Abstract
OBJECTIVES
Vitamin K is thought to be involved in both bone health and maintenance of neuromuscular function. We tested the effect of vitamin K2 supplementation on postural sway, falls, healthcare costs, and indices of physical function in older people at risk of falls.
DESIGN
Parallel‐group double‐blind randomized placebo‐controlled trial.
SETTING
Fourteen primary care practices in Scotland, UK.
PARTICIPANTS
A total of 95 community‐dwelling participants aged 65 and older with at least two falls, or one injurious fall, in the previous year.
INTERVENTION
Once/day placebo, 200 μg or 400 μg of oral vitamin K2 for 1 year.
MEASUREMENTS
The primary outcome was anteroposterior sway measured using sway plates at 12 months, adjusted for baseline. Secondary outcomes included the Short Physical Performance Battery, Berg Balance Scale, Timed Up & Go Test, quality of life, health and social care costs, falls, and adverse events.
RESULTS
Mean participant age was 75 (standard deviation [SD] = 7) years. Overall, 58 of 95 (61%) were female; 77 of 95 (81%) attended the 12‐month visit. No significant effect of either vitamin K2 dose was seen on the primary outcome of anteroposterior sway (200 μg vs placebo: −.19 cm [95% confidence interval [CI] −.68 to .30; P = .44]; 400 μg vs placebo: .17 cm [95% CI −.33 to .66; P = .50]; or 400 μg vs 200 μg: .36 cm [95% CI −.11 to .83; P = .14]). Adjusted falls rates were similar in each group. No significant treatment effects were seen for other measures of sway or secondary outcomes. Costs were higher in both vitamin K2 arms than in the placebo arm.
CONCLUSION
Oral vitamin K2 supplementation did not improve postural sway or physical function in older people at risk of falls. J Am Geriatr Soc 67:2102–2107, 2019
Keywords: falls, vitamin K, postural sway, health economic analysis, recruitment
Current evidence suggests that multifactorial falls interventions (combining strength and balance training, home environment modification, calcium and vitamin D supplementation, cataract extraction, and medication review) reduce falls in selected patients,1 but the magnitude of effect is modest. Furthermore, such interventions are expensive,2 labor intensive, and appear less successful when applied to a general older population3, 4 than when applied to more highly selected groups of at‐risk individuals referred to falls clinics. New simple and inexpensive ways of reducing falls in the wider population of older people are therefore needed.
Vitamin K is an essential cofactor for gamma carboxylation, required for the effective function of a range of proteins5 including those involved in bone remodeling, vascular calcification, glucose handling, inflammation, and neuromuscular function.6, 7, 8, 9, 10, 11, 12 Low levels of dietary vitamin K intake are very common and associated with a higher risk of cardiovascular disease and osteoporosis.13, 14
In our previous work, a nonsignificant improvement in postural sway was seen with 6 months of once/day supplementation with 100 μg vitamin K2 in participants aged 70 and older with vascular disease.15 Before conducting a large trial to evaluate whether vitamin K supplementation can reduce falls in older people at high risk, it is necessary to test which dose of vitamin K is effective (using a surrogate marker of falls risk) and to test whether recruitment to such a trial is feasible. The aims of this trial were therefore first to establish the optimum vitamin K dose to improve postural sway in older people, second to test recruitment strategy and likely effect size, and third to conduct preliminary health economic analyses to inform the design of future large multicenter trials of vitamin K to reduce falls in at‐risk older people.
METHODS
Trial Design
This was a randomized double‐blind parallel‐group placebo‐controlled trial. Ethics approval was obtained from East of Scotland Research Ethics committee (approval number 15/ES/0197), and the trial was registered at http://isrctn.com (ISRCTN18436190). Written informed consent was obtained from all participants at the screening visit.
Population and Recruitment
We recruited community‐dwelling participants aged 65 and older with either two or more falls in the previous 12 months, or at least one fall resulting in hospitalization in the last 12 months. Exclusion criteria were an inability to give written informed consent; unable to stand without human assistance; atrial fibrillation (because this group should usually be taking warfarin); taking warfarin or other coumadin derivatives; taking more than 100 μg vitamin K supplement per day; known contraindication to vitamin K; currently enrolled in, or within 30 days of completing another trial; currently undertaking physiotherapy or another time‐limited supervised nonpharmacologic intervention to reduce falls risk; and intolerance to soy products. All participants were recruited via primary care from three Health Board areas (Tayside, Grampian, and Fife). Telephone prescreening was conducted, and those eligible at this stage were invited to a combined screening and baseline visit.
Intervention and Comparator
Matching tablets containing either 200 μg vitamin K2 (MK7 subtype), 400 μg vitamin K2 (MK7 subtype), or placebo were manufactured and bottled by Legosan AB (Kumla, Sweden) and distributed to sites by Tayside Pharmaceuticals (Dundee, Scotland). The trial product was provided in identical bottles with a unique trial identifier on each bottle to ensure masking to participants, clinicians, and researchers. Participants were asked to take one tablet each day for the 12 months of the trial. No clear evidence exists to favor vitamin K1 or K2, or to favor a particular subtype of vitamin K2. However, MK7 was the subtype used in our previous trial that suggested possible benefit on postural sway.15 Thus this subtype was selected for use in the current trial but at higher doses than we used previously.
Randomization and Allocation Concealment
Randomization was performed in a 1:1:1 ratio by an online‐based randomization system, run by the Health Informatics Centre, University of Dundee, to ensue allocation concealment. A minimization algorithm with a small random element was used to ensure balance across recruitment centers and key baseline measures. Minimization factors were trial center (Tayside, Grampian, or Fife), age (>80 or ≤80 y), and baseline anteroposterior (AP) sway (>3 cm or ≤3 cm).
Outcomes
The primary outcome was the between‐group difference in AP sway at 12 months, measured using the AMTI AccuSway sway platform (AMTI, Watertown, MA, USA). Sway was measured with participants standing with feet together on the platform; three 30‐second runs were performed with eyes open, followed by three 30‐second runs with eyes closed. Sampling rate was 100 Hz, and proprietary software (AMTI Balance Clinic) was used to derive the outcome measures from raw center of pressure data. The mean value of each set of three runs was taken as the outcome measure. AP sway was previously shown to predict future falls in a range of populations.16, 17, 18 The secondary outcomes were other markers of postural sway (mediolateral sway, 95% ellipse, total path length) compared between groups at 6 and 12 months; Berg Balance Scale,19 the Short Physical Performance Battery,20 Timed Up & Go Test,21 falls frequency collected using monthly falls diaries,22 and desphospho‐undercarboxylated matrix Gla protein (dp‐ucMGP) levels as a measure of the biological effect of vitamin K.23 The dp‐ucMGP was measured using a semi‐commercial enzyme‐linked immunosorbent assay by VitaK (Maastricht, Netherlands).24 Office blood pressure was measured using an OMRON HEM‐705 oscillometric device (OMRON). Participants were rested in a supine position for a minimum of 5 minutes, supine blood pressure was measured three times, and the mean of the second and third readings was used. Postural drop in blood pressure was measured as the largest drop between supine blood pressure and standing blood pressure measured immediately on standing, at 1 minute, and 3 minutes. We also collected health and social care utilization at each visit, and quality of life using the EuroQoL EQ5D‐5 L and the Investigating Choice Experiments for the preferences of older people Capability Tool for Older People (ICECAP‐O) tools.25, 26 Adherence was measured by tablet count, comparing the number returned with the number expected to be returned at each study visit. All assessments were conducted by research nurses masked to treatment allocation.
Analysis
All analyses were conducted according to a prespecified statistical analysis plan, using SPSS software v.22 (IBM, Armonk, NY, USA). A two‐sided P value <.015 was taken as significant for all analyses. Details of the analysis and sample size calculations are provided in Supplementary Material S1.
RESULTS
We sent study information to 4145 individuals identified from screening 14 primary care practices (6 in Tayside, 4 in Grampian, and 4 in Fife). A total of 444 expressed interest in the trial, of whom 99 attended a screening visit and 95 were randomized between June 28, 2016, and July 4, 2017. Baseline details of those randomized are given in Table 1; Supplementary Material S1 shows the Consolidated Standards of Reporting Trials diagram for participant flow through the trial. No significant difference in adherence was found between groups: 90% (SD = 20) in the 200 μg vitamin K group, 82% (SD = 27) in the 400 μg vitamin K group, and 88% (SD = 33) in the placebo group (P > .05 for all comparisons).
Table 1.
Baseline Characteristics
| Placebo (n = 32) | Vitamin K 200 μg (n = 32) | Vitamin K 400 μg (n = 31) | |
|---|---|---|---|
| Mean age, y (SD) | 75.0 (6.9) | 74.7 (7.4) | 75.1 (6.5) |
| Female sex (%) | 18 (56) | 21 (66) | 19 (61) |
| Median number of falls in last year (IQR) | 3 (2‐7) | 3 (2‐6) | 3 (2‐4) |
| Uses walking aid (%) | 21 (66) | 15 (47) | 12 (39) |
| Previous myocardial infarction (%) | 2 (6) | 1 (3) | 1 (3) |
| Chronic heart failure (%) | 0 (0) | 1 (3) | 0 (0) |
| Parkinsonian syndrome (%) | 0 (0) | 0 (0) | 0 (0) |
| Previous stroke (%) | 2 (6) | 2 (6) | 3 (10) |
| Hypertension (%) | 17 (53) | 15 (47) | 18 (58) |
| Diabetes mellitus (%) | 6 (19) | 1 (3)* | 8 (26) |
| Peripheral neuropathy (%) | 4 (13) | 1 (3) | 2 (6) |
| Previous fragility fracture (%) | 8 (25) | 14 (44) | 12 (39) |
| Osteoarthritis (%) | 15 (47) | 19 (59) | 18 (58) |
| Chronic obstructive pulmonary disease (%) | 4 (13) | 7 (22) | 6 (19) |
| Cataracts (%) | 9 (28) | 15 (47) | 12 (39) |
| Retinopathy (%) | 2 (6) | 1 (3) | 3 (10) |
| Median no. of medications (IQR) | 5 (4‐8) | 6 (3‐10) | 6 (4‐9) |
| ACEi/ARB (%) | 14 (44) | 12 (38) | 12 (39) |
| Other antihypertensive or antianginal (%) | 13 (41) | 14 (44) | 15 (48) |
| Vitamin D or analog (%) | 4 (13) | 12 (38)* | 12 (39)* |
| Bisphosphonate (%) | 1 (3) | 5 (16) | 5 (16) |
| Antidepressant (%) | 8 (25) | 8 (25) | 7 (23) |
| Hypnotic (%) | 5 (16) | 0 (0)* | 1 (3) |
| Opioid (%) | 13 (41) | 10 (31) | 8 (26) |
| Mean body mass index, kg/m2 (SD) | 29.7 (4.7) | 29.4 (5.1) | 30.8 (7.9) |
| Anteroposterior sway, cm (SD) | 2.74 (1.01) | 2.95 (.88) | 2.93 (1.21) |
Abbreviations: ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; IQR, interquartile range; SD, standard deviation.
*P < .05 compared with placebo.
Figure 1 and Table 2 show the primary outcome results. No significant differences in AP sway were seen between groups at 12 months, either adjusted for baseline or adjusted for minimization variables. Table 2 and Supplementary Material S1 show the secondary outcomes. No significant differences were seen between groups for other measures of sway, for blood pressure, or for measures of physical performance. Dp‐ucMGP levels were significantly lower, however, in the 200 μg and 400 μg vitamin K2 groups than in the placebo group, confirming that vitamin K was producing the expected dose‐dependent effect on this marker of vitamin K biological activity. Supplementary Material S1 contains the results of falls analyses. The treatment groups did not show a statistically significantly different falls rate to placebo, although falls rates were higher in the 200‐μg arm. Time to first fall was also not significantly different from placebo in the treatment groups. There were more adverse events in the 200‐μg arm than in the placebo or 400‐μg arms as shown in Supplementary Material S1. This was driven by a slightly higher rate of gastrointestinal adverse events and a higher injury rate, paralleling the higher falls rate seen in the 200‐μg arm.
Figure 1.
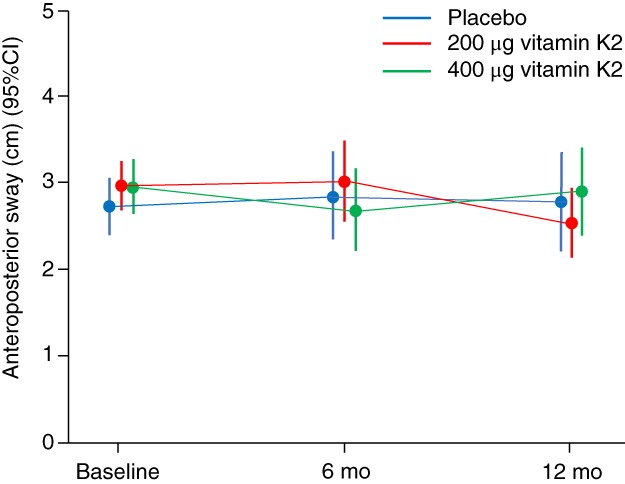
Anteroposterior sway at each time point by treatment group. CI, confidence interval.
Table 2.
Anteroposterior Sway and Physical Performance Measures
| Baseline | 6 mo | 12 mo | ||
|---|---|---|---|---|
| Unadjusted anteroposterior sway, cm (SD) | Placebo | 2.74 (1.01) | 2.84 (1.35) | 2.75 (1.38) |
| Vitamin K 200 μg | 2.95 (.88) | 3.01 (1.33) | 2.51 (1.02) | |
| Vitamin K 400 μg | 2.93 (1.21) | 2.67 (1.22) | 2.88 (1.20) | |
| Treatment effect at 12 mo, cm (95% CI) | p | |||
| Adjusted for baseline | 200 μg vs placebo | −.19 (−.68 to .30) | .44 | |
| 400 μg vs placebo | .17 (−.33 to .66) | .50 | ||
| 200 μg vs 400 μg | .36 (−.11 to .83) | .14 | ||
| Fully adjusteda | 200 μg vs placebo | −.09 (−.53 to .35) | .69 | |
| 400 μg vs placebo | .19 (−.25 to .63) | .40 | ||
| 200 μg vs 400 μg | .28 (−.15 to .70) | .20 | ||
| Multiple imputationa | 200 μg vs placebo | −.20 (−.66 to .26) | .39 | |
| 400 μg vs placebo | .04 (−.44 to .51) | .88 | ||
| 200 μg vs 400 μg | .24 (−.22 to .67) | .31 | ||
| Repeated measuresa | 200 μg vs placebo | −.07 (−.43 to .30) | .72 | |
| 400 μg vs placebo | −.08 (−.44 to .28) | .66 | ||
| 200 μg vs 400 μg | −.02 (−.37 to .34) | .93 | ||
| Baseline | 6 mo | 12 mo | ||
| Unadjusted SPPB (mean, SD) | Placebo | 7.7 (2.3) | 8.0 (2.7) | 7.9 (3.0) |
| Vitamin K 200 μg | 7.4 (2.6) | 7.8 (2.7) | 7.8 (2.7) | |
| Vitamin K 400 μg | 7.1 (2.7) | 7.2 (2.8) | 6.7 (3.1) | |
| Treatment effect at 12 mo | P | |||
| SPPB treatment effecta (95% CI) | 200 μg vs placebo | −.2 (−1.1 to .7) | .63 | |
| 400 μg vs placebo | −.4 (−1.3 to .5) | .37 | ||
| 200 μg vs 400 μg | −.2 (−1.1 to .7) | .66 | ||
| Baseline | 6 mo | 12 mo | ||
| Unadjusted Timed Up & Go (median, IQR) | Placebo | 12.9 [9.7‐17.1] | 9.9 [8.5‐16.2] | 11.2 [9.1‐17.2] |
| Vitamin K 200 μg | 14.2 [9.2‐21.3] | 11.7 [8.4‐18.1] | 12.5 [8.9‐19.0] | |
| Vitamin K 400 μg | 14.1 [9.4‐20.4] | 12.9 [8.4‐17.9] | 13.3 [9.6‐18.7] | |
| Treatment effect at 12 mo | P | |||
| Log10 Timed Up & Go Test treatment effecta (95% CI) | 200 μg vs placebo | .02 (−.03 to .07) | .44 | |
| 400 μg vs placebo | .03 (−.02 to .09) | .23 | ||
| 200 μg vs 400 μg | .01 (−.04 to .06) | .64 | ||
| Baseline | 6 mo | 12 mo | ||
| Unadjusted Berg Balance Scale (median, IQR) | Placebo | 50 [45‐53] | 51 [38‐53] | 52 [41‐54] |
| Vitamin K 200 μg | 48 [42‐53] | 52 [46‐54] | 50 [47‐54] | |
| Vitamin K 400 μg | 48 [44‐54] | 47 [42‐54] | 49 [40‐54] | |
| Treatment effect at 12 mo | P | |||
| Log10 Berg Balance Scale treatment effecta (95% CI) | 200 μg vs placebo | .00 (−.00 to .02) | .31 | |
| 400 μg vs placebo | −.00 (−.02 to .01) | .80 | ||
| 200 μg vs 400 μg | −.01 (−.03 to .00) | .20 | ||
Abbreviation: CI, confidence interval; IQR, interquartile range; SD, standard deviation; SPPB, Short Physical Performance Battery.
Adjusted for age, sex, baseline anteroposterior sway, and trial center.
Costs were lowest over 1 year among participants randomized to the placebo group (mean cost = £764 per participant). Costs were lower among participants in the low‐dose vitamin K2 group, relative to high‐dose vitamin K2 (£896‐£902 vs £1033‐£1044). Supplementary Material S1 combines the data just cited to show the incremental costs and quality‐adjusted life years for the 200 μg and 400 μg vitamin K2 arms compared with placebo. Neither dose of vitamin K2 produced any improvement relative to placebo. Supplementary Material S1 shows the Expected Value of Sample Information graph. Based on the assumptions used, the optimal sample size for the most cost‐effective future trial would be 131 participants per group, but such a trial would remain cost effective up to a sample size of 1100 per group.
DISCUSSION
There are a number of possible explanations for the lack of observed benefit of vitamin K supplementation that merit consideration. Adherence was good, and the Dp‐ucMGP results confirm that the vitamin K had a measurable biological effect, but it is still possible that an even larger dose is required to improve sway or that vitamin K has to be given for longer to achieve an effect. The study population was at risk of falls, as shown by the average of 1.7 falls per participant during the trial. However, postural sway was relatively low in the trial population. Thus a ceiling effect may have limited the ability of the trial interventions to produce a measurable change. A more challenging balance task than standing on a firm level surface may be required to allow any benefit of the intervention to be observed in this participant group. Although previous studies found that many older people have low dietary vitamin K intakes, we did not examine dietary intake of vitamin K via food diaries in this trial, and so we cannot be sure if the trial population had particularly low vitamin K intakes. However, the fact that both 200‐μg and 400‐μg doses of vitamin K significantly lowered dp‐ucMGP levels suggests that participants were not vitamin K replete at baseline.
One previous trial examined the effect of vitamin K supplementation on postural sway; this trial did not find a significant benefit of lower dose vitamin K supplementation on this outcome.15 Previous trials suggest, but do not establish conclusively, a beneficial effect on vascular health of vitamin K. A recent meta‐analysis7 showed statistically significant improvements in vascular calcification but not in vascular stiffness, arguably a more important functional measure of vascular health and one that has been linked to orthostatic hypotension, a potential mediator of falls. It is possible that an even longer duration of therapy is required to produce benefit from vitamin K supplementation; one trial in postmenopausal women did not find significant benefit before the third year of supplementation.27
Our trial has a number of strengths. The groups were balanced at baseline, the trial recruited participants at an elevated risk of falls, and both retention and intervention adherence were good. The involvement of multiple recruitment and assessment center improves the generalizability of our results. A number of additional limitations require comment. Effects in people with different ethnicity or diet cannot be ascertained because the trial population were overwhelmingly white, and dietary information was not collected. The trial was not powered to detect a difference in falls rates, and thus an effect on falls rates cannot be excluded; such an effect could be mediated by mechanisms other than improvements in postural sway or orthostatic hypotension that we did not measure in this trial.
It is possible that populations at higher risk (eg, those in nursing homes, falls clinics, or those with very low vitamin K intake) could still potentially benefit, and future research could usefully target these groups. At present, however, insufficient evidence exists to recommend use of vitamin K supplementation in community‐dwelling older adults at risk of falls.
Supporting information
Supplementary Material S1: Supplementary methods (analysis and sample size calculation), results tables and figures
ACKNOWLEDGMENTS
With thanks to NHS Support for Science for supporting the trial, the participants for agreeing to take part, and NHS Research Scotland Primary Care Network for their help recruiting participants. We thank Petra Rauchhaus for statistical advice during preparation of the statistical analysis plan for this trial and Tayside Clinical Trials Unit for their assistance with the management of the trial. The full protocol, statistical, and health economic analysis plans are available from the corresponding author on request. Professor Witham acknowledges support from the NIHR Newcastle Biomedical Research Centre.
Financial Disclosure: This work was supported by the Chief Scientist Office, Scottish Government, grant number CZH/4/1100.
Conflicts of Interest: None to declare.
Author Contributions: Study design: Witham, Price, Band, Fulton, Clarke, Donnan, McNamee, Cvoro, and Soiza. Study management: All authors. Analysis of results: Witham, Hannah, Donnan, and McNamee. Interpretation of results: All authors. Drafting of manuscript: Witham and McNamee. Critical revision of manuscript: All authors.
Sponsor's Role: The sponsor approved the protocol, but the design, results analysis, and manuscript drafting were all performed independently with no input from the sponsor (Tayside Academic Sciences Centre, a joint enterprise between University of Dundee and NHS Tayside)
This work was presented at the British Geriatrics Society Spring scientific meeting, held in Cardiff, UK, April 10, 2019. Trial Registration: ISRCTN18436190.
REFERENCES
- 1. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Irvine L, Conroy SP, Sach T, et al. Cost‐effectiveness of a day hospital falls prevention programme for screened community‐dwelling older people at high risk of falls. Age Ageing. 2010;39:710–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gates S, Fisher JD, Cooke MW, Carter YH, Lamb SE. Multifactorial assessment and targeted intervention for preventing falls and injuries among older people in community and emergency care settings: systematic review and meta‐analysis. BMJ. 2008;336:130–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Finnegan S, Bruce J, Skelton DA, Withers EJ, Lamb SE, PreFIT Study Group . Development and delivery of an exercise programme for falls prevention: the prevention of falls injury trial (PreFIT). Physiotherapy. 2018;104:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shearer MJ, Okano T. Key pathways and regulators of vitamin K function and intermediary metabolism. Annu Rev Nutr. 2018;38:127–151. [DOI] [PubMed] [Google Scholar]
- 6. Harshman SG, Shea MK. The role of vitamin K in chronic aging diseases: inflammation, cardiovascular disease, and osteoarthritis. Curr Nutr Rep. 2016;5:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lees JS, Chapman FA, Witham MD, Jardine AG, Mark PB. Vitamin K status, supplementation and vascular disease: a systematic review and meta‐analysis. Heart. 2019;105(2):938–945. [DOI] [PubMed] [Google Scholar]
- 8. Manna P, Kalita J. Beneficial role of vitamin K supplementation on insulin sensitivity, glucose metabolism, and the reduced risk of type 2 diabetes: a review. Nutrition. 2016;32:732–739. [DOI] [PubMed] [Google Scholar]
- 9. Brandenburg VM, Schurgers LJ, Kaesler N, et al. Prevention of vasculopathy by vitamin K supplementation: can we turn fiction into fact? Atherosclerosis. 2015;240:10–16. [DOI] [PubMed] [Google Scholar]
- 10. Viegas CS, Rafael MS, Enriquez JL, et al. Gla‐rich protein acts as a calcification inhibitor in the human cardiovascular system. Arterioscler Thromb Vasc Biol. 2015;35:399–408. [DOI] [PubMed] [Google Scholar]
- 11. Shafit‐Zagardo B, Gruber RC, DuBois JC. The role of TAM family receptors and ligands in the nervous system: from development to pathobiology. Pharmacol Ther. 2018;188:97–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin X, Brennan‐Speranza TC, Levinger I, Yeap BB. Undercarboxylated osteocalcin: experimental and human evidence for a role in glucose homeostasis and muscle regulation of insulin sensitivity. Nutrients. 2018;10:E847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thane CW, Bolton‐Smith C, Coward WA. Comparative dietary intake and sources of phylloquinone (vitamin K1) among British adults in 1986‐7 and 2000‐1. Br J Nutr. 2006;96:1105–1115. [DOI] [PubMed] [Google Scholar]
- 14. Rees K, Guraewal S, Wong YL, et al. Is vitamin K consumption associated with cardio‐metabolic disorders? A systematic review. Maturitas. 2010;67:121–128. [DOI] [PubMed] [Google Scholar]
- 15. Fulton RL, McMurdo ME, Hill A, et al. Effect of vitamin K on vascular health and physical function in older people with vascular disease—a randomised controlled trial. J Nutr Health Aging. 2016;20:325–333. [DOI] [PubMed] [Google Scholar]
- 16. Pajala S, Era P, Koskenvuo M, Kaprio J, Törmäkangas T, Rantanen T. Force platform measures as predictors of indoor and outdoor falls in community‐dwelling older women aged 63‐76 years. J Gerontol A Biol Sci Med Sci. 2008;63A:171–178. [DOI] [PubMed] [Google Scholar]
- 17. Piirtola M, Era P. Force platform measurements as predictors of falls among older people—a review. Gerontology. 2006;52:1–16. [DOI] [PubMed] [Google Scholar]
- 18. Maki BE, Holliday PJ, Topper AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J Gerontol. 1994;49:M72–M84. [DOI] [PubMed] [Google Scholar]
- 19. Berg KO, Wood‐Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(suppl 2):S7–S11. [PubMed] [Google Scholar]
- 20. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 21. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 22. Lamb SE, Jorstad‐Stein EC, Hauer K, Becker C, Prevention of Falls Network Europe and Outcomes Consensus Group . Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53:1618–1622. [DOI] [PubMed] [Google Scholar]
- 23. Riphagen IJ, Keyzer CA, Drummen NEA, et al. Prevalence and effects of functional vitamin K insufficiency: the PREVEND study. Nutrients. 2017;9:E1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cranenburg EC, Koos R, Schurgers LJ, et al. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104:811–822. [DOI] [PubMed] [Google Scholar]
- 25. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five‐level version of EQ‐5D (EQ‐5D‐5L). Qual Life Res. 2011;20:1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flynn T, Chan P, Coast J, Peters TJ. Assessing quality of life among British older people using the ICEPOP CAPability (ICECAP‐O) measure. Appl Health Econ Health Policy. 2011;9:317–329. [DOI] [PubMed] [Google Scholar]
- 27. Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer C. Menaquinone‐7 supplementation improves arterial stiffness in healthy postmenopausal women. A double‐blind randomised clinical trial. Thromb Haemost. 2015;113:1135–1144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material S1: Supplementary methods (analysis and sample size calculation), results tables and figures


