Abstract
Introduction
Differences in treatment and outcome have been reported for persons with haemophilia (PWH) on intermediate‐dose (Dutch) and high‐dose (Swedish) prophylaxis, but the potential influence of sports participation has not been considered.
Aim
To compare sports participation and clinical outcome between adult Dutch and Swedish PWH.
Methods
Self‐reported sports participation (type and frequency per week), physical functioning (SF‐36PF: 100‐0), joint status (HJHS: 0‐144), perceived limitations (HALsum: 100‐0) and physical activity (IPAQ) were recorded. Sports were classified according to National Haemophilia Foundation classification (5 categories, highest two were classified as high‐risk sports). Sports participation and clinical outcome were compared according to country and age (18‐22, 23‐29, 30‐40 years) using non‐parametric tests and Spearman correlations (rho).
Results
Seventy‐one adult PWH (NL: 43, SWE: 28) completed sports questionnaires (mean age: 26 years). All participants engaged in sports, including 59.2% in high‐risk sports (33.9% twice weekly). Dutch PWH showed a significant age‐related decline in (high‐risk) sports participation (7x/wk in PWH 18‐22 years to 2x/wk in PWH 30‐40 years, P < 0.05), joint health (HJHS: median 2‐15.5, P < 0.01) and physical functioning (SF‐36PF: median 100 to 77.5, P < 0.01), while Swedish did not. Sports participation was not associated with bleeding (Spearman's rho = −0.119).
Conclusion
All participants reported sports participation, including 59.2% in high‐risk sports. Dutch PWH treated with intermediate‐dose prophylaxis showed an age‐related decline in sports participation, joint status and physical functioning, whereas Swedish PWH on high‐dose prophylaxis did not. Sports participation was not associated with bleeding.
Keywords: factor consumption, haemarthrosis, prophylaxis, sports participation, treatment strategies
What is already known:
With modern treatment strategies, sports participation is part of daily life for PWH.
PWH on a high‐dose regimen report fewer bleeds than PWH on intermediate‐dose, but similar quality of life.
Data on sports participation and physical activity in adults with severe haemophilia are lacking
What this study adds:
All participants were active in sports, including high‐risk sports.
An age‐related decline in sports participation, joint status and HRQoL was observed in Dutch PWH, but not in Swedish PWH.
No associations between sports participation and bleeding or clotting factor consumption were observed.
1. INTRODUCTION
Sports participation and an active lifestyle are important aspects of daily life for persons with haemophilia (PWH).1 The increased availability of recombinant clotting factor (FVIII/IX) and the introduction of prophylactic replacement therapy have promoted sports participation and an active lifestyle for PWH. In fact, several studies have reported that PWH are just as physically active as the general population.2, 3 With the increased sports participation in PWH, sports participation and physical activity (PA) seem to have become relevant outcome parameters for PWH. However, most studies assessing sports participation in PWH showed conflicting results4 and reported on children and adolescents only.2, 4, 5
In a previous study, Fischer et al (2013) compared costs and outcome of intermediate‐dose prophylaxis in the Netherlands (46 IU/kg/wk) and high‐dose prophylaxis in Sweden (88 IU/kg/wk).6 Besides differences on factor dosage, costs and clinical outcome (joint function), this study reported a non‐significant trend towards higher PA in Dutch PWH. However, no detailed overview of sports participation data or its association with outcome was provided. Sports participation, especially high‐risk sports, may be associated with bleeding and/or treatment strategy. Perceived differences in bleeding risk according to different types of sports are reflected in the risk categories that were formulated by the National Hemophilia Foundation (NHF).7 These categories run from 1 (safe) via 1.5 (safe to moderate), 2 (moderate risk), 2.5 (moderate to dangerous) to 3 (dangerous). The first category contains low impact sports with a low collision risk like walking and swimming, while sports in category 2.5 (eg soccer) and category 3 (eg boxing and rugby) are considered to be high‐risk sports. This current analysis explored age‐related differences in sports participation and high‐risk sports between adult PWH and age‐matched peers in two countries with different treatment strategies. Furthermore, associations between PA and perceived limitations, physical functioning, joint status, treatment and bleeding data were investigated.
2. METHODS
2.1. Design and setting
The data analysed in this study were collected as a prespecified part of a prospective observational study comparing cost and outcome of intermediate‐dose vs high‐dose prophylaxis.6 Details of study design and methods have been reported elsewhere.6 The study was performed at the haemophilia treatment centres of the University Medical Centre Utrecht, the Netherlands (Van Creveldkliniek); the Karolinska University Hospital in Stockholm, Sweden; and the Skåne University Hospital in Malmö, Sweden. Dutch and Swedish PWH were selected based on comparable socioeconomic, demographic 8, 9 and cultural aspects.10, 11
2.2. Participants
The participants in the original study consisted of two birth cohorts of PWH with severe haemophilia A and B (FVIII/IX, <1 IU/dL) without a history of inhibitors from the age of 12 onwards. Because the aim of this study was to assess sports participation in adult PWH, only adult PWH who completed the sports questionnaires were included in the current analysis. The participants were divided into three age groups of approximately equal size: 18‐22 (“youngest”), 23‐29 (“intermediate”) and 30‐40 (“oldest”). Data and questionnaires were collected during regular outpatient consultations. All participants provided written informed consent before being included in this study. To be able to compare patients’ sports participation with a group of healthy, age‐matched peers, all participating PWH were requested to ask two male friends, colleagues or neighbours (maximum age difference: 3 years) to complete the same sports questionnaire. Not all adult participants included in the original study completed the sports questionnaire. To assess potential selection bias, responders and non‐responders were compared with regard to clinical and self‐reported scores and bleeding data.
2.3. Data collected
Sports participation, bleeding and treatment history, clinical and self‐reported outcomes were collected for participating PWH. Bleeding and treatment data were collected from medical files, patient logs and hospital records. This included the total number of (joint) bleeds, treatment regimen and annual clotting factor for the last 3 years.
All participants completed a specifically developed sports questionnaire, listing 23 sports (see Table 1), on which they marked the sports they had been performing during the last 12 months. Furthermore, they were asked to indicate how often they would perform this sport during a typical week in May. This month was chosen because the European weather in May is generally optimal for performing sports outdoors. Comparisons in sports participation had to be made at country level, as most popular sports were different according to country.12, 13 Joint health was assessed by performing the Haemophilia Joint Health Scale (HJHS1.0, optimum score 0, worst score 144).14, 15 Self‐reported limitations in activities were assessed using the Hemophilia Activities Lists (HAL sum score).16, 17, 18 Additionally, physical functioning was assessed by means of the SF‐36 (domain: physical functioning: SF‐36PF).19, 20, 21 Both the HAL and the SF‐36 have a score range of 0‐100, with a higher score indicating less limitations or better physical functioning.
Table 1.
Sports included in the sports questionnaire with the injury risk category used
| Sport | NHF risk category | |
|---|---|---|
| 1. | Swimming | 1 |
| 2. | Walking | 1 |
| 3. | Cycling | 1.5 |
| 4. | Aerobicsa | 2 |
| 5. | Fitnessa | 1.5 |
| 6. | Billiardsa | 1 |
| 7. | Bowling | 2 |
| 8. | Golf | 1 |
| 9. | Football | 2.5 |
| 10. | Indoor Footballa | 2.5 |
| 11. | Tennis | 2 |
| 12. | Squasha | 2 |
| 13. | Jogging/Running | 2 |
| 14. | Ice hockey | 3 |
| 15. | Field hockey | 3 |
| 16. | Indoor hockey | 3 |
| 17. | Table tennisa | 1 |
| 18. | Basketball | 2.5 |
| 19. | Volleyball | 2.5 |
| 20. | Skiing (downhill) | 2.5 |
| 21. | Skiing (cross country) | 2 |
| 22. | Ice skating | 2.5 |
| 23. | Skateboarding | 2.5 |
Not included in the NHF classification. These sports were classified according to their impact and collision risk.
To facilitate the comparison of HJHS, HALsum and SF‐36PF scores, the outcomes for HALsum and SF‐36PF scores are presented as the proportional deviation from the optimal score (100). A HJHS score of 10 was used as a cut‐off value to be able to distinguish between patients with significant joint damage (HJHS ≥ 10) and patients without significant joint damage (HJHS < 10).6
To assess potential associations between participation in high‐risk sports and bleeding (risk), sports were classified into risk categories according to the NHF classification.7 This classification runs from 1 (safe) via 1.5 (safe to moderate), 2 (moderate risk), 2.5 (moderate to dangerous) to 3 (dangerous). The injury risk for several sports was classified over a range of risk categories (eg: soccer 2 [moderate]—3 [dangerous]).7 In these cases, the median value of the indicated range was applied in the analysis. Sports that were not included in the NHF classification were classified according to their estimated impact and collision risk. Throughout the text, whenever “football” is mentioned, this refers “soccer,” not American football. Sports in categories 2.5 (eg: soccer, skiing) and 3 (eg: boxing, rugby) are considered to be high‐risk sports. The highest risk category in which participants were engaged at least twice per week was determined to assess how often participants were exposed to high‐risk sports. PA was assessed with the International Physical Activity Questionnaire (IPAQ), which was converted into, and expressed as Metabolic Equivalent of Task (METs)22
2.4. Statistics
The primary aim of this study was to compare sports participation according to treatment strategy and age in Dutch and Swedish PWH. This was done by assessing and comparing age‐related sports participation based on country of origin for PWH. All age‐related comparisons were made for three age groups based on approximately equal size: 18‐22 (“youngest”), 23‐29 (“intermediate”) and 30‐40 years (“oldest”).
For sports participation, the weekly number of different sports, frequency and high‐risk sports were assessed. Participation in high‐risk sports was compared by means of chi‐square testing. Secondary outcome parameters included physical activity (IPAQ), bleeding data, joint health status, limitations in activities (HALsum) and health‐related quality of life (SF‐36PF). To be able to present clinical outcomes in a similar fashion, data from HAL and SF‐36 are presented as the relative deviation from the optimal score (100%—test result). Differences between PWH and peers according to country and age categories were analysed using Mann‐Whitney U test or Kruskal‐Wallis tests. Associations between sports participation and bleeding characteristics, treatment history and clinical data were analysed with Spearman's rank correlation testing. Correlation coefficients were regarded small (rho = 0.1), medium (rho = 0.3) or large (rho = 0.5).23
All results are being presented as median and interquartile range (IQR). Significance levels were set at 5% (P < 0.05). Statistical analyses were performed using SPSS statistical software (version 22; IBM Corp., Armonk, NY).
3. RESULTS
3.1. Participants
Figure 1 shows an overview of the participants (patients and peers) and the outcome parameters available. The original study sample included 103 adult PWH, of which 71 (68.9%), 43/62 Dutch (69.4%) and 28/41 Swedish (68.3%), completed the sports questionnaire. Dutch PWH provided 46 healthy peers, while 27 Swedish provided 27 peers who completed the sports questionnaire. These numbers combined into a total number of 144 participants.
Figure 1.
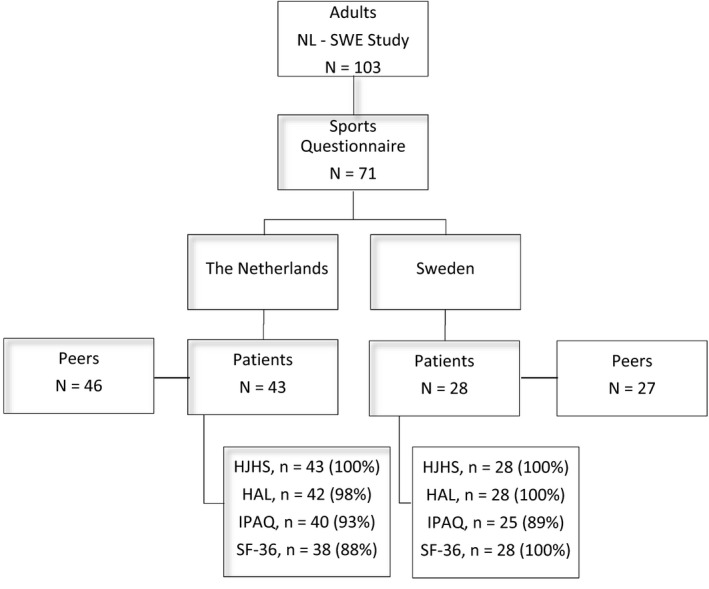
Overview of available participants and data response
A comparison of adult PWH who did (n = 71; 68.9%) and did not (n = 32; 31.1%) complete sports questionnaires showed similar treatment, joint status and self‐reported outcome (HAL, IPAQ, SF‐36), but increased bleeding: non‐responders had significantly more soft tissue bleeds (median 1 [IQR: 1.0‐2.3] vs 0 [IQR: 0‐2.0]; P < 0.05), joint bleeds (2.0 [IQR: 0.8‐3.0] vs 1.0 [IQR: 0‐2.0]; P < 0.05) and 5‐year joint bleeds (15.5 [IQR: 4.5‐21.3] vs 9.8 [IQR: 1.0‐15.0]; P < 0.05) than responders.
3.2. Participant characteristics and treatment
Table 2 shows participant characteristics and treatment data of the PWH in this study. The average age for all participants was 26 years (IQR 23‐30, range: 18‐40), in both Dutch and Swedish participants, and included 62 (87%) with severe haemophilia A and 9 (13%) with severe haemophilia B. Similar to the original study, <10% of PWH in both cohorts was HIV positive while the proportion of PWH with HCV was significantly higher in Dutch PWH (53.5% vs 25.0%). Treatment was significantly less intensive in the Netherlands: prophylaxis was started later (at median age 5.0 in Dutch vs median 1.7 years in Swedish PWH) and current weekly prophylactic dosing was lower at 41 vs 82 IU/kg/wk. Clinical outcome showed more joint damage in Dutch PWH (HJHS 9.0 vs 4.5), more limitations in activities (HALsum: 94 vs 99) and lower quality of life scores (SF36PF: 95 vs 100).
Table 2.
Patient characteristics and treatment history
| N | Dutch PWH | Swedish PWH |
|---|---|---|
| 43 | 28 | |
| Median (IQR) or number (%) | ||
| Age (y) | 27 (22‐30) | 25 (22‐31) |
| Haem type A (%) | 37 (86%) | 25 (89%) |
| HIV positive (%) | 4 (9.3%) | 1 (3.6%) |
| HCV positive (%) | 23 (53.5%) | 7 (25.0%) |
| Age start prophylaxis (y) | 5.0* (3.3‐5.6) | 1.7 (1.3‐3.0) |
| Weekly dose (IU/kg) | 41* (31‐51) | 82 (59‐96) |
| Joint health (HJHS, 0‐144) | 9* (2‐18) | 4.5 (2.3‐7) |
| Limitations (HAL, 100‐0) | 94* (82‐98) | 99 (94‐100) |
| Physical functioning (SF‐36PF, 100‐0) | 95‡ (75‐100) | 100 (95‐100) |
Dutch PWH score significantly higher than Swedish PWH with regard to treatment history (* P < 0.01), joint health (* P < 0.01), self‐reported limitations (* P < 0.01) and physical functioning (‡ P < 0.05), but have lower weekly treatment dose (* P < 0.01).
3.3. Clinical and self‐reported outcome according to age
Treatment and outcome according to age and country are shown in Table 3. Consistent with the previous publication, treatment was significantly less intensive for Dutch PWH in all age groups.6 Dutch PWH reported more joint bleeds than Swedish PWH in all age categories. Concomitantly, Dutch PWH showed an age‐related increase in HJHS score (median 2 [IQR: 1.0‐12.3] to 15.5 [8.8‐22.5], P for trend: <0.01), indicating a trend of decreasing joint health and physical functioning (SF‐36PF: median 100 [90‐100] to 77.5 [42.5‐95.0], P for trend: <0.01) with age, while Swedish PWH (100 [91.3‐100] vs 97.5 [IQR 95.0‐100], P = 0.767) remained stable (see Figure 2). As in the original study,6 Dutch PWH reported more limitations (HALsum) than Swedish PWH, although this difference was not statistically significant (P = 0.223).
Table 3.
Treatment and outcome according to age and country
| Age group | Dutch patients | Swedish patients | ||||
|---|---|---|---|---|---|---|
| 18‐22 y | 23‐29 y | 30‐40 y | 18‐22 y | 23‐29 y | 30‐40 y | |
| N | 11 | 14 | 18 | 8 | 12 | 8 |
| Median (IQR) or number (%) | ||||||
| Age start prophylaxis (y) | 4.3 (3.4‐5.1) | 3.6 (2.1‐5.9) | 5.3 (4.4‐8.4)* | 1.4 (1.2‐1.7) | 1.5 (1.2‐5.1) | 2.7 (2.0‐4.2) |
| Weekly dose (IU/kg) | 50 (38.5‐64.4) | 42.9 (24.1‐53.8) | 35.7 (29.4‐47.1)* | 91 (81.9‐108.5) | 63.0 (51.4‐106.0) | 81 (52.7‐92.0) |
| Annual number of joint bleeds | 1 (1‐2) | 1 (0.8‐2) | 2 (1‐3.5) | 0.3 (0.0‐1.0) | 0 (0.0‐1.0) | 0 (0.0‐2.5) |
| Total number of joint bleeds (during 5‐y follow‐up) | 8 (4‐15) | 11 (3‐17)* | 10.5 (6.5‐21.3) | 1.5 (0.3‐7.8) | 1 (0.0‐5.5) | 0.5 (0.0‐19.3) |
| Joint health (HJHS, 0‐144) | 2.0 (1‐12.3) | 6.5 (1.8‐11.8) | 15.5 (8.8‐22.5)‡ | 3.0 (3.0‐6.8) | 4.5 (3.0‐6.0) | 6.5 (1.3‐18.5) |
| HJHS ≥ 10 (N[%]) | 3 (27.3) | 4 (28.6) | 11 (61.1) | 1 (12.5) | 1 (8.3) | 2 (25) |
| Limitations (HALsum, 100‐0) | 95 (76‐98) | 92 (82‐100) | 94 (87‐98) | 98.5 (91‐100) | 100 (93‐100) | 99.5 (96‐100) |
| Physical functioning (SF‐36PF, 100‐0) | 100 (90‐100) | 100 (91.3‐100) | 77.5 (42.5‐95)‡ | 100 (92.5‐100) | 100 (91.3‐100) | 97.5 (95‐100) |
| Physical activity (×1000 METS/wk) | 5.8 (1.1‐15.1) | 5.0 (0.7‐14.9) | 2.6 (1.1‐12.1) | 3.5 (1.2‐7.9) | 4.5 (1.3‐12.0) | 1.8 (0.5‐12.6) |
Dutch PWH started using prophylaxis later than Swedish PWH and use lower weekly doses (* P < 0.001). Dutch PWH report more (annual) joint bleeds than Swedish PWH in all age categories. Dutch PWH show an age‐related decline in joint health (‡ P for trend <0.01). Joint health could not be assessed independently from age for Swedish PWH due to a lack of variation. Dutch 30 to 40 y PWH show an age‐related decrease in physical functioning (‡ P < 0.01) and report more limitations and lower physical functioning
(‡ P < 0.01) than Swedish PWH. Dutch PWH show a trend towards more PA than Swedish in all age groups.
Figure 2.
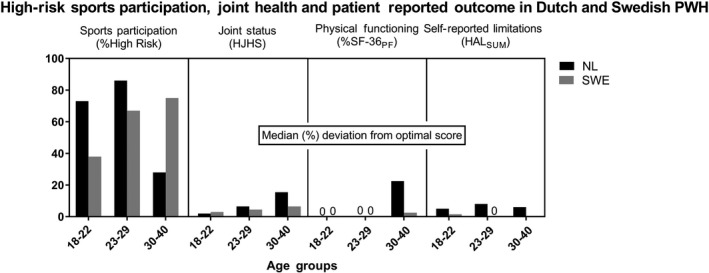
High‐risk sports participation and relative deviations from optimal score for joint status, physical functioning and self‐reported limitations in Dutch and Swedish PWH. Patient‐reported outcomes are presented as median deviation (%) from the optimal score. Dutch PWH aged 30‐40 showed less high‐risk sports participation and worse outcome than younger Dutch PWH and Swedish PWH of all age categories
3.4. Sports participation according to country
All 71 PWH who completed the sports questionnaire indicated to be active in some kind of sports. 59.2% of PWH was engaged in high‐risk sports (NHF > 2), of which 33.9% (NL: 27.9%; SWE: 42.9%; P < 0.05) at least twice weekly. Dutch and Swedish PWH showed different 10 most frequently reported sports. In both countries, the 10 most frequently reported sports in PWH (Dutch PWH: cycling, swimming, walking, fitness, bowling, skiing, billiards, ice skating, football and jogging; Swedish PWH: cycling, swimming, walking, fitness, jogging, bowling, skiing, football, billiards and skating) were similar to their peers (data available on request).
3.5. Sports participation according to age
Sports participation according to age and country is shown in Table 4. A clear decrease in sports participation (number of sports, frequency per week and high‐risk sports per week) according to age was observed in Dutch PWH but not in Swedish PWH. An age‐related decline in the proportion of PWH engaged in high‐risk sports (NHF > 2) in Dutch PWH was observed: from 73% (18‐22 years old) and 86% (23‐29 years old) in the youngest groups to 28% for 30‐ to 40‐year‐old PWH. Proportionally, more Swedish PWH aged 30‐40 (75%) were engaged in high‐risk sports than Dutch PWH (28%) aged 30‐40.
Table 4.
Sports participation according to age and country
| Dutch PWH | Swedish PWH | |||||
|---|---|---|---|---|---|---|
| 18‐22 | 23‐29 | 30‐40 | 18‐22 | 23‐29 | 30‐40 | |
| N | 11 | 14 | 18 | 8 | 12 | 8 |
| Median (IQR) | ||||||
| Sports participation: Number of sports | 5.0 (2.0‐8.0) | 4.0 (1.8‐8.0) | 3.0 (1.0‐3.0) | 3.0 (2‐5.8) | 4.5 (2.0‐7.0) | 4.0 (1.0‐5.8) |
| Sports participation: Times/wk | 3.0 (2.0‐5.8) | 7.0 (3.0‐13.0) | 2.0 (1.0‐6.5) | 5.5 (3.5‐6.5) | 6.0 (2.0‐11.0) | 5.0 (2.0‐14.0) |
| Sports participation high‐risk sports: Times/wk | 1.5 (1.0‐4.3) | 3.0 (1.0‐8.0) | 0 (0.0‐6.5) | 3.5 (0.5‐7.3) | 5.0 (3.0‐6.0) | 2.0 (1.8‐3.8) |
| Number of participants involved in HR sports (n[%]) | ||||||
| High‐Risk Sports (NHF > 2) | 8 (73%) | 12 (86%) | 5 (28%)a | 3 (38%) | 8 (67%) | 6 (75%)b |
Dutch PWH show an age‐related decrease in sports participation and high‐risk sports participation. High‐risk sports were defined as sports in categories 2.5 (moderate risk to dangerous) or 3 (dangerous) of the NHF classification. Participants in HR sports are those active in one of these sports at least once per week.
30 to 40 y Dutch PWH are proportionally less involved in HR sports (*) than young Dutch PWH (18‐22 and 23‐29 y) and proportionally more involved in safe than in (moderately) dangerous sports (§).
Swedish 30 to 40 y PWH are proportionally more involved in HR sports than 30 to 40 y Dutch PWH.
3.6. Sports participation in relation to clinical outcome
Spearman rank correlation showed a negative correlation between joint status (HJHS), number of sports (rho = −0.46; P < 0.01) and number of sports per week (rho = −0.36; P < 0.05) for Dutch PWH. However, due to the low correlation coefficients (rho > −0.5),23 these correlations do not seem to be clinically relevant.
3.7. Treatment and bleeding parameters
Because of the assumed increased bleeding risk associated with engaging in high‐risk sports,24 the potential association between performing high‐risk sports and joint bleeding (annual and 5‐year joint bleeding) was analysed. Spearman rank correlation coefficient testing showed no associations (rho < 0.5)23 between high‐risk sports participation per week and bleeding data (NL: rho = −0.25, P = 0.27, SWE: rho = 0.08, P = 0.76) in Dutch or Swedish PWH.
4. DISCUSSION
This is the first study to report on sports participation according to age for two groups of adult PWH under different treatment strategies. All participants indicated to be active in some kind of sports, with 59.2% of PWH engaged in high‐risk sports. A similar proportion was participating in high‐risk sports in the Netherlands and Sweden. The main difference identified was a trend towards significantly worse joint status combined with less (high‐risk) sports participation in Dutch PWH aged 30‐40, but not in Swedish. Dutch PWH aged 30‐40 reported more limitations than Swedish. Sports participation was not associated with bleeding.
4.1. Internal and external validity
This analysis included a Dutch and Swedish birth cohort (1970‐1991).These groups were chosen because of similar socio‐economic status, 8 cultural norms 10, 11 and lifetime differences in treatment, enabling to explore potential associations between treatment intensity and sports participation. The current data may represent an overestimation of sports participation in PWH. The questionnaire was not completed by 31% of PWH. Analysis showed that non‐responders had significantly more joint bleeds. Data on bleeds were retrieved from hospital records and are based on patient reports. Even if this would result in underestimation of bleeding events, this potential bias occurred both in Swedish and Dutch patients and is therefore unlikely to affect the comparison between both groups. The data collected with the sports questionnaire were limited to type and frequency of sports, without considering duration or intensity and without any objective confirmation. This could potentially lead to overestimation of total PA.25, 26 All participating PWH were asked to invite two healthy, age‐matched peers to complete the sports questionnaire. This procedure may induce selection bias, as peers are more likely to perform similar sports/activities. The sample size in this study was limited, especially in the Swedish sample, limiting the power to address age‐related trends. Due to a lack of a valid, standardized tool, data on sports participation were collected by means of a self‐devised, non‐validated questionnaire. Recently, the Modifiable Activity Questionnaire (MAQ) was validated for adults.27, 28 This questionnaire can be used in prospective studies to document sports participation and exposure. Recently, the HEP test Questionnaire was developed and validated in adults and children with haemophilia.29, 30 This instrument assesses self‐reported physical activity and fitness including four domains: physical status, mobility, strength and coordination, and endurance and body perception.
4.2. Comparison with other studies
The key observation of the present study was a significant reduction in sports participation for 30‐ to 40‐year‐old Dutch PWH, which was not observed in Swedish PWH. A similar trend has been reported in the general population: sports participation seems to decline with increasing age in both the Dutch13 and the Swedish12 general population. The high sports participation observed is corroborated by previous publications reporting that PWH were just as active as healthy subjects.2, 3, 31, 32, 33, 34, 35, 36 However, of these studies only the studies by Heijnen et al (2001) and Khawaji et al (2011) involved adult PWH.3, 33
4.3. Clinical relevance and future research
This study showed a trend towards an age‐related decrease in sports participation and clinical and functional outcomes in Dutch PWH, but not in Swedish PWH This suggests that differences in outcome only become apparent in the fourth decade of life and may be associated with changes in sports participation. However, it is yet unknown whether this decrease is related to differences in treatment strategy.
To enable use of the present results on sports participation in clinical counselling, more details on duration, frequency and intensity of sports performed are needed. More information is needed regarding the balance between benefits and burdens of PA in PWH, particularly regarding injuries and bleeding risk. The present study did not find an association between sports participation and bleeding risk, but this may have been affected by selection bias, as the participants showed a potential overrepresentation of low sports participation and a low number of bleeds.
Ideally, assessment of sports participation should include information on frequency, duration and intensity of physical activity; the MAQ27, 28 and the use of accelerometers37 are potential, validated tools for this purpose. Furthermore, assessment of sports participation should be accompanied by assessment of sports‐related injuries, with sufficient follow‐up to include seasonal differences. To combine information on sports‐related injury risk with treatment intensity, data on sports injuries should be combined with information on FVIII/IX levels at the time of injury similar to the study by Broderick et al24 This information is especially valuable in the context of extended half‐life FVIII/IX concentrates, which result in more time with lower FVIII/IX activity levels.38
5. CONCLUSION
In conclusion, all Dutch and Swedish PWH in this study were actively engaged in sports, the majority including high‐risk sports. The main finding of this study is an age‐related decrease in (high‐risk) sports participation and joint health and a decrease in physical functioning in Dutch patients, who received less intensive treatment. No associations were observed between bleeding data and sports participation. To support counselling on sports participation, future research should focus on prospective studies for sports participation, exposure and should include sports injuries.
DISCLOSURES
OV has reported no interests which might be perceived as posing a conflict or bias. EB has reported no interests which might be perceived as posing a conflict or bias. PP has been a paid consultant to Baxter/Shire, Bayer, Sobi and Roche. RL has during the last 5 years received speakers’ or consultancy fees from Baxter/Shire, CSL Behring, Novo Nordisk, Octapharma, Pfizer—however not related to this paper. JA has served as a consultant for Shire, CSL Behring, Bayer, Novo Nordisk, Sobi, Pfizer, Spark Therapeutics, Unique, Octapharma and has research grants from Bayer, Shire and CSL Behring. None of these is considered to compete with this paper's content and message. MH has reported no interests which might be perceived as posing a conflict or bias. PK has reported no interests which might be perceived as posing a conflict or bias. KF has received speaker's fees from Bayer, Baxter/Shire, Biotest, CSL Behring, Octapharma, Pfizer, Novo Nordisk; performed consultancy for Bayer, Baxter/Shire, Biogen, CSL Behring,Freeline, Novo Nordisk, Pfizer, Roche and SOBI; and has received research support from Bayer, Pfizer, Baxter/Shire and Novo Nordisk.
AUTHOR CONTRIBUTION
EB, PP, RL, JA, MH, PK and KF designed and performed the study. OV and KF analysed the data and wrote the paper. All authors reviewed and approved the final version of the paper.
Versloot O, Berntorp E, Petrini P, et al. Sports participation and physical activity in adult Dutch and Swedish patients with severe haemophilia: A comparison between intermediate‐ and high‐dose prophylaxis. Haemophilia. 2019;25:244–251. 10.1111/hae.13683
Funding information
Data collection and analysis were performed with two independent, unrestricted grants from Bayer.
REFERENCES
- 1. Von Mackensen S. Quality of life and sports activities in patients with haemophilia. Haemophilia. 2007;13:38‐43. [DOI] [PubMed] [Google Scholar]
- 2. Groen WG, Takken T, Van Der Net J, Helders P, Fischer K. Habitual physical activity in Dutch children and adolescents with haemophilia. Haemophilia. 2011;17:906‐912. [DOI] [PubMed] [Google Scholar]
- 3. Heijnen L, Mauser‐Bunschoten EP, Roosendaal G. Participation in sports by Dutch persons with haemophilia. Haemophilia. 2000;6:537‐546. [DOI] [PubMed] [Google Scholar]
- 4. Broderick CR, Herbert RD, Latimer J, van Doorn N. Patterns of physical activity in children with haemophilia. Haemophilia. 2013;19:59‐64. [DOI] [PubMed] [Google Scholar]
- 5. Fromme A, Dreeskamp K, Pollmann H, et al. Participation in sports and physical activity of haemophilia patients. Haemophilia. 2007;13:323‐327. [DOI] [PubMed] [Google Scholar]
- 6. Fischer K, Steen Carlsson K, Petrini P, et al. Intermediate‐dose versus high‐dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. Blood. 2013;122:1129‐1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson A, Forsyth A. Playing it Safe: Bleeding Disorders, Sports and Exercise. New York, NY: National Hemophilia Foundation; 2017. [Google Scholar]
- 8. EuroStat. Quality of Life - Facts and Views [Internet]. Quality of Life - facts and views. Luxembourg: Publications Office of the European Union, 2015; 2015. https://ec.europa.eu/eurostat/web/products-statistical-books/-/KS-05-14-073. Accessed January 16, 2019.
- 9. United States Census Bureau . International Database. 2011. https://www.census.gov/data-tools/demo/idb/region.php?N=Results&T=13&A=separate&RT=0&Y=2018&R=-1&C=NL,SW. Accessed September 6, 2018.
- 10. Wilson TC. The Paradox of social class and sports involvement. The roles of cultural and economic capital. Int Rev Sociol Sport. 2002;37:5‐16. [Google Scholar]
- 11. van Tuyckom C, Scheerder J, Bracke P. Gender and age inequalities in regular sports participation: a cross‐national study of 25 European countries. J Sports Sci. 2010;28:1077‐1084. [DOI] [PubMed] [Google Scholar]
- 12. Norberg JR. Statens stöd till idrotten. Uppföljning 2015 [Internet]. 2016. https://centrumforidrottsforskning.se/wp-content/uploads/2016/05/Statens-stod-till-idrotten-uppfoljning-2015.pdf. Accessed January 15, 2019.
- 13. Tiessen-Raaphorst (ed.) A. Rapportage Sport 2014 [Internet]. The Hague; 2015. https://www.scp.nl/Publicaties/Alle_publicaties/Publicaties_2015/Rapportage_Sport_2014. Accessed January 15, 2019.
- 14. Hilliard P, Funk S, Zourikian N, et al. Hemophilia joint health score reliability study. Haemophilia. 2006;12:518‐525. [DOI] [PubMed] [Google Scholar]
- 15. Feldman BM, Funk SM, Bergstrom BM, et al. Validation of a new pediatric joint scoring system from the international hemophilia prophylaxis study group: validity of the hemophilia joint health score. Arthritis Care Res. 2011;63:223‐230. [DOI] [PubMed] [Google Scholar]
- 16. van Genderen FR, van Meeteren N, van der Bom JG, et al. Functional consequences of haemophilia in adults: the development of the haemophilia activities list. Haemophilia. 2004;10:565‐571. [DOI] [PubMed] [Google Scholar]
- 17. Van Genderen FR, Westers P, Heijnen L, et al. Measuring patients’ perceptions on their functional abilities: validation of the haemophilia activities list. Haemophilia. 2006;12:36‐46. [DOI] [PubMed] [Google Scholar]
- 18. Brodin E, Baghaei F, Elfvinger P, Lindvall K, Sunnerhagen KS. The Swedish version of the haemophilia activity list. Haemophilia. 2011;17:662‐668. [DOI] [PubMed] [Google Scholar]
- 19. Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36) 1. Conceptual framework and item selection. Med Care. 1992;30:473‐483. [PubMed] [Google Scholar]
- 20. McHorney CA, Johne W, Anastasiae R. The MOS 36‐Item Short‐Form Health Survey (SF‐ 36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247‐263. [DOI] [PubMed] [Google Scholar]
- 21. Brazier JE, Harper R, Jones NM, et al. Validating the SF‐36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12‐Country reliability and validity. Med Sci Sports Exerc. 2003;35:1381‐1395. [DOI] [PubMed] [Google Scholar]
- 23. Cohen J. A power primer. Psychol Bull. 1992;112:155‐159. [DOI] [PubMed] [Google Scholar]
- 24. Broderick CR, Herbert RD, Latimer J, et al. Association between physical activity and risk of bleeding in children with hemophilia. JAMA. 2012;308:1452‐1459. [DOI] [PubMed] [Google Scholar]
- 25. Klesges R, Eck L, Mellon M, et al. The accuracy of self‐reports of physical activity. Med Sci Sports Exerc. 1990;22:690‐697. [DOI] [PubMed] [Google Scholar]
- 26. Nusser SM, Beyler NK, Welk GJ, et al. Modeling errors in physical activity recall data. J Phys Act Heal. 2012;9(Suppl 1):S56‐67. [DOI] [PubMed] [Google Scholar]
- 27. Momenan AA, Delshad M, Sarbazi N, et al. Reliability and validity of the modifiable activity questionnaire (MAQ) in an Iranian urban adult population. Arch Iran Med. 2012;15:279‐282. [PubMed] [Google Scholar]
- 28. Tonoli C, Heyman E, Roelands B, et al. Validation and reliability of the Dutch language version of the Modifiable Activity Questionnaire in healthy subjects. Sport Sci Health. 2013;9:139‐144. [Google Scholar]
- 29. von Mackensen S, Czepa D, Herbsleb M, Hilberg T. Development and validation of a new questionnaire for the assessment of subjective physical performance in adult patients with haemophilia–the HEP‐Test‐Q. Haemophilia. 2010;16:170‐178. [DOI] [PubMed] [Google Scholar]
- 30. Khair K, Littley A, Will A, von Mackensen S. The impact of sport on children with haemophilia. Haemophilia. 2012;18:898‐905. [DOI] [PubMed] [Google Scholar]
- 31. Köiter J, Van genderen FR, Brons P, Nijhuis‐van der sanden M. Participation and risk‐taking behaviour in sports in children with haemophilia. Haemophilia. 2009;15(3):686‐694. [DOI] [PubMed] [Google Scholar]
- 32. Buxbaum NP, Ponce M, Saidi P, Michaels LA. Psychosocial correlates of physical activity in adolescents with haemophilia. Haemophilia. 2010;16:656‐661. [DOI] [PubMed] [Google Scholar]
- 33. Khawaji M, Astermark J, Akesson K, Berntorp E. Physical activity and joint function in adults with severe haemophilia on long‐term prophylaxis. Blood Coagul Fibrinolysis. 2011;22:50‐55. [DOI] [PubMed] [Google Scholar]
- 34. Cuesta‐Barriuso R, Torres‐Ortuño A, Pérez‐Alenda S, et al. Sporting activities and quality of life in children with hemophilia. Pediatr Phys Ther. 2016;28:453‐459. [DOI] [PubMed] [Google Scholar]
- 35. González LM, Peiró‐Velert C, Devís‐Devís J, et al. Comparison of physical activity and sedentary behaviours between young haemophilia A patients and healthy adolescents. Haemophilia. 2011;17:676‐682. [DOI] [PubMed] [Google Scholar]
- 36. Schoenmakers M, Gulmans V, Helders P, Van Den Berg HM. Motor performance and disability in Dutch children with haemophilia: a comparison with their healthy peers. Haemophilia. 2001;7:293‐298. [DOI] [PubMed] [Google Scholar]
- 37. Westerterp KR. Reliable assessment of physical activity in disease: an update on activity monitors. Curr Opin Clin Nutr Metab Care. 2014;17:401‐406. [DOI] [PubMed] [Google Scholar]
- 38. Collins PW, Fischer K, Morfini M, Blanchette VS, Björkman S. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011;17:2‐10. [DOI] [PubMed] [Google Scholar]


