Abstract
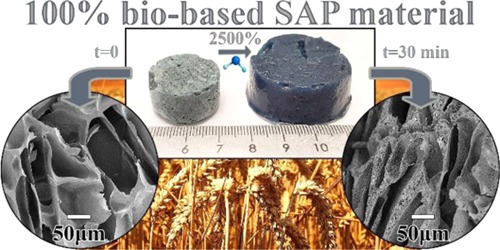
The development of fully natural wheat gluten foams showing rapid and high uptake of water, sheep blood, and saline solution, while maintaining high mechanical stability in the swollen state, is presented. Genipin was added as a natural and polar cross-linker to increase the polarity of the protein chains, whereas cellulose nanofibers (CNFs) were added as a reinforcement/stiffener of the foams, alone or in combination with the genipin. The presence of only genipin resulted in a foam that absorbed up to 25 g of water per gram of foam and a more than 15 g uptake in only 8 min. In contrast, with CNF alone, it was not possible to maintain the mechanical stability of the foam during the water uptake and the protein foam disintegrated. The combination of CNF and genipin yielded a material with the best mechanical stability of the tested samples. In the latter case, the foam could be compressed repeatedly more than 80% without displaying any structural damage. The results revealed that a strong network had formed between the wheat gluten matrix, genipin, and cellulose in the foam structure. A unique feature of the absorbent/foam, in contrast to commercial superabsorbents, was that it was able to rapidly absorb nonpolar liquids (here, n-heptane) due to the open-cell structure. The capillary-driven absorption due to the open-cell structure, the high liquid absorption in the cell walls, and the mechanical properties (both in dry and swollen states) of these natural foams make them interesting as a sustainable replacement for a range of petroleum-based foam materials, including absorbent hygiene products such as sanitary pads.
1. Introduction
Wheat gluten (WG) protein is obtained as an industrial product stream that stands out among proteins due to its high molecular weight and unique ability to form stable cross-linked networks, even in its plasticized state.1−5 WG has been extensively evaluated for its properties, showing its potential as an interesting natural-based viscoelastic material that is possible to be processed with traditional plastic-processing techniques, e.g., extrusion and injection/compression molding.6−13 Its high cohesion/melt strength also makes WG suitable for expansion into stable foams.13−15 We have in previous works shown that stiff or soft (glycerol-plasticized) WG foams absorb large amounts of polar liquids and are suitable as sponges/absorbent materials.15,16 Besides capillary-driven uptake, these foams also absorb a high volume of water in the cell walls, leading to an uptake of more than 30 times its initial weight, providing superabsorbent properties.15,17 The capillary action of the open cells in freeze-dried WG foams also led to a rapid and relatively high uptake of nonpolar liquids.15 The mechanical stability/integrity of the dry and swollen WG-based foam can be improved by the use of compounds that cross-link the protein network, in addition to what is achieved by internal disulfide cross-links in the protein itself. The most commonly used cross-link agents for proteins are dialdehydes such as glutaraldehyde, glyoxal, and formaldehyde.18−21 These are, however, toxic unless fully reacted in the protein network, and they are typically not biobased. This is unfortunate since the development of a formulation based on solely nontoxic elements would facilitate the development of WG as a fully sustainable and safe material.
Thermally insulating and flame-retardant WG foams have previously been made with the addition of in situ polymerized silica.13 Conductive WG foams have been obtained using carbon nanotubes (CNTs) and nanosized carbon black.14 In addition, cellulose nanofibers (CNFs, ∼1 wt %),22 cellulose nanocrystals (CNCs, 10 wt %)23 and CNTs (>10 wt %)14 were demonstrated to increase the stiffness of the WG material by a factor of up to 10. The CNFs and CNCs are interesting as fillers since they provide reinforcement for a fully biobased and biodegradable material.24−29 Also, CNF and CNC hydrogels with high swelling capacity have been previously reported, demonstrating the capability of these materials to function as fillers in absorbent biobased materials.30−32 Hence, it has been demonstrated that WG as a raw material has the potential to produce materials with tunable properties, which are sufficient to compete with fossil-oil-based plastics, e.g., absorbent products.33 Life-cycle analysis of protein-based plastics (WG) was previously compared to four common oil-based plastics, polypropylene, high- and low-density polyethylene, and polyamide 6,6, demonstrating a reduction of greenhouse gases, when using WG, in the range of 1–8 kg gas per kg material.34
The development of highly liquid-absorbing, fully natural WG foams with mechanical stability in the swollen state is presented here. The liquids tested for absorption were water, 0.9 wt % NaCl aqueous salt solution, and defibrinated sheep blood, which are important in the context of evaluating absorbent hygiene products. Genipin (GEN), a naturally occurring and harmless cross-linker extracted from the fruit of the Gardenia jasminoidesEllis plant,35−37 was used as an alternative to dialdehyde cross-linkers. In addition, cellulose nanofibers were added to stiffen/reinforce the foam. Surprisingly, the addition of CNF alone led to foams that disintegrated in the liquids while showing the highest compression modulus in the dry state. On the other hand, the combination of CNF and genipin yielded a foam with high mechanical stability in the swollen state, resembling commercial foams encountered in daily-care products. Because of its open-cell structure and contributions from capillary actions, the foam could also rapidly and readily absorb nonpolar n-heptane.
2. Results and Discussion
2.1. Foam Morphology
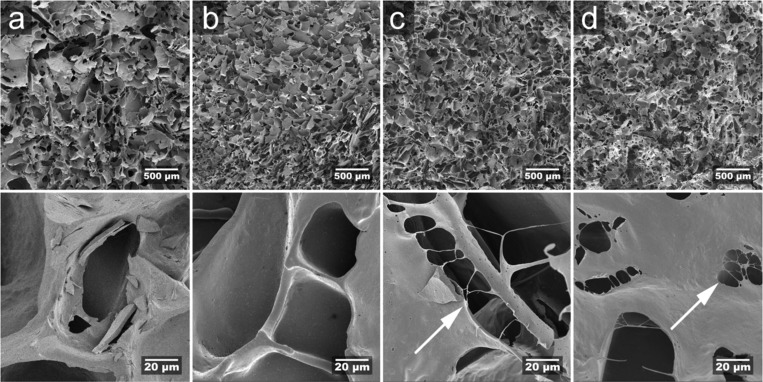
Figure 1 shows the surfaces of the cryofractured dry foams. The porous structure of the WG reference foam was similar to that of foams investigated previously, consisting mainly of open cells (Figure 1a).13−15,22 As shown in Table 1 and in the upper images in Figure 1, the cells were larger in the WG reference foam than in the other three foams containing cellulose nanofibers, genipin, or a mixture of these, where the cells showed relatively similar size. The density was also the highest and the porosity consequently the lowest in the WG foam (Table 1). The porosity range of the four foams was 86–90%. As shown in Figure 1, the cell walls often had holes (least frequent in WG and WG/CNF and most frequent in WG/CNF/GEN). They were located close to the cell wall edges, as in the WG/GEN sample (Figure 1c) or close to the middle of the cell walls, as in the WG/CNF/GEN sample (Figure 1d). Note the fibrillar strands that bridge the holes in the samples containing genipin (Figure 1c,d). These features are a consequence of the consistency of the dispersion and its effect on the formation of ice and cells in the freeze-drying process. The dispersion apparent viscosity observed during mixing was the lowest for WG and WG/CNF and increased significantly when genipin was added, especially in the presence of cellulose nanofibers. The homogenized foam (air/liquid dispersion mixture) was stable for more than an hour in the presence of genipin, but without genipin, the foamed suspension collapsed during cooling from 90 °C. No clear border separating the foam above the liquid part and the liquid existed when genipin was used.
Figure 1.
Scanning electron micrographs (SEM) of low (top) and high (bottom) magnification showing dry (a) WG, (b) WG/CNF, (c) WG/GEN, and (d) WG/CNF/GEN. The arrows indicate holes with fibrillar strands in the cell walls.
Table 1. Foam Characteristics.
| sample | cell size (μm) | density (kg/m3) | porosity (%) |
|---|---|---|---|
| WG | 286 ± 67 | 182 ± 11 | 86 ± 1 |
| WG/CNF | 166 ± 37 | 159 ± 1 | 88 ± 0 |
| WG/GEN | 167 ± 30 | 146 ± 10 | 89 ± 1 |
| WG/GEN/CNF | 186 ± 44 | 135 ± 1 | 90 ± 0 |
The WG/GEN dispersion changed color during the stirring from yellow/light brown to green, indicating a reaction between genipin and amino acids/protein in the presence of oxygen.38 This, together with the observed increase in viscosity and stability of the foam dispersion, indicated that genipin formed cross-links and/or bulky polar side groups grafted onto the protein chain. Since the dispersion viscosity was the highest and the color change greatest among the other samples in the presence of both genipin and CNF, a strong molecular network seemed to form between the WG, genipin, and CNF. To verify that there was a reaction not only between WG and genipin but also between genipin and CNF, the latter two were mixed together following the protocol for making WG/CNF/GEN but without WG. The change in color to green of the whitish CNF dispersion when the white genipin powder was added indicated a reaction between genipin and CNF (Figure S1, Supporting Information). When the supernatant was removed by centrifugation, it was seen that the CNF pellet had changed color from white to light yellow/brown (Figure S1d). In addition, a comparison of the IR spectra of neat CNF and CNF reacted with genipin indicated that the −OH concentration was lower in the latter. This, together with the absence of the intrinsic genipin carbonyl band in this sample indicated that a reaction had occurred between CNF and GEN (Figure S2, Supporting Information).
2.2. Liquid Absorption in the Foams
The materials swollen in water and saline solution using the tea-bag method became gels, as illustrated for water in Figure 2. The exception was the WG/CNF foam, which rapidly disintegrated into a slurry without any mechanical integrity. As shown in Figure 3, the uptake of water and saline solution was the lowest in this sample. The presence of cellulose nanofibers alone apparently reduced the cohesion of the protein material in the wet state. However, together with genipin, the foam containing CNF showed high cohesion properties (Figure 2d) with a high mechanical stability in the swollen state, leading to a lower liquid uptake than that of WG/GEN (Figure 3). The highest apparent uptake of water after 16 h swelling was observed for WG and WG/GEN, with, respectively, 18 and 25 g of water per gram of dry foam, corresponding to weight increases of, respectively, ca. 2000 and 2900% (Figure 3a).
Figure 2.
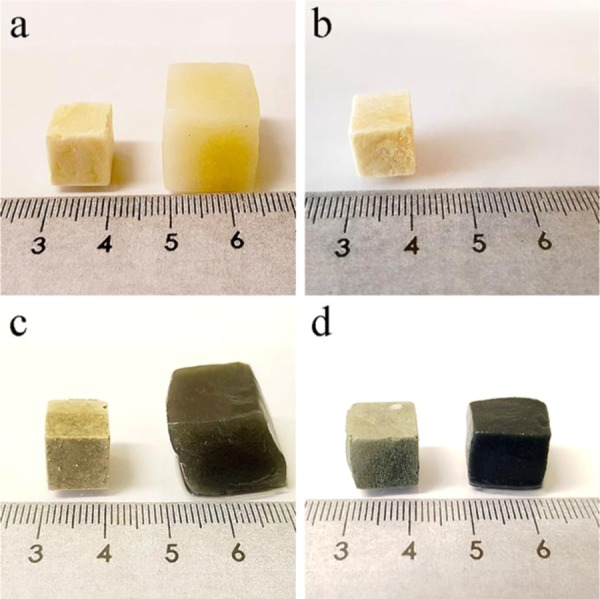
Appearance of the cuboid foams before (left) and after 16 h of swelling in water (right): (a) WG, (b) WG/CNF, (c) WG/GEN, and (d) WG/CNF/GEN.
Figure 3.
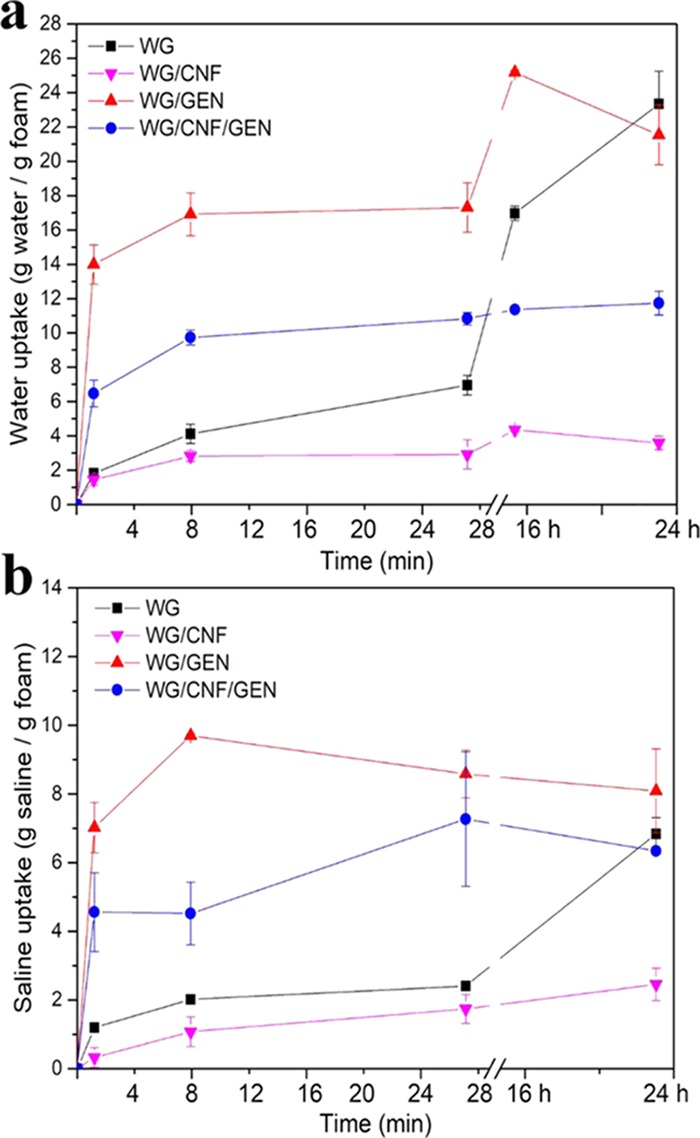
Free swelling capacity in water (a) and saline (b) using the tea-bag method.
The saline absorption in the WG and WG/GEN foams was reduced by 72 and 63%, respectively, compared to their maximum uptake in water. This decrease in the uptake of saline solution was due to the combined decrease in osmotic pressure and charge repulsion effects when the liquid contained mobile ions.39,40 The difference in uptake between water and saline solution was smaller for WG/CNF/GEN and WG/CNF (Figure 3), and the saline absorption in the WG, WG/GEN, and WG/CNF/GEN foams was in the range that made them interesting as fully biobased alternatives in absorbent hygiene products.17,33 The blood swelling tests revealed that the WG/CNF/GEN material was able to swell ca. 7 g/g of blood within the first 10 min, corresponding to 50% of the blood swelling obtained for foams taken from a commercial sanitary pad. The lower absorption of the protein foam compared to the commercial foam is likely due the pore sizes observed for the latter, which were overall 10 times smaller than those of the WG/CNF/GEN material (Figure S3, Supporting Information). However, the protein-based material revealed the ability to behave in a manner similar to that of the commercial sanitary foam, i.e., it was possible to repeatedly squeeze the blood out of the material and reabsorb the fluid within seconds (Video S1, Supporting Information).
The effects of capillary action were evaluated by a rapid absorption test in a solvent that is not absorbed into the foam cell walls.15,16Table 2 shows that the 1 s absorption of n-heptane was by far the highest in WG/GEN/CNF. This can be explained by the numerous holes in the cell walls, giving this sample a greater porosity than the other foams (Figure 1d). If all of the cells are completely filled with n-heptane (Qnh), the uptake can be calculated from the known density of n-heptane (ρnh = 684 kg/m3)41 and solid WG (ρWG, 1300 kg/m3) and the foam porosity (vc, Table 1), using eq 1
| 1 |
Table 2 shows that, in contrast to the other foams, almost all of the cells were filled in the WG/GEN/CNF foam during the 1 s immersion. This material also showed the highest 1 s water uptake, although the differences were smaller between the samples than in the case of n-heptane.
Table 2. Short-Term Liquid Uptake (g Liquid/g Dry Foam)a.
| sample | uptake after 1 s in n-heptane | uptake after 1 s in water | predicted uptake in cells (n-heptane) |
|---|---|---|---|
| WG | 2.6 | 1.8 | 3.2 |
| WG/CNF | 2.6 | 2.3 | 3.8 |
| WG/GEN | 2.4 | 2.3 | 4.2 |
| WG/GEN/CNF | 4.2 | 2.4 | 4.5 |
Dry foam is in this case a foam equilibrated at 23 °C and 50% relative humidity (RH).
The short-term uptake of water in a commercial superabsorbent material was also determined for comparison. It is noteworthy that, with a 1 s value of ca. 1.5 g/g, its short-term uptake was lower than that of all of the WG foams (Table 2).
The Voigt equation (eq 3) was fitted to the water uptake data for the first 1600 s (27 min), which included the rapid initial uptake within the first 1 min and the following levelling off (Figure 3a). Hence, the further sizable uptake of water in longer times, which was large for WG and WG/GEN, was not included. The very high rapid uptake in the WG/GEN foam resulted in the shortest characteristic swelling time (τ = 44 s) and the highest pre-exponential factor (Qo = 17 g/g) (Table 3). The longest τ was observed for WG (506 s), explained by this having the highest density, whose τ was in the same range as that reported earlier for water in cottonseed protein grafted with a synthetic acrylic acid monomer, but it was not as small as in the present WG/GEN foam.42,43 Although the R2 value for this sample was lower for the saline uptake than for water, the most rapid and high uptake of saline was observed for WG/GEN (τ = 51 s and Qo = 9.1 g/g, Table 3). This foam became a large gel within the first minute, which is explained by the high polarity of genipin remaining in the reacted/cross-linked state. The highly alkaline conditions used for the foam preparation could also induce hydrolysis of the methyl ester group present in the genipin molecule, inducing the formation of neutralized carboxylic acid groups that provide higher electrostatic repulsion and explain the rapid water swelling hereby obtained.33,44,45 A small τ and high Qo were also observed for the WG/CNF/GEN, which shows the importance of having genipin in the formulation if a fast and high uptake of both water and saline is desired.
Table 3. Fitted Parameters in the Voigt Equation.
| sample | solution | Qo (g liquid/g foam) | τ (s) | R2 |
|---|---|---|---|---|
| WG | water | 7.1 ± 0.9 | 506 ± 175 | 0.9546 |
| WG | saline | 2.2 ± 0.1 | 100 ± 26 | 0.9687 |
| WG/CNF | water | 2.9 ± 0.02 | 107 ± 4 | 0.9994 |
| WG/CNF | saline | 1.8 ± 0.1 | 521 ± 74 | 0.9928 |
| WG/GEN | water | 17.1 ± 0.1 | 44 ± 2 | 0.9994 |
| WG/GEN | saline | 9.1 ± 0.4 | 51 ± 11 | 0.9836 |
| WG/CNF/GEN | water | 10.3 ± 0.4 | 76 ± 12 | 0.9877 |
| WG/CNF/GEN | saline | 5.89 ± 0.9 | 50.6 ± 40 | 0.7916 |
Beyond ca. 28 min, the additional water uptake was small for WG/CNF and WG/CNF/GEN (Figure 3a). The WG foam showed, on the other hand, a greater increase in uptake of both water and saline between 28 min and 24 h. The situation for WG/GEN was more complex. In water, a similar uptake pattern as with WG was observed, except that a decrease in weight was observed toward the swelling time above 16 h. In saline, the weight of the WG/GEN foam decreased already beyond 8 min of swelling. The decrease in weight, coupled with the blue coloring of the water and saline during the later stages of swelling (above ca. 16 h), indicated that genipin reacted to a certain degree with water/saline-soluble peptides/amino acids, which were slowly leached from the foam to the solution. Genipin was considered to be grafted onto the protein chains without always forming a cross-link. With all of the genipin-containing foams, the liquid turned slightly blue and the actual weight change pattern at longer times depended on whether uptake or loss dominated. It should be emphasized that the long-term swelling behavior is irrelevant for several absorbent applications, especially those related to hygiene products.
The capillary-driven uptake was not the only absorption mechanism since the maximum swelling was greater than the expected uptake if only the pores were filled with liquid and no uptake occurred in the cell wall material (Tables 2 and 3).15 The swelling in saline also indicated that the expansion of the foams was affected by absorption in the cell walls since it was 50% lower than when pure water was used, i.e., the cell wall expansion was limited due to the presence of salt ions.31,33,46
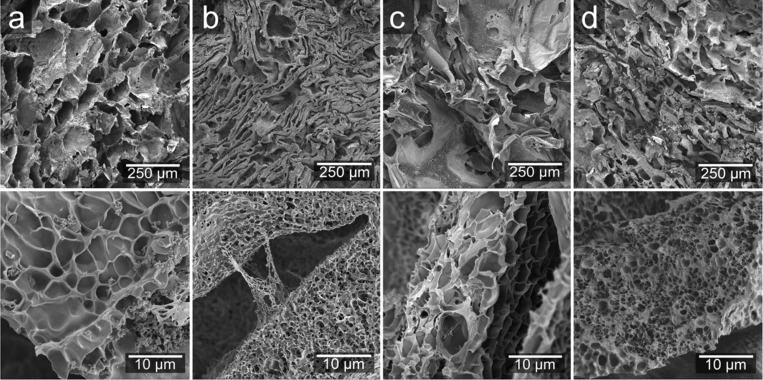
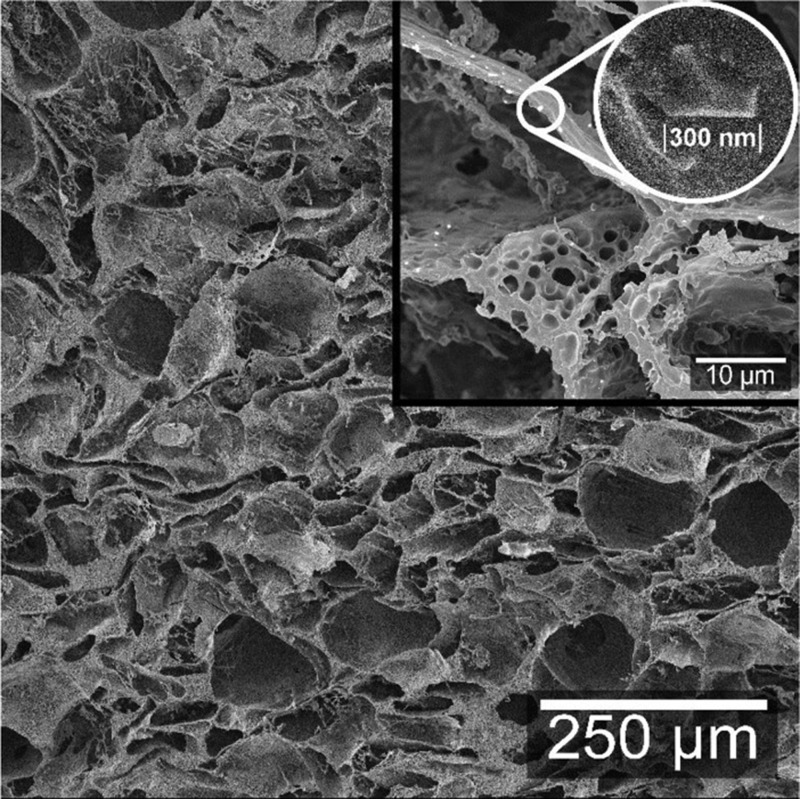
The uptake in the cell wall was revealed by a second freeze-drying of the swollen foam, which is also a way of obtaining a higher-porosity bimodal cell size system.15 This was done on foams that had been subjected to 24 h swelling in water and contained in the tea bags. The swollen materials were frozen in liquid nitrogen while contained in the tea bag prior to the surface analysis. The cryofractured surfaces of these revealed numerous small cells (≤10 μm) inside the cell wall, which was a consequence of ice formation and subsequent sublimation (Figure 4). The structure of the freeze-dried WG/CNF water-swollen foam was very different from that of the other foams (lower-magnification images in Figure 4b with Figure 4a,c,d). The cells developed during the first freeze-drying collapsed during subsequent swelling in water, probably due to the poor cohesion and mechanical stability of this foam. The saline-swollen foam structures were similar to those of the water-swollen foams (see WG foam in Figure 5), but with the second freeze-drying, it was also possible to see that salt ions had entered the cell walls since salt crystals were observed embedded within the cell wall (Figure 5 inset). Nonetheless, the absence of a secondary porous structure in the foams taken from a commercial sanitary pad and swollen in the exact same way as the WG foams suggested that the majority of the swelling in the former material is solely due to capillary-driven actions (Figure S3b, Supporting Information). This represents an advantage over commercial non-bio-based foams since the swelling is due to both capillary-driven absorption and cell wall expansion.
Figure 4.
SEM micrographs of (a) WG, (b) WG/CNF, (c) WG/GEN, and (d) WG/CNF/GEN after 24 h immersion in water.
Figure 5.
Structure of the WG foam after 24 h immersion in saline solution. An embedded salt crystal is shown in the inset.
2.3. Mechanical Properties
The modulus of the dry WG foam was 13 MPa, which was higher than previously reported values due to the higher density of the present WG foam.13−15 The cellulose nanofibers (1 wt %) resulted in an increase in the modulus of the WG foam by ca. 85% (WG/CNF, Table 4). In addition, the compression modulus of WG/CNF was at least twice as high as that previously reported for reinforced WG-based foams with the same densities as the materials developed here.13−15 The strength (σs/σ10) was, however, only slightly improved by adding CNF since this foam (WG/CNF) was more brittle than the other foams and fragmented completely during the deformation (Figure 6a) long before 80% strain was reached. An additional reason for the only slight improvement in strength was that its density was lower than that of the pure WG foam. The addition of genipin (WG/GEN) did not increase the stiffness, but it improved the strength of the foam. The density was, however, lower than those of both the WG and WG/CNF foams. The combination of CNF and genipin (WG/CNF/GEN) yielded a foam with an only marginally higher stiffness than that without CNF (WG/GEN), and it had the lowest yield/σ10 strength of all of the foams studied (Table 4). This is explained by the fact that WG/CNF/GEN had the lowest density of the tested materials (Table 1). The stress at the maximum strain (80%) increased with increasing initial density (WG/CNF/GEN < WG/GEN < WG) (Figure 6a).
Table 4. Mechanical Properties.
| dry sample | E (MPa) | σs (MPa) | σ10 (MPa) |
|---|---|---|---|
| WG | 13.0 ± 1.6 | 0.42 ± 0.06 | 0.48 ± 0.05 |
| WG/CNF | 20.6 ± 0.8 | 0.48 ± 0.06 | 0.48 ± 0.07 |
| WG/GEN | 12.3 ± 1.1 | 0.63 ± 0.24 | 0.71 ± 0.22 |
| WG/CNF/GEN | 13.2 ± 2.4 | 0.31 ± 0.04 | 0.32 ± 0.03 |
| wet sample | E (kPa) | σs (kPa) |
|---|---|---|
| WG | 3.7 ± 1.5 | 0.31 ± 0.09 |
| WG/GEN | 1.3 ± 0.1 | 0.14 ± 0.04 |
| WG/CNF/GEN | 1.9 ± 0.5 | 0.22 ± 0.04 |
Figure 6.
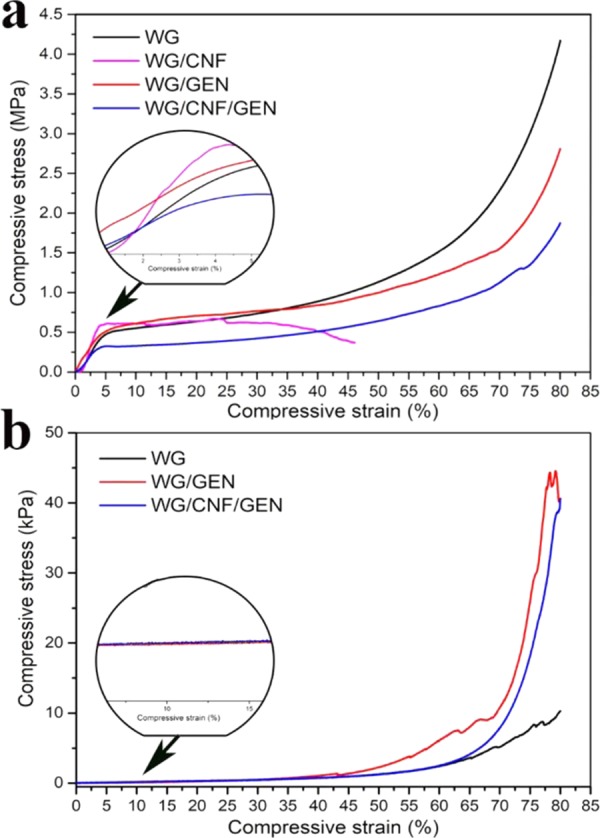
Representative compressive stress–strain curves of foams (a) conditioned at 50% RH and (b) swollen for 16 h in water.
The stiffness and strength of the wet foams were considerably lower (3–4 orders of magnitude) than those of the dry foams, showing the effect of water as a plasticizing agent (Table 4). Since the addition of CNF (WG/CNF) resulted in a foam that fragmented in water, mechanical testing could not be performed. It should be noted that the foams containing genipin were very soft and, in the case of WG/CNF/GEN, retained their shape with no apparent plastic deformation or partial fracture after exposure to a high compressive strain (Figure 6b). The swollen WG/CNF/GEN foam recovered ca. 75% of its original size/shape during the first 20 s after repeated high compression and release (Video S2, Supporting Information). The recovery was smaller for the wet WG/GEN and wet WG foams, where significant damage occurred before the maximum strain was reached. To conclude, the combination of genipin and cellulose nanofibers yielded a natural-based foam with remarkably high gel strength.
2.4. Molecular Structure
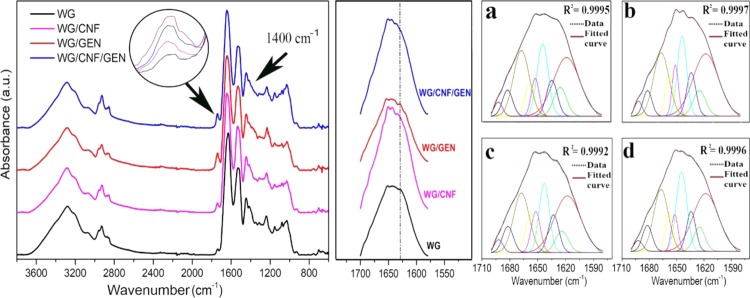
The IR spectra of the samples are shown in Figure 7. The carbonyl peak at 1730–1750 cm–1 was higher for WG/GEN than for the other foams, a consequence of the ester group in the genipin molecule. In addition, the increase in the peak intensity at 1720 cm–1 and 1440–1320 cm–1 for WG/GEN (and also, but to a lesser extent, for WG/GEN/CNF) suggests the formation of carboxylic acid (−COOH/–COO–) functional groups.47,48 This group formation could be a consequence of the hydrolysis of the methyl ester group in the genipin molecule due to the alkali conditions used for the foam preparation, also explaining the high/rapid swelling obtained for the WG/GEN specimen.44,45 The deconvolution of the nine peaks in the amide I region is shown in Figure 7 and Table 5. The peak positions were fixed and were the same as in previous publications.49−52 When the R2 value for the fitting reached 0.9992 or higher, the peaks were allowed to move a maximum of ±1 cm–1 from their initial position, and the fitting iteration process continued until an R2 value above 0.9995 was obtained. This was possible for all samples except WG/GEN. Overall, the variation in the content of the different secondary structures was relatively small (Table 5). The content of strongly hydrogen-bonded β-sheets was the highest for WG/CNF and the lowest for WG/GEN, indicating that the CNF promoted more protein aggregation than genipin. Less aggregated proteins should show a higher content of α-helices and unordered chains,9,12,52,53 which is in line with the higher values for the genipin-containing samples. It appears that the grafting and cross-linking using genipin resulted in a more constrained molecular structure, reducing the degree of aggregation compared to the other foams. Both CNF and genipin, at least in its unreacted form, absorb infrared radiation in the amide I region at 1630 cm–1 (CNF)54 and 1680/1621 cm–1 (unreacted genipin). However, the changes in the amide I spectra [Figure 7 (middle)] when the two additives were present could not be explained by the presence of their individual peaks, probably due to the small content of the two, even though changes in the carbonyl peak (Figure 7, left, inset) were readily observed for WG/GEN. This IR peak is, however, very sensitive to carbonyl content. Hence, the deconvolution and peak fitting were made, ignoring any contribution from CNF and genipin.
Figure 7.
Full IR spectra (left), amide I region (middle), and deconvoluted amide I region with the corresponding resolved/fitted curves of (a–d) WG, WG/CNF, WG/GEN, and WG/CNF/GEN, respectively.
Table 5. Resolved Peaks from Deconvoluted IR Spectra in the Amide I Region.
| Gaussian
component (%) |
|||||
|---|---|---|---|---|---|
| λ (cm–1) | assignment | WG | WG/CNF | WG/GEN | WG/CNF/GEN |
| 1618, 1625 | β-sheets, strongly H-bonded | 37.2 | 38.4 | 35.5 | 36.8 |
| 1634, 1681 | β-sheets, weakly H-bonded | 12.9 | 12.7 | 13.6 | 13.0 |
| 1644, 1651, 1658 | α-helices and unordered material | 27.4 | 27.6 | 30.1 | 29.4 |
| 1667, 1692 | β-turns | 22.5 | 21.8 | 20.9 | 20.9 |
The extraction buffer and procedure used for the size-exclusion high-performance liquid chromatography (SE-HPLC) analysis are known to extract 100% of the proteins that are present in the raw WG powder and also in WG foams prepared by denaturing the protein in a pH 11 aqueous dispersion heated to 75 °C.13,22 However, denaturing the protein at 90 °C, as performed here, increases the degree of cross-linking/polymerization of the protein. The total extraction was ca. 70% of that of the foams previously made at 75 °C.13 The WG/CNF, WG/GEN, and WG/CNF/GEN foams had a total relative extraction of 110–150% compared to the WG foam (Figure 8a), which is the result of changes in the protein structure (protein chain folding and/or changes in the protein chemical structure) leading to different light absorption/reflectance patterns, which affect the HPLC detector signal and the HPLC output data, as previously observed.22,55 It is, nevertheless, possible to detect certain trends in the extraction data. Proteins were generally more easily extractable from the WG/CNF foam than from the other foams (more from Ext 1 and 2 than from Ext 3), whereas the IR spectrum indicated a more ordered structure. Thus, the addition of CNF seemed to contribute with a greater number of β-sheet aggregates but of relatively smaller sizes, making them readily extractable. Both GEN and CNF, alone or in combination, led to an increase in the polymer-to-monomer ratio of the extracted protein, indicating an increase in the overall protein polymer size compared to that of the WG foam (Figure 8b).
Figure 8.
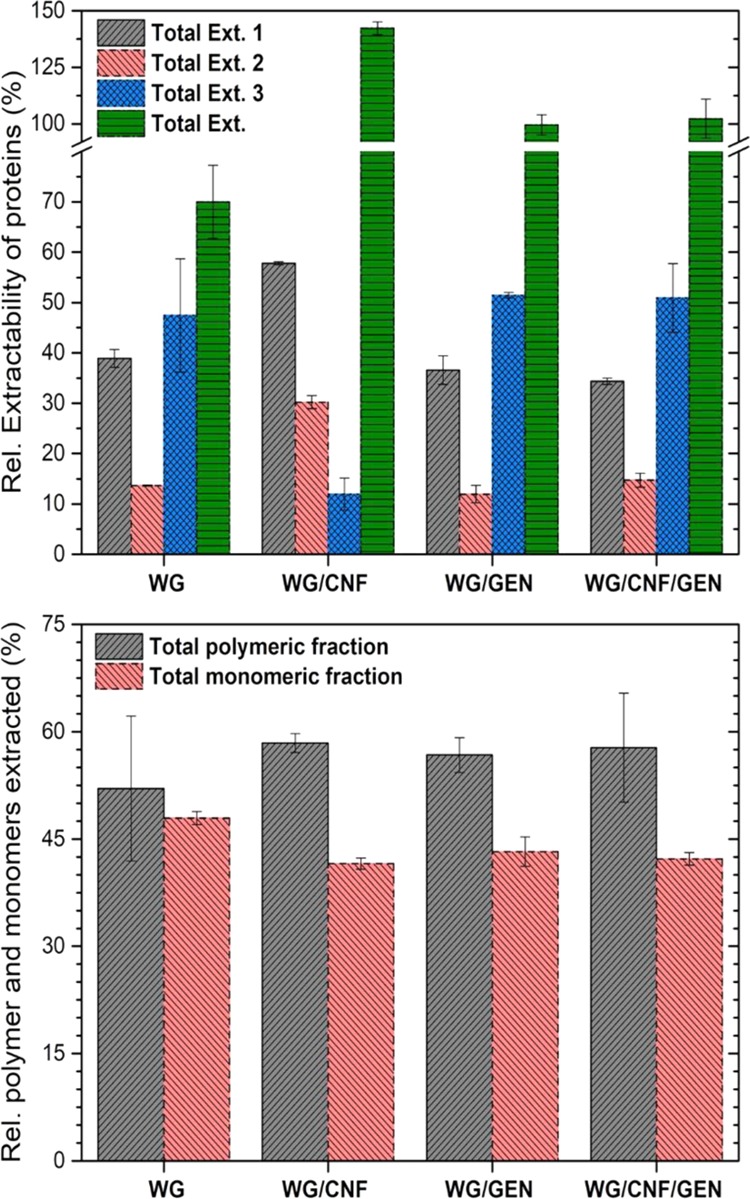
Relative extractability of proteins using the three-step SE-HPLC method. The total extractable proteins were normalized to WG assuming a total protein extraction of 70%.
The amount of genipin grafted onto the protein chains depends not only on the amount of genipin added (only 1 wt % here) but also on the groups available for reaction, which are mainly the amine groups of the lysine residue. In addition, for a covalent cross-link to be formed, two grafted lysine residues each with a genipin molecule have to meet.36,56 WG has a relatively low lysine content compared to proteins where a high degree of genipin cross-linking has been observed, e.g., soy protein.35,57,58 Nevertheless, the low content of genipin used here led to a suitable mix of grafted and cross-linked polar molecules, which provided rapid liquid uptake and a foam with high mechanical stability in the swollen state, especially where CNF was also present. Future work should concern the optimization of the genipin content relative to the protein reactive sites to further tailor the properties of these biofoams toward promising substitutes for several applications nowadays dominated by petroleum-based absorbent/porous materials.
3. Conclusions
A fully natural and biobased foam of wheat gluten, containing genipin and cellulose nanofibrils, was demonstrated as a porous material with superabsorbent (up to 25 times its initial weight in water) and rapid swelling properties. The porous WG/CNF/GEN material absorbed ca. 10 times its own weight in both water and saline, and about 7 g/g of blood, while maintaining a high mechanical integrity, which was in contrast to the behavior when CNF was used alone as a foam filler. The wet foam could be repeatedly compressed to a high strain value (80%), showing essentially no damage to the foam after compression. A suggested structure involving WG and genipin giving rise to the specific properties of the foam, with and without CNF, is shown in Figure 9. A unique property of the present absorbent/foam compared to that of commercial superabsorbents is that, besides the high absorption of polar liquids, it also rapidly absorbed the nonpolar n-heptane as a result of the capillary forces associated with the open-cell structure.
Figure 9.
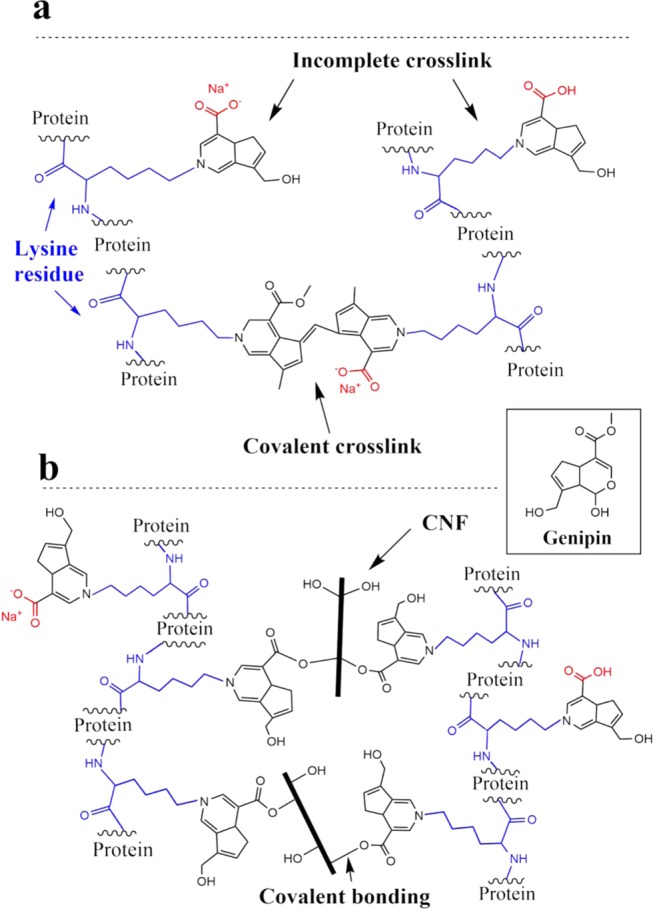
Suggested reactions of the (a) WG/GEN and (b) WG/CNF/GEN systems. The average size of the CNF used was approximately 2–3 μm in length and 10 nm in diameter.
It was also demonstrated that the mechanical properties and absorption/swelling behavior of foams could be tuned by the addition of solely natural additives: a polar cross-linker (genipin) and cellulose nanofibers. The results pave the way for future uses of protein superabsorbents in a range of products including hygiene applications where foams are used, e.g., sanitary pads.
4. Experimental Section
4.1. Materials
Wheat gluten (WG) powder with a protein content of 77.7 wt % (modified NMKL N6, Kjeltec, Nx5.7) was provided by Lantmännen Reppe AB, Sweden. The total starch content in the WG was 5.8 wt %, as determined by the Ewers polarimetric method (ISO 10520:1997). The lipid content was 1.2 wt % (Soxtec, Lidfett.OA.19, Tecator AN 301). Genipin (>98% HPLC Grade) was purchased from Zhixin Biotechnology, China. The cellulose nanofibers (CNFs) were purchased as a commercial type of Nata de Coco before their extraction (see Section 4.2.3).
4.2. Foam Preparation
4.2.1. WG Foam Preparation
The WG foams were prepared as previously described by Wu et al.13−15 Briefly, 20 g of WG powder was poured into a beaker containing 100 mL of Milli-Q water under magnetic stirring. The dispersion was adjusted to pH 11 by adding 1 M NaOH (Sigma-Aldrich, Puriss, p.a. ACS >98%). The dispersion was stirred until the pH became stable at 11, which took ca. 10 min. Thereafter, additional Milli-Q water was added until a volume of 120 mL was reached. To achieve protein denaturation and intermolecular disulfide cross-linking, the WG dispersion was heated in a silicone oil bath to 90 °C. While being heated, the wheat gluten dispersion was homogenized and foamed at 9500 rpm, using an IKA Yellow Line Di 25 homogenizer equipped with an S25N-18G dispersion tool. After reaching 90 °C and a volume increase of ca. 300%, the mixture was removed from the bath and cooled to room temperature at ambient conditions and thereafter poured into cuboid silicone molds [10 × 10 × 11 mm]. The filled molds were then stored at −25 °C for 12 h and subsequently freeze-dried for 48 h. This sample is referred to as the WG foam.
4.2.2. WG/GEN Preparation
The WG/Genipin foam was prepared by the same procedure as the pure WG, but when the cooling WG dispersion reached 40 °C, 200 mg of genipin powder was slowly added to give a final foam with 1 wt % genipin. The dispersion was then stirred for another 2 h at 40 °C, whereupon the mixture gradually turned green in color. It was then frozen and freeze-dried in the same way as the WG foam. This is referred to as the WG/GEN foam.
4.2.3. WG/CNF and WG/CNF/GEN Preparation
The cellulose nanofibers (CNFs) were extracted from bacterial cellulose cubes as described by Liu et al.59 The CNF had a specific surface area of 160 m2/g, an average length of 2–3 μm, and a diameter below 10 nm. The extracted CNF was available as a 0.4 wt % aqueous dispersion (neutral pH). To prepare the foam containing 1 wt % cellulose nanofibers (CNFs), 20 g of WG was added to an aqueous dispersion containing 200 mg of CNF. The foam was then prepared in the same way as with WG alone. This foam is referred to as WG/CNF. In the foam that contained 1 wt % CNF and 1 wt % genipin, the preparation was the same as with WG/CNF, except that, when the material had cooled to 40 °C, 200 mg of genipin powder was added. As with the WG/GEN, the mixture was stirred for another 2 h before it was freeze-dried. This foam is referred to as WG/CNF/GEN.
4.3. Density Determination
The density was calculated from the mass and volume of five foam specimens. The porosity was calculated assuming a WG solid density of 1300 kg/m3.9
4.4. Short Immersion in Water and n-Heptane
The foam was immersed in Milli-Q water or n-heptane (97%) for 1 s and then weighed on an analytical balance. Loosely attached water/n-heptane on the surface of the foam was removed before weighing by touching a corner of the cuboid foam with a tissue paper.
4.5. Water and Liquid Swelling Measurement
The absorption of water, saline solution (0.9 wt % NaCl), and defibrinated sheep blood in the foams was assessed using the “Tea-bag method” described in the EDANA NWSP 240.0.R2 standard. The defibrinated sheep blood herein used was provided by the Disease Vector Group, Department of Plant Protection Biology, Swedish University of Agricultural Sciences, Alnarp, Sweden. For the swelling test, bags were produced from two 40 × 60 mm2 sheets of a 408-mesh nylon fabric. The bags were heat-sealed along three edges and stored in a desiccator with silica gel for at least 3 days before use. For the absorption test, a cuboid foam sample, which had been dried in a desiccator with silica gel for at least 30 days, was placed inside the bag, which was then heat-sealed along the fourth edge. The nylon bag containing the foam was first weighed and then placed in the liquid for different time periods. When the bag was removed from the liquid, it was allowed to hang for 10 s and then placed onto a piece of tissue paper for 5 s to remove the excess liquid before being weighed again. The liquid absorption was calculated as
| 2 |
where Wi is the weight of the wet sample and bag, We the weight of the dry bag, kblank the correction factor for absorption of liquid in the bag material itself, and Wd the weight of the dry sample.
| 3 |
The Voigt equation (eq 3) was fitted to the short-term liquid uptake data, following the procedure of Zhang et al.42 In eq 3, Q (g liquid/g dry foam) is the absorption at time t (s), Qo is the final absorption (30 min value), and τ (s) is a “characteristic” swelling time.
4.6. Scanning Electron Microscopy (SEM)
The sample morphology was investigated using a Hitachi S-4800 field-emission scanning electron microscope (FE-SEM). The specimens were frozen in liquid nitrogen for ca. 5 min and then fractured into small pieces. The SEM images of the swollen materials were obtained from frozen fragments rescued from the nylon bags. These specimens were fixed onto FE-SEM sample holders using a conductive carbon paste. The samples were coated with palladium/platinum in an Agar high-resolution sputter coater (model 208RH) for 30 s. The average cell diameter of each foam was obtained from 50 measurements in SEM micrographs. The cell size diameter was taken as the largest distance between two opposite cell walls.
4.7. Infrared (IR) Spectroscopy
The samples were dried in a desiccator with silica gel for 1 week before the IR measurements. The IR spectroscopy data were obtained with a PerkinElmer Spectrum 100 instrument, equipped with a Golden Gate unit (Single-reflection ATR, Graseby Specac Ltd.) and a triglycine sulfate detector. The scanning step was 1.0 cm–1 with a resolution of 4.0 cm–1. The spectrum was based on 16 consecutive scans between 4000 and 600 cm–1. The peak deconvolution in the amide I region was performed as reported by Cho et al.9 using the PerkinElmer Spectrum software [version 10.5.1 (2015)] with an enhancement factor (γ) of 2 and a smoothing filter of 70%. The subsequent Gaussian peak curve fitting was performed in Origin and MATLAB.
4.8. Size-Exclusion High-Performance Liquid Chromatography (SE-HPLC)
The protein solubility and relative molecular size were evaluated by means of size-exclusion high-performance liquid chromatography (SE-HPLC) in a Waters 2690 Separations Module and a Waters 996 Photodiode Array Detector (Waters). The three-step extraction procedure implemented here was the same as that reported by Gällstedt et al.60 Briefly, a solution of 0.5 wt % sodium dodecyl sulfate (SDS) and 0.05 M NaH2PO4 (pH 6.9) was used as a buffer in combination with multiple ultrasonication steps. For the tests, all of the foams were ground using a mortar. The first extraction (Ext. 1) was obtained from the supernatant (SN) of a centrifuged dispersion after 5 min of shaking at 2000 rpm (16.000 RCF of 16 mg of the powders in 1.4 mL buffer). In the second extraction (Ext. 2), the pellet from Ext. 1 was resuspended in 1.4 mL of SDS–phosphate solution followed by 30 s ultrasonication and centrifugation for obtaining the SN. The third extraction (Ext. 3) consisted of the SN obtained from centrifuging the pellet from Ext. 2 after dispersing the pellet with fresh SDS–phosphate solution and applying repeated ultrasonication intervals (30 + 60 + 60 s). Three replicates were used. The total protein extractability from the three extraction steps of a ground pure WG foam treated at 90 °C was used as a reference. The amount of protein extracted from the other foams was normalized with respect to that of the WG foam (total extraction from the three extraction steps) and assuming a total protein extraction of 70% as obtained in previous work.13 SE-HPLC chromatography was performed using 0.2 mL/min of an isocratic flow consisting of 50% acetonitrile, 50% Millipore water and 0.1% trifluroacetic acid.
4.9. Mechanical Compression Tests
Compression testing of the dry and wet cuboids (swollen in water at ambient conditions for 16 h, slightly less than the maximum swelling time obtained for these materials) was performed in an Instron 5944 Universal Testing Machine, equipped with a 500 N load cell. The “dry” specimens were conditioned at 50% RH for at least 72 h before testing. The dimensions of the materials were measured at three different positions using a Mitutoyo micrometer. A compression rate of 10 mm/min was used in all cases. The compressive strength was taken as the stress at yield, if the yield occurred before 10% strain, according to ASTM D1621-16. The compression modulus (E) was obtained from the slope of the linear region (typically below 5% strain) according to the same standard. In addition, the stress at 10% strain and at the deflection point in the stress–strain curve, from which the densification of the porous foam starts, was determined for the dry foams. The compression tests were performed at 23 °C and 50% RH using 3–4 specimens per sample with the longest side of the cuboid foam in the strain direction.
Acknowledgments
This work was financed by VINNOVA (2015-03506), Lantmännen Ekonomisk Förening, and Essity AB. We especially acknowledge Annelie Moldin (Lantmännen) and Malin Lundman (Essity) for their valuable input/support and Maria-Luisa Prieto Linde for her help in obtaining the SE-HPLC data. The Disease Vector Group, Swedish University of Agricultural Sciences, is acknowledged for providing the defibrinated sheep blood used in this work.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02271.
CNF suspensions treated with genipin following the standard protocol but without wheat gluten (Figure S1); FTIR spectra of the neat CNF, GEN, and CNF reacted with GEN (Figure S2); and SEM micrographs of a commercial sanitary foam before and after water swelling (Figure S3) (PDF)
Swelling behavior of the WG/CNF/GEN foam in sheep blood (MOV)
Elastic recovery of the WG/CNF/GEN foams after 24 h swelling (MOV)
The authors declare no competing financial interest.
Supplementary Material
References
- Johansson E.; Prieto-Linde M. L.; Jönsson J. Ö. Effects of Wheat Cultivar and Nitrogen Application on Storage Protein Composition and Breadmaking Quality. Cereal Chem. J. 2001, 78, 19–25. 10.1094/CCHEM.2001.78.1.19. [DOI] [Google Scholar]
- Kuktaite R.; Larsson H.; Johansson E. Variation in protein composition of wheat flour and its relationship to dough mixing behaviour. J. Cereal Sci. 2004, 40, 31–39. 10.1016/j.jcs.2004.04.007. [DOI] [Google Scholar]
- Wieser H. Chemistry of gluten proteins. Food Microbiol. 2007, 24, 115–119. 10.1016/j.fm.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Singh H.; MacRitchie F. Application of Polymer Science to Properties of Gluten. J. Cereal Sci. 2001, 33, 231–243. 10.1006/jcrs.2000.0360. [DOI] [Google Scholar]
- Song Y.; Zheng Q. Dynamic rheological properties of wheat flour dough and proteins. Trends Food Sci. Technol. 2007, 18, 132–138. 10.1016/j.tifs.2006.11.003. [DOI] [Google Scholar]
- Ullsten N. H.; Cho S.-W.; Spencer G.; Gällstedt M.; Johansson E.; Hedenqvist M. S. Properties of Extruded Vital Wheat Gluten Sheets with Sodium Hydroxide and Salicylic Acid. Biomacromolecules 2009, 10, 479–488. 10.1021/bm800691h. [DOI] [PubMed] [Google Scholar]
- Türe H.; Gallstedt M.; Kuktaite R.; Johansson E.; Hedenqvist M. S. Protein network structure and properties of wheat gluten extrudates using a novel solvent-free approach with urea as a combined denaturant and plasticiser. Soft Matter 2011, 7, 9416–9423. 10.1039/c1sm05830d. [DOI] [Google Scholar]
- Pietsch V. L.; Emin M. A.; Schuchmann H. P. Process conditions influencing wheat gluten polymerization during high moisture extrusion of meat analog products. J. Food Eng. 2017, 198, 28–35. 10.1016/j.jfoodeng.2016.10.027. [DOI] [Google Scholar]
- Cho S. W.; Gällstedt M.; Johansson E.; Hedenqvist M. S. Injection-molded nanocomposites and materials based on wheat gluten. Int J. of Biol. Macromol. 2011, 48, 146–152. 10.1016/j.ijbiomac.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Zárate-Ramírez L. S.; Martínez I.; Romero A.; Partal P.; Guerrero A. Wheat gluten-based materials plasticised with glycerol and water by thermoplastic mixing and thermomoulding. J. Sci. Food Agric. 2011, 91, 625–633. 10.1002/jsfa.4224. [DOI] [PubMed] [Google Scholar]
- Cuq B.; Boutrot F.; Redl A.; Lullien-Pellerin V. Study of the Temperature Effect on the Formation of Wheat Gluten Network: Influence on Mechanical Properties and Protein Solubility. J. Agric. Food Chem. 2000, 48, 2954–2959. 10.1021/jf991339b. [DOI] [PubMed] [Google Scholar]
- Rasheed F.; Hedenqvist M. S.; Kuktaite R.; Plivelic T. S.; Gällstedt M.; Johansson E. Mild gluten separation – A non-destructive approach to fine tune structure and mechanical behavior of wheat gluten films. Ind. Crops Prod. 2015, 73, 90–98. 10.1016/j.indcrop.2015.04.007. [DOI] [Google Scholar]
- Wu Q.; Andersson R. L.; Holgate T.; Johansson E.; Gedde U. W.; Olsson R. T.; Hedenqvist M. S. Highly porous flame-retardant and sustainable biofoams based on wheat gluten and in situ polymerized silica. J. Mater. Chem. A 2014, 2, 20996–21009. 10.1039/C4TA04787G. [DOI] [Google Scholar]
- Wu Q.; Sundborg H.; Andersson R. L.; Peuvot K.; Guex L.; Nilsson F.; Hedenqvist M. S.; Olsson R. T. Conductive biofoams of wheat gluten containing carbon nanotubes, carbon black or reduced graphene oxide. RSC Adv. 2017, 7, 18260–18269. 10.1039/C7RA01082F. [DOI] [Google Scholar]
- Wu Q.; Yu S.; Kollert M.; Mtimet M.; Roth S. V.; Gedde U. W.; Johansson E.; Olsson R. T.; Hedenqvist M. S. Highly Absorbing Antimicrobial Biofoams Based on Wheat Gluten and Its Biohybrids. ACS Sustainable Chem. Eng. 2016, 4, 2395–2404. 10.1021/acssuschemeng.6b00099. [DOI] [Google Scholar]
- Alander B.; Capezza A. J.; Wu Q.; Johansson E.; Olsson R. T.; Hedenqvist M. S. A facile way of making inexpensive rigid and soft protein biofoams with rapid liquid absorption. Ind. Crops Prod. 2018, 119, 41–48. 10.1016/j.indcrop.2018.03.069. [DOI] [Google Scholar]
- Haar J. P. Jr.; Ross R. J.. Super-Absorbing Polymeric Network. U.S. Patent US59984921999.
- Zhang X.; Hoobin P.; Burgar I.; Do M. D. Chemical Modification of Wheat Protein-Based Natural Polymers: Cross-Linking Effect on Mechanical Properties and Phase Structures. J. Agric. Food Chem. 2006, 54, 9858–9865. 10.1021/jf061597q. [DOI] [PubMed] [Google Scholar]
- Reddy N.; Tan Y.; Li Y.; Yang Y. Effect of Glutaraldehyde Crosslinking Conditions on the Strength and Water Stability of Wheat Gluten Fibers. Macromol. Mater. Eng. 2008, 293, 614–620. 10.1002/mame.200800031. [DOI] [Google Scholar]
- Micard V.; Belamri R.; Morel M. H.; Guilbert S. Properties of Chemically and Physically Treated Wheat Gluten Films. J. Agric. Food Chem. 2000, 48, 2948–2953. 10.1021/jf0001785. [DOI] [PubMed] [Google Scholar]
- Nishi C.; Nakajima N.; Ikada Y. In vitro evaluation of cytotoxicity of diepoxy compounds used for biomaterial modification. J. Biomed. Mater. Res. 1995, 29, 829–834. 10.1002/jbm.820290707. [DOI] [PubMed] [Google Scholar]
- Blomfeldt T. O. J.; Kuktaite R.; Johansson E.; Hedenqvist M. S. Mechanical Properties and Network Structure of Wheat Gluten Foams. Biomacromolecules 2011, 12, 1707–1715. 10.1021/bm200067f. [DOI] [PubMed] [Google Scholar]
- Rafieian F.; Shahedi M.; Keramat J.; Simonsen J. Mechanical, thermal and barrier properties of nano-biocomposite based on gluten and carboxylated cellulose nanocrystals. Ind. Crops Prod. 2014, 53, 282–288. 10.1016/j.indcrop.2013.12.016. [DOI] [Google Scholar]
- Moriana R.; Vilaplana F.; Ek M. Cellulose Nanocrystals from Forest Residues as Reinforcing Agents for Composites: A Study from Macro- to Nano-Dimensions. Carbohydr. Polym. 2016, 139, 139–149. 10.1016/j.carbpol.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Oinonen P.; Krawczyk H.; Ek M.; Henriksson G.; Moriana R. Bioinspired composites from cross-linked galactoglucomannan and microfibrillated cellulose: Thermal, mechanical and oxygen barrier properties. Carbohydr. Polym. 2016, 136, 146–153. 10.1016/j.carbpol.2015.09.038. [DOI] [PubMed] [Google Scholar]
- López D. V.; Larsson P. A.; Wågberg L. Chemical modification of cellulose-rich fibres to clarify the influence of the chemical structure on the physical and mechanical properties of cellulose fibres and thereof made sheets. Carbohydr. Polym. 2018, 182, 1–7. 10.1016/j.carbpol.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Yildirim N.; Shaler S. M.; Gardner D. J.; Rice R.; Bousfield D. W. Cellulose nanofibril (CNF) reinforced starch insulating foams. Cellulose 2014, 21, 4337–4347. 10.1007/s10570-014-0450-9. [DOI] [Google Scholar]
- Svagan A. J.; Samir M. A.; Berglund L. A. Biomimetic Foams of High Mechanical Performance Based on Nanostructured Cell Walls Reinforced by Native Cellulose Nanofibrils. Adv. Mater. 2008, 20, 1263–1269. 10.1002/adma.200701215. [DOI] [Google Scholar]
- Oksman K.; Aitomäki Y.; Mathew A. P.; Siqueira G.; Zhou Q.; Butylina S.; Tanpichai S.; Zhou X.; Hooshmand S. Review of the recent developments in cellulose nanocomposite processing. Composites, Part A 2016, 83, 2–18. 10.1016/j.compositesa.2015.10.041. [DOI] [Google Scholar]
- Benselfelt T.; Wågberg L. Unidirectional Swelling of Dynamic Cellulose Nanofibril Networks: A Platform for Tunable Hydrogels and Aerogels with 3D Shapeability. Biomacromolecules 2019, 20, 2406–2412. 10.1021/acs.biomac.9b00401. [DOI] [PubMed] [Google Scholar]
- Karlsson R.-M. P.; Larsson P. T.; Hansson P.; Wågberg L. Thermodynamics of the Water-Retaining Properties of Cellulose-Based Networks. Biomacromolecules 2019, 20, 1603–1612. 10.1021/acs.biomac.8b01791. [DOI] [PubMed] [Google Scholar]
- Nascimento D. M.; Nunes Y. L.; Figueirêdo M. C. B.; de Azeredo H. M. C.; Aouada F. A.; Feitosa J. P. A.; Rosa M. F.; Dufresne A. Nanocellulose nanocomposite hydrogels: technological and environmental issues. Green Chem. 2018, 20, 2428–2448. 10.1039/C8GC00205C. [DOI] [Google Scholar]
- Capezza A. J.; Newson W. R.; Olsson R. T.; Hedenqvist M. S.; Johansson E. Advances in the Use of Protein-Based Materials: Toward Sustainable Naturally Sourced Absorbent Materials. ACS Sustainable Chem. Eng. 2019, 7, 4532–4547. 10.1021/acssuschemeng.8b05400. [DOI] [Google Scholar]
- Renewable Plastics Materials of Wheat Gluten, Scaling Up and Purification to Meet Industrial Demands, Vinnova-UDI-S1; Vinnova, July 19, 2019; p 98.
- Song F.; Zhang L.-M. Gelation Modification of Soy Protein Isolate by a Naturally Occurring Cross-Linking Agent and Its Potential Biomedical Application. Ind. Eng. Chem. Res. 2009, 48, 7077–7083. 10.1021/ie801372f. [DOI] [Google Scholar]
- Butler M. F.; Ng Y.-F.; Pudney P. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci., Part A: Polym. Chem. 2003, 41, 3941–3953. 10.1002/pola.10960. [DOI] [Google Scholar]
- Chang C.; Nickerson M. T. Effect of plasticizer-type and genipin on the mechanical, optical, and water vapor barrier properties of canola protein isolate-based edible films. Eur. Food Res. Technol. 2014, 238, 35–46. 10.1007/s00217-013-2075-x. [DOI] [PubMed] [Google Scholar]
- Wu S.; Gregory H.. Genipin-Rich Material and Its Use. U.S. Patent USRE46314E1, Nov 07, 2011.
- Zhang M.; Cheng Z.; Zhao T.; Liu M.; Hu M.; Li J. Synthesis, Characterization, and Swelling Behaviors of Salt-Sensitive Maize Bran–Poly(acrylic acid) Superabsorbent Hydrogel. J. Agric. Food Chem. 2014, 62, 8867–8874. 10.1021/jf5021279. [DOI] [PubMed] [Google Scholar]
- Wågberg L.; Decher G.; Norgren M.; Lindström T.; Ankerfors M.; Axnäs K. The Build-Up of Polyelectrolyte Multilayers of Microfibrillated Cellulose and Cationic Polyelectrolytes. Langmuir 2008, 24, 784–795. 10.1021/la702481v. [DOI] [PubMed] [Google Scholar]
- Dymond J. H.; Malhotra R.; Isdale J. D.; Glen N. F. (p, ϱ, T) of n-heptane, toluene, and oct-1-ene in the range 298 to 373 K and 0.1 to 400 MPa and representation by the Tait equation. J. Chem. Thermodyn. 1988, 20, 603–614. 10.1016/0021-9614(88)90090-0. [DOI] [Google Scholar]
- Zhang B.; Cui Y.; Yin G.; Li X.; Liao L.; Cai X. Synthesis and swelling properties of protein-poly(acrylic acid-co-acrylamide) superabsorbent composite. Polym. Compos. 2011, 32, 683–691. 10.1002/pc.21077. [DOI] [Google Scholar]
- Zhang B.; Cui Y.; Yin G.; Li X.; You Y. Synthesis and Swelling Properties of Hydrolyzed Cottonseed Protein Composite Superabsorbent Hydrogel. Int. J. Polym. Mater. 2010, 59, 1018–1032. 10.1080/00914031003760709. [DOI] [Google Scholar]
- Tsuchiya K.; Numata K. Chemical Synthesis of Multiblock Copolypeptides Inspired by Spider Dragline Silk Proteins. ACS Macro Lett. 2017, 6, 103–106. 10.1021/acsmacrolett.7b00006. [DOI] [PubMed] [Google Scholar]
- Tonami H.; Uyama H.; Kobayashi S.; Rettig K.; Ritter H. Chemoenzymatic synthesis of a poly(hydroquinone). Macromol. Chem. Phys. 1999, 200, 1998–2002. . [DOI] [Google Scholar]
- Hwang D.-C.; Damodaran S. Synthesis and properties of fish protein-based hydrogel. J. Am. Oil Chem. Soc. 1997, 74, 1165–1171. 10.1007/s11746-997-0041-0. [DOI] [Google Scholar]
- De Palma R.; Peeters S.; Van Bael M. J.; Van den Rul H.; Bonroy K.; Laureyn W.; Mullens J.; Borghs G.; Maes G. Silane Ligand Exchange to Make Hydrophobic Superparamagnetic Nanoparticles Water-Dispersible. Chem. Mater. 2007, 19, 1821–1831. 10.1021/cm0628000. [DOI] [Google Scholar]
- Papageorgiou S. K.; Kouvelos E. P.; Favvas E. P.; Sapalidis A. A.; Romanos G. E.; Katsaros F. K. Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. 10.1016/j.carres.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Mangavel C.; Barbot J.; Popineau Y.; Guéguen J. Evolution of Wheat Gliadins Conformation during Film Formation: A Fourier Transform Infrared Study. J. Agric. Food Chem. 2001, 49, 867–872. 10.1021/jf0009899. [DOI] [PubMed] [Google Scholar]
- Georget D. M.; Belton P. S. Effects of Temperature and Water Content on the Secondary Structure of Wheat Gluten Studied by FTIR Spectroscopy. Biomacromolecules 2006, 7, 469–475. 10.1021/bm050667j. [DOI] [PubMed] [Google Scholar]
- Wellner N.; Mills E. N. C.; Brownsey G.; Wilson R. H.; Brown N.; Freeman J.; Halford N. G.; Shewry P. R.; Belton P. S. Changes in Protein Secondary Structure during Gluten Deformation Studied by Dynamic Fourier Transform Infrared Spectroscopy. Biomacromolecules 2005, 6, 255–261. 10.1021/bm049584d. [DOI] [PubMed] [Google Scholar]
- Ye X.; Hedenqvist M. S.; Langton M.; Lendel C. On the role of peptide hydrolysis for fibrillation kinetics and amyloid fibril morphology. RSC Adv. 2018, 8, 6915–6924. 10.1039/C7RA10981D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newson W. R.; Rasheed F.; Kuktaite R.; Hedenqvist M. S.; Gallstedt M.; Plivelic T. S.; Johansson E. Commercial potato protein concentrate as a novel source for thermoformed bio-based plastic films with unusual polymerisation and tensile properties. RSC Adv. 2015, 5, 32217–32226. 10.1039/C5RA00662G. [DOI] [Google Scholar]
- Hernández E.; Neira-Velazquez M.; Ramos L.; Ponce A.; Weinkauf D. Changing the Surface Characteristics of CNF, from Hydrophobic to Hydrophilic, Via Plasma Polymerization with Acrylic Acid. J. Nano Res. 2010, 9, 45–53. 10.4028/www.scientific.net/JNanoR.9.45. [DOI] [Google Scholar]
- Olabarrieta I.; Cho S.-W.; Gällstedt M.; Sarasua J.-R.; Johansson E.; Hedenqvist M. S. Aging Properties of Films of Plasticized Vital Wheat Gluten Cast from Acidic and Basic Solutions. Biomacromolecules 2006, 7, 1657–1664. 10.1021/bm0600973. [DOI] [PubMed] [Google Scholar]
- Ninh C.; Iftikhar A.; Cramer M.; Bettinger C. Diffusion–reaction models of genipin incorporation into fibrin networks. J. Mater. Chem. B 2015, 3, 4607–4615. 10.1039/C4TB02025A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woychik J. H.; Boundy J. A.; Dimler R. J. Wheat Gluten Proteins, Amino Acid Composition of Proteins in Wheat Gluten. J. Agric. Food Chem. 1961, 9, 307–310. 10.1021/jf60116a020. [DOI] [Google Scholar]
- Wolf R. B.; Cavins J. F.; Kleiman R.; Black L. T. Effect of temperature on soybean seed constituents: Oil, protein, moisture, fatty acids, amino acids and sugars. J. Am. Oil Chem. Soc. 1982, 59, 230–232. 10.1007/BF02582182. [DOI] [Google Scholar]
- Liu D.; Wu Q.; Andersson R. L.; Hedenqvist M. S.; Farris S.; Olsson R. T. Cellulose nanofibril core-shell silica coatings and their conversion into thermally stable nanotube aerogels. J. Mater. Chem. A 2015, 3, 15745–15754. 10.1039/C5TA03646A. [DOI] [Google Scholar]
- Gällstedt M.; Mattozzi A.; Johansson E.; Hedenqvist M. S. Transport and Tensile Properties of Compression-Molded Wheat Gluten Films. Biomacromolecules 2004, 5, 2020–2028. 10.1021/bm040044q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.