Abstract
Support for the “biotic resistance hypothesis,” that species‐rich communities are more successful at resisting invasion by exotic species than are species‐poor communities, has long been debated. It has been argued that native–exotic richness relationships (NERR) are negative at small spatial scales and positive at large scales, but evidence for the role of spatial scale on NERR has been contradictory. However, no formal quantitative synthesis has previously examined whether NERR is scale‐dependent across multiple studies, and previous studies on NERR have not distinguished spatial grain and extent, which may drive very different ecological processes. We used a global systematic review and hierarchical mixed‐effects meta‐analysis to provide a comprehensive quantitative assessment of the patterns of NERR over a range of spatial grain sizes and spatial extents, based on 204 individual cases of observational (non‐experimental) NERRs from 101 publications. We show that NERR was indeed highly scale dependent across studies and increased with the log of grain size. However, mean NERR was not negative at any grain size, although there was high heterogeneity at small grain sizes. We found no clear patterns of NERR across different spatial extents, suggesting that extent plays a less important role in determining NERR than does grain, although there was a complex interaction between extent and grain size. Almost all studies on NERR were conducted in North America, western Europe, and a few other regions, with little information on tropical or Arctic regions. We did find that NERR increased northward in temperate regions and also varied with longitude. We discuss possible explanations for the patterns we found, and caution that our results do not show that invasive species are benign or have no negative consequences for biodiversity preservation. This study represents the first global quantitative analysis of scale‐based NERR, and casts doubt on the existence of an “invasion paradox” of negative NERR at small scales and positive correlations at large scales in non‐experimental studies.
Keywords: biotic resistance, extent, grain size, invasion paradox, native–exotic species richness relationships, scale, spatial patterns
Introduction
The invasion of exotic species into natural habitats is a global ecological and environmental issue that has been a major focus of ecological research for the past 25 yr (Vitousek et al. 1997, Richardson and Pyšek 2008, Lowry et al. 2013). Identifying the ecological determinants that make environments more or less invasible is crucial for predicting the spatial distribution of exotic species and managing biodiversity conservation and is also of fundamental importance for understanding constraints on and factors supporting biodiversity.
Many ecologists have suggested that the relationship between the number of native species and the number of invasive species is negative at small spatial scales and positive at large spatial scales (Levine 2000, Shea and Chesson 2002, Stohlgren et al. 2003, 2006a, Fridley et al. 2004, 2007), but results have been ambiguous and controversial. Elton (1958) hypothesized that species‐rich communities are less vulnerable to the biological invasions than species‐poor communities, and this view, known as the “biotic resistance hypothesis,” was long taken to imply that there should be a negative correlation between native and non‐native species richness in ecological communities (e.g., recently reviewed by Jeschke et al. 2018). Subsequently, theoretical, experimental, and observational studies have reported negative relationships between diversity and invasibility in many ecosystem types including riparian corridors (Brown and Peet 2003), tall grass prairie and sagebrush (Stohlgren et al. 2006b), forest (Byrne et al. 2010), subtropical wetlands (Boughton et al. 2011), grasslands (Harrison et al. 2015), and agricultural sites (Peltzer and MacLeod 2014). However, positive native–exotic relationships (NER, a general term for any measure on which the diversity–invasibility relationship is based, including, e.g., species richness, biomass, density, and dominance) have also been reported in many field studies, suggesting the opposite pattern: habitats with more native species may be more readily invaded by exotic species (Stohlgren et al. 2003, Brummer et al. 2016, Hui and Richardson 2017, Jeschke et al. 2018). These studies are generally based on observational data that span large spatial scales such as nature reserves and islands (Lonsdale 1999, Stohlgren et al. 2003, Fridley et al. 2004). Therefore, scale has been considered to be an important factor in explaining this “invasion paradox” (Brown and Peet 2003, Stohlgren et al. 2003, 2006b, Fridley et al. 2004, 2007). Shea and Chesson (2002) constructed a conceptual framework examining correlations between native and exotic species richness and suggested that negative NER at smaller spatial scales (“clusters” of points, possibly representing sites or sampled areas) and positive NER at larger spatial scales (across such “clusters”) might be caused by extrinsic homogeneity of environmental conditions at smaller scales, leading to poorer “niche opportunities” for exotics, but greater variation in extrinsic environmental factors across larger scales (an example of Simpson's paradox). If extrinsic factors favor high numbers of both native and exotic species, mean NER will increase with spatial scale as relationships are averaged across “clusters.”
Although several narrative reviews on scale‐dependent NER have been carried out in recent decades (Levine and D'Antonio 1999, Lonsdale 1999, Herben et al. 2004, Fridley et al. 2007), no quantitative synthesis has previously been published on NER patterns from a global perspective. We carried out a systematic review and meta‐analysis to provide a comprehensive assessment of global patterns of the correlation between native and exotic plant species richness over a range of spatial scales. We focused on the native–exotic richness relationship (NERR), a more specific relationship than the more general NER. While NERR is concerned specifically with species richness relationships, NER may also include various measures of species abundances or other measures of diversity. We examined the evidence for whether NERR is negative at small spatial scales and positive at large spatial scales, and quantitatively tested whether unmanipulated (non‐experimental) patterns of NERR are indeed scale dependent by synthesizing and comparing the results from the published literature.
Generally speaking, spatial scale usually includes two aspects, grain size and extent. Grain size represents the smallest area for which data are available, which is often sampling plot area in field studies, while extent is the total spatial area included in the study (Turner 1989, Wiens 1989). Interactions between individual plants are thought to operate largely at small grain sizes where competitive exclusion operates, reducing the number of invasive species relative to native species, and in which the environment within plots can be regarded as relatively homogenous. Thus, biotic resistance is believed to be more likely to be detected in relatively small plots, leading to a negative relationship between diversity and invasibility and negative correlations between native and exotic species richness at small grain sizes (Levine 2000, Fridley et al. 2007). In contrast, at grain sizes larger than local neighborhoods, extrinsic factors are thought to become more important than biotic interactions in determining NER. Such a pattern of “biotic acceptance” might be explained by a favorable environment or environmental heterogeneity. The favorable condition hypothesis assumes that exotic and native species respond similarly to favorable environmental conditions that support higher levels of both native and exotic diversity (Levine and D'Antonio 1999, Naeem et al. 2000). The spatial heterogeneity hypothesis relies on the observation that larger sampling units have the potential to contain higher environmental heterogeneity than small plots, allowing more species of both natives and exotics to coexist (Davies et al. 2005, Kumar et al. 2006).
Extent is less widely considered than grain size, and frequently in the discussion of NER extent is not clearly distinguished from grain. In fact, both grain size and extent could play important roles affecting the directions and magnitudes of NER, because variation in environmental conditions exists both within plots and between plots. Variation along environmental gradients among sampled plots can include heterogeneity in abiotic conditions, resource availability, degree of disturbance and distance to propagule sources (Fridley et al. 2007, Hulme et al. 2008). We considered the effects of grain and extent on NERR separately in our study. We focused on NERR for plants because by far the most information is available for plants, and the argument about the scale‐dependent NERR has largely concerned data on plants.
The primary goals of our systematic review were to (1) conduct a broad search and qualitatively summarize the literature on scale‐dependent NERR, including publication information, study location, grain size, extent, and habitat investigated; (2) evaluate the limitations of existing studies on scale‐dependent NERR and propose areas for future research; and (3) identify papers suitable for a meta‐analysis of NERR. In our meta‐analysis, we address the following questions: (1) Does the relationship between native and exotic species richness change with grain size, and if it does, is it negative at small grain sizes and positive at large grain sizes? Is this relationship, if it exists, discrete, or does NERR vary continuously with grain size? If NERR is negative at small grain sizes and positive at large grain sizes, across all systems, on average, at what grain size does the relationship reverse sign? (2) Is NERR significantly different between small and large extents, and how does extent affect the grain size‐dependent NERR? (3) Does the pattern of NERR with grain size differ between various habitat types and geographic locations, particularly with latitude and longitude? We note that, while it might be very interesting to evaluate the effects of total species richness on the patterns, we were not able to do so because this information is almost invariably missing from the primary research papers whose results we synthesized.
Clarifying relationships among these factors can address fundamental questions about the limits to and determinants of biodiversity and may provide information to policy makers for better prioritization of management efforts for exotic species invasions, a rather remarkable omission.
Materials and Methods
Systematic review
A systematic review was conducted in February 2017 for scale‐dependent NERR in plants using the keywords “biodiversity or diversity” AND “plant and inva*” in Web of Science (ISI) and Google Scholar from 1986 to 2016, limiting our search results to relevant research fields of “Ecology,” “Plant sciences,” and “Biodiversity conservation.” The Chinese National Knowledge Infrastructure (CNKI) database was also searched for relevant papers but, regrettably, no articles found in CNKI were suitable. We then screened the titles, abstracts, and results to select studies based on the following criteria: (1) Studies were observational or experimental. Literature reviews, syntheses and mathematical simulation models were excluded. (2) Studies reported NER using species richness; studies reporting other response indices (e.g., species cover, density, or abundance; biomass of one or several exotic species) were excluded. Relevant publications were further screened using the full text. We also cross‐checked studies derived from the reference lists of relevant reviews and articles we previously identified to investigate whether there were additional publications. For our systematic review, we extracted descriptive information from each study to catalog its characteristics (Table 1). See Appendix S2 for details on extracting latitude and longitude. Few studies provided explicit values for spatial extent. To approximate the extent of study areas, we selected the four most distant points sampled in each study at the cardinal directions of the study area, calculated their distances with ImageJ software, and estimated the rectangular area (Schneider et al. 2012; available online).4 Extent estimates were grouped into seven categories (Table 1).
Table 1.
Classification scheme of factors selected for systematic review
| Case characteristics | Levels |
|---|---|
| Publication journal and year | |
| Grain size | classified into six categories: (0, 1], (1, 10], (10, 100], (100, 500], (500, 1,000] and (1,000) m2 |
| Country | |
| Study area within country | |
| Geographic coordinates | midpoint of study area (latitude, longitude) |
| Spatial extent | (0, 10), [10, 100), [102, 103), [103, 104), [104, 105), [105, 106) and [106) km2 |
| Research type | observational or experimental |
| Habitat type | forest, grassland, shrubland, wetland, riparian, savanna, agricultural habitat (refers to the non‐crop semi‐natural areas in an agricultural landscape), urban, many habitat types included in study, and miscellaneous other habitats (e.g., freshwater, old fields) |
| Climatic zone | tropical, temperate, or polar |
Meta‐analysis
Our meta‐analysis used a subset of studies from the systematic review for which the correlation between native and exotic (or alien/invasive) species richness was explicitly reported or could be calculated across multiple grain sizes. We extracted Pearson's product–moment correlation coefficients (r) directly from the study, if available, or we calculated r using native and exotic species richness if reported for multiple plots, extracting data points from figures if needed with Getdata 2.26 (available online).5
Because almost all of the experimental studies included in our systematic review were conducted in grasslands at small grain sizes and extents, we limited the meta‐analysis to natural (i.e., unmanipulated) communities rather than including experimental plant communities. Some studies incorporated more than one NERR in different habitat types, locations, or at different spatial extents. For those studies, these NERR values were included as separate individual observations (cases). We only considered neophytes to be exotic (Deutschewitz et al. 2003). We did not extract data from state species lists or other sources of this type directly, because those data do not provide information on grain sizes (Vitousek et al. 1997, Wu et al. 2010).
Statistical analyses
The Pearson's product–moment correlation coefficient (r) from each case was normalized using Fisher's z transformation as an effect size, where the sample size was the number of plots for which of exotic and native species numbers were calculated (see Appendix S2 for details). Initial data exploration was conducted by using OpenMEE software (Wallace et al. 2017; software available online).6 Full analyses were conducted in R version 3.3.3 using the metafor package (Viechtbauer 2010, R Core Team 2017). Meta‐analysis assumes that individual studies are statistically independent; thus, obtaining several observations (e.g., cases in different habitats) from one publication could violate the assumption of independence and create a hierarchical dependence structure among the effect size estimates (Stevens and Taylor 2009). Therefore, we accounted for this hierarchical structure in the model by modeling variance with both among‐study and within‐study (among‐case) components, in addition to the within‐case variance (sampling error). We constructed an inverse‐variance‐weighted hierarchical mixed‐effects meta‐regression of z as a function of covariates of interest, estimating within‐study and among‐study variance (model detailed in Appendix S2). The explained heterogeneity Q statistic (Q M) was also calculated to test for significance in single covariate meta‐regressions. Effect sizes were considered significant if the 95% confidence intervals (CIs) did not overlap zero. All parameters in the meta‐analytical models were estimated using maximum likelihood, which is preferred when fitting hierarchical mixed‐effects models (Zuur et al. 2009). We examined the possibility of publication bias and temporal change in effect size using a funnel plot and cumulative meta‐analysis, respectively (Appendix S2).
We selected the following covariates a priori to be incorporated in the hierarchical mixed‐effect meta‐regression models to explain heterogeneity in effect sizes: (1) grain size (grain size on a natural logarithm scale), (2) extent, (3) habitat type, (4) latitude (absolute value), (5) longitude, (6) grain size × extent (interaction), and (7) grain size × habitat type. Habitat types were classified as forest, grassland (including savannas and prairies), shrubland, wetland (e.g., riparian, freshwater), urban, agricultural habitat (e.g., old fields; cultivated croplands were excluded), and many (more than one habitat). Latitude and longitude are proxies of climate and biogeographic history. Longitude is not a commonly used covariate in ecology and biogeography, but is valuable in placing data in the context of the climatic effects of atmospheric and oceanic circulation patterns and vicariant relationships, in order to map patterns spatially in a more quantitative manner.
In order to select a model that best explains the heterogeneity in effect sizes, we fit hierarchical mixed‐effects multiple meta‐regressions of z using all combinations of first‐order covariates, though longitude was always fit with linear and quadratic terms. Model fit was compared using Akaike's information criterion corrected for small sample size (AICc).
Results
Systematic review
We found 124 studies, of which 101 could be used for meta‐analysis. The remaining 23 studies were either experimental studies or observational studies with no available original data to analyze (Appendix S1, Appendix S6, Data S1). The 124 papers incorporated in our review were published from 1988 to 2016, with the majority published from 2003 to 2013 (Appendix S3: Fig. S1). There were more than 150 locations where NERR was studied. Geographically, most study areas were located in North America (72%), followed by Europe (18%), Australia and New Zealand (4%), Asia (3%), South America (2%), and Africa (1%).
More than 50% of cases examined NERR at grain sizes ≤10 m2, including 32% with grain ≤1 m2 in size, while 15% of cases were explored at larger grain size (>1,000 m2; Appendix S3: Fig. S2a). Most cases evaluated NERR at local extents (<10 km2, 34% of cases), and only 7% of cases involved extents that were larger than 106 km2 (Appendix S3: Fig. S2b). Nearly 90% of studies (110 studies) were observational. The largest numbers of cases were conducted in grassland (26%) and forest (28%) habitats, while the remaining cases were conducted in savanna (3%), riparian (12%), shrubland (6%), agricultural (2%), wetland (4%), urban (2%), other (4%), or across many different habitats (13%; Appendix S3: Fig. S2c). Moreover, more than 90% of publications were conducted in temperate regions, with a relative paucity of data from the tropics (Yu et al. 2016) and polar regions.
Meta‐analysis
Our database of 101 studies included 204 individual cases (Data S2). The effect size increased with grain size, and the intercept of the regression was positive (with grain on a natural log scale, slope = 0.0373, 95% CI [0.0247, 0.0498], P < 0.0001; intercept = 0.1294, 95% CI [0.0193, 0.2395]). There was a great deal of heterogeneity in effect sizes at small grain sizes, with NERR either negative or positive. Heterogeneity decreased as grain sizes become larger and, at larger grain sizes, NERR was always positive (Fig. 1, Table 2). When we assessed the sensitivity of the results to potentially disproportionately influential effect sizes by removing the four largest data points with Fisher's z > 1.500, the slope and intercept estimates were somewhat smaller (slope = 0.0360; intercept = 0.1230), but the regression remained significant.
Figure 1.
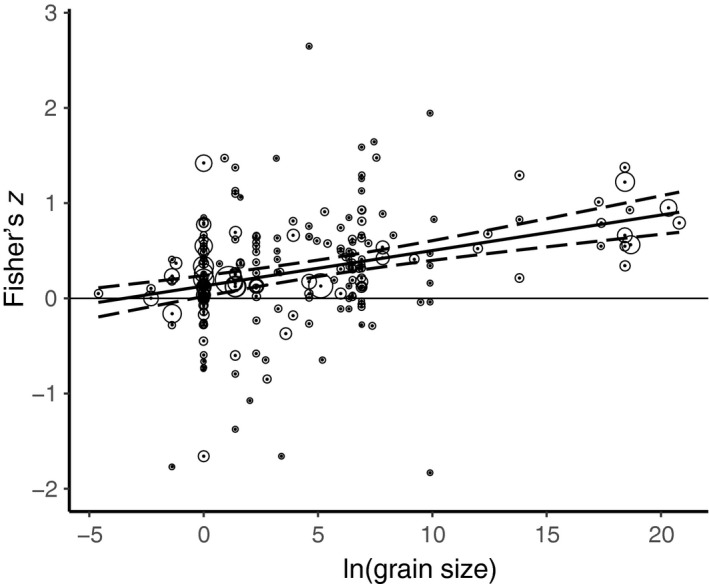
The relationship between Fisher's z (native–exotic richness relationship) and the natural log of grain size (m2) showing the calculated hierarchical mixed‐effects meta‐regression slope (solid line) and 95% confidence intervals (dashed lines). Open circles around points have radii relative to the inverse variance of each case, i.e., studies with larger circles have lower variance in z.
Table 2.
Model fit statistics from single covariate meta‐regressions
| Covariates | τ2 | ω2 | Q M | P (Q M) | I 2 |
|---|---|---|---|---|---|
| Grain size | 0.1072 | 0.0847 | 33.8306 | <0.0001 | 98.2476 |
| Ordered (extent) | 0.1535 | 0.0899 | 4.8876 | 0.5583 | 98.4084 |
| Habitat (full) | 0.1357 | 0.0884 | 13.4746 | 0.0361 | 98.3925 |
| Habitat | 0.1314 | 0.0946 | 8.9418 | 0.1114 | 98.2869 |
| Latitude | 0.1446 | 0.0941 | 3.7841 | 0.0517 | 98.5886 |
| Longitude2 | 0.1118 | 0.0927 | 19.6765 | <0.0001 | 98.3092 |
Habitat (full) represents the model including “many” group. τ2 and ω2 represent study‐level and case‐level variance, respectively. I 2 is a metric quantifying the heterogeneity relative to sampling variance. Longitude2 indicates that the values for longitude were squared (see text). Values shown in boldface type represent significant effects (P ≤ 0.05).
Spatial extent did not have a large influence on variation in NERR. We found no significant effect of extent on the NERR when extent was fitted as an ordered covariate (Fig. 2a; Table 2). When grain size, extent (as an ordered covariate), and their interaction were fit in a regression, there was a significant interaction between grain size and extent (Q M = 13.54, P = 0.04), suggesting heterogeneity in the slope of the grain size relationship between extent categories. This relationship was complex and did not exhibit a straightforward increase in the influence of grain as extent decreased (Appendix S3: Fig. S3). The slope of the relationship between grain size and effect size varied linearly and quadratically with increasing extent size, with significantly negative linear and positive quadratic interaction coefficients (Appendix S4: Table S3). Increasing grain size had a non‐linear effect on the influence of extent on NERR (Appendix S4: Table S3). The interaction of grain size with habitat types was not significant (Q M = 5.5064, P = 0.4807).
Figure 2.
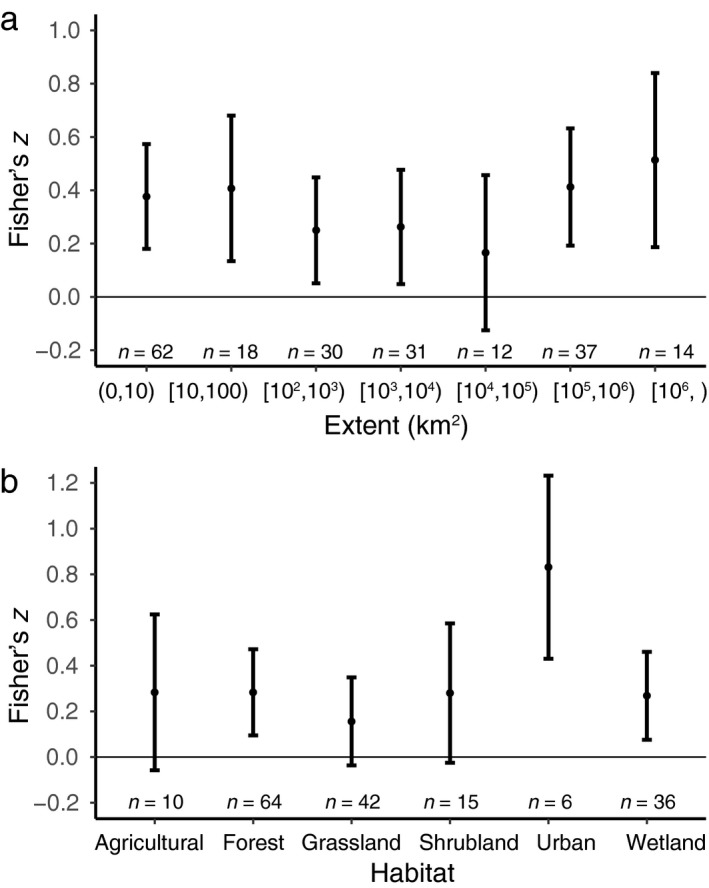
Native–exotic richness relationship (NERR; Fisher's z) grouped by categorical covariates. Means and 95% confidence intervals for NERR for (a) seven extent categories and (b) six habitat types. The number of cases in each category is shown on the x‐axis. Brackets indicate “greater than and including” and parentheses indicate “up to”.
The difference in NERR among habitat types was not significant (Table 2). Native–exotic richness relationships appear to be larger in urban habitats compared to the other habitat types, which differ only slightly from one another (Appendix S4: Table S2). To assess the sensitivity of the effect of differences in habitat types to the catch‐all category that included several habitat types, we excluded that mixed category and found no difference in the results (Fig. 2b; Table 2).
Native–exotic richness relationships increased linearly with latitude (slope = 0.0136, 95% CI [−0.0001, 0.0274], Fig. 3, Table 2). Longitude had a significant quadratic relationship with effect size, where the correlation was most strongly positive at lower longitudes (Europe and Africa), and was closer to zero near the Americas, East Asia, and Australia (Fig. 4, Table 2).
Figure 3.
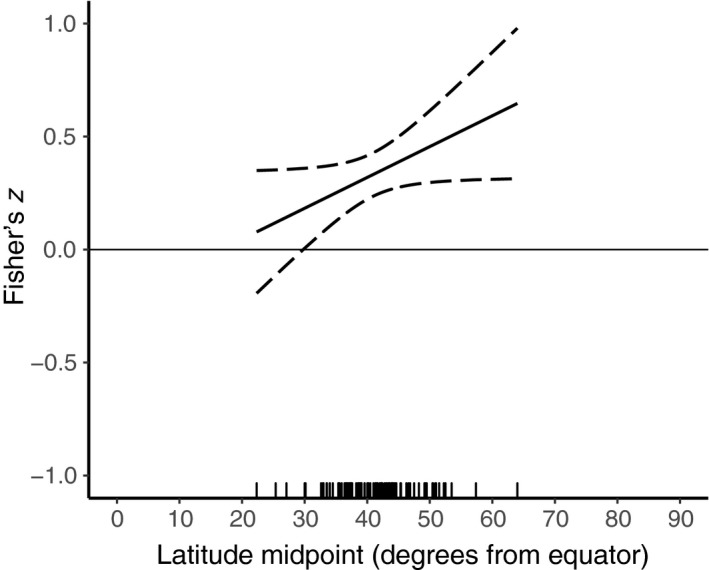
Mixed‐effects meta‐regression of Fisher's z as a function of latitude midpoint, expressed as degrees latitude away from the equator (solid line). The 95% confidence intervals are shown as dashed lines. A frequency rug is shown on the x‐axis.
Figure 4.
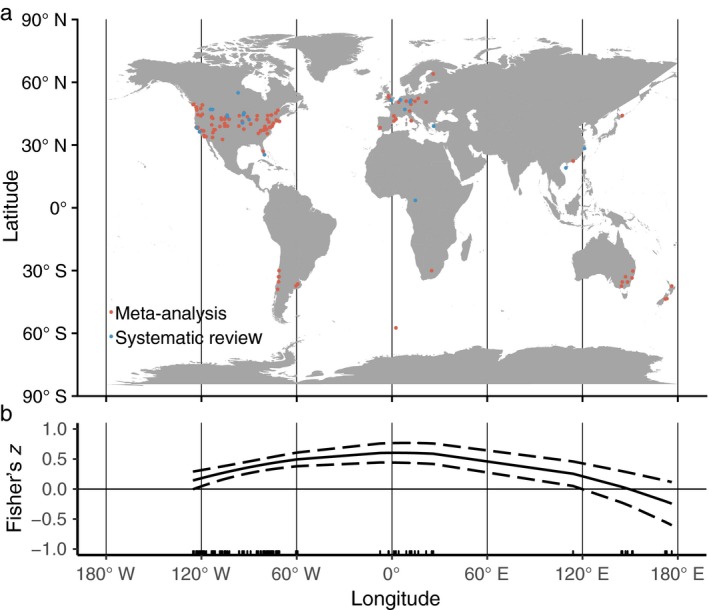
(a) The locations of cases, expressed as latitude and longitude midpoints. Red points represent cases included in both the meta‐analysis and systematic review databases; blue points represent locations of cases only included in the systematic review database. (b) Mixed‐effects meta‐regression of Fisher's z (relationship of NERR and grain) as a function of longitude including linear and quadratic terms with a 95% confidence interval. A frequency rug is shown on the x‐axis. [Color figure can be viewed at http://www.wileyonlinelibrary.com]
Among our candidate regression models, the model including grain size and latitude, and the model including grain size, latitude, and longitude were best, with the lowest AICc (Appendix S4: Tables S1, S4). To test whether NERR increases with grain size solely because of sampling error as a consequence of species area curves, we conducted a simulation and confirmed that the relationship between NERR and grain size is not merely a statistical artifact and is therefore likely to be a consequence of biology (see Appendix S2 for details).
We did not observe any strong indication of publication bias (Appendix S5: Fig. S1). However, mean effect sizes were significantly negative in early years and gradually become positive and stable in recent years (Appendix S5: Fig. S2).
Discussion
Effects of scale
Despite long debate on the relationship between NER or NERR and spatial scale, no previous studies have synthesized results across a wide range of studies. There have been many observations of negative NERRs in individual studies reported in publications, and many attempts to account for the hypothesized pattern of negative NERR at small spatial scales and positive NERR at large spatial scales using both conceptual and mathematical arguments (Stohlgren et al. 1999, 2006b, Fridley et al. 2004, Herben et al. 2004, Davies et al. 2005, Hill and Fischer 2014, Tarasi and Peet 2017).
We found a positive linear relationship between grain size and NERR across a wide range of plant species, grain sizes, and geographic locations in a meta‐analysis, when spatial grain is expressed on a natural logarithm scale. The regression for this relationship is never negative, and there is no indication of any nonlinearity such that there are negative values at small grain sizes and positive values at large grain sizes. We caution that the data and patterns do not show that invasive species are benign or have no negative consequences for the preservation of biodiversity, because the nature of the data we analyzed is inadequate to address this, and the studies we synthesized were not designed to determine whether invasive species have negative effects on native species. Variance in NERR is high at small grain sizes, as might be expected from sampling error in small plots, with fewer individual plants. In the meta‐regression of NERR and grain size (Fig. 1), 51 of the 52 points within the 95% CI and 61 of the 63 points within the 99% CI of the meta‐regression were positive, emphasizing the result that native and exotic species richness are positively correlated when examined across multiple studies, regardless of grain size (in non‐experimental studies).
The NERR of experimental studies was generally negative (Data S3; mean effect size = −0.77, 95% CI [−1.30, −0.23]). The results of observational studies may not be comparable to those in experimentally manipulated studies for several reasons. They address fundamentally different questions and employ different methods than observational studies (Fridley et al. 2007, and see Jeschke and Heger 2018). Experiments are designed explicitly to test hypotheses about the effects of native species richness on invasive species, while observational studies include all of the correlated abiotic and biotic factors that determine both native and invasive species numbers, including environmental heterogeneity at whatever scale the observational studies are conducted. It might be that the most important differences between large scale observational studies and small‐scale experimental studies are those between manipulative experiments designed to test hypotheses, and natural patterns revealed by observational studies (J. Levine, personal communication). These differences cannot be determined by the current meta‐analysis, because the data to evaluate them do not exist. We note also that experimental studies are typically carried out only at small spatial grain and extent (Stohlgren 2002, and confirmed here) and conducted for short periods of time, whereas observational studies report on patterns that might have taken many decades to establish. In addition, experimental results are typically reported in terms of cover, biomass, and density rather than species richness, which is reasonable for these generally short‐term studies in which richness is experimentally manipulated. In our systematic review, we found only 15 experimental studies that reported NERR, and these ranged in grain size from 0.1 to 9 m2. Because of the high heterogeneity at small spatial scales and the small number of experimental studies that report NERR (Data S3), the negative values in experimental studies may also simply be merely an artifact of small numbers. Experimental manipulations in protected environments cannot fully simulate natural environmental fluctuations and may not be representative of natural landscapes that experience various types of disturbance, including fire and grazing (Stohlgren et al. 1999). In addition, many experimental communities are random selections from a species pool (as in Knops et al. 1999, Fargione and Tilman 2005). However, natural landscapes have more complicated spatial structure and interspecific relationships, which may affect the availability of resources and their supply rates (Huston and DeAngelis 1994, Stohlgren et al. 1999). Finally and perhaps most compellingly, any attempt to compare NERR in natural and experimental communities is truly an “apples and oranges” comparison, because while “invader” refers to alien (exotic or nonindigenous) species in observational studies, in experimental studies, it is commonly simply species that were not part of the original seed mixture on a given plot. In other words, the “invader” in experimental studies often includes both natives and exotics (Tilman 1997).
Spatial extent did not influence NERR, although we had only limited and approximate information on extents. Few previous papers on spatial scale and NERR have considered grain and extent separately (Sandel and Corbin 2010, Symonds and Pither 2012), although these two components of spatial scale likely represent very different kinds of biotic interactions and abiotic influences on species richness. There was no general pattern of negative NERR at any spatial extent.
Many biological explanations have been proposed for factors contributing to differences in NERR as spatial scale increases. It has been suggested that small grain size is the scale of strong individual interactions (Stohlgren et al. 1999, Herben et al. 2004, Davies et al. 2005), in which competitive exclusion might reduce the number of exotic species relative to native species. Other studies have suggested that structurally complex communities could provide more microenvironments for exotic species due to different microbial communities and available root depths, although overall environmental conditions within a plot at small grain size tend to be homogeneous (Palmer and Maurer 1997). The “spatial heterogeneity” and “favorable conditions” hypotheses are usually used to explain positive NERR at larger spatial scales. The “spatial heterogeneity” hypothesis is based on the observation that at larger spatial scales, there is likely to be greater resource heterogeneity, rather than just the increase in any one resource, allowing more different species to coexist (Wright et al. 1993, Hawkins et al. 2003). Increasing NERR as spatial grain increases might be attributable to general overall favorability, such as an increase in available resources (de Albuquerque et al. 2011).
Due to the complexity of ecological processes and their interactions, it is reasonable to believe that NERRs are determined by the net outcome of multiple interacting ecological processes operating at each grain size (Stachowicz and Byrnes 2006). Even at larger grain sizes, we cannot exclude competitive effects, while at small grain sizes, the role of favorable environmental conditions and greater resource availability also cannot be ignored. Our results strongly support the idea that communities are not niche “saturated,” but rather, confirm the generalization that exotic species richness is positively correlated with native species richness.
In theory, regional processes can interact with local processes to affect NERR patterns. Spatial extent has not received much explicit attention in the discussion of scale and NERR, but several studies suggest that extent may be important in determining NERR patterns (Sandel and Corbin 2010, Symonds and Pither 2012). Our results, however, indicated that spatial extent plays a less important and less clear role than does grain. Our analyses were limited by limited data reporting on extent in most primary studies. The interaction between grain and extent was complex, and surprisingly, the slope of NERR with respect to grain was steepest in studies with the largest and the smallest extents when extent was fitted as a categorical covariate (non‐ordered; Appendix S3: Fig. S3, Appendix S4: Table S3; Kolasa and Rollo 1991, Stein et al. 2014). Previous researchers have suggested that at small extents, NERR patterns may be determined largely by the processes operating within plots, and shifts from biotic interactions to extrinsic factors would result in larger NERR as grain size increases, while at broad extents, the differences in environmental conditions between plots become greater as the distance between plots gets greater (Siefert et al. 2012, Tomasetto et al. 2013), and consequently, this higher heterogeneity between plots may balance the role of ecological processes within plots. However, our results do not support these predictions.
In addition, according to the results of our cumulative meta‐analysis (Appendix S2), we found that earlier studies initially appeared to confirm the biotic resistance hypothesis (negative NERR), whereas later studies on average find the opposite result. The phenomenon, which has been referred to as a “decline effect” of declining support for an original hypothesis over time (Lehrer 2010, Schooler 2011, Jeschke and Heger 2018) is well known in meta‐analysis (Koricheva et al. 2013). This result is consistent with the findings in the non‐meta‐analytic review of Jeschke et al. (2018).
Other influences on NERR and scale
We were unfortunately not able to evaluate the influence of species richness per se on the patterns, because few of the papers we used in this synthesis reported total species richness or the richness of native or exotic species. Despite the clear relationship of NERR to grain size, we found very high heterogeneity among studies (Table 2, Fig. 1). This is a very common finding in ecological meta‐analyses, where heterogeneous populations are purposefully included so that broad generalizations can be found, and where the goal, as is ours here, is to find the factors of commonality among studies and identify broad patterns rather than achieve a clear accounting of all variation among studies (Gurevitch et al. 2018). We examined the influence of covariates representing several major hypotheses for variation in NERR in addition to spatial grain and extent. Geographic location (longitude and latitude) was the most important factors influencing NERR when grain was taken into account. Differences among habitats had a small influence on the relationship, with urban habitats demonstrating larger NERRs than other habitats. We used absolute, rather than relative, units of grain and extent in this analysis for comparability among many studies in different locations, though the grain size at which individuals interact or the grain size at which environmental heterogeneity acts are likely habitat dependent. For example, grain and extent sizes may have different significance in grassland vs. forest habitats; however, we compared the relationship between NERR and grain size between studies conducted in forests and grasslands, and did not find a significant difference (Appendix S3: Fig. S4). On average, studies in forests had positive NERR at all grains while studies in grasslands had negative NERR at very small grains.
Overall, covariates relating to geographical information are important in explaining variation in NERR in hierarchical mixed‐effects multiple meta‐regressions. Global latitudinal variation and longitudinal variation in the NERR patterns have not been previously quantified, but we found higher correlations as latitude increases. However, we caution that the available literature on NERR is geographically biased as it is in almost all in temperate regions (a form of research bias; Gurevitch and Hedges 1999), and essentially excludes both tropics and polar regions (Pyšek et al. 2008, Lowry et al. 2013, Yu et al. 2016, Jeschke et al. 2018). While it has long been suggested that tropical plant communities are less invaded than temperate plant communities (Rejmánek 1996), data on invasions in tropical environments are very limited, and our conclusions apply only to the temperate regions where we have sufficient data. The quadratic relationship between longitude and NERR in Europe is much higher than that in other areas. Europe has been a long‐time center for international trade for many centuries, which has introduced a large number of exotic species (Keller et al. 2011).
Conclusions and Recommendations
We confirmed the long‐held observation that the native–exotic species richness relationship is highly scale dependent when quantified across many studies, and found that there is no grain size at which NERR is consistently negative. At small grain sizes, there is high heterogeneity in NERR, with a wide range of both positive and negative values in individual studies, while at larger grain sizes, NERRs are always positive. Extent appears to play a much less important and less consistent role in determining NERR. Latitude and longitude also appear to affect NERR, although there is very limited information on NERRs outside of temperate systems. Where our study falls acutely short is particularly in having very limited information on areas outside of temperate regions of western Europe, North America, and the southeast coast of Australia. We strongly encourage more studies on the questions addressed here in other parts of the world. In addition, we recommend that authors of primary papers report their data more completely and transparently, particularly for native, exotic and total species richness, that more researchers consider (and report) the effects of spatial extent, and distinguish between spatial grain and extent in discussions of patterns of scale on NERR.
Data Availability
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.59kv753
Supporting information
Acknowledgments
We appreciate the help and valuable discussions of Morodoluwa Akin‐Fajiye, Shanxin Gong, Eric Mensah, and Isabela Kernin. This project was funded by the National Natural Science Foundation of China (31030015), Chinese Scholarship Council and the Scientific Research Foundation, Hongda Zhang, and Sun Yat‐sen University. The co‐corresponding author on this paper is Shaolin Peng, State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat‐sen University, Guangzhou 510275, China, “lsspsl@mail.sysu.edu.cn”. We are grateful to the Department of Ecology and Evolution of Stony Brook University for hosting Shijia Peng for a year‐long visit during which this paper was researched and written.
Peng, S. , Kinlock N. L., Gurevitch J., and Peng S.. 2019. Correlation of native and exotic species richness: a global meta‐analysis finds no invasion paradox across scales. Ecology 100(1):e02552 10.1002/ecy.2552
Corresponding Editor: Jonathan M. Levine.
Notes
Literature Cited
- Boughton, E. H. , Quintana‐Ascencio P. F., Nickerson D., and Bohlen P. J.. 2011. Management intensity affects the relationship between non‐native and native species in subtropical wetlands. Applied Vegetation Science 14:210–220. [Google Scholar]
- Brown, R. L. , and Peet R. K.. 2003. Diversity and invasibility of southern Appalachian plant communities. Ecology 84:32–39. [Google Scholar]
- Brummer, T. J. , Byrom A. E., Sullivan J. J., and Hulme P. E.. 2016. Alien and native plant richness and abundance respond to different environmental drivers across multiple gravel floodplain ecosystems. Diversity and Distributions 22:823–835. [Google Scholar]
- Byrne, K. M. , Lauenroth W. K., and McManus L.. 2010. Impacts of nonnative plant species on production and diversity in the front range of Colorado. Western North American Naturalist 70:288–295. [Google Scholar]
- Davies, K. F. , Chesson P., Harrison S., Inouye B. D., Melbourne B. A., and Rice K. J.. 2005. Spatial heterogeneity explains the scale dependence of the native–exotic diversity relationship. Ecology 86:1602–1610. [Google Scholar]
- de Albuquerque, F. S. , Castro‐Díez P., Rodríguez M. Á., and Cayuela L.. 2011. Assessing the influence of environmental and human factors on native and exotic species richness. Acta Oecologica 37:51–57. [Google Scholar]
- Deutschewitz, K. , Lausch A., Kühn I., and Klotz S.. 2003. Native and alien plant species richness in relation to spatial heterogeneity on a regional scale in Germany. Global Ecology and Biogeography 12:299–311. [Google Scholar]
- Elton, C. S. 1958. The ecology of invasions by animals and plants. Methuen and Company, London, UK. [Google Scholar]
- Fargione, J. E. , and Tilman D.. 2005. Diversity decreases invasion via both sampling and complementarity effects. Ecology Letters 8:604–611. [Google Scholar]
- Fridley, J. D. , Brown R. L., and Bruno J. F.. 2004. Null models of exotic invasion and scale‐dependent patterns of native and exotic species richness. Ecology 85:3215–3222. [Google Scholar]
- Fridley, J. D. , Stachowicz J. J., Naeem S., Sax D. F., Seabloom E. W., Smith M. D., Stohlgren T. J., Tilman D., and Holle B. V.. 2007. The invasion paradox: reconciling pattern and process in species invasions. Ecology 88:3–17. [DOI] [PubMed] [Google Scholar]
- Gurevitch, J. , and Hedges L. V.. 1999. Statistical issues in ecological meta‐analyses. Ecology 80:1142–1149. [Google Scholar]
- Gurevitch, J. , Koricheva J., Nakagawa S., and Stewart G.. 2018. Meta‐analysis and the science of research synthesis. Nature 555:175–182. [DOI] [PubMed] [Google Scholar]
- Harrison, S. , Cornell H., and Grace J. B.. 2015. Retracted: Does natural variation in diversity affect biotic resistance? Journal of Ecology 103:1099–1106. [Google Scholar]
- Hawkins, B. A. , et al. 2003. Energy, water, and broad‐scale geographic patterns of species richness. Ecology 84:3105–3117. [Google Scholar]
- Herben, T. , Mandák B., Bímová K., and Münzbergová Z.. 2004. Invasibility and species richness of a community: a neutral model and a survey of published data. Ecology 85:3223–3233. [Google Scholar]
- Hill, K. C. , and Fischer D. G.. 2014. Native‐exotic species richness relationships across spatial scales in a prairie restoration matrix. Restoration Ecology 22:204–213. [Google Scholar]
- Hui, C. , and Richardson D. M.. 2017. Invasion dynamics. Oxford University Press, Oxford, UK. [Google Scholar]
- Hulme, P. E. , et al. 2008. Grasping at the routes of biological invasions: a framework for integrating pathways into policy. Journal of Applied Ecology 45:403–414. [Google Scholar]
- Huston, M. A. , and DeAngelis D. L.. 1994. Competition and coexistence the effects of resource transport and supply rates. American Naturalist 144:954–977. [Google Scholar]
- Jeschke, J. M. , Debille S., and Lortie C. J.. 2018. Biotic resistance and island susceptibility hypotheses Pages 60–70 in Jeschke J. M. and Heger T., editors. Invasion biology: hypotheses and evidence. CABI, Wallingford, UK. [Google Scholar]
- Jeschke, J. M. , and Heger T., editors. 2018. Invasion biology: hypotheses and evidence. CABI, Wallingford, UK. [Google Scholar]
- Keller, R. P. , Geist J., Jeschke J. M., and Kühn I.. 2011. Invasive species in Europe: ecology, status, and policy. Environmental Sciences Europe 23:23. [Google Scholar]
- Knops, J. M. H. , et al. 1999. Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecology Letters 2:286–293. [DOI] [PubMed] [Google Scholar]
- Kolasa, J. , and Rollo C. D.. 1991. Introduction: the heterogeneity of heterogeneity: a glossary Pages 1–23 in Kolasa J. and Pickett S. T. A., editors. Ecological heterogeneity. Springer‐Verlag, New York, New York, USA. [Google Scholar]
- Koricheva, J. , Jennions M. D., and Lau J.. 2013. Temporal trends in effect sizes: causes, detection and implications Pages 236–254 in Koricheva J., Gurevitch J., and Mengersen K., editors. Handbook of meta‐analysis in ecology and evolution. Princeton University Press, Princeton, New Jersey, USA. [Google Scholar]
- Kumar, S. , Stohlgren T. J., and Chong G. W.. 2006. Spatial heterogeneity influences native and nonnative plant species richness. Ecology 87:3186–3199. [DOI] [PubMed] [Google Scholar]
- Lehrer, J. 2010. The truth wears off. The New Yorker, December 13, 229:52–57. [Google Scholar]
- Levine, J. M. 2000. Species diversity and biological invasions: relating local process to community pattern. Science 288:852–854. [DOI] [PubMed] [Google Scholar]
- Levine, J. M. , and D'Antonio C. M.. 1999. Elton revisited: a review of evidence linking diversity and invasibility. Oikos 87:15–26. [Google Scholar]
- Lonsdale, W. M. 1999. Global patterns of plant invasions and the concept of invasibility. Ecology 80:1522–1536. [Google Scholar]
- Lowry, E. , Rollinson E. J., Laybourn A. J., Scott T. E., Aiello‐Lammens M. E., Gray S. M., Mickley J., and Gurevitch J.. 2013. Biological invasions: a field synopsis, systematic review, and database of the literature. Ecology and Evolution 3:182–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem, S. , Knops J. M. H., Tilman D., Howe K. M., Kennedy T., and Gale S.. 2000. Plant diversity increases resistance to invasion in the absence of covarying extrinsic factors. Oikos 91:97–108. [Google Scholar]
- Palmer, M. W. , and Maurer T. A.. 1997. Does diversity beget diversity? A case study of crops and weeds. Journal of Vegetation Science 8:235–240. [Google Scholar]
- Peltzer, D. A. , and MacLeod C. J.. 2014. Weeds and native plant species are negatively associated along grassland and kiwifruit land management intensity gradients. Austral Ecology 39:39–49. [Google Scholar]
- Pyšek, P. , Richardson D. M., Pergl J., Jarošík V., Sixtová Z., and Weber E.. 2008. Geographical and taxonomic biases in invasion ecology. Trends in Ecology & Evolution 23:237–244. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.r-project.org [Google Scholar]
- Rejmánek, M. 1996. Species richness and resistance to invasions Pages 153–172 in Orians G. H., Dirzo R., and Cushman J. H., editors. Biodiversity and ecosystem processes in tropical forests. Ecological studies. Volume 122. Springer‐Verlag, Berlin, Germany. [Google Scholar]
- Richardson, D. M. , and Pyšek P.. 2008. Fifty years of invasion ecology–the legacy of Charles Elton. Diversity and Distributions 14:161–168. [Google Scholar]
- Sandel, B. , and Corbin J. D.. 2010. Scale, disturbance and productivity control the native‐exotic richness relationship. Oikos 119:1281–1290. [Google Scholar]
- Schneider, C. A. , Rasband W. S., and Eliceiri K. W.. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9:671–675. (Open access.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler, J. 2011. Unpublished results hide the decline effect: some effects diminish when tests are repeated. Jonathan Schooler says being open about findings that don't make the scientific record could reveal why. Nature 470:437–438. [DOI] [PubMed] [Google Scholar]
- Shea, K. , and Chesson P.. 2002. Community ecology theory as a framework for biological invasions. Trends in Ecology & Evolution 17:170–176. [Google Scholar]
- Siefert, A. , et al. 2012. Scale dependence of vegetation‐environment relationships: a meta‐analysis of multivariate data. Journal of Vegetation Science 23:942–951. [Google Scholar]
- Stachowicz, J. J. , and Byrnes J. E.. 2006. Species diversity, invasion success, and ecosystem functioning: disentangling the influence of resource competition, facilitation, and extrinsic factors. Marine Ecology Progress Series 311:251–262. [Google Scholar]
- Stein, A. , Gerstner K., and Kreft H.. 2014. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecology Letters 17:866–880. [DOI] [PubMed] [Google Scholar]
- Stevens, J. R. , and Taylor A. M.. 2009. Hierarchical dependence in meta‐analysis. Journal of Educational & Behavioral Statistics 34:46–73. [Google Scholar]
- Stohlgren, T. J. 2002. Beyond theories of plant invasions: lessons from natural landscapes. Comments on Theoretical Biology 7:355–379. [Google Scholar]
- Stohlgren, T. J. , Binkley D., Chong G. W., Kalkhan M. A., Schell L. D., Bull K. A., Otsuki Y., Newman G., Bashkin M., and Son Y.. 1999. Exotic plant species invade hot spots of native plant diversity. Ecological Monographs 69:25–46. [Google Scholar]
- Stohlgren, T. J. , Barnett D. T., and Kartesz J. T.. 2003. The rich get richer: patterns of plant invasions in the United States. Frontiers in Ecology and the Environment 1:11–14. [Google Scholar]
- Stohlgren, T. J. , Barnett D., Flather C., Fuller P., Peterjohn B., Kartesz J., and Master L. L.. 2006a. Species richness and patterns of invasion in plants, birds, and fishes in the United States. Biological Invasions 8:427–447. [Google Scholar]
- Stohlgren, T. J. , Jarnevich C., Chong G. W., and Evangelista P. H.. 2006b. Scale and plant invasions: a theory of biotic acceptance. Preslia 78:405–426. [Google Scholar]
- Symonds, J. E. , and Pither J.. 2012. Multi‐scale analyses of exotic and native plant species diversity within Canada's endangered antelope‐brush ecosystem. Plant Ecology 213:1263–1275. [Google Scholar]
- Tarasi, D. D. , and Peet R. K.. 2017. The native‐exotic species richness relationship varies with spatial grain of measurement and environmental conditions. Ecology 98:3086–3095. [DOI] [PubMed] [Google Scholar]
- Tilman, D. 1997. Community invasibility, recruitment limitation, and grassland biodiversity. Ecology 78:81–92. [Google Scholar]
- Tomasetto, F. , Duncan R. P., and Hulme P. E.. 2013. Environmental gradients shift the direction of the relationship between native and alien plant species richness. Diversity and Distributions 19:49–59. [Google Scholar]
- Turner, M. G. 1989. Landscape ecology: the effect of pattern on process. Annual Review of Ecology and Systematics 20:171–197. [Google Scholar]
- Viechtbauer, W. 2010. Conducting meta‐analyses in R with the metafor Package. Journal of Statistical Software 36:1–48. [Google Scholar]
- Vitousek, P. M. , D'Antonio C. M., Loope L. L., Rejmánek M., and Westbrooks R.. 1997. Introduced species: a significant component of human‐caused global change. New Zealand Journal of Ecology 21:1–16. [Google Scholar]
- Wallace, B. C. , Lajeunesse M. J., Dietz G., Dahabreh I. J., Trikalinos T. A., Schmid C. H., and Gurevitch J.. 2017. OpenMEE: intuitive, open‐source software for meta‐analysis in ecology and evolutionary biology. Methods in Ecology and Evolution 8:941–947. [Google Scholar]
- Wiens, J. A. 1989. Spatial scaling in ecology. Functional Ecology 3:385–397. [Google Scholar]
- Wright, D. H. , Currie D. J., and Maurer B. A.. 1993. Energy supply and patterns of species richness on local and regional scales Pages 66–74 in Ricklefs R. E. and Schluter D., editors. Species diversity in ecological communities: historical and geographical perspectives. University of Chicago Press, Chicago, Illinois, USA. [Google Scholar]
- Wu, S.‐H. , Sun H.‐T., Teng Y.‐C., Rejmánek M., Chaw S.‐M., Yang T.‐Y. A., and Hsieh C.‐F.. 2010. Patterns of plant invasions in China: taxonomic, biogeographic, climatic approaches and anthropogenic effects. Biological Invasions 12:2179–2206. [Google Scholar]
- Yu, F. , Akin‐Fajiye M., Magar K. T., Ren J., and Gurevitch J.. 2016. A global systematic review of ecological field studies on two major invasive plant species, Ageratina adenophora and Chromolaena odorata . Diversity and Distributions 22:1174–1185. [Google Scholar]
- Zuur, A. F. , Ieno E. N., Walker N. J., Saveliev A. A., and Smith G. M.. 2009. Mixed effects modelling for nested data Pages 101–142 in Zuur A., Ieno E. N., Walker N., Saveliev A. A., and Smith G. M., editors. Mixed effects models and extensions in ecology with R. Springer, New York, New York, USA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.59kv753


