Abstract
We developed a Hidden Markov mark–recapture model (R package marked) to examine sex‐specific demography in Magellanic Penguins (Spheniscus magellanicus). Our model was based on 33 yr of resightings at Punta Tombo, Argentina, where we banded ~44,000 chicks from 1983 to 2010. Because we sexed only 57% of individuals over their lifetime, we treated sex as an uncertain state in our model. Our goals were to provide insight into the population dynamics of this declining colony, to inform conservation of this species, and to highlight the importance of considering sex‐specific vital rates in demographic seabird studies. Like many other seabirds, Magellanic Penguins are long‐lived, serially monogamous, and exhibit obligate biparental care. We found that the non‐breeding‐season survival of females was lower than that of males and that the magnitude of this bias was highest for juveniles. Biases in survival accumulated as cohorts aged, leading to increasingly skewed sex ratios. The survival bias was greatest in years when overall survival was low, that is, females fared disproportionality worse when conditions were unfavorable. Our model‐estimated survival patterns are consistent with independent data on carcasses from the species’ non‐breeding grounds, showing that mortality is higher for juveniles than for adults and higher for females than for males. Juveniles may be less efficient foragers than adults are and, because of their smaller size, females may show less resilience to food scarcity than males. We used perturbation analysis of a population matrix model to determine the impact of sex‐biased survival on adult sex ratio and population growth rate at Punta Tombo. We found that adult sex ratio and population growth rate have the greatest proportional response, that is, elasticity, to female pre‐breeder and adult survival. Sex bias in juvenile survival (i.e., lower survival of females) made the greatest contribution to population declines from 1990 to 2009. Because starvation is a leading cause of morality in juveniles and adults, precautionary fisheries and spatial management in the region could help to slow population decline. Our data add to growing evidence that knowledge of sex‐specific demography and sex ratios are necessary for accurate assessment of seabird population trends.
Keywords: adult sex ratio, bands, demography, female‐biased mortality, Magellanic Penguins, multi‐event model, perturbation analysis, seabirds, sexing, sexual size dimorphism
Introduction
Although males and females of most seabirds are monomorphic, they may vary in foraging behavior (Bearhop et al. 2006, Weimerskirch et al. 2015), parental care (Jacobs et al. 2013), site fidelity (Becker et al. 2008), or other ecological traits that influence their demographic rates. Survival or apparent survival, which is a combined estimate of true survival and permanent emigration, is higher in males than females of many bird taxa (Liker et al. 2005). Sex‐biased survival leads to skewed adult sex ratios (ASR; measured relative to the proportion of breeding‐aged males, see Eq. (3); Donald 2007, Veran and Beissinger 2009, Székely et al. 2014a , b, Ancona et al. 2017). Male‐skewed sex ratios can have important consequences in both monogamous and polygamous populations, as males become mate limited, generating Allee effects and increasing population extinction risk (Engen et al. 2003, Bessa‐Gomes et al. 2004, Lee et al. 2011, Haridas et al. 2014, Berec et al. 2018, Kuparinen 2018).
In long‐lived organisms like seabirds, sex biases in survival can accumulate with age and time, resulting in increasingly skewed sex ratios. Furthermore, most seabirds are serially monogamous with obligate biparental care and strong natal site fidelity. Therefore, deviation from a balanced sex ratio causes a reduction in effective population size, the number of chicks produced, and ultimately, the number of breeders that recruit to the colony (Bessa‐Gomes et al. 2004, Jenouvrier et al. 2010, Eberhart‐Phillips et al. 2017).
The causes and effects of skewed ASR are of growing interest (Schacht et al. 2017, Eberhart‐Phillips et al. 2018), but this issue was largely overlooked in three recent reviews of seabird conservation (Croxall et al. 2012, Lewison et al. 2012, Paleczny et al. 2015). Even though early long‐term studies of seabirds reported higher survival of males than females (e.g., in Yellow‐eyed Penguins, Megadyptes antipodes [Richdale 1957]; in Adélie Penguins, Pygoscelis adeliae [Ainley and DeMaster 1980]), only 20% of recent (2005–2015) seabird mark–recapture studies test for sex‐specific demography (Appendix S1: Text S1). Developing reliable sexing techniques based on genetics (Sabo et al. 1994, Faux et al. 2014), morphometrics (Boersma 1977, Hanners and Patton 1985, Boersma and Davies 1987, Cappello and Boersma 2018), and/or behavioral traits has long been a focus of study in the seabird community, but many individuals remain unsexed throughout even long‐term seabird studies (Ancona et al. 2017). Fortunately, there are now statistical approaches to estimating sex‐specific demography when the sex of some individuals is unknown (Nichols et al. 2004, Choquet et al. 2009, Pradel 2009, Genovart et al. 2012, Johnson et al. 2016).
Magellanic Penguin case study
We examine sex‐specific demography in the Magellanic Penguin (Spheniscus magellanicus) colony at Punta Tombo, Argentina (Fig. 1). We sought to understand this colony's population dynamics, to inform conservation of this species, and to highlight the importance of considering sex‐specific vital rates in demographic studies. Magellanic Penguins exhibit life history traits characteristic of many seabirds. They are long‐lived, serially monogamous, and exhibit biparental care and high site fidelity. We have studied the focal colony intensively since 1983 (Boersma 2008) and documented a population decline of over 40% since 1987 (Boersma et al. 1990, Rebstock et al. 2016).
Figure 1.
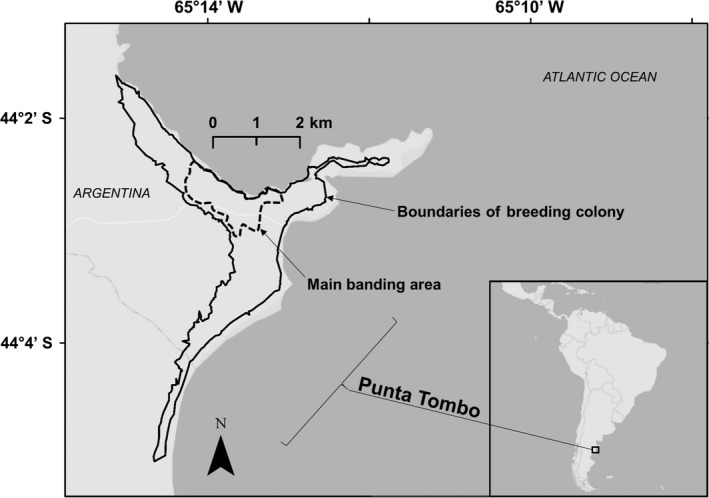
The Magellanic Penguin colony at Punta Tombo was once the largest breeding colony of this species in the world. Located in the Chubut Province of Argentina, the colony covers approximately 400 ha (boundaries of breeding colony). Although we search the entire colony, most banding and resighting efforts are concentrated in a core area (main banding area). Approximately 44,000 chicks were banded at the colony from 1983 to 2010.
We used Hidden Markov mark–recapture models developed with the package marked (Laake 2013, Laake et al. 2013, Johnson et al. 2016), 33 yr of banding data for known‐aged penguins, and perturbation of population matrix models to examine sex‐specific demography in this species. Because mortality is highest, and our knowledge of this species is lowest, during the non‐breeding season (Stokes et al. 2014), our analysis focused on this period. We used our long‐term and detailed data set to examine sex‐specific survival in Magellanic Penguins by addressing the following questions: (1) Does non‐breeding season survival vary significantly with sex? (2) If so, to what extent does age, time (i.e., year), and breeding state influence sex‐specific survival? and (3) What is the contribution of sex‐specific survival to the known population decline and biased sex ratio at the colony? In addressing these questions, we add to the growing understanding of how sex‐biased survival impacts population growth in monogamous seabirds (e.g., Jenouvrier et al. 2010), many of which are increasingly threatened (Paleczny et al. 2015).
Materials and Methods
Study system
The Punta Tombo colony of Magellanic Penguins (44°02′ S, 65°02′ S) was established in the 1920s and was once the largest colony of this species, reaching its peak size in the 1960s–1970s (Boersma et al. 1990). The colony currently occupies 400 ha and consists of approximately 200,000 breeding pairs that nest in bushes, burrows, or in the open (Rebstock et al. 2016). Male Magellanic Penguins return to Punta Tombo from non‐breeding grounds in September, approximately one week before females, to secure nest sites. Natal colony fidelity (Boersma 2008) and nest site fidelity are high in this species; males show a year‐to‐year nest site fidelity of 70% (Boersma et al. 2013). Mate fidelity is also high but is slightly lower for females (83%) than for males (86%) and extra‐pair copulations are uncommon (Boersma et al. 2013). Starvation is the leading cause of chick mortality, but reproductive success exhibits strong interannual variability and is increasingly influenced by extreme weather events (Boersma 2008, Boersma and Rebstock 2014). Females generally lay two eggs and very rarely re‐lay if they lose their eggs or chicks (Boersma et al. 2013). Males and females take turns foraging during incubation and chick rearing.
During the non‐breeding season (April–August), Magellanic Penguins travel an average of 2,000 km north of Punta Tombo, following the seasonal migration of Argentina anchovy (Engraulis anchoita; Stokes et al. 2014). Their non‐breeding season diet consists of anchovy, Brazilian sardine (Sardinella brasiliensis), and squid (Loligo spp. and Illex spp.; Silva et al. 2014, Marques et al. 2018). Trip distance varies among colonies and interannually, possibly due to variation in oceanographic conditions and food availability (Boersma and Rebstock 2009a, Boersma et al. 2009, García‐Borboroglu et al. 2010, Stokes et al. 2014, Rebstock and Boersma 2018), and the species’ non‐breeding range is expanding northward (Dantas et al. 2014). Juveniles leave the colony earlier and migrate further north than adults do (Stokes et al. 2014).
Starvation, oiling, bycatch, and algal toxins are the most common causes of Magellenic Penguin mortality, which is highest during the non‐breeding season (Gandini et al. 1994, Shumway et al. 2003, García‐Borboroglu et al. 2006, Cardoso et al. 2011, Trathan et al. 2015). Evidence from carcasses that wash up within the non‐breeding range suggest that juveniles have higher mortality than adults and that females have higher mortality than males (Vanstreels et al. 2011, 2013, Stokes et al. 2014, Altrão et al. 2017). Like many other penguin populations (e.g., Yellow‐eyed Penguin [Richdale 1957], Adélie Penguin [Ainley and DeMaster 1980]; King Penguin, Aptenodytes patagonicus [Olsson and Van der Jeugd 2002]; Galápagos Penguin, Spheniscus mendiculus [Boersma et al. 2013]; Southern Rockhopper Penguin, Eudyptes chrysocome [Morrison et al. 2015]; African Penguin, Spheniscus demersus [Spelt and Pichegru 2017]), the sex ratio of Magellanic Penguins at Punta Tombo is male biased (Boersma et al. 2013).
In most cases, the driver of biased ASR in penguin populations has not been formally tested, that is, using population matrix model perturbation analysis. Examples of biased hatching sex ratios (African Penguin; Spelt and Pichegru 2017) and of survival bias in juveniles (Southern Rockhopper Penguin; Dehnhard et al. 2014) and in breeding‐aged individuals (Yellow‐eyed Penguin [Richdale 1957]; Adélie Penguin [Ainley and DeMaster 1980]; King Penguin [Olsson and Van der Jeugd 2002]) have all been documented in penguins. However, highly skewed hatching or fledging sex ratios are rare in wild bird populations (Donald 2007, Booksmythe et al. 2017) and there is no evidence that sex ratios are skewed at hatching or fledging in Magellanic Penguins (Koehn et al. 2016; P. D. Boersma, unpublished data). We therefore assumed that fledgling sex ratio was 0.5 in our population. It is important to note that ASR shows low sensitivity to hatching sex ratio in seabirds (Eberhart‐Phillips et al. 2018) and neither hatching nor fledging sex ratios predict ASR among birds (Székely et al. 2014a, Komdeur et al. 2017).
Quantifying sex‐specific survival
We banded between 197 and 3,941 chicks at Punta Tombo (Fig. 1) annually from 1983 to 2010 (total of 44,374). We used stainless‐steel bands marked with a unique five‐digit identification number, which we custom fitted to the left flippers of penguins. We conducted daily searches for banded individuals throughout each breeding season, from settlement (September) through fledging (February), and aggregated resightings by breeding season (i.e., no secondary occasions). Here and throughout, we use the term “resighted” instead of “recaptured,” because individuals were sometimes resighted in the colony or on the beaches but not captured.
We developed a Hidden Markov mark–recapture model using the mvmscjs function in the R package marked (R Version 3.5.1, marked Version 1.2.2; Johnson et al. 2016) for the Punta Tombo population of Magellanic Penguins based on the 44,374 recapture histories. The statistical foundation of marked is detailed in Laake (2013), Laake et al. (2013), and Johnson et al. (2016). Our model included two states: sex and breeding state. In addition to estimating survival (Φ or Phi), recapture (p), and state transition (Ψ or Psi) parameters, marked includes a parameter for the probability that an individual's state is assessed with certainty (δ or delta; Appendix S1: Text S2). We assumed an equal number of male and female chicks at fledging, all of which started in the pre‐breeding state.
Resighting and state assignment probabilities are occasion parameters aggregated by breeding season, which is when individuals are present at the colony and resighted and/or assigned to a state. Therefore, when discussing these parameters, year refers to the breeding season that started in that year (e.g., “1984” is the September 1984–February 1985 breeding season and the hatch year of the 1984 cohort). Survival is an interval parameter, that is, it occurs between breeding periods, so we discuss year in terms of the year during which that non‐breeding period took place (e.g., “1984” refers to April 1984–August 1984 and to first year survival of the 1983 cohort). As true of most published mark–recapture models, our survival estimates represent “apparent survival,” that is, we cannot separate true survival and permanent emigration (Lebreton et al. 1992). For brevity, we refer to model estimated survival as “survival.”
We treated sex and breeding state as independent states with assignment uncertainty. We only defined sex and breeding state in years when we assessed them with certainty because marked does not allow for misclassification or for the use of prior knowledge. Although this approach increases the complexity of the model, it provides information to estimate the uncertainty parameter delta (Nichols et al. 2004, Johnson et al. 2016). We sexed individuals using one of four methods, all of which we assumed were certain: (1) genetic testing, (2) cloaca size around egg laying (Boersma and Davies 1987), (3) breeding behavior (copulation, arrival, nest defense, etc.), or (4) bill size (male bill depth ≥ 2.24; females < 2.24; Appendix S1: Text S2). Of the 3,296 individuals sighted after fledging, we sexed 1,893 with certainty; 471 were female and 1,422 were male (sex ratio = 0.75).
We considered transitions among five breeding states: pre‐breeder (individuals that have not yet bred), unobservable pre‐breeder (pre‐breeders not present at the colony), breeder (individuals currently breeding), non‐breeder (individuals that have bred but are not currently breeding), and unobservable non‐breeder (individuals that skipped breeding and are not present at the colony) (Appendix S1: Text S2; Appendix S3: Fig. S1). Unobservable states were included to address temporary emigrants, which have a recapture rate of zero and therefore violate the assumption that individuals do not vary in their recapture rates (Lebreton et al. 1992, Cooch and White 2013). Temporary emigration is a common feature in seabird populations (Fujiwara and Caswell 2002), and Magellanic Penguins may skip breeding seasons if they are in too poor body condition to breed (Rebstock et al. 2016). It is important to note that pre‐breeder always refers to individuals that have not bred, but that this definition varies with age, that is, individuals <4 yr of age are always pre‐breeders, whereas pre‐breeders ≥4 yr are of breeding age but have not bred.
We selected models in three phases (Appendix S1: Text S3; Appendix S3: Fig. S2), with each phase starting with the best‐supported model from the previous phase. We held the specification of Psi and delta constant; the transition parameter Psi varied only with breeding state, sex assignment certainty varied with sex, and breeding state assignment certainty varied with sex and breeding state (Appendix S1: Text S3). We assessed goodness‐of‐fit for a modified version of the most general model in the program MARK (Version 6.2; Appendix S1: Text S3). During the first phase of model selection, we compared a suite of models with additive variation in recapture with sex, year, and/or breeding state and additive variation in survival with sex, year, and/or age. Based on clear age‐specific trends in survival during the first phase, we divided specification of Phi into age classes for the second phase of model selection. These age classes included juveniles (fledging through the first year at sea), adults (age 2–18), and elder adults (age ≥ 19). In the final stage of model selection, we tested for variation in survival due to breeding state (Appendix S1: Text S3).
The impact of sex‐specific survival
We developed a two‐sex, nonlinear, age‐based, matrix model that was parameterized using vital rates from the best‐supported mark–recapture model and from previous publications on the population dynamics of Punta Tombo (Appendix S1: Text S4; Appendix S2: Table S3). The matrix model was age structured, but we used averaged survival rates within the following groups: female juveniles, male juveniles, female pre‐breeders (1–5 yr of age), male pre‐breeders (1–6 yr of age), female breeders <19 yr of age, male breeders <19 yr of age, female breeders ≥19 yr of age, and male breeders ≥19 yr of age (Appendix S2: Table S3). We developed two population matrix models: (1) a year‐specific model (Mφ t) used to back‐simulate population trends from 1990 to 2009 and (2) a year‐averaged model (Mφ) used to estimate the stable stage distribution and to conduct perturbation analyses. We constrained the simulation to 1990–2009 to allow for the use of unbiased estimates for the juvenile and adult age classes (see Results). In both cases, we defined the projection matrix as
| (1) |
where n is a vector of population sizes distributed across age classes and the two sexes and M is a matrix containing sex‐specific fecundity and year‐specific (Mφ t) or year‐averaged (Mφ) survival rates (Appendix S3: Fig. S3).
Data from daily nest check areas at Punta Tombo suggest that nearly all reproductively mature females that return to the colony breed (P. D. Boersma, unpublished data), as also seen in long‐term studies of Yellow‐eyed Penguin (Richdale 1957) and Adélie Penguin (Ainley et al. 1983) colonies with skewed sex ratios. The dominance mating function assumes that 100% of reproductively mature individuals of the less abundant sex breed, defined here based on the sex‐specific average first breeding age (Veran and Beissinger 2009, Jenouvrier et al. 2010, Ballerini et al. 2015). This function was therefore appropriate for modeling the mating process of this monogamous population
| (2) |
where are the number of breeding‐age males and are the number of breeding‐age females and is reproductive success. Because we were not able to estimate year‐ or age‐specific breeding state transitions using our mark–recapture model, we assumed all breeding‐aged individuals were available to mate (Appendix S2: Table S3). We defined ASR as the proportion of breeding‐aged males present at any given period
| (3) |
where are the number of breeding‐aged males and are the number of breeding‐aged females.
For Mφ t, we used year‐ and sex‐specific survival rates for juveniles and year‐ and sex‐specific, age‐averaged rates for the three remaining groups. We developed a complementary matrix model () to examine how assuming equal male and female survival would influence the projected population change over 20 yr (1990–2009). This matrix assumed equal‐weighted, sex‐averaged survival that was year and age class specific. If a mark–recapture model of a population with a skewed sex ratio ignores sex, apparent survival estimates are biased toward the more abundant sex (i.e., there are more recapture histories for the more abundant sex). Because the average would be skewed toward the male survival rate, the difference between average and female survival would be greater in this scenario than a scenario using a rate halfway between males and females, as done in our study.
We compared trends in the projected number of breeding pairs 1990–2009 to temporal trends in the number of active nests during that period. During annual October surveys (settlement/incubation period), we counted the number of nests and their contents (females, males, and/or eggs) within 100‐m2 permanently staked plots spaced 100 m apart throughout the colony (Rebstock et al. 2016). We defined an active nest as any nest with an egg or penguin present, so active nests serve as an upper estimate of the number of breeding pairs, that is, the actual decline in breeding pairs may exceed that of active nests.
Our model may overestimate juvenile mortality because we did not separately estimate permanent emigration. We know that some penguins from Punta Tombo emigrate to other colonies (Bouzat et al. 2009, Pozzi et al. 2015; P. D. Boersma, unpublished data) and that emigration rates are generally highest in juveniles (e.g., as seen for related African Penguins; Sherley et al. 2014, Weller et al. 2014) and may be sex specific (e.g., in Willow Warbler, Phylloscopus trochilus; Morrison et al. 2016). We addressed this issue with a fitness landscape analysis (e.g., Ballerini et al. 2015) of sex‐specific and sex‐averaged projections. For this analysis, we increased male and/or female juvenile survival sequentially by 0.1 (increased by up to 0.5). We used model‐estimated juvenile survival rates (i.e., instead of adjusted juvenile survival rates) for all perturbation analyses.
We conducted retrospective and prospective analyses using population metrics calculated at the stable stage distribution, that is, after 100 yr of simulation (Appendix S1: Text S4). Retrospective analyses, or life table response experiments (LTREs), showed how past variation in demographic parameters influenced population metrics. Retrospective analyses depend on historical conditions, so cannot inform future population dynamics (Caswell 2000, 2001). We used prospective analyses, that is, perturbed the matrix model across a wide range of vital rates irrespective of past variation, to explore the impacts of hypothetical changes in demographic parameters.
The prospective analyses we used included calculations of sensitivity and elasticity. We relied on the spline‐based perturbation methods presented in Veran and Beissinger (2009) and Eberhart‐Phillips et al. (2017, 2018). Sensitivity was rescaled to elasticity using a ratio of the parameter to the stable stage population metric, allowing for comparison across parameters. The formula we used to test the elasticity of lambda to demographic change was
| (4) |
where e(θ) is the elasticity of the population metric to the demographic parameter of interest, based on the sensitivity . We estimated the sensitivity of the equilibrium values of the population metrics lambda and ASR to the following parameters: male survival of each age class and breeding state, female survival of each age class and breeding state, hatching sex ratio, and reproductive success. We used the sensitivity of each population metric to conduct an LTRE, defining the contribution of each parameter C(θ) as
| (5) |
For the LTRE, we compared the projection based on the sex‐specific survival matrix Mφ t (the “treatment matrix”) with a matrix (the “control matrix”) where female survival was assumed to equal male survival. In both the treatment and control matrices, hatching sex ratio was assumed to equal 0.5 and reproductive success was based on year‐specific estimates (Appendix S1: Text S4; Appendix S2: Table S3). To conduct the LTRE, we calculated sensitives for a prime matrix (M′), a matrix populated with demographic values averaged between the treatment and control matrices (Veran and Beissinger 2009, Eberhart‐Phillips et al. 2017). We also used Eqs. (4) and 5 to calculate the elasticity of ASR and the contribution to ASR for each demographic parameter by replacing lambda with ASR in each equation.
Results
Quantifying sex‐specific survival
In the best‐supported model resulting from the final phase (Phase 3) of model development, the probability of being resighted depended on additive variation in sex, year, and breeding state (Appendix S2: Tables S1, S2). Sex was included as an additive variable in the best‐supported specification of Phi, which also included variables specific to each age class. The survival rate of juveniles varied with year. Adult survival (ages 2–18) varied with breeding state, year, and age and elder adult survival (ages ≥ 19) varied with breeding state and as a linear, decreasing function of age (Appendix S2: Table S2). It should be noted that all models were additive on the logit scale (i.e., logit parallelism) due to concerns of parameter identifiability, but this does not necessitate linear parallelism (i.e., interaction may still occur on the real scale; Cooch and White 2013).
Resighting rates increased gradually until 2005 and then stabilized. Increased resighting rates over time reflect the fact that we found and marked nests of banded penguins each year of the study. We had a high chance of resighting birds in marked nests in ensuring years because site fidelity is high in Magellanic Penguins (Boersma et al. 2013).
Resighting rates (P) were high for male (P = 0.97 ± 0.02 [mean ± SD]) and female breeders (P = 0.89 ± 0.08). Non‐breeding males (P = 0.77 ± 0.16) had lower resighting rates than breeding males and, because non‐breeding males often remain at the colony to secure nest sites for subsequent breeding seasons, they had higher resighting rates than did female non‐breeders (P = 0.47 ± 0.21). Pre‐breeders have less frequent colony attendance than breeders and, when they do attend the colony, are often on the beach (Pozzi 2015) where resighting efforts are lower and bands are harder to read. Accordingly, pre‐breeders had the lowest resighting rates (females, P = 0.12 ± 0.11; males, P = 0.35 ± 0.20). The probability of sexing a resighted female with certainty was greater than the probability of sexing a resighted male with certainty (Table 1).
Table 1.
The probability of assessing an individual's sex or breeding state with certainty
| Sex | Breeding state | a,b |
|
|
|---|---|---|---|---|
| Female | breeder | 0.30 (0.29–0.30) | 0.52 (0.49–0.55) | |
| Female | non‐breeder | 0.30 (0.29–0.30) | 0.14 (0.12–0.17) | |
| Male | breeder | 0.25 (0.25–0.25) | 0.46 (0.43–0.49) | |
| Male | non‐breeder | 0.25 (0.25–0.25) | 0.11 (0.10–0.12) |
The 95% confidence interval for each estimate is shown in parentheses.
Approximately 57% of the banded population has been sexed with certainty at some point during their lifetime.
Breeding state assignment rates were state‐specific and sex‐specific and were higher for breeders than for non‐breeders (Table 1). Pre‐breeders had a 0.10 probability of becoming breeders each year and, once breeding, had a 0.81 probability of remaining a breeder (Appendix S3: Fig. S5). Similarly, non‐breeders were most likely to remain non‐breeders the following year (0.68). Individuals had a low probability of entering unobservable states (i.e., skipping breeding), particularly once they became breeders (Appendix S3: Fig. S4).
Annual juvenile survival rates showed high variability, ranging from 0.007 to 0.32 for females and from 0.01 to 0.44 for males (Fig. 2). Survival rates of adults were higher and less variable than juvenile survival rates and were lower for pre‐breeders than for breeders and non‐breeders. Adult survival showed substantial interannual variability, ranging from 0.61 to 0.99 for females and from 0.71 to 0.99 for males. Although male survival was higher than female survival, the confidence intervals of adult males and adult females overlapped in all years (Fig. 3). Survival rate decreased linearly with age by 0.05 a year for both male and female elder breeders (aged ≥ 19 yr).
Figure 2.
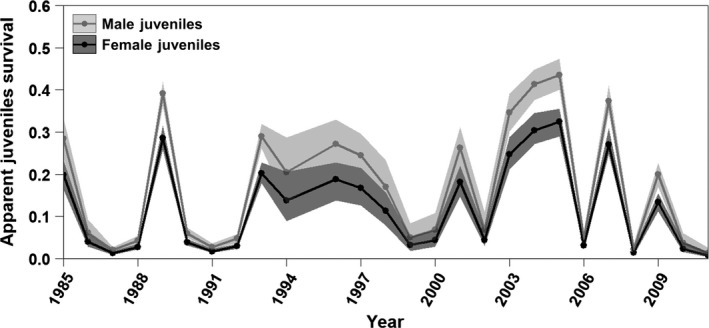
Apparent non‐breeding‐season survival of Magellanic Penguin juveniles (fledging to age 1) is highly variable among years but generally low. On average, juvenile survival is 33% higher for males than for females. The 95% confidence intervals (semi‐transparent ribbons) of the sexes overlap in some years. Juvenile survival for the 1983 cohort (1984 non‐breeding season) was not included in this figure because it had confidence intervals spanning [0,1].
Figure 3.
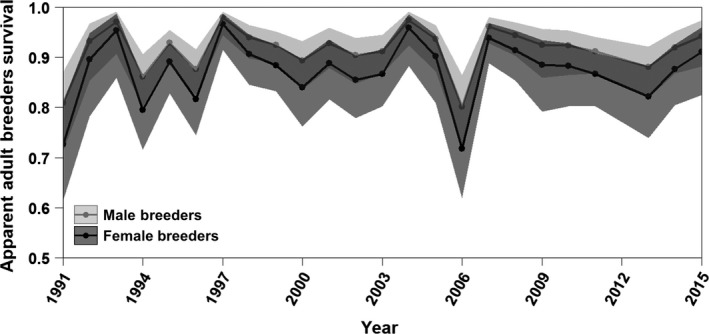
When averaged across ages, apparent non‐breeding‐season survival rates of breeding adult Magellanic Penguins (ages 4–18) are comparable to previous studies on this and other seabird species. On average, survival was 5% higher for males than for females, and the 95% confidence intervals of the two sexes (semi‐transparent ribbons) overlapped in all years. Non‐breeding adults showed the same survival rates as breeding adults. Due to the sparseness of data on breeding adults prior to 1991, survival estimates had confidence intervals spanning [0,1] and are not included in this figure.
The magnitude of the sex bias varied with age, year, and breeding state (Fig. 4). Juveniles showed the greatest average sex bias, with average survival rates 33.3% higher in males than in females (Fig. 4). As compared to females of the same age class and breeding state, the average survival sex bias favoring males was 5.5% in pre‐breeders aged <19, 13.5% in pre‐breeders aged ≥ 19, 4.7% in breeders aged <19, and 11.3% in breeders aged ≥ 19. The sex bias toward adult males was greatest in years when overall survival was lowest (Appendix S3: Fig. S4).
Figure 4.
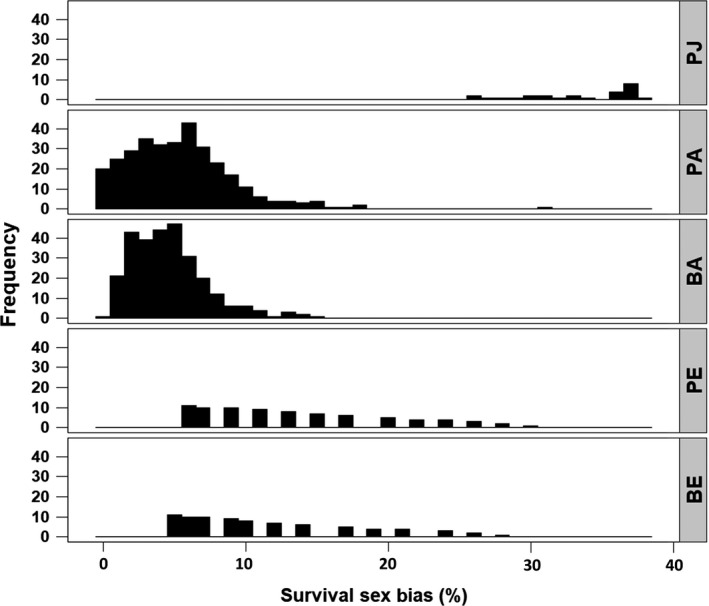
In the Magellanic Penguin population studied, survival rates were higher for males than for females of the same age class and breeding state. The survival sex bias is the percent difference between female and male survival and is relative to male survival. This bias was highest in juveniles and in elder adults. Among adults and elder adults, pre‐breeders (individuals that had not yet bred) had a higher survival bias than did breeders and non‐breeders (individuals that had bred at least once but were not breeding that year). The first letter of the x‐axis category refers to breeding state (P, pre‐breeder; B, breeder or non‐breeder) while the second letter refers to age class (J, juvenile [fledging to age 1]; A, adult [age 1 to 18]; E, elder [age ≥ 19]). Frequency was measured as unique age by time combinations, so sample size is smallest for juveniles (1 age, 27 yr) and largest for adults (18 ages, 27 yr).
Sex ratio increased, that is, became more male biased, as each cohort aged. Here, we use the 1989 cohort as an example of the accumulation in age‐specific sex bias, because it is the earliest cohort with fully estimable age‐specific parameters (Fig. 5). We assume age‐specific pre‐breeder survival rates to calculate the sex ratio of immature individuals and age‐specific breeder survival rates to calculate ASR. For the 1989 cohort, the sex ratio increased from 0.61 at age one to 0.84 at age 25, the last age of this cohort we studied. The sex ratio deviated significantly from parity from ages one (χ2 = 4.27; df = 1; P = 0.04) to 24 (χ2 = 4.41; df = 1; P = 0.04); at age 25, the χ2 test resulted in a P value of 0.05 due to low sample size (14 individuals remaining).
Figure 5.
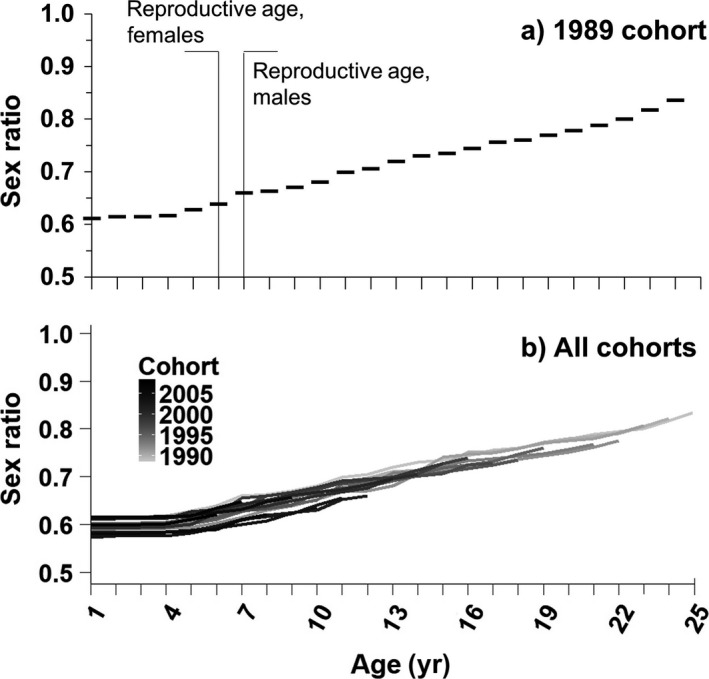
Survival sex bias accumulates with age in Magellanic Penguins, leading to increasingly skewed sex ratios. (a) The 1989 cohort; (b) trends in sex ratio for the 1989–2009 cohorts. All cohorts studied showed a similar pattern of an increase in sex ratio with age, but the rate of this increase varied. By the time cohorts reached average male maturity (age 7; Boersma et al. 2013), the sex ratio ranged from 0.61 (2004 cohort) to 0.70 (2005 cohort), with an average of 0.60.
Across all 19 cohorts studied (hatch years 1989–2008), an average of 136 individuals survived to age 1, with a maximum of 423 individuals surviving to age 1 in the 1992 cohort (out of 1,725 banded chicks). All cohorts showed a similar trend of increasing male bias from fledging to reproductive age, but the exact patterns varied, with sex ratios ranging from 0.61 to 0.70 by the age of male maturation (age 7; Boersma et al. 2013; Fig. 5). The sex ratio deviated significantly from parity (i.e., equal numbers of males and females) by age 1 in 8 of 19 cohorts and by age 6 in 12 of 19 cohorts (χ2 test; df = 1; P < 0.05). In 7 of 19 cohorts, sex ratio never significantly deviated from parity, probably because of low sample sizes. All seven of these cohorts had fewer than 50 individuals surviving to age one, because a small number of individuals were banded and/or juvenile survival was low that year.
The impact of sex‐specific survival
The number of active nests at Punta Tombo declined linearly from 1990 to 2009 (r 2 = 0.52, P < 0.001), with a trend‐estimated population decline of 23%. Simulated trends based on population matrix projections using year‐variant, sex‐specific survival did not match survey‐estimated trends and indicated that the number of breeding females declined by 85% from 1990 to 2009. Because we defined active nests as any nest with an egg or penguin present, they serve as an upper estimate of the number of breeding pairs. Therefore, the actual decline in breeding pairs would exceed that of active nests if, as the model outputs and data from the colony suggest, ASR became increasingly skewed from 1990 to 2009. The mismatch between survey‐estimated trends and matrix model‐estimated trends also indicates that immigration to the colony is likely occurring.
For population matrix model projections to match survey trends, we had to increase female juvenile survival by at least 0.40 and male juvenile survival by at least 0.20 (Appendix S2: Table S4). Projections based on sex‐averaged survival rates always led to a larger effective population than projections based on sex‐specific survival rates. For example, when we increased male and female juvenile survival rates by 0.40, the projection based on sex‐specific survival rates predicted a 24% decline in the number of breeding pairs while the projection based on sex‐averaged survival rates predicted a 16% increase in the number of breeding pairs (Appendix S2: Table S4).
The asymptotic population growth rate has a greater elasticity to the survival of females, the less abundant sex, than to that of males (Fig. 6). Specifically, population growth shows the greatest elasticity to survival of female pre‐breeders (e = 0.36) and female adult breeders (e = 0.46). Population growth rate showed a low and similar response to hatching sex ratio, reproductive success, and juvenile survival (all e = 0.07).
Figure 6.
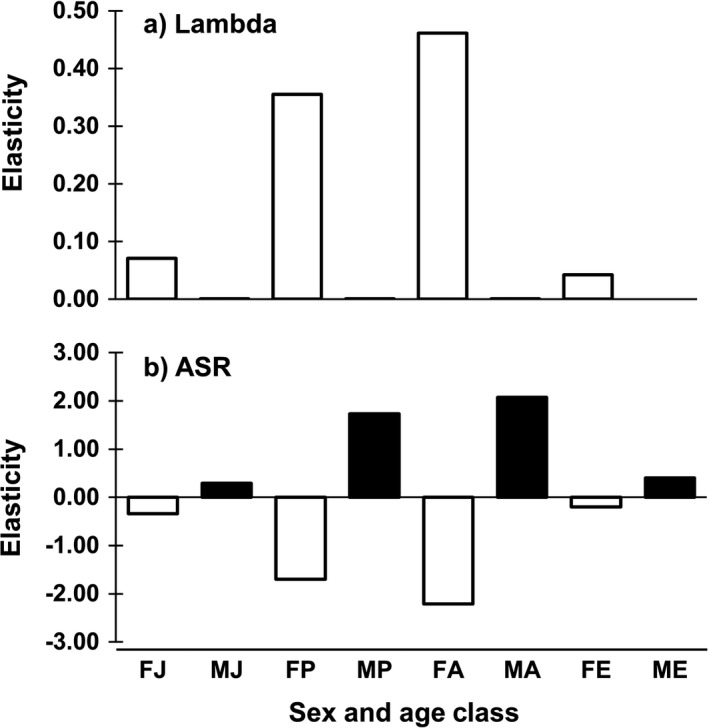
(a) The population growth rate of Magellanic Penguins showed the greatest elasticity to variation in female pre‐breeder and adult survival. Elasticity of lambda to male survival of all age classes was close to zero because of the colony's skewed sex ratio and the use of a dominance mating function. The first letter of the x‐axis category refers to sex (F, female; M, male) while the second letter refers to the age and breeding class (J, juvenile [fledging to age 1]; P, pre‐breeder [age 1–6 for females, age 1–7 for males]; A, adult breeder/non‐breeder [age 7–18 for females, age 8–18 for males]; E, elder breeder/non‐breeder [age ≥ 19]). (b) Like lambda, adult sex ratio (ASR) responded most strongly to changes in apparent survival of the pre‐breeder and adult age classes. However, because the juvenile age class showed the greatest sex bias, this age class made the greatest contribution to population declines and skewed adult sex ratio at the colony from 1990 to 2009.
Although population growth rate showed a low elasticity to juvenile survival, this demographic parameter showed the greatest sex bias. As a result, the model showed that the sex bias in juvenile survival made the greatest contribution (C) to population declines from 1990 to 2009 (C = 0.027). The contribution of sex bias in juvenile survival was 2 times greater than that in pre‐breeders (C = 0.013), 1.4 times greater than that in adult breeders (C = 0.019), and 9 times greater than that in female elder breeders (C = 0.003).
The asymptotic ASR showed similar‐magnitude elasticity to both sexes, but an increase in female survival reduces ASR because it results in a greater number of females and therefore a larger denominator in the ASR calculation (Fig. 6). Like the population growth rate, ASR showed higher elasticity to pre‐breeder (e = −1.70 for females; e = 1.73 for males) and adult age classes (e = −2.21 for females; e = 2.07 for males) than to juvenile (e = −0.34) and elder age classes (e = −0.20) and low elasticity to hatching sex ratio and reproductive success (both e = −0.05; Fig. 6).
Because population growth rate and ASR are sensitive to the same demographic parameters, these population metrics may covary. However, ASR has a greater elasticity, so it shows a greater proportional response to demographic change. Though the contribution is based on the sensitivity of ASR to female survival, and is thus negative, we report positive values for clarity (i.e., the increase in ASR caused by female‐biased mortality). As seen for population growth rate, sex bias in juvenile survival made the greatest contribution (C) to increased ASR from 1990 to 2009 at the Punta Tombo colony (C = 0.094). This contribution was 2.1 times greater than it was for pre‐breeders (C = 0.044), 1.4 times greater than it was for breeding adults (C = 0.065), and 9.1 times greater than it was for elder breeders (C = 0.01).
Discussion
Reports of skewed sex ratios at penguin colonies started with the earliest long‐term studies of these species, though these studies did not link skewed sex ratios to population decline (Richdale 1957, Ainley et al. 1983). In his study of a Yellow‐eyed Penguin population from 1936 to 1954, Richdale (1957) estimated that sex ratio reaches 0.67 (two males per female) in adults of this species and that, consequently, males spend one‐third of their lives unmated. Using resightings of chicks banded from 1962 to 1970, Ainley and DeMaster (1980) found that sex ratio increased even more rapidly with age in Adélie Penguins than in Yellow‐eyed Penguins. In the population studied by Ainley and DeMaster (1980), less than 8% of birds sighted aged 10 or older were females. Male‐skewed sex ratios have since been documented at colonies of several other penguin species, including King Penguins (Olsson and Van der Jeugd 2002), Galápagos Penguins (Boersma et al. 2013), Southern Rockhopper Penguins (Morrison et al. 2015), and African Penguins (Spelt and Pichegru 2017). Regardless, sex is often ignored in contemporary research on penguin demographic rates (e.g., Sherley et al. 2014 on African Penguins), and the extent that sex‐biased survival influences ASR and population growth rate in these populations remains poorly understood.
We found that males have higher survival rates than females at Punta Tombo, Argentina, a declining colony of Magellanic Penguins known to have a skewed ASR. Our findings suggest that lower female survival is a driver of population decline and an increasingly skewed ASR at Punta Tombo. In Magellanic Penguins and other seabirds, sex‐biased survival creates a bottleneck that limits effective population size (Jenouvrier et al. 2005, 2010; this study). Accounting for sex‐specific demography therefore provides valuable insight into the conservation of Magellanic Penguins and species with similar life history traits.
Quantifying sex‐specific survival
Survival of Magellanic Penguins varied with sex, age class, breeding state, and year. When averaged across ages and years, we found average annual survival rates of breeding adults (females, 0.85 ± 0.13; males, 0.89 ± 0.11) similar to those estimated by Boersma and Rebstock (2010) for individuals banded as adults at Punta Tombo (0.87 for both sexes). Our average adult survival rates were also within the range of the 10 penguin species (0.62–0.94; average 0.83 ± 0.09) reported in Schreiber and Burger (2001). They were, however, considerably lower than that found by Pozzi (2015) for Magellanic Penguins greater than two years of age (0.96). Pozzi's (2015) mark–recapture model used the same data set as ours (i.e., Punta Tombo individuals banded as chicks) but did not consider sex or breeding state. The interannual variability we found in adult survival was unexpected, as adult survival rates are generally high and relatively constant in long‐lived species (Sæther and Bakke 2000, Jenouvrier et al. 2005).
Juvenile survival also showed high interannual variability, suggesting high variability in cohort recruitment (Φ♀J = 0.12 ± 0.11 SD; Φ♂J = 0.17 ± 0.14 [mean ± SD]). Because juvenile seabirds may prospect at other colonies and have lower colony attendance than do mature individuals, robust estimates of juvenile survival are rare (available for <2% of seabird species; Lewison et al. 2012). Juvenile survival for this population was considerably lower than an average across 32 diverse seabird species (e.g., eiders, gulls, albatrosses, penguins), based on data collated by Horswill and Robinson (2015) and Sæther and Bakke (2000) (0.47 ± 0.27 SD), though Horswill and Robinson (2015) noted that many of the estimates they included were of poor quality. Juvenile survival rates for this population are, however, comparable to those seen for highly perturbed populations of African Penguins (e.g., <0.25 at the Robben Island colony, South Africa in 2004–2010 [Sherley et al. 2014], average 0.19 ± 0.12 across years/colonies [Sherley et al. 2018]).
Males had higher survival rates than females, but confidence intervals overlapped for adults in all years and the magnitude of the sex bias in survival varied with age class and year (Fig. 4). Male survival ranged from 1% to 38% higher than females of the same age and breeding state; the bias was greatest in the juvenile age class. Because Magellanic Penguins are long lived, even small sex biases in survival accumulate and sex ratio becomes increasingly skewed as a cohort ages (Fig. 5). Similarly, in long‐term studies of Yellow‐eyed and Adélie Penguins (Ainley and DeMaster 1980), ASR steadily increased with age. In contrast to these studies, however, we found that the largest increase in sex ratio occurred prior to breeding age in Magellanic Penguins, suggesting that sex‐specific survival did not result from high reproduction costs to females.
In Adélie Penguins, breeding individuals have lower survival rates than do non‐breeding individuals, and the sex bias in survival is higher among breeders than non‐breeders (Ainley and DeMaster 1980). The greater breeding cost in Adélie Penguins compared to Magellanic Penguins may in part be due to higher predation rates by seals in the former, as Ainley and DeMaster (1980) estimated that breeders pass by seals 10 times more often than non‐breeders in a season. In our study, the most parsimonious model grouped breeders and non‐breeders, suggesting that their survival rates do not significantly differ. Interestingly, as for Yellow‐eyed and Adélie Penguins (Richdale 1957, Ainley et al. 1983), the sex with lower survival rates (female) starts to breed at a younger average age than the sex with higher survival (male) in Magellanic Penguins (Boersma et al. 2013).
The survival patterns we estimated coincide with data on beached carcasses within the species’ non‐breeding habitat. Beached carcasses are biased toward juveniles and females of all age classes, and thus suggest that true survival is age and sex biased. In the most extreme example, Mäder et al. (2010) found that 97.5% of carcasses along a stretch of the Southern Brazil shoreline were juveniles. Several of the years for which juvenile carcass counts were anomalously high were also low juvenile survival years in our study (1990, 1998, 2008; Mäder et al. 2010).
Vanstreels et al. (2013) examined 119 beached carcasses from southern Brazil from 2005 to 2007 and found a sex ratio of 0.26 in juveniles (455 individuals) and adults (73 individuals). This sex bias was not evident in stranded oiled penguins, indicating that it did not result from sex‐specific non‐breeding habitat use (Vanstreels et al. 2013). Two recent studies with more limited sample sizes also found female‐biased mortality: Nunes et al. (2015) found a sex ratio of 0.35 among 43 carcasses washed up in southern Brazil, most of which were juveniles. Altrão et al. (2017) examined parasitic nematodes from 36 carcasses washed up along the coast of southern Brazil, which had a sex ratio of 0.38 among juveniles and of 0.29 among adults. Carcasses therefore suggest that sex‐biased mortality results in sex ratios ranging from 0.62 to 0.74 among surviving individuals, comparable to those we found by age 7 in our population (0.61–0.70 depending on cohort).
Intraspecific variation in foraging and survival
Adult and juvenile survival rates were significantly correlated across years, suggesting similar drivers of mortality during the non‐breeding season. Food availability is a known determinant of seabird survival (Cairns 1988), and demography and foraging ecology are tightly linked in seabirds (Weimerskirch 2018). Furthermore, starvation is a documented major cause of non‐breeding‐season mortality in Magellanic Penguins of all age classes (Gandini et al. 1994, García‐Borboroglu et al. 2006, Boersma 2008, Vanstreels et al. 2013). During years of low food availability, individuals must forage over greater distances, increasing starvation risk and reducing survival (García‐Borboroglu et al. 2010, Stokes et al. 2014). The sex bias in Magellanic Penguin survival was greatest in low survival years (Appendix S3: Fig. S4) and age classes (i.e., juveniles or elder birds; Fig. 4). Among seabirds, vulnerable subsets of the population often fair disproportionally worse during poor environmental conditions (King Penguin [Olsson and Van der Jeugd 2002]; Galápagos Penguin [Boersma 1977, 1998], Emperor Penguin, Aptenodytes forsteri [Barbraud and Weimerskirch 2001, Jenouvrier et al. 2005]; Snow Petrel, Pagodroma nivea [Jenouvrier et al. 2005]).
Intraspecific variation in foraging behavior and energetics determine how individuals respond to changes in food availability. Juvenile seabirds often forage less efficiently than do older, more experienced conspecifics (e.g., Brown Pelican, Pelecanus occidentalis [Orians 1969]; African Penguins [Wilson 1985]; Wandering Albatross, Diomedea exulans [Riotte‐Lambert and Weimerskirch 2013]; Northern Gannet, Morus bassanus [Votier et al. 2017]), decreasing their probability of surviving the non‐breeding season. Survival rates increase dramatically between fledglings and 1‐yr‐olds in Magellanic Penguins (this study). In Wandering Albatross, the foraging skills of fledglings improve rapidly over the first few months at sea, after which they are comparable to those of adults (Weimerskirch 2018). We found that survival decreased again for individuals ≥19 yr of age, potentially because of senescence‐related reductions in foraging efficiency (e.g., Zimmer et al. 2011) or to increased investment in reproduction with age (Cerchiara et al. 2017). Interestingly, Rebstock and Boersma (2018) found that egg size increased with female Magellanic Penguin age until age 18 and then began to decline, another indication that senescence begins around age 19 in females of this species.
Sex‐specific energetics and foraging behavior provide a potential explanation for the lower survival rates of female Magellanic Penguins. Studies have shown that male Magellanic Penguin chicks are fed at a higher trophic level (i.e., higher δ15N) than female chicks (Forero et al. 2002). The proportion of fish in the diet influences size at fledging in Adélie Penguins (Whitehead et al. 2015), and males of several species of penguin fledge at a larger size or in better body condition than do females (King Penguins in some years [Bordier et al. 2014]; Adélie Penguins [Jennings et al. 2016]; African Penguin [Spelt and Pichegru 2017]). Because of their smaller size and less favorable surface area to volume ratio, female Magellanic Penguins may fledge at an energetic disadvantage to males, lowering their juvenile survival rates.
On average, adult male Magellanic Penguins are 17% heavier than adult females and have bills that are 8% longer and 15% deeper (Boersma et al. 2013). Relative to males, female Magellanic Penguins may experience (1) shallower or less frequent diving due to a lower oxygen storage capacity (Walker and Boersma 2003), (2) higher drag due to a greater surface area to volume ratio, (3) less resilience to periods of food scarcity due to a lower food storage capacity (Colchero et al. 2017), and (4) less prey size flexibility due to smaller bills (e.g., Vanstreels et al. 2013, Silva et al. 2014, Ciancio et al. 2018). Evidence from non‐breeding areas suggests that female Magellanic Penguins are more susceptible to starvation. Females are less likely than males to survive rehabilitation (Vanstreels et al. 2013) and, unlike males, their body condition when returning to Punta Tombo is significantly correlated with non‐breeding‐season oceanographic conditions (Rebstock and Boersma 2018).
Studies into sex‐specific foraging behavior and diet of adult Magellanic Penguins are inconclusive and suggest that variation in foraging behavior is context dependent. Boersma and Rebstock (2009a) found no difference in female and male foraging distance during the breeding season (205 penguins, 1996–2006), while Scioscia et al. (2016; 126 penguins, 2006–2008) and Raya Rey et al. (2012; 56 penguins, 2003–2005) found that females had a larger foraging range than males during incubation. Pütz et al. (2007) found that females and males used similar non‐breeding habitat and Silva et al. (2014) and Marques et al. (2018) found that females and males had similar isotope signatures for the period representing the non‐breeding season. Silva et al. (2014) did find that males had larger isotopic niches than did females, suggesting greater foraging diversity among individuals of this sex. Males’ larger size allows them to dive deeper (Walker and Boersma 2003, Raya Rey et al. 2012) and take a larger size range of prey (Forero et al. 2002), giving them greater diet flexibility. Even if females and males have similar diets, females may have to work harder to maintain body condition. For example, recent studies suggest that males provision more food to Magellanic Penguin chicks than do females (Ciancio et al. 2018), despite similar duration foraging trips during the breeding season.
Banding considerations
The impact of flipper bands is an ongoing debate in the seabird community, and the studies suggest that their impacts are highly dependent on the type of band used, how the band is fitted, and the species of interest (Jackson and Wilson 2002). For example, in a double banding study, Boersma and Rebstock (2010) sighted Magellanic Penguins injured or killed by aluminum bands but not by stainless‐steel bands. Carrying two stainless‐steel bands for 15 yr did not reduce average male survival (0.87) as compared to males with two web tags, but did reduce average female survival (0.79 banded vs. 0.87 unbanded; Boersma and Rebstock 2010). Boersma and Rebstock (2010) did not determine the impact of single bands on female survival, but a study of single‐banded Magellanic Penguins found no impact of bands on the foraging trip duration of males or females during chick rearing (Boersma and Rebstock 2009b).
In their review of banding impacts on penguins, Jackson and Wilson (2002) noted that the effects of bands are generally greatest in the initial year of banding. For example, Ainley et al. (1983) found that aluminum bands had the largest impact on Adélie Penguin survival the first year after they were banded and estimated a higher survival rate for unbanded than for banded fledged chicks (0.51 vs. 0.37). In contrast, Boersma and Rebstock (2010) found females with two bands laid eggs of similar size to the eggs they laid the year before they were banded.
We cannot discount the possibility that there are sex‐specific banding effects in Magellanic Penguins that are exacerbated during the first year at sea, reducing juvenile female survival more than that of males. However, carcasses of unbanded individuals found in non‐breeding areas also suggest female‐biased mortality. Furthermore, annual surveys conducted at Punta Tombo during the late chick‐rearing period, when both males and females are intermittently present and take long foraging trips, suggest increasing ASR throughout the colony (ASR of 0.59 ± 0.05 from 1986 to 1989; ASR of 0.70 ± 0.04 from 2013 to 2016; P. D. Boersma, unpublished data). Only a small portion of the Punta Tombo population is banded, so sex‐specific banding effects cannot explain the increasing ASR.
Conservation implications for Magellanic Penguins
The growth rate and ASR of the Magellanic Penguin population at Punta Tombo are highly sensitive to female survival rates, particularly those of pre‐breeders and adults. Other long‐lived species also show this pattern (adult survival [Sæther and Bakke 2000], pre‐breeder survival/recruitment [Jenouvrier et al. 2015]). However, because juvenile survival rates had the greatest sex bias, juveniles made the greatest contribution to population declines and increases in ASR at the colony from 1990 to 2009. Studies of shorebirds (Eberhart‐Phillips et al. 2018) and parrots (Veran and Beissinger 2009) likewise found that sex‐biased juvenile survival made the largest contributions to ASR.
Studies on closely related African Penguins are relevant to the conservation of Magellanic Penguins because African Penguins show female‐biased mortality, a skewed sex ratio (Pichegru and Parsons 2014), and male‐biased sexual size dimorphism (males are 15% heavier; Pichegru et al. 2013). Starvation is the most common cause of mortality in this endangered species (Pichegru and Parsons 2014), which shows high overlap with fisheries in terms of foraging area and size of fish selected (Pichegru et al. 2009). Climate change and fisheries have altered fish availability in once‐productive African Penguin foraging habitat, creating ecological traps and reducing juvenile survival (Sherley et al. 2017). Female African Penguins show higher foraging effort than do males and the overlap between male and female foraging habitat is lower in years of low food availability (Pichegru et al. 2013). Unlike at Punta Tombo, juvenile and adult survival have shown temporal declines at African Penguin colonies (Sherley et al. 2014). Sex‐specific survival rates have not yet been estimated for African Penguins.
Due to the rapid decline of African Penguin populations over the 20th century, there have been several efforts to increase food availability in the region using fisheries closures. These closures have focused on protecting foraging habitat near breeding colonies of the species. Their effectiveness remains uncertain, however, due in part to the small area protected and potential lag times of protected area benefits in long‐lived species (Sherley et al. 2018). Pichegru et al. (2012) found that fisheries closures near the St. Croix colony temporarily reduced foraging effort of African Penguins and that juvenile survival increased with an increase in fish biomass. Fisheries closures also had positive impacts on reproductive success (18% increase in chick survival) at the Robben Island colony, but the population continued to decline due to high adult mortality (Sherley et al. 2015). A system dynamics model of the Dyer Island colony suggests little population benefit of fisheries closures (~1% gain for a 20‐year closure) where other pressures, in this case seal predation and juvenile emigration, are high enough (Weller et al. 2016).
Several insights relevant to Magellanic Penguins can be gained from recent efforts to conserve African Penguins. First, although it is easiest to focus conservation efforts on relatively well‐defined breeding areas, reversing Magellanic Penguin population decline is likely to require improvements to adult survival during the non‐breeding season. Our finding that the population growth rate of Magellanic Penguins at Punta Tombo shows low elasticity to reproductive success supports this assertion. A second and related conclusion is that small‐scale fisheries closures that focus on breeding season foraging habitat are likely to have little conservation benefit for this species. Effective conservation of Magellanic Penguins would require spatial management at a much larger scale than current marine protected areas (Boersma et al. 2015) and/or than is complemented by other conservation measures, such as bycatch reduction or lower catch limits for forage fish (Sherley et al. 2015, 2018, Weller et al. 2016).
Forage fish make up the bulk of the Magellanic Penguin diet during the species long non‐breeding migration (Silva et al. 2014, Marques et al. 2018), and research has suggested forage fish biomass thresholds under which adult survival begins to decline rapidly (e.g., in African Penguins; Weller et al. 2016). The size of anchovy selected by Magellanic Penguins (mean = 15.8 cm; Marques et al. 2018) overlaps with the size selected with anchovy fisheries in South Africa (6–12 cm; Pichegru et al. 2009), suggesting likely competition between growing commercial fisheries for anchovy in Brazil and migrating Magellanic Penguins. High spatial overlap between Magellanic Penguin non‐breeding habitat and fisheries has been noted elsewhere in their range (Skewgar et al. 2014). If, as our study suggests, juvenile and female Magellanic Penguins are particularly sensitive to food shortage, boosting food availability during the species non‐breeding season would help to balance ASR and slow population decline. Given the importance of anchovy to the region's marine food web, a precautionary approach to their management should be taken (Skewgar et al. 2007).
The last key insight provided by studies on African Penguins is the importance of emigration as a driver of local population change (Weller et al. 2016). Emigration and true survival are confounded in our analysis and sex‐specific dispersal patterns may have influenced our results. Variation in seabird movement patterns can drive local population dynamics more than adult survival rates or recruitment (Szostek et al. 2014) and rates of dispersal can vary with environmental conditions (Dugger et al. 2010). Furthermore, female birds may be more likely to emigrate than males, particularly in declining populations (Morrison et al. 2016), and sex‐biased dispersal can lead to skewed ASRs (Becker et al. 2008, Pipoly et al. 2015, Komdeur et al. 2017).
The panmictic genetic structure and metapopulation dynamics of Magellanic Penguins in the South Atlantic indicates inter‐colony movement (Boersma 2008, Bouzat et al. 2009, Pozzi et al. 2015, Dantas et al. 2018). Pozzi et al.'s (2015) analysis suggested that internal dynamics could describe Punta Tombo's population trajectory, but this required assuming a high juvenile survival rate of 0.65 and the use of a one‐sex matrix model. Across all years studied, the highest juvenile survival rates we estimated were 0.32 for females and 0.44 for males (2003 cohort, 2004 non‐breeding season), and the average across cohorts was only 0.12 for females and 0.17 for males.
It is likely that there is emigration from and immigration to Punta Tombo that we could not account for with our model. Growth rates of increasing colonies of this species are too high to be explained by internal dynamics, so emigrants from Punta Tombo may be acting as a source for these populations (Pozzi et al. 2015). However, to match recorded population trends from 1990 to 2009, we had to increase juvenile survival at Punta Tombo, so there may be immigration to the colony as well. It is important to note that Punta Tombo's effective population size is probably declining faster than suggested by our survey data. We counted any nest with a penguin present as active, and an increasing proportion of those nests are likely to represent unmated males.
A better understanding of the metapopulation dynamics of Magellanic Penguins is a key conservation priority. Genetic analysis highlights the importance of the metapopulation dynamics to this species (Bouzat et al. 2009, Dantas et al. 2018). Several northern colonies in the South Atlantic are growing rapidly (Boersma et al. 2013, Pozzi et al. 2015), but the status of the overall population is unknown. Furthermore, there is no published information on the sex ratio of growing colonies of Magellanic Penguins. It is possible that juvenile females are emigrating from Punta Tombo to growing colonies at higher rates than are males.
Sex‐biased survival and seabird conservation
Considerations of sex‐specific survival are likely to be important to the monitoring and conservation of many seabird colonies. If sexual size dimorphism is a driver, sex‐biased survival may be more prominent among seabirds than currently recognized, as males of most seabird species are larger than females (true of 74% of 96 seabirds reviewed by Schreiber and Burger 2001). Donald (2007) showed that populations of threatened species tended to have more skewed ASRs than non‐threatened populations, suggesting that ASR and population health covary. For Magellanic Penguins at Punta Tombo, ASR is sensitive to the same demographic parameters (i.e., female pre‐breeder and adult survival) as the population growth rate, but its response to these parameters is more dramatic. Colony sex ratio trends may therefore act as an early warning system for deteriorating foraging conditions in this species and other seabirds. Reproductive success is the most commonly used indicator of changing environmental conditions in seabirds because it responds rapidly and dramatically to environmental fluctuations (Boersma 1978, Oro 2014), but it has low impact on population growth in Magellanic Penguins (this study) and other long‐lived species (Sæther and Bakke 2000).
Accounting for ASR also provides a more accurate assessment of population trends. We estimated trends in Punta Tombo's Magellanic Penguin population using the number of nests with adults or eggs present and, because a decreasing proportion of these nests represented breeding pairs, we systematically underestimated the populations’ decline. Likewise, in migratory terrestrial birds, singing males are more detectible than females and many unmated males sing throughout the breeding season, making population declines easy to miss (Morrison et al. 2016).
Considering sex in demographic studies will become increasingly important in the face of climate change, which will result in reductions and shifts in global primary productivity and prey availability (Behrenfeld et al. 2006, Sherley et al. 2017). Seabirds show some flexibility in response to fishing or climate‐induced shifts in prey availability, but there are physiological limits to this flexibility (Grémillet and Boulinier 2009). For seabirds in which females are already close to these physiological limits (e.g., foraging distances approach those that result in no energetic gain), small shifts in prey availability may have greater impacts on female than male survival, resulting in a skewed ASRs and declines in effective population size.
Conclusions
Magellanic Penguins at Punta Tombo, Argentina declined by over 40% since 1987. We found that apparent survival rates during the non‐breeding season were lower for females than for males, in agreement with data collected on carcasses from the species’ non‐breeding range. The long lifespan of Magellanic Penguins means that even small sex biases in survival accumulate with age and time to result in increasingly male‐biased sex ratios. Sex‐biased survival, particularly among juveniles, has reduced the effective population size at Punta Tombo. Our findings are relevant to other species with life history traits similar to Magellanic Penguins, that is, that are long‐lived and show serial monogamy and biparental care. Effective conservation of Magellanic Penguins will require improved knowledge of the species’ metapopulation dynamics and a precautionary approach to fisheries management in the region. Knowledge of sex‐biased demography and ASRs may provide an early warning system for population decline, as ASR is sensitive to the same demographic parameters as population growth rate.
Supporting information
Acknowledgments
The Center for Ecosystem Sentinels at the University of Washington has collected data at Punta Tombo since 1982. Financial support to P. D. Boersma for field work and data analysis was provided by Wildlife Conservation Society, Exxonmobil Foundation, the Pew Fellows Program in Marine Conservation, the Disney Worldwide Conservation Fund, the Chase, Cunningham, CGMK, Offield, Peach, Thorne, Tortuga, and Kellogg Foundations, the Wadsworth Endowed Chair in Conservation Science, and Friends of the Penguins. We thank the Wildlife Conservation Society and the Global Penguin Society for logistical and permit support, particularly Pablo García‐Borboroglu, William Conway, Graham Harris, and Pat Harris. We are grateful to the La Regina family for allowing us to work on their land. We owe great appreciation to J. Laake, who developed marked and provided statistical support. Discussions with G. Orians, G. Rebstock, K. Holt, C. Cappello, L. Koehn, J. Smith, J. Cerchiara, and O. Woods and comments from P. Dayton and anonymous reviewers greatly improved earlier versions of this manuscript. All research was conducted under approval of the University of Washington IACUC (Protocol #2213‐02) and with permission of the Province of Chubut, Offices of Turismo and Flora and Fauna. P. D. Boersma collected the bulk of the data and N. J. Gownaris analyzed the data and led the writing of the manuscript. Both P. D. Boersma and N. J. Gownaris conceived of the manuscript, contributed critically to manuscript drafts, and gave final approval for publication. Field volunteers and students collected the data used in this study for 35 yr and C. Gravelle and O. Woods helped access the database.
Gownaris N. J., and Boersma P. D.. 2019. Sex‐biased survival contributes to population decline in a long‐lived seabird, the Magellanic Penguin. Ecological Applications 29(1):e01826 10.1002/eap.1826
Corresponding Editor: Paul K. Dayton.
Literature Cited
- Ainley, D. G. , and DeMaster D. P.. 1980. Survival and mortality in a population of Adélie Penguins. Ecology 61:522–530. [Google Scholar]
- Ainley, D. G. , LeResche R. E., and Sladen W. J. L.. 1983. Breeding biology of the Adélie penguin. University of California Press, Berkeley, California, USA. [Google Scholar]
- Altrão, C. S. , de Paula A. A., Tavares M., Ott P. H., and Silva‐Souza Â. T.. 2017. Population structure of the nematode Contracaecum pelagicum Johnston & Mawson, 1942 during the winter migration of the Magellanic penguin Spheniscus magellanicus (Forster, 1781) in southern Brazil. Oecologia Australis 21:62–71. [Google Scholar]
- Ancona, S. , Dénes F. V., Krüger O., Székely T., and Beissinger S. R.. 2017. Estimating adult sex ratios in nature. Philosophical Transactions of the Royal Society B: Biological Sciences. 372:20160313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini, T. , Tavecchia G., Pezzo F., Jenouvrier S., and Olmastroni S.. 2015. Predicting responses of the Adélie penguin population of Edmonson Point to future sea ice changes in the Ross Sea. Frontiers in Ecology and Evolution 3:Article 8. [Google Scholar]
- Barbraud, C. , and Weimerskirch H.. 2001. Emperor penguins and climate change. Nature 411:183–186. [DOI] [PubMed] [Google Scholar]
- Bearhop, S. , Phillips R. A., McGill R., Cherel Y., Dawson D. A., and Croxall J. P.. 2006. Stable isotopes indicate sex‐specific and long‐term individual foraging specialisation in diving seabirds. Marine Ecology Progress Series 311:157–164. [Google Scholar]
- Becker, P. H. , Ezard T. H., Ludwigs J. D., Sauer‐Gürth H., and Wink M.. 2008. Population sex ratio shift from fledging to recruitment: consequences for demography in a philopatric seabird. Oikos 117:60–68. [Google Scholar]
- Behrenfeld, M. J. , O'Malley R. T., Siegel D. A., McClain C. R., Sarmiento J. L., Feldman G. C., Milligan A. J., Falkowski P. G., Letelier R. M., and Boss E. S.. 2006. Climate‐driven trends in contemporary ocean productivity. Nature 444:752–755. [DOI] [PubMed] [Google Scholar]
- Berec, L. , Kramer A. M., Bernhauerová V., and Drake J. M.. 2018. Density‐dependent selection on mate search and evolution of Allee effects. Journal of Animal Ecology 87:24–35. [DOI] [PubMed] [Google Scholar]
- Bessa‐Gomes, C. , Legendre S., and Clobert J.. 2004. Allee effects, mating systems and the extinction risk in populations with two sexes. Ecology Letters 7:802–812. [Google Scholar]
- Boersma, P. 1977. An ecological and behavioral study of the Galápagos Penguin. Living Bird 15:43–93. [Google Scholar]
- Boersma, P. D. 1978. Breeding patterns of Galápagos penguins as an indicator of oceanographic conditions. Science 200:1481–1483. [DOI] [PubMed] [Google Scholar]
- Boersma, P. D. 1998. Population trends of the Galápagos penguin: impacts of El Niño and La Niña. Condor 100:245–253. [Google Scholar]
- Boersma, P. D. 2008. Penguins as marine sentinels. BioScience 58:597–607. [Google Scholar]
- Boersma, P. D. , and Davies E. M.. 1987. Sexing monomorphic birds by vent measurements. Auk 104:779–783. [Google Scholar]
- Boersma, P. D. , and Rebstock G. A.. 2009a. Foraging distance affects reproductive success in Magellanic penguins. Marine Ecology Progress Series 375:263–275. [Google Scholar]
- Boersma, P. D. , and Rebstock G. A.. 2009b. Flipper bands do not affect foraging‐trip duration of Magellanic penguins. Journal of Field Ornithology 80:408–418. [Google Scholar]
- Boersma, P. D. , and Rebstock G. A.. 2010. Effects of double bands on Magellanic penguins. Journal of Field Ornithology 81:195–205. [Google Scholar]
- Boersma, P. D. , and Rebstock G. A.. 2014. Climate change increases reproductive failure in Magellanic penguins. PLoS ONE 9:e85602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma, P. , Stokes D., Yorio P., Davis L., and Darby J.. 1990. Reproductive variability and historical change of Magellanic penguins (Spheniscus magellanicus) at Punta Tombo, Argentina. Penguin Biology 7:15–44. [Google Scholar]
- Boersma, P. D. , Rebstock G. A., Frere E., and Moore S. E.. 2009. Following the fish: penguins and productivity in the South Atlantic. Ecological Monographs 79:59–76. [Google Scholar]
- Boersma, P. D. , et al. 2013. Magellanic penguin Pages 233–263 in Borboroglu P. G. and Boersma P. D., editors. Penguins: natural history and conservation. University of Washington Press, Seattle, Washington, USA. [Google Scholar]
- Boersma, P. D. , Rebstock G. A., and García‐Borboroglu P.. 2015. Marine protection is needed for Magellanic penguins in Argentina based on long‐term data. Biological Conservation 182:197–204. [Google Scholar]
- Booksmythe, I. , Mautz B., Davis J., Nakagawa S., and Jennions M. D.. 2017. Facultative adjustment of the offspring sex ratio and male attractiveness: a systematic review and meta‐analysis. Biological Reviews 92:108–134. [DOI] [PubMed] [Google Scholar]
- Bordier, C. , Saraux C., Viblanc V. A., Gachot‐Neveu H., Beaugey M., Le Maho Y., and Le Bohec C.. 2014. Inter‐annual variability of fledgling sex ratio in king penguins. PLoS ONE 9:e114052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzat, J. L. , Walker B. G., and Boersma P. D.. 2009. Regional genetic structure in the Magellanic penguin (Spheniscus magellanicus) suggests metapopulation dynamics. Auk 126:326–334. [Google Scholar]
- Cairns, D. 1988. Seabirds as indicators of marine food supplies. Biological Oceanography 5:261–271. [Google Scholar]
- Cappello, C. D. , and Boersma P. D.. 2018. Sexing Galápagos penguins Spheniscus mendiculus by morphological measurements. Endangered Species Research 35:169–173. [Google Scholar]
- Cardoso, L. G. , Bugoni L., Mancini P. L., and Haimovici M.. 2011. Gillnet fisheries as a major mortality factor of Magellanic penguins in wintering areas. Marine Pollution Bulletin 62:840–844. [DOI] [PubMed] [Google Scholar]
- Caswell, H. 2000. Prospective and retrospective perturbation analyses: their roles in conservation biology. Ecology 81:619–627. [Google Scholar]
- Caswell, H. 2001. Matrix population models: construction, analysis, and interpretation. Sinauer Associates, Sunderland, Massachusetts, USA. [Google Scholar]
- Cerchiara, J. A. , Risques R. A., Prunkard D., Smith J. R., Kane O. J., and Boersma P. D.. 2017. Magellanic penguin telomeres do not shorten with age with increased reproductive effort, investment, and basal corticosterone. Ecology and Evolution 7:5682–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet, R. , Rouan L., and Pradel R.. 2009. Program E‐SURGE: a software application for fitting multievent models Pages 845–865 in Thomas D. L., Cooch E. G., and Conroy M. J., editors. Modeling demographic processes in marked populations. Environmental and ecological statistics. Volume 3. Springer, Boston, Massachusetts, USA. [Google Scholar]
- Ciancio, J. E. , Yorio P., Wilson R., and Frere E.. 2018. Food provisioning in Magellanic penguins as inferred from stable isotope ratios. Rapid Communications in Mass Spectrometry 32:489–494. [DOI] [PubMed] [Google Scholar]
- Colchero, F. , Aliaga A. E., Jones O., and Conde D. A.. 2017. Individual heterogeneity determines sex differences in mortality in a monogamous bird with reversed sexual dimorphism. Journal of Animal Ecology 86:899–907. [DOI] [PubMed] [Google Scholar]
- Cooch, E. , and White G.. 2013. Program MARK: a gentle introduction. 13th edition Cornell University, Ithaca, New York, USA. [Google Scholar]
- Croxall, J. P. , Butchart S. H., Lascelles B., Stattersfield A. J., Sullivan B., Symes A., and Taylor P.. 2012. Seabird conservation status, threats and priority actions: a global assessment. Bird Conservation International 22:1–34. [Google Scholar]
- Dantas, G. P. M. , et al. 2014. Evidence for northward extension of the winter range of Magellenic penguins along the Brazilian coast. Marine Ornithology 41:195–197. [Google Scholar]
- Dantas, G. P. M. , Maria G. C., Marasco A. C. M., Castro L. T., Almeida V. S., Santos F. R., Oliveira L. R., Crespo E., Frere E., and Milliones A.. 2018. Demographic history of the Magellanic penguin (Spheniscus magellanicus) on the Pacific and Atlantic coasts of South America. Journal of Ornithology 159:1–13. [Google Scholar]
- Dehnhard, N. , Poisbleau M., Demongin L., Ludynia K., and Quillfeldt P.. 2014. High juvenile annual survival probabilities in Southern Rockhopper penguins Eudyptes chrysocome are independent of individual fledging traits. Ibis 156:548–560. [Google Scholar]
- Donald, P. F. 2007. Adult sex ratios in wild bird populations. Ibis 149:671–692. [Google Scholar]
- Dugger, K. M. , Ainley D. G., Lyver P. O. B., Barton K., and Ballard G.. 2010. Survival differences and the effect of environmental instability on breeding dispersal in an Adélie penguin meta‐population. Proceedings of the National Academy of Sciences USA 107:12375–12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart‐Phillips, L. J. , Küpper C., Miller T. E., Cruz‐López M., Maher K. H., Dos Remedios N., Stoffel M. A., Hoffman J. I., Krüger O., and Székely T.. 2017. Sex‐specific early survival drives adult sex ratio bias in snowy plovers and impacts mating system and population growth. Proceedings of the National Academy of Sciences USA 114:E5474–E5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart‐Phillips, L. J. , Küpper C., Carmona‐Isunza M. C., Vincze O., Zefania S., Cruz‐López M., Kosztolányi A., Miller T. E., Barta Z., and Cuthill I. C.. 2018. Demographic causes of adult sex ratio variation and their consequences for parental cooperation. Nature Communications 9:Article 1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engen, S. , Lande R., and SÆther B.‐E.. 2003. Demographic stochasticity and Allee effects in populations with two sexes. Ecology 84:2378–2386. [Google Scholar]
- Faux, C. E. , McInnes J. C., and Jarman S. N.. 2014. High‐throughput real‐time PCR and melt curve analysis for sexing Southern Ocean seabirds using fecal samples. Theriogenology 81:870–874. [DOI] [PubMed] [Google Scholar]
- Forero, M. G. , Hobson K. A., Bortolotti G. R., Donázar J. A., Bertellotti M., and Blanco G.. 2002. Food resource utilisation by the Magellanic penguin evaluated through stable‐isotope analysis: segregation by sex and age and influence on offspring quality. Marine Ecology Progress Series 234:289–299. [Google Scholar]
- Fujiwara, M. , and Caswell H.. 2002. A general approach to temporary emigration in mark–recapture analysis. Ecology 83:3266–3275. [Google Scholar]
- Gandini, P. , Boersma P. D., Frere E., Gandini M., Holik T., and Lichtschein V.. 1994. Magellanic penguins (Spheniscus magellanicus) affected by chronic petroleum pollution along coast of Chubut, Argentina. Auk 111:20–27. [Google Scholar]
- García‐Borboroglu, P. , Boersma P. D., Ruoppolo V., Reyes L., Rebstock G. A., Griot K., Heredia S. R., Adornes A. C., and Da Silva R. P.. 2006. Chronic oil pollution harms Magellanic penguins in the Southwest Atlantic. Marine Pollution Bulletin 52:193–198. [DOI] [PubMed] [Google Scholar]
- García‐Borboroglu, P. , Boersma P. D., Ruoppolo V., Pinho‐da‐Silva‐Filho R., Corrado‐Adornes A., Conte‐Sena D., Velozo R., Myiaji‐Kolesnikovas C., Dutra G., and Maracini P.. 2010. Magellanic penguin mortality in 2008 along the SW Atlantic coast. Marine Pollution Bulletin 60:1652–1657. [DOI] [PubMed] [Google Scholar]
- Genovart, M. , Pradel R., and Oro D.. 2012. Exploiting uncertain ecological fieldwork data with multi‐event capture–recapture modelling: an example with bird sex assignment. Journal of Animal Ecology 81:970–977. [DOI] [PubMed] [Google Scholar]
- Grémillet, D. , and Boulinier T.. 2009. Spatial ecology and conservation of seabirds facing global climate change: a review. Marine Ecology Progress Series 391:121–137. [Google Scholar]
- Hanners, L. A. , and Patton S. R.. 1985. Sexing Laughing Gulls using external measurements and discriminant analysis. Journal of Field Ornithology 56:158–164. [Google Scholar]
- Haridas, C. , Eager E. A., Rebarber R., and Tenhumberg B.. 2014. Frequency‐dependent population dynamics: effect of sex ratio and mating system on the elasticity of population growth rate. Theoretical Population Biology 97:49–56. [DOI] [PubMed] [Google Scholar]
- Horswill, C. , and Robinson R.. 2015. Review of seabird demographic rates and density dependence. Joint Nature Conservation Committee, Peterborough, UK. [Google Scholar]
- Jackson, S. , and Wilson R. P.. 2002. The potential costs of flipper‐bands to penguins. Functional Ecology 16:141–148. [Google Scholar]
- Jacobs, S. R. , Elliott K. H., and Gaston A. J.. 2013. Parents are a drag: long‐lived birds share the cost of increased foraging effort with their offspring, but males pass on more of the costs than females. PLoS ONE 8:e54594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings, S. , Varsani A., Dugger K. M., Ballard G., and Ainley D. G.. 2016. Sex‐based differences in Adélie penguin (Pygoscelis adeliae) chick growth rates and diet. PLoS ONE 11:e0149090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenouvrier, S. , Barbraud C., and Weimerskirch H.. 2005. Long‐term contrasted responses to climate of two antarctic seabird species. Ecology 86:2889–2903. [Google Scholar]
- Jenouvrier, S. , Caswell H., Barbraud C., and Weimerskirch H.. 2010. Mating behavior, population growth, and the operational sex ratio: a periodic two‐sex model approach. American Naturalist 175:739–752. [DOI] [PubMed] [Google Scholar]
- Jenouvrier, S. , Péron C., and Weimerskirch H.. 2015. Extreme climate events and individual heterogeneity shape life‐history traits and population dynamics. Ecological Monographs 85:605–624. [Google Scholar]
- Johnson, D. S. , Laake J. L., Melin S. R., and DeLong R. L.. 2016. Multivariate state Hidden Markov models for mark‐recapture data. Statistical Science 31:233–244. [Google Scholar]
- Koehn, L. E. , Hard J. J., Akst E. P., and Boersma P. D.. 2016. Natural selection on morphology varies among years and by sex in Magellanic Penguins (Spheniscus magellanicus). Auk 133:783–805. [Google Scholar]
- Komdeur, J. , Székely T., Long X., and Kingma S. A.. 2017. Adult sex ratios and their implications for cooperative breeding in birds. Philosophical Transactions of the Royal Society B 372:20160322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuparinen, A. 2018. The mechanistic basis of demographic Allee effects: the search for mates. Journal of Animal Ecology 87:4–6. [DOI] [PubMed] [Google Scholar]
- Laake, J. L. 2013. Capture‐recapture analysis with Hidden Markov Models. Alaska Fisheries Science Center Processed Report 2013‐04. NOAA National Marine Fisheries Service, Seattle, Washington, USA. [Google Scholar]
- Laake, J. L. , Johnson D. S., and Conn P. B.. 2013. marked: An R package for maximum likelihood and Markov Chain Monte Carlo analysis of capture–recapture data. Methods in Ecology and Evolution 4:885–890. [Google Scholar]
- Lebreton, J.‐D. , Burnham K. P., Clobert J., and Anderson D. R.. 1992. Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecological Monographs 62:67–118. [Google Scholar]
- Lee, A. M. , Sæther B.‐E., and Engen S.. 2011. Demographic stochasticity, Allee effects, and extinction: the influence of mating system and sex ratio. American Naturalist 177:301–313. [DOI] [PubMed] [Google Scholar]
- Lewison, R. , Oro D., Godley B., Underhill L., Bearhop S., Wilson R., Ainley D., Arcos J., Boersma P., and Borboroglu P.. 2012. Research priorities for seabirds: improving conservation and management in the 21st century. Endangered Species Research 17:93–121. [Google Scholar]
- Liker, A. , Székely T., and Tregenza T.. 2005. Mortality costs of sexual selection and parental care in natural populations of birds. Evolution 59:890–897. [PubMed] [Google Scholar]
- Mäder, A. , Sander M., and Casa G. Jr. 2010. Ciclo sazonal de mortalidade do pinguim‐de‐magalhães, Spheniscus magellanicus influenciado por fatores antrópicos e climáticos na costa do Rio Grande do Sul, Brasil. Revista Brasileira de Ornitologia 18:228–233. [Google Scholar]
- Marques, F. P. , Cardoso L. G., Haimovici M., and Bugoni L.. 2018. Trophic ecology of Magellanic penguins (Spheniscus magellanicus) during the non‐breeding period. Estuarine, Coastal and Shelf Science 210:109–122. [Google Scholar]
- Morrison, K. W. , Battley P. F., Sagar P. M., and Thompson D. R.. 2015. Population dynamics of Eastern Rockhopper Penguins on Campbell Island in relation to sea surface temperature 1942–2012: current warming hiatus pauses a long‐term decline. Polar Biology 38:163–177. [Google Scholar]
- Morrison, C. A. , Robinson R. A., Clark J. A., and Gill J. A.. 2016. Causes and consequences of spatial variation in sex ratios in a declining bird species. Journal of Animal Ecology 85:1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, J. D. , Kendall W. L., Hines J. E., and Spendelow J. A.. 2004. Estimation of sex‐specific survival from capture–recapture data when sex is not always known. Ecology 85:3192–3201. [Google Scholar]
- Nunes, G. T. , da Rosa Leal G., da Silva Barreto J., Mäder A., de Freitas T. R. O., Lopes D. D., and Fernández G. P.. 2015. Razão sexual assimétrica entre carcaças de Spheniscus magellanicus na costa norte do Rio Grande do Sul. Ornithologia 8:75–77. [Google Scholar]
- Olsson, O. , and Van der Jeugd H. P.. 2002. Survival in king penguins Aptenodytes patagonicus: temporal and sex‐specific effects of environmental variability. Oecologia 132:509–516. [DOI] [PubMed] [Google Scholar]
- Orians, G. H. 1969. Age and hunting success in the Brown Pelican (Pelecanus occidentalis). Animal Behaviour 17:316–319. [Google Scholar]
- Oro, D. 2014. Seabirds and climate: knowledge, pitfalls, and opportunities. Frontiers in Ecology and Evolution 2:Article 79. [Google Scholar]
- Paleczny, M. , Hammill E., Karpouzi V., and Pauly D.. 2015. Population trend of the world's monitored seabirds, 1950‐2010. PLoS ONE 10:e0129342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichegru, L. , and Parsons N.. 2014. Female‐biased mortality in African Penguins. African Journal of Marine Science 36:279–282. [Google Scholar]
- Pichegru, L. , Ryan P. G., Le Bohec C., van der Lingen C. D., Navarro R., Petersen S., Lewis S., van der Westhuizen J., and Grémillet D.. 2009. Overlap between vulnerable top predators and fisheries in the Benguela upwelling system: implications for marine protected areas. Marine Ecology Progress Series 391:199–208. [Google Scholar]
- Pichegru, L. , Ryan P. G., van Eeden R., Reid T., Grémillet D., and Wanless R.. 2012. Industrial fishing, no‐take zones and endangered penguins. Biological Conservation 156:117–125. [Google Scholar]
- Pichegru, L. , Cook T., Handley J., Voogt N., Watermeyer J., Nupen L., and McQuiad C. D.. 2013. Sex‐specific foraging behavior and a field sexing technique for Endangered African penguins. Endangered Species Research 19:255–264. [Google Scholar]
- Pipoly, I. , Bókony V., Kirkpatrick M., Donald P. F., Székely T., and Liker A.. 2015. The genetic sex‐determination system predicts adult sex ratios in tetrapods. Nature 527:91–94. [DOI] [PubMed] [Google Scholar]
- Pozzi, L. M. 2015. Dinámica poblacional del pinüino de Magallanes (Speniscus magellanicus) en el norte de Patagonia Argentina. Universidad Nacional del Comahue, Neuquén, Argentina. [Google Scholar]
- Pozzi, L. M. , Borboroglu P. G., Boersma P. D., and Pascual M. A.. 2015. Population regulation in Magellanic penguins: what determines changes in colony size? PLoS ONE 10:e0119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel, R. 2009. The stakes of capture–recapture models with state uncertainty Pages 781–795 in Thomas D. L., Cooch E. G., and Conroy M. J., editors. Modeling demographic processes in marked populations. Environmental and ecological statistics. Volume 3. Springer, Boston, Massachusetts, USA. [Google Scholar]
- Pütz, K. , Schiavini A., Rey A. R., and Lüthi B. H.. 2007. Winter migration of Magellanic penguins (Spheniscus magellanicus) from the southernmost distributional range. Marine Biology 152:1227–1235. [Google Scholar]
- Raya Rey, A. , Pütz K., Scioscia G., Lüthi B., and Schiavini A.. 2012. Sexual differences in the foraging behaviour of Magellanic penguins related to stage of breeding. Emu 112:90–96. [Google Scholar]
- Rebstock, G. A. , and Boersma P. D.. 2018. Oceanographic conditions in wintering grounds affect arrival date and body condition in breeding female Magellanic penguins. Marine Ecology Progress Series 601:253–267. [Google Scholar]
- Rebstock, G. A. , Boersma P. D., and García‐Borboroglu P.. 2016. Changes in habitat use and nesting density in a declining seabird colony. Population Ecology 58:105–119. [Google Scholar]
- Richdale, L. E. 1957. A population study of penguins. Oxford University Press, Oxford, UK. [Google Scholar]
- Riotte‐Lambert, L. , and Weimerskirch H.. 2013. Do naive juvenile seabirds forage differently from adults? Proceedings of the Royal Society B 280:20131434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo, T. J. , Kesseli R., Halverson J. L., Nisbet I. C., and Hatch J. J.. 1994. PCR‐based method for sexing roseate terns (Sterna dougallii). Auk 111:1023–1027. [Google Scholar]
- Sæther, B.‐E. , and Bakke Ø.. 2000. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 81:642–653. [Google Scholar]
- Schacht, R. , Kramer K. L., Székely T., and Kappeler P. M.. 2017. Adult sex ratios and reproductive strategies: a critical re‐examination of sex differences in human and animal societies. Philosophical Transactions of the Royal Society B 19:20160309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, E. A. , and Burger J.. 2001. Biology of marine birds. CRC Press, Taylor and Francis Group, Boca Raton, Florida, USA. [Google Scholar]
- Scioscia, G. , Rey A. R., and Schiavini A.. 2016. Breeding biology of Magellanic penguins (Spheniscus magellanicus) at the Beagle Channel: interannual variation and its relationship with foraging behaviour. Journal of Ornithology 157:773–785. [Google Scholar]
- Sherley, R. B. , Abadi F., Ludynia K., Barham B. J., Clark A. E., and Altwegg R.. 2014. Age‐specific survival and movement among major African penguin Spheniscus demersus colonies. Ibis 156:716–728. [Google Scholar]
- Sherley, R. B. , Winker H., Altwegg R., van der Lingen C. D., Votier S. C., and Crawford R. J.. 2015. Bottom‐up effects of a no‐take zone on endangered penguin demographics. Biology Letters 11:20150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherley, R. B. , Ludynia K., Dyer B. M., Lamont T., Makhado A. B., Roux J.‐P., Scales K. L., Underhill L. G., and Votier S. C.. 2017. Metapopulation tracking juvenile penguins reveals an ecosystem‐wide ecological trap. Current Biology 27:563–568. [DOI] [PubMed] [Google Scholar]
- Sherley, R. B. , Barham B. J., Barham P. J., Campbell K. J., Crawford R. J., Grigg J., Horswill C., McInnes A., Morris T. L., and Pichegru L.. 2018. Bayesian inference reveals positive but subtle effects of experimental fishery closures on marine predator demographics. Proceedings of the Royal Society B 285:20172443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway, S. E. , Allen S. M., and Boersma P. D.. 2003. Marine birds and harmful algal blooms: sporadic victims or under‐reported events? Harmful Algae 2:1–17. [Google Scholar]
- Silva, L. , Saporit F., Vales D., Tavares M., Gandini P., Crespo E. A., and Cardona L.. 2014. Differences in diet composition and foraging patterns between sexes of the Magellanic penguin (Spheniscus magellanicus) during the non‐breeding period as revealed by δ13C and δ15N values in feathers and bone. Marine Biology 161:1195–1206. [Google Scholar]
- Skewgar, E. , Boersma P., Harris G., and Caille G.. 2007. Anchovy fishery threat to Patagonian ecosystem. Science 315:45. [DOI] [PubMed] [Google Scholar]
- Skewgar, E. , Boersma P. D., and Simeone A.. 2014. Winter migration of Magellanic penguins (Spheniscus magellanicus) along the southeastern Pacific. Waterbirds 37:203–209. [Google Scholar]
- Spelt, A. , and Pichegru L.. 2017. Sex allocation and sex‐specific parental investment in an endangered seabird. Ibis 159:272–284. [Google Scholar]
- Stokes, D. L. , Boersma P. D., de Casenave J. L., and García‐Borboroglu P.. 2014. Conservation of migratory Magellanic penguins requires marine zoning. Biological Conservation 170:151–161. [Google Scholar]
- Székely, T. , Liker A., Freckleton R. P., Fichtel C., and Kappeler P. M.. 2014a. Sex‐biased survival predicts adult sex ratio variation in wild birds. Proceedings of the Royal Society B 281:20140342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Székely, T. , Weissing F., and Komdeur J.. 2014b. Adult sex ratio variation: implications for breeding system evolution. Journal of Evolutionary Biology 27:1500–1512. [DOI] [PubMed] [Google Scholar]
- Szostek, K. L. , Schaub M., and Becker P. H.. 2014. Immigrants are attracted by local pre‐breeders and recruits in a seabird colony. Journal of Animal Ecology 83:1015–1024. [DOI] [PubMed] [Google Scholar]
- Trathan, P. N. , García‐Borboroglu P., Boersma D., Bost C. A., Crawford R. J., Crossin G. T., Cuthbert R. J., Dann P., Davis L. S., and De La Puente S.. 2015. Pollution, habitat loss, fishing, and climate change as critical threats to penguins. Conservation Biology 29:31–41. [DOI] [PubMed] [Google Scholar]
- Vanstreels, R. E. T. , Adornes A. C., Ruoppolo V., Canabarro P. L., Silva‐Filho R. P., and Catao‐Dias J. L.. 2011. Gender determination from morphometrics in migrating Magellanic Penguins (Spheniscus magellanicus). Marine Ornithology 39:215–220. [Google Scholar]
- Vanstreels, R. E. T. , Adornes A. C., Canabarro P. L., Ruoppolo V., Amaku M., da Silva‐Filho R. P., and Catão‐Dias J. L.. 2013. Female‐biased mortality of Magellanic penguins (Spheniscus magellanicus) on the wintering grounds. Emu 113:128–134. [Google Scholar]
- Veran, S. , and Beissinger S. R.. 2009. Demographic origins of skewed operational and adult sex ratios: perturbation analyses of two‐sex models. Ecology Letters 12:129–143. [DOI] [PubMed] [Google Scholar]
- Votier, S. C. , Fayet A. L., Bearhop S., Bodey T. W., Clark B. L., Grecian J., Guilford T., Hamer K. C., Jeglinski J. W., and Morgan G.. 2017. Effects of age and reproductive status on individual foraging site fidelity in a long‐lived marine predator. Proceedings of the Royal Society B 284:20171068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, B. G. , and Boersma P. D.. 2003. Diving behavior of Magellanic penguins (Spheniscus magellanicus) at Punta Tombo, Argentina. Canadian Journal of Zoology 81:1471–1483. [Google Scholar]
- Weimerskirch, H. 2018. Linking demographic processes and foraging ecology in wandering albatross‐conservation implications. Journal of Animal Ecology 87:945–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimerskirch, H. , Delord K., Guitteaud A., Phillips R. A., and P. Pinet . 2015. Extreme variation in migration strategies between and within wandering albatross populations during their sabbatical year, and their fitness consequences. Scientific Reports 5:8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller, F. , Cecchini L.‐A., Shannon L., Sherley R. B., Crawford R. J., Altwegg R., Scott L., Stewart T., and Jarre A.. 2014. A system dynamics approach to modelling multiple drivers of the African penguin population on Robben Island, South Africa. Ecological Modelling 277:38–56. [Google Scholar]
- Weller, F. , Sherley R. B., Waller L. J., Ludynia K., Geldenhuys D., Shannon L. J., and Jarre A.. 2016. System dynamics modelling of the Endangered African penguin populations on Dyer and Robben islands, South Africa. Ecological Modelling 327:44–56. [Google Scholar]
- Whitehead, A. L. , Lyver P. O. B., Ballard G., Barton K., Karl B. J., Dugger K. M., Jennings S., Lescroël A., Wilson P. R., and Ainley D. G.. 2015. Factors driving Adélie penguin chick size, mass and condition at colonies of different sizes in the Southern Ross Sea. Marine Ecology Progress Series 523:199–213. [Google Scholar]
- Wilson, R. 1985. The Jackass penguin (Spheniscus demersus) as a pelagic predator. Marine Ecology Progress Series 25:219–227. [Google Scholar]
- Zimmer, I. , Ropert‐Coudert Y., Kato A., Ancel A., and Chiaradia A.. 2011. Does foraging performance change with age in female little penguins (Eudyptula minor)? PLoS ONE 6:e16098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


