Abstract
Background
The association of Lewy bodies (LBs) with olfactory dysfunction was investigated in community-dwelling elders without clinical Parkinson’s disease (PD) using the 12-item Brief Smell Identification Test (BSIT), a standard measure of odor identification.
Methods
280 participants in the Rush Memory and Aging Project completed the BSIT annually. Lewy bodies were detected in 13 brain regions by immunohistochemistry and assigned to the Braak PD stages 1 to 6.
Results
Of the 280 participants, 101 (36.1 %) had LBs which were maximal in the olfactory bulb and tract (85.1%) and least in Heschl’s cortex (21.8%). Due to the small number of cases in Braak PD stages 2, 3 and 5, the distribution of LBs in the 6 Braak PD stages was contracted into 3 main LB stages: 1) LBs in olfactory bulbs and dorsal motor nucleus of vagus, 2) further extension of LBs to limbic and other brainstem regions and 3) additional extension of LBs to neocortical areas. MMSE, global cognition and odor test scores were lower and frequency of dementia was higher at the time of the last valid BSIT, in cases with LBs as compared to those without LBs. Linear regression analyses showed that LBs were associated with impaired olfaction. However, on stratification of LBs into 3 stages, only the stage 3 cases were independently associated with impaired olfaction.
Conclusion
Although LB pathology was detected in olfactory bulbs in the early stage of LB progression (stage 1), the strongest association of LBs with olfactory dysfunction was observed in the late pathological stage (stage 3) when LBs extended to neocortical areas.
Keywords: Aging, Alzheimer’s disease, Brief smell identification test, Cognition, Lewy bodies, Odor identification test, Olfaction, Olfactory bulbs, Parkinson’s disease
Introduction
Olfactory dysfunction in the elderly develops with normal aging [1, 2], and 6-16% of presumed healthy elderly are affected [3]. A population-based study of elders reported a prevalence of impaired olfaction of 24.5% with the prevalence increasing with age to 62.5% in 80-97 year-olds [4]. Olfactory deficits are also a common early clinical finding in many neurodegenerative diseases [5] with most attention being directed to sporadic Parkinson’s disease (PD), dementia with Lewy bodies (DLB) [6-9], and Alzheimer’s disease (AD) [9-13]. In sporadic PD, olfactory dysfunction is reported in approximately 96% of early-stage PD preceding the onset of movement and cognitive dysfunction [6, 14, 15].
Pathological studies of the olfactory bulb and tract in PD demonstrate phosphorylated α-synuclein immunoreactive neuronal intracytoplasmic Lewy bodies (LBs) and dystrophic Lewy neurites [16-19]. Localization of LB pathology in the olfactory bulb is also reported in a majority of cases of DLB [20] and incidental LB disease, (ILBD) [16, 20-24] in which the clinical features of PD are not present and which may represent the preclinical form of PD [7, 25, 26]. Extension of LB pathology beyond the olfactory bulb, in primary olfactory cortices including the olfactory tubercle, the frontal and temporal piriform cortices has also been reported in PD and ILBD [27, 27]. Presence of LBs in the secondary olfactory region (orbitofrontal cortex) is reported to be associated with clinical PD [28].
Several internationally recognized staging schemes are available to assess the distribution of LBs in the cerebrum, brainstem and olfactory bulbs. In sporadic PD, the Braak 6-stage scheme [16] proposes that LB pathology begins in the anterior olfactory nucleus and dorsal IX/X motor nucleus (IX/XMNuc) (stage 1) and additional involvement of other medullary regions and midbrain occur in PD stages 2-3 while additional involvement of medial temporal structures (entorhinal cortex and amygdala) occur in PD stage 4. Additional involvement of cortical neurons occurs in the end stages of 5 and 6. Clinical symptoms of PD are typically noted during stages 4 to 6 [16]. Another staging system for classification of all LB disorders, although different in details still proposes that olfactory bulb involvement is early and in this staging scheme the highest stage (stage IV) shows involvement of the neocortex similar to the Braak PD stages of 5 and 6 [21]. The original Newcastle-McKeith criteria [29] for DLB were revised to include an olfactory only and amygdala predominant stage, in addition to their original brainstem, limbic and neocortical stages [30]. However, the clinical significance of the presence of LBs in the olfactory system remains uncertain since none of these postmortem studies linked LBs with olfactory function as detected by a functional test such as the University of Pennsylvania Smell Identification Test (UPSIT) [31].
The link between the presence of LBs in the olfactory system and decreased olfactory function was evident in 10 cases with a clinicopathologic diagnosis of sporadic PD and 13 cases of ILBD, using UPSIT [7], though tissue sampling in the latter cases was limited to the olfactory bulbs, amygdala and the entorhinal cortex. A previous study by our group [32] reported a strong association between LBs in neocortical areas with olfactory dysfunction in elders without PD using the Brief Smell Identification Test (BSIT). However, in the latter study the olfactory bulbs or dorsal IX/XMNuc, 2 regions where LBs were reported to occur early, were not evaluated.
Since olfactory system dysfunction has been reported to be an early event in PD, our hypothesis was that in elders without PD, LBs may occur early in the olfactory bulbs and be associated with olfactory dysfunction. This hypothesis was tested in community dwelling elders in the Rush Memory and Aging Project (MAP), in whom olfaction was assessed with BSIT. At death, a detailed neuropathologic examination was done which included detection of LBs in 13 brain regions allowing application of the Braak PD staging scheme.
Materials and methods
Participants
The analyses are based on older persons who participated in MAP, a longitudinal clinical-pathologic study that involves uniform, annual, clinical evaluations and brain donation at death [33]. This study was approved by the Institutional Review Board of Rush University Medical Center. When MAP began in 1997 only 5 brain areas and substantia nigra were assessed for LBs. Since 2013, a total of 13 areas were assessed for LBs as described below. 280 consecutive participants with this enhanced LB collection were included in this study after excluding 8 cases with a clinical diagnosis of PD. All participants completed the BSIT (see below), had a mean age of 89.8 years (SD 6.1) at the time of the last olfactory testing and completed a mean of 14.6 (SD 2.9) years of schooling. 75.7% were women and 97.5 % were white and non-Hispanic.
Odor Identification
The Brief Smell Identification Test (BSIT) [34, 35] which was derived from UPSIT assesses the ability to recognize 12 familiar odors. This test was administered as part of the baseline clinical evaluation and was subsequently added to the annual follow-up evaluations. On each item, the participant was asked to match an odor with one of four choices. The score for the test was the number of correct choices plus 0.25 assigned for each missing response to a maximum of 2 as described previously [13]. The odor test score was treated as missing if more than 2 item responses were missing. BSIT was administered over a mean period of 8.2 (SD 3.8) years and the number of tests done in each participant varied from 1 to 7, however, the last BSIT was done a mean of 1.2 (SD 1.6) years prior to death and this score was used in analyses. In previous research, the olfactory score was shown to correlate with the 40-item UPSIT from which it was derived [31] and to have adequate short-term temporal stability [34].
Clinical Evaluation
The annual clinical evaluation of all participants included a medical history, neurological examination and detailed cognitive performance testing which was evaluated at baseline and at each follow-up evaluation using a standardized battery of 19 cognitive performance tests (Table 1). The Mini-Mental State Examination (MMSE) was used for descriptive purposes and Complex Ideational material was only used for diagnostic classification. The remaining 19 tests were selected to assess 5 domains of cognitive function, including episodic, semantic, and working memory, perceptual speed and visuospatial ability. The z scores of the 19 tests were averaged to compute the global cognitive function score as described previously [36]. Scores were reviewed by a neuropsychologist to diagnose cognitive impairment. Next, participants were evaluated by a clinician who used all cognitive and clinical data to classify cognitive function as previously described [37]. After death, a board-certified neurologist, blinded to autopsy data, reviewed all available cognitive and clinical data to assign final clinical diagnoses and dementia using the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the AD and Related Disorders Association [38].
Table 1.
Neuropsychological test scores in cognitive domains by Lewy body pathology
| Cognitive Domains | Neuropsychological Tests |
LB Pathology | p-value | |
|---|---|---|---|---|
| Not
present n=179 mean, SD |
Present n=98 mean, SD |
|||
| Episodic Memory | Word list I – immediate | 13.8 (6.1) | 11.7 (7.3) | 0.012 |
| Word List II – delayed | 3.4 (2.8) | 2.8 (2.9) | 0.132 | |
| Word List III – recognition | 8.0 (3.1) | 6.9 (3.5) | 0.009 | |
| East Boston Story – immediate | 7.6 (3.4) | 5.9 (4.0) | <0.001 | |
| East Boston Story – delayed recall | 6.5 (4.2) | 4.9 (4.4) | 0.002 | |
| Logical memory Ia | 9.2 (5.8) | 6.9 (5.7) | <0.001 | |
| Logical memory IIa | 7.6 (6.0) | 5.3 (5.4) | <0.001 | |
| Semantic Memory | Boston Naming | 12.9 (2.6) | 11.2 (4.5) | <0.001 |
| Category fluency | 22.1 (10.9) | 19.6 (13.4) | 0.115 | |
| Reading test NART | 7.6 (2.6) | 6.5 (3.3) | 0.003 | |
| Working Memory | Digits forwards | 7.1 (2.6) | 6.1 (3.1) | 0.004 |
| Digits Backwards | 4.9 (2.4) | 4.3 (2.6) | 0.034 | |
| Digital Ordering | 6.0 (2.1) | 4.9 (2.7) | 0.002 | |
| Perceptual Speed | Symbol Digit Modalities | 25.5 (14.3) | 19.8 (16.2) | 0.003 |
| Number comparison | 18.0 (8.7) | 15.6 (9.2) | 0.034 | |
| Stroop – Word Reading | 35.4 (15.0) | 32.8 (15.4) | 0.178 | |
| Stroop – Color Naming | 11.3 (7.3) | 10.0 (7.9) | 0.191 | |
| Visuospatial Ability | Line Orientation | 8.9 (3.7) | 8.1 (4.1) | 0.113 |
| Progressive matrices | 10.0 (3.4) | 9.3 (3.7) | 0.084 | |
p-value derived from t-test
Clinical evaluation also included a modified version of the Unified Parkinson’s Disease Rating Scale which included 26-items to assess four parkinsonian signs (gait disturbance, bradykinesia, rigidity and tremor) [39]. The scores of these 4 signs were averaged to provide a summary global parkinsonian score as described previously [39]
Neuropathological Evaluation
After a mean post-mortem interval of 9.5 (SD 8.0) hrs, neuropathological evaluation was performed following a standard protocol and blinded to all clinical data. Following macroscopic examination of the brain, blocks were taken from 13 regions to assign subjects to the 6 Braak PD stages of LB distribution in brain [16]. Blocks taken were the dorsal IX/XMNuc (medulla), the olfactory bulb and tract, locus ceruleus (rostral pons), nucleus gigantocellularis (pontomedullary junction), substantia nigra, pedunculopontine nucleus (caudal midbrain), amygdala, entorhinal, midfrontal, midtemporal, inferior parietal, motor and Heschl’s cortices as described previously [23]. Blocks processed using standard techniques were embedded in paraffin and 6μm sections stained with hematoxylin and eosin were examined to ensure that the required anatomic structures were included in the block.
AD pathology was assessed in five regions (midfrontal, midtemporal, inferior parietal and entorhinal cortices and CA1 sector of the hippocampus) in sections stained by the modified Bielschowsky silver stain. Manual counts of neuritic and diffuse plaques and neurofibrillary tangles in a 1 mm2 area having the highest density of these structures were used to create a summary measure of AD pathology [40], for use in analyses.
A semiquantitative estimate of neuronal loss in the substantia nigra was obtained from hematoxylin-eosin stained hemisections (6 μm) of the midbrain at the level of the exiting third nerve fibers. Results were graded as none, mild, moderate and severe as described previously [41].
Immunohistochemical evaluation
Immunohistochemistry was done using a Leica-Bond Max autostainer (Leica Microsystems Inc., New Buffalo, IL). Paraffin sections (6μm) of the 13 regions were used to localize LBs (Fig.1) using an anti-phosphorylated α-synuclein antibody (1:20,000, Wako Chem. USA, Richmond, VA) following antigen retrieval by heat-induced epitope retrieval in citrate buffer (pH 6.0). The Bond™ Polymer Refine Red Detection kit utilizing alkaline phosphatase with a Fast Red chromogen was used. LBs in each region were recorded and analyzed as dichotomous variables.
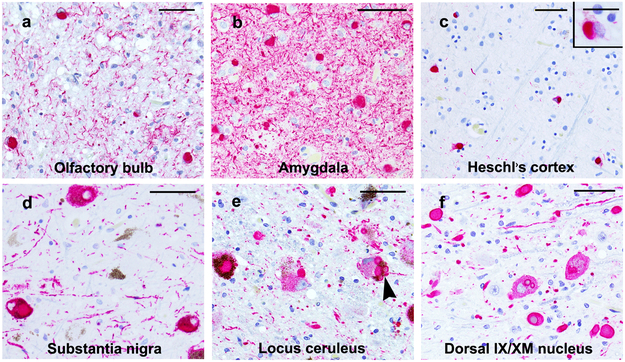
Fig. 1.
Lewy bodies and Lewy neurites, detected by α-synuclein immunostaining are present in the olfactory bulbs (a), amygdala (b), Heschl’s cortex (c), substantia nigra (d), locus ceruleus (e) and dorsal motor nucleus of the vagus nerve (dorsal IX/XM Nucleus) (f). A Lewy body in Heschl’s cortex (c) is shown at higher magnification in the inset. (e) A neuron in the locus ceruleus shows 4 LBs (arrowhead). a-f, scale bar = 50 μm, inset scale bar = 20 μm
Statistical Analyses
Clinical and pathological characteristics of participants without LBs and those with LBs were compared using ANOVA or chi-square. Correlation between the last valid smell test and the MMSE scores or the global cognition scores was tested using Pearson’s correlation coefficient. Linear regression analyses were used to evaluate the association of LBs in all 13 regions and separately LBs in the stages 1 to 3 (described below) with the last valid odor test score as the outcome. Each stage was contrasted with the no LB reference group. All models controlled for age, gender and education and the time from olfactory testing to death. Sensitivity analyses were done to control for AD pathology, substantia nigra neuronal loss, dementia at the time of the last valid olfactory score as well as other potential confounders including smoking, head injuries and vascular risk factors such as hypertension and diabetes. All analyses were performed using SAS software, version 9.3 of the SAS system for Linux (SAS Institute, Cary, NC). Model assumptions were validated with graphic and analytic techniques to check for possible nonlinearity and interactions. A threshold of p< 0.01 was used for statistical significance since multiple models were used to test the hypothesis.
Results
Lewy Body Pathology
Lewy body pathology was in the form of α-synuclein positive spherical, neuronal cytoplasmic inclusions and neurite positivity in the affected regions (Fig. 1). Single LBs were smaller in cortical neurons, particularly those in Hesch’s gyrus (Fig. 1c) as compared to those in brainstem nuclei (Fig. 1 d-f). However, brainstem nuclei occasionally showed multiple small cytoplasmic LBs (Fig. 1e). Of the 280 participants, 101 (36.1%) had LBs which were most frequent in the olfactory bulbs and tracts (85.1%) and least (21.8%) in Heschl’s cortex (Table 2). On assigning the 101 LB positive cases to the Braak PD stages, most cases (n=42) were Braak PD 6, followed by Braak PD 4 (n=34) and then Braak PD 1 (n=14). Since the Braak PD stages 2, 3 and 5 had fewer cases their numbers being 2, 4 and 5, respectively, the six Braak PD stages were contracted to three as follows: Stage 1 (olfactory and brainstem) corresponded to Braak PD stage 1 and included LBs in the olfactory bulb and tract and those in the dorsal IX/XMNuc (Table 2). Stage 2 (limbic and brainstem) included LBs in Braak PD stages 2 to 4 while Stage 3 (neocortical) included LBs in Braak PD stages 5 and 6. These revised stages were used in statistical analyses. The frequency of cases in stages 1 to 3 was 13.9, 39.6 and 46.5 %, respectively. In all 3 stages, frequency of LBs was highest in the olfactory bulb and tract (Table 2) while amygdala was the next most common site of LBs. The frequency of LBs in all regions was maximal in the stage 3 cases.
Table 2.
Frequency of Lewy bodies by region and stage in 280 subjects
| Brain and brainstem regions | Regional Frequency of LBs |
Revised LB Stages | |||
|---|---|---|---|---|---|
| No LBs (n=179) |
LBs n,
% (n=101) |
Stage I Olfactory and IX/XMNuc (n=14), n, % |
Stage 2 Limbic and Brainstem (n=40), n, % |
Stage
3 Neocortical (n=47) n, % |
|
| Olfactory bulb and tract | 0 | 86 (85.1) | 10 (71.4) | 33 (82.5) | 43 (91.5) |
| Dorsal motor nucleus of vagus | 0 | 53 (52.5) | 7 (50.0) | 9 (22.5) | 37 (78.7) |
| Nucleus gigantocellularis | 0 | 44 (43.6) | 10 (25.0) | 34 (72.3) | |
| Locus ceruleus | 0 | 38 (37.6) | 9 (22.5) | 29 (61.7) | |
| Pedunculopontine nucleus | 0 | 36 (35.6) | 5 (12.5) | 31 (66.0) | |
| Substantia nigra | 0 | 59 (58.4) | 18 (45.0) | 41 (87.2) | |
| Amygdala | 0 | 74 (73.3) | 30 (75.0) | 44 (93.6) | |
| Entorhinal cortex | 0 | 71 (70.3) | 27 (67.5) | 44 (93.6) | |
| Midtemporal cortex | 0 | 42 (41.6) | 42 (89.4) | ||
| Midfrontal cortex | 0 | 29 (28.7) | 29 (61.7) | ||
| Inferior parietal cortex | 0 | 29 (28.7) | 29 (61.7) | ||
| Motor cortex | 0 | 32 (31.7) | 32 (68.1) | ||
| Heschl’s cortex | 0 | 22 (21.8) | 22 (46.8) | ||
Clinical Pathologic Findings
The scores obtained using the 19 cognitive performance tests in those with and without LBs are shown in Table 1. There were no statistical differences in the age, sex and education of participants with and without LBs (Table 3). At the time of the last valid BSIT, frequency of no cognitive impairment and mild cognitive impairment decreased while the frequency of dementia was greater in those with LBs as compared to those without LBs. The Pearson correlation coefficients showed a moderate correlation between the last valid BSIT score and the MMSE (r = 0.35, p<0.001) and global cognition scores (r = 0.41, p<0.001). The MMSE and global cognition scores at the time of the last valid BSIT were lower in cases with LBs (p=0.029 and 0.017, respectively) compared to those without LBs. There was no difference in the global parkinsonian summary score at the time of the last valid BSIT in participants without and with LBs (p=0.748). The frequency of nigral neuronal loss was higher in those with LBs particularly stage 2 and 3 cases compared to those without LBs. The frequency of a pathologic diagnosis of AD was similar in those without and with LBs.
Table 3.
Demographic, clinical and pathologic characteristics of 280 participants by LB stage
| Characteristics | LB Pathology | p-value | |||
|---|---|---|---|---|---|
| Not
present n=179 |
Stage 1 n=14 |
Stage 2 n= 40 |
Stage 3 n=47 |
||
| Age at death, y, mean (SD) | 91.3 (6.2) | 89.5 (6.4) | 90.0 (6.2) | 90.8 (6.0) | 0.494 † |
| Female, n (%) | 139 (77.7) | 10 (71.4) | 30 (75.0) | 33 (70.2) | 0.731 |
| Education, mean (SD) | 14.5 (3.0) | 15.2 (2.4) | 14.3 (3.2) | 14.9 (2.9) | 0.647 † |
| Odor test score*, mean (SD) | 8.0 (2.4) | 6.8 (2.9) | 7.3 (2.7) | 4.9 (2.8) | <0.001 |
| No cognitive impairment**, n (%) | 75 (41.9) | 4 (28.6) | 13 (32.5) | 11 (23.4) | 0.032 |
| Mild cognitive impairment**, n (%) | 48 (26.8) | 2 (14.3) | 10 (25.0) | 9 (19.2) | |
| Dementia*, n (%) | 56 (31.3) | 8 (57.1) | 17 (42.5) | 27 (57.5) | |
| MMSE score*, mean (SD) | 22.2 (8.5) | 20.8 (6.9) | 18.8 (11.3) | 18.2 (10.2) | 0.029 † |
| Global cognition score*, mean (SD) | −0.56 (0.9) | −1.0 (0.9) | −0.91 (1.3) | −1.06 (1.2) | 0.017 † |
| Global parkinsonism summary score*, mean (SD) | 14.1 (9.4) | 13.5 (8.5) | 12.3 (8.5) | 15.1 (11.3) | 0.748 † |
| Substantia nigra neuronal loss (mild-severe), n (%) | 56 (31.2) | 3 (21.4) | 18 (45.0) | 38 (80.9) | <0.001 |
| Alzheimer’s Disease (NIA-Reagan), n (%) | 132 (73.7) | 7 (50.0) | 31 (77.5) | 34 (72.3) | 0.240 |
Last valid odor test score
These values were obtained at the time of the last valid odor test.
p-value derived from ANOVA† or chi-square.
LBs and Olfactory Function
The mean odor test score at a mean of 1.19 (SD 1.6) years prior to death, in those with LBs was 6.3 (SD 1.3) and 8.0 (SD 2.4) in those without LBs (p<0.001). In a linear regression model that controlled for demographics, and time from olfactory testing to death, the presence of LBs was associated with a lower level of odor identification (Table 4, Model A). This association persisted when a term for AD pathology was added to the model (Table 4, Model B) and when terms for both AD pathology and dementia were added to the model (Table 4, Model C).
Table 4.
Relation of pathological measures and dementia to odor identification in all regions studied in 280 participants
| Model terms | Estimate, SE, P value | ||
|---|---|---|---|
| Model A | Model B | Model C | |
| LB’s (all regions) | −2.85,
0.52 <0.001 |
−2.87,
0.51 <0.001 |
−2.56,
0.50 <0.001 |
| AD pathology | −0.94,
0.26 <0.001 |
−0.53, 0.26 0.044 |
|
| Dementia at the time of the last valid odor test | −1.47, 0.33 <0.001 |
||
Odor identification scores estimated from separate linear regressions, all adjusted for age at death, sex, and education. Cell entries are estimate, standard error (SE), and probability value.
The mean last valid odor test score in the 3 LB stages was 6.8 (SD 2.9) in the stage 1 group, 7.3 (SD 2.7) in the stage 2 group and 4.9 (SD 2.8) in the stage 3 group (Table 3). To determine whether the association of LBs with olfaction differed between stages, analyses were repeated with separate indicators for the 3 LB stages (Table 5). In a linear regression model which controlled for demographics, stage 3 cases had a significantly lower level of odor identification (Table 5, Model A). On addition of a term to control for AD pathology (Table 5, Model B) and terms for both AD pathology and nigral neuronal loss (Table 5, Model C), LBs in stage 3 cases remained associated with a lower level of odor identification. Cognitive impairment is known to be associated with LBs [42, 43] and olfactory identification [44]. Therefore, analyses were repeated to control for the presence of dementia at the time of the last valid olfactory score. In this model, stage 3 cases remained associated with a lower level of odor identification (Table 5, Model D). Additional linear regression models showed an association between olfactory dysfunction as determined by the odor identification test and global cognition and the domains of episodic, semantic and working memory, perceptual speed and visuospatial abilities (p<0.001).
Table 5.
Relation of pathological measures and dementia to odor identification in the three Lewy Body stages
| Model terms | Estimate, SE, P value | |||
|---|---|---|---|---|
| Model A | Model B | Model C | Model D | |
| Stage 1* Olfactory and IX/XMNuc |
−1.37, 0.70 0.052 |
−1.37, 0.69 0.048 |
−1.40, 0.69 0.044 |
−1.05, 0.68 0.122 |
| Stage 2* Limbic and brainstem |
−0.96, 0.46 0.037 |
−0.81, 0.46 0.077 |
−0.76, 0.46 0.098 |
−0.62, 0.45 0.164 |
| Stage 3* Neocortical |
−2.94, 0.44 <0.001 |
−2.9,
0.43 <0.001 |
−2.61,
0.48 <0.001 |
−2.43,
0.47 <0.001 |
| AD pathology | −0.83, 0.25 <0.001 |
−0.84, 0.25 <0.001 |
−0.50, 0.26 0.059 |
|
| Substantia nigra neuronal loss | −0.28, 0.26 0.277 |
−0.16, 0.26 0.537 |
||
| Dementia at the time of the last valid odor test | −1.29, 0.34 <0.001 |
|||
Odor identification scores estimated from separate linear regressions, all adjusted for age at death, sex, and education. Cell entries are estimate, standard error (SE), and probability value. IX/XMNuc= motor nucleus of vagus.
represents contrasts with Stage 0 (no LBs)
No association was observed between the last valid BSIT score and years of education (p=0.712), smoking (p=0.426), head injury (p=0.394) or vascular risk factors (p=0.295) such as hypertension or diabetes in cases in any of the LB stages.
Discussion
This clinical-pathologic study of community dwelling older decedents without a clinical diagnosis of PD focuses on the association of odor identification, a marker of olfactory function, with LBs in 13 brain regions and LBs in 3 defined stages. LBs were detected early in the olfactory bulbs (stage 1), and the presence of LBs in all the regions studied was associated with impaired olfaction. However, on stratification of LBs into 3 stages, and adjustment for pathologic AD, dementia and nigral neuronal loss, only the cases with additional LBs in neocortical areas (stage 3) were independently associated with impaired olfaction.
In this study, 13 brain regions were sampled to assign cases to the Braak PD LB stages. Although initial pathological studies [17, 45] supported the Braak PD LB staging system, subsequent studies [46-49] reported that up to 50 % of cases were unclassifiable using this staging scheme. Progression of LBs in the Braak PD LB staging scheme is partially supported by an experimental study [50] in which preformed α-synuclein fibrils were injected into one olfactory bulb of wild-type mice and their propagation through the brain was documented. At month 1, α-synuclein pathology was found in the ipsilateral piriform and entorhinal cortices, the cortical amygdaloid nucleus and ipsilateral and contralateral anterior olfactory nuclei; at month 3, it was detected in the hippocampus, basal amygdaloid nucleus, the insular, entorhinal, and orbitofrontal cortices; at month 6 there was further extension to the thalamus and hypothalamus while at month 12, there was extension to the associative neocortex and occasionally brainstem nuclei. In this study, since the Braak PD LB stages 2, 3 and 5 had only few cases, the Braak PD stages were contracted to 3 LB stages. Stage 1 corresponded to the original Braak PD stage 1 with LBs in the olfactory bulbs and dorsal IX/XMNuc. In stage 2 there was further extension of LBs to limbic and brainstem sites while in stage 3 there was further LB positivity in neocortical areas.
The widespread sampling for LBs resulted in a high frequency of LBs, which in this study was 36.1% as compared to a frequency of 12.9 % [32] and 18% [42] in our previous studies of community dwelling elders in whom LB assessment was limited to 6 regions. Frequency of LBs was highest in the olfactory bulb in all stages and the finding that 71% of stage 1 cases show olfactory bulb LBs supports the hypothesis that LB formation occurs early in this region. The latter finding is supported by other pathological studies which report early involvement of the olfactory bulbs in PD, Dementia with Lewy bodies [16, 20, 51] and incidental LB disease [16, 20, 28]. The early appearance of LBs in the olfactory bulbs is the basis of the hypothesis that environmental insults might contribute to the initiation of protein aggregation in the olfactory bulb, which then triggers the spread of LB pathology by a templating mechanism in a prion-like manner as demonstrated in experimental animals [52]. Given the early appearance of LB pathology in the olfactory bulbs, if in the future a test becomes available to diagnose LBs in the olfactory bulb during life, one would still be unable to predict the extent of LB disease in the brain.
The olfactory system is complex with widespread connections with ipsilateral and contralateral brain structures [53-55]. The first order neurons (olfactory epithelium) in the nasal cavity connect the olfactory bulbs (second order neurons) to the ipsilateral primary olfactory cortex (piriform cortex, anterior cortical nucleus of the amygdala, the periamygdaloid cortex and the anterior entorhinal cortex). The primary olfactory cortex receives input from the brainstem and midbrain nuclei and sends projections to the secondary olfactory region which encompasses the hippocampus, hypothalamus, thalamus and orbitofrontal cortex. In this study, the high frequency of LBs in stage 3 cases was not only present in the olfactory bulbs but in components of the primary olfactory system such as the entorhinal cortex, amygdala, and brainstem nuclei.
In the present study, linear regression analyses showed an association between LBs in all regions and olfactory dysfunction, supporting our previous study [32] in which this association was demonstrated using only 26 cases with LBs. The present study differs from our previous study [32] in several respects. The mean age of participants is about 5 years older, the mean interval between olfactory testing and death is approximately 2 years less than in the previous study. In addition, the modified Braak PD LB staging system used in this study is a cumulative staging system which differs from that used previously in which LBs were grouped into 3 mutually exclusive categories of nigral, limbic and neocortical. In the present study, after controlling for pathologic AD, nigral neuronal loss and dementia, impaired olfaction was only associated with stage 3 cases in whom LBs extended to neocortical areas thus providing the strongest evidence to date that significant olfactory dysfunction is mainly confined to the neocortical stage of LB disease.
In this study, there was positive correlation between the last valid BSIT scores and the global cognition and MMSE scores as observed previously [42, 43]. Further, linear regression analyses showed an association between olfactory dysfunction and global cognition and the domains of episodic, semantic and working memory, perceptual speed and visuospatial abilities. Olfactory dysfunction detected by BSIT requires proficiency in executive functioning and semantic memory for odor discrimination and identification performance [54, 56]. Since this test employs brain functions that are controlled by regions beyond the olfactory bulbs, it is not surprising that olfactory dysfunction as detected by the BSIT test is only significant when LB pathology extends beyond the olfactory bulbs to involve the neocortex (stage 3). This also implies that presence of LB pathology in the olfactory bulbs alone is not sufficient for olfactory dysfunction.
Several risk factors have been associated with olfactory dysfunction [57]. In this study, there was lack of association between the last valid BSIT score and smoking or head injury. Studies on the use of tobacco products on olfactory function are not conclusive showing an association [4] or no effect [58]. Olfactory disorders have been reported in 5-15 % of cases with head injury [59, 60]. The former study reported that damage to the frontal lobes and olfactory bulbs as shown in the brain magnetic resonance images and hypoperfusion in the frontal, left parietal, and left temporal lobes in the semiquantitative single photon emission computed tomography images corresponded to post-traumatic anosmia. In our studies, the history of head injury with loss of consciousness was self-reported. Possibly, the head injury was not severe enough to cause olfactory bulb damage therefore no association was observed between impaired olfaction and head injury.
The main effect of LBs whether toxic or protective to cells harboring them remains uncertain. Under pathological conditions, α-synuclein becomes misfolded and aggregates into soluble oligomeric species and later forms fibrils [61], which are the main component of LBs. Both α-synuclein oligomers and fibrils have been shown to be toxic in vitro and in vivo [62, 63]. Furthermore, preformed fibrils of α-synuclein can induce the formation of intracellular inclusions in vitro [64] and in vivo [65], the latter leading to neurodegeneration with neurologic dysfunction in experimental animals [65]. On the other hand, LBs may be protective since they are produced by active processes and in specific structures such as the insoluble prOtein deposits or aggresomes, which under physiological conditions, isolate harmful species from the cytoplasm [66]. Detailed evidence relating to the toxic and/or protective effects of LBs is beyond the scope of this article and readers are referred to reviews in the literature [61, 65-67].
Strengths of this study include the large numbers of community dwelling, elderly men and women who were not demented when they enrolled in this study. There was a high rate of clinical follow-up and odor identification was assessed with a standard test. There was a high autopsy rate and uniform post mortem examination with blinding to clinical data thus minimizing important sources of error. Because impaired odor identification is well documented in AD [12, 13, 68], the linear regression models used in this study controlled for AD pathology.
There are several limitations in this study. The findings are based on a selected group of older decedents who differ in education from the general population. Clinical assessments were not performed by a Movement Disorders specialist therefore the clinical diagnosis of PD may be an underestimate. Since only one section per region was analysed for presence of LBs, cases with mild LB pathology may have been missed, therefore, the percentage of LB positive cases may be an underestimate. Since the number of stage 1 cases is small, the lack of association with impaired olfaction will have to be confirmed by a larger number of cases.
Conclusion
Although LB pathology was detected in olfactory bulbs in the early stage of LB progression (stage 1), the strongest association of LBs with olfactory dysfunction was observed in the late pathological stage (stage 3) when LBs extended to neocortical areas.
Acknowledgments
We thank all the participants of the Rush Memory and Aging Project and the staff of Rush Alzheimer’s Disease Center including Er-Yun Chen and Alysha Hodges.
Funding
This work was supported by National Institutes of Health, National Institute on Aging (R01AG017917, R01NS078009, R01AG047976) and the Illinois Department of Public Health. The funding organizations had no role in the design or conduct of the study, collection, management, analysis or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Availability of data and materials
All data associated with this work is available upon request from the RADC Research Resource Sharing Hub (https:/www.radc.rush.edu).
Conflict of interest Authors declare that they have no competing financial interests.
Ethics approval and consent to participate
Autopsied participants were from a longitudinal clinical-pathologic study of aging and dementia, the Rush Memory and Aging Project. A signed, informed consent was obtained from each participant for an annual clinical evaluation and for brain donation. This study was approved by the Institutional Review Board of Rush University Medical Center and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
References
- 1.Boyce JM, Shone GR (2006) Effects of ageing on smell and taste. Postgrad Med J 82:239–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landis BN, Konnerth CG, Hummel T (2004) A study on the frequency of olfactory dysfunction. Laryngoscope 114:1764–1769 [DOI] [PubMed] [Google Scholar]
- 3.Rouby C, Thomas-Danguin T, Vigouroux M, Ciuperca G, Jiang T, Alexanian J, Barges M, Gallice I, Degraix JL, Sicard G (2011) The lyon clinical olfactory test: validation and measurement of hyposmia and anosmia in healthy and diseased populations. Int J Otolaryngol 2011:203805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM (2002) Prevalence of olfactory impairment in older adults. JAMA 288:2307–2312 [DOI] [PubMed] [Google Scholar]
- 5.Attems J, Walker L, Jellinger KA (2014) Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol 127:459–475 [DOI] [PubMed] [Google Scholar]
- 6.Doty RL (2012) Olfactory dysfunction in Parkinson disease. Nat Rev Neurol 8:329–339 [DOI] [PubMed] [Google Scholar]
- 7.Driver-Dunckley E, Adler CH, Hentz JG, Dugger BN, Shill HA, Caviness JN, Sabbagh MN, Beach TG (2014) Olfactory dysfunction in incidental Lewy body disease and Parkinson’s disease. Parkinsonism Relat Disord 20:1260–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkes CH, Shephard BC, Daniel SE (1997) Olfactory dysfunction in Parkinson’s disease. J Neurol Neurosurg Psychiatry 62:436–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mesholam RI, Moberg PJ, Mahr RN, Doty RL (1998) Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol 55:84–90 [DOI] [PubMed] [Google Scholar]
- 10.Doty RL, Reyes PF, Gregor T (1987) Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res Bull 18:597–600 [DOI] [PubMed] [Google Scholar]
- 11.Koss E, Weiffenbach JM, Haxby JV, Friedland RP (1988) Olfactory detection and identification performance are dissociated in early Alzheimer’s disease. Neurology 38:1228–1232 [DOI] [PubMed] [Google Scholar]
- 12.Kovacs T, Cairns NJ, Lantos PL (2001) Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. Neuroreport 12:285–288 [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA (2007) The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry 78:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doty RL, Deems DA, Stellar S (1988) Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 38:1237–1244 [DOI] [PubMed] [Google Scholar]
- 15.Hawkes C (2006) Olfaction in neurodegenerative disorder. Adv Otorhinolaryngol 63:133–151 [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Del TK, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211 [DOI] [PubMed] [Google Scholar]
- 17.Jellinger KA (2003) Alpha-synuclein pathology in Parkinson’s and Alzheimer’s disease brain: incidence and topographic distribution--a pilot study. Acta Neuropathol 106:191–201 [DOI] [PubMed] [Google Scholar]
- 18.Kranick SM, Duda JE (2008) Olfactory dysfunction in Parkinson’s disease. Neurosignals 16:35–40 [DOI] [PubMed] [Google Scholar]
- 19.Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW (2003) Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol Appl Neurobiol 29:503–510 [DOI] [PubMed] [Google Scholar]
- 20.Beach TG, White CL III, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J, Akiyama H, Adler CH (2009) Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol 117:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL III, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117:613–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloch A, Probst A, Bissig H, Adams H, Tolnay M (2006) Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 32:284–295 [DOI] [PubMed] [Google Scholar]
- 23.Buchman AS, Nag S, Leurgans SE, Miller J, VanderHorst VGJM, Bennett DA, Schneider JA (2018) Spinal Lewy body pathology in older adults without an antemortem diagnosis of Parkinson’s disease. Brain Pathol 28:560–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sengoku R, Saito Y, Ikemura M, Hatsuta H, Sakiyama Y, Kanemaru K, Arai T, Sawabe M, Tanaka N, Mochizuki H, Inoue K, Murayama S (2008) Incidence and extent of Lewy body-related alpha-synucleinopathy in aging human olfactory bulb. J Neuropathol Exp Neurol 67:1072–1083 [DOI] [PubMed] [Google Scholar]
- 25.Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE (2008) Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol 115:437–444 [DOI] [PubMed] [Google Scholar]
- 26.Jellinger KA (2012) Neuropathology of sporadic Parkinson’s disease: evaluation and changes of concepts. Mov Disord 27:8–30 [DOI] [PubMed] [Google Scholar]
- 27.Silveira-Moriyama L, Holton J, Kingsbury A, Ayling H, Petrie A, Sterlacci W, Poewe W, Maier H, Lees AJ, Revesz T (2007) The primary olfactory cortex in idiopathic Parkinson’s disease (IPD) and incidental Lewy body disease (ILBD). Mov Disord 22:S 91 [Google Scholar]
- 28.Hubbard PS, Esiri MM, Reading M, McShane R, Nagy Z (2007) Alpha-synuclein pathology in the olfactory pathways of dementia patients. J Anat 211:117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del ST, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872 [DOI] [PubMed] [Google Scholar]
- 30.Outeiro TF, Koss DJ, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D, Donaghy P, Morris C, Taylor JP, Thomas A, Attems J, McKeith I (2019) Dementia with Lewy bodies: an update and outlook. Mol Neurodegener 14:5 doi: 10.1186/s13024-019-0306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doty RL, Shaman P, Dann M (1984) Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav 32:489–502 [DOI] [PubMed] [Google Scholar]
- 32.Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA (2011) Lewy bodies and olfactory dysfunction in old age. Chem Senses 36:367–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA (2018) Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 64:S161–S189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doty RL, Frye RE, Agrawal U (1989) Internal consistency reliability of the fractionated and whole University of Pennsylvania Smell Identification Test. Percept Psychophys 45:381–384 [DOI] [PubMed] [Google Scholar]
- 35.Doty RL, Marcus A, Lee WW (1996) Development of the 12-item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope 106:353–356 [DOI] [PubMed] [Google Scholar]
- 36.Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, Schneider JA (2015) Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 77:942–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA (2006) Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology 67:441–445 [DOI] [PubMed] [Google Scholar]
- 38.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944 [DOI] [PubMed] [Google Scholar]
- 39.Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS (1997) Metric properties of nurses’ ratings of parkinsonian signs with a modified Unified Parkinson’s Disease Rating Scale. Neurology 49:1580–1587 [DOI] [PubMed] [Google Scholar]
- 40.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA (2004) Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 62:1148–1155 [DOI] [PubMed] [Google Scholar]
- 41.Buchman AS, Shulman JM, Nag S, Leurgans SE, Arnold SE, Morris MC, Schneider JA, Bennett DA (2012) Nigral pathology and parkinsonian signs in elders without Parkinson disease. Ann Neurol 71:258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA (2012) Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain 135:3005–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA (2010) Neurodegenerative basis of age-related cognitive decline. Neurology 75:1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson RS, Arnold SE, Tang Y, Bennett DA (2006) Odor identification and decline in different cognitive domains in old age. Neuroepidemiology 26:61–67 [DOI] [PubMed] [Google Scholar]
- 45.Jellinger KA (2004) Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm (Vienna ) 111:1219–1235 [DOI] [PubMed] [Google Scholar]
- 46.Attems J, Jellinger KA (2008) The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease. Neuropathol Appl Neurobiol 34:466–467 [DOI] [PubMed] [Google Scholar]
- 47.Jellinger KA (2008) A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol 116:1–16 [DOI] [PubMed] [Google Scholar]
- 48.Parkkinen L, Pirttila T, Alafuzoff I (2008) Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 115:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG (2008) Patterns and stages of alpha-synucleinopathy: Relevance in a population-based cohort. Neurology 70:1042–1048 [DOI] [PubMed] [Google Scholar]
- 50.Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VM, Brundin P (2016) Widespread transneuronal propagation of alpha-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med 213:1759–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Braak H, Del TK (2017) Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J Parkinsons Dis 7:S71–S85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rey NL, Wesson DW, Brundin P (2018) The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis 109:226–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cersosimo MG (2018) Propagation of alpha-synuclein pathology from the olfactory bulb: possible role in the pathogenesis of dementia with Lewy bodies. Cell Tissue Res 373:233–243 [DOI] [PubMed] [Google Scholar]
- 54.Doty RL (2012) Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis 46:527–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel RM, Pinto JM (2014) Olfaction: anatomy, physiology, and disease. Clin Anat 27:54–60 [DOI] [PubMed] [Google Scholar]
- 56.Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T (2010) Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol 32:1062–1067 [DOI] [PubMed] [Google Scholar]
- 57.Yang J, Pinto JM (2016) The Epidemiology of Olfactory Disorders. Curr Otorhinolaryngol Rep 4:130–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinto JM, Wroblewski KE, Kern DW, Schumm LP, McClintock MK (2015) The Rate of Age-Related Olfactory Decline Among the General Population of Older U.S. Adults. J Gerontol A Biol Sci Med Sci 70:1435–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atighechi S, Salari H, Baradarantar MH, Jafari R, Karimi G, Mirjali M (2009) A comparative study of brain perfusion single-photon emission computed tomography and magnetic resonance imaging in patients with post-traumatic anosmia. Am J Rhinol Allergy 23:409–412 [DOI] [PubMed] [Google Scholar]
- 60.Collet S, Grulois V, Bertrand B, Rombaux P (2009) Post-traumatic olfactory dysfunction: a cohort study and update. B-ENT 5 Suppl 13:97–107 [PubMed] [Google Scholar]
- 61.Pieri L, Madiona K, Melki R (2016) Structural and functional properties of prefibrillar alpha-synuclein oligomers. Sci Rep 6:24526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bengoa-Vergniory N, Roberts RF, Wade-Martins R, Alegre-Abarrategui J (2017) Alpha-synuclein oligomers: a new hope. Acta Neuropathol 134:819–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pieri L, Madiona K, Bousset L, Melki R (2012) Fibrillar alpha-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys J 102:2894–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM (2009) Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci U S A 106:20051–20056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steiner JA, Quansah E, Brundin P (2018) The concept of alpha-synuclein as a prion-like protein: ten years after. Cell Tissue Res 373:161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chartier S, Duyckaerts C (2018) Is Lewy pathology in the human nervous system chiefly an indicator of neuronal protection or of toxicity? Cell Tissue Res 373:149–160 [DOI] [PubMed] [Google Scholar]
- 67.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM (2012) Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med 209:975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Attems J, Lintner F, Jellinger KA (2005) Olfactory involvement in aging and Alzheimer’s disease: an autopsy study. J Alzheimers Dis 7:149–157 [DOI] [PubMed] [Google Scholar]