Abstract
Bladder cancer is the ninth most common cause of cancer-related deaths worldwide. Although cisplatin is used routinely in treating bladder cancer, refractory disease remains lethal for many patients. The recent addition of immunotherapy has improved patient outcomes, however a large cohort of patients do not respond to these treatments. Therefore, identification of innovative molecular targets for bladder cancer is crucial. Apurinic/apyrimidinic endonuclease 1/ redox factor-1 (APE1/Ref-1) is a multifunctional protein involved in both DNA repair and activation of transcription factors through reduction-oxidation (redox) regulation. High APE1/Ref-1 expression is associated with shorter patient survival time in many cancer types. In this study, we found high APE1/Ref-1 expression in human bladder cancer tissue relative to benign urothelium. Inhibition of APE1/Ref-1 redox signaling using APE1/Ref-1 specific inhibitors attenuates bladder cancer cell proliferation in monolayer, in 3D cultures, and in vivo. This inhibition corresponds with an increase in apoptosis and decreased transcriptional activity of NFκB and STAT3, transcription factors known to be regulated by APE1/Ref-1, resulting in decreased expression of downstream effectors survivin and Cyclin D1 in vitro and in vivo. We also demonstrate that in vitro treatment of bladder cancer cells with APE1/Ref-1 redox inhibitors in combination with standard-of care chemotherapy cisplatin is more effective than cisplatin alone at inhibiting cell proliferation. Collectively, our data demonstrate that APE1/Ref-1 is a viable drug target for the treatment of bladder cancer, provide a mechanism of APE1/Ref-1 action in bladder cancer cells, and support the use of novel redox-selective APE1/Ref-1 inhibitors in clinical studies.
Keywords: Bladder Cancer, APE1/Ref-1, STAT3 Signaling, NFκB Signaling, Redox Regulation
INTRODUCTION
Bladder cancer (BCa) is the fourth most common malignancy and eighth leading cause of cancer-related death of men in the United States and the ninth most common cancer worldwide, with an estimated 430,000 new cases and 165,000 deaths annually (1, 2). Twenty-three percent of bladder cancer patients die within 5 years of diagnosis from the disease (2). Bladder cancer deaths are primarily caused by forms of the disease that are inherently refractory, or become refractory, to the current standard-of-care options such as platinum-based chemotherapy for muscle invasive bladder cancers (3). In the last five years, there has been the development of PD-1/PD-L1 immunotherapy that has led to a 20-30% response in cisplatin refractory disease (4). Despite this, there is still a large cohort of refractory disease in which no effective therapy options exist. Consequently, there is a need to discover new therapeutic drug options and combinations that target and kill BCa cells with this resistant phenotype.
Apurinic/apyrimidinic endonuclease 1/ redox factor 1 (APE1/Ref-1) is a multifunctional protein involved in both DNA repair and reduction-oxidation (redox) regulation of transcription factors (5, 6). APE1/Ref-1 is a major enzyme in the DNA base excision repair (BER) pathway responsible for the repair of oxidative and alkylation DNA damage (5, 6). This protein also plays a critical role in transcription factor function by regulating the redox signaling of transcription factors (TFs) via reduction of cysteine residues that affect the ability of TFs to bind to DNA and activate gene expression (7). Additionally, APE1/Ref-1 has been shown to interact with NPM1, directly acting upon RNA quality control mechanisms (8). Subsequently, APE1/Ref-1 protein performs multiple major functions in cells that affect a number of cellular processes including cell proliferation and cell survival.
APE1/Ref-1 expression is associated with shorter median patient survival time in many cancer types (8-12). While a basal level of APE1/Ref-1 expression is indispensable to all cells due to its critical role in DNA repair, expression is upregulated in many tumor types, including bladder cancer (9, 13). As the DNA repair function requires nuclear localization, it is of note that cytosolic localization of APE1/Ref-1 is inversely-correlated with tumor sensitivity to radiation and chemotherapy (12, 14, 15). This suggests the importance of cellular localization for the protein in cancer and non-repair functions. Redox regulation of TFs has been implicated in the development and progression of various cancer types, and inhibition of APE1/Ref-1 redox activity has been shown to reduce inflammatory, angiogenesis, growth-promoting, and anti-apoptotic activity in cancer cells (16, 17). Redox-specific inhibition by small molecule inhibitors APX3330 or APX2009 of APE1/Ref-1 has been shown to attenuate the proliferation of breast, colon, pancreatic, and prostate cancers (18-23).
Two of the primary TF targets of APE1/Ref-1 redox regulation are the ubiquitous factors NFκB and STAT3 (7, 24). These two central transcription factors have been shown to regulate proliferation and survival in multiple cancers, as well as playing a role in cancer progression, signaling within the microenvironment, and resistance to chemotherapy (25). NFκB and STAT3 drive the expression of a number of critical signaling molecules in cell proliferation and cell survival, including cyclin D1, myc, Bcl-2, Mcl-1 and the Inhibitor of Apoptosis (IAP) family member, survivin. Therefore, reduction of the activity of NFκB and STAT3 has dynamic effects on cancer cells, and targeting APE1/Ref-1 as a master regulator of the activity of these pathways is a promising approach in cancer therapy as APE1/Ref-1 inhibitior, APX3330 has recently completed Phase I clinical trials () (26, 27).
Patients with urinary bladder cancer exhibit increased levels of APE1/Ref-1 in their serum and their urine, and APE1/Ref-1 levels correlated with tumor stage and grade suggesting APE1/Ref-1 may act as a biomarker in bladder cancer (9, 13). However, a characterization of APE1/Ref-1 expression and activity in bladder cancer tissue has not been reported. To address this deficiency, we examined APE1/Ref-1 expression in control benign bladder tissue and bladder cancer tissue as well as constructing a tissue array from over 36 patients with cisplatin-refractory bladder cancer from cystectomy specimens. APE1/Ref-1 expression was robust in the majority of patient tumor samples compared to benign. Assessment of potency and efficacy of APE1/Ref-1 redox-selective inhibitors in preclinical experimental bladder cancer models is lacking, leaving APE1/Ref-1 inhibition as an untapped opportunity in bladder cancer therapy. Here, we report that inhibition of APE1/Ref-1 redox-specific signaling attenuates bladder cancer cell proliferation in monolayer, in 3D cultures and in vivo, induces apoptosis and blocks cancer cell proliferation, decreases the transcriptional activity of NFκB and STAT3, and thereby decreases expression of key survival proteins in vitro and in vivo. One of these proteins was survivin. Survivin is known to be induced during bladder cancer (28-31), and over the past year has become the focus of intense screening as a functional biomarker for the disease. Finally, we show that in vitro treatment with APE1/Ref-1 redox inhibitors in combination with the current standard-of care, cisplatin, is more effective than cisplatin alone.
MATERIALS AND METHODS
Human Specimens
We used two sets of paraffin-embedded human specimens for assessment of APE1/Ref-1 and target protein assessment by histology. In Set one (Figure 1B, C, Supplemental Figure 1), used for APE1/Ref-1 Immunofluorescence, specimens were obtained from patients undergoing cystectomy for muscle invasive bladder cancer, and controls were obtained as freshly harvested cadaveric specimens (Figure 1A, n=12). All human specimens were collected with written informed consent, and with appropriate minimal risk institutional review board approval according to the approval and guidelines at Indiana University School of Medicine, and in accordance with the ethical standards of the Declaration of Helsinki. These controls (average age 68 ± 8yrs) were age-matched to the bladder cancer specimens (64± 8yrs) and were confirmed by histology to be free from malignant or inflammatory bladder disease. The controls used in this analysis were verified by pathology to be void of bladder cancer or bladder inflammatory diseases. Specimens were fixed in 10% buffered formalin, processed routinely through ethanol and xylene gradients and into paraffin, and embedded in paraffin blocks as previously described. Sections were made at 5 μm via microtome cutting. All human specimens were stained with APE1/Ref-1 antibodies and known target proteins of APE1/Ref-1 signaling for immunofluorescence or immunohistochemistry, as described below. In addition, basic histology of these specimens was performed by hematoxylin and eosin (H&E) staining; this was used to assess any underlying inflammation and the pathological features of bladder tumors or any underlying pathology that may have been present in the controls, which disqualified them from use.
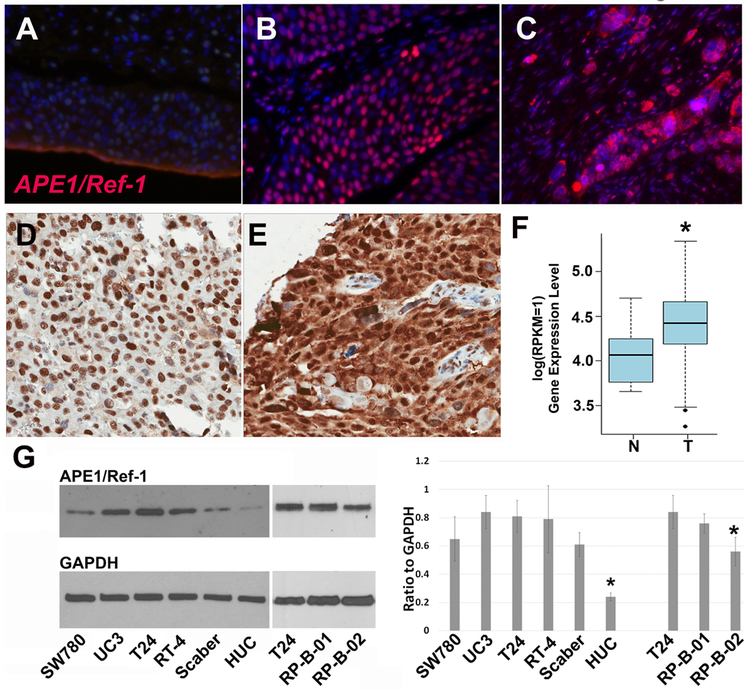
Figure 1. APE1/Ref-1 is expressed in bladder cancer tissue as well as bladder cancer cell lines including patient-derived xenograft lines, RP-B-01 and RP-B-02.
APE1/Ref-1 (red) is expressed at a low level in benign bladder urothelium (A), but is highly expressed in urothelial carcinoma (B,C) as determined by IF. Expression is primarily nuclear in non-invasive tumors (B), but exhibits both nuclear and cytosolic pattern in invasive lesions (C). Immunohistochemistry on cisplatin-refractory patient samples also demonstrated nuclear localization of APE1/Ref-1 in urothelial-confined tumor (D) and a shift to both cytosolic and nuclear expression in invasive tumor (E). (F), Samples within The Cancer Genome Atlas (TCGA) show that expression of APE1/Ref-1 mRNA (APEX1) is significantly upregulated in bladder cancer patients compared to matched control (p=1.68e-05, Mann Whitney test). Bladder cancer cell lines SW-780, UC3, T24, RT-4, Scaber, as well as the PDX lines B01 and B02 all expressed robust levels of APE1/Ref-1 in comparison to HUC (human urothelial cells), justifying their use for inhibitor study. (G *- p<0.05, ANOVA). Established bladder cancer lines exhibit significantly higher expression than the benign HUC cell line.
A second set of patient samples was collected from bladder tumors in patients with muscle invasive bladder cancer (Figure 1D, E). A human cisplatin-refractory bladder cancer tissue microarray (CisR-TMA) was created from this cohort of 36 patients that were noted to have residual disease at time of cystectomy despite preoperative neoadjuvant cisplatin-combination chemotherapy. As with the human sample set described above, specimens for the CisRef-TMA were obtained with proper written informed consent and appropriate minimal risk institutional review board approval according to the approval and guidelines at Indiana University School of Medicine, and in accordance with the ethical standards of the Declaration of Helsinki. Bladder tumor specimens for the CisR-TMA were obtained in duplicate 3mm cores from formalin fixed paraffin embedded cystectomy samples. A total of 36 separate patient samples were obtained, all of which were treated with preoperative cisplatin-combination chemotherapy. The average age was 64±8 yrs, 19% were female, and cystectomy surgery was performed between 2007-2017. Median survival after cystectomy was 33±12 months, with greater than 70% of patients undergoing additional systemic treatments in the adjuvant setting or at time of relapse. Tissues were fixed at room temperature overnight in 10% NBF (neutral buffered formalin), and processed routinely through ethanol into Xylene. Processed tissues were embedded in paraffin and cut on a microtome into 5-micron sections, mounted on positively charged slides and baked at 60°C. Sections from the CisRef-TMA were stained with the APE1/Ref-1 antibody by the Indiana University School of Medicine Research Immunohistochemistry Facility (Indianapolis, IN) and quantified using the HALO image analysis platform (Indica Labs) by Dr. George Sandusky. Basic histology of these specimens was performed by H&E staining; this was used to assess any underlying inflammation and the pathological features of bladder tumors or any underlying pathology that may have been present in the controls, which disqualified them from use. H&E analysis revealed that the tumors exhibited mostly epithelially confined loci of tumors, but all had some elements of invasive lesion formation consistent with clinical expectiations of specimens from patients that have failed cisplatin therapy.
Immunofluorescence and Immunohistochemistry (IHC)
Immunofluorescence protocols were followed as previously described (32). Sections were processed routinely and subjected to heat-induced antigen retrieval in 10 mM citrate buffer. Sections were blocked at ambient temperature with a bovine serum albumin (BSA)-Donkey serum blocking medium for 2-4 hours, and incubated with the same blocking medium containing the indicated primary antibodies overnight at 4 ˚C. Primary antibodies and dilutions included rabbit survivin (1:100, Cell Signaling Technologies), mouse APE1/Ref-1 (1:200, Novus Biologicals), rabbit BrdU (1:200, Cell Signaling Technologies), and mouse PanCK (1:200, Cell Signaling Technologies). After incubation, all sections were washed with 1X PBS -Tween and incubated with blocking medium containing with the species-specific (Invitroge) IgG Alexa 488 and IgG Alexa 594-conjugated secondary antibody targeting rabbit or mouse for 1 hour at room temperature at a dilution of 1:200 as previously optimized (32). Nuclei were stained by incubation with Hoechst 33258 nuclear stain Sigma) at a concentration of 1 μg/ml. Tissues were again washed and covered with aqueous medium and glass coverslips. All specimens were visualized using immunofluorescence intensity with the Leica 6000 epifluorescence/confocal microscope. IHC was conducted as previously published for APE1/Ref-1 (10).
Drugs
APX3330, which was previously named E3330, was synthesized and used as previously described (22). APX2009 and APX2014 were kind gifts from Apexian Pharmaceuticals LLC (Indianapolis, IN). Synthesis, description, and molecular target verification of APX2009, APX2014, and RN7-58 have been previously described (16, 33, 34). The concentrations defined in this study are within the specific activivity concentration range of each inhibitor, as described in previous studies (11, 16, 18, 19, 21, 33). Additionally, the concentrations are within the achieveable levels in patients. Molecular target confirmation is routinely done via assessment of protein or gene expression of known pathway targets (18, 21, 24, 35, 36).
Cells in culture
The following cell lines were obtained from the ATCC in 2016 and have been maintained in our laboratory since: Grade 3/4 transitional cell carcinoma (TCC) line UC3; Grade 3 papillary urothelial carcinoma T24; Grade 1 TCC line SW-780; Grade 1 papillary urothelial RT-4; squamous bladder cancer line SCaBER; and non-cancerous human urothelial cell (HUC). Cells were grown in RPMI medium supplemented with antibiotic and 10% fetal bovine serum (FBS) for 2 passages, after which stocks were made and deep frozen in liquid nitrogen. From these laboratory stocks, cells for all experiments have been recultured, with a maximum of 10 passages performed before returning to the laboratory stocks for fresh cultures. All cells are authenticated regularly (1x per year) to verify cell line integrity at the University of Arizona Genetics Core: (https://uagc.arl.arizona.edu/cell-line-authentication), and all are routinely tested and verified as mycoplasma-free.
Alamar blue assay
Bladder cancer cell lines (BLCAb001 (RP-B-01) and BLCAb002 (RP-B-02) (37, 38)) maintained in RPMI growth medium with 10% FBS were plated at 4,000 cells/well in poly-D-lysine treated 96-well clear bottom black plates and grown overnight in 5% CO2 at 37°C. Cells in monolayer were then treated with increasing concentrations of redox-specific inhibitor APX3330, APX2014 or APX2009 and serially diluted 1:2 in a 5-point dose scheme. For combination studies, cells were treated with both cisplatin and APX compounds to determine efficacy. After 72 hours, fresh RPMI medium with 5% FBS was exchanged, and a fluorescent metabolic indicator, Alamar Blue was added to each well at 10% final concentration. After a 4 hour incubation, plates were read on a Synergy H4 (Bio-Tek) plate reader. For each drug dose, background was subtracted and then further normalized to media alone.
BrdU labeling and proliferation quantification
To determine the proliferation rate of cells in response to APE1/Ref-1 redox inhibitors, cells were grown in the culture conditions described above and treated with inhibitors as described above, in chamber slides. After 24 hours in culture with inhibitors or vehicle, all cells were treated with 3.1 μg/ml BrdU (in sterile PBS; 0.1%) for one hour. Cells were fixed and permeablized with 4 % paraformaldehyde and stained for BrdU incorporation with the antibody and methodology described in the Roche BrdU labeling Kit, (Risch-Rotkreuz, Switzerland), using secondary antibodies as described previously (39). Positive cells were captured on a Leica 6000 fluorescent microscope and were quantified relative to Hoechst positive nuclei (total live cells).
Apoptosis labeling via Incucyte Caspase-3/7 reagent
UC3 and T24 cells were plated in 96-well plates at 3,500 cell/well and allowed to attach overnight. Increasing amounts of APX2009 or APX2014 were added to each well along with 1μM of the caspase reagent (Caspase-3/7 Red, Essen Bioscience) and then the cells were allowed to recover for 2 hours prior to beginning imaging with the Incucyte system (Essen Bioscience). Each well was imaged for phase contrast as well as red fluorescence every 2 hours for 96 hours. The Incucyte software generated movies of the cells following treatment as well as real-time imaging data with red fluorescence normalized to the percent confluency of the well.
Transfection of APE1/Ref-1 siRNA
All siRNA transfections in T24, UC3, RP-B-01 and RP-B-02 cells were performed using the Lipofectamine RNAimax Reagent (Thermofisher) protocol as previously described (10, 11, 21, 24). The APE1/Ref-1 siRNA sequences were: #1) GTCTGGTACGACTGGAGTA and #2) Life Technologies Cat #s1446 CAGATATACTGTGCCTTCA. Twenty four hours post transfection, cells (1,500/well) were replated in xCELLigence plates, and growth was measured in real time using the xCELLigence RTCA system (18, 24). For the RP-B-02 cell line, 1,500 cells/well were replated in 96-well black plates and cell growth determined over five days using the Alamar blue assay. Alamar blue assay was used with the RP-B-02 cells due to the fact that they did not attach and proliferate proficiently on the xCELLigence plates. Samples for western blotting were collected 72 and 144 hours post transfection of cancer cells with APE1/Ref-1 siRNA and scrambled siRNA control.
Western blot
Whole cell extracts were prepared using RIPA (radioimmunoprecipitation) buffer containing protein inhibitors (1:100 PMSF, 1:100 orthovanadate and 1:100 protease inhibitor). Total protein concentration was determined via Lowry or BCA assay. 10-50 μg/well of each lysate was separated by SDS-PAGE using a 12% SDS-polyacrylamide gel. Blots were blocked with 5% nonfat dry milk in 1xTBS for 1 h and incubated overnight with primary antibodies to either APE1/Ref-1 (1:1000 dilution, Novus, NB-100-116), survivin (1:1000, Cell Signaling), Cyclin D1 (1:500, Abcam), PARP-1 (1:1000, Cell Signaling) or GAPDH (1:5000, Cell Signaling). After blots were washed three - six times with TBS-Tween, blots were incubated with HRP-conjugated secondary mouse antibodies (1:5000, Pierce). After washing three - six times with TBS-Tween, blots were visualized by enhanced chemiluminescence (West Pico/West Femto, Pierce).
Three-dimensional (3D) Spheroid Growth Assays
RP-B-01 and RP-B-02 cells were resuspended in normal growth media containing 3% Reduced Growth Factor Matrigel (BD Biosciences) at a cell density of 1,500 and 3,000 cells/well, respectively and plated in ultra-low adherence 96-well plates (Corning). After spheroid formation, APE1Ref-1 inhibitors were added on days 4, 8, and 12 as described in Arpin, et al (40). On Day 15, Alamar blue reagent (LifeTechnologies) was added to each well (10 μL/well) and all wells were incubated for 24 hours. IC50 values were calculated for each compound and the linear regression model was employed to generate a line of best fit, wherein the percent survival equaled 50% (n=3-4).
In vivo subcutaneous tumor
107 T24 human bladder cancer cells were grown in conditions described above and harvested with 0.05% Trypsin, centrifuged, and resuspended in a 50:50 solution of Matrigel: RPMI medium. For each subcuetaneous tumor, a 100 μl volume of this suspension was implanted in the hind flank of male athymic nude male and female mice. Previous characterization of the T24 model indicated that log phase was entered when tumor volumes reached 65 to 150 mm3 (between 2-4 weeks post-implant to reach log phase). At this point and individual for each animal, the animals were treated with either 50 mg/kg APX3330, 25 mg/kg IP APX2009 or vehicle (both in Cremophor ethanol (4% in PBS)) every 12 hours for up to 12 days. BrdU was injected into the animals 2 hours prior to sacrifice and tumor tissues were harvested and split into either snap frozen tissues for protein harvest and molecular analysis or fixed in formalin and processed for histological analysis, and then analyzed for survivin, Cyclin D1 levels (immunofluorescence and immunoblotting) for target protein assessment and BrdU incorporation (immunofluorescence).
Statistical analysis
IC50 values for all dose response curves were calculated using ANOVA with Tukey post hoc analysis of all cell lines in monolayer and 3D cultures. The caspase time course curves were analyzed using Prism 6 software and generating linear regression curves for each treatment. The linear regression data indicated that all slopes were significantly different from each other and from vehicle control (p<0.05, n=2-4).
RESULTS
APE1/Ref-1 is highly expressed in bladder cancer compared to benign bladder urothelium and in multiple bladder cancer cells lines.
To investigate APE1/Ref-1 redox signaling in bladder cancer, we obtained bladder cancer patient samples and stained for APE1/Ref-1 via immunofluorescence (IF, Figure 1, A-C) as well as immunohistochemistry (IHC, Figure 1D, E). Benign bladder urothelium has low nuclear staining in the urothelium (Figure 1A, representative of n=12). Strong nuclear fluorescence is observable in the tumor cell epithelia in all bladder cancer cases examined (Figure 1B, C), representative of twelve specimens from bladder cancer, See Supplemental Figure 1 for additional staining). Expression is primarily nuclear in non-invasive tumors as determined by the presence of satellite lesions in the muscularis of the tissue by H&E (Figure. 1B), but exhibits both nuclear and a stronger cytosolic pattern in invasive lesions where microsatellite growths occur in detrusor muscle (Figure. 1C, Supplementary Figure 1). In calculation, 77±11% of cells in muscle-invaded satellite lesions exhibit nuclear staining while 2.8±0.6% exhibit any detectable cytosolic staining by ImageJ.
APE1/Ref-1 protein expression in cisplatin-refractory patient samples.
We obtained 36 cisplatin-refractory patient samples, constructed a TMA (tissue microarray), and performed IHC staining of APE1/Ref-1. Figure 1D & E confirm the intense nuclear staining in the tumor cells within samples in the TMA, as well as the nuclear localization of APE1/Ref-1 in urothelial-confined tumor (Figure. 1D) with a stronger cytosolic expression in invasive tumor in addition to nuclear APE1/Ref-1 expression (Fig.1E, n=36). This trend of an increase in cytosolic APE1/Ref-1 expression has also been observed in ovarian and prostate cancer (12, 14). Samples within The Cancer Genome Atlas (TCGA) confirmed that APE1/Ref-1 mRNA (APEX1) is significantly upregulated in bladder cancer patients compared to matched control (Figure 1F, p=1.68e-05, Mann Whitney test). RNA-seq V2 data of the TCGA BLCA data were used for the analysis. Mann Whitney test was used for the differential gene expression test.
Human bladder cancer cell lines including SW-780, UM-UC3, T24, as well as the patient-derived xenograft lines RP-B-01 (B01) and RP-B-02 (B02) all express robust levels of APE1/Ref-1 protein (Figure 1G). High levels of APE1/Ref-1 in patient samples as well as patient-derived cell lines support the investigation of APE1/Ref-1 as a novel target in bladder cancer. Furthermore, the RP-B-01 have been characterized as more cisplatin-resistant than the RP-B-02 cells (37) which we confirmed in vitro in Figure 7. RP-B-01 are at least 3-fold more resistant to cisplatin than RP-B-02 cells, providing us with representative BCa cell lines to study APE1/Ref-1 signaling and response to inhibition.
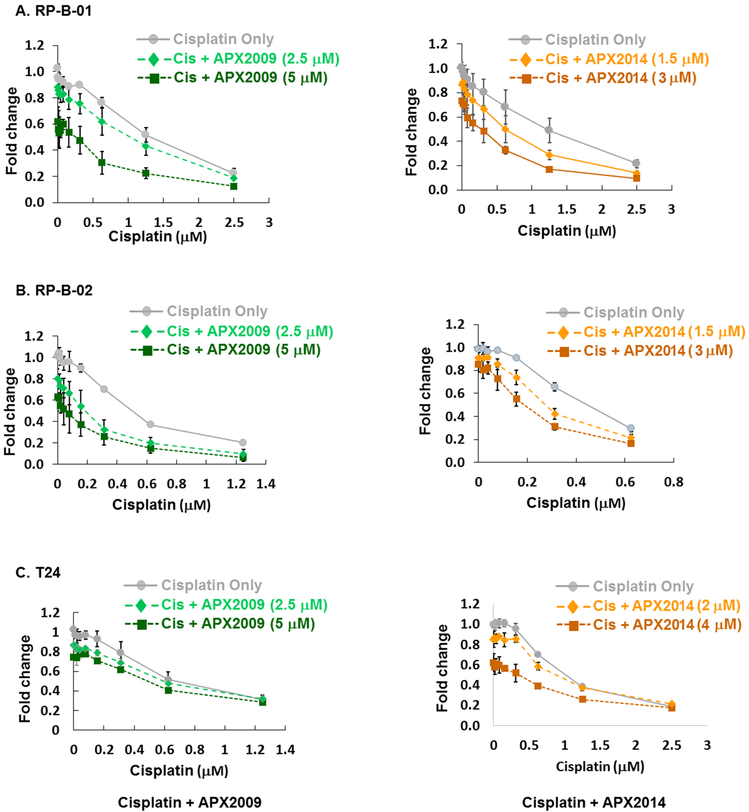
Figure 7. Combination treatment with cisplatin and APX inhibitors resulted in an enhancement of cisplatin-induced cytotoxicity.
RP-B-01 (A), RP-B-02 (B), and T24 (C) cell lines were treated with increasing concentrations of cisplatin in combination with a single dose of either APX2009 or APX2014 as indicated (n=3±SE). Cell proliferation was normalized to saline control and expressed as Fold Change. Combination index (CI) values calculated with Compusyn for combination of APX inhibitors and cisplatin. CI values indicated mainly additivity (0.95 – 1.2) to synergy (0.8 – 0.94).
Bladder cancer cell proliferation is inhibited and apoptosis is induced by potent, selective redox inhibitors of APE1/Ref-1.
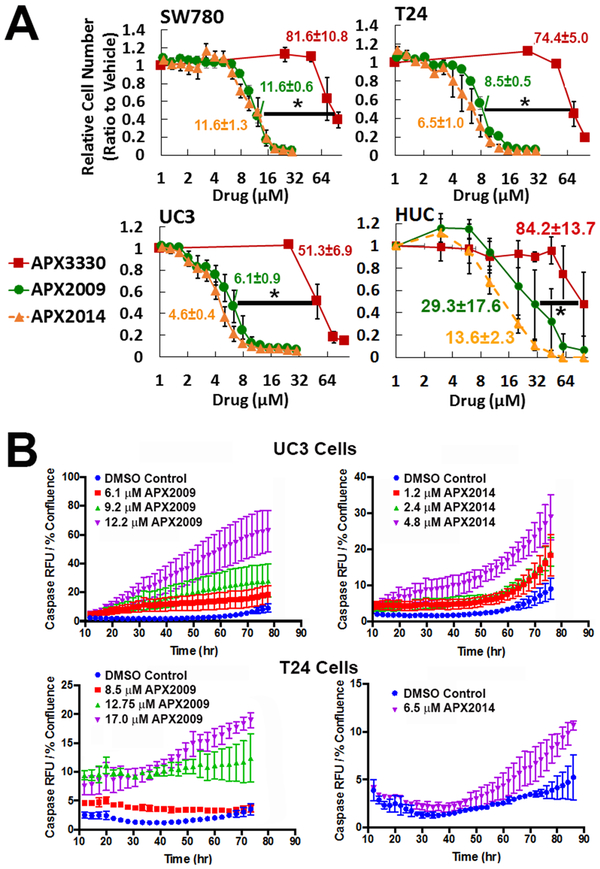
We used a panel of bladder cancer cell lines including SW780, T24, UC3, and noncancerous HUC cells to investigate the effects of parent compound APX3330 and more potent analogs, APX2009 and APX2014 on bladder cancer cell proliferation and apoptosis. Data in Figure 2A clearly demonstrates that the inhibition of APE1/Ref-1 redox activity potently and significantly reduces bladder cancer cell number in vitro. Analogs, APX2009 and APX2014 were significantly more potent in all cell lines tested (p<0.0001) compared to APX3330. The IC50 values for APX2009 and APX2014 were 7-11-fold lower than the IC50s for APX3330. Notably, the noncancerous HUC cell line exhibited substantially less response to APE1/Ref-1 redox-selective inhibitors (Figure 2A). To characterize further this effect, we determined if this reduced cell number is due to decreases in proliferation, increases in apoptosis, or both. BrdU incorporation assay demonstrated that the number of BrdU-positive cells was reduced from 11.2 ± 0.84% to 7.8% ± 0.64 after treatment with IC50 concentration of APX3330, to 6.9% ± 0.58 by the IC50 concentration of APX2009, and to 7.2% ± 0.49 by the IC50 concentration of APX2014 (all p<0.05 by ANOVA; n=4). Along with the observed decrease in proliferation, an increase in apoptosis was seen in both UC3 and T24 cells (Figure 2B). To assay for caspase-3/7 mediated apoptosis, we monitored the increase in red fluorescence over time following the addition of APX2009 and APX2014. Representative images of the vehicle- and APX- treated cells at 48 hours are shown in Supplementary Figure 3, while the time lapse video of the cells during treatment is in Supplementary Videos 1-4. A dose-dependent increase in caspase activation was also accompanied by an increase in PARP-1 cleavage (Supplemental Figure 3B).
Figure 2. Treatment with APE1/Ref-1 inhibitors potently blocks bladder cancer cell proliferation and activated Caspase 3/7.
SW780, T24, UC3, and HUC cell lines (A) were treated with increasing concentrations of redox-specific inhibitor APX3330 (RED square), as well as the more potent analogs APX2009 (GREEN circle) and APX2014 (orange triangle). The IC50 values for A were determined using the methylene blue assay, n=4. IC50 values were calculated and compared between the drugs, and are listed next to their curves in the figure; * denotes p<0.05 APX3330 IC50 versus both APX analogs. (B) T24 and UC3 cells were treated with APX2009 and APX2014 at the IC50 and IC90 and monitored over time for activation of capsase 3/7 by an increase in red fluorescence. Time course graphs at 48 hr are shown. The curves are all significantly different from DMSO (p<0.05).
Blockade of APE1/Ref-1 inhibits PDX bladder cancer cell growth in monolayer and in 3D culture model.
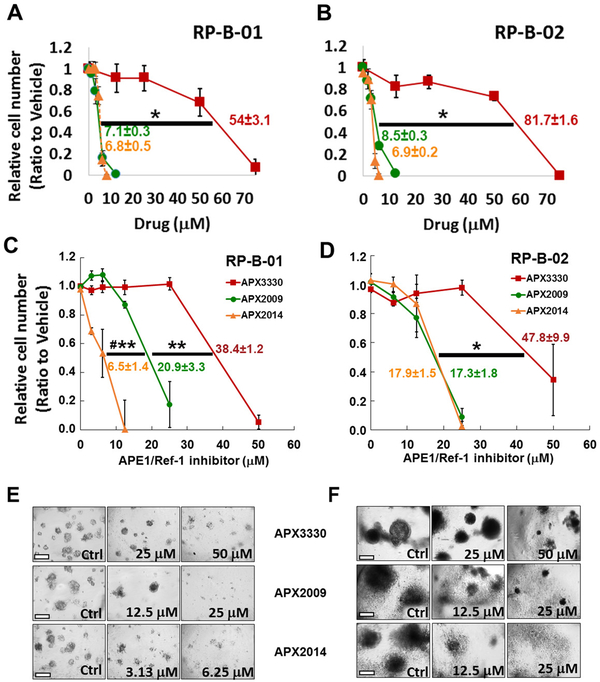
APE1/Ref-1 redox inhibition was also characterized in two additional bladder cancer cell lines that were derived from a patient-derived xenograft (PDX) model (37). Patient-derived cells demonstrated similar sensitivity to APE1/Ref-1 redox inhibition as established bladder cancer cell lines and were similarly more sensitive to analogs, APX2009 and 2014 (Figure 3A,B). In order to mimic tumor growth more accurately in a relevant microenvironment and in a more robust model for predicting response to treatment, we utilized a three-dimensional (3D) culture model of patient-derived RP-B-01 and RP-B-02 bladder cancer cells. Both RP-B-01 and RP-B-02 cell lines are transitional cell carcinoma staged as T4bN1Mx and T2bN0Mx Grade III tumors, respectively (37). Using this 3D culture model, we observed a dose-dependent decrease in spheroid growth with all three APE1/Ref-1 inhibitors with a similar increase in potency for the new analogs, APX2009 and APX2014 as seen in monolayer (Figure 3 C,D). One difference that was observed with the 3D culture compared to monolayer was that the RP-B-01 cells were significantly more sensitive to APX2014 than APX2009 (Figure 3C,E, p<0.01). APX2009 and APX2014 were more potent in the RP-B-02 cells than parent compound APX3330 (p<0.05), but were not significantly different from each other (Figure 3D, F). Both PDX cell lines exhibited similar expression patterns of both APE1/Ref-1 and its target STAT3 in 3D and in monolayer conditions, with no significant changes in these two critical proteins between either culturing conditions (Supplementary Figure 4).
Figure 3. Inhibition of APE1/Ref-1 in monolayer and in three-dimensional (3D) culture using PDX-derived cells RP-B-01 and RP-B-02 blocks tumor growth.
RP-B-01 and RP-B-02 cells were grown first in monolayer cell culture (A, B, n=3), and then in 3D culture (C-F) and treated with APX3330, APX2009 and APX2014 over the course of 15 days (n=3-4±SE). Monolayer and spheroid growth was measured via Alamar blue and normalized to the fluorescence of media control. Representative images of spheroids treated with APX inhibitors are shown in E and F. The IC50 values were determined (n=3,4 ± SE) and compared between the drugs using ANOVA with Tukey post hoc test: * p<0.05, **p<0.001 comparison of IC50 of APX3330 versus APX analog; while # denotes p<0.01 APX2009 vs APX2014.
Blockade of APE1/Ref-1 via siRNA similarly reduces the ability of the bladder cancer cells to proliferate.
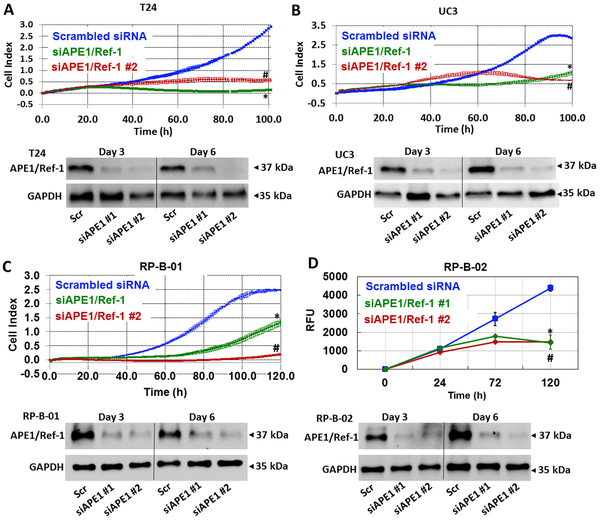
To confirm the effects of APE1/Ref-1 inhibition on bladder cancer cell proliferation, we transfected bladder cancer cells with APE1/Ref-1 siRNA and quantified the proliferative capacity following APE1/Ref-1 knockdown. Using two siRNAs that are specific to APE1/Ref-1, we effectively reduced the levels of APE1/Ref-1 protein to greater than 70% over a 6-day period (Figure 4). T24, UC3, RP-B-01 and RP-B-02 cell lines were transfected with APE1/Ref-1 siRNAs, and growth of cells with reduced APE1/Ref-1 levels was compared to the Scrambled siRNA-transfected cells over time. The xCELLigence system was used to monitor cell attachment, proliferation, and morphology in real time. Bladder cancer cells transfected with APE1/Ref-1 siRNA grew at a significantly slower rate compared to those transfected with the Scrambled control siRNA (Figure 4 A-D, p<0.05 compared to Scrambled control, at t=100 h). Western blotting was performed Day 3 and Day 6 post transfection, and APE1/Ref-1 levels were found to be decreased compared to scrambled control at all timepoints tested.
Figure 4. Reducing APE1/Ref-1 levels via siRNA dramatically slows down the proliferation of bladder cancer cells.
T24 (A), UC3 (B), RP-B-01 (C) and RP-B-02 (D) were transfected with two distinct sequences of APE1/Ref-1 siRNA (50 nM) and growth was compared to Scrambled control (n = 3, *p < 0.05 (Scr vs siAPE/Ref-1 #1), # p < 0.05 (Scr vs siAPE/Ref-1 #2) at 100 h). Cell index was monitored via xCELLigence system in A-C and fluorescence using Alamar blue assay was monitored over time in D. Western analysis confirmed the reduction in APE1/Ref-1 protein levels and GAPDH was used as a loading control.
Redox-specific APE1/Ref-1 inhibition with APX2009 and APX2014 reduced the transcriptional activity of NFκB and STAT3 promoters as well as downstream expression of NFκB- and STAT3- regulated genes.
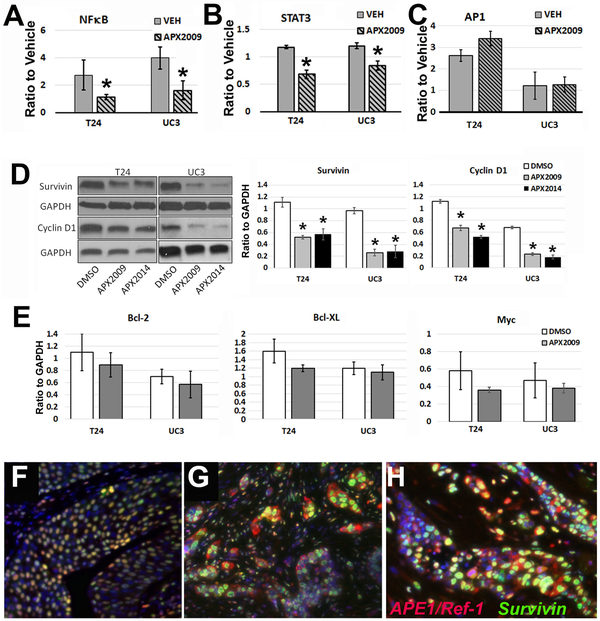
The transcriptional activity of NFκB, STAT3, and AP-1 is under redox control by APE1/Ref-1 (24). Therefore, we quantified NFκB, STAT3, or AP-1 transcriptional activity following treatment with APE1/Ref-1 redox inhibitors (Figure 5A-C). In T24 and UC3 cells induced with the NFκB activator TNFα, APX2009 significantly reduced NFκB-driven luciferase activity 2-fold (*-p<0.05 APX treated vs vehicle, n=4). Similarly STAT3 activity was significantly blocked in T24 and UC3 cells following induction with STAT3 activator IL-6, and treatment with APX2009 (Figure 5B, *-p<0.05 APX treated vs vehicle, n=4). No effect on the transcription factor AP-1 was observed in these cell lines at the timepoints tested (Figure 5C). These data show that following inhibition of APE1/Ref-1, the expected decrease in NFκB and STAT3 activity is observed.
Figure 5. Redox-specific APE1/Ref-1 inhibition with APX2009 and APX2014 reduced the transcriptional activity of NFκB and STAT3 resulting in a decrease in expression of Survivin and Cyclin D1.
NFκB-driven luciferase (A), STAT3-driven luciferase (B), and AP-1-driven luciferase (C) were quantified and normalized to Renilla in cytokine-induced T24 and UC3 cells following treatment with the corresponding cell growth-inhibitory IC50 concentrations. T24 cells were treated with APX2009 (8.5 μM) and APX2014 (6.5 μM), and UC3 cells were treated with APX2009 (6.1 μM) and APX2014 (4.6 μM) for 24 h. (*-p<0.05 APX treated vs vehicle, n=4). APX2009 and APX2014 treatment also decreased expression of two verified NFκB and STAT3 targets, survivin and Cyclin D1 (D), as indicated by densitometry quantified immunoblots (right panel, *-p<0.05 APX treated vs vehicle, n=4). In panels F and G, we analyzed co-expression of survivin and APE1/Ref-1 in superficial (F) and invasive human bladder tumors (G) in patients, and found nearly universal overlap of positivity per cell in both in human samples, though cellular localization of APE1/Ref-1 was cytoplasmic in invasive lesions. This was also true in cisplatin-refractory patients from our tissue microarray specimens (H).
For further confirmation of a decrease in NFκB and STAT3 transcriptional activity, levels of survivin and Cyclin D1 were examined. Both survivin and Cyclin D1 are known to be downstream targets of NFκB and STAT3. Following treatment with APX2009 and APX2014, the expression of two proteins, survivin and Cyclin D1, were significantly down-regulated, as shown in Figure 5D. We did not observe a change in expression from other cell proliferation or cell survival proteins including c-Myc, Bcl-2, or Bcl-XL (Figure 5E). We also analyzed co-expression of APE1/Ref-1 and survivin in superficial (n=12, Figure 5F) and invasive human bladder tumors (n=12, Figure 5G), and found a nearly universal overlap of positivity in both, as over 99% of cells positive for APE1/Ref-1 also demonstrated high levels of survivin. However, unlike APE1/Ref-1, survivin expression was primarily nuclear in both urothelial confined and invasive lesions. This result held true in dual fluorescence staining of the cisplatin-resistant TMA specimens (Figure 5H).
In vivo blockade of APE1/Ref-1 redox signaling decreases tumor growth and proliferation with a corresponding decrease in the protein levels of NFκB/STAT3 target, survivin.
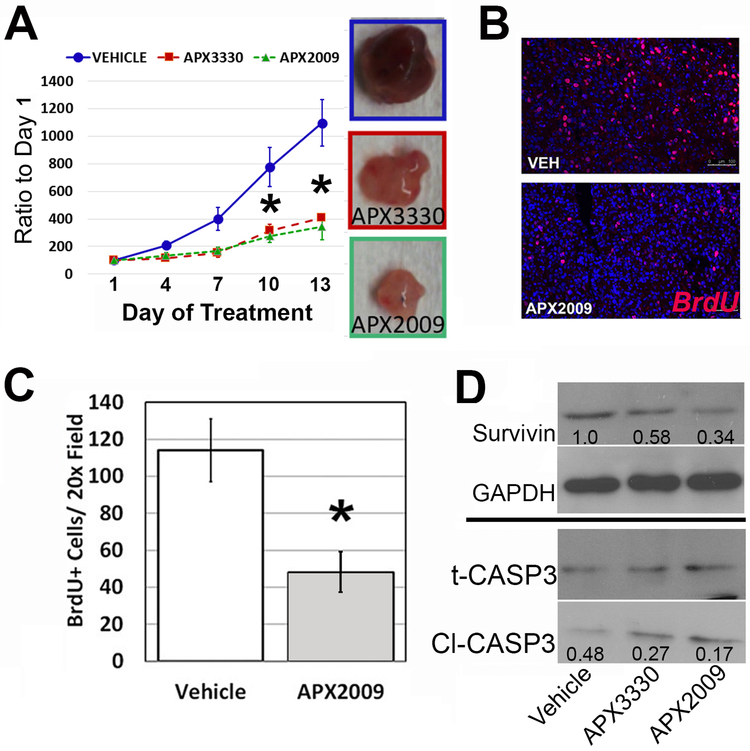
Thus far, we have demonstrated that APE1/Ref-1 is highly expressed in human bladder cancer as well as PDX and established cell lines and that blockade of this critical redox protein inhibits bladder cancer cell growth and reduces cell survival proteins such as survivin and Cyclin D1 in vitro. Figure 6 demonstrates in vivo efficacy following inhibition of APE1/Ref-1 redox activity in subcutaneous T24 tumor growth (Figure 6A), as mice treated throughout the growth period exhibited reduced graft tumor growth compared to vehicle-treated controls. At sacrifice, vehicle-treated animals bore tumors of 1.0 ± 0.2 g on average, while tumors from animals treated with APX3330 were 0.43 ± 0.10 g, and APX2009-treated tumors averaged 0.37 ± 0.14 g (both p<0.05 compared to vehicle control). Due to the observed increase in potency of APX2009, we were able to achieve similar tumor growth reduction to parent compound APX3330 at half the dose (APX3330 50mg/kg and APX2009 25 mg/kg, twice daily as previously reported (21).) Prior to sacrifice, mice were injected with BrdU and BrdU incorporation was visualized and quantitated via red fluorescence. There is a dramatic 60% decrease in BrdU+ cells following treatment with APX2009 (Figure 6B, C; *p<0.05) confirming that inhibition of APE1/Ref-1 redox activity similarly reduces bladder cancer cell proliferation in vivo. Mechanistically, we proposed that the regulation of the transcriptional activity of NFκB and STAT3 by APE1/Ref-1 played a role in the observed tumor efficacy. In support of this, decreased expression of survival protein, survivin was observed in vitro as well as in vivo and could at least partially explain the decrease in tumor cell survival. Immunoblotting for survivin protein in tumor tissues following in vivo treatment with APE1/Ref-1 inhibitors also demonstrated a significant reduction in survivin levels when compared to control tumors (Figure 6D, p=0.016). Harvested tumors demonstrated a 42% reduction in survivin expression by APX3330 and a 64% reduction by APX2009, p<0.05, each inhibitor compared to vehicle. In addition, harvested tumors from mice treated with APX3330 and APX2009 demonstrated a increase in the ratio of cleaved caspase 3 (Cl-CASP3) to total caspase 3 by immunoblotting, (p<0.05, each inhibitor compared to vehicle).
Figure 6. Treatment with APX3330 and APX2009 exhibited significantly reduced tumor growth and proliferation in vivo compared to vehicle control.
Tumor growth delay following treatment with APX compounds in T24 flank xenografts with images of representative size of tumors shown in A. Mice were treated with APX3330 (50 mg/kg, ip) and APX2009 (25mg/kg, ip) twice daily for the duration of the experiment. Tumor growth was normalized to the tumor volume on Day 1, when treatment was started (A, *p<0.05, n=8 animals per group). Proliferation following treatment as determined by BrdU labeling and IF is pictured in B, with quantification shown in C (n=8; p<0.05). Harvested tumors also showed a reduction in survivin expression by both APX3330 (42%) and APX2009 (64%) with densitometry ratios to GAPDH shown in the blots, and a reduction in the ratio of cleaved caspase 3 to total caspase 3, as demonstated by the densitometry data included in the blots as the ratio of cleaved to total caspase 3 (D, n=8, p=0.03). Representative immunoblots shown, and the average densitometry of 8 tumors is inset.
APE1/Ref-1 inhibition enhances cisplatin’s therapeutic effect.
Cisplatin is the standard of care for bladder cancer, but many patients’ tumors are refractory to this therapy. Therefore, we evaluated whether the combined treatment of cisplatin and APE1/Ref-1 inhibitors was more effective at reducing bladder cancer cell proliferation. To determine this, we treated PDX lines, RP-B-01 and RP-B-02 as well as established cell line, T24 with increasing concentrations of cisplatin in combination with APX compounds (Figure 7). Cell proliferation was assessed using Alamar blue 72 h after drug treatment. First, preferential sensitivity of RP-B-02 cells was confirmed in our laboratory by demonstrating that the RP-B-02 cells exhibit an IC50 to cisplatin of 0.5 μM while the RP-B-01 cells exhibited an IC50 to cisplatin of 1.44 μM (n=3; p<0.05). At the doses used in combination treatment, minimal reduction in bladder cancer cell proliferation was observed with APX2014 and APX2009 alone in all cell lines (~10-30%). However, when combined with increasing concentrations of cisplatin we observed a further reduction in cellular proliferation with combination treatment. Interestingly, the effects of APE1/Ref-1 on cisplatin-induced cytotoxicity was more prevalent in the PDX lines (Figure 7A, B) than the T24 cells (Figure 7C). Using the Chou-Talalay method, we determined that APX2009 and APX2014 have primarily an additive effect when combined with cisplatin. Combination index (CI) values were calculated with Compusyn for combination of APX inhibitors and cisplatin, and CI values ranged from 0.82 – 1.2. indicative of synergy to additivity with this combination. Importantly, the combination of cisplatin with APE1/Ref-1 inhibition is potently killing more cells than either agent alone.
DISCUSSION
Bladder cancer is one of the leading causes of cancer-related deaths in men in the United States. The current chemotherapeutic standard-of-care is cisplatin-combination therapy. However, the majority of patients are refractory to cisplatin treatment (1, 3, 41, 42). Our results indicate that APE1/Ref-1 is highly expressed in patient samples of bladder cancer, with much lower staining in benign epithelium. These data corroborate previous studies indicating an increase of APE1/Ref-1 in the urine and serum of patients with bladder cancer (9, 13), and support the rationale for targeting APE1/Ref-1 in bladder cancer. In addition, we observe strong nuclear staining of APE1/Ref-1 in epithelial-confined tumors, while invasive lesions within the detrusor exhibit nuclear and increased cytosolic staining of this protein. This was observed previously in prostate and ovarian tumors (12, 14, 15), and this suggested to us that APE1/Ref-1 might have enhanced its redox activity in these tumors, as its redox targets are both cytosolic and nuclear while its repair activity predominantly occurs in the nucleus. Because of this, we investigated redox activity inhibitors and their activity in models of bladder cancer cell growth.
APX3330, APX2009, and APX2014 are known to inhibit APE1/Ref-1 redox signaling by binding to the redox active region (16, 18, 20, 22-24). APE1/Ref-1 adopts locally unfolded conformations near the critical cysteine residues responsible for redox activity. APX3330 can then bind to the protein, facilitate disulfide bond formation and block the redox active cysteine 65 responsible for redox activity (23). In this study, we show that bladder cancer cell proliferation can be attenuated through the inhibition of redox signaling by APE1/Ref-1 with APX compounds. We also establish the downregulation of transcriptional activity of key transcription factors, STAT3 and NFkB and downstream targets of these TFs (cyclin D1 and survivin) following treatment with APE1/Ref-1 inhibitors. These pathways have been shown to be key players in proliferation and chemoresistance in BCa as well as other cancer types. Consistent with that, we demonstrate that cisplatin-resistant human PDX cells are sensitive to APE1/Ref-1 redox-selective inhibition, and we demonstrate that inhibition of APE1/Ref-1 redox activity sensitizes bladder cancer cell lines to cisplatin. Future in vivo studies assessing the efficacy APE1/Ref-1 inhibitors in treating cisplatin-resistant tumors in combination with cisplatin are warranted.
APE1/Ref-1 was originally discovered as an AP endonuclease that functions in base excision repair and is conserved from E. coli to humans (5). APE1/Ref-1 is responsible for repairing oxidative and alkylated DNA damage by nicking the DNA backbone at the AP site with further processing by β-polymerase and DNA ligase (6). It was later discovered that APE1/Ref-1 plays a role in redox signaling by perturbing DNA binding capabilities of key TFs such as NFκB and STAT3. These transcriptional activators are known to play an important part in the regulation of proliferation in many cancers (24, 25, 31, 40, 43). Here we show that the transcriptional activity of NFκB and STAT3, but not AP-1 are both attenuated in the presence of novel redox-selective APE1/Ref-1 inhibitors in established bladder cancer cell lines.
We assessed the expression of several known downstream targets of TFs whose DNA binding activity are under redox control by APE1/Ref-1 including Cyclin D1, c-Myc, Bcl-2, Bcl-XL, Mcl-1, and survivin. Importantly, many of these critical proteins have binding sites for STAT3 and NFκB in their promoters indicating that the targeting of APE1/Ref-1 and thereby blockade of STAT3 and NFκB impacts upon the expression of survival and proliferation proteins in bladder cancer cells. Redox-selective APE1/Ref-1 blockade showed a significant reduction in cyclin D1 expression (2-fold, p<0.05), as well as a substantial reduction in survivin levels (3-4-fold, p<0.01). Interestingly, we did not observe a universal decrease in the NFκB/STAT3 targets we investigated, nor a reduction in AP-1 activity at the timepoints tested. This suggests that redox signaling through the APE1/Ref-1 axis is a specific transcription factor-dependent, as well as a gene and cancer type-specific event. The caveats to these conclusions remain that it is possible the timing of the reduction in expression is different between each of these proteins or we are seeing induction of a potential feedback loop. Both of these options are being explored in future studies. We also observe a potential effect on the vascularization of the bladder grafts (Figure 6A). APE1/Ref-1 regulation of STAT3 and HIF1-α potentially may decrease the levels of VEGF leading to an effect on angiogenesis. APE1/Ref-1 role in angiogenesis has been confirmed in a multitude of other studies (16, 17, 19, 20) and will be investigated in vivo in the future.
Survivin is a bifunctional protein that has been shown to have both anti-apoptotic and pro-proliferative effects in bladder cancer cells (28-31, 44-47). Because survivin is known to be regulated by both NFκB and STAT3 and is regulated by APE1/Ref-1 in prostate cancer (21, 28, 45) we included this protein target as a marker of APE/Ref-1 inhibition in our studies in bladder cancer and as a mechanistic explanation for the decrease in cell growth. We found a near universal co-expression of APE1/Ref-1 and high survivin levels in human bladder cancer specimens. Importantly, we found that survivin is the most reduced of all the known target proteins of APE1/Ref-1-regulated transcription factor activity, a reduction in protein levels of more than 50%, both in vitro and in vivo (Figure 5D & 6D). Survivin is a member of the XIAP family of apoptosis inhibitors and is a known inhibitor of caspase 3 function, supporting cell survival (45). It is especially active in cancer tissues. However, survivin also has pro-cell proliferation functions in cancer, as it is known to interact with microtubules and chromatin, promoting cell proliferation (45). In addition, survivin is known to be induced during bladder cancer (28, 29, 44, 48), and recently both survivin and APE1/Ref-1 have been implicated as functional biomarkers for the disease. Our data support future mechanistic analysis of APE1/Ref-1 activity that is centered on survivin and supports investigation of the APE1/Ref-1 axis that is driving survivin expression as a target in bladder cancer cell survival and proliferation.
Thus far, targeting survivin in cancer has proved difficult. For example, survivin inhibitor YM-155 affects the expression of additional proteins through a mechanism involving Sp1 which contributes to off-target effects (49). In spite of this, inhibition of survivin is a viable approach to killing bladder cancer cells as knockdown of survivin or a reduction in survivin protein levels in bladder cancer cells results in a decrease in cell proliferation and increase in apoptosis. Inhibition of survivin with small molecule inhibitors also causes bladder cancer cells to enter cell arrest or apoptosis (28, 31, 45), again pointing to its importance in bladder cancer cell survival. Yet, how it functions and how it is regulated in bladder cancer is unknown. Our data presented here clearly demonstrate that inhibition of APE1/Ref-1 redox signaling activity dramatically reduces survivin expression in both in vitro and in vivo models of bladder cancer and circumvents the need for a direct survivin inhibitor. Survivin as a key mediator of bladder cancer cell response following perturbation of the APE1/Ref-1 signaling axis is a novel finding with important clinical implications.
Preclinical studies demonstrating the validity of APE1/Ref-1 redox function as a drug target in cancer led to a phase I clinical trial assessing the safety of APX3330 and recommended phase II dose for future treatment of solid tumors () (26, 27). With the advancement of APX3330 to the clinic, we are also focusing on the development of more potent second-generation compounds such as APX2009 and APX2014. Our work here extends APE1/Ref-1 targeting into bladder cancer and is the first demonstration of APE1 as a potential bladder cancer target. Our ultimate goal is to provide a therapeutic option in bladder tumors that are inherently resistant to cisplatin. Many bladder cancer patients will recur post initial treatment, and up to 20% of them will be administered cisplatin-based chemotherapy (3), Our work therefore provides justification to move forward with a clinical evaluation of redox-specific inhibition of APE1/Ref-1 in bladder cancer patients, as it may provide a potential option for treating cisplatin-refractory bladder cancer patients.
Our data demonstrate a critical role for APE1/Ref-1 redox activity in bladder cancer cells and confirm its use as a putative urinary bladder cancer tumor marker. In addition, our work shows, for the first time, the feasibility of targeting APE1/Ref-1 therapeutically for the treatment of bladder cancer and for combination therapy with standard-of-care drug, cisplatin. This is supported by the additive effect that APE1/Ref-1 inhibition exhibits on bladder cancer growth and cell death in combination with cisplatin. Our work also demonstrates a mechanistic target for APE1/Ref-1 redox function in bladder cancer: the induction of the critical cell regulator, survivin. Our work supports the use of novel redox-selective inhibitors in clinical studies for patients with bladder cancer.
Supplementary Material
SIGNIFICANCE: This work identifies a critical mechanism for APE1/Ref-1 in bladder cancer growth, and provides compelling preclinical data using selective redox activity inhibitors of APE1/Ref-1 in vitro and in vivo.
ACKNOWLEDGEMENTS:
We would like to thank Olivia Babb for technical support on the Westerns. The AngioBio Core through IU Simon Cancer Center provided the Incucyte system and technical support for the data collection and analysis. Drs. Fenil Shah and Harlan Shannon also aided in the analysis of the caspase data.
Financial Information
Funding for this project included support from the following grants:
The department of defense Prostate Cancer Research Program: DOD-PCRP (PI: Travis J. Jerde) W81XWH-14-1-0525
The National Institutes of Health; National Cancer Institute: NIH RO1 CA205166-02 (Mark R. Kelley and Jill C. Fehrenbacher PIs) and NIH R01 CA167291-06 (Mark R. Kelley and Melissa L. Fishel, PIs)
Footnotes
DISCLOSURE OF POTENTIAL CONFLICT OF INTEREST: Mark R. Kelley has licensed APX3330 through Indiana University Research and Technology Corporation to Apexian Pharmaceuticals LLC. APX2009 is a second generation compound from Apexian Pharmaceuticals. Apexian Pharmaceuticals had neither control nor oversight of the studies, interpretation, or presentation of the data in this manuscript.
REFERENCES
- 1.Antoni S FJ, Soerjomataram I1, Znaor A1, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71(1):96–108. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL MK, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Massari F SM, Ciccarese C, Brunelli M, Conti A, Santini D, Montironi R, Cascinu S, Tortora G. Emerging concepts on drug resistance in bladder cancer: Implications for future strategies. Crit Rev Oncol Hematol. 2015;96(1):81–90. [DOI] [PubMed] [Google Scholar]
- 4.Rouanne M, Roumiguie M, Houede N, Masson-Lecomte A, Colin P, Pignot G, Larre S, Xylinas E, Roupret M, and Neuzillet Y Development of immunotherapy in bladder cancer: present and future on targeting PD(L)1 and CTLA-4 pathways. World J Urol 2018. [DOI] [PubMed] [Google Scholar]
- 5.Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461(2):83–108. [DOI] [PubMed] [Google Scholar]
- 6.Vascotto C, Cesaratto L, Zeef LA, Deganuto M, D’Ambrosio C, Scaloni A, Romanello M, Damante G, Taglialatela G, Delneri D, Kelley MR, Mitra S, Quadrifoglio F, Tell G. Genome-wide analysis and proteomic studies reveal APE1/Ref-1 multifunctional role in mammalian cells. Proteomics. 2009;9(4):1058–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah F, Logsdon D, Messmann RA, Fehrenbacher JC, Fishel ML, Kelley MR. Exploiting the Ref-1-APE1 node in cancer signaling and other diseases: from bench to clinic. npj Precision Oncology. 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan X WL, Li Y, Lou L, Liu W, Zhang J. The expression profile and prognostic value of APE/Ref-1 and NPM1 in high-grade serous ovarian adenocarcinoma. APMIS. 2017;25(10):857–62. [DOI] [PubMed] [Google Scholar]
- 9.Choi S, Shin JH, Lee YR, Joo HK, Song KH, Na YG, Chang SJ, Lim JS, Jeon BH. Urinary APE1/Ref-1: A Potential Bladder Cancer Biomarker. Dis Markers. 2016;2016:7276502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y, Zhou S, Sandusky GE, Kelley MR, Fishel ML. Reduced expression of DNA repair and redox signaling protein APE1/Ref-1 impairs human pancreatic cancer cell survival, proliferation, and cell cycle progression. Cancer Investigation. 2010;28(9):885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fishel ML, He Y, Reed AM, Chin-Sinex H, Hutchins GD, Mendonca MS, Kelley MR. Knockdown of the DNA repair and redox signaling protein Ape1/Ref-1 blocks ovarian cancer cell and tumor growth. DNA Repair (Amst). 2008;7(2):177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore DH, Michael H, Tritt R, Parsons SH, Kelley MR. Alterations in the expression of the DNA repair/redox enzyme APE/ref-1 in epithelial ovarian cancers. Clin Cancer Res. 2000;6(2):602–9. [PubMed] [Google Scholar]
- 13.Shin JH, Choi S, Lee YR, Park MS, Na YG, Irani K, Lee SD, Park JB, Kim JM, Lim JS, Jeon BH. APE1/Ref-1 as a Serological Biomarker for the Detection of Bladder Cancer. Cancer Res Treat. 2015;47(4):823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley MR, Cheng L, Foster R, Tritt R, Jiang J, Broshears J, Koch M. Elevated and altered expression of the multifunctional DNA base excision repair and redox enzyme Ape1/ref-1 in prostate cancer. Clin Cancer Res. 2001;7(4):824–30. [PubMed] [Google Scholar]
- 15.McIlwain DW, Fishel ML, Boos A, Kelley MR, Jerde TJ. APE1/Ref-1 redox-specific inhibition decreases survivin protein levels and induces cell cycle arrest in prostate cancer cells. Oncotarget. 2018;9:10962–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sardar Pasha SPB, Sishtla K, Sulaiman RS, Park B, Shetty T, Shah F, Fishel ML, Wikel JH, Kelley MR, Corson TW. Ref-1/APE1 Inhibition with Novel Small Molecules Blocks Ocular Neovascularization. J Pharmacol Exp Ther. 2018;367(1):108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Liu X, Zhou T, Kelley MR, Edwards PA, Gao H, Qiao X. Suppression of choroidal neovascularization through inhibition of APE1/Ref-1 redox activity. Investigative ophthalmology & visual science. 2014;55(7):4461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishel ML, Jiang Y, Rajeshkumar NV, Scandura G, Sinn AL, He Y, Shen C, Jones DR, Pollok KE, Ivan M, Maitra A, Kelley MR. Impact of APE1/Ref-1 redox inhibition on pancreatic tumor growth. Mol Cancer Ther. 2011;10(9):1698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang A, Gao H, Kelley MR, Qiao X. Inhibition of APE1/Ref-1 Redox Activity with APX3330 Blocks Retinal Angiogenesis in vitro and in vivo. Vision Res. 2011;51:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo M, Delaplane S, Jiang A, Reed A, He Y, Fishel M, Nyland RL 2nd, Borch RF, Qiao X, Georgiadis MM, Kelley MR. Role of the multifunctional DNA repair and redox signaling protein Ape1/Ref-1 in cancer and endothelial cells: small-molecule inhibition of the redox function of Ape1. Antioxid Redox Signal. 2008;10(11):1853–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIlwain DW, Fishel ML, Boos A, Kelley MR & Jerde TJ APE1/Ref-1 redox-specific inhibition decreases survivin protein levels and induces cell cycle arrest in prostate cancer cells. . Oncotarget. 2017;9:10962–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nyland RL, Luo M, Kelley MR, Borch RF. Design and synthesis of novel quinone inhibitors targeted to the redox function of apurinic/apyrimidinic endonuclease 1/redox enhancing factor-1 (Ape1/ref-1). J Med Chem. 2010;53(3):1200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su DG, Delaplane S, Luo M, Rempel DL, Vu B, Kelley MR, Gross ML, Georgiadis MM. Interactions of APE1 with a redox inhibitor: Evidence for an alternate conformation of the enzyme. Biochemistry. 2011;50:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cardoso AA, Jiang Y, Luo M, Reed AM, Shahda S, He Y, Maitra A, Kelley MR, Fishel ML. APE1/Ref-1 Regulates STAT3 Transcriptional Activity and APE1/Ref-1-STAT3 Dual-Targeting Effectively Inhibits Pancreatic Cancer Cell Survival. PLoS ONE. 2012;7(10):e47462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grivennikov SI KM. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21(1):11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahda S, Lakhani NJ, O’Neil B, Rasco DW, Wan J, Mosley AL, Liu H, Kelley MR, Messmann RA, editors. A phase I study of APE1 protein inhibitor APX3330 in patients with advanced solid tumors. ASCO; 2019; Chigago, IL. [Google Scholar]
- 27.Chu L, Anderson AKL, Mark Andrew Landers MA, Wang Y, Kelley MR, Messmann RA, editors. CTC enumeration and characterization as a pharmacodynamic marker in the phase I clinical study of APX3330, an APE1/Ref-1 inhibitor, in patients with advanced solid tumors. ASCO; 2019. [Google Scholar]
- 28.Akhtar M, Gallagher L, Rohan S. Survivin: role in diagnosis, prognosis, and treatment of bladder cancer. Adv Anat Pathol. 2006;13(3):122–6. [DOI] [PubMed] [Google Scholar]
- 29.Shariat SF, Ashfaq R, Karakiewicz PI, Saeedi O, Sagalowsky AI, Lotan Y. Survivin expression is associated with bladder cancer presence, stage, progression, and mortality. Cancer. 2007;109(6):1106–13. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Li P, Fu D, Wei Z, Xu S, Xu F, Tian F, Ge J, Zhang Z, Cheng W. Combined use of urinary Survivin detection and liquid-based cytology for the early diagnosis of bladder urothelial carcinoma. Oncol Lett. 2018;15(5):7739–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang B, Lu Z, Hou Y, Hu J, Wang C. The effects of STAT3 and Survivin silencing on the growth of human bladder carcinoma cells. Tumour Biol. 2014;35(6):5401–7. [DOI] [PubMed] [Google Scholar]
- 32.McIlwain DW, Zoetemelk M, Myers JD, Edwards MT, Snider BM, Jerde TJ. Coordinated induction of cell survival signaling in the inflamed microenvironment of the prostate. Prostate. 2016;76(8):722–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley MR, Luo M, Reed A, Su D, Delaplane S, Borch RF, Nyland RL, Gross ML, Georgiadis MM. Functional Analysis of Novel Analogs of E3330 That Block the Redox Signaling Activity of the Multifunctional AP Endonuclease/Redox Signaling Enzyme APE1/Ref-1. Antioxid Redox Signal. 2011;14(8):1387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelley MR, Wikel JH, Guo C, Pollok KE, Bailey BJ, Wireman R, Fishel ML, Vasko MR. Identification and Characterization of New Chemical Entities Targeting Apurinic/Apyrimidinic Endonuclease 1 for the Prevention of Chemotherapy-Induced Peripheral Neuropathy. J Pharmacol Exp Ther. 2016;359(2):300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fishel ML, Wu X, Devlin CM, Logsdon DP, Jiang Y, Luo M, He Y, Yu Z, Tong Y, Lipking KP, Maitra A, Rajeshkumar NV, Scandura G, Kelley MR, Ivan M. Apurinic/apyrimidinic endonuclease/redox factor-1 (APE1/Ref-1) redox function negatively regulates NRF2. J Biol Chem. 2015;290(5):3057–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logsdon DP, Grimard M, Luo M, Shahda S, Jiang Y, Tong Y, Yu Z, Zyromski N, Schipani E, Carta F, Supuran CT, Korc M, Ivan M, Kelley MR, Fishel ML. Regulation of HIF1alpha under Hypoxia by APE1/Ref-1 Impacts CA9 Expression: Dual Targeting in Patient-Derived 3D Pancreatic Cancer Models. Mol Cancer Ther. 2016;15(11):2722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei L, Chintala S, Ciamporcero E, Ramakrishnan S, Elbanna M, Wang J, Hu Q, Glenn ST, Murakami M, Liu L, Gomez EC, Sun Y, Conroy J, Miles KM, Malathi K, Ramaiah S, Anbarasu A, Woloszynska-Read A, Johnson CS, Conroy J, Liu S, Morrison CD, Pili R. Genomic profiling is predictive of response to cisplatin treatment but not to PI3K inhibition in bladder cancer patient-derived xenografts. Oncotarget. 2016;7(47):76374–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciamporcero E, Shen H, Ramakrishnan S, Yu Ku S, Chintala S, Shen L, Adelaiye R, Miles KM, Ullio C, Pizzimenti S, Daga M, Azabdaftari G, Attwood K, Johnson C, Zhang J, Barrera G, Pili R. YAP activation protects urothelial cell carcinoma from treatment-induced DNA damage. Oncogene. 2016;35(12):1541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L ZM, Chitteti BR, Ratliff TL, Myers JD, Srour EF, Broxmeyer H, Jerde TJ. Expansion of prostate epithelial progenitor cells after inflammation of the mouse prostate. Am J Physiol Renal Physiol. 2015;308(12):F1421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arpin CC MS, Jiang Y, Cheng H, Grimard M, Page BD, Kamocka MM, Haftchenary S, Su H, Ball DP, Rosa DA, Lai PS, Gómez-Biagi RF, Ali AM, Rana R, Hanenberg H, Kerman K, McElyea KC, Sandusky GE, Gunning PT, Fishel ML. Applying Small Molecule Signal Transducer and Activator of Transcription-3 (STAT3) Protein Inhibitors as Pancreatic Cancer Therapeutics. Mol Cancer Ther. 2016;15(5):794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee IL, Melquist J, Bondaruk J, Majewski T, Zhang S, Pretzsch S, Baggerly K, Siefker-Radtke A, Czerniak B, Dinney CP, McConkey DJ. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sjödahl G, Jackson CL, Bartlett JM, Siemens DR, Berman DM. Molecular profiling in muscle‐invasive bladder cancer: more than the sum of its parts. The Journal of Pathology. 2019; 274(5): 563–73. [DOI] [PubMed] [Google Scholar]
- 43.Chen CL, Cen L, Kohout J, Hutzen B, Chan C, Hsieh FC, Loy A, Huang V, Cheng G, Lin J. Signal transducer and activator of transcription 3 activation is associated with bladder cancer cell growth and survival. Mol Cancer. 2008;7:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen D, Xu J, Zhang Q. Detection of survivin expression in bladder cancer and renal cell carcinoma using specific monoclonal antibodies. Oncol Rep. 2018;39(6):2817–28. [DOI] [PubMed] [Google Scholar]
- 45.Garg H SP, Gupta JC, Talwar GP, Dubey S. Survivin: a unique target for tumor therapy. Cancer Cell Int. 2016;16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, Zhang B, Mani AM, Wu Z, Fan Y, Li W, Wu ZH. Survivin inhibitors mitigate chemotherapeutic resistance in breast cancer cells by suppressing genotoxic NF-kappaB activation. J Pharmacol Exp Ther. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Zhang Y, Liu X, Fang A, Li P, Li Z, Liu T, Yang Y, Du L, Wang C. MicroRNA-203 Is a Prognostic Indicator in Bladder Cancer and Enhances Chemosensitivity to Cisplatin via Apoptosis by Targeting Bcl-w and Survivin. PLoS One. 2015;10(11):e0143441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong D, Jiang W, Lei J, Chen L, Liu X, Ge J, Che B, Xi X, Shao J. Ubiquitin-like protein FAT10 promotes bladder cancer progression by stabilizing survivin. Oncotarget. 2016;7(49):81463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sachita K, Yu HJ, Yun JW, Lee JS, Cho SD. YM155 induces apoptosis through downregulation of specificity protein 1 and myeloid cell leukemia-1 in human oral cancer cell lines. J Oral Pathol Med. 2015;44(10):785–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.