Summary
A gold-catalyzed 6-endo-dig carbocyclization of alkyne with the pendent diazo group is reported. It provides an expeditious approach for the synthesis of multi-functionalized naphthalene derivatives under mild conditions. Mechanistic studies suggest that a vinyl gold carbene is generated as the key intermediate in this cascade transformation that smoothly delivers naphthalene products through an unprecedented stepwise aromatization or an intermolecular aromatic substitution process. The unique endocyclic vinyl species is inaccessible with other precursors; thus, novel carbene cascade transformations could be envisioned with the current catalytic model. Functional groups, such as alkenyl, hydroxyl, amino, and carboxyl groups, remain untouched under these conditions. In addition, the utility of these generated 2-carboxyl naphthalenes is illustrated by the synthesis of chiral 1,2′-binaphthalene ligands and π-conjugated polycyclic hydrocarbons (CPHs).
Subject Areas: Catalysis, Organic Synthesis, Organic Reaction
Graphical Abstract
Highlights
-
•
A general method for the construction of multi-functionalized naphthalenes
-
•
First 6-endo-dig diazo-yne carbocyclization leading to the vinyl gold carbene
-
•
Expeditious access to naphthalenes with broad functional group compatibility
-
•
Applications for the synthesis of chiral 1,2′-binaphthalene ligands and CPHs
Catalysis; Organic Synthesis; Organic Reaction
Introduction
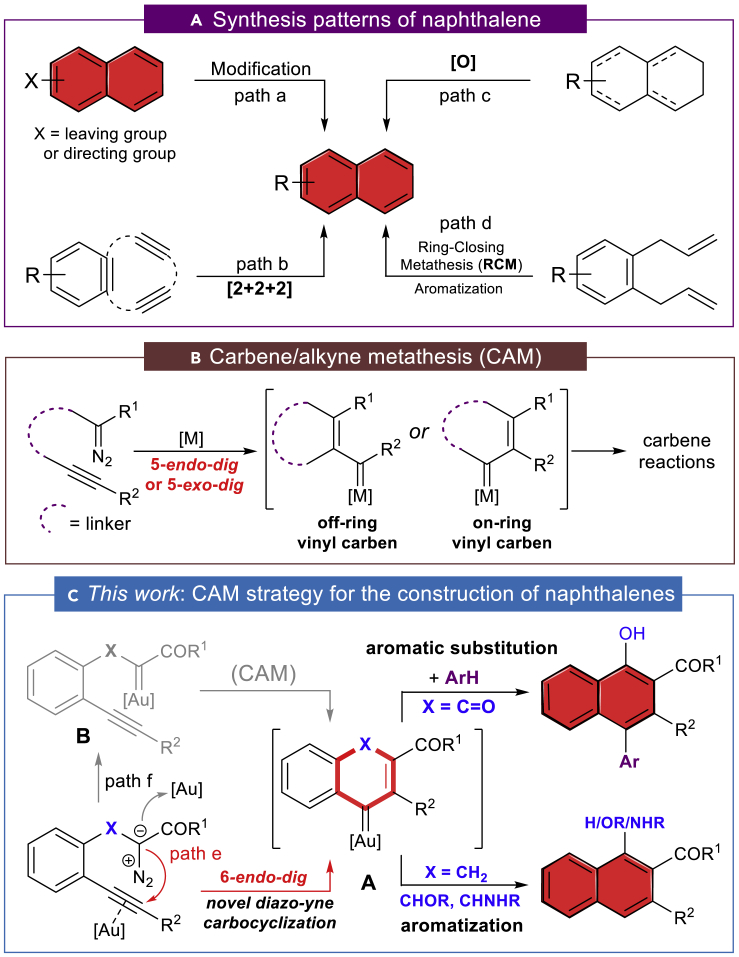
Naphthalene derivatives are one of the most prevalent key motifs in π-conjugated polycyclic hydrocarbons (CPHs) (Frederickson et al., 2017), and polycyclic systems related to naphthalene derivatives have shown versatile applications in physical organic chemistry (Frederickson et al., 2017, Huang et al., 2016), organometallic chemistry (Edelmann, 2017), materials science (Cao et al., 2015), and bioactive molecules (Stockdale and Williams, 2015). During the past decades, a variety of approaches for aromatic ring modification either through metal- (Tanaka, 2013, Phipps and Gaunt, 2009, Meng et al., 2017, Zhu et al., 2016, Della Ca’ et al., 2016) or organo-catalysis (Qi et al., 2018) have been reported. Nevertheless, most of these methods are less efficient for sterically hindered substrates including naphthalenes and usually require a pre-installation of leaving groups or directional groups (Figure 1A, path a). On the other hand, transition-metal-catalyzed aromatization reactions provide a convenient way in the practical construction of substituted naphthalenes, including but not limited to the [2+2+2]-cycloaddition of benzyne intermediates with alkynes (path b) (Pérez et al., 2013), oxidative dehydrogenation of cyclic hydrocarbons (path c) (Iosub and Stahl, 2016, Wu and Jiang, 2012), ring-closing metathesis followed by aromatization (path d) (Donohoe et al., 2006, van Otterlo and de Koning, 2009), the electrocyclization reactions, and others (Tanaka, 2013). Recently, alkyne benzannulation has emerged as a straightforward approach for accessing densely functionalized naphthalene compounds, complementing the above-described methods (Hein et al., 2017). Despite these advances, polyfunctionalized naphthalenes of interest are still challenging to prepare and many of the substitution patterns are beyond the scope of the current synthetic methods; these include the methods for accessing naphthalenes with versatile functional groups such as the hydroxyl, amino, and carboxyl groups (Izawa et al., 2011, Hein et al., 2017, Raviola et al., 2016). These groups not only act as the key pharmacophores in pharmaceuticals (Stockdale and Williams, 2015) but also can be used for further transformations in preparing other complex molecules. Therefore, the development of novel and practical synthetic methods to construct naphthalenes with broad functional group compatibility still remains highly desirable and appealing.
Figure 1.
Strategy for Constructing Aromatic Rings
(A) General synthesis patterns of naphthalene.
(B) Carbene/alkyne metathesis (CAM).
(C) This work: CAM strategy for the construction of naphthalenes.
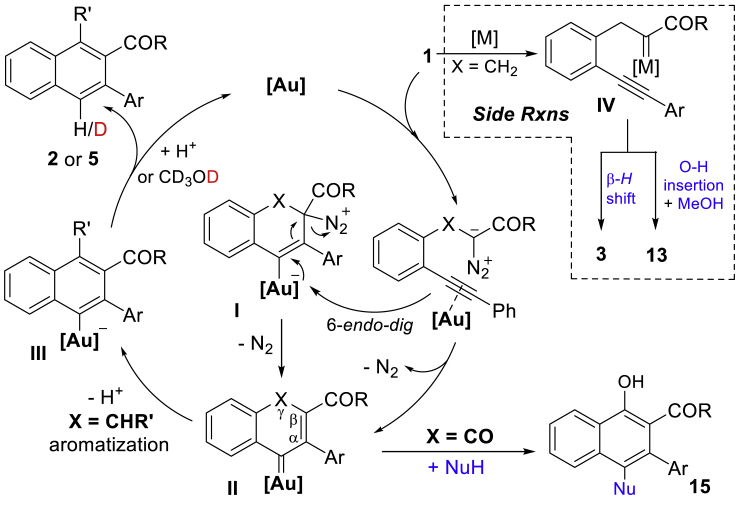
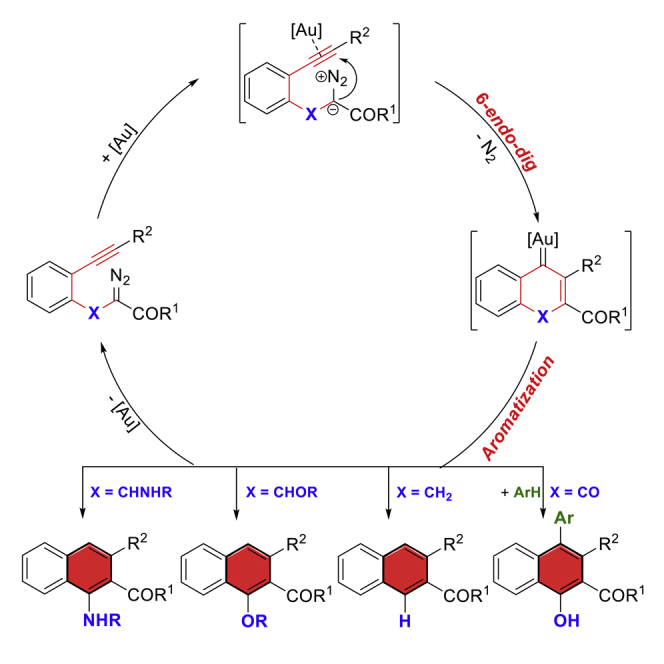
In the last two decades, gold-catalyzed alkyne carbocyclizations have experienced explosive development in the construction of cyclic molecules with structural complexity (Pflästerer and Hashmi, 2016, Chen et al., 2018, Gorin and Toste, 2007, Dorel and Echavarren, 2015, Zheng et al., 2016). After the first reports of Hashmi on benzene ring formation by gold catalysis (Hashmi et al., 2000, Hashmi et al., 2001, Hashmi et al., 2002, Zeiler et al., 2015), another early example of 5-exo-dig diazo-yne carbocyclization was disclosed by Toste for the synthesis of indanone derivatives (Witham et al., 2007, Padwa et al., 1993, Mueller et al., 1993). Recently, Hashmi (Nösel et al., 2013) and Tang (Liu et al., 2013) have reported on the catalytic oxidative diyne 5-exo-dig cyclization in the presence of gold and rhodium catalysts, respectively. Although the catalytic 6-endo-dig carbocyclization of alkynes has also been studied (Yuan et al., 2016), no example of analogous diazo-yne cyclization has been reported for the construction of 6-membered carbocyclic rings. Inspired by these advances, and as the continuation of our interest in the carbene/alkyne metathesis (CAM) transformations (Figure 1B) (Pei et al., 2018, Le and May, 2015, González-Rodríguez et al., 2015, Torres et al., 2015, Zheng et al., 2015, Dong et al., 2018, Hashmi et al., 2008), we are intrigued by the possibility that nucleophilic addition of the diazo compound onto the gold-activated alkyne through an unprecedented 6-endo-dig diazo-yne carbocyclization followed by the expulsion of dinitrogen can be used to generate the endocyclic vinyl gold carbene species A (Figure 1C, path e) (Hashmi et al., 2000, Lu et al., 2010). With this concept, the side reactions in general carbene/alkyne metathesis through carbene species B (path f), and in particular the β-H shift process of α-alkyl carbene intermediate B (X = CHR) (Lonca et al., 2017, Goto et al., 2011, Zhang et al., 2019), can be avoided, which would substantially expand the chemistry of the CAM process (Lauterbach et al., 2013, Lauterbach et al., 2015, Rode et al., 2018). Herein, we report our recent results in this direction: the first example of gold-catalyzed 6-endo-dig diazo-yne carbocyclization and the generated key intermediate A that is a versatile synthon for the construction of naphthalene frameworks via a stepwise aromatization or an intermolecular electrophilic aromatic substitution (Figure 1C). As a result of this new synthetic approach, naphthalene structures with a variety of functional groups were uncovered, such as alkenyl, hydroxyl, amino, and carboxyl groups, remaining untouched under these conditions.
Results and Discussion
To test the feasibility of our proposed approach for the construction of naphthalene frameworks, o-alkynylphenyl diazoacetate 1a was used as a model substrate in the presence of various metal catalysts in 1,2-dichloroethane (DCE) at 25°C (Table 1). With its sterically demanding ligand, JohnPhos(CH3CN)AuSbF6 exhibited superior reactivity for the selective formation of naphthalene 2a in 91% isolated yield (entry 1). The corresponding chloride salt, JohnPhosAuCl, showed very low reactivity, and most of 1a was recovered (entry 2). All of the other metal catalysts including Rh-, Cu-, Pd-, and Ag-catalysts predominantly delivered the β-H shift product 3a (entries 3–6). Further investigation of the ligands and counterions of the gold catalysts indicated that the steric effect of the ligand plays a crucial role in the selectivity control (entry 8 versus 9), and the gold catalyst bearing a triphenylphosphine ligand catalyzed the reaction to predominantly form the β-H shift product alkene 3a (entries 9 and 10). For the extensive examination of the ligand effect, see Table S2. On the other hand, the counter anion of these catalysts shows no obvious effects on the reaction outcomes (entries 7–10) (Schießl et al., 2018a, Schießl et al., 2018b). Notably, the observed 6-endo-dig diazo-yne cyclization process shows a unique effect for the gold catalysis that preferentially activates the C-C triple bond in the presence of a diazo group (Zheng et al., 2015). Reactions with other metal catalysts formed the β-H shift product 3a as the major/only product, indicating that the reaction mechanism of this gold-catalyzed carbocyclization is distinctly different and that initial catalytic decomposition of the diazo group to form the corresponding carbenoid intermediate does not occur in this case.
Table 1.
Reaction Optimization
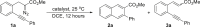 | |||
|---|---|---|---|
| Entry | Catalyst | 2a (%)a | 3a (%)a |
| 1 | JohnPhosAu(CH3CN)SbF6 | 94 (91)b | <5 |
| 2c | JohnPhosAuCl | – | <10 |
| 3c | Rh2(OAc)4 | <5 | <5(70)d |
| 4 | Cu(CH3CN)4BF4 | <5 | 91 |
| 5 | Pd2(dba)3 | 22 | 47 |
| 6 | AgSbF6 | <5 | 96(90)b |
| 7 | JohnPhosAuCl + AgSbF6 | 92 | <5 |
| 8 | JohnPhosAuCl + AgNTf2 | 90 | <5 |
| 9 | PPh3AuNTf2 | <5 | 87 |
| 10 | PPh3AuSbF6 | <5 | 86 |
Optimization conditions: 1a (58 mg, 0.2 mmol) and catalyst (0.01 mmol) in DCE (1,2-dichloroethane, 2.0 mL) at 25°C for 12.0 h, unless otherwise stated.
Yield determined by proton NMR using 1,3,5-trimethoxybenzene as the internal standard.
Isolated yields.
Most of 1a (>90%) was recovered.
The results in parentheses is the reaction that was conducted at 60°C instead of 25°C.
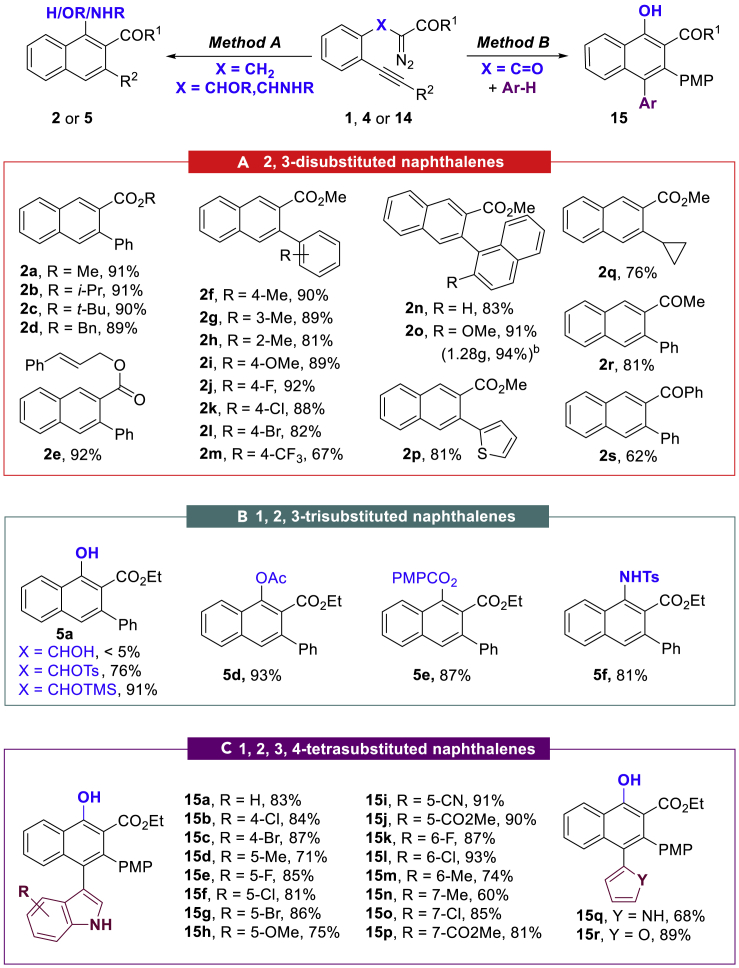
Under the optimal reaction conditions, we investigated the scope of this unprecedented 6-endo-dig diazo-yne cyclization for the synthesis of 2,3-disubstituted naphthalenes (Scheme 1A). The impact of the ester part was examined first. It was found that the reaction could be applied to alkyl, benzyl, and 3-phenylallyl esters without a noticeable yield deterioration (2a-2e, 89%–92% yields). Then, the nature of the alkyne terminus was investigated. The electronic effects and the position of the substituent groups on the phenyl group of the substrates had little influence, and the corresponding products 2f–2m were produced in high to excellent yields (67%–92%). Moreover, the 1-naphthyl-, 2-thienyl-, and alkyl-substituted substrates underwent the reaction smoothly, leading to naphthalene products 2n–2q in >76% yields. Subsequently, diazoketones were used instead of diazoacetates, and it was found that they were also tolerated under these conditions. The corresponding products 2r and 2s were isolated in 81% and 62% yields, respectively. In addition, the reaction performed well on a gram scale with 94% isolated yield (note b, on 4.0 mmol).
Scheme 1.
Scope for the Synthesis of Naphthalenes
Reaction conditions: Method A: 1 or 4 (0.2 mmol), JohnPhosAuSbF6 (0.01 mmol) in DCE (1,2-dichloroethane, 2.0 mL) at 25°C,12 h, and yields are given in isolated yields. Method B: 14 (0.2 mmol), Ar-H (0.3 mmol), JohnPhosAuSbF6 (0.01 mmol) in DCE (1,2-dichloroethane, 2.0 mL) at 60°C, 3 h, and yields are given in isolated yields.
The reaction was conducted on a 4.0-mmol scale.
(A) Synthesis of 2,3-disubstituted naphthalenes.
(B) Synthesis of 1,2,3-trisubstituted naphthalenes.
(C) Synthesis of 1,2,3,4-tetrasubstituted naphthalenes.
Encouraged by these promising results, we envisioned that this catalytic system may also facilitate other challenging substrates for the synthesis of multi-functionalized naphthalene derivatives, with the results from these investigations summarized in Scheme 1B. Initially, α-hydroxyl substrate 4a was used under the method A; however, no corresponding naphthalene product was observed. To our delight, when the substrates protected either with the Ts (4b) or TMS (4c) groups were used under the optimized reaction conditions, the reaction directly delivered the deprotected α-naphthol product 5a in 76% and 91% yields, respectively. The ester variants, 4d and 4e, also provided the corresponding 1,2,3-trisubstituted naphthalenes in high yields (>87%). Notably, naphthylamine 5f was isolated in 81% yield under the current conditions from 4f.
To further demonstrate the generality of the present transformation, 1,3-dicarbonyl diazo compound 14 without α-methylene linkage was prepared and the interception reaction of the corresponding vinyl carbene intermediate A (Figure 1C, X = CO) was envisioned. To our delight, the C(sp2)-H insertion products 15 were isolated in good to excellent yields (Scheme 1C, 53%–94% yields) when the reaction was performed at 60°C in the presence of nucleophiles such as indoles, furan, and pyrrole.
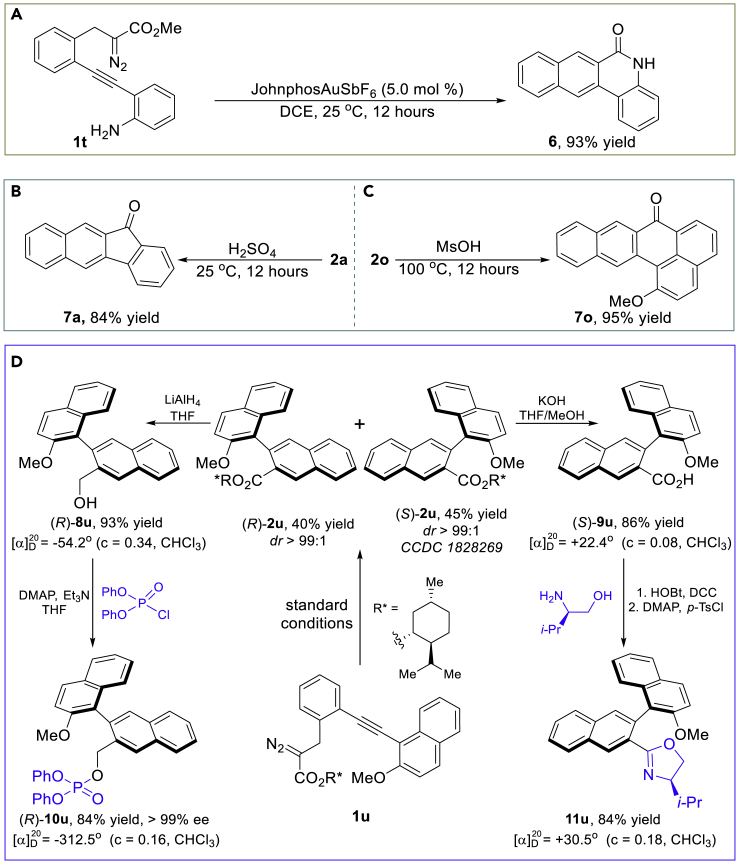
To demonstrate the synthetic utility of the present method, further transformations of carbocyclization products 2 were conducted for the synthesis of π-CPHs. For example, the tetracyclic fused lactam 6 was generated in one-pot from 1t after the ester-amide exchange reaction with the internal amino group (Figure 2A). In addition, these 2-carbonyl naphthalenes were smoothly converted under acidic conditions to polycyclic hydrocarbons (Figures 2B and 2C), with 7a and 7o isolated in 84% and 95% yields, respectively.
Figure 2.
Synthesis of π-Conjugated Polycyclic Hydrocarbons (CPHs) and Chiral 1,2′-Dinaphthalene Ligands
(A) Synthesis of polycyclic lactam 6.
(B) Synthesis of polycyclic ketone 7a.
(C) Synthesis of polycyclic ketone 7o.
(D) Synthesis of chiral 1,2’-dinaphthalene ligands.
Notably, chiral 1,2′-binaphthalene products could be prepared in high yield and 1:1 dr with (−)-L-menthol derived diazo compound 1u (Figure 2D). The two diastereoisomers were separated by column chromatography with the (S)-isomer confirmed by single-crystal X-ray analysis. Moreover, the optically pure chiral phosphate derivative (R)-10u and chiral oxazole ligand 11u with 1,2′-binaphthalene frameworks were synthesized in high yields. Although ligands with the 1,1′-binaphthalene skeletons have been studied well and have broad applications, chiral ligands derived from 1,2′-binaphthalene motifs are rare, mainly because of the limited methods for access to this class of compounds (Lotter et al., 2016).
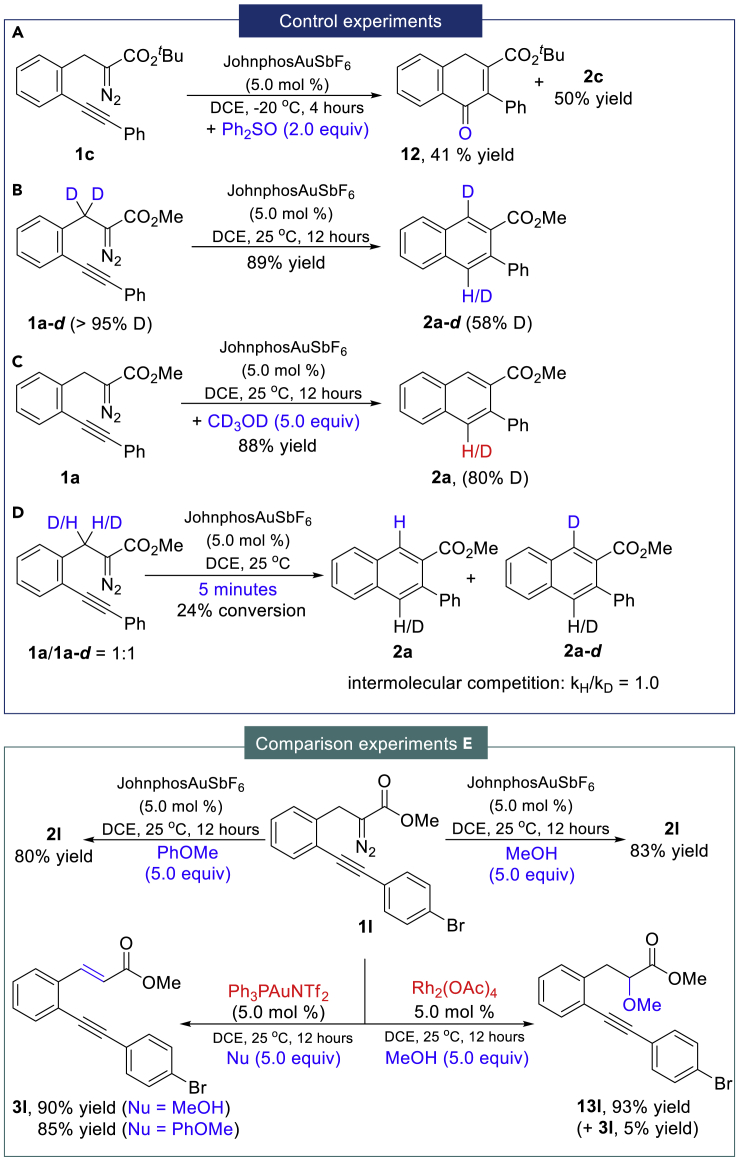
Control experiments were conducted to investigate the mechanism of this reaction. To verify the existence of the vinyl gold carbene intermediate, the interception reaction with 1c in the presence of diphenyl sulfoxide was carried out at −20°C and the corresponding ketone product 12 was isolated in 41% yield combined with 2c in 50% yield (Figure 3A) (Witham et al., 2007, Padwa et al., 1993, Mueller et al., 1993, Nösel et al., 2013, Liu et al., 2013). Evidence for the stepwise aromatization and protodeauration process was confirmed by an isotope-labeling experiment (Figure 3B, 2a-d with 58% D). Moreover, the deuterated product 2a was also obtained when the reaction was carried out in the presence of CD3OD under standard conditions (Figure 3C, with 80% D). In addition, an intermolecular kinetic isotope effect (KIE) experiment (Figure 3D, kH/kD = 1.0) demonstrated that the deprotonation process is not the rate-limiting step. Based on these results and previously studied gold-catalyzed transformations (Witham et al., 2007, Padwa et al., 1993, Mueller et al., 1993, Nösel et al., 2013, Liu et al., 2013, Yuan et al., 2016, Pei et al., 2018, Le and May, 2015, González-Rodríguez et al., 2015, Torres et al., 2015, Zheng et al., 2015, Dong et al., 2018, Hashmi et al., 2008, Lu et al., 2010 Lonca et al., 2017, Goto et al., 2011, Qiu et al., 2016, Hashmi, 2010), a possible reaction mechanism is proposed in Figure 4. Initially, the gold catalyst coordinates the π-bond of alkyne to form a gold π-complex followed by a 6-endo-dig cyclization with the carbon on the diazo group to generate intermediate I that delivered the vinyl gold carbene II followed by a stepwise aromatization (deprotonation, X = CHR) and protodeauration process to form the naphthalene product 2 or 5 via III. Alternatively, direct formation of the key intermediate III from I through deprotonation with synchronous dinitrogen extrusion is also possible. Intermolecular electrophilic aromatic substitution interception of the vinyl gold carbene II would lead to the formation of C(sp2)-H insertion product 15. It should be noted that the reaction pathway through direct catalytic gold carbene formation followed by cyclopropenation and gold-catalyzed rearrangement of the cyclopropene to form the vinyl gold carbene intermediate II was ruled out in this reaction (Hashmi et al., 2000, Hashmi et al., 2001, Hashmi et al., 2002, Zeiler et al., 2015, Archambeau et al., 2015, Hoye et al., 1990, Bauer et al., 2008, Xu et al., 2013), which is consistent with the results of the comparison experiments (Figure 3E). For example, no corresponding direct carbene insertion product via IV was obtained when the reaction was carried out in the presence of MeOH or anisole, and the carbocyclization product 2l was isolated as the only product in 83% and 80% yields, respectively. In addition, using the catalysts that prefer to activate the diazo group instead of the alkyne part, such as PPh3AuNTf2 and Rh2(OAc)4, the formation of carbene intermediate IV would occur initially (Figure 4, side reactions in dotted box), and the β-H shift product 3l and O-H insertion product 13l were generated predominantly via common carbene intermediate IV (Figure 3E).
Figure 3.
Control Experiments and Comparison Experiments
(A) Control reaction in the presence of dimethylsulfoxide.
(B) Control reaction with deuterated reagent.
(C) Control reaction in the presence of CD3OD.
(D) Intermolecular kinetic isotope effect (KIE) experiment.
(E) The comparison experiments of carbene vs non carbene process.
Figure 4.
Proposed Reaction Mechanism
Conclusion
In summary, we have developed a gold-catalyzed 6-endo-dig carbocyclization of alkyne with the pendent diazo groups that provides an expeditious approach for the synthesis of multi-functionalized naphthalene derivatives in high to excellent yields under mild conditions with broad substrate scope; functional groups, such as alkenyl, hydroxyl, amino, and carboxyl groups, are well tolerated under current conditions. The generated 2-carboxyl naphthalenes are useful for further diversification, as exemplified by the synthesis of chiral 1,2′-binaphthalene ligands and π-CPHs. Mechanistic studies indicate that the naphthyl-gold complex and the vinyl gold carbene species are the key intermediates in this cascade transformation, and side reactions in the usual carbene/alkyne metathesis process can be avoided under the current conditions, particularly for the β-H shift process. Synthetic applications based on the interception of these unique on-ring vinyl carbene intermediates, including the development of novel cascade reactions and synthesis of aromatic products with structural diversity, could be expected in due course.
Limitations of the Study
The asymmetric version for the formal C-H insertion reaction has not been realized, which is the main challenge in gold catalysis.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This project was supported by National Natural Science Foundation of China (21602148), and the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (No. 2016ZT06Y337) is greatly acknowledged.
Author Contributions
X.X. supervised the project. C.Z., K.H., S.D., C.P., X.Z., and C.H. conducted the experimental work. C.Z., W.H., and X.X. co-wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: November 22, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.10.042.
Contributor Information
Wenhao Hu, Email: huwh9@mail.sysu.edu.cn.
Xinfang Xu, Email: xinfangxu@suda.edu.cn.
Data and Code Availability
The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 1828269 ((S)-2u) and can be obtained free of charge from www.ccdc.cam.ac.uk/getstructures.
Supplemental Information
References
- Archambeau A., Miege F., Meyer C., Cossy J. Intramolecular cyclopropanation and C–H insertion reactions with metal carbenoids generated from cyclopropenes. Acc. Chem. Res. 2015;48:1021–1031. doi: 10.1021/acs.accounts.5b00016. [DOI] [PubMed] [Google Scholar]
- Bauer J.T., Hadfield M.S., Lee A. Gold catalysed reactions with cyclopropenes. Chem. Commun. 2008;44:6405–6407. doi: 10.1039/b815891f. [DOI] [PubMed] [Google Scholar]
- Cao J., London G., Dumele O., Rekowski V.W.M., Trapp N., Ruhlmann L., Boudon C., Stanger A., Diederich F. The impact of antiaromatic subunits in [4n+2] π-systems: bispentalenes with [4n+2] π-electron perimeters and antiaromatic character. J. Am. Chem. Soc. 2015;137:7178–7188. doi: 10.1021/jacs.5b03074. [DOI] [PubMed] [Google Scholar]
- Chen L., Chen K., Zhu S. Transition-metal-catalyzed intramolecular nucleophilic addition of carbonyl groups to alkynes. Chem. 2018;4:1208–1262. [Google Scholar]
- Della Ca’ N., Fontana M., Motti E., Catellani M. Pd/Norbornene: a winning combination for selective aromatic functionalization via C−H bond activation. Acc. Chem. Res. 2016;49:1389–1400. doi: 10.1021/acs.accounts.6b00165. [DOI] [PubMed] [Google Scholar]
- Dong K., Pei C., Zeng Q., Wei H., Doyle M.P., Xu X. Selective C(sp3)–H bond insertion in carbene/alkyne metathesis reactions. enantioselective construction of dihydroindoles. ACS Catal. 2018;8:9543–9549. [Google Scholar]
- Donohoe T.J., Orr A.J., Bingham M. Ring-closing metathesis as a basis for the construction of aromatic compounds. Angew. Chem. Int. Ed. 2006;45:2664–2670. doi: 10.1002/anie.200503512. [DOI] [PubMed] [Google Scholar]
- Dorel R., Echavarren A.M. Gold(I)-catalyzed activation of alkynes for the construction of molecular complexity. Chem. Rev. 2015;115:9028–9072. doi: 10.1021/cr500691k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann F.T. Lanthanides and actinides: Annual survey of their organometallic chemistry covering the year 2016. Coord. Chem. Rev. 2017;338:27–140. [Google Scholar]
- Frederickson C.K., Rose B.D., Haley M.M. Explorations of the indenofluorenes and expanded quinoidal analogues. Acc. Chem. Res. 2017;50:977–987. doi: 10.1021/acs.accounts.7b00004. [DOI] [PubMed] [Google Scholar]
- González-Rodríguez C., Suárez J.M., Varela J.A., Saá C.C. Nucleophilic addition of amines to ruthenium carbenes: ortho−(alkynyloxy)benzylamine cyclizations towards 1,3−benzoxazines. Angew. Chem. Int. Ed. 2015;54:2724–2728. doi: 10.1002/anie.201410284. [DOI] [PubMed] [Google Scholar]
- Gorin D.J., Toste F.D. Relativistic effects in homogeneous gold catalysis. Nature. 2007;446:395–403. doi: 10.1038/nature05592. [DOI] [PubMed] [Google Scholar]
- Goto T., Takeda K., Shimada N., Nambu H., Anada M., Shiro M., Ando K., Hashimoto S. Highly enantioselective cyclopropenation reaction of 1−alkynes with α−alkyl−α−diazoesters catalyzed by dirhodium(II) carboxylates. Angew. Chem. Int. Ed. 2011;50:6803–6808. doi: 10.1002/anie.201101905. [DOI] [PubMed] [Google Scholar]
- Hashmi A.S.K. Homogeneous gold catalysis beyond assumptions and proposals-characterized intermediates. Angew. Chem. Int. Ed. 2010;49:5232–5241. doi: 10.1002/anie.200907078. [DOI] [PubMed] [Google Scholar]
- Hashmi A.S.K., Frost T.M., Bats J.W. Highly selective gold-catalyzed arene synthesis. J. Am. Chem. Soc. 2000;122:1553–11554. [Google Scholar]
- Hashmi A.S.K., Frost T.M., Bats J.W. Gold catalysis: on the phenol synthesis. Org. Lett. 2001;3:3769–3771. doi: 10.1021/ol016734d. [DOI] [PubMed] [Google Scholar]
- Hashmi A.S.K., Frost T.M., Bats J.W. Homogeneous gold-catalyzed synthesis of biphenyls and furfuryl-substituted arenes. Catal. Today. 2002;72:19–27. [Google Scholar]
- Hashmi A.S.K., Rudolph M., Siehl H.-U., Tanaka M., Bats J.W., Frey W. Gold catalysis: deuterated substrates as the Key for an experimental insight into the mechanism and selectivity of the phenol synthesis. Chem. Eur. J. 2008;14:3703–3708. doi: 10.1002/chem.200701795. [DOI] [PubMed] [Google Scholar]
- Hein S.J., Lehnherr D., Arslan H., Uribe-Romo F.J., Dichtel W.R. Alkyne benzannulation reactions for the synthesis of novel aromatic architectures. Acc. Chem. Res. 2017;50:2776–2788. doi: 10.1021/acs.accounts.7b00385. [DOI] [PubMed] [Google Scholar]
- Hoye T.R., Dinsmore C.J., Johnson D.S., Korkowski P.F. Alkyne insertion reactions of metal-carbenes derived from enynyl α-diazo ketones [R′CN2COCR2CH2C≡C(CH2)n−2CH=CH2] J. Org. Chem. 1990;55:4518–4520. [Google Scholar]
- Huang R., Phan H., Herng T.S., Hu P., Zeng W., Dong S.-Q., Das S., Shen Y., Ding J., Casanova D., Wu J. Higher order π-conjugated polycyclic hydrocarbons with open-shell singlet ground state: nonazethrene versus nonacene. J. Am. Chem. Soc. 2016;138:10323–10330. doi: 10.1021/jacs.6b06188. [DOI] [PubMed] [Google Scholar]
- Iosub A.V., Stahl S.S. Palladium-catalyzed aerobic dehydrogenation of cyclic hydrocarbons for the synthesis of substituted aromatics and other unsaturated products. ACS Catal. 2016;6:8201–8213. doi: 10.1021/acscatal.6b02406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa Y., Pun D., Stahl S.S. Palladium−catalyzed aerobic dehydrogenation of substituted cyclohexanones to phenols. Science. 2011;333:209–213. doi: 10.1126/science.1204183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach T., Gatzweiler S., Nçsel P., Rudolph M., Rominger F., Hashmia A.S.K. Carbene transfer - a new pathway for propargylic esters in gold catalysis. Adv. Synth. Catal. 2013;355:2481–2487. [Google Scholar]
- Lauterbach T., Higuchi T., Hussong M.W., Rudolph M., Rominger F., Mashima K., Hashmi A.S.K. Gold-catalyzed carbenoid transfer reactions of diynes-pinacol rearrangement versus retro-Buchner reaction. Adv. Synth. Catal. 2015;357:775–781. [Google Scholar]
- Le P.Q., May J.A. Hydrazone-initiated carbene/alkyne cascades to form polycyclic products: ring-fused cyclopropenes as mechanistic intermediates. J. Am. Chem. Soc. 2015;137:12219–12222. doi: 10.1021/jacs.5b08157. [DOI] [PubMed] [Google Scholar]
- Liu R., Winston-McPherson G.N., Yang Z.-Y., Zhou X., Song W., Guzei I.A., Xu X., Tang W. Generation of rhodium(I) carbenes from ynamides and their reactions with alkynes and alkenes. J. Am. Chem. Soc. 2013;135:8201–8204. doi: 10.1021/ja4047069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonca G.H., Tejo C., Chan H.L., Chiba S., Gagosz F. Gold(I)−catalyzed 6−endo−dig azide–yne cyclization: efficient access to 2H−1,3−oxazines. Chem. Commun. 2017;53:736–739. doi: 10.1039/c6cc08397h. [DOI] [PubMed] [Google Scholar]
- Lotter D., Neuburger M., Rickhaus M., Häussinger D., Sparr C. Stereoselective arene−forming aldol condensation: synthesis of configurationally stable oligo−1,2−naphthylenes. Angew. Chem. Int. Ed. 2016;55:2920–2923. doi: 10.1002/anie.201510259. [DOI] [PubMed] [Google Scholar]
- Lu B., Li C., Zhang L. Gold−catalyzed highly regioselective oxidation of C-C triple bonds without acid additives: propargyl moieties as masked α, β-unsaturated carbonyls. J. Am. Chem. Soc. 2010;132:14070–14072. doi: 10.1021/ja1072614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q.-Y., Gao X.-W., Lei T., Liu Z., Zhan F., Li Z.-J., Zhong J.-J., Xiao H., Feng K., Chen B. Identifying key intermediates generated in situ from Cu(II) salt–catalyzed C–H functionalization of aromatic amines under illumination. Sci. Adv. 2017;3:e1700666. doi: 10.1126/sciadv.1700666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P.H., Kassir J.M., Semones M.A., Weingarten M.D., Padwa A. Rhodium carbenoid mediated cyclizations. Intramolecular cyclopropanation and C-H insertion reactions derived from type II o-alkynyl substituted α-diazoacetophenones. Tetrahedron Lett. 1993;34:4285–4288. [Google Scholar]
- Nösel P., Comprido L.N.S., Lauterbach T., Rudolph M., Rominger F., Hashmi A.S.K. 1,6-Carbene transfer: gold-catalyzed oxidative diyne cyclizations. J. Am. Chem. Soc. 2013;135:15662–15666. doi: 10.1021/ja4085385. [DOI] [PubMed] [Google Scholar]
- Padwa A., Chiacchio U., Fairfax D.J., Kassir J.M., Litrico A., Semones M.A., Xu S.L. A comparative study of the decomposition of o-alkynyl-substituted aryl diazo ketones. synthesis of polysubstituted β-naphthols via arylketene intermediates. J. Org. Chem. 1993;58:6429–6437. [Google Scholar]
- Pei C., Zhang C., Qian Y., Xu X. Catalytic carbene/alkyne metathesis (CAM): a versatile strategy for alkyne bifunctionalization. Org. Biomol. Chem. 2018;16:8677–8685. doi: 10.1039/c8ob02420k. [DOI] [PubMed] [Google Scholar]
- Pérez D., Peña D., Guitián E. Aryne cycloaddition reactions in the synthesis of large polycyclic aromatic compounds. Eur. J. Org. Chem. 2013;2013:5981–6013. [Google Scholar]
- Pflästerer D., Hashmi A.S.K. Gold catalysis in total synthesis−recent achievements. Chem. Soc. Rev. 2016;45:1331–1367. doi: 10.1039/c5cs00721f. [DOI] [PubMed] [Google Scholar]
- Phipps R.J., Gaunt M.J. A meta-selective copper-catalyzed C-H bond arylation. Science. 2009;323:1593–1597. doi: 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]
- Qi L.-W., Mao J.-H., Zhang J., Tan B. Organocatalytic asymmetric arylation of indoles enabled by azo groups. Nat. Chem. 2018;10:58–64. doi: 10.1038/nchem.2866. [DOI] [PubMed] [Google Scholar]
- Qiu H., Srinivas H.D., Zavalij P.Y., Doyle M.P. Unprecedented intramolecular [4 + 2]-cycloaddition between a 1,3−diene and a diazo ester. J. Am. Chem. Soc. 2016;138:1808–1811. doi: 10.1021/jacs.5b12877. [DOI] [PubMed] [Google Scholar]
- Raviola C., Protti S., Ravelli D., Fagnoni M. (Hetero)aromatics from dienynes, enediynes and enyne–allenes. Chem. Soc. Rev. 2016;45:4364–4390. doi: 10.1039/c6cs00128a. [DOI] [PubMed] [Google Scholar]
- Rode N., Marinelli F., Arcadi A., Adak T., Rudolph M., Rominger F., Hashmi A.S.K. Sequential gold-catalyzed carbene transfer/ring closure: oxidative cyclization of β-(2-alkynylphenyl)-α,β-ynones to indenofuranones. Adv. Synth. Catal. 2018;360:4790–4794. [Google Scholar]
- Schießl J., Schulmeister J., Doppiu A., Wörner E., Rudolph M., Karch R., Hashmi A.S.K. An industrial perspective on counter anions in gold catalysis: on alternative counter anions. Adv. Synth. Catal. 2018;360:3949–3959. [Google Scholar]
- Schießl J., Schulmeister J., Doppiu A., Wörner E., Rudolph M., Karch R., Hashmi A.S.K. An industrial perspective on counter anions in gold catalysis: underestimated with respect to “ligand effects”. Adv. Synth. Catal. 2018;360:2493–2502. [Google Scholar]
- Stockdale T.P., Williams C.M. Pharmaceuticals that contain polycyclic hydrocarbon scaffolds. Chem. Soc. Rev. 2015;44:7737–7763. doi: 10.1039/c4cs00477a. [DOI] [PubMed] [Google Scholar]
- Tanaka K. John Wiley & Sons, Hoboken, Wiley-VCH; 2013. Transition-metal-mediated Aromatic Ring Construction. [Google Scholar]
- Torres Ó., Parella T., Solà M., Roglans A., Pla-Quintana A. Enantioselective rhodium(I) donor carbenoid−mediated cascade triggered by a base−free decomposition of arylsulfonyl hydrazones. Chem. Eur. J. 2015;21:16240–16245. doi: 10.1002/chem.201502909. [DOI] [PubMed] [Google Scholar]
- van Otterlo W.A.L., de Koning C.B. Metathesis in the synthesis of aromatic compounds. Chem. Rev. 2009;109:3743–3782. doi: 10.1021/cr900178p. [DOI] [PubMed] [Google Scholar]
- Witham C.A., Mauleón P., Shapiro N.D., Sherry B.D., Toste F.D. Gold(I)-catalyzed oxidative rearrangements. J. Am. Chem. Soc. 2007;129:5838–5839. doi: 10.1021/ja071231+. [DOI] [PubMed] [Google Scholar]
- Wu W., Jiang H. Palladium-catalyzed oxidation of unsaturated hydrocarbons using molecular oxygen. Acc. Chem. Res. 2012;45:1736–1748. doi: 10.1021/ar3000508. [DOI] [PubMed] [Google Scholar]
- Xu X., Zavalij P.Y., Doyle M.P. A donor–acceptor cyclopropene as a dipole source for a silver(I) catalyzed asymmetric catalytic [3+3]-cycloaddition with nitrones. Chem. Commun. 2013;49:10287–10289. doi: 10.1039/c3cc46415f. [DOI] [PubMed] [Google Scholar]
- Yuan Z., Cheng R., Chen P., Liu G., Liang S.H. Efficient pathway for the preparation of aryl(isoquinoline)iodonium(III) salts and synthesis of radiofluorinated isoquinolines. Angew. Chem. Int. Ed. 2016;55:11882–11886. doi: 10.1002/anie.201606381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler A., Ziegler M.J., Rudolph M., Rominger F., Hashmi A.S.K. Scope and limitations of the intermolecular furan-yne cyclization. Adv. Synth. Catal. 2015;357:1507–1514. [Google Scholar]
- Zhang C., Li H., Pei C., Qiu L., Hu W., Bao X., Xu X. Selective vinylogous reactivity of carbene intermediate in gold-catalyzed alkyne carbocyclization: synthesis of indenols. ACS Catal. 2019;9:2440–2447. [Google Scholar]
- Zheng Y., Mao J., Weng Y., Zhang X., Xu X. Cyclopentadiene construction via Rh-catalyzed carbene/alkyne metathesis terminated with intramolecular formal [3 + 2] cycloaddition. Org. Lett. 2015;17:5638–5641. doi: 10.1021/acs.orglett.5b02912. [DOI] [PubMed] [Google Scholar]
- Zheng Z., Wang Z., Wang Y., Zhang L. Au-Catalysed oxidative cyclisation. Chem. Soc. Rev. 2016;45:4448–4458. doi: 10.1039/c5cs00887e. [DOI] [PubMed] [Google Scholar]
- Zhu R.-Y., Farmer M.E., Chen Y.-Q., Yu J.-Q. A simple and versatile amide directing group for C−H functionalizations. Angew. Chem. Int. Ed. 2016;55:10578–10599. doi: 10.1002/anie.201600791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The crystallography data have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession number CCDC: 1828269 ((S)-2u) and can be obtained free of charge from www.ccdc.cam.ac.uk/getstructures.