Abstract
Aims
Rifaximin (RFX), a non‐systemic antibiotic, improves liver/neuropsychological functions in patients with hepatic encephalopathy (HE). We aimed to investigate the clinical profiles associated with gut bacterial loads using exploratory data analysis and the effects of RFX on the gut microbiota of patients with HE.
Methods
We analyzed the data from 17 patients with HE who underwent fecal microbiota examination in phase II/III trials in Japan. Profiles associated with genera Streptococcus, Veillonella, and Lactobacillus loads were analyzed using classification and regression trees (CART). Changes in gut microbial consortia of seven patients with HE were then assessed 2 weeks after RFX treatment by principal component analysis.
Results
In the CART, the first and second divergence variables for each higher bacterial load were as follows: (i) in Streptococcus, the number connection test‐A ≥39.55 s and presence of portal‐systemic shunt; (ii) in Veillonella, serum potassium levels <4.75 mEq/L and total cholesterol level <129.5 mg/dL; and (iii) in Lactobacillus, white blood cell counts ≥3.4 × 103/μL and aspartate aminotransferase level ≥44.5 U/L. There was no significant change in total bacterial load before and after RFX treatment; however, there was a decrease in Streptococcus, Veillonella, and Lactobacillus counts after RFX treatment.
Conclusion
We report clinical profiles associated with gut bacterial loads in patients with HE, and showed that RFX altered gut microbiota components associated with liver/neuropsychological functions. Thus, RFX could improve liver/neuropsychological functions through the regulation of the gut microbial consortia in patients with HE.
Keywords: gut flora, hepatic encephalopathy, liver cirrhosis, rifaximin, Veillonella
Introduction
The gut microbiota plays a crucial role in disease progression in patients with liver cirrhosis.1 Veillonella, Streptococcus, Clostridium, and Prevotella have been found to be enriched in feces from patients with liver cirrhosis, and patients with high loads of these bacteria show higher Child–Pugh scores.2 In addition, the bacterial loads of Veillonella and Streptococcus negatively correlate with cognitive function in patients with liver cirrhosis.3 Moreover, the colonic mucosal microbiota is enriched for Enterococcus, Veillonella, Megasphaera, and Burkholderia in cirrhotic patients with hepatic encephalopathy (HE).4 Thus, accumulating evidence suggests that changes in the gut microbiota are associated with liver/neuropsychological function in patients with liver cirrhosis. However, the gut microbiota may be altered by environmental factors, including diet.5 Associations between enriched bacterial loads and liver/neuropsychological functions remain unclear in cirrhotic patients with HE in Japan.
Exploratory data analysis (EDA) is a statistical approach proposed by Tukey, which is able to discover meaningful hypotheses (or models) or productive knowledge through the use of a graphical approach.6 Exploratory data analysis has previously been utilized in the field of computer learning, and is now applied in the medical field.7 Classification and regression tree (CART), random forest (RF), and principle component analysis (PCA) are useful tools for EDA. Classification and regression tree is a statistical method that uses a constructed model to repeatedly recursively divide the explanatory variable involved in the response. The factorial structure of the underlying data is visually represented by a “tree”.8 Random forest is useful technique for prediction problems. Random forest is applied bootstrap samples and random feature selection to individual CART for prediction. This analysis provides a high level of predictive accuracy and can be used to estimate the relative importance of each factor.9 Principal component analysis is a method that provides a primarily graphical representation of 2‐Dordinates based on linear transformation of several variables. Although EDA is useful for revealing the pathological role of microbiota components, to date it has never been used to investigate associations between bacterial load and liver/neuropsychological functions in patients with HE.
Rifaximin (RFX) is a non‐systemic antibiotic that reduces the risk of HE recurrence and HE‐related hospitalization.10 Rifaximin also reduces the risk of spontaneous bacterial peritonitis and variceal bleeding, and prolongs overall survival in patients with HE.11, 12 As RFX inhibits ammonia‐producing bacterial genera such as Clostridium and Streptococcus,13 it is thought to exert beneficial effects by modulating the bacterial composition of the gut microbiota.10 However, recent studies reported that treatment with RFX has only minor effects on intestinal bacterial composition in patients with decompensated liver cirrhosis and non‐alcoholic steatohepatitis.14, 15, 16 Thus, the effects of RFX on gut microbiota composition remain unclear.
The aims of this study were to investigate: (i) the clinical profiles associated with bacterial loads using EDA; and (ii) the effects of RFX on gut microbiota composition in cirrhotic patients with HE.
Methods
Study design and ethics
We undertook a stratification analysis of phase II/III clinical studies of RFX 17 to analyze associations between bacterial load (genera Streptococcus, Veillonella, and Lactobacillus) and liver/neuropsychological function, and changes in the gut microbiota 2 weeks after RFX treatment. Our protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by the prior approval of each institution's review board.
Subjects
Subjects who participated in this study underwent periodic nutritional consultations with a dietitian. The nutritional consultation recommended 30–35 kcal with 1.0–1.5 g protein per kg ideal body weight/day, according to the guideline of the Japanese Nutritional Study Group for Liver Cirrhosis 2012.17
Patients with liver cirrhosis or idiopathic portosystemic shunting patients who met the following three inclusion criteria were enrolled: (i) 20–74 years old; (ii) grade I or II HE according to the criteria of the Inuyama Symposium in Japan;18 and (iii) hyperammonemia (peripheral venous blood ammonia concentration ≥80 μg/dL). Patients who met any of the following exclusion criteria were excluded: (i) grade III or more HE; (ii) psychiatric comorbidities; (iii) biochemical examinations (total bilirubin ≥5.0 mg/dL, hemoglobin ≤8 g/dL, serum potassium ≤2.5 mEq/L, blood urea nitrogen ≥25 mg/dL, and serum creatinine ≥2.0 mg/dL); and (iv) any comorbidity or medical history of acute hepatitis, acute liver failure, or acute exacerbation of chronic hepatitis.
In analysis 1, we analyzed 17 patients with HE from five medical institutions in Japan, who had undergone fecal microbiota examination before RFX treatment in phase II/III clinical studies of RFX between 2013 and 2015. In analysis 2, we analyzed seven patients from analysis 1, who had undergone fecal microbiota examinations both before and 2 weeks after RFX treatment.
Data collection
The following variables were recorded at the start of the clinical trial: age, sex, body weight, white blood cell count (WBC), platelet count, prothrombin time/international normalized ratio, and serum levels of aspartate aminotransferase (AST), alanine aminotransferase, alkaline phosphatase, γ‐glutamyl transpeptidase, total protein, albumin, total bilirubin, and creatinine, and branched‐chain amino acids‐to‐tyrosine ratio (BTR). Presence of portal systemic shunt, esophageal varix, and ascites was evaluated by abdominal images including ultrasonography, computed tomography, magnetic resonance imaging, and endoscopy. Asterixis score was determined as previously described:17 score 0, no asterixis observed; score 1, no spontaneous asterixis observed, with slight occurrence when the subject is in a tremor‐producing posture; score 2, no spontaneous asterixis observed, but it occurs easily when the subject is in a tremor‐producing posture; score 3, asterixis occurs without the subject being in a tremor‐producing posture; and score 4, asterixis occurs almost continuously without the subject being in a tremor‐producing posture.
Portal systemic encephalopathy (PSE) index
The PSE index was calculated before and 2 weeks after treatment of RFX using the following formula as previously described:17 PSE index = (3 × HE coma score + blood ammonia score + asterixis score + number connection test A [NCT‐A] score)/24.
Neuropsychological functions
Neuropsychological functions were evaluated both before and 2 weeks after RFX treatment by means of NCT‐A and NCT‐B and the digit symbol test (DST) using neuropsychological test system software.19 The software is distributed by the Japan Society of Hepatology. The hardware consists of a touch screen tablet, such as an iPad (Apple, Cupertino, CA, USA).
Fecal collection procedure
The whole feces were collected by the subject before and 2 weeks after treatment with RFX. The feces were immediately placed in a specialized container. The fecal specimens were kept in a refrigerator (4°C) until analysis.
Gut microbiota component
Components of the fecal gut microbiota were evaluated using a culture method carried out at LSI Medience (Tokyo, Japan).20 The following microbes were analyzed: aerobes (Enterobacteriaceae, glucose non‐fermentative Gram‐negative rods (NFR), Staphylococcus, Streptococcus, Enterococcus, Bacillus, Corynebacterium, and yeast) and anaerobes (Bacteroides, Fusobacterium, Clostridium, Clostridium difficile, Veillonella, Megasphaera, Bifidobacterium, Eubacterium, Peptostreptococcus, and Lactobacillus).
Statistics
Data are expressed as number, mean ± standard deviation, and median (range). Differences between groups were estimated using their mean difference and two‐sided 95% confidence intervals, as previously described.21 Associations between bacterial load and liver/neuropsychological function were evaluated by EDA. The statistical methods are described in detail below. Statistical analysis was carried out using the R software package (http://www.R-project.org/).9
Classification and regression trees
Classification and regression trees was constructed to reveal profiles associated with the fecal load of Streptococcus, Veillonella, and Lactobacillus (analysis 1), and changes in NCT‐A 2 weeks after RFX treatment (analysis 2), as previously described.8, 9 The tree plot was visualized with a regression tree model for each fecal load and the box plot shows the distribution of observations for nodes and leaves. The preconditions for splitting with CART were as follows: the complexity parameter set to 0.001, with eight and three observations as the minimum number per node for analyses 1 and 2, respectively.
Random forest analysis
Exploratory data analysis can identify factors theoretically associated with an objective variable, and can then generate hypotheses for future confirmatory study. An RF analysis was used to identify factors that distinguished between high and low Streptococcus, Veillonella, and Lactobacillus load on an ordinal scale, as previously described.8, 9 The variable importance value, which reflects the relative contribution of each variable to the model, was measured from increases in mean squared error following random permutation of explanatory variables and recalculating the predictive accuracy of the model. The analysis also included all effects arising from interaction of the variables.
Principal component analysis
The PCA was generated in accordance with the Euclidean distance matrix calculated from each sample, as previously described.22 The PCA results were represented as principle component scores in a biplot for each sample, which indicated first and second principle components. The rate of contribution for each of the principle components was also calculated.
Results
Analysis 1
Patient characteristics
Patient characteristics are summarized in Table 1. Briefly, the mean age of the subjects was 64.4 years, 58.8% of subjects suffered recurrence of HE, 76.5% of subjects developed HE grade I, and the mean PSE index was 0.34. The mean NCT‐A, NCT‐B, and DST were 49.9 s, 94.2 s, and 10.4 counts, respectively. The mean blood ammonia concentration and BTR were 129.9 μg/dL and 2.44, respectively.
Table 1.
Characteristics of patients with hepatic encephalopathy (HE) included in analysis 1
| Characteristic | |
|---|---|
| n | 17 |
| Age, years | 64.4 (8.6) |
| Sex, male/female; n (%) | 4 (23.5)/13 (76.5) |
| Body weight, kg | 62.6 (11.7) |
| Onset of HE, new onset/recurrent; n (%) | 7 (41.2)/10 (58.8) |
| HE grade I/II; n (%) | 13 (76.5)/4 (23.5) |
| Asterixis score, 0/1/2; n (%) | 9 (52.9)/7 (41.2)/1 (5.9) |
| PSE index | 0.34 (0.09) |
| Neuropsychological tests | |
| NCT‐A, s | 49.9 (16.2)† |
| NCT‐B, s | 94.2 (44.5)‡ |
| Digit symbol test, count | 10.4 (4.3) |
| Etiology of HE, n (%) | |
| Hepatitis C virus | 8 (47.1) |
| Alcohol | 3 (17.6) |
| Autoimmune hepatitis | 2 (11.8) |
| Primary biliary cholangitis | 1 (5.9) |
| Non‐alcoholic steatohepatitis | 2 (11.8) |
| Idiopathic portal hypertension | 1 (5.9) |
| Child–Pugh classification, A/B/C | 1 (5.9)/7 (41.2)/9 (52.9) |
| BCAA supplementation, yes/no | 6 (35.3)/11 (64.7) |
| Presence of portal systemic shunt | 11 (64.7) |
| Presence of esophageal varix | 9 (52.9) |
| Presence of ascites | 7 (41.2) |
| Biochemical examinations | |
| Blood ammonia concentration, μg/dL | 129.9 (38.3) |
| BTR | 2.44 (0.99) |
| Platelet count, ×104/μL | 8.86 (4.01) |
| Total protein, g/dL | 6.7 (0.9) |
| Albumin, g/dL | 2.8 (0.5) |
| Total bilirubin, mg/dL | 2.4 (1.1) |
| PT‐INR | 1.38 (0.18) |
| AST, U/L | 55.1 (24.2) |
| ALT, U/L | 35.5 (18.4) |
| ALP, U/L | 426.9 (121.7) |
| γ‐GTP, U/L | 43.1 (39.6) |
| Creatinine, mg/dL | 0.73 (0.21) |
n = 16.
n = 15.
Data are expressed as mean (standard deviation) or n (%), as indicated.
γ‐GTP, γ‐glutamyl transpeptidase; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCAA, branched‐chain amino acid; BTR, BCAA‐to‐tyrosine ratio; NCT, number connection test; PSE, portal systemic encephalopathy index; PT‐INR, prothrombin time – international normalized ratio.
Microbiome in feces of patients with HE
The microbiome in the feces of 17 patients with HE is shown in Table 2. The total bacterial count was 10.78 (9.53–11.18) log/g. Genus Bifidobacterium was the most abundant. Corynebacterium, NFR, and Megasphaera were below the lower limit of quantitation in all patients.
Table 2.
Fecal microbiome constituents in patients with hepatic encephalopathy (n = 17)
| Name | Log/g feces | |
|---|---|---|
| Median | Range | |
| Lactobacillus | 8.90 | 6.00–9.98 |
| Streptococcus | 7.78 | 2.00–9.72 |
| Veillonella | 8.15 | 2.00–9.92 |
| Bifidobacterium | 10.30 | 8.08–11.08 |
| Bacteroides | 10.26 | 2.00–10.68 |
| Eubacterium | 9.20 | 2.00–10.15 |
| Peptostreptococcus | 8.90 | 2.00–10.08 |
| Enterobacteriaceae | 8.73 | 5.15–10.08 |
| Enterococcus | 8.64 | 2.00–10.60 |
| Clostridium | 8.04 | 3.60–9.30 |
| Staphylococcus | 3.82 | 2.00–6.40 |
| Yeast | 3.51 | 2.00–6.00 |
| Bacillus | 2.30 | 2.00–6.15 |
| Fusobacterium | 2.00 | 2.00–8.78 |
| Glucose non‐fermentative Gram‐negative rods | 2.00 | – |
| Corynebacterium | 2.00 | – |
| Megasphaera | 2.00 | – |
| Clostridium difficile | 2.00 | 2.00–7.60 |
| Total | 10.78 | 9.53–11.18 |
Values are expressed as median (range) in decreasing order of magnitude. Data below the lower limit of quantification was interpreted as 2.00 Log/g.
–, not applicable.
Classification and regression trees for clinical profiles associated with high bacterial load of Streptococcus, Veillonella, and Lactobacillus
Streptococcus
In all patients, Streptococcus load was 7.78 (2.00–9.72) log/g feces. The NCT‐A was selected as the variable for the initial split, and Streptococcus load was 8.80 (7.26–9.72) log/g feces in patients with NCT‐A ≥39.55 s (Fig. 1a). In patients with NCT‐A ≥ 39.55 s, portal systemic shunt was selected as the variable for the second split; Streptococcus load was 9.15 (8.78–9.72) log/g feces in patients with portal systemic shunt.
Figure 1.
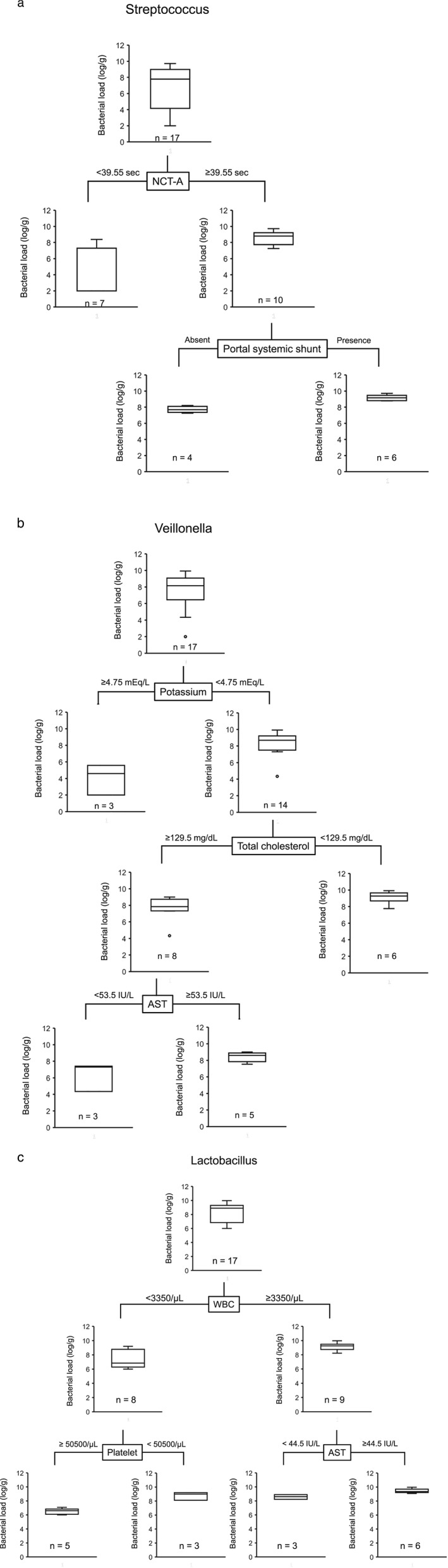
Classification and regression tree for clinical profiles associated with high bacterial load of (a) Streptococcus, (b) Veillonella, and (c) Lactobacillus in 17 Japanese patients with hepatic encephalopathy. AST, aspartate aminotransferase; NCT‐A, number connection test‐A; WBC, white blood cell count.
Veillonella
In all patients, Veillonella load was 8.15 (2.00–9.92) log/g feces. Potassium level was selected as the variable for the initial split, and Veillonella load was 8.69 (4.34–9.92) log/g feces in patients with potassium <4.75 mEq/L (Fig.1b). In patients with potassium <4.75 mEq/L, total cholesterol was selected as the variable for the second split; Veillonella load was 9.28 (7.76–9.92) log/g feces in patients with total cholesterol <129.5 mg/dL.
Lactobacillus
In all patients, Lactobacillus load was 8.90 (6.00–9.98) log/g of feces. WBC was selected as the variable for the initial split, and Lactobacillus load was 9.26 (8.23–9.98) log/g of feces in patients with WBC ≥3350/μL (Fig. 1c). In patients with WBC ≥3350/μL, AST level was selected as the variable for the second split; Lactobacillus load was 9.36 (9.08–9.98) log/g of feces in patients with AST ≥44.5 U/L.
Random forest analysis for factors that distinguished between high and low gut bacterial load
Streptococcus
The results of RF analysis are summarized in rank order in Figure 2(a). The analysis indicated that NCT‐A was the highest ranked variable for distinguishing between the high and low Streptococcus load groups. This was followed by DST and NCT‐B (Fig. 2a).
Figure 2.
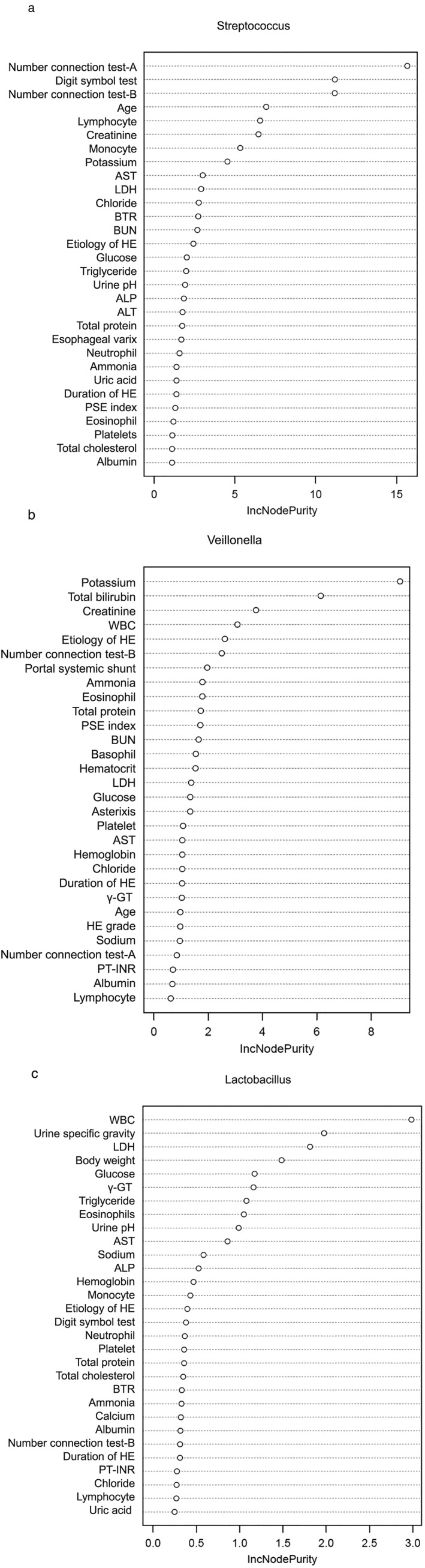
Random forest analysis for factors distinguishing between high and low (a) Streptococcus, (b) Veillonella, and (c) Lactobacillus bacterial load in 17 Japanese patients with hepatic encephalopathy (HE). γ‐GTP, γ‐glutamyl transpeptidase; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BTR, branched‐chain amino acid‐to‐tyrosine ratio; BUN, blood urea nitrogen; LDH, lactate dehydrogenase; PSE, portal systemic encephalopathy index; PT‐INR, prothrombin time – international normalized ratio; WBC, white blood cell count.
Veillonella
The results of the RF analysis are summarized in rank order in Figure 2(b). The analysis indicated that serum potassium level was the highest ranked variable for distinguishing between the high and low Veillonella load groups. This was followed by total bilirubin level and creatinine level (Fig. 2b).
Lactobacillus
The results of the RF analysis are summarized in rank order in Figure 2(c). The analysis indicated that serum WBC was the highest ranked variable for distinguishing between the high and low Lactobacillus load groups. This was followed by urine specific gravity and lactate dehydrogenase level (Fig. 2c).
Analysis 2
Patient characteristics
Patient characteristics are summarized in Table 3. Briefly, the mean age of the subjects was 64.3 years, 57.1% of subjects suffered recurrence of HE, and 42.9% of subjects developed HE grade I.
Table 3.
Characteristics of patients with hepatic encephalopathy (HE) included in analysis 2, assessed before the 2 weeks after rifaximin treatment
| Characteristic | Value |
|---|---|
| n | 7 |
| Age, years | 64.3 (10.6) |
| Sex, male/female; n (%) | 3 (42.9)/4 (57.1) |
| Body weight, kg | 66.7 (14.7) |
| Onset of HE, new onset/recurrent; n (%) | 3 (42.9)/4 (57.1) |
| HE grade I/II; n (%) | 3 (42.9)/4 (57.1) |
| Etiology of HE, n (%) | |
| Hepatitis C virus | 4 (57.1) |
| Alcohol | 2 (28.6) |
| Non‐alcoholic steatohepatitis | 1 (14.3) |
| Child–Pugh classification, A/B/C | 1 (14.3)/3 (42.9)/3 (42.9) |
| BCAA supplementation, yes/no | 2 (28.6)/5 (71.4) |
| Presence of portal systemic shunt | 3 (42.9) |
| Presence of esophageal varix | 4 (57.1) |
| Presence of ascites | 3 (42.9) |
Data are expressed as mean (standard deviation) or n (%), as indicated.
BCAA, branched‐chain amino acid.
Effects of RFX on liver/neuropsychological functions in patients with HE
Two weeks after RFX treatment, improvement was seen in NCT‐A, DST, and PSE index (Table 4).
Table 4.
Effects of rifaximin (RFX) on liver/neuropsychological functions in patients with hepatic encephalopathy
| Parameter | Baseline | Two weeks after RFX treatment | Mean difference (95% CI) |
|---|---|---|---|
| n | 7 | 7 | |
| NCT‐A, s | 49.7 (19.5) | 35.3 (11.3) | −14.4 (−23.67, −5.08)† |
| NCT‐B, s | 104.3 (56.7) | 77.0 (52.6)‡ | −14.7 (−35.321, 6.021) |
| Digit symbol test, counts | 10.1 (3.8) | 13.1 (4.7) | 3.0 (0.80, 5.20)† |
| Asterixis score, 0/1/2 | 3 (42.9%)/ | 6 (85.7%)/ | |
| 3 (42.9%)/ | 1 (14.3%)/ | ||
| 1 (14.3%) | 0 (0.0%) | ||
| PSE index | 0.37 (0.12.) | 0.26 (0.06) | −0.1 (−0.210, −0.007)† |
| MELD score | 9.6 (4.04) | 10.7 (4.27) | 1.14 (−2.39, 0.10) |
| PT‐INR | 1.39 (0.26) | 1.45 (0.29) | 0.1 (0.001, 0.119)† |
| Platelet count, ×104/μL | 9.33 (4.29) | 8.40 (3.99) | −0.93 (−0.683, 2.54) |
| Blood ammonia concentration, μg/dL | 119.7 (34.6) | 132.1 (35.0) | 12.4 (−34.54, 59.39) |
| Total protein, g/dL | 6.9 (1.2) | 6.8 (0.8) | −0.1 (−0.54, 0.40) |
| Albumin, g/dL | 3.0 (0.6) | 3.1 (0.5) | 0.1 (−0.19, 0.36) |
| Total bilirubin, mg/dL | 2.1 (1.1) | 2.1 (1.5) | 0.0 (−0.75, 0.69) |
| AST, U/L | 51.9 (33.2) | 55.7 (37.8) | 3.9 (−3.59, 11.30) |
| ALT, U/L | 34.0 (24.4) | 34.7 (25.7) | 0.7 (−3.71, 5.14) |
| ALP, U/L | 387.0 (111.6) | 359.9 (96.7) | −27.1 (−94.97, 40.68) |
| γ‐GTP, U/L | 48.9 (53.8) | 43.6 (46.7) | −5.3 (−12.54, 1.97) |
| Creatinine, mg/dL | 0.79 (0.25) | 0.86 (0.27) | 0.07 (−0.12, −0.02)† |
| BCAA to tyrosine ratio | 2.69 (1.04) | 2.38 (0.66) | −0.3 (−1.029, 0.403) |
Data are expressed as mean (standard deviation or rate of subjects).
95% confidence interval (CI) does not include zero.
n = 6.
γ‐GTP, γ‐glutamyl transpeptidase; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCAA, branched‐chain amino acid; MELD, Model for End‐stage Liver Disease; NCT, number connection test; PSE, portal systemic encephalopathy; PT‐INR, prothrombin time – international normalized ratio.
Changes in gut microbiota 2 weeks after RFX treatment in patients with HE
No significant change in total fecal bacterial load before (10.78 [9.6–11.1] log/g) and after (10.00 [9.3–10.4] log/g) treatment with RFX was seen. However, changes to the gut microbiota 2 weeks after RFX treatment in each case of HE is displayed in Figure 3. Streptococcus, Veillonella, and Lactobacillus populations were reduced 2 weeks after, compared to those before RFX treatment (Fig. 3).
Figure 3.
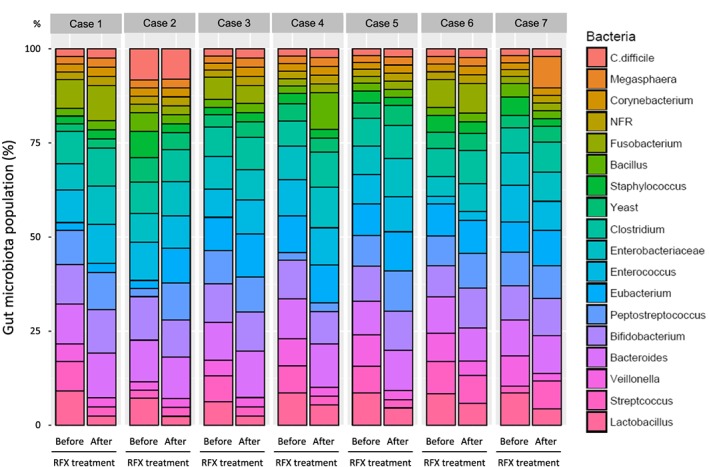
Gut microbiota composition prior to, and 2 weeks after, rifaximin (RFX) treatment in seven Japanese patients with hepatic encephalopathy. C., Clostridium; NFR, glucose non‐fermentative Gram‐negative rods. [Color figure can be viewed at http://wileyonlinelibrary.com]
Changes to the gut microbiota were also assessed by PCA. The cumulative contribution rate of the first and second principal components was 52.0%. The PCA showed that the component consisting of aerobic bacteria, including Streptococcus, Veillonella, and Lactobacillus, was decreased whereas the anaerobic bacteria component, including Bacteroides and Enterobacteriaceae, was increased 2 weeks after RFX treatment (Fig. 4).
Figure 4.
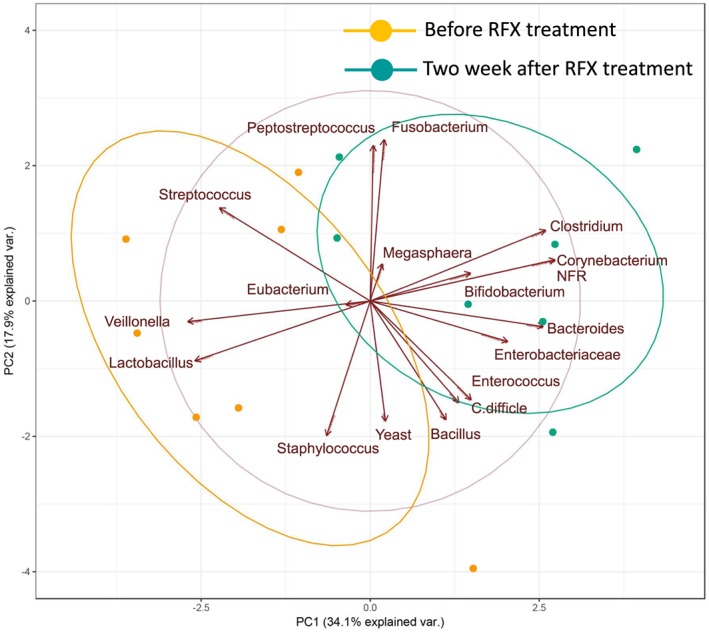
Principal component (PC) analysis of components of the gut microbiota before and 2 weeks after rifaximin (RFX) treatment in seven Japanese patients with hepatic encephalopathy. var., variable. [Color figure can be viewed at http://wileyonlinelibrary.com]
Classification and regression tree for bacterial profiles associated with changes of NCT‐A 2 weeks after RFX treatment in patients with HE
We undertook a CART analysis to identify bacterial profiles associated with changes in NCT‐A 2 weeks after RFX treatment. Changes in the proportion of Enterobacteriaceae load was selected as the variable for the initial split for changes in NCT‐A (Fig. 5). Patients with more than 2.399% of increase in the proportion of Enterobacteriaceae load showed marked improvement of NCT‐A after 2 weeks of RFX treatment.
Figure 5.
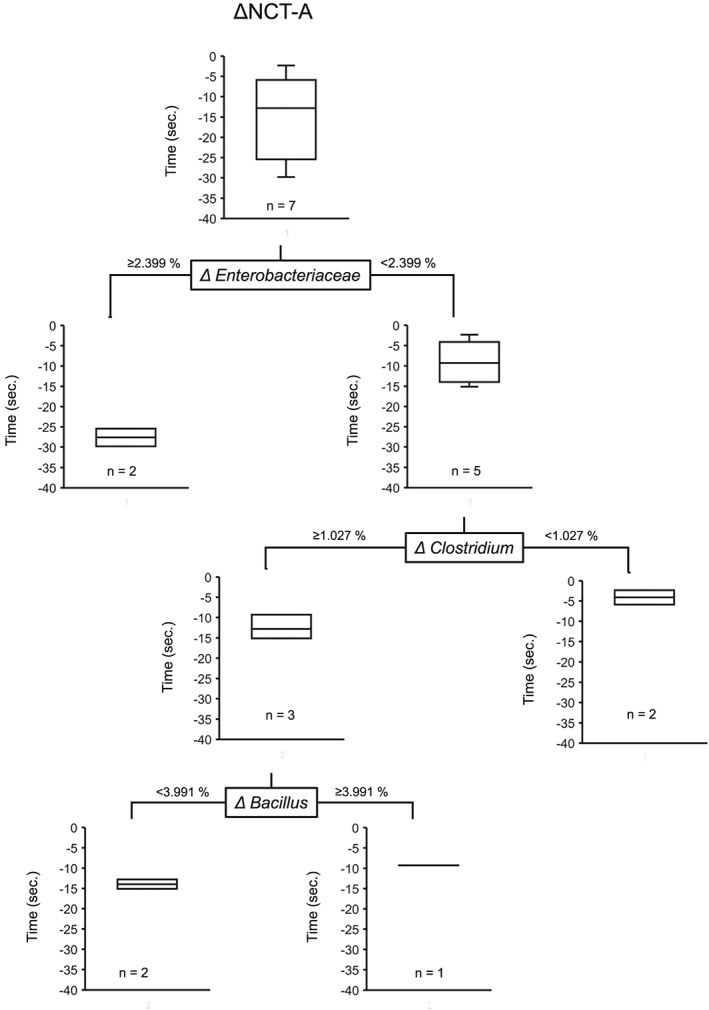
Classification and regression tree for the proportion of bacterial load associated with number connection test‐A (NCT‐A) in seven Japanese patients with hepatic encephalopathy.
Discussion
In this study, we reported the clinical profiles associated with bacterial loads of the genera Streptococcus, Veillonella, and Lactobacillus in patients with HE. We also showed that RFX altered gut microbiota associated with liver/neuropsychological functions. Thus, RFX could ameliorate the decline in these functions through the regulation of the gut microbial consortia in patients with HE.
Genus Bifidobacterium is reported to be one of main intestinal bacteria in healthy Japanese subjects.20 In addition, oral species such as Veillonella and Streptococcus are reported to be detected in the feces of patients with cirrhosis in the USA and China.2, 23 In our study, Bifidobacterium was the most abundant genus in the feces of patients with HE, and Veillonella and Streptococcus were also detected in the feces of patients with HE in Japan. There were no changes between healthy subjects and patients with HE in terms of their main intestinal bacteria. In addition, oral species were also detected in Japanese patients with HE.
In this study, CART and RF analyses were used. We showed that an impairment of cognitive function was associated with fecal Streptococcus load. Previous research showed that fecal Streptococcus load is higher in patients with chronic liver disease.3 Thus, our findings are in good agreement with the results of this report. Streptococcus salivarius has urease activity, and fecal Streptococcus load is positively correlated with ammonia accumulation in cirrhotic patients with covert HE.3 Moreover, Inoue et al. recently reported an increase in the urease gene mainly encoded by viridans group streptococci during disease progression in patients with chronic hepatitis C virus infection.24 Taken together, Streptococcus could impair cognitive function through upregulation of ammonia production.
As with Streptococcus, fecal Veillonella load is reported to be high in cirrhotic patients2 and correlated with cognitive function in patients with HE.25 However, fecal Veillonella load was not associated with neuropsychological parameters such as NCT‐A/B or DST in this study. We were the first to show that fecal Veillonella load is associated with lower serum potassium level in this study. Veillonella is known to have a potassium uptake system, which is involved in the maintenance of its cytoplasmic pH.26 This potassium uptake system might be a possible mechanism explaining the lower serum potassium level in our study. Moreover, Zuccalà et al. reported that a low serum potassium level is an independent factor associated with cognitive impairment in patients with heart failure.27 Thus, Veillonella might be associated with cognitive impairment through modification of potassium levels.
In our study, fecal Lactobacillus load was associated with higher WBC counts and AST levels. In general, Lactobacillus species are commonly used as probiotics, and Lactobacillus GG is reported to suppress endotoxemia and inflammation in patients with cirrhosis.28 In contrast to the genus’ beneficial functions, some Lactobacillus species are associated with the development of disease.29 Lactobacillus fermentum is a known producer of ethanol through sugar fermentation and is thought to be responsible for the development of hepatic inflammation in patients with non‐alcoholic fatty liver disease.30 Although we did not evaluate individual bacterial species in this study, ethanol‐producing Lactobacillus might be associated with elevated WBC counts and AST levels.
Rifaximin is known to improve hyperammonemia;17 however, blood ammonia level was not significantly decreased 2 weeks after RFX treatment in analysis 2. The reason for this discrepancy remains unclear. A possible reason is that, although RFX inhibited ammonia‐producing bacterial genera such as Streptococcus in analysis 2, approximately 85% of subjects were classified as Child–Pugh class B or C and BTR was significantly low, suggesting that the detoxication ability for ammonia was insufficient to decrease blood ammonia levels in patients enrolled in analysis 2.
In this study, there was no significant difference in total bacterial load before and after RFX treatment; however, PCA showed a decrease in Streptococcus, Veillonella, and Lactobacillus populations 2 weeks after RFX treatment. The proportion of species of oral origin, such as Veillonella and Streptococcus, is reported to be high in the feces of cirrhotic patients.2, 23 Rifaximin shows high antibacterial activity against Veillonella (minimal inhibitory concentration 50, 2 μg/mL)31 and Streptococcus (minimal inhibitory concentration 50, ≤0.03–0.12 μg/mL)32 and several studies have reported that RFX treatment decreases the relative abundances of the genera Veillonella and Streptococcus.13, 14, 23 Our findings on Streptococcus and Veillonella are in good agreement with these previous reports. However, RFX treatment has also been reported to increase the proportion of Lactobacillus in animal and human studies,33, 34 which is at odds with the results of our study. Although the reason for this discrepancy remains unclear, the proportion of Lactobacillus salivarius, a species of oral origin, is increased in the feces of cirrhotic patients.2 As RFX inhibits bacterial translocation in animal models of colitis and acute pancreatitis,35, 36 RFX could be inhibiting the translocation of oral origin Lactobacillus, consequently reducing the fecal proportion of Lactobacillus in patients with HE. These modulatory effects of RFX on the composition of the gut microbiota might, in part, contribute to the improvement in liver/neuropsychological functions in patients with HE.
In this study, patients with an increased proportion of Enterobacteriaceae load showed marked improvement of NCT‐A after 2 weeks of RFX treatment. Unlike our results, Enterobacteriaceae is reported to be associated with cognitive impairment in patients with HE.25 Rifaximin possesses potent activity against species of Staphylococcus, Streptococcus, and Enterococcus but less activity against species of Enterobacteriaceae.37 Thus, an increased proportion of Enterobacteriaceae could be relative to a decreased proportion of the ammonia‐producing bacterial genera such as Staphylococcus, Streptococcus, and Enterococcus.
There are limitations in this study. First, our data are based on a small number of patients. Second, components of the fecal gut microbiota were evaluated by genus level and, therefore, no information is available for species of the fecal gut microbiota. Finally, we did not evaluate fecal pH. Inoue et al. showed a high fecal pH level in patients with chronic hepatitis C.24 As RFX is known to inhibit urease production,38 RFX might reduce fecal pH level. Thus, large sample size, measurement of fecal pH, and evaluation of bacterial species using the real‐time quantitative polymerase chain reaction method are required in future studies.
In conclusion, we showed that fecal Streptococcus load was associated with neuropsychological functions in patients with HE. Moreover, we showed that RFX did not reduce the total load of gut microbiota, but altered gut microbiota components that are related to liver/neuropsychological functions. Thus, RFX might improve liver/neuropsychological functions by modulating the gut microbiota in patients with HE.
Acknowledgments
This study was conducted as a clinical trial by ASKA Pharmaceutical Co., Ltd.
Kawaguchi, T. , Suzuki, F. , Imamura, M. , Murashima, N. , Yanase, M. , Mine, T. , Fujisawa, M. , Sato, I. , Yoshiji, H. , Okita, K. , and Suzuki, K. (2019) Rifaximin‐altered gut microbiota components associated with liver/neuropsychological functions in patients with hepatic encephalopathy: An exploratory data analysis of phase II/III clinical trials. Hepatol Res, 49: 404–418. 10.1111/hepr.13300.
Conflict of interest: Masaki Fujisawa and Ikuya Sato are employees of ASKA Pharmaceutical Co., Ltd. Takumi Kawaguchi received lecture fees from Mitsubishi Tanabe Pharma Corporation. The other authors have no conflicts of interest.
Financial support: This study was conducted as a clinical trial by ASKA Pharmaceutical Co., Ltd.
References
- 1. Gorham J, Gleeson M. Cirrhosis and dysbiosis: new insights from next‐generation sequencing. Hepatology 2016; 63: 336–338. [DOI] [PubMed] [Google Scholar]
- 2. Qin N, Yang F, Li A et al Alterations of the human gut microbiome in liver cirrhosis. Nature 2014; 513: 59–64. [DOI] [PubMed] [Google Scholar]
- 3. Zhang Z, Zhai H, Geng J et al Large‐scale survey of gut microbiota associated with MHE via 16S rRNA‐based pyrosequencing. Am J Gastroenterol 2013; 108: 1601–1611. [DOI] [PubMed] [Google Scholar]
- 4. Bajaj JS, Hylemon PB, Ridlon JM et al Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol 2012; 303: G675–G685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee S, Sung J, Lee J, Ko G. Comparison of the gut microbiotas of healthy adult twins living in South Korea and the United States. Appl Environ Microbiol 2011; 77: 7433–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoaglin D, John W. Tukey and data analysis. Stat Sci 2003; 18: 311–318. [Google Scholar]
- 7. Hendricks B, Mark‐Carew M. Using exploratory data analysis to identify and predict patterns of human Lyme disease case clustering within a multistate region, 2010‐2014. Spat Spatiotemporal Epidemiol 2017; 20: 35–43. [DOI] [PubMed] [Google Scholar]
- 8. Kawaguchi T, Inokuchi T, Honma T et al Factors associated with advanced hepatic fibrosis in patients with various internal diseases: a multicenter community‐based survey. Hepatol Res 2018; 48: 882–892. [DOI] [PubMed] [Google Scholar]
- 9. Kawaguchi T, Tokushige K, Hyogo H et al A data mining‐based prognostic algorithm for NAFLD‐related hepatoma patients: a nationwide study by the Japan Study Group of NAFLD. Sci Rep 2018; 8: 10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bajaj JS. Review article: potential mechanisms of action of rifaximin in the management of hepatic encephalopathy and other complications of cirrhosis. Aliment Pharmacol Ther 2016; 43(Suppl. 1): 11–26. [DOI] [PubMed] [Google Scholar]
- 11. Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long‐term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol 2013; 28: 450–455. [DOI] [PubMed] [Google Scholar]
- 12. Kang SH, Lee YB, Lee JH et al Rifaximin treatment is associated with reduced risk of cirrhotic complications and prolonged overall survival in patients experiencing hepatic encephalopathy. Aliment Pharmacol Ther 2017; 46: 845–855. [DOI] [PubMed] [Google Scholar]
- 13. Zuo Z, Fan H, Tang XD et al Effect of different treatments and alcohol addiction on gut microbiota in minimal hepatic encephalopathy patients. Exp Ther Med 2017; 14: 4887–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaji K, Takaya H, Saikawa S et al Rifaximin ameliorates hepatic encephalopathy and endotoxemia without affecting the gut microbiome diversity. World J Gastroenterol 2017; 23: 8355–8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kimer N, Pedersen JS, Tavenier J et al Rifaximin has minor effects on bacterial composition, inflammation, and bacterial translocation in cirrhosis: a randomized trial. J Gastroenterol Hepatol 2018; 33: 307–314. [DOI] [PubMed] [Google Scholar]
- 16. Cobbold JFL, Atkinson S, Marchesi JR et al Rifaximin in non‐alcoholic steatohepatitis: an open‐label pilot study. Hepatol Res 2018; 48: 69–77. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki K, Endo R, Takikawa Y et al Efficacy and safety of rifaximin in Japanese patients with hepatic encephalopathy: a phase II/III, multicenter, randomized, evaluator‐blinded, active‐controlled trial and a phase III, multicenter, open trial. Hepatol Res 2018; 48: 411–423. [DOI] [PubMed] [Google Scholar]
- 18. Mochida S, Takikawa Y, Nakayama N et al Classification of the etiologies of acute liver failure in Japan: a report by the Intractable Hepato‐Biliary Diseases Study Group of Japan. Hepatol Res 2014; 44: 365–367. [DOI] [PubMed] [Google Scholar]
- 19. Kawaguchi T, Konishi M, Kato A et al Updating the neuropsychological test system in Japan for the elderly and in a modern touch screen tablet society by resetting the cut‐off values. Hepatol Res 2017; 47: 1335–1339. [DOI] [PubMed] [Google Scholar]
- 20. Mitsuoka T. Establishment of intestinal bacteriology. Biosci Microbiota Food Health 2014; 33: 99–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wasserstein RL, Lazar NA. The ASA's statement on P‐values: context, process, and purpose. Am Stat 2016; 70: 129–133. [Google Scholar]
- 22. Attri S, Sharma K, Raigond P, Goel G. Colonic fermentation of polyphenolics from Sea buckthorn (Hippophae rhamnoides) berries: assessment of effects on microbial diversity by principal component analysis. Food Res Int 2018; 105: 324–332. [DOI] [PubMed] [Google Scholar]
- 23. Bajaj JS, Heuman DM, Sanyal AJ et al Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One 2013; 8: e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Inoue T, Nakayama J, Moriya K et al Gut dysbiosis associated with hepatitis C virus infection. Clin Infect Dis 2018; 67: 869–877. [DOI] [PubMed] [Google Scholar]
- 25. Bajaj JS, Ridlon JM, Hylemon PB et al Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol 2012; 302: G168–G175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Do T, Sheehy EC, Mulli T, Hughes F, Beighton D. Transcriptomic analysis of three Veillonella spp. present in carious dentine and in the saliva of caries‐free individuals. Front Cell Infect Microbiol 2015; 5: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zuccalà G, Marzetti E, Cesari M et al Correlates of cognitive impairment among patients with heart failure: results of a multicenter survey. Am J Med 2005; 118: 496–502. [DOI] [PubMed] [Google Scholar]
- 28. Bajaj JS, Heuman DM, Hylemon PB et al Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther 2014; 39: 1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Million M, Maraninchi M, Henry M et al Obesity‐associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii . Int J Obes (Lond) 2012; 36: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30. Elshaghabee FM, Bockelmann W, Meske D et al Ethanol production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Front Microbiol 2016; 7: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang FD, Liao CH, Lin YT, Sheng WH, Hsueh PR. Trends in the susceptibility of commonly encountered clinically significant anaerobes and susceptibilities of blood isolates of anaerobes to 16 antimicrobial agents, including fidaxomicin and rifaximin, 2008–2012, northern Taiwan. Eur J Clin Microbiol Infect Dis 2014; 33: 2041–2052. [DOI] [PubMed] [Google Scholar]
- 32. Hoover WW, Gerlach EH, Hoban DJ, Eliopoulos GM, Pfaller MA, Jones RN. Antimicrobial activity and spectrum of rifaximin, a new topical rifamycin derivative. Diagn Microbiol Infect Dis 1993; 16: 111–118. [DOI] [PubMed] [Google Scholar]
- 33. Xu D, Gao J, Gillilland M 3rd et al Rifaximin alters intestinal bacteria and prevents stress‐induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014; 146: 484–96.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ponziani FR, Scaldaferri F, Petito V et al The role of antibiotics in gut microbiota modulation: the eubiotic effects of rifaximin. Dig Dis 2016; 34: 269–278. [DOI] [PubMed] [Google Scholar]
- 35. Fiorucci S, Distrutti E, Mencarelli A, Barbanti M, Palazzini E, Morelli A. Inhibition of intestinal bacterial translocation with rifaximin modulates lamina propria monocytic cells reactivity and protects against inflammation in a rodent model of colitis. Digestion 2002; 66: 246–256. [DOI] [PubMed] [Google Scholar]
- 36. Marotta F, Geng TC, Wu CC, Barbi G. Bacterial translocation in the course of acute pancreatitis: beneficial role of nonabsorbable antibiotics and lactitol enemas. Digestion 1996; 57: 446–452. [DOI] [PubMed] [Google Scholar]
- 37. Gillis JC, Brogden RN. Rifaximin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic potential in conditions mediated by gastrointestinal bacteria. Drugs 1995; 49: 467–484. [DOI] [PubMed] [Google Scholar]
- 38. Ricci A, Coppo E, Barbieri R, Debbia EA, Marchese A. The effect of sub‐inhibitory concentrations of rifaximin on urease production and on other virulence factors expressed by Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa and Staphylococcus aureus . J Chemother 2017; 29: 67–73. [DOI] [PubMed] [Google Scholar]


