Summary
Obesity before and during pregnancy leads to reduced offspring cardiometabolic health. Here, we systematically reviewed animal experimental evidence of maternal obesity before and during pregnancy and offspring anthropometry and cardiometabolic health. We systematically searched Embase and Medline from inception until January 2018. Eligible publications compared offspring of mothers with obesity to mothers with a normal weight. We performed meta‐analyses and subgroup analyses. We also examined methodological quality and publication bias. We screened 2543 publications and included 145 publications (N = 21 048 animals, five species). Essential methodological details were not reported in the majority of studies. We found evidence of publication bias for birth weight. Offspring of mothers with obesity had higher body weight (standardized mean difference (SMD) 0.76 [95% CI 0.60;0.93]), fat percentage (0.99 [0.64;1.35]), systolic blood pressure (1.33 [0.75;1.91]), triglycerides (0.64 [0.42;0.86], total cholesterol (0.46 [0.18;0.73]), glucose level (0.43 [0.24;0.63]), and insulin level (0.81 [0.61;1.02]) than offspring of control mothers, but similar birth weight. Sex, age, or species did not influence the effect of maternal obesity on offspring's cardiometabolic health. Obesity before and during pregnancy reduces offspring cardiometabolic health in animals. Future intervention studies should investigate whether reducing obesity prior to conception could prevent these detrimental programming effects and improve cardiometabolic health of future generations.
Keywords: Cardiometabolic health, obesity, pregnancy, systematic review
Abbreviations
- BF%
Body fat percentage
- BMI
Body mass index
- CI
Confidence interval
- DBP
Diastolic blood pressure
- FM
Fat mass
- HDL
High‐density lipoprotein
- HOMA‐IR
Homeostatic model assessment for insulin resistance
- IQR
Inter quartile range
- LDL
Low‐density lipoprotein
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐analysis
- SBP
Systolic blood pressure
- SD
Standard deviations
- SE
Standard error
- SMD
Standardized mean difference
- SYRCLE
SYstematic Review Center for Laboratory animal Experimentation
1. INTRODUCTION
Obesity has reached epidemic proportions, and its worldwide prevalence has nearly tripled since 1975.1 Estimates from the World Health Organization indicated that, in 2016, more than 1.9 billion adults were overweight, of whom over 650 million were obese.1 The rise in obesity prevalence has been most prominent in women of reproductive age.2, 3 Obesity in pregnancy increases maternal and neonatal morbidity including preterm birth, congenital anomalies, gestational diabetes, preeclampsia, and increased caesarean section rate.4, 5, 6 More recently, epidemiological studies have shown that offspring of mothers with obesity are themselves at increased risk of obesity, cardiometabolic morbidity, and all‐cause mortality, which is thought to occur independently of genetic transmission of poor health.7, 8 In maternal obesity, the intrauterine environment is hypothesized to play a key role in the mediation of these effects on offspring's health—a concept that has been termed developmental programming.
However, it is still unclear whether maternal obesity is causal to diminished offspring health, primarily because associations from observational studies are subject to confounding. For example, various socio‐demographic, nutritional, lifestyle, and genetic factors may determine both maternal pre‐pregnancy body mass index (BMI) and offspring's disease risk.9 Therefore, animal models, which employ a common genetic background, carefully controlled dietary and activity conditions, and controlled postnatal environments are imperative for examining how obesity before and during pregnancy increases the development of obesity, insulin resistance, and cardiometabolic disease in the offspring.
Narrative reviews of animal experiments have described the detrimental effects of maternal obesity on offspring's health.10, 11, 12 While valuable, they can be hindered by subjective selection and lack of appropriate publication bias assessment.13 Existing systematic reviews of animal experiments focused on the effect of an obesogenic diet at any time during gestation instead of focusing on the effects of maternal obesity already present prior to conception.14, 15 Animals models where obesity is present before and during pregnancy are more reflective of the offspring effects in conceiving women with obesity. Our objective was to systematically review the available evidence provided by animal experiments on the effect of maternal obesity before and during pregnancy on offspring's anthropometry, cardiovascular, and metabolic outcomes.
2. METHODS
The conduct and reporting of this review adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) statement.16 Furthermore, this review was conducted in collaboration with SYstematic Review Center for Laboratory animal Experimentation (SYRCLE).
2.1. Study protocol
The review protocol (first version at 28 August 2015 and updated version at 3 January 2017) was registered at the website of SYRCLE on 11 January 2017 and can be accessed via the website: http://www.syrcle.nl. A few amendments were applied to the study protocol, as outlined in table S1.
2.2. Literature search
A medical information specialist (J.L.) performed a systematic search in OVID MEDLINE (including Epub Ahead of Print, In‐Process, and Other Non‐Indexed Citations) and OVID EMBASE from inception to 30 January 2018 (final update) using both controlled terms (i.e., MESH) and text words. To keep the search broad, so relevant articles would not be missed, we did not include outcomes in our search, but searched for the following concepts: (1) animals; (2) prenatal/maternal exposure (including maternal/intrauterine AND offspring); and (3) obese (including high fat diet and maternal/body weight/mass). Reviews, editorials, and conference abstracts (the latter only in EMBASE) were excluded. No further restrictions were applied. We cross‐checked the reference lists and the citing studies via Web of Science for relevant publications and review studies. The bibliographic records retrieved were imported and de‐duplicated in Endnote. The complete search strategies are presented in file S1.
2.3. Selection process
Two reviewers independently screened all identified studies for eligibility using Covidence.17 We first screened titles and abstracts of all unique studies for eligibility in duplo (M.M. screened all; C.vd.B. screened 70% and S.M. and C.F. screened 15% each). Secondly, we performed eligibility screening of full text of studies deemed possibly eligible after title and abstract screening (M.M. and S.M.). Disagreements were resolved by discussion or by consulting a third reviewer (R.P.).
2.4. Eligibility criteria
We only included original animal studies. There was no limitation in animal species. Studies were eligible if they compared anthropometry and/or cardiometabolic outcomes of offspring born to females that were obese before and during pregnancy to offspring born to females that had a normal weight before and during pregnancy. Maternal obesity was defined as a statistically significant higher body weight or a higher fat mass of experimental females compared with control females. For a study to be eligible, higher body weight and/or fat mass needed to be present before pregnancy (defined as prior to mating or at mating), to ensure that offspring were exposed to maternal obesity during the entire gestational period. Authors were contacted if they stated that maternal weight or fat mass was measured prior to conception but weight/fat mass was not reported in the study. The outcome variables in the offspring were birth weight, body weight, body fat percentage (BF%), fat mass (FM), systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), triglycerides, total cholesterol, high‐density lipoprotein (HDL) cholesterol, low‐density lipoprotein (LDL) cholesterol, glucose, insulin, and homeostatic model assessment for insulin resistance (HOMA‐IR).
We excluded studies for the following reasons: (1) different postnatal environments between the intervention and control group; (2) no normal or chow diet of the offspring from weaning onwards; (3) an additional disease factor in the mother such as severe diabetes; (4) interventions potentially interfering with the primary effect of maternal obesity (eg, postnatal leptin injections); (5) lack of a control group with a normal weight and normal diet (as defined by the authors of the study); (6) only data on molecular, epigenetic, or fetal effects; (7) mothers who were made obese by genetic factors or selective breeding; and (8) reviews, editorials, conference abstracts, and interviews. When data were published in duplicate (eg, identical values in multiple journals), we included the data from the first published study only.
2.5. Data extraction
Two reviewers (initial search: S.M. and C.F.; update search: S.M., M.M., and E.F.) extracted data using a piloted data‐extraction form. Due to limited resources, we extracted 10% of the studies in duplo, in which there was minimal discrepancy (2%). We extracted the following data: (1) general characteristics of the study; (2) animal species and strain; (3) obesity generating model; (4) mating age; (5) dietary information of the parental animals and their offspring; (6) sex of the offspring; (7) litter size adjustment; and (8) cross‐fostering of the offspring. We present a summary of these characteristics in table S2.
Outcome values were extracted for data analyses in terms of means, standard deviations (SDs) or standard errors (SEs), number of animals (N), and age at time of outcome assessment. If an outcome was measured at multiple time points in the same animal, we extracted the last measurement because our focus was on the long‐term effects of maternal obesity on the offspring. If results were only displayed graphically, we read the outcomes as precisely as possible using a digital screen ruler (Adobe Acrobat XI pro), extracting the most conservative estimate. If the relevant values were not extractable, we contacted the authors for more information.
2.6. Quality assessment and risk of publication bias
The methodologic quality of all selected studies was evaluated by evaluating six of the 10 questions of the SYRCLE risk of bias tool for animal studies. The SYRCLE risk of bias tool is based on the Cochrane Risk of Bias tool and has been adjusted for aspects of bias that play a specific role in animal intervention studies.18 First, we determined whether three key characteristics of scientific reporting were mentioned in the study (reporting questions): “randomization,” “blinding,” and “power calculation.” If studies only reported “blinding” or “power calculation” concerning outcomes not of interest to our study, we scored it as “no.” When the key characteristics were mentioned in the study, we hand searched the full text for the answers to the corresponding risk of bias questions of the SYRCLE Risk of Bias tool. We did not exclude studies based on poor quality. The answers to all questions were displayed separately, and no aggregated quality was determined (table S3). We performed Duval and Tweedie's Trim and Fill analysis to investigate possible publication bias, when a minimum of 15 studies were available on any particular outcome.19 We used the inverse of the standard error as precision estimate.20
2.7. Data analysis
The statistical analyses and forest plots were conducted using Review Manager (RevMan, Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We performed meta‐analyses for each outcome with more than two studies available. Studies were excluded from meta‐analyses when not all outcome data (mean, SD, and N) could be obtained. We calculated the standardized mean difference (SMD) and 95% confidence interval (95% CI) for each separate intervention‐control comparison group with Hedges' g correction.21 If a study contained more than one experimental group of animals with obesity with similar methods of obesity induction that were compared with the control group, then the experimental groups were pooled. In the meta‐analysis, the individual SMDs were pooled to obtain an overall SMD and 95% CI for the respective outcome. Due to anticipated heterogeneity, we used a random effects model for the meta‐analyses. Heterogeneity between studies was assessed by the I2 statistics.22 We accepted any degree of heterogeneity for meta‐analyses. We defined low, moderate, and high heterogeneity according to I2 cut‐offs of 25%, 50%, and 75%, respectively.23 We performed pre‐defined subgroup analyses for sex (male, female, overall), age (infancy, juvenile, adult), and species (rodents, non‐rodents) provided the subgroups contained a minimum of three independent studies. We considered P‐values of less than 0.05 as statistically significant. For the subgroup analyses, we adjusted our significance level according to the conservative Bonferroni method to account for multiple analyses (p* number of comparisons).24 If the direction of the effect between subgroups were statistically significantly different from each other, we considered the subgroups to (partly) explain the observed heterogeneity.
3. RESULTS
3.1. Search results
The systematic literature search yielded 2543 unique references (Figure 1, PRISMA flowchart). Three studies that appeared eligible after title and abstract screening were not retrievable, also after contacting the authors. Of the remaining 540, 396 studies failed to meet the inclusion criteria and were excluded (reasons indicated in Figure 1). We included one additional study after searching the references of relevant publications. Eventually, we included 145 studies for data extraction (see supplementary file 2). There were a total of 21 048 animals in 474 comparisons across 13 outcomes included in meta‐analyses.
Figure 1.
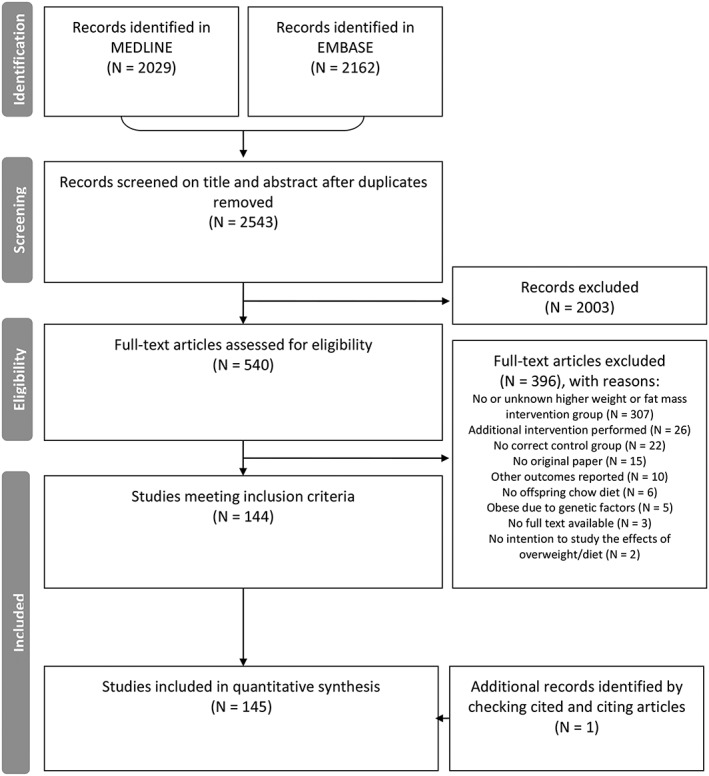
PRISMA flowchart
3.2. Study characteristics
A general description of the included studies is provided in table S2. Most studies reported on rodents (rats N = 76, mice N = 59). Other studies reported on sheep (N = 5), pigs (N = 3), and non‐human primates (N = 2). The median time of obesity induction was 6 weeks before mating (inter quartile range [IQR] 5.0; 8.7, N = 136 reported). Median age at mating was 13 weeks (IQR 11.2; 16.0, N = 107 reported). Maternal obesity was defined as an increased maternal body weight in the intervention group relative to the control group in most studies (N = 138, 95%), and as higher fat mass in a minority of studies (N = 7, 5%).
Outcomes reported on in studies were offspring's anthropometry (N = 139, 96%), blood pressure (N = 11, 8%), glucose homeostasis (including glucose and insulin levels) (N = 87, 60%), and lipid profile (N = 51, 35%). Five studies (3%) reported on all four outcome categories. The largest number of studies reported on offspring's body weight (N = 123, 85%) and insulin (N = 70, 48%), while a limited number of studies reported on HDL cholesterol (N = 5), DBP (N = 4), and LDL cholesterol (N = 4). The largest number of offspring was included in the meta‐analysis on birth weight (N = 6530 animals, N = 63 studies), and the smallest number on LDL cholesterol (N = 78 animals, N = 4 studies). Figure 2 provides overall effect estimates of maternal obesity for each offspring outcome.
Figure 2.
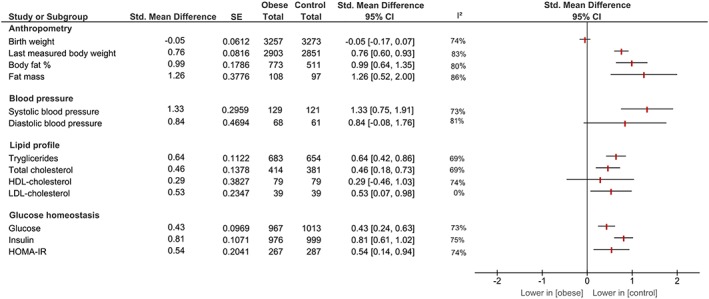
Effect estimates of maternal obesity before and during pregnancy on offspring outcomes [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.3. Anthropometry
Birth weight was obtained from N = 63 studies (N = 6530 offspring), one study was included twice in meta‐analysis since they reported on two separate cohorts.25 In six studies, data on birth weight was not extractable.26, 27, 28, 29, 30, 31 There was no difference in birth weight between offspring of mothers with obesity and offspring of mothers with a normal weight, SMD −0.05 [95% CI −0.17; 0.07], I2 = 74% (figure S1).
Body weight of offspring was obtained from N = 123 studies (N = 5772 offspring), two studies were included twice in the meta‐analysis since they reported on two separate cohorts.25, 32 Body weight was measured at age 4 days up to 13 months. We were unable to extract outcome data from four studies.26, 31, 33, 34 Offspring's body weight was increased following maternal obesity, SMD 0.76 [95% CI 0.60; 0.93], I2 = 86% (figure S2).
BF% was obtained from N = 32 studies (N = 1284 animals) and measured from birth up to 22 months of age. We were unable to extract data from one study.35 The meta‐analysis showed higher BF% in offspring of mothers with obesity, SMD 0.99 [95% CI 0.64; 1.35], I2 = 83% (figure S3). Data obtained from 10 studies showed higher FM in offspring of mothers with obesity, SMD 1.26 [95% CI 0.52; 2.00], I2 = 80% (figure S4).
3.4. Blood pressure
Blood pressure was measured via a tail cuff (N = 6 studies) or invasively by remote radio‐telemetry after surgical implantation of carotid artery probes (N = 5 studies).36, 37, 38, 39, 40 Effect estimates did not differ substantially between both methods.
SBP was obtained from nine studies, including N = 251 animals between 8 and 24 weeks of age. Offspring of mothers with obesity had higher SBP compared with offspring of control mothers, SMD 1.33 [95% CI 0.75; 1.91], I2 = 73% (figure S5). Offspring's DBP was only reported in four studies, including N = 129 animals between 12 and 23 weeks of age, and the difference between offspring of mothers with obesity and offspring of mothers with a normal weight did not achieve statistical significance, SMD 0.84 [95% CI −0.08; 1.74], I2 = 81% (figure S6). MAP was measured invasively, and one of two studies reported a statistically significantly higher MAP in offspring of mothers with obesity.39, 40
3.5. Lipid profile
Data on offspring triglyceride levels were available from N = 46 studies, including N = 1337 animals between 1 day and 12 months of age. We could not obtain data from one study.41 Offspring of mothers with obesity had higher triglycerides levels compared with offspring of control mothers, SMD 0.64 [95% CI 0.42; 0.86], I2 = 69% (figure S7).
Data from N = 27 articles, including N = 795 animals between 21 days and 12 months of age, showed that offspring of mothers with obesity had higher total cholesterol levels compared with offspring of control mothers, SMD 0.46 [95% CI 0.18; 0.73], I2 = 69% (figure S8).
No difference in HDL cholesterol between offspring of mothers with obesity and offspring of mothers with a normal weight was observed in five studies, including N = 158 animals between 28 days and 12 months of age, with a SMD of 0.29 [95% CI −0.46; 1.03], I2 = 74% (figure S9).
Four studies reporting on offspring LDL cholesterol, N = 78 animals between 28 days and 12 months of age, indicated that maternal obesity increased offspring LDL cholesterol levels, SMD 0.53 [0.07; 0.98], I2 = 0% (figure S10).
3.6. Glucose homeostasis
Sixty‐eight studies, including N = 1980 animals, reported on offspring's glucose levels at birth until 12 months of age. One study was included twice in the meta‐analysis since they reported on two different cohorts.25 In five studies, we were unable to extract outcome data.33, 34, 42, 43, 44 Glucose was higher in offspring of mothers with obesity compared with offspring of control mothers, SMD 0.43 [95% CI 0.24; 0.63], I2 = 73% (figure S11).
Data from N = 70 studies, including N = 1975 animals from birth to 12 months of age, showed higher insulin levels in offspring of mothers with obesity, SMD 0.81 [95% CI 0.61; 1.02], I2 = 75% (figure S12). We could not extract offspring insulin levels from one study.42
Thirteen studies reported on HOMA‐IR, including N = 554 animals between 20 days and 6 months of age. HOMA‐IR was higher in offspring of mothers with obesity compared with control mothers, SMD 0.54 [95% CI 0.14; 0.94], I2 = 74% (figure S13).
3.7. Subgroup analyses
To account for the possible moderating effects of offspring sex (male, female, overall; table S5 and figure S14‐S24), age (infancy, juvenile, adult; table S6 and figure S25‐S29), and species (rodents, non‐rodents; table S7 and figure S30‐S32), we performed subgroup analyses for these determinants for all of the outcome variables (as predefined in our protocol). Effect estimates were similar for subgroups, indicating no differences based on offspring's sex, age, or species (Figure 3A‐C).
Figure 3.
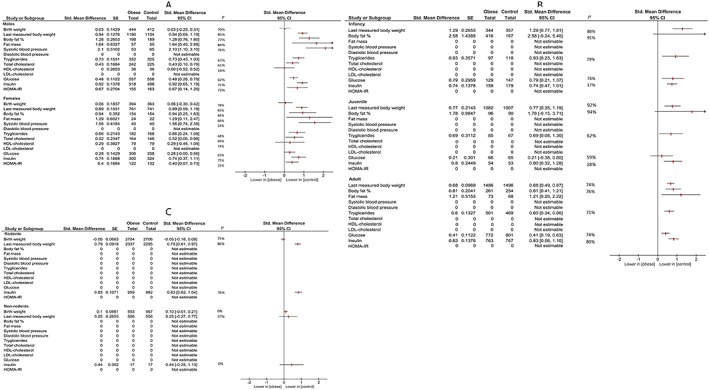
Effect estimates of maternal obesity before and during pregnancy on offspring outcomes per sex (A), age (B), and species (C) subgroups [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.8. Study quality and publication bias
The results of the quality assessment of the included studies are shown in table S3. Of the 71 (49%) studies reporting on randomization, none reported the method of randomization. Five studies (3%) reported on blinding, of which only one specified blinding of outcome assessment,45 and one reported the investigators were not blinded.46 Four studies (3%) reported that a power calculation for one of the outcomes used in our meta‐analyses had been performed.
Publication bias was analysed by inspection of the funnelplots and trim and fill analyses. Only for birth weight publication bias was suggested (figure S33). Inspection of the funnelplot of birthweight showed significant asymmetry and trim and fill imputed 19 “missing” studies (red dots). The adjusted overall effect size for birthweight is −0.35 [95% CI −0.48; −0.22] (note: the SMD in the trim and fill analysis is based on a different precision estimate, i.e., 1/√N instead of SE).20
4. DISCUSSION
In this meta‐analysis examining animal experiments of maternal obesity before and during pregnancy, we found that maternal obesity induced poorer cardiometabolic health in the offspring. More specifically, based on data of 145 included studies (N = 21 048 animals of five species), we conclude that maternal obesity leads to higher adiposity and systolic blood pressure and negatively affects lipid, insulin, and glucose homeostasis in the offspring. Maternal obesity does not influence offspring birth weight. The effect of maternal obesity on offspring cardiometabolic health was independent of offspring sex, age, or species.
Our findings are in line with a systematic review of animal experiments showing that a maternal obesogenic diet during pregnancy negatively affects offspring's body weight, body composition, and glucose homeostasis.15 Effect sizes from a meta‐regression of maternal high‐fat diet during pregnancy on offspring adult body weight, adiposity, total cholesterol, triglycerides, and insulin were in the same direction and magnitude as our findings; however, in contrast to our findings, there was no statistically significant difference for glucose in males.14 Similar to these animal systematic reviews,14, 15 but in contrast to human studies,47, 48 no effect of maternal obesity on birth weight was found. This could be due to the fact that rodents are born relatively immature compared with humans,49 and in humans fetal adiposity increased most rapidly in the last weeks of gestation.50, 51
This systematic review adds to the evidence that not only an obesogenic diet during pregnancy but also obesity induced before pregnancy has negative effects on offspring cardiometabolic health. Also, since all offspring were fed a normal diet after weaning, we were able to show that the diminished offspring health was independent of offspring's own diet. Similar to our findings, observational data of human cohort studies show that children from mothers with obesity have higher risks of infant and childhood overweight/obesity,47, 52 and cardiometabolic disease later in life.53, 54 However, in human observational studies, causality cannot be inferred because it is difficult to differentiate direct effects from residual confounding.55 Animal experiments overcome this difficulty. The results of this systematic review of animal experiments—maternal obesity is causal to offspring adverse cardiometabolic health—do suggest that maternal obesity is indeed likely causal to poorer offspring cardiometabolic health in humans.53
4.1. Potential mechanisms
The effect of maternal obesity on adverse cardiometabolic health in the offspring may be explained by several mechanisms, including epigenetic changes, metabolic factors, inflammatory pathways and direct structural effects, or a combination of these.4
Epigenetic mechanisms describe altered expression of genes without altering the DNA sequence. Mostly animal experiments indicate that fetal exposure to maternal obesity induces variance in microRNAs, DNA methylation, and histone modification.56, 57, 58 These processes, which influence gene expression, are largely established during fetal development and affected by the intrauterine enviroment.59 In turn, these epigenetic changes were associated with increased hepatic triglycerides in primates and altered mouse brain dopamine and opioid gene expression related to food behavior.60, 61 Evidence from a limited number of human observation studies indicate that similar mechanisms may be at work in the human situations; while no global effect on methylation in obesity was seen, there were several associations between specific methylation sites at birth and obesity later in life.62
Second, accelerated fetal pancreatic maturation, induced by maternal obesity‐related hyperglycemia, may lead to premature loss of ß‐cells and consequently lead to permanently impaired glucose and insulin homeostasis in offspring.63 Additionally, both high fetal or neonatal insulin, leptin, or lipid levels may in part induce hypothalamic programming,64, 65 leading to hyperphagia and altered satiety response.63, 66, 67 Also, maternal obesity during pregnancy may induce fetal adipocyte hypertrophy and by upregulating lipoprotein lipase and peroxisome proliferator‐activated receptor gamma (PPARy) activity in the adipocyte modify their fatty acid composition.10, 68, 69 This could lead to obesity and metabolic disturbances in the offspring later in life.
Third, the low‐grade inflammation seen in obesity could also contribute to offspring's risks of cardiometabolic disease, since upregulation of inflammatory markers is seen in children of mothers with obesity, independent of offspring's BMI.70, 71
Lastly, direct structural effects of maternal obesity include fetal cardiac hypertrophy72, 73 and aortic stiffness,74 each of which predispose to hypertension. This risk of hypertension is further augmented by selective leptin resistance and increased sympathetic nervous system activity in the offspring.75
4.2. Clinical relevance
No animal model perfectly represents the human situation, and this limits the generalizability of our results to the human situation. For example, there is no animal model that fully resembles human placentation.76 Additionally, in the vast majority of the included animal experiments, maternal obesity was induced by high‐fat diets. While these diets do induce obesity and metabolic disorders that resemble human obesity, there is heterogeneity in their composition and lack of standardization.77 Moreover, human obesity may be more strongly related to high carbohydrate intake than fat intake, and the role of (low) physical activity is often not accounted for in animal studies.78, 79 Nevertheless, for most outcomes, there was a large evidence base containing multiple species with comparable direction of effects increasing our confidence that maternal obesity before and during pregnancy causes obesity and hypertension and negatively affects lipid and glucose homeostasis also in human offspring.
Animal experiments have suggested a transgenerational effect of obesity: maternal obesity may not only affect the offspring, but the negative effects may be carried over to subsequent generations.80, 81, 82 For example, the second generation of obese overnourished ewes showed increased adiposity and higher blood glucose and insulin levels compared with controls, independent of their mother's weight.81 Thus, our findings have a profound public health implication. Our results could be useful in developing strategies to prevent adverse health outcomes among children of mothers with obesity.53 Whether interventions aimed at reducing obesity in women of childbearing age could improve maternal and child's health needs to be investigated further. Some animal experiments provide evidence that exercise or dietary interventions during pregnancy could reverse the detrimental effects of maternal obesity on the offspring,83, 84, 85, 86, 87 with potential sex differences.85, 87 In humans however, only a limited amount of studies examined the consequences of reducing maternal obesity on offspring health. In observational studies, maternal weight loss through bariatric surgery was associated with lower adiposity and improved lipids in offspring.88, 89 However, only two lifestyle intervention showed a favorable effect on infants,90, 91 while others found no effect on childhood anthropometry and cardiometabolic health.92
4.3. Strengths and limitations
Strengths of this research are that we performed a broad and inclusive search of the available literature. Second, we only included studies where maternal obesity was present before and during pregnancy, which more closely resembles human conditions. Third, we were able to exclude possible confounding postnatal effects; i.e., we included only the offspring fed a normal diet after weaning.
However, there are several methodological limitations that may impact the generalizability and validity of our findings. First, the vast majority of the studies used rodent models. Larger animal models could be of closer parallel to human development.93 Subgroup analysis of rodents versus other species was only possible for selected outcomes (birth weight, body weight, insulin), due to limited numbers of non‐rodent models. While these subgroup analyses showed a similar direction of effect, future studies including non‐rodent models are needed to establish whether non‐human primates for example do in fact display the same responses to maternal obesity as rodents.94
Second, studies reported poorly on important methodological details (randomization, blinding, and power calculation). This hampered reliable risk of bias assessment and may reduce the reliability of our conclusions. Therefore, consistent reporting of essential details regarding experimental design, as described for example in the ARRIVE guidelines,95 is needed in future animal studies.
Third, although we accounted for heterogeneity by using a random rather than a fixed effect model for meta‐analysis, variation between the studies was high. A recent study has suggested evidence of sex‐dependent differences in pathways related to CVD development.96 Our subgroup analyses based on offspring sex showed no differences in effects; however, not all studies could be included because of missing data on males and females separately. Further, we performed pre‐specified subgroup analyses for age and species of the offspring, but these factors also did not explain the heterogeneity. Other factors, such as the maternal diet during lactation and timing of exposure, may explain the variation observed.14 Standardizing dietary composition and duration and intensity of maternal obesity induction might reduce this form of heterogeneity and may help unravel modifying factors of the effect of maternal obesity on the offspring.77
Fourth, we only observed potential publication bias for the outcome birth weight by trim and fill analysis. When we adjusted for publication bias, we observed a significant lower birth weight in offspring of mothers with obesity. Since the trim and fill method may inappropriately adjust for publication bias when there is substantial between‐study heterogeneity,97, 98 the true effect of maternal obesity on offspring's birth weight remains uncertain.
5. CONCLUSION
In conclusion, this systematic review of animal experiments provides strong evidence that maternal obesity before and during pregnancy causes obesity and hypertension and negatively affects lipid and glucose homeostasis in the offspring in animals. These findings are important since the incidence of obesity among women of child bearing age is increasing. Targeting obesity in women in the pre‐pregnancy period could play a pivotal role to improve health in future generations and warrants intervention studies. Yet, issues need to be addressed, including the translation of the results to human populations.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
R.P. conceived the study. J.L. performed the systematic search. M.M., S.M., C.vd.B., and C.F. performed the screening and data extraction process. M.M. and S.M. performed the analyses with methodological help of C.H., A.v.D., and R.P. and drafted the initial versions of the manuscript, which were revised critically by the other authors C.vd.B., S.O., J.L., T.R., C.H., A.v.D. All authors approved the final version of the manuscript.
FUNDING
This work was supported by a grant of the Dutch Heart Foundation (2013T085), DynaHealth H2020 grant (agreement no 633595), and by a grant of Netherlands Organization for Health Research and Development (project number 114024105). The funders had no role in the design, conduct, and interpretation of the study.
Supporting information
Supplementary table 1. Amendments of the study protocol
Supplementary table 2. Study characteristics of the included studies
Supplementary table 3. Reporting of randomization, blinding and power calculation and risk of bias in the included studies
Supplementary table 4. Effect estimates of maternal obesity before and during pregnancy on offspring outcomes.
Supplementary table 5. Effect estimates of maternal obesity before and during pregnancy on offspring outcomes with subgroup analyses of sex
Supplementary table 6. Effect estimates of maternal obesity before and during pregnancy on offspring outcomes with subgroup analyses of age
Supplementary table 7. Effect estimates of maternal obesity before and during pregnancy on offspring outcomes with subgroup analyses of species
Figure S1. Birth weight forest plot
Figure S2. Body weight forest plot
Figure S3. Body fat percentage forest plot
Figure S4. Fat mass forest plot
Figure S5. Systolic blood pressure forest plot
Figure S6. Diastolic blood pressure forest plot
Figure S7. Triglycerides forest plot
Figure S8. Total cholesterol forest plot
Figure S9. HDL‐cholesterol forest plot
Figure S10. LDL‐cholesterol forest plot
Figure S11. Glucose forest plot
Figure S12. Insulin forest plot
Figure S13. HOMA‐IR forest plot
Figure S14. Birth weight with sex subgroup forest plot
Figure S15. Body weight with sex subgroup forest plot
Figure S16. Body fat percentage with sex subgroup forest plot
Figure S17. Fat mass with sex subgroup forest plot
Figure S18. Systolic blood pressure with sex subgroup forest plot
Figure S19. Triglycerides with sex subgroup forest plot
Figure S20. Total cholesterol with sex subgroup forest plot
Figure S21. HDL‐cholesterol with sex subgroup forest plot
Figure S22. Glucose with sex sub group forest plot
Figure S23. Insulin with sex subgroup forest plot
Figure S24. HOMA‐IR with sex subgroup forest plot
Figure S25. Body weight with age sex group forest plot
Figure S26. Body fat percentage with age sex group forest plot
Figure S27. Triglycerides with age sex group forest plot
Figure S28. Glucose with age sex group forest plot
Figure S29. Insulin with age subgroup forest plot
Figure S30. Birth weight with species subgroup forest plot
Figure S31. Body weight with species subgroup forest plot
Figure S32. Insulin with species subgroup forest plot
Figure S33. Birth weight with trim and fill funnel plot
Supplementary file 1. Systematic search strategies and hits
Supplementary file 2. List of studies included in the quantitative synthesis
ACKNOWLEDGEMENTS
The authors thank the authors who have provided us information about their study and therefore contributed to the meta‐analysis. We are thankful for the methodological guidance provided by SYRCLE. We also thank Eefje van den Nieuwenhof (EN) for her help in the screening and data‐extraction phase.
Menting MD, Mintjens S, van de Beek C, et al. Maternal obesity in pregnancy impacts offspring cardiometabolic health: Systematic review and meta‐analysis of animal studies. Obesity Reviews. 2019;20:675–685. 10.1111/obr.12817
REFERENCES
- 1. WHO . Obesity and Overweight Fact Sheet. World Health Organisation; 2017. [Google Scholar]
- 2. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989‐2007. Int J Obes (Lond). 2010;34(3):420‐428. [DOI] [PubMed] [Google Scholar]
- 4. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: a systematic review and meta‐analysis. JAMA. 2014;311(15):1536‐1546. [DOI] [PubMed] [Google Scholar]
- 6. Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta‐analysis. JAMA. 2009;301(6):636‐650. [DOI] [PubMed] [Google Scholar]
- 7. Stuebe AM, Forman MR, Michels KB. Maternal‐recalled gestational weight gain, pre‐pregnancy body mass index, and obesity in the daughter. Int J Obes (Lond). 2009;33:743‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reynolds RM, Allan KM, Raja EA, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow‐up of 1 323 275 person years. BMJ. 2013;347(aug13 1):f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mamun AA. Maternal obesity during pregnancy is associated with adult offspring cardiovascular morbidity and mortality but may represent confounding by other factors. Evid Based Med. 2014;19(3):111. [DOI] [PubMed] [Google Scholar]
- 10. Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010;140(3):387‐398. [DOI] [PubMed] [Google Scholar]
- 11. Li M, Sloboda DM, Vickers MH. Maternal obesity and developmental programming of metabolic disorders in offspring: evidence from animal models. Exp Diabetes Res. 2011;2011:592408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zambrano E, Nathanielsz PW. Mechanisms by which maternal obesity programs offspring for obesity: evidence from animal studies. Nutr Rev. 2013;71(Suppl 1):S42‐S54. [DOI] [PubMed] [Google Scholar]
- 13. Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126(5):376‐380. [DOI] [PubMed] [Google Scholar]
- 14. Ribaroff GA, Wastnedge E, Drake AJ, Sharpe RM, Chambers TJG. Animal models of maternal high fat diet exposure and effects on metabolism in offspring: a meta‐regression analysis. Obesity Reviews. 2017;18(6):673‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ainge H, Thompson C, Ozanne SE, Rooney KB. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond). 2011;35(3):325‐335. [DOI] [PubMed] [Google Scholar]
- 16. Moher D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264. [DOI] [PubMed] [Google Scholar]
- 17. Covidence . Covidence Systematic Review Software. Melbourne, Australia: Veritas Health Innovation; Available at http://www.covidence.org. [Google Scholar]
- 18. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes‐Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56(2):455‐463. [DOI] [PubMed] [Google Scholar]
- 20. Zwetsloot PP, Van Der Naald M, Sena ES, et al. Standardized mean differences cause funnel plot distortion in publication bias assessments. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hedges LV, Olkin I. Statistical Methods for Meta‐Analysis. New York Academic Press; 1985. [Google Scholar]
- 22. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539‐1558. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dunn OJ. Multiple comparisons among means. J Am Stat Assoc. 1961;56(293):52‐64. [Google Scholar]
- 25. Chin EH, Schmidt KL, Martel KM, et al. A maternal high‐fat, high‐sucrose diet has sex‐specific effects on fetal glucocorticoids with little consequence for offspring metabolism and voluntary locomotor activity in mice. PLoS One [Electronic Resource]. 2017;12(3):e0174030‐e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bruce KD, Cagampang FR, Argenton M, et al. Maternal high‐fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50(6):1796‐1808. [DOI] [PubMed] [Google Scholar]
- 27. Rajia S, Chen H, Morris MJ. Maternal overnutrition impacts offspring adiposity and brain appetite markers‐modulation by postweaning diet. J Neuroendocrinol. 2010;22:905‐914. [DOI] [PubMed] [Google Scholar]
- 28. Rolls BJ, Rowe EA. Pregnancy and lactation in the obese rat: effects on maternal and pup weights. Physiol Behav. 1982;28(3):393‐400. [DOI] [PubMed] [Google Scholar]
- 29. Segovia SA, Vickers MH, Zhang XD, Gray C, Reynolds CM. Maternal supplementation with conjugated linoleic acid in the setting of diet‐induced obesity normalises the inflammatory phenotype in mothers and reverses metabolic dysfunction and impaired insulin sensitivity in offspring. J Nutr Biochem. 2015;26(12):1448‐1457. [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Ji J, Yu Y, et al. Neonatal overfeeding in female mice predisposes the development of obesity in their male offspring via altered central leptin signalling. J Neuroendocrinol. 2015;27(7):600‐608. [DOI] [PubMed] [Google Scholar]
- 31. Wankhade UD, Zhong Y, Kang P, et al. Enhanced offspring predisposition to steatohepatitis with maternal high‐fat diet is associated with epigenetic and microbiome alterations. PLoS One [Electronic Resource]. 2017;12:e0175675‐e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Balsevich G, Baumann V, Uribe A, Chen A, Schmidt MV. Prenatal exposure to maternal obesity alters anxiety and stress coping behaviors in aged mice. Neuroendocrinology. 2016;103(3‐4):354‐368. [DOI] [PubMed] [Google Scholar]
- 33. Akyol A, McMullen S, Langley‐Evans SC. Glucose intolerance associated with early‐life exposure to maternal cafeteria feeding is dependent upon post‐weaning diet. Br J Nutr. 2012;107(07):964‐978. [DOI] [PubMed] [Google Scholar]
- 34. Thompson MD, Cismowski MJ, Trask AJ, et al. Enhanced steatosis and fibrosis in liver of adult offspring exposed to maternal high‐fat diet. Gene Expr. 2016;17(1):47‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Franco JG, Fernandes TP, Rocha CP, et al. Maternal high‐fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J Physiol. 2012;590(21):5503‐5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Samuelsson AM, Matthews PA, Argenton M, et al. Diet‐induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension. 2008;51(2):383‐392. [DOI] [PubMed] [Google Scholar]
- 37. Samuelsson AM, Matthews PA, Jansen E, Taylor PD, Poston L. Sucrose feeding in mouse pregnancy leads to hypertension, and sex‐linked obesity and insulin resistance in female offspring. Front Physiol. 2013;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fernandes C, Grayton H, Poston L, et al. Prenatal exposure to maternal obesity leads to hyperactivity in offspring. Mol Psychiatry. 2012;17(12):1159‐1160. [DOI] [PubMed] [Google Scholar]
- 39. Samuelsson AM, Morris A, Igosheva N, et al. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension. 2010;55(1):76‐82. [DOI] [PubMed] [Google Scholar]
- 40. Zhang YP, Huo YL, Fang ZQ, et al. Maternal high‐fat diet acts on the brain to induce baroreflex dysfunction and sensitization of angiotensin II‐induced hypertension in adult offspring. Am J Physiol Heart Circ Physiol. 2018;314(5):H1061‐H1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alfaradhi MZ, Kusinski LC, Fernandez‐Twinn DS, et al. Maternal obesity in pregnancy developmentally programs adipose tissue inflammation in young, lean male mice offspring. Endocrinology. 2016;157(11):4246‐4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu Z, Zhao J, Xu H, et al. Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur J Nutr. 2014;53(8):1669‐1683. [DOI] [PubMed] [Google Scholar]
- 43. Long NM, Ford SP, Nathanielsz PW. Maternal obesity eliminates the neonatal lamb plasma leptin peak. J Physiol. 2011;589(6):1455‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet‐induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151(8):4039‐4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ford SP, Zhang L, Zhu M, et al. Maternal obesity accelerates fetal pancreatic beta‐cell but not alpha‐cell development in sheep: prenatal consequences. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R835‐R843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zaborska KE, Wareing M, Edwards G, Austin C. Loss of anti‐contractile effect of perivascular adipose tissue in offspring of obese rats. [erratum appears in Int J Obes (Lond). 2017 Jun;41(6):997; PMID: 28358003]. Int J Obes (Lond). 2016;40(8):1205‐1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre‐pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta‐analysis. PLoS One. 2013;8(4):e61627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kalliala I, Markozannes G, Gunter MJ, et al. Obesity and gynaecological and obstetric conditions: umbrella review of the literature. BMJ. 2017;359:j4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Swanson AM, David AL. Animal models of fetal growth restriction: considerations for translational medicine. Placenta. 2015;36(6):623‐630. [DOI] [PubMed] [Google Scholar]
- 50. Anglim B, Farah N, O'Connor C, Daly N, Kennelly MM, Turner MJ. The relationship between maternal body composition in early pregnancy and foetal mid‐thigh soft‐tissue thickness in the third trimester in a high‐risk obstetric population. J Obstet Gynaecol. 2017;37(5):591‐594. [DOI] [PubMed] [Google Scholar]
- 51. Larciprete G, Valensise H, Vasapollo B, et al. Fetal subcutaneous tissue thickness (SCTT) in healthy and gestational diabetic pregnancies. Ultrasound Obstet Gynecol. 2003;22(6):591‐597. [DOI] [PubMed] [Google Scholar]
- 52. Gaudet L, Ferraro ZM, Wen SW, Walker M. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta‐analysis. Biomed Res Int. 2014;2014:640291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gaillard R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol. 2015;30(11):1141‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. O'Reilly JR, Reynolds RM. The risk of maternal obesity to the long‐term health of the offspring. Clin Endocrinol (Oxf). 2013;78(1):9‐16. [DOI] [PubMed] [Google Scholar]
- 55. Richmond RC, Al‐Amin A, Smith GD, Relton CL. Approaches for drawing causal inferences from epidemiological birth cohorts: a review. Early Hum Dev. 2014;90(11):769‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Drummond EM, Gibney ER. Epigenetic regulation in obesity. Curr Opin Clin Nutr Metab Care. 2013;16:392‐397. [DOI] [PubMed] [Google Scholar]
- 57. Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R711‐R722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5(7):401‐408. [DOI] [PubMed] [Google Scholar]
- 59. Seki Y, Williams L, Vuguin PM, Charron MJ. Minireview: epigenetic programming of diabetes and obesity: animal models. Endocrinology. 2012;153(3):1031‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aagaard‐Tillery KM, Grove K, Bishop J, et al. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol. 2008;41(2):91‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM. Maternal high‐fat diet alters methylation and gene expression of dopamine and opioid‐related genes. Endocrinology. 2010;151(10):4756‐4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS. Members of Epi S. Epigenetics and human obesity. Int J Obes (Lond). 2015;39(1):85‐97. [DOI] [PubMed] [Google Scholar]
- 63. Nathanielsz PW, Poston L, Taylor PD. In utero exposure to maternal obesity and diabetes: animal models that identify and characterize implications for future health. Obstet Gynecol Clin North Am. 2007;34. 201−+ [DOI] [PubMed] [Google Scholar]
- 64. Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848(1‐2):114‐123. [DOI] [PubMed] [Google Scholar]
- 65. Mina TH, Lahti M, Drake AJ, et al. Maternal lipids in pregnancy are associated with increased offspring cortisol reactivity in childhood. Psychoneuroendocrinology. 2017;83:79‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Desai M, Han G, Ross MG. Programmed hyperphagia in offspring of obese dams: altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite. 2016;99:193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guarino D, Nannipieri M, Iervasi G, Taddei S, Bruno RM. The role of the autonomic nervous system in the pathophysiology of obesity. Front Physiol. 2017;8:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dhasarathy A, Roemmich JN, Claycombe KJ. Influence of maternal obesity, diet and exercise on epigenetic regulation of adipocytes. Mol Aspects Med. 2017;54:37‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lecoutre S, Breton C. Maternal nutritional manipulations program adipose tissue dysfunction in offspring. Front Physiol. 2015;6:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leibowitz KL, Moore RH, Ahima RS, et al. Maternal obesity associated with inflammation in their children. World J Pediatr. 2012;8(1):76‐79. [DOI] [PubMed] [Google Scholar]
- 71. Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol. 2010;30(7):441‐446. [DOI] [PubMed] [Google Scholar]
- 72. Fernandez‐Twinn DS, Blackmore HL, Siggens L, et al. The programming of cardiac hypertrophy in the offspring by maternal obesity is associated with hyperinsulinemia, AKT, ERK, and mTOR activation. Endocrinology. 2012;153(12):5961‐5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Porrello ER, Widdop RE, Delbridge LM. Early origins of cardiac hypertrophy: does cardiomyocyte attrition programme for pathological ‘catch‐up’ growth of the heart? Clin Exp Pharmacol Physiol. 2008;35(11):1358‐1364. [DOI] [PubMed] [Google Scholar]
- 74. Armitage JA, Lakasing L, Taylor PD, et al. Developmental programming of aortic and renal structure in offspring of rats fed fat‐rich diets in pregnancy. J Physiol. 2005;565(1):171‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Taylor PD, Samuelsson AM, Poston L. Maternal obesity and the developmental programming of hypertension: a role for leptin. Acta Physiol (Oxf). 2014;210(3):508‐523. [DOI] [PubMed] [Google Scholar]
- 76. Carter AM. Animal models of human placentation—a review. Placenta. 2007;28(Suppl A):S41‐S47. [DOI] [PubMed] [Google Scholar]
- 77. Buettner R, Scholmerich J, Bollheimer LC. High‐fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring). 2007;15(4):798‐808. [DOI] [PubMed] [Google Scholar]
- 78. Nicklas TA, Baranowski T, Cullen KW, Berenson G. Eating patterns, dietary quality and obesity. J Am Coll Nutr. 2001;20(6):599‐608. [DOI] [PubMed] [Google Scholar]
- 79. Caballero B. The global epidemic of obesity: an overview. Epidemiol Rev. 2007;29(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 80. Benyshek DC. The “early life” origins of obesity‐related health disorders: new discoveries regarding the intergenerational transmission of developmentally programmed traits in the global cardiometabolic health crisis. Am J Phys Anthropol. 2013;152(Suppl 57):79‐93. [DOI] [PubMed] [Google Scholar]
- 81. Shasa DR, Odhiambo JF, Long NM, Tuersunjiang N, Nathanielsz PW, Ford SP. Multigenerational impact of maternal overnutrition/obesity in the sheep on the neonatal leptin surge in granddaughters. Int J Obes (Lond). 2015;39(4):695‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Aiken CE, Ozanne SE. Breaking the cycle of intergenerational obesity. J Physiol. 2017;595(5):1443‐1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fernandez‐Twinn DS, Gascoin G, Musial B, et al. Exercise rescues obese mothers' insulin sensitivity, placental hypoxia and male offspring insulin sensitivity. Sci Rep. 2017;7(1):44650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Moser VC, McDaniel KL, Woolard EA, Phillips PM, Franklin JN, Gordon CJ. Impacts of maternal diet and exercise on offspring behavior and body weights. Neurotoxicol Teratol. 2017;63:46‐50. [DOI] [PubMed] [Google Scholar]
- 85. Gallou‐Kabani C, Vige A, Gross MS, et al. Resistance to high‐fat diet in the female progeny of obese mice fed a control diet during the periconceptual, gestation, and lactation periods. Am J Physiol Endocrinol Metab. 2007;292(4):E1095‐E1100. [DOI] [PubMed] [Google Scholar]
- 86. Zambrano E, Martinez‐Samayoa PM, Rodriguez‐Gonzalez GL, Nathanielsz PW. Dietary intervention prior to pregnancy reverses metabolic programming in male offspring of obese rats. J Physiol. 2010;588(10):1791‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Vega CC, Reyes‐Castro LA, Bautista CJ, Larrea F, Nathanielsz PW, Zambrano E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int J Obes (Lond). 2015;39(4):712‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Barisione M, Carlini F, Gradaschi R, Camerini G, Adami GF. Body weight at developmental age in siblings born to mothers before and after surgically induced weight loss. Surg Obes Relat Dis. 2012;8(4):387‐391. [DOI] [PubMed] [Google Scholar]
- 89. Smith J, Cianflone K, Biron S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009;94(11):4275‐4283. [DOI] [PubMed] [Google Scholar]
- 90. Patel N, Godfrey KM, Pasupathy D, et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy. Int J Obes (Lond). 2017;41(7):1018‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vesco KK, Leo MC, Karanja N, et al. One‐year postpartum outcomes following a weight management intervention in pregnant women with obesity. Obesity (Silver Spring). 2016;24(10):2042‐2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dalrymple KV, Martyni‐Orenowicz J, Flynn AC, Poston L, O'Keeffe M. Can antenatal diet and lifestyle interventions influence childhood obesity? A systematic review. Matern Child Nutr. 2018;e12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cox LA, Olivier M, Spradling‐Reeves K, Karere GM, Comuzzie AG, VandeBerg JL. Nonhuman Primates and translational research‐cardiovascular disease. ILAR J. 2017;58(2):235‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. True C, Dean T, Takahashi D, Sullivan E, Kievit P. Maternal high‐fat diet effects on adaptations to metabolic challenges in male and female juvenile nonhuman primates. Obesity (Silver Spring). 2018;26(9):1430‐1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lomas‐Soria C, Reyes‐Castro LA, Rodriguez‐Gonzalez GL, et al. Maternal obesity has sex‐dependent effects on insulin, glucose and lipid metabolism and the liver transcriptome in young adult rat offspring. J Physiol. 2018;596(19):4611‐4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Terrin N, Schmid CH, Lau J, Olkin I. Adjusting for publication bias in the presence of heterogeneity. Stat Med. 2003;22(13):2113‐2126. [DOI] [PubMed] [Google Scholar]
- 98. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Performance of the trim and fill method in the presence of publication bias and between‐study heterogeneity. Stat Med. 2007;26(25):4544‐4562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1. Amendments of the study protocol
Supplementary table 2. Study characteristics of the included studies
Supplementary table 3. Reporting of randomization, blinding and power calculation and risk of bias in the included studies
Supplementary table 4. Effect estimates of maternal obesity before and during pregnancy on offspring outcomes.
Supplementary table 5. Effect estimates of maternal obesity before and during pregnancy on offspring outcomes with subgroup analyses of sex
Supplementary table 6. Effect estimates of maternal obesity before and during pregnancy on offspring outcomes with subgroup analyses of age
Supplementary table 7. Effect estimates of maternal obesity before and during pregnancy on offspring outcomes with subgroup analyses of species
Figure S1. Birth weight forest plot
Figure S2. Body weight forest plot
Figure S3. Body fat percentage forest plot
Figure S4. Fat mass forest plot
Figure S5. Systolic blood pressure forest plot
Figure S6. Diastolic blood pressure forest plot
Figure S7. Triglycerides forest plot
Figure S8. Total cholesterol forest plot
Figure S9. HDL‐cholesterol forest plot
Figure S10. LDL‐cholesterol forest plot
Figure S11. Glucose forest plot
Figure S12. Insulin forest plot
Figure S13. HOMA‐IR forest plot
Figure S14. Birth weight with sex subgroup forest plot
Figure S15. Body weight with sex subgroup forest plot
Figure S16. Body fat percentage with sex subgroup forest plot
Figure S17. Fat mass with sex subgroup forest plot
Figure S18. Systolic blood pressure with sex subgroup forest plot
Figure S19. Triglycerides with sex subgroup forest plot
Figure S20. Total cholesterol with sex subgroup forest plot
Figure S21. HDL‐cholesterol with sex subgroup forest plot
Figure S22. Glucose with sex sub group forest plot
Figure S23. Insulin with sex subgroup forest plot
Figure S24. HOMA‐IR with sex subgroup forest plot
Figure S25. Body weight with age sex group forest plot
Figure S26. Body fat percentage with age sex group forest plot
Figure S27. Triglycerides with age sex group forest plot
Figure S28. Glucose with age sex group forest plot
Figure S29. Insulin with age subgroup forest plot
Figure S30. Birth weight with species subgroup forest plot
Figure S31. Body weight with species subgroup forest plot
Figure S32. Insulin with species subgroup forest plot
Figure S33. Birth weight with trim and fill funnel plot
Supplementary file 1. Systematic search strategies and hits
Supplementary file 2. List of studies included in the quantitative synthesis


