseline to MrOS Visit 2 (approximately 4–5 years). Based on previous work in the MrOS cohort [16] and elsewhere [11, 12], we hypothesized that we would observe dietary patterns similar to the Prudent and Western patterns described in previous studies. Further, we hypothesized that greater adherence to a Prudent dietary pattern would be associated with reduced BMD loss (as measured by %ΔBMD) and that greater adherence to a Western dietary pattern would be associated with increased BMD loss over time.
Materials and methods
Study participants
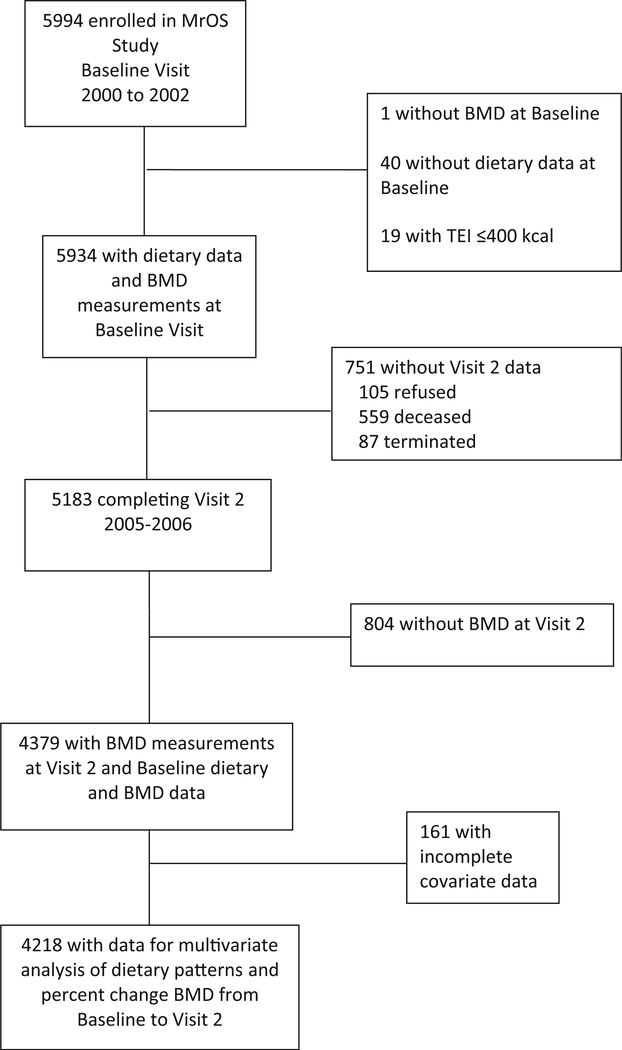
Participants in the MrOS cohort study include community-dwelling ambulatory men ≥ 65 years old recruited at six sites in the USA (Birmingham, AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; and San Diego, CA). Men were enrolled from March 2000 through April 2002 (n = 5994), and Visit 2 took place from March 2005 through May 2006 (n = 5202). Men who were unable to walk without assistance, as well as those with a history of bilateral hip replacement, which precludes hip BMD measurement, were ineligible to participate [15, 18]. For the present analysis, we included data from men with complete dietary data at baseline and BMD measurements at baseline and MrOS Visit 2. Men with implausibly low energy intake (≤ 400 kcal; n =19) were excluded from this analysis. The final sample size was 4379 participants for this analytic cohort; additionally, 4218 participants had complete covariate data for multivariate analysis (Fig. 1). All participants provided written informed consent, and the Institutional Review Board at each clinic site approved the study.
Fig. 1.
Recruitment and inclusion of participants. BMD, bone mineral density; TEI, total energy intake
Dietary intake patterns
Dietary assessment
Dietary data were collected using the Block 98.2 food frequency questionnaire (FFQ), which was a brief FFQ modified for the MrOS study to capture the most frequently consumed sources of calcium, vitamin D, and other selected nutrients influencing risk of osteoporosis and prostate cancer in US men. The Block 98.2 FFQ was also used to collect dietary data during MrOS Visit 1, and though this specific instrument did not undergo validation, other similar abbreviated Block questionnaires have been validated against two 7-day food records in older men [16, 19, 20]. The FFQs were analyzed by Block Dietary Data Systems (NutritionQuest, Berkeley, CA, USA). Details of this nutritional assessment have been described elsewhere [16]. Briefly, there were 69 individual food item questions, 14 questions pertaining to nutritional supplements and 13 questions about food preparation and low-fat foods. For each type of food or beverage, there were nine response categories for frequency (i.e., how often in the past year the participant consumed each item), and there were four response categories for portion sizes; participants were provided with a graphic representation of standard portion sizes to aid their responses [20].
Dietary pattern derivation
Using dietary data from the FFQs, food groups were constructed using individual food variables based on nutrient similarities, culinary use, and previous studies. To derive dietary patterns, the PROC FACTOR procedure in SAS was used to determine the number of dietary factors, which was based on robustness, interpretability, and eigenvalues on a scree plot. An eigenvalue cut-point of 3 was employed, and varimax (orthogonal) rotation of the data as performed in order to obtain uncorrelated factors with better interpretability. Factor loadings for each food group were calculated across the dietary patterns, and factor scores were calculated for each participant for the dietary patterns. The factor score is a measure of common variation and indicates strength and direction of association; a higher factor score indicates a higher correlation of foods with high factor loadings. Dietary patterns were named based on factor loadings that contributed the most to each pattern.
In this report, we use the term “adherence” to describe how closely participants’ usual intakes aligned with the derived dietary patterns. Dietary patterns were divided into quartiles, with quartile 1 representing the lowest adherence and quartile 4 representing the greatest adherence to the pattern [7, 10, 12, 13].
Other measurements
In a comprehensive questionnaire administered at the baseline visit, participants self-reported demographic information (race, education, marital status), medical diagnoses (stroke, diabetes mellitus, myocardial infarction, Parkinson’s disease, congestive heart failure (CHF), chronic obstructive pulmonary disorder (COPD), hypertension, osteoporosis), and lifestyle habits (smoking and alcohol use). Prescription and over-the-counter medications (including dietary supplements) used in the past 30 days were assessed with a computerized medication coding directory [21], and prescription medication data were stored electronically in a medication inventory database (San Francisco Coordinating Center, San Francisco, CA). The Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) was used to match each medication to its ingredients. The Physical Activity Scale for the Elderly (PASE) was administered to assess physical activity [22]. A wall-mounted stadiometer was used to measure each participant’s height without shoes, and body weight was measured with a standard, regularly calibrated balance beam scale or digital scale. Body mass index (BMI; kg/m2) was calculated for each participant based on weight and height measurements.
Estimation of change in BMD
Dual-energy x-ray absorptiometry (DXA) (QDR 4500W; Hologic Inc., Bedford, MA) was used to measure BMD (g/cm2) of the total hip and femoral neck at baseline and MrOS Visit 2. Percentage change in BMD (%ΔBMD) was calculated by subtracting BMD at baseline from BMD at Visit 2 and dividing by BMD at baseline. A central quality control lab, certification of DXA operators, and standardized procedures for scanning were used to insure reproducibility of DXA measurements. At baseline, a set of hip and linearity phantoms were circulated and measured at the six clinical sites. The variability across clinics was within acceptable limits, and cross-calibration correction factors were not required. However, to adjust for inter-clinic differences, statistical models include indicator variables for the individual scanners. Each clinic scanned a hip phantom throughout the study to monitor longitudinal changes, and correction factors for longitudinal changes were applied to participant data as appropriate. For scans at the San Diego clinic, a correction factor of 1.0179 was applied to scans performed between 12/9/03 and 1/4/2005, and a correction factor of 1.0112 was applied to scans performed on or after 1/5/2005 [23]. The precision of DXA scans of the total hip and femoral neck is 1–2% [24, 25].
Statistical analyses
Participant characteristics were compared across quartiles of consumption of each major dietary pattern using analysis of variance (ANOVA) for continuous normally distributed variables, Kruskal-Wallis tests for skewed continuous variables, and chi-square tests for categorical variables. These results later guided covariate selection in multivariable models. Additionally, intake of energy, macronutrients, selected micronutrients (calcium and vitamin D), and servings of vegetables, fruits, and dairy products were compared across quartiles of consumption of each major dietary pattern using ANOVA.
Box plots were constructed in order to visualize the shape of associations between quartiles of each dietary pattern and distribution of %ΔBMD before adjustment for other variables. Generalized linear regression models were then used to estimate least square (LS) means of % ΔBMD in quartiles of dietary pattern factor scores adjusted for potential confounding factors. p for trend was calculated. Models were initially adjusted for age and clinical center, and multivariate models were further adjusted for smoking (yes/no) calcium supplement use (yes/no), self-reported hypertension (yes/no), and self-reported diabetes (yes/no). These covariates were chosen using backward selection by selecting participant characteristic variables that were significantly different across quartiles of dietary pattern at p <0.10 (Table 1). Variables that remained in the model at p < 0.05 were included in the multivariate models. Although we anticipated that BMI might be an important covariate [12], in this approach, BMI did not remain in the model after backward selection and therefore was not included in the multivariate models. Furthermore, because adjustment for baseline health status may introduce bias and lead to spurious results [26], we did not adjust for baseline BMD in this analysis. Additionally, because both dietary patterns were correlated with total energy intake (r =0.39, p <0.001 for Prudent; r = 0.78, p <0.001 for Western; data not shown), we also added total energy intake (TEI) to the multivariate models.
Table 1.
Characteristics of participants based on quartiles of consumption of (a) Prudent and (b) Western dietary patterns
| Characteristic | Q1 (lowest consumption) (n = 1094) |
Q2 (n = 1095) | Q3 (n = 1095) | Q4 (highest consumption) (n = 1095) |
p value |
|---|---|---|---|---|---|
| a | |||||
| Age (years), mean ± SD | 72.6 ± 5.4 | 73 ± 5.4 | 73 ± 5.5 | 72.9 ± 5.5 | 0.292 |
| African American, n (%) | 43 (3.9) | 41 (3.7) | 30 (2.7) | 32 (2.9) | 0.314 |
| < High school education, n (%) | 74 (6.8) | 51 (4.7) | 56 (5.1) | 51 (4.7) | 0.088 |
| Married, n (%) | 893 (81.6) | 930 (84.9) | 941 (85.9) | 907 (82.8) | 0.026 |
| Glucocorticoid use, n (%) | 82 (7.8) | 106 (10.1) | 91 (8.6) | 83 (7.9) | 0.208 |
| Calcium supplement, n (%) | 307 (29.3) | 353 (33.2) | 405 (38.7) | 422 (39.7) | < 0.0001 |
| BMI (kg/m2), mean ± SD | 27.9 ± 3.8 | 27.4 ± 3.4 | 27.4 ± 3.9 | 26.9 ± 3.9 | < 0.0001 |
| PASE score, mean ± SD | 150.5 ± 66.5 | 149.7 ± 64.3 | 149.6 ± 67.2 | 157.7 ± 71.1 | 0.011 |
| Current smoker, n (%) | 60 (5.5) | 30 (2.7) | 17 (1.6) | 25 (2.3) | < 0.0001 |
| Drinks per day > 2, n (%) | 147 (13.5) | 128 (11.7) | 131 (12) | 104 (9.5) | 0.038 |
| Self-reported medical conditions, mean ± SD | |||||
| Stroke | 66±6 | 50 ± 4.6 | 42 ± 3.8 | 46 ± 4.2 | 0.077 |
| Diabetes | 86 ± 7.9 | 105 ± 9.6 | 104 ± 9.5 | 118 ± 10.8 | 0.138 |
| Parkinson’s disease | 6±0.6 | 6±0.6 | 6±0.6 | 4±0.4 | 0.908 |
| COPD | 117 ± 10.7 | 118 ± 0.8 | 110 ± 10.1 | 84 ± 7.7 | 0.049 |
| Myocardial Infarction | 129 ± 11.8 | 125 ± 11.4 | 130 ± 11.9 | 143 ± 13.1 | 0.666 |
| CHF | 47 ± 4.3 | 36 ± 3.3 | 47 ± 4.3 | 45 ± 4.2 | 0.577 |
| Hypertension | 430 ± 39.3 | 454 ± 41.5 | 455 ± 41.6 | 441 ± 40.18 | 0.670 |
| Osteoporosis | 28 ± 2.6 | 37 ± 3.4 | 37 ± 3.4 | 34 ± 3.1 | 0.653 |
| b | |||||
| Age (years), mean ± SD | 73.4 ± 5.6 | 73.2 ± 5.6 | 72.7 ± 5.3 | 72.1 ± 5.2 | < 0.0001 |
| African American, n (%) | 30 (2.7) | 35 (3.2) | 31 (2.8) | 50 (4.6) | 0.064 |
| < High school education, n (%) | 46 (4.2) | 48 (4.4) | 57 (5.2) | 81 (7.4) | 0.003 |
| Married, n (%) | 900 (82.3) | 927 (84.7) | 929 (84.8) | 915 (83.6) | 0.333 |
| Glucocorticoid use, n (%) | 98 (9.3) | 91 (8.8) | 76 (7.2) | 97 (9.3) | 0.2702 |
| Calcium supplement, n (%) | 431 (40.9) | 380 (36.1) | 355 (33.7) | 321 (30.4) | < 0.0001 |
| BMI (kg/m2), mean ± SD | 26.3 ± 3.5 | 27.2 ± 3.6 | 27.6 ± 3.6 | 28.5 ± 4.0 | < 0.0001 |
| PASE score, mean ± SD | 146.5 ± 67.3 | 152.9 ± 64.8 | 152.4 ± 66.9 | 155.7 ± 70 | 0.013 |
| Current smoker, n (%) | 12 (1.1) | 21 (1.9) | 33 (3) | 66 (6.0) | < 0.0001 |
| Drinks per day > 2, n (%) | 107 (9.8) | 131 (12) | 142 (13) | 130 (11.9) | 0.127 |
| Self-reported medical conditions, mean ± SD | |||||
| Stroke | 54 ± 4.9 | 48 ± 4.4 | 42 ± 3.8 | 60 ± 5.5 | 0.295 |
| Diabetes | 78 ± 7.1 | 99±9 | 111 ± 10.1 | 125 ± 11.4 | 0.005 |
| Parkinson’s disease | 6±0.6 | 5±0.5 | 7±0.6 | 4±0.4 | 0.822 |
| COPD | 95 ± 8.7 | 96 ± 8.8 | 99 ± 9 | 139 ± 12.7 | 0.003 |
| Myocardial infarction | 160 ± 14.6 | 124 ± 11.3 | 130 ± 11.9 | 113 ± 10.3 | 0.015 |
| CHF | 41 ± 3.8 | 37 ± 3.4 | 48 ± 4.4 | 49 ± 4.5 | 0.504 |
| Hypertension | 407 ± 37.2 | 431 ± 39.4 | 461 ± 42.1 | 481 ± 43.9 | 0.008 |
| Osteoporosis | 35 ± 3.2 | 32 ± 2.9 | 40 ± 3.7 | 29 ± 2.7 | 0.571 |
BMI body mass index, CHF congestive heart failure, COPD chronic obstructive pulmonary disorder, PASE physical activity scale for the elderly
Unless otherwise noted, two-sided significance levels were set at p < 0.05, and all analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The analysis cohort was comprised of 4379 primarily Caucasian (97%) men with an average age of 72.9 ± 5.5 years at baseline and an average BMI of 27.4± 3.8 kg/m2. Mean follow-up was 4.57 ± 0.35 years.
With an eigenvalue cut-point of 3, results of the scree plot suggested two major dietary patterns (Online Resource 1). The first dietary pattern, which we named “Western,” was characterized by high factor loadings for hamburger, fries, processed meats, gravy, cheese and cheese dishes, ice cream, butter, doughnuts/pastries, mayonnaise, and white bread. The second dietary pattern, which we named “Prudent,” had the highest factor loadings for carrots, broccoli, spinach, green beans, green salad, cabbage, baked beans, tomatoes, non-fried fish, and vegetable soup (Online Resource 2). Since dairy products are rich in many nutrients beneficial to bone [27], we also compared the factor loadings specifically for dairy products by dietary pattern. The Western pattern loaded highest in ice cream, cheese dishes, butter, cheese, whole milk, and 2% milk. The Prudent pattern loaded highest in yogurt/frozen yogurt non-fat milk and cheese dishes (Online Resource 3).
Participant characteristics were stratified by quartile of scores of the Prudent dietary pattern (Table 1 (a)) and Western dietary pattern (Table 1 (b)). Compared to men with lower adherence (quartile 1), men with greater adherence (quartile 4) to the Prudent dietary pattern were more likely to be married and users of calcium supplements and have lower BMI and a higher PASE score; they were less likely to be current smokers, consume more than two alcoholic drinks per day, or to have self-reported COPD. Men did not differ across quartiles of the Prudent dietary pattern in age, race, education, glucocorticoid use, or other self-reported medical conditions (Table 1 (a)). Compared to men with lower adherence to the Western dietary pattern, men with greater adherence were more likely to be younger, less educated, have a higher BMI and PASE score, and more likely to currently smoke. Additionally, men with greater adherence to the Western dietary pattern were more likely to report diabetes, COPD, and hypertension and were less likely to use calcium supplements or to report a myocardial infarction compared to men with lower adherence to the Western dietary pattern. There were no differences across quartiles of Western dietary pattern scores in race, marital status, glucocorticoid use, alcoholic drinks per day, stroke, Parkinson’s disease, CHF, and osteoporosis.
Significant differences across quartiles of dietary patterns for selected nutritional variables were observed for both dietary patterns (Table 2). Men with greater adherence to the Prudent dietary pattern had lower intakes of energy from fat and greater intakes of energy from carbohydrate and protein, calcium, vitamin D and servings of vegetables, fruit, and (mostly lower fat) dairy products compared to men with lower adherence to the Prudent dietary pattern (Table 2 (a) and Online Resource 3). Men with greater adherence to the Western dietary pattern had lower intakes of energy from carbohydrate and protein and lower intake of fruit, but greater intakes of energy from fat, calcium, vitamin D, vegetables, and (mostly higher-fat) dairy products compared to men with lower adherence to the Western dietary pattern (Table 2 (b) and Online Resource 3). In addition, there were significant differences in TEI across quartiles of both dietary patterns, with the highest quartiles consuming significantly more energy than the lowest quartiles (Table 2).
Table 2.
Daily nutritional intake per quartile of (a) Prudent and (b) Western dietary pattern scores, mean ± SD
| Q1 (lowest consumption) (n = 1094) |
Q2 (n = 1095) | Q3 (n = 1095) | Q4 (highest consumption) (n = 1095) |
p value | |
|---|---|---|---|---|---|
| a | |||||
| Energy intake (kcal) | 1369.1 ± 552.6 | 1479.7 ± 536.9 | 1644.1 ± 545.7 | 1991.4 ± 682.6 | < 0.0001 |
| Energy from fat (%) | 40 ± 7.7 | 37.2 ± 7.7 | 35.4 ± 7.6 | 33.2 ± 7.8 | < 0.0001 |
| Energy from carbohydrates (%) | 46.9 ± 8.7 | 49.5 ± 8.3 | 51.1 ± 8.2 | 53.1 ± 8.6 | < 0.0001 |
| Energy from protein (%) | 15.3 ± 2.8 | 15.9 ± 2.8 | 16.5 ± 2.8 | 17.1 ± 2.8 | < 0.0001 |
| Dietary calcium intake (mg) | 669.9 ± 333.6 | 734.9 ± 342.1 | 813.4 ± 344.2 | 1016.1 ± 424.2 | < 0.0001 |
| Dietary vitamin D intake (IU) | 150.1 ± 111.2 | 153 ± 106 | 170 ± 117.9 | 196.8 ± 131 | < 0.0001 |
| Servings of vegetables | 1.5 ± 0.8 | 2.4 ± 1 | 3.4 ± 1.2 | 5.8 ± 2.3 | < 0.0001 |
| Servings of fruits and fruit juices | 1.1 ± 0.7 | 1.6 ± 0.8 | 1.9 ± 0.9 | 2.3 ± 1 | < 0.0001 |
| Servings of milk/yogurt/cheese | 1.4 ± 1 | 1.4 ± 1 | 1.5 ± 1 | 1.7 ± 1.2 | < 0.0001 |
| b | |||||
| Energy intake (kcal) | 1164.8 ± 407.2 | 1380.8 ± 369 | 1643.6 ± 361.5 | 2295 ± 651.9 | < 0.0001 |
| Energy from fat (%) | 29.3 ± 7.5 | 35.9 ± 6.8 | 38.9 ± 6.2 | 41.8 ± 5.9 | < 0.0001 |
| Energy from carbohydrates (%) | 57.3 ± 8.4 | 51 ± 7.3 | 47.7 ± 6.9 | 44.7 ± 6.6 | < 0.0001 |
| Energy from protein (%) | 17.1 ± 3 | 16.1 ± 2.7 | 15.9 ± 2.8 | 15.7 ± 2.8 | < 0.0001 |
| Dietary calcium intake (mg) | 749.9 ± 409 | 746.3 ± 356.6 | 789.3 ± 328.3 | 949 ± 406.1 | < 0.0001 |
| Dietary vitamin D intake (IU) | 166.1 ± 125.1 | 155.4 ± 114.1 | 158.5 ± 112.2 | 189.9 ± 118.5 | < 0.0001 |
| Servings of vegetables | 3 ± 2.3 | 2.9 ± 1.9 | 3.1 ± 1.9 | 4.1 ± 2.3 | < 0.0001 |
| Servings of fruits and fruit juices | 1.9 ± 1.0 | 1.7 ± 0.9 | 1.6 ± 0.9 | 1.6 ± 0.9 | < 0.0001 |
| Servings of milk/yogurt/cheese | 1.4 ± 1.1 | 1.5 ± 1 | 1.5 ± 1 | 1.7 ± 1.1 | < 0.0001 |
Box plots depicting unadjusted associations between BMD and quartiles of each dietary pattern are presented in Fig. 2. Adjusted associations between quartiles of the Prudent dietary pattern and %ΔBMD at the total hip are presented in Table 3 (a). There was a significant inverse association between adherence to the Prudent pattern and total hip %ΔBMD in model 1, which adjusted for age and clinic site. This association was attenuated after further adjustment for smoking calcium supplement use, self-reported hypertension, and self-reported diabetes (model 2) but regained significance when the multivariate model was adjusted for TEI (model 3). Associations between quartiles of the Prudent dietary pattern and femoral neck %ΔBMD are presented in Table 3 (b), and associations between quartiles of the Western dietary pattern and %ΔBMD are presented in Table 4. These associations were inconsistent and did not reach statistical significance.
Fig. 2.
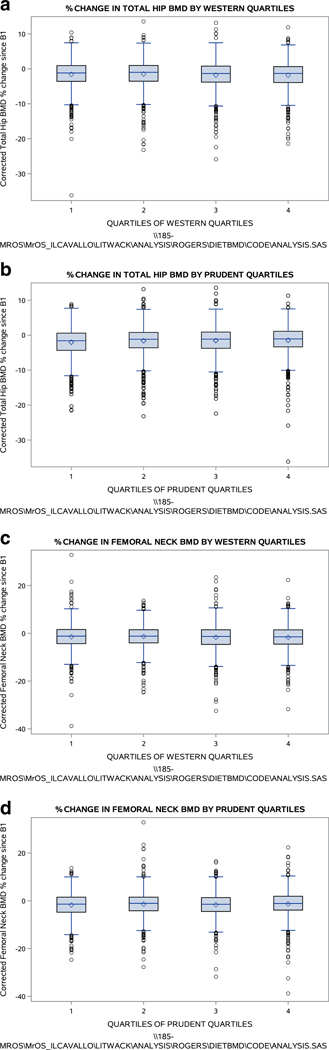
Box plots of BMD and dietary patterns, unadjusted (n = 4379). a Percent change in total hip BMD by quartile of Western dietary pattern. b Percent change in total hip BMD by quartile of Prudent dietary pattern. c Percent change in femoral neck BMD by quartile of Western dietary pattern. d Percent change in femoral neck BMD by quartile of Prudent dietary pattern
Table 3.
Associations between Prudent dietary pattern and percent change in (a) totael hip BMD and in (b) femoral neck BMD in older men
| Model 1 age and site adjusted, n = 4379 |
Model 2 MV adjusted, n = 4218 |
Model 3 MV + TEI adjusted, n = 4218 |
||||
|---|---|---|---|---|---|---|
| LS means (95%CI) | p-trend | LS means (95%CI) | p-trend | LS means (95%CI) | p-trend | |
| a | ||||||
| Q1 | − 1.96 (− 2.19, − 1.73) | 0.028 | − 1.91 (− 2.14, − 1.67) | 0.060 | − 1.93 (− 2.18, − 1.69) | 0.033 |
| Q2 | − 1.57 (− 1.80, − 1.34) | − 1.54 (− 1.77, − 1.31) | − 1.55 (− 1.78, − 1.32) | |||
| Q3 | − 1.59 (− 1.82, − 1.36) | − 1.60 (− 1.83, − 1.36) | − 1.59 (− 1.82, − 1.36) | |||
| Q4 | − 1.56 (− 1.79, − 1.33) | − 1.55 (− 1.78, − 1.32) | − 1.51 (− 1.75, − 1.27) | |||
| b | ||||||
| Q1 | − 1.75 (− 2.06, − 1.45) | 0.136 | − 1.70 (− 2.01, − 1.39) | 0.215 | − 1.75 (− 2.07, − 1.44) | 0.090 |
| Q2 | − 1.38 (− 1.68, − 1.08) | − 1.37 (− 1.67, − 1.07) | − 1.40 (− 1.70, − 1.09) | |||
| Q3 | − 1.67 (− 1.97, − 1.36) | − 1.66 (− 1.97, − 1.36) | − 1.66 (− 1.96, − 1.35) | |||
| Q4 | − 1.31 (− 1.61, − 1.01) | − 1.31 (− 1.61, − 1.00) | − 1.23 (− 1.55, − 0.91) | |||
Multivariate (MV) model adjusted for age, clinic site, smoking, calcium supplement use, self-reported hypertension, and self-reported diabetes TEI total energy intake
Table 4.
Associations between Western dietary pattern and percent change in (a) total hip BMD and in (b) femoral neck BMD in older men
| Model 1 age and site adjusted, n = 4379 |
Model 2 MV adjusted, n = 4218 |
Model 3 MV + TEI adjusted, n = 4218 |
||||
|---|---|---|---|---|---|---|
| LS means (95%CI) | p-trend | LS means (95%CI) | p-trend | LS means (95%CI) | p-trend | |
| a | ||||||
| Q1 | − 1.63 (− 1.86, − 1.40) | 0.176 | − 1.70 (− 2.01, − 1.39) | 0.591 | − 1.65 (− 1.91, − 1.39) | 0.593 |
| Q2 | − 1.50 (− 1.73, − 1.27) | − 1.37 (− 1.67, − 1.07) | − 1.50 (− 1.74, − 1.26) | |||
| Q3 | − 1.77 (− 2.00, − 1.54) | − 1.66 (− 1.97, − 1.36) | − 1.74 (− 1.98, − 1.51) | |||
| Q4 | − 1.79 (− 2.02, − 1.55) | − 1.31 (− 1.61, − 1.00) | − 1.69 (− 1.98, − 1.40) | |||
| b | ||||||
| Q1 | − 1.42 (− 1.72, − 1.11) | 0.095 | − 1.46 (− 1.77, − 1.15) | 0.381 | − 1.49 (− 1.83, − 1.15) | 0.657 |
| Q2 | − 1.33 (− 1.63, − 1.03) | − 1.37 (− 1.67, − 1.06) | − 1.38 (− 1.70, − 1.07) | |||
| Q3 | − 1.66 (− 1.96, − 1.36) | − 1.63 (− 1.93, − 1.32) | − 1.63 (− 1.93, − 1.32) | |||
| Q4 | − 1.70 (− 2.01, − 1.40) | − 1.58 (− 1.89, − 1.27) | − 1.54 (− 1.92, − 1.15) | |||
Multivariate (MV) model adjusted for age, clinic site, smoking, calcium supplement use, self-reported hypertension and self-reported diabetes TEI total energy intake
Discussion
As we hypothesized, the two major dietary patterns derived from this cohort were similar to the Prudent and Western dietary patterns described in previous studies [11, 12]. In the present study, the Prudent dietary pattern loaded most heavily in vegetables and non-fried fish, and the Western dietary pattern had highest factor loadings for red and processed meats, cheese, fats, and sweets. In partial support of our hypothesis, there was a modest association between adherence to the Prudent dietary pattern and attenuated total hip BMD loss (%ΔBMD). Contrary to our hypothesis, the Prudent dietary pattern was not significantly associated with %ΔBMD at the femoral neck, and the Western dietary pattern was not associated with %ΔBMD at the total hip or femoral neck. The observational nature of these data preclude establishment of a causative relationship, but our results support adherence to a Prudent dietary pattern as a potential lifestyle strategy for minimizing total hip bone loss in older men.
The synergistic effects of antioxidants in fruit and vegetables [28] and other nutrients for bone health [29] have been previously described; therefore, we speculate that the high factor loadings of vegetables and non-fried fish in the Prudent dietary pattern may have contributed to reduced total hip BMD decline in the present analysis in a multifactorial manner. Key micronutrients for bone health that are found in vegetables include potassium, magnesium, vitamin C, vitamin K, lycopene, folate, and carotenoids [6, 30]. Antioxidants in vegetables reduce oxidative stress, which has been shown to increase bone turnover and to be negatively associated with BMD [31]. Intake of vegetables may also provide potassium and bicarbonate to buffer excess dietary metabolic acids, promote a pH range conducive to calcium balance, reduce bone turnover, and improve bone mineral accretion and BMD [32–35]. Additionally, high-fiber foods such as vegetables may enhance the diversity of gut microbiota and subsequently increase absorption of calcium and magnesium [36]. Calcium is also present in vegetables such as spinach but may have limited bioavailability due to binding by oxalates [37, 38]. The polyunsaturated fatty acids (PUFA) present in fish have known anti-inflammatory effects and are involved in calcium homeostasis and bone metabolism [39]; PUFA intake has also been associated with lower risk of fracture in some [40, 41] but not all studies [42].
To our knowledge, the present study is the first to report associations between dietary patterns derived by factor analysis with %ΔBMD in a cohort of older men in the USA. Our results are in alignment with a recent scoping review which concluded that a “healthy” dietary pattern (rich in fruit, vegetables, whole grains, poultry, fish, nuts, legumes, and low-fat dairy and low in soda, fried food, meat, processed foods, refined grains, and sweets) appears beneficial to bone health [6]. However, in contrast to the null results between the Western dietary pattern and % ΔBMD in our analysis, this review concluded that a Western dietary pattern is potentially detrimental to bone [6]. Differences in statistical methodology and participant populations make it challenging to directly compare results of the present analysis with other studies that have examined dietary patterns and bone outcomes.
Among other studies using factor analysis, a dietary pattern high in fruit and vegetables, whole grains, dairy, and nuts was positively associated with BMD of the whole leg, whole pelvis, lumbar spine, and whole body in middle-aged Korean men [9], and a dietary pattern rich in legumes, seafood, seeds, nuts, wine rice, and vegetables was positively associated with BMD at the lumbar spine and total hip in Australian women [43]. In the Canadian Multicenter Osteoporosis Study, greater adherence to a nutrient-dense (Prudent) dietary pattern was associated with higher BMD in younger men after adjustment for BMI; however, there were no consistent relationships between dietary patterns and BMD without adjusting for BMI [12].
In studies of other bone outcomes besides BMD, greater adherence to a Prudent diet was associated with reduced bone resorption (C-telopeptide of type 1 collagen, CTX) in Canadian women, as well as increased 25-hydroxy vitamin D (25[OH]D) and reduced parathyroid hormone (PTH) in Canadian men, whereas greater adherence to a Western diet was associated with increased bone-specific alkaline phosphatase (BSAP) and reduced 25(OH)D in women and increased CTX in men [7]. Also in the Canadian population, the Prudent dietary pattern was associated with reduced risk of fractures in men and women, independent of BMI, BMD, falls, and demographics, but the Western pattern was not related to fracture risk [11]. Interestingly, among elderly Japanese men and women, a dietary pattern high in vegetables, seaweed, mushrooms, and soy was associated with an increased risk of fracture, and a there was a trend for reduced fracture risk with a dietary pattern high in chicken, pork, beef, processed meat, and seafood [44]. Furthermore, there were no associations between the Prudent or Western dietary patterns and risk of hip fractures study in women enrolled in the Nurses’ Health Study and men in the Health Professionals Follow-up Study [14].
In the present analysis, after adjustment for covariates related to lifestyle (smoking and calcium supplement use) and comorbidities (self-reported hypertension and diabetes), the association between adherence to the Prudent dietary pattern and total hip %ΔBMD was attenuated (Table 3 (a), model 2) but regained significance when TEI was added to the model (Table 3 (a), model 3). Supplement use is a marker of positive health behaviors [45], and calcium supplement use has been shown to attenuate BMD loss in older men [46], while smoking has been established as a risk factor for bone loss and is likely a proxy of other unhealthy and unmeasured risk factors [47–50]. Associations between hypertension and bone loss [51], as well as diabetes and osteoporosis [52], have also been reported. Further research is needed to corroborate our observations and to elucidate potential biological mechanisms.
Our findings, particularly in light of the conflicting results reported in the literature, illustrate the complexity of studying diet-disease relationships in the context of realistic (rather than recommended) dietary patterns, the intricacy of nutrition and lifestyle factors in relation to health outcomes, the necessity of interpreting data with regard to population differences, and the need for additional research. The present analysis advances the field by informing of the associations between dietary intake patterns and %ΔBMD at the total hip and femoral neck over approximately 4 years in older, predominately white, American men. Furthermore, results support adherence to a Prudent dietary pattern for improved bone health in this population [6].
This study has a number of strengths. The MrOS cohort is well-characterized for dietary patterns, BMD, and numerous potentially confounding factors such as comorbidities and lifestyle habits. The same DXA machines were used at each visit, thus providing good quality control for BMD measurements. Advantages of factor analysis include the creation of a continuous variable, which provides increased power to examine diet-disease relationships, and the ability to examine dietary patterns that exist in free-living subjects without depending on preconceived ideas of diet-disease relationships [6, 43, 53]. However, factor analysis is exploratory and involves subjective decisions on handling the data, such as the eigenvalue cut-point, adjustment for BMI or total energy, and transformation of the data [53]. In addition, the factor analysis method is more difficult to reproduce compared to diet indices and scoring [6]. This study was also limited by the underrepresentation of minorities in MrOS cohort, as well as the use of a brief FFQ, which was approximately two thirds the length of a standard FFQ [44] and may have underestimated energy and macronutrients [14]. Despite these limitations, the present analysis addresses a current knowledge gap [54] as one of the few longitudinal, data-driven studies of dietary patterns and bone outcomes using factor analysis with exclusively older male participants.
In the future, dietary data from MrOS and other cohorts could be used to examine longitudinal associations with other musculoskeletal outcomes such as fracture incidence, bone turnover, muscle mass, and BMD ofthe hip (as well as lumbar spine) assessed by high-resolution peripheral quantitative computed tomography (HRpQCT), rather than DXA. Direct, as well as indirect, causal pathways should also be further explored.
Conclusions
These results suggest that greater adherence to a Prudent dietary pattern (rich in vegetables and non-fried fish) is modestly associated with attenuated BMD loss (as measured by %ΔBMD) at the total hip in older men.
Supplementary Material
Acknowledgments
Funding The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health (NIH) funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, UL1 TR000128, U01 AR45580, U01 AR45614, U01 AR45632, AR45647, AR45654, AR45583, and AG18197. Additional funding was provided by NIH/NIAMS grant P50 AR063043.
Footnotes
Compliance with ethical standards
Conflicts of interest None.
Ethical approval All procedures performed in the MrOS study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board at each clinic site, and all participants provided written informed consent. For this type of retrospective analysis, additional formal consent was not required.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00198–018-4388-x) contains supplementary material, which is available to authorized users.
References
- 1.Lappe JM, Heaney RP (2012) Why randomized controlled trials of calcium and vitamin D sometimes fail. Dermatoendocrinol 4(2): 95–100. 10.4161/derm.19833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tucker KL, Chen H, Hannan MT, Cupples LA, Wilson PW, Felson D, Kiel DP (2002) Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr 76(1):245–252 [DOI] [PubMed] [Google Scholar]
- 3.Edwards MH, Jameson K, Denison H, Harvey NC, Sayer AA, Dennison EM, Cooper C (2013) Clinical risk factors, bone density and fall history in the prediction of incident fracture among men and women. Bone 52(2):541–547. https://doi.org/10.1016Zj.bone.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardcastle AC, Aucott L, Fraser WD, Reid DM, Macdonald HM (2011) Dietary patterns, bone resorption and bone mineral density in early post-menopausal Scottish women. Eur J Clin Nutr 65(3): 378–385. 10.1038/ejcn.2010.264 [DOI] [PubMed] [Google Scholar]
- 5.Ward KA, Prentice A, Kuh DL, Adams JE, Ambrosini GL (2016) Life course dietary patterns and bone health in later life in a British birth cohort study. J Bone Miner Res 31(6):1167–1176. 10.1002/jbmr.2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Movassagh EZ, Vatanparast H (2017) Current evidence on the association of dietary patterns and bone health: a scoping review. Adv Nutr 8(1):1–16. 10.3945/an.116.013326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langsetmo L, Barr SI, Dasgupta K, Berger C, Kovacs CS, Josse RG, Adachi JD, Hanley DA, Prior JC, Brown JP, Morin SN, Davison KS, Goltzman D, Kreiger N (2016) Dietary patterns in men and women are simultaneously determinants of altered glucose metabolism and bone metabolism. Nutr Res 36(4):328–336. 10.1016/j.nutres.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 8.Isanejad M, Sirola J, Mursu J, Rikkonen T, Kroger H, Tuppurainen M, Erkkila AT (2017) Association of the Baltic Sea and Mediterranean diets with indices of sarcopenia in elderly women, OSPTRE-FPS study. Eur J Nutr 10.1007/s00394-017-1422-2 [DOI] [PubMed] [Google Scholar]
- 9.Shin S, Sung J, Joung H (2015) A fruit, milk and whole grain dietary pattern is positively associated with bone mineral density in Korean healthy adults. Eur J Clin Nutr 69(4):442–448. 10.1038/ejcn.2014.231 [DOI] [PubMed] [Google Scholar]
- 10.Judd SE, Letter AJ, Shikany JM, Roth DL, Newby PK (2014) Dietary patterns derived using exploratory and confirmatory factor analysis are stable and generalizable across race, region, and gender subgroups in the REGARDS Study. Front Nutrition 1:29 10.3389/fnut.2014.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langsetmo L, Hanley DA, Prior JC, Barr SI, Anastassiades T, Towheed T, Goltzman D, Morin S, Poliquin S, Kreiger N (2011) Dietary patterns and incident low-trauma fractures in postmenopausal women and men aged >/= 50 y: a population-based cohort study Am J Clin Nutr 93(1):192–199. 10.3945/ajcn.110.002956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langsetmo L, Poliquin S, Hanley DA, Prior JC, Barr S, Anastassiades T, Towheed T, Goltzman D, Kreiger N (2010) Dietary patterns in Canadian men and women ages 25 and older: relationship to demographics, body mass index, and bone mineral density. BMC Musculoskelet Disord 11:20 10.1186/1471-2474-11-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shikany JM, Safford MM, Newby PK, Durant RW, Brown TM, Judd SE (2015) Southern dietary pattern is associated with hazard of acute coronary heart disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circulation 132(9):804–814. 10.1161/circulationaha.114.014421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung TT, Feskanich D (2015) Dietary patterns and risk of hip fractures in postmenopausal women and men over 50 years. Osteoporos Int 26(6):1825–1830. 10.1007/s00198-015-3081-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K (2005) Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials 26(5): 569–585. 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 16.Shannon J, Shikany JM, Barrett-Connor E, Marshall LM, Bunker CH, Chan JM, Stone KL, Orwoll E (2007) Demographic factors associated with the diet quality of older US men: baseline data from the Osteoporotic Fractures in Men (MrOS) study. Public Health Nutr 10(8):810–818. 10.1017/s1368980007258604 [DOI] [PubMed] [Google Scholar]
- 17.Shikany JM, Barrett-Connor E, Ensrud KE, Cawthon PM, Lewis CE, Dam TT, Shannon J, Redden DT (2014) Macronutrients, diet quality, and frailty in older men. J Gerontol A Biol Sci Med Sci 69(6):695–701. 10.1093/gerona/glt196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR (2005) Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials 26(5):557–568. 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 19.Block G, Hartman AM, Naughton D (1990) A reduced dietary questionnaire: development and validation. Epidemiology 1(1): 58–64 [DOI] [PubMed] [Google Scholar]
- 20.Langsetmo L, Shikany JM, Burghardt AJ, Cawthon PM, Orwoll ES, Cauley JA, Taylor BC, Schousboe JT, Bauer DC, Vo TN, Ensrud KE (2017) High dairy protein intake is associated with greater bone strength parameters at the distal radius and tibia in older men: a cross-sectional study. Osteoporos Int 29(1):69–77. https://doi.org/10.!007/s00198-017-4261-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P (1994) Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10(4):405–411 [DOI] [PubMed] [Google Scholar]
- 22.Washburn RA, Smith KW, Jette AM, Janney CA (1993) The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 46(2):153–162 [DOI] [PubMed] [Google Scholar]
- 23.Cawthon PM, Ewing SK, Mackey DC, Fink HA, Cummings SR, Ensrud KE, Stefanick ML, Bauer DC, Cauley JA, Orwoll ES (2012) Change in hip bone mineral density and risk of subsequent fractures in older men. J Bone Miner Res 27(10):2179–2188. 10.1002/jbmr.1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings SR, Bates D, Black DM (2002) Clinical use of bone densitometry: scientific review. JAMA 288(15):1889–1897 [DOI] [PubMed] [Google Scholar]
- 25.Shepherd JA, Fan B, Lu Y, Lewiecki EM, Miller P, Genant HK (2006) Comparison of BMD precision for Prodigy and Delphi spine and femur scans. Osteoporos Int 17(9):1303–1308. 10.1007/s00198-006-0127-9 [DOI] [PubMed] [Google Scholar]
- 26.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM (2005) When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol 162(3):267–278. 10.1093/aje/kwi187 [DOI] [PubMed] [Google Scholar]
- 27.Rizzoli R (2014) Dairy products, yogurts, and bone health. Am J Clin Nutr 99(5 Suppl):1256s-1262s. 10.3945/ajcn.113.073056 [DOI] [PubMed] [Google Scholar]
- 28.Liu RH (2003) Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr 78(3 Suppl):517s–520s [DOI] [PubMed] [Google Scholar]
- 29.Sahni S, Cupples LA, McLean RR, Tucker KL, Broe KE, Kiel DP, Hannan MT (2010) Protective effect of high protein and calcium intake on the risk of hip fracture in the Framingham offspring cohort. J Bone Miner Res 25(12):2770–2776. 10.1002/jbmr.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byberg L, Bellavia A, Orsini N, Wolk A, Michaelsson K (2015) Fruit and vegetable intake and risk of hip fracture: a cohort study of Swedish men and women. J Bone Miner Res 30(6):976–984. 10.1002/jbmr.2384 [DOI] [PubMed] [Google Scholar]
- 31.Zeng FF, Wu BH, Fan F, Xie HL, Xue WQ, Zhu HL, Chen YM (2013) Dietary patterns and the risk of hip fractures in elderly Chinese: a matched case-control study. J Clin Endocrinol Metab 98(6):2347–2355. 10.1210/jc.2013-1190 [DOI] [PubMed] [Google Scholar]
- 32.Lemann J Jr, Gray RW, Pleuss JA (1989) Potassium bicarbonate, but not sodium bicarbonate, reduces urinary calcium excretion and improves calcium balance in healthy men. Kidney Int 35(2):688–695 [DOI] [PubMed] [Google Scholar]
- 33.Macdonald HM, New SA, Fraser WD, Campbell MK, Reid DM (2005) Low dietary potassium intakes and high dietary estimates of net endogenous acid production are associated with low bone mineral density in premenopausal women and increased markers of bone resorption in postmenopausal women. Am J Clin Nutr 81(4):923–933 [DOI] [PubMed] [Google Scholar]
- 34.New SA, MacDonald HM, Campbell MK, Martin JC, Garton MJ, Robins SP, Reid DM (2004) Lower estimates of net endogenous non-carbonic acid production are positively associated with indexes of bone health in premenopausal and perimenopausal women. Am J Clin Nutr 79(1): 131–138 [DOI] [PubMed] [Google Scholar]
- 35.Bushinsky DA (1996) Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am J Phys 271(1 Pt 2):F216–F222 [DOI] [PubMed] [Google Scholar]
- 36.Jeffery IB, O’Toole PW (2013) Diet-microbiota interactions and their implications for healthy living. Nutrients 5(1):234–252. 10.3390/nu5010234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heaney RP, Weaver CM, Recker RR (1988) Calcium absorbability from spinach. Am J Clin Nutr 47(4):707–709 [DOI] [PubMed] [Google Scholar]
- 38.Noonan SC, Savage GP (1999) Oxalate content of foods and its effect on humans. Asia Pac J Clin Nutr 8(1):64–74 [PubMed] [Google Scholar]
- 39.Kruger MC, Coetzee M, Haag M, Weiler H (2010) Long-chain polyunsaturated fatty acids: selected mechanisms of action on bone. Prog Lipid Res 49(4):438–449. 10.1016/j.plipres.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 40.Orchard TS, Cauley JA, Frank GC, Neuhouser ML, Robinson JG, Snetselaar L, Tylavsky F, Wactawski-Wende J, Young AM, Lu B, Jackson RD (2010) Fatty acid consumption and risk of fracture in the Women’s Health Initiative. Am J Clin Nutr 92(6):1452–1460. 10.3945/ajcn.2010.29955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farina EK, Kiel DP, Roubenoff R, Schaefer EJ, Cupples LA, Tucker KL (2011) Dietary intakes of arachidonic acid and alpha-linolenic acid are associated with reduced risk of hip fracture in older adults. J Nutr 141(6):1146–1153. 10.3945/jn.110.133728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virtanen JK, Mozaffarian D, Cauley JA, Mukamal KJ, Robbins J, Siscovick DS (2010) Fish consumption, bone mineral density, and risk of hip fracture among older adults: the cardiovascular health study. J Bone Miner Res 25(9):1972–1979. 10.1002/jbmr.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McNaughton SA, Wattanapenpaiboon N, Wark JD, Nowson CA (2011) An energy-dense, nutrient-poor dietary pattern is inversely associated with bone health in women. J Nutr 141(8):1516–1523. 10.3945/jn.111.138271 [DOI] [PubMed] [Google Scholar]
- 44.Monma Y, Niu K, Iwasaki K, Tomita N, Nakaya N, Hozawa A, Kuriyama S, Takayama S, Seki T, Takeda T, Yaegashi N, Ebihara S, Arai H, Nagatomi R, Tsuji I (2010) Dietary patterns associated with fall-related fracture in elderly Japanese: a population based prospective study. BMC Geriatr 10:31 10.1186/1471-2318-10-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT (2013) Why US adults use dietary supplements. JAMA Intern Med 173(5): 355–361. 10.1001/jamainternmed.2013.2299 [DOI] [PubMed] [Google Scholar]
- 46.Peacock M, Liu G, Carey M, McClintock R, Ambrosius W, Hui S, Johnston CC (2000) Effect of calcium or 25OH vitamin D3 dietary supplementation on bone loss at the hip in men and women over the age of 60. J Clin Endocrinol Metab 85(9):3011–3019. 10.1210/jcem.85.9.6836 [DOI] [PubMed] [Google Scholar]
- 47.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP (2000) Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 15(4):710–720. 10.1359/jbmr.2000.15.4.710 [DOI] [PubMed] [Google Scholar]
- 48.Slemenda CW, Christian JC, Reed T, Reister TK, Williams CJ, Johnston CC Jr (1992) Long-term bone loss in men: effects of genetic and environmental factors. Ann Intern Med 117(4):286–291 [DOI] [PubMed] [Google Scholar]
- 49.Burger H, de Laet CE, van Daele PL, Weel AE, Witteman JC, Hofman A, Pols HA (1998) Risk factors for increased bone loss in an elderly population: the Rotterdam Study. Am J Epidemiol 147(9):871–879 [DOI] [PubMed] [Google Scholar]
- 50.Willcox BJ, He Q, Chen R, Yano K, Masaki KH, Grove JS, Donlon TA, Willcox DC, Curb JD (2006) Midlife risk factors and healthy survival in men. JAMA 296(19):2343–2350. 10.1001/jama.296.19.2343 [DOI] [PubMed] [Google Scholar]
- 51.Farhat GN, Strotmeyer ES, Newman AB, Sutton-Tyrrell K, Bauer DC, Harris T, Johnson KC, Taaffe DR, Cauley JA (2006) Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: the health, aging, and body composition study. Calcif Tissue Int 79(2):102–111. 10.1007/s00223-006-0052-0 [DOI] [PubMed] [Google Scholar]
- 52.Antonopoulou M, Bahtiyar G, Baneiji MA, Sacerdote AS (2013) Diabetes and bone health. Maturitas 76(3):253–259. 10.1016/j.maturitas.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 53.Newby PK, Muller D, Hallfrisch J, Andres R, Tucker KL (2004) Food patterns measured by factor analysis and anthropometric changes in adults. Am J Clin Nutr 80(2):504–513 [DOI] [PubMed] [Google Scholar]
- 54.Hannan MT, Mangano KM, Sahni S (2015) Do nutrients influence bone health? A commentary on new findings in the field. J Bone Miner Res 30(6):967–969. 10.1002/jbmr.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.