Abstract
Gel-based injectable biomaterials have significant potential for treating vocal fold defects such as scarring. An ideal injectable for vocal fold lamina propria restoration should mimic the microenvironment of the lamina propria to induce scarless wound healing and functional tissue regeneration. Most current synthetic or natural injectable biomaterials do not possess the same level of complex, tissue-specific constituents as the natural vocal fold lamina propria. In this study we present a newly-developed injectable gel fabricated from decellularized bovine vocal fold lamina propria. Blyscan assay and mass spectrometry indicated that the vocal fold-specific gel contained a large amount of sulfated glycosaminoglycans and over 250 proteins. Gene Ontology overrepresentation analysis revealed that the proteins in the gel dominantly promote antifibrotic biological process. In vivo study using a rabbit vocal fold injury model showed that the injectable gel significantly reduced collagen density and decreased tissue contraction of the lamina propria in vocal folds with chronic scarring. Furthermore, this acellular gel only elicited minimal humoral immune response after injection. Our findings suggested that the tissue-specific, injectable extracellular matrix gel could be a promising biomaterial for treating vocal fold scarring, even after the formation of mature scar.
Keywords: Xenogeneic biomaterial, Laryngeal regenerative medicine, Fibrosis, Proteomics, Gene Ontology
1. Introduction
Vocal fold lamina propria defects such as scarring and sulcus vocalis remain among the foremost therapeutic challenges in the management of patients with voice disorders. The vocal fold lamina propria has proven difficult to restore, regenerate, or remove and replace due to its unique biomechanical properties and vulnerability to scarring [1,2]. Conventional approaches to repair and regenerate damaged vocal fold lamina propria have limitations [3,4]. For example, surgical interventions may cause secondary scarring and yield inconsistent results while voice therapy does not address the fundamental histological changes of the scarred vocal folds [1,2]. Regenerative medicine approaches such as cell-based therapy, development of synthetic or biological materials, and growth factor therapy have attracted significant research interest for the prevention and treatment of vocal fold scarring [1,5]. However, most of these approaches have only been tested as a means to treat acute injuries rather than chronic vocal fold scar [1].
In a chronic vocal fold scarring model, treatment is given after mature scar tissue has already formed, posing a more challenging clinical scenario. It is commonly agreed that the scarred vocal folds reach a mature phase of wound repair or chronic scarring stage 6 months post injury in rabbit and canine models based on the dynamic nature of the wound healing and scarring process [1,6-10]. For the rat model, due to its rapid wound healing process, Tateya and colleagues proposed a 2-month postoperative time frame for the studies of mature vocal fold scars instead [11]. If these mature scar formation time frames were strictly followed, 5 published animal studies were truly about treating chronically scarred vocal folds, to the best of our knowledge. Growth factors were used in 3 reports [12-14], the fourth tested stem cell therapy [15], and more recently Serpinh1-siRNA was shown to reverse scar-associated collagen accumulation [16]. In addition, hepatocyte growth factor [17] and adipose-derived stromal vascular fraction [18] have been shown to have efficacy in early clinical trials. In those reports, biomaterials (e.g., gelatin hydrogel) were only used as a delivery system for bioactive factors or as control [14,15].
The current concept of developing an ideal biomaterial for vocal fold lamina propria restoration is to mimic the microenvironment of the lamina propria to induce scarless wound healing and functional tissue regeneration [1]. Major constituents of vocal fold lamina propria extracellular matrix (ECM) include fibrous proteins (i.e., collagen and elastin), interstitial proteins (e.g., decorin, fibronectin), and glycosaminoglycans [19-21]. Hundreds of proteins in human and animal vocal fold lamina propria ECM have been identified [22,23]. In contrast, most biomaterials being studied for vocal fold regeneration usually comprise just one or two kinds of macromolecules (e.g., collagen, hyaluronic acid derivatives, or fibrin) [1]. The discrepancy in biochemical composition between these biomaterials and the natural vocal fold ECM they try to mimic can limit their therapeutic efficacy. Culturing cells on biomaterials may ameliorate the problem. For example, Ling et al [24] were able to significantly increase the number of proteins in the polymerized type I collagen by co-culturing primary human vocal fold fibroblasts and epithelial cells on the construct. On the other hand, they also reported that engineered mucosa did not show lamina propria fiber complexity equivalent to that of native mucosa.
The most biomimetic materials are acellular ECM manufactured from allogenic or xenogeneic tissues. These extracellular matrix-derived biomaterials have been used in tissue engineering and regenerative medicine of many tissue types including heart valves, skin, nerves, blood vessels, tendon, and ligament [25,26]. In previous work, we successfully developed a biodegradable ECM scaffold from bovine vocal fold lamina propria using a novel saline-based, detergent-free decellularization approach [27]. In vivo and in vitro studies also indicated it was biocompatible, facilitated cell infiltration, and was able to deliver growth factor in vivo [28-30]. In the present study, we fabricated the acellular ECM scaffold into an injectable gel form to minimize possible injury to vocal fold caused by surgical implantation and to conform to any three-dimensional shape. The resulting vocal fold ECM gel or VF-ECM gel was characterized in terms of proteome and sulfated glycosaminoglycans (sGAG) content. In a rabbit model of chronic vocal fold scarring, the VF-ECM gel was injected into one vocal fold with the contralateral vocal fold serving as sham control. The thickness of the regenerated vocal fold lamina propria and levels of key lamina propria constituents, including collagen, elastin, hyaluronic acid and sGAG, were examined. Vocal fold vibratory amplitude was assessed by high speed imaging and videokymography, and mucosal wave was graded. Finally, the host humoral response to the VF-ECM gel was measured and compared to that of immunized rabbits.
2. Materials and methods
2.1. Preparation of acellular bovine vocal fold lamina propria ECM gel
The choice of donor source was based on the high homology between bovine and human type I collagen [27], the ready availability of large numbers of fresh bovine larynges at a low cost, and the size of their vocal folds. Their ample supply made it feasible to generate sufficient quantities of bioengineered biomaterial for this study.
Bovine larynges from healthy, about 30-month-old steers and heifers were procured from a local abattoir. The larynges were excised immediately postmortem. Vocal fold specimens consisted of the epithelium and the lamina propria were dissected from the larynges using microsurgical instruments. All vocal folds included in this study were examined by a laryngologist and appeared normal. Decellularization was performed using a previously described saline-based osmotic approach [27-29] which is able to completely remove native cell nuclei [27,31] and more than 99% of double-stranded DNA [27]. In brief, the vocal fold covers were mounted on plastic frames to mimic in situ tissue tension followed by sequential incubation in 3 M sodium chloride solution, isotonic phosphate buffered saline (PBS) solution with DNAse and RNAse, and 75% ethanol. After decellularization, samples were freeze-dried in a Labconco FreeZone 4.5 Plus Liter Benchtop Freeze Dry System (Kansas City, MO) overnight. For in vivo studies, freeze-dried acellular lamina propria ECM were sterilized using ethylene oxide (750 mg/h for 16 h).
Numerous methods have been reported to transform decellularized biological tissues into hydrogels [32]. In this study, pepsin was used to mediate solubilization of the acellular vocal fold ECM because it is able to cleave the terminal telopeptide of the collagen, resulting in significantly reduced antigenicity and increased solubility [33]. In brief, 5 mg pulverized ECM was submerged into 0.5 ml 0.04 N HCl solution with 0.1% pepsin (Sigma–Aldrich, St Louis, MO) and kept under mechanical agitation for ~ 24 h. Once the acellular ECM was solubilized, NaOH was added to bring the pH of the final ECM gel to 7. The resultant VF-ECM gel is shown in Fig. 1A.
Fig. 1.
(A) VF-ECM gel derived from decellularized bovine vocal fold lamina propria. (B) VF-ECM gel being injected into the left vocal fold of a rabbit cadaver. Gel was stained with green food dye to improve visualization. (C) A histological coronal section of the rabbit hemilarynx stained with H&E, showing the ECM gel in vocal fold lamina propria immediately after injection. Arrow indicates the gel. This trial run demonstrated the feasibility of precisely delivering the VF-ECM gel into rabbit vocal fold lamina propria. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.2. Sulfated glycosaminoglycan and total protein quantifications
The sulfated glycosaminoglycan content in the VF-ECM gel was measured with the Blyscan assay (Biocolor, Carrickfergus, UK), a quantitative dye-binding method using 1, 9-dimethylmethylene blue, per manufacturer’s instructions. The method is able to detect sulfated polysaccharide component of proteoglycans or the protein-free sulfated glycosaminoglycan chains without interference from small sulfated disaccharide fragments. Six VF-ECM gel samples derived from 6 animals were run in duplicate for the sGAG measurement. Total protein content in each sample was also quantified using a BCA (bicinchoninic acid) protein assay kit (Thermo Fisher Scientific, Waltham, MA). The measured sGAG content was expressed as micrograms of sGAG per milligram of total protein.
2.3. SDS-PAGE and mass spectrometry
A sequential protein extraction methodology described by Mayr and colleagues [34,35] was employed prior to SDS-PAGE and mass spectrometry with slight modifications. In brief, VF-ECM gel samples were mixed with protein extraction buffer (4 M guanidine hydrochloride, 50 mM sodium acetate, 5 mM ethylenediaminetetraacetic acid or EDTA, and protease inhibitor cocktail, pH = 5.8) and kept under mechanical agitation for 24 hr. Subsequently, the extracts were transferred to cold ethanol and kept at −80 °C for 24 hr. Proteins were then precipitated by centrifugation at 24,000×g for 30 min at 4 °C. After removing the ethanol, the pellets were redissolved in deglycosylation buffer (pH = 6.8) which consisted of 150 mM NaCl, 50 mM sodium acetate, 10 mM EDTA, and protease inhibitor cocktail. Deglycosylation was achieved by adding chondroitinase ABC and endo-b-galactosidase (Sigma–Aldrich) to samples followed by incubation at 37 °C for overnight under mechanical agitation. Following deglycosylation, the solutions were clarified with centrifugation (14,000×g for 10 min at 4 °C) to ensure that the samples were free of turbidity. For SDS-PAGE, deglycosylated samples were first mixed with Laemmli reducing sample buffer and heat denatured at 96 °C for 5 min, followed by running into 4–15% precast polyacrylamide gel (Bio-rad, Hercules, CA) along with pre-stained protein marker (New England Biolabs, Ipswich, MA). After the proteins were resolved by SDS-PAGE, the gel was fixed then stained with Coomassie blue G-250 dye (Thermo Fisher Scientific) (Fig. 2). The segments of gel containing proteins or protein fragments with mass larger than 7 KDa were excised for protein identification using mass spectrometry.
Fig. 2.

SDS-PAGE separation of the VF-ECM gel protein extract in a 4–15% gradient polyacrylamide gel, stained with Coomassie blue. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To prepare for mass spectrometry, diced polyacrylamide gels were digested overnight with mass spectrometry grade trypsin (Thermo Fisher Scientific) following reduction and alkylation with DL-dithiothreitol and iodoacetamide (Sigma-Aldrich), respectively. The samples then underwent solid-phase extraction cleanup with Oasis HLB plates (Waters, Milford, MA) and the resulting samples were analyzed by LC/MS/MS, using an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific) coupled to an Ultimate 3000 RSLC-Nano liquid chromatography systems (Dionex, Sunnyvale, CA). Samples were injected onto a 75 μm i.d., 75-cm long EasySpray column (Thermo Fisher Scientific), and eluted with a gradient from 0 to 28% buffer B over 90 min. Buffer A contained 2% (v/v) acetonitrile and 0.1% formic acid in water, and buffer B contained 80% (v/v) acetonitrile, 10% (v/v) trifluoroethanol, and 0.1% formic acid in water. The mass spectrometer operated in positive ion mode with a source voltage of 2.4 kV and an ion transfer tube temperature of 275 °C. MS scans were acquired at 120,000 resolution in the Orbitrap and up to 10 MS/MS spectra were obtained in the ion trap for each full spectrum acquired using higher-energy collisional dissociation (HCD) for ions with charges 2–7. Dynamic exclusion was set for 25 s after an ion was selected for fragmentation. Resulting raw MS data files were converted to a peak list format and analyzed using the central proteomics facilities pipeline (CPFP), version 2.0.3 [36,37]. Peptide identification was performed using the X!Tandem [38], and open MS search algorithm (OMSSA) [39] search engines against the bovine protein database from UniProtKB [40]. Fragment and precursor tolerances of 20 ppm and 0.5 Da were specified, and three missed cleavages were allowed. Carbamidomethylation of Cys was set as a fixed modification and oxidation of Met was set as a variable modification. A label-free, normalized spectral index quantitation method was performed by SINQ software [41] to estimate the absolute quantity of proteins in the mixture samples.
Gene Ontology (GO) overrepresentation analysis was performed using the PANTHER overrepresentation testing tool [42] at http://pantherdb.org with GO Ontology database (released 2019-01-01). UniProt accession numbers of the proteins detected in all 6 VF-ECM gel samples were used as input data. Fisher’s exact test with Benjamini-Hochberg False Discovery Rate (FDR) correction was employed to determine whether there was a statistical overrepresentation or underrepresentation of the GO terms in molecular function and biological process relative to the Bos Taurus genome. The resulting significantly overrepresented GO terms (FDR corrected p-value < 0.05) were summarized and reduced-redundancy using SimRel semantic similarity measure in REVIGO [43] with allowed similarity set to 0.5. Finally, interactive graphs of the non-redundant GO terms were generated using REVIGO and Cytoscape 3.7.1 [44].
2.4. Animal surgery
Animal experiment protocols including antibody production were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center, in accordance with the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act. For demonstration of feasibility and technique development, VF-ECM gel was injected into the vocal folds of a rabbit cadaver (Fig. 1B) before the animal study. The gel was precisely delivered to the vocal fold lamina propria as demonstrated by histology (Fig. 1C).
Paired experimental design (i.e., treating one vocal fold in each animal while using the contralateral vocal fold as control) was selected in this study to achieve greater statistical power over two-group study design [45,46]. Five adult female New Zealand white rabbits, each weighing around 2.5 kg, underwent the first surgery to create bilateral vocal fold injuries. The animals were anesthetized with intramuscular administration of ketamine (35 mg/kg) and xylazine (3 mg/kg). Buprenorphine (0.02 mg/kg) was administered after induction of general anesthesia and prior to laryngoscopy. Suspension laryngoscopy was performed using a no. 1 Miller blade with the animal in supine position. Vocal fold injury was conducted under visual guidance of a 2.7-mm, 30° Hopkins telescope (Karl Storz, Tuttlingen, Germany) connected to a CCD camera (Toshiba, Tokyo, Japan) and a video monitor. Vocal fold injury was created as a biopsy of the mid-membranous vocal fold using 1-mm up-angled microcup forceps. The small size of the cup adequately controlled for the depth of injury. Six months post injury, the rabbits underwent a second anesthesia, at which time 0.05 ml VF-ECM gel or saline was injected with a 25 G needle subepithelially into the right or left vocal fold, respectively. The volume of 0.05 ml was chosen because some injectant was likely to leak out of the puncture site [47]. All animals recovered without complications. Three months after the injection, the animals were euthanized and larynges harvested.
2.5. Excised larynx phonation, high speed digital imaging, and videokymography
Immediately following euthanasia, the larynges were harvested and subjected to excised larynx phonation as previously described [48]. In brief, soft tissue superior to the vocal folds were removed to provide an unobstructed view of the vocal folds for high speed digital imaging. The vocal folds were adducted by suturing the arytenoids together. The larynx was then mounted on a custom built excised larynx phonation apparatus and subglottal airflow was slowly increased until audible phonation occurred. Once phonation began, high-speed images were captured at 6000 frames per second using a Phantom V311 high-speed camera (Vision Research, Wayne, NJ) and a Nikkon micro lens (Melville, NY) mounted over the larynx. After the high-speed videos were captured, the larynges were processed for histology.
The videos were inspected to identify the earliest segment of stable vibratory behavior following phonation onset. Videokymographic images were generated by a custom MATLAB program from a transverse line taken from the high-speed images at the location of the greatest amplitude during vocal fold vibration, which was typically at the mid-membranous vocal fold. The videokymographic images were then manually analyzed using NIH ImageJ software (Bethesda, MD) to measure and compare the amplitude of vibration [49] between the gel-injected vocal fold and the saline-injected control. In a separate analysis, two speech pathologist raters with extensive experience in rating laryngeal videostroboscopic exams reviewed the high speed videos and were asked to determine for each larynx if “the right side has obviously more mucosal wave”, “the left side has obviously more mucosal wave”, or “no obvious difference”.
2.6. Histology and quantitative image analysis
Larynges were fixed in 10% neutral buffered formalin followed by decalcification in 0.35 M EDTA. After decalcification, larynges were embedded in paraffin and 5 μm thick coronal sections at the mid-fold location were prepared. In addition to routine Hematoxylin and Eosin (H&E) staining, picrosirius red, Hart’s elastin, and Alcian blue staining were used to identify collagen, elastin, and glycosaminoglycans especially sulfated glycosaminoglycans in the samples, respectively [45]. Considering Alcian blue staining for hyaluronic acid (HA) can be affected by the fixation method [50], HA was examined by immunostaining with biotinylated hyaluronic acid binding protein (Sigma-Aldrich) as the primary antibody and horseradish peroxidase-conjugated streptavidin (Thermo Fisher Scientific) as the secondary antibody following the procedures previously described [28,29]. A Leica DM200 upright compound microscope (Buffalo Grove, IL) equipped with a Jenoptik Gryphax camera (Jena, Thuringia, Germany) was used to capture digital histological images of the slides. Identical microscope and camera settings were used for the slides with the same staining to ensure consistency in image acquisition conditions across samples.
To determine the degree of scar contraction, thickness of the lamina propria (distance between the free edge and muscle layer) of each vocal fold was measured at five different locations approximately equally positioned between the superior and inferior edges of the mid-membranous vocal fold. The relative amounts or densities of the molecules of interest were calculated as the fraction of the positively stained area to the total cross-section area of the vocal fold lamina propria by digital image analysis using ImageJ as previously reported [28,29,45]. Image analysis was carried out in a blinded fashion by two examiners to minimize measurement bias. Histological figures of a normal rabbit vocal fold were used as comparison. Images taken at a lower magnification (25×) of the same sample were published in one of our previous reports [45]. Since comparison of ECM constituent levels is most accurate when the experimental and the control vocal folds are on the same slide and processed identically, and the normal vocal fold was not used as a control in this paired design study, the comparison between normal and treated vocal folds was only descriptive instead of quantitative except for the thickness of lamina propria, which is not affected by staining condition, camera setting, etc.
Finally, to identify possible remnant of the VF-ECM gel in the rabbit vocal folds, slides were incubated in proteinase K (Sigma–Aldrich) epitope retrieval buffer for 15 min at 37 °C, followed by incubation in peroxidase suppressor solution then 5% bovine serum albumin (BSA) blocking buffer with serial washings in between each step. Bovine collagen I in the samples was examined by immunostaining with biotinylated anti-bovine collagen I antibody (Thermo Fisher Scientific) as the primary antibody and horseradish peroxidase-conjugated streptavidin as the secondary antibody as previously described [48].
2.7. VF-ECM gel specific antibody determination
Humoral immune response to the xenogeneic VF-ECM gel was determined by the production of gel-specific antibody. After implantation, a serum sample was collected from each rabbit weekly. Sera collected before injections (denoted as week 0) served as negative control and provided the baseline levels of the VF-ECM gel specific antibodies. Three additional rabbits were immunized by subcutaneous injection with 1 ml acellular ECM mixed with 1 ml complete Freund’s adjuvant (Sigma–Aldrich), followed by a booster injection of the acellular ECM and incomplete Freund’s adjuvant (Sigma–Aldrich) mixture 28 days later. Sera from these three immunized rabbits were pooled and served as positive control. All serum samples were stored at −20 °C until testing.
Modified sandwich ELISA was used to assess the presence of antibodies in sera reacting to VF-ECM gel. Samples were run in triplicate. Briefly, aliquots of 50 μl carbonate-bicarbonate coating buffer containing 175 ng ECM gel protein were inoculated into Immulon 96-well microtiter plates (Thermo Fisher Scientific) and incubated at 4 °C overnight. After a series of washing, the plates were blocked with 1% BSA in PBS. Serum samples were diluted at 1:200 in 1% BSA and aliquots (100 μl) incubated in microtiter wells at 4 °C overnight. Plates were then rinsed three times with 0.05% Tween-20 in PBS, and incubated for 2 h at room temperature with sheep anti-rabbit IgG (H+L) highly cross adsorbed-peroxidase antibody (Sigma-Aldrich). For detection, plates were rinsed and incubated with o-phenylenediamine dihydrochloride (Sigma-Aldrich) for 30 min, followed by measurement at 450 nm in a microwell plate reader (BioTek. Winooski, VT).
2.8. Statistical analysis
Two-tailed paired Student’s t tests were applied to the analyses of vibrational amplitudes from high-speed imaging, relative densities of key ECM constituents, and thickness of lamina propria. The level of significance (alpha) was set at 0.05.
3. Results
3.1. Tissue-specific vocal fold ECM gel characterization
Retained vocal fold lamina propria-specific biochemical cues in the VF-ECM gel including proteins and sulfated glycosaminoglycans were confirmed using SDS-PAGE, mass spectrometry and Blyscan assay. Fig. 2 shows SDS-PAGE separation of the VF-ECM gel protein extract in a 4–15% polyacrylamide gel. The numerous high-molecular weight bands confirmed the complex mixture nature of the acellular ECM gel. Only the part of polyacrylamide gel that contained proteins with mass larger than 7 KDa was used for protein identification by mass spectrometry, to exclude small protein fragments and peptides from proteomic characterization.
A total of 266 proteins were identified in 6 bovine VF-ECM gel samples, each of which was fabricated from one animal. Among them, 75 proteins (Table 1) were found in all six samples. Besides variations among individual donors, the lower similarity of proteins identified across 6 samples were mainly due to the fact that mass spectrometry is not an all-inclusive technique, which means proteins with only trace amount in the mixture might not be identified in each test. In this study, we focus on these 75 major proteins, which made up 94.78 ± 3.29% of the total proteins in the VF-ECM gel. These proteins could be sorted into 5 classes: collagens, proteoglycans and glycoproteins, ECM-associated proteins and secreted factors, non-matrisome proteins, and others (i.e., uncharacterized proteins). Fig. 3 shows the number of proteins in each class as well as their relative abundance compared to total protein based on estimated absolute quantity. The result of relative abundance or intensity showed that core matrisome proteins (collagens, glycoproteins, and proteoglycans) were well preserved following decellularization and solubilization.
Table 1.
List of the 75 proteins identified in all six vocal fold ECM gel samples, each of which was derived from one animal. Proteins are listed in the order of descending intensity in each category.
| Protein name | UniProt accession number | Molecular mass (kDa) |
|---|---|---|
| Collagens | ||
| Collagen alpha-1(I) chain | P02453 | 139 |
| Collagen alpha-2(I) chain | P02465 | 129 |
| Collagen alpha-1(III) chain | P04258 | 94 |
| Collagen alpha-1(III) chain | F1MXS8 | 139 |
| Collagen type VI alpha 2 chain | F1MKG2 | 110 |
| Collagen type VI alpha 3 chain | E1BB91 | 342 |
| Collagen type V alpha 2 chain | F1N2Y2 | 145 |
| Collagen type VI alpha 1 chain | E1BI98 | 109 |
| Collagen type V alpha 1 chain | G3MZI7 | 199 |
| Collagen alpha-1(II) chain | P02459 | 142 |
| Collagen type VII alpha 1 chain | E1B7H2 | 294 |
| Collagen alpha-1(XII) chain | F1N401 | 340 |
| Collagen type V alpha 3 chain | F1MJQ6 | 178 |
| Collagen type XIV alpha 1 chain | E1BA17 | 194 |
| COL8A1 protein | A7E303 | 73 |
| Proteoglycans and glycoproteins | ||
| Fibrillin-1 | P98133 | 312 |
| Lumican | Q05443 | 39 |
| Dermatopontin | P19427 | 24 |
| Fibrillin 2 | F1MTZ4 | 403 |
| Prolargin | F1MX63 | 44 |
| EGF-containing fibulin-like extracellular matrix protein 1 | A2VE41 | 55 |
| Decorin | P21793 | 40 |
| Fibrinogen alpha chain | P02672 | 67 |
| ABI family, member 3 (NESH) binding protein | A1L573 | 39 |
| Periostin | D1Z308 | 93 |
| Mimecan | P19879 | 34 |
| Elastin microfibril interfacer 1 | E1BLS8 | 107 |
| Latent transforming growth factor beta binding protein 4 | E1BA44 | 173 |
| Laminin subunit gamma 1 | F1MD77 | 178 |
| Latent transforming growth factor beta binding protein 1 | F1N444 | 186 |
| Fibulin-5 | Q5EA62 | 50 |
| Fibulin 2 | E1BEB4 | 124 |
| Fibrinogen beta chain | P02676 | 53 |
| Fibrinogen gamma-B chain | F1MGU7 | 50 |
| Latent transforming growth factor beta binding protein 4 | G3MXC3 | 136 |
| Fibronectin | P07589 | 272 |
| Laminin subunit alpha 5 | F1MC13 | 396 |
| Versican core protein | F1N6I5 | 262 |
| Heparan sulfate proteoglycan 2 | F1MER7 | 466 |
| Elastin microfibril interfacer 2 | E1BIP4 | 118 |
| Tenascin XB | F1MPK6 | 439 |
| Laminin subunit beta 1 | F1MNT4 | 199 |
| ECM-associated proteins or secreted factors | ||
| Serum albumin | P02769 | 69 |
| Annexin A2 | P04272 | 39 |
| Annexin A1 | P46193 | 39 |
| Galectin-1 | P11116 | 15 |
| Multiple EGF like domains 6 | E1BGE0 | 164 |
| Non-matrisome proteins | ||
| Histone H2B | E1BGW2 | 14 |
| Keratin, type II cytoskeletal 5 | Q5XQN5 | 63 |
| KRT4 protein | A4IFP2 | 58 |
| KRT15 protein | Q17QL7 | 49 |
| Vimentin | P48616 | 54 |
| Histone H2A.Z | P0C0S4 | 14 |
| Keratin, type I cytoskeletal 14 | F1MC11 | 52 |
| Keratin, type I cytoskeletal 10 | P06394 | 55 |
| Lamin A/C | F1MYG5 | 74 |
| Keratin 1 | G3N0V2 | 63 |
| Desmoplakin | E1BKT9 | 332 |
| Histone H2A | Q17QG8 | 15 |
| Histone H2A type 2-C | A1A4R1 | 14 |
| Myosin heavy chain 11 | F1MYM9 | 228 |
| Myosin heavy chain 9 | F1MQ37 | 227 |
| Core histone macro-H2A | Q2HJ65 | 40 |
| Keratin, type I cytoskeletal 17 | A1L595 | 49 |
| Keratin 24 | F1MFW9 | 51 |
| Actin, alpha skeletal muscle | P68138 | 42 |
| Histone H1.2 | P02253 | 21 |
| Sushi repeat containing protein X-linked | F1MQX1 | 51 |
| Heterogeneous nuclear ribonucleoprotein K | Q3T0D0 | 51 |
| Keratin, type I cytoskeletal 19 | P08728 | 44 |
| Myosin-10 | Q27991 | 229 |
| Other | ||
| Uncharacterized protein | E1BHG5 | 33 |
| Uncharacterized protein | M0QVY0 | 61 |
| Uncharacterized protein | E1BDK6 | 196 |
| Uncharacterized protein | G8JKW7 | 46 |
Fig. 3.

Proteins identified in all six ECM gel samples sorted by protein class: filled columns are the number of proteins in each class while the empty columns are the corresponding relative abundance (n = 6). The error bars represent variance in relative abundance for each category across the samples.
As a major constituent of the vocal fold ECM, collagen makes up about 76.6% of the VF-ECM gel (Fig. 3). Multiple collagen subtypes I, II, III, V, VI, VII, VIII, XII, and XIV were found in all samples. Collagens I and III, which are the dominant collagens in vocal fold lamina propria of difference species [11,48,51,52], were the most abundant collagen types in our tissue-specific VF-ECM gel. Primarily found in basement membrane zone along with collagen V [51,52], collagen IV was only identified in 1 out of 6 VF-ECM gel samples. However, it was found in all acellular scaffold samples before solubilization (data not shown). Collagen types VI, VII, XII, XIV were also identified in human vocal fold mucosa by mass spectrometry [31]. High sensitivity of the mass spectrometry allows for detecting collagen subtypes which are not found previously using immunoassays. Another fibrous protein, elastin, was not found in the ECM gel even though it was present in decellularized bovine vocal fold lamina propria prior to solubilization (data not shown). On the other hand, VF-ECM gel contained fibulin-2 and fibulin-5, which are glycoproteins associated with microfibril of elastic fibers [53], and a large amount of fibrillins, which are primary microfibrillar components [53]. Including fibrillins and fibulins, a total of 27 proteoglycans and glycoproteins (e.g., heparan sulfate, decorin, versican, fibronectin, and fibrinogen) were detected across 6 gel samples. Finally, mass spectrometry data indicated that remnant cellular proteins only constituted a small portion (≈9%) of the total protein content in the VF-ECM gel. 9 out of these 24 non-matrisome proteins belongs to the keratin family which very likely came from epithelial cells.
To further characterize the VF-ECM gel, Gene Ontology (GO) overrepresentation analysis was conducted on the 75 proteins found in all gel samples. 80 biological process terms and 14 molecular function terms were found significantly overrepresented (FDR corrected p < 0.05) (Supplementary Tables S1 and S2). No terms were found to be significantly underrepresented. Notably, the most enriched (>300 folds) biological process term was “negative regulation of collagen fibril organization” which refers to any process that stops, prevents or reduces the frequency, rate or extent of collagen fibril organization. The most enriched (>200 folds) molecular function term was “platelet-derived growth factor binding” and platelet-derived growth factor has been shown to stimulate production of hyaluronic acid [54]. Fig. 4 summarizes the GO terms in interactive graphs. The main clusters of overrepresented biological process terms were associated with organization of ECM, wound healing, development, and regulatory processes. Terms such as cell-substrate adhesion and biogenesis were also highly enriched (Fig. 4A). Overrepresented molecular function GO terms indicated the VF-ECM gel proteins were largely related to binding, such as protein binding, cell adhesion molecule binding, growth factor binding, and glycosaminoglycan binding. The term “structural molecule activity” was also significantly enriched (Fig. 4B).
Fig. 4.
Interactive graphs summarizing overrepresented Gene Ontology (A) biological process terms and (B) molecular function terms associated with the VF-ECM gel proteome. Terms are depicted as nodes and highly similar GO terms are linked by edges in the graph, where the line width indicates the degree of similarity. Node size is proportional to the frequency of the GO term in the underlying Bos taurus GO annotation database, whereas node color represents the FDR corrected p-value corresponding to the overrepresentation of the term. More similar nodes are placed closer together on the graphs.
The average sGAG content in VF-ECM gel samples was approximately 15.67 ± 8.99 μg/mg total protein (n = 6). The results were in excellent agreement with data reported by Hahn and colleagues: 15–22 μg sGAG/mg protein was found in human, canine, porcine and ferret membranous vocal folds [55].
3.2. Histological results
Fig. 5 shows representative coronal sections of the larynx harvested 3 months after injection and coronal sections of a normal rabbit hemilarynx. The thickness of lamina propria, indicated in Fig. 5A by left-right arrows, was significantly greater in the gel-treated vocal folds (150.76 ± 85.26 μm) than in the saline-injected vocal folds (113.83 ± 72.98 μm) (p = 0.004). H&E stained coronal sections of the other 4 rabbit larynges are shown in Fig. S1. According to Fig. 5F and our unpublished data, the thickness of normal vocal fold lamina propria in similar-sized adult female New Zealand white rabbit is approximately 210 μm, which agrees very well with a previous report [56]. Therefore, the VF-ECM gel was able to reduce tissue contraction of the chronically scarred lamina propria to maintain approximately 70% of its original thickness. This effect was comparable to that induced by hepatocyte growth factor hydrogel [14].
Fig. 5.
(Left panels, A-E) Representative coronal sections of chronically scarred rabbit vocal folds harvested 3 month after treatment, with gel-treated vocal fold on the right, and sham control vocal fold on the left within each panel: (A) H&E staining with left-right arrows showing the thickness of the lamina propria of the treated vocal fold was increased compared to the control vocal fold; (B) picrosirius red staining for collagen. Decrease in collagen density (arrow) is evident in the gel-injected vocal fold lamina propria compared to saline-injected vocal fold; (C) Hart’s elastin staining with arrows pointing to the regenerated subepithelial elastin band in both vocal folds; (D) immunostaining for hyaluronic acid with arrow pointing to an area in the sham control vocal fold where HA was largely absent; (E) Alcian blue staining with arrow pointing to the deep aspect of the treated vocal fold where staining for sGAG is strong. Laryngeal airway was narrowed to reduce image size. (Right panels, F-J) Coronal sections of a normal rabbit vocal fold: (F) H&E staining, (G) picrosirius red staining, (H) Hart’s elastin staining, (I) immunostaining for hyaluronic acid with arrows pointing to the band of HA, and (J) Alcian blue staining. Total magnification = 100×. HA = hyaluronic acid; sGAG = sulfated glycosaminoglycans. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2 lists the means and standard deviations of the relative densities of major extracellular matrix constituents in gel-injected and control vocal folds. Data from individual specimens are plotted in Fig. 6. Unlike normal, uninjured rabbit vocal fold lamina propria, which has no visible longitudinal collagen bundles (Fig. 5G), thick collagen fiber bundles (red/pink in picrosirius red-stained sections) were present through the entire thickness of the lamina propria in the sham control vocal fold. In contrast, thick collagen fibers only appeared in the deeper aspect of the treated lamina propria and its superficial portion contained small amount of much thinner fibers (Fig. 5B) similar to those found in normal vocal fold lamina propria. Collagen deposition was significantly decreased in the VF-ECM gel-treated vocal folds (p = 0.008). In normal rabbit vocal folds, staining for elastin is rather sparse except for the intensively stained “subepithelial elastin band” [45,57] (Fig. 5H). Due to chronic scarring, elastin concentration was significantly elevated in both treated and control vocal folds (Fig. 5C). Both treated and control vocal folds were also able to regenerate the subepithelial elastin band. Fig. 5D demonstrates the difference in hyaluronic acid staining pattern between the VF-ECM gel-treated and sham control vocal folds. The levels of hyaluronic acid, a major component of the lamina propria ECM and the major determinant of tissue rheological properties [20,58], were not significantly higher in the lamina propria of gel-treated vocal folds (Table 2). Moreover, none of the treated or control vocal folds regenerated the previously reported “band of hyaluronic acid” commonly seen in normal rabbit vocal folds [45,48] (Fig. 5I). Compared to the sham control vocal fold, Alcian blue staining was more intense in the deep aspects of the treated vocal fold lamina propria (Fig. 5E). However, similar to its effect on hyaluronic acid, VF-ECM gel was only able to slightly elevate the average density of sGAG in chronically scarred vocal folds (Table 2).
Table 2.
Relative densities of major extracellular matrix constituents in gel-injected and control vocal folds. The p-values were calculated using two-tailed paired Student’s t tests.
| ECM constituent | Relative density (%) | p-value | |
|---|---|---|---|
| Gel-injected | Sham control | ||
| Collagen | 63.14 ± 11.68 | 71.23 ± 14.70 | 0.008 |
| Elastin | 53.16 ± 18.49 | 57.11 ± 23.51 | 0.564 |
| Hyaluronic acid | 48.81 ± 13.73 | 43.66 ± 23.78 | 0.590 |
| Sulfated glycosaminoglycans | 57.04 ± 27.09 | 53.75 ± 19.73 | 0.663 |
Fig. 6.

Relative densities of major extracellular matrix constituents at the mid-fold location in rabbit vocal folds injected with VF-ECM gel versus those in the contralateral sham control vocal folds (n = 5). The two data points from each animal are connected. *p < 0.05 for differences between the two groups.
Bovine collagen I, the most abundant protein in the VF-ECM gel injectant, was not identified in any section (Fig. S2), suggesting complete degradation or resorption of the VF-ECM gel. This result is consistent with our previous finding where decellularized vocal fold scaffold was fully disintegrated within 3 month post implantation [28,29].
3.3. High-speed imaging and videokymography
Stable excised larynx phonation was obtained for 4 out of the 5 larynges. A representative kymograph is shown in Fig. 7. There was no statistically significant difference in the vibrational amplitude between the ECM gel-injected (0.50 ± 0.19 mm) and the saline-injected (0.41 ± 0.15 mm) vocal folds (p = 0.09). In perceptual ratings, the gel-injected vocal fold was judged to have better mucosal wave than the saline control in 2 of the 4 larynges, whereas the mucosal wave was determined to be not different between the two sides in the other 2 larynges. The interrater agreement was 100% between the two raters in this small sample.
Fig. 7.
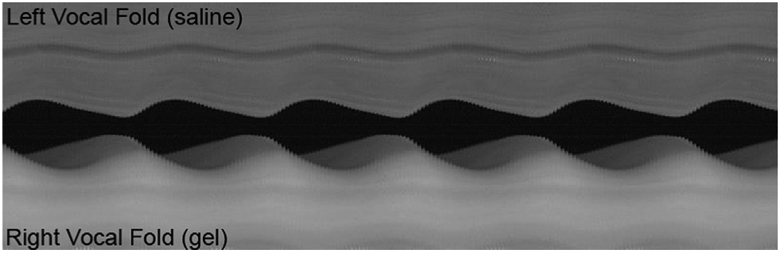
A representative videokymograph from high-speed imaging of excised larynx phonation with the left vocal fold injected with saline (top) and the right vocal fold injected with VF-ECM gel (bottom).
3.4. Humoral immune response to the VF-ECM gel
All five animals displayed minimal increase in IgG antibody titers from baseline levels during the 3-month post-implantation period (Fig. 8). Compared to the sharp 59-fold increase in the serum concentration of specific IgG 3 weeks after booster shot in the immunized rabbits, the VF-ECM gel only induced an average 0.74-fold increase at the same time. In addition, throughout the 3-month post injection period, VF-ECM gel specific IgG increased 0.91 ± 0.79 folds (n = 5) compared to preimplant levels. Hence, systemic response triggered by the xenogeneic bovine vocal fold ECM gel was minimal.
Fig. 8.

VF-ECM gel-specific IgG levels in each animal over three months after injection. The absorbance values at 450 nm (1:100 dilution) are plotted against duration of injection. Pooled sera of the 3 immunized rabbits were used as positive control.
4. Discussion
Previous work in the area of vocal fold tissue repair and regeneration has largely focused on biomaterials developed from one specific ECM component such as hyaluronic acid or collagen. Recent work has also introduced composite biomaterials (e.g., fibrin-collagen, fibrin-HA, collagen-HA, and collagen I-III) generated by combining two or three ECM components or by incorporating ECM constituents into synthetic materials to enhance matrix complexity and accommodate functional requirements [1,59,60]. However, histological or functional improvements achieved by these biomaterials in acute injury models have not been shown in chronic models [1,61]. Given that these “bottom-up” approaches have yet to generate a biomaterial able to treat chronically scarred vocal fold, in this study we employed a “top-down” approach and derived a vocal fold tissue-specific injectable biomaterial in order to closely mimic the lamina propria ECM composition.
The importance of biomaterials being tissue-specific lies in the fact that ECM from different tissues has compositions that are presumably tailored to tissue function [25,62]. The particular composition of vocal fold lamina propria ECM imparts unique biomechanical properties suitable for phonation [20,21,58]. Although the potential of acellular ECM biological material to treat vocal fold lamina propria defects has been demonstrated in multiple reports, most of these studies utilized acellular biological products made from tissues other than vocal fold, such as small intestine submucosa (SIS), urinary bladder submucosa (UBM), and liver [26,57,63-67]. Until now, therapeutic efficacy of decellularized ECM fabricated from non-site specific tissues has not been reported in a chronic vocal fold scarring model (6 months postinjury for rabbits and dogs or 2 months for rats). Additionally, SIS gel was tested in acute vocal fold wound healing models by Choi and colleagues but was not able to promote scarless vocal fold regeneration [66,67]. The tissue-specific vocal fold ECM gel in this study was able to reduce collagen levels and increase the thickness of the lamina propria in chronically scarred vocal folds. This histological difference may be due to the differences in the ECM composition, with the vocal fold-derived ECM gel being more favorable. Our finding adds to the growing evidence [68-70] that tissue-specific ECM-derived biomaterials have improved efficacy as compared to non-tissue matched ECM sources for regenerative applications owing to the unique properties of the ECM in every tissue. Future studies to compare VF-ECM gel with non-tissue specific biologic materials such as SIS derived hydrogel and collagen gel will be necessary to understand the mechanisms underlying the novel properties of the vocal fold site-specific biomaterial.
In contrast to synthetic materials in which the molecular makeup is well-defined, biological ECM materials consist of a complex mixture of molecules that mediate structural and biological functions. For this reason, mass spectrometry is gaining popularity in studies of acellular biomaterials for its ability to generate a comprehensive protein profile of the sample. However, concern had been raised previously that the enzymatic digestion involved in ECM solubilization made it difficult to apply mass spectrometry approaches to the ECM gel or pregel solution [71]. To address that, we first performed SDS-PAGE of the VF-ECM gel and only the portion of the polyacrylamide gel that contained proteins or protein fragments larger than 7 KDa was included for mass spectrometry to remove interfering peptides. The mass spectrometry result indicated that the acellular ECM gel retained a large number of proteins from the normal vocal fold lamina propria ECM such as various subtypes of collagens, proteoglycans, and glycoproteins. Several of these functional and/or structural macromolecules are known to directly relate to vocal fold wound healing and regeneration. For example, decorin, which is significantly reduced in scarred vocal folds, modulates transforming growth factor β and alters collagen organization to keep it sparse and oriented [20,72]; versican is thought to be a dominant source of chondroitin sulfate in the vocal fold lamina propria and its level also likely decreases in scarring [73]. It should be noted that the biological activities of these proteins could be affected by the preparation procedure and protein-protein interaction. Further studies are needed to characterize the functional proteins in the VF-ECM gel.
The results of the Gene Ontology-based analysis were remarkably consistent with our histological findings and support the potential anti-scarring properties of the VF-ECM gel. For example, even though GO terms such as “collagen fibril organization” and “elastic fiber assembly” were highly enriched, “negative regulation of collagen fibril organization” was by far the most enriched term associated with the VF-ECM gel. It suggests that the VF-ECM gel could possibly reverse the homeostasis altered by injury in vocal fold lamina propria. Further, “sequestering of TGFbeta in extracellular matrix” was one of the most overrepresented biological process terms for being enriched 155 folds in the VF-ECM gel proteome. With the potential to negatively regulate transforming growth factor beta receptor signaling pathway, the VF-ECM gel could counter the effects of elevated TGF-β1 levels in injured vocal folds [74], which has been identified as a target for therapies to prevent or treat vocal fold fibrosis [47,75,76].
The composition of biological material also varies depending on the fabrication processes including decellularization [77]. In this study, the decellularization procedure was saline-based and detergent free [27]. Further, no detergent was used during scaffold solubilization. While the addition of a detergent treatment step to our decellularization protocol was shown to preserve sGAG and collagen [31], other groups have reported that the inclusion of detergents such as SDS or Triton X-100 in decellularization can cause complete washout of glycosaminoglycans [70,78]. Our results confirmed the preservation of key ECM components including sGAG in the VF-ECM gel, which could bind and sequester soluble growth factors, regulating their availability and activity [28,79].
In addition to the proteins and sGAG identified in the VF-ECM gel, considerable amount of smaller protein fragments and peptides existed in the gel mainly due to the solubilization procedure. It is known that limited enzymatic cleavage of ECM proteins and glycosaminoglycans can result in the release of biologically active fragments with activities different from those of the full-length molecules [80]. Among these bioactive fragments, called matrikines and matricryptins [80], Arg-Gly-Asp (RGD) may be the best known. Pepsin digestion, which is not expected to cleave in the RGD sequence [81], can facilitate the release of RGD fragments from proteins like collagens and fibronectins. Given that some major vocal fold ECM constituents (e.g., collagens, proteoglycans, and elastin) are well-known sources of matrikines and matricryptins [80], future studies of the bioactive protein fragments in the VF-ECM gel are warranted.
Part of the therapeutic mechanism of the decellularized ECM biomaterials may be attributed to their ability to recruit [82,83] endogenous cells to repair or regenerate damaged tissue [84]. This represents an alternative or complementary approach to incorporating exogenous cells or growth factors in biomaterials. For instance, basic fibroblast growth factor and hepatocyte growth factor have been shown to have potential to treat chronic vocal fold scarring [12-14] but repeated application or a drug delivery system is almost always required for their administration [85]. The tissue-specific, decellularized ECM approach presumably takes advantage of the dynamic interrelationship between resident vocal fold cells and the engineered ECM. This concept is supported by previous finding in cell culture that fibroblasts in recellularized vocal fold mucosa seemed to respond to regional cues and engage in remodeling [27,31]. To understand the host responses at the cellular level that could contribute to tissue remodeling, including immune response mediated by T lymphocytes and macrophages, shorter time points will be required. In addition, the rabbit only has one IgG subclass [86] and may not be an ideal animal model to differentiate Th1 vs. Th2 lymphocyte response by comparing the serum concentration of IgG1 and IgG2. A different animal model may be preferable to investigate how the tissue-specific, decellularized ECM biomaterials interact with host cells.
High-speed digital imaging showed that the gel-injected vocal folds vibrated with mean amplitude about 20% greater than that of control vocal folds. However, the difference was not statistically significant (p = 0.09) based on the 4 out of 5 larynges tested (one larynx failed to vibrate on the excised phonation apparatus). We recently reported that in order to detect 33–50% improvement in the rheological properties of the scarred rabbit vocal folds (i.e., reduction in viscoelastic shear modulus and dynamic viscosity), 7 to 21 animals are needed in a paired study design [46]. Because rheological properties of vocal fold lamina propria critically determine their vibratory characteristics [87], it is likely the current sample size was insufficient to detect a statistically significant treatment effect using videokymography. Another limitation of the study is the lack of rheometric testing, which requires dissection of the vocal fold lamina propria and epithelium from the larynges. In order to reduce the number of animals involved in this preliminary study, only excised larynx phonation with videokymography was used for functional outcome evaluation. Given that rheometric testing has been shown to be reliable [87,88] with high fidelity across samples [89], future studies with both rheometric test and ex vivo phonation test with proper sample size are necessary.
5. Conclusions
More than 250 proteins and a large amount of sulfated glycosaminoglycans were found in the VF-ECM gel. Gene Ontology overrepresentation analysis revealed that the proteins in the gel dominantly promote wound healing and antifibrotic biological process. Preliminary animal study showed that this vocal fold-specific gel was able to significantly reduce tissue contraction and decrease collagen levels in chronically scarred vocal folds. In addition, the VF-ECM gel only elicited minimal humoral immune response after injection. Overall, these results supported the promise of the tissue-specific, injectable acellular gel in vocal fold regenerative medicine.
Supplementary Material
Statement of Significance.
Vocal fold lamina propria scarring remains among the foremost therapeutic challenges in the management of patients with voice disorders. Surgical excision of scar may cause secondary scarring and yield inconsistent results. The present study reports an extracellular matrix-derived biomaterial that demonstrated antifibrotic effect on chronic scarring in vocal fold lamina propria. Its injectability minimizes the invasiveness of the delivery procedure and the degree of mucosal violation. In this work we also describe a new methodology which can more accurately identify proteins from the complex mixture of an acellular extracellular matrix gel by excluding interfering peptides produced during the enzymatic digestion in gel fabrication.
Acknowledgements
This work was supported by the National Institutes of Health (grants R03DC011145, R21DC013363, and OMIC administrative supplement R21DC013363-01A1S1). The authors would like to thank the following core facilities, labs, and colleagues for equipment provision and assistance in various technical aspects of the work: UT Southwestern Proteomic Core (Hamid Mirzaei, Andrew Lemoff, Viswanadham Sridhara, and David Trudgian), Histo Pathology Core (John Shelton, Jessica Williams, Alejandro Daniel, and Anne Starling), Animal Resource Center, Qing-jun Zhang, Chan Lab (Roger Chan, Elhum McPherson, Mindy Du, and Miwako Kimura), Pawlowski Lab (Karen Pawlowski and Paula Timmons), Siegwart Lab (Daniel Siegwart, Qiang Cheng, and Tuo Wei), Liu lab (Xin Liu, Xin Yang, and Siming Chen), Bo Ci, Peiying Liu, Guojin Huang, Welch lab (Tre Welch, Jamie Wright, and Amy Goodfriend), Gao Lab (Jinming Gao, Chensu Wang, and Yang Li), Lehrman lab (Mark Lehrman and Ningguo Gao), Petroll lab (Matthew Petroll and Miguel Miron Mendoza), Lishu Zhang, Yisheng Fang, Isamu Tachibana, Hao-Min Pan, and Keerthan Somanath.
Footnotes
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.actbio.2019.08.025.
References
- [1].Li L, Stiadle JM, Lau HK, Zerdoum AB, Jia X, Thibeault SL, Kiick KL, Tissue engineering-based therapeutic strategies for vocal fold repair and regeneration, Biomaterials 108 (2016) 91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hirano S, Current treatment of vocal fold scarring, Curr. Opin. Otolaryngol. Head Neck Surg. 13 (3) (2005) 143–147. [DOI] [PubMed] [Google Scholar]
- [3].Dailey SH, Ford CN, Surgical management of sulcus vocalis and vocal fold scarring, Otolaryngol. Clin. North Am 39 (1) (2006) 23–42. [DOI] [PubMed] [Google Scholar]
- [4].Welham NV, Choi SH, Dailey SH, Ford CN, Jiang JJ, Bless DM, Prospective multi-arm evaluation of surgical treatments for vocal fold scar and pathologic sulcus vocalis, The Laryngoscope 121 (6) (2011) 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Long JL, Tissue engineering for treatment of vocal fold scar, Curr. Opin. Otolaryngol. Head Neck Surgery 18 (6) (2010) 521–525. [DOI] [PubMed] [Google Scholar]
- [6].Kishimoto Y, Welham NV, Hirano S, Implantation of atelocollagen sheet for vocal fold scar, Curr. Opin. Otolaryngol. Head Neck Surgery 18 (6) (2010) 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rousseau B, Hirano S, Scheidt TD, Welham NV, Thibeault SL, Chan RW, Bless DM, Characterization of vocal fold scarring in a canine model, The Laryngoscope 113 (4) (2003) 620–627. [DOI] [PubMed] [Google Scholar]
- [8].Rousseau B, Hirano S, Chan RW, Welham NV, Thibeault SL, Ford CN, Bless DM, Characterization of chronic vocal fold scarring in a rabbit model, J. Voice: Off. J. Voice Found. 18 (1) (2004) 116–124. [DOI] [PubMed] [Google Scholar]
- [9].Kutty JK, Webb K, Tissue engineering therapies for the vocal fold lamina propria, Tissue Eng. Part B, Rev. 15 (3) (2009) 249–262. [DOI] [PubMed] [Google Scholar]
- [10].Hansen JK, Thibeault SL, Current understanding and review of the literature: vocal fold scarring, J. Voice: Off. J. Voice Found. 20 (1) (2006) 110–120. [DOI] [PubMed] [Google Scholar]
- [11].Tateya T, Tateya I, Sohn JH, Bless DM, Histologic characterization of rat vocal fold scarring, Ann. Otol. Rhinol. Laryngol 114 (3) (2005) 183–191. [DOI] [PubMed] [Google Scholar]
- [12].Welham NV, Montequin DW, Tateya I, Tateya T, Choi SH, Bless DM, A rat excised larynx model of vocal fold scar, J. Speech Lang Hear Res. 52 (4) (2009) 1008–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tateya I, Tateya T, Sohn JH, Bless DM, Histological effect of basic fibroblast growth factor on chronic vocal fold scarring in a rat model, Clin Exp Otorhinolaryngol 9 (1) (2016) 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kishimoto Y, Hirano S, Kitani Y, Suehiro A, Umeda H, Tateya I, Kanemaru S, Tabata Y, Ito J, Chronic vocal fold scar restoration with hepatocyte growth factor hydrogel, The Laryngoscope 120 (1) (2010) 108–113. [DOI] [PubMed] [Google Scholar]
- [15].Valerie A, Vassiliki K, Irini M, Nikolaos P, Karampela E, Apostolos P, Adipose-derived mesenchymal stem cells in the regeneration of vocal folds: a study on a chronic vocal fold scar, Stem Cells Int 2016 (2016) 9010279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kishimoto Y, Yamashita M, Wei A, Toya Y, Ye S, Kendziorski C, Welham NV, Reversal of vocal fold mucosal fibrosis using siRNA against the collagen-specific chaperone serpinh1, Mol. Ther. Nucleic Acids 16 (2019) 616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hirano S, Kawamoto A, Tateya I, Mizuta M, Kishimoto Y, Hiwatashi N, Kawai Y, Tsuji T, Suzuki R, Kaneko M, Naito Y, Kagimura T, Nakamura T, Kanemaru SI, A phase I/II exploratory clinical trial for intracordal injection of recombinant hepatocyte growth factor for vocal fold scar and sulcus, J. Tissue Eng. Regen. Med 12 (4) (2018) 1031–1038. [DOI] [PubMed] [Google Scholar]
- [18].Mattei A, Magalon J, Bertrand B, Grimaud F, Revis J, Velier M, Veran J, Dessi P, Sabatier F, Giovanni A, Autologous adipose-derived stromal vascular fraction and scarred vocal folds: first clinical case report, Stem Cell Res. Ther. 9 (1) (2018) 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pawlak AS, Hammond T, Hammond E, Gray SD, Immunocytochemical study of proteoglycans in vocal folds, Ann. Otol. Rhinol. Laryngol 105 (1) (1996) 6–11. [DOI] [PubMed] [Google Scholar]
- [20].Gray SD, Titze IR, Chan R, Hammond TH, Vocal fold proteoglycans and their influence on biomechanics, The Laryngoscope 109 (6) (1999) 845–854. [DOI] [PubMed] [Google Scholar]
- [21].Gray SD, Titze IR, Alipour F, Hammond TH, Biomechanical and histologic observations of vocal fold fibrous proteins, Ann. Otol. Rhinol. Laryngol 109 (1) (2000) 77–85. [DOI] [PubMed] [Google Scholar]
- [22].Welham NV, Chang Z, Smith LM, Frey BL, Proteomic analysis of a decellularized human vocal fold mucosa scaffold using 2D electrophoresis and high-resolution mass spectrometry, Biomaterials 34 (3) (2013) 669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Welham NV, Yamashita M, Choi SH, Ling C, Cross-sample validation provides enhanced proteome coverage in rat vocal fold mucosa, PLoS One 6 (3) (2011) e17754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ling C, Li Q, Brown ME, Kishimoto Y, Toya Y, Devine EE, Choi KO, Nishimoto K, Norman IG, Tsegyal T, Jiang JJ, Burlingham WJ, Gunasekaran S, Smith LM, Frey BL, Welham NV, Bioengineered vocal fold mucosa for voice restoration, Sci Transl Med 7 (314) (2015) 314ra187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chan BP, Leong KW, Scaffolding in tissue engineering: general approaches and tissue-specific considerations, Eur. Spine J. 17 (Suppl 4) (2008) 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gilbert TW, Agrawal V, Gilbert MR, Povirk KM, Badylak SF, Rosen CA, Liver-derived extracellular matrix as a biologic scaffold for acute vocal fold repair in a canine model, The Laryngoscope 119 (9) (2009) 1856–1863. [DOI] [PubMed] [Google Scholar]
- [27].Xu CC, Chan RW, Tirunagari N, A biodegradable, acellular xenogeneic scaffold for regeneration of the vocal fold lamina propria, Tissue Eng 13 (3) (2007) 551–566. [DOI] [PubMed] [Google Scholar]
- [28].Xu CC, Chan RW, Weinberger DG, Efune G, Pawlowski KS, Controlled release of hepatocyte growth factor from a bovine acellular scaffold for vocal fold reconstruction, J. Biomed. Mater. Res. Part A 93 (4) (2010) 1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Xu CC, Chan RW, Weinberger DG, Efune G, Pawlowski KS, A bovine acellular scaffold for vocal fold reconstruction in a rat model, J. Biomed. Mater. Res. Part A 92 (1) (2010) 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xu CC, Chan RW, Pore architecture of a bovine acellular vocal fold scaffold, Tissue Eng. Part A 14 (11) (2008) 1893–1903. [DOI] [PubMed] [Google Scholar]
- [31].Li Q, Chang Z, Oliveira G, Xiong M, Smith LM, Frey BL, Welham NV, Protein turnover during in vitro tissue engineering, Biomaterials 81 (2016) 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF, Extracellular matrix hydrogels from decellularized tissues: Structure and function, Acta Biomater 49 (2017) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lynn AK, Yannas IV, Bonfield W, Antigenicity and immunogenicity of collagen, J. Biomed. Mater. Res. B Appl. Biomater 71 (2) (2004) 343–354. [DOI] [PubMed] [Google Scholar]
- [34].Didangelos A, Yin X, Mandal K, Baumert M, Jahangiri M, Mayr M, Proteomics characterization of extracellular space components in the human aorta, Mol. Cell. Proteomics 9 (9) (2010) 2048–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Barallobre-Barreiro J, Didangelos A, Schoendube FA, Drozdov I, Yin X, Fernandez-Caggiano M, Willeit P, Puntmann VO, Aldama-Lopez G, Shah AM, Domenech N, Mayr M, Proteomics analysis of cardiac extracellular matrix remodeling in a porcine model of ischemia/reperfusion injury, Circulation 125 (6) (2012) 789–802. [DOI] [PubMed] [Google Scholar]
- [36].Trudgian DC, Mirzaei H, Cloud CPFP: a shotgun proteomics data analysis pipeline using cloud and high performance computing, J. Proteome Res. 11 (12) (2012) 6282–6290. [DOI] [PubMed] [Google Scholar]
- [37].Trudgian DC, Thomas B, McGowan SJ, Kessler BM, Salek M, Acuto O, CPFP: a central proteomics facilities pipeline, Bioinformatics 26 (8) (2010) 1131–1132. [DOI] [PubMed] [Google Scholar]
- [38].Craig R, Beavis RC, TANDEM: matching proteins with tandem mass spectra, Bioinformatics 20 (9) (2004) 1466–1467. [DOI] [PubMed] [Google Scholar]
- [39].Geer LY, Markey SP, Kowalak JA, Wagner L, Xu M, Maynard DM, Yang X, Shi W, Bryant SH, Open mass spectrometry search algorithm, J. Proteome Res. 3 (5) (2004) 958–964. [DOI] [PubMed] [Google Scholar]
- [40].UniProt C, Ongoing and future developments at the Universal Protein Resource, Nucleic Acids Res. 39 (Database issue) (2011) D214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Trudgian DC, Ridlova G, Fischer R, Mackeen MM, Ternette N, Acuto O, Kessler BM, Thomas B, Comparative evaluation of label-free SINQ normalized spectral index quantitation in the central proteomics facilities pipeline, Proteomics 11 (14) (2011) 2790–2797. [DOI] [PubMed] [Google Scholar]
- [42].Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD, PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools, Nucleic Acids Res. 47 (D1) (2019) D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Supek F, Bosnjak M, Skunca N, Smuc T, REVIGO summarizes and visualizes long lists of gene ontology terms, PLoS One 6 (7) (2011) e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T, Cytoscape: a software environment for integrated models of biomolecular interaction networks, Genome Res. 13 (11) (2003) 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Xu CC, Gao A, Zhang S, An investigation of left-right vocal fold symmetry in rheological and histological properties, The Laryngoscope 128 (10) (2018) E359–E364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Xu CC, Li D, Mau T, McPherson E, Du M, Zhang S, Paired versus two-group experimental design for rheological studies of vocal fold tissues, J. Biomech 83 (2019) 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Duflo S, Thibeault SL, Li W, Shu XZ, Prestwich G, Effect of a synthetic extracellular matrix on vocal fold lamina propria gene expression in early wound healing, Tissue Eng 12 (11) (2006) 3201–3207. [DOI] [PubMed] [Google Scholar]
- [48].Mau T, Du M, Xu CC, A rabbit vocal fold laser scarring model for testing lamina propria tissue-engineering therapies, The Laryngoscope 124 (10) (2014) 2321–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Svec JG, Sram F, Schutte HK, Videokymography in voice disorders: what to look for?, Ann Otol. Rhinol. Laryngol 116 (3) (2007) 172–180. [DOI] [PubMed] [Google Scholar]
- [50].Lin W, Shuster S, Maibach HI, Stern R, Patterns of hyaluronan staining are modified by fixation techniques, J. Histochem. Cytochem 45 (8) (1997) 1157–1163. [DOI] [PubMed] [Google Scholar]
- [51].Hahn MS, Kobler JB, Zeitels SM, Langer R, Quantitative and comparative studies of the vocal fold extracellular matrix II: collagen, Ann. Otol. Rhinol. Laryngol 115 (3) (2006) 225–232. [DOI] [PubMed] [Google Scholar]
- [52].Tateya T, Tateya I, Bless DM, Collagen subtypes in human vocal folds, Ann. Otol. Rhinol. Laryngol 115 (6) (2006) 469–476. [DOI] [PubMed] [Google Scholar]
- [53].Kielty CM, Sherratt MJ, Shuttleworth CA, Elastic fibres, J. Cell Sci 115 (Pt 14) (2002) 2817–2828. [DOI] [PubMed] [Google Scholar]
- [54].Ward PD, Thibeault SL, Gray SD, Hyaluronic acid: its role in voice, J. Voice 16 (3) (2002) 303–309. [DOI] [PubMed] [Google Scholar]
- [55].Hahn MS, Kobler JB, Zeitels SM, Langer R, Midmembranous vocal fold lamina propria proteoglycans across selected species, Ann. Otol. Rhinol. Laryngol 114 (6) (2005) 451–462. [DOI] [PubMed] [Google Scholar]
- [56].Hertegard S, Cedervall J, Svensson B, Forsberg K, Maurer FH, Vidovska D, Olivius P, Ahrlund-Richter L, Le Blanc K, Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection, The Laryngoscope 116 (7) (2006) 1248–1254. [DOI] [PubMed] [Google Scholar]
- [57].Pitman MJ, Kurita T, Powell ME, Kimball EE, Mizuta M, Chang S, Garrett CG, Rousseau B, Vibratory function and healing outcomes after small intestinal submucosa biomaterial implantation for chronic vocal fold scar, The Laryngoscope 128 (4) (2018) 901–908. [DOI] [PubMed] [Google Scholar]
- [58].Chan RW, Gray SD, Titze IR, The importance of hyaluronic acid in vocal fold biomechanics, Otolaryngol.–Head Neck Surgery: Off. J. Am. Acad. Otolaryngol. – Head Neck Surg 124 (6) (2001) 607–614. [DOI] [PubMed] [Google Scholar]
- [59].Latifi N, Asgari M, Vali H, Mongeau L, A tissue-mimetic nano-fibrillar hybrid injectable hydrogel for potential soft tissue engineering applications, Sci. Rep 8 (1) (2018) 1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Jimenez-Vergara AC, Munoz-Pinto DJ, Becerra-Bayona S, Wang B, Iacob A, Hahn MS, Influence of glycosaminoglycan identity on vocal fold fibroblast behavior, Acta Biomater 7 (11) (2011) 3964–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Chhetri DK, Mendelsohn AH, Hyaluronic acid for the treatment of vocal fold scars, Curr. Opin. Otolaryngol. Head Neck Surg 18 (6) (2010) 498–502. [DOI] [PubMed] [Google Scholar]
- [62].Eskin SG, Horbett TA, McIntire LV, Mitchell RN, Ratner BD, Schoen FJ, Yee A, Some background concepts, in: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (Eds.), Biomaterials Science: An Introduction to Materials in Medicine Academic Press, San Diego, CA, 2004, pp. 237–291. [Google Scholar]
- [63].Kitamura M, Hirano S, Kanemaru S, Kitani Y, Ohno S, Kojima T, Nakamura T, Ito J, Rosen CA, Gilbert TW, Glottic regeneration with a tissue-engineering technique, using acellular extracellular matrix scaffold in a canine model, J. Tissue Eng. Regen. Med 10 (10) (2016) 825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Huber JE, Spievack A, Simmons-Byrd A, Ringel RL, Badylak S, Extracellular matrix as a scaffold for laryngeal reconstruction, Ann. Otol. Rhinol. Laryngol 112 (5) (2003) 428–433. [DOI] [PubMed] [Google Scholar]
- [65].Pitman MJ, Cabin JA, Iacob CE, Small intestinal submucosa implantation for the possible treatment of vocal fold scar, sulcus, and superficial lamina propria atrophy, Ann. Otol. Rhinol. Laryngol 125 (2) (2016) 137–144. [DOI] [PubMed] [Google Scholar]
- [66].Choi JW, Park JK, Chang JW, Kim DY, Kim MS, Shin YS, Kim CH, Small intestine submucosa and mesenchymal stem cells composite gel for scarless vocal fold regeneration, Biomaterials 35 (18) (2014) 4911–4918. [DOI] [PubMed] [Google Scholar]
- [67].Choi JS, Lee S, Kim DY, Kim YM, Kim MS, Lim JY, Functional remodeling after vocal fold injury by small intestinal submucosa gel containing hepatocyte growth factor, Biomaterials 40 (2015) 98–106. [DOI] [PubMed] [Google Scholar]
- [68].Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL, Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering, Biomaterials 30 (29) (2009) 5409–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zhang Y, He Y, Bharadwaj S, Hammam N, Carnagey K, Myers R, Atala A, Van Dyke M, Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype, Biomaterials 30 (23–24) (2009) 4021–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Robb KP, Shridhar A, Flynn LE, Decellularized matrices as cell-instructive scaffolds to guide tissue-specific regeneration, ACS Biomater. Sci. Eng (2017). [DOI] [PubMed] [Google Scholar]
- [71].Pouliot RA, Link PA, Mikhaiel NS, Schneck MB, Valentine MS, Kamga Gninzeko FJ, Herbert JA, Sakagami M, Heise RL, Development and characterization of a naturally derived lung extracellular matrix hydrogel, J. Biomed. Mater. Res. Part A 104 (8) (2016) 1922–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jetté ME, Thibeault SL, Vocal fold extracellular matrix and wound healing, in: Rubin J, Sataloff RT, Korovin G (Eds.), Diagnosis and Treatment of Voice Disorders, Plural Publishing San Diego, CA, 2014, pp. 79–94. [Google Scholar]
- [73].Hahn MS, Jao CY, Faquin W, Grande-Allen KJ, Glycosaminoglycan composition of the vocal fold lamina propria in relation to function, Ann. Otol. Rhinol. Laryngol 117 (5) (2008) 371–381. [DOI] [PubMed] [Google Scholar]
- [74].Ohno T, Hirano S, Rousseau B, Gene expression of transforming growth factor-beta1 and hepatocyte growth factor during wound healing of injured rat vocal fold, The Laryngoscope 119 (4) (2009) 806–810. [DOI] [PubMed] [Google Scholar]
- [75].Thibeault SL, Bless DM, Gray SD, Interstitial protein alterations in rabbit vocal fold with scar, J. Voice 17 (3) (2003) 377–383. [DOI] [PubMed] [Google Scholar]
- [76].Vyas B, Ishikawa K, Duflo S, Chen X, Thibeault SL, Inhibitory effects of hepatocyte growth factor and interleukin-6 on transforming growth factor-beta1 mediated vocal fold fibroblast-myofibroblast differentiation, Ann. Otol. Rhinol. Laryngol 119 (5) (2010) 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Badylak SF, Xenogeneic extracellular matrix as a scaffold for tissue reconstruction, Transpl. Immunol 12 (3–4) (2004) 367–377. [DOI] [PubMed] [Google Scholar]
- [78].Roosens A, Somers P, De Somer F, Carriel V, Van Nooten G, Cornelissen R, Impact of detergent-based decellularization methods on porcine tissues for heart valve engineering, Ann. Biomed. Eng 44 (9) (2016) 2827–2839. [DOI] [PubMed] [Google Scholar]
- [79].Raman R, Sasisekharan V, Sasisekharan R, Structural insights into biological roles of protein-glycosaminoglycan interactions, Chem. Biol 12 (3) (2005) 267–277. [DOI] [PubMed] [Google Scholar]
- [80].Ricard-Blum S, Salza R, Matricryptins and matrikines: biologically active fragments of the extracellular matrix, Exp. Dermatol 23 (7) (2014) 457–463. [DOI] [PubMed] [Google Scholar]
- [81].Beynon RJ, Bond JS, Proteolytic Enzymes: A Practical Approach, Oxford University Press, 2001. [Google Scholar]
- [82].Beattie AJ, Gilbert TW, Guyot JP, Yates AJ, Badylak SF, Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds, Tissue Eng. Part A 15 (5) (2009) 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Brennan EP, Tang XH, Stewart-Akers AM, Gudas LJ, Badylak SF, Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells, J. Tissue Eng. Regen. Med 2 (8) (2008) 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Badylak SF, The extracellular matrix as a biologic scaffold material, Biomaterials 28 (25) (2007) 3587–3593. [DOI] [PubMed] [Google Scholar]
- [85].Hirano S, Regeneration of the Vocal Fold, Regenerative Medicine in Otolaryngology, Springer; (2015) 171–195. [Google Scholar]
- [86].Titzard IR, Veterinary Immunology, ninth ed., Saunders, St. Louis, Missouri, 2012. [Google Scholar]
- [87].Chan RW, Rodriguez ML, A simple-shear rheometer for linear viscoelastic characterization of vocal fold tissues at phonatory frequencies, J. Acoust. Soc. Am 124 (2) (2008) 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Xu CC, Chan RW, Sun H, Zhan X, A mixed-effects model approach for the statistical analysis of vocal fold viscoelastic shear properties, J. Mech. Behav. Biomed. Mater 75 (2017) 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Krishna P, Regner M, Palko J, Liu F, Abramowitch S, Jiang J, Wells A, The effects of decorin and HGF-primed vocal fold fibroblasts in vitro and ex vivo in a porcine model of vocal fold scarring, The Laryngoscope 120 (11) (2010) 2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





