Abstract
Additive and bidirectional effects of executive control and hypothalamic–pituitary–adrenal (HPA) axis regulation on children’s adjustment were examined, along with the effects of low income and cumulative risk on executive control and the HPA axis. The study utilized longitudinal data from a community sample of preschool age children (N = 306, 36–39 months at Time 1) whose families were recruited to overrepresent low-income contexts. We tested the effects of low income and cumulative risk on levels and growth of executive control and HPA axis regulation (diurnal cortisol level), the bidirectional effects of executive control and the HPA axis on each other, and their additive effects on children’s adjustment problems, social competence and academic readiness. Low income predicted lower Time 4 executive control, and cumulative risk predicted lower Time 4 diurnal cortisol level. There was little evidence of bidirectional effects of executive control and diurnal cortisol. However, both executive control and diurnal cortisol predicted Time 4 adjustment, suggesting additive effects. There were indirect effects of income on all three adjustment outcomes through executive control, and of cumulative risk on adjustment problems and social competence through diurnal cortisol. The results provide evidence that executive control and diurnal cortisol additively predict children’s adjustment and partially account for the effects of income and cumulative risk on adjustment.
Keywords: adjustment, cumulative risk, early childhood executive control, hypothalamic–pituitary–adrenal axis, income
The deleterious effects of economic disadvantage and its associated adversity in early childhood on children’s social, emotional, and academic adjustment are well established (Barbain et al., 2006; Brooks-Gunn & Duncan, 1997; Evans, 2003; Kim, Conger, Elder, & Lorenz, 2003; McLoyd, 1998; Mistry, Vandewater, Huston, & McLoyd, 2002; Petterson & Albers, 2001) and are shown to impact health and mental health well into adulthood (e.g., Duncan, Ziol-Guest, & Kalil, 2010; McEwen, 2012). Disruptions to neurobiological systems underlying self-regulation are a purported core mechanism in the lifelong effects of adverse childhood experiences (McLaughlin, Green, Gruber, Sampson, & Zaslavski, 2012). Two indicators of neurobiologically based, stress-sensitive systems underlying behavioral and emotional regulation were examined in this study: executive control indicating activity in the prefrontal cortex (PFC), and diurnal cortisol indicating regulation of the neuroendocrine stress system (hypothalamic–pituitary–adrenal [HPA] axis). The accumulation of stress early in development may disrupt the development of children’s self-regulatory systems, which are thought to account for the effects of adversity on children’s developmental outcomes (e.g., Gunnar, 2016). However, how disruptions in each of these aspects of self-regulation simultaneously contribute to children’s adjustment or impact each other over time is less well understood. It is possible that there are additive and bidirectional effects of systems of self-regulation, which in turn have implications for children’s adjustment in the face of adversity. This study tested the effects of income and cumulative risk on children’s executive control and diurnal cortisol level, the bidirectional effects of executive control and diurnal cortisol level on each other, and their joint prediction of preschool-age children’s adjustment.
Low Income and Cumulative Risk
Low income is a marker for the potential presence of a number of risk factors, including negative life events, residential instability, family conflict and disorganization, parental mental health problems, and many other factors that often co-occur and have cumulative effects on children’s adjustment (Ackerman, Brown, & Izard, 2004; Evans, 2003; Linver, Brooks-Gunn, & Kohen, 2002; Mistry et al., 2002), predicting children’s academic achievement, social competencies, and externalizing and internalizing problems, among other developmental outcomes (Evans, Li, & Whipple, 2013). Not all children in contexts of low family income experience multiple risk factors, and most risk factors are not specific to low-income contexts (e.g., negative life events, marital status transitions, family conflict, and parental mental health problems). However, there is a higher likelihood of the presence of multiple risk factors when families live in a low-income context. In particular, cumulative risk characterizes the burden of risk that may be associated with low income and poverty, and that stress and adversity associated with experiencing multiple risk factors is expected to play a role in children’s adjustment beyond the effects of any one individual risk factor.
Evidence suggests that there are both direct and indirect effects of income on child adjustment (e.g., Linver et al., 2002). Consistent with a family stress model (Conger & Elder, 1994), children’s experiences of family risk factors and the burden of stress associated with the accumulation of risk factors might account for the effects of low income on children’s self-regulation (Raver, Blair, & Willoughby, 2013). A growing body of evidence indicates that the accumulation of poverty-related risk factors partially accounts for or mediates the effects of poverty on children’s behavioral and neurobiological indicators of self-regulation (Buckner, Mezzacappa, & Beardslee, 2003; Evans & English, 2002; Evans et al., 2013), and specifically with lower effortful or executive control (Hughes & Ensor, 2009; Lengua, Honirado, & Bush, 2007; Lengua et al., 2014; Mistry, Benner, Biesanz, Clark, & Howes, 2010; Raver et al., 2013; Sektnan, McClelland, Acock, & Morrison, 2010) and disrupted HPA axis regulation (Zalewski et al., 2012).
It is possible that lack of resources related to low income might have a different effect on children’s self-regulation than the adversity associated with the presence of multiple family and contextual risk factors that might increase levels of stress, chaos, and conflict. Thus, low income and cumulative risk might have differential effects on neurobiological systems underlying self-regulation. As an example, it is suggested that experiences of deprivation might impact different neural processes, those related to language, executive function, and memory, than experiences of threat, which impact salience processing systems associated with the amygdala (McLaughlin, Sheridan, & Lambert, 2014). In a similar manner, it is possible that low income, when controlling for cumulative risk, might index the experience of the absence of resources and uncertainty about meeting basic needs, whereas an index of cumulative risk, when controlling for income, might indicate family stress and adversity. Further, income and cumulative risk may have distinct associations with potential mediators such as parenting. For example, low income was associated with less scaffolding and consistent limit setting, that is, control aspects of parenting, whereas, cumulative risk was associated with greater parental negativity, that is, an affective aspect of parenting (Lengua et al., 2014). For these reasons, the unique effects of income and cumulative risk on children’s executive control and diurnal cortisol levels were tested in this study to test the possibility of unique pathways from low income and cumulative risk to children’s adjustment outcomes.
Executive Control and HPA Axis Indicators of Self-Regulation
Self-regulation is a broad term encompassing constructs including behavioral control, emotion regulation, executive functioning, and attentional processes, and can be assessed at multiple levels including behavioral styles, objective task performance, and neurophysiological responses. In children, self-regulation has been defined as biobehavioral systems that enable control of attention and emotional arousal (Blair, 2010) composed of behavioral, cognitive, and physiological components that are reciprocally integrated, including top-down (executive and other cognitive) and bottom-up (stress and emotion physiology) processes that influence behavior (Blair & Raver, 2012). In this study, we focus on top-down (executive control) and bottom-up (HPA axis) stress-sensitive neurobiological indicators of self-regulation that are relevant for predicting children’s responses to socioeconomic and contextual adversity.
Diurnal cortisol, contextual risk, and adjustment
Diurnal cortisol is an indicator of the regulation of the HPA axis system. The end product of the system is cortisol, which is a useful, noninvasive marker of the system’s activity. Cortisol follows a diurnal rhythm, with levels reaching their peak about 30 min after awakening and decreasing throughout the day (Kirschbaum et al., 1990). Well-regulated HPA axis activity can be indicated by diurnal cortisol patterns characterized by higher morning levels and a steep declining slope across the day. In general, a higher morning cortisol level is thought to be essential for mobilizing metabolic and cognitive processes (e.g., de Kloet, 1991). Similarly, a strong diurnal rhythm (high morning levels that decrease to low evening levels) is considered normative, and a weak or absent diurnal rhythm is considered a sign of wear and tear on the system (Ross, Murphy, Adam, Chen, & Miller, 2014). Together, low morning levels and flat diurnal slopes, a pattern sometimes referred to as a blunted pattern, suggest a dysregulated HPA axis system (e.g., Adam & Gunnar, 2001). Although these indicators are often examined separately, together they may provide a more robust indicator of regulation of the system in that the low, flat diurnal cortisol pattern is more completely characterized. That is, in the current study, the diurnal slope is calculated by subtracting the evening level from the morning level so that a higher value reflects a steeper slope. Combining morning level and diurnal slope might provide a more complete picture of the system’s regulation with the highest scores indicating both a higher morning level and a steeper slope, whereas the lowest scores indicate low morning levels that remain low through the day.
Stress, particularly severe or chronic adversity, can disrupt diurnal patterns or levels of cortisol (e.g., Miller, Chen, & Zhou, 2007). A meta-analysis showed that acute stress is associated with elevations in cortisol, whereas chronic stress is related to blunted levels in adults (Miller et al., 2007), and other research indicates that low income is most consistently related to low or blunted diurnal cortisol in adults (Dowd, Simanek, & Aiello, 2009). In children, low income and cumulative risk or stress have been found to relate to both elevated cortisol levels (Blair et al., 2011; Laurent et al., 2013) and lower or blunted levels (Badanes, Watamura, & Hankin, 2011; Fernald, Burke, & Gunnar, 2008; Zalewski, Lengua, Fisher, et al., 2012). These inconsistent findings may be due to age or methodological differences across studies (see Zalewski, Lwngua, Kiff, & Fisher, 2012) or potential nonlinear effects (e.g., Bush, Obradović, Adler, & Boyce, 2011, Gustafsson, Ankarsäter, Lichtenstein, Nelson, & Gustafsson, 2010, Marsman et al., 2012). However, a blunted diurnal cortisol pattern, characterized by low morning levels and a flat diurnal slope, is most likely to emerge in relation to exposure to pervasive, chronic adversity (Gunnar & Vazquez, 2001). The regulation of this system, in turn, relates to children’s adjustment. There is evidence that higher cortisol is related to lower social competence (Gunnar, Tout, de Haan, Pierce, & Stansbury, 1997), higher internalizing (Ashman, Dawson, Panagiotides, Yamada, & Wilkson, 2002) and externalizing problems (Gunnar, Sebanc, Tout, Donzella, & van Dulmen, 2003). There is also evidence that blunted (lower level and diurnal slope) cortisol is related to higher adjustment problems (Alink et al., 2008). In sum, we expect low income and cumulative risk to be associated with lower, flat (or blunted) levels of cortisol, consistent with evidence regarding chronic, pervasive stress, and in turn, blunted cortisol levels will predict children’s adjustment problems, partially accounting for the effects of low income and cumulative risk.
Executive control, contextual risk, and adjustment
Executive control includes attention regulation, inhibitory control, and flexible set shifting, which are core aspects of executive function (Best & Miller, 2010). Executive function is a broader construct that includes additional components including working memory, planning, and problem solving that were not assessed in the current study. Executive control is a robust predictor of lower externalizing (Hughes & Ensor, 2009; Lengua, 2003) and internalizing problems (Kiff, Lengua, & Bush, 2011; Muris, van der Pennen, Sigmond, & Mayer, 2008), and of higher academic (Blair & Razza, 2007; Obradovic et al., 2009) and social–emotional competence (e.g., Eisenberg et al., 2003; Lengua et al., 2014; Raver, Blackburn, Bancroft, & Torp, 1999). Executive control reflects activity in the PFC, which has been identified as highly sensitive to the effects of stress (Arnsten, 2009), with evidence of structural changes in the PFC after just a week of stress exposure (Brown, Henning, & Wellman, 2005). These changes can be understood through the structural and functional connections with stress-response systems, and evidence of plasticity of the PFC has led to increased focus on the role of experience in shaping executive control. Low income and cumulative risk are related to lower executive control (e.g., Li-Grining, 2007; Mezzacappa, 2004), and in previous analyses with this sample, executive control mediated the effects of low income on children’s adjustment (Lengua et al., 2014).
Bidirectional effects of diurnal cortisol and executive control
There is a call for research that simultaneously examines multiple indicators of self-regulation or stress responses (Quas et al., 2014), which is critical as dysregulation in one system may have cascading effects on other systems. Although prior studies have examined associations between cortisol levels and executive control, few studies have examined longitudinal relations across self-regulation indicators. For example, elevated infant cortisol predicted lower preschool executive function (Blair et al., 2011), whereas in our data lower morning cortisol at 36 months prospectively predicted lower executive control (Lengua, Zalewski, Fisher, & Moran, 2013). More important, few if any studies have examined bidirectional effects across multiple indicators of self-regulation. As mentioned above, self-regulation consists of both top-down, effortful (executive) processes and bottom-up, automatic stress physiology processes that might be mutually influential. In particular, the medial PFC plays a role in both the stimulation and the inhibition of the HPA axis (Ulrich-Lai & Herman, 2009) in a negative feedback circuit that is mediated through the binding of cortisol to glucocorticoid receptors in the PFC, which is rich in such receptors (Furay, Bruestle, & Herman, 2008; Ulrich-Lai & Herman, 2009). While in the short term, PFC activity might operate to lower cortisol levels in response to acute stress, in a context characterized by chronic or pervasive stress, prolonged activation of the stress response system would likely lead to a downregulation or blunting of HPA axis activity. An individual with higher or more efficient executive control might be better able to manage attention and emotions in a way that facilitates less stress arousal, and hence, have a lower likelihood of blunting of the HPA axis system over time. This process illustrates “top-down” regulation, with cortisol mediating the effect of the PFC on other areas involved in stress reactivity, including the amygdala and hippocampus. However, we are not aware of any previous study examining executive control as a predictor of changes in diurnal cortisol levels over time. Thus, the current study may elucidate important developmental mechanisms in which exposure to chronic adversity and stressors shapes children’s developing top-down regulatory capacities, which in turn, may alter HPA axis regulation.
Conversely, it has been proposed that prolonged exposure to abnormal levels of cortisol reduces top-down HPA inhibition by the PFC, thereby facilitating excessive amygdala activity (Liberzon et al., 2007; Reser, 2016). The HPA axis, through glucocorticoid secretion, affects activity in the PFC (Cerqueira, Mailliet, Almeida, Jay, & Sousa, 2007). Moreover, there are direct neural connections between the PFC and other areas of the brain involved in stress reactivity, including the amygdala and hippocampus. Thus, there may be both structural and functional mechanisms underlying the association of HPA axis dysregulation and deficits in executive control over time. For example, it has been shown that chronic stress results in decreased synaptic density or dendritic loss in the PFC, while also promoting dendritic expansion in the amygdala (McEwen, 2012; Reser, 2016). This may be associated with a weakening of abilities centralized in the PFC while strengthening structures that coordinate stress reactivity, potentially resulting in a predominant bottom-up pattern of reactivity in the context of chronic stress (Arnsten, 2009; Veer et al., 2012). In most studies examining associations between cortisol and executive control, cortisol is examined as a predictor of executive control (Blair et al., 2011; Davis, Bruce, & Gunnar, 2002). It follows that dysregulation of the HPA axis due to adversity might contribute to the lower levels of executive control and adjustment observed among children. In this study, we go beyond these unidirectional tests to examine possible bidirectional effects of diurnal cortisol levels and executive control, each predicting changes in the other, in an attempt to map the unfolding of dysregulation in multiple indicators. We know little about how changes in self-regulation systems might shape each other over time, and such bidirectional effects between indicators of self-regulation in the contexts of low income and adversity would suggest that disruptions in these systems might have cascading effects on each other.
This Study
This study tested the effects of income and cumulative risk on executive control and HPA axis regulation and the bidirectional effects of executive control and HPA axis regulation over time. We tested the possibility that disruptions in each indicator of self-regulation would prospectively predict changes in the other above the effects of income and cumulative risk. Further, we examined the additive effects of executive control and HPA axis regulation on children’s social–emotional and academic adjustment in preschool. Rarely have executive control and the HPA axis been examined simultaneously in a longitudinal, developmental study, particularly during preschool, when executive control capacities are developing markedly. We hypothesized that (a) low income and cumulative risk would predict lower executive control and lower diurnal cortisol levels, (b) lower diurnal cortisol level would predict lower executive control over time, (c) lower executive control would predict lower diurnal cortisol level over time, (d) executive control and diurnal cortisol level would additively predict children’s adjustment problems, social–emotional competence, and academic readiness, and (e) income and cumulative risk would have indirect effects on children’s adjustment through executive control and diurnal cortisol level.
Method
Participants
Participants were 306 mothers and their 36- to 40-month-old children (M = 37, SD = 0.84) who were recruited from a university hospital birth register, daycares, preschools, health clinics, and charitable agencies. Families at these sites received information forms about the study and could indicate their interest in participating in the study on the forms. Recruitment sites, other than the birth register, received an honorarium of $100 when 90% or more of their families returned forms, regardless of the number of families indicating interest in participating. If a site returned 75% or 50% of the forms, the site received $75 or $50, respectively.
Families were recruited to obtain equal representation across income levels. The 2009/2010 Federal HHS Poverty Guidelines (US Department of Health & Human Services, 2010), in place at Time 1 (T1), which is an income-to-needs ratio based on the family’s income from all sources and the number of people in the home, was used to recruit families and to describe the income levels represented in the sample. The distribution included 29% of the sample at or near poverty (N = 90 at or below 150% of the federal poverty threshold), 28% lower income (N = 84 between 150% poverty and the local median income of $58,000), 25% middle income (N = 77 between the median income and $100,000), and 18% upper income (N = 54 above $100,000). To participate, families were required to have reasonable proficiency in English (self-determined) to comprehend the assessment procedures, and children diagnosed with a developmental disability were excluded. Participants included 50% girls. The racial and ethnic composition of the sample of children included 64% European American, 10% Latino or Hispanic, 9% African American, 3% Asian American, 2% Native or American Indian, and 12% multiple racial and ethnic backgrounds. Mothers’ educational distribution included 3% mothers with some high school attainment; 6% with high school degree; 34% with some college, technical school, or professional school; 30% college graduates; and 27% with postgraduate education. Eighty-one percent of mothers were married or had stable live-in partners, 12% were never married, and 7% were separated, divorced, or widowed and were single heads-of-household.
Substantial efforts were employed to minimize study attrition, including arranging assessments at the convenience of participants, providing transportation and childcare as needed, conducting home assessments when barriers to lab visits existed, completing phone assessments when families moved out of the state, and providing research assistants with extensive training so that families experienced a respectful, skillful research team. Analyses suggested that minimal bias was introduced as a result of missing data. Complete data were available on 222 cases (73%), with 53 cases missing one variable (17%), 13 cases missing two variables (4%), and 18 cases (6%) missing three or more. All participants had complete T1 income and cumulative risk data. Complete executive control data were available for 88% of participants at T1, 95% at Time 2 (T2), 94% at Time 3 (T3), and 94% at Time 4 (T4). Cortisol data were available for 89% of participants at T1, 86% of participants at T2, 88% of participants at T3, and 85% of participants at T4. T4 Teacher reports of child adjustment were available for 77% of participants. Missingness was related to lower income, higher cumulative risk, and lower executive control. However, the effect sizes of the associations of missingness were modest, average r = .19, range = .01–.28, and did not reach suggested thresholds for introducing substantial bias (i.e., r > .40; Collins, Schafer, & Kam, 2001). Thus, all analyses were conducted using missing data estimation and were based on the complete sample of 306.
Procedures
Families were assessed in offices on a university campus. They were assessed at four time points separated by 9 months each when children were 36–40, 45–49, 54–58, and 63–67 months. With approval by the Human Subjects Institutional Review Board at the University of Washington (Approval #32596, “Low Income, Family Disruption, and the Development of Effortful Control”), both active parental consent and child assent were secured prior to data collection. Assessments included behavioral, neuropsychological, and questionnaire measures administered by trained experimenters. Children completed neuropsychological and behavioral measures of executive control while mothers completed questionnaire measures in a separate room. Families received $70 for their first assessment and compensation increased by $20 for each of the three subsequent assessments. With parental consent, children’s teachers were mailed a questionnaire and asked to complete measures about children’s adjustment once they had the children in their classrooms for at least a month. Teachers received $15 for returning the questionnaires.”
Across all four time points, mothers were trained in the collection of child cortisol and were given a home-collection kit and instructions to collect the saliva samples at home. Specifically, mothers were instructed to collect their child’s saliva 30 min after the child woke in the morning and 30 min prior to bedtime, for 3 consecutive days. Mothers were to place a sorbette (Salimetrics, LLC State College, PA) under the child’s tongue for 1 min and then place the sorbettes into a prelabeled swab storage tube. Mothers repeated this process with another sorbette to ensure adequate saliva volume. A staff member called families on the first night to ensure proper collection and answer questions. A reminder call was placed on the third evening to prompt mothers to return the packets via the mail. Mailing saliva has been shown to have no influence on saliva collection (Clements & Parker, 1998), and this method has been successfully used in childhood samples (Bruce, Davis, & Gunnar, 2002). Parents were paid an additional $30 for all cortisol packets returned. Families were invited to collect cortisol regardless of their ability to attend the laboratory visit at that time point.
Measures
Income
Mothers reported on household income from all sources on a 14-point Likert scale that provided a fine-grained breakdown of income at the lower levels facilitating identification of families at the federal poverty cutoff using an income-to-needs ratio (e.g., 1 = $14,570 or less, 2 = $14,571–$18,310, 3 = $18,311–$22,050, etc.). Families were recruited into the study to equally represent the full range of income, and as a result, family income and the income-to-needs ratio were highly correlated (r = .92). Therefore, the 14-point variable representing the full range of income was used for analyses (M = 8.75 [≈$38,000–$39,000], SD = 3.93, Range = 1.00 [$14,570 or less]–14.00 [above $150,000]. Correlations among T1–T4 income ranged from .80 to .88. Given the high stability in income, only T1 income was analyzed.
Cumulative risk
Cumulative risk was assessed at T1 and included eight risk factors: adolescent parent, low education, single parent, residential instability, family structure transitions, household density, negative events, and maternal depression, which represent risk factors commonly included in cumulative risk indices. There are numerous approaches to calculating a cumulative risk index, including efforts to avoid artificially dichotomizing continuous variables (Evans et al., 2013). Dichotomous risk factors (adolescent parent, education, single parent, residential instability, and divorce) were scored as 0 = not present, 1 = present. Continuous risk factor scores (household density, negative events, and depression) were converted into proportions of the total possible score so that each score ranged from 0 to 1, and thus, were on a similar scale as the dichotomous variables. This was done to avoid arbitrary selection of a cutoff score and the artificial categorization of a continuous variable for which there are not well-established cutoffs for risk. Using this approach compared to dichotomizing the variables to create the cumulative risk score had little or no impact on the associations of cumulative risk with other variables as the rank order of participants’ scores was maintained across scoring approaches. The total cumulative risk score was the sum of all component factors.
Mothers reported their age at the time of the study child’s birth, and 3% were adolescent parents (≤19 years) when the child was born. Mothers reported on their education. Risk was indicated by mothers’ not graduating from high school (3% of the sample at T1). Mothers reported on marital status and were identified as single parents if they indicated being never married; currently widowed, separated, or divorced; or having a live-in partner for <1 year (19% at T1). Residential instability was indicated by the family changing households more than three times in the previous 3 years at T1 (10%). Family structure transitions were indicated by mothers reporting being divorced in the child’s lifetime at T1 (3%). Household density was calculated as the number of individuals living in the home divided by the number of rooms in the home. At T1 the ratio ranged from .18 to 1.75, with a mean of .52, suggesting that on average, there were twice as many rooms as individuals in the home. The score was converted to a proportion of the highest score in the sample.
Negative life events were assessed with parent report on the General Life Events Schedule for Children (Sandler, Ramirez, & Reynolds, 1986). The 29 events include moderate to major negative events, including changing schools, death of a family member or friend, parental arrest, and loss of friends. Parents reported whether events occurred in the previous 9 months, and total scores were the number of events. The average number of life events at T1 was 5.3, SD = 4.0, range 0–26. The total score was converted into a proportion of the possible 29 events.
Mothers reported on their depressive symptoms over the previous month using the 20-item Center for Epidemiological Studies–Depression Scale (Radloff, 1977), designed to measure depressive symptoms in the general population. Participants indicated whether each symptom was present on a scale of 0 (rarely/never) to 3 (most of the time), and the items were summed for a total score. Internal consistency was 0.88. The T1 average score was 10.01, SD = 8.38, range 0–46.67. The total score was converted into a proportion of the total possible score of 60. Correlations among T1–T4 cumulative risk scores ranged from .60 to .79. Given the high stability in cumulative risk, only the T1 score was analyzed.
Given how cumulative risk is scored in this study, that is, using proportion scores for continuous variables, and excluding income so that it could be examined separately, the scores do not provide a clear indication of the level of risk in the sample. For descriptive purposes, we also calculated the number of risk factors present using 1.5 SD cutoffs for continuous scores. This cumulative risk score using this alternate scoring was correlated .92 with the variable used in the present study. Further, when including poverty status in the risk count, the distribution of risk in our sample is nearly identical to that reported for children growing up in low income (<200% of the federal poverty threshold) by the National Center for Children in Poverty for 2014 (consistent with those cited for 2010 in Evans et al., 2013). That is, 39% have no risk factors, 46% have one or two risk factors and 16% have three or more risk factors.
Executive control
Executive control was assessed at all four times with identical measures of attention, inhibitory control, and flexible set shifting. We use the term executive control to reflect these core aspects of executive function, while acknowledging that we have not assessed other aspects of executive function such as working memory, planning, and problem solving. Modeled after traditional cognitive tests, measures were selected to be of varying difficulty for children across childhood so that identical measures could be used over time. Although some of the measures were normed for children older than those in the sample, there was variability in performance even at early time points. Conversely, some measures were developed for younger children, and as a result showed less variability at the later time points. Averaging across these test scores resulted in adequate variability at each time point. Proportion scores were used so that scores were on a comparable scale. Descriptive and psychometric statistics for each task at each time point are reported elsewhere (Lengua et al., 2013; 2014).
Executive control was assessed using six tasks. The inhibition and auditory attention subscales of the NEPSY developmental neuropsychological assessment battery (Korkman, Kirk, & Kemp, 1998) were administered. The inhibition subtest assesses the ability to inhibit a dominant response to enact a novel response. Children are shown an array of circles and squares and asked to label each shape in an opposite manner (e.g., say “circle” when shown a square). Auditory attention is a continuous performance test that assesses the ability to be vigilant and to maintain and shift selective sets. Children are required to listen to a series of words and respond only when they hear a target word, refraining from responses to other words. Scores on the inhibition and auditory attention subscales were the proportion of correct responses.
Behavioral inhibitory control was assessed using Bear-Dragon (an appealing monkey puppet was substituted in this study; Kochanska, Murray, Jacques, Koenig, & Vandegeest, 1996), which requires the child to perform actions when the directive is given by the monkey puppet, but not when given by a dragon puppet. Children’s actions during monkey trials were scored as performing no movement (0), wrong movement (1), partial movement (2), or complete movement (3); behaviors for dragon trials were scored in the reverse of monkey trials, such that a no movement was scored a 3 and a complete movement was scored 0. Trial scores were summed across both monkey and dragon trials (10 trials), and the total scores were converted into the proportion of the sum of trials to the total possible score.
Cognitive inhibitory control was assessed using Day-Night (Gerstadt, Hong, & Diamond, 1994), which requires the child to say “day” when shown a picture of moon/stars and “night” when shown a picture of the sun. Responses were scored 1 = correct nondominant response or 0 = dominant response. Total scores were the proportion of correct responses out of 16 trials.
The Dimensional Change Card Sort (Zelazo, Muller, Frye, & Marcovitch, 2003) assesses working memory, cognitive inhibitory control, attention focusing, and set shifting. In this task, children were introduced to two boxes with slots in the top. Target cards were attached to the front of each box. The target cards included a silhouetted figure on a colored background (star on blue, truck on red). Children were instructed to sort cards first according to shape (6 trials) then according to color (6 trials). The experimenter stated sorting rules before each trial and presented a card labeled according to the current dimension (e.g., on a shape trial, “Here’s a truck. Where does it go?”). Children advanced to the next level in which the target cards integrated the sorting properties. Target cards consisted of a colored figure on a white background (blue star, red truck), and children were again instructed to sort according to shape (6 trials), then color (6 trials). If they correctly sorted >75% of the cards, children advanced to the next level in which they were instructed to sort by color if the card had a border on it and by shape if the card lacked the border (12 trials). The score was the proportion of correct trials out of the total 36 possible trials.
Head–Toes–Knees–Shoulders integrates attention and inhibitory control (Ponitz et al., 2008). Children are asked to follow the experimenter’s instructions, but to enact the opposite of the direction (e.g., touch toes when asked to touch head). Behaviors were coded as 0 = touched the directed body part, 1 = self-corrected, or 2 = correctly touched the opposite. Scores were the proportion of the sum of the item scores across 20 trials to the total possible score.
An executive control score was computed at each time point as the mean of the proportion scores of the individual tasks and was considered missing if ≥50% of the component scores were missing (intraclass correlation = 0.83, T1 α = 0.67, T2 α = 0.70, T3 α = 0.74, T4 α = 0.70).
Diurnal cortisol
Details of salivary cortisol sample collection and processing are reported elsewhere (Zalewski, Lengua, Kiff, & Fisher, 2012; Zalewski, Lengua, Thompson, & Kiff, 2015), and summarized briefly here. Saliva samples were assayed using the High-Sensitivity Cortisol Salivary Enzyme Immunoassay Kit provided by Salimetrics LLC (State College, PA). The sensitivity of this kit ranges from .005 ug/dL to 2.5 ug/dL. All samples from the same subject for each set of saliva were included in the same assay batch to minimize inter-assay within-subject variability. Each time point was assayed after all cortisol had been collected. At T1, the intra-assay cortisol value (CV) = 3.98% and the inter-assay CV = 2.78%; T2 intra-assay CV = 3.82% and inter-assay CV = 4.9%; T3 intra-assay CV = 3.35% and inter-assay CV = 4.15%; and T4 intra-assay CV = 3.73% and inter-assay CV = 4.0%, all acceptable values. Assay results that were over 2.0 ug/dl were deemed biologically implausible and the values were not used, consistent with methods used in other studies (Ashman et al., 2002). Values in samples that had been collected 90 min after wake-up or prior to bedtime were also discarded. Within each time point, the associations of raw morning and evening values were examined to determine if it was appropriate to average across days, as has been done in other studies to create a more reliable measure (Bruce, Fisher, Pears, & Levine, 2009). For morning levels within a time point, cortisol values were all significantly correlated, with an average r = .35 (14–.48, all p < .05). For evening levels, all but two associations were significant, with an average r = .26 (.08–.56). As such, at each time point, cortisol levels refer to the average across the 3 days of sampling.
Average morning collection times ranged between 8 a.m. to 8:12 a.m. across T1–T4, respectively (SDrange in collection times = 56–60 min). Samples that had been collected 90 min after wake-up or prior to bedtime were discarded, with 74 samples excluded, where a sample represents a single instance of sampling on 1 day of any of the 3 days across the four time points. Average latencies to collect these morning samples ranged from 27 to 40 min after awakening across T1–T4 (SDrange = 10–22 min). Average evening collection times were 8:19 p.m. to 8:27 p.m. across T1–T4 (SDrange in collection times = 59–72 min). On average, these samples were collected between 31 and 49 min before bed across T1–T4 (SDrange = 12–19 min). In prior analyses with this sample, latency to collect from waking or evening collection times were largely uncorrelated with cortisol levels and had little or no impact on cortisol associations with other variables (Zalewski et al., 2015). As such, they were not included in the current analyses given the complexity of the models being tested. As is common with cortisol data, values were positively skewed, and log transformations were applied to average morning variables. All data used in analyses were conducted with log-transformed values. Diurnal slope was calculated as the average evening level subtracted from the average morning level, such that higher scores reflect a steeper diurnal slope. A composite of morning level and diurnal slope was created to provide a more robust measure of a regulated diurnal cortisol pattern. For both morning level and diurnal slope, a higher value suggests a better regulated system, and correlations of morning level with diurnal slope ranged from .37 to .51 across all study time points. In addition, their correlations with other study variables were predominantly in the same direction and with similar magnitude, suggesting that they could be combined to index a regulated cortisol pattern. The diurnal cortisol variable was computed as the average of morning level and diurnal slope values, with higher scores indicating a more regulated pattern.
Use of steroid medications or inhaler, health, and food intake have been shown to affect cortisol levels. Mothers completed a daily questionnaire regarding children’s wake-up and bed times, sampling times, and their children’s health, medication use, and eating times on sampling days. Variables indicating the time of sampling and the latency from children’s wake-up time to morning collection and from evening collection to bedtime were calculated from mother’s reports. The questionnaires were reviewed to ensure compliance. In addition, mothers were given a phone call on the first evening of collection to review the collection procedures and answer any questions. Mothers were reminded to avoid sampling when their children were using steroid-based medications or were ill. Mothers were mailed additional materials if they accidently sampled when the child was ill. Prior analyses indicated that these factors had little or no impact on the association of cortisol with other study variables, and were therefore not included further in this study.
Child adjustment
At T4, teachers rated children’s academic readiness, social competence, and total adjustment problems. Teachers rated children’s academic readiness using the School Readiness Survey (O’Donnell, 2008) in which teachers report on 9 items indicating children’s ability to identify colors and letters, count, write their names, hold a pencil correctly, produce intelligible speech, and recognize letter sounds. Items were aggregated as the mean-weighted sum (average of the items present multiplied by the total number of items) and the α was 0.71. Teachers rated children’s social competence and total problems using the preschool teacher-report form of the Social Skills Rating System (Gresham & Elliot, 1990). Teachers rated children’s cooperation (e.g., puts away toys and helps with tasks; 12 items), assertiveness (e.g., self-confident and introduces self; 8 items), and self-control (e.g., controls temper and attends to instructions; 10 items) for a social competence score (30 items). Teachers rated children’s externalizing problems (7 items), internalizing problems (6 items), and hyperactivity (6 items) for a total adjustment problems score (19 items). Scores were the mean-weighted sum of the items, and αs for the composite Social Skills Rating System scales were 0.91 for social competence and 0.87 for total adjustment problems.
Results
Analytic plan
Parallel process and autoregressive latent trajectory modeling were used to examine potential additive and bidirectional effects of executive control and HPA axis regulation, and models were tested in Mplus 6.0 (Muthén & Muthén, 2010) using full information maximum likelihood estimation to address missing data. The overall low level of missing data and modest effect sizes of missingness suggested that the data set would be robust to the assumptions of full information maximum likelihood estimation to generate unbiased parameter estimates (Enders & Bandalos, 2001). Therefore, analyses included all 306 families. First, simultaneous growth models were tested for their effects on the outcomes. In these models, executive control and diurnal cortisol growth factors were conditioned on child gender, T1 income, and T1 cumulative risk and were examined as predictors of T4 adjustment problems, social competence, and academic readiness. Second, autoregressive and cross-lagged paths and correlated residuals were added to the model to determine improvement in model fit and to test potential time-specific bidirectional effects above the between-person variance captured in the intercept and slope growth factors. Latent intercept factors were specified to indicate T4 levels of executive control and diurnal cortisol, which were tested as predictors of T4 adjustment problems, social competence, and academic readiness. Third, possible curvilinear effects of cortisol level on child adjustment were tested given prior evidence that both high and low levels of cortisol were related to adjustment problems. Fourth, indirect effects of income and cumulative risk on outcomes were tested to determine whether executive control and diurnal cortisol accounted for the effects of income and cumulative risk on children’s adjustment.
Preliminary analyses
Descriptive statistics and correlations among the study variables are reported in Table 1. Average level of executive control increased across the time points, whereas average diurnal cortisol levels remained relatively consistent. Lower income was related to lower levels of executive control at all time points, and to lower levels of diurnal cortisol only at T4. Cumulative risk was related to lower executive control and lower diurnal cortisol at most time points. Both lower income and higher cumulative risk were associated with lower academic readiness and social competence and higher total problems at T4. Correlations between higher executive control and higher diurnal cortisol levels were significant at some time points. The pattern of correlations suggested that the hypothesized model was plausible.
Table 1.
Descriptive statistics and correlations among study variables
| M | SD | INC | T1 | T1 | T2 | T3 | T4 | T1 | T2 | T3 | T4 | Academic readiness | Social comp. | Total problems | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Child gendera,b | – | – | −.06 | −.01 | −.09 | −.13* | −.11* | −.10 | −.04 | −.06 | −.04 | −.13* | −.10 | −.19* | .18* |
| Family income | 8.75 | 3.93 | – | −.59* | .19* | .24* | .24* | .24* | .11 | .00 | .11 | .19* | .22* | .17* | −.27* |
| CR T1 | 0.90 | 0.81 | – | −.16* | −.21* | −.25* | −.21* | −.16* | −.06 | −.14* | −.25* | −.22* | −.21* | .27* | |
| EC T1 | 0.29 | 0.15 | – | .50* | .53* | .42* | .00 | .13* | .05 | .10 | .29* | .18* | −.10 | ||
| EC T2 | 0.49 | 0.20 | – | .61* | .48* | .04 | .05 | .05 | .18* | .41* | .21* | −.22* | |||
| EC T3 | 0.68 | 0.17 | – | .65* | .05 | .14* | .08 | .15* | .52* | .25* | −.24* | ||||
| EC T4 | 0.79 | 0.15 | – | .03 | .15* | .14* | .18* | .54* | .30* | −.25* | |||||
| DC T1 | 0.08 | 0.27 | – | .16* | .16* | .27* | .18* | .10 | −.08 | ||||||
| DC T2 | 0.04 | 0.27 | – | .17* | .24* | .20* | .12 | −.05 | |||||||
| DC T3 | 0.00 | 0.28 | – | .26* | .12 | .05 | −.09 | ||||||||
| DC T4 | 0.04 | 0.37 | – | .10 | .15* | −.18* |
Note:
Child gender coded 0 = girl, 1 = boy.
Point biserial correlations are reported for dichotomous variables.
INC, income. T1–T4, Time 1–Time 4. CR, cumulative risk. EC, executive control. DC, diurnal cortisol.
p < .05.
Test of the effects of income and cumulative risk on executive control and diurnal cortisol, their bidirectional effects, and prediction of adjustment
A series of nested models was examined to test the extent to which income and cumulative risk predicted the growth factors (intercept and slope) of executive control and diurnal cortisol, whether there were bidirectional effects between these variables, and whether ultimate levels of executive control and diurnal cortisol predicted adjustment.
Unconditional executive control and diurnal cortisol growth models
Unconditional growth models of executive control and diurnal cortisol were tested first to identify models that reflected the form of growth of each variable. These were specified with the intercept reflecting T4 levels, and linear growth factors specified by the T1–T4 indicators. A linear growth model provided an acceptable fit for executive control, χ2 (5) = 25.97, root mean square error of approximation (RMSEA) = .08, comparative fit index (CFI) = .95. Executive control demonstrated an intercept significantly different than 0 (0.79, SE = 0.01, p < .001) with significant variance (0.02, SE = 0.002, p < .001), and a significant slope (0.10, SE = 0.002, p < .001) and significant variance in the slope (0.001, SE < 0.001, p < .001), indicating individual differences in rates of growth. A linear growth model fit the diurnal cortisol data poorly, χ2 (5) = 21.83, RMSEA = .11, CFI = .68, as would be expected given the observed mean values at each time point. A model that included a quadratic growth term also fit the data poorly, χ2 (1) = 10.99, RMSEA = .19, CFI = .81. Empirically based growth factor loadings were specified to represent the values of the observed means at each time point relative to the last time point while modeling unidirectional change (i.e., −1, −0.25, −0.2, 0) which resulted in an adequate fit to the data, χ2 (5) = 8.53, RMSEA = .05, CFI = .93. The intercept was significantly different than 0 (0.04, SE = 0.015, p = .008) with significant variance (0.023, SE = 0.007, p = .001). The average slope was significantly different than 0 (0.12, SE = 0.021, p < .001), with a trend toward significant variance in slope values (0.06, SE = 0.034, p = .07). Although the variance of the slope factor was not significant, we tested predictors of the cortisol slope as the additional degrees of freedom in these models and clarification of systematic variance by controlling for covariates can sometimes provide additional power to detect effects.1
Tests of bidirectional effects of executive control and diurnal cortisol
A parallel growth model was specified in which correlated growth factors of executive control and diurnal cortisol were conditioned on T1 income, cumulative risk, and child gender, and the slopes and intercepts (i.e., T4 levels) of executive control and diurnal cortisol predicted T4 total adjustment problems, social competence, and academic readiness. This model demonstrated adequate fit to the data, χ2 (64) = 98.91, RMSEA = .04, CFI = .96. To test for potential bidirectional effects, directional effects were added in which the intercept of executive control was conditioned on the slope of diurnal cortisol, and the intercept of diurnal cortisol was conditioned on the slope of executive control. The addition of these effects, χ2 (60) = 89.98, RMSEA = .04, CFI = .96, resulted in a trend toward a significant improvement to the fit of the model, χ2 difference (4) = 8.93, p = .06; however, the effects of the slopes on intercepts were not significant, suggesting that the executive control and diurnal cortisol slope factors were not significant predictors of the T4 levels of the other variable.
Autoregressive and cross-lagged effects of the T1–T4 residuals of executive control and diurnal cortisol and within-time residual correlations were included in the model, with within-construct autoregressive paths and between-construct, within-time residual correlations constrained to be equal, χ2 (59) = 78.14, RMSEA = .03, CFI = .98. This resulted in an improvement in model fit to the data, χ2 difference (5) = 20.77, p < .001. Autoregressive paths indicated modest stability of residuals for executive control and diurnal cortisol above the variance accounted for by the growth factors. Next, the cross-lagged paths from each time point of executive control and diurnal cortisol to the subsequent time point of the other variable were freed to vary to test whether there were time-specific bidirectional effects of executive control and diurnal cortisol, χ2 (55) = 68.45, RMSEA = .03, CFI = .99. This resulted in a significant improvement to the fit of the model, χ2 difference (4) = 9.69, p = .045. Only one cross-lagged path was significant, and that was for T1 executive control predicting T2 diurnal cortisol (β = .15, p = .01), although there was a trend toward an effect of T3 executive control on T4 diurnal cortisol (β = .06, p = .08) and a trend toward an effect of T3 diurnal cortisol on T4 executive control (β = .08, p = .08).
Effects of income and cumulative risk on executive control and diurnal cortisol
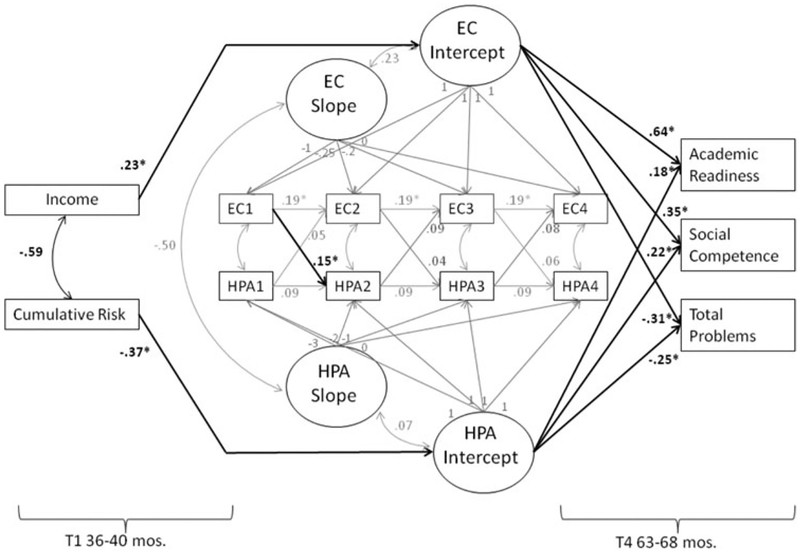
Based on the final model including directional slope effects, autoregressive and cross-lagged paths, and correlated residuals, we examined the effects of income and cumulative risk on executive control and diurnal cortisol intercept and slope factors (Figure 1). Neither the slope of executive control nor diurnal cortisol was predicted by income or cumulative risk. A higher intercept of executive control was predicted by higher income (b = 0.01, β = .23, p = .01), but not cumulative risk (b = −0.02, β = −.14, p = .09). A higher intercept of diurnal cortisol was predicted by lower cumulative risk (b = −0.05, β = −.37, p = .02), but not income (b = 0.03, β = .10, ns).
Figure 1.
Model with standardized coefficients of the effects of income and cumulative risk on executive control and diurnal cortisol level, the bidirectional effects of executive control and diurnal cortisol, and their effects on children’s academic readiness, social competence, and adjustment problems. Effects of child gender were covaried but are not depicted. *p < .05.
Effects of executive control and diurnal cortisol on adjustment
Above the effects of diurnal cortisol, the intercept of executive control significantly predicted lower T4 adjustment problems (b = −22.72, β = −.31, p < .001), higher social competence (b = 37.41, β = .35, p < .001), and higher academic readiness (b = 25.47, β = .64, p < .001). Above the effects of executive control, the intercept of diurnal cortisol predicted lower adjustment problems (b = −25.04, β = −.25, p = .01), higher social competence (b = 25.82, β = .22, p = .02), and higher academic readiness (b = 5.91, β = .18, p = .01). Adjustment outcomes were not predicted by the executive control or diurnal cortisol slope factors.2 The possibility that both high and low levels of diurnal cortisol might be related to child adjustment was tested by examining the effects of a quadratic T4 diurnal cortisol term on child adjustment above the linear effects indicated by the intercept factor. These effects were nonsignificant.
Indirect effects of income and cumulative risk on adjustment
The indirect effects of income and cumulative risk through the intercepts of executive control and diurnal cortisol were tested to identify potential specific effects of income and cumulative risk. Given that the effects of income and cumulative risk on the slope factors were nonsignificant, these were not examined. Income had a significant indirect effect through the intercept of executive control on social competence (β = .07, p =.04), academic readiness (β = .15, p = .02), and a trend toward an indirect effect on adjustment problems (β = −.06, p = .07), whereas the indirect effects of income on adjustment through diurnal cortisol were all nonsignificant. Cumulative risk had significant indirect effects through diurnal cortisol on adjustment problems (β = .17, p = .01) and social competence (β = −.12, p = .04), but not on academic readiness, and the indirect effects of cumulative risk on adjustment through executive control were nonsignificant.
Discussion
In this study, we investigated the hypothesis that the effects of low income and cumulative risk on young children’s adjustment would be accounted for by the additive effects of executive control and HPA axis regulation. Further, we hypothesized that disruptions in one aspect of self-regulation would have cascading effects on other aspects of self-regulation, with these bidirectional effects further accounting for the effects of income and cumulative risk on adjustment. In particular, exposure to low-income contexts and their associated chronic adversity were expected to predict lower executive control and dysregulation of the HPA axis, which we proposed would shape each other over time. There was little support for the hypothesis of bidirectional effects. However, executive control and HPA axis functioning each contributed uniquely to children’s adjustment outcomes over and above the effects of the other, supporting the hypothesis that they have additive effects on adjustment. The results point to the value of examining multiple indicators of neurobiological systems of self-regulation simultaneously and support the presence of additive effects on children’s adjustment, accounting for the effects of income and cumulative risk. There was also evidence of unique pathways of the effects of income and cumulative risk on children’s adjustment through executive control and diurnal cortisol.
This study provided evidence that both executive control and diurnal cortisol independently contributed to children’s adjustment above the effects of each other, implying they have additive effects on children’s adjustment. This highlights the importance of considering multiple indicators of self-regulation simultaneously and supports the conjecture that income and cumulative risk have effects on children’s adjustment in part through their impact on multiple self-regulatory systems. In the future, it would be useful to examine whether the effects of executive control and diurnal cortisol on adjustment problems are accounted for by distinct mechanisms, such as emotional versus behavioral dysregulation, or account for distinct adjustment problems, such as distinguishing internalizing and externalizing problems.
Interesting also was the suggestion in the results of unique pathways in the prediction of adjustment outcomes by income and cumulative risk. When cumulative risk is accounted for, income appears to be specifically related to children’s executive control and has an indirect effect on children’s adjustment through this effect. In addition, when income is accounted for, cumulative risk appears to be more relevant in the prediction of diurnal cortisol and has indirect effects on children’s adjustment through this effect. It is important to note that these effect sizes, although significant, were small in magnitude. However, the distinct pathways highlight the value of examining low income and cumulative risk separately, as they appear to operate differently in relation to specific aspects of self-regulation. This is consistent with suggestions that different forms of stress or adversity can have differing effects on neurobiological systems underlying self-regulation (e.g., McLaughlin et al., 2014). In addition, income and cumulative risk appear to have different effects on other potential mediating variables, such as parenting, that might account for their differential effects on aspects of self-regulation (Lengua et al., 2014). In this study, when accounting for cumulative risk, which indexes contextual and family adversity and disruptions, the net effect of low income might capture few resources and greater uncertainty in meeting basic needs, including deficits in nutrition, cognitive and interpersonal experiences, and potentially less instrumental support from parents. Conversely, cumulative risk captures pervasive, chronic stress in the context that may also lead to greater negativity and conflict with parents, which may overwhelm the neuroendocrine stress response system resulting in a downregulation or blunting of the system. The specific effects of low income and cumulative risk on these potential mediating factors and other aspects of self-regulation should be examined in the future.
There is now ample evidence of the associations of low income and cumulative risk with lower executive control (Li-Grining, 2007; Mezzacappa, 2004) and disrupted diurnal cortisol (Badanes et al., 2011; Fernald et al., 2008; Zalewski, Lengua, Fisher, et al., 2012). This study expands the evidence in this area by considering a developmental model of self-regulation and by demonstrating how the effects of risk on adjustment might unfold over time through their effects on self-regulation. Specifically, it appears that levels of executive control and diurnal cortisol regulation attained in early childhood account for children’s adjustment and readiness at school entry. These processes might represent a canalization of lower executive control and disrupted HPA axis regulation in early childhood with potential implications for later adjustment.
Few, if any, previous studies have examined the bidirectional, prospective associations of executive control and diurnal cortisol in young children. The results of this study provide minimal evidence of potential bidirectional effects once growth, average levels, and contextual factors were taken into account. Neither the slope of executive control nor of diurnal cortisol predicted the ultimate level of the other. However, there was some evidence of time-specific effects of the variables on each other. Adding the time-specific cross-lagged effects significantly improved the model fit, and albeit modest in magnitude, there was one significant and two trends toward significant cross-lagged effects. Most prior studies investigating these associations have tested cortisol as a predictor of executive control (e.g., Blair et al., 2011). The results of this study suggest the possibility that executive control affects HPA axis regulation as well. Initial executive control predicted changes in diurnal cortisol at the subsequent time point, with a trend toward an effect of executive control on diurnal cortisol, and vice versa, at later time points. However, given the modest effect sizes, these effects should be interpreted with caution. The results do point to the possibility that early executive control takes precedence in predicting diurnal cortisol in 3-year-old children. It is possible that young children who show higher executive control are better regulated in the face of stress or adversity, potentially managing their arousal, emotions, and behaviors more effectively, and they may have neuroendocrine stress systems that are more regulated as a result. These findings provide evidence of top-down regulation of HPA axis activity (Blair & Raver, 2012).
There are several possible explanations for the minimal support for the hypothesized bidirectional effects. It is possible that executive control and diurnal cortisol levels are already established at this age or represent inherited individual differences, so that once accounting for the levels and stability, there was little variance remaining to be accounted for. Although possible, this is unlikely as other analysis with this data set demonstrate predictors of “state” levels of diurnal cortisol that represent specific time variations from trait levels (e.g., redacted for review, 2018), and other variables, specifically parenting, predict growth in executive control (e.g., Lengua et al., 2014). It is also possible that any potential bidirectional effects were accounted for by the presence of contextual risk factors, particularly if those risk factors represent pervasive and chronic risk, such as indicated by low income and cumulative risk. In this case, contextual risk might operate as a third variable accounting for the associations and potential bidirectional effects between executive control and diurnal cortisol levels. This might parallel the recent findings that longitudinal effects of delay of gratification on adjustment were reduced when a wide range of family contextual, parenting, child cognitive ability, and temperament variables were controlled (Watts, Duncan, & Quan, 2018). Another possible explanation is that executive control and diurnal cortisol developmental processes operate on different time scales from each other, or on a time scale not captured in the assessments in this study, which were separated by 9 months. Finally, it is possible that cortisol levels have distinct and nuanced associations with specific aspects of executive control, as suggested by evidence regarding associations of acute stress and cortisol with specific executive functions (Shields, Sazma, & Yonelinas, 2016). These alternative possibilities can be investigated in the future to contribute to our understanding about whether bidirectional effects between executive control and diurnal cortisol level exist. Further, there may be alternative processes or associations between executive control and diurnal cortisol that were not examined in this study. For example, it is possible that they interact such that they exacerbate each other’s effects on children’s adjustment, a possibility that should be examined in the future.
It is also interesting to note that the intercepts, and not the slopes, of executive control and diurnal cortisol were related to the predictors and outcomes in this study. For diurnal cortisol, this might reflect the fact that there was not significant individual variation around the average slope, although there was a trend toward significant variance. In addition, the pattern of change in diurnal cortisol levels was not well characterized by either a linear or a quadratic term, suggesting that there might not be a systematic pattern of change in diurnal cortisol levels. However, this does not explain the lack of significant associations with the executive control slope, which had both a significant linear slope pattern and significant variance around the average slope. The lack of association of income with the executive control growth is consistent with the results of another study that examined the relation of income to growth in executive function across early to middle childhood (Hughes et al., 2010). This might suggest that income has its effects on level of effortful control earlier in development, and that other factors not examined in this study, such as parenting and family relationships, might predict growth (for executive control) or time-specific levels (for diurnal cortisol), as indicated above.
However, the lack of association of executive control growth is inconsistent with prior evidence with preadolescent and adolescent children demonstrating that initial levels as well as rates of growth of effortful control were relevant to children’s behavioral, social, and emotional adjustment (Bridget & Mayes, 2011; Hughes & Ensor, 2009; King et al., 2013). Thus, it is possible that the rate of developmental change is more relevant to children’s adjustment when children are older and required to navigate their contexts more independently than during the preschool period, in which case a more accelerated rate of growth would be beneficial. In early childhood, it is possible that, for both executive control and diurnal cortisol, the level attained is more relevant than variation in growth rates, with children generally maintaining their rank order over time.
Notable strengths of the study include the longitudinal assessment of executive control and diurnal cortisol at multiple time points across the preschool period. Another strength of the study is the use of a relatively large sample that equally represented all levels of income, allowing robust tests of the effects of income and cumulative risk. In addition, multiple methods of assessment were utilized, including physiological, task performance, and parent and teacher reports, minimizing the likelihood that method variance or bias accounted for the observed effects.
However, the study also includes several limitations. First, only diurnal cortisol patterns were assessed in this study, whereas the inclusion of cortisol reactivity or awakening responses would have allowed clarification of the effects of adversity on the regulation of the neuroendocrine stress system and facilitated comparisons of findings with other related studies. Second, a cumulative risk score was used to capture income-related stress and adversity, in part to simplify already complicated models. Although there is value in utilizing a cumulative risk score to capture the burden of stress and adversity often associated with low income, there is also value in examining the specific effects of individual risk factors included in the cumulative risk score. Third, this study was not able to address the role that racial or ethnic background might play in experiences of low income and cumulative risk, as the study was not designed to adequately examine this. Low income was confounded with minority status, particularly for African American families, and we did not assess experiences of discrimination to facilitate understanding of additional sources of stress for families with diverse racial and ethnic backgrounds. Future studies should carefully recruit participants with representation across the range of income and risk levels for each ethnic and racial group included, such that the effects of income, cumulative risk, and race or ethnicity can be clarified. Fourth, we adopted a latent growth curve and autoregressive latent trajectory modeling framework to test the potential bidirectional effects between executive control and cortisol levels. However, latent growth curve models may not have been the optimal analytic approach for the cortisol data. Although the minimal evidence of bidirectional effects should be replicated, it also points to the need to examine the relations between cortisol levels and executive control in models that capture alternative temporal relations between them to specify the developmental processes that account for their association.
Future research could clarify the effects of low income and cumulative risk on children’s developing self-regulation systems by following children from birth or even prenatally, to map the early effects of adversity on the neurobiological systems underlying self-regulation and the unfolding of self-regulation over time. For example, there is evidence of the association of prenatal stress exposure with later mental health problems (e.g., Class et al., 2014; Li, Olsen, Vestergaard, & Obel, 2010; Phillips, Hammen, Brennan, Najman, & Bor, 2005), and disruptions to self-regulation, such as HPA axis functioning (Huzink et al., 2008), might account for these effects. In addition, it would be valuable to follow children into preadolescence and adolescence to examine the long-term differential effects of executive control and diurnal cortisol on emerging emotional, behavioral, and mental health problems in adolescence (e.g., Martin, Davies, Cummings, & Cicchetti, 2017). Finally, examination of additional aspects of self-regulation, including emotion regulation and physiological reactivity, could elucidate potential cascading effects of disruptions in multiple self-regulation systems in the context of risk (e.g., El-Sheikh, Erath, Buckhalt, Granger, & Mize, 2008).
In sum, the results of this study indicate that executive control and diurnal cortisol additively contribute to children’s adjustment problems, accounting for the effects of income and adversity on children’s outcomes, and point to modest bidirectional effects. In terms of translational implications, interventions aimed at improving child outcomes that address one aspect of children’s self-regulation, for example, only promoting executive function through training in executive function tasks or activities, might be hampered by failure to address other aspects of self-regulation simultaneously. It might be more fruitful to consider interventions that simultaneously promote multiple aspects of self-regulation. For example, prior research has shown that aspects of parenting, such as scaffolding and consistent limit setting, promote the development of executive function (Devine, Bignardi, & Hughes, 2016; Lengua et al., 2014), while other aspects of parenting, such as negativity, might predict disruptions to diurnal cortisol regulation (Zalewski, Lengua, Kiff, et al., 2012). Most behavioral parenting programs target both reducing negativity and increasing warmth and consistent limit setting, thus, potentially impacting both executive control and HPA axis regulation. The results of this study highlight that both executive control and diurnal cortisol regulation impact children’s adjustment, and interventions that target multiple aspects of self-regulation and the factors that shape them are ultimately more likely have a meaningful effect on children’s adjustment.
Acknowledgments.
The authors thank the families who participated in this study.
Financial support. Support for this research was provided by National Institute of Child Health and Human Development Grant R01HD054465 awarded to the first author.
Footnotes
We examined the results of the final model excluding slope effects. The pattern of associations remains similar with the exception that the effects of the cortisol intercept factor on total adjustment problems (β = −.16) and social competence (β = .15) are reduced with a trend toward significance.
To explore the possibility that cortisol morning levels and diurnal slopes operated differently, the final model was tested for each separately. The cortisol morning level intercept, but not the slope, was significantly predicted by cumulative risk (β = −.44), but not income. A higher morning level intercept predicted lower total adjustment problems (β = −.35) and higher social competence (β = .26). The cortisol diurnal slope intercept, but not the slope, was significantly predicted by cumulative risk (β = −.38), but not income. A higher diurnal slope intercept predicted lower total adjustment problems (β = −.18). When examined separately for cortisol morning level and diurnal slope, evidence of bidirectional effects between HPA axis activity and executive control remained consistent with the those for the combined cortisol variable. For cortisol morning level, T1 executive control predicted T2 morning level (β = .14), and T3 cortisol morning level predicted T4 executive control (β = .08). For diurnal slope, T1 executive control predicted T2 diurnal slope (β = .18), and T2 diurnal slope predicted T3 executive control at a trend level (β = .07). Thus, the general pattern of associations for cortisol morning level and diurnal slope were largely similar to the pattern of associations when the two cortisol indicators were combined.
References
- Ackerman BP, Brown ED, & Izard CE (2004). The relations between contextual risk, earned income, and the school adjustment of children from economically disadvantaged families. Developmental Psychology, 40, 204. [DOI] [PubMed] [Google Scholar]
- Adam EK, & Gunnar MR (2001). Relationship functioning and home and work demands predict individual differences in diurnal cortisol patterns in women. Psychoneuroendocrinology, 26(2), 189–208. [DOI] [PubMed] [Google Scholar]
- Alink LR, van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, & Koot HM (2008). Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental psychobiology, 50, 427–450. [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10, 410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman SB, Dawson G, Panagiotides H, Yamada E, & Wilkson CW (2002). Stress hormone levels of children of depressed mothers. Development and Psychopathology, 14, 333–349. [DOI] [PubMed] [Google Scholar]
- Badanes LS, Watamura SE, & Hankin BL (2011). Hypocortisolism as a potential marker of allostatic load in children: Associations with family risk and internalizing disorders. Developmental Psychopathology, 23, 881–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbain O, Bryant D, McCandies T, Burchinal M, Early D, Clifford R, … Howes C (2006). Children enrolled in public pre-K: The relation of family life, neighborhood quality, and socioeconomic resources to early competence. American Journal of Orthopsychiatry, 76, 265–276. [DOI] [PubMed] [Google Scholar]
- Best JR, & Miller PH (2010). A developmental perspective on executive function. Child Development, 81, 1641–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C (2010). Stress and the development of self-regulation in context. Child Development Perspectives, 4, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, … FLP Investigators. (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82, 1970–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2012). Individual development and evolution: Experiential canalization of self-regulation. Developmental Psychology, 48, 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Razza R (2007). Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development, 78, 647–663. [DOI] [PubMed] [Google Scholar]
- Bridgett DJ, & Mayes LC (2011). Development of inhibitory control among prenatally cocaine exposed and non-cocaine exposed youths from late childhood to early adolescence: The effects of gender and risk and subsequent aggressive behavior. Neurotoxicology and Teratology, 33, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, & Duncan GJ (1997). The effects of poverty on children. Future of Children: Children and Poverty, 7, 55–71. [PubMed] [Google Scholar]
- Brown SM, Henning S, & Wellman CL (2005). Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cerebral Cortex, 15, 1714–1722. [DOI] [PubMed] [Google Scholar]
- Bruce J, Davis EP, & Gunnar MR (2002). Individual differences in children’s cortisol response to the beginning of a new school year. Psychoneuroendocrinology, 27, 635–650. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, & Levine S (2009). Morning cortisol Levels in preschool-aged foster children: Differential effects of maltreatment type. Developmental Psychobiology, 51, 14–23. doi: 10.1002/dev.20333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JC, Mezzacappa E, & Beardslee WR (2003). Characteristics of resilient youths living in poverty: The role of self-regulatory processes. Development and Psychopathology, 15, 139–162. [DOI] [PubMed] [Google Scholar]
- Bush NR, Obradović J, Adler N, & Boyce WT (2011). Kindergarten stressors and cumulative adrenocortical activation: The “first straws” of allostatic load? Development and Psychopathology, 23, 1089–1106. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, & Sousa N (2007). The prefrontal cortex as a key target of the maladaptive response to stress. Journal of Neuroscience, 27, 2781–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Class QA, Abel KM, Khashan AS, Rickert ME, Dalman C, Larsson H, … D’Onofrio BM (2014). Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychological Medicine, 44, 71–84. doi: 10.1017/S0033291713000780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AD, & Parker RC (1998). The relationship between salivary cortisol concentrations in frozen versus mailed samples. Psychoneuroendocrinology, 23, 613–616. [DOI] [PubMed] [Google Scholar]
- Collins LM, Schafer JL, & Kam CM (2001). A comparison of inclusive and restrictive strategies in modeling missing data procedures. Psychological Methods, 6, 330–351. [PubMed] [Google Scholar]
- Conger RD, & Elder GH Jr. (1994). Families in troubled times. New York: de Gruyter. [Google Scholar]
- Davis EP, Bruce J, & Gunnar MR (2002). The anterior attention network: Associations with temperament and neuroendocrine activity in 6-year-old children. Developmental Psychobiology, 40, 43–56. [DOI] [PubMed] [Google Scholar]
- De Kloet ER (1991). Brain Corticosteroid receptor balance and homeostatic control. Frontiers in Neuroendocrinology, 12, 95–64. [DOI] [PubMed] [Google Scholar]
- Devine RT, Bignardi G, & Hughes C (2016). Executive function mediates the relations between parental behaviors and children’s early academic ability. Frontiers in Psychology, 7, 1902. doi: 10.3389/fpsyg.2016.01902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Simanek AM, & Aiello AE (2009). Socio-economic status, cortisol and allostatic load: A review of the literature. International Journal of Epidemiology, 38, 1297–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Ziol-Guest KM, & Kalil A (2010). Early-childhood poverty and adult attainment, behavior, and health. Child Development, 81, 306–325. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Fabes R, Smith C, Reiser M, Shepard S, … Cumberland AJ (2003). The relations of effortful control and ego control to children’s resiliency and social functioning. Developmental Psychology, 39, 761–776. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, & Mize J (2008). Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology, 36, 601–611. [DOI] [PubMed] [Google Scholar]
- Enders CK, & Bandalos DL (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8, 430–457. 10.1207/S15328007SEM0803_5 [DOI] [PubMed] [Google Scholar]
- Evans GW (2003). A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology, 39, 761–776. [DOI] [PubMed] [Google Scholar]
- Evans GW, & English K (2002). The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioeconomic adjustment. Child Development, 73, 1238–1248. [DOI] [PubMed] [Google Scholar]
- Evans GW, Li D, & Whipple SS (2013). Cumulative risk and child development. Psychological Bulletin, 139, 1342. [DOI] [PubMed] [Google Scholar]
- Fernald LC, Burke HM, & Gunnar MR (2008). Salivary cortisol levels in children of low-income women with high depressive symptomatology. Developmental Psychopathology, 20, 423–436. [DOI] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, & Herman JP (2008). The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology, 149, 5482–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstadt CL, Hong YL, & Diamond A (1994). The relationship between cognition and action: Performance of children 3.5–7 years old on a Stroop-like day-night test. Cognition, 53, 129–153. [DOI] [PubMed] [Google Scholar]
- Gresham FM, & Elliot SN (1990). Social Skills Rating System. Circle Pines, MN: American Guidance Service. [Google Scholar]
- Gunnar M (2016). Early life stress: What is the human chapter of the mammalian story? Child Development Perspectives, 10, 178–183. [Google Scholar]
- Gunnar MR, Sebanc AM, Tout K, Donzella B, & van Dulmen MMH (2003). Peer rejection, temperament, and cortisol activity in preschoolers. Developmental Psychobiology, 43, 346–358. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Tout K, de Haan M, Pierce S, & Stansbury K (1997). Temperament, social competence, and adrenocortical activity in preschoolers. Developmental Psychobiology, 31, 65–85. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Vazquez DM (2001). Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology, 13, 515–538. doi: 10.1017/S0954579401003066 [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Anckarsäter H, Lichtenstein P, Nelson N, & Gustafsson PA (2010). Does quantity have a quality all its own? Cumulative adversity and up-and down-regulation of circadian salivary cortisol levels in healthy children. Psychoneuroendocrinology, 35, 1410–1415. [DOI] [PubMed] [Google Scholar]
- Hughes C, & Ensor R (2009). Independence and interplay between maternal and child risk factors for preschool problem behaviors? International Journal of Behavioral Development, 33, 312–322. [Google Scholar]
- Hughes C, Ensor R, Wilson A, & Graham A (2010). Tracking executive function across the transition to school: A latent variable approach. Developmental Neuropsychology, 35, 1, 20–36. doi: 10.1080/87565640903325691 [DOI] [PubMed] [Google Scholar]
- Huizink AC, Bartels M, Rose RJ, Pulkkinen L, Eriksson CJP, & Kaprio J (2008). Chernobyl exposure as stressor during pregnancy and hormone levels in adolescent offspring. Journal of Epidemiology and Community Health, 62, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff CJ, Lengua LJ, & Bush N (2011). Temperament variations in sensitivity to parenting: Predicting changes in depression and anxiety. Journal of Abnormal Child Psychology, 39, 1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Conger RD, Elder GH, & Lorenz FO (2003). Reciprocal influences between stressful life events and adolescent internalizing and externalizing problems. Child Development, 74, 127–143. [DOI] [PubMed] [Google Scholar]
- King K, Lengua L, & Monahan K (2013). Differentiating executive and motivational components of self-regulation: Differences in trajectories, predictors and adjustment. Journal of Abnormal Child Psychology, 41, 57–69, doi: 10.1007/s10802-012-9665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Steyer R, Eid M, Patalla U, Schwenkmezger P, & Hellhammer DH (1990). Cortisol and behavior: Application of a latent state-trait model to salivary cortisol. Psychoneuroendocrinology, 15, 297–307. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, Jacques T, Koenig A, & Vandegeest K (1996). Inhibitory control in young children and its role in emerging internalization. Child Development, 67, 490–507. [PubMed] [Google Scholar]
- Korkman M, Kirk U, & Kemp S (1998). NEPSY: A Developmental Neuropsychological Assessment. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Laurent HK, Neiderhiser JM, Natsuaki MN, Shaw DS, Fisher PA, Reiss D, & Leve LD (2013). Stress system development from age 4.5 to 6: Family environment predictors and adjustment implications of HPA activity stability versus change. Developmental Psychobiology, 56, 340–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ (2003). Associations among emotionality, self-regulation, adjustment problems and positive adjustment in middle childhood. Journal of Applied Developmental Psychology, 24, 595–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Honorado E, & Bush N (2007). Contextual risk and parenting as predictors of effortful control and social competence in preschool children. Journal of Applied Developmental Psychology, 28, 40–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Kiff C, Moran L, Zalewski M, Thompson S, Cortes R, & Ruberry E (2014). Parenting mediates the effects of income and cumulative risk on the development of effortful control. Social Development, 23, 631–649. [Google Scholar]
- Lengua LJ, Moran LR, Zalewski M, Ruberry E, Kiff C, & Thompson S (2014). Relations of growth in effortful control to family income, cumulative risk, and adjustment in preschool-age children. Journal of Abnormal Child Psychology. 10.1007/s10802-014-9941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Zalewski M, Fisher P, & Moran L (2013). Does HPA-axis dysregulation account for the effects of income on effortful control and adjustment in preschool children? Infant and Child Development, 22, 439–458. DOI: 10.1002/icd.1805, NIHMS599969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, & Obel C (2010). Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: A nationwide follow-up study in Denmark. European Journal of Child and Adolescent Psychiatry, 19, 747–753. [DOI] [PubMed] [Google Scholar]
- Liberzon I, King AP, Britton JC, Phan KL, Abelson JL, & Taylor SF (2007). Paralimbic and medial prefrontal cortical involvement in neuroendocrine responses to traumatic stimuli. American Journal of Psychiatry, 164, 1250–1258. [DOI] [PubMed] [Google Scholar]
- Li-Grining CP (2007). Effortful control among low-income preschoolers in three cities: Stability, change, and individual differences. Developmental Psychology, 43, 208–221. [DOI] [PubMed] [Google Scholar]
- Linver M, Brooks-Gunn J, & Kohen D (2002). Family processes as pathways from income to young children’s development. Developmental Psychology, 38, 719–734. [PubMed] [Google Scholar]
- Marsman R, Nederhof E, Rosmalen JG, Oldehinkel AJ, Ormel J, & Buitelaar JK (2012). Family environment is associated with HPA-axis activity in adolescents. The TRAILS study. Biological Psychology, 89, 460–466. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Davies PT, Cummings EM, & Cicchetti D (2017). The mediating roles of cortisol reactivity and executive functioning difficulties in the pathways between childhood histories of emotional insecurity and adolescent school problems. Development and Psychopathology, 29, 1483–1498. doi:S0954579417000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2012). Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences, 109(Suppl. 2), 17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, & Zaslavski AM (2012). Child hood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoyd VC (1998). Socioeconomic disadvantage and child development.American Psychologist, 53, 185–204. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E (2004). Alerting, orienting, and executive attention: Developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children. Child Development, 75, 1373–1386. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. [DOI] [PubMed] [Google Scholar]
- Mistry R, Benner A, Biesanz J, Clark S, & Howes C (2010). Family and social risk, and parental investments during the early childhood years as predictors of low-income children’s school readiness outcomes. Early Childhood Research Quarterly, 25, 432–449. [Google Scholar]
- Mistry RS, Vandewater EA, Huston AC, & McLoyd VC (2002). Economic well-being and children’s social adjustment: The role of family process in an ethnically diverse low-income sample. Child Development, 73, 935–951. [DOI] [PubMed] [Google Scholar]
- Muris P, van der Pennen E, Sigmond R, & Mayer B (2008). Symptoms of anxiety, depression, and aggression in non-clinical children: Relationships with self-report and performance-based measures of attention and effortful control. Child Psychiatry & Human Development, 39, 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2010). Mplus user’s guide (6th ed.). Los Angeles: Author. [Google Scholar]
- Obradovic J, Long JD, Cutuli JJ, Chan C, Hinz E, Heistad D, & Masten AS (2009). Academic achievement of homeless and highly mobile children in an urban school district: Longitudinal evidence on risk, growth, and resilience. Development and Psychopathology, 21, 493–518. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Portilla XA, & Ballard PJ (2016). Biological sensitivity to family income: Differential effects on early executive functioning. Child Development, 87, 374–384. [DOI] [PubMed] [Google Scholar]
- O’Donnell K (2008). Parents’ Reports of the School Readiness of Young Children from the National Household Education Surveys Program of 2007 (NCES 2008–051). Washington, DC: US Department of Education, National Center for Education Statistics, Institute of Education Sciences. [Google Scholar]
- Ponitz CE, McClelland MM, Jewkes AM, Conner CM, Farris CL, & Morrison FJ (2008). Touch your toes! developing a direct measure of behavioral regulation in early childhood. Early Childhood Research Quarterly, 23, 141–158. [Google Scholar]
- Petterson SM, & Albers AB (2001). Effects of poverty and maternal depression on early child development. Child Development, 72, 1794–1813. [DOI] [PubMed] [Google Scholar]
- Phillips NK, Hammen CL, Brennan PA, Najman JM, & Bor W (2005). Early adversity and the prospective prediction of depressive and anxiety disorders in adolescents. Journal of Abnormal Child Psychology, 33, 13–24. [DOI] [PubMed] [Google Scholar]
- Quas JA, Yim IA, Oberlander TF, Nordstokke D, Essex MJ, Armstrong JM, … Boyce WT (2014). The symphonic structure of childhood stress reactivity: Patterns of sympathetic, parasympathetic and adrenocortical responses to psychological challenge. Development and Psychopathology, 26, 963–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. [Google Scholar]
- Raver CC, Blackburn EK, Bancroft M, & Torp N (1999). Relations between effective emotional self-regulation, attentional control, and low-income preschoolers’ social competence with peers. Early Education and Development, 10, 333–350. [Google Scholar]
- Raver CC, Blair C, & Willoughby M (2013). Poverty as a predictor of 4-year-olds’ executive function: New perspectives on models of differential susceptibility. Developmental Psychology, 49, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reser JE (2016). Chronic stress, cortical plasticity and neuroecology. Behavioural Processes, 129, 105–115. [DOI] [PubMed] [Google Scholar]
- Ross KM, Murphy ML, Adam EK, Chen E, & Miller GE (2014). How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology, 39, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler I, Ramirez R, & Reynolds K (1986). General life events schedule for children. Paper presented at the annual meeting of the American Psychological Association, Washington, DC. [Google Scholar]
- Sektnan M, McClelland M, Acock A, & Morrison F (2010). Relations between early family risk, children’s behavioral regulation, and academic achievement. Early Childhood Research Quarterly, 25, 464–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, & Yonelinas AP (2016). The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Neuroscience Biobehavioral Review, 68, 651–668. doi: 10.1016/j.neubiorev.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, & Herman JP (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10, 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health & Human Services. (2010). Poverty guidelines, research, and measurement. Retrieved from http://aspe.hhs.gov/poverty/
- Veer IM, Oei NYL, Spinhoven P, van Buchem MA, Elzinga BM, & Rombouts SARB (2012). Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology, 37, 1039–1047. [DOI] [PubMed] [Google Scholar]
- Watts TW, Duncan GJ, & Quan H (2018). Revisiting the marshmallow test: A conceptual replication investigating links between early delay of gratification and later outcomes. Psychological Science, 29, 1159–1177. doi: 10.1177/0956797618761661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski M, Lengua LJ, Fisher P, Trancik A, Bush NR, & Meltzoff AN (2012). Poverty and single parenting: Relations with preschoolers’ cortisol and effortful control. Infant and Child Development, 21, 537–554. doi: 10.1002/icd.1759 [DOI] [Google Scholar]
- Zalewski M, Lengua LJ, Kiff CJ, & Fisher PA (2012). Understanding the relation of low income to HPA-axis functioning in preschool children: Cumulative family risk and parenting as pathways to disruptions in cortisol. Child Psychiatry & Human Development, 43, 924–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski M, Lengua LJ, Thompson S, & Kiff CJ (2015). Income, cumulative risk and longitudinal profiles of hypothalamic-picuitary-adrenal axis activity in preschool age children. Development and Psychopathology, 28, 341–353. doi: 10.1017/S0954579415000474 [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Muller U, Frye D, & Marcovitch S (2003). The development of executive function in early childhood. Monographs of Society for Research in Child Development, 68(3, Serial No. 274). [DOI] [PubMed] [Google Scholar]