Short abstract
Background
In the past, the airway epithelium was thought to be primarily an inert physical barrier. We now know that the upper airway epithelium plays a critical role in both innate and adaptive immunity, and that epithelial dysfunction is strongly associated with inflammatory airway disease. The pathogenesis of chronic rhinosinusitis is poorly understood, but growing evidence supports a key role for the airway epithelium in the pathophysiology of the disease.
Objective
The purpose of this study is to explore our current understanding of how dysfunction in human sinonasal epithelial cells (HSNECs) contributes to the pathogenesis of chronic rhinosinusitis with nasal polyps (CRSwNP) and to examine how current and developing therapies affect epithelial cell functions.
Methods
A literature review of papers published in English pertaining to epithelial cell dysfunction in patients with CRSwNP was performed using the PubMed database. The search utilized combinations of the following key words: sinusitis, polyps, epithelium, pathophysiology, barrier function, dendritic cells, eosinophils, T cells, complement, mucociliary clearance, vitamin D, cytokines, chemokines, taste receptors, steroids, saline, and therapy.
Results
HSNEC mucociliary clearance, barrier function, secretion of cytokines, influence on dendritic cells, influence on T-cells, regulation of eosinophils, vitamin D metabolism, complement production, and taste receptor function are altered in patients with CRSwNP and contribute to the pathogenesis of the disease. Current therapies utilized to manage CRSwNP counteract the effects of HSNEC dysfunction and relieve key symptoms of the disease.
Conclusion
HSNECs are key players in both innate and adaptive immunity, and altered epithelial functions are closely intertwined with the pathogenesis of CRSwNP. Our review supports further investigation of altered HSNEC function in patients with CRSwNP and supports development of novel epithelial-targeted therapies for its management.
Keywords: sinusitis, nasal polyp, epithelial cell, dysfunction, pathogenesis, therapy, cytokine, chemokine, eosinophil, immunity
Introduction
Chronic rhinosinusitis (CRS) is a chronic inflammatory disease of the upper airway that affects approximately 16% of the U.S. population and up to 27.1% of certain European countries.1 CRS is commonly divided into 2 distinct phenotypes based on the absence or presence of nasal polyps: CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP). Although there is some overlap, CRSsNP is typically characterized by a type 1 inflammatory profile, and CRSwNP demonstrates a type 2 inflammatory profile and eosinophilia.2 Type 2 cytokines can have significant effects on both innate and adaptive immunity and drive many of the physical symptoms of CRSwNP including rhinorrhea, mucus production, and tissue remodeling.3,4 CRSwNP is the most difficult form of the disease to treat, with reported surgical revision rates ranging from 3.5% to 50%,5–10 and will be the focus of this review. This article will describe how dysfunction of the airway epithelium contributes to the pathogenesis of CRSwNP and further will discuss the impact of current and developing therapies on epithelial cell functions.
Roles of the Upper Airway Epithelium
Mucociliary Clearance
Mucociliary clearance (MCC) is an innate immune mechanism in which ciliated epithelial cells drive liquid on the surface of the airway, primarily mucus that traps pathogens and other inhaled irritants, toward the throat where it can be swallowed or expectorated.11 HSNECs play a fundamental role in this process, and effective, coordinated, ciliary beating is essential to effective MCC.12 Multiple studies have demonstrated that CRS is associated with a reduction in MCC.13–15 Although basal ciliary beat frequency (CBF) is shown to be comparable between CRS patients and control subjects, CBF is blunted in response to environmental stimuli in patients with CRS.16–18 Impaired MCC can lead to mucostasis, bacterial colonization, biofilm formation, and even CRS itself.16 One study showed that genetically engineered mice lacking functional cilia, and thus lacking effective MCC, all developed severe CRS.19 Collectively, these studies suggest that the blunted epithelial CBF response and reduced MCC associated with CRS may play a significant role in its pathophysiology.
Barrier Function
The upper airway epithelium consists of pseudostratified, ciliated, columnar, epithelial cells that serve as a physical barrier between the external environment and cells in the subepithelial space below.20,21 Apical tight junctions (TJs), underlying adherens junctions, gap junctions, and desmosomes form multiprotein complexes that, through cell-cell contact, help to establish cell polarity, form a barrier, and regulate the paracellular flow of electrolytes and macromolecules.20–23 Both trans-tissue resistance and trans-epithelial resistance (TER) have been shown to be significantly reduced in patients with CRSwNP, as compared to healthy subjects.24 Epithelial tissue from patients with CRSwNP has also been shown to have a severely disrupted TJ layer with significantly decreased expression of occludin, ZO-1, claudin-1, DSG1, and DSG2.23,24 Exposure to pro-inflammatory cytokines IL-4, IL-13, and IFN-, which are elevated in CRSwNP, has been shown to disrupt epithelial integrity and reduce TER in human sinonasal epithelial cells (HSNECs) cultured at the air–liquid interface.24,25 Disturbances to integral epithelial structures create a leaky barrier that allows an increased number of inhaled irritants to infiltrate and activate immune cells in the subepithelial space.26 Thus, the disrupted epithelial barrier evident in CRSwNP may play a key role in the pathogenesis of the disease.
Secretion of Cytokines
HSNECs release inflammatory cytokines when exposed to foreign substances such as microbes and allergens.20 Of the most notable pro-inflammatory mediators produced by HSNECs are IL-25, thymic stromal lymphopoietin (TSLP), and IL-33. IL-25 has been shown to be overexpressed in patients with CRSwNP as compared to controls, and increased IL-25 has been shown to correlate with increased serum eosinophils and worse computed tomography (CT) scores.27 TSLP messenger RNA (mRNA) expression has been shown to be increased in CRSwNP nasal tissue28 and has been shown to be an important initiator of type 2 inflammation.29 Overexpression of IL-33 is associated with CRSwNP, and in murine models, blocking IL-33 reduces the activation of Th2 cells.30 Collectively, these studies demonstrate that HSNEC expression of inflammatory cytokines is altered in patients with CRSwNP, which is thought to contribute to disease pathogenesis.
HSNEC Influence on Dendritic Cells
Dendritic cells (DCs) are immune cells that process and present antigens to naïve T-cells, and thus play a major role in directing the adaptive immune response.31 HSNECs regulate DCs through their own epithelial derived cytokines and chemokines, which include IL-6, GM-CSF, PGE2, TSLP, CCL2, and CCL20.32 Prior studies have demonstrated that independent of in vitro stimulation with a fungal antigen, CRSwNP HSNECs can recruit and activate DCs to initiate Th2 skewing. Furthermore, CRSwNP HSNECs are hyperresponsive to fungal antigen exposure and activate DCs more robustly than control subject derived HSNECs.32
The HSNEC barrier also blocks inhaled irritants from reaching and stimulating the maturation of DCs in the subepithelial space. In patients with CRSwNP, epithelial integrity is significantly disrupted which can lead to heightened DC stimulation.33 Overstimulation of DCs due to compromised epithelial integrity is noteworthy due to the ability of DCs to drive Th1/Th2 responses after exposure to exogenous antigens.32 Abnormal HSNEC modulation of DCs affects adaptive immunity and may contribute to the altered immune profile seen in patients with CRSwNP.
HSNEC influence on T-cells
Elevation of T-cells in patients with nasal polyps is well established1,34,35 with HSNECs being hypothesized to play a role in the modulation of T-cell functions. The elevated presence of T-cell attracting chemokines in nasal polyps may contribute to a continual trafficking of T-cells to the inflamed sinonasal mucosa.36 For example, studies have shown that T-cell recruiting chemokines CXCL9 and CCL17 are significantly elevated in nasal polyp tissues.37,38 HSNECs also express B7 costimulatory molecules which can interact with the T-cell Receptor, affecting T-cell activation, survival, and differentiation.39–41 As previously mentioned, CRSwNP HSNECs also contribute to an altered T-cell response by influencing DCs to promote Th2 skewing.32,41 Therefore, it is clear that dysfunction of HSNECs can contribute to altered recruitment, activation, and differentiation of T-cells, which may drive the skewed adaptive immune response evident in CRSwNP.
HSNEC Regulation of Eosinophils
The majority of patients with CRSwNP in the United States and Europe show pronounced infiltration of eosinophils in nasal polyp tissue.42 Eosinophils play a multifaceted role in immunity by releasing toxic granules, releasing 35 different cytokines, recruiting lymphocytes, presenting antigen to T-cells, and regulating DCs.43,44 CRS patients with high tissue eosinophilia have been shown to have significantly worse endoscopic scores,45 decreased olfactory function,46 and worse postoperative outcomes.47,48
Sinonasal tissue from patients with CRS shows significantly elevated expression of eotaxin-1, -2, and -3, which are known to attract circulating eosinophils into local tissues.37,49 As compared to control turbinate tissue, elevated expression of all 3 eotaxins in nasal polyp tissue has been shown to correlate to the degree of eosinophilia,50 although it is important to note that the use of control turbinate tissue rather than control sinus tissue may have influenced these findings. As mentioned previously in this review, IL-25, IL-33, and TSLP have been shown to be overexpressed in CRSwNP.27,28,30 This is significant given that one recent study demonstrated that mRNA expression of IL-25 and IL-33 correlates with a high degree of tissue eosinophilia,51 and another study found that TSLP and its receptors are expressed at higher levels in patients with eosinophilic CRS.52 CRSwNP HSNECs also show elevated expression of Cystatin SN (CST1), which promotes the recruitment and activation of eosinophils.53,54 These studies suggest that the altered epithelial expression of eotaxins, cytokines, and CST1 may drive the high degree of sinonasal tissue eosinophilia found in patients with CRSwNP, which is significant given the negative association between high tissue eosinophilia and endoscopic scores, olfactory function, and postoperative outcomes.45–48
Sinonasal Production of 1,25(OH)2D3
Vitamin D3 (VD3) plays a complex role in immunity, having a wide range of functional effects on innate and adaptive immune responses. In the skin, pro-VD3 is converted to pre-VD3 which then binds to vitamin D binding protein for transport to the liver where it is converted to 25(OH)D3. Finally, 25(OH)D3 is converted to its active metabolite, 1,25(OH)2D3, by 1α-hydroxylase.55 In the sinonasal mucosa, 80% of 1α-hydroxylase positive cells are HSNECs.56 However, studies have shown that patients with CRSwNP have reduced sinonasal 1α‐hydroxylase and 1,25(OH)2D3 levels as compared to controls, and that reduction in 1α‐hydroxylase is associated with worse SNOT22 scores.57 In addition to serving as a major source of local 1,25(OH)2VD3, HSNEC can also respond to it. One study showed 1,25(OH)2D3 reduced HSNEC secretion of TNFα, IL-6, and IL-8 in response to a H1N1 infection,58 while another study demonstrated that 1,25(OH)2D3 is able to block cigarette smoke extract-induced secretion of IL-6, IL-8, and CCL20 by HSNECs.56 Thus, the reduced HSNEC synthesis of 1,25(OH)2D3 in CRSwNP may be a key contributor to sinonasal inflammation.
Complement Production
Traditionally, the observed effect of complement factors and receptors on various cell types was thought to be mediated solely by complement activation fragments generated in the serum, the lymph, or interstitial fluids.59,60 Although liver-generated circulating C3 and C5 are required for the detection and removal of pathogens,59,60 an emerging paradigm suggests that local, cell-derived, complement activation is key in driving and modulating specific immune responses.61–64 The complement cascade can be activated by 3 distinct pathways, but each pathway converges at the central protein of the complement system, C3.65 C3 has a wide range of inflammatory effects, and its cleavage products have been shown to influence Th2 reactions in asthma and allergy.66 C3a increases small vessel permeability, vasodilation, and histamine release, thus enhancing inflammatory status. C3a is also a chemoattractant for eosinophils, while C3a and C3b can both activate eosinophils.66,67 As eosinophilia is a major feature of CRSwNP, complement proteins produced by HSNEC could play an important role in eosinophilic CRSwNP.36,68
A study published by Lane et al. in 2006 utilized immunohistochemical analysis to demonstrate that C3 is present on HSNECs.68 Another study has also shown a 4- to 5-fold increase in C3 gene transcriptional activity in sinus tissue samples of patients with CRSwNP as compared to controls.65 Recently, CRSwNP HSNECs have been shown to have elevated intracellular stores of C3 and C3a compared to control subjects, and elevated nasal mucus C3 levels have been shown to correlate with worse subjective disease severity.69 The same study demonstrated that C3aR deficiency and C3aR antagonism reduced sinonasal epithelial injury and inflammation, and decreased epithelial thickness, inflammatory cell infiltrates, and eosinophil numbers in a murine model,69 suggesting that blunting sinonasal complement activity reduces sinonasal inflammation.
Bitter and Sweet Taste Receptors
Ciliated epithelial cells and non-ciliated solitary chemosensory cells (SCCs) in the nasal mucosa express both Taste Receptor Family 2 (T2R) and Taste Receptor Family 1 (T1R) receptors which are commonly known for their role in bitter and sweet taste perception, respectively. They share a common G Protein signaling pathway in which binding of a bitter or sweet ligand triggers a downstream signaling cascade that generates an action potential (AP). The AP triggers the release of ATP which activates taste sensory receptors, ultimately resulting in the perception of taste.12 However, recent studies demonstrate that the role of T1R and T2R receptors extends beyond taste perception.12,70–76
T2R and T1R receptor isoforms have been implicated in sinonasal innate immunity and the pathophysiology of CRS. One extensively studied T2R isoform, T2R38, is expressed on ciliated HSNEC and responds specifically to bitter bacterial compounds.12 Upon activation, T2R38 drives the production of nitric oxide (NO).73 NO contributes to the innate immune response by directly inducing intracellular damage to infectious microbes. NO also increases CBF which accelerates both the dispersion of antimicrobial peptides (AMPs) in the sinonasal mucosa and the removal of pathogens trapped in mucus.73,76,77 Recent studies have demonstrated that a polymorphism of the T2R38 gene, which renders the receptor inactive, correlates with a decreased ability of upper respiratory cells to eliminate bacteria, increased gram-negative bacterial infections, and worse surgical outcomes for affected CRS patients.70,72 The nonfunctional T2R38 genotype has also been shown to be overrepresented in patients with medically recalcitrant CRS.71 The T2R and T1R receptors expressed on SCCs in the sinonasal mucosa also play a role in the innate immune response. The activation of SCC T2R triggers the release of AMPs such as beta defensins 1 and 2. On the other hand, the activation of SCC T1R antagonizes SCC T2R signaling, inhibiting the immune response to inhaled microbes.12 Recent studies have implicated hyporesponsive T2R receptors and hyperresponsive T1R receptors in the decreased sinonasal innate immune response to pathogens in patients with CRS.74,75 These studies demonstrated a significantly decreased sensitivity to the bitter compound quinine75 and increased sensitivity to the sweet compound sucrose74 in patients with CRSwNP. Therefore, altered function of taste receptors expressed on HSNECs may contribute to the pathogenesis of CRSwNP.
Targeting Epithelial Cells for the Treatment of CRSwNP
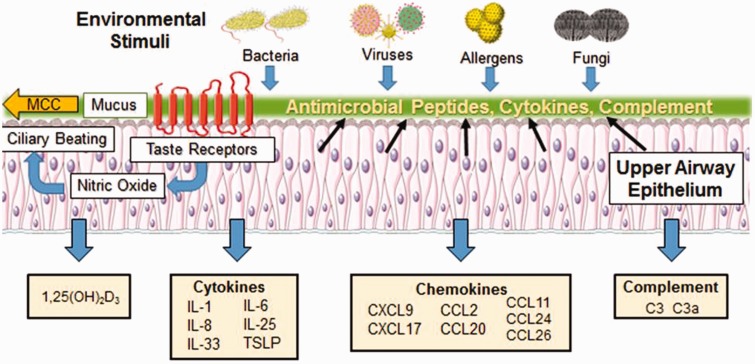
Treatment of CRSwNP is complicated by the fact that no singular pathogen or gene has been identified as causing CRSwNP, as its causes are likely multifactorial in nature.2,78 As such, ideal therapeutic targets will likely need to modulate both innate and adaptive arms of the immune system. HSNEC line the sinus cavity and play important roles in orchestrating innate and adaptive immune responses (summarized in Figure 1). Given their immunomodulatory properties and anatomical location, they are ideal targets for topically delivered therapeutic agents. The impact of current and developing therapies on HSNEC function is described below.
Figure 1.
Immune functions of the upper airway epithelium. The upper airway epithelium is a key player in both innate and adaptive immunity and serves as the first line of defense against allergens, pathogens, and pollutants inhaled from the external environment. The epithelium kills or neutralizes inhaled microorganisms through the release of AMPs and coordinates the clearance of inhaled irritants trapped by mucus via ciliary beating in the process of mucociliary clearance (MCC). Stimulation of taste receptors expressed on epithelial cells by bitter bacterial compounds enhances the release of AMPs and triggers the production of nitric oxide which increases ciliary beat frequency, enhancing the dispersion of AMPS and improving MCC. The pseudostratified ciliated columnar epithelial cells joined by tight junctions, adherens junctions, gap junctions, and desmosomes also serve as a physical barrier that blocks inhaled irritants from passing through to the subepithelial space below. Exposure to foreign substances triggers epithelial release of inflammatory cytokines that influence T-cell, dendritic cell (DC), and eosinophil function. The epithelium is also a rich source of numerous chemokines that modulate T-cell, DC, and eosinophil activity. In addition, the epithelium is a local source of 1,25(OH)D3 and complement proteins C3 and C3a which play critical roles in innate and adaptive immune responses. Alterations to these epithelial functions are closely intertwined with the pathogenesis of chronic rhinosinusitis with nasal polyps.
Steroids
Topical and oral steroids are cornerstones of CRSwNP treatment regimens.79 Numerous studies have shown that intranasal steroid treatments have a positive effect on polyp size, polyp reoccurrence, nasal airflow, and symptoms.1,80 Steroids reduce the activation and viability of eosinophils and can reduce the expression of epithelial-derived inflammatory cytokines such as IL‐1β, IL‐6, IL‐8, tumor necrosis factor‐α (TNFα), and granulocyte macrophage-colony stimulating factor (GM‐CSF).81,82 Glucocorticoids (GCs) also activate anti-inflammatory genes that encode mitogen-activated kinase phosphatase-1 (MKP-1), IL-10, inhibitor of NFκB (I-κBα), and glucocorticoid-induced leucine zipper protein.83 GCs exert there anti-inflammatory effects through the glucocorticoid receptor (GCR), which in sinonasal tissue, is present in all cells, but predominantly in HSNECs.83,84 Four isoforms of the GCR are known to exist: GCRα, GCRβ, GCRδ, and GCRγ. GCRα is the active isoform that mediates GC action. GCRβ is unable to bind steroid ligands; however, it is able to bind DNA, and thus competitively blocks the GC-GCRα complex from binding DNA and exerting anti-inflammatory effects.83 Variable expression of GCRα and GCRβ may play a significant role in the efficacy of GC treatments in managing CRSwNP. One study found that GCRβ expression is elevated in nasal polyp tissues of GC-insensitive patients, and that GCRα:GCRβ ratios in nasal polyp tissues are decreased in GC-insensitive patients.85 Other studies have also found decreased sinonasal expression of GCRα and/or increased sinonasal expression of GCRβ to be associated with decreased clinical efficacy of GC treatment.86–88 On the other hand, some studies have found that no correlation exists between differential expression of GCRα and GCRβ and GC treatment sensitivity.89,90 The relationship between GCR isoform expression and GC treatment efficacy thus requires further investigation.
Saline Rinse
Nasal saline irrigation has been shown to be effective in relieving symptoms of CRS both on its own and as a treatment adjunct.91 Hypertonic saline solution has been shown to significantly increase TER values in HSNECs derived from patients with CRS.92 Utilization of saline rinse is also associated with a significant reduction in microbial antigens and a reduction in microbial burden.93 Conversely, another study demonstrated that saline rinses reduce the levels of AMPs in nasal secretions, thus compromising the innate immune response of the sinonasal epithelium.94 However, the same study also showed that while saline irrigation initially reduced levels of lysozyme and lactoferrin in nasal secretions, low-salt solution stimulated an increase of AMP secretion.94 These studies suggest that the clinical efficacy of saline rinse may be related to its effects on epithelial integrity and AMP secretion.
Anti-IL-25 Therapies
IL-25 produced by HSNECs can significantly affect type 2 immunity, and it can contribute to upper airway remodeling and pathological changes in patients with CRSwNP.95 Furthermore, in the upper airway, SCCs have been found to be the primary source of IL-25.96 IL-25 overexpression in CRSwNP is also associated with worse CT and endoscopy scores.97 A study published by Shin et al. in 2015 demonstrated that in murine models of CRSwNP, anti-IL-25 therapy reduced the number of polyps, collagen deposition, infiltration of inflammatory cells such as eosinophils and neutrophils, and expression of local inflammatory cytokines such as IL-4 and IFN-γ.98 A number of other studies have also demonstrated that blocking IL-25 reduces type 2 inflammatory cytokines and improves clinical conditions in other type 2 inflammatory disorders such as asthma and ulcerative colitis.99,100 Despite these findings, to date, no clinical trials using anti-IL-25 antibodies for treatment of CRSwNP have been conducted. Given the evidence of the association between IL-25 and the pathogenesis of CRSwNP and other type 2 inflammatory disorders, anti-IL-25 therapy appears to be a promising avenue for the development of novel therapies for patients with CRSwNP.
Conclusions
HSNECs from patients with CRSwNP have altered functions including increased permeability and cytokine/chemokine production and are hyperresponsive to antigen stimulation. As such, HSNECs are major contributors to the pathogenesis of CRSwNP. Gaining a greater insight into the specific mechanisms driving epithelial cell dysfunctions may lead to the identification of novel therapeutic pathways that can ameliorate CRSwNP.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Mulligan is supported by a grant from the National Institute of Health (R01AI34698).
References
- 1.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; 50:1–12. [DOI] [PubMed] [Google Scholar]
- 2.Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015; 64:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015; 15:271–282. [DOI] [PubMed] [Google Scholar]
- 4.Khalmuratova R, Park JW, Shin HW. Immune cell responses and mucosal barrier disruptions in chronic rhinosinusitis. Immune Netw. 2017; 17:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkins C, Browne JP, Slack R, et al. The national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Clin Otolaryngol. 2006; 31:390–398. [DOI] [PubMed] [Google Scholar]
- 6.Stein NR, Jafari A. Revision rates and time to revision following endoscopic sinus surgery: a large database analysis. 2018;128:31–36. [DOI] [PMC free article] [PubMed]
- 7.Wynn R, Har-El G. Recurrence rates after endoscopic sinus surgery for massive sinus polyposis. Laryngoscope. 2004; 114:811–813. [DOI] [PubMed] [Google Scholar]
- 8.Calus L, Van Bruaene N, Bosteels C, et al. Twelve-year follow-up study after endoscopic sinus surgery in patients with chronic rhinosinusitis with nasal polyposis. Clin Transl Allergy. 2019; 9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philpott C, Hopkins C, Erskine S, et al. The burden of revision sinonasal surgery in the UK-data from the Chronic Rhinosinusitis Epidemiology Study (CRES): a cross-sectional study. BMJ Open. 2015; 5:e006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miglani A, Divekar RD, Azar A, et al. Revision endoscopic sinus surgery rates by chronic rhinosinusitis subtype. Int Forum Allergy Rhinol. 2018; 8:1047–1051. [DOI] [PubMed] [Google Scholar]
- 11.Ganesan S, Comstock AT, Sajjan US. Barrier function of airway tract epithelium. Tissue Barriers. 2013; 1:e24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maina IW, Workman AD, Cohen NA. The role of bitter and sweet taste receptors in upper airway innate immunity: recent advances and future directions. World J Otorhinolaryngol Head Neck Surg. 2018; 4:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passali D, Ferri R, Becchini G, et al. Alterations of nasal mucociliary transport in patients with hypertrophy of the inferior turbinates, deviations of the nasal septum and chronic sinusitis. Eur Arch Otorhinolaryngol. 1999; 256:335–337. [DOI] [PubMed] [Google Scholar]
- 14.Hua X, Naselsky WC, Bennett WD, et al. Adenosine increases nasal mucociliary clearance rate in mice through A2A and A2B adenosine receptors. Laryngoscope. 2013; 123:306–310. [DOI] [PubMed] [Google Scholar]
- 15.Elwany S, Hisham M, Gamaee R. The effect of endoscopic sinus surgery on mucociliary clearance in patients with chronic sinusitis. Eur Arch Otorhinolaryngol. 1998; 255:511–514. [DOI] [PubMed] [Google Scholar]
- 16.Hamilos DL. Host-microbial interactions in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014; 133:640–653.e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen B, Shaari J, Claire SE, et al. Altered sinonasal ciliary dynamics in chronic rhinosinusitis. Am J Rhinol. 2006; 20:325–329. [DOI] [PubMed] [Google Scholar]
- 18.Zhao K-Q, Cowan AT, Lee RJ, et al. Molecular modulation of airway epithelial ciliary response to sneezing. FASEB J. 2012; 26(8):3178–3187. [DOI] [PubMed] [Google Scholar]
- 19.Ostrowski LE, Yin W, Rogers TD, et al. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am J Respir Cell Mol Biol. 2010; 43:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang N, Van Crombruggen K, Gevaert E, et al. Barrier function of the nasal mucosa in health and type-2 biased airway diseases. Allergy. 2016; 71:295–307. [DOI] [PubMed] [Google Scholar]
- 21.Kojima T, Go M, Takano K, et al. Regulation of tight junctions in upper airway epithelium. BioMed Res Int. 2013;2013:947072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology (Carlton, Vic). 2003; 8:432–446. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Wang X, Wang R, et al. The expression of epithelial intercellular junctional proteins in the sinonasal tissue of subjects with chronic rhinosinusitis: a histopathologic study. ORL J Otorhinolaryngol Relat Spec. 2014; 76:110–119. [DOI] [PubMed] [Google Scholar]
- 24.Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol. 2012; 130:1087–1096.e1010. [DOI] [PubMed] [Google Scholar]
- 25.Wise SK, Laury AM, Katz EH, et al. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int Forum Allergy Rhinol. 2014; 4:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao J, Wang M, Duan S, et al. Transforming growth factor-β1 decreases epithelial tight junction integrity in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018; 141:1160–1163.e1169. [DOI] [PubMed] [Google Scholar]
- 27.Lam M, Hull L, McLachlan R, et al. Clinical severity and epithelial endotypes in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013; 3:121–128. [DOI] [PubMed] [Google Scholar]
- 28.Nagarkar DR, Poposki JA, Tan BK, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013; 132:593–600.e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitajima M, Lee HC, Nakayama T, et al. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011; 41:1862–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Jiang LL, Cao ZW. Interleukin-33 promotes the inflammatory reaction in chronic rhinosinusitis with nasal polyps by NF-kappaB signaling pathway. Eur Rev Med Pharmacol Sci. 2017; 21:4501–4508. [PubMed] [Google Scholar]
- 31.Cao PP, Shi LL, Xu K, et al. Dendritic cells in inflammatory sinonasal diseases. Clin Exp Allergy. 2016; 46:894–906. [DOI] [PubMed] [Google Scholar]
- 32.Mulligan JK, Mulligan RM, Atkinson C, et al. Human sinonasal epithelial cells direct dendritic function and T-cell T helper 1/T helper 2 skewing following Aspergillus exposure. Int Forum Allergy Rhinol. 2011; 1:268–274. [DOI] [PubMed] [Google Scholar]
- 33.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014; 134:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derycke L, Eyerich S, Van Crombruggen K, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014; 9:e97581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Zele T, Claeys S, Gevaert P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; 61:1280–1289. [DOI] [PubMed] [Google Scholar]
- 36.Hulse KE, Stevens WW, Tan BK, et al. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015; 45:328–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens WW, Ocampo CJ, Berdnikovs S, et al. Cytokines in chronic rhinosinusitis. role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med. 2015; 192:682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho DY, Nayak JV, Bravo DT, et al. Expression of dual oxidases and secreted cytokines in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013; 3:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang P, Martin M, Yang QB, et al. Role of B7 costimulatory molecules in immune responses and T-helper cell differentiation in response to recombinant HagB from Porphyromonas gingivalis. Infect Immun. 2004; 72:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Myers AC, Chen L, et al. Constitutive and inducible expression of b7 family of ligands by human airway epithelial cells. Am J Respir Cell Mol Biol. 2005; 33:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schleimer RP, Kato A, Kern R, et al. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007; 120:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fokkens W, Lund V, Mullol J. European position paper on rhinosinusitis and nasal polyps 2007. Rhinol Suppl. 2007; 20:1–136. [PubMed] [Google Scholar]
- 43.Wen T, Rothenberg ME. The regulatory function of eosinophils. Microbiol Spectr. 2016; 4:10.1128/microbiolspec.MCHD-0020-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davoine F, Lacy P. Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity. Front Immunol. 2014; 5:570–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snidvongs K, Lam M, Sacks R, et al. Structured histopathology profiling of chronic rhinosinusitis in routine practice. Int Forum Allergy Rhinol. 2012; 2:376–385. [DOI] [PubMed] [Google Scholar]
- 46.Hauser LJ, Chandra RK, Li P, et al. Role of tissue eosinophils in chronic rhinosinusitis-associated olfactory loss. Int Forum Allergy Rhinol. 2017; 7:957–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soler ZM, Sauer D, Mace J, et al. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg. 2010; 142:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tosun F, Arslan HH, Karslioglu Y, et al. Relationship between postoperative recurrence rate and eosinophil density of nasal polyps. Ann Otol Rhinol Laryngol. 2010; 119:455–459. [DOI] [PubMed] [Google Scholar]
- 49.Kato A. Immunopathology of chronic rhinosinusitis. Allergol Int. 2015; 64:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olze H, Forster U, Zuberbier T, et al. Eosinophilic nasal polyps are a rich source of eotaxin, eotaxin-2 and eotaxin-3. Rhinology. 2006; 44:145–150. [PubMed] [Google Scholar]
- 51.Lam M, Hull L, Imrie A, et al. Interleukin-25 and interleukin-33 as mediators of eosinophilic inflammation in chronic rhinosinusitis. Am J Rhinol Allergy. 2015; 29:175–181. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang Y, Fan E, Li Y, et al. Clinical characteristics and expression of thymic stromal lymphopoetin in eosinophilic and non-eosinophilic chronic rhinosinusitis. ORL J Otorhinolaryngol Relat Spec. 2013; 75:37–45. [DOI] [PubMed] [Google Scholar]
- 53.Magister S, Kos J. Cystatins in immune system. J Cancer. 2012; 4:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan B, Lou H, Wang Y, et al. Epithelium-derived cystatin-SN enhances eosinophil activation and infiltration via interleukin-5 in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2019; 144(2): 455–469. doi:10.1016/j.jaci.2019.03.026 [DOI] [PubMed]
- 55.Mulligan JK, Pasquini WN, Carroll WW, et al. Dietary vitamin D3 deficiency exacerbates sinonasal inflammation and alters local 25(OH)D3 metabolism. PLoS One. 2017; 12:e0186374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mulligan JK, Nagel W, O’Connell BP, et al. Cigarette smoke exposure is associated with vitamin D3 deficiencies in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014; 134:342–349. [DOI] [PubMed] [Google Scholar]
- 57.Schlosser RJ, Carroll WW, Soler ZM, et al. Reduced sinonasal levels of 1alpha-hydroxylase are associated with worse quality of life in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2016; 6:58–65. [DOI] [PubMed] [Google Scholar]
- 58.Khare D, Godbole NM, Pawar SD, et al. Calcitriol [1, 25[OH]2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cells. Eur J Nutr. 2013; 52:1405–1415. [DOI] [PubMed] [Google Scholar]
- 59.Walport MJ. Complement. First of two parts. N Engl J Med. 2001; 344:1058–1066. [DOI] [PubMed] [Google Scholar]
- 60.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001; 344:1140–1144. [DOI] [PubMed] [Google Scholar]
- 61.Kolev M, Le Friec G, Kemper C. The role of complement in CD4(+) T cell homeostasis and effector functions. Semin Immunol. 2013; 25:12–19. [DOI] [PubMed] [Google Scholar]
- 62.Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012; 217:216–224. [DOI] [PubMed] [Google Scholar]
- 63.Heeger PS, Lalli PN, Lin F, et al. Decay-accelerating factor modulates induction of T cell immunity. J Exp Med. 2005; 201:1523–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liszewski MK, Kolev M, Le Friec G, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013; 39:1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlosser RJ, Mulligan RM, Casey SE, et al. Alterations in gene expression of complement components in chronic rhinosinusitis. Am J Rhinol Allergy. 2010; 24:21–25. [DOI] [PubMed] [Google Scholar]
- 66.Jun SW, Kim TH, Lee HM, et al. Overexpression of the anaphylatoxin receptors, complement anaphylatoxin 3a receptor and complement anaphylatoxin 5a receptor, in the nasal mucosa of patients with mild and severe persistent allergic rhinitis. J Allergy Clin Immunol. 2008; 122:119–125. [DOI] [PubMed] [Google Scholar]
- 67.Shamri R, Xenakis JJ, Spencer LA. Eosinophils in innate immunity: an evolving story. Cell Tissue Res. 2011; 343:57–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lane AP, Truong-Tran QA, Myers A, et al. Serum amyloid A, properdin, complement 3, and toll-like receptors are expressed locally in human sinonasal tissue. Am J Rhinol. 2006; 20:117–123. [PMC free article] [PubMed] [Google Scholar]
- 69.Mulligan JK, Patel K, Williamson T, et al. C3a receptor antagonism as a novel therapeutic target for chronic rhinosinusitis. Mucosal Immunol. 2018; 11:1375–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adappa ND, Farquhar D, Palmer JN, et al. TAS2R38 genotype predicts surgical outcome in nonpolypoid chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016; 6:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adappa ND, Zhang Z, Palmer JN, et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014; 4:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012; 122:4145–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee RJ, Xiong G, Kofonow JM, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012; 122:4145–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Workman AD, Brooks SG, Kohanski MA, et al. Bitter and sweet taste tests are reflective of disease status in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2018; 6:1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Workman AD, Maina IW, Brooks SG, et al. The role of quinine-responsive taste receptor family 2 in airway immune defense and chronic rhinosinusitis. Front Immunol. 2018; 9:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carey RM, Workman AD, Yan CH, et al. Sinonasal T2R-mediated nitric oxide production in response to Bacillus cereus. Am J Rhinol Allergy. 2017; 31:211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li D, Shirakami G, Zhan X, et al. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am J Respir Cell Mol Biol. 2000; 23:175–181. [DOI] [PubMed] [Google Scholar]
- 78.Halderman A, Lane AP. Genetic and immune dysregulation in chronic rhinosinusitis. Otolaryngol Clin N Am. 2017; 50:13–28. [DOI] [PubMed] [Google Scholar]
- 79.Cain RB, Lal D. Update on the management of chronic rhinosinusitis. Infect Drug Resist. 2013; 6:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rudmik L, Hoy M, Schlosser RJ, et al. Topical therapies in the management of chronic rhinosinusitis: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2013; 3:281–298. [DOI] [PubMed] [Google Scholar]
- 81.Mullol J, Xaubet A, Gaya A, et al. Cytokine gene expression and release from epithelial cells. A comparison study between healthy nasal mucosa and nasal polyps. Clin Exp Allergy. 1995; 25:607–615. [DOI] [PubMed] [Google Scholar]
- 82.Xaubet A, Mullol J, Lopez E, et al. Comparison of the role of nasal polyp and normal nasal mucosal epithelial cells on in vitro eosinophil survival. Mediation by GM-CSF and inhibition by dexamethasone. Clin Exp Allergy. 1994; 24:307–317. [DOI] [PubMed] [Google Scholar]
- 83.Grzanka A, Misiolek M, Golusinski W, et al. Molecular mechanisms of glucocorticoids action: implications for treatment of rhinosinusitis and nasal polyposis. Eur Arch Otorhinolaryngol. 2011; 268:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee SH. Mechanisms of glucocorticoid action in chronic rhinosinusitis. Allergy Asthma Immunol Res. 2015; 7:534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li P, Li Y, Li YQ, et al. Glucocorticoid receptor expression and glucocorticoid therapeutic effect in nasal polyps. Clin Invest Med. 2010; 33:E181–E188. [DOI] [PubMed] [Google Scholar]
- 86.Watanabe S, Suzaki H. Changes of glucocorticoid receptor expression in the nasal polyps of patients with chronic sinusitis following treatment with glucocorticoid. In vivo (Athens, Greece). 2008; 22:37–42. [PubMed] [Google Scholar]
- 87.Hamilos DL, Leung DY, Muro S, et al. GRbeta expression in nasal polyp inflammatory cells and its relationship to the anti-inflammatory effects of intranasal fluticasone. J Allergy Clin Immunol. 2001; 108:59–68. [DOI] [PubMed] [Google Scholar]
- 88.Valera FC, Scrideli C, Queinoz R, et al. NF-kappaB expression predicts clinical outcome for nasal polyposis. Rhinology. 2010; 48:408–441. [DOI] [PubMed] [Google Scholar]
- 89.Pujols L, Alobid I, Benítez P, et al. Regulation of glucocorticoid receptor in nasal polyps by systemic and intranasal glucocorticoids. Allergy. 2008; 63(10):1377–1386. [DOI] [PubMed] [Google Scholar]
- 90.Choi B-R, Kwon JH, Gong S-J, et al. Expression of glucocorticoid receptor mRNAs in glucocorticoid-resistant nasal polyps. Exp Mol Med. 2006; 38(5):466–473. [DOI] [PubMed] [Google Scholar]
- 91.Harvey R, Hannan SA, Badia L, et al. Nasal saline irrigations for the symptoms of chronic rhinosinusitis. Cochrane Database Syst Rev. 2007;(3):CD006394. [DOI] [PubMed] [Google Scholar]
- 92.Ramezanpour M, Rayan A, Smith JLP, et al. The effect of topical treatments for CRS on the sinonasal epithelial barrier. Rhinology. 2017; 55:161–169. [DOI] [PubMed] [Google Scholar]
- 93.Principi N, Esposito S. Nasal irrigation: an imprecisely defined medical procedure. Int J Environ Res Public Health. 2017; 14:E516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Woods CM, Tan S, Ullah S, et al. The effect of nasal irrigation formulation on the antimicrobial activity of nasal secretions. Int Forum Allergy Rhinol. 2015; 5:1104–1110. [DOI] [PubMed] [Google Scholar]
- 95.Park SK, Jin YD, Park YK, et al. IL-25-induced activation of nasal fibroblast and its association with the remodeling of chronic rhinosinusitis with nasal polyposis. PLoS One. 2017; 12:e0181806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kohanski MA, Workman AD, Patel NN, et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018; 142:460–469.e467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hong HY, Chen FH, Sun YQ, et al. Local IL-25 contributes to Th2-biased inflammatory profiles in nasal polyps. Allergy. 2018; 73:459–469. [DOI] [PubMed] [Google Scholar]
- 98.Shin HW, Kim DK, Park MH, et al. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015; 135:1476–1485.e1477. [DOI] [PubMed] [Google Scholar]
- 99.Camelo A, Barlow JL, Drynan LF, et al. Blocking IL-25 signalling protects against gut inflammation in a type-2 model of colitis by suppressing nuocyte and NKT derived IL-13. J Gastroenterol. 2012; 47:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gregory LG, Jones CP, Walker SA, et al. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax. 2013; 68:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]