ABSTRACT
The ‘Age of Mammals’ began in the Paleocene epoch, the 10 million year interval immediately following the Cretaceous–Palaeogene mass extinction. The apparently rapid shift in mammalian ecomorphs from small, largely insectivorous forms to many small‐to‐large‐bodied, diverse taxa has driven a hypothesis that the end‐Cretaceous heralded an adaptive radiation in placental mammal evolution. However, the affinities of most Paleocene mammals have remained unresolved, despite significant advances in understanding the relationships of the extant orders, hindering efforts to reconstruct robustly the origin and early evolution of placental mammals. Here we present the largest cladistic analysis of Paleocene placentals to date, from a data matrix including 177 taxa (130 of which are Palaeogene) and 680 morphological characters. We improve the resolution of the relationships of several enigmatic Paleocene clades, including families of ‘condylarths’. Protungulatum is resolved as a stem eutherian, meaning that no crown‐placental mammal unambiguously pre‐dates the Cretaceous–Palaeogene boundary. Our results support an Atlantogenata–Boreoeutheria split at the root of crown Placentalia, the presence of phenacodontids as closest relatives of Perissodactyla, the validity of Euungulata, and the placement of Arctocyonidae close to Carnivora. Periptychidae and Pantodonta are resolved as sister taxa, Leptictida and Cimolestidae are found to be stem eutherians, and Hyopsodontidae is highly polyphyletic. The inclusion of Paleocene taxa in a placental phylogeny alters interpretations of relationships and key events in mammalian evolutionary history. Paleocene mammals are an essential source of data for understanding fully the biotic dynamics associated with the end‐Cretaceous mass extinction. The relationships presented here mark a critical first step towards accurate reconstruction of this important interval in the evolution of the modern fauna.
Keywords: Placentalia, evolution, phylogeny, Palaeogene, radiation, Condylarthra, palaeontology
I. INTRODUCTION
The Cretaceous–Palaeogene (hereafter K/Pg) mass extinction represents one of the largest global ecological turnovers in the history of life. Occurring 66 million years ago, it was the second largest mass extinction of all time, during which some 75% of terrestrial species were extinguished (Jablonski & Chaloner, 1994), dramatically altering both terrestrial and marine ecosystems (Vajda, Raine & Hollis, 2001; Sessa et al., 2012). Palaeontologists usually reconstruct this point as the beginning of the so‐called ‘Age of Mammals’; prior to the K/Pg boundary, mammals were mainly small, terrestrial‐to‐arboreal insectivores with low ecological disparity (Goswami, 2012; Grossnickle & Polly, 2013), albeit with a few notable exceptions (Luo, 2007). By contrast, Palaeogene mammals include the first large‐bodied herbivores, specialised carnivores, and later, radiations of gliding, flying, and fully aquatic organisms, with a corresponding increase in diversity (Darroch et al., 2014).
This apparently sudden increase in ecospace occupation has been interpreted as an adaptive radiation, particularly in placental mammals (Osborn, 1902; Simpson, 1953; Alroy, 1999; Raia et al., 2013). However, macroevolutionary studies of placental mammals of this period are limited by the lack of a comprehensive phylogeny for Paleocene placentals. With the exception of Primates (Russell, 1964), Rodentia (Jepsen, 1937), and Carnivora (Fox, Scott & Rankin, 2010), no extant order of placental mammal has an unambiguous representative during the Paleocene, minimally leaving a 10 million year gap between the K/Pg mass extinction and the origin of most extant orders. Pertinent to the question of when placental mammals diversified are the currently unresolved phylogenetic relationships of the majority of Paleocene mammals; they occur during the period of rapid ecological diversification for placental mammals, but pre‐date the definitive first appearances of most of the extant orders.
Many previous studies have assessed the timing of the origin of placental mammals (Bininda‐Emonds et al., 2007; O'Leary et al., 2013), or examined changes in rates of evolution of body size or diversification across the K/Pg boundary (Springer et al., 2003; Venditti, Meade & Pagel, 2011; Slater, 2013). All, however, have used data sets that mostly or entirely excluded Paleocene taxa, and therefore lack data from the important period during which an adaptive radiation would seem, from a strict reading of the fossil record, to have occurred. These analyses, which have mostly used divergence estimates from molecular dating techniques, have tended to favour a ‘mid’ to Late Cretaceous origin of placental orders and superorders (Springer et al., 2003; Bininda‐Emonds et al., 2007; dos Reis et al., 2012). However, despite numerous suggestions of Cretaceous placentals, no Cretaceous eutherian mammal has been unambiguously resolved within the placental crown (Wible et al., 2009; Goswami et al., 2011). The earliest definitive members of crown orders are mostly known from the Late Paleocene or Eocene. A Cretaceous origin would therefore require the existence of long ghost lineages. Additionally, it has been suggested that clock models suffer from artefacts resulting from historical changes in evolutionary rate (Beck & Lee, 2014). Estimating the date of origin of placental mammals and reconstructing their response to the end‐Cretaceous mass extinction are therefore highly contingent on method and data set.
Addition of fossil data has also been shown to change results of analyses significantly in a wide range of macroevolutionary studies (Tarver & Donoghue, 2011; Pyron & Burbrink, 2012; Slater, Harmon & Alfaro, 2012; Wood et al., 2013; Raj Pant, Goswami & Finarelli, 2014). The inclusion of fossil data is, however, only possible where the phylogenetic relationships of those fossil forms is understood.
The phylogenetic relationships among extant placental mammals have a long history of study based on morphological data, with some degree of stability in tree topology for several decades (Gregory, 1910; Simpson, 1945; McKenna, 1975; Novacek, 1992). This traditional topology accommodated many of the Paleocene mammal clades in a relatively straightforward manner, such as ‘condylarths’ being identified as stem ungulates (Fig. 1A). However, towards the latter half of the 20th century, questions were raised about some of these groupings, such as the traditional clades of ‘Insectivora’ (insectivorous mammals) and ‘Ungulata’ (hoofed mammals) (see Asher, Geisler, & Sanchez‐Villagra, 2008 for a review of these). For instance, the distinction between the Afrotherian golden mole Chrysochloris and European moles was suggested, based on entirely morphological data, as early as the 19th century (Cope, 1884c ). The advent of molecular sequencing and its application to mammalian phylogenetics confirmed the suggestions that Insectivora and Ungulata were polyphyletic (Stanhope et al., 1998). With the division of Insectivora into Eulipotyphla and Afroinsectivora, and Ungulata into Perissodactyla, Artiodactyla, and Paenungulata, several fossil taxa were left without a well‐supported position in the placental tree of life. In particular, ‘Condylarthra’, historically thought to be ancestral to ‘Ungulata’, was reduced to the status of ‘wastebasket taxon’, into which any generically bunodont, unguligrade mammal from the Palaeogene has been consigned (Archibald, 1998). Patently, these taxa must have ancestors, and extant orders likely evolved from some of the ambiguous Paleocene taxa, but the nature of their relationships remains perplexing. Indeed, every ‘condylarth’ family‐level clade has been suggested to be related to one or more extant clade, with several hypotheses existing for each (Fig. 1B). For the most part, these ‘condylarth’ clades themselves are considered monophyletic, but the relationships among them, and between any ‘condylarth’ clade and extant orders are unknown.
Figure 1.
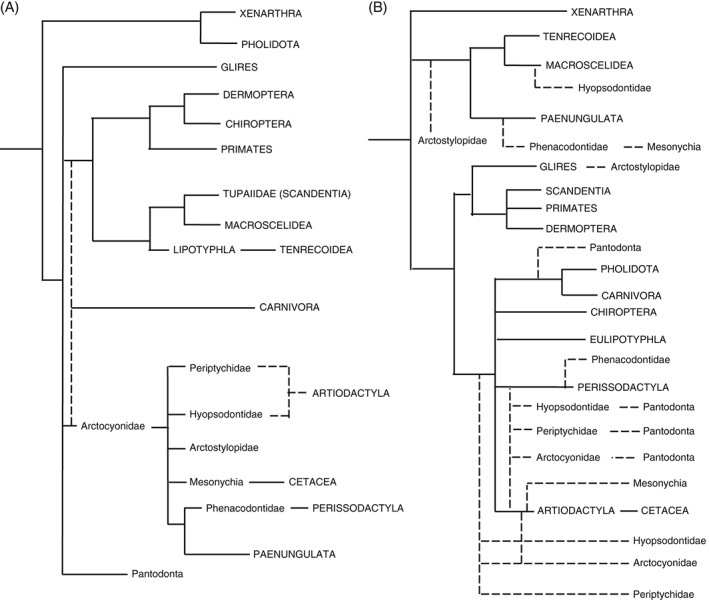
A depiction, in broad terms, of changes in understanding of the relationships of placental mammals over the last 20 years. (A) Broad understanding of placental mammal relationships prior to the advent of molecular data. ‘Condylarth’ families were considered basal to Ungulata, a number of plesiomorphic taxa were grouped together as Insectivora, whales were considered a separate order, and pangolins were joined with xenarthrans in Edentata. (B) The current consensus on placental mammal phylogeny. While the relationships of extant groups are clear – Cetacea is a subgroup of Artiodactyla, Carnivora and Pholidota are sister taxa, Ungulata and Insectivora are polyphyletic – the relationships of Paleocene taxa have become far more uncertain. In both, dotted lines represent uncertainty.
Identifying the phylogenetic position of these enigmatic Palaeogene taxa with respect to extant orders and Cretaceous groups is therefore essential to understanding the timing of divergence of extant orders. Distinct Paleocene mammals first appear less than a million years after the K/Pg boundary, and if they are crown‐placental mammals, this timing would imply that speciation between extant orders most likely occurred cryptically during the Cretaceous. Establishing how many mammalian lineages span the K/Pg boundary also allows for greater accuracy in assessing the role of mass extinctions in evolutionary dynamics more generally. Ascertaining the phylogenetic relationships of fossil forms will facilitate robust, inclusive studies of character evolution that directly sample taxa from the relevant intervals and better represent true clade diversity. Ultimately, a phylogeny of Paleocene mammals is sorely needed, but has not been forthcoming, despite a great deal of energy directed towards study of the end‐Cretaceous mass extinction and its aftermath.
II. AIMS
Here, we present the results of the largest cladistic analysis of Palaeogene mammals to date, with the aim of resolving the relationships of some enigmatic groups of eutherian mammals. Inclusion of key representative taxa from this important period in placental evolution in a wide‐reaching study is a crucial requirement for future analysis of the early evolution of this clade. Below, we provide a detailed overview of the early fossil record of placental mammals, followed by the new cladistic analysis and a discussion of its implications for resolving internal placental relationships and the origins of the group.
III. OVERVIEW OF THE FOSSIL RECORD OF EARLY PLACENTAL MAMMALS
(1). ‘Condylarthra’
By far the largest component of the mammalian biota in the Paleocene is the collection of ‘archaic ungulates’ known as ‘condylarths’. While this grouping is almost certainly an anachronistic grade of largely terrestrial, bunodont, herbivorous‐to‐omnivorous mammals, there are several well‐defined families which fall within ‘Condylarthra’.
(a). Arctocyonidae
(i). Composition
Arctocyonidae is composed of approximately 20 genera, including some of the best known of the ‘archaic ungulates’, such as the type species Arctocyon primaevus, discovered in 1841 (de Blainville, 1841), and the arboreal mammal Chriacus. Other genera include Mentoclaenodon, Lambertocyon, Thryptacodon, Anacodon, and Claenodon. It has been disputed whether Claenodon is considered a separate genus in its own right (Rose, 1981), a synonym of Arctocyon (Rigby, 1980), or a synonym of Arctocyonides (Van Valen, 1978). Some recent studies (Williamson & Carr, 2007; De Bast & Smith, 2013) have hinted that the subfamilies of Arctocyonidae may be polyphyletic, with the Oxyclaeninae occurring at the base of crown placentals (although all taxa sampled in those analyses are certain or probable laurasiatheres), Arctocyoninae as a sister group to the Mesonychia and Triisodontidae, and Loxolophinae closest to a broader clade comprising ancestors of Artiodactyla and Perissodactyla. According to De Bast & Smith (2013, p. 971), arctocyonids have ‘been used as a wastebasket for basal condylarths’; making arctocyonids particularly enigmatic, as the general consensus is that ‘Condylarthra’ is itself a wastebasket taxon (Archibald, 1998).
(ii). Proposed relationships
The arctocyonids are a primarily European group (Russell, 1964) which has variously been considered ancestral to Carnivora (Van Valen, 1969), specifically to miacid carnivorans (see Van Valen, 1978), to artiodactyls (Rose, 1996), and as part of the ancestral ungulate group (Kondrashov & Lucas, 2004; De Bast & Smith, 2013). Arctocyonidae was one of the two groups (the other being Oxyclaenidae) that was assigned to the most primitive groupings of ‘archaic ungulate’ – the Procreodi – by Matthew (1915). Simpson (1937) preferred to amalgamate Oxyclaenidae and Arctocyonidae, the latter taking precedence as the name for the whole group. McKenna & Bell (1997), however, placed Arctocyonidae within Procreodi once more, and divided the family into three main subgroups – the Arctocyoninae, Loxolophinae, and Oxyclaeninae. Some debate has concerned whether the particularly primitive but ungulate‐grade organism Protungulatum, excluded from Arctocyonidae by Prothero, Manning & Fischer (1988), should be included within the family.
(iii). Ecology
The species Arctocyon primaevus was originally described as being aquatic (de Blainville, 1841), and has been variously hypothesised as being terrestrial (Russell, 1964), fossorial (Kondrashov, 2009), and scansorial–arboreal (Argot, 2013) by subsequent authors. More consistent in interpretation, Chriacus is considered to be arboreal (Rose, 1987) on the basis of features of its tarsal bones, among others. The climbing of Chriacus is largely inferred from various skeletal features of an almost complete postcranial specimen, which includes all but portions of the femur and a number of vertebrae (Rose, 1987). The shape of the acromion process in Chriacus, combined with the extensive deltopectoral ridge of the humerus is indicative of powerful forelimb musculature, and the presence of large forefeet with curved claws suggests either climbing or burrowing ability. Comparisons may be made between the humerus of Chriacus and that of various arboreal carnivorans such as the coati, civets and red pandas (Rose, 1987). There is a great diversity of locomotor styles within Procreodi/Arctocyonidae; it has been suggested that some members of the group – Arctocyon mumak and Anacodon – might have been semifossorial, based on morphological features of the astragalus (Gould & Rose, 2014). In general, the arctocyonids possess teeth adapted for some degree of carnivory, even if it is not as extensive as that displayed by the creodonts and true carnivorans, lacking as they do any specialised shearing carnassials. Large canines are often present, and the premolars are relatively sharp, but the molars are bunodont, suggesting a largely omnivorous dietary niche.
(b). Periptychidae
(i). Composition
Periptychidae is composed of approximately 15 genera, divided among the subfamilies Periptychinae, Anisonchinae, and Conacodontinae (Archibald, Schoch & Rigby, 1983), the latter of which comprises Conacodon and Oxyacodon. According to Archibald et al. (1983), the most ‘primitive’ periptychid is Mimatuta, although Van Valen (1978) recognised five lineages of periptychids and suggested that they all descended directly from Protungulatum, the Cretaceous–Palaeogene proto‐ungulate‐grade eutherian.
(ii). Proposed relationships
Periptychids are represented by several North American genera, such as Periptychus, Anisonchus, Ectoconus, and others, and, in the case of Periptychus, are among the earliest known crown‐placental mammals (Prothero, 1998; Lofgren et al., 2004). They first appear in the San Juan Basin as immigrants (Clemens, 2010; Wilson, 2014) approximately 500000 years after the end‐Cretaceous mass‐extinction event, and are, as a result, usually thought to be basal among ‘condylarths’ (Prothero, 1994).
(iii). Ecology
Periptychid condylarths are characterised by highly bunodont, square molars which are of roughly equal size along the tooth row. As they are considered to be both temporally early and phylogenetically basal, they show the first adaptations to a herbivorous lifestyle.
(c). Hyopsodontidae
(i). Composition
Depending on interpretation, Hyopsodontidae may be treated as synonymous with Mioclaenidae (Williamson & Weil, 2011). Some authors prefer Mioclaenidae to be an entirely separate group, an interpretation backed up with some cladistic evidence (Ladevèze, Missiaen & Smith, 2010). In that analysis, both groups were represented by only a single genus, and the sample size of the entire analysis was not sufficient to draw an informed judgement on the relative positions of all ‘condylarth’ lineages. In total, there are 15 genera of hyopsodontid. The type genus, Hyopsodus is primarily Eocene, and is found across all Laurasian land masses.
(ii). Proposed relationships
Hyopsodontids are typical of the archaic ungulate families in that they have been considered ancestral to many different groups of ungulate‐grade mammal. Traditionally, they were considered to be early ancestors of artiodactyls (Simpson, 1937; Schaeffer, 1947), but later hypotheses placed them with either afrotheres – whether hyracoids (Godinot, Smith & Smith, 1996) or macroscelideans (Tabuce et al., 2001) – or with the enigmatic South American ungulates (Cifelli, 1983; de Muizon & Cifelli, 2000). The characters that have linked hyopsodontids to this large variety of clades are mostly dental, although in the case of the macroscelidean relationships this depends on the assumption that apheliscid ‘condylarths’ fall within Hyopsodontidae, and is supported primarily by the morphology of the tarsals. Since both teeth and tarsals have morphologies that are highly tied to their ecology (diet and locomotor ability, respectively), the conflicting evidence suggests that hyopsodontids are convergent in one or both of these regions. This is problematic when there is little in the way of postcranial remains of hyopsodontid ‘condylarths’.
(iii). Ecology
Hyopsodontid condylarths are one of the most widespread groups of archaic ungulate. They are found from the Middle Paleocene, with the earliest representatives found across North and South America. Eocene representatives are known from Europe and Asia, and they are a remarkably cosmopolitan group until they disappear during the Eocene (Hooker & Dashzeveg, 2003).
Analysis of the braincase of Hyopsodus lepidus has indicated that it possessed strong abilities to detect the positions of acoustic stimuli accurately, due to an enlarged inferior colliculus. This has been interpreted as implying an ability to echolocate in a similar way to some burrowing and nocturnal shrews and tenrecs (Orliac, Argot & Gilissen, 2012a ). However, because the postcranium of Hyopsodus is not strongly adapted for digging, and as the genus is more usually reconstructed as a scansorial herbivore (Williamson & Lucas, 1992), the more likely interpretation is of at least a nocturnal habit, although some digging ability – as well as the possibility of living in vacated burrows – cannot be discounted.
(d). Mioclaenidae
(i). Composition
Those that subscribe to the viewpoint that mioclaenids are a separate family place all 10 mioclaenid genera in the subfamily Mioclaeninae (e.g. Zack et al., 2005b ). Regardless of phylogenetic topology, mioclaenids are considered by all to be monophyletic.
(ii). Proposed relationships
Mioclaenidae are a little known group of archaic ungulates considered by some to be a subgroup or synonym of Hyopsodontidae (Williamson & Weil, 2011). Originally erected as a monospecific family (Osborn & Earle, 1895), it includes several taxa from across North America and Europe, as well as a few in South America.
(iii). Ecology
The most recent summary of the characters which define Mioclaenidae was a reanalysis of two genera – Bomburia and Ellipsodon – by Williamson & Carr (2007), where the family was rediagnosed as 'Ungulate with P4 metacone absent, upper molar postcingulum continuous with metastyle, lower molar metaconid nearly lingual to protoconid, ratio of m3 length/m2 length between 0.9 and 1.1'. Lack of a metacone aside, these traits are relatively primitive for a large number of groups of ‘archaic ungulates’ (see Prothero et al., 1988). Mioclaenids also have relatively enlarged premolars, similar to periptychids, which are thought to indicate a diet consisting of tough vegetation (Rose, 2006).
(e). Pleuraspidotheriidae
(i). Composition
Pleuraspidotheriidae is a small group sometimes placed within Meniscotheriidae, Hyopsodontidae or Phenacodontidae, and recently affined to the early arctocyonids. It is exclusively found in Europe, mainly in northern France and Belgium, and consists of three genera – Pleuraspidotherium, Orthaspidotherium and the enigmatic Turkish fossil Hilalia.
(ii). Proposed relationships
Ladevèze et al. (2010) hypothesised that Pleuraspidotheriidae are closest relatives to arctocyonids such as Chriacus, in a group also including the basal artiodactyls, but their taxonomic sampling was very low, and only very few representatives of each supposed group were present. Since the majority of the ‘condylarth’ material has been collected from North America, or are parts of families that are present in North America with representatives elsewhere, the phylogenetic position of a clade from another continent is of interest when considering biogeographic questions regarding the origin of modern orders, and little has been proposed concerning the relationships of this family.
(iii). Ecology
The basicranial morphology of pleuraspidotheres is similar to that of the early artiodactyls such as Gobiohyus, their teeth resemble the previously mentioned ‘condylarth’ groups, and their tarsal morphology is basal in appearance, with little in the way of unambiguous synapomorphies. Preservation of the two better‐known genera is very good, with an almost complete skeleton known for Pleuraspidotherium, and a complete skull with assorted postcranial material known for Orthaspidotherium (Ladevèze et al., 2010). The pseudohypocone that characterised the square molars of the Pleuraspidotheriidae mark the difference between this morphology and the superficially similar molars of perissodactyls (Ladevèze et al., 2010).
(f). Phenacodontidae
(i). Composition
Phenacodontidae is composed of 14 North American genera, the best known and most complete of which are the phenacodontine phenacodontids Phenacodus, Tetraclaenodon and Copecion (Osborn, 1898; Thewissen, 1990; Kondrashov & Lucas, 2012). Meniscotheriidae, once considered separate, is now generally included within Phenacodontidae (Rose, 2006) as the subfamily Meniscotheriinae (Simpson, 1937), and includes Ectocion and Meniscotherium.
(ii). Proposed relationships
Like the apheliscid ‘condylarths’, phenacodontids have been suggested to be closely related to afrotherian and laurasiatherian orders. In particular, Phenacodontidae was resolved by Tabuce et al. (2001) as being part of a clade comprising Paenungulata, Phenacodontidae and Perissodactyla, while Kondrashov & Lucas (2012) found phenacodontids to be paraphyletic to Perissodactyla, Paenungulata, and Hyracoidea. While Perissodactyla is certainly not closely related to Paenungulata, being consistently resolved as being in a totally different superorder by molecular methods (Springer et al., 2004), Phenacodontidae represent the phenotype that was previously thought to link the two groups ancestrally. It is not clear whether support for the affinity was driven largely by one order or another – both Paenungulata (e.g. Rose et al., 2014) and Perissodactyla (e.g. Kondrashov & Lucas, 2012) have been considered the closest living relatives of phenacodontids in recent years, whether phenacodontids are considered paraphyletic or monophyletic. Indeed, Phenacodus and Meniscotherium have also been found to be close relatives of Artiodactyla (Wible et al., 2007).
(iii). Ecology
Phenacodontids are superficially similar to the modern groups of ungulate mammals, with a herbivorous diet, and generalised, often slightly cursorial limbs, especially in more derived forms (Thewissen, 1990). Upper molars are bunodont and square, with the presence of a hypocone being relatively derived. Lower molars, however, are reduced in the number of cusps, with the paraconid having been lost. The forelimb of Tetraclaenodon has been described as having features associated with both terrestriality and climbing, although these attributes are weakly developed, and it has been suggested that Tetraclaenodon behaved in such a way that it was facultatively terrestrial, but able to scale trees for food or safety (Kondrashov & Lucas, 2012). This hypothesis is borne out by the morphology of the hind limb, which is far more specialised for terrestriality, although not cursoriality (Kondrashov & Lucas, 2012). The third trochanter on the femur is a cursorial adaptation, as is the weakening of the deltopectoral crest of the humerus.
(g). South American native ungulates (SANUs)
(i). Composition
The placental fauna of South America, with the exception of the native xenarthrans and later invasions of African and North American groups, include three to five orders of ‘ungulate’ (McKenna, 1975). These orders – Xenungulata, Notoungulata, Litopterna, and, if they are considered separate, Pyrotheria and Astrapotheria – are highly enigmatic with respect to their relationships with extant placental orders. They first appear in South America during the Paleocene (de Muizon & Cifelli, 2000), surviving into the Late Pleistocene (MacFadden & Shockey, 1997). Across all five orders, there are well over 200 named genera, many of which are known from multiple species (McKenna & Bell, 1997).
(ii). Proposed relationships
Although some hypotheses have suggested that SANUs are more closely related to Afrotheria (Agnolin & Chimento, 2011), or descended from ‘condylarths’ (de Muizon & Cifelli, 2000), recent analysis of protein sequences from subfossil material (Buckley, 2015; Welker et al., 2015) has indicated that the closest extant relatives of both Notoungulata (represented by Toxodon) and Litopterna (represented by Macrauchaenia) are stem Perissodactyla, a result which is consistent with a recent morphological analysis incorporating Notoungulata (Beck & Lee, 2014), which returned close relationships between notoungulates and perissodactyls. As morphological analyses have been inconsistent in terms of the relationships of these unusual taxa, this particular topology raises many biogeographical questions. Cladistic analysis of the morphology of SANU postcrania has supported a relationship between a notoungulate–litoptern clade and phenacodontids (Horovitz, 2004), with astrapotheres most closely related to periptychids. Relationships among orders of SANU are unclear, with Billet (2010) finding a notoungulate–astrapothere clade and non‐monophyletic Litopterna.
(iii). Ecology
Ecologically, the SANUs are remarkably diverse, with analogues of several artiodactyl and perissodactyl clades, most clearly emphasised in the similarity between litopterns, artiodactyl camelids, and perissodactyl equids (Bond et al., 2006). It is their especially derived morphology and geographical isolation that presents problems when determining their closest relatives, despite a relatively good fossil record from the Late Paleocene onwards.
(2). Other placental non‐ungulate clades
In addition to the condylarths, there are several other controversial and enigmatic mammal groups represented by Paleocene fossils. These include two groups, Leptictida and Cimolestidae, variously considered to be stem to the placental lineage, or ancestral to an extant order or group of orders (Lopatin, 2006; Wible et al., 2007). Both show relatively basal general morphology, but also bear specialisations that have driven hypotheses of relationships to extant clades.
(a). Leptictida
(i). Composition
Leptictida was first identified as a superorder by McKenna (1975), in which it was proposed to be a clade of crown‐group placental mammals with unclear affinities, whose closest relatives were the Kennalestidae. Novacek (1986) provided an extensive and comprehensive morphological characterisation of the group, reduced the rank to ordinal status, and proposed a position within Insectivora. In McKenna & Bell (1997), Leptictida was a diverse assemblage of taxa including several additional Cretaceous mammals such as Zhelestes, Gypsonictops, Lainodon and Gallolestes, and also Kennalestidae. The traditional leptictid forms such as Prodiacodon and Leptictis were also included in Leptictida, as well as the European Pseudorhynchocyonidae.
In light of further analysis, the taxonomic composition of Leptictida has been revised as a more restricted group. Archibald, Averianov & Ekdale (2001) provided evidence that Gypsonictops was part of a separate clade from Zhelestes and its kin, implying that Leptictida was at least paraphyletic. In that study, both clades were resolved within the placental crown, with Gypsonictops closer to Glires, and Zhelestes to Ungulata (represented in this case solely by Protungulatum, whose placement as a crown ungulate is questionable). Kielan‐Jaworowska, Cifelli & Luo (2004) maintained the presence of Gypsonictopidae in Leptictida, but considered that Leptictida was, as had originally been suggested, within Insectivora, as a sister group to Lipotyphla. The composition of Gypsonictopidae was also reduced by the removal of Zhelestes, as well as other forms previously allied to Leptictida such as Lainodon. The newly constructed Zhelestidae was allied with Ungulatomorpha, well within the crown of placental mammals.
An extensive study of Cretaceous mammalian affinities by Wible et al. (2007) further modified the position of Leptictida. Here, rather than being crown‐group placental mammals, Leptictis and Gypsonictops were placed at the crownward end of the placental stem, more derived than Zalambdalestidae but less than Protungulatum. Meehan & Martin (2010), however, favoured inclusion of Leptictida in the abandoned grouping ‘Insectivora’. They noted that the morphology of leptictidans was highly convergent to that of extant macroscelideans, due to similar ecological specialisations to insectivory, digging, and saltatory locomotion.
Recently, the European Eocene–Miocene Pseudorhynchocyonidae – a subgroup of Leptictida sensu McKenna & Bell (1997) – has been separated from Leptictida entirely, instead placed closer to Palaeanodonta and Pantodonta (Hooker, 2013). However, an unrooted version of the tree from this analysis is entirely consistent with a monophyletic Leptictida to the exclusion of Palaeanodonta and Pantodonta. The order Leptictida currently consists of three families: Gypsonictopidae, a monogeneric family containing only Gypsonictops; Leptictidae, consisting of several North American genera; and Pseudorhynchocyonidae. In total, the clade contains 16 genera. Although there is largely a consensus on the taxonomic composition of Leptictida, the precise position of their relationships to extant orders of mammals remains under question, and they occupy a crucial position in the temporal story of eutherian mammal evolution.
(ii). Proposed relationships
The leptictids are one of the few orders of mammals definitively to cross the K/Pg boundary. A few representatives from the Cretaceous, such as Gypsonictops, hint at an early branching from the placental mammal tree, although some analyses have preferred to place them within the crown (Kielan‐Jaworowska et al., 2004; Meehan & Martin, 2010). Leptictida, therefore, are one of the key groups for understanding the timescale of placental evolution. Their presence on both sides of the K/Pg boundary means that, were they to fall within the placental radiation, it would provide conclusive proof of the early (pre K/Pg) origin of placental mammals.
The initial discovery of Leptictis haydeni was in Dakota, identified along with Ictops dakotensis (now known as Leptictis dakotensis) as two genera of ‘insectivorous mammals, which appear to be peculiar, but related to the hedge‐hogs’ (Leidy, 1868, p. 315). Leidy placed them within the order Insectivora, and they were first identified as a separate family with the name Leptictidae by Gill (1872).
(iii). Ecology
Leptictida are a specialised Laurasian group, occurring throughout northern North America from the Cretaceous to the Oligocene, with some examples – the Mongolian Praolestes (Matthew, Granger & Simpson, 1929), European pseudorhynchocyonids such as Pseudorhynchocyon (Filhol, 1892), and a Spanish specimen of Leptictis (Crusafont‐Pairo & Golpe Posse, 1975) – from the Eocene of Europe and Paleocene of Asia. They are characterised by their long hind limbs, superficially resembling jerboas and sengis, although this is probably an entirely convergent adaptation to a similar ecological niche of a hopping insectivore–omnivore (Rose, 1999b ).
(b). Cimolestidae
(i). Composition
There are 13 genera within Cimolestidae, seven of which are monospecific. Five species of Cimolestes (C. magnus, C. cerberoides, C. incisus, C. stirtoni, and C. propalaeoryctes), as well as Batodon tenuis and Maelestes gobiensis are found in the Cretaceous – the former two in North America, and the latter in Mongolia, spanning the Judithian and Lancian North American faunal stages (83.3 to 65.5 Ma). Of these, Cimolestes is unusual in that it is a genus spanning the K/Pg boundary, and is found in the Puercan of North America, equivalent‐aged rocks in Bolivia, and the Thanetian of Morocco. Nonetheless, it must be pointed out that the monophyly of Cimolestes has at times been questioned. Paleocene and Cretaceous forms of Cimolestes may not be the same genus, with some concluding that Carnivora and Creodonta were independently derived from Cimolestes (Lillegraven, 1969; McKenna, 1975).
(ii). Proposed relationships
The cimolestids are a second group whose phylogenetic placement should inform strongly on the date of origin of the major clades of placental mammals. They too are hypothesised to occupy a variety of phylogenetic positions, as well as crossing the K/Pg boundary. It is disputed whether the Pantodonta are part of this clade (see differences between McKenna & Bell, 1997; Wible et al., 2007), but even excluding the pantodonts, the cimolestids are a highly diverse and probably monophyletic lineage (Archibald, 2011).
The Cretaceous cimolestids include only the three genera mentioned above. By the earliest Paleocene, the group had diversified to include the South American Alcidedorbignya, and the Laurasian Puercolestes. Some also include the Paleocene taxon Procerberus in the cimolestid lineage (Williamson, Weil & Standhardt, 2011), although Procerberus has also been considered to be a very basal eutherian (Kielan‐Jaworowska, Bown & Lillegraven, 1979), and still others favour a relationship with Leptictida (Sloan & Van Valen, 1965).
Considering Procerberus as a stem eutherian mammal need not necessarily remove Procerberus from the cimolestids, however, because evidence has suggested that cimolestids might be a group of stem placental mammals as well, although others have likened them to the hypothesised ancestors of modern carnivorans and creodonts (Hunt & Tedford, 1993). Given that Carnivora is a group nested well within crown Eutheria, the placement of Cimolestidae is one which impinges strongly on the timescale of placental diversification. If Cimolestidae are indeed closer to Carnivora than to many other Laurasiatherian groups, this would demonstrate that the diversification of the placental mammal lineages occurred at least before the earliest cimolestid material, which is from the Middle Campanian Foremost Formation (approximately 80 Ma), probably significantly earlier. If, however, Cimolestidae are shown to be basal to crown Eutheria, along with the other clades that originate in the Cretaceous, it would be strongly suggestive of a Paleocene diversification event within placental mammals.
Anatomical features consistent with a basal position include the presence of an unusual morphological trait – the prootic canal – found only in Asioryctitheria, Zhelestidae and Cimolestidae among eutherians (Archibald et al., 2001; Ekdale, Archibald & Averianov, 2004). Because both Asioryctitheria are uncontroversially Cretaceous stem placental mammals, it is not unreasonable to suppose that Cimolestidae are also close to the base of placental mammals. Within Placentalia, only Solenodon possesses a prootic canal, which appears to be a result of convergence (Wible et al., 2009).
(iii). Ecology
Cimolestids have in general been considered to have incipiently carnassial teeth (Rana & Wilson, 2003), and as such have been inferred to be faunivorous, if not carnivorous. Indeed, it is the dental similarities that have led to the attribution of this group to the stem of Carnivora. The presence of steep shearing wear marks on the molars of cimolestids (Butler, 1972) illustrates that their teeth were capable of slicing actions, and thus adapted for this diet, but this would be convergent with Carnivora if they are resolved as members of the placental stem.
Cimolestids are relatively primitive in their postcrania, and, like the majority of Cretaceous mammals, their ankle bones suggest a scansorial habit (Szalay & Decker, 1974).
(c). Pantodonta
(i). Composition
Pantodonta is composed of several families, the most diverse of which is the Coryphodontidae, which includes 18 species from seven genera. Although the relationships among these families are not well established, there is evidence for the existence of a grouping of exclusively North American pantodonts, the Pantolambdoidea, which includes Cyriacotheriidae, Pantolambdodontidae, Pastoralodontidae, and Titanoideidae (Simons, 1960). In total, Pantodonta includes approximately 35 genera (McKenna & Bell, 1997).
(ii). Proposed relationships
The pantodonts, for the purposes of this introduction, are considered separately from the Cimolestidae, although they are regarded as a suborder in McKenna & Bell (1997). Superficially, pantodonts are distinct from the majority of the rest of the supposed cimolestids, being large, ground‐dwelling and herbivorous, as opposed to small, scansorial, and carnivorous or insectivorous. Additionally, this classification is a departure from the more traditional interpretations of pantodonts being related to either an assortment of unusual South American ungulates or Paenungulata – the Afrotherian lineage including proboscideans, sirenians and hyracoids. Pantodonts appeared in the Early Paleocene, with a largely global distribution, and survive to the Middle to Late Eocene, whereupon they became extinct approximately 33 Ma.
(iii). Ecology
Pantodonts include some of the largest terrestrial mammals of the period – the coryphodontids – enormous rhinoceros‐like herbivores which lived from the Arctic to the southern edge of North America (Dawson, 2012), as well as in the Palaeogene of eastern Asia (Ting et al., 2003). They are extremely abundant components of North American Eocene faunas, being common enough to be a stratigraphic indicator for several North American Land Mammal Ages (NALMAs) (Robinson et al., 2004), but have smaller representation throughout the Paleocene.
(d). Creodonta
(i). Composition
The status of the 16 genera of Creodonta has long been considered controversial, and the precise composition of the group has changed radically across the history of the literature. Whether the two major groups within Creodonta – Oxyaenidae and Hyaenodontidae – are sister taxa to one another (in other words, whether Creodonta can be considered monophyletic) is not clear (Morlo, Gunnell & Polly, 2009; Zack, 2011). Indeed, their affinity with Carnivora has been suggested to be an artefact of convergent evolution – the superficially similarly shaped carnassial teeth are, developmentally, different teeth (Van Valkenburgh, 1999), suggesting that Carnivora could not have evolved directly from a creodont without significant developmental repatterning.
(ii). Proposed relationships
Originally described as a group within ‘Insectivora’ (Cope, 1884c ), Creodonta has been through several iterations, including being related to mesonychians, arctocyonids, carnivorans, palaeoryctids, and even briefly being abandoned as a group completely (for a summary, see Gunnell & Gingerich, 1991). While there has been considerable confusion over what defines a creodont (Polly, 1994; Morlo et al., 2009), the consensus today is that Creodonta is likely a close relative of, although not ancestral to, Carnivora.
(iii). Ecology
Creodonts were arguably the most specialised placental carnivores, with some members of the group achieving a hypercarnivorous state and modifying their entire molar row to carnassials (Stucky & Hardy, 2007). Members of Creodonta can be considered analogues of several carnivoran clades, with examples of dog‐like, civet‐like, and cat‐like forms (Van Valkenburgh, 1999). The ecological niches exploited by Creodonta are similar enough to Carnivora that hypotheses of competitive exclusion have been invoked to explain the eventual replacement of the former by the latter (Wesley‐Hunt, 2005).
(e). Mesonychia
(i). Composition
Mesonychia was traditionally composed of the Asian family Didymoconidae, the semiaquatic Hapalodectidae, and the most speciose member of the clade, Mesonychidae, which includes 19 of the 29 mesonychian genera (Carroll, 1988). Didymoconidae has subsequently been removed from the clade (Meng, Suyin & Schiebout, 1995; Lopatin, 2001), but both hapalodectids and mesonychids remain, with hapalodectids considered more derived than mesonychids. The best known of the mesonychians, Sinonyx, Mesonyx and Dissacus, are all mesonychid mesonychians. The giant mammal Andrewsarchus has historically been considered to be a mesonychian or a close relative (Osborn, 1924; Tabuce, Clavel & Antunes, 2011), but competing hypotheses have suggested that Andrewsarchus may in fact be a cetancodontomorph artiodactyl (Spaulding, O'Leary & Gatesy, 2009) or an arctocyonid ‘condylarth’ (Van Valen, 1978).
(ii). Proposed relationships
Mesonychians are an enigmatic group of ‘archaic ungulate’, often considered separate from the ‘condylarths’. Mesonychians were considered, on the basis of shared simplification of the dentition, to be related to whales, but this hypothesis was overturned by the discovery of early whale postcrania, particularly the double pulley astragalus that cemented the position of Cetacea within Artiodactyla, separate from mesonychians (Gingerich et al., 2001; Thewissen et al., 2001). The precise position of the mesonychids with respect to extant clades has been unclear; they have been thought of as stem artiodactyls (Theodor & Foss, 2005) or stem to the clade comprising Artiodactyla and Perissodactyla (Spaulding et al., 2009). Conservatively, they have been grouped with triisodontids and oxyclaenids in the basal placental group Acreodi, and sister to the arctocyonid ‘condylarths’ (Tabuce et al., 2011).
(iii). Ecology
Apart from a few mesonychians such as Hapalodectes, which lack specialised running features of the humerus (O'Leary, 1998), mesonychians have been described as having an ecological niche similar to wolves – that of a cursorially adapted predator (O'Leary & Rose, 1995).
(3). Paleocene representatives of extant placental clades
(a). Afrotheria (elephants, hyraxes, dugongs, aardvarks, tenrecs, sengis, golden moles)
The earliest afrotherians known from the fossil record are found in the Middle Paleocene. The species Ocepeia daouiensis is known from the Selandian (61.6 to 59.2 Ma) of Morocco, and possesses a mosaic of characters suggesting that it is close to the divergence of Paenungulata and Afroinsectiphilia (Gheerbrant et al., 2014). Its presence in Africa during this time suggests that Afrotheria arose, or at least initially diverged, in Africa, in contrast to some hypotheses which have suggested that at least some afrotherian groups arose in North America (Zack et al., 2005a ).
(b). Xenarthra (sloths, armadillos, anteaters)
The location and phylogenetic affinities of the earliest xenarthran is controversial. The earliest member of the crown group that is not disputed is the already highly derived Late Paleocene or Early Eocene armadillo Utaetus, which is found from the Casamayoran of Argentina (Ameghino, 1902; Rose, 2006). More controversial is the inclusion of the Asian Paleocene genus Ernanodon (Ding, 1987), supposedly part of the suborder Ernanodonta (McKenna & Bell, 1997). This controversy is in part because it occurs on a different continent from other xenarthrans, with the exception of the only other putative xenarthran from Guangdong, Asiabradypus. This taxon, however, was considered by Rose et al. (2005) to be an animal of unknown affinity, ‘irrelevant to xenarthran origins’. Xenarthrans have historically been grouped together on the basis of simplistic characters such as a lack of teeth. There are few examples of positive characters, with the exception of the additional articulations of the vertebrae which characterise them as xenarthrous, and a relatively highly variable vertebral number (Asher et al., 2011). No clear affinity with typical xenarthran characters has been definitively shown for Ernanodon, and Gaudin (1999, p. 30) suggested that the articulations of the vertebrae of Ernanodon ‘only vaguely resembles that characteristic of most true xenarthrans’.
(c). Euarchontoglires (rodents, rabbits, pikas, primates, tree shrews, flying lemurs)
The fossil record of Euarchontoglires in the Paleocene is limited to rodents and primates. The earliest lagomorph (rabbits, hares, pikas) and scandentian (tree shrews) fossils are known from the early to mid Eocene of China and Mongolia (Yongsheng, 1988; Lopatin & Averianov, 2008), while the earliest dermopteran (flying lemur) is known from the Late Eocene of Thailand (Rage et al., 1992).
(i). Rodentia (mice, squirrels, porcupines, guinea pigs, beavers, voles)
The earliest definitive rodents are known from the Paleocene, with Tribosphenomys a close outgroup to Rodentia (Meng & Wyss, 2001) and Paramys. Both are known entirely from tooth fragments, but contain crucial synapomorphies that allow identification to their respective positions. Both have the definitive rodent pattern of a single pair of continuously growing incisors with enamel only on the anterior edge.
(ii). Primates (apes, monkeys, lemurs, lorises, tarsiers)
While there are no definitive crown primates in the Paleocene, there is strong evidence of the presence of plesiadapiforms. These taxa are considered by most to be ancestral to primates, and are arboreal specialists, consistent with the interpretation of many primate features as adaptations for an arboreal lifestyle – for example, grasping hands and a good depth of vision (Rose, 2006). Purgatorius is a putative plesiadapiform based on teeth (Clemens, 2004), and recently, tarsal material (Chester et al., 2015). However, its relationships to modern forms has been controversial, with some analyses reconstructing Purgatorius outside of Placentalia (Wible et al., 2009). If the Cretaceous Indian genus Deccanolestes is, as some have suggested, closely related to purported euarchontans, such as nyctitheres (Hooker, 2001, 2014) or adapisoriculids (Smith, De Bast & Sige, 2010), then Deccanolestes would represent a Cretaceous occurrence of a euarchontan. However, while Deccanolestes has been shown to be more closely related to adapisoriculids (Goswami et al., 2011), neither group fell within Placentalia. Nyctitheres, by contrast, appear to be more closely related to eulipotyphlans (Manz et al., 2015).
(d). Laurasiatheria
(i). Carnivora (cats, dogs, bears, otters, badgers, mongooses, hyaenas)
The earliest stem carnivorans are the genera Ravenictis and Pristinictis, which are from the earliest Paleocene (Fox & Youzwyshyn, 1994). These earliest forms have relatively unspecialised molars, suggesting a generalised omnivorous diet with only limited specialisation to true carnivory, although Pristinictis has been considered a primitive member of Viverravidae. Miacidae and Viverravidae, both relatively derived carnivorans, are both known from the Late Paleocene (Meehan & Wilson, 2002; Sole & Smith, 2013).
Diversification into the major two groups of extant carnivorans – caniforms and feliforms – occurred in the Eocene, but the precise position is dependent on the phylogenetic placement of some enigmatic members of the miacid carnivorans (Tomiya, 2011).
(ii). Pholidota (pangolins)
Pholidotans are known from the middle Eocene of Europe, being represented by the two genera Eomanis and Eurotamandua, both from the Messel Pits of Germany (Storch, 1978; Rose et al., 2005). Already relatively derived, a relationship with the Paleocene palaeanodonts has been proposed (Rose, 1999a ; Gaudin, Emry & Wible, 2009).
(iii). Eulipotyphla (shrews, hedgehogs, moles)
Eulipotyphla include much of what once was ‘Insectivora’, the basalmost wastebasket taxon of placental mammals from which all others were supposed to have derived (McKenna, 1975). Now recognised as a derived group within Laurasiatheria, if morphologically plesiomorphic, the split between Eulipotyphla and Scrotifera is generally considered to be the basalmost division within Laurasiatheria (Waddell et al., 1999; Nishihara, Hasegawa & Okada, 2006; Zhou et al., 2012), although some earlier molecular analyses support a sister relationship between Eulipotyphla and Chiroptera (Onuma et al., 2000). Combined morphological and molecular analyses consistently have been able to distinguish the ‘true’ insectivores – which comprise moles, shrews, hedgehogs and kin – from the African insectivores – elephant shrews and tenrecs, now known to be members of Afrotheria (Stanhope et al., 1998; Tabuce, Asher & Lehmann, 2008).
If nyctitheres are eulipotyphlans (Manz et al., 2015), the earliest eulipotyphlans in the fossil record are the earliest Paleocene nyctitheres such as Leptacodon (Van Valen & Sloan, 1965), with putative but controversial members of the group in the latest Cretaceous (Antunes, Sigogneau‐Russell & Russell, 1986). Other than nyctitheres, the first eulipotyphlans known from the fossil record are from the Late Paleocene, by which time some division into the erinaceids and soricids had taken place (Rose, 1981).
(iv). Chiroptera (bats)
The first chiropteran fossils are of already relatively derived bats from the Green River Formation of the Early Eocene of Wyoming – Onychonycteris finneyi (Simmons et al., 2008) and Icaronycteris index (Jepsen, 1966). Morphologically, they were capable of true flight, but unable to echolocate (Simmons et al., 2008). Other dissimilarities with modern bats include a relatively large tail, and, in the case of Onychonycteris, the presence of claws on all forelimb digits. No earlier fossil material is attributable to either the crown or stem of bats, making their origins difficult to determine.
(v). Perissodactyla (horses, rhinoceroses, tapirs)
Of the five main clades of Perissodactyla – Equidae (horses), Tapiridae (tapirs), Rhinocerotidae (rhinoceroses), Brontotheriidae, and Chalicotheriidae – all are known in the earliest Eocene with superficially similar, small, browsing forest‐dwelling forms (Eberle, Rybczynski & Greenwood, 2014). The earliest equid, Hyracotherium, underwent a dramatic taxonomic revision in 2002 (Froehlich, 2002), with the separation of the genus into many new (and resurrected) genera. Tapirs and rhinoceroses, which are monophyletic to the exclusion of equids (Froehlich, 1999), are represented in the earliest Eocene by Heptodon (Radinsky, 1965) and Hyrachyus, respectively. Heptodon is known primarily from North America, where the majority of perissodactyl evolution occurred, although there are reports of the genus from China (Chow & Li, 1965). Hyrachyus is known from Europe and Asia, but has also been reported from the Caribbean (Domning et al., 1997). Along with these crown members of the perissodactyl families are early members linking the lineages, such as Mesolambdolophus setoni, which appears to be close to the base of the tapiromorphs (Holbrook & Lapergola, 2011).
(vi). Artiodactyla (cattle, deer, giraffes, camels, pigs, hippopotamuses, whales)
Artiodactyla is another extant order whose first members appear at the base of the Eocene (Rose, 1996), with the basal group Dichobunidae, a speciose northern hemisphere group whose best‐known member is the genus Diacodexis. Represented by near‐complete specimens (Rose, 1982b ; Orliac, Benoit & O'Leary, 2012b ), Diacodexis is known from layers immediately above the Paleocene–Eocene boundary (Smith, Smith & Sudre, 1996). It was a cursorial animal capable of high speeds and agile turns, as evidenced by the morphology of the semicircular canals (Orliac et al., 2012b ) and postcranium (Rose, 1982b ).
IV. NEW PHYLOGENETIC ANALYSIS OF CRETACEOUS AND PALAEOGENE PLACENTAL MAMMALS
(1). Materials
(a). Taxonomic sample
A broad sample of 177 eutherian taxa was selected in order to evaluate robustly hypotheses of relationships across Placentalia. For both extant orders and extinct groups of unknown affinity, taxa were selected based on several criteria, with particular preference for the most basal members of each lineage. For groups with a limited fossil record, such as all xenarthran groups, dermopterans, and scandentians, and groups where the early relationships and character polarities are not clear, such as in Eulipotyphla, extant taxa were used to supplement fossil material. The reason for preferring fossil taxa over extant forms is that extensive evolutionary change has inevitably occurred within each clade over the last 66 million years. By taking the basalmost and/or earliest members of an order, the chances that key synapomorphies of that group have been obscured through convergence or reversal are far lower.
Terminals were coded at genus rather than species level, to increase character completeness for fossil taxa. Certain genera which have been considered both as separate and synonymous (for example, Arctocyon and Claenodon, and Hyracotherium and Eohippus), are treated separately to minimise the potential issue of including poorly supported genera. Cimolestes has been suspected to be polyphyletic – Scott (2010, p. 197) states ‘evidence for the monophyly of (Cimolesta) is weak, as is evidence for monophyly of Cimolestes Marsh, 1889 itself’ – but is here treated as a single terminal. For the purposes of this study, and lacking any conclusive evidence as regards the monophyly or otherwise of Cimolestes, all species assigned to this genus have been considered to represent Cimolestes, and are coded into the same terminal to maximise completeness of this important taxon. Completeness and quality of fossil material was also taken into account in taxonomic sampling, with preference for taxa with a higher proportion of codable characters. With the exception of the problematic South American meridiungulate groups of Notoungulata and Litopterna, each group was represented by multiple taxa, to avoid apomorphies being taken as plesiomorphic for a higher clade. In total, 904 specimens and casts were examined in international museum and university collections, supplemented by character data from the published literature, including character state data matrices, scans, and photographs (see online Supporting information, Appendix S1). In total, 177 genera were coded, comprising 130 Palaeogene, 29 Cretaceous, and 18 extant taxa.
(b). Characters
In total, 680 morphological characters – 48 of which are continuous – were coded for the 177 taxa, resulting in two matrices, one traditionally discrete (Appendix S2), and one with continuous characters treated as such (Appendix S3).
Characters were derived from four major sources – the PhD thesis of Zack (2009), which studied postcranial and dental morphology of largely Paleocene mammals, but excluded cranial characters from the supplied data matrix and included several terminals that were composites of multiple genera; a matrix from Williamson et al. (2011) focusing on the Cretaceous–Palaeogene group Cimolestidae, which ultimately descends from the Wible matrix for Cretaceous eutherians (Wible et al., 2007, 2009); a matrix used for establishing the relationships of the Palaeogene ‘ungulate’ mesonychians (Geisler & McKenna, 2007); and a matrix containing several ‘archaic ungulate’ characters, with particular focus on the enigmatic Pleuraspidotheriidae (Ladevèze et al., 2010). Characters were modified such that they were consistently applicable, easily interpreted, and divisions between character states were better and more consistently defined. This resulted in a final list of 680 morphological characters, consisting of 235 dental, 264 cranial, and 181 postcranial characters (Appendix S4).
(2). Methods
(a). Treatment of continuous characters
There is much debate over the benefits of using continuous traits in morphological phylogenetic analysis (Rae, 1998; Wiens, 2001; Goloboff, Mattoni & Quinteros, 2006). While more objective than the traditional division of character states in discretized continuous traits, issues arise when determining the relative weighting of a continuous trait. Here, we weight the continuous characters such that the difference between the maximum and minimum values for the trait is equivalent to a single step. Coded values for each terminal taxon were generated through measurement of multiple specimens where possible (Appendix S1) and calculation of the mean value of those measurements. This approach treats the character as effectively equivalent to a binary discrete trait, with the variation in between represented by decimal places within that range. As a result of this treatment of the characters, the steps that take place along the branches of the phylogeny are necessarily on average shorter than when the trait is discretized, which means that the trees are also concomitantly shorter. As a result, it is not possible directly to compare the accuracy of the topology by tree length alone when comparing data sets with and without continuous traits. However, to make sure that the difference in length was exclusively due to the alternate methods of coding particular characters, discrete, ordered, multistate characters were also weighted such that the entire range represented a single step. For binary characters, this requires no weighting, but a three‐state character would be weighted at 0.5 the value of a binary character, since it takes two changes to get from one endpoint to another. We modified the weights of continuous and discretized characters using TNT (Goloboff et al., 2006; Goloboff, Farris & Nixon, 2008).
(b). Constraints on tree topology
Placentalia is known to display a high level of morphological homoplasy, with adaptive radiations in different groups leading to occupation of similar niches (Madsen et al., 2001), with concomitant morphological similarities. As a result, relationships derived from solely morphological data have often been in conflict with those derived from molecular data, with homoplasy overriding phylogenetic signal at higher phylogenetic levels (Lee & Camens, 2009). As noted above, this issue of homoplasy has long been appreciated, with certain traditional placental groupings (ungulates and insectivores) identified as being particularly suspect (Asher et al., 2008). The most obvious failure of morphological phylogenetic analyses of Placentalia is the lack of support for the major placental ‘superorders’ – Afrotheria, Xenarthra, Euarchontoglires, and Laurasiatheria, each of which are very well supported in most molecular studies (Stanhope et al., 1998; Springer et al., 2003; Bininda‐Emonds et al., 2007; Prasad et al., 2008; dos Reis et al., 2012; Morgan et al., 2013). In order to incorporate the uncontentious aspects of topology for living placentals provided by molecular studies, we constrained the relationships among extant clades with a topological scaffold that is consistent with the vast majority of molecular analyses of placental mammals (Fig. 2). Using molecular constraints can help to correct for morphological homoplasy, and allow the truly synapomorphic morphological features to have a stronger effect.
Figure 2.
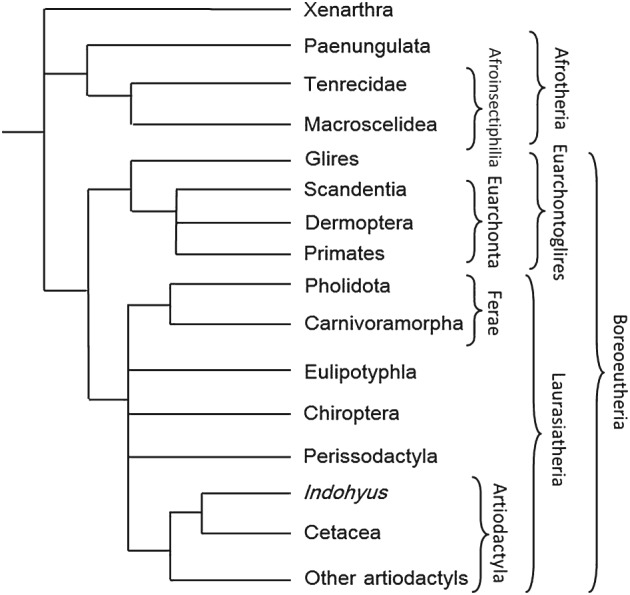
The constraint applied to all analyses, derived from the molecular understanding of the relationships of extant placental mammal groups. In CM and DM analyses, Xenarthra was composed of Chaetophractus, Bradypus and Tamandua; Paenungulata: Eritherium and Procavia; Tenrecidae: Potamogale; Macroscelidea: Chambius and Rhynchocyon. Glires was composed of Tribosphenomys, Paramys and Gomphos; Scandentia: Tupaia and Ptilocercus; Dermoptera: Cynocephalus; Primates: Saxonella , Cantius, and Adapis. Pholidota was composed of Eomanis and Eurotamandua, Carnivoramorpha by Miacis and Viverravus. Eulipotyphla was represented by Domnina, Oreotalpa, Blarina, Solenodon and Echinosorex. Chiroptera was represented by Pteropus, Perissodactyla by Eohippus and Hyracotherium, Cetacea by Rodhocetus, and other artiodactyls by Gobiohyus, Poebrotherium, Leptomeryx and Elomeryx. In the CF and DF analyses, additional taxa were, for Xenarthra, Utaetus; Dilambdogale was added in a polytomy with Macroscelidea and Tenrecidae within Afroinsectiphilia; for Glires, Rhombomylus; for Dermoptera Elpidophorus, Worlandia and Plagiomene; for Primates, Elphidotarsius, Plesiadapis and Notharctus; for Carnivoramorpha, Didymictis, Vulpavus, Protictis and Uintacyon; for Eulipotyphla, Litocherus, Uropsilus and Centetodon; for Chiroptera, Onychonycteris and Icaronycteris; for Perissodactyla, Heptodon, Homogalax, Litolophus and Lambdotherium; and for Cetacea, Pakicetus. In the CP and DP analyses, Purgatorius was further constrained within Primates.
Rather than constraining taxa, many phylogenetic analyses have used a total‐evidence approach to explore the relationships of groups, incorporating both molecular and morphological data. While this tactic would be possible for these fossil groups, the aim of this study was to elucidate the relationships among the placental mammals of the Paleocene and their affinities with extant orders. Total‐evidence analyses require a substantial proportion of extant taxa. As noted above, we have included earlier and more basal members of extant clades as representatives, as they are less morphologically derived, and are therefore more likely to possess more useful synapomorphies relevant to the diversification of Placentalia, and concomitantly fewer homoplastic traits.
In applying molecular constraints, we allowed for areas of uncertainty such as the topology of the Laurasiatherian orders (Hu, Zhang & Yu, 2012) and the relationships between Boreoeutheria, Atlantogenata and Xenarthra (Murphy et al., 2007; Nishihara, Maruyama & Okada, 2009), discussed further below, by treating them as unresolved polytomies within the scaffold. Two levels of constraint were implemented; one imposed a ‘minimum’ constraint, including a limited subset of exemplar taxa as representatives of the extant orders. For example, while both Pakicetus and Rodhocetus are undoubtedly closest relatives in this data set, both being stem cetaceans, only Rodhocetus was included in the constraint. This approach minimises the degree to which constraints are allowed to affect the data, and is a test that known relationships can still be recovered from the morphological data with a minimal constraint. Exemplar taxa were selected on the basis of both morphological completeness and the level of support for inclusion within a group. For example, within Chiroptera, the extant Pteropus was included in the minimum constraint due to high completeness relative to Onychonycteris and Icaronycteris (both of which are indisputably bats), while within Carnivoramorpha, the genera Miacis and Viverravus were selected as representatives of Miacidae and Viverravidae respectively. The second approach constrained all taxa which are unequivocally accepted as stem members of the extant orders (Fig. 2) in recent analyses focusing on those groups, in order to ensure that well‐established and evidenced relationships were maintained in these analyses.
Each node that has been constrained is justified on the basis of multiple previous phylogenetic analyses. The ‘four‐clade model’ of placental relationships is now well established (Asher, 2007; Murphy et al., 2007; Wible et al., 2007), but the nature of the relationship between Xenarthra, Afrotheria, and Boreoeutheria, the well‐accepted grouping of Laurasiatheria and Euarchontoglires (Delsuc et al., 2002; Asher & Helgen, 2010) is unclear (Hallstrom et al., 2007; Murphy et al., 2007; Morgan et al., 2013; Teeling & Hedges, 2013). As a result, the scaffold is constrained to allow any topology between these three groups. Within Afrotheria, the basal separation of ‘ungulate‐grade’ from ‘insectivore‐grade’ organisms is well established (Tabuce et al., 2008; Asher & Helgen, 2010), and therefore was also constrained. The division between Glires and Euarchonta is supported by a number of analyses, but the relationships within the orders of Euarchonta differ among them (Waddell, Kishino & Ota, 2001; Nie et al., 2008). As a result, Primates, Dermoptera, and Scandentia, all certainly monophyletic, were allowed to vary in their interrelationships within Euarchonta. Within Laurasiatheria, there is no clear consensus for the relationships of the orders (Hallstrom et al., 2011; Hu et al., 2012), except that Carnivora and Pholidota are likely to be sister taxa (Nishihara et al., 2006; Zhou et al., 2012). Raoellid artiodactyls (including Indohyus) are considered to be closer relatives of cetaceans than other artiodactyls in this analysis (Bajpai, Thewissen & Sahni, 2009), and as a result, the topologies within Artiodactyla were also constrained to reflect this basal division.
Further, there remains particular doubt as to the status of the enigmatic genus Purgatorius. This early Paleocene genus has been allied by many to the plesiadapiforms (Clemens, 2004; Fox & Scott, 2011), with the implication that it represents an early stem primate. Alternative topologies have placed Purgatorius on the stem of Placentalia, due to the conservativeness of its morphology (Wible et al., 2009; Rook & Hunter, 2014), although counterarguments suggest that this more basal position has resulted from inadequate sampling of plesiadapiforms and early Primates (Chester et al., 2015), which could also potentially affect its positioning in this analysis. In order to accommodate these alternative hypotheses, which are both substantially supported on the basis of tarsal and dental similarities, respectively, Purgatorius was constrained along with Primates and their kin in a further analysis, and left unconstrained in the others.
In implementing these constraints, all taxa involved in the constraint were set as ‘non‐floaters’ in TNT, while all others were set as ‘floaters’, meaning that they are able to invade an otherwise constrained topology. Peramus, Deltatheridium, and Bobolestes were set as sequential outgroup taxa in the constraint, as all are unambiguous stem eutherians (McKenna & Bell, 1997), in order to ensure that trees were rooted appropriately.
In total, we used three different constraints with two types of data, as well as running an unconstrained analysis with both data matrices, resulting in eight separate sets of most parsimonious trees (MPTs). For brevity, we refer to these eight analyses using the following abbreviations: CU and DU represent the continuous and discretised unconstrained analyses. CM and DM the minimum constraints; CF and DF the full constraints in which all unambiguously placed fossil taxa are included, and CP and DP the constraints equivalent to CF and DF, but with Purgatorius constrained with the Primates.
(c). Phylogenetic analysis
Maximum parsimony phylogenetic analysis was carried out in the freeware program TNT (Goloboff et al., 2008), using the New Technology Search algorithms. The consensus was stabilized twice with factor 75, employing random and exclusive sectorial searches, drift (rejection factor 50) and tree fusing, dumping fused trees for computational ease due to the size of the data set. This was followed by a round of traditional tree bisection and reconnection (TBR) searching, using the MPTs from the New Technology Search as starting trees for the TBR analyses, following Mannion et al. (2013). Analyses each took approximately 350–500 h of computing time. Multistate characters were treated as ordered where meristic or where they represented a morphological sequence in which one or more states are discrete intermediates between end‐member states (Wilkinson, 1992). For example, character 325 describes the position of the palatine foramen, and has the ordered states ‘within palatine’, ‘between palatine and maxilla’ and ‘within maxilla’ (see Appendix S4). Due to the precision of continuous and weighted discrete analyses of multiple decimal places, near‐optimal trees which were less than a step longer than the MPTs were also recovered.
(d). Templeton's tests
Templeton's test (Templeton, 1983) allows alternative, suboptimal topologies to be compared in order to ascertain whether the additional length is significantly longer than the optimal topology, and therefore can be rejected as unsupported by the data. Where two competing hypotheses for the phylogenetic placement of a taxon exist, Templeton's tests are therefore a useful way to determine whether a data set supports one hypothesis strongly over another. Pairwise Templeton's tests were conducted on each set of MPTs for both discrete and continuous data sets using Microsoft Excel.
(e). Bremer support
Relative Bremer supports were calculated by searching for suboptimal trees at increasing levels of suboptimality until the storage limit of 99999 trees was reached in TNT, calculating relative support, and subsequently pruning out those taxa that were causing local reduction in support due to their instability. Relative Bremer supports measure the degree to which topologies supporting a clade outnumber those invalidating a clade within a set of trees, and give a corresponding value between −100 and 100, where −100 represents topologies that are never supported, and a score of 100 clades that are always present. For example, a score of 50 would indicate that the number of trees that contradicted the clade was half that of the number that supported the clade. Values of 0 or below result in the node being collapsed, as they are contradicted by a majority of trees. Relative Bremer support holds the advantage over absolute Bremer support of taking into account contradictory and favourable evidence for a clade, rather than just favourable evidence (Goloboff & Farris, 2001). Additionally, because they vary within the same scale, measuring a ratio, results can be directly comparable across trees. As a result, it has been argued that relative Bremer support is a superior metric of node support to absolute Bremer support (Goloboff & Farris, 2001).
V. RESULTS
(1). Phylogenetic topology
The MPTs resulting from the six constrained analyses were generally consistent with each other. For clarity, only the results from three analyses will be discussed in detail – the discrete, unconstrained tree (DU), and both continuous and discrete trees, with the full constraint applied (CF and DF). Details of the differences between these trees and those derived from alternative constraints (the ‘minimum’ constraint and full constraint incorporating Purgatorius) may be found in Appendix S5. Numbers of MPTs, number of suboptimal trees within a single step of the MPTs, and tree metrics are summarised in Table 1.
Table 1.
Numbers of most parsimonious trees (MPTs), their lengths, the number of suboptimal trees within a single step, and consistency (CI) and retention (RI) indices. Across all analyses, homoplasy is extremely high. Lengths of trees that are not whole numbers are due to the presence and weighting of continuous characters or discretised and reweighted continuous characters
| MATRIX: | CU | CM | CF | CP | DU | DM | DF | DP |
|---|---|---|---|---|---|---|---|---|
| No MPTs | 5 | 4 | 2 | 8 | 79 | 60 | 480 | 10 |
| Length | 7820.16876 | 8009.03713 | 8017.90619 | 8059.26802 | 8330.75 | 8471.9 | 8521.8 | 8528.23 |
| No Suboptimal | 4163 | 39516 | 6672 | 20448 | 8506 | 3950 | 8884 | 1054 |
| CI | 0.111 | 0.108 | 0.108 | 0.108 | 0.111 | 0.109 | 0.108 | 0.108 |
| RI | 0.448 | 0.431 | 0.432 | 0.429 | 0.446 | 0.434 | 0.431 | 0.430 |
(a). Unconstrained analyses
The topology of the unconstrained analysis (Fig. 3, see online Fig. S1) contained many of the groupings that have been generally supported by previous morphological analyses, and failed to recover Eulipotyphla as a monophyletic group to the exclusion of other laurasiatherians. Afrotheria was recovered as polyphyletic and Chiroptera allied with a reduced Euarchontoglires. Aspects of the topology such as these demonstrate the need for constraining relationships among extant clades to those that are well supported by both molecular and phenotypic data sets, such as the four superorders. Results were consistent between discretised and continuous characters.
Figure 3.
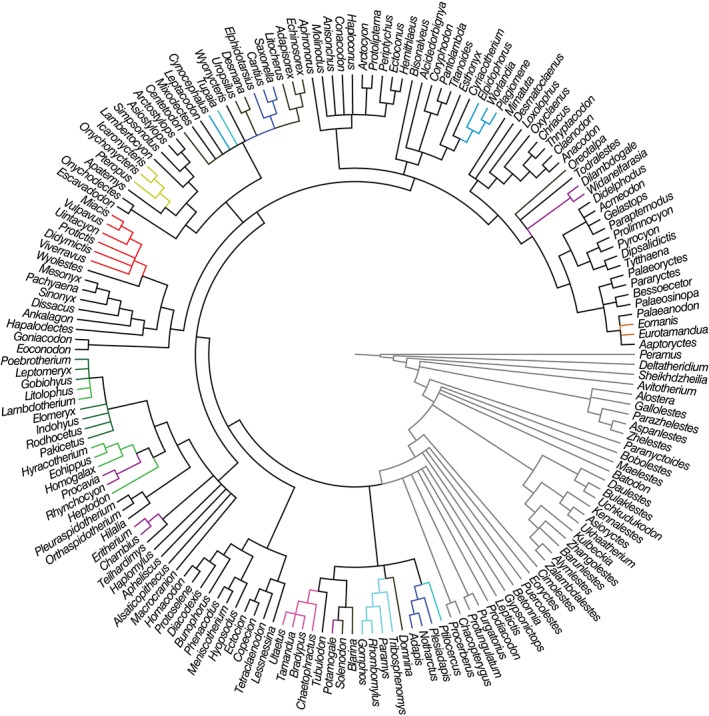
Strict consensus of all discrete, unconstrained (DU) trees within one step of the most parsimonious trees. Colours represent members of extant orders as follows: pink, Xenarthra; purple, Afrotheria; sky blue, Glires; light blue, Scandentia; mid‐blue, Dermoptera; royal blue, primates; brown, Eulipotyphla; dark green, Artiodactyla; light green, Perissodactyla; yellow, Chiroptera; orange, Pholidota; red, Carnivora.
(b). Fully constrained analyses
When full constraints were implemented as described in Section IV.2b , the precise topology of extant clades varied where there was uncertainty, for example in the relationships among the laurasiatherian orders (Fig. 4, see online Figs S2–S6). Nonetheless, topological relationships of the clades of interest were generally consistent among all constrained analyses. The retention and consistency indices were similar in the constrained and unconstrained analyses (Table 1), indicating that the level of homoplasy in the unconstrained tree was almost as high as when relationships were constrained.
Figure 4.
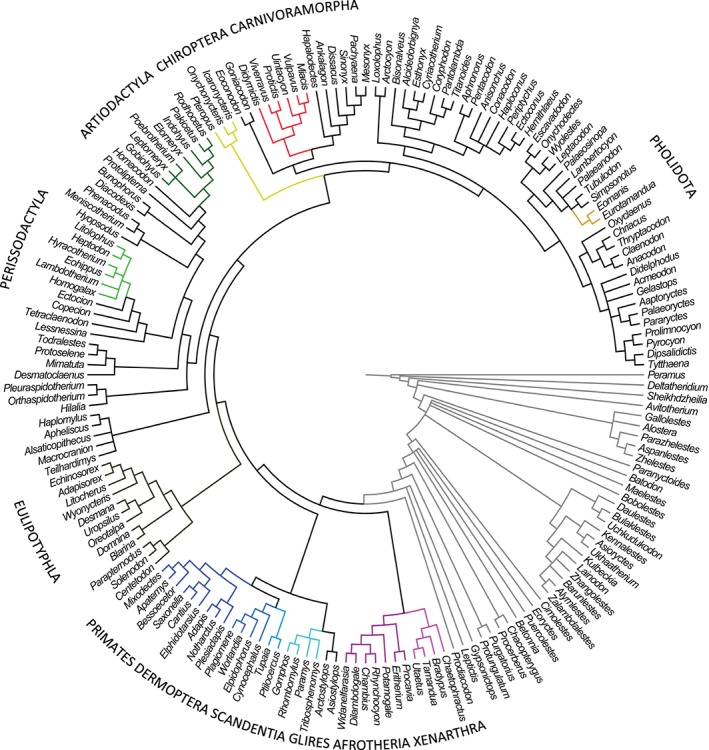
Strict consensus of trees derived from the discrete data set with the full constraints applied (DF analysis). Colours are as in Fig. 3.
Below, we discuss relationships of clades of interest, and the synapomorphies that support them. Full lists of synapomorphies for major recovered clades are found in Appendix S6.
(c). Stem placentals
In all analyses, Protungulatum was most parsimoniously reconstructed as a non‐placental eutherian, contrary to previous suggestions that it represented the earliest crown placental, or that it was an arctocyonid ‘condylarth’. Purgatorius was found consistently as sister taxon to Protungulatum, as in Wible et al. (2007), with both taxa immediately stemward of a paraphyletic Leptictida. Zhelestidae was recovered in a basal stem‐placental position as opposed to being a stem member of an ‘ungulate’ clade within the crown. The monophyly of Zhelestidae was supported by the presence of a metaconid on p4 (Character 131), the separation of a relatively small paraconid from the metaconid (19, 20, 214), a hypoconulid close to the entoconid (236) and a mandibular ramus that deepens below the molars (263). Zalambdalestidae, too, was recovered as a monophyletic group of stem placentals, and was supported by several unambiguous synapomorphies, including the development of an enlarged, procumbent lower first incisor with an extensive root (72, 74, 76), a more anterior position of the posteriormost mental foramen (261), the lack of an ectoflexus on any upper molar (160), an uneven distribution of enamel on the incisors (256), and the presence of a separate metaconid on the fourth premolar (131).
(d). Cimolestidae
Cimolestids were found to be diphyletic in all analyses; one group, consisting of Cimolestes, Procerberus, Chacopterygus, Betonnia and Puercolestes, was consistently placed in a relatively basal position on the eutherian stem. However, the cimolestid Gelastops was reconstructed as part of the broadly carnivorous radiation of mammals including palaeoryctidans, creodonts, and Ferae, falling out specifically with the palaeoryctidan Acmeodon and the mesonychid Wyolestes. The inconsistency in the composition of this group means that there are few synapomorphies that are supported across all analyses. However, a premaxilla that extends beyond the canine (293), sharp, gracile molar cusps (138), and a small ectopterygoid process (387) are consistently reconstructed where this broadly carnivorous grouping is recovered as monophyletic.
(e). Leptictida
The three leptictidans were not recovered as monophyletic in any analysis, but Gypsonictops and Leptictis were found to be sister taxa in all analyses, with synapomorphies including prominent premolar conules (112), a developed cristid obliqua (230), and the presence of a hypoconule on upper molars (206). In the CF and DF analyses, Prodiacodon was found to be the sister taxon to crown Placentalia, with Gypsonictops and Leptictis the next closest relatives, rendering Leptictida paraphyletic with respect to Placentalia.
(f). The placental root and higher‐level relationships
Molecular and morphological analyses have been equivocal in support for the three prevailing hypotheses for the placental root topology (e.g. Churakov et al., 2009). Here, in all constrained analyses, a split between Atlantogenata and Boreoeutheria was favoured as the root of placental mammals, rather than either Xenarthra or Afrotheria being most basal among placental superorders, as has previously been hypothesised (Gaudin et al., 1996; Waddell et al., 2001). This result is consistent with many recent genetic and genomic analyses of placental mammals (Hallstrom et al., 2007; Murphy et al., 2007; Kuntner, May‐Collado & Agnarsson, 2011). Morphological synapomorphies for Atlantogenata were inconsistent across different analyses, due to the shifting relationships of other taxa. Those which remain across the majority of analyses were dental – typically related to the simplification of the molars – which poses problems for identifying these traits in edentulous taxa such as the majority of Xenarthra. Commonly reconstructed synapomorphies of Atlantogenata included loss of the pre‐ and postcingula (109, 110), as well as a vertical lingual face of the protocone (191), and the presence of a hypoconid on the second lower molar (232). In the DF and CF analyses, 26 and 25 synapomorphies, respectively, supported Atlantogenata, of which 21 were common to both (Appendix S6). Examples of these are postcranial characters including an increase in the number of thoracic vertebrae (524), a rounded rather than ovoid radial head (560), and a shortened astragalar neck (630), as well as many additional losses in cheek tooth complexity. However, no taxon was consistently resolved on the stem of either Atlantogenata or Boreoeutheria.
Although Laurasiatheria and Euarchontoglires were each constrained to form monophyletic clades in all analyses, they were nonetheless supported by a number of unambiguous synapomorphies. Character transitions which consistently occurred at the base of Laurasiatheria include the movement of the foramen ovale to a medial position relative to the glenoid fossa (393), the opening of the cavum epiptericum (419), the loss of the hypotympanic sinus (452), and, where present, more distal re‐entrant grooves on the molars (257). Eulipotyphla was supported as the most basal extant order within Laurasiatheria in all constrained analyses, but the relationships among other laurasiatherian orders were more variable. With the exception of the minimum constraints analyses, the next most basal division within Laurasiatheria was between Euungulata (Artiodactyla and Perissodactyla) and a Chiroptera–Ferae (Carnivora and Pholidota) clade. Characters supporting Euungulata include a flattened ulnar facet on the radial head (560), the lack of a paraconid on the lower molars (213) or a preparacrista on the upper molars (164), and an elongate calcaneal tubercle (656), while the Chiroptera–Ferae clade was supported by a loss of a postpalatine torus (328), a laterally exposed mastoid region (508), three sacral vertebrae (527), and an inferior petrosal sinus that was housed between the petrosal, basisphenoid and the basicranium (476).
Euarchontoglires was supported unambiguously by an extended ectopterygoid process of the alisphenoid (386), an anteriorly expanded tegmen tympani (447), a small and shallow stapedius fossa (463), and a reduction to three sacral vertebrae from four (527). The most common division within Euarchonta is a Scandentia–Dermoptera clade to the exclusion of Primates; this is supported by five consistent and unambiguous synapomorphies. These are a reduction in the number of lower incisors (57), a loss of contact between the jugal and lacrimal (339), the absence of a sagittal crest (372), the presence of the interparietal (373), and a fused scaphoid and lunate (575).
(g). ‘Condylarths’
All major ‘archaic ungulate’ groups were resolved within Laurasiatheria, with a division between broadly herbivorous taxa on the one hand and carnivorous–insectivorous ones on the other. Phenacodontidae was not recovered as monophyletic. One group (Tetraclaenodon, Copecion and Ectocion) was consistently resolved as paraphyletic with respect to Perissodactyla; this was one of the best‐supported relationships, even being recovered in unconstrained analyses. However, Phenacodus and Meniscotherium were found among hyopsodontids in all analyses. With the exception of the presence of a mesostyle (148), there was no single synapomorphy that was unambiguously associated with a node subtending perissodactyls and the three phenacodontids when all analyses were considered. Nonetheless, several character states, such as a strong metalophid (221), highly molarised premolars (118, 119) and the loss of upper molar conular cristae (184) are synapomorphies in a majority of analyses. Pleuraspidotheriidae was also consistently included towards the base of an ungulate group including Perissodactyla, and, sometimes, Artiodactyla.
Contrary to suggestions that Apheliscidae is related to Macroscelidea, apheliscids were here recovered in a basal position within Laurasiatheria, sister to Scrotifera (the clade comprising all laurasiatherian orders except Eulipotyphla) in all analyses except CM and DP. Hyopsodontids are placed, in all analyses except CM and DM, as the sister taxon to Artiodactyla. Periptychidae and Pantodonta are consistently found to be sister taxa, more closely related to Ferae and Chiroptera than to other Laurasiatherian orders. Arctocyonidae was polyphyletic in the DF and CF analyses, with Arctocyon and Loxolophus sister to the Pantodonta–Periptychidae clade, Goniacodon and Eoconodon sister to a Carnivora–Mesonychia clade, and the remaining genera allied with creodonts and palaeoryctidans.
(h). Other Paleocene taxa
The close relationship between Creodonta and Carnivora was consistently supported, with other pseudocarnivorous genera such as Gelastops, Acmeodon, Wyolestes and Didelphodus, as well as Palaeoryctidae, also placed as close relatives to this grouping. Moreover, palaeanodonts are found to be sister taxa to Pholidota (represented here by Eomanis and Eurotamandua), supported by a strong teres tubercle (544), a central process of the radial head (559), a shallow olecranon fossa (573), and no iliopubic eminence (587).
The enigmatic South American meridiungulates are represented in this study by the henricosbornid notoungulate Simpsonotus and the early litoptern Protolipterna. Protolipterna was resolved alongside archaic dichobunid artiodactyls in most analyses. Simpsonotus, however, had a less consistent position, being found next to Palaeanodonta in DF, CM and CP, but on the atlantogenatan stem with Arctostylopidae in DP, on the chiropteran stem with Arctostylopidae in CF, and close to Artiodactyla in DM. As the sampling in this study does not adequately capture the diversity of meridiungulates, which include at least five distinct and unusual clades, further work focusing on this group is certainly required to clarify their affinities. The relationships presented here provide a starting point from which a more detailed analysis of this group can proceed, by including these potential close relatives of the South American ungulates. Neither Protolipterna nor Simpsonotus were resolved as close relatives of Perissodactyla, contrary to recent evidence from protein sequences (Welker et al., 2015).
The relationships of Arctostylopidae are extremely poorly understood (Zack, 2004), but this group has been thought to be related to Glires, Notoungulata, or Artiodactyla (Cifelli, Schaff & McKenna, 1989; McKenna & Bell, 1997). Affiliation with Glires, supported by the DF analysis, was supported by mandibular and postcranial characters such as a single mental foramen (259), a space between m3 and the coronoid process (264), and a rotated sustentacular facet of the astragalus (626). A relationship with Notoungulata was supported in the CF and DP analyses by reduction of the metacone and protocone (9, 12), as well as smaller canines (83, 86), reduced protocristid (223), and a more even‐sized tooth row (137). A more focused study of this enigmatic and rare family is required to resolve the character conflict between this hypothesis and that implied by an Arctostylopidae–Glires clade.
(2). Templeton's tests
Templeton's tests were used to compare all pairwise combinations of MPTs – a total of 42 comparisons. The lengths of all constrained topologies were found to be significantly longer than those of unconstrained trees for a given data set (Table 2), with the latter bearing no relationship to the known topologies of placental mammal phylogenies derived from molecular data. This result suggests that morphological data cannot of itself accurately reconstruct placental phylogeny without application of constraints, due to substantial differences between topologies resulting from constrained and unconstrained analyses. Nonetheless, the constraints that were applied are based upon well‐established relationships that are consistently retrieved from both molecular and combined morphological–molecular analyses, and so are justified in this context.
Table 2.
Results of Templeton's tests, comparing each set of topologies under both discrete and continuous data sets. Of all constrained topologies, the only comparison that was considered to be significantly different is that between the discrete (DM) and continuous (CM) minimum constraints under the discrete data set
| Data set | Shorter topology | Longer topology | W | n | z | P value (two‐tailed) |
|---|---|---|---|---|---|---|
| Continuous | CU | CF | 22398 | 406 | 4.73 | <0.0001 |
| Continuous | CU | CP | 20772 | 414 | 4.26 | <0.0001 |
| Continuous | CU | CM | 23174 | 421 | 4.64 | <0.0001 |
| Continuous | CU | DF | 20294 | 423 | 4.03 | 0.0001 |
| Continuous | CU | DP | 24412 | 429 | 4.75 | <0.0001 |
| Continuous | CU | DM | 19465 | 407 | 4.1 | <0.0001 |
| Continuous | CM | CF | 1071 | 327 | 0.31 | 0.7566 |
| Continuous | CM | CP | 1451 | 345 | 0.39 | 0.6965 |
| Continuous | CM | DF | 342 | 353 | 0.09 | 0.9283 |
| Continuous | CM | DP | 2856 | 353 | 0.74 | 0.4593 |
| Continuous | CM | DM | 2733 | 327 | 0.8 | 0.4237 |
| Continuous | CF | CP | 1184 | 294 | 0.41 | 0.6816 |
| Continuous | CF | DF | 1838 | 313 | 0.57 | 0.5687 |
| Continuous | CF | DP | 2207 | 305 | 0.72 | 0.4715 |
| Continuous | CF | DM | 2009 | 329 | 0.58 | 0.5619 |
| Continuous | CP | DF | 370 | 201 | 0.22 | 0.8259 |
| Continuous | CP | DP | 1842 | 305 | 0.6 | 0.5485 |
| Continuous | CP | DM | 999 | 358 | 0.25 | 0.8026 |
| Continuous | DF | DM | 2389 | 360 | 0.6 | 0.5485 |
| Continuous | DP | DM | 5259 | 380 | 1.23 | 0.2187 |
| Continuous | DF | DP | 161 | 301 | 0.05 | 0.9601 |
| Discrete | DU | DF | 35176 | 410 | 7.33 | <0.0001 |
| Discrete | DU | DP | 37655 | 414 | 7.73 | <0.0001 |
| Discrete | DU | DM | 34198 | 425 | 6.75 | <0.0001 |
| Discrete | DU | CF | 40483 | 421 | 8.1 | <0.0001 |
| Discrete | DU | CP | 38274 | 420 | 7.69 | <0.0001 |
| Discrete | DU | CM | 41344 | 430 | 8.02 | <0.0001 |
| Discrete | DM | DF | 4359 | 348 | 1.16 | 0.246 |
| Discrete | DM | DP | 5911 | 370 | 1.44 | 0.1499 |
| Discrete | DM | CF | 5980 | 324 | 1.77 | 0.0767 |
| Discrete | DM | CP | 5623 | 348 | 1.5 | 0.1336 |
| Discrete | DM | CM | 7540 | 321 | 2.27 | 0.0232 |
| Discrete | DF | DP | 1049 | 296 | 0.36 | 0.7188 |
| Discrete | DF | CF | 439 | 306 | 0.14 | 0.8887 |
| Discrete | DF | CP | 1751 | 194 | 1.12 | 0.2627 |
| Discrete | DF | CM | 3334 | 339 | 0.92 | 0.3576 |
| Discrete | DP | CF | 1397 | 302 | 0.46 | 0.6455 |
| Discrete | DP | CP | 2756 | 302 | 0.91 | 0.3628 |
| Discrete | DP | CM | 1865 | 346 | 0.5 | 0.6171 |
| Discrete | CF | CP | 1613 | 284 | 0.58 | 0.5619 |
| Discrete | CF | CM | 2282 | 316 | 0.7 | 0.4839 |
| Discrete | CM | CP | 1274 | 334 | 0.36 | 0.7188 |
p‐values significant at an alpha level of 0.05 are in bold.
Among the constrained topologies, no significant differences were found in either discrete or continuous character optimisations using the same constraints (Table 2), and for each data set, no particular constraint resulted in significantly longer trees than any other constraint. When different constraints and data types were compared in concert, significant differences were found between DM and CM under the discrete data set. In this case, the continuous topologies were significantly longer due to the trees derived from the discrete data set better fitting the discretised data. All other differences in length between combinations of data set and constraint were not significant.
(3). Relative Bremer support
Relative Bremer support was low for several nodes across all trees (see online Figs S7–S12). The lack of support is in large part due to a few very unstable taxa, as confirmed by application of the ‘Pruned trees’ option in TNT, which identifies taxa that, when removed, result in the resolution of polytomies (Table 3). When relative Bremer supports were calculated after pruning these unstable taxa from the suboptimal topologies, the level of support increased markedly. In the CF analysis, many higher‐level relationships were strongly supported, being found in all suboptimal topologies to the storage limit of 99999 trees. Well‐supported relationships included the placement of all ‘condylarths’ within Laurasiatheria, the monophyly of Euungulata, the closest relatives of Perissodactyla being phenacodontids, and the affinity of Hyopsodontidae with Artiodactyla. Additionally, the placement of triisodontids with Arctocyonidae as a sister clade to Carnivora and Creodonta was very strongly supported. When Eoryctes was excluded, Atlantogenata was supported 100% of the time, as was the paraphyletic relationship of Leptictida with respect to crown Placentalia.
Table 3.
Unstable taxa pruned from each topology for the calculation of representatives
| Level of Constraint | Continuous | Discrete |
|---|---|---|
| Unconstrained | n/a | Lainodon, Pentacodon |
| Minimum | Hilalia, Lainodon, Oxyclaenus | Lainodon, Oreotalpa |
| Full | Centetodon | Molinodus |
| Purgatorius | Prodiacodon | Eoryctes, Molinodus, Lainodon |
In the CM analysis, node support was in general weaker, although monophyly of many Paleocene clades was conserved. Ectocion, Copecion and Tetraclaenodon were still strongly supported as sequential closest relatives to perissodactyls. After excluding unstable taxa (Lainodon, Oxyclaenus and Hilalia) from the CM analysis, Atlantogenata was well supported, but laurasiatherian clades generally received poorer support than in the other analyses (see online Fig. S11).
In the DF analysis, support values were generally higher than other analyses, even before pruning unstable taxa, with relatively good support for a Hyopsodontidae–Artiodactyla relationship, and very high support for an arctocyonid–creodont–palaeanodontan clade. Atlantogenata, the laurasiatherian affinity for ‘condylarths’, the relationship between Perissodactyla and some phenacodontids, and a Triisodontidae‐Carnivora relationship were also notably all supported. The DM and DP analyses were very stable once unstable taxa (Lainodon and Oreotalpa in the former; Eoryctes, Molinodus and Lainodon in the latter) had been excluded from the strict consensus tree, with strong support for many higher‐level relationships within the phylogeny.
Supports for nodes across crown Placentalia were weaker when Purgatorius was constrained as a primate. In all analyses, the weakest area of support was within Ferae, where relative Bremer indicated almost equivocal support for the presence or absence of any given clade. The best‐supported topologies across the entire eutherian tree were found in the DF analysis (Fig. 4, see online Fig. S7).
VI. DISCUSSION
(1). Comparison with previous studies
This study represents a significant methodological improvement over all previous analyses that have sought to understand the affinities of enigmatic Paleocene groups. The only study to approach similar numbers of Palaeogene taxa (130 in this analysis) was the PhD thesis of Zack (2009). However, several of the terminals in that analysis are composites of multiple genera, which therefore may not represent the character distributions of any actual organism, and may result in topologies supported by none of the data from each constituent taxon (Malia, Lipscomb & Allard, 2003). Further, the Zack (2009) analyses presented trees derived primarily from dental and postcranial material. While cranial material, a rich source of data, was included in a single analysis, this was unconstrained to a backbone of known relationships, and as a result contains highly improbable topologies that contradict the body of scientific work to date. Excluding cranial data in all other analyses effectively ignores a rich source of phylogenetic information which may be more reliable than dental data in phylogenetic analyses (Sansom, 2014) due to atomisation of characters and strong functional correlations among occluding teeth. Otherwise, all other studies of mammal phylogenetics have included few, if any, Paleocene taxa, and those that do have focussed primarily on ordinal‐ or family‐level clades (e.g. Missiaen et al., 2012; Chester & Bloch, 2013). While adequate for understanding relationships within groups, these smaller analyses are unable to test all competing hypotheses of placental interrelationships. For instance, by studying apheliscid and louisinid ‘condylarths’, but only including single members of Macroscelidea, Amphelimuridae, Adapisoriculidae, and outgroups (as in Hooker & Russell, 2012), it is possible to study the interrelationships of apheliscids and louisinids, but not possible to test alternative hypotheses of the relationships robustly between these taxa and the rest of the placental tree. The inclusion of a combination of living and fossil members of Placentalia in the present analysis advances our understanding of placental mammal origins, providing additional data, new resolution, and novel perspectives on the long‐debated affinities of several enigmatic clades.
(2). Resolving placental relationships
Atlantogenata was strongly supported over Exafroplacentalia or Epitheria for the first time in an exclusively morphological analysis – albeit one incorporating constraints in other portions of the tree. While Atlantogenata has been supported by a wide range of molecular studies (e.g. Hallstrom & Janke, 2008; Prasad et al., 2008; Song et al., 2012; Morgan et al., 2013), analyses including morphology have tended to favour a xenarthran root (Gaudin et al., 1996; O'Leary et al., 2013). The concordance between topologies derived from previous molecular studies and this morphological study suggests that a solution to the conflict between data sources may be possible, despite the degree of convergence that is clearly present in placental mammal morphology. Although molecular constraints were implemented in this tree, the topology at the root of Placentalia was left unconstrained, and Atlantogenata was recovered in all analyses. That there are dental synapomorphies at the node subtending Atlantogenata despite the extremely simplified dentition of xenarthrans can be explained by noting that the majority of dental characters that support Atlantogenata are concerned with structures that are lost in both Afrotheria and Xenarthra.
We found that a broad division can be drawn within Scrotifera between a loosely ‘ungulatomorph’ clade, including Artiodactyla, Perissodactyla, Hyopsodontidae, Phenacodontidae and Pleuraspidotheriidae, and a remaining group of more insectivorous, omnivorous, and carnivorous taxa, including Chiroptera, Mesonychia, Palaeanodonta, Pholidota, Carnivora, Creodonta, Palaeoryctidae and Arctocyonidae, associated with Periptycidae and Pantodonta.
The polyphyly of ‘Condylarthra’ and its status as a wastebasket taxon are supported by a large number of morphological features. Several enigmatic Paleocene groups within this wastebasket, including Periptychidae, Pantodonta and Mesonychia, as well as, in a majority of analyses, Arctocyonidae, are resolved as monophyletic sister taxa to extant clades. Others, such as Leptictida and Cimolestidae are recovered as paraphyletic with respect to extant clades. The polyphyly of Hyopsodontidae, which has long been suspected (Cifelli, 1983; Archibald, 1998; Zack et al., 2005a ), is also supported here, with Pleuraspidotheriidae, Apheliscidae and the ‘true’ hyopsodontids Hyopsodus and allies found to be entirely separate lineages (Fig. 4).
Although Phenacodontidae is here returned as diphyletic, the consistent support for the presence of phenacodontids as closest relatives of Perissodactyla is in agreement with the majority of the literature. The original definition of ‘Condylarthra’ was as a subgroup of Perissodactyla, and was largely composed of phenacodontids (Cope, 1884a ; Rose, 2006); phenacodontids and perissodactyls have been considered close relatives since (Radinsky, 1966; Thewissen, 1990; Zack, 2009; Ladevèze et al., 2010), although sometimes Phenacodontidae has been identified as the sister group of Altungulata (Kondrashov & Lucas, 2012), a problematic grouping of perissodactyls and several afrotherian ‘ungulates’, which is in conflict with molecular topologies.
The placement of creodonts closer to pangolins than to carnivorans has not been recovered in previous phylogenetic analyses. While Creodonta has been suggested to be paraphyletic relative to Carnivora (Flynn & Wesley‐Hunt, 2005), the distinctive carnivoran carnassial teeth are composed of the upper fourth premolar and the lower first molar, rather than solely molars as seen in Creodonta (Colbert, 1933; Colbert & Morales, 1991; Goswami, 2010; Ungar, 2010), suggesting a possible convergent acquisition of this phenotype. Additionally, monophyly of Creodonta is not always recovered (Polly, 1996; Morlo et al., 2009; Sole et al., 2009). A position within Ferae – the clade uniting Carnivora and Pholidota – is accepted (MacIntyre, 1966; Smith & Smith, 2001), but the relative positions of Pholidota, Carnivora, and Creodonta have been unclear.
While many relationships presented in this study are consistent across analyses, several remain poorly supported. Although a posteriori pruning of unstable taxa removes some of the uncertainty in relationships, there are several aspects of the tree that still remain to be clarified with additional data, including the position of apheliscine condylarths and the topology of the enigmatic groups here resolved on the stem of Ferae. Indeed, evidence is accruing that the diversification of the laurasiatherian orders occurred extremely rapidly, (Hallstrom &Janke, 2008; Zhou et al., 2012) such that incomplete lineage sorting has been invoked as an explanation for lack of resolution in early placental relationships (Hallstrom & Janke, 2010).
Clearly, there is little consensus for the majority of the relationships of Paleocene placental mammals, and many of the relationships supported herein will continue to be debated. Many of the results from this analysis actually resurrect previous hypotheses from recent and more distant studies. The nature of a wastebasket taxon, particularly one with such a long history as ‘Condylarthra’, is that many hypotheses of internal and external relationships have been and are being put forward, based upon different lines of evidence. This study, in presenting relationships supported by a broad skeleto‐dental matrix combined with molecularly derived constraints, supports topologies which are largely consistent with at least some of the literature and provides new resolution to the placental tree.
With the inclusion of Paleocene taxa into a broad phylogenetic tree for eutherian mammals, we have a window into the time during which the placental diversification was occurring, and are able to break the methodological constraints of using only extant data to peer back at events whose effects on the genome, have, over time, been overwritten and obscured. Use of molecular constraints which can overcome problems of homoplasy in morphological data help to reveal past patterns (Davalos et al., 2014), meaning that integration of palaeontological and neontological data is essential to answer questions of ancient relationships. This analysis represents an important step in untangling the relationships of these extinct clades, and understanding the evolutionary and ecological context of the radiation of placental mammals. Ongoing work is focused on robustly dating the topologies produced in this phylogenetic analysis, and investigating the rates of morphological character evolution and changes in morphological disparity over the K/Pg boundary in order to ascertain whether the end‐Cretaceous mass extinction had a discernible effect on the macroevolutionary patterns within eutherian mammals.
(3). Implications for the timing of placental origins
As noted in previous studies including Protungulatum (Wible et al., 2007; Archibald et al., 2011; O'Leary et al., 2013), the phylogenetic position of that taxon is critical to the interpretation of the oldest known members of crown Placentalia. Protungulatum (and Purgatorius) were most parsimoniously resolved on the stem of Placentalia, except where Purgatorius was constrained. However, Templeton's tests do not reject alternative phylogenetic positions for either Protungulatum or Purgatorius within the crown. Protungulatum is known from both Paleocene and Cretaceous formations (Archibald et al., 2011), and as such, the presence of this taxon within crown Placentalia would be evidence that the origin of placental mammals pre‐dates the K/Pg boundary. Where Protungulatum is resolved as a stem placental, the conclusions are more equivocal, as neither a Cretaceous nor a Paleocene origin for placental mammals can be ruled out. Nevertheless, given that the earliest ‘condylarths’ are known from the first few hundred thousand years of the Paleocene (Lofgren et al., 2004), and are consistently resolved not just within Placentalia but within Laurasiatheria, an explosive increase in evolutionary rate would be necessary for a radiation to occur entirely within the Paleocene. Estimates from extant taxa require a fivefold increase in background rates of morphological evolution to allow the placental mammal origin to be less than 66 million years ago (Beck & Lee, 2014). This new topology, with broad sampling of temporally relevant taxa, makes it possible to analyse the timing and rate of placental mammal divergences robustly.
Identifying the sister taxon to crown Placentalia is also relevant for dating its origin and estimating the effect of the mass extinction on mammal diversity. The sister taxon to crown Placentalia varied among analyses in this study, but was either a member of the now‐paraphyletic Leptictida in DP and CP, or a Protungulatum–Purgatorius clade in analyses where Purgatorius was not constrained as a stem primate. Gypsonictops is known from the Late Cretaceous, while the other two genera are Paleocene. The presence of Cimolestidae and Leptictis on the placental stem implies that, minimally, three lineages of eutherian mammals (Placentalia, Cimolestidae, and a subgroup of Leptictida) survived the end‐Cretaceous mass extinction; more if Placentalia had already begun to diverge in the Late Cretaceous.
Additionally, there are several well‐supported relationships within Placentalia that provide minimum estimates for the divergence of orders which, based simply on first‐appearance dates, differ markedly in some cases from previous estimates. The oldest perissodactyl, Hyracotherium, is known from the earliest Eocene (e.g. Smith & Smith, 2003), giving a minimum divergence date of Perissodactyla from its nearest relatives of 56 Ma. However, the earliest phenacodontid, Tetraclaenodon, is known from the Torrejonian (e.g. Scott, Spivak, & Sweet, 2013). With a close relationship found here between Perissodactyla and some members of Phenacodontidae, including Tetraclaenodon, the minimum divergence date of Perissodactyla from its closest extant relatives would be 63 Ma, in the Early Paleocene. Such changes to internal estimates of divergence dates will impact further on the predicted date of divergence of crown Placentalia. The deep nesting within Laurasiatheria of Periptychidae, one of the earliest definitively crown placental clades from the first faunal substage of the Paleocene, would seem to support the hypothesis that either a rapid increase in evolutionary rate took place, or the origin of placental mammals pre‐dated the end‐Cretaceous mass extinction.
VII. CONCLUSIONS
The majority of members of Cimolestidae and Leptictida are here resolved as stem eutherians, and both are paraphyletic with respect to crown Placentalia. The hypothesised relationship between Cimolestidae and Pantodonta is therefore not supported here.
All ‘condylarth’ taxa are laurasiatherian, with no taxa favoured as a stem paenungulate. The origin of the afrotherian ‘ungulates’ therefore remains unresolved, and although some ‘ungulate’ taxa such as Simpsonotus are occasionally recovered on the stem of Afrotheria, the pattern is inconsistent and requires further analysis with a more representative sample of South American ungulates.
The closest relatives of crown perissodactyls are consistently found to be phenacodontids, although Phenacodus and Meniscotherium are more parsimoniously resolved with members of Hyopsodontidae, which are sometimes closer to Artiodactyla.
Periptychidae and Pantodonta are sister taxa, and are more closely related to Ferae and Chiroptera than to Perissodactyla or Artiodactyla.
Where there is lack of support for the relationships of Paleocene mammals, this is in large part due to the behaviour of a few highly unstable taxa such as Lainodon.
An Atlantogenata–Boreoeutheria split is favoured over Epitheria or Exafroplacentalia at the root of Placentalia. While relatively common in molecular systematics, this topology is rarely supported using maximum parsimony and morphological data.
No definitive crown‐placental mammal has yet been found from the Cretaceous, as Protungulatum is resolved as a stem eutherian, and therefore the Cretaceous occurrence of Protungulatum cannot be considered definitive proof of a Cretaceous origin for placental mammals.
Supporting information
Fig. S1. Consensus topology deriving from the CU analysis.
Fig. S2. Consensus topology deriving from the DM analysis.
Fig. S3. Consensus topology deriving from the DP analysis.
Fig. S4. Consensus topology deriving from the CF analysis.
Fig. S5. Consensus topology deriving from the CM analysis.
Fig. S6. Consensus topology deriving from the CP analysis.
Fig. S7. Bremer support tree from the DF analysis.
Fig. S8. Bremer support tree from the DM analysis.
Fig. S9. Bremer support tree from the DP analysis.
Fig. S10. Bremer support tree from the CF analysis.
Fig. S11. Bremer support tree from the CM analysis.
Fig. S12. Bremer support tree from the CP analysis.
Appendix S1. Specimens used in the coding of this matrix, complete with specimen number, genus and species‐level affiliation, and the literature from which codings were assessed, where relevant, including the figure number.
Appendix S2. Matrix used for the phylogenetic analyses which resulted in the DU, DF, DM and DP sets of most‐parsimonious trees, formatted to be openable in TNT.
Appendix S3. Matrix used for the phylogenetic analyses which resulted in the CU, CF, CM and CP sets of most‐parsimonious trees, formatted to be openable in TNT.
Appendix S4. List of all 680 characters with descriptions of each character state, the proximal and ultimate sources of that character, and the treatment of that character – whether it is continuous or discrete, and ordered or unordered.
Appendix S5. Additional topologies recovered in the DM, CM, DP and CP analyses.
Appendix S6. List of unambiguous synapomorphies of commonly recovered clades.
VIII. ACKNOWLEDGEMENTS
Thanks are due to the Willi Hennig Society for their continued support of TNT. For access to specimens, we thank Dr Michael Brett‐Surman (National Museum of Natural History, Washington DC), Dr Pip Brewer (Natural History Museum, London), Judy Galkin (American Museum of Natural History, New York), Dr Jerry Hooker (Natural History Museum, London), Dr Mat Lowe (University Museum of Zoology, Cambridge), Dr Emma‐Louise Nicholls (Grant Museum of Zoology, London), Dr Christopher Norris (Yale Peabody Museum, New Haven), Dr William Sanders (University of Michigan Museum of Paleontology, Ann Arbor), Dr Kevin Seymour (Royal Ontario Museum, Toronto), and Dr William Simpson (Field Museum of Natural History, Chicago). We thank Dr Mark Bell and Dr Pablo Goloboff for assistance with computer programs. Advice was gladly received from Dr John Alroy, Dr Mary Silcox, members of the ADaPTiVE discussion group, and the anonymous reviewers that provided useful criticism for this and previous versions of this manuscript. T.J.D.H. was funded by Natural Environment Research Council (NERC) Award NE/J/500136/1.
IX. REFERENCES
References marked with asterisk have been cited within the supporting information
- Agnolin, F. L. & Chimento, N. R. (2011). Afrotherian affinities for endemic South American “ungulates”. Mammalian Biology – Zeitschrift für Säugetierkunde 76, 101–108. [Google Scholar]
- Alroy, J. (1999). The fossil record of North American mammals: evidence for a Paleocene evolutionary radiation. Systematic Biology 48, 107–118. [DOI] [PubMed] [Google Scholar]
- Ameghino, F. (1902). Notices préliminaires sur des ongulés nouveaux des terrains crétacés de Patagonie. Boletín Academia Nacional de Ciencias (Argentina) 16, 350–426. [Google Scholar]
- Antunes, M. T. , Sigogneau‐Russell, D. & Russell, D. E. (1986). Sur quelques dents de Mammifères du Crétacé supérieur de Taveiro, Portugal (Note préliminaire). Comptes Rendus de l'Académie des Sciences à Paris, Série II 303, 1247–1250. [Google Scholar]
- Archibald, J. D. (1998). Archaic ungulates (“Condylarthra”) In Evolution of Tertiary Mammals of North America. Terrestrial Carnivores, Ungulates, and Ungulate‐like Mammals (eds Janis C. M., Scott K. M. and Jacobs L. L.), pp. 292–331. Cambridge University Press, Cambridge. [Google Scholar]
- Archibald, J. D. (2011). Extinction and Radiation: How the Fall Of Dinosaurs Led to the Rise Of Mammals. Johns Hopkins University Press, Baltimore, Maryland, USA. [Google Scholar]
- *Archibald, J. D. & Averianov, A. O. (2001). Paranyctoides and allies from the late Cretaceous of North America and Asia. Acta Palaeontologica Polonica 46, 533–551. [Google Scholar]
- *Archibald, J. D. & Averianov, A. O. (2006). Late Cretaceous asioryctitherian eutherian mammals from Uzbekistan and phylogenetic analysis of Asioryctitheria. Acta Palaeontologica Polonica 51, 351–376. [Google Scholar]
- *Archibald, J. D. & Averianov, A. O. (2011). Phylogenetic analysis, taxonomic revision and dental ontogeny of the Cretaceous Zhelestidae (Mammalia: Eutheria). Zoological Journal of the Linnean Society 164, 361–426. [Google Scholar]
- Archibald, J. D. , Averianov, A. O. & Ekdale, E. G. (2001). Late Cretaceous relatives of rabbits, rodents, and other extant eutherian mammals. Nature 414, 62–65. [DOI] [PubMed] [Google Scholar]
- Archibald, J. D. , Schoch, R. M. & Rigby, J. K. Jr. (1983). A new subfamily, Conacodontinae, and new species, Conacodon kohlbergeri, of the Periptychidae (Condylarthra, Mammalia). Postilla 191, 1–24. [Google Scholar]
- Archibald, J. D. , Zhang, Y. , Harper, T. & Cifelli, R. L. (2011). Protungulatum, confirmed Cretaceous occurrence of an otherwise Paleocene eutherian (Placental?) mammal. Journal of Mammalian Evolution 18, 153–161. [Google Scholar]
- Argot, C. (2013). Postcranial analysis of a carnivoran‐like archaic ungulate: the case of Arctocyon primaevus (Arctocyonidae, Mammalia) from the late Paleocene of France. Journal of Mammalian Evolution 20, 83–114. [Google Scholar]
- Asher, R. J. (2007). A web‐database of mammalian morphology and a reanalysis of placental phylogeny. BMC Evolutionary Biology 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher, R. J. , Geisler, J. H. & Sanchez‐Villagra, M. R. (2008). Morphology, paleontology, and placental mammal phylogeny. Systematic Biology 57, 311–317. [DOI] [PubMed] [Google Scholar]
- Asher, R. J. & Helgen, K. M. (2010). Nomenclature and placental mammal phylogeny. BMC Evolutionary Biology 10, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher, R. J. , Lin, K. H. , Kardjilov, N. & Hautier, L. (2011). Variability and constraint in the mammalian vertebral column. Journal of Evolutionary Biology 24, 1080–1090. [DOI] [PubMed] [Google Scholar]
- *Asher, R. J. , McKenna, M. C. , Emry, R. J. , Tabrum, A. R. & Kron, D. G. (2002). Morphology and relationships of Apternodus and other extinct, zalambdodont, placental mammals. Bulletin of the American Museum of Natural History 273, 1–117. [Google Scholar]
- *Asher, R. J. , Meng, J. , Wible, J. R. , McKenna, M. C. , Rougier, G. W. , Dashzeveg, D. & Novacek, M. J. (2005). Stem lagomorpha and the antiquity of Glires. Science 307, 1091–1094. [DOI] [PubMed] [Google Scholar]
- *Asher, R. J. , Novacek, M. J. & Geisler, J. H. (2003). Relationships of endemic African mammals and their fossil relatives based on morphological and molecular evidence. Journal of Mammalian Evolution 10, 131–194. [Google Scholar]
- *Averianov, A. O. (1997). New late Cretaceous mammals of Southern Kazakhstan. Acta Palaeontologica Polonica 42, 243–256. [Google Scholar]
- *Averianov, A. O. (2000). Mammals from the Mesozoic of Kirgizstan, Uzbekistan, Kazakhstan, and Tadzhikistan In The Age of Dinosaurs in Russia and Mongolia (eds Benton M. J., Shishkin M. A., Unwin D. M. and Kurochkin E. N.), pp. 627–652. Cambridge University Press, Cambridge. [Google Scholar]
- *Averianov, A. O. & Archibald, J. D. (2013). New material and reinterpretation of the late Cretaceous eutherian mammal Paranyctoides from Uzbekistan. Acta Palaeontologica Polonica 58, 17–23. [Google Scholar]
- *Bai, B. , Wang, Y. & Meng, J. (2010). New craniodental materials of Litolophus gobiensis (Perissodactyla, “Eomoropidae”) from Inner Mongolia, China, and phylogenetic analyses of Eocene chalicotheres. American Museum Novitates 3688, 1–27. [Google Scholar]
- Bajpai, S. , Thewissen, J. G. M. & Sahni, A. (2009). The origin and early evolution of whales: macroevolution documented on the Indian Subcontinent. Journal of Biosciences 34, 673–686. [DOI] [PubMed] [Google Scholar]
- *Barnes, L. G. (1984). Fossil odontocetes (Mammalia: Cetacea) from the Almejas Formation, Isla Cedros, Mexico. PaleoBios 42, 1–46. [Google Scholar]
- *Beard, K. C. & Dawson, M. R. (2009). Early Wasatchian mammals of the Red Hot local fauna, uppermost Tuscahoma Formation, Lauderdale County, Mississippi. Annals of the Carnegie Museum 78, 193–243. [Google Scholar]
- *Beard, K. C. , Wang, Y. Q. , Meng, J. , Ni, X.‐J. , Gebo, D. L. & Li, C. K. (2010). Paleocene Hapalodectes (Mammalia, Mesonychia) from Subeng, Nei Mongol: further evidence of “East of Eden” dispersal at the Paleocene‐Eocene boundary. Vertebrata PalAsiatica 48, 375–389. [Google Scholar]
- Beck, R. M. D. & Lee, M. S. Y. (2014). Ancient dates or accelerated rates? Morphological clocks and the antiquity of placental mammals. Proceedings of the Royal Society B: Biological Sciences 281 (doi: 10.1098/rspb.2014.1278). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billet, G. (2010). New observations on the skull of Pyrotherium (Pyrotheria, Mammalia) and new phylogenetic hypotheses on South American ungulates. Journal of Mammalian Evolution 17, 21–59. [Google Scholar]
- Bininda‐Emonds, O. R. P. , Cardillo, M. , Jones, K. E. , MacPhee, R. D. E. , Beck, R. M. D. , Grenyer, R. , Price, S. A. , Vos, R. A. , Gittleman, J. L. & Purvis, A. (2007). The delayed rise of present‐day mammals. Nature 446, 507–512. [DOI] [PubMed] [Google Scholar]
- de Blainville, H. M. D. (1841). Ostéographie et description iconographique des Mammifères récents et fossiles (Carnivores), J.B. Baillière et Fils, Paris. [Google Scholar]
- *Bloch, J. I. , Secord, R. & Gingerich, P. D. (2004). Systematics and phylogeny of late Paleocene and early Eocene Palaeoryctinae (Mammalia, Insectivora) from the Clarks Fork and Bighorn basins, Wyoming. Papers in the Earth and Atmospheric Sciences 187, 119–154. [Google Scholar]
- Bond, M. , Reguero, M. A. , Vizcaino, S. F. & Marenssi, S. A. (2006). A new 'South American ungulate' (Mammalia : Litopterna) from the Eocene of the Antarctic Peninsula. Cretaceous‐Tertiary High‐Latitude Palaeoenvironments: James Ross Basin Antartica 258, 163–176. [Google Scholar]
- *Boyer, D. M. & Georgi, J. A. (2007). Cranial morphology of a pantolestid eutherian mammal from the Eocene Bridger Formation, Wyoming, USA: implications for relationships and habitat. Journal of Mammalian Evolution 14, 239–280. [Google Scholar]
- Buckley, M. (2015). Ancient collagen reveals evolutionary history of the endemic South American 'ungulates'. Proceedings of the Royal Society B: Biological Sciences 282 (doi: 10.1098/rspb.2014.2671). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, P. M. (1972). Some functional aspects of molar evolution. Evolution 26, 474. [DOI] [PubMed] [Google Scholar]
- Carroll, R. L. (1988). Vertebrate Paleontology and Evolution. W.H. Freeman and Company, New York. [Google Scholar]
- Chester, S. G. B. & Bloch, J. I. (2013). Systematics of Paleogene Micromomyidae (Euarchonta, Primates) from North America. Journal of Human Evolution 65, 109–142. [DOI] [PubMed] [Google Scholar]
- Chester, S. G. B. , Bloch, J. I. , Boyer, D. M. & Clemens, W. A. (2015). Oldest known euarchontan tarsals and affinities of Paleocene Purgatorius to Primates. Proceedings of the National Academy of Sciences of the United States of America 112, 1487–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, M. & Li, C.‐K. (1965). Homogalax and Heptodon of Shantung. Vertebrata PalAsiatica 9, 15–22. [Google Scholar]
- Churakov, G. , Kriegs, J. O. , Baertsch, R. , Zemann, A. , Brosius, J. & Schmitz, J. (2009). Mosaic retroposon insertion patterns in placental mammals. Genome Research 19, 868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cifelli, R. (1982). The petrosal structure of Hyopsodus with respect to that of some other ungulates, and its phylogenetic implications. Journal of Vertebrate Paleontology 56, 795–805. [Google Scholar]
- Cifelli, R. (1983). The origin and affinities of the South American Condylarthra and early Tertiary Litopterna (Mammalia). American Museum Novitates 2772, 1–49. [Google Scholar]
- *Cifelli, R. (1990). Cretaceous mammals of Southern Utah IV: eutherian mammals from the Wahweap (Aquilan) and Kaiparowits (Judithian) Formations. Journal of Vertebrate Paleontology 3, 346–360. [Google Scholar]
- Cifelli, R. L. , Schaff, C. R. & McKenna, M. C. (1989). The relationships of the Arctostylopidae (Mammalia): new data and interpretation. Bulletin of the Museum of Comparative Zoology 152, 1–44. [Google Scholar]
- Clemens, W. A. (2004). Purgatorius (Plesiadapiformes, Primates?, Mammalia), a Paleocene immigrant into northeastern Montana: stratigraphic occurrences and incisor proportions. Bulletin of Carnegie Museum of Natural History 36, 3–13. [Google Scholar]
- Clemens, W. A. (2010). Were immigrants a significant part of the Earliest Paleocene mammalian fauna of the North American Western Interior? Vertebrata PalAsiatica 48, 285–307. [Google Scholar]
- Colbert, E. H. (1933). The skull of Dissopsalis carnifex pilgrim, a Miocene creodont from India. American Museum Novitates 603, 1–8. [Google Scholar]
- Colbert, E. H. & Morales, M. (1991). Creodonts and Carnivores In Evolution of the Vertebrates (eds Colbert E. H. and Morales M.). Wiley‐Liss, New York, 373–390. [Google Scholar]
- *Coombs, M. C. & Coombs, W. P. Jr. (1977). Dentition of Gobiohyus and a re‐evaluation of the Helohyidae (Artiodactyla). Journal of Mammalogy 58, 291–308. [Google Scholar]
- *Coombs, M. C. & Coombs, W. P. Jr. (1982). Anatomy of the ear region of four Eocene artiodactyls: Gobiohyus, ?Helohyus, Diacodexis and Homacodon . Journal of Vertebrate Paleontology 2, 219–236. [Google Scholar]
- *Cooper, L. N. , Thewissen, J. G. M. , Bajpai, S. & Tiwari, B. N. (2012). Postcranial morphology and locomotion of the Eocene raoellid Indohyus (Artiodactyla: Mammalia). Historical Biology 24, 279–310. [Google Scholar]
- Cope, E. D. (1884a). The Condylarthra. The American Naturalist 18, 790–805. [Google Scholar]
- *Cope, E. D. (1884b). The Condylarthra (continued). The American Naturalist 18, 892–906. [Google Scholar]
- Cope, E. D. (1884c). The Creodonta. The American Naturalist 18, 255–267. [Google Scholar]
- Crusafont‐Pairo, M. & Golpe Posse, J. M. (1975). Les prosimiens de l'Eocene de la region preaxiale pyrenaique II: Adapidae. Colloque International CNRS: Problemes Actuels de Paleontologie‐Evolution des Vertebres 218, 851–860. [Google Scholar]
- Darroch, S. A. F. , Webb, A. E. , Longrich, N. & Belmaker, J. (2014). Palaeocene‐Eocene evolution of beta diversity among ungulate mammals in North America. Global Ecology and Biogeography 23, 757–768. [Google Scholar]
- Davalos, L. M. , Velazco, P. M. , Warsi, O. M. , Smits, P. D. & Simmons, N. B. (2014). Integrating incomplete fossils by isolating conflicting signal in saturated and non‐independent morphological characters. Systematic Biology 63, 582–600. [DOI] [PubMed] [Google Scholar]
- Dawson, M. R. (2012). Coryphodon, the northernmost Holarctic Paleogene pantodont (Mammalia), and its global wanderings. Swiss Journal of Palaeontology 131, 11–22. [Google Scholar]
- De Bast, E. & Smith, T. (2013). Reassessment of the small arctocyonid Prolatidens waudruae from the early Paleocene of Belgium, and its phylogenetic relationships with ungulate‐like mammals. Journal of Vertebrate Paleontology 33, 964–976. [Google Scholar]
- Delsuc, F. , Scally, M. , Madsen, O. , Stanhope, M. J. , de Jong, W. W. , Catzeflis, F. M. , Springer, M. S. & Douzery, E. J. P. (2002). Molecular phylogeny of living xenarthrans and the impact of character and taxon sampling on the placental tree rooting. Molecular Biology and Evolution 19, 1656–1671. [DOI] [PubMed] [Google Scholar]
- Ding, S.‐Y. (1987). A Paleocene edentate from Nanxiong Basin, Guangdong. Palaeontologica Sinica New Series C 173, 1–118. [Google Scholar]
- Domning, D. P. , Emry, R. J. , Portell, R. W. , Donovan, S. K. & Schindler, K. S. (1997). Oldest West Indian land mammal: rhinocerotoid ungulate from the Eocene of Jamaica. Journal of Vertebrate Paleontology 17, 638–641. [Google Scholar]
- Eberle, J. J. , Rybczynski, N. & Greenwood, D. R. (2014). Early Eocene mammals from the Driftwood Canyon Provincial Park, northern British Columbia. Journal of Vertebrate Paleontology 34, 739–746. [Google Scholar]
- Ekdale, E. G. , Archibald, J. D. & Averianov, A. O. (2004). Petrosal bones of placental mammals from the Late Cretaceous of Uzbekistan. Acta Palaeontologica Polonica 49, 161–176. [Google Scholar]
- Filhol, H. (1892). Note sur un insectivore nouveau. Bulletin de la Société Philomathique de Paris 8, 134. [Google Scholar]
- *Flynn, J. J. & Galiano, H. (1982). Phylogeny of early Tertiary Carnivora, with a description of a new species of Protictis from the Middle Eocene of Northwestern Wyoming. American Museum Novitates 2725, 1–64. [Google Scholar]
- Flynn, J. J. & Wesley‐Hunt, G. D. (2005). Phylogeny and early diversification of the Carnivora In The Rise of Placental Mammals: Origins and Relationships of the Major Extant Clades (eds Archibald J. D. and Rose K.). John Hopkins University Press, Baltimore, 171–198. [Google Scholar]
- *Fox, R. C. (1984). First North American record of the Paleocene primate Saxonella . Journal of Paleontology 66, 499–520. [Google Scholar]
- *Fox, R. C. (1991). Saxonella (Plesiadapiformes, ?Primates) in North America: S. naylori, sp. nov., from the Late Paleocene of Alberta, Canada. Journal of Vertebrate Paleontology 11, 334–349. [Google Scholar]
- *Fox, R. C. (1994). Composition of the holotype of the North American Late Cretaceous mammal Cimolestes cerberoides Lillegraven 1969. Journal of Paleontology 68, 910–911. [Google Scholar]
- *Fox, R. C. (2011). An unusual early primate from the Paleocene Paskapoo Formation, Alberta, Canada. Acta Palaeontologica Polonica 56, 1–10. [Google Scholar]
- Fox, R. C. & Scott, C. S. (2011). A new, early Puercan (Earliest Paleocene) species of Purgatorius (Plesiadapiformes, Primates) from Saskatchewan, Canada. Journal of Paleontology 85, 537–548. [Google Scholar]
- Fox, R. C. , Scott, C. S. & Rankin, B. D. (2010). New early carnivoran specimens from the Puercan (Earliest Paleocene) of Saskatchewan, Canada. Journal of Paleontology 84, 1035–1039. [Google Scholar]
- Fox, R. C. & Youzwyshyn, G. P. (1994). New primitive carnivorans (Mammalia) from the Paleocene of Western Canada, and their bearing on relationships of the order. Journal of Vertebrate Paleontology 14, 382–404. [Google Scholar]
- Froehlich, D. J. (1999). Phylogenetic systematics of basal perissodactyls. Journal of Vertebrate Paleontology 19, 140–159. [Google Scholar]
- Froehlich, D. J. (2002). Quo vadis Eohippus? The systematics and taxonomy of the early Eocene equids (Perissodactyla). Zoological Journal of the Linnean Society 134, 141–256. [Google Scholar]
- Gaudin, T. J. (1999). The morphology of xenarthrous vertebrae (Mammalia: Xenarthra). Fieldiana Geology 41, 1–38. [DOI] [PubMed] [Google Scholar]
- Gaudin, T. J. , Emry, R. J. & Wible, J. R. (2009). The phylogeny of living and extinct pangolins (Mammalia, Pholidota) and associated taxa: a morphology based analysis. Journal of Mammalian Evolution 16, 235–305. [Google Scholar]
- Gaudin, T. J. , Wible, J. R. , Hopson, J. A. & Turnbull, W. D. (1996). Reexamination of the morphological evidence for the cohort Epitheria (Mammalia, Eutheria). Journal of Mammalian Evolution 3, 31–79. [Google Scholar]
- *Geisler, J. H. (2001). New morphological evidence for the phylogeny of Artiodactyla, Cetacea, and Mesonychidae. American Museum Novitates 3344, 1–53. [Google Scholar]
- Geisler, J. H. & McKenna, M. C. (2007). A new species of mesonychian mammal from the lower Eocene of Mongolia and its phylogenetic relationships. Acta Palaeontologica Polonica 52, 189–212. [Google Scholar]
- *Geisler, J. H. & Luo, Z. X. (1996). The petrosal and inner ear of Herpetocetus sp. (Mammalia, Cetacea) and their implications for the phylogeny and hearing of archaic mysticetes. Journal of Paleontology 70, 1045–1066. [Google Scholar]
- *Geisler, J. H. & Luo, Z. X. (1998). Relationships of Cetacea to terrestrial ungulates and the evolution of cranial vasculature in Cete In The Emergence of Whales (ed. Thewissen J. G. M.), pp. 163–212. Springer, New York. [Google Scholar]
- *Gentry, A. W. & Hooker, J. J. (1988). The phylogeny of the Artiodactyla In The Phylogeny and Classification of the Tetrapods (ed. Benton M. J.), pp. 235–272. Clarendon Press, Oxford. [Google Scholar]
- *George, S. B. , Choate, J. R. & Genoways, H. H. (1986). Blarina brevicauda. Mammalogy Papers: University of Nebraska State Museum 95, 1–9. [Google Scholar]
- *Gheerbrant, E. (1991). Todralestes variabilis n.g. n.sp., nouveau proteuthérien (Eutheria, Todralestidae fam. nov.) du Paléocène du Maroc. Comptes Rendus de l'Académie des Sciences à Paris Série II 312, 1249–1255. [Google Scholar]
- *Gheerbrant, E. (2009). Paleocene emergence of elephant relatives and the rapid radiation of African ungulates. Proceedings of the National Academy of Sciences of the United States of America 106, 10717–10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheerbrant, E. , Amaghzaz, M. , Bouya, B. , Goussard, F. & Letenneur, C. (2014). Ocepeia (Middle Paleocene of Morocco): the oldest skull of an afrotherian mammal. PLoS One 9, e89739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Gheerbrant, E. & Astibia, H. (1994). Un nouveau mammifère du Maastrichtien de Laño (Pays Basques espagnol). Comptes Rendus de l'Académie des Sciences à Paris, Série II 318, 1125–1131. [Google Scholar]
- Gill, T. (1872). Arrangement of the Families of Mammals with Analytical Tables. Smithsonian Institution, Washington. [Google Scholar]
- *Gingerich, P. D. (1980). Tytthaena parrisi, oldest known oxyaenid (Mammalia, Creodonta) from the Late Paleocene of Western North America. Journal of Paleontology 54, 570–576. [Google Scholar]
- *Gingerich, P. D. (1981). Radiation of early Cenozoic Didymoconidae (Condylarthra, Mesonychia) in Asia, with a new genus from the early Eocene of Western North America. Journal of Mammalogy 62, 526–538. [Google Scholar]
- *Gingerich, P. D. (1982). Aaptoryctes (Palaeoryctidae) and Thelysia (Palaeoryctidae?): new insectivorous mammals from the late Paleocene and early Eocene of Western North America. Contributions from the Museum of Paleontology, University of Michigan 26, 37–47. [Google Scholar]
- *Gingerich, P. D. (1983a). Systematics of early Eocene Miacidae (Mammalia, Carnivora) in the Clark's Fork Basin, Wyoming. Contributions from the Museum of Paleontology, University of Michigan 26, 197–225. [Google Scholar]
- *Gingerich, P. D. (1983b). New Adapisoricidae, Pentacodontidae, and Hyopsodontidae (Mammalia, Insectivora and Condylarhtra) from the late Paleocene of Wyoming and Colorado. Contributions from the Museum of Paleontology, University of Michigan 26, 227–255. [Google Scholar]
- *Gingerich, P. D. (1987). Early Eocene bats (Mammalia, Chiroptera) and other vertebrates in freshwater limestones of the Willwood Formation, Clark's Fork Basin, Wyoming. Contributions from the Museum of Paleontology, University of Michigan 27, 275–320. [Google Scholar]
- *Gingerich, P. D. (1990). Prediction of body mass in mammalian species from long bone lengths and diameters. Contributions from the Museum of Paleontology, University of Michigan 28, 79–92. [Google Scholar]
- *Gingerich, P. D. (1994). New species of Apheliscus, Haplomylus and Hyopsodus (Mammalia, Condylarthra) from the late Paleocene of Southern Montana and early Eoceneof Northwestern Wyoming. Contributions from the Museum of Paleontology, University of Michigan 29, 119–134. [Google Scholar]
- *Gingerich, P. D. & Deutsch, H. A. (1989). Systematics and evolution of early Eocene Hyaenodontidae (Mammalia, Creodonta) in the Clark's Fork Basin, Wyoming. Contributions from the Museum of Paleontology, University of Michigan 27, 319–327. [Google Scholar]
- Gingerich, P. D. , ul Haq, M. , Zalmout, I. S. , Khan, I. H. & Malkani, M. S. (2001). Origin of whales from early artiodactyls: hands and feet of Eocene Protocetidae from Pakistan. Science 293, 2239–2242. [DOI] [PubMed] [Google Scholar]
- *Gingerich, P. D. , Raza, S. M. , Arif, M. , Anwar, M. & Zhou, X. (1994). New whale from the Eocene of Pakistan and the origin of cetacean swimming. Nature 368, 844–847. [Google Scholar]
- *Gingerich, P. D. & Russell, D. E. (1981). Pakicetus inachus, a new archaeocete (Mammalia, Cetacea) from the early‐Middle Eocene Kuldana Formation of Kohat (Pakistan). Contributions from the Museum of Paleontology, University of Michigan 25, 235–246. [Google Scholar]
- Godinot, M. , Smith, T. & Smith, R. (1996). Life habitat and affinities of Paschatherium (Condylarthra, Hyopsodontidae) based on tarsal examination. Palaeovertebrata (Montpellier) 25, 225–242. [Google Scholar]
- Goloboff, P. A. & Farris, J. S. (2001). Methods for quick consensus estimation. Cladistics 17, S26–S34. [Google Scholar]
- Goloboff, P. A. , Farris, J. S. & Nixon, K. C. (2008). TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786. [Google Scholar]
- Goloboff, P. A. , Mattoni, C. I. & Quinteros, A. S. (2006). Continuous characters analyzed as such. Cladistics 22, 589–601. [DOI] [PubMed] [Google Scholar]
- Goswami, A. (2010). Introduction to Carnivora In Carnivoran evolution: new views on phylogeny, form and function [Cambridge Studies in Morphology and Molecules: New Paradigms in Evolutionary Biology] (eds Goswami A. and Friscia A.), pp. 1–24. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Goswami, A. (2012). A dating success story: genomes and fossils converge on placental mammal origins. EvoDevo 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami, A. , Prasad, G. V. R. , Upchurch, P. , Boyer, D. M. , Seiffert, E. R. , Verma, O. , Gheerbrant, E. & Flynn, J. J. (2011). A radiation of arboreal basal eutherian mammals beginning in the late Cretaceous of India. Proceedings of the National Academy of Sciences of the United States of America 108, 16333–16338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, F. D. H. & Rose, K. D. (2014). Gnathic and postcranial skeleton of the largest known arctocyonid 'condylarth' Arctocyon mumak (Mammalia, Procreodi) and ecomorphological diversity in Procreodi. Journal of Vertebrate Paleontology 34, 1180–1202. [Google Scholar]
- Gregory, W. K. (1910). The Orders of Mammals. Bulletin of the American Museum of Natural History 27, 1–556. [Google Scholar]
- Grossnickle, D. M. & Polly, P. D. (2013). Mammal disparity decreases during the Cretaceous angiosperm radiation. Proceedings of the Royal Society B: Biological Sciences 280 (doi: 10.1098/rspb.2013.2110). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnell, G. F. & Gingerich, P. D. (1991). Systematics and evolution of late Paleocene and early Eocene Oxyaenidae (Mammalia, Creodonta) in the Clark's Fork Basin, Wyoming, USA. Contributions from the Museum of Paleontology University of Michigan 28, 141–179. [Google Scholar]
- Hallstrom, B. M. & Janke, A. (2008). Resolution among major placental mammal interordinal relationships with genome data imply that speciation influenced their earliest radiations. BMC Evolutionary Biology 8 (doi: 10.1186/1471-2148-8-162). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrom, B. M. & Janke, A. (2010). Mammalian evolution may not be strictly bifurcating. Molecular Biology and Evolution 27, 2804–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallstrom, B. M. , Kullberg, M. , Nilsson, M. A. & Janke, A. (2007). Phylogenomic data analyses provide evidence that Xenarthra and Afrotheria are sister groups. Molecular Biology and Evolution 24, 2059–2068. [DOI] [PubMed] [Google Scholar]
- Hallstrom, B. M. , Schneider, A. , Zoller, S. & Janke, A. (2011). A genomic approach to examine the complex evolution of Laurasiatherian mammals. PLoS One 6(12), e28199 (doi: 10.1371/journal.pone.0028199). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Heinrich, R. E. (1997). Referral of Miacis jepseni Guthrie to Oödectes Wortman, and an assessment of phylogenetic relationships among early Eocene Miacidae (Mammalia:Carnivora). Journal of Paleontology 71, 1172–1178. [Google Scholar]
- *Holbrook, L. T. (1999). The phylogeny and classification of tapiromorph perissodactyls (Mammalia). Cladistics 15, 331–350. [DOI] [PubMed] [Google Scholar]
- Holbrook, L. T. & Lapergola, J. (2011). A new genus of perissodactyl (Mammalia) from the Bridgerian of Wyoming, with comments on basal perissodactyl phylogeny. Journal of Vertebrate Paleontology 31, 895–901. [Google Scholar]
- Hooker, J. J. (2001). Tarsals of the extinct insectivoran family Nyctitheriidae (Mammalia): evidence for archontan relationships. Zoological Journal of the Linnean Society 132, 501–529. [Google Scholar]
- Hooker, J. J. (2013). Origin and evolution of the Pseudorhyncocyonidae, a European Paleogene family of insectivorous placental mammals. Palaeontology 56, 807–835. [Google Scholar]
- Hooker, J. J. (2014). New postcranial bones of the extinct mammalian family Nyctitheriidae (Paleogene, UK): primitive euarchontans with scansorial locomotion. Palaeontologica Electronica 17(3), 47A. [Google Scholar]
- Hooker, J. J. & Dashzeveg, D. (2003). Evidence for direct mammalian faunal interchange between Europe and Asia near the Paleocene‐Eocene boundary. Geological Society of America, Special Paper 369, 479–500. [Google Scholar]
- Hooker, J. J. & Russell, D. E. (2012). Early Palaeogene Louisinidae (Macroscelidea, Mammalia), their relationships and north European diversity. Zoological Journal of the Linnean Society 164, 856–936. [Google Scholar]
- *Horovitz, I. (2000). The tarsus of Ukhaatherium nessovi (Eutheria, Mammalia) from the Late Cretaceous of Mongolia: an appraisal of the evolution of the ankle in basal therians. Journal of Vertebrate Paleontology 20, 547–560. [Google Scholar]
- *Horovitz, I. (2003). Postcranial skeleton of Ukhaatherium nessovi (Eutheria, Mammalia) from the Late Cretaceous of Mongolia. Journal of Vertebrate Paleontology 23, 857–868. [Google Scholar]
- Horovitz, I. (2004). Eutherian mammal systematics and the origins of South American ungulates as based on postcranial osteology. Bulletin of Carnegie Museum of Natural History 36, 63–79. [Google Scholar]
- *Horovitz, I. & Sánchez‐Villagra, M. R. (2003). A morphological analysis of marsupial mammal higher‐level phylogenetic relationships. Cladistics 19, 181–212. [Google Scholar]
- *Horovitz, I. , Storch, G. & Martin, T. (2005). Ankle structure in Eocene pholidotan mammal Eomanis krebsi and its taxonomic implications. Acta Palaeontologica Polonica 50, 545–548. [Google Scholar]
- Hu, J. , Zhang, Y. & Yu, L. (2012). Summary of Laurasiatheria (Mammalia) Phylogeny. Zoological Research 33, E65–E74. [DOI] [PubMed] [Google Scholar]
- Hunt, R. M. & Tedford, R. H. (1993). Phylogenetic relationships within the aeluroid Carnivora and implications of their temporal and geographic distribution In Mammal Phylogeny:Placentals (eds Szalay F. S., Novacek M. J. and McKenna M. C.), pp. 53–73. Springer‐Verlag, New York, USA. [Google Scholar]
- Jablonski, D. & Chaloner, W. G. (1994). Extinctions in the fossil record. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences 344, 11–16. [Google Scholar]
- Jepsen, G. L. (1937). A Paleocene rodent, Paramys atavus . Proceedings of the American Philosophical Society 78, 291–301. [Google Scholar]
- Jepsen, G. L. (1966). Early Eocene bat from Wyoming. Science 154, 1333. [DOI] [PubMed] [Google Scholar]
- *Kalthoff, D. C. , Rose, K. D. & von Koenigswald, W. (2011). Dental microstructure in Palaeanodon and Tubulodon (Palaeanodonta) and bioerosional tunnelling as a widespread phenomenon in fossil mammal teeth. Journal of Vertebrate Paleontology 31, 1303–1313. [Google Scholar]
- *Kielan‐Jaworowska, Z. (1968). Preliminary data on the Upper Cretaceous eutherian mammals from Bayn Dzak, Gobi Desert. Acta Palaeontologica Polonica 19, 171–191. [Google Scholar]
- *Kielan‐Jaworowska, Z. (1975). Preliminary description of two new eutherian genera from the Late Cretaceous of Mongolia. Acta Palaeontologica Polonica 33, 1–13. [Google Scholar]
- Kielan‐Jaworowska, Z. , Bown, T. M. & Lillegraven, J. A. (1979). Eutheria In Mesozoic Mammals: the First Two‐Thirds of Mammalian History (eds Lillegraven J. A., Kielan‐Jaworowska Z. and Clemens W. A.), pp. 221–258. University of California Press, Berkeley. [Google Scholar]
- Kielan‐Jaworowska, Z. , Cifelli, R. L. & Luo, Z.‐X. (2004). Mammals from the Age of Dinosaurs: Origin, Evolutions, and Structure. Columbia University Press, New York. [Google Scholar]
- *Kihm, A. J. & Schumaker, K. K. (2008). Domnina (Mammalia, Soricomorpha) from the latest Eocene (Chadronian) Medicine Pole Hills Local Fauna of North Dakota. Paludicola 7, 26–36. [Google Scholar]
- Kondrashov, P. (2009). Postcranial adaptations of European arctocyonids (Mammalia, Arctocyonidae). Journal of Vertebrate Paleontology 29, 128A. [Google Scholar]
- Kondrashov, P. E. & Lucas, S. G. (2004). Oxyclaenus from the Early Paleocene of New Mexico and the status of the Oxyclaeninae (Mammalia, Arctocyonidae). Bulletin of the New Mexico Museum of Natural History and Science 26, 21–32. [Google Scholar]
- *Kondrashov, P. E. & Lucas, S. G. (2006). Early Paleocene (Puercan and Torrejonian) archaic ungulates (Condylarthra, Procreodi and Acreodi) of the San Juan Basin, New Mexico In Fossils from Federal Lands, New Mexico Museum of Natural History and Science Bulletin (eds Lucas S. G., Spielmann J. A., Hester P. M., Kenworthy J. P. and Santiccui V. L.), pp. 84–97. New Mexico Museum of Natural History, New Mexico. [Google Scholar]
- Kondrashov, P. E. & Lucas, S. G. (2012). Nearly complete skeleton of Tetraclaenodon (Mammalia, Phenacodontidae) from the early Paleocene of New Mexico: morpho‐functional analysis. Journal of Paleontology 86, 25–43. [Google Scholar]
- *Korth, W. W. (1988). Paramys compressidens Peterson and the systematic relationships of the species of Paramys (Paramyinae, Ischyromyidae). Journal of Paleontology 62, 468–471. [Google Scholar]
- Kuntner, M. , May‐Collado, L. J. & Agnarsson, I. (2011). Phylogeny and conservation priorities of afrotherian mammals (Afrotheria, Mammalia). Zoologica Scripta 40, 1–15. [Google Scholar]
- Ladevèze, S. , Missiaen, P. & Smith, T. (2010). First skull of Orthaspidotherium edwardsi (Mammalia, “Condylarthra”) from the Late Paleocene of Berru (France) and phylogenetic affinities of the enigmatic European family Pleuraspidotheriidae. Journal of Vertebrate Paleontology 30, 1559–1578. [Google Scholar]
- Lee, M. S. Y. & Camens, A. B. (2009). Strong morphological support for the molecular evolutionary tree of placental mammals. Journal of Evolutionary Biology 22, 2243–2257. [DOI] [PubMed] [Google Scholar]
- Leidy, J. (1868). Notice of some remains of extinct Insectivora from Dakota. Proceedings of the Academy of Natural Sciences of Philadelphia 20, 196–197. [Google Scholar]
- *Lessertisseur, J. & Saban, R. (1967). Squelette appendiculaire In Traité de Zoologie I (ed. Grassé P.‐P.). Masson & Cie, Paris, 709–1078. [Google Scholar]
- *Lihoreau, F. , Ducrocq, S. , Antoine, P.‐O. , Vianey‐Liaud, M. , Rafaÿ, S. , Garcia, G. & Valentin, X. (2009). First complete skulls of Elomeryx crispus (Gervais 1849) and of Protaceratherium albingense (Roman 1912) from a new Oligocene locality near Moissac (SW France). Journal of Vertebrate Paleontology 29, 242–253. [Google Scholar]
- Lillegraven, J. A. (1969). Latest Cretaceous mammals of the upper part of the Edmonton Formation of Alberta, Canada, and review of the marsupial‐placental dichotomy in mammalian evolution. University of Kansas Paleontological Contributions 50, 1–122. [Google Scholar]
- *Lillegraven, J. A. (1976). A new genus of therian mammal from the Late Cretaceous “El Gallo Formation”, Baja California, Mexico. Journal of Paleontology 50, 437–443. [Google Scholar]
- *Lillegraven, J. A. , McKenna, M. C. & Krishtalka, L. (1981). Evolutionary relationships of Middle Eocene and younger species of Centetodon (Mammalia, Insectivora, Geolabididae) with a description of the dentition of Ankylodon (Adapisoricidae). University of Wyoming Publications 45, 1–115. [Google Scholar]
- *Lloyd, K. J. & Eberle, J. J. (2008). A new talpid from the late Eocene of North America. Acta Palaeontologica Polonica 53, 539–543. [Google Scholar]
- Lofgren, D. L. , Lillegraven, J. A. , Clemens, W. A. , Gingerich, P. D. & Williamson, T. E. (2004). Paleocene biochronology: the Puercan through Clarkforkian land mammal ages In Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology (ed. Woodburne M. O.), pp. 43–105. Columbia University Press, New York. [Google Scholar]
- Lopatin, A. V. (2001). The skull structure of Archaeoryctes euryalis sp. nov. (Didymoconidae, Mammalia) from the Paleocene of Mongolia, with comments on the the taxonomic position of the family. Paleontological Journal 3, 97–107. [Google Scholar]
- Lopatin, A. V. (2006). Early Paleogene insectivore mammals of Asia and establishment of the major groups of Insectivora. Paleontological Journal 40, S205–S405. [Google Scholar]
- Lopatin, A. V. & Averianov, A. O. (2008). The earliest lagomorph (Lagomorpha, Mammalia) from the basal Eocene of Mongolia. Proceedings of the Academy of Sciences of the USSR, Biological Sciences 419, 131–132. [DOI] [PubMed] [Google Scholar]
- *López‐Martinez, N. & Peláez‐Campomanus, P. (1999). New mammals from south central Pyrenees (Tremp Formation, Spain) and their bearing on Late Paleocene marine‐continental correlations. Bulletin du Sociéte Géologique de la France 170, 681–696. [Google Scholar]
- *Lucas, S. G. & O'Neill, F. M. (1981). Occurrence of Pantolambda (Mammalia, Pantodonta) in the Torrejonian Deltatherium “Zone”, San Juan Basin, New Mexico. American Journal of Science 281, 187–191. [Google Scholar]
- *Luo, Z. X. (1991). Variability of dental morphology and the relationships of the earliest arctocyonid species. Journal of Vertebrate Paleontology 11, 452–471. [Google Scholar]
- Luo, Z. X. (2007). Transformation and diversification in early mammal evolution. Nature 450, 1011–1019. [DOI] [PubMed] [Google Scholar]
- *Luo, Z. X. & Wible, J. R. (2005). A Late Jurassic digging mammal and early mammalian diversification. Science 308, 103–107. [DOI] [PubMed] [Google Scholar]
- *Maas, M. C. , Thewissen, J. G. M. , Sen, S. , Kazanci, N. & Kappelman, J. (2001). Enigmatic new ungulates from the early middle Eocene of central Anatolia, Turkey. Journal of Vertebrate Paleontology 21, 578–590. [Google Scholar]
- MacFadden, B. J. & Shockey, B. J. (1997). Ancient feeding ecology and niche differentiation of Pleistocene mammalian herbivores from Tarija, Bolivia: morphological and isotopic evidence. Paleobiology 23, 77–100. [Google Scholar]
- MacIntyre, G. T. (1966). The Miacidae (Mammalia, Carnivora): the systematics of Ictidopappus and Protictis . Bulletin of the American Museum of Natural History 131, 117–209. [Google Scholar]
- *MacPhee, R. D. E. (1994). Morphology, adaptations, and relationships of Plesiorycteropus, and a diagnosis of a new order of eutherian mammals. Bulletin of the American Museum of Natural History 220, 1–214. [Google Scholar]
- Madsen, O. , Scally, M. , Douady, C. J. , Kao, D. J. , DeBry, R. W. , Adkins, R. , Amrine, H. M. , Stanhope, M. J. , de Jong, W. W. & Springer, M. S. (2001). Parallel adaptive radiations in two major clades of placental mammals. Nature 409, 610–614. [DOI] [PubMed] [Google Scholar]
- Malia, M. J. , Lipscomb, D. L. & Allard, M. W. (2003). The misleading effects of composite taxa in supermatrices. Molecular Phylogenetics and Evolution 27, 522–527. [DOI] [PubMed] [Google Scholar]
- Mannion, P. D. , Upchurch, P. , Barnes, R. N. & Mateus, O. (2013). Osteology of the Late Jurassic Portuguese sauropod dinosaur Lusotitan atalaiensis (Macronaria) and the evolutionary history of basal titanosauriforms. Zoological Journal of the Linnean Society 168, 98–206. [Google Scholar]
- Manz, C. L. , Chester, S. G. B. , Bloch, J. I. , Silcox, M. T. & Sargis, E. J. (2015). New partial skeletons of Palaeocene Nyctitheriidae and evaluation of proposed euarchontan affinities. Biology Letters 11, 20140911–20140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew, W. D. (1915). A revision of the lower Eocene Wasatch and Wind River faunas: part I – Order Ferae (Carnivora) Suborder Creodonta. Bulletin of the American Museum of Natural History 34, 4–103. [Google Scholar]
- Matthew, W. D. , Granger, W. & Simpson, G. G. (1929). Additions to the fauna of the Gashato Formation of Mongolia. American Museum Novitates 376, 1–12. [Google Scholar]
- *McKenna, M. C. (1968). Leptacodon, an American Paleocene nyctithere (Mammalia, Insectivora). American Museum Novitates 2317, 1–12. [Google Scholar]
- McKenna, M. C. (1975). Towards a phylogenetic classification of the Mammalia In Phylogeny of the Primates: a Multidisciplinary Approach (eds Luckett W. and Szalay F.), pp. 21–46. Plenum, New York. [Google Scholar]
- McKenna, M. C. & Bell, S. K. (1997). Classification of Mammals Above the Species Level. Columbia University Press, New York. [Google Scholar]
- Meehan, T. J. & Martin, L. D. (2010). New leptictids (Mammalia: lnsectivora) from the early Oligocene of Nebraska, USA. Neues Jahrbuch Fur Geologie Und Palaontologie‐Abhandlungen 256, 99–107. [Google Scholar]
- Meehan, T. J. & Wilson, R. W. (2002). New viverravids from the Torrejonian (Middle Paleocene) of Kutz Canyon, New Mexico and the oldest skull of the Order Carnivora. Journal of Paleontology 76, 1091–1101. [Google Scholar]
- Meng, J. , Suyin, T. & Schiebout, J. A. (1995). The cranial morphology of an early Eocene didymoconid (Mammalia, Insectivora). Journal of Vertebrate Paleontology 14, 534–551. [Google Scholar]
- Meng, J. & Wyss, A. R. (2001). The morphology of Tribosphenomys (Rodentiaformes, Mammalia): phylogenetic implications for basal Glires. Journal of Mammalian Evolution 8, 1–71. [Google Scholar]
- *Mehta, S. K. & Jolly, A. (1989). Leptomeryx, an Oligocene artiodactyl from the Lower Murree of Sial Sui (Kalakot Tehsil), District Rajauri, Jammu and Kashmir. Current Science 58, 625–627. [Google Scholar]
- *Meng, J. , Hu, Y. & Li, C. (2003). The osteology of Rhombomylus (Mammalia, Glires): implications for phylogeny and evolution of Glires. Bulletin of the American Museum of Natural History 275, 1–247. [Google Scholar]
- *Meng, J. , Wyss, A. R. , Dawson, M. R. & Zhai, R. (1994). Primitive fossil rodent from Inner Mongolia and its implications for mammalian phylogeny. Nature 370, 134–136. [DOI] [PubMed] [Google Scholar]
- *Middleton, M. D. & Dewar, E. W. (2004). New mammals from the early Paleocene Littleton Fauna (Denver Formation, Colorado). New Mexico Museum of Natural History Science Bulletin 26, 51–80. [Google Scholar]
- Missiaen, P. , Escarguel, G. , Hartenberger, J.‐L. & Smith, T. (2012). A large new collection of Palaeostylops from the Paleocene of the Flaming Cliffs area (Ulan‐Nur Basin, Gobi Desert, Mongolia), and an evaluation of the phylogenetic affinities of Arctostylopidae (Mammalia, Gliriformes). Geobios 45, 311–322. [Google Scholar]
- Morgan, C. C. , Foster, P. G. , Webb, A. E. , Pisani, D. , McInerney, J. O. & O'Connell, M. J. (2013). Heterogeneous models place the root of the placental mammal phylogeny. Molecular Biology and Evolution 30, 2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlo, M. , Gunnell, G. & Polly, P. D. (2009). What, if not nothing, is a creodont? Phylogeny and classification of Hyaenodontida and other former creodonts. Journal of Vertebrate Paleontology 29, 152A–152A. [Google Scholar]
- de Muizon, C. & Cifelli, R. L. (2000). The “condylarths” (archaic Ungulata, Mammalia) from the early Palaeocene of Tiupampa (Bolivia): implications on the origin of the South American ungulates. Geodiversitas 22, 47–48. [Google Scholar]
- *de Muizon, C. & Marshall, L. G. (1992). Alcidedorbignya inopinata (Mammalia: Pantodonta) from the early Paleocene of Bolivia: phylogenetic and paleobiogeographic implications. Journal of Paleontology 66, 499–520. [Google Scholar]
- Murphy, W. J. , Pringle, T. H. , Crider, T. A. , Springer, M. S. & Miller, W. (2007). Using genomic data to unravel the root of the placental mammal phylogeny. Genome Research 17, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Nessov, L. A. , Archibald, J. D. & Kielan‐Jaworowska, Z. (1998). Ungulate‐like mammals from the late Cretaceous of Uzbekistan and a phylogenetic analysis of Ungulatomorpha. Bulletin of the Carnegie Museum of Natural History 34, 40–88. [Google Scholar]
- Nie, W. , Fu, B. , O'Brien, P. C. M. , Wang, J. , Su, W. , Tanomtong, A. , Volobouev, V. , Ferguson‐Smith, M. A. & Yang, F. (2008). Flying lemurs – the 'flying tree shrews'? Molecular cytogenetic evidence for a Scandentia‐Dermoptera sister clade. BMC Biology 6, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara, H. , Hasegawa, M. & Okada, N. (2006). Pegasoferae, an unexpected mammalian clade revealed by tracking ancient retroposon insertions. Proceedings of the National Academy of Sciences of the United States of America 103, 9929–9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihara, H. , Maruyama, S. & Okada, N. (2009). Retroposon analysis and recent geological data suggest near‐simultaneous divergence of the three superorders of mammals. Proceedings of the National Academy of Sciences of the United States of America 106, 5235–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novacek, M. J. (1986). The skull of leptictid insectivorans and the higher‐level classification of eutherian mammals. Bulletin of the American Museum of Natural History 183, 1–111. [Google Scholar]
- *Novacek, M. J. (1987). Auditory features and affinities of the Eocene bats Icaronycteris and Palaeochiropteryx (Microchiroptera, incertae sedis). American Museum Novitates 2877, 1–18. [Google Scholar]
- Novacek, M. J. (1992). Mammalian phylogeny – shaking the tree. Nature 356, 121–125. [DOI] [PubMed] [Google Scholar]
- *Novacek, M. J. , Bown, T. M. & Schankler, D. (1985). On the classification of the early Tertiary Erinaceomorpha (Insectivora, Mammalia). American Museum Novitates 2813, 1–22. [Google Scholar]
- *Novacek, M. J. & Wyss, A. R. (1986). Higher‐level relationships of the recent eutherian orders: morphological evidence. Cladistics 2, 257–287. [DOI] [PubMed] [Google Scholar]
- *Nummela, S. , Taseer Hussain, S. & Thewissen, J. G. M. (2006). Cranial anatomy of Pakicetidae (Cetacea, Mammalia). Journal of Vertebrate Paleontology 26, 746–759. [Google Scholar]
- O'Leary, M. A. (1998). Morphology of the humerus of Hapalodectes (Mammalia, Mesonychia). American Museum Novitates 3242, 1–6. [Google Scholar]
- O'Leary, M. A. , Bloch, J. I. , Flynn, J. J. , Gaudin, T. J. , Giallombardo, A. , Giannini, N. P. , Goldberg, S. L. , Kraatz, B. P. , Luo, Z. X. , Meng, J. , Ni, X. J. , Novacek, M. J. , Perini, F. A. , Randall, Z. S. , Rougier, G. W. , Sargis, E. J. , Silcox, M. T. , Simmons, N. B. , Spaulding, M. , Velazco, P. M. , Weksler, M. , Wible, J. R. & Cirranello, A. L. (2013). The placental mammal ancestor and the post‐K‐Pg radiation of placentals. Science 339, 662–667. [DOI] [PubMed] [Google Scholar]
- *O'Leary, M. A. & Gatesy, J. (2008). Impact of increased character sampling on the phylogeny of Cetartiodactyla (Mammalia): combined analyses including fossils. Cladistics 24, 397–442. [DOI] [PubMed] [Google Scholar]
- *O'Leary, M. A. & Geisler, J. H. (1999). The position of Cetacea within Mammalia: phylogenetic analysis of morphological data from extinct and extant taxa. Systematic Biology 48, 455–490. [DOI] [PubMed] [Google Scholar]
- O'Leary, M. A. & Rose, K. D. (1995). Postcranial skeleton of the early Eocene mesonychid Pachyaena (Mammalia: Mesonychia). Journal of Vertebrate Paleontology 15, 401–430. [Google Scholar]
- Onuma, M. , Cao, Y. , Hasegawa, M. & Kusakabe, S. (2000). A close relationship of Chiroptera with Eulipotyphla (core insectivora) suggested by four mitochondrial genes. Zoological Science 17, 1327–1332. [Google Scholar]
- Orliac, M. J. , Argot, C. & Gilissen, E. (2012a). Digital cranial endocast of Hyopsodus (Mammalia, “Condylarthra”): a case of Paleogene terrestrial echolocation? PLoS One 7, e30000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orliac, M. J. , Benoit, J. & O'Leary, M. A. (2012b). The inner ear of Diacodexis, the oldest artiodactyl mammal. Journal of Anatomy 221, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn, H. F. (1898). Remounted skeleton of Phenacodus primaevus; comparison with Euprotogonia . Bulletin of the American Museum of Natural History 10, 159–165. [Google Scholar]
- Osborn, H. F. (1902). The law of adaptive radiation. American Naturalist 36, 353–363. [Google Scholar]
- Osborn, H. F. (1924). Andrewsarchus, giant mesonychid of Mongolia. American Museum Novitates 146, 1–5. [Google Scholar]
- Osborn, H. F. & Earle, C. (1895). Fossil mammals of the Puerco bed. Collection of 1892. Bulletin of the American Museum of Natural History 7, 1–70. [Google Scholar]
- Polly, P. D. (1994). What, if anything, is a creodont? Journal of Vertebrate Paleontology 14, 42A. [Google Scholar]
- Polly, P. D. (1996). The skeleton of Gazinocyon vulpeculus gen. et comb. nov. and the cladistic relationships of Hyaenodontidae (Eutheria, Mammalia). Journal of Vertebrate Paleontology 16, 303–319. [Google Scholar]
- Prasad, A. B. , Allard, M. W. , Green, E. D. & Program, N. C. S. (2008). Confirming the phylogeny of mammals by use of large comparative sequence data sets. Molecular Biology and Evolution 25, 1795–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prothero, D. R. (1994). The Eocene‐Oligocene Transition: Paradise Lost, Cambridge University Press, New York, New York, USA. [Google Scholar]
- Prothero, D. R. (1998). The chronological, climatic, and paleogeographic background to North American mammalian evolution In Evolution of Tertiary Mammals of North America, Terrestrial Carnivores, Ungulates, and Ungulatelike Mammals (Volume 1, eds Janis C. M., Scott K. M. and Jacobs L. L.), pp. 9–36. Cambridge University Press, Cambridge. [Google Scholar]
- Prothero, D. R. , Manning, E. M. & Fischer, M. (1988). The Phylogeny of the Ungulates In The Phylogeny and Classification of the Tetrapods, Mammals (Volume II, ed. Benton M. J.), pp. 201–234. Clarendon, Oxford. [Google Scholar]
- Pyron, R. A. & Burbrink, F. T. (2012). Trait‐dependent diversification and the impact of palaeontological data on evolutionary hypothesis testing in New World ratsnakes (tribe Lampropeltini). Journal of Evolutionary Biology 25, 497–508. [DOI] [PubMed] [Google Scholar]
- *Qi, T. , Zong, G. & Wang, Y. (1989). Discovery of Lushilagus and Miacis in Jiangsu and its zoogeographical significance. Vertebrata PalAsiatica 29, 59–63. [Google Scholar]
- Radinsky, L. B. (1965). Evolution of the tapiroid skeleton from Heptodon to Tapirus . Bulletin of the Museum of Comparative Zoology, Harvard University 134, 69–106. [Google Scholar]
- Radinsky, L. B. (1966). Adaptive radiation of phenacodontid condylarths and origin of Perissodactyla. Evolution 20, 408. [DOI] [PubMed] [Google Scholar]
- Rae, T. C. (1998). The logical basis for the use of continuous characters in phylogenetic systematics. Cladistics 14, 221–228. [DOI] [PubMed] [Google Scholar]
- Rage, J. C. , Buffetaut, E. , Buffetauttong, H. , Chaimanee, Y. , Ducrocq, S. , Jaeger, J. J. & Suteethorn, V. (1992). A colubrid snake in the Late Eocene of Thailand ‐ the oldest known Colubridae (Reptilia, Serpentes). Comptes Rendus de l'Académie des Sciences à Paris, Serie II 314, 1085–1089. [Google Scholar]
- Raia, P. , Carotenuto, F. , Passaro, F. , Piras, P. , Fulgione, D. , Werdelin, L. , Saarinen, J. & Fortelius, M. (2013). Rapid action in the Palaeogene, the relationship between phenotypic and taxonomic diversification in Coenozoic mammals. Proceedings of the Royal Society B: Biological Science 280, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj Pant, S. , Goswami, A. & Finarelli, J. A. (2014). Complex body size trends in the evolution of sloths (Xenarthra: Pilosa). BMC Evolutionary Biology 14, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana, R. S. & Wilson, G. P. (2003). New Late Cretaceous mammals from the Intertrappean beds of Rangapur, India and paleobiogeographic framework. Acta Palaeontologica Polonica 48, 331–348. [Google Scholar]
- dos Reis, M. , Inoue, J. , Hasegawa, M. , Asher, R. J. , Donoghue, P. C. J. & Yang, Z. (2012). Phylogenomic datasets provide both precision and accuracy in estimating the timescale of placental mammal phylogeny. Proceedings of the Royal Society B: Biological Sciences 279, 3491–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby, J. K. Jr. (1980). Swain quarry of the Fort Union Formation, middle Paleocene (Torrejonian), Carbon County, Wyoming: geologic setting and mammalian fauna. Evolutionary Monographs 3, 1–178. [Google Scholar]
- Robinson, P. , Gunnell, G. F. , Walsh, S. L. , Clyde, W. C. , Storer, J. E. , Stucky, R. K. , Froehlich, D. J. , Ferrusquia‐Villafranca, I. & McKenna, M. C. (2004). Wasatchian through Duchesnean biochronology In Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology (ed. Woodburne M. O.), pp. 106–155. Columbia University Press, New York. [Google Scholar]
- Rook, D. L. & Hunter, J. P. (2014). Rooting around the eutherian family tree: the origin and relations of the Taeniodonta. Journal of Mammalian Evolution 21, 75–91. [Google Scholar]
- Rose, K. D. (1981). The Clarkforkian Land Mammal Age and mammalian faunal composition across the Paleocene‐Eocene boundary. Museum of Paleontology Papers on Paleontology 26, 1–197. [Google Scholar]
- *Rose, K. D. (1982a). Anterior dentition of the early Eocene plagiomenid dermopteran Worlandia . Journal of Mammalogy 63, 179–183. [Google Scholar]
- Rose, K. D. (1982b). Skeleton of Diacodexis, oldest known artiodactyl. Science 216, 621–623. [DOI] [PubMed] [Google Scholar]
- Rose, K. D. (1987). Climbing adaptations in the early Eocene mammal Chriacus and the origin of Artiodactyla. Science 236, 314–316. [DOI] [PubMed] [Google Scholar]
- Rose, K. D. (1996). On the origin of the order Artiodactyla. Proceedings of the National Academy of Sciences of the United States of America 93, 1705–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, K. D. (1999a). Eurotamandua and Palaeanodonta: convergent or related? Palaeontologische Zeitschrift 73, 395–401. [Google Scholar]
- Rose, K. D. (1999b). Postcranial skeleton of Eocene Leptictidae (mammalia), and its implications for behavior and relationships. Journal of Vertebrate Paleontology 19, 355–372. [Google Scholar]
- Rose, K. D. (2006). The Beginning of the Age of Mammals. The John Hopkins University Press, Baltimore. [Google Scholar]
- *Rose, K. D. , Chew, A. E. , Dunn, R. H. , Kraus, M. J. , Fricke, H. C. & Zack, S. P. (2012). Earliest Eocene mammalian fauna from the Paleocene‐Eocene Thermal Maximum at Sand Creek Divide, Southern Bighorn Basin, Wyoming. University of Michigan Papers on Paleontology 36, 1–122. [Google Scholar]
- Rose, K. D. , Emry, R. J. , Gaudin, T. J. & Storch, G. (2005). Xenarthra and Pholidota In The Rise of Placental Mammals: Origins and Relationships of the Major Extant Clades (eds Rose K. D. and Archibald J. D.), pp. 106–126. John Hopkins University Press, Baltimore. [Google Scholar]
- Rose, K. D. , Holbrook, L. T. , Rana, R. S. , Kumar, K. , Jones, K. E. , Ahrens, H. E. , Missiaen, P. , Sahni, A. & Smith, T. (2014). Early Eocene fossils suggest that the mammalian order Perissodactyla originated in India. Nature Communications 5, 5570. [DOI] [PubMed] [Google Scholar]
- *Rose, K. D. & Krause, D. W. (1982). Cyriacotheriidae, a new family of early Tertiary Pantodonts from Western North America. Proceedings of the American Philosophical Society 126, 26–50. [Google Scholar]
- *Rose, K. D. & Lucas, S. G. (2000). An early Paleocene Palaeanodont (Mammalia, ?Pholidota) from New Mexico, and the origin of Palaeanodonta. Journal of Vertebrate Paleontology 20, 139–156. [Google Scholar]
- *Rose, K. D. & Walker, A. (1985). The skeleton of early Eocene Cantius, oldest lemuriform primate. American Journal of Physical Anthropology 66, 73–89. [DOI] [PubMed] [Google Scholar]
- *Rougier, G. W. , Wible, J. R. & Novacek, M. J. (1998). Implications of Deltatheridium specimens for early marsupial history. Nature 396, 459–463. [DOI] [PubMed] [Google Scholar]
- Russell, D. E. (1964). The Paleocene mammals of Europe. Mémoires du Musée National d'Histoire Naturelle Série C 13, 1–324. [Google Scholar]
- *Sánchez‐Villagra, M. R. , Horovitz, I. & Motokawa, M. (2006). A comprehensive morphological analysis of talpid moles (Mammalia) phylogenetic relationships. Cladistics 22, 59–88. [DOI] [PubMed] [Google Scholar]
- Sansom, R. S . (2014). Dental morphology of mammals is less reliable than osteology: phylogenetic differences align with taphonomic biases. In 62nd Symposium for Vertebrate Palaeontology and Comparative Anatomy, York, United Kingdom.
- Schaeffer, B. (1947). Notes on the origin and function of the artiodactyl tarsus. American Museum Novitates 1356, 1–24. [Google Scholar]
- *Scott, W. B. (1891). On the osteology of Pœbrotherium: a contribution to the phylogeny of the Tylopoda. Journal of Morphology 5, 1–78. [Google Scholar]
- Scott, C. S. (2010). New cyriacotheriid pantodonts (Mammalia, Pantodonta) from the Paleocene of Alberta, Canada, and the Relationships of Cyriacotheriidae. Journal of Paleontology 84, 197–215. [Google Scholar]
- Scott, C. S. , Spivak, D. N. & Sweet, A. R. (2013). First mammals from the Paleocene Porcupine Hills Formation of southwestern Alberta, Canada. Canadian Journal of Earth Sciences 50, 355–378. [Google Scholar]
- *Seiffert, E. R. (2010). The oldest and youngest records of afrosoricid placentals from the Fayum Depression of Northern Egypt. Acta Palaeontologica Polonica 55, 599–616. [Google Scholar]
- *Seiffert, E. R. & Simons, E. L. (2000). Widanelfarasia, a diminutive placentalfrom the Late Eocene of Egypt. Proceedings of the National Academy of Sciences of the United States of America 97, 2646–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Seiffert, E. R. , Simons, E. L. , Ryan, T. M. , Bown, T. M. & Attia, Y. (2007). New remains of Eocene and Oligocene Afrosoricida (Afrotheria) from Egypt, with implications for the origin of afrosoricid zalambdodonty. Journal of Vertebrate Paleontology 27, 963–972. [Google Scholar]
- Sessa, J. A. , Bralower, T. J. , Patzkowsky, M. E. , Handley, J. C. & Ivany, L. C. (2012). Environmental and biological controls on the diversity and ecology of Late Cretaceous through early Paleogene marine ecosystems in the U.S. Gulf Coastal Plain. Paleobiology 38, 218–239. [Google Scholar]
- *Silcox, M.T. (2001). A phylogenetic analysis of Plesiadapiformes and their relationship to Euprimates and other archontans. PhD Thesis: John Hopkins University.
- Simmons, N. B. , Seymour, K. L. , Habersetzer, J. & Gunnell, G. F. (2008). Primitive early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature 451, 818–822. [DOI] [PubMed] [Google Scholar]
- Simons, E. L. (1960). The Paleocene Pantodonta. Transactions of the American Philosophical Society 50, 1–81. [Google Scholar]
- Simpson, G. G. (1937). The Fort Union of the Crazy Mountain field, Montana, and its mammalian faunas. Bulletin of the United States National Museum 169, 1–287. [Google Scholar]
- Simpson, G. G. (1945). The principles of classification and a classification of mammals. Bulletin of the American Museum of Natural History 85, 1–350. [Google Scholar]
- Simpson, G. G. (1953). The Major Features of Evolution. Columbia University Press, New York. [Google Scholar]
- *Simpson, G. G. (1957). Fossil mammals from the type area of the Puerco and Nacimiento Strata, Paleocene of New Mexico. American Museum Novitates 1957, 1–22. [Google Scholar]
- Slater, G. J. (2013). Phylogenetic evidence for a shift in the mode of mammalian body size evolution at the Cretaceous‐Palaeogene boundary. Methods in Ecology and Evolution 4, 734–744. [Google Scholar]
- Slater, G. J. , Harmon, L. J. & Alfaro, M. E. (2012). Integrating fossils with molecular phylogenies improves inference of trait evolution. Evolution 66, 3931–3944. [DOI] [PubMed] [Google Scholar]
- Sloan, R. E. & Van Valen, L. (1965). Cretaceous mammals from Montana. Science 148, 220. [DOI] [PubMed] [Google Scholar]
- Smith, T. , De Bast, E. & Sige, B. (2010). Euarchontan affinity of Paleocene Afro‐European adapisoriculid mammals and their origin in the late Cretaceous Deccan Traps of India. Naturwissenschaften 97, 417–422. [DOI] [PubMed] [Google Scholar]
- Smith, T. & Smith, R. (2001). The creodonts (Mammalia, Ferae) from the Paleocene‐Eocene transition in Belgium (Tienen Formation, MP7). Belgian Journal of Zoology 131, 117–135. [Google Scholar]
- Smith, T. & Smith, R. (2003). Terrestrial mammals as biostratigraphic indicators in Upper Paleocene‐Lower Eocene marine deposits of the southern North Sea Basin. Geological Society of America, Special Paper 369, 513–520. [Google Scholar]
- Smith, R. , Smith, T. & Sudre, J. (1996). Diacodexis gigasei n. sp., the oldest Belgian artiodactyl (Mammalia), found near the Palaeocene‐Eocene transition. Bulletin de l'Institut Royal des Sciences Naturelles de Belgique: Sciences de la Terre 66, 177–196. [Google Scholar]
- Sole, F. , Gheerbrant, E. , Amaghzaz, M. & Bouya, B. (2009). Further evidence of the African antiquity of hyaenodontid ('Creodonta', Mammalia) evolution. Zoological Journal of the Linnean Society 156, 827–846. [Google Scholar]
- Sole, F. & Smith, T. (2013). Dispersals of placental carnivorous mammals (Carnivoramorpha, Oxyaenodonta & Hyaenodontida) near the Paleocene‐Eocene boundary: a climatic and almost worldwide story. Geologica Belgica 16, 254–261. [Google Scholar]
- Song, S. , Liu, L. , Edwards, S. V. & Wu, S. (2012). Resolving conflict in eutherian mammal phylogeny using phylogenomics and the multispecies coalescent model. Proceedings of the National Academy of Sciences of the United States of America 109, 14942–14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding, M. , O'Leary, M. A. & Gatesy, J. (2009). Relationships of Cetacea (Artiodactyla) among mammals: increased taxon sampling alters interpretations of key fossils and character evolution. PLoS One 4, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, M. S. , Murphy, W. J. , Eizirik, E. & O'Brien, S. J. (2003). Placental mammal diversification and the Cretaceous‐Tertiary boundary. Proceedings of the National Academy of Sciences of the United States of America 100, 1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, M. S. , Stanhope, M. J. , Madsen, O. & de Jong, W. W. (2004). Molecules consolidate the placental mammal tree. Trends in Ecology & Evolution 19, 430–438. [DOI] [PubMed] [Google Scholar]
- Stanhope, M. J. , Waddell, V. G. , Madsen, O. , de Jong, W. , Hedges, S. B. , Cleven, G. C. , Kao, D. & Springer, M. S. (1998). Molecular evidence for multiple origins of Insectivora and for a new order of endemic African insectivore mammals. Proceedings of the National Academy of Sciences of the United States of America 95, 9967–9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *St. Clair, E. M. , Boyer, D. M. , Bloch, J. I. & Krause, D. W. (2010). First records of a triisodontine mammal (Goniacodon levisanus) in the late Paleocene of the Northern Great Plains, North America. Journal of Vertebrate Paleontology 30, 604–608. [Google Scholar]
- *Stock, C. (1934). Microsyopinæ and Hyopsodontinæ in the Sespe Upper Eocene, California. Geology 20, 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch, G. (1978). Messel fossil finds part 14: Eomanis waldi (new genus, new species), a pangolin from the middle Eocene of the messel pit near darmstadt, West Germany (Mammalia, Pholidota). Senckenbergiana Lethaea 59, 503–530. [Google Scholar]
- Stucky, R. K. & Hardy, T. G. (2007). A new large, hypercarnivorous oxyaenid (Mammalia, Creodonta) from the middle Eocene of the Wind River Formation, Natrona County, Wyoming. Bulletin of Carnegie Museum of Natural History 39, 57–65. [Google Scholar]
- *Szalay, F. S. (1969). Mixodectidae, Microsyopidae, and the insectivore‐primate transition. Bulletin of the American Museum of Natural History 140, 195–330. [Google Scholar]
- Szalay, F. S. & Decker, R. L. (1974). Origins, evolution and function of the tarsus in Late Cretaceous Eutheria and Paleocene primates In Primate Locomotion (ed. Jenkins F. A.), pp. 223–259. Illustrated Academic Press, New York. [Google Scholar]
- *Tabuce, R. , Antunes, M. T. , Smith, R. & Smith, T. (2006). Dental and tarsal morphology of the European Palaeocene/Eocene “condylarth” mammal Microhyus . Acta Palaeontologica Polonica 51, 37–52. [Google Scholar]
- Tabuce, R. , Asher, R. J. & Lehmann, T. (2008). Afrotherian mammals: a review of current data. Mammalia 72, 2–14. [Google Scholar]
- Tabuce, R. , Clavel, J. & Antunes, M. T. (2011). A structural intermediate between triisodontids and mesonychians (Mammalia, Acreodi) from the earliest Eocene of Portugal. Naturwissenschaften 98, 145–155. [DOI] [PubMed] [Google Scholar]
- Tabuce, R. , Coiffait, B. , Coiffait, P. E. , Mahboubi, M. & Jaeger, J. J. (2001). A new genus of Macroscelidea (Mammalia) from the Eocene of Algeria: a possible origin for elephant‐shrews. Journal of Vertebrate Paleontology 21, 535–546. [Google Scholar]
- *Tabuce, R. , Marivaux, L. , Adaci, M. , Bensalah, M. , Hartenberger, J.‐L. , Mahboubi, M. , Mebrouk, F. , Tafforeau, P. & Jaeger, J.‐J. (2007). Early Tertiary mammals from North Africa reinforce the molecular Afrotheria clade. Proceedings of the Royal Society B: Biological Science 274, 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarver, J. E. & Donoghue, P. C. J. (2011). The trouble with topology: phylogenies without fossils provide a revisionist perspective of evolutionary history in topological analyses of diversity. Systematic Biology 60, 700–712. [DOI] [PubMed] [Google Scholar]
- Teeling, E. C. & Hedges, S. B. (2013). Making the impossible possible: rooting the tree of placental mammals. Molecular Biology and Evolution 30, 1999–2000. [DOI] [PubMed] [Google Scholar]
- Templeton, A. R. (1983). Phylogenetic inference from restriction endonuclease cleavage site maps with particular reference to the evolution of humans and the apes. Evolution 37, 221–244. [DOI] [PubMed] [Google Scholar]
- Theodor, J. M. & Foss, S. E. (2005). Deciduous dentitions of Eocene cebochoerid artiodactyls and cetartiodactyl relationships. Journal of Mammalian Evolution 12, 161–181. [Google Scholar]
- Thewissen, J. G. M. (1990). Evolution of Paleocene and Eocene Phenacodontidae (Mammalia, Condylarthra). Museum of Paleontology Papers on Paleontology III‐VIII, 1–107. [Google Scholar]
- *Thewissen, J. G. M. (1991). Limb osteology and function of the primitive Paleocene ungulate Pleuraspidotherium with notes on Tricuspiodon and Dissacus (Mammalia). Geobios 24, 483–495. [Google Scholar]
- *Thewissen, J. G. M. (1994). Phylogenetic aspects of cetacean origins: a morphological perspective. Journal of Mammalian Evolution 2, 157–183. [Google Scholar]
- *Thewissen, J. G. M. , Cooper, L. N. , Clementz, M. T. , Bajpai, S. & Tiwari, B. N. (2007). Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature 450, 1190–1194. [DOI] [PubMed] [Google Scholar]
- *Thewissen, J. G. M. & Domning, D. G. (1992). The role of phenacodontids in the origin of the modern orders of ungulate mammals. Journal of Vertebrate Paleontology 12, 494–504. [Google Scholar]
- *Thewissen, J. G. M. & Gingerich, P. D. (1989). Skull and endocranial cast of Eoryctes melanus, a new palaeoryctid (Mammalia, Insectivora) from the early Eocene of Western North America. Journal of Vertebrate Paleontology 9, 459–470. [Google Scholar]
- Thewissen, J. G. M. , Williams, E. M. , Roe, L. J. & Hussain, S. T. (2001). Skeletons of terrestrial cetaceans and the relationship of whales to artiodactyls. Nature 413, 277–281. [DOI] [PubMed] [Google Scholar]
- Ting, S. Y. , Bowen, G. J. , Koch, P. L. , Clyde, W. C. , Wang, Y. , Wang, Y. & McKenna, M. C. (2003). Biostratigraphic, chemostratigraphic, and magnetostratigraphic study across the Paleocene‐Eocene boundary in the Hengyang Basin, Hunan, China. Geological Society of America, Special Paper 369, 521–535. [Google Scholar]
- *Ting, S. Y. & Li, C. K. (1987). The skull of Hapalodectes (Acreodi, Mammalia), with notes on some Chinese Paleocene mesonychids. Vertebrata PalAsiatica 25, 161–186. [Google Scholar]
- Tomiya, S. (2011). A new basal caniform (Mammalia: Carnivora) from the middle Eocene of North America and Remarks on the Phylogeny of early Carnivorans. PLoS One 6(9), e24146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar, P. S. (2010). Mammal Teeth: Origin, Evolution, and Diversity. John Hopkins University Press, Baltimore. [Google Scholar]
- Vajda, V. , Raine, J. I. & Hollis, C. J. (2001). Indication of global deforestation at the Creataceous‐Tertiary boundary by New Zealand fern spike. Science 294, 1700–1702. [DOI] [PubMed] [Google Scholar]
- Van Valen, L. (1969). The multiple origins of the placental carnivores. Evolution 23, 118–130. [DOI] [PubMed] [Google Scholar]
- Van Valen, L. (1978). The beginning of the age of Mammals. Evolutionary Theory 4, 46–80. [Google Scholar]
- Van Valen, L. & Sloan, R. E. (1965). The earliest Primates. Science 150, 743. [DOI] [PubMed] [Google Scholar]
- Van Valkenburgh, B. (1999). Major patterns in the history of carnivorous mammals. Annual Review of Earth and Planetary Sciences 27, 463–493. [Google Scholar]
- Venditti, C. , Meade, A. & Pagel, M. (2011). Multiple routes to mammalian diversity. Nature 479, 393–396. [DOI] [PubMed] [Google Scholar]
- Waddell, P. J. , Cao, Y. , Hauf, J. & Hasegawa, M. (1999). Using novel phylogenetic methods to evaluate mammalian mtDNA, including amino acid invariant sites LogDet plus site stripping, to detect internal conflicts in the data, with special reference to the positions of hedgehog, armadillo, and elephant. Systematic Biology 48, 31–53. [DOI] [PubMed] [Google Scholar]
- Waddell, P. J. , Kishino, H. & Ota, R. (2001). A phylogenetic foundation for comparative mammalian genomics. Genome Informatics International Conference on Genome Informatics 12, 141–154. [PubMed] [Google Scholar]
- *Webb, S. D. & Taylor, B. E. (1980). The phylogeny of hornless ruminants and a description of the cranium of Archaeomeryx . Bulletin of the American Museum of Natural History 167, 121–157. [Google Scholar]
- Welker, F. , Collins, M. J. , Thomas, J. A. , Wadsley, M. , Brace, S. , Cappellini, E. , Turvey, S. T. , Reguero, M. , Gelfo, J. N. , Kramarz, A. , Burger, J. , Thomas‐Oates, J. , Ashford, D. A. , Ashton, P. D. , Rowsell, K. , Porter, D. M. , Kessler, B. , Fischer, R. , Baessmann, C. , Kaspar, S. , Olson, J. V. , Kiley, P. , Elliott, J. A. , Kelstrup, C. D. , Mullin, V. , Hofreiter, M. , Willerslev, E. , Hublin, J.‐J. , Orlando, L. , Barnes, I. & MacPhee, R. D. E. (2015). Ancient proteins resolve the evolutionary history of Darwin's South American ungulates. Nature 522, 81–84. [DOI] [PubMed] [Google Scholar]
- Wesley‐Hunt, G. D. (2005). The morphological diversification of carnivores in North America. Paleobiology 31, 35–55. [Google Scholar]
- *West, R. M. (1970). Tetraclaenodon puercensis (Mammalia: Phenacodontidae), Goler Formation, Paleocene of California, and distribution of the genus. Journal of Paleontology 44, 852–857. [Google Scholar]
- *Wible, J. R. , Novacek, M. J. & Rougier, G. W. (2004). New data on the skull and dentition in the Mongolian late Cretaceous Eutherian mammal Zalambdalestes . Bulletin of the American Museum of Natural History 281, 1–144. [Google Scholar]
- Wible, J. R. , Rougier, G. W. & Novacek, M. J. (2005). Anatomical evidence for superordinal/ordinal eutherian taxa in the Cretaceous In The Rise of Placental Mammals: Origins and Relationships of the Major Extant Clades (eds Rose K. D. and Archibald J. D.). John Hopkins University Press, Baltimore, 15–36. [Google Scholar]
- Wible, J. R. , Rougier, G. W. , Novacek, M. J. & Asher, R. J. (2007). Cretaceous eutherians and Laurasian origin for placental mammals near the K/T boundary. Nature 447, 1003–1006. [DOI] [PubMed] [Google Scholar]
- Wible, J. R. , Rougier, G. W. , Novacek, M. J. & Asher, R. J. (2009). The eutherian mammal Maelestes gobiensis from the late cretaceous of mongolia and the phylogeny of Cretaceous Eutheria. Bulletin of the American Museum of Natural History 327, 1–123. [Google Scholar]
- Wiens, J. J. (2001). Character analysis in morphological phylogenetics: problems and solutions. Systematic Biology 50, 689–699. [DOI] [PubMed] [Google Scholar]
- Wilkinson, M. (1992). Ordered versus unordered characters. Cladistics‐the International Journal of the Willi Hennig Society 8, 375–385. [DOI] [PubMed] [Google Scholar]
- Williamson, T. E. & Carr, T. D. (2007). Bomburia and Ellipsodon (Mammalia : Mioclaenidae) from the early Paleocene of new Mexico. Journal of Paleontology 81, 966–985. [Google Scholar]
- Williamson, T. E. & Lucas, S. G. (1992). Meniscotherium (Mammalia: “Condylarthra”) from the Paleocene‐Eocene of Western North America. New Mexico Museum of Natural History Scientific Bulletin 1, 1–75. [Google Scholar]
- Williamson, T. E. & Weil, A. (2011). A new puercan (early Paleocene) hyopsodontid “condylarth” from New Mexico. Acta Palaeontologica Polonica 56, 247–255. [Google Scholar]
- Williamson, T. E. , Weil, A. & Standhardt, B. (2011). Cimolestids (Mammalia) from the early Paleocene (Puercan) of New Mexico. Journal of Vertebrate Paleontology 31, 162–180. [Google Scholar]
- Wilson, G. P. (2014). Mammalian extinction, survival, and recovery dynamics across the Cretaceous‐Palaeogene boundary in north‐eastern Montana, USA. Geological Society of America Special Papers 503, 365–392. [Google Scholar]
- Wood, H. M. , Matzke, N. J. , Gillespie, R. G. & Griswold, C. E. (2013). Treating fossils as terminal taxa in divergence time estimation reveals ancient vicariance patterns in the palpimanoid spiders. Systematic Biology 62, 264–284. [DOI] [PubMed] [Google Scholar]
- Yongsheng, T. (1988). Fossil tree shrews from the eocene Hetaoyuan Formation of Xichuan, Henan. Vertebrata PalAsiatica 26, 214–220. [Google Scholar]
- Zack, S. P. (2004). An early eocene arctostylopid (Mammalia: Arctostylopida) from the Green River Basin, Wyoming. Journal of Vertebrate Paleontology 24, 498–501. [Google Scholar]
- Zack, S. P. (2009). The phylogeny of eutherian mammals: a new analysis emphasizing dental and postcranial morphology of paleogene taxa. PhD Thesis: John Hopkins University.
- Zack, S. P. (2011). New Species of the rare early Eocene creodont Galecyon and the radiation of early Hyaenodontidae. Journal of Paleontology 85, 315–336. [Google Scholar]
- Zack, S. P. , Penkrot, T. A. , Bloch, J. I. & Rose, K. D. (2005a). Affinities of 'hyopsodontids' to elephant shrews and a Holarctic origin of Afrotheria. Nature 434, 497–501. [DOI] [PubMed] [Google Scholar]
- Zack, S. P. , Penkrot, T. A. , Krause, D. W. & Maas, M. C. (2005b). A new apheliscine “condylarth” mammal from the late Paleocene of Montana and Alberta and the phylogeny of “hyopsodontids”. Acta Palaeontologica Polonica 50, 809–830. [Google Scholar]
- *Zan, S. , Wood, C. B. , Rougier, G. W. , Jin, L. , Chen, J. & Schaaf, C. R. (2006). A new “middle” Cretaceous zalambdalestid mammal, from a new locality in Jilin Province, Northeastern China. Journal of the Paleontological Society of Korea 22, 153–172. [Google Scholar]
- *Zheng, J. J. (1979). Notoungulata from the Paleocene of Jiangsu, South China In Mesozoic and Cenozoic Red Beds of South China (eds IVPP ), pp. 387–394. Science Press, Marrickville. [Google Scholar]
- Zhou, X. , Xu, S. , Xu, J. , Chen, B. , Zhou, K. & Yang, G. (2012). Phylogenomic analysis resolves the interordinal relationships and rapid diversification of the laurasiatherian mammals. Systematic Biology 61, 150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Zhou, X. , Zhai, R. , Gingerich, P. D. & Chen, L. (1995). Skull of a new mesonychid (Mammalia: Mesonychia) from the late Paleocene of China. Journal of Vertebrate Paleontology 15, 387–400. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Consensus topology deriving from the CU analysis.
Fig. S2. Consensus topology deriving from the DM analysis.
Fig. S3. Consensus topology deriving from the DP analysis.
Fig. S4. Consensus topology deriving from the CF analysis.
Fig. S5. Consensus topology deriving from the CM analysis.
Fig. S6. Consensus topology deriving from the CP analysis.
Fig. S7. Bremer support tree from the DF analysis.
Fig. S8. Bremer support tree from the DM analysis.
Fig. S9. Bremer support tree from the DP analysis.
Fig. S10. Bremer support tree from the CF analysis.
Fig. S11. Bremer support tree from the CM analysis.
Fig. S12. Bremer support tree from the CP analysis.
Appendix S1. Specimens used in the coding of this matrix, complete with specimen number, genus and species‐level affiliation, and the literature from which codings were assessed, where relevant, including the figure number.
Appendix S2. Matrix used for the phylogenetic analyses which resulted in the DU, DF, DM and DP sets of most‐parsimonious trees, formatted to be openable in TNT.
Appendix S3. Matrix used for the phylogenetic analyses which resulted in the CU, CF, CM and CP sets of most‐parsimonious trees, formatted to be openable in TNT.
Appendix S4. List of all 680 characters with descriptions of each character state, the proximal and ultimate sources of that character, and the treatment of that character – whether it is continuous or discrete, and ordered or unordered.
Appendix S5. Additional topologies recovered in the DM, CM, DP and CP analyses.
Appendix S6. List of unambiguous synapomorphies of commonly recovered clades.


