Abstract
Background
Lower socioeconomic status (SES) and psychosocial stress during pregnancy have been associated with adverse birth outcomes. While hypothalamic-pituitary-axis activation is thought to be the primary driver, oxidative stress may also be involved mechanistically. We used data from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort (N=476) to examine associations between self-reported psychosocial stress measures, SES indicators, and urinary oxidative stress biomarker concentrations, hypothesizing that women with lower SES and increased psychosocial stress would have elevated oxidative stress biomarkers.
Methods
Maternal age, education, marital status, insurance status, alcohol use and smoking status were obtained via self-reported questionnaires and were used as indicators of SES. Perceived stress, depression, negative life experiences, neighborhood perceptions, and social support were self-reported in questionnaires administered during pregnancy. Responses were grouped into tertiles for analysis, where the highest tertile corresponded to highest level of psychosocial stress. Urinary concentrations of 8-iso-prostaglandin F2α (8-iso-PGF2α) and its primary metabolite were measured at three study visits (median 18, 24, 28 weeks gestation) and averaged to reflect oxidative stress across pregnancy. Linear models were used to examine associations between SES indicators, tertiles of psychosocial stress and oxidative stress biomarkers.
Results
Average levels of 8-iso-PGF2α and the 8-iso-PGF2α metabolite were higher among pregnant women who were younger, who had public compared to private insurance, and who were unemployed compared to employed. However, no associations were observed between psychosocial stress measures and biomarker concentrations in adjusted analyses.
Conclusions
Psychosocial stress during pregnancy, as indicated by self-reported questionnaire measures, was not associated with biomarkers of oxidative stress in the PROTECT study. However, results suggest that these biomarkers are elevated among women of lower SES, which is typically associated with stress. Notably, compared to other populations, self-reported psychosocial stress measures were lower in PROTECT compared to other populations.
Keywords: psychosocial stress, oxidative stress, pregnancy, social determinants, isoprostanes
Graphical Abstract
Introduction
Decreasing socioeconomic status (SES) and increasing psychosocial stress have been associated with adverse pregnancy outcomes.1 One potential pathway linking these factors to adverse birth outcomes may be elevated oxidative stress. Thus, understanding the origins of oxidative stress during pregnancy may lead to improved intervention strategies for preventing poor pregnancy endpoints, such as preterm birth.2
Certain health behaviors frequently associated with lower SES may contribute to higher oxidative stress levels. For example, relative to higher SES individuals, smoking, alcohol use, and unhealthy diets are more common among those with lower SES.3,4 These behaviors are known to increase oxidative stress.5,6 Additionally, individuals of lower SES may be disproportionately exposed to environmental contaminants, such as air pollution and phthalates,7 which have been associated with increased concentrations of oxidative stress biomarkers.8,9
Individuals who are socioeconomically disadvantaged may also experience higher levels of psychosocial stress, in part due to experiencing a greater number of daily hassles, anxiety, and poorer living conditions.1 Studies in non-pregnant populations have shown that individuals with higher levels of psychosocial stress, such as those who experience stressful life events and symptoms of depression and anxiety, have elevated oxidative stress biomarkers compared to their less stressed counterparts.10–12 Few studies, however, have been conducted among pregnant women13,14 where it may be particularly important. Furthermore, these studies have been limited by a small number of scales used to measure psychosocial stress.11,14 Thus, we sought to examine the relationships between SES, 5 measures of psychosocial stress, and oxidative stress biomarkers in a cohort of pregnant women residing in Puerto Rico. We hypothesized that increased psychosocial stress and indicators of SES disadvantage would be associated with elevated oxidative stress levels.
Methods
Study Population
Pregnant women included in this analysis delivered between August 2012 and April 2017 and are a subset of women enrolled to date in the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) cohort. Recruitment methods have been previously described in detail.15,16 Inclusion criteria for PROTECT were as follows: maternal age between 18–40 years, residence in the Northern Karst aquifer region, and no known obstetric and medical complications (e.g., diabetes). Women who used in vitro fertilization to become pregnant and who used oral contraceptives for 3 months prior to conception were excluded. Participants completed up to 3 study visits, targeted at approximately 20±2, 24±2, and 28±2 weeks gestation, and demographic information is obtained via questionnaire at the first visit. Spot urine samples were obtained at each study visit. All women provided written informed consent prior to participating and the Institutional Review Board at all participating locations (University of Georgia, University of Michigan, Northeastern University, University of Puerto Rico) approved this study.
We included the following categorical covariates as indicators of SES or as sociodemographic characteristics in our analyses: maternal age in years (18–24, 25–29, 30–34, ≥35), marital status (single, married, living together and unmarried), maternal education (<high school, high school degree or equivalent, some college or technical school, ≥college degree), employment status (unemployed, employed), insurance status (public, private, uninsured), alcohol use (never, before pregnancy, current at visit 1) and smoking status (never, before pregnancy, current at visit 1).
Psychosocial Stress
Five questionnaire measures of psychosocial stress were administered to participants in PROTECT. The Life Experience Survey17 and two questions assessing perceptions of neighborhood safety and quality18 were administered at the 2nd visit as indicators of negative life experiences and neighborhood perceptions, respectively. The 10-item Perceived Stress Scale,19 20-item Center for Epidemiologic Studies-Depression scale (CES-D),20 and 7-item ENRICHD Social Support Instrument21 were administered at the 3rd visit.
Responses to individual questions on each scale were summed to create continuous measures of stress. If the response to any individual question on the scale was missing, the overall scale was coded as missing for that individual. Continuous measures for each scale were grouped into tertiles (i.e., low, medium, high stress) for analyses, where the highest tertile corresponded to high stress for all scales, except the ENRICHD Social Support Instrument where the lowest tertile indicated low social support (i.e., high stress). Additional information regarding classification of psychosocial stress measures is provided elsewhere.22
Oxidative Stress Biomarker Assessment
The Eicosanoid Core Laboratory at Vanderbilt University Medical Center (Nashville, TN) analyzed free 8-isoprostane-prostaglandin-F2α (8-iso-PGF2α) as a biomarkers of oxidative stress using stable isotype dilution gas chromatography-negative ion chemical ionization-mass spectrometry in 476 participants (N= 272 samples at visit 1, N= 345 samples at visit 2, N= 221 samples at visit 3).23 The major 8-iso-PGF2α metabolite, 2,3-dinor-5,6-dihydro-15-F2t-isoprostanes, was also measured, as is thought to be a superior biomarker of oxidative stress than 8-iso-PGF2α in urine.24 We additionally measured prostaglandin-F2α (PGF2α) which can be used to distinguish whether 8-iso-PGF2α derive from chemical or enzymatic pathways.25 As a sensitivity analysis, we quantified the proportion of 8-iso-PGF2α derived from chemical and enzymatic fractions using the ratio of PGF2α to 8-iso-PGF2α, as calculated by a custom interface for the R package “Constrained Linear Mixed Effects (CLME)”, and examined associations with these endpoints.25 For all biomarkers measured, values below the limit of detection (LOD; 0.101 ng/mL) were replaced by LOD/the square root of 2.
Urinary specific gravity (SpG) was measured using a digital handheld refractometer to indicate urine dilution. All urinary oxidative stress biomarker concentrations were corrected for SpG using the equation Oxc = Ox[(1.019–1)/(SpG-1)], where 1.019 is the median SpG in the PROTECT population, Ox is the measured oxidative stress concentration, and Oxc is the SpG-corrected measure. We then took the geometric mean of the available SpG-corrected oxidative stress concentrations across visits to reflect pregnancy averages. All averages were natural log transformed for normality in statistical models.
Statistical Analysis
Descriptive statistics were used to summarize participant sociodemographic characteristics. Linear regression models were used to calculate crude estimates and 95% confidence intervals (CI) for the associations between SES indicators, psychosocial stress and each oxidative stress biomarker pregnancy average. Adjusted estimates were also obtained from linear regression models in which psychosocial stress was the exposure. QQ-plots were examined for each model to check linear regression assumptions, including linearity, normality, and homoscedasticity. Beta estimates were converted to percent difference in oxidative stress biomarker concentration in association with SES indicators and psychosocial stress. Tests for linear trend across tertiles were conducted using the Cochrane Armitage test.26 SES indicators retained in adjusted models changed point estimates by ≥10%.
Missing data for psychosocial stress measures and covariates (<10% for each imputed variable) was imputed using multiple imputation via chained equations (MICE), which was implemented using the ‘mice’ package in R. Oxidative stress biomarker concentrations were not included as predictors in the imputation procedure. All analyses were conducted in R Version 3.5.0 and SAS 9.4 (Cary, NC).
Results
This analytic sample included 476 women who had at least one urine sample analyzed for oxidative stress biomarkers. Most women were between 18–24 years of age (39.7%), were married (53.8%), had a college degree or higher (41.6%), and were non-smokers (82.6%; Table 1). The geometric mean of 8-iso-PGF2α and the 8-iso-PGF2α metabolite was 1.84 (geometric standard deviation [SD]=1.66) and 0.88 (geometric SD=1.72), respectively.
Table 1.
Distribution of demographic characteristics in the PROTECT study population (N=476).
| N (%) | |
|---|---|
| Maternal Age, years | |
| 18–24 | 189 (39.7) |
| 25–29 | 148 (31.1) |
| 30–34 | 85 (17.9) |
| ≥35 | 54 (11.3) |
| Maternal Education | |
| <High school | 35 (7.35) |
| High school or equivalent | 72 (15.1) |
| Some college or technical school | 171 (35.9) |
| ≥College degree | 198 (41.6) |
| Employment Status | |
| Employed | 295 (62.0) |
| Unemployed | 181 (38.0) |
| Marital Status | |
| Single | 91 (19.1) |
| Married | 256 (53.8) |
| Living together | 129 (27.1) |
| Alcohol Use | |
| Never | 210 (44.1) |
| Before pregnancy | 241 (50.6) |
| Current | 25 (5.25) |
| Smoking | |
| Never | 392 (82.4) |
| Before pregnancy | 65 (13.7) |
| Current | 19 (4.00) |
| Insurance Status | |
| Private | 293 (61.6) |
| Public | 177 (37.2) |
| Uninsured | 6 (1.26) |
Note: percentages may not sum to 100 due to rounding. The number of imputed values for each variable were as follows: maternal age=0, maternal education=4, employment status=5,marital status=2, alcohol use=6, smoking=2, insurance status=17.
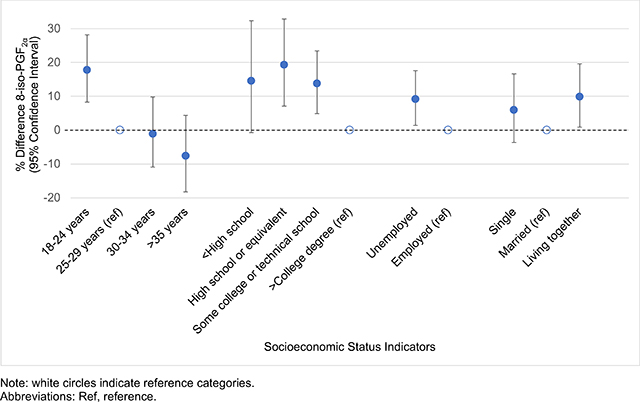
In bivariate analyses, indicators of lower SES were associated with elevated pregnancy averages of both oxidative stress biomarkers (Table 2). For example, compared to women who were employed, women who were unemployed had 9.19% (95% CI=1.40–17.6) and 14.3% (95% CI=5.71–23.7) higher 8-iso-PGF2α and 8-iso-PGF2α metabolite levels, respectively. Women with public insurance also had elevated levels of 8-iso-PGF2α (% difference=14.2, 95% CI=6.01–23.0) and 8-iso-PGF2α metabolite (% difference=18.1, 95% CI=9.23–27.8) compared to women with private insurance. Additionally, women who had less than a college education and who were between 18–24 years of age had elevated levels of both biomarkers compared to those who had a college education and who were between 25–29. Associations between SES indicators and PGF2α and the chemical and enzymatic fractions of 8-iso-PGF2α were similar to those observed with 8-iso-PGF2α and the 8-iso-PGF2α metabolite (Table S1).
Table 2.
Associations between specific gravity corrected urinary oxidative stress concentrations (ng/mL) and selected demographic characteristics (N=476).
| 8-iso-prostaglandin-F2α | 8-iso-prostaglandin-F2α metabolite | |||
|---|---|---|---|---|
| % Difference (95% CI) | p | % Difference (95% CI) | p | |
| Maternal Age, years | ||||
| 18–24 | 17.8 (8.31, 28.2) | <0.01 | 22.5 (12.0, 33.9) | <0.01 |
| 25–29 | Ref | Ref | Ref | Ref |
| 30–34 | −1.10 (−10.9, 9.78) | 0.84 | −1.99 (−12.3, 9.50) | 0.72 |
| ≥35 | −7.62 (−18.2, 4.37) | 0.20 | 0.88 (−11.4, 14.9) | 0.89 |
| Maternal Education | ||||
| <High school | 14.6 (−0.73, 32.3) | 0.06 | 21.1 (4.29, 40.7) | 0.01 |
| High school or equivalent | 19.3 (7.11, 32.9) | <0.01 | 28.1 (14.5, 43.4) | <0.01 |
| Some college or technical school | 13.8 (4.90, 23.4) | <0.01 | 18.7 (8.98, 29.3) | <0.01 |
| ≥College degree | Ref | Ref | Ref | Ref |
| Employment Status | ||||
| Unemployed | 9.19 (1.40, 17.6) | 0.02 | 14.3 (5.71, 23.7) | <0.01 |
| Employed | Ref | Ref | Ref | Ref |
| Marital Status | ||||
| Single | 6.01 (−3.64, 16.6) | 0.23 | 11.1 (0.49, 22.8) | 0.04 |
| Married | Ref | Ref | Ref | Ref |
| Living together | 9.86 (0.89, 19.6) | 0.03 | 18.7 (8.61, 29.8) | <0.01 |
| Alcohol Use | ||||
| Never | Ref | Ref | Ref | Ref |
| Before pregnancy | −2.43 (−9.42, 5.10) | 0.52 | −2.36 (−9.77, 5.66) | 0.55 |
| Current | −5.90 (−20.8, 11.8) | 0.49 | −2.59 (−18.8, 16.8) | 0.78 |
| Smoking | ||||
| Never | Ref | Ref | Ref | Ref |
| Before pregnancy | 8.62 (−2.36, 20.8) | 0.13 | 5.07 (6.11, 17.6) | 0.39 |
| Current | 12.6 (−6.30, 35.4) | 0.21 | 2.67 (−15.7, 25.1) | 0.79 |
| Insurance Status | ||||
| Private | Ref | Ref | Ref | Ref |
| Public | 14.2 (6.01, 23.0) | <0.01 | 18.1 (9.23, 27.8) | <0.01 |
| Uninsured | 30.0 (−6.62, 80.9) | 0.12 | 36.6 (−2.31, 91.0) | 0.07 |
Note: associations are unadjusted. The number of imputed values for each variable were as follows: maternal age=0, maternal education=4, employment status=5,marital status=2, alcohol use=6, smoking=2, insurance status=17.
Abbreviations: CI, confidence interval; Ref, reference.
In crude models of the associations between psychosocial stress and 8-iso-PGF2α, women with high compared to low scores on the Life Experience Survey had increased 8-iso-PGF2α (% difference=9.59, 95% CI=0.24–19.8; p-trend=0.04). High compared to low scores on the CES-D were also somewhat associated with elevated 8-iso-PGF2α in crude models (% difference=6.70, 95% CI=−3.09–17.5). Maternal age, education, and marital status were retained as covariates in final adjusted models. No associations were observed in adjusted models examining associations between psychosocial stress measures and 8-iso-PGF2α (Table 3).
Table 3.
Associations between specific gravity corrected urinary 8-iso-prostaglandin-F2α concentrations (ng/mL) and psychosocial stress measures (N=476).
| Crude | Adjusted1 | ||||
|---|---|---|---|---|---|
| % Difference (95% CI) | p | % Difference (95% CI) | p | ||
| ENRICHD Social Support Instrument | |||||
| High | Ref | Ref | Ref | Ref | |
| Medium | 2.41 (−6.83, 12.6) | 0.62 | −2.44 (−13.1, 9.48) | 0.68 | |
| Low | 4.26 (−5.03, 14.5) | 0.38 | −1.77 (−11.7, 9.32) | 0.74 | |
| p trend | 0.37 | 0.75 | |||
| Perceived Stress Scale | |||||
| Low | Ref | Ref | Ref | Ref | |
| Medium | −3.17 (−11.5, 5.98) | 0.48 | −5.65 (−13.7, 3.15) | 0.20 | |
| High | 2.09 (−7.05, 12.1) | 0.67 | −2.11 (−10.8, 7.41) | 0.65 | |
| p trend | 0.71 | 0.61 | |||
| CES-D | |||||
| Low | Ref | Ref | Ref | Ref | |
| Medium | −1.18 (−9.66, 8.10) | 0.80 | −1.62 (−9.95, 7.48) | 0.72 | |
| High | 6.70 (−3.09, 17.5) | 0.19 | 2.89 (−6.50, 13.2) | 0.56 | |
| p trend | 0.21 | 0.60 | |||
| Life Experience Survey | |||||
| Low | Ref | Ref | Ref | Ref | |
| Medium | 6.85 (−2.39, 17.0) | 0.15 | 5.13 (−3.82, 14.9) | 0.27 | |
| High | 9.59 (0.24, 19.8) | 0.04 | 7.74 (−1.36, 17.7) | 0.10 | |
| p trend | 0.04 | 0.09 | |||
| Neighborhood Perceptions | |||||
| Low | Ref | Ref | Ref | Ref | |
| Medium | −1.38 (−9.73, 7.74) | 0.76 | 0.16 (−8.15, 9.23) | 0.97 | |
| High | 0.84 (−9.89, 12.8) | 0.88 | −1.35 (−11.8, 10.3) | 0.81 | |
| p trend | 0.99 | 0.86 | |||
Models are adjusted for maternal age, maternal education, and marital status.
Note: the number of imputed values for each variable were as follows: ENRICHD Social Support Instrument=36, Perceived Stress Scale=37, CES-D=45, Life Experience Survey=35, Neighborhood Perceptions=17.
Abbreviations: CI, confidence interval; Ref, reference; CES-D, Center for Epidemiologic Studies-Depression.
For the 8-iso-PGF2α metabolite, point estimates were generally elevated among women in the upper two tertiles of each stress scale compared to reference groups in crude and adjusted models (Table 4). In crude models, medium and high levels of the ENRICHD Social Support Instrument were associated with an 9.46% (95% CI=−0.18–20.0) and 12.3% (95% CI=1.87–23.7) increase in the 8-iso-PGF2α metabolite relative to women in the lowest tertile (p-trend=0.01). Women with high compared to low scores on the Perceived Stress Scale also had moderately increased levels of the metabolite (% difference=7.83, 95% CI=−2.43–19.2; p-trend=0.14). However, no associations between psychosocial stress measures and the 8-iso-PGF2α metabolite were observed in adjusted models (Table 4). These associations, as well as associations between psychosocial stress measures and 8-iso-PGF2α, were similar in unimputed data (data not shown). No associations between psychosocial stress measures and PGF2α were observed in crude or adjusted models (Table S2). Additionally, our sensitivity analysis revealed no associations between psychosocial stress measures and the chemical and enzymatic fractions of 8-iso-PGF2α (Table S3–S4).
Table 4.
Associations between specific gravity corrected urinary 8-iso-prostaglandin-F2α metabolite concentrations and psychosocial stress measures (N=476).
| Crude | Adjusted1 | ||||
|---|---|---|---|---|---|
| % Difference (95% CI) | p | % Difference (95% CI) | p | ||
| ENRICHD Social Support Instrument | |||||
| High | Ref | Ref | Ref | Ref | |
| Medium | 9.46 (−0.18, 20.0) | 0.06 | 1.68 (−8.84, 13.4) | 0.77 | |
| Low | 12.3 (1.87, 23.7) | 0.02 | 3.31 (−7.23, 15.0) | 0.55 | |
| p trend | 0.01 | 0.55 | |||
| Perceived Stress Scale | |||||
| Low | Ref | Ref | Ref | Ref | |
| Medium | 3.72 (−5.68, 14.1) | 0.45 | 0.46 (−8.51, 10.3) | 0.92 | |
| High | 7.83 (−2.43, 19.2) | 0.14 | 1.93 (−7.67, 12.5) | 0.70 | |
| p trend | 0.14 | 0.71 | |||
| CES-D | |||||
| Low | Ref | Ref | Ref | Ref | |
| Medium | 0.36 (−8.62, 10.2) | 0.94 | −0.14 (−8.81, 9.36) | 0.98 | |
| High | 7.22 (−2.96, 18.5) | 0.17 | 1.87 (−7.74, 12.5) | 0.71 | |
| p trend | 0.18 | 0.73 | |||
| Life Experience Survey | |||||
| Low | Ref | Ref | Ref | Ref | |
| Medium | −0.98 (−10.0, 8.99) | 0.84 | −3.57 (−12.1, 5.82) | 0.44 | |
| High | 2.73 (−6.59, 13.0) | 0.58 | 0.30 (−8.61, 10.8) | 0.95 | |
| p trend | 0.62 | 0.97 | |||
| Neighborhood Perceptions | |||||
| Low | Ref | Ref | Ref | Ref | |
| Medium | −0.52 (−9.38, 9.20) | 0.91 | 0.84 (−7.84, 10.3) | 0.86 | |
| High | 4.53 (−7.20, 17.8) | 0.47 | 1.78 (−9.42, 14.4) | 0.77 | |
| p trend | 0.58 | 0.75 | |||
Models are adjusted for maternal age, maternal education, and marital status.
Note: the number of imputed values for each variable were as follows: ENRICHD Social Support Instrument=36, Perceived Stress Scale=37, CES-D=45, Life Experience Survey=35, Neighborhood Perceptions=17.
Abbreviations: CI, confidence interval; Ref, reference; CES-D, Center for Epidemiologic Studies-Depression
Discussion
We examined associations between indicators of SES as well as self-reported psychosocial stress and oxidative stress biomarkers during pregnancy. Overall, our results suggest that women at a socioeconomic disadvantage have higher levels of oxidative stress, but we did not observe associations between self-reported psychosocial stress and oxidative stress biomarkers.
Our finding that women of lower SES have higher oxidative stress biomarker concentrations is consistent with the literature. For example, another pregnancy cohort found that women who had less than a college degree and were unmarried had elevated 8-iso-PGF2α in the 3rd trimester.14 Additionally, in a population of pregnant women from Boston, MA, participants with higher education levels had lower 8-iso-PGF2α compared to women with a high school education only.13
Other evidence has shown that 8-iso-PGF2α levels are elevated among pregnant and nonpregnant individuals who experience extreme stressful life events and elevated levels of perceived stress.12,14 Although our results do not support this hypothesis, other studies in nonpregnant populations have shown that psychosocial stress is associated with other oxidative stress biomarkers, specifically 8-hydroxy-2’-deoxyguanosine (8-OH-dG), a measure of oxidative DNA damage. For example, women who experienced anxiety and anger had elevated 8-OH-dG concentrations compared to women with lower scores on these scales.27 An additional study found that increasing workplace social support was associated with decreasing 8-OH-dG.28
We did not observe any associations between psychosocial stress measures and oxidative stress biomarkers in adjusted analyses. Some of our previous work has shown that the mean levels of self-reported psychosocial stress questionnaires in PROTECT are lower than mean stress levels observed in other cohorts.22 For example, in PROTECT, the mean CES-D score was 11 as compared to 24.4 among women in the Boston Puerto Rico Health Study29 or 21.8 among women recruited from primary care clinics in San Juan, PR.30 The lower levels of reported stress in our population may be a result of differences in self-report across scales or due to features of the cohort’s institutional context. For example, participants in PROTECT receive multiple prenatal care visits as a result of their participation in the study.
An important limitation of our study was that our scales only measured psychosocial stress during pregnancy. We had no measure prior to pregnancy and some literature suggests that it is the accumulation of stress across the life course that is more relevant for adverse health outcomes.31 Additionally, oxidative stress biomarkers measured in our study are markers of lipid peroxidation only. Future research should examine associations between SES, psychosocial stress, and other biomarkers of oxidative stress, such as metabolites of DNA or RNA oxidation, as SES and psychosocial stress may be influencing oxidative stress through different pathways. Nonetheless, our study has many strengths, as we examined multiple indices of psychosocial stress and SES. We also had repeated measures of oxidative stress biomarkers across pregnancy, which were averaged across study visits and allowed us to obtain a more stable measure of oxidative stress. Lastly, 8-iso-PGF2α and its metabolite are considered to be the best biomarkers of oxidative stress32 and these biomarkers were quantified using a highly sensitive and specific mass-spectrometry method which is preferred over immunoassay-based methods.33
Conclusions
In conclusion, demographics indicative of a socioeconomic disadvantage were associated with elevated oxidative stress biomarkers in our analyses. However, we did not observe any associations between psychosocial stress and oxidative stress biomarkers. It is possible that women enrolled in PROTECT experience lower levels of psychosocial stress or respond to these questionnaires differently than other cohorts, which could explain our inability to detect associations. Future research should explore other pathways through which psychosocial stress may lead to adverse pregnancy outcomes.
Supplementary Material
Highlights.
Oxidative stress levels were elevated among women with lower socioeconomic status
Self-reported psychosocial stress was not associated with urinary oxidative stress biomarkers
Self-reported stress levels in this population are lower than other cohorts
Acknowledgments
Funding: This work was supported by the National Institute of Environmental Health Sciences grants P42ES017198 and P50ES026049, the National Institutes of Health Office of the Director grants UG3OD023251 and UH3OD023251, and grant U54MD007600 from the National Institute on Minority Health and Health Disparities. This work was also supported in part by the Intramural Research Program of the NIEHS, NIH.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wadhwa PD, Entringer S, Buss C, Lu MC. The Contribution of Maternal Stress to Preterm Birth: Issues and Considerations. Clinics in perinatology. 2011;38(3):351–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson KK, McElrath TF, Chen YH, Loch-Caruso R, Mukherjee B, Meeker JD. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. American journal of obstetrics and gynecology. 2015;212(2):208.e201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M. Socioeconomic status and smoking: a review. Annals of the New York Academy of Sciences. 2012;1248(1):107–123. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. Journal of studies on alcohol. 1997;58(5):464–473. [DOI] [PubMed] [Google Scholar]
- 5.van ‘t Erve TJ, Kadiiska MB, London SJ, Mason RP. Classifying oxidative stress by F2-isoprostane levels across human diseases: A meta-analysis. Redox biology. 2017;12:582–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vetrani C, Costabile G, Di Marino L, Rivellese AA. Nutrition and oxidative stress: a systematic review of human studies. International journal of food sciences and nutrition. 2013;64(3):312–326. [DOI] [PubMed] [Google Scholar]
- 7.Kobrosly RW, Parlett LE, Stahlhut RW, Barrett ES, Swan SH. Socioeconomic factors and phthalate metabolite concentrations among United States women of reproductive age. Environmental research. 2012;115:11–17. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson KK, Chen YH, VanderWeele TJ, McElrath TF, Meeker JD, Mukherjee B. Mediation of the Relationship between Maternal Phthalate Exposure and Preterm Birth by Oxidative Stress with Repeated Measurements across Pregnancy. Environmental health perspectives. 2017;125(3):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to airborne particulate matter and adverse perinatal outcomes: a biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environmental health perspectives. 2006;114(11):1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salim S Oxidative Stress and Psychological Disorders. Current Neuropharmacology. 2014;12(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimanoe C, Hara M, Nishida Y, et al. Perceived Stress, Depressive Symptoms, and Oxidative DNA Damage. Psychosom Med. 2018;80(1):28–33. [DOI] [PubMed] [Google Scholar]
- 12.Aschbacher K, O’Donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E. Good Stress, Bad Stress and Oxidative Stress: Insights from Anticipatory Cortisol Reactivity. Psychoneuroendocrinology. 2013;38(9):1698–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environmental health perspectives. 2015;123(3):210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eick SM, Barrett ES, van ‘t Erve TJ, et al. Association between prenatal psychological stress and oxidative stress during pregnancy. Paediatric and perinatal epidemiology. 2018;32(4):318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental science & technology. 2013;47(7):3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferguson KK, Rosario Z, McElrath TF, et al. Demographic risk factors for adverse birth outcomes in Puerto Rico in the PROTECT cohort. PloS one. 2019;14(6):e0217770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. Journal of consulting and clinical psychology. 1978;46(5):932–946. [DOI] [PubMed] [Google Scholar]
- 18.Eunice Kennedy Shriver National Institute of Child Health and Human Development. National Children’s Study (NCS). 2017; https://www.nichd.nih.gov/research/supported/NCS. Accessed Septembr 21, 2018.
- 19.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 20.Radloff LS. The CES-D Scale. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 21.Mitchell PH, Powell L, Blumenthal J, et al. A short social support measure for patients recovering from myocardial infarction: the ENRICHD Social Support Inventory. Journal of cardiopulmonary rehabilitation. 2003;23(6):398–403. [DOI] [PubMed] [Google Scholar]
- 22.Eick SM, Meeker JD, Swartzendruber A, et al. Psychosocial Factors During Pregnancy and Preterm Birth in Puerto Rico. Submitted. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nature protocols. 2007;2(1):221–226. [DOI] [PubMed] [Google Scholar]
- 24.Dorjgochoo T, Gao YT, Chow WH, et al. Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. The American journal of clinical nutrition. 2012;96(2):405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van ‘t Erve TJ, Lih FB, Kadiiska MB, Deterding LJ, Eling TE, Mason RP. Reinterpreting the best biomarker of oxidative stress: The 8-iso-PGF(2alpha)/PGF(2alpha) ratio distinguishes chemical from enzymatic lipid peroxidation. Free radical biology & medicine. 2015;83:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armitage P Tests for Linear Trends in Proportions and Frequencies. Biometrics. 1955;11(3):375–386. [Google Scholar]
- 27.Irie M, Asami S, Nagata S, Ikeda M, Miyata M, Kasai H. Psychosocial factors as a potential trigger of oxidative DNA damage in human leukocytes. Japanese journal of cancer research : Gann. 2001;92(3):367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takaki J Associations of job stress indicators with oxidative biomarkers in Japanese men and women. International journal of environmental research and public health. 2013;10(12):6662–6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falcon LM, Todorova I, Tucker K. Social support, life events, and psychological distress among the Puerto Rican population in the Boston area of the United States. Aging & mental health. 2009;13(6):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattei J, Tamez M, Ríos-Bedoya CF, Xiao RS, Tucker KL, Rodríguez-Orengo JF. Health conditions and lifestyle risk factors of adults living in Puerto Rico: a cross-sectional study. BMC Public Health. 2018;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Maternal and child health journal. 2003;7(1):13–30. [DOI] [PubMed] [Google Scholar]
- 32.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free radical biology & medicine. 2000;28(4):505–513. [DOI] [PubMed] [Google Scholar]
- 33.Il’yasova D, Morrow JD, Ivanova A, Wagenknecht LE. Epidemiological marker for oxidant status: comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3-dinor-5,6-dihydro-15-F2t-isoprostane. Ann Epidemiol. 2004;14(10):793–797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


