Abstract
Watermelon (Citrullus lanatus (Thunb.) Matsum. &Nakai) is an economic crop, which is widely cultivated around the world. The ploidy study of watermelon has an important role in field breeding and production, therefore, timely and convenient ploidy detection is necessary to accelerate its application. Traditionally, the ploidy of watermelon was determined by a series of time-consuming, expensive, and less efficient methods. In this study, we developed a more efficient method to simplify and accelerate the polyploidy identification in watermelons. We first confirmed the ploidy of watermelon by traditional tetraploid morphological features and well-established flow cytometry (FCM). Then we developed a reliable real-time quantitative PCR (qPCR) technique by quantifying the highly conserved 5S rDNA sequence and its copy numbers. This technique requires less sample collection and has comparable accuracy to FCM, it accelerates the analysis process and provides a new method for the identification of polyploidy of watermelon.
Keywords: watermelon, diploid, tetraploid, flow cytometry, qPCR
1. Introduction
Watermelon is an annual vine herb of the Cucurbitaceae watermelon species. It is one of the important economic crops which is widely cultivated for its sweet dessert fruit or “the king fruit in summer”. Typically, the diploid watermelon (2n = 2× = 22) is more common in nature, while the autopolyploids make the triploid watermelon (3n = 3× = 33) and tetraploid watermelon (4n = 4× = 44) possible [1]. The triploid watermelon is a hybrid generation of common diploid and tetraploid watermelon, and the triploid watermelon is famous for the high quality of seedless fruit. Autopolyploids have excellent characteristics such as diverse DNA contents, high secondary metabolite organisms, large tissues and organs, improved yield, and high tolerance to abiotic and biotic stresses [2,3]. For example, the tetraploid watermelon contains an enlarged leaf, thicker vine and peel, larger pistil and stamen flower organs, and bigger seeds. Additionally, the tetraploid watermelon has: a higher chlorophyll content; the total β-carotene, lycopene, fructose, and glucose content is higher than diploid [4,5]; and it is more tolerant to salt stress compared to diploid [6]. Although with respect to those benefits, the low frequency of autopolyploids and the obscure observation and selection limit the application to commercial cultivation.
Nowadays, the successful usage of colchicine-induced Datura tetraploid by Blakeslee and Avery in 1937 [1], promotes the artificial induction of polyploids. For instance, the tetraploid parents were produced with the treatment of colchicine on newly emerged diploid seedlings [7]. The tetraploid inbred line can also be propagated by seed and tissue culture [8,9,10]. After induction of polyploids, the observation and selection become more important. Several approaches have been used to identify ploidy level in watermelon. The most intuitive method is morphological characterization. Previous studies have found that leaf length and width, ovary diameter, male flower petals, and pollen sac diameter are good indicators for identification [11,12]. The number of chloroplasts in watermelon leaf guard cells is positively correlated with plant ploidy, which can be used as a rapid and effective method as well [13,14]. Chromosome counting is also a common method for determining ploidy, but the small size of the watermelon chromosome makes it difficult to achieve accurate results, and this method is not practical for non-dividing cells in differentiated tissues, such as leaves [15]. Therefore, an efficient and reliable ploidy measurement technique is required for accelerating the tetraploid breeding of watermelon. Commonly, chromosome number increases or decreases will result in the increase or decrease of nuclear DNA content, respectively. Flow cytometry (FCM) is a fast and efficient method for identifying ploidy by rapidly measuring the size of the nucleus and the total amount of DNA in the nucleus, which has been well established in watermelon [16,17,18,19,20]. It can apply to tissues grown in the field or in the greenhouse. But the choice of the organization is limited, tissues that are young and have poor cell division are better detected by FCM. In addition, conventional flow cytometers are expensive and not common in routine laboratories. qPCR is a mature method for gene expression detection and copy number analysis [21,22]. After ploidy induction, the chromosomes were doubled in single cells, which would inevitably double the copy number of genes [1]. Thus, we developed a qPCR method for watermelon ploidy detection that quantifies the copy numbers of the highly conserved 5S rDNA sequence, which was also compared with the FCM method to evaluate the efficiency of the new method.
2. Results
2.1. Tetraploid Induction and Morphological Characterization in Watermelon
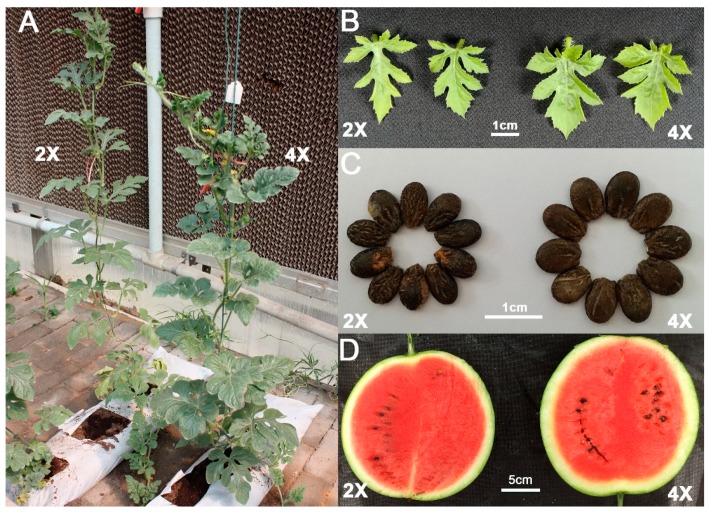
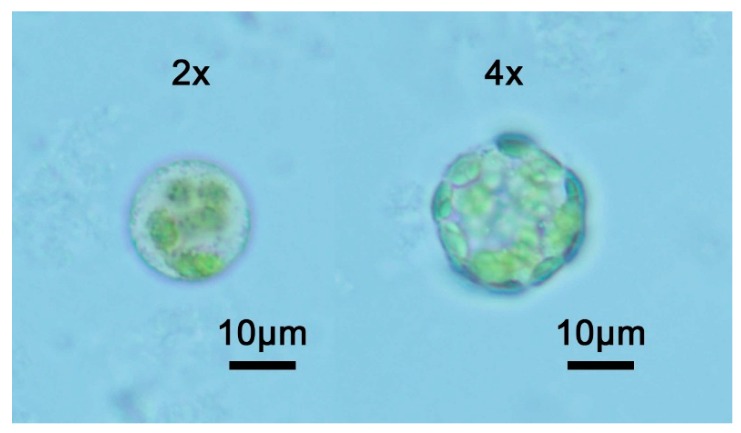
Diploid watermelons (E46, 2n) were induced by colchicine and a total of 20 tetraploid watermelons (yE46, 4n) were obtained. Six generations of continuous planting and self-crossing screened out high-quality watermelon. As shown in Figure 1, the tetraploid watermelon leaves, seeds, and fruits are larger than in diploid. Statistical analysis was performed on the vertical diameter, cross diameter, ratio of the fruit, and the area of the seed. Tetraploid watermelon seeds are significantly larger than diploid (Table 1). Protoplast preparation was carried out on the leaves. The results of microscopic examination and statistical analysis showed that the protoplasts of tetraploid watermelon were significantly larger than diploid (Figure 2, Table 1).
Figure 1.
Plants (A), young leaves (B), seeds (C), and fruits (D) of diploid (E46, 2 ×) and tetraploid (yE46, 4 ×) watermelon.
Table 1.
Morphological comparison between diploid and tetraploid watermelon.
| Genotype | Fruit | Seed Area (cm2) |
Protoplast Area (μm2) | ||
|---|---|---|---|---|---|
| Vertical Diameter (cm) | Cross Diameter (cm) | Aspect Ratio | |||
| E46 | 16.44 ± 0.61 | 16.08 ± 0.44 | 1.02 ± 0.02 | 0.30 ± 0.01b | 258.06 ± 10.87b |
| yE46 | 17.66 ± 0.23 | 17.84 ± 0.68 | 0.99 ± 0.03 | 0.37 ± 0.01a | 591.02 ± 83.11a |
Different letters show the significant differences between the average values according to the t-test at the 5% level.
Figure 2.
Microscopic observation of protoplasts of different ploidy watermelons. Bars 10 μm.
2.2. Tetraploid Characterization by Flow Cytometric Analysis
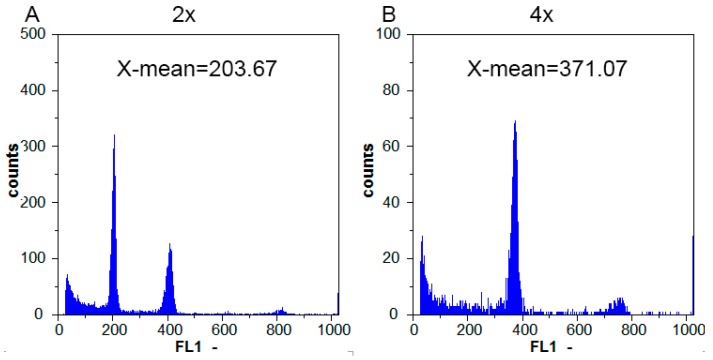
The ploidy was judged by the fluorescence intensity X-mean of the sample. The results showed that the fluorescence intensity of the main peak of diploid E46 plant was 203.46, and the fluorescence intensity of the main peak of tetraploid yE46 plant was about 371.07, which was about twice that of E46, indicating that yE46 plant cells DNA content was 1.8-times that of the E46 and was a tetraploid plant (Figure 3).
Figure 3.
Polyploidy detection through flow cytometry. The DNA ploidy level of tetraploid watermelon (B, E46, 2×) was evaluated based on the DNA ploidy level of diploid watermelon (A, E46, 4×).
2.3. 5S rDNA Sequences in Watermelon Genome
A fragment of about 100 bp was obtained by primer amplification (Figure 4). The fragment was TA cloned and picked for monoclonal sequencing. The result showed that the length was 111 bp and the base sequence was as follows: 5′-CGATCATACCAGCACTAATGCACCGGATCCAACCAGAACTCCGCAGTTAAGCGTGCTGGGACGAGAGTAGTACTAAGTTGGGTGACCACTTGGGAAGTCCTCGTGTTGCAC-3′
Figure 4.
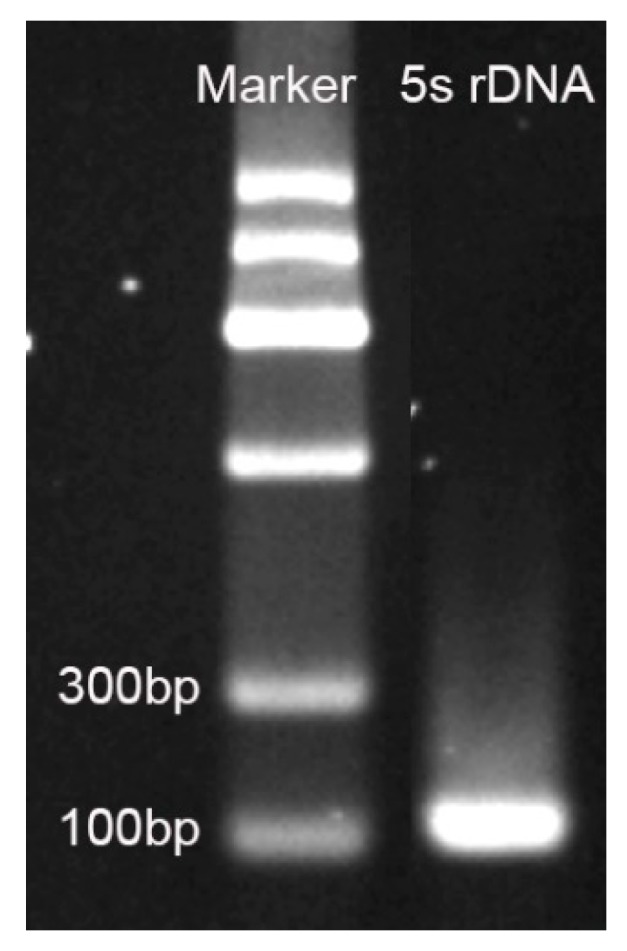
5S rDNA homologous sequence amplification results.
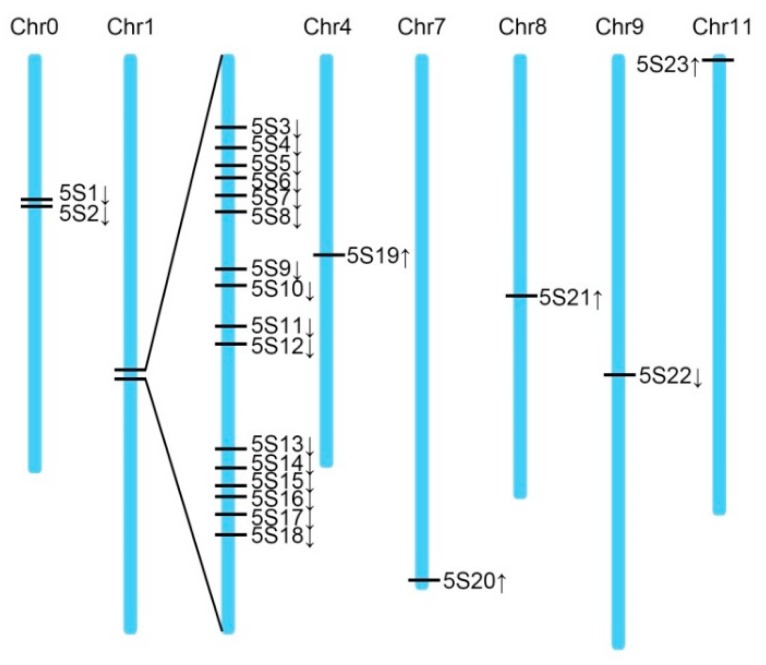
Sequence alignment was performed using Blastn. After deleting redundant data, 23 homologous sequences of 5S rDNA fragments were obtained (Table 2), and the sequence was cloned according to its position on the chromosome. The sequence cloned in the first section was named 5S4. A physical map of the 5S rDNA fragment was drawn based on the position of each sequence on the chromosome, wherein a cluster of 16 copies was present on chromosome 1 (Figure 5).
Table 2.
5S rDNA fragment homologous sequence information.
| ID | Chromosome | Location | Length (bp) |
|---|---|---|---|
| 5s1 | Chr0 | 8960268–8960378(+) | 110 |
| 5s2 | Chr0 | 8960616–8960726(+) | 110 |
| 5s3 | Chr1 | 20672252–20672358(+) | 106 |
| 5s4 | Chr1 | 20672524–20672634(+) | 110 |
| 5s5 | Chr1 | 20672797–20672907(+) | 110 |
| 5s6 | Chr1 | 20673032–20673142(+) | 110 |
| 5s7 | Chr1 | 20673317–20673424(+) | 107 |
| 5s8 | Chr1 | 20673590–20673700(+) | 110 |
| 5s9 | Chr1 | 20674311–20674421(+) | 110 |
| 5s10 | Chr1 | 20674587–20674697(+) | 110 |
| 5s11 | Chr1 | 20675133–20675243(+) | 110 |
| 5s12 | Chr1 | 20675409–20675519(+) | 110 |
| 5s13 | Chr1 | 20676875–20676985(+) | 110 |
| 5s14 | Chr1 | 20677151–20677261(+) | 110 |
| 5s15 | Chr1 | 20677427–20677537(+) | 110 |
| 5s16 | Chr1 | 20677612–20677722(+) | 110 |
| 5s17 | Chr1 | 20677885–20677995(+) | 110 |
| 5s18 | Chr1 | 20678161–20678271(+) | 110 |
| 5s19 | Chr4 | 11951587–11951479(−) | 108 |
| 5s20 | Chr7 | 30808653–30808545(−) | 108 |
| 5s21 | Chr8 | 16056311–16056201(−) | 110 |
| 5s22 | Chr9 | 23004927–23005033(+) | 102 |
| 5s23 | Chr11 | 1932259–1932150(−) | 110 |
Figure 5.
Distribution of 5S rDNA fragment homologous sequences on the chromosome.
2.4. Tetraploid Characterization by RT-qPCR
There are 23 copies of the 5S rDNA fragment in the watermelon genome. The watermelon genome size is known to be 425 Mb [23], and 1 ng DNA contains 9.80 × 1011 base pairs [24]. Therefore, the copy number of 5S rDNA fragments in 1 ng of watermelon DNA was 53,035.29. The above results indicate that the 5S rDNA fragment can meet the qPCR detection requirement in the lower concentration watermelon DNA solution.
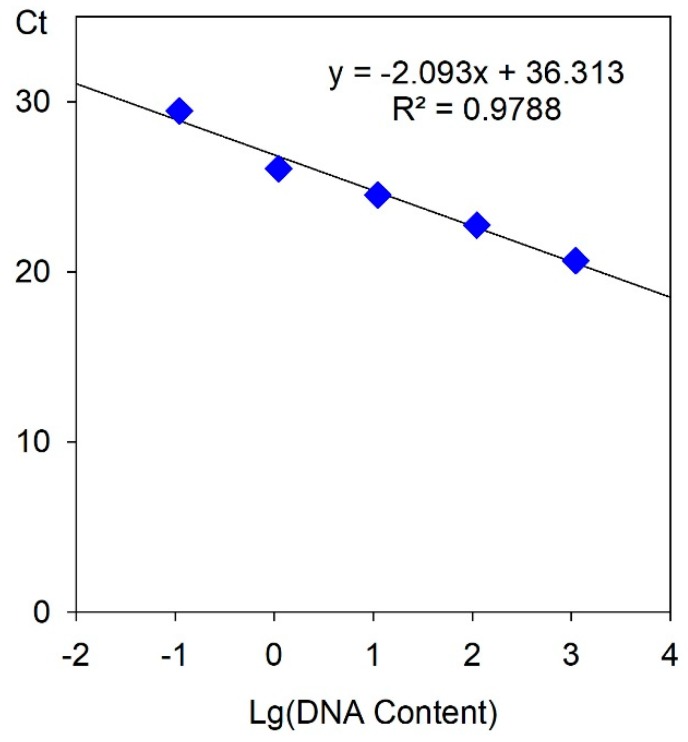
The concentration of total DNA extracted was measured by NanoDrop 2000C (Thermo Scientific, Wilmington, USA), wherein the E46 DNA concentration was 110.7 ng/μL and the yE46 DNA concentration was 196.1 ng/μL, which was about 2-times different. A standard curve between the cycle threshold (Ct) value after qPCR reaction and the total amount of template in the reaction system was constructed with reference to the DNA solution of E46. 10 μL, 1 μL, 0.1 μL, 0.01 μL, and 0.001 μL of E46 DNA solution were sequentially added to different reaction systems, and the total amount of DNA in the reaction system was 1107 ng, 110.7 ng, 11.07 ng, 1.107 ng, and 0.1107 ng, respectively. After the reaction was completed, the Ct values of the respective concentration samples were converted to 20.6610, 22.7435, 24.5173, 26.0610, and 29.44673 in order to draw a standard curve, as shown in Figure 6. The regression equation is y = −2.093x + 26.875, R2 = 0.9788, indicating that the Ct value is highly linearly related to DNA concentration.
Figure 6.
The standard curve between the Ct value in the qPCR reaction and the DNA contents in the reaction system.
The results of qPCR detection of 0.2 μL of E46 and yE46 DNA solutions are shown in Table 3. After completion of the reaction, the Ct values of E46 and yE46 were 24.0873 and 23.6052, respectively, and the total amount of DNA in the E46 and yE46 reaction systems was converted according to the standard curve. They are 21.4740 ng and 36.4961 ng, respectively. The above results indicate that there is a fold relationship between the DNA concentration and the total amount in the DNA mother liquor of E46 and yE46, that is, the total amount of DNA in yE46 is about twice that of E46. The total amount of protoplasts extracted from the two sample DNAs was equal, indicating that the amount of DNA in yE46 single cells was about 1.7-times that in E46, indicating that E46 is diploid and yE46 is tetraploid. At the same time, this result is the same as the total amount of DNA measured after protoplast extraction of DNA, and consistent with the results measured by FCM.
Table 3.
DNA concentration measured by NanoDrop and DNA concentration converted by the qPCR method.
| Sample | NanoDrop | qPCR | ||||
|---|---|---|---|---|---|---|
| DNA Conc. | Ploidy | Ct | Total Amount of DNA | DNA Conc. | Ploidy | |
| E46 | 110.7 ng/μL | 2 × | 24.0873 | 21.4740 ng | 1.0737 ng/μL | 2 × |
| yE46 | 196.1 ng/μL | 4 × | 23.6052 | 36.4961 ng | 1.8248 ng/μL | 4 × |
3. Discussion
3.1. Method for Identification of Watermelon Polyploid
In the process of inducing polyploidy, accurate and timely identification of polyploid plants can shorten the culture period and improve the efficiency of polyploid breeding. Several methods such as evaluation of stomatal size, chloroplast number of the guard cells, morphological, FCM, and chromosome counts can be used to determine polyploidy, which can be used for preliminary screening and judgment of polyploid [14,25]. Polyploid plants have obvious differences in their external morphological characteristics with diploids due to chromosome doubling, mainly in the shape and size of roots, stems, leaves, flowers, and fruits. The diameter of the ovary, the petals and anther diameter of the male flower, the leaf length and the leaf width ratio are good signs of the ploidy level of the watermelon [11]. Morphological parameters are often used as the first primary selection criteria for polyploids but are generally not completely reliable [26]. For example, in Lagerstroemia indica, plants were selected based on morphological parameters typical for induced tetraploids (increased width-to-length leaf ratios, thicker stems, higher number of chloroplasts per guard cell, and larger stomata) and afterward confirmed by FCM [27]. Surprisingly, only 50% of the morphologically screened tetraploid plants were confirmed to be tetraploids [27]. FCM quickly detects the ploidy of a large number of plants and analyzes different types of tissues and cell layers [18,28]. It allows polyploid plants to be analyzed at an early stage, which saves space and time [29,30]. However, the FCM method requires more accurate samples, e.g., the detection is tissue-specific and cell division is under control. The technique also requires expensive equipment. Counting chromosomes is the most direct and most accurate method to identify ploidy. However, chromosome counting is time-consuming and laborious, and it is necessary to make good chromosomal compression and actively divide tissue, otherwise counting is difficult [31]. Brown compared these techniques and proposed that FCM is the most effective technique [32].
3.2. RT-qPCR Could be Used for Tetraploid Characterization
Even if the above methods can successfully detect ploidy levels [14], they all have different shortcomings. Theoretically, FCM detects DNA and correlates DNA content with ploidy numbers. Inspired with this idea, we develop a widely used qPCR method. The ribosomal DNA gene (rDNA gene) is a highly repetitive and transcriptionally active gene family in the genome of eukaryotes, and the DNA encoding 45S and 5S rRNA is the most important housekeeping gene. Its tandem organization and high copy number are specific markers for determining the chromosome and karyotype of different plant species [22,33,34]. A close relationship between rDNA copy number and genome size was verified [35]. Although the process of the qPCR method is more complicated than the FCM method, which requires three steps of enzymatic hydrolysis and DNA extraction, the qPCR method requires lower samples and is not tissue-specific. In addition, the qPCR has comparable efficiency to FCM and can perform statistical analysis on a large number of cells. In our results, these two methods are quite accurate. In addition, because it requires only conventional fluorescent quantitative PCR instruments and commercial reagents, the requirements for instruments and equipment are lower and less expensive. It is equipped in conventional laboratories and the related reagent consumables are inexpensive. In contrast, the price of the conventional flow cell instrument is much higher than that of the conventional fluorescence quantitative PCR instrument. It is widely used in medical testing. The conventional plant research laboratory is less equipped, and the price of related reagents is higher, and the daily management and maintenance is more complicated. Thus supplementing the method of watermelon ploidy identification.
4. Materials and Methods
4.1. Tetraploid Induction and Morphological Characterization
The material is homologous diploid (E46, 2n) and tetraploid (yE46, 4n) watermelon. Tetraploid watermelon is induced by homologous diploid watermelon by colchicine (Sigma Chemical Company, MO, USA) [36], the diploid drying seeds were soaked in warm water at 50 °C for 30 min, soaked in 0.5% KMnO4 (Sinopharm Chemical Reagent Company, Shanghai, China) for 30 min, then washed with water and soaked in water for 6 h. Germination at 28 °C for 24–36 h. Seeded in a 72-well format tray (Grass soil:Perlite = 3:1), 25 °C. When the cotyledons of the seedlings were flattened, the new leaves of the growing point were removed, and the growth point was treated with a 0.3% colchicine, which was treated twice every day at 8:00 and 18:00 for 7 days. A total of 598 strains were treated.
The morphological characteristics of the plants in the field were observed, including leaf, seed, and fruit size. The number of chloroplasts of the lower epidermis was examined by a microscope (Olympus, Tokyo, Japan), and 10 stomatal guard cells were counted per plant to identify ploidy. The number of tetraploid strains was 35, artificial pollination followed, and finally, 20 watermelons were collected.
4.2. Flow Cytometric Analysis
The ploidy of E46 and yE46 was detected by FCM (Partec CyFlow Space, Muenster, Germany), and a small number of young leaves were taken and processed according to the instructions of the Partec CyStain UV Precise P kit (Partec, Muenster, Germany). The ploidy is judged by the fluorescence intensity X-mean of the sample. Taking the known diploid watermelon as a control, if the diploid peak is at 200, the tetraploid will be at 400.
4.3. 5S rDNA Sequence Retrieval
The 5S rDNA sequence in plants is highly conserved. Primers (Table 4) have previously reported homologous sequence cloning in the total DNA of Agave tequila [22]. The watermelon 5S rDNA fragment was used as a search sequence, and the watermelon genome sequence was searched to determine its copy number in the genome. Downloaded the Blast program software in the NCBI database and performed a local Blast search after installation (ftp://ftp.ncbi.nlm.nih.gov/blast/executables/blast+/LATEST/). The watermelon genome sequence was downloaded from the Cucurbitaceae genome database (http://cucurbitgenomics.org/). Sequence alignment was performed using Blastn.
Table 4.
5S rDNA homologous sequence clone primers.
| Gene | Forward 5′–3′ | Reverse 5′–3′ | Size (bp) | Temperature (°C) | Efficiency % |
|---|---|---|---|---|---|
| 5S | CGATCATACCAGCACTAAAGCACC | ATGCAACACGAGGACTTCCCAG | 111 | 60 | 104 |
4.4. Protoplast and DNA Isolation
Protoplasts were isolated from diploid (E46, 2 ×) and tetraploid (yE46, 4 ×) watermelon leaves (Figure 1). The leaves were grown for 2 weeks or more and were cut into 10 mm × 0.1 mm thin strips, placed in a petri dish, and 10 mL of the enzyme solution was added, and then placed in a 40 rpm shaker at 25 °C under a light-shielding condition for 4–6 h. The enzyme solution was 1.25% cellulase R10, 0.3% eductase R10, 0.4 M mannitol, 20 mM potassium chloride, and 10 mM calcium chloride, and the pH was adjusted to 5.7 [35]. After the reaction was completed, the protoplasts were collected by centrifugation at 100 g and examined by microscopy (Figure 2).
The protoplasts of different ploidy watermelons were adjusted to the same concentration by a hemocytometer, and the total DNA was extracted from an equal volume of protoplast suspension. Total DNA was extracted using the Tiangen Efficient Plant Genomic DNA Extraction Kit (DP350, Beijing, China), and the total DNA adsorbed by the adsorption column was eluted with an equal amount of TB (50 μL) buffer. The extraction process needs to strictly control the volume of the addition solution, and try to avoid the change of the total amount of DNA caused by the difference in volume of the extract.
4.5. RT-qPCR Analysis
The concentration of total DNA extracted from 4.4 was detected by NanoDrop 2,000C, and a standard curve between the Ct value after qPCR reaction and the total amount of template in the reaction system was constructed with reference to the DNA solution of E46. The qPCR instrument is the ABI QuantStudio 6 Flex real-time PCR system (Applied Biosystems, CA, USA), and the reaction system and procedure referred to the reported method [37]. Then, 0.2 μL of E46 and yE46 DNA solutions were used for qPCR detection to determine the Ct value of the template DNA. Each sample was set to 3 technical replicates and 3 biological replicates, and the reaction system and procedure were the same as the standard curve.
4.6. Statistical Analysis
Experimental data were analyzed by analysis of variance (ANOVA) and t-test using SPSS 11.5 (SPSS, Chicago, Illinois, USA).
Acknowledgments
We would like to thank Thomas Gbokie Jr. from Nanjing Agricultural University (Nanjing 210095, China) for his suggestions on experimental design.
Author Contributions
Conceptualization, N.Z. and X.H.; formal analysis, N.Z., Y.B. and Z.X.; investigation, N.Z., Y.B., X.H., Y.S., G.F., H.Z., J.R., Y.L., J.X., W.C. and C.Y.; writing—original draft preparation, N.Z., Y.B. and Z.X.; writing—review and editing, X.H.; supervision, M.T.; funding acquisition, N.Z. and G.F.
Funding
This research was funded by the National Key R&D Program of China (2018YFD0201300) and Hubei Natural Science Foundation (2018CFB686).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Blakeslee A., Avery A.G. Methods of inducing doubling of chromosomes in plants: By treatment with colchicine. J. Hered. 1937;28:393–411. doi: 10.1093/oxfordjournals.jhered.a104294. [DOI] [Google Scholar]
- 2.Soltis D.E., Misra B.B., Shan S., Chen S., Soltis P.S. Polyploidy and the proteome. Biochim. Biophys. Acta. 2016;1864:896–907. doi: 10.1016/j.bbapap.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Godfree R.C., Marshall D.J., Young A.G., Miller C.H., Mathews S. Empirical evidence of fixed and homeostatic patterns of polyploid advantage in a keystone grass exposed to drought and heat stress. R. Soc. Open Sci. 2017;4:170934. doi: 10.1098/rsos.170934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaskani M.J., Kwon S.W., Kim D.H. Comparative study on vegetative, reproductive and qualitative traits of seven diploid and tetraploid watermelon lines. Euphytica. 2005;145:259–268. doi: 10.1007/s10681-005-1644-x. [DOI] [Google Scholar]
- 5.Liu W., Zhao S., Cheng Z., Wan X., Yan Z., King S. Lycopene and citrulline contents in watermelon (Citrullus lanatus) fruit with different ploidy and changes during fruit development. Acta Hortic. 2010;871:543–550. [Google Scholar]
- 6.Zhu H., Zhao S., Lu X., He N., Gao L., Dou J., Bie Z., Liu W. Genome duplication improves the resistance of watermelon root to salt stress. Plant Physiol. Biochem. 2018;133:11–21. doi: 10.1016/j.plaphy.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Tan S.Y., Huang X.Q., Liu J.W., Liu W.G. Raising the frequency of inducing tetraploid watermelon by treating with colchicine. Acta Hort. 1995;402:18–22. [Google Scholar]
- 8.Zhang X. Tetraploid Watermelons Producing Small Fruits. 8742208. U.S. Patent. 2014 Mar 6;
- 9.Compton M.E., Gray D.J. Shoot organogenesis on cotyledons of watermelon. Hortscience A Publ. Am. Soc. Hortic. Sci. 1991;26:772. [Google Scholar]
- 10.Krug M.G.Z., Stipp L.C.L., Rodriguez A.P.M., Mendes B.M.J. In vitro organogenesis in watermelon cotyledons. Pesqui. Agropecuária Bras. 2005;40:1678–3921. doi: 10.1590/S0100-204X2005000900004. [DOI] [Google Scholar]
- 11.Compton M.E., Gray D.J., Elmstrom G.W. Identification of tetraploid regenerants from cotyledons of diploid watermelon culturedin vitro. Euphytica. 1996;87:165–172. doi: 10.1007/BF00023744. [DOI] [Google Scholar]
- 12.Rhodes B., Zhang X.P. Hybrid seed production in watermelon. New Seed. 1999;1:69–88. doi: 10.1300/J153v01n03_03. [DOI] [Google Scholar]
- 13.McCuistion F., Elmstrom G.W. Identifying polyploids of various cucurbits by various guard cell chloroplast numbers. Proc. Fla. State Hort. Soc. 1993;106:155–157. [Google Scholar]
- 14.Sari N., Abak K., Pitrat M. Comparison of ploidy level screening methods in watermelon: Citrullus lanatus (Thunb.) Matsum. and Nakai. Sci. Hortic. Amst. 1999;82:265–277. doi: 10.1016/S0304-4238(99)00077-1. [DOI] [Google Scholar]
- 15.Fahleson J., Dixelius J., Sundberg E., Glimelius K. Correlation between flow cytometric determination of nuclear DNA content and chromosome number in somatic hybrids within Brassiceae. Plant Cell Rep. 1988;7:74–77. doi: 10.1007/BF00272983. [DOI] [PubMed] [Google Scholar]
- 16.Liu W., Ming W., Yan A. AFLP Analysis of the Genetic Diversity between Diploid and Autopoly-ploidy Watermelon. J. Fruit Sci. 2004;21:46–49. [Google Scholar]
- 17.Fox M.H., Galbraith D.W. Application of flow cytometry and sorting to higher plant systems. Flow Cytom. Sorting. 1990:633–650. [Google Scholar]
- 18.Arumuganathan K., Earle E.D. Estimation of nuclear DNA content of plants by flow cutometry. Plant Mol. Biol. Report. 1991;9:221–231. doi: 10.1007/BF02672017. [DOI] [Google Scholar]
- 19.Koh G.C. Tetraploid production of Moodeungsan watermelon. J. Kor. Soc. Hortic. Sci. 2002;43:671–676. [Google Scholar]
- 20.Jaskani M.J., Kwon S.W., Koh G.C., Huh Y.C., Ko B.R. Induction and characterization of tetraploid watermelon. J. Kor. Soc. Hort. Sci. 2004;45:60–65. [Google Scholar]
- 21.Fleige S., Pfaffl M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Asp. Med. 2006;27:126–139. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Rubio-Piña J., Quiroz-Moreno A., Sánchez-Teyer L.F. A quantitative PCR approach for determining the ribosomal DNA copy number in the genome of Agave tequila Weber. Electron. J. Biotechnol. 2016;22:9–15. doi: 10.1016/j.ejbt.2016.05.002. [DOI] [Google Scholar]
- 23.Guo S., Zhang J., Sun H., Salse J., Lucas W.J., Zhang H., Zheng Y., Mao L., Ren Y., Wang Z., et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013;45:51–58. doi: 10.1038/ng.2470. [DOI] [PubMed] [Google Scholar]
- 24.Bennett M.D., Leitch I.J. Nuclear DNA amounts in angiosperms: Progress, problems and prospects. Ann. Bot. 2005;95:45–90. doi: 10.1093/aob/mci003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren X., Wang X., Xiao F., Zhou Y., Wei L. Polyploidy Induction and Identification of Hibiscus syriacus. J. West China For. Sci. 2019;48:119–125. [Google Scholar]
- 26.Norrmann G., Quarin C., Keeler K. Evolutionary implications of meiotic chromosome behavior, reproductive biology and hybridization in 6× and 9×cytotypes of Andropogon gerardii (Poaceae) Am. J. Bot. 1997;84:201–208. doi: 10.2307/2446081. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q.Y., Luo F.X., Liu L., Guo F. In vitro induction of tetraploids in crape myrtle (Lagerstroemia indica L.) Plant Cell Tissue Organ Cult. 2010;101:41–47. doi: 10.1007/s11240-009-9660-5. [DOI] [Google Scholar]
- 28.Leus L., Van Laere K., Dewitte A., Van Huylenbroeck J. Flow clytometry for plant breeding. Acta Hortic. 2009;836:221–226. doi: 10.17660/ActaHortic.2009.836.31. [DOI] [Google Scholar]
- 29.Väinölä A. Polyploidization and early screening of Rhododendron hybrids. Euphytica. 2000;112:239–244. doi: 10.1023/A:1003994800440. [DOI] [Google Scholar]
- 30.Montijn M.B., Houtsmuller A.B., Ten Hoopen R., Oud J.L., Nanninga N. The 5S rRNA gene clusters have a defined orientation toward the nucleolus in Petunia hybrida and Crepis capillaries. Chromosome Res. 1999;7:387–399. doi: 10.1023/A:1009272115295. [DOI] [PubMed] [Google Scholar]
- 31.De Laat A.M.M., Göhde W., Vogelzang M.J.D.C. Determination of ploidy of single plants and plants population by flow cytometry. Plant Breed. 1987;99:303–307. doi: 10.1111/j.1439-0523.1987.tb01186.x. [DOI] [Google Scholar]
- 32.Brown S.C., Devaux P., Marie D., Bergounioux C., Petit P.X. Cytométrie enflux: Application à l’analyse de la ploidie chez les végétaux. Biofuture. 1991;105:2–16. [Google Scholar]
- 33.Dhar M.K., Kaul S., Friebe B., Gill B.S. Chromosome identification in Plantago ovata Forsk. Through C-banding and FISH. Curr. Sci. 2002;83:150–152. [Google Scholar]
- 34.Murata M., Heslop-Harrison J.S., Motoyoshi F. Physical mapping of the 5S ribosomal RNA genes in Arabidopsis thaliana by multi-color fluorescence in situ hybridization with cosmid clones. Plant J. 1997;12:31–37. doi: 10.1046/j.1365-313X.1997.12010031.x. [DOI] [PubMed] [Google Scholar]
- 35.Tian S., Jiang L., Gao Q., Zhang J., Zong M., Zhang H., Ren Y., Guo S., Gong G., Liu F., et al. Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep. 2017;36:399–406. doi: 10.1007/s00299-016-2089-5. [DOI] [PubMed] [Google Scholar]
- 36.Zhang N., Zeng H.X., Shi X.F., Ren J., Cheng W.S., Yang Y.X., Li Y.H., Sun Y.H. Selection of Tetraploid of a Yellow Flesh Mini-Watermelon Using Oryzalin. Adv. Mater. Res. 2013;838:2449–2454. doi: 10.4028/www.scientific.net/AMR.838-841.2449. [DOI] [Google Scholar]
- 37.Huang X., Chen J., Bao Y., Liu L., Jiang H., An X., Dai L., Wang B., Peng D. Transcript Profiling Reveals Auxin and Cytokinin Signaling Pathways and Transcription Regulation during In Vitro Organogenesis of Ramie (Boehmeria nivea L. Gaud) PLoS ONE. 2014;9:e113768. doi: 10.1371/journal.pone.0113768. [DOI] [PMC free article] [PubMed] [Google Scholar]