Abstract
Background:
It is questionable as to whether total serum 25 hydroxyvitamin D (T25D) levels are lower in African Americans. We measured serum T25D, free 25hydroxyvitamin D (F25D), and serum parathyroid hormone (PTH) in African American and Caucasian women and studied the effect of vitamin D dosing to determine if differences by race or age occur.
Methods:
Healthy young and older Caucasian and African American women who were vitamin D insufficient were randomized in two clinical trials to escalating daily doses of vitamin D from 400 IU - 4800IU and placebo for 12 months.
Results:
Baseline F25D and T25D were significantly lower in young but not older African American compared to Caucasian women. At baseline the rate of change (slope) in F25D with T25D was significantly greater in younger women than in older women, but difference in the rate of change (slope) in F25D with T25D is similar in African American and Caucasian women.
After vitamin D supplementation there was an increase in F25D, and the dose response was not significantly different by age or race. The ratio of F25D/T25D decreased in all groups once T25D exceeded ~ 60nmol/L. There was a progressive decrease in serum PTH with increasing vitamin D doses and the percent change was similar for F25D and T25D.
Conclusion:
Serum F25D and T25D are lower in younger African American women and since dietary vitamin D is similar in the groups, it is likely that the cause of low serum 25OHD in African American women is due to reduced UV exposure and reduced skin production of vitamin D.
Keywords: Vitamin D supplementation, Serum 25 hydroxyvitamin D, Free Serum 25OHD
Graphical Apstract
INTRODUCTION
Serum 25 hydroxyvitamin D (25OHD) represents the input of vitamin D from diet and from vitamin D synthesized by skin in response to UV light; it is the best biomarker of vitamin D nutritional status. Serum 25OHD levels increase in summer and decrease in winter particularly in the Northern latitudes when the sun is low over the horizon and UV light is unable to penetrate atmospheric pollution and generate vitamin D in skin. It is likely that sunlight accounts for 80–90 percent of circulating serum 25OHD in summer (1). Although vitamin D deficiency is common in many parts of the world it is not common in countries where there is fortification of food and dairy products with vitamin D. In the recent NHANES analysis of 7000 people in North America only 9 percent of Caucasians had serum 25OHD levels below 37.5nmol/L in winter and 4 percent in summer, but in African Americans the number was much larger, with 42 percent in winter and 36 percent in summer (2). The Institute of Medicine had set 37.5nmol/L as a goal for 50 percent of the population (EAR) or estimated average requirement and 50nmol/L for 97.5 percent of population (3). It has been reported previously in population-based studies that serum 25OHD levels are lower in African American compared to Caucasians (4–6).
A recent paper challenged the idea that serum 25OHD levels really were lower in African Americans by calculating bioavailable serum 25OHD and showing that levels were similar African American and Caucasians (7).
Serum 25OHD is bound to vitamin D binding protein (DBP), but there are problems with measuring DBP. Measurement of DBP can vary considerably depending on the antibody used in the assay, because a monoclonal antibody gives different values compared to those assays that use a polyclonal antibody. There are genetic polymorphisms of DBP that differ in their affinity for 25OHD and these polymorphisms are associated with ethnicity. The vitamin DBP genotype rs7041 has a T allele that is more common in African Americans, whereas, the G allele is more common in Caucasians. Another DBP genotype rs4588 also shows an ethnic variant with the A allele being more common in African Americans and C allele in Caucasians. Since these genotypes may produce different levels of serum 25OHD there may be an advantage in measuring the free 25OHD.
In a recent study, (8) the measurement of lower serum DBP in African Americans compared to Caucasians were lower when measured with the monoclonal antibody, which measures a limited epitopes of DBP, in contrast to polyclonal antibodies that measure multiple epitopes (7). There was no difference in serum DBP levels between African American and Caucasian subjects when DBP was measured by proteomics (9). Nevertheless, these measurements do not address the issue of affinity of 25OHD for DBP, so it may be important to measure free serum 25OHD (F25D), and not a calculated free or bioavailable F25D. The free hormone hypothesis predicts that only free 25(OH)D is converted to active 1,25(OH)2D which is the biologically active hormone.
In an earlier study, we measured total serum 25D after supplementation with multiple vitamin D doses and showed an increase in serum T25D with a plateau ~ 110 nmol/L on doses of 4000–4800 IU daily (10). The advent of an assay that measures F25D allowed us to repeat the measurements on stored samples. To address the issue of ethnicity and serum 25OHD, we measured F25D and T25D in African American and Caucasian women and studied the effect of vitamin D dosing on F25D and T25D in Caucasian and African American women, across young and old age ranges.
METHODS
Subjects
Volunteers were recruited from the local population by advertising the study in local newspapers and church bulletins. Those who called in were contacted by the recruiter and underwent a telephone screening interview for exclusion and inclusion criteria. If they qualified, women were told to stop taking multivitamins containing vitamin D prior to a defined washout period of 3 months. The main inclusion criteria were a screening serum 25OHD level between 12.5–50nmol/L measured by the Diasorin assay. Exclusion criteria were as follows: serum 25OHD< 12.5nmol/L which was considered clinically too low not to treat, significant co-morbidities, history of cancer except skin cancer within the last 10 years, terminal illness, previous hip fracture, hemiplegia, uncontrolled type I diabetes ± significant proteinuria or persistent fasting blood sugar >140 mg in type II diabetes, active kidney stone disease or kidney stones > 2 times in lifetime, chronic renal failure (serum creatinine >1.4 mg/dl), evidence of chronic liver disease including alcoholism, any severe physical conditions such as rheumatoid arthritis, osteoarthritis and heart failure, on bisphosphonates for more than 3 months in the past, any history of taking fluoride, previous treatment within the last 6 months with PTH, calcitonin or estrogen, chronic high dose corticosteroid therapy (> 10 mg/d) for over 6 months, or currently on anticonvulsants (Dilantin, Phenobarbital), high dose thiazide therapy (> 37.5 mg/d) and any drugs interfering with vitamin D metabolism.
Randomization and treatment:
The study design for both randomized clinical trials was parallel dose groups and placebo. The primary outcomes of both studies have been published previously (10–12). Older women, Caucasian and African American, were randomly assigned equally to one of eight groups, vitamin D3 doses of 400, 800, 1600, 2400, 3200, 4000, or 4800 IU per day or matching placebo for vitamin D3. Younger women, Caucasian and African American, were randomly assigned to one of four vitamin D dose groups 400, 800, 1600, or 2400 IU/d or placebo. Both studies were double-blind; i.e., all the study participants as well as staff that assessed primary outcomes were blinded to the treatment, and only the statistician had access to the treatment code. The randomization method was randomized blocks, stratified by race and screening serum 25OHD level (<37.5nmol/L versus >37.5nmol/L for Caucasians and <30nmol/L versus >30nmol/L for African Americans). The study statistician generated the randomization list with SAS software (SAS Institute Inc., Cary, NC, USA). Screening occurred throughout the year from January 2008 to January 2010, and most women were recruited in wintertime during January, February, March and early April.
Vitamin D3, 400 IU, 800 IU, 1600 IU, 2400 IU, 3600 IU, 4000 IU, 4800 IU capsules and matching placebo capsules were manufactured specifically for these studies by Douglas Labs, Pittsburgh, PA. The actual vitamin D3 concentrations in the capsules were measured independently in Dr. Hector DeLuca’s laboratory located at the University of Wisconsin (Madison, WI) every 12 months over 4 years. The average of the analyses of the vitamin D3 tablets for each dose are given (in brackets); 400 IU (503 IU), 800 IU (910 IU), 1600 IU (1532 IU), 2400 IU (2592 IU), 3200 IU (2947 IU), 4000 IU (4209 IU), 4800 IU (4937 IU). There was no significant change in potency during the study time period. Calcium tablets contained 200 mg of elemental calcium (Citracal; Bayer HealthCare, Morristown, NJ) and were given to maintain a total calcium intake of ~1200 mg/d based on baseline calcium intake estimated from a 7-day food diary. The average calcium supplement during study was 600 mg at 12 months.
Every participant was advised to take one vitamin D3 tablet in the morning after breakfast and calcium twice daily. Adherence was measured at 3, 6, 9, and 12 months as a percentage (number of pills supplied- number of pills returned)/number of pills dispensed x100%). The average of the adherence at 3, 6, 9, and 12 months was calculated to give an overall adherence value for 12 months. Mean adherence averaged over 12 months was 94% for vitamin D3 and 91% for calcium.
Fasting blood samples were collected at all visits – baseline, 3, 6, 9 and 12 months between 7AM and 10AM for a basic metabolic panel measured in the University Clinical Chemistry Laboratory. Serum 25OHD and serum PTH were collected at baseline, 6 and 12 months. After collection, serum samples were stored in multiple aliquots and frozen at –70 C until analysis.
This study was approved by the institutional review board at Creighton University, Omaha, Nebraska and informed consent was obtained from all participants prior to randomization.
Laboratory Methods
Serum total 25OHD (T25D) was measured by 2 methods; initially serum 25OHD was measured by radioimmunoassay (RIA) after an acetonitrile extraction in the Bone Metabolism Laboratory using kits manufactured by Diasorin, Inc. (Stillwater, MN), in 2010 this assay did not detect vitamin D2. The Bone Metabolism Laboratory participates in the vitamin D external quality assessment scheme (DEQAS), which is a program that monitors the accuracy and precision of 25OHD assays (8); our results were within +/−1 standard deviation (SD) of the all-laboratory trimmed mean. We also incorporated National Institute of Standards and Technology standards (NIST) (9). The inter assay variation for NIST standards was as follows: level 1 −8.4%; level 2 −9%; level 3 −7.7%; and level 4 −10.5. The limit of detection in the Diasorin assay is 5 nmol/L.
Samples were re-measured later by Liquid Chromatography Mass spectrophotometry (LCMS) (13). The inter assay variation for LCMS was 4%, intra assay variation was 7%. DEQAS standards were −2% below the trimmed mean and NIST standards showed a mean bias of −4%. Only LCMS measurements are used in this paper. A comparison between the measurements performed by the Diasorin assay and LCMS is shown in supplementary figure 1. There is a systematic difference when total serum 25OHD level is below 50–55nmol/L with Diasorin assay reading about 10 nmol/L lower than LCMS, above 50–55nmol/L the two measurements are comparable.
Free 25(OH)D (F25D) was measured by Elisa competitive immunoassay using an antivitamin D antibody pre-coated onto a microplate (14). The antibody recognizes both 25OHD3 as well as 25OHD2. The cross-reactivity with D2 is estimated to be 75 percent. Free 25(OH)D is captured during this first incubation step, and after washing, a second incubation with biotin-labeled 25(OH)D analog reacts with non-occupied antibody binding sites, then after incubating with a streptavidin- peroxidase conjugate, absorbance [A450nm] is measured using a spectrophotometer. The limit of detection (LOD) for blank serum is 0.7 pg/ml. At 5.02 pg/mL, the between-run coefficient of variation (CV) is 6.2% and between-day CV is 4.5%. Assays were performed at Future Diagnostics B.V. This assay was validated by comparison with measurements using equilibrium dialysis at 37C in 15 normal samples yielding a correlation of 0.83. The lower limit of detection was 1.9 pg/ml, and assay precision was ≤6%.
Serum intact PTH was measured by Diasorin immunoradiometric assay (Stillwater, MN). The limit for serum PTH detection range in our laboratory was 1.0pg/ml. The inter-assay variation is 4.1% and intra-assay variation 2.9% (10). None of the laboratory measures were below the limit of detection.
Outcomes:
The original primary outcomes of this clinical dose ranging trial were changes in total serum 25OHD (Diasorin assay) and serum PTH. The measurement of total 25OHD by LCMS and Free 25OHD and were part of a post hoc analysis of original stored serum at −70 F not previously thawed.
Statistical methods:
Comparisons by race and age groups at baseline were conducted using t-tests. Dose response of F25D to vitamin D was calculated with linear mixed models, including dose as a continuous variable, time, race, and random subject effect. The relationship between serum F25D, T25D and PTH was assessed at baseline using linear regression and correlation. Multiple linear regression was used to look at baseline levels of F25D as the dependent variable with T25D as an independent variable, including age group (young vs. old), race, and interactions between T25D, age, and race in the model. Pairwise comparisons were adjusted for multiple comparisons with Tukey’s method. P-values less than 0.05 are considered to be statistically significant. SAS software version 9.4 was used for data analysis (SAS Institute Inc., Cary, NC).
RESULTS
Baseline characteristics are summarized by age and race in table 1. The mean age of the older women was 66.8 (±7.4) years and that of younger women 36.7 (±5.6) years. BMI and serum PTH were significantly higher in both older and younger African American women. Serum 1,25(OH)2D was significantly higher in young African American women. Dietary calcium intake was significantly lower in both young and older African American women. Serum F25D levels were similar in older African American and Caucasian women, but T25D was significantly lower in older African American women compared to older Caucasian women. In younger women T25D and F25D were both significantly lower (p< 0.001) in African American women. There was a high correlation between serum free 25OHD and total 25OHD measured by LCMS, r=0.73 at baseline and r=0.86 at 12 months (Figure 1).
Table 1.
Baseline characteristics
| Older women | Younger women | |||
|---|---|---|---|---|
|
| ||||
| Caucasian (n=140) | African American (n=88) | Caucasian (n=91) | African American (n=39) | |
| Age (years) | 66.9 (7.4) | 66.7 (7.4) | 38.3 (5.1) | 35.4 (6.3)** |
| BMI (kg/m2) | 30.5 (6.0) | 32.6 (7.3) ^^ | 29.0 (6.2) | 31.4 (5.8)* |
| Serum 25 OHD (nmol/L) | 50.9 (13.2) | 47.5 (14.1) ^ | 55.7 (13.8) | 35.8 (10.3)** |
| Free 25OHD (nmol/L) | 9.21 (2.88) | 8.81 (3.20) | 11.82 (3.32) | 9.24 (2.90)* |
| %Free 25D/total 25OHD | 0.019 (0.004) | 0.018 (0.005) | 0.021 (0.004) | 0.025 (0.005)** |
| Serum 1,25 (OH)2D (nmol/L) | 109.0 (36.9) | 105.9 (32.0) | 113.9 (40.7) | 131.4 (47.1)* |
| Serum PTH (pg/ml) | 36.9 (13.6) | 43.8 (20.3) ^^ | 33.5 (10.3) | 43.2 (17.1)** |
| Dietary vitamin D intake (IU/day) | 118.7 (76.9) | 113.4 (89.0) | 109.8 (68.5) | 95.7 (79.8) |
| Dietary calcium intake (mg/day) | 694.0 (225.8) | 555.9 (226.4)^^ | 789.0 (265.1) | 502.4 (164.8)** |
Table contains Means (Standard Deviations)
Dietary vitamin D intake only available for Omaha site
p<0.05
p<0.001 comparing by race in older women
p<0.05
p<0.001 comparing by race in younger women
Figure 1.
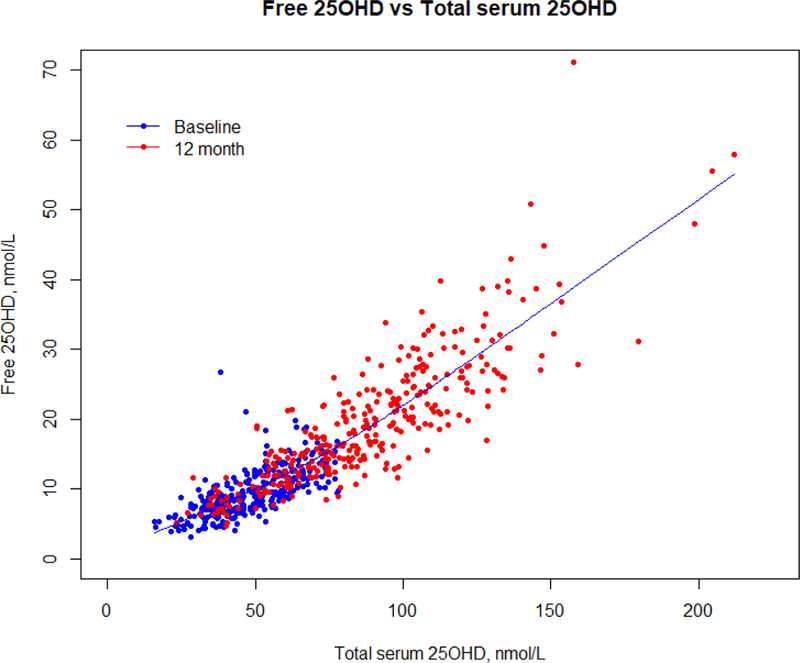
Relationship between serum free 25OHD and total serum 25OHD at baseline and 12 months using a spline.
Serum F25D and T25D
The relationship between F25D and T25D was assessed using multiple linear regression, adjusting for BMI (Figure 2). The three-way interaction between T25D, age group, and race was not significant and removed from the model. There was significant interaction between T25D and age group (p=0.0021), but not between T25D and race (p=0.16). In younger women, a 1nmol/L increase in T25D results in a 0.18 nmol/L increase in F25D. Whereas, in older women, a 1nmol/L increase in T25D results in a 0.13 nmol/L increase in F25D. The difference in slope for the two races was negligible at 0.008 nmol/L. The mean proportion of serum F25D/ T25D in younger African Americans was 0.025% compared to 0.021% in younger Caucasians (p=0.0002). In older women the mean proportion of F25D/T25D was 0.018% in African Americans versus 0.019% in Caucasians (p=0.98).
Figure 2.
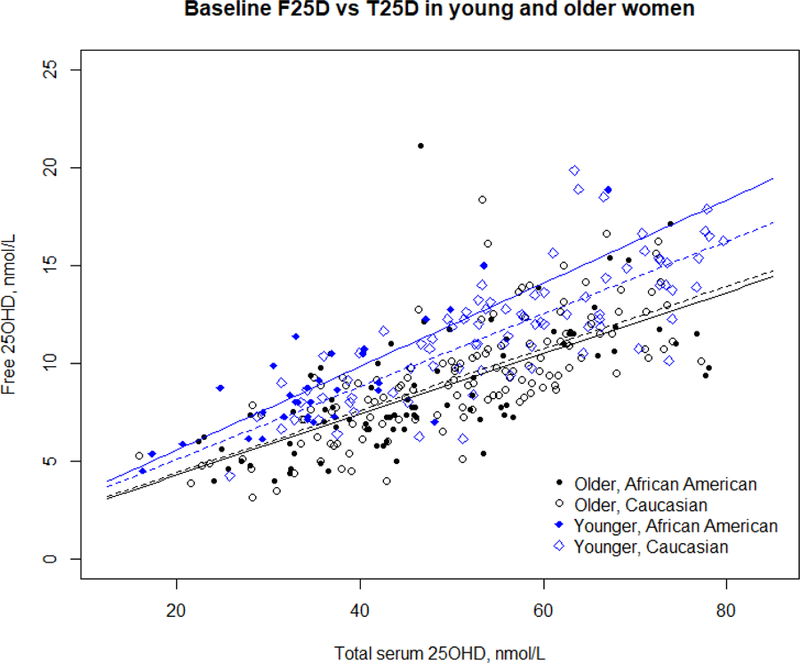
Baseline comparison of serum free 25OHD and total serum 25OHD by age and race. No difference in was observed by race in older women, but significant age differences are observed as well as race difference in younger women.
Effect of vitamin D dosing on serum F25D.
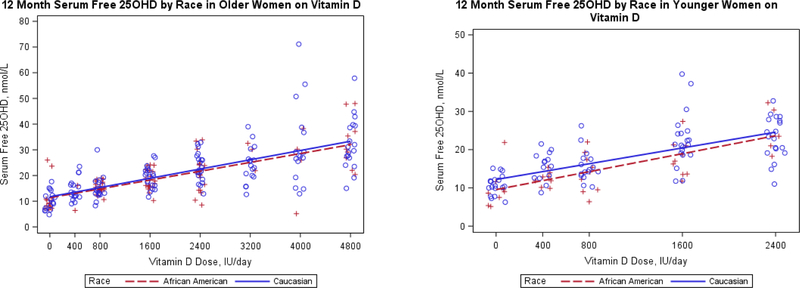
As observed in the regression analysis, baseline F25D was higher relative to T25D in younger women (Table 1). The effect of increasing vitamin D dose on F25D is shown in figure 3a, there is no difference in the response of older African American versus older Caucasian women (p>0.5). In younger women, the dose response curve for African Americans is significantly lower than Caucasians (p=0.038), but the slope of the dose response curve is the same (p=0.96), figure 3b and supplementary figure 2. The slope of the regression lines for young and older women are similar (p=0.58), for every 1000 IU/day increase in Vitamin D dose, F25D increases on average by 5.2pmol/L in younger women and by 4.5pmol/L in older women.
Figure 3.
12 month serum free 25OHD according to vitamin D dose levels by race in a) older women and b) younger women.
Serum PTH
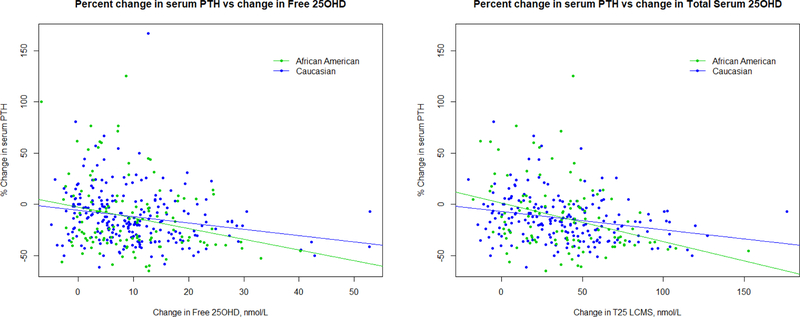
Baseline mean serum PTH was higher in the older African American women 44(±21) compared to Caucasians 38 (±14) pg/ml (p=0.03). In young African American women serum PTH was higher 44(±18) than Caucasian women 34(±10) (p<0.01). Percent change in PTH from baseline to 12 months, decreased with increasing vitamin D dose and increasing percent change in serum T25D and F25D (Figure 4). The decrease in PTH was 6% for each 1% increase in F25D. The percent change in PTH slope compared to levels of serum T25D was steeper in African Americans than Caucasian (p=0.014) but not different by age (p=0.41). The percent decrease in PTH was 13% in African Americans and 5% in Caucasians for each 1% increase in T25D. The percent change in PTH corresponding to percent change in serum T25D or F25D, is similar in Caucasian young and older women at 5–6%. African American women showed a larger percent decrease in PTH due to T25D at 13% than F25D at 6%. However, this difference may be partially explained by regression to the mean considering that the African American had higher levels of PTH at baseline as well as lower levels of T25D, allowing for greater change.
Figure 4.
Percent change in PTH from baseline to 12 months by the change in a) serum free 25OHD and b) total serum 25OHD in African American and Caucasian women.
DISCUSSION
The measurement of F25D has emerged recently as a potentially useful test. Although it is possible to estimate bioavailable 25OHD based on DBP, albumin and the dissociation constants the correlation between bioavailable and F25D shows a wide variance, the changes on vitamin D are different and a direct measurement of F25D is more accurate. (15)
In a recent cross-sectional multicenter study of 1661 subjects, F25D was measured in 279 normal subjects, 90 patients with cirrhosis, 20 pregnant, 479 pre-diabetes and 79 nursing home patients. (16) This study showed that mean levels of F25D were 10.75pmol/L in normal subjects and were significantly lower compared to patients with cirrhosis, with a mean 17.75pmol/L and pre-diabetes, with a mean 13.75pnol/L.
There are a few studies that show F25D to be a better clinical measurement than T25D. In a short 12-week study of weekly 50,000IU D2 or D3 the decrease in serum PTH at 4 weeks was more highly correlated with F25D than T25D but after 8–12 weeks of treatment this was no longer true (17). A recent paper in 1387 Chinese patients with proven coronary disease showed that F25D, but not T25D was highly correlated with coronary disease and mortality during a 6.7 year follow up suggesting that F25D may have an important clinical role in cardiovascular disease (18). In a comparison of baseline physical tests of grip strength and gait speed there were significant correlations with F25D but not T25D.(19) A study of the effect of vitamin D on calcium absorption did not show any significant correlation with F25D nor T25D (8). In a previous paper on the effect of vitamin D on bone density and bone markers, in this same study group described in this paper, we did not find any significant correlation between changes in BMD or bone markers with serum F25D or T25D (20). In this paper vitamin D supplementation caused a decrease in serum PTH, but neither F25D or T25D were superior compared to the change in serum PTH.
There are no previous placebo-controlled studies that compare the change in F25D on vitamin D treatment in both younger and older African American and Caucasian women. In younger women baseline F25D was significantly lower in African American 9.2pmol/L compared to 11.8pmol/L in Caucasian women, and T25D was significantly lower in young African American compared to Caucasian women 36nmol/L and 56nmol/L respectively. But in older African American and Caucasian women there was no difference in F25D, and only a small difference of 3.4nmol/L in T25D. After vitamin D there was a linear response in serum F25D increasing from a baseline mean of 9.25nmol/L and 9.75nmol/L to 21nmol/L and 23.25nmol/L on vitamin D 2400 IU, and to 33nmol/L and 32.5nmol/L on vitamin D 4800 IU in African American and Caucasian women respectively. Similarly, for T25D, African Americans versus Caucasians, in young women baseline means increased from 45nmol/L and 50nmol/L to 96.75nmol/L and 110nmo/L on 2400IU and 128nmol/L (or 132nmol/L respectively on 4800IU. Thus, after vitamin D supplementation there was no significant difference in the 12-month treatment responses for serum F25D, T25D among young, old, African American and Caucasian women.
At baseline before treatment with vitamin D when serum 25OHD levels were low, the proportion of F25D/T25D was significantly higher in younger women compared to older women. In younger women the increase in F25D with T25D (slope of the regression line) was significantly higher in younger African Americans than Caucasians (Figure 2). However, in the older African American and Caucasian women the increase in F25D with T25D at baseline was similar, showing almost identical slopes and intercepts. After vitamin D treatment the proportion of F25D/T25D was similar in all groups. In subjects with vitamin D insufficiency we suggest that the higher proportion of F25D to T25D may be due to increased bioavailability of F25D as 25OHD levels decrease, possibly higher circulating PTH associated with vitamin D insufficiency or deficiency could increase the bioavailability of F25D.
Most African American people have the DBP genotype Gc1F that one study reported to have higher affinity for 25OHD (21) but others did not find the same result (22) which is more in keeping with our results in this study. We do not have the genotypes available in this study.
Although a previous study reported lower levels of serum DBP in African American people (7) we know that the explanation for this result was due to the monoclonal antibody used in the assay that did not detect all of the DBP genotypes whereas the polyclonal antibody detects all DBP genotypes (16). It has been reported that vitamin D showed a small non-significant decrease in serum DBP levels in a study in Norway of vitamin D 20,000 IU given weekly. (15)
In summary, in this normal population of African American and Caucasian women the effect of vitamin D on F25D and T25D were highly correlated. There was no significant difference in the response of F25D or T25D to vitamin D supplementation in younger, older, African American and Caucasian women. Also, we confirm that F25D and T25D levels are lower in some African American women, and since dietary intake of vitamin D is similar amongst groups, this implies that low levels in African American women are most likely due to reduced UV exposure.
A further question is whether there is any clinical advantage in the use of F25D over T25D. In normal subjects the use of serum T25D which is widely available is the optimal screening test for assessment of vitamin D insufficiency and deficiency. There may be a role for the use of F25D, based on a recent study (18) that showed F25D was more closely associated with cardiovascular mortality than with T25D, however, this result needs confirmation in other clinical outcomes studies.
Supplementary Material
Supplemental figure 1.Comparison of serum 25OHD measured by immunoassay ( Diasorin) and LCMS
Supplemental figure 2.Mean serum free 25OHD (± SD) in young and old women after 12 month treatment with vitamin D (all doses combined)
Acknowledgements
This work was supported by grants from NIH AG28168 and Department of Defense (DOD) (W81XWH-07-1-201). Clinical trial registration:
We greatly appreciate the assistance and support of Future Diagnostics and Leon Swinkels for measurement of Free 25OHD and appreciate the measurement of serum 25OHD using LCMS by Martin Kaufmann and Glenville Jones.
Financial Support – This study was supported by National Institute on Aging -AG28168 and Office of Dietary Supplements and by Department of Defense (DOD) (W81XWH-07-1-201)
Footnotes
Conflict of interest: The authors have no conflict of interest to report.
Disclosures: The authors have nothing to disclose.
References
- 1.Hansen L, Tjønneland A, Køster B, Brot C, Andersen R, Cohen AS, Frederiksen K, Olsen A.Vitamin D Status and Seasonal Variation among Danish Children and Adults: A Descriptive Study. Nutrients 2018. November 20;10(11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schleicher RL, Sternberg MR, Looker AC, Tetley EA, Lacher DA, Sempos CT, Taylor CL, Durazo-Arvizu RA, Maw KL, Chaudhary-Webb M, Johnson CL, Pfeiffer CM. National Estimates of Serum Total 25-Hydroxyvitamin D and Metabolite Concentrations Measured by Liquid Chromatography-Tandem Mass Spectrometry in the US Population during 2007–2010. J Nutr 2016. May;146(5):1051–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96(1):53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engelman CD, Fingerlin TE, Langefeld CD, Hicks PJ, Rich SS, Wagenknecht LE, Bowden DW, Norris JM. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab 2008. September;93(9):3381–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr 2008. December;88(6):1519–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durazo-Arvizu RA, et al. Serum 25-Hydroxyvitamin D in African-origin populations at varying latitudes challenges the construct of a physiologic norm. Am J Clin Nutr 2014. September;100(3):908–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powe C, Karumanchi S, Thadhani R. Vitamin D-binding protein and vitamin D in blacks and whites. N Engl J Med 2014;370(9):880–1. [DOI] [PubMed] [Google Scholar]
- 8.Aloia J, Dhaliwal R, Mikhail M, Shieh A, Stolberg A, Ragolia L, Fazzari M, Abrams SA. Free 25(OH)D and Calcium Absorption, PTH, and Markers of Bone Turnover. J Clin Endocrinol Metab 2015;100(11):4140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoofnagle AN, Eckfeldt JH, Lutsey PL.Vitamin D-Binding Protein Concentrations Quantified by Mass Spectrometry. N Engl J Med 2015. October 8;373(15):1480–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallagher J, Sai A, Templin T, Smith L. Dose Response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med 2012;156:425–37. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher JC, Peacock M, Yalamanchili V, Smith L. Effects of vitamin D supplementation in older African American women. J Clin Endocrinol Metab 2013;98:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher JC, Jindal PS, Smith LM. Vitamin D supplementation in young White and African American women. Journal of Bone and Mineral Research 2014. January;29(1):173–81. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann M, Gallagher JC, Peacock M, Schlingmann KP, Konrad M, DeLuca HF, Sigueiro R, Lopez B, Mourino A, Maestro M, St-Arnaud R, Finkelstein JS, Cooper DP, Jones G. Clinical Utility of Simultaneous Quantitation of 25-Hydroxyvitamin D and 24,25-Dihydroxyvitamin D by LC-MS/MS Involving Derivatization With DMEQ-TAD. J Clin Endocrinol Metab 2014. July;99(7):2567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heureux N, Lindhout E, Swinkels LA Direct Assay for Measuring Free 25-Hydroxyvitamin D. J AOAC Int 2017. September 1;100(5):1318–1322. [DOI] [PubMed] [Google Scholar]
- 15.Sollid ST, Hutchinson MY, Berg V, Fuskevag OM, Figenschau Y, Thorsby PM, Jorde R. Effects of vitamin D binding protein phenotypes and vitamin D supplementation on serum total 25(OH)D and directly measured free 25(OH)D. Eur J Endocrinol 2016;174(4):445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz JB, Gallagher JC, Berg V, Eastell R, Evans AL, Naylor KE, Jones KS, Schoenmakers I, Holick M, Orwoll E, Nielson C, Kaufman M, Jones G, Bouillon R, Lai J, Verotta D, Bikle D.Determination of free 25(OH)D concentrations and their relationships to total 25(OH)D in multiple clinical populations. J Clin Endocrinol Metab 2018;103(9):3278–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shieh A, Chun RF, Ma C, Witzel S, Meyer B, Rafison B, Swinkels L, Huijs T, Pepkowitz S, Holmquist B, Hewison M, Adams JS. Effects of High-Dose Vitamin D2 Versus D3 on Total and Free 25-Hydroxyvitamin D and Markers of Calcium Balance. J Clin Endocrinol Metab 2016. August;101(8):3070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu C, Xue H, Wang L, Chen Q, Chen X, Zhang Y, Hu G, Ling W.Serum Bioavailable and Free 25-Hydroxyvitamin D Levels, but Not Its Total Level, Are Associated With the Risk of Mortality in Patients With Coronary Artery Disease. Circ Res 2018. September 28;123(8):996–1007. [DOI] [PubMed] [Google Scholar]
- 19.Aloia J, Dhaliwal R, Mikhail M, Shieh A, Stolberg A, Ragolia L, Fazzari M, Abrams SA.Free 25(OH)D and Calcium Absorption, PTH, and Markers of Bone Turnover. J Clin Endocrinol Metab 2015. November;100(11):4140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith LM, Gallagher JC, Kaufmann M, Jones G. Effect of increasing doses of vitamin D on bone mineral density and serum N-terminal telopeptide in elderly women: a randomized controlled trial. J Intern Med 2018. December;284(6):685–693. [DOI] [PubMed] [Google Scholar]
- 21.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Human genetics 1993;92:183–8. [DOI] [PubMed] [Google Scholar]
- 22.Boutin B, Galbraith RM, Arnaud P. Comparative affinity of the major genetic variants of human group-specific component (vitamin D-binding protein) for 25-(OH) vitamin D. Journal of Steroid Biochemistry 1989;32(1A):59–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1.Comparison of serum 25OHD measured by immunoassay ( Diasorin) and LCMS
Supplemental figure 2.Mean serum free 25OHD (± SD) in young and old women after 12 month treatment with vitamin D (all doses combined)