Abstract
Major depressive disorder (MDD) is one of the most disabling psychiatric disorders. Approximately one‐third of the patients with MDD are treatment resistant to the current antidepressants. There is also a significant therapeutic time lag of weeks to months. Furthermore, depression in patients with bipolar disorder (BD) is typically poorly responsive to antidepressants. Therefore, there exists an unmet medical need for rapidly acting antidepressants with beneficial effects in treatment‐resistant patients with MDD or BD. Accumulating evidence suggests that the N‐methyl‐D‐aspartate receptor (NMDAR) antagonist ketamine produces rapid and sustained antidepressant effects in treatment‐resistant patients with MDD or BD. Ketamine is a racemic mixture comprising equal parts of (R)‐ketamine (or arketamine) and (S)‐ketamine (or esketamine). Because (S)‐ketamine has higher affinity for NMDAR than (R)‐ketamine, esketamine was developed as an antidepressant. On 5 March 2019, esketamine nasal spray was approved by the US Food and Drug Administration. However, preclinical data suggest that (R)‐ketamine exerts greater potency and longer‐lasting antidepressant effects than (S)‐ketamine in animal models of depression and that (R)‐ketamine has less detrimental side‐effects than (R,S)‐ketamine or (S)‐ketamine. In this article, the author reviews the historical overview of the antidepressant actions of enantiomers of ketamine and its major metabolites norketamine and hydroxynorketamine. Furthermore, the author discusses the other potential rapid‐acting antidepressant candidates (i.e., NMDAR antagonists and modulators, low‐voltage‐sensitive T‐type calcium channel inhibitor, potassium channel Kir4.1 inhibitor, negative modulators of γ‐aminobutyric acid, and type A [GABAA] receptors) to compare them with ketamine. Moreover, the molecular and cellular mechanisms of ketamine’s antidepressant effects are discussed.
Keywords: gut microbiota; (R)‐ketamine (or arketamine), (S)‐ketamine (or esketamine), (S)‐norketamine
Major depressive disorder (MDD) is a chronic and debilitating mood disorder that affects approximately 320 million people worldwide. Its incidence increased by >18% between 2005 and 2015.1 In addition, approximately 800 000 individuals take their own life annually, which indicates that suicide is also a serious problem for public health. The currently available antidepressants have certain limitations (e.g., long time lags and low response rates). Approximately 30% of MDD patients are refractory to the current antidepressants.2 Thus, there exists an unmet medical need for rapidly acting novel antidepressants that are also effective in treatment‐resistant patients and in patients with suicide ideation.
Bipolar disorder (BD) is a major psychiatric disorder that exhibits extreme mood swings (i.e., mania or hypomania and depression). Most patients with BD spend a significant amount of their time experiencing either subsyndromal depression or major depression.3, 4 Bipolar depression is typically poorly responsive to antidepressants, with a risk of switch to mania with antidepressant treatment being extremely high.5 A cohort study suggests that the treatment failure rates of bipolar depression are higher than those of MDD.6 Therefore, there is also an unmet medical need for treatment options for refractory patients with bipolar depression, indicating one of the most important issues faced by psychiatrists and researchers.4
Accumulating evidence has revealed the robust antidepressant effects and anti‐suicidal effects of ketamine, the N‐methyl‐D‐aspartate receptor (NMDAR) antagonist, in treatment‐resistant patients with MDD or BD.7, 8, 9, 10 The discovery of the robust antidepressant actions of ketamine is regarded as the greatest advancement in mood disorder research in the past 60 years.11 In this article, the author reviews the historical overview and the molecular and cellular mechanisms of ketamine’s antidepressant actions. Furthermore, the antidepressant actions of ketamine and its major metabolites are discussed. Moreover, the other potential rapid‐acting antidepressant candidates (i.e., other NMDAR antagonists, NMDAR modulators, low‐voltage‐sensitive T‐type calcium channel inhibitor, potassium channel Kir4.1 inhibitor, negative modulators of γ‐aminobutyric acid type A [GABAA] receptors) are also discussed. Finally, the author discusses the future perspective of ketamine and its metabolites.
Brief history of phencyclidine and ketamine
Phencyclidine (PCP; formerly known as ‘CI‐395’ and ‘Sernyl’; Ki = 0.06 μM for NMDAR; Fig. 1 and Table 1)12 was synthesized in 1956 at the pharmaceutical company Park Davis. From 1957 to 1958, PCP was administered as an anesthetic compound in humans undergoing surgery. It was considered that PCP was a safe compound in humans; however, some patients treated with PCP exhibited severe and prolonged post‐surgery delirium. Subsequently, Luby et al.14 investigated the effects of PCP in healthy control subjects and in schizophrenia patients. They found that PCP was an excellent drug model of schizophrenia as PCP could produce schizophrenia‐like symptoms in healthy subjects.14 Currently, the PCP model of schizophrenia has been accepted by several scientists.15, 16, 17 With more clinical experience, it became clear that PCP was not useful as an anesthetic agent in humans.
Figure 1.
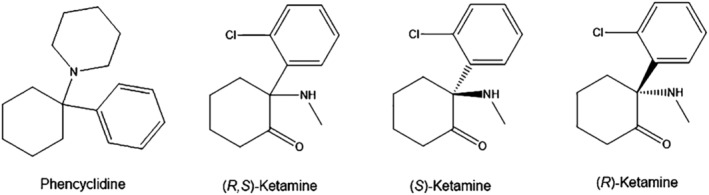
Chemical structure of phencyclidine, (R,S)‐ketamine, and enantiomers of ketamine.
Table 1.
Binding affinities of (+)‐MK‐801, phencyclidine, enantiomers of ketamine, and its metabolites to the NMDA receptor
| Compounds | Ki (μM): Ebert et al.12 | Ki (μM): Moaddel et al.13 |
|---|---|---|
| (+)‐MK‐801 | 0.0019 | 0.0047 |
| Phencyclidine | 0.06 | ND |
| (R,S)‐Ketamine | 0.53 | 1.06 |
| (S)‐Ketamine | 0.3 | 0.69 |
| (R)‐Ketamine | 1.4 | 2.57 |
| (S)‐Norketamine | 1.7 | 2.25 |
| (R)‐Norketamine | 13 | 26.46 |
| (2S,6S)‐Hydroxynorketamine | ND | 21.19 |
| (2R,6R)‐Hydroxynorketamine | ND | >100 |
ND, not determined.
Subsequently, scientists at Park Davis synthesized a series of short‐acting derivatives of PCP. The compound CI‐581 (known as ‘ketamine’: Ki = 0.53 μM12 or Ki = 1.06 μM13 for NMDAR; Fig. 1 and Table 1) was synthesized in 1962. In 1964, Dr Edward F. Domino and colleagues (University of Michigan) conducted the first clinical study of ketamine in humans.18 In 1970, ketamine was introduced as a short‐acting anesthetic drug for humans;17, 18, 19 however, ketamine produced certain side‐effects, such as psychotomimetic effects and dissociation in humans.20, 21 Several ketamine users showed derealization, including visual hallucinations, a sense of unity with external environmental factors, and out‐of‐body experiences.22 Ketamine has been widely used as a recreational drug worldwide,17, 18, 23, 24 although it is also suggested to be useful for substance use disorders.25 Importantly, ulcerative cystitis in ketamine users is one of most serious concerns.26, 27
Ketamine is a racemic mixture of equal amounts of the two enantiomers: (R)‐enantiomer and (S)‐enantiomer. (S)‐ketamine (Ki = 0.30 μM12 or Ki = 0.69 μM13 for NMDAR) has a three‐ to fourfold greater affinity for the NMDAR than (R)‐ketamine (Ki = 1.4 μM12 or Ki = 2.57 μM13 for NMDAR; Fig. 1 and Table 1). Furthermore, the IC50 values for (S)‐ketamine were similar among the four subtypes (GluN2A, GluN2B, GluN2C, GluN2D) of NMDAR, whereas the IC50 for (R)‐ketamine varied slightly among these subtypes.28 In humans, (S)‐ketamine as an anesthetic is twofold more potent than (R,S)‐ketamine and approximately threefold more potent than (R)‐ketamine.29 Therefore, (S)‐ketamine has been widely used in some countries as an anesthetic drug.
Ketamine’s antidepressant effects in humans
MDD
In a review article, Domino described his experience of a patient, an abuser of PCP and ketamine, in the late 1970s or early ‘80s: ‘She stated that ketamine and PCP worked quickly and were much better antidepressants but they did not last as long so she took them again and again.18
In 2000, Berman and colleagues (Yale University) reported the rapidly acting antidepressant effects of ketamine in MDD patients. This was the first double‐blinded, placebo‐controlled trial showing the rapidly acting antidepressant effects of ketamine in patients with MDD.30 Subsequent studies replicated the robust antidepressant actions of ketamine in refractory MDD patients.31, 32, 33, 34 The seminal study of Krystal et al.21 reported dissociative and psychotomimetic effects of ketamine at the dose of 0.5 mg/kg. These side‐effects gradually disappeared within 60–120 min after a single infusion. Several previous studies have used placebo control for randomized, double‐blinded, clinical trials. However, Murrough et al.35 used the other anesthetic agent midazolam as an active control. The ketamine‐treated patients showed greater improvement in depressive symptoms than the midazolam‐treated patients. Furthermore, the score for suicide ideation in the ketamine‐treated patients was lower than in the midazolam‐treated patients, indicating that ketamine could be useful for suicide ideation.36 In addition, unlike midazolam, adjunctive treatment with ketamine significantly reduces suicidal ideation in patients with depression.37
Response rates of ketamine ranged from 25% to 85% at 24 h post‐injection and from 14% to 70% at 72 h post‐injection.38 Murrough et al.39 gave six ketamine infusions over a 12‐day period in treatment‐resistant MDD patients, and 70.8% of these patients were responders to ketamine. Moreover, the repeated infusions were tolerated, without serious side‐effects.39 Singh et al.40 demonstrated that twice‐ and thrice‐a‐week infusions of ketamine maintained the antidepressant efficacy in treatment‐resistant MDD patients.
In addition to intravenous infusions, other multiple routes of ketamine administration, such as intramuscular, nasal, oral, rectal, subcutaneous, and sublingual administrations, have been used.41, 42 The bioavailability of ketamine administered via intramuscular injection was slightly lower (93%) than intravenous infusion (100%, by definition; Table 2).41 Due to the first‐pass metabolism, oral ketamine’s bioavailability was reportedly low (17%–29%) and that via intranasal, rectal, and sublingual administrations was 8%–45%, 11%–25%, and 24%–30%, respectively (Table 2).41, 42 Thus, it seems that low bioavailability of intranasal administration causes lower efficacy compared to intravenous and intramuscular administrations.
Table 2.
Pharmacokinetic profiles of (R,S)‐ketamine in humans
An open‐label study of oral ketamine (0.5 mg/kg/day, 28 days) improved the depressive symptoms in depressed patients receiving hospice care; however, significant antidepressant effects were not shown until Day 14 of administration.43 Furthermore, the oral ketamine (150 mg/day for 6 weeks) group had significantly lower depressive scores compared to the diclofenac group.44 Moreover, oral ketamine (150 mg/day for 6 weeks) decreased the time lag for antidepressant actions of sertraline in MDD patients.45 Thus, low oral bioavailability of ketamine might contribute to its slow‐acting antidepressant effects in depressed patients. Collectively, it seems that oral administration of ketamine produces lower efficacy than intravenous and intramuscular administrations.
Bipolar depression
In 2010, Diazgranados and colleagues (US National Institutes of Health [NIH]) reported that ketamine showed rapid antidepressant actions in refractory BD patients.46 Subsequently, Zarate et al.47 replicated the robust antidepressant effects of ketamine in refractory patients with BD. In addition, six injections of ketamine produced antidepressant and anti‐suicidal effects in Chinese patients with MDD and BD.48
The switch to mania from depression is an important side‐effect of using antidepressants to treat bipolar depression.49, 50 Although the use of ketamine might produce adverse reactions (i.e., dissociative symptoms) in BD patients, the risk of switch from depression to mania remains to be reported.51, 52 However, two case reports showed ketamine‐induced switch from depression to mania in treatment‐resistant patients.53, 54 Nonetheless, it is noteworthy that ketamine has robust antidepressant effects for bipolar depression.
Comparison of ketamine enantiomers in healthy control subjects
Mathisen et al.55 reported that both (S)‐ketamine (0.45 mg/kg) and (R)‐ketamine (1.8 mg/kg) caused side‐effects, such as blurred vision, altered hearing, dizziness, proprioceptive disturbances, and illusions, in patients with oral pain. The incidence of psychotomimetic side‐effects of the (S)‐ketamine group was higher than that of the (R)‐ketamine group, although the dose of (S)‐ketamine (0.45 mg/kg) was lower than that of (R)‐ketamine (1.8 mg/kg).55 Furthermore, it is well known that experiencing illusion and alterations in hearing, vision, and proprioception is attributable to (S)‐ketamine’s actions.56 Vollenweider et al.57 administered 15 mg of (S)‐ or (R)‐ketamine by intravenous injection and measured its effect on psychological state and cerebral glucose metabolism. Whereas (S)‐ketamine caused psychotic reactions, including depersonalization and hallucinations, the same dose of (R)‐ketamine did not produce any psychotic symptoms in the same subjects and most of them experienced a state of relaxation.57 Positron emission tomography showed that (S)‐ketamine markedly increased the glucose utilization in the frontal cortex and thalamus whereas (R)‐ketamine significantly suppressed glucose metabolic rate in several brain areas.57 These findings suggest that (S)‐ketamine contributes to the acute side‐effects of ketamine, whereas (R)‐ketamine may not be associated with these side‐effects.58
(S)‐ketamine
As NMDAR inhibition was believed to play a crucial role in ketamine’s robust antidepressant effects in MDD patients, the Janssen Pharmaceutical Company selected the (S)‐enantiomer of ketamine as an antidepressant candidate (Table 3). An intravenous infusion of (S)‐ketamine is reported to elicit rapid‐acting and sustained antidepressant actions in refractory patients with MDD.60 The most common adverse events (i.e., headache, nausea, dissociation) of (S)‐ketamine were transient.60
Table 3.
List of ketamine enantiomers, ketamine metabolites, and NMDAR‐related compounds as novel antidepressants
| Drug | Company or institute | Mechanisms | Status |
|---|---|---|---|
| (S)‐Ketamine (Esketamine) | Janssen/J&J | NMDAR antagonist | Approved |
| (R)‐Ketamine (Arketamine, PCN 101) | Perception Neuroscience | NMDAR antagonist | Phase 1 in 2019 |
| (2R,6R)‐Hydroxynorketamine | NIMH, USA | AMPAR activator | Phase 1 in 2019 |
| Rapastinel | Allergan | NMDAR modulator | Phase 3 (negative of three phase 3 trials) |
| AV‐101 | VistaGen Therapeutics | NMDAR antagonist | Phase 2 (negative Phase 2 trials) |
| (L‐4‐chlorokynurenine) | |||
| NRX‐101 | NeuroRx Pharma | NMDAR modulator plus | Phase 2 |
| (D‐cycloserine plus lurasidone) | 5‐HT2A receptor antagonist | ||
| AGN‐241751 | Allergan | NMDAR modulator | Phase 1 |
| AXS‐05 (dextromethorphan plus bupropion) | Axsome Therapeutics | NMDAR antagonist plus norepinephrine and dopamine reuptake inhibitor | Phase 1 |
From Reardon59 with a slight modification. AMPAR, AMPA receptor; NIMH, National Institute of Mental Health; NMDAR, NMDA receptor.
The intranasal single injection of (S)‐ketamine produced a decline of cognitive performance in healthy control subjects 40 min after a single dose (84 mg).61 There was no difference in the driving performance in healthy control subjects at 8 h after the intranasal single injection of (S)‐ketamine (84 mg).62 The Janssen Pharmaceutical Company reported the results of a phase 2 study of (S)‐ketamine. The intranasal injections of (S)‐ketamine (28, 56, or 84 mg twice a week) showed good efficacy for the rapid reduction in the depressive symptoms in treatment‐resistant MDD patients. Changes in depressive scores in all three (S)‐ketamine groups were superior to those in the placebo group.63 Subsequently, Canuso et al.64 reported the beneficial effects of intranasal (S)‐ketamine infusions in depressed patients with imminent risks for suicide. (S)‐ketamine significantly decreased the depressive symptoms and suicidality at both 4 and 24 h after the initial injection, although these beneficial effects were not shown after 4 weeks of treatment.64
The Janssen Pharmaceutical Company presented the five phase 3 trials of intranasal administration of (S)‐ketamine in treatment‐resistant patients. Only two of the five phase 3 trials demonstrated positive results. On 12 February 2019, the majority of panelists of the US Food and Drug Administration (FDA) voted to recommend to approve an (S)‐ketamine nasal spray, though two voted against it because of its insufficient efficacy and potential for abuse.65 On 5 March 2019, the US FDA approved (S)‐ketamine nasal spray for treatment‐resistant patients. Because of the risk of serious adverse effects, it is only available through a restricted distribution system, under the Risk Evaluation and Mitigation Strategy.66 At present, the clinical trial of (R)‐ketamine in depressed patients with MDD or BD remains to be reported.
Predictable biomarkers of ketamine’s antidepressant effects in MDD patients
Ketamine can produce antidepressant effects in approximately two‐thirds of refractory MDD patients, with effects lasting for >7 days.38 However, the detrimental side‐effects (i.e., psychotomimetic effects and dissociation) were evident after a single injection. Therefore, predictable biomarkers that distinguish responders or non‐responders to ketamine are necessary.10
The Val/Val carrier (rs6265) in the brain‐derived neurotrophic factor (BDNF) gene in MDD patients shows a higher ketamine response than the Met carriers,67 suggesting that the Val66Met polymorphism in the BDNF gene might be a potential genetic biomarker for ketamine’s responder. The Met allele in Asian populations occurs at a frequency of 40%–50%, whereas it occurs at a frequency of 20%–30% in Caucasian populations.68 However, this finding was not replicated from the samples in a Taiwanese population.34
In 2015, we reported that baseline interleukin‐6 (IL‐6) could be a predictive blood biomarker for the rapid antidepressant effects of ketamine.69 Furthermore, there is a positive correlation between the decrease in levels of tumor necrosis factor‐α 40 min post‐injection of ketamine and ketamine’s antidepressant actions.70 Thus, the rapid anti‐inflammatory actions of ketamine might contribute to its rapid antidepressant effects. Conversely, changes in the IL‐6 levels after ketamine infusion were not associated with the antidepressant response.71 Furthermore, several pro‐inflammatory cytokines exhibited significant changes in the blood of treatment‐resistant MDD patients after ketamine injection.72 Moreover, the low baseline levels of fibroblast growth factor 2 might be related to ketamine’s antidepressant response.72 Nonetheless, future studies using a large sample size are necessary to confirm the potential of these predictable biomarkers for ketamine responders.73
In 2018, Kadriu et al.74 reported increased blood levels of osteoprotegerin (OPG)/receptor activator of nuclear factor kB Ligand (RANKL) ratio and osteopontin after ketamine infusion, suggesting that the OPG/RANK/RANKL system might be implicated in ketamine’s antidepressant effects. Unlike (S)‐ketamine and (2R,6R)‐hydroxynorketamine (HNK), (R)‐ketamine attenuated the higher blood levels of RANKL in a chronic social‐defeat stress (CSDS) model.75, 76 There was also a positive correlation between ketamine‐induced behavioral outcome (i.e., anhedonia) and OPG/RANKL ratio. In addition, (R)‐ketamine, but not (2R,6R)‐HNK, significantly improved the decreased bone mineral density of CSDS‐susceptible mice.76 Collectively, it is likely that the bone turnover markers might be involved in the antidepressant effects of (R)‐ketamine.75, 76 Furthermore, (R)‐ketamine would be a potential therapeutic drug for abnormalities in bone metabolism in depressed patients.76
A metabolomics analysis showed that the ketamine responder group showed a decrease in blood levels of kynurenine and an increase in the bioavailability of arginine compared to the non‐responder group.77 Further studies focusing on the kynurenine and arginine pathways for ketamine’s antidepressant actions are thus deemed necessary.
Shaw et al.78 reported that ketamine (0.5 mg/kg) increased β amplitudes, decreased the peak γ frequency in the visual cortex, and that ketamine could amplify γ band amplitudes in the visual cortex of healthy control subjects. Subsequently, Nugent et al.79 reported the effects of ketamine on γ power in healthy control subjects and MDD patients. Both groups showed the increased resting γ power after a single infusion of ketamine. The γ power at baseline in MDD patients might influence the relationship between post‐ketamine γ power and ketamine’s antidepressant effects. Interestingly, higher γ power after ketamine injection was related to better antidepressant response in MDD patients with lower baseline γ. These findings were replicated by the same group.80 Collectively, it is likely that baseline γ power could be a predictable biomarker for ketamine’s beneficial actions in MDD patients, although further study is needed.
Enantiomers of ketamine and its metabolites
Antidepressant effects of (R)‐ketamine and (S)‐ketamine in rodents
Based on an assumption that the effect of ketamine is mediated by the NMDAR antagonisms, several drugs inhibiting NMDAR have been developed as new antidepressants (Table 3). However, the antidepressant actions of ketamine in MDD patients are more potent than those of non‐ketamine NMDAR antagonists,7, 8 which suggests that NMDAR inhibition does not play a major role in ketamine’s antidepressant effects in humans. In early 2010, we hypothesized that (R)‐ketamine would produce more potent antidepressant actions compared to (S)‐ketamine. To confirm this hypothesis, we purified two enantiomers from (R,S)‐ketamine using the recrystallization method because two enantiomers of ketamine were not available. In 2014, we first demonstrated that (R)‐ketamine produced longer‐lasting antidepressant actions than (S)‐ketamine in mice after neonatal dexamethasone exposure.81, 82, 83 In 2015, we confirmed more potent antidepressant actions of (R)‐ketamine in the other rodent models, such as mouse CSDS and rat learned helplessness (LH) models, compared to (S)‐ketamine.84 In 2016, Zanos and colleagues (University of Maryland and NIH, USA) confirmed our findings on (R)‐ketamine versus (S)‐ketamine in rodent models.85 Subsequently, Fukumoto and colleagues reported that, unlike (S)‐ketamine, (R)‐ketamine and (R,S)‐ketamine (24 h after a single injection) significantly reversed depression‐like behavior in rats after repeated corticosterone treatments.86 Importantly, the levels of both the enantiomers in the brain and blood after a single injection were the same, which suggests that the differences in the potency of two enantiomers are not correlated to the differences in their pharmacokinetic profiles.85, 86 The potent NMDAR antagonist (+)‐MK‐801 caused rapid antidepressant effects in CSDS‐susceptible mice, although (+)‐MK‐801 did not elicit a long‐lasting antidepressant effect,87 supporting the previous reports.88, 89 Cumulatively, it is unlikely that the potent NMDAR inhibition might be involved in the long‐lasting antidepressant actions of ketamine.
Brain regions for the antidepressant actions of ketamine
Ketamine could attenuate the decreased levels of BDNF and PSD‐95 (postsynaptic density protein 95) in the prefrontal cortex (PFC), dentate gyrus (DG), and CA3 of the hippocampus from CSDS‐susceptible mice.90, 91 Conversely, ketamine exhibited no effect against the increased levels of BDNF, and PSD‐95 in the nucleus accumbens (NAc) from CSDS‐susceptible mice.90, 91 Interestingly, (R)‐ketamine induces more potent therapeutic effects on reduced synaptogenesis (i.e., dendritic spine density, PSD‐95) and BDNF–tropomyosin receptor kinase B (TrkB) cascade in the PFC and hippocampus from CSDS‐susceptible mice in comparison with (S)‐ketamine.84
Fuchikami and colleagues reported that ketamine infusion into the infralimbic region of the medial PFC (mPFC) reproduced the antidepressant‐like actions of systemic ketamine administration, and that optogenetic stimulation of the infralimbic region of mPFC showed ketamine‐like antidepressant‐like effects in control‐naive rats.92 Subsequently, Shirayama et al.93 demonstrated that direct injection of (R)‐ketamine into the infralimbic region of the mPFC or hippocampus (i.e., CA3 and DG) of LH rats produced antidepressant effects. Conversely, direct injection of (R)‐ketamine into other brain regions (i.e., the prelimbic cortex of mPFC, the shell and core of the NAc, central nucleus of the amygdala, and basolateral amygdala) had no effect.93 In addition, we also showed that increased BDNF–TrkB signaling in the NAc might contribute to depression‐like phenotypes after withdrawal from repeated methamphetamine administration and that ketamine did not improve depression‐like phenotypes in these mice.94 Taken together, it seems that the NAc might not play a key role in the therapeutic effects of ketamine93, 94, 95 although further study is needed.
In contrast, ketamine restored a decreased dopamine neuron population activity and synaptic plasticity in the NAc and hippocampus pathway in Wistar‐Kyoto rats exposed to inescapable, uncontrollable footshocks.96 Furthermore, Yao et al.97 reported that long‐term potentiation (LTP) in the NAc of control naive mice was disrupted 24 h after ketamine injection, and that ketamine‐induced LTP loss in the NAc was maintained for 7 days. It is currently unclear whether ketamine‐induced LTP loss in the NAc is associated with its antidepressant actions. A pilot clinical study using magnetic resonance imaging showed that ketamine may normalize larger left NAc volume in MDD patients.98 It is also unclear whether ketamine‐induced normalization of left NAc volume in MDD patients is related to its antidepressant actions. Further detailed study using a large sample size is necessary to assess the role of NAc in the antidepressant actions of ketamine.
In 2018, Yang et al.99 demonstrated that ketamine‐induced blockade of NMDAR‐dependent bursting activity in the lateral habenula (LHb) might mediate its rapid‐acting antidepressant effects in rodents with depression‐like phenotype, suggesting that rapid‐acting antidepressant effects of ketamine are dependent on ketamine’s action in the LHb, with a primary site of action.100 In contrast, the dose (25 μg/each side) of ketamine99 was higher than that of the previous report (2 μg/each side of (R)‐ketamine in the mPFC of LH rats93). It may be, therefore, of interest to investigate whether direct injection of (R)‐ketamine in the LHb from LH rats shows antidepressant effects.
Side‐effects of ketamine
Although the psychotomimetic effects and dissociative symptoms in humans after ketamine infusion are well known, off‐label ketamine treatment for depression has become popular in the USA.101, 102, 103 In 2018, an estimated 300 clinics were providing off‐label ketamine treatment to patients with depression, although the US FDA had not approved ketamine for depression.59 This situation is expected to change due to the recent approval of esketamine nasal spray by the US FDA.
(S)‐ketamine‐induced locomotor activity in mice was reported to be higher than that of (R)‐ketamine,84, 104 although one study showed that these two enantiomers exerted equipotent hyperlocomotion at the same dose in rats and mice.86 Antidepressant dose (10 mg/kg) of (R)‐ketamine did not increase locomotor activity of rats and mice.84, 86 (S)‐ketamine, but not (R)‐ketamine, caused prepulse inhibition (PPI) deficits in mice.84 Furthermore, the ED50 of (R)‐ketamine (6.33 mg/kg) for PPI deficits in rats was higher than that of (S)‐ketamine (2.86 mg/kg),105 indicating that (R)‐ketamine disrupts PPI with 2.5‐fold lower potency than (S)‐ketamine. In a conditioned place preference (CPP) test, (S)‐ketamine increased the CPP score in mice.84 However, (R)‐ketamine did not alter the CPP score in mice.84 Therefore, an antidepressant dose of (R)‐ketamine may not produce side‐effects (i.e., psychotomimetic and dissociative effects and abuse liability) in humans.84 In collaboration with Dr Hideo Tsukada (Hamamatsu Photonics Co., Ltd., Japan), we demonstrated a significant reduction in the dopamine D2/3 receptor binding potential in the monkey striatum after a single intravenous injection of (S)‐ketamine (0.5 mg/kg for 40‐min), but not (R)‐ketamine (0.5 mg/kg for 40‐min).106 This positron emission tomography study suggests that (S)‐ketamine‐induced marked dopamine release from the presynaptic terminal may contribute to acute side‐effects (i.e., psychotomimetic and dissociation) in humans.
In 1989, Olney and colleagues (Washington University) reported that the NMDAR antagonists (i.e., PCP, ketamine, and (+)‐MK‐801) caused neuropathological changes in the retrosplenial cortex of rat brains.107 These neuropathological changes are well known as ‘Olney’s lesion.’ The order of potency of NMDAR antagonist‐induced neuropathological changes is known to be correlated with the potency of these compounds for NMDAR.107 Furthermore, it is reported that these NMDAR antagonists induce heat shock protein HSP‐70 protein (a marker of reversible neuronal injury) in the retrosplenial cortex.108, 109, 110 (R,S)‐ketamine, (S)‐ketamine, and (+)‐MK‐801 produced HSP‐70 protein in the rat retrosplenial cortex whereas (R)‐ketamine did not produce HSP‐70 protein in this brain region.111
The reduction of parvalbumin (PV)‐immunoreactivity in the PFC is associated with psychosis.112 A single dose of (S)‐ketamine produced the reduction of PV‐immunoreactivity in the mPFC.84 In contrast, a single dose of (R)‐ketamine did not cause the reduction of PV‐immunoreactivity in the same brain region.84 Furthermore, repeated injections with (S)‐ketamine, but not with (R)‐ketamine, caused a significant reduction in PV‐immunoreactivity in the mPFC.113 Thus, it seems that reduction of PV‐immunoreactivity in the PFC after injection of (S)‐ketamine might be involved in the risk of psychosis.
Very recently, we compared antidepressant effects and side‐effects in mice after intranasal administration of (R,S)‐ketamine and its two enantiomers.114 The order of potency of antidepressant effects was (R)‐ketamine > (R,S)‐ketamine > (S)‐ketamine. In contrast, the order of side‐effects was (S)‐ketamine > (R,S)‐ketamine > (R)‐ketamine. Thus, intranasal administration of (R)‐ketamine might be a safer antidepressant than (R,S)‐ketamine and (S)‐ketamine.
Taken all together, these preclinical and clinical studies suggest a possibility that (R)‐ketamine may be a safer antidepressant than (S)‐ketamine and (R,S)‐ketamine.114, 115, 116, 117, 118, 119
(2R,6R)‐hydroxynorketamine
Ketamine undergoes extensive metabolism via N‐demethylation to norketamine by the cytochrome P450 (CYP) enzymes in the liver initially. Following this N‐demethylation, norketamine is further metabolized to HNK (Fig. 2) and dehydronorketamine.58 Several metabolites of HNK, including (2R,6R;2S,6S)‐HNK and (2S,6R; 2R,6S)‐HNK, were detected in human plasma following ketamine infusion.120, 121
Figure 2.
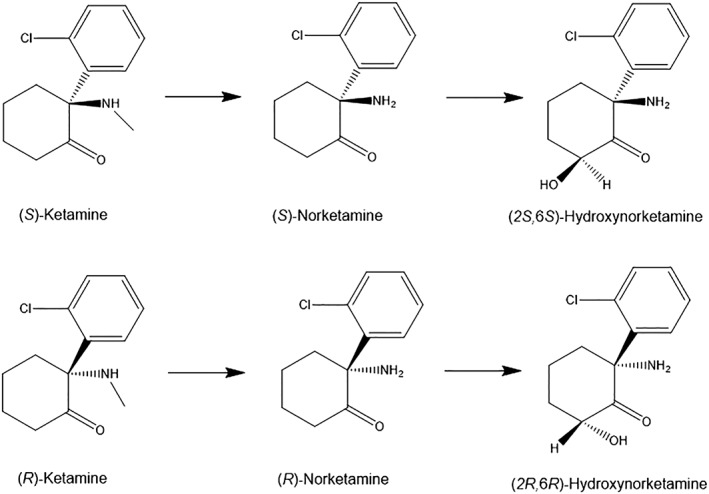
Major metabolites norketamine and hydroxynorketamine of (S)‐ketamine and (R)‐ketamine.
In 2016, Zanos and colleagues demonstrated that the generation of (2R,6R)‐HNK (Ki >10 μM for NMDAR; Table 1 13) in the body was essential for (R,S)‐ketamine’s antidepressant actions in rodents.85 Other researchers have also supported this finding: Chou et al.122 showed reversal of inescapable shock‐induced depression‐like behaviors following intra‐vIPAG (ventrolateral periaqueductal gray) infusion of (2R,6R)‐HNK in the modified rat LH paradigm. Pham et al.123 reported that intracortical administration of (2R,6R)‐HNK exerted antidepressant‐like effects in control naive mice. Fukumoto et al.124 also reported that (2R,6R)‐HNK displayed sustained antidepressant‐like activity in socially isolated mice. These findings are consistent with the initial report by Zanos and colleagues.85
It is reported that (2R,6R)‐HNK inhibits LTP in the NAc97 similarly to ketamine, and (2R,6R)‐HNK could enhance structural plasticity in mouse mesencephalic and human iPSC‐derived dopaminergic neurons via AMPA receptor (AMPAR)‐dependent BDNF.125 Furthermore, (2R,6R)‐HNK promoted dendrite outgrowth in human iPSC‐derived neurons through AMPAR.126 Although (2R,6R)‐HNK can affect synaptic plasticity in the NAc and dopaminergic neurons, it is unknown whether these actions of (2R,6R)‐HNK are associated with its antidepressant actions. Further study is necessary to assess the role of dopaminergic system in the antidepressant actions of ketamine and (2R,6R)‐HNK. Furthermore, Wray et al.127 reported that ketamine and (2R,6R)‐HNK may mediate antidepressant‐like actions through the translocation of GαS from lipid rafts.
On the other hand, however, we reported that (2R,6R)‐HNK did not produce antidepressant effects in CSDS and lipopolysaccharide (LPS)‐induced models although (R)‐ketamine elicited rapid and long‐lasting antidepressant effects in the same models.128 Furthermore, neither (R)‐norketamine nor (2R,6R)‐HNK, which are the major metabolites of (R)‐ketamine, showed any antidepressant effect in a rat LH model, although its parent compound (R)‐ketamine demonstrated potent antidepressant actions in the same model.129
Intracerebroventricular (i.c.v.) injection of (R)‐ketamine revealed rapid and long‐lasting antidepressant actions in a CSDS model. Conversely, the i.c.v. injection of (2R,6R)‐HNK did not produce antidepressant actions in the same model, although the brain level of (2R,6R)‐HNK after i.c.v. injection of (2R,6R)‐HNK was higher than that after i.c.v. injection of (R)‐ketamine.130 In addition, direct injection of (R)‐ketamine into the brain regions (i.e., the infralimbic region of the mPFC, CA3, and the DG of the hippocampus) demonstrated long‐lasting antidepressant actions in a rat LH model.93 Together, it seems that (R,S)‐ketamine (or (R)‐ketamine) itself in the brain could exert antidepressant actions in rodents.
Zanos et al.85 also reported the deuterium isotope effects of metabolism and the antidepressant effects of (R,S)‐ketamine. Using (R)‐d2‐ketamine, we replicated the deuterium isotope effects of metabolism of (R)‐d2‐ketamine to (2R,6R)‐d1‐HNK. In contrast, both (R)‐ketamine and (R)‐d2‐ketamine produced similar rapid and long‐lasting antidepressant effects in CSDS‐susceptible mice.131 These findings suggest no deuterium isotope effect in the antidepressant actions of (R)‐ketamine.
Ketamine is reportedly metabolized mainly by CYP enzymes in the liver.58, 85 To prevent the metabolism of (R)‐ketamine to (2R,6R)‐HNK, we used the CYP inhibitors (e.g., ticlopidine hydrochloride and 1‐aminobenzotriazole). Treatment with these two CYP inhibitors before (R)‐ketamine (3 mg/kg) injection increased the blood (R)‐ketamine levels, whereas (2R,6R)‐HNK was not detected in the blood. In the presence of CYP inhibitors, (R)‐ketamine (3 mg/kg) produced antidepressant actions although the dose did not show antidepressant effects in the absence of CYP inhibitors. These results suggest that the production to (2R,6R)‐HNK from (R)‐ketamine is not necessary for its antidepressant effects.132 Furthermore, we did not detect any sex difference in the pharmacokinetic profile and the antidepressant actions of (R)‐ketamine in an LPS‐induced mouse model of depression.133
These findings suggest that production of (2R,6R)‐HNK from (R,S)‐ketamine is not essential for the antidepressant effects of (R,S)‐ketamine (or (R)‐ketamine). A recent study by Zanos et al.134 demonstrated that (R)‐ketamine may exert antidepressant actions partly via conversion to (2R,6R)‐HNK. Thus, the word essential was changed to the word partly in their conclusion.85, 134
Although the reasons underlying these discrepancies remain unclear, variations in the strain, species, or experimental conditions may contribute to these discrepancies.124 More interestingly, healthy control subjects showed significant increases in depressive symptom for up to 1 day after a single ketamine infusion,80 suggesting that ketamine does not produce antidepressant effects in healthy control subjects. Collectively, the use of rodents with or without depression‐like phenotypes may contribute to these discrepancies.135
(2R,6R)‐HNK is a final metabolite of (R)‐ketamine; however, the antidepressant action of (2R,6R)‐HNK as a compound independent from (R,S)‐ketamine (or (R)‐ketamine) needs to be studied.136, 137, 138 Furthermore, the intravenous (or intranasal) injections of (S)‐ketamine can produce rapid and sustained antidepressant effects in treatment‐resistant MDD patients.60, 63, 64 It is also possible that (2R,6R)‐HNK is not necessary for (R,S)‐ketamine’s antidepressant effects as (2R,6R)‐HNK is not generated from (S)‐ketamine in humans. Furthermore, a recent study showed that (2R,6R)‐HNK has favorable oral bioavailability in three species (mouse, rat, dog).139 In 2019, a clinical trial of (2R,6R)‐HNK in humans will be performed at NIH, USA (Table 3). Therefore, it is of great interest to compare the antidepressant actions of (R)‐ketamine and (2R,6R)‐HNK in MDD patients.
(S)‐norketamine
Ketamine‐induced side‐effects are suggested to be associated with NMDAR inhibition.59, 102, 103, 140 (S)‐ketamine is metabolized to (S)‐norketamine (Ki = 1.70 μM12 or Ki = 2.25 μM13 for NMDAR) by the CYP enzymes (Table 1 and Fig. 2). (S)‐norketamine shows rapid and sustained antidepressant actions in CSDS and LPS models of depression. Potency of antidepressant actions of (S)‐norketamine is similar to its parent compound (S)‐ketamine although (S)‐norketamine’s antidepressant actions are less potent than (R)‐ketamine. Interestingly, unlike (S)‐ketamine, (S)‐norketamine did not produce behavioral and biochemical abnormalities (i.e., rewarding effects, PPI deficits, increases in baseline γ‐oscillations, and reduction of PV‐immunoreactivity in the mPFC) in mice.141 Thus, it is possible that side‐effects of (S)‐norketamine in humans are significantly lower than (S)‐ketamine (Table 1). Taken all together, (S)‐norketamine and its prodrugs would be safer antidepressants than (S)‐ketamine in humans.10, 141, 142 Notably, unlike (S)‐ketamine, it should be noted that (S)‐norketamine is not a schedule compound.
Other NMDAR antagonists and modulators
Inspired by the rapid antidepressant effects of ketamine, application of other NMDAR antagonists for treatment of depression has also been studied. Memantine (Ki = approximately 1.0 μM for NMDAR) is a low‐to‐moderate‐affinity NMDAR antagonist that is currently used in the treatment of Alzheimer’s disease. Unlike ketamine, memantine does not cause psychotomimetic effects at therapeutic doses.143 In a double‐blind, placebo‐controlled study, memantine (5–20 mg/day for 8 weeks) was not effective in the treatment of MDD.144 Although the precise mechanism(s) underlying the differences are currently unknown, the differential effects of ketamine and memantine at intracellular signaling coupled to NMDAR at rest may contribute to the difference.145
Lanicemine (AZD6765; Ki = 0.56–1.5 μM for NMDAR) is also a low‐trapping NMDAR antagonist that shares several pharmacological profiles of ketamine at the NMDAR.146, 147, 148 A phase 2b trial using a large sample size showed that lanicemine (50 or 100 mg/day, three infusions a week for 12 weeks) did not show antidepressant actions in treatment‐resistant MDD patients.148 Furthermore, ketamine significantly increased the average PFC global brain connectivity with global signal regression (GBCr) during infusion and at 24 h post‐treatment, whereas lanicemine showed no significant effects on GBCr compared with the placebo,149 suggesting differences between ketamine and lanicemine. Moreover, (R)‐ketamine demonstrated antidepressant actions in a CSDS model, whereas lanicemine did not produce any antidepressant effect.150 Hence, the development of lanicemine was discontinued.
Rapastinel (formerly GLYX‐13; Table 3) had antidepressant‐like actions in rodents, and this compound does not have ketamine‐like side‐effects.151, 152 Furthermore, the 5‐hydroxytryptamine subtype‐2 receptor‐mediated synaptic and behavioral actions of rapastinel may be associated with the lack of psychotomimetic side‐effects of this compound.153 A recent study suggests that the positive modulation of NMDAR by rapastinel might show rapid and sustained antidepressant effects.154 Furthermore, (R)‐ketamine elicited longer‐lasting antidepressant actions than rapastinel in a CSDS model.155 Intravenous administration of rapastinel (5 or 10 mg/kg) showed antidepressant actions in depressed patients who did not respond to another antidepressant.156 However, on 6 March 2019, Allergan announced that three acute pivotal phase 3 studies of rapastinel in MDD patients did not meet their primary endpoint (Table 3).157 AGN‐241751 (Table 3), an orally bioavailable positive modulator of NMDAR, is being developed as a rapid and sustained antidepressant.158
A clinical study of AV‐101 (L‐4‐chlorokynurenine: a potent antagonist at the glycine site of NMDAR), NRX‐101 (D‐cycloserine plus lurasidone), and AXS‐05 (dextromethorphan plus bupropion) is also underway (Table 3).59, 159 On 3 May 2019, the company VistaGen stated that AV‐101 did not show antidepressant actions in depressed patients (Table 3).
Low‐voltage‐sensitive T‐type calcium channel inhibitor and potassium channel Kir4.1 inhibitor
In 2018, Yang and colleagues (Zhejiang University, China) demonstrated that the blockade of NMDAR‐dependent bursting activity by ketamine in the LHb promotes its rapid‐acting antidepressant effects in rodents. Furthermore, LHb bursting requires both NMDAR and low‐voltage‐sensitive T‐type calcium channels (T‐VSCC). Interestingly, the T‐VSCC inhibitor ethosuximide (200 mg/kg) could show rapid antidepressant actions in rodents99; however, the data are lacking in the control (no stress) group, analysis of dose dependency, and positive control (i.e., ketamine) group. In contrast, unlike (R)‐ketamine, ethosuximide (100, 200, 400 mg/kg) did not produce any antidepressant action in CSDS‐susceptible mice.160 Therefore, T‐VSCC inhibitors might not exert ketamine‐like robust antidepressant actions. Nonetheless, it would be interesting to investigate whether ethosuximide can produce ketamine‐like rapid antidepressant actions in patients with depression since ethosuximide is an anticonvulsant commonly used for absence seizures in adults and children. A clinical study of ethosuximide in treatment‐resistant patients with MDD is underway at Zhejiang University (NCT03887624).
The inwardly rectifying potassium channel Kir4.1 in astroglia is reportedly responsible for potassium buffering.161 The same group demonstrated upregulation of astroglial Kir4.1 in the LHb of rats with depression‐like phenotype.162 In addition, astrocyte‐specific gain and loss function of Kir4.1 in the LHb could regulate neuronal bursting and depression‐like phenotypes, suggesting that Kir4.1 in the LHb may be a therapeutic target for depression.162 We reported that the levels of Kir4.1 and GABAB (γ‐aminobutyric acid, type B) receptor subunit 1 in the parietal cortex were higher in the MDD group than in the control group.163 Interestingly, there was a positive correlation between Kir4.1 protein and GABAB receptor subunit 1 in the parietal cortex in the control group, but not in the MDD group.163 Thus, increased expression of Kir4.1 might be involved in the pathophysiology of MDD. However, unlike (R)‐ketamine, non‐specific Kir4.1 inhibitors (i.e., quinacrine and sertraline) did not produce any antidepressant action in CSDS‐susceptible mice. Therefore, Kir4.1 channel inhibitors might not produce ketamine‐like antidepressant effects; however, future studies using potent and selective Kir4.1 channel inhibitors are warranted.164
Negative modulators of α5 GABAA receptors
The GABAA (γ‐aminobutyric acid, type A) receptors play an important role in several psychiatric disorders, such as MDD, as the regulation of GABAA receptors reportedly influences glutamate neurotransmission.165, 166 Two negative allosteric modulators (NAM; MRK‐016 and L‐655,708) of α5 GABAA receptors showed rapid‐acting antidepressant actions in chronic‐restraint and chronic unpredictable mild stress (CUMS) models.167 Furthermore, MRK‐016 produced ketamine‐like rapid‐acting antidepressant actions, through AMPAR‐dependent increase coherent neuronal activity.168 Furthermore, L‐655,708 and another GABAB receptor, MAM RY‐080, had antidepressant‐like effects in rodents.169, 170 However, unlike (R)‐ketamine, MRK‐016 did not produce an antidepressant effect 7 days after a single dose in a CSDS model although MRK‐016 elicited rapid‐acting antidepressant effects in the same model. Conversely, L‐655,708 did not produce any antidepressant effect in the same model.171
Molecular and cellular mechanisms of ketamine’s antidepressant actions
The molecular and cellular mechanisms underlying ketamine’s antidepressant effects have been proposed in several preclinical studies.11, 172, 173, 174, 175, 176, 177, 178
AMPAR activation
In 1997, Moghaddam and colleagues (Yale University) reported that low doses (10–30 mg/kg) of ketamine can increase extracellular levels of glutamate in the rat PFC, suggesting that ketamine might stimulate glutamatergic neurotransmission in the PFC.179 It is reported that the activation of AMPAR is required for ketamine’s antidepressant effects as the pretreatment of AMPAR antagonist blocked the ketamine’s antidepressant effects in rodents.88 Subsequently, the role of AMPAR activation in the antidepressant effects of ketamine and its two enantiomers has been replicated.89, 180, 181, 182, 183, 184
Although the molecular and cellular mechanisms underlying robust antidepressant actions of ketamine remain unclear, the rapid antidepressant actions of ketamine are believed to occur via the blockade of NMDAR located at inhibitory interneurons, which causes the disinhibition of the pyramidal cells, thereby resulting in a burst of glutamatergic transmission. Collectively, AMPAR activation plays a key role in the antidepressant actions of ketamine and its enantiomers.11, 84, 174 Conversely, AMPAR activation may not be necessary for the antidepressant effects of (S)‐norketamine in a CSDS model.141 A clinical study using AMPAR antagonist perampanel, an FDA‐approved drug, is underway at Yale University to assess the role of AMPAR in the antidepressant action of ketamine (NCT03367533).
Mammalian target of the rapamycin complex 1 signaling
The mammalian target of the rapamycin complex 1 (mTORC1) is a ubiquitous protein kinase involved in protein synthesis and synaptic plasticity. In 2010, Li and colleagues (Yale University) reported that the mTORC1 signaling plays a key role in ketamine’s antidepressant effects as the pretreatment (i.c.v. or direct injection into mPFC) of rapamycin (an mTOR inhibitor) blocked the antidepressant effects of ketamine in rats.180 Ketamine could increase the numbers of synaptic proteins and increase the numbers and functions of new spine synapses in the rat PFC through activation of the mTORC1 signaling.180, 185 Subsequent preclinical studies have supported the role of mTORC1 signaling in the antidepressant actions of ketamine.186, 187 Conversely, Autry and colleagues (University of Texas Southwestern Medical Center) reported that systemic administration of rapamycin (1.0 mg/kg, intraperitoneal [i.p.]) did not block the antidepressant actions of ketamine and that the phosphorylation of mTOR in the mPFC was not detected by ketamine injection.89 Thus, the role of mTORC1 in the antidepressant actions of ketamine remains controversial. One reason underlying the discrepancy is due to the different route of treatment (i.e., i.c.v. or direct injection into the mPFC vs i.p.). However, it is reported that rapamycin (10 mg/kg, i.p.) improved social interaction deficits in mouse models of tuberous sclerosis complex,188 indicating that the dose (10 mg/kg) is effective in the brain disorders. In addition, the role of mTORC1 in the antidepressant actions of (R)‐ketamine and (S)‐ketamine remains unclear. Recently, we showed that mTORC1 signaling in the PFC plays a role in the antidepressant actions of (S)‐ketamine, but not of (R)‐ketamine, and that extracellular signal‐regulated kinase (ERK) signaling might be involved in the antidepressant effects of (R)‐ketamine, but not of (S)‐ketamine (Fig. 3).189
Figure 3.
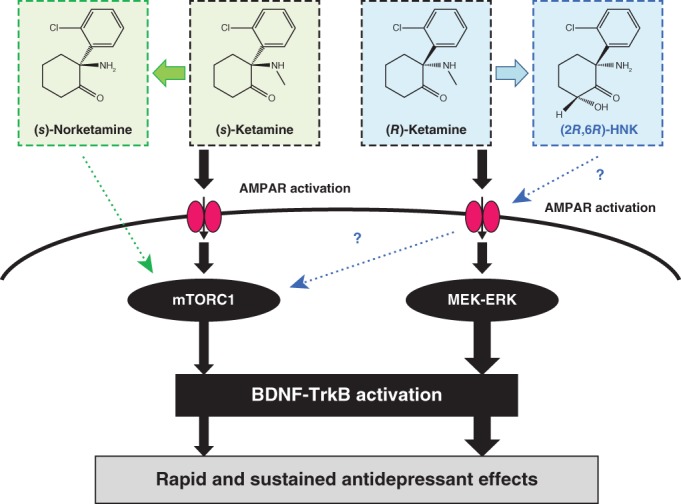
Proposed cellular mechanisms of (S)‐ketamine, (R)‐ketamine, (S)‐norketamine, and (2R,6R)‐hydroxynorketamine (HNK) for antidepressant effects. Both (S)‐ketamine and (R)‐ketamine activate AMPA receptors (AMPAR). Subsequently, (S)‐ketamine and (R)‐ketamine activate mammalian target of the rapamycin complex 1 (mTORC1) signaling and MAPK/ERK kinase (MEK)–extracellular signal‐regulated kinase (ERK) signaling, respectively, and then activate brain‐derived neurotrophic factor (BDNF)–tropomyosin receptor kinase B (TrkB) signaling, resulting in antidepressant effects. Antidepressant effects of (R)‐ketamine are more potent than (S)‐ketamine, although the precise mechanisms underlying the different efficacies of two enantiomers are currently unknown.189 In contrast, (S)‐norketamine, a major metabolite of (S)‐ketamine, may not activate AMPAR. (S)‐norketamine activates mTORC1 signaling and then activates BDNF–TrkB signaling, resulting in antidepressant effects.141 Zanos et al.85 demonstrated that metabolism of (2R,6R)‐HNK from (R,S)‐ketamine is essential for ketamine’s antidepressant actions, and that AMPAR activation and mTORC1 signaling may play a role in the antidepressant effects of (2R,6R)‐HNK. However, our data do not support the conclusion of Zanos et al.85 In addition our data suggest that, unlike (R)‐ketamine, (2R,6R)‐HNK does not have robust antidepressant actions in rodents with depression‐like phenotype.
Interestingly, Denk et al.190 reported the first evidence of increased phosphorylated mTOR protein in the blood from an MDD patient after a single dose of (S)‐ketamine. Subsequently, Yang et al.191 also demonstrated that ketamine showed rapid antidepressant actions with acute increases in the mTOR expression in the blood from MDD patients. It is, therefore, interesting to investigate whether (R)‐ketamine can influence the ERK (or mTOR) and their phosphorylation in the blood from patients with MDD or BD.
Very recently, Abdallah and colleagues (Yale University) demonstrated that pretreatment with rapamycin did not block the rapid and sustained antidepressant effects of ketamine in treatment‐resistant MDD patients. Unexpectedly, rapamycin prolonged the sustained antidepressant actions of ketamine in these patients. There was a significantly higher response rate in the rapamycin plus ketamine group (41%) than that in the placebo plus ketamine group (13%).192 These data do not support the previous preclinical report180 from the same university; however, the treatment route of these two studies was different (i.e., oral administration for human study vs i.c.v. or direct injection into mPFC of rodents). Therefore, further detailed studies on the role of mTORC1 signaling in ketamine’s antidepressant actions are warranted.
BDNF–TrkB signaling
Multiple lines of evidence suggest that BDNF and its receptor TrkB play a crucial role in depression and in the mechanisms of antidepressants.95, 193, 194, 195, 196 In 2011, it was reported that ketamine’s rapid‐acting antidepressants depend on the rapid synthesis of BDNF as ketamine does not elicit antidepressant‐like effects in inducible BDNF knock‐out mice.89 Subsequent studies have supported the key role of the BDNF–TrkB cascade in the antidepressant actions of ketamine.183, 197 ANA‐12 (a TrkB inhibitor) significantly blocked the rapid and long‐lasting antidepressant actions of both (R)‐ketamine and (S)‐ketamine in CSDS‐susceptible mice.84 (R)‐ketamine produced more potent beneficial effects on reduced synaptogenesis and BDNF–TrkB cascade in the PFC and hippocampus (i.e., CA3 and DG) from CSDS‐susceptible mice than (S)‐ketamine.84 Furthermore, it is reported that the regulation of glutamate transporter 1 on astrocytes through TrkB activation is involved in the beneficial effects of ketamine on behavioral abnormalities and morphological changes in the hippocampus of CUMS‐exposed rats.198 Collectively, the long‐lasting activation of BDNF–TrkB cascade in the PFC and hippocampus might be implicated in the long‐lasting antidepressant effects of ketamine and its enantiomers.
Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) plays a role in stress and stress‐related disorders, including depression.199, 200 Viral‐mediated hippocampal knockdown of VEGF produced depressive‐like behaviors and decrease in hippocampal neurogenesis in rats, which partially recovered after injection of ketamine. This study suggests that ketamine‐induced VEGF expression might partially contribute to neurogenesis in the hippocampus and the antidepressant‐like effects of ketamine.201
In 2019, Deyama and colleagues showed that antidepressant‐like effects of ketamine in control naive rats were blocked by forebrain excitatory neuron‐specific deletion of either VEGF or its receptor Flk‐1 or by intra‐mPFC injection of a VEGF neutralizing antibody.202 Furthermore, intra‐mPFC infusions of VEGF are sufficient to produce rapid ketamine‐like behavioral actions, and these effects are blocked by neuron‐specific Flk‐1 deletion. Moreover, inhibition of neuronal VEGF signaling blocks the neurotrophic and synaptogenic effects of ketamine.202 This study suggests that neuronal VEGF‐Flk‐1 cascade in the mPFC might be implicated in ketamine’s antidepressant actions although further study using rodents with depression‐like phenotype is needed.
In addition, co‐infusion of a VEGF neutralizing antibody blocked the rapid and sustained antidepressant‐like actions of intra‐mPFC BDNF, and neuron‐specific mPFC deletion of VEGF blocked the antidepressant‐like actions of BDNF. This study suggests that antidepressant‐like and neurotrophic effects of BDNF may require VEGF signaling.203
Hyperpolarization‐activated cyclic nucleotide‐gated channel
The inhibition or deletion of the hyperpolarization‐activated cyclic nucleotide‐gated channel 1 (HCN1) is reported to occlude completely the synaptic and behavioral actions of ketamine,204 suggesting that the reduced activity of presynaptic HCN1 channels by ketamine‐induced blockade of NMDAR may be involved in the antidepressant actions of ketamine. Furthermore, the lentiviral‐mediated knockdown of HCN1 protein prevented depression‐like behaviors after CUMS.205 The systemic administration of HCN channel inhibitor cilobradine (DK‐AH269) produced rapid and sustained antidepressant actions in a CSDS model.206 Therefore, HCN1 inhibitors may prove to be novel potential antidepressants; however, further studies on their direct comparison with ketamine are needed.
Glycogen synthase kinase‐3
Ketamine is reported to robustly increase the serine phosphorylation of both glycogen synthase kinase‐3 (GSK‐3)α and GSK‐3β in the cerebral cortex and hippocampus. The ketamine’s antidepressant actions in the LH paradigm were completely absent in GSK3 knock‐in mice with seine‐to‐alanine mutations, suggesting that the phosphorylation of GSK3 by ketamine is essential for its antidepressant action.207 Furthermore, the synaptogenic and antidepressant‐like actions of ketamine in rats were potentiated when administered together with lithium (a nonselective GSK‐3 inhibitor) or a GSK‐3β inhibitor SB216763,208, 209 suggesting the role of the GSK‐3 pathway in the antidepressant actions of ketamine. However, unlike ketamine, SB216763 did not produce any rapid and long‐lasting antidepressant effect in a CUMS model.210 Therefore, future studies elucidating the role of GSK‐3β in ketamine’s antidepressant effects are necessary.
P11
The protein p11 (also known as ‘S100A10’) is widely expressed in several brain regions that are involved in depression.211, 212 Ketamine could improve the decreased expressions of BDNF and p11 in the hippocampus of CUMS rats.213 Interestingly, ketamine did not produce sustained antidepressant effects in rats after the knockdown of hippocampal p11.213 Collectively, hippocampal p11 may be involved in the sustained antidepressant effects of ketamine.
Opioid receptor system
Previous reports have demonstrated interactions between ketamine and opioid receptors. The order of affinity for opioid receptor subtypes is mu > kappa > delta. (S)‐ketamine is known to bind approximately two‐ to fourfold stronger to mu and kappa receptors than does (R)‐ketamine.214, 215 In 2018, Williams and colleagues (Stanford University) demonstrated that pretreatment with naltrexone (the opioid receptor antagonist) markedly diminished the ketamine‐induced antidepressant effects in treatment‐resistant MDD patients.216 The lack of impact of naltrexone on dissociative experiences suggests that opiate receptors are not central to ketamine’s dissociative effects; this report concludes that the opioid system activation is necessary to produce rapid‐acting antidepressant effects of ketamine.216 However, there has been much debate about this paper.217, 218, 219, 220, 221 In contrast, pilot data from Yale University demonstrated that pretreatment with naltrexone did not affect the antidepressant actions of ketamine in patients with depression and alcohol use disorder.222, 223, 224 Further clinical trials using a large sample size are needed to better understand whether opioid receptor activation is necessary for ketamine’s antidepressant actions in patients with depression.
Recently, we reported that naltrexone did not block the rapid‐acting and sustained antidepressant effects of ketamine in a CSDS model or LPS‐induced inflammation model.225 If opioid receptors are implicated in the antidepressant actions of ketamine, (S)‐ketamine must be more potent than (R)‐ketamine in animal models of depression. Therefore, the opioid receptor system might not play a role in ketamine’s antidepressant actions.225
microRNA
The microRNA (miRNA) are a class of non‐coding RNA (approximately 22 nts) that regulate the translation of mRNA by binding to the 3′ untranslated region of mRNA in a sequence‐specific manner. Repeated electroconvulsive shock therapy and ketamine possessed miRNA‐598‐5p as a common target.226 Yang et al.227 reported that miR‐206 is a crucial gene for BDNF expression in the hippocampus of control naive rats after ketamine injection. Furthermore, the treatment with an antagonist to miR‐448‐3p could diminish the antidepressant effects of ketamine in the LH paradigm, suggesting that the upregulation of miR448‐3p could have an antidepressant action.228 A new study has demonstrated that miR‐29b‐3p in the PFC plays a role in the antidepressant effects of ketamine in a CUMS model.229 Collectively, miRNA may play a role in the antidepressant effects; however, further detailed studies are needed to confirm this role.
Role of gut microbiota
Multiple lines of evidence suggest that gut microbiota might be involved in the pathophysiology of depression and in the antidepressant‐like effects of certain potential candidates.230, 231, 232, 233, 234, 235, 236, 237, 238 Recently, we investigated the possible role of gut microbiota in the antidepressant effects of (R)‐ketamine in CSDS‐susceptible mice. At the class level, (R)‐ketamine, but not (S)‐ketamine, could attenuate the reduced levels of Mollicutes in the susceptible mice. At the genus level, (R)‐ketamine was more potent than (S)‐ketamine in reducing the levels of Butyricimonas in the susceptible mice.239 Furthermore, (R)‐ketamine, unlike lanicemine, could attenuate the altered levels of Bacteroidales, Clostridiales, and Ruminococcaceae in the susceptible mice.150 Moreover, the phylum Actinobacteria and the class Coriobacteriia might be associated with ketamine’s antidepressant effects in the LPS model.240 At the genus level, ketamine could amplify Lactobacillus, Turicibacter, and Sarcina by 3.3‐, 26‐, and 42‐fold, respectively. In contrast, ketamine reduced the opportunistic pathogens Mucispirillum and Ruminococcus. 241 Cumulatively, the antidepressant actions of (R)‐ketamine (or ketamine) might be, in part, mediated through the gut–brain axis, although further studies on the role of the gut–brain axis are essential.
Concluding remarks and future directions
The development of rapid‐acting antidepressants for treatment‐resistant patients with MDD or BD is an unmet medical need. Multiple lines of evidence have demonstrated the robust antidepressant and anti‐suicidal effects of ketamine in treatment‐resistant patients with MDD or BD. Thus, the discovery of ketamine’s antidepressant effects in these patients is serendipity in the field of mood disorders.242 On 5 March 2019, the US FDA approved (S)‐ketamine nasal spray (Spravato) for treatment‐resistant depression (Table 3). A phase 1 study of (R)‐ketamine and (2R,6R)‐HNK was initiated in early 2019 (Table 3). Therefore, it will be very interesting to perform a direct comparison of (R)‐ketamine, (S)‐ketamine, and (2R,6R)‐HNK in patients with depression.
Considering the high attrition rates, substantial costs, and the slow pace of development of novel drugs, old drug repurposing is increasingly becoming an attractive approach.243 (R)‐ketamine was discarded for a long period when (S)‐ketamine was prepared from (R,S)‐ketamine to provide (S)‐ketamine as an anesthetic drug in several countries. If (R)‐ketamine could elicit rapid and sustained antidepressant effects without side‐effects in depressed patients with MDD or BD, it will exemplify drug repurposing from a racemic mixture in the field of psychiatric disorders. Finally, the identification of novel molecular and cellular targets responsible for the rapid and sustained antidepressant effects of ketamine and its enantiomers will thus be useful for the development of novel antidepressants without the detrimental side‐effects of ketamine.
Disclosure statement
Dr Hashimoto is the inventor of filed patent applications on ‘The use of R‐ketamine in the treatment of psychiatric diseases’ and ‘(S)‐norketamine and salt thereof as pharmaceutical’ by Chiba University. Dr Hashimoto also declares that he has received research support and consultant fees from Dainippon Sumitomo, Otsuka, and Taisho.
Author contributions
K.H. wrote the article.
Acknowledgments
I would like to thank my collaborators who are listed as the co‐authors of our papers in the reference list. I would also like to thank Professor Edward F. Domino (University of Michigan) who has encouraged our (R)‐ketamine projects. This study was supported by grants from the Japan Agency for Medical Research and Development (AMED; to K.H.; JP19dm0107119).
References
- 1. World Health Organization . Depression. [Cited 7 April 2017]. Available from URL: https://www.who.int/mental_health/management/depression/en/
- 2. Trivedi MH, Rush AJ, Wisniewski SR et al Evaluation of outcomes with citalopram for depression using measurement‐based care in STAR*D: Implications for clinical practice. Am. J. Psychiatry 2006; 163: 28–40. [DOI] [PubMed] [Google Scholar]
- 3. Kupka RW, Altshuler LL, Nolen WA et al Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord. 2007; 9: 531–535. [DOI] [PubMed] [Google Scholar]
- 4. Hidalgo‐Mazzei D, Berk M, Cipriani A et al Treatment‐resistant and multi‐therapy‐resistant criteria for bipolar depression: Consensus definition. Br. J. Psychiatry 2019; 214: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tondo L, Vázquez GH, Baldessarini RJ. Options for pharmacological treatment of refractory bipolar depression. Curr. Psychiatry Rep. 2014; 16: 431. [DOI] [PubMed] [Google Scholar]
- 6. Li CT, Bai YM, Huang YL et al Association between antidepressant resistance in unipolar depression and subsequent bipolar disorder: Cohort study. Br. J. Psychiatry 2012; 200: 45–51. [DOI] [PubMed] [Google Scholar]
- 7. Newport DJ, Carpenter LL, McDonald WM et al Ketamine and other NMDA antagonists: Early clinical trials and possible mechanisms in depression. Am. J. Psychiatry 2015; 172: 950–966. [DOI] [PubMed] [Google Scholar]
- 8. Kishimoto T, Chawia JM, Hagi K et al Single‐dose infusion ketamine and non‐ketamine N‐methyl‐D‐aspartate receptor antagonists for unipolar and bipolar depression: A meta‐analysis of efficacy, safety and time trajectories. Psychol. Med. 2016; 46: 1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilkinson ST, Ballard ED, Bloch MH et al The effect of a single dose of intravenous ketamine on suicidal ideation: A systematic review and individual participant data meta‐analysis. Am. J. Psychiatry 2018; 175: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang K, Hashimoto K. An update on ketamine and its two enantiomers as rapid‐acting antidepressants. Expert Rev. Neurother. 2018; 19: 83–92. [DOI] [PubMed] [Google Scholar]
- 11. Duman RS. Ketamine and rapid‐acting antidepressants: A new era in the battle against depression and suicide. F1000Res 2018; 7: 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non‐competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur. J. Pharmacol. 1997; 333: 99–104. [DOI] [PubMed] [Google Scholar]
- 13. Moaddel R, Abdrakhmanova G, Kozak J et al Sub‐anesthetic concentrations of (R,S)‐ketamine metabolites inhibit acetylcholine‐evoked currents in α7 nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2013; 698: 228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luby ED, Cohen BD, Rosenbaum G, Gottlieb JS, Kelley R. Study of a new schizophrenic drug sernyl. Arch. Neurol. Psychiatry 1959; 81: 363–369. [DOI] [PubMed] [Google Scholar]
- 15. Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 1991; 148: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 16. Javitt DC, Zukin SR, Heresco‐Levy U, Umbricht D. Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr. Bull. 2012; 38: 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Domino EF, Luby ED. Phencyclidine/schizophrenia: One view toward the past, the other to the future. Schizophr. Bull. 2012; 38: 914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Domino EF. Taming the ketamine tiger. Anesthesiology 2010; 113: 678–684. [DOI] [PubMed] [Google Scholar]
- 19. Tyler MW, Yourish HB, Ionescu DF, Haggarty SJ. Classics in chemical neuroscience: Ketamine. ACS Chem. Nerosci. 2017; 8: 1122–1134. [DOI] [PubMed] [Google Scholar]
- 20. Domino EF, Chodoff P, Corssen G. Pharmacological effects of CF‐581, a new dissociative anesthetic, in man. Clin. Pharmacol. Ther. 1965; 6: 279–291. [DOI] [PubMed] [Google Scholar]
- 21. Krystal JH, Karper LP, Seibyl JP et al Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry 1994; 51: 199–214. [DOI] [PubMed] [Google Scholar]
- 22. Muetzelfeldt L, Kamboj SK, Rees H, Taylor J, Morgan CJ, Curran HV. Journey through the K‐hole: Phenomenological aspects of ketamine use. Drug Alcohol Depend. 2008; 95: 219–229. [DOI] [PubMed] [Google Scholar]
- 23. Sassano‐Higgins S, Baron D, Juarez G, Esmaili N, Gold M. A review of ketamine abuse and diversion. Depress. Anxiety 2016; 33: 718–727. [DOI] [PubMed] [Google Scholar]
- 24. Liao Y, Tang YL, Hao W. Ketamine and international regulations. Am. J. Drug Alcohol Abuse 2017; 43: 495–504. [DOI] [PubMed] [Google Scholar]
- 25. Jones JL, Mateus CF, Malcolm RJ, Brady KT, Back SE. Efficacy of ketamine in the treatment of substance use disorders: A systematic review. Front. Psych. 2018; 9: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shahani R, Streutker C, Dickson B, Stewart RJ. Ketamine‐associated ulcerative cystitis: A new clinical entity. Urology 2007; 69: 810–812. [DOI] [PubMed] [Google Scholar]
- 27. Myers FA Jr, Bluth MH, Cheung WW. Ketamine: A cause of urinary tract dysfunction. Clin. Lab. Med. 2016; 36: 721–744. [DOI] [PubMed] [Google Scholar]
- 28. Yamakura T, Sakimura K, Shimoji K. The stereoselective effects of ketamine isomers on heteromeric N‐methyl‐D‐aspartate receptor channels. Anesth. Analg. 2000; 91: 225–229. [DOI] [PubMed] [Google Scholar]
- 29. White PF, Schüttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br. J. Anaesth. 1985; 57: 197–203. [DOI] [PubMed] [Google Scholar]
- 30. Berman RM, Cappiello A, Anand A et al Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000; 47: 351–354. [DOI] [PubMed] [Google Scholar]
- 31. Zarate CA, Singh JB, Carlson PJ et al A randomized trial of an N‐methyl‐D‐aspartate antagonist in treatment‐resistant major depression. Arch. Gen. Psychiatry 2006; 63: 856–864. [DOI] [PubMed] [Google Scholar]
- 32. Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment‐resistant depression. Biol. Psychiatry 2009; 66: 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Larkin GL, Beautrais AL. A preliminary naturalistic study of low‐dose ketamine for depression and suicide ideation in the emergency department. Int. J. Neuropsychopharmacol. 2011; 14: 1127–1131. [DOI] [PubMed] [Google Scholar]
- 34. Su TP, Chen MH, Li CT et al Dose‐related effects of adjunctive ketamine in Taiwanese patients with treatment‐resistant depression. Neuropsychopharmacology 2017; 42: 2482–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murrough JW, Iosifescu DV, Chang LC et al Antidepressant efficacy of ketamine in treatment‐resistant major depression: A two‐site randomized controlled trial. Am. J. Psychiatry 2013; 170: 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murrough JW, Soleimani L, DeWilde KE et al Ketamine for rapid reduction of suicidal ideation: A randomized controlled trial. Psychol. Med. 2015; 45: 3571–3580. [DOI] [PubMed] [Google Scholar]
- 37. Grunebaum MF, Galfalvy HC, Choo TH et al Ketamine for rapid reduction of suicidal thoughts in major depression: A midazolam‐controlled randomized clinical trial. Am. J. Psychiatry 2018; 175: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. aan het Rot M, Zarate CA Jr, Charney DS, Mathew SJ. Ketamine for depression: Where do we go from here? Biol. Psychiatry 2012; 72: 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murrough JW, Perez AM, Pillemer S et al Rapid and longer‐term antidepressant effects of repeated ketamine infusions in treatment‐resistant major depression. Biol. Psychiatry 2013; 74: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh JB, Fedgchin M, Daly EJ et al A double‐blind, randomized, placebo‐controlled, dose‐frequency study of intravenous ketamine in patients with treatment‐resistant depression. Am. J. Psychiatry 2016; 173: 816–826. [DOI] [PubMed] [Google Scholar]
- 41. Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front. Hum. Neurosci. 2016; 10: 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peltoniemi MA, Hagelberg NM, Olkkola KT, Saari T. Ketamine: A review of clinical pharmacokinetics and pharmacodynamics in anesthesia and pain therapy. Clin. Pharmacokinet. 2016; 55: 1059–1077. [DOI] [PubMed] [Google Scholar]
- 43. Irwin SA, Iglewicz A, Nelesen RA et al Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: A 28‐day open‐label proof‐of‐concept trial. J. Palliat. Med. 2013; 16: 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jafarinia M, Afarideh M, Tafakhori A et al Efficacy and safety of oral ketamine versus diclofenac to alleviate mild to moderate depression in chronic pain patients: A double‐blind, randomized, controlled trial. J. Affect. Disord. 2016; 204: 1–8. [DOI] [PubMed] [Google Scholar]
- 45. Arabzadeh S, Hakkikazazi E, Shahmansouri N et al Does oral administration of ketamine accelerate response to treatment in major depressive disorder? Results of a double‐blind controlled trial. J. Affect. Disord. 2018; 235: 236–241. [DOI] [PubMed] [Google Scholar]
- 46. Diazgranados N, Ibrahim L, Brutsche NE et al A randomized add‐on trial of an N‐methyl‐D‐aspartate antagonist in treatment‐resistant bipolar depression. Arch. Gen. Psychiatry 2010; 67: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zarate CA Jr, Brutsche NE, Ibrahim L et al Replication of ketamine's antidepressant efficacy in bipolar depression: A randomized controlled add‐on trial. Biol. Psychiatry 2012; 71: 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zheng W, Zhou YL, Liu WJ et al Rapid and longer‐term antidepressant effects of repeated‐dose intravenous ketamine for patients with unipolar and bipolar depression. Psychiatry Res. 2018; 106: 61–68. [DOI] [PubMed] [Google Scholar]
- 49. Viktorin A, Lichtenstein P, Thase ME et al The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone and in combination with a mood stabilizer. Am. J. Psychiatry 2014; 171: 1067–1073. [DOI] [PubMed] [Google Scholar]
- 50. Vieta E, Salagre E, Grande I et al Early intervention in bipolar disorder. Am. J. Psychiatry 2018; 175: 411–426. [DOI] [PubMed] [Google Scholar]
- 51. Niciu MJ, Luckenbaugh DA, Ionescu DF, Mathews DC, Richards EM, Zarate CA Jr. Subanesthetic dose ketamine does not induce an affective switch in three independent samples of treatment‐resistant major depression. Biol. Psychiatry 2013; 74: e23–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Romeo B, Choucha W, Fossati P, Rotge JY. Meta‐analysis of short‐ and mid‐term efficacy of ketamine in unipolar and bipolar depression. Psychiatry Res. 2015; 230: 682–688. [DOI] [PubMed] [Google Scholar]
- 53. Banwari G, Desai P, Patidar P. Ketamine‐induced affective switch in a patient with treatment‐resistant depression. Indian J. Pharmacol. 2015; 47: 454–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McInnes LA, James‐Myers MB, Turner MS. Possible affective switch associated with intravenous ketamine treatment in a patient with bipolar I disorder. Biol. Psychiatry 2016; 79: e71–e72. [DOI] [PubMed] [Google Scholar]
- 55. Mathisen LC, Skjelbred P, Skoglund LA, Oye I. Effect of ketamine, an NMDA receptor inhibitor, in acute and chronic orofacial pain. Pain 1995; 61: 215–220. [DOI] [PubMed] [Google Scholar]
- 56. Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: Evidence for a role of N‐methyl‐D‐aspartate receptors. J. Pharmacol. Exp. Ther. 1992; 260: 1209–1213. [PubMed] [Google Scholar]
- 57. Vollenweider FX, Leenders KL, Oye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)‐ and (R)‐ketamine in healthy volunteers using positron emission tomography (PET). Eur. Neuropsychopharmacol. 1997; 7: 25–38. [DOI] [PubMed] [Google Scholar]
- 58. Zanos P, Moaddel R, Morris PJ et al Ketamine and ketamine metabolite pharmacology: Insights into therapeutic mechanisms. Pharmacol. Rev. 2018; 70: 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reardon S. ‘Party drug’ turned antidepressant approaches approval. Nat. Rev. Drug Discov. 2018; 17: 773–775. [DOI] [PubMed] [Google Scholar]
- 60. Singh JB, Fedgchin M, Daly E et al Intravenous esketamine in adult treatment‐resistant depression: A double‐blind, double‐randomization, placebo‐controlled study. Biol. Psychiatry 2016; 80: 424–431. [DOI] [PubMed] [Google Scholar]
- 61. Morrison RL, Fedgchin M, Singh J et al Effect of intranasal esketamine on cognitive functioning in healthy participants: A randomized, double‐blind, placebo‐controlled study. Psychopharmacology 2018; 235: 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Van de Loo AJAE, Bervoets AC, Mooren L et al The effects of intranasal esketamine (84 mg) and oral mirtazapine (30 mg) on on‐road driving performance: A double‐blind, placebo‐controlled study. Psychopharmacology 2017; 234: 3175–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Daly EJ, Singh JB, Fedgchin M et al Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment‐resistant depression: A randomized clinical trial. JAMA Psychiatry 2018; 75: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Canuso CM, Singh JB, Fedgchin M et al Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: Results of a double‐blind, randomized, placebo‐controlled study. Am. J. Psychiatry 2018; 175: 620–630. [DOI] [PubMed] [Google Scholar]
- 65. Reardon S. Antidepressant based on party drug gets backing from FDA advisory group. Nature 2019. 10.1038/d41586-019-00559-2 [DOI] [Google Scholar]
- 66. Food and Drug Administration . FDA News Release at March 5, 2019: FDA approves new nasal spray medication for treatment‐resistant depression; available only at a certified doctor's office or clinic. [Cited 30 March 2019]. Available from URL: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm632761.htm
- 67. Laje G, Lally N, Mathews D et al Brain‐derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol. Psychiatry 2012; 72: e27–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hashimoto K. A BDNF Val66Met polymorphism and ketamine‐induced rapid antidepressant action. Clin. Psychopharmacol. Neurosci. 2012; 10: 59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang JJ, Wang N, Yang C, Shi JY, Yu HY, Hashimoto K. Serum interleukin‐6 is a predictive biomarker for ketamine's antidepressant effect in treatment‐resistant patients with major depression. Biol. Psychiatry 2015; 77: e19–e20. [DOI] [PubMed] [Google Scholar]
- 70. Chen MH, Li CT, Lin WC et al Rapid inflammation modulation and antidepressant efficacy of a low‐dose ketamine infusion in treatment‐resistant depression: A randomized, double‐blind control study. Psychiatry Res. 2018; 269: 207–211. [DOI] [PubMed] [Google Scholar]
- 71. Park M, Newman LE, Gold PW et al Change in cytokine levels is not associated with rapid antidepressant response to ketamine in treatment‐resistant depression. J. Psychiatr. Res. 2017; 84: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kiraly DD, Horn SR, Van Dam NT et al Altered peripheral immune profiles in treatment‐resistant depression: Response to ketamine and prediction of treatment outcome. Transl. Psychiatry 2017; 7: e1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hashimoto K. Inflammatory biomarkers as differential predictors of antidepressant response. Int. J. Mol. Sci. 2015; 16: 7796–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kadriu B, Gold PW, Luckenbaugh DA et al Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol. Psychiatry 2018; 23: 1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang K, Ma M, Dong C, Hashimoto K. Role of inflammatory bone markers in the antidepressant actions of (R)‐ketamine in a chronic social defeat stress model. Int. J. Neuropsychopharmacol. 2018; 21: 1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Xiong Z, Fujita Y, Zhang K et al Beneficial effects of (R)‐ketamine, but not its metabolite (2R,6R)‐hydroxynorketamine, in the depression‐like phenotype, inflammatory bone markers, and bone mineral density in a chronic social defeat stress model. Behav. Brain Res. 2019; 368: 111904. [DOI] [PubMed] [Google Scholar]
- 77. Moaddel R, Shardell M, Khadeer M et al Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology 2018; 235: 3017–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Shaw AD, Saxena N, Jackson LE, Hall JE, Singh KD, Muthukumaraswamy SD. Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. Eur. Neuropsychopharmacol. 2015; 25: 1136–1146. [DOI] [PubMed] [Google Scholar]
- 79. Nugent AC, Ballard ED, Gould TD et al Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol. Psychiatry 2018; 24: 1040–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nugent AC, Wills KE, Gilbert JR, Zarate CA Jr. Synaptic potentiation and rapid antidepressant response to ketamine in treatment‐resistant major depression: A replication study. Psychiatry Res. Neuroimaging 2019; 283: 64–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li SX, Fujita Y, Zhang JC et al Role of the NMDA receptor in cognitive deficits, anxiety and depressive‐like behavior in juvenile and adult mice after neonatal dexamethasone exposure. Neurobiol. Dis. 2014; 62: 124–134. [DOI] [PubMed] [Google Scholar]
- 82. Li SX, Zhang JC, Wu J, Hashimoto K. Antidepressant effects of ketamine on depression‐like behavior in juvenile mice after neonatal dexamethasone exposure. Clin. Neuropharmacol. Neurosci. 2014; 12: 124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang J, Li S, Hashimoto K. R (−)‐ketamine shows greater potency and longer lasting antidepressant effects than S (+)‐ketamine. Pharmacol. Biochem. Behav. 2014; 116: 137–141. [DOI] [PubMed] [Google Scholar]
- 84. Yang C, Shirayama Y, Zhang JC et al R‐ketamine: A rapid‐onset and sustained antidepressant without psychotomimetic side effects. Transl. Psychiatry 2015; 5: e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zanos P, Moaddel R, Morris PJ et al NMDAR inhibition‐independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Fukumoto K, Toki H, Iijima M et al Antidepressant potential of (R)‐ketamine in rodent models: Comparison with (S)‐ketamine. J. Pharmacol. Exp. Ther. 2017; 361: 9–16. [DOI] [PubMed] [Google Scholar]
- 87. Yang B, Ren Q, Ma M, Chen QX, Hashimoto K. Antidepressant effects of (+)‐MK‐801 and (−)‐MK‐801 in the social defeat stress model. Int. J. Neuropsychopharmacol. 2016; 19: pyw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Maeng S, Zarate CA Jr, Du J et al Cellular mechanisms underlying the antidepressant effects of ketamine: Role of α‐amino‐3‐hydroxy‐5‐methylisoxazole‐4‐propionic acid receptors. Biol. Psychiatry 2008; 63: 349–352. [DOI] [PubMed] [Google Scholar]
- 89. Autry AE, Adachi M, Nosyreva E et al NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang JC, Yao W, Dong C et al Comparison of ketamine, 7,8‐dihydroxyflavone, and ANA‐12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology 2015; 232: 4325–4335. [DOI] [PubMed] [Google Scholar]
- 91. Dong C, Zhang JC, Yao W et al Rapid and sustained antidepressant action of the mGlu2/3 receptor antagonist MGS0039 in the social defeat stress model: Comparison with ketamine. Int. J. Neuropsychopharmacol. 2017; 20: 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fuchikami M, Thomas A, Liu R et al Optogenetic stimulation of infralimbic PFC reproduces ketamine's rapid and sustained antidepressant actions. Proc. Natl. Acad. Sci. U. S. A. 2015; 112: 8106–8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Shirayama Y, Hashimoto K. Effects of a single bilateral infusion of R‐ketamine in the rat brain regions of a learned helplessness model of depression. Eur. Arch. Psychiatry Clin. Neurosci. 2017; 267: 177–182. [DOI] [PubMed] [Google Scholar]
- 94. Ren Q, Ma M, Yang C, Zhang JC, Yao W, Hashimoto K. BDNF‐TrkB signaling in the nucleus accumbens shell of mice has key role in methamphetamine withdrawal symptoms. Transl. Psychiatry 2015; 5: e666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhang JC, Yao W, Hashimoto K. Brain‐derived neurotrophic factor (BDNF) – TrkB signaling in inflammation‐related depression and potential therapeutic targets. Curr. Neuropharmacol. 2016; 14: 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Belujon P, Grace AA. Restoring mood balance in depression: Ketamine reverses deficit in dopamine‐dependent synaptic plasticity. Biol. Psychiatry 2014; 76: 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K. Ketamine and its metabolite (2R,6R)‐hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol. Psychiatry 2018; 23: 2066–2077. [DOI] [PubMed] [Google Scholar]
- 98. Abdallah CG, Jackowski A, Salas R et al The nucleus accumbens and ketamine treatment in major depressive disorder. Neuropsychopharmacology 2017; 42: 1739–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Yang Y, Cui Y, Sang K et al Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 2018; 554: 317–322. [DOI] [PubMed] [Google Scholar]
- 100. Cui Y, Hu S, Hu H. Laternal habenular burst firing as a target of the rapid antidepressant effects of ketamine. Trends Neurosci. 2019; 42: 179–191. [DOI] [PubMed] [Google Scholar]
- 101. Singh I, Morgan C, Curran V, Nutt D, Schlag A, McShane R. Ketamine treatment for depression: Opportunities for clinical innovation and ethical foresight. Lancet Psychiatry 2017; 4: 419–426. [DOI] [PubMed] [Google Scholar]
- 102. Wilkinson ST, Toprak M, Turner MS, Levine SP, Katz RB, Sanacora G. A survey of the clinical, off‐label use of ketamine as a treatment for psychiatric disorders. Am. J. Psychiatry 2017; 174: 695–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Short B, Fong J, Galvez V, Shelker W, Loo CK. Side‐effects associated with ketamine use in depression: A systematic review. Lancet Psychiatry 2018; 5: 65–78. [DOI] [PubMed] [Google Scholar]
- 104. Ryder S, Way WL, Trevor AJ. Comparative pharmacology of the optical isomers of ketamine in mice. Eur. J. Pharmacol. 1978; 49: 15–23. [DOI] [PubMed] [Google Scholar]
- 105. Halberstadt AL, Slepak N, Hyun J, Buell MR, Powell SB. The novel ketamine analog methoxetamine produces dissociative‐like behavioral effects in rodents. Psychopharmacology 2016; 233: 1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hashimoto K, Kakiuchi T, Ohba H, Nishiyama S, Tsukada H. Reduction of dopamine D2/3 receptor binding in the striatum after a single administration of esketamine, but not R‐ketamine: A PET study in conscious monkeys. Eur. Arch. Psychiatry Clin. Neurosci. 2017; 267: 173–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science 1989; 244: 1360–1362. [DOI] [PubMed] [Google Scholar]
- 108. Sharp FR, Jasper P, Hall J, Noble L, Sagar SM. MK‐801 and ketamine induce heat shock protein HSP72 in injured neurons in posterior cingulate and retrosplenial cortex. Ann. Neurol. 1991; 30: 801–809. [DOI] [PubMed] [Google Scholar]
- 109. Hashimoto K, Tomitaka S, Narita N, Minabe Y, Iyo M, Fukui S. Induction of heat shock protein (HSP)‐70 in posterior cingulate and retrosplenial cortex of rat brain by dizocilpine and phencyclidine: Lack of protective effects of sigma receptor ligands. Addict. Biol. 1996; 1: 61–70. [DOI] [PubMed] [Google Scholar]
- 110. Tomitaka S, Hashimoto K, Narita N, Sakamoto A, Minabe Y, Tamura A. Memantine induces heat shock protein HSP‐70 in the posterior cingulate cortex, retrosplenial cortex and dentate gyrus of rat brain. Brain Res. 1996; 740: 1–5. [DOI] [PubMed] [Google Scholar]
- 111. Tian Z, Dong C, Fujita A, Fujita Y, Hashimoto K. Expression of heat shock protein HSP‐70 in the retrosplenial cortex of rat brain after administration of (R,S)‐ketamine and (S)‐ketamine, but not (R)‐ketamine. Pharmacol. Biochem. Behav. 2018; 172: 17–21. [DOI] [PubMed] [Google Scholar]
- 112. Gonzalez‐Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol. Psychiatry 2015; 77: 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Yang C, Han M, Zhang JC, Ren Q, Hashimoto K. Loss of parvalbumin‐immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R‐ketamine. Psychiatric Res. 2016; 239: 281–283. [DOI] [PubMed] [Google Scholar]
- 114. Chang L, Zhang K, Pu Y et al Comparison of antidepressant and side effects in mice after intranasal administration of (R,S)‐ketamine, (R)‐ketamine, and (S)‐ketamine. Pharmacol. Biochem. Behav. 2019; 181: 53–59. [DOI] [PubMed] [Google Scholar]
- 115. Hashimoto K. The R‐stereoisomer of ketamine as an alternative for ketamine for treatment‐resistant major depression. Clin. Psychopharmacol. Neurosci. 2014; 12: 72–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hashimoto K. R‐ketamine: A rapid‐onset and sustained antidepressant without risk of brain toxicity. Psychol. Med. 2016; 46: 2449–2451. [DOI] [PubMed] [Google Scholar]
- 117. Hashimoto K. Ketamine's antidepressant action: Beyond NMDA receptor inhibition. Expert Opin. Ther. Targets 2016; 20: 1389–1392. [DOI] [PubMed] [Google Scholar]
- 118. Hashimoto K. Detrimental side effects of repeated ketamine infusions in the brain. Am. J. Psychiatry 2016; 173: 1044–1045. [DOI] [PubMed] [Google Scholar]
- 119. Hashimoto K. Rapid antidepressant activity of ketamine beyond NMDA receptor In: Hashimoto K. (ed.). The NMDA Receptors. Humana Press, New York, NY, 2017; 69–81. [Google Scholar]
- 120. Moaddel R, Venkata SL, Tanga MJ et al A parallel chiral‐achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta 2010; 82: 1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zarate CA Jr, Brutsche N, Laje G et al Relationship of ketamine's plasma metabolites with response, diagnosis, and side effects in major depression. Biol. Psychiatry 2012; 72: 331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Chou D, Peng HY, Lin TB et al (2R,6R)‐hydroxynorketamine rescues chronic stress‐induced depression‐like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology 2018; 139: 1–12. [DOI] [PubMed] [Google Scholar]
- 123. Pham TH, Defaix C, Xu X et al Common neurotransmission recruited in (R,S)‐ketamine and (2R,6R)‐hydroxynorketamine‐induced sustained antidepressant‐like effects. Biol. Psychiatry 2018; 84: e3–e6. [DOI] [PubMed] [Google Scholar]
- 124. Fukumoto K, Fogaça MV, Liu RJ et al Activity‐dependent brain‐derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)‐hydroxynorketamine. Proc. Natl. Acad. Sci. U. S. A. 2018; 116: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cavalleri L, Merio Pich E, Millan MJ et al Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC‐derived dopaminergic neurons via AMPAR‐driven BDNF and mTOR signaling. Mol. Psychiatry 2018; 23: 812–823. [DOI] [PubMed] [Google Scholar]
- 126. Collo G, Cavalleri L, Chiamulera C, Merlo Pich E. (2R,6R)‐Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell‐derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport 2018; 29: 1425–1430. [DOI] [PubMed] [Google Scholar]
- 127. Wray NH, Schappi JM, Singh H, Senese NB, Rasenick MM. NMDAR‐independent, cAMP‐dependent antidepressant actions of ketamine. Mol. Psychiatry 2018. 10.1038/s41380-018-0083-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yang C, Qu Y, Abe M, Nozawa D, Chaki S, Hashimoto K. (R)‐ketamine shows greater potency and longer lasting antidepressant effects than its metabolite (2R,6R)‐hydroxynorketamine. Biol. Psychiatry 2017; 82: e43–e44. [DOI] [PubMed] [Google Scholar]
- 129. Shirayama Y, Hashimoto K. Lack of antidepressant effects of (2R,6R)‐hydroxynorketamine in a rat learned helplessness model: Comparison with (R)‐ketamine. Int. J. Neuropsychopharmacol. 2018; 21: 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhang K, Fujita Y, Hashimoto K. Lack of metabolism in (R)‐ketamine's antidepressant actions in a chronic social defeat stress model. Sci. Rep. 2018; 8: 4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang K, Toki H, Fujita Y et al Lack of deuterium isotope effects in the antidepressant effects of (R)‐ketamine in a chronic social defeat stress model. Psychopharmacology 2018; 235: 3177–3185. [DOI] [PubMed] [Google Scholar]
- 132. Yamaguchi JI, Toki H, Qu Y et al (2R,6R)‐Hydroxynorketamine is not essential for the antidepressant actions of (R)‐ketamine in mice. Neuropsychopharmacology 2018; 43: 1900–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chang L, Toki H, Qu Y et al No sex‐specific differences in the acute antidepressant actions of (R)‐ketamine in an inflammation model. Int. J. Neuropsychopharmacol. 2018; 21: 932–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zanos P, Highland JH, Liu X et al (R)‐ketamine exerts antidepressant actions partly via conversion to (2R,6R)‐hydroxynorketamine, while causing adverse effects at sub‐anaesthetic doses. Br. J. Pharmacol 2019; 176: 2573–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hashimoto K, Shirayama Y. What are the causes for discrepancies of antidepressant actions of (2R,6R)‐hydroxynorketamine? Biol. Psychiatry 2018; 84: e7–e8. [DOI] [PubMed] [Google Scholar]
- 136. Chaki S. Is metabolism of (R)‐ketamine essential for the antidepressant effects? Int. J. Neuropsychopharmacol. 2018; 21: 154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chaki S, Yamaguchi JI. Now is the time for (2R,6R)‐hydroxynorketamine to be viewed independently from its parent drug. Pharmacol. Biochem. Behav. 2018; 175: 24–26. [DOI] [PubMed] [Google Scholar]
- 138. Chaki S, Yamaguchi JI. Is the history repeated? Can (2R,6R)‐hydroxynorketamine be another antidepressant? J. Exp. Neurosci. 2018. 10.1177/1179069518815445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Highland JN, Morris PJ, Zanos P et al Mouse, rat, and dog bioavailability and mouse oral antidepressant efficacy of (2R,6R)‐hydroxynorketamine. J. Psychopharmacol. 2018; 33: 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Yang C, Hashimoto K. Rapid antidepressant effects and abuse liability of ketamine. Psychopharmacology 2014; 231: 2041–2042. [DOI] [PubMed] [Google Scholar]
- 141. Yang C, Kobayashi S, Nakao K et al AMPA receptor activation‐independent antidepressant actions of ketamine metabolite (S)‐norketamine. Biol. Psychiatry 2018; 84: 591–600. [DOI] [PubMed] [Google Scholar]
- 142. Hashimoto K, Yang C. Is (S)‐norketamine an alternative antidepressant for esketamine? Eur. Arch. Psychiatry Clin. Neurosci. 2018. 10.1007/s00406-018-0922-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Parsons CG, Danysz W, Quack G. Memantine is a clinical well tolerated N–methyl‐D‐aspartate (NMDA) receptor antagonist—A review of preclinical data. Neuropharmacology 1999; 38: 735–767. [DOI] [PubMed] [Google Scholar]
- 144. Zarate CA Jr, Singh JB, Quiroz JA et al A double‐blind, placebo‐controlled study of memantine in the treatment of major depression. Am. J. Psychiatry 2006; 463: 153–155. [DOI] [PubMed] [Google Scholar]
- 145. Gideons ES, Kavalali ET, Monteggia LM. Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proc. Natl. Acad. Sci. U. S. A. 2014; 111: 8649–8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zarate CA Jr, Mathews D, Ibrahim L et al A randomized trial of a low‐trapping nonselective N‐methyl‐D‐aspartate channel blocker in major depression. Biol. Psychiatry 2013; 74: 257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sanacora G, Smith MA, Pathak S et al Lanicemine: A low‐trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol. Psychiatry 2014; 19: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sanacora G, Johnson MR, Khan A et al Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: A randomized, placebo‐controlled study. Neuropsychopharmacology 2017; 42: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Abdallah CG, Dutta A, Averill CL et al Ketamine, but not the NMDAR antagonist lanicemine, increases prefrontal global connectivity in depressed patients. Chronic Stress 2018. 10.1177/2470547018796102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Qu Y, Yang C, Ren Q, Ma M, Dong C, Hashimoto K. Comparison of (R)‐ketamine and lanicemine on depression‐like phenotype and abnormal composition of gut microbiota in a social defeat stress model. Sci. Rep. 2017; 7: 15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Burgdorf J, Zhang XL, Nicholson KL et al GLYX‐13, a NMDA receptor glycine‐site functional partial agonist, induces antidepressant‐like effects without ketamine‐like side effects. Neuropsychopharmacology 2013; 38: 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Moskal JR, Burgdorf JS, Stanton PK et al The development of rapastinel (formerly GLYX‐13); a rapid acting and long lasting antidepressant. Curr. Neuropharmacol. 2017; 15: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Liu RJ, Duman C, Kato T et al GLYX‐13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology 2017; 42: 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Donello JE, Banerjee P, Li YX et al Positive N‐methyl‐D‐aspartate receptor modulation by rapastinel promotes rapid and sustained antidepressant‐like effects. Int. J. Neuropsychopharmacol. 2019; 22: 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Yang B, Zhang JC, Han M et al Comparison of R‐ketamine and rapastinel antidepressant effects in the social defeat stress model of depression. Psychopharmacology 2016; 233: 3647–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Preskorn S, Macaluso M, Mehra DO et al Randomized proof of concept trial of GLYX‐13, an N‐methyl‐D‐aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 2015; 21: 140–149. [DOI] [PubMed] [Google Scholar]
- 157. Allergan . Allergan announces phase 3 results for rapastinel as an adjunctive treatment of major depressive disorder (MDD). [Cited 30 March 2019]. Available from URL: https://www.allergan.com/news/news/thomson-reuters/allergan-announces-phase-3-results-for-rapastinel
- 158. Donello J, Li YX, Banerjee P et al. AGN‐241751, an orally bioavailable positive NMDA receptor modulator, exhibits rapid and sustained antidepressant‐like effects in rodents. Poster M124 presented at the 57th Annual Meeting of the American College of Neuropsychopharmacology (ACNP); December 9–13, 2018; Hollywood, FL.
- 159. Wilkinson ST, Sanacora G. A new generation of antidepressants: An update on the pharmaceutical pipeline for novel and rapid‐acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov. Today 2018; 24: 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Tian Z, Dong C, Zhang K, Chang L, Hashimoto K. Lack of antidepressant effects of low‐voltage‐sensitive T‐type calcium channel blocker ethosuximide in a chronic social defeat stress model: Comparison with (R)‐ketamine. Int. J. Neuropsychopharmacol. 2018; 21: 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ohno Y. Astrocytic Kir4.1 potassium channels as a novel therapeutic target for epilepsy and mood disorders. Neural. Regen. Res. 2018; 13: 651–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Cui Y, Yang Y, Ni Z et al Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 2018; 554: 323–327. [DOI] [PubMed] [Google Scholar]
- 163. Xiong Z, Zhang K, Ren Q, Chang L, Chen J, Hashimoto K. Increased expression of inwardly rectifying Kir4.1 channel in the parietal cortex from patients with major depressive disorder. J. Affect. Disord. 2018; 245: 265–269. [DOI] [PubMed] [Google Scholar]
- 164. Xiong Z, Zhang K, Ishima T et al Lack of rapid antidepressant effects of Kir4.1 channel inhibitors in a chronic social defeat stress model: Comparison with (R)‐ketamine. Pharmacol. Biochem. Behav. 2018; 176: 57–62. [DOI] [PubMed] [Google Scholar]
- 165. Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 2011; 16: 383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Rudolph U, Knoflach F. Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2011; 10: 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM. Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of alpha5‐containing GABAA receptors. Neuropsychopharmacology 2015; 40: 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zanos P, Nelson ME, Highland JN et al A negative allosteric modulator for α5 subunit‐containing GABA receptors exerts a rapid and persistent antidepressant‐like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro 2017. 10.1523/ENEURO.0285-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Carreno FR, Collins GT, Frazer A, Lodge DJ. Selective pharmacological augmentation of hippocampal activity produces a sustained antidepressant‐like response without abuse‐related or psychotomimetic effects. Int. J. Neuropsychopharmacol. 2017; 20: 504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Xu NZ, Ernst M, Treven M et al Negative allosteric modulation of alpha 5‐containing GABAA receptors engenders antidepressant‐like effects and selectively prevents age‐associated hyperactivity in tau‐depositing mice. Psychopharmacology 2018; 235: 1151–1161. [DOI] [PubMed] [Google Scholar]
- 171. Xiong Z, Zhang K, Ishima T et al Comparison of rapid and long‐lasting antidepressant effects of negative modulators of α5‐containing GABAA receptors and (R)‐ketamine in a chronic social defeat stress model. Pharmacol. Biochem. Behav. 2018; 175: 139–145. [DOI] [PubMed] [Google Scholar]
- 172. Duman RS, Aghajanian GK. Synaptic dysfunction in depression: Potential therapeutic targets. Science 2012; 338: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: Progress and prospects. Nat. Rev. Drug Discov. 2017; 16: 472–486. [DOI] [PubMed] [Google Scholar]
- 174. Abdallah CG, Sanacora G, Duman RS, Krystal JH. The neurobiology of depression, ketamine and rapid‐acting antidepressants: Is it glutamate inhibition or activation? Pharmacol. Ther. 2018; 190: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Gerhard DM, Duman RS. Rapid‐acting antidepressants: Mechanistic insights and future directions. Curr. Behav. Neurosci. Rep. 2018; 5: 36–47. [PMC free article] [PubMed] [Google Scholar]
- 176. Witkin JM, Knutson DE, Rodriguez GJ, Shi S. Rapid‐acting antidepressants. Curr. Pharm. Des. 2018; 24: 2556–2563. [DOI] [PubMed] [Google Scholar]
- 177. Pałucha‐Poniewiera A. The role of glutamatergic modulation in the mechanism of action of ketamine, a prototype rapid‐acting antidepressant drug. Pharmacol. Rep. 2018; 70: 837–846. [DOI] [PubMed] [Google Scholar]
- 178. Gould TD, Zarate CA Jr, Thompson SM. Molecular pharmacology and neurobiology of rapid‐acting antidepressants. Annu. Rev. Pharmacol. Toxicol. 2019; 59: 213–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997; 17: 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Li N, Lee B, Liu RJ et al mTOR‐dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Walker AK, Budac DP, Bisulco S et al NMDA receptor blockade by ketamine abrogates lipopolysaccharide‐induced depressive‐like behavior in C57BL/6J mice. Neuropsychopharmacology 2013; 38: 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant‐like effects of ketamine in animal models of depression. Behav. Brain Res. 2011; 224: 107–111. [DOI] [PubMed] [Google Scholar]
- 183. Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine‐induced antidepressant effects are associated with AMPA receptors‐mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur. Psychiatry 2014; 29: 419–423. [DOI] [PubMed] [Google Scholar]
- 184. Fukumoto K, Iijima M, Chaki S. The antidepressant effects of an mGlu2/3 receptor antagonist and ketamine require AMPA receptor stimulation in the mPFC and subsequent activation of the 5‐HT neurons in the DRN. Neuropsychopharmacology 2016; 41: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Hashimoto K. Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine. Expert Rev. Neurother. 2011; 11: 33–36. [DOI] [PubMed] [Google Scholar]
- 186. Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant‐like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 2011; 61: 1419–1423. [DOI] [PubMed] [Google Scholar]
- 187. Yang C, Hu YM, Zhou ZQ, Zhang GF, Yang JJ. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups. J. Med. Sci. 2013; 118: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Sato A, Kasai S, Kobayashi T et al Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nat. Commun. 2012; 3: 1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Yang C, Ren Q, Qu Y et al Mechanistic target of rapamycin‐independent antidepressant effects of (R)‐ketamine in a social defeat stress model. Biol. Psychiatry 2018; 83: 18–28. [DOI] [PubMed] [Google Scholar]
- 190. Denk MC, Rewerts C, Holsboer F, Erhardt‐Lehmann A, Turck CW. Monitoring ketamine treatment response in a depressed patient via peripheral mammalian target of rapamycin activation. Am. J. Psychiatry 2011; 168: 751–752. [DOI] [PubMed] [Google Scholar]
- 191. Yang C, Zhou Z, Gao Z, Shi JY, Yang JJ. Acute increases in plasma mammalian target of rapamycin, glycogen synthase kinase‐3β, and eukaryotic elongation factor 2 phosphorylation after ketamine treatment in three depressed patients. Biol. Psychiatry 2013; 73: e35–e36. [DOI] [PubMed] [Google Scholar]
- 192. Abdallah CG, Averill LA, Gueorguieva R et al Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate of ketamine at 2 weeks following treatment. A double‐blind, placebo‐controlled, cross‐over, randomized clinical trial. bioRxiv 2018. 10.1101/500959 [DOI] [Google Scholar]
- 193. Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002; 34: 13–25. [DOI] [PubMed] [Google Scholar]
- 194. Hashimoto K, Shimizu E, Iyo M. Critical role of brain‐derived neurotrophic factor in mood disorders. Brain Res. Rev. 2004; 45: 104–114. [DOI] [PubMed] [Google Scholar]
- 195. Duman RS, Monteggia LM. A neurotrophic model for stress‐related mood disorders. Biol. Psychiatry 2006; 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 196. Hashimoto K. Brain‐derived neurotrophic factor as a biomarker for mood disorders: An historical overview and future directions. Psychiatry Clin. Neurosci. 2010; 64: 341–357. [DOI] [PubMed] [Google Scholar]
- 197. Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int. J. Neuropsychopharmacol. 2014; 18: pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Liu WX, Wang J, Xie ZM et al Regulation of glutamate transporter 1 via BDNF‐TrkB signaling plays a role in the anti‐apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology 2016; 233: 405–415. [DOI] [PubMed] [Google Scholar]
- 199. Clark‐Raymond A, Halaris A. VEGF and depression: A comprehensive assessment of clinical data. J. Psychiatr. Res. 2013; 47: 1080–1087. [DOI] [PubMed] [Google Scholar]
- 200. Pisoni A, Strawbridge R, Hodsoll J et al Growth factor proteins and treatment‐resistant depression: A place on the path to precision. Front. Psych. 2018; 9: 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Choi M, Lee SH, Chang HL, Son H. Hippocampal VEGF is necessary for antidepressant‐like behaviors but not sufficient for antidepressant‐like effects of ketamine in rats. Biochim. Biophys. Acta 1862; 2016: 1247–1254. [DOI] [PubMed] [Google Scholar]
- 202. Deyama S, Bang E, Wohleb ES et al Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am. J. Psychiatry 2019; 176: 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Deyama S, Bang E, Kato T, Yuan X, Duman RS. Neurotrophic and antidepressant actions of brain‐derived neurotrophic factor require vascular endothelial growth factor. Biol. Psychiatry 2018; 141: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Zhang K, Xu T, Yuan Z et al Essential roles of AMPA receptor GluA1 phosphorylation and presynaptic HCN channels in fast‐acting antidepressant responses of ketamine. Sci. Signal. 2016; 9: ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Kim CS, Brager DH, Johnston D. Perisomatic changes in h‐channels regulate depressive behaviors following chronic unpredictable stress. Mol. Psychiatry 2017; 23: 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zhu Y, Stanly MR, Ku S et al HCN channel inhibitor induces a rapid and sustained reversal of social deficit in a chronic social defeat stress model of depression. Poster M123 presented at the 57th Annual Meeting of the American College of Neuropsychopharmacology (ACNP); December 9–13, 2018; Hollywood, FL.
- 207. Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase‐3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol. Psychiatry 2011; 16: 1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK‐3 inhibition potentiates the synaptogenic and antidepressant‐like effects of subthreshold doses of ketamine. Neuropsychopharmacology 2013; 38: 2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Chiu CT, Scheuing L, Liu G et al The mood stabilizer lithium potentiates the antidepressant‐like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int. J. Neuropsychopharmacol. 2014; 18: pyu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Ma XC, Dang YH, Jia M et al Long‐lasting antidepressant action of ketamine, but not glycogen synthase kinase‐3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One 2013; 8: e56053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Svenningsson P, Chergui K, Rachleff I et al Alterations in 5‐HT1B receptor function by p11 in depression‐like states. Science 2006; 311: 77–80. [DOI] [PubMed] [Google Scholar]
- 212. Svenningsson P, Kim Y, Warner‐Schmidt J, Oh YS, Greengard P. p11 and its role in depression and therapeutic responses to antidepressants. Nat. Rev. Neurosci. 2013; 14: 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Sun HL, Zhou ZQ, Zhang GF et al Role of hippocampal p11 in the sustained antidepressant effect of ketamine in the chronic unpredictable mild stress model. Transl. Psychiatry 2016; 6: e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Hirota K, Lambert DG. Ketamine: Its mechanism(s) of action and unusual clinical uses. Br. J. Anesth. 1996; 77: 441–444. [DOI] [PubMed] [Google Scholar]
- 215. Kohrs R, Durieux ME. Ketamine: Teaching an old drug new tricks. Anesth. Analg. 1998; 87: 1186–1193. [DOI] [PubMed] [Google Scholar]
- 216. Williams NR, Heifets BD, Blasey C et al Attenuation of antidepressant effects of ketamine by opioid receptor antagonisms. Am. J. Psychiatry 2018; 175: 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Sanacora G. Caution against overinterpreting opiate receptor stimulation as mediating antidepressant effects of ketamine. Am. J. Psychiatry 2019; 176: 249. [DOI] [PubMed] [Google Scholar]
- 218. Heifets BD, Williams NR, Blasey C, Sudheimer K, Rodriguez CI, Schatzberg AF. Interpreting ketamine's opioid receptor dependent effect: Response to Sanacora. Am. J. Psychiatry 2019; 176: 249–250. [DOI] [PubMed] [Google Scholar]
- 219. Amiaz R. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism: Is it a ketamine‐specific effect? Am. J. Psychiatry 2019; 176: 250–251. [DOI] [PubMed] [Google Scholar]
- 220. Heifets BD, Williams NR, Blasey C, Sudheimer K, Rodriguez CI, Schatzberg AF. Target population, dose, and timing considerations for understanding naltrexone's subjective effect: Response to Amiaz. Am. J. Psychiatry 2019; 176: 251–252. [DOI] [PubMed] [Google Scholar]
- 221. Wang M, Kaplin A. Explaining naltrexone's interference with ketamine's antidepressant effect. Am. J. Psychiatry 2019; 176: 410–411. [DOI] [PubMed] [Google Scholar]
- 222. Yoon G, Petrakis IL, Krystal JH. Association of combined naltrexone and ketamine with depressive symptoms in a case series of patients with depression and alcohol use disorder. JAMA Psychiatry 2019; 76: 337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Heifets BD, Williams NR, Bentzley BS, Schatzberg AF. Rigorous trial design is essential to understand the role of opioid receptors in ketamine's antidepressant effect. JAMA Psychiatry 2019; 76: 657. [DOI] [PubMed] [Google Scholar]
- 224. Krystal JH, Yoon G, Petrakis IL. Rigorous trial design is essential to understand the role of opioid receptors in ketamine's antidepressant effect—Reply. JAMA Psychiatry 2019; 76: 658. [DOI] [PubMed] [Google Scholar]
- 225. Zhang K, Hashimoto K. Lack of opioid system in the antidepressant actions of ketamine. Biol. Psychiatry 2018; 85: e25–e27. [DOI] [PubMed] [Google Scholar]
- 226. O'Connor RM, Grenham S, Dinan TG, Cryan JF. microRNAs as novel antidepressant targets: Converging effects of ketamine and electroconvulsive shock therapy in the rat hippocampus. Int. J. Neuropsychopharmacol 2013; 16: 1885–1892. [DOI] [PubMed] [Google Scholar]
- 227. Yang X, Yang Q, Wang X et al MicroRNA expression profile and functional analysis reveal that miR‐206 is a critical novel gene for the expression of BDNF induced by ketamine. Neuromolecular Med. 2014; 16: 594–605. [DOI] [PubMed] [Google Scholar]
- 228. Grieco SF, Velmeshev D, Magistri M et al Ketamine up‐regulates a cluster of intronic miRNAs within the serotonin receptor 2C gene by inhibiting glycogen synthase kinase‐3. World J. Biol. Psychiatry 2017; 18: 445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Wan YQ, Feng JG, Li M et al Prefrontal cortex miR‐29b‐3p plays a key role in the antidepressant‐like effect of ketamine in rats. Exp. Mol. Med. 2018; 50: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Jiang H, Ling Z, Zhang Y et al Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015; 48: 186–194. [DOI] [PubMed] [Google Scholar]
- 231. Wong ML, Inserra A, Lewis MD et al Inflammasome signaling affects anxiety‐ and depressive‐like behavior and gut microbiome composition. Mol. Psychiatry 2016; 21: 797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Zheng P, Zeng B, Zhou C et al Gut microbiome remodeling induces depressive‐like behaviors through a pathway mediated by the host's metabolism. Mol. Psychiatry 2016; 21: 786–796. [DOI] [PubMed] [Google Scholar]
- 233. Burokas A, Arboleya S, Moloney RD et al Targeting the microbiota‐gut‐brain axis: Prebiotics have anxiolytic and antidepressant‐like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry 2017; 82: 472–487. [DOI] [PubMed] [Google Scholar]
- 234. Ho P, Ross DA. More than a gut feeling: The implications of the gut microbiota in psychiatry. Biol. Psychiatry 2017; 81: e35–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Yang C, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci. Rep. 2017; 7: 45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Zhang JC, Yao W, Dong C et al Blockade of interleukin‐6 receptor in the periphery promotes rapid and sustained antidepressant actions: A possible role of gut‐microbiota‐brain axis. Transl. Psychiatry 2017; 7: e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Macedo D, Filho AJ, Soares de Sousa CN et al Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J. Affect. Disord. 2017; 208: 22–32. [DOI] [PubMed] [Google Scholar]
- 238. Yang C, Fang X, Zhan G et al Key role of gut microbiota in anhedonia‐like phenotype in rodents with neuropathic pain. Transl. Psychiatry 2019; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Yang C, Qu Y, Fujita Y et al Possible role of gut‐microbiota in the antidepressant effects of (R)‐ketamine in a social defeat stress model. Transl. Psychiatry 2017; 7: 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Huang N, Hua D, Zhan G et al Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol. Biochem. Behav. 2018; 176: 93–100. [DOI] [PubMed] [Google Scholar]
- 241. Getachew B, Aubee JI, Schottenfeld RS, Csoka AB, Thompson KM, Tizabi Y. Ketamine interactions with gut‐microbiota in rats: Relevance to its antidepressant and anti‐inflammatory properties. BMC Microbiol. 2018; 18: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Krystal JH, Abdallah CG, Sanacora G, Charney DS, Duman RS. Ketamine: A paradigm shift for depression research and treatment. Neuron 2019; 101: 774–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Pushpakom S, Iorio F, Eyers PA et al Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019; 18: 41–58. [DOI] [PubMed] [Google Scholar]


