Abstract
Cell–collagen interactions are crucial for cell migration and invasion during cancer development and progression. Heat shock protein 47 (HSP47) is an endoplasmic reticulum–resident molecular chaperone that facilitates collagen maturation and deposition. It has been previously shown that HSP47 expression in cancer cells is crucial for cancer invasiveness. However, exogenous collagen cannot rescue cell invasion in HSP47-silenced cancer cells, suggesting that other HSP47 targets contribute to cancer cell invasion. Here, we show that HSP47 expression is required for the stability and cell-surface expression of discoidin domain–containing receptor 2 (DDR2) in breast cancer tissues. HSP47 silencing reduced DDR2 protein stability, accompanied by suppressed cell migration and invasion. Co-immunoprecipitation results revealed that HSP47 binds to the DDR2 ectodomain. Using a photoconvertible technique and total internal reflection fluorescence microscopy, we further demonstrate that HSP47 expression significantly sustains the membrane localization of the DDR2 protein. These results suggest that binding of HSP47 to DDR2 increases DDR2 stability and regulates its membrane dynamics and thereby enhances cancer cell migration and invasion. Given that DDR2 has a crucial role in the epithelial-to-mesenchymal transition and cancer progression, targeting the HSP47–DDR2 interaction might be a potential strategy for inhibiting DDR2-dependent cancer progression.
Keywords: heat shock protein (HSP), protein stability, protein–protein interaction, cell invasion, protein dynamic
Introduction
Adhesion of cells to the extracellular matrix (ECM)4 is essential for cell migration, proliferation, and differentiation (1). Collagen is the most abundant ECM protein, and 28 different types of collagen have been characterized in mammals. The basic unit of collagen is the triple-helical structure, which is formed by the repeating motif Gly-Pro-Xaa (2). HSP47 was identified as a collagen-specific chaperone that regulates maturation of collagen molecules. Binding of Hsp47 to the triple helix region inhibits the aggregation of collagen in the endoplasmic reticulum (ER), thereby facilitating its secretion and deposition (3).
Fibroblasts are considered the major source of collagen in stroma. Consistently, HSP47, encoded by serpin family H member 1 (SERPINH1) gene, is highly expressed in fibroblasts (4–6). Interestingly, we and others show that HSP47 expression in cancer cells is up-regulated during cancer development and progression (7–10). HSP47 silencing suppresses cancer cell migration and invasion in vitro and in vivo. However, the addition of collagen cannot fully rescue cell migration and invasion in HSP47-silenced cells (7). HSP47 is a heat shock protein that is produced when cells are exposed to stressful conditions (11–13). These results suggest that HSP47 has additional targets during cancer progression and in response to stressful conditions. Identification of HSP47 targets may advance our understanding how HSP47 expression promotes cell migration and provide new insights into function of HSP47 in cancer progression.
Cell–collagen interactions are mainly mediated by integrins and discoidin domain-containing receptors (DDRs), and dysregulation of the collagen-receptor interaction contributes to cancer development and progression (14, 15). DDRs, including DDR1 and DDR2, are receptor tyrosine kinases. DDRs consist of an extracellular discoidin domain, a transmembrane region, a cytoplasmic juxtamembrane region, and a catalytic domain (16). Binding of collagen to the ectodomain induces DDR phosphorylation in the cytoplasmic domain and activation of downstream signaling (17). A 3–5% incidence of DDR2 point mutations has been detected in lung squamous cell carcinoma, suggesting that the DDR2 gene is a potential oncogenic target (14). Although DDR2 mutation has not been detected in breast cancer, roles of DDR2 in breast cancer metastasis are well-established. It has been shown that DDR2 expression is induced during epithelial–mesenchymal transition (EMT) (15, 18, 19). Enhanced DDR2 activities in the EMT cells stabilize Snail protein by stimulating ERK2 activity and subsequently promote cancer cell invasion and colonization at distant organs (15). Therefore, determining how DDR2 protein expression is regulated may identify potential strategies to target this oncogene and suppress cancer progression.
In the present study, we have identified DDR2 as a new target of HSP47. Binding of HSP47 is crucial for the stability and cell-surface localization of DDR2 protein. We also show that introducing exogenous DDR2 rescues cell migration and invasion in HSP47-silenced cells. These results suggest that the function of HSP47 in cancer progression is at least partially mediated by DDR2.
Results
HSP47 regulates DDR2 expression at the protein level
Our previous study showed that HSP47 expression is induced during breast cancer development and progression. Silencing HSP47 in breast cancer cells suppressed cancer cell migration and invasion (7). However, the molecular mechanism by which HSP47 regulates cell migration and invasion remains to be determined. Using gene co-expression analysis, we showed that mRNA levels of HSP47 and DDR2 were significantly correlated in human breast cancer tissues (Fig. 1A).
Figure 1.
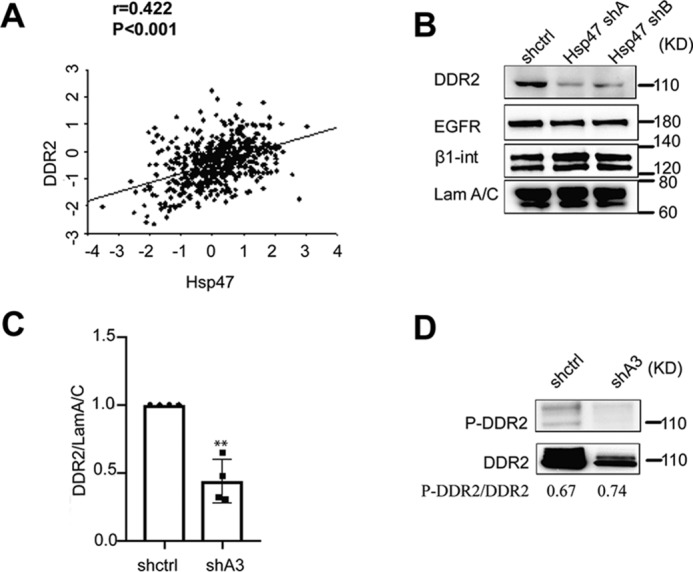
DDR2 expression is regulated by HSP47. A, spearman correlation analysis showed that mRNA levels of HSP47 and DDR2 were significantly correlated in human breast cancer tissues (TCGA; n = 532). B and C, silencing HSP47 significantly reduced DDR2 protein levels in MDA-MB-231 cells. Western blotting data were quantified by ChemImage (C), and DDR2 levels in HSP47-silenced cells (shA3) were normalized to control (shctrl; n = 3). Displayed are the means with S.E. **, p < 0.01, two-tailed Student's t test. D, total DDR2 and Tyr phosphorylation of DDR2 were assessed after DDR2 was immunoprecipitated from control and HSP47-silenced cells.
The correlated gene expression suggests a functional connection between HSP47 and DDR2; therefore, we examined DDR2 expression in HSP47-silenced breast cancer cells. We found that DDR2 protein levels were reduced in HSP47-silenced MDA-MB-231 cells (Fig. 1, B and C), which is associated with reduced cell migration and invasion (7). In addition, knockdown of HSP47 had little effect on protein expression of β1-integrin and epidermal growth factor receptor. To assess whether DDR2 activity is regulated by HSP47, we analyzed DDR2 phosphorylation in control and HSP47-silenced cells using immunoprecipitation experiments. Total protein DDR2 levels were reduced in HSP47-silenced cells; however, silence of HSP47 had little effect on the relative levels of tyrosine phosphorylated DDR2 (Fig. 1D). It has been shown that DDR2 activation in breast cancer cells promotes cell migration and invasion (18, 20, 21). These results suggest that DDR2 is the potential HSP47 target that mediates its function in regulating cell migration and invasion.
HSP47 binds to DDR2 and enhances its protein stability
To understand how HSP47 regulates DDR2 expression, we first determined whether DDR2 is regulated by HSP47 at the transcription level. Quantitative RT-PCR data showed that silencing HSP47 had little effect on DDR2 mRNA levels (Fig. 2A). Next, we examined whether the protein stability of DDR2 is regulated by HSP47. Control and HSP47-silenced MDA-MB-231 cells were treated with cycloheximide, and DDR2 protein levels were assessed by Western blotting at different time points. We found that DDR2 protein levels decreased much faster in HSP47-silenced cells compared with the levels in control cells (Fig. 2B). These results indicate that silencing HSP47 reduced DDR2 protein stability, which may lead to the reduction in DDR2 protein levels.
Figure 2.
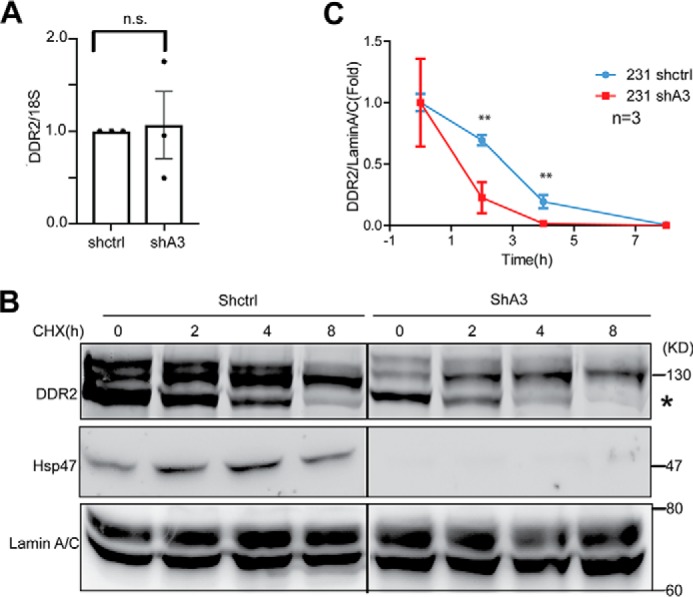
DDR2 exhibits low stability in HSP47 silenced cells. A, DDR2 mRNA levels were measured by real-time RT-PCR in control and HSP47-silenced MDA-MB-231 cells. n.s., no significance. B, DDR2 stability was analyzed by Western blotting in control cells (shctrl) and HSP47-silenced (shA3) cells treated with cycloheximide (CHX, 100 μg/ml) at 0, 2, 4, and 8 h. C, quantification for B. **, p < 0.01, two-tailed Student's t test (n = 3). Displayed are the means ± S.D.
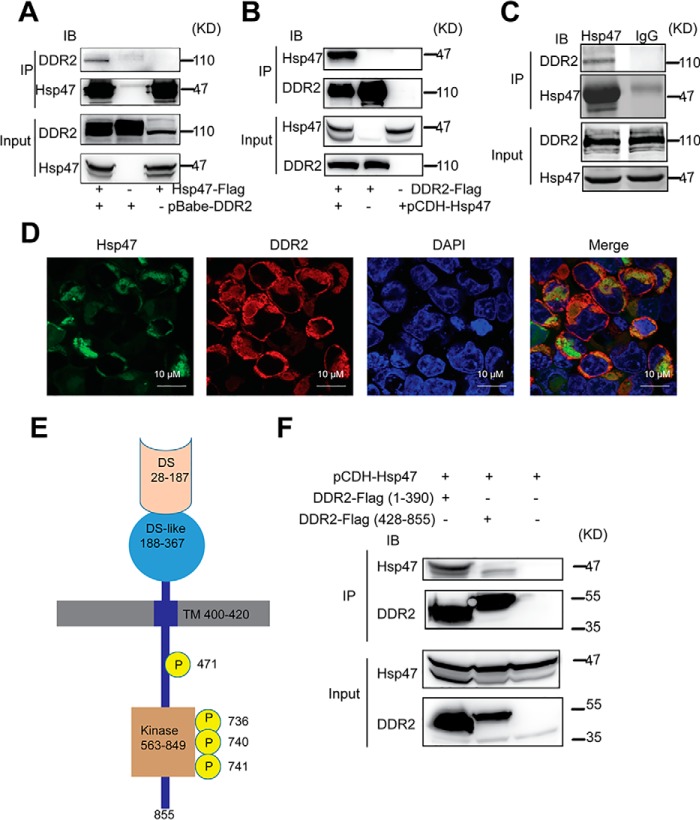
HSP47 is a molecular chaperone that facilitates protein maturation through protein–protein interaction (22, 23). We asked whether HSP47 binds to DDR2 and enhances its stability. An expression construct containing FLAG-tagged HSP47 gene was introduced in HEK 293 cells, and protein complexes were immunoprecipitated with anti-FLAG M2 beads. The DDR2–HSP47 protein complex was detected in the IP products (Fig. 3A). In another experiment, the protein complexes were immunoprecipitated from cells expressing FLAG–DDR2, and binding of HSP47 to DDR2 was also detected in the complexes (Fig. 3B). To confirm the DDR2 and HSP47 interaction in breast cancer cells, we performed co-IP experiments using endogenous protein extracted from HS578 cells. DDR2 was detected in the protein complexes immunoprecipitated by the antibody against HSP47, but not in the IgG control group (Fig. 3C). In addition, confocal microscope imaging analysis showed that DDR2 protein was mainly localized at cell surface and in cytoplasm. The co-localization of HSP47 and DDR2 was mainly detected in the cytoplasm (Fig. 3D).
Figure 3.
HSP47 binds to DDR2 at the DS and DS-like domains. A, co-IP analysis was performed in HEK293 cells transfected with DDR2 and/or FLAG-HSP47 expression constructs. Protein complexes were pulled down with M2 beads and analyzed by Western blotting. B, co-IP analysis was performed in HEK293 expressing FLAG-DDR2 and/or HSP47. C, endogenous co-IP was performed in HS578 cells. Protein complexes were pulled down with HSP47 antibody and protein A/G beads. D, confocal microscopy imaging analyzing localization of HSP47 (green) and DDR2 (red) in HEK 293 cells. Nuclei was staining with (DAPI; blue). E, the cartoon showing the DDR2 protein structure. F, co-IP analysis was performed in HEK 293 cells expressing FLAG-HSP47 and truncated DDR2 (EX-domain and In-Domain). IB, immunoblotting.
DDR2 is composed of extracellular discoidin homology (DS) and DS-like domain and an intracellular domain (Fig. 3E). To determine which domain is involved in the DDR2–HSP47 interaction, we generated constructs containing the DDR2 extracellular region, DS domain, DS-like domain, or the intracellular domain. Results from co-IP experiments showed that HSP47 bound to DS domain and DS-like domain but had no detectable interaction with the intracellular region (Fig. 3F). These results suggest that binding of HSP47 to DS domain and DS-like domain contributes to the DDR2 protein maturation and stabilization.
Dynamics of membrane DDR2 are regulated by HSP47
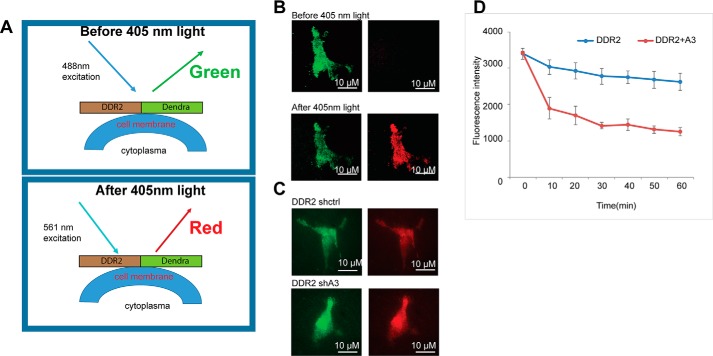
DDR2 is synthesized in the ER and traffics to the cell surface as a collagen receptor (24). We asked whether binding of HSP47 regulates DDR2 translocation and dynamic at cell surface. The photoconvertible fluorescent protein, Dendra2, has recently been used to track protein translocation and dynamics (25). Dendra2 is synthesized as a fluorescent protein, with excitation at 488 nm. Exposure to 405 nm light can photoconvert Dendra2 from green to red emission. Combining this technique with total internal reflection fluorescence microscopy (TIRF), we can monitor the translocation and dynamics of Dendra2 fusion proteins located on the plasma membrane (Fig. 4A).
Figure 4.
HSP47 expression prolongs membrane localization of DDR2 protein. A, a scheme showing how the DDR2–Dendra fused protein was photoconverted with 405-nm light. B, DDR2–Dendra2 expressing HEK293 cells were photoconverted within 5–10 s, and images were taken with TIRF microcopy under green and red channels immediately after photoconversion. C, TIRF images showed the membrane localization of DDR2–Dendra2 expressing in control and HSP47-silenced HEK293 cells. D, the red fluorescence intensity was quantified and normalized with ImageJ; the live cell images were taken with TIRF microcopy every 10 min after photoconversion. The experiment was repeated twice, and the fluorescence intensity was quantified in total 50 cells. Displayed are the means ± S.E.
An expression vector containing DDR2–Dendra2 fusion gene was generated, and the photoconversion of the fused protein was tested in HEK 293 cells. Exposure of the fusion protein-expressing HEK293 cells to 405-nm light successfully converted the fluorescence from green to red (Fig. 4B). To determine whether HSP47 regulates the translocation of DDR2 to cell surface, the DDR2–Dendra2 construct was transfected in control and HSP47-silenced epithelial cells. DDR2–Dendra2 on the cell surface was selectively converted using a 405-nm laser in a TIRF configuration. Red emission was monitored overtime in TIRF. A decay in fluorescence corresponds to endocytosis of DDR2 moving it out of the TIRF focal volume. TIRF imaging analysis showed that silencing HSP47 did not block translocation of DDR2 to the cell surface (Fig. 4C). Next, we monitored the dynamics or stability of DDR2–Dendra2 fusion protein on cell surface after photoconversion by quantifying the red florescence intensity with TIRF. We found that the half-life of the fusion protein at the cell surface was significantly shorter in HSP47-silenced cells compared with that in control cells (Fig. 4D). These results suggest that HSP47 regulates cell membrane dynamics of DDR2 protein.
DDR2 mediates function of HSP47 in enhancing cancer cell invasion
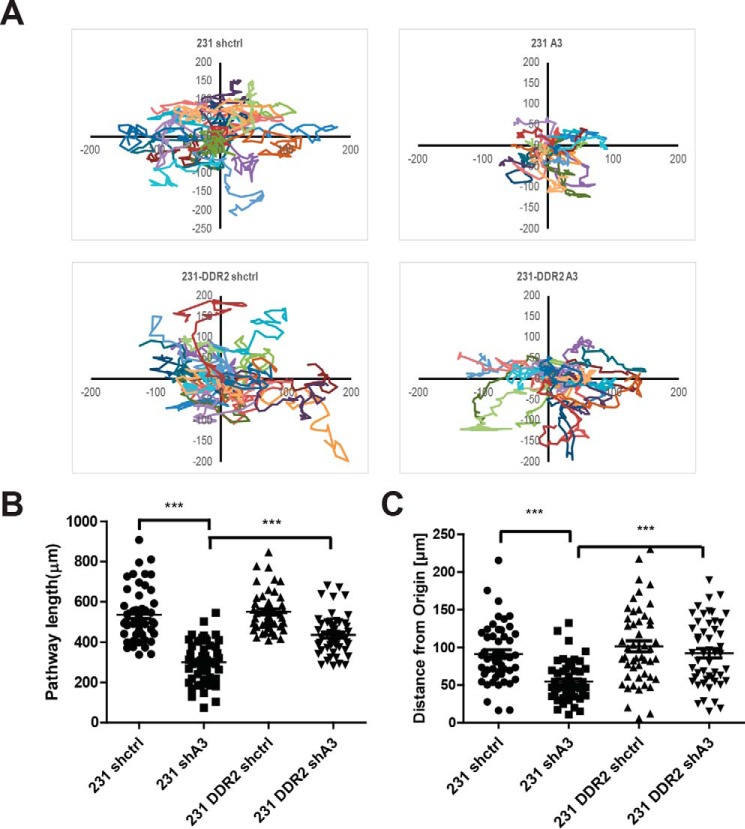
It has been shown that DDR2 expression promotes EMT and enhances cell migration and invasion (15). To determine whether HSP47 promotes cancer cell migration and invasive phenotypes through DDR2, we have introduced exogenous DDR2 in HSP47-silenced cells. By tracking single-cell movement with live cell imaging, we showed that silencing HSP47 in MDA-MB-231 cells significantly inhibited cell migration as previously described (Fig. 5, A and B). Overexpression of DDR2 in HSP47-silenced cells at least partially rescued cell migration (Fig. 5, A and B).
Figure 5.
DDR2 rescues cell migration in HSP47-silenced MDA-MB 231 cells. A, single-cell migration was performed in DDR2 overexpression or not MDA-MB-231 shctrl cells and MDA-MB-231 shA3 cells for 8 h. The migration pathway were analyzed by Excel with 50 single cells for each group. B and C, quantification analysis of single cells' pathway length and distance from the origin for each group (n = 50). Displayed are the means ± S.E. ***, p < 0.0001, one-way ANOVA test.
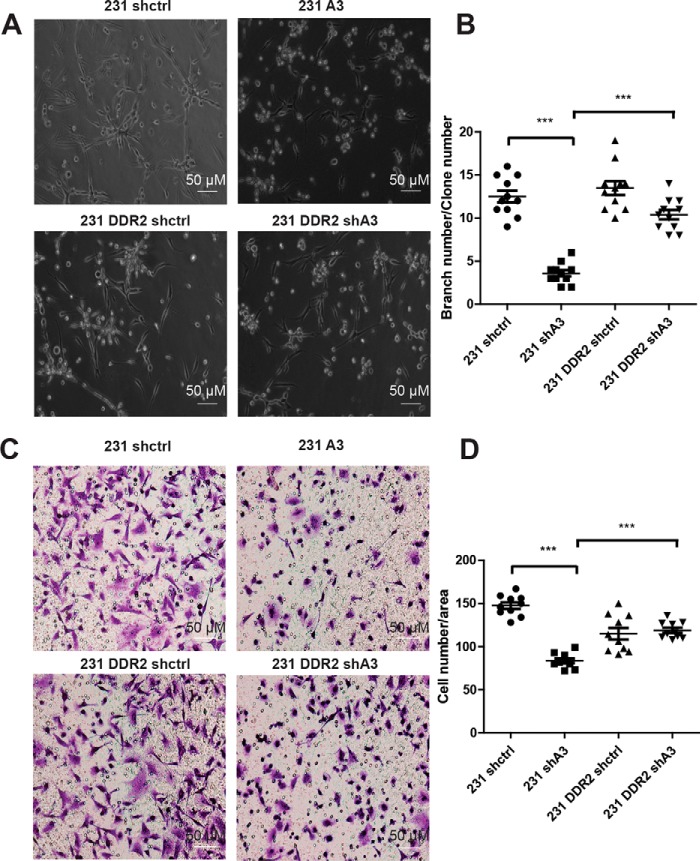
3D tissue culture assay provides a physiologically relevant microenvironment to investigate cell–ECM interaction and cancer progression. MDA-MB-231 cells forms invasive stellate structures in 3D culture (26, 27). Knockdown of HSP47 significantly reduced the invasive branching, and expression of DDR2 partially restored the invasive phenotype of MDA-MB-231 cells in 3D culture (Fig. 6, A and B). We further confirmed that DDR2 expression rescued invasion in HSP47-silenced MDA-MB-231 cells using the Transwell assay (Fig. 6, C and D). These results indicate that HSP47 promotes cancer cell migration and invasion at least partially through enhancing DDR2 protein stability.
Figure 6.
DDR2 rescues cell invasion in HSP47-silenced MDA-MB-231 cells. A and B, phase images and quantification data showing the invasive growth of control and HSP47-silenced MDA-MB-231 cells in the presence or absence of exogenous DDR2 in 3D culture (n = 11). Displayed are the means ± S.E. ***, p < 0.0001, one-way ANOVA test. The 3D culture assay was repeated twice. C and D, phase images and quantification of cancer cell invasion in Transwell analysis. Control and HSP47-silenced MDA-MB-231 cells in the presence or absence of exogenous DDR2 were plated on Matrigel-coated Transwell and invaded cells were quantified under phase contrast microcopy (n = 10). Displayed are the means ± S.E. ***, p < 0.0001, one-way ANOVA test.
Discussion
HSP47 was considered a collagen-specific chaperone; however, IRE1a, decorin, and lumican have recently been identified as HSP47 targets (28, 29). Binding of HSP47 to decorin and lumican is crucial for protein maturation and secretion (28). HSP47 directly binds to the ER luminal domain of IRE1a, and this interaction is crucial for the negative regulation of ER stress (29). Here, we identified DDR2 as a new HSP47 target that mediates HSP47 function in cell migration and invasion.
We found that the presence of HSP47 enhanced the DDR2 protein stability. The co-IP data showed that HSP47 bound to the extracellular region of DDR2. Photoconversion and TIRF imaging analyses showed that binding of HSP47 was not required for the cell surface translocation of DDR2 protein; however, HSP47 expression significantly enhanced the half-life of DDR2 at cell surface. Interestingly, confocal images showed that HSP47 and DDR2 were mainly co-localized in cytoplasm but not at the cell surface. DDR2 is synthesized and matures in the ER, and the ectodomain locates in the ER lumen during synthesis. HSP47 mainly localizes in the ER; therefore, we predict that HSP47 binds to DDR2 in the ER during DDR2 synthesis and maturation.
Protein stability is regulated by post-translational modification, such as glycosylation, hydroxylation, and phosphorylation (30–32). Silencing HSP47 induced a band shift of DDR2 in the Western blotting analysis (Fig. S1), suggesting that HSP47 regulates DDR2 post-translational modification. N-Glycosylation has been characterized in DDR2 (33). We have some data showing that knockdown of HSP47 reduced DDR2 glycosylation (Fig. S2). Therefore, HSP47 may regulate DDR2 stability by facilitating its glycosylation.
It has been shown that expression of DDR2 is induced during EMT (34, 35). EMT is characterized by enhanced cell migration and invasion. Aberrant activation of EMT promotes metastatic spread in many solid tumors (36). The collagen/DDR2 axis plays crucial roles in regulating EMT and promoting breast cancer metastasis (15, 37, 38). Activation of DDR2 increases the level and activity of Snail in breast cancer cells and subsequently promotes cell invasion and cancer metastasis (15). We also detected increased expression of HSP47 during EMT. HSP47 promotes EMT phenotypes in breast cancer cells, such as cell migration and invasion (7). DDR2 overexpression at least partially rescued cell migration and invasion in HSP47-silenced cancer cells, suggesting that HSP47 contributes to the EMT process by enhancing collagen/DDR2 signaling.
DDR2 has been identified as an oncogene (14); however, the regulation of DDR2 during breast cancer progression largely remains to be determined. We found that expression of HSP47 and DDR2 are associated during breast cancer development and progression. Importantly, HSP47 binds to DDR2 and enhances its protein stability. Given the crucial function of DDR2 in EMT and cancer progression, targeting the HSP47–DDR2 interaction may be a potential strategy to inhibit DDR2-dependent cancer progression.
Materials and methods
Antibodies and reagents
Dulbecco's modified Eagle's medium (DMEM) and DMEM/Ham's F-12 medium were obtained from Sigma–Aldrich. Fetal bovine serum, transferrin, β-estradiol, and prolactin were obtained from Sigma. Insulin was from Roche Applied Science (Indianapolis, IN), and sodium selenite, hydrocortisone, and epidermal growth factor were from BD Biosciences (Bedford, MA). Cycloheximide was purchased from Abcam (ab120093). Anti-FLAG M2 affinity gel (A2220) and 3× FLAG peptide (F4799) were from Sigma, and protease inhibitor cocktails were from EMD Millipore (539131). Matrigel® and type I collagen were purchased from BD Bioscience (354230; San Diego, CA), and 4′,6′-diamino-2-phenylindole was from Molecular Probes (D1306). Pierce glycoprotein staining kit was purchased from Thermo Scientific (24562)
Antibodies against the following proteins were obtained from the following companies: DDR2 (R&D, AF2538) and HSP47 (Santa Cruz, sc-5293). Antibodies against DDR2 or HSP47 were verified by protein overexpression or gene silence approaches (Fig. S3). FLAG (Sigma, F1804); tubulin (Millipore Sigma, 05-661); DyLight 680–conjugated goat anti-rabbit IgG secondary antibody (Thermo Fisher Scientific, 35569), DyLight 800–conjugated goat anti-mouse IgG secondary antibody (Thermo Fisher Scientific, SA5-35521)
2D and 3D cell culture
MDA-MB-231 (ATCC) cells were cultured in DMEM/Ham's F-12 medium (Sigma), Hs578T, and HEK293FT in DMEM/high glucose (Sigma), all supplemented with 10% fetal bovine serum, 10 units/ml of penicillin, and 0.1 mg/ml of streptomycin (Invitrogen). In 3D culture, the cells were plated on Matrigel®-coated dishes and maintained in the culture medium containing 10% Matrigel (26).
Plasmids construct and lentivirus generation
Full-length DDR2 cDNA clone was purchased from Addgene. DDR2 full-length, truncated DDR2 (1–390), and truncated DDR2(428–855) were cloned into the pCDH-EF1-MCS-T2A-Puro (PCDH) expression vectors (System Biosciences). The fluorescent protein Dendra2 was incorporated in to the C terminus of full-length pCDH–DDR2. All plasmids were verified by sequencing.
For lentivirus generation, 293FT cells were transfected with short hairpin RNA plasmids (Sigma) plus packaging vectors (PSPAX + PMD2) or pCDH, pCDH–DDR2, pCDH–DDR2–Dendra plus packaging vector (PLP1 + PLP2 + PLPVSVG) using FuGENE HD transfection reagent (Promega) for 48 h to produce virus stock. Then MDA-MB-231 cells were infected with lentivirus and selected by puromycin after 48 h of infection.
Immunoprecipitation assay
293FT cells were transfected with DDR2 and HSP47 expression plasmids using FuGENE HD transfection reagent (Promega). Following 48 h of transfection, the cells were washed with PBS then lysed with ice-cold hypotonic gentle lysis buffer (10 mm Tris-HCl, pH 7.5, 10 mm NaCl, 2 mm EDTA, 0.5% Triton X-100, Protease Inhibitor Mixture Set 1 (Calbiochem)) and centrifuged at 4 °C for 15min. The cell lysates were incubated with 20 μl of anti-FLAG M2 affinity gel (Sigma) for 4 h at 4 °C. The protein complexes were eluted with 3× FLAG peptide and analyzed by Western blotting. Each IP experiment was repeated at least twice.
Western blotting analysis
The cells were lysed with 2% SDS in PBS buffer containing phosphatase and protease inhibitor cocktails. Protein concentration was measured using DCTM protein assay (Bio-Rad). Equal amounts of protein lysates were subjected to SDS gel electrophoresis, immunoblotted, and detected with an ECL system (Pierce). Exposures were acquired and quantified using a FluroChem HD2 (Alpha Innotech).
Quantitative RT-PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen). cDNA synthesis was performed with SuperScript III first-strand synthesis system (Invitrogen) according to the protocol. Quantitative RT-PCRs were carried out using SYBR Green PCR master mix reagents on an ABI 7500 Fast real-time PCR system (Applied Biosystems). Thermal cycling was conducted at 95 °C for 30 s, followed by 40 cycles of amplification at 95 °C for 5 s, 55 °C for 30 s, and 72 °C for 15 s. The following primers were used to amplify: DDR2 forward, 5′-CCAGTCAGTGGTCAGAGTCCA-3′; and DDR2 reverse, 5′-GGGTCCCCACCAGAGTGATAA-3′. The quantitative RT-PCR experiments were performed three times. DDR2 Ct value was subtracted by corresponding 18s value, which produced the ΔCt of DDR2. The ΔCt value of each group was subtracted by ΔCt value of shctrl to generate the ΔΔCt value.
Single-cell migration assay
Control and HSP47-silenced MDA-MB-231 cells (0.04 × 106) with or without DDR2 overexpression were plated on type I collagen precoated 35-mm dishes in DMEM/Ham's F-12 medium containing 2% fetal bovine serum and 4 ng/ml epidermal growth factor. After 2 h of incubation at 37 °C, the images were taken by live cell/incubator imaging system (Nikon Biostation IMQ) every 10 min for 8 h. The cell migration distance and velocity were quantified as previously described (27).
Invasion assay
The Transwell (Corning) were coated with 60 μl of 1 mg/ml Matrigel and incubated for 30 min at 37 °C. The cells were plated in 200 μl on top of the Transwell filter and incubated in 37 °C 5% CO2 for 24 h. The invaded cells on the bottom face of the filter were fixed by methanol and stained with 8% crystal violet.
Immunofluorescence staining
293FT cells were cultured on glass slide, then fixed with 4% paraformaldehyde, and permeabilized with 0.5% Triton X-100. After blocking with 10% goat serum at room temperature for 60 min, specimens were incubated with primary antibody (anti-DDR2 antibody, 1:200 dilution; anti-HSP47 antibody, 1:100 dilution) and fluorochrome-conjugated secondary antibody (1:500 dilution) at 4 °C. Stained samples were imaged with an Eclipse 80i microscope (Nikon) or an Olympus FV1000 confocal microscope.
Stability assay
Control and HSP47-silenced MDA-MB-231 cells were plated with a density of 2 × 106 cells. The cells were treated with cycloheximide (100 μg/ml) for 0, 2, 4, and 8 h. The protein levels of DDR2 were analyzed with Western blotting (39).
Total internal reflection fluorescence microscopy
Control and HSP47-silenced HEK293 cells were transfected with the DDR2–Dendra2 expression plasmid and then were plated on a 35-mm Matrigel-coated glass-bottomed dish for 24 h. Before imaging, the growth medium was replaced with Leibovitz's L-15 imaging medium. TIRF images of selected cells were taken before Dendra2 photoconversion using both 561-nm and 488-nm excitation. This verified the absence of any fluorescence in the red emission channel prior to photoconversion. These cells were then exposed to TIRF-oriented 405-nm laser (∼16.5 milliwatts at the objective) excitation for 3 s. Real-time TIRF images were then acquired for both 561-nm and 488-nm excitation to collect emission in both the red and green channels at 10 min intervals for a total of 7 h. Image collection was initiated 20 min after photoconversion to allow for the clearance of endosomes. The cells were kept at 37 °C for the duration of the experiment using a stage top incubator to avoid any temperature induced changes in endocytosis rates.
Statistical analysis
The correlation expression of HSP47 and DDR2 was assessed with Spearman correlation analysis using the microarray data set generated from human breast cancer tissues (TCGA). All experiments were repeated at least twice. Results were reported as means ± S.E.; significance of difference was assessed by an independent Student t test or one-way ANOVA test. A p value of 0.05 represented statistical significance, and a p value of 0.01 represented high statistical significance.
Author contributions
J. C., S. W., and Z. Z. data curation; J. C. and Z. Z. formal analysis; J. C., S. W., and Z. Z. investigation; J. C. methodology; S. W. writing-original draft; C. I. R. and R. X. conceptualization; C. I. R. and R. X. writing-review and editing; R. X. supervision; R. X. funding acquisition.
Supplementary Material
This work was supported by Grants 1R01CA207772 and 1R01CA215095 from NCI, National Institutes of Health (to R. X.) and Department of Defense Grant W81XWH-15-1-0052 (to R. X.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S3 and supporting text.
- used are: ECM
- extracellular matrix
- HSP47
- heat shock protein 47
- ER
- endoplasmic reticulum
- co-IP
- co-immunoprecipitation
- TIRF
- total internal reflection fluorescence
- DDR
- discoidin domain-containing receptor
- EMT
- epithelial–mesenchymal transition
- DMEM
- Dulbecco's modified Eagle's medium
- ANOVA
- analysis of variance
- DS
- discoidin homology.
References
- 1. Xu R., Boudreau A., and Bissell M. J. (2009) Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metastasis Rev. 28, 167–176 10.1007/s10555-008-9178-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shoulders M. D., and Raines R. T. (2009) Collagen structure and stability. Annu. Rev. Biochem. 78, 929–958 10.1146/annurev.biochem.77.032207.120833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hendershot L. M., and Bulleid N. J. (2000) Protein-specific chaperones: the role of hsp47 begins to gel. Curr. Biol. 10, R912–R915 10.1016/S0960-9822(00)00850-2 [DOI] [PubMed] [Google Scholar]
- 4. Ito S., and Nagata K. (2019) Roles of the endoplasmic reticulum-resident, collagen-specific molecular chaperone Hsp47 in vertebrate cells and human disease. J. Biol. Chem. 294, 2133–2141 10.1074/jbc.TM118.002812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuroda K., Tsukifuji R., and Shinkai H. (1998) Increased expression of heat-shock protein 47 is associated with overproduction of type I procollagen in systemic sclerosis skin fibroblasts. J. Invest. Dermatol. 111, 1023–1028 10.1046/j.1523-1747.1998.00437.x [DOI] [PubMed] [Google Scholar]
- 6. Chen J. J., Zhao S., Cen Y., Liu X. X., Yu R., and Wu D. M. (2007) Effect of heat shock protein 47 on collagen accumulation in keloid fibroblast cells. Br. J. Dermatol. 156, 1188–1195 10.1111/j.1365-2133.2007.07898.x [DOI] [PubMed] [Google Scholar]
- 7. Zhu J., Xiong G., Fu H., Evers B. M., Zhou B. P., and Xu R. (2015) Chaperone Hsp47 drives malignant growth and invasion by modulating an ECM gene network. Cancer Res. 75, 1580–1591 10.1158/0008-5472.CAN-14-1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hebert C., Norris K., Della Coletta R., Reynolds M., Ordóñez J., and Sauk J. J. (1999) Cell surface colligin/Hsp47 associates with tetraspanin protein CD9 in epidermoid carcinoma cell lines. J. Cell Biochem. 73, 248–258 [DOI] [PubMed] [Google Scholar]
- 9. Jiang X., Zhou T., Wang Z., Qi B., and Xia H. (2017) HSP47 promotes glioblastoma stemlike cell survival by modulating tumor microenvironment extracellular matrix through TGF-β pathway. ACS Chem. Neurosci. 8, 128–134 10.1021/acschemneuro.6b00253 [DOI] [PubMed] [Google Scholar]
- 10. Wu Z. B., Cai L., Lin S. J., Leng Z. G., Guo Y. H., Yang W. L., Chu Y. W., Yang S. H., and Zhao W. G. (2016) Heat shock protein 47 promotes glioma angiogenesis. Brain Pathol. 26, 31–42 10.1111/bpa.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagata K., Saga S., and Yamada K. M. (1986) A major collagen-binding protein of chick embryo fibroblasts is a novel heat shock protein. J. Cell Biol. 103, 223–229 10.1083/jcb.103.1.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirayoshi K., Kudo H., Takechi H., Nakai A., Iwamatsu A., Yamada K. M., and Nagata K. (1991) HSP47: a tissue-specific, transformation-sensitive, collagen-binding heat shock protein of chicken embryo fibroblasts. Mol. Cell Biol. 11, 4036–4044 10.1128/MCB.11.8.4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takechi H., Hirayoshi K., Nakai A., Kudo H., Saga S., and Nagata K. (1992) Molecular cloning of a mouse 47-kDa heat-shock protein (HSP47), a collagen-binding stress protein, and its expression during the differentiation of F9 teratocarcinoma cells. Eur. J. Biochem. 206, 323–329 10.1111/j.1432-1033.1992.tb16930.x [DOI] [PubMed] [Google Scholar]
- 14. Hammerman P. S., Sos M. L., Ramos A. H., Xu C., Dutt A., Zhou W., Brace L. E., Woods B. A., Lin W., Zhang J., Deng X., Lim S. M., Heynck S., Peifer M., Simard J. R., et al. (2011) Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discovery 1, 78–89 10.1158/2159-8274.CD-11-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang K., Corsa C. A., Ponik S. M., Prior J. L., Piwnica-Worms D., Eliceiri K. W., Keely P. J., and Longmore G. D. (2013) The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 15, 677–687 10.1038/ncb2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Itoh Y. (2018) Discoidin domain receptors: Microenvironment sensors that promote cellular migration and invasion. Cell Adh. Migr. 12, 378–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leitinger B. (2014) Discoidin domain receptor functions in physiological and pathological conditions. Int. Rev. Cell Mol. Biol. 310, 39–87 10.1016/B978-0-12-800180-6.00002-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonzalez M. E., Martin E. E., Anwar T., Arellano-Garcia C., Medhora N., Lama A., Chen Y. C., Tanager K. S., Yoon E., Kidwell K. M., Ge C., Franceschi R. T., and Kleer C. G. (2017) Mesenchymal stem cell-induced DDR2 mediates stromal–breast cancer interactions and metastasis growth. Cell Rep. 18, 1215–1228 10.1016/j.celrep.2016.12.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maeyama M., Koga H., Selvendiran K., Yanagimoto C., Hanada S., Taniguchi E., Kawaguchi T., Harada M., Ueno T., and Sata M. (2008) Switching in discoid domain receptor expressions in SLUG-induced epithelial–mesenchymal transition. Cancer 113, 2823–2831 10.1002/cncr.23900 [DOI] [PubMed] [Google Scholar]
- 20. Ren T., Zhang W., Liu X., Zhao H., Zhang J., Zhang J., Li X., Zhang Y., Bu X., Shi M., Yao L., and Su J. (2014) Discoidin domain receptor 2 (DDR2) promotes breast cancer cell metastasis and the mechanism implicates epithelial–mesenchymal transition programme under hypoxia. J. Pathol. 234, 526–537 10.1002/path.4415 [DOI] [PubMed] [Google Scholar]
- 21. Xie B., Lin W., Ye J., Wang X., Zhang B., Xiong S., Li H., and Tan G. (2015) DDR2 facilitates hepatocellular carcinoma invasion and metastasis via activating ERK signaling and stabilizing SNAIL1. J Exp Clin. Cancer Res. 34, 101 10.1186/s13046-015-0218-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Widmer C., Gebauer J. M., Brunstein E., Rosenbaum S., Zaucke F., Drögemüller C., Leeb T., and Baumann U. (2012) Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc. Natl. Acad. Sci. U.S.A. 109, 13243–13247 10.1073/pnas.1208072109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Satoh M., Hirayoshi K., Yokota S., Hosokawa N., and Nagata K. (1996) Intracellular interaction of collagen-specific stress protein HSP47 with newly synthesized procollagen. J. Cell Biol. 133, 469–483 10.1083/jcb.133.2.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vogel W., Gish G. D., Alves F., and Pawson T. (1997) The discoidin domain receptor tyrosine kinases are activated by collagen. Mol. Cell 1, 13–23 10.1016/S1097-2765(00)80003-9 [DOI] [PubMed] [Google Scholar]
- 25. Zhang Z., Heidary D. K., and Richards C. I. (2018) High resolution measurement of membrane receptor endocytosis. J. Biol. Methods 5, e105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiong G., Wang C., Evers B. M., Zhou B. P., and Xu R. (2012) RORα suppresses breast tumor invasion by inducing SEMA3F expression. Cancer Res. 72, 1728–1739 10.1158/0008-5472.CAN-11-2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang H., Fredericks T., Xiong G., Qi Y., Rychahou P. G., Li J. D., Pihlajaniemi T., Xu W., and Xu R. (2018) Membrane associated collagen XIII promotes cancer metastasis and enhances anoikis resistance. Breast Cancer Res. 20, 116 10.1186/s13058-018-1030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishikawa Y., Rubin K., Bächinger H. P., and Kalamajski S. (2018) The endoplasmic reticulum-resident collagen chaperone Hsp47 interacts with and promotes the secretion of decorin, fibromodulin, and lumican. J. Biol. Chem. 293, 13707–13716 10.1074/jbc.RA117.000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sepulveda D., Rojas-Rivera D., Rodríguez D. A., Groenendyk J., Köhler A., Lebeaupin C., Ito S., Urra H., Carreras-Sureda A., Hazari Y., Vasseur-Cognet M., Ali M. M. U., Chevet E., Campos G., Godoy P., et al. (2018) Interactome screening identifies the ER luminal chaperone Hsp47 as a regulator of the unfolded protein response transducer IRE1α. Mol. Cell 69, 238–252.e7 10.1016/j.molcel.2017.12.028 [DOI] [PubMed] [Google Scholar]
- 30. Jayaprakash N. G., and Surolia A. (2017) Role of glycosylation in nucleating protein folding and stability. Biochem. J. 474, 2333–2347 10.1042/BCJ20170111 [DOI] [PubMed] [Google Scholar]
- 31. Zurlo G., Guo J., Takada M., Wei W., and Zhang Q. (2016) New insights into protein hydroxylation and its important role in human diseases. Biochim. Biophys. Acta 1866, 208–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bertoletti F., Cea V., Liang C. C., Lanati T., Maffia A., Avarello M. D. M., Cipolla L., Lehmann A. R., Cohn M. A., and Sabbioneda S. (2017) Phosphorylation regulates human poleta stability and damage bypass throughout the cell cycle. Nucleic Acids Res. 45, 9441–9454 10.1093/nar/gkx619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Phan T. N., Wong E. L., Sun X., Kim G., Jung S. H., Yoon C. N., and Yang B. S. (2013) Low stability and a conserved N-glycosylation site are associated with regulation of the discoidin domain receptor family by glucose via post-translational N-glycosylation. Biosci. Biotechnol. Biochem. 77, 1907–1916 10.1271/bbb.130351 [DOI] [PubMed] [Google Scholar]
- 34. Walsh L. A., Nawshad A., and Medici D. (2011) Discoidin domain receptor 2 is a critical regulator of epithelial–mesenchymal transition. Matrix Biol. 30, 243–247 10.1016/j.matbio.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeisberg M., and Neilson E. G. (2009) Biomarkers for epithelial–mesenchymal transitions. J. Clin. Invest. 119, 1429–1437 10.1172/JCI36183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heerboth S., Housman G., Leary M., Longacre M., Byler S., Lapinska K., Willbanks A., and Sarkar S. (2015) EMT and tumor metastasis. Clin. Transl. Med. 4, 6 10.1186/s40169-015-0048-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Medici D., and Nawshad A. (2010) Type I collagen promotes epithelial–mesenchymal transition through ILK-dependent activation of NF-κB and LEF-1. Matrix Biol. 29, 161–165 10.1016/j.matbio.2009.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen Q. K., Lee K., Radisky D. C., and Nelson C. M. (2013) Extracellular matrix proteins regulate epithelial–mesenchymal transition in mammary epithelial cells. Differentiation 86, 126–132 10.1016/j.diff.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xiong G., Stewart R. L., Chen J., Gao T., Scott T. L., Samayoa L. M., O'Connor K., Lane A. N., and Xu R. (2018) Collagen prolyl 4-hydroxylase 1 is essential for HIF-1α stabilization and TNBC chemoresistance. Nat. Commun. 9, 4456 10.1038/s41467-018-06893-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.