Abstract
Cellulolytic clostridia use a highly efficient cellulosome system to degrade polysaccharides. To regulate genes encoding enzymes of the multi‐enzyme cellulosome complex, certain clostridia contain alternative sigma I (σI) factors that have cognate membrane‐associated anti‐σI factors (RsgIs) which act as polysaccharide sensors. In this work, we analyzed the structure‐function relationship of the extracellular sensory elements of Clostridium (Ruminiclostridium) thermocellum and Clostridium clariflavum (RsgI3 and RsgI4, respectively). These elements were selected for comparison, as each comprised two tandem PA14‐superfamily motifs. The X‐ray structures of the PA14 modular dyads from the two bacterial species were determined, both of which showed a high degree of structural and sequence similarity, although their binding preferences differed. Bioinformatic approaches indicated that the DNA sequence of promoter of sigI/rsgI operons represents a strong signature, which helps to differentiate binding specificity of the structurally similar modules. The σI4‐dependent C. clariflavum promoter sequence correlates with binding of RsgI4_PA14 to xylan and was identified in genes encoding xylanases, whereas the σI3‐dependent C. thermocellum promoter sequence correlates with RsgI3_PA14 binding to pectin and regulates pectin degradation‐related genes. Structural similarity between clostridial PA14 dyads to PA14‐containing proteins in yeast helped identify another crucial signature element: the calcium‐binding loop 2 (CBL2), which governs binding specificity. Variations in the five amino acids that constitute this loop distinguish the pectin vs xylan specificities. We propose that the first module (PA14A) is dominant in directing the binding to the ligand in both bacteria. The two X‐ray structures of the different PA14 dyads represent the first reported structures of tandem PA14 modules.
Keywords: anti‐sigma factors, biomass sensing, cellulosome, crystallography, RsgI, sigI, sigma factors
Abbreviations
- CBL
calcium‐binding loop
- CBM
carbohydrate‐binding module
- GH
glycoside hydrolase
- RsgI
anti‐σI factor
- sigI
sigma I (σI) factor
1. INTRODUCTION
Cellulolytic clostridia, notably Clostridium (Ruminiclostridium) thermocellum, have been extensively studied for their remarkable ability to ferment plant‐derived polysaccharides.1, 2 The biomass degradation is performed by a highly efficient multi‐enzyme system called cellulosome.3 Genomes of cellulosome‐producing bacteria encode a large variety of saccharolytic enzymes, including cellulases, hemicellulases, and pectin‐degrading enzymes.3, 4 During the saccharolytic process, the type(s) of enzymes that are incorporated into the cellulosome are adjusted to suit the type(s) of polysaccharide present in the biomass.5, 6, 7
In an effort to better understand the mechanisms that govern the adjustment of the enzymatic composition of the cellulosome, our research group discovered in C. thermocellum a collection of eight RNA polymerase alternative sigI (σI) factors (Figure 1).8, 9 Six of these σIs (σI1 to σI6) have cognate membrane‐associated anti‐σI factors (RsgI1‐RsgI6) that contain C‐terminal sensory elements, such as carbohydrate‐binding modules (eg, CBM3 and CBM2), sugar‐binding elements (eg, PA14 motif) or a glycoside hydrolase (eg, GH10 and GH5 families). The RsgI‐borne sensory elements are displayed on the cell surface, suggesting that RsgIs may act as polysaccharide sensors.8 Furthermore, the expression of σI1 to σI6 was shown to be affected by the presence of different polysaccharides in the growth medium (eg, cellulose and xylan).9 Recent publications have shown that these sensing systems are also present in other cellulosome‐producing bacteria, namely, Clostridium (Ruminiclostridium) clariflavum (Figure 1), Clostridium (Ruminiclostridium) straminisolvens, Clostridium sp. Bc‐iso‐3, Acetivibrio cellulolyticus, and Bacteroides (Pseudobacteroides) cellulosolvens.10, 11
Figure 1.
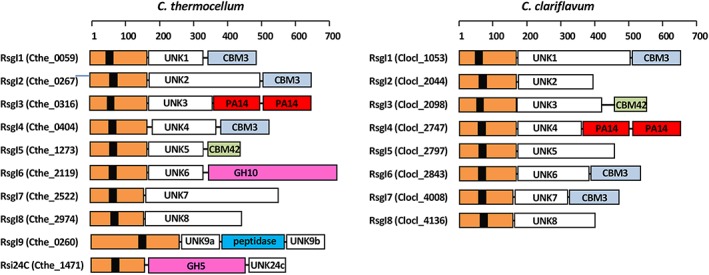
Schematic representation of the modular organization of RsgI proteins in C. thermocellum and C. clariflavum. Each multi‐modular protein has a RsgI domain (orange) with an N‐terminal transmembrane region (black). Additional modules are marked in different colors as follows: PA14, red; GH10, magenta; GH5, magenta; CBM3, light blue; CBM42, mint; UNK, divergent domains of unknown function/s having no sequence identity to other proteins, white; peptidase, blue. See main text for details. The linkers between the modules are indicated as lines. The ruler above indicates the length of the proteins (number of amino acids) [Color figure can be viewed at http://wileyonlinelibrary.com]
Alternative σ factors are highly promoter specific.12, 13, 14 In this regard, a recent study showed that the C. thermocellum σI3 factor is involved in the regulation of genes encoding pectin‐degrading enzymes by using highly stringent promoter sequences.10 These observations correlate with the binding capacities of the sensory element of the anti‐σI3 factor, which comprises two tandem PA14 motifs that bind pectin.8
The PA14 motif is a conserved protein module, possessing a core β‐barrel topology, shared by a wide variety of bacterial and eukaryotic proteins.15, 16, 17, 18, 19 PA14 occurs either as a single module, a doublet, or even a triplet in different proteins. However, the biological significance of these duplications has not been clear.20 PA14 modules appear as components of carbohydrate‐active enzymes,17, 21, 22 epithelial adhesins,15, 16, 18, 19 flocculins,18 and bacterial toxins, such as the anthrax protective antigen (PA), which is also a component of the anthrax toxin complex of known 3D structure.23 According to Rigden et al,20 PA14 was predicted to be a putative CBM. Only the structures of single PA14 modules have been determined to date.
In the present work, we combined biochemical analysis, computational biology and structural methods to better understand the relationship between the comparative binding specificities of RsgI‐based PA14 modular dyads and the respective regulons of their cognate σI factors in two different clostridial species. The term, dyad, as used in this article, differs from the crystallographic meaning as dimers. In this article, dyad refers to a modular element comprising two parts and is consistent with the term modular dyad as used previously.24, 25, 26, 27 By using bioinformatic approaches, we showed that the σI4 of C. clariflavum appears to be involved in the regulation of genes encoding mainly xylanases, as opposed to σI3 of C. thermocellum that was previously shown to be involved in the regulation of genes encoding mainly pectinases. These results correlate well with the binding capacities of the sensory elements that comprise PA14 dyads in their cognate RsgIs.
The crystal structures of the PA14 modular dyads in both C. thermocellum and C. clariflavum showed a high degree of similarity to the single PA14 modules from yeast that bind either galactosyl or mannosyl carbohydrates.18, 28 The rare DcisD calcium‐binding motif was detected in the RsgI_PA14. The potential carbohydrate‐binding mechanism of RsgI_PA14 was proposed, based on the comparison between the RsgI_PA14 modules and PA14 modules derived from yeast. Differences in primary structures of the calcium‐binding loop 2 (CBL2) of the RsgI_PA14s appear to play an important role in the recognition of their target polysaccharide.
2. MATERIALS AND METHODS
2.1. Cloning
DNA fragments encoding the PA14 dyad (PA14AB) of RsgI4 (Clocl_2747) (GenBank accession No. WP_014255852) were amplified by PCR from C. clariflavum DSM 19732 genomic DNA. DNA fragments encoding RsgI3 (Cthe_0316) PA14 dyad (PA14AB; GenBank accession No. KM504391), were amplified by PCR from C. thermocellum ATCC 27405 genomic DNA. Genomic DNA was isolated as described by Murray and Thompson.29 For the cloning of C. clariflavum PA14A: PA14A_C.cla_For (NdeI) 5′‐GGAATTCCATATGGGATTAAGAGGCGATTA‐3′ and PA14A_C.cla_Rev (XhoI) 5′‐CCGCTCGAGTCACGCCGGATACAGGCATTCA‐3′ were used; for PA14B: PA14B_C.cla_For (NdeI) 5′‐GGAATTCCATATGGGCTTGTTCTATGAGTA‐3′ and PA14B_C.cla_Rev (XhoI) 5′‐CCGCTCGAGTTATCTTGGATATAAACAGCT‐3′; for the cloning of the PA14AB: PA14A_C.cla_For and PA14B_C.cla_Rev. For the cloning of C. thermocellum PA14A: PA14A_C.the_For (NcoI) 5′‐CCATGGCGCCAACCGTCAGAAACGG‐3′ and PA14A_C.the_Rev (NotI) 5′‐GCGGCCGCGGAAGGATACAGTTGACTTG‐3′ were used; for PA14B: PA14B_C.the_For (NcoI) 5′‐CCATGGACGGCCCGCTGCCTCAG‐3′ and PA14B_C.the_Rev (NotI) 5′‐GCGGCCGCATCTGCAAACAAATTTTTTGAAG‐3′; for the cloning of the PA14AB: PA14A_C.the_For and PA14B_C. the_Rev. To obtain constructs with N′ terminal hexahistidine (His) tag, primers PA14AB_C.the_For (NdeI) 5′‐CATATGGGACTTAGGGGAGAGTATTAC‐3′ and PA14AB_C.the_Rev (XhoI) 5′‐CTCGAGATCTGCAAACAAATTTTTTGAAGG‐3′ were used for the cloning of C. thermocellum PA14AB. The PCR products were purified, cloned into pGEM‐T easy vector according to the manufacturer's instructions (Promega, Madison, WI), cleaved with the indicated restriction enzymes, and inserted into the pET‐28a(+) expression vector (Novagen, Madison, WI). The obtained constructs, that is, pET‐PA14AB_C.cla, containing the PA14 dyad, pET‐PA14A_C.cla, and pET‐PA14B_C.cla, containing both single PA14 modules, contained an N‐terminal His tag and thrombin cleavage site. Constructs pET‐PA14AB_C.the_C′, pET‐PA14AB_C.the_N′, containing the PA14 dyad and pET‐PA14A_C.the and pET‐PA14B_C.the, contained either C‐terminal or N‐terminal His tags as indicated.
2.2. Protein expression
Overnight cultures of E. coli BL21(DE3)/pET28a(+), bearing pET‐PA14AB_C.cla, pET‐PA14A_C.cla, pET‐PA14B_C.cla, pET‐PA14AB_C.the_C′, pET‐PA14AB_C.the_N′, pET‐PA14A_C.the, and pET‐PA14B_C.the were diluted to an absorbance at 600 nm of 0.1 in LB broth (Lennox, Difco, BD Diagnostics, Sparks, MD), containing kanamycin (50 μg/mL), and shaken vigorously at 37°C. When cultures reached an absorbance at 600 nm, isopropyl‐β‐d‐thiogalactopyranoside (IPTG; Sigma, St. Louis, MO) was added to a final concentration of 0.5 mM. The cells were grown overnight at ~20°C and harvested by centrifugation.
2.3. Protein purification
The cell pellet was resuspended in sonication buffer (50 mM sodium phosphate buffer pH 7.6 containing 300 mM NaCl, glycerol 10%, and imidazole 10‐20 mM), and the suspension was sonicated in an ultrasonic processor (Misonics, Seoul, Korea) until the suspension clarified. The sonicate was centrifuged at 5000g for 45 min at 4°C. The recombinant His‐tagged protein was first isolated by metal‐chelate affinity chromatography, whereby the supernatant fluids were loaded onto a HisTrap FF Ni Sepharose column (GE Healthcare Bio‐Sciences AB, Uppsala, Sweden), equilibrated with the sonication buffer, washed thoroughly with buffer, and eluted with buffer containing 500 mM imidazole. Further purification was accomplished by fast protein liquid chromatography on a Superdex 75 16/60 column using an ÄKTA prime system (GE Healthcare, Chicago, IL), equilibrated with a solution of 50 mM Tris‐HCl at pH 7.5 or 8.4, 150 mM NaCl, and sodium azide 0.05%. The eluted protein (the major peak eluted as monomer) was collected.
For crystallization, C. clariflavum PA14AB was subsequently cleaved by thrombin (Novagen) according to manufacturer's instructions, in buffer containing 20 mM Tris‐HCl pH 8.4, 150 mM NaCl, 2.5 mM CaCl2, incubated at 15°C overnight. The reaction mixture was dialyzed against buffer containing 50 mM Tris‐HCl, pH 7.6, concentrated, and subjected to anion exchange chromatography using a Mono Q anion exchange chromatography column (GE Healthcare) in the same buffer. The protein failed to bind to the column and eluted at the void volume. The thrombin‐cleaved protein contained three additional residues (GSH) that originated from the cleavage site of the enzyme at its N‐terminus. Protein purity was evaluated by SDS‐PAGE (15%). C. clariflavum PA14AB was concentrated to 15 mg/mL in buffer containing 50 mM Tris‐HCl, pH 7.6, using Centriprep YM‐10 centrifugal filter devices (Amicon Bioseparation, Millipore, Billerica, MA), and C. thermocellum PA14AB was concentrated to 18 mg/mL in buffer containing 50 mM Tris‐HCl, pH 8.4, 150 mM NaCl, and the resultant proteins were subjected to crystallization screening.
While 2.5 mM CaCl2 was included in the thrombin cleavage buffer for C. clariflavum PA14AB, no calcium was added to C. thermocellum proteins during any of the purification steps.
For carbohydrate‐binding assays, the uncleaved C. clariflavum PA14AB protein was used. C. thermocellum PA14AB proteins containing both N′ and C′ terminal His tags were used with similar results. Protein concentration was determined by measuring the UV absorbance at 280 nm, using the calculated extinction coefficient of the protein, as determined by the ProtParam tool (http://www.expasy.org/tools/protparam.html).
2.4. SDS‐PAGE‐based carbohydrate‐binding assay
Qualitative carbohydrate‐binding assays were performed as described previously.30, 31, 32 In brief, protein samples (50 μg in a total volume of 200 μL in buffer containing 50 mM Tris‐HCl, pH 8.4, and 150 mM NaCl) were mixed with various polysaccharides (5 mg; Sigma Chem. Co., St Louis, MO): microcrystalline cellulose (Avicel), 1% amorphous cellulose.33 The respective contents of the xylans from different sources were as follows (Sigma): Oat spelt xylan (10% arabinose, 15% glucose, and 70% xylose), birchwood xylan (90% xylose), and beechwood xylan (90% xylose). Pectin from apple (70‐75% esterification), polygalacturonic acid, lichenan, and starch were also purchased from Sigma. CaCl2 (10 mM) was included in assays containing pectin and polygalacturonic acid in order to precipitate the polysaccharides, since both are soluble in the absence of calcium. In specified assays, including xylan, 10 mM EDTA was included in the mixture. The mixtures were maintained at room temperature for 60 min with gentle rotation, then centrifuged at 12 000g for 5 min to sediment the polysaccharide and bound proteins. The supernatant (containing unbound proteins) was recovered and applied to SDS‐PAGE gels. The polysaccharide precipitates were also washed four times with 1‐mL aliquots of buffer. After centrifugation, the polysaccharides were resuspended into 200 μL of the same buffer and placed in a boiling water bath for 10 min. After centrifugation, the supernatant was recovered, and the proteins were subjected to SDS‐PAGE. Each assay was repeated at least three times.
2.5. Crystallization and data collection
Crystals were grown at 293 K by the hanging‐drop vapor‐diffusion method {McPherson, 1982 #89}. Crystals of C. thermocellum PA14A appeared in condition number 35 of Crystal screen 1 (0.1M HEPES pH 7.5, 0.8M sodium phosphate monobasic monohydrate, and 0.8M potassium phosphate monobasic). The crystals of PA14B, appeared in condition number 10 of Crystal Screen 1 (0.2M ammonium acetate, 0.1M sodium acetate pH 4.6, and 30% PEG 4000). Finally, the crystals of PA14AB appeared in condition B7 from Wizard 3 (0.2M ammonium nitrate and 20% PEG 3350). Crystals of C. clariflavum PA14A appeared after 2 days in condition No. 2 of PEG ION 2 kit from Hampton Research (0.2M sodium malonate pH 4 and 20% w/v polyethylene glycol 3350). The crystals of PA14B and PA14AB appeared after a month in condition No. 7 of PEG ION 1(0.2M calcium chloride dihydrate and 20% PEG 3350) and in condition No. 22 of Crystal Screen 2 (0.1M MES pH 6.5, 12% PEG 20000), respectively.
The crystals were harvested from the crystallization drop using a MiTeGen micromount (http://www.mitegen.com) made of polyimide and transferred for several seconds into cryostabilization solution, composed of equal volumes of a twofold‐concentrated solution of the crystallization components and a solution consisting of 18% (w/v) sucrose, 16% (w/v) glycerol, 16% (w/v) ethylene glycol, and 4% (w/v) glucose. For data collection, crystals were mounted on the MiTeGen micromount, plunged into liquid nitrogen, and placed in pucks for mounting at the European Synchrotron Radiation Facility (ESRF, Grenoble, France). Diffraction data were collected on beamline BM14 for PA14A and PA14B from C. thermocellum and C. clariflavum, respectively, beamline ID23‐1 for PA14B, and beamline ID29 for PA14A, PA14AB from C. thermocellum and C. clariflavum, respectively. X‐ray data were collected on a Dectris PILATUS 6M pixel detector at 100 K.
2.6. Structure determination and refinement
The crystal structure of the C. thermocellum PA14A was determined by molecular replacement using Molrep implemented in the CCP4i suite,34, 35 using the atomic coordinates from the anthrax protective antigen, PDB entry code 1ACC, as a search model. Although there is only 25.6% sequence identity between the two proteins, the structure of PA14A was successfully resolved. The model of PA14A was refined using REFMAC36 as implemented in CCP4. The iterative model building and correlation to the electron density maps were conducted via COOT.37 Water molecules were added by ARP/wARP.38 The structure of the PA14B was determined by molecular replacement using PA14A as a search model. The structure of the PA14AB was determined by two‐step molecular replacement (MR), using Molrep34 from the CCP4 program suite.35 In the first step, the coordinates of C. thermocellum PA14A were used as a search model for molecular replacement. Consequently, the PA14A coordinates were used as a fixed model, and the coordinates of PA14B were used as a search model. The initial coordinates that were obtained (including both PA14A and PA14B) were subjected to ARP/wARP38 for model building, followed by further refinement with CCP4, and manually rebuilt using Coot, until convergence (Table 1). The structure of the C. clariflavum PA14AB was determined and refined via a similar procedure as that used for C. thermocellum PA14A. However, anisotropic temperature factors were refined due to the high resolution (Table 1). The refined structures and the corresponding amplitudes of the structure factors have been deposited in the PDB with accession codes 6QDI and 6QE7.
Table 1.
Crystallographic data collection and refinement statistics
| PA14ccl_AB | PA14ct_AB | |
|---|---|---|
| ESRF beamline | ID29 | ID29 |
| Wavelength (Å) | 0.98 | 0.972 |
| PDB entry | 6QDI | 6QE7 |
| Space group | C2 | P21 |
| Cell unit | a = 104.60 | a = 65.34 |
| b = 41.43 | b = 121.67 | |
| c = 73.31 | c = 65.94 | |
| β = 100.35 | β = 106.39 | |
| Resolution range (Last Shell) Å | 45.97‐1.13 (1.159‐1.13) | 63.26‐2.06 (2.113‐2.06) |
| Unique reflections | 111 927 (7515) | 60 326 (4500) |
| Redundancy | 2.6 (2.5) | 3.0 (3.0) |
| R sym(I) | 5.1 (42.7) | 3.7 (36.4) |
| Completeness | 96.67 (92.54) | 95.73 (97.49) |
| I/σ | 9.9 (1.9) | 13.5 (2.5) |
| CC(1/2) | 99.9 (81.0) | 99.9 (89.7) |
| Number of protein atoms | 2436 | 7117 |
| Number of Ca atoms | 4 | 6 |
| Number of ethylene glycol (EDO) atoms | 8 | — |
| Number of solvent atoms | 388 | 463 |
| R factor, % | 13.4 (23.2) | 18.7 (30.1) |
| R free, % | 16.5 (26.7) | 25.5 (35.2) |
| Avg. B factor, Å2 | ||
| Protein | 11.53 | 43.36 |
| Ca+2 | 7.30 | 34.86 |
| EDO | 11.92 | — |
| Solvent | 23.26 | 45.47 |
| MolProbity Score (percentile) | 1.69 (49th) | 1.94 (84th) |
| rms deviation from ideality | ||
| Bond length, Å | 0.018 | 0.015 |
| Bond angle, ° | 1.85 | 2.01 |
| Ramachandran Plot | ||
| Favored, % | 95.9 | 93.7 |
| Allowed, % | 4.1 | 5.9 |
| Outlier, % | 0 | 0.5 |
Note: Quantities in the parentheses are defined for the last shell.
2.7. Bioinformatics
Protein structure databases were searched for similar structures with the PA14A and PA14B modules as queries.39 Phylogenetic analysis of σIs was conducted using the MEGA7 program40 (http://www.megasoftware.net/) and multi‐sequence analysis of σIs was performed using the MUSCLE41 algorithm, implemented by MEGA7. The evolutionary history of 66 nucleotide sequences was inferred using the Neighbor‐Joining method42 with 2000 bootstrap replicates.43 The evolutionary distances were computed using the Maximum Composite Likelihood method.44 The phylogenetic tree was visualized and annotated with the online tool Interactive Tree Of Life (iTOL; available at: http://itol.embl.de/).45
DNA promoter motif searchers were carried out using the program Pattern Locator46 (http://www.cmbl.uga.edu/software/patloc.html) and analyzed with the Jalview software.47 Multiple DNA sequence alignments were performed using the T‐Coffee algorithm48 provided by Jalview. DNA sequence logos were generated with the program WebLogo49 (http://weblogo.berkeley.edu/logo.cgi). The sequences used to build the phylogenetic tree and their accession numbers are given in Supporting Information.
3. RESULTS AND DISCUSSION
3.1. Binding properties of PA14AB dyads from C. thermocellum and C. clariflavum
The alternative anti‐sigma factors, C. thermocellum RsgI3 (Cthe_0316) and C. clariflavum RsgI4 (Clocl_2747), each possesses a C‐terminal polysaccharide sensory element, composed of two tandem PA14‐superfamily motifs, that were herein named PA14A (N‐terminal) and PA14B (C‐terminal). The PA14 modular dyads share high sequence similarity of 66% with an identity of 38.6%. However, the respective PA14A‐PA14B dyads (PA14AB) from C. thermocellum RsgI3 and C. clariflavum RsgI4 appear to have different binding preferences. In a previous study, the C. thermocellum PA14AB dyad was examined for its binding to various polysaccharides and was found to bind strongly to pectin as well as to polygalacturonic acid8 (Figure 2A). During the present work, we reproduced the previous approach with the C. thermocellum dyad to determine which of the subunits is/are responsible for the binding. Indeed, we verified that PA14AB binds pectin. Moreover, the single PA14A module (without PA14B) also bound pectin, whereas PA14B exhibited negligible levels of binding to pectin and polygalacturonic acid and no binding to xylan (Figure 2A).
Figure 2.
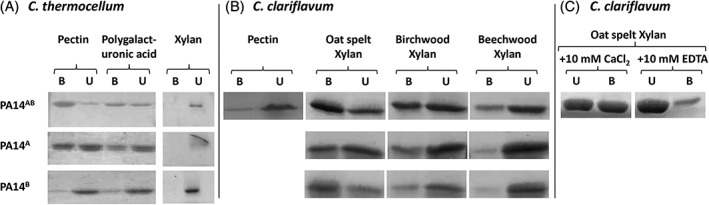
Binding of RsgI‐borne PA14 modules from (A) C. thermocellum and (B) C. clariflavum to complex polysaccharides. Proteins were incubated with the insoluble polysaccharide, followed by centrifugation to separate pellet and supernatant, both of which were subjected to SDS‐PAGE. The bound protein fraction (B) settled with the polysaccharide‐containing pellet, whereas the unbound, noninteracting fraction (U) remained in the supernatant fluids. (C) Binding of C. clariflavum PA14AB to oat spelt xylan in the presence of calcium or EDTA
Similar to the RsgI‐borne PA14 motifs in C. thermocellum, we also examined the C. clariflavum PA14 modules for binding to the same insoluble polysaccharides. The results (Figure 2B) show that C. clariflavum PA14AB binds xylan and to a much lesser extent pectin derivatives. The individual C. clariflavum PA14 modules, PA14A, PA14B (or PA14AB) were able to bind all three types of xylan tested in this work, that is, oat spelt xylan, birchwood xylan, and beechwood xylan (Figure 2B), with a preference for oat spelt xylan and less for birchwood and then beechwood xylans. Binding of xylan by PA14AB was abolished by addition of a metal chelator EDTA to the reaction mixture (Figure 2C; for details see section 3.6).
3.2. The conservation of σI sequences represents a strong signature for differentiating the binding specificity of PA14 modules
To reveal the consequences of the different binding preferences of the two very similar PA14 dyads of the respective anti‐σI factor (RsgI), we performed a phylogenetic analysis of the respective σI genes from available complex cellulosome‐producing bacteria. It can be observed that C. thermocellum σI3 clusters together with B. cellulosolvens σI11, A. cellulolyticus σI6, Clostridium sp. Bc‐iso‐3, and C. straminisolvens σI3 factors (light blue‐highlighted clade in Figure 3). C. clariflavum σI4 and similar σI factors from other cellulolytic clostridia, namely, A. cellulolyticus σI3 (WP_010247803.1) and B. cellulosolvens σI1 (Bccel_0204), cluster together (light green‐highlighted clade in Figure 3) at a branch relatively remote from that of the C. thermocellum σI3‐like proteins. The C‐terminal sensory elements of the C. thermocellum RsgI3‐like proteins would presumably sense pectin as demonstrated by the binding assays performed with PA14AB of C. thermocellum (Figure 2A) and the PA14‐CBM35 of B. cellulosolvens RsgI11 (data not shown). The C‐terminal sensory elements of the C. clariflavum RsgI4‐like proteins presumably sense xylan, as demonstrated by the binding assays performed with the PA14AB of C. clariflavum (Figure 2B) and the PA14AB dyad of B. cellulosolvens RsgI1 (data not shown). These results suggest that sequence conservation of the σI factors represents a signature that reflects the specificities of the PA14 sensory elements, located on their cognate RsgI.
Figure 3.
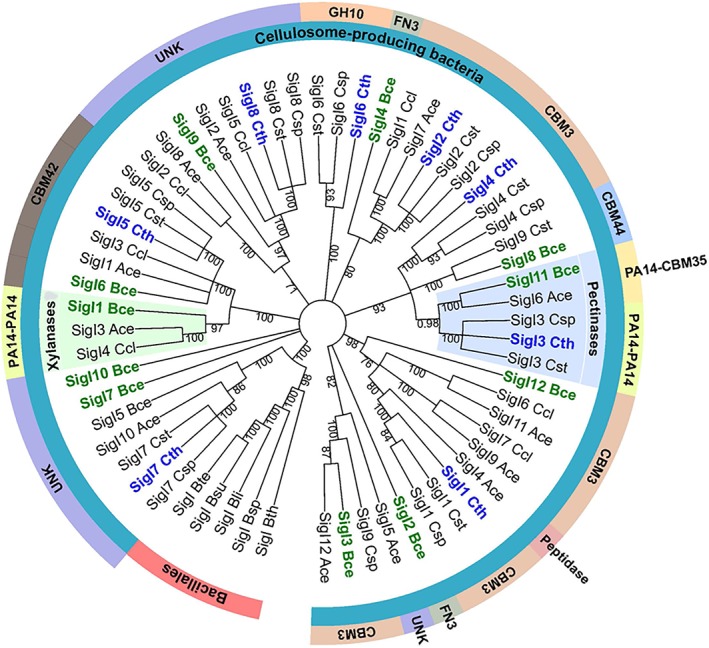
Evolutionary relationships of σI factors, derived from cellulosome‐producing clostridia that harbor multiple σIs. Branches corresponding to partitions reproduced in <50% bootstrap replicates are collapsed. Corresponding putative sensory elements of cognate RsgIs are indicated by the outside labels. The clade of σI factors, containing the B. cellulosolvens σI11 and C. thermocellum σI3, involved in regulating pectinase genes, is highlighted in light blue. The clade of σI factors, containing B. cellulosolvens σI1, presumably involved in regulating xylanase genes, is highlighted in light green. Ace, Acetivibrio cellulolyticus; Bce, Bacteroides cellulosolvens; Bli, Bacillus licheniformis; Bsp, Bacillus sp. NRRL B14911; Bsu, Bacillus subtilis; Bte, Bacillus tequilensis; Bth Bacillus thuringiensis; Cce, Clostridium cellobioparum; Ccl, Clostridium clariflavum; ChBD3, chitin‐binding domain; Csp, Clostridium sp. Bc‐iso‐3; Cst, Clostridium straminisolvens; Cte, Clostridium termitidis; Cth, Clostridium thermocellum; FN3, fibronectin type 3 domain; GH, glycoside hydrolase; UNK, unknown [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. The promoter sequence of the sigI/rsgI operon represents a strong signature for differentiating the binding specificity of the PA14 modules
Previous work indicated that C. thermocellum σI3 and σI11 from B. cellulosolvens use highly conserved promoter sequences to regulate genes encoding pectin‐degrading enzymes.50
In order to identify the conserved promoter motif for genes encoding xylan‐degrading enzymes, we performed bioinformatics analysis to identify the putative regulon of C. clariflavum σI4. We first compared the promoter regions of C. clariflavum sigI4 and A. cellulolyticus sigI4 to the previously predicted σI‐dependent promoter of B. cellulosolvens sigI1.50 As shown in Figure 4A, the predicted −35 region of the putative σI promoter of C. clariflavum sigI4 has a conserved AAAA tetrad, and a conserved CGA triad three nucleotides upstream of the latter tetrad. A conserved CGAAT pentad can be observed in the predicted −10 region (Figure 4A). The spacer between the −35 and −10 region is 13 nucleotides (Figure 4A). Assuming that C. clariflavum sigI4 is autoregulated, as has already been shown in other alternative σI factor genes,9, 50 we searched for putative σI4‐dependent promoters in the genome of C. clariflavum by using the conserved motifs of the predicted σI‐dependent promoter of C. clariflavum sigI4 (Figure 4A). During the search, we allowed a distance of 12‐14 nucleotides between the AAAA tetrad and the CGAAT pentad. Additionally, we allowed one mismatch in each of the −35 and −10 promoter sequences. As can be observed in Figure 4B, two of the four putative C. clariflavum σI4‐dependent promoters identified in the present work correspond to genes encoding modules of a family 10 glycoside hydrolase (GH10) and/or family 11 glycoside hydrolase (GH11). According to the Carbohydrate‐Active enZYmes (CAZy) Database, the GH11 family is composed only of xylanases and members of the GH10 family are mainly xylanases.
Figure 4.
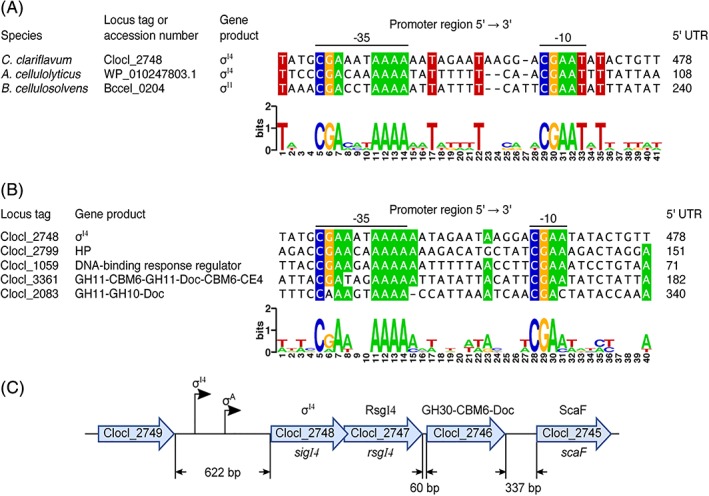
Identification of conserved elements of the C. clariflavum σI4‐dependent promoter sequences. (A) Alignment of putative σI‐dependent promoters of the C. clariflavum σI4, A. cellulolyticus σI4, and B. cellulosolvens σI1 genes. Predicted −35 and −10 promoter elements are indicated. Distances between the promoter region and the first codon of the corresponding genes are shown in the column labeled 5′‐UTR (5′‐untranslated region). The WebLogo was generated with the sequence shown in the alignment. (B) Alignment of the putative C. clariflavum σI4‐dependent promoter sequences. CBM6, family 6 carbohydrate‐binding module; CE4, family 4 carbohydrate esterase; Doc, dockerin; GH10, family 10 glycoside hydrolase; GH11, family 11 glycoside hydrolase; HP, hypothetical protein. (C) Organization of the genomic context of the C. clariflavum sigI4‐rsgI4 operon. GH30, family 30 glycoside hydrolase; ScaF, scaffoldin F [Color figure can be viewed at http://wileyonlinelibrary.com]
In C. clariflavum, the cellulosomal component genes are mainly scattered on the chromosome. Interestingly, 60 bp downstream of the C. clariflavum sigI4‐rsgI4 operon, there is a gene (Clocl_2746) encoding a dockerin‐containing family 30 glycoside hydrolase (GH30; Figure 4C). We analyzed the intergenic region between C. clariflavum rsgI4 and Clocl_2746 and the region upstream of sigI4, in order to search for putative σI‐ and σA‐dependent promoters (σA is the housekeeping σ factor). During this analysis, we identified a putative σA‐dependent promoter upstream of sigI4 that has the sequence TTGAAA‐17N‐TATAAA, but we did not detect any σI‐ or σA‐dependent promoter in the intergenic region between sigI4 and Clocl_2746 (Figure 4C). These results indicate that the C. clariflavum sigI4‐rsgI4 pair of genes forms an operon with Clocl_2746, which is under the control of both σI4 and σA. Interestingly, a recent report of C. clariflavum showed that Clocl_2746 has xylanolytic activity and that this enzyme was highly expressed, as measured by protein abundance in cellulosomes, upon growth of cells on different polysaccharide substrates including acid‐pretreated switch‐grass.51
The results of the above‐described bioinformatic analysis indicate that C. clariflavum σI4 most likely regulates genes encoding xylan‐degrading enzymes, and hence, C. clariflavum RsgI4 likely senses xylan. This correlates well with the binding capacities of the C. clariflavum RsgI4 PA14AB dyad. We have thus concluded that the promoter sequence of the sigI/rsgI operon represents a strong signature which can be used to identify binding specificities of similar modules.
3.4. Crystal structures of C. thermocellum and C. clariflavum PA14AB modules
In order to study the mode of action of the C. thermocellum RsgI3 and C. clariflavum RsgI4 to the target polysaccharides, we determined the X‐ray crystal structures of both dyads from each of the two clostridial species. It is important to mention that the term, dyad, used here does not follow the crystallographic definition of dyad as having a twofold axis that forms a dimer. The C. thermocellum PA14AB dyad was crystallized and diffracted to a resolution of 2.06 Å (Figure 5A), and the C. clariflavum PA14AB dyad crystal diffracted to 1.13 Å (Figure 5D). The statistics and data collection are given in Table 1. The structures were submitted to the Protein Data Bank (PDB: 6QDI and 6QE7).
Figure 5.
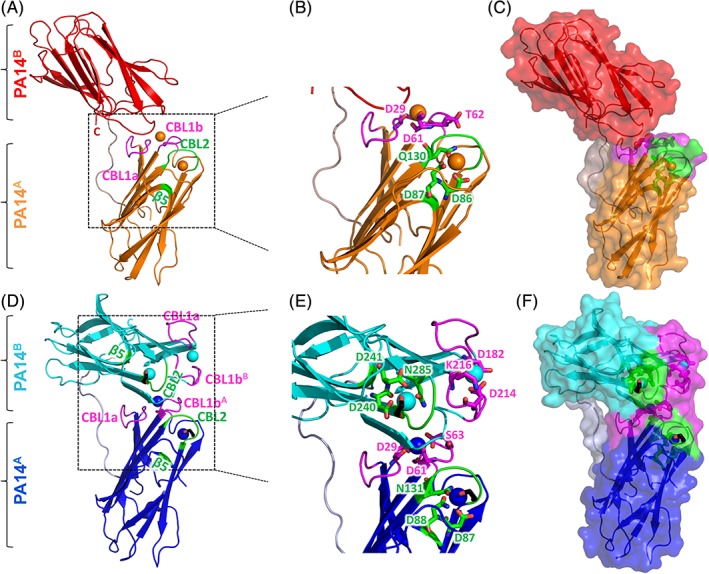
Crystal structures of the C. thermocellum (A‐C) and C. clariflavum (D‐F) PA14AB modules. (A/D) Cartoon diagrams of the three‐dimensional structures of the C. thermocellum RsgI PA14AB and C. clariflavun RsgI PA14AB dyads. Ca2+ atoms are represented as spheres. The first calcium atom of PA14 is coordinated by calcium‐binding loop 1a (CBL1a) and CBL1b (magenta). The second Ca2+atom is anchored by CBL2 and β‐strand 5 (green). C and N termini are indicated. (A) The PA14A module is in orange and PA14B is in red. Only the PA14A module possesses two calcium atoms, whereas PA14B is void of calcium. (D) The PA14A module is in blue and PA14B is in cyan. Both PA14A and PA14B possess two Ca2+atoms each. (B/E) Close‐up view of the calcium‐binding loops in C. thermocellum PA14A and C. clariflavum PA14AB. The calcium‐binding residues of CBL1a and CBL1b are presented as magenta sticks, and the calcium‐binding residues of CBL2 and β5 are presented as green sticks. (C/F) Transparent surface views of the C. thermocellum RsgI PA14AB and C. clariflavum RsgI PA14AB dyads. PA14A of the two structures were first superimposed and then the structures were separated for clarity. CBL1a and CBL1b are colored magenta, and CBL2 and β5 are colored green. The Ethylene Glycol molecules are presented as black sticks [Color figure can be viewed at http://wileyonlinelibrary.com]
The C. thermocellum and C. clariflavum RsgI‐PA14 modules exhibit β‐barrel structures, typical to those displayed by other PA14 modules, consisting of a 10‐stranded anti‐parallel β‐sandwich fold. One of the β‐sheets of the β‐sandwich is formed by strands 1, 2, 4, 9, 6, and 7 while the other sheet comprises anti‐parallel strands 3, 10, 5, and 8.
Clostridium thermocellum, PA14A contains two calcium ions. The first is coordinated by calcium‐binding loop 1a (CBL1a) by D29 and calcium‐binding loop 1b (CBL1b) by D61 and T62 (Figure 5A–C). CBL1a is positioned between the β strands 1 and 2, while CBL1b is positioned between strands 3 and 4 (Figure 6). The second calcium ion is coordinated by residue Q130 of CBL2 together with residues D86 and D87 of β‐strand 5. CBL2 is located between β7 and β8 (Figure 6). Unlike PA14A, C. thermocellum PA14B lacks calcium in its structure. In contrast, in the C. clariflavum PA14AB dyad, two calcium ions are present in both modules—one by CBL1a and CBL1b and the second by CBL2 and β5 (Figures 5D,E and 6). In C. clariflavum PA14A, the first calcium atom is coordinated by D29 (CBL1a) and D61, S63 (CBL1b), and the second is coordinated by D87, N88 (CBL2), and N131 (β5). In C. clariflavum PA14B, the first calcium atom is coordinated by D182 (CBL1a) and D214, K216 (CBL1b), and the second calcium is coordinated by D240, D241, and N285 (Figure 4E). The second calcium ion in each C. clariflavum module is bound to an ethylene glycol molecule, which presumably originated from the cryo‐protectant used for mounting the crystals (Figure 5D‐F, black sticks). Figure 5F clearly represents the ethylene glycol molecule bound to the potential carbohydrate‐binding site of PA14AB from C. clariflavum.
Figure 6.
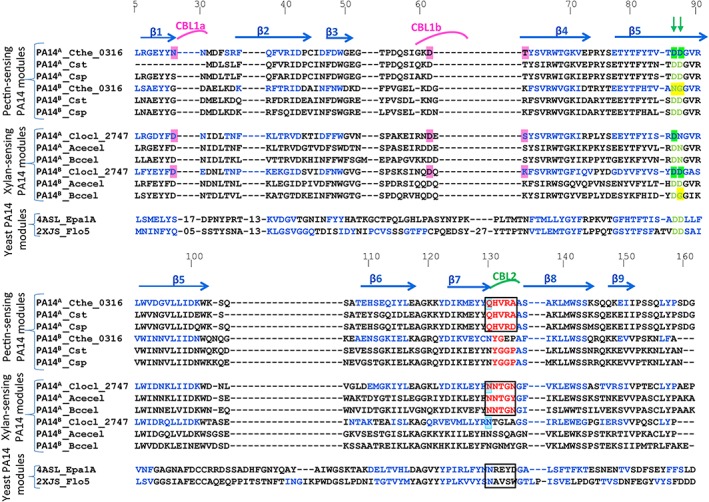
Sequence‐based alignment of PA14 protein modules. β‐Strand residues (arrows) at homologous positions are indicated in blue font. Amino acids responsible for Ca2+binding in CBL1a and CBL1b are highlighted in magenta. Amino acids responsible for Ca2+binding in CBL2 are highlighted in green. Amino acids of CBL2 are colored red, outlined by a black square. For clarity, the positions of selected residues in the sequence are enumerated. C. clariflavum PA14A and PA14B (Clocl_2747), C. thermocellum PA14A (Cthe_0316), PA14B (Cthe_0316) vs RsgI_PA14 modules of C. straminisolvens (Cst), Clostridium sp. Bc‐iso‐3 (Csp), Acetivibrio cellulolyticus (Acecel), Bacteroides cellulosolvens (Bccel), and yeast PA14 modules: Epa1A—Candida glabrata adhesin and Flo5—Saccharomyces cerevisiae flocculin [Color figure can be viewed at http://wileyonlinelibrary.com]
Superposition of the PA14A and PA14B modules resulted in an rmsd of 0.63 Å over 95/95 residues and in 0.563 Å over 115/115 residues for C. clariflavum and C. thermocellum respectively, as calculated using Cα atoms in PyMOL. Overall, the structures of both modules are very similar, with some minor differences in the loops. The superposition of PA14A domains from both bacteria revealed that PA14B is located 150° relative to PA14A in C. thermocellum and about 90° relative to PA14A in C. clariflavum (Figure 5C,F).
The PA14A and PA14B modules are separated by a flexible linker (Figure 5A,D, gray). It is known that linkers may adopt alternative conformations, in accordance with their natural environmental conditions.52 The linkers between the PA14A and PA14B modules (residues 160‐174 in C. clariflavum and 159‐170 in C. thermocellum) are long and presumably flexible, and the two modules may possess different orientations towards each other. The two different crystal structures presented here may represent only part of the possible conformations, where the modules may be distant from one another (as in C. thermocellum) or in close proximity (as in C. clariflavum). Their position may depend, for instance, on the presence of an environmental signal, such as their carbohydrate substrate. The presence of the PA14 dyads and their interaction with the substrate may, in fact, play a regulatory role in signal transduction through the cell envelope.
3.5. Clostridial RsgI_PA14s display similarity to PA14 modules from yeast
In order to better understand the mechanism of action of the RsgI_PA14 modules, we searched for proteins which share structural similarity. The Protein Data Bank was therefore searched with C. thermocellum and C. clariflavum PA14A/ PA14B (monomers) as queries, using the DALI server,39 and several similar structures were found. High structural similarity of 2.2 and 2.3 Å was found with Saccharomyces cerevisiae flocculin (Flo) proteins (2XJS, 4AIL, and 4LHL‐A) and Candida glabrata epithelial adhesins (Epa) (4ASL), respectively.16, 18, 53, 54, 55 The RsgI_PA14 modules and the PA14 modular structures of the flocculin and adhesin proteins superimpose well at the core part of the β‐sandwich region, although the adhesin (Figure 7A, gray) and flocculin (Figure 7A, purple) proteins are decorated by additional structural regions, which are absent from the RsgI PA14 modules. In both cases, the latter PA14 modules primarily exist in the single state in the parent proteins and not as modular dyads.
Figure 7.
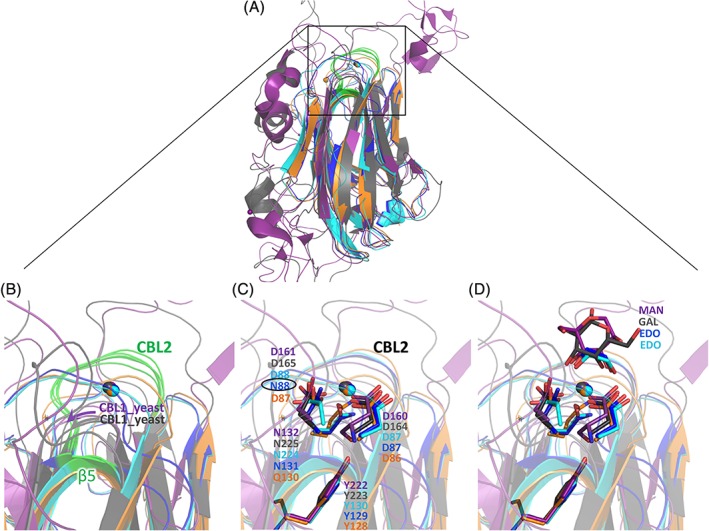
Structural comparison of C. clariflavum and C. thermocellum RsgI_PA14 with PA14 modules from yeast. C. clariflavum PA14A (blue), C. clariflavum PA14B (cyan), C. thermocellum PA14A (orange), Ca. glabrata epithelial adhesin (4ASL, gray), and S. cerevisiae flocculin (2XJS, purple). (A) Comparison of C. clariflavum PA14A, C. clariflavum PA14B and C. thermocellum PA14A with representative PA14 modules from yeast. Ca2+atoms are presented as spheres. CBL2 and β‐strand 5, which coordinate the Ca2+atom, are colored green in all species. (B) Close‐up view of CBL2 and β‐strand 5 from RsgI_PA14 (green), and CBL1 from yeast (gray and purple), that interact with the calcium atom. (C) Structural superposition of the calcium‐binding DD‐N triad of members of the PA14 superfamily. N88 of the DcisN motif of C. clariflavum PA14A is designated by a black circle. The four functionally important tyrosine residues in the various PA14 modules are shown. (D) The ethylene glycol molecules (cyan and blue) superimpose well with the hydroxyls of the sugar rings bound by yeast CBL2
S. cerevisiae flocculins and Ca. glabrata adhesins16, 18, 53, 55 rely on the DcisD‐N (AspcisAsp‐Asn) motif for complexation of the Ca2+ ions, which contribute to their carbohydrate‐binding properties as part of sugar‐binding elements.56 The DcisD‐N calcium‐binding signatures are found in over 85% of the 200 Epa adhesin‐like domains that are present in the known fungal genome sequences57 and also in the clostridial RsgI_PA14 modules as described herein. In the case of the clostridial PA14s, the conserved DcisD motif is located on β‐strand 5, which is equivalent in location to CBL1 from yeast (Figure 7B), while the asparagine of the DcisD‐N signature is located in CBL2, similar to that of the yeast. Whereas in the yeast PA14, both CBL1 and CBL2 are involved in binding of the same calcium atom, in the clostridial PA14s, CBL1a together with CBL1b bind the first calcium atom and CBL2, together with β5, bind the second. The CBL1a and CBL1b loops of RsgI_PA14 modules comprise a unique feature of biomass‐sensing RsgI_PA14 modules and are absent from the structures of other PA14. The biological role of these loops is unclear.
The two aspartate residues (DcisD) positioned in cis orientation are rare and assume a structurally unfavorable conformation. In proteins for which the DcisD motif has been described,18 the motif is highly conserved. (Figure 6, green arrows). However, in some of the RsgI_PA14 modules, like in C. clariflavum PA14A, the second Asp is replaced by Asn (N88), which can be considered a comparatively minor replacement (Figure 6, Figure 7C, black circle). In RsgI_PA14, the CBL2 of both modules (res.132‐137) links strands β7 and β8, and residues in β‐strand 5 adopt a conserved Asp‐cis‐Asp (Figure 7C, D160, D161, cyan) in PA14B and Asp‐cis‐Asn motif (Figure 7C, D87, N88, blue) in PA14A. Both Asp and Asn interact with the calcium ion via their carbonyl group. The unfavorable cis‐peptide configuration necessary for calcium binding is stabilized by a hydrogen bond to the hydroxyl group of the highly conserved C. clariflavum Y129 (in PA14A) and Y283 (in PA14B), similar to the equivalent tyrosines in the yeast, that is, Y222 in Flo5A (flocculin) and Y223 in Epa1A (adhesin; Figure 7C). Moreover, CBL2 contributes to calcium binding via the side chain of a highly conserved polar side residue: Asn (N131 in PA14A and N285 in PA14B) in C. clariflavum and Asn Q130 in PA14A of C. thermocellum (Figure 6C). Carbonyl groups of the CBL2 main chain also contribute to calcium binding: T133 and N135 in C. clariflavum PA14A, G287 and A289 in PA14B, V132 and A134 in C. thermocellum PA14A. Interestingly, in C. thermocellum RsgI_PA14, only the PA14A module possesses the DcisD calcium‐binding motif (Figure 6, indicated in green arrows). In the amino acid sequence of C. thermocellum PA14B, however, the first aspartate is substituted by asparagine and the second by glycine (Figure 6, yellow‐highlighted), thereby precluding the presence of the DcisD motif and preventing calcium binding, as demonstrated by its absence in the crystal structure (Figure 5A). These results are in good agreement with substrate‐binding experiments, which show that C. thermocellum PA14B is unable to bind pectin on its own, indicating that a calcium ion is essential for the ability to coordinate the polysaccharide.
3.6. The calcium ion of CBL2 is involved in direct interaction with the ligand in RsgI_PA14 modules
Structural comparison of the clostridial PA14 modules with the yeast PA14 modules revealed that the position of the calcium ion in CBL2 is highly conserved between the Ca. glabrata epithelial adhesins, S. cerevisiae flocculins, and clostridial RsgI_PA14 modules (Figure 7B). This is in addition to the presence of the conserved DcisD motif (Figure 7C). Therefore, we assumed that the substrate‐binding site of RsgI_PA14 is similar to that of the PA14 modules of adhesins and flocculins, and the carbohydrates may directly bind to the calcium ion via hydroxyls 3 and 4 of their xylose residues. In support of this hypothesis, the structures of both PA14A and PA14B modules in C. clariflavum have ethylene glycol molecules, bound to the calcium ion via hydroxyls (Figure 7D; EDO colored cyan, EDO colored blue), which superimpose perfectly onto the sugar ring hydroxyls, bound to the calcium ions in the structures of both Epa1a and Flo5 (Figure 7D MAN, GAC). Unfortunately, attempts to crystallize the RsgI_PA14 modules in the presence of xylose, xylobiose, and xylohexaose, failed to yield positive results. The ethylene glycol capture of the carbohydrate‐binding site may have blocked the binding region under the standard cryoprotective conditions.
To verify the direct involvement of calcium in carbohydrate binding by RsgI_PA14, we tested the ability of C. clariflavum PA14AB to bind xylan in the absence of calcium. EDTA was added to the carbohydrate‐binding assay reaction mixture (Figure 2C). Chelation of calcium by EDTA completely abolished the binding of PA14AB to xylan, demonstrating the involvement of calcium in carbohydrate binding. Parallel assays were not conducted for C. thermocellum PA14 binding to pectin, because calcium is necessary to keep the pectin insoluble for this assay. We propose, however, based on high structural similarity, that the role of calcium in C. thermocellum PA14AB is the same as in C. clariflavum modules.
Based on structural comparisons in C. clariflavum, the presence of ethylene glycol at the carbohydrate‐binding site and the results for binding capacities in the presence and absence of calcium, we propose that in the C. clariflavum RsgI_PA14 modules, the xylose ring binds directly to the calcium ion at CBL2.
3.7. The protein sequence of CBL2 represents a signature for differentiation of xylan vs pectin binding by RsgI_PA14
Bioinformatics helped us to understand that the sigI/rsgI promoter sequence and the sequence of the sigI factor itself together dictate which kind of polysaccharide will be bound to the cognate RsgI PA14 module. In addition, review of the structural literature for PA14 modules revealed that prior to publishing the first PA14 modular structures, Zupancic et al19 performed sequence swapping between various epithelial adhesins (Epas) and mapped a five amino acid region important for substrate binding specificity, within the PA14 module. In the 3D structure of Epa1a (PDB 4ASL), this region comprises the CBL2, which was shown to be directly involved in the interaction with the carbohydrate substrate (Figure 5 black square). Mutation of some key amino acids in this loop, specifically E227 and Y228 (first two amino acids of CBL2) affects the specificity of carbohydrate recognition by Epa1a.28 These results would imply that the CBL2 sequence defines substrate recognition in B. cellulosolvens PA14A (Figure 6 PA14A_Bccel) and Acetivibrio cellulolyticus PA14A (Figure 6, PA14A_AceCel). Their CBL2s comprise residues NNTGN and NNTGY respectively, which are very similar to CBL2 from PA14A of C. clariflavum (NNTGN; Figure 6, black square). Three of them were predicted to bind xylan according to the promoter sequences of their respective genes and were verified experimentally.50 The supporting observation that the sequence of CBL2 is conserved within the group of pectin‐binding bacteria was also detected in the sequences of CBL2 from C. straminisolvens and C. sp., that comprise residues QHVRA and QHVRD, respectively, which are very similar to CBL2 from PA14A of C. thermocellum (QHVRA) (Figure 6, black square). The sequences of CBL2 in both pectin‐sensing and xylan‐sensing RsgI PA14 bacterial groups are very different from each other. This variation, we believe, explains the divergence of carbohydrate‐binding specificity between the two species. The sequence conservation of the CBL2 would thus constitute an additional control signature for substrate binding specificity.
From the sequence conservation in the PA14A modules, it follows that PA14A is more responsible for leading RsgI binding to its substrate as opposed to the PA14B modules. Thus, the sequences of the CBL2 of the PA14B modules within the members of the bacterial group that bind xylan are not conserved completely. In contrast, these sequences in members of the pectin‐sensing bacterial group are conserved, although it was shown that CBL2 of C. thermocellum cannot bind substrate alone. The role of the conservation of CBL2 PA14B sequence within the pectin‐binding bacterial group is still unclear.
3.8. Summary
Since the recombinant PA14 dyad from an RsgI of C. thermocellum (Cthe_0316) binds pectin strongly, it was thus initially expected that a similar PA14 dyad, discovered in an RsgI (Clocl_2747) of a related bacterium, C. clariflavum, would also bind pectin. Surprisingly, despite the strong sequence and structural similarity to the C. thermocellum protein, the C. clariflavum RsgI_PA14 was found to bind mainly xylan. Our findings therefore represent a fine‐tuned structure‐function relationship in sugar binding. The main function of oligosaccharide binding was preserved in the PA14 dyads from both bacteria; however, the type of target sugar differed.
A clearer understanding of this phenomenon became possible after comparing the relevant promoter regions and the protein sequences of the sigma I factors of the different species. The promoter and the sigma I factor of RsgI_PA14 from C. thermocellum possess conserved sequences associated with pectin sensing, whereas RsgI_PA14 from C. clariflavum possesses conserved sequences associated with xylan sensing. Structural similarity between the PA14 modules from yeast and those of the RsgI_PA14 served to pinpoint residues on CBL2 that appear responsible for substrate binding. Minor changes in the yeast sequence interfere with the binding capacity. Sequence divergence in this loop of PA14 pectin‐binding dyads vs xylan‐binding dyads thus implies that this loop is important for binding specificity.
It is important to consider comparison between PA14 and CBM modules. Most CBMs possess calcium atoms in their structures, however calcium generally plays a structural role, and its removal by chelation does not affect its carbohydrate‐binding ability.31, 32 However, several CBM families, which, according to classification by Boraston et al,58 belong to CBM type C, utilize calcium for recognition of their substrate polysaccharides, by either direct interaction of calcium with the hydroxyls of the sugar ring or with carboxylate moieties on a relevant carbohydrate.59 These families are CBM35, CBM36, CBM60, CBM6, and CBM62.37, 60, 61, 62, 63 Despite the fact that selected PA14s clearly bind carbohydrates, and in this particular case, calcium is directly involved in the interaction with the carbohydrate substrate, PA14 has yet to be classified as a bona fide CBM family.
4. CONCLUSIONS
Redundancy is a common phenomenon in nature. Here, we detected three types of signature that allow differentiation of the function of the different sigI/rsgI pairs for targeting pectin vs xylan substrates, using very similar PA14 dyads as biosensors. The first relies on the DNA sequence of promoter region of the respective sigI/rsgI operon. This is a strong tool to predict the actual target of apparently similar protein biosensors. The second signature is based on the high similarity of the DNA sequence of related sigma factors, as reflected in their phylogenetic relationship. The third is based on the sequence similarity of specific regions within the CBL2 of the PA14A modules. Therefore, the same binding specificity data is “coded” at several biological levels and not only derived from protein structure. The present study provides a simplified method for predicting sugar binding specificity of newly discovered PA14 modules from sigI/rsgI operons.
Supporting information
Appendix S1. FASTA sequences used during the analysis of the evolutionary relationships of σI factors from cellulolytic clostridia shown in Figure 3.
ACKNOWLEDGEMENTS
The authors thank the staff scientists of ESRF for their remarkable assistance. This research was supported by the Israel Science Foundation (ISF; Grant nos. 293/08 to FF and 1349/13 to EAB). Additional support was obtained by a grant (No. 24/11) issued to RL by the Sidney E. Frank Foundation through the ISF. The authors also acknowledge a research grant from the Israel Science Foundation (ISF) (No. 2566/16) ‐ National Natural Science Foundation of China (NSFC) (No. 31661143023). IM‐G is grateful for the award of a Martin Kushner Schnur Post‐Doctoral Fellowship at the Weizmann Institute. LOO was supported by the “Consejo Nacional de Ciencia y Tecnología ‐ México” with a PhD scholarship (440354). EAB is the incumbent of The Maynard I. and Elaine Wishner Chair of Bio‐organic Chemistry. We thank the staff of ESRF, Grenoble, for their outstanding maintenance and upgrading the facility. We thank Eleanor Gafni for her help with the characterization of C. thermocellum RsgI_PA14.
Grinberg IR, Yaniv O, de Ora LO, et al. Distinctive ligand‐binding specificities of tandem PA14 biomass‐sensory elements from Clostridium thermocellum and Clostridium clariflavum . Proteins. 2019;87:917–930. 10.1002/prot.25753
Funding information Israel Science Foundation, Grant/Award Numbers: 1349/13, 24/11, 2566/16, 293/08; National Natural Science Foundation of China, Grant/Award Number: 31661143023; Consejo Nacional de Ciencia y Tecnología ‐ México, Grant/Award Number: 440354
REFERENCES
- 1. Akinosho H, Yee K, Close D, Ragauskas A. The emergence of Clostridium thermocellum as a high utility candidate for consolidated bioprocessing applications. Front Chem. 2014;2:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tracy BP, Jones SW, Fast AG, Indurthi DC, Papoutsakis ET. Clostridia: the importance of their exceptional substrate and metabolite diversity for biofuel and biorefinery applications. Curr Opin Biotechnol. 2012;23(3):364‐381. [DOI] [PubMed] [Google Scholar]
- 3. Artzi L, Bayer EA, Morais S. Cellulosomes: bacterial nanomachines for dismantling plant polysaccharides. Nat Rev Microbiol. 2017;15(2):83‐95. [DOI] [PubMed] [Google Scholar]
- 4. Zverlov VV, Kellermann J, Schwarz WH. Functional subgenomics of Clostridium thermocellum cellulosomal genes: identification of the major catalytic components in the extracellular complex and detection of three new enzymes. Proteomics. 2005;5(14):3646‐3653. [DOI] [PubMed] [Google Scholar]
- 5. Gold ND, Martin VJ. Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis. J Bacteriol. 2007;189(19):6787‐6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raman B, Pan C, Hurst GB, et al. Impact of pretreated Switchgrass and biomass carbohydrates on Clostridium thermocellum ATCC 27405 cellulosome composition: a quantitative proteomic analysis. PLoS One. 2009;4(4):e5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei H, Fu Y, Magnusson L, et al. Comparison of transcriptional profiles of Clostridium thermocellum grown on cellobiose and pretreated yellow poplar using RNA‐Seq. Front Microbiol. 2014;5:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahel‐Raifer H, Jindou S, Bahari L, et al. The unique set of putative membrane‐associated anti‐sigma factors in Clostridium thermocellum suggests a novel extracellular carbohydrate‐sensing mechanism involved in gene regulation. FEMS Microbiol Lett. 2010;308(1):84‐93. [DOI] [PubMed] [Google Scholar]
- 9. Nataf Y, Bahari L, Kahel‐Raifer H, et al. Clostridium thermocellum cellulosomal genes are regulated by extracytoplasmic polysaccharides via alternative sigma factors. Proc Natl Acad Sci USA. 2010;107(43):18646‐18651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Munoz‐Gutierrez I, Ortiz de Ora L, Rozman Grinberg I, et al. Decoding biomass‐sensing regulons of Clostridium thermocellum alternative sigma‐I factors in a heterologous Bacillus subtilis host system. PLoS One. 2016;11(1):e0146316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ortiz de Ora L, Munoz‐Gutierrez I, Bayer EA, Shoham Y, Lamed R, Borovok I. Revisiting the regulation of the primary Scaffoldin gene in Clostridium thermocellum . Appl Environ Microbiol. 2017;83(8):e03088‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campagne S, Allain FH, Vorholt JA. Extra cytoplasmic function sigma factors, recent structural insights into promoter recognition and regulation. Curr Opin Struct Biol. 2015;30:71‐78. [DOI] [PubMed] [Google Scholar]
- 13. Feklistov A, Darst SA. Promoter recognition by bacterial alternative sigma factors: the price of high selectivity? Genes Dev. 2009;23(20):2371‐2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rhodius VA, Segall‐Shapiro TH, Sharon BD, et al. Design of orthogonal genetic switches based on a crosstalk map of sigmas, anti‐sigmas, and promoters. Mol Syst Biol. 2013;9:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Groot PW, Klis FM. The conserved PA14 domain of cell wall‐associated fungal adhesins governs their glycan‐binding specificity. Mol Microbiol. 2008;68(3):535‐537. [DOI] [PubMed] [Google Scholar]
- 16. Ielasi FS, Decanniere K, Willaert RG. The epithelial adhesin 1 (Epa1p) from the human‐pathogenic yeast Candida glabrata: structural and functional study of the carbohydrate‐binding domain. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 3):210‐217. [DOI] [PubMed] [Google Scholar]
- 17. Silipo A, Larsbrink J, Marchetti R, Lanzetta R, Brumer H, Molinaro A. NMR spectroscopic analysis reveals extensive binding interactions of complex xyloglucan oligosaccharides with the Cellvibrio japonicus glycoside hydrolase family 31 alpha‐xylosidase. Chemistry. 2012;18(42):13395‐13404. [DOI] [PubMed] [Google Scholar]
- 18. Veelders M, Bruckner S, Ott D, Unverzagt C, Mosch HU, Essen LO. Structural basis of flocculin‐mediated social behavior in yeast. Proc Natl Acad Sci USA. 2010;107(52):22511‐22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zupancic ML, Frieman M, Smith D, Alvarez RA, Cummings RD, Cormack BP. Glycan microarray analysis of Candida glabrata adhesin ligand specificity. Mol Microbiol. 2008;68(3):547‐559. [DOI] [PubMed] [Google Scholar]
- 20. Rigden DJ, Mello LV, Galperin MY. The PA14 domain, a conserved all‐beta domain in bacterial toxins, enzymes, adhesins and signaling molecules. Trends Biochem Sci. 2004;29(7):335‐339. [DOI] [PubMed] [Google Scholar]
- 21. Yoshida E, Hidaka M, Fushinobu S, et al. Role of a PA14 domain in determining substrate specificity of a glycoside hydrolase family 3 beta‐glucosidase from Kluyveromyces marxianus . Biochem J. 2010;431(1):39‐49. [DOI] [PubMed] [Google Scholar]
- 22. Zmudka MW, Thoden JB, Holden HM. The structure of DesR from Streptomyces venezuelae, a beta‐glucosidase involved in macrolide activation. Protein Sci. 2013;22(7):883‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385(6619):833‐838. [DOI] [PubMed] [Google Scholar]
- 24. Jindou S, Xu Q, Kenig R, et al. Novel architecture of family‐9 glycoside hydrolases identified in cellulosomal enzymes of Acetivibrio cellulolyticus and Clostridium thermocellum . FEMS Microbiol Lett. 2006;254(2):308‐316. [DOI] [PubMed] [Google Scholar]
- 25. Levy‐Assaraf M, Voronov‐Goldman M, Rozman Grinberg I, et al. Crystal structure of an uncommon cellulosome‐related protein module from Ruminococcus flavefaciens that resembles papain‐like cysteine peptidases. PLoS One. 2013;8(2):e56138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Noach I, Levy‐Assaraf M, Lamed R, Shimon LJ, Frolow F, Bayer EA. Modular arrangement of a cellulosomal scaffoldin subunit revealed from the crystal structure of a cohesin dyad. J Mol Biol. 2010;399(2):294‐305. [DOI] [PubMed] [Google Scholar]
- 27. Rincon MT, Cepeljnik T, Martin JC, et al. A novel cell surface‐anchored cellulose‐binding protein encoded by the sca gene cluster of Ruminococcus flavefaciens . J Bacteriol. 2007;189(13):4774‐4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ielasi FS, Verhaeghe T, Desmet T, Willaert RG. Engineering the carbohydrate‐binding site of Epa1p from Candida glabrata: generation of adhesin mutants with different carbohydrate specificity. Glycobiology. 2014;24(12):1312‐1322. [DOI] [PubMed] [Google Scholar]
- 29. Murray MG, Thompson WF. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980;8(19):4321‐4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xu Q, Morrison M, Nelson KE, Bayer EA, Atamna N, Lamed R. A novel family of carbohydrate‐binding modules identified with Ruminococcus albus proteins. FEBS Lett. 2004;566(1–3):11‐16. [DOI] [PubMed] [Google Scholar]
- 31. Yaniv O, Shimon LJ, Bayer EA, Lamed R, Frolow F. Scaffoldin‐borne family 3b carbohydrate‐binding module from the cellulosome of Bacteroides cellulosolvens: structural diversity and significance of calcium for carbohydrate binding. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 6):506‐515. [DOI] [PubMed] [Google Scholar]
- 32. Yaniv O, Jindou S, Frolow F, Lamed R, Bayer EA. A simple method for determining specificity of carbohydrate‐binding modules for purified and crude insoluble polysaccharide substrates. Methods Mol Biol. 2012;908:101‐107. [DOI] [PubMed] [Google Scholar]
- 33. Lamed R, Setter E, Bayer EA. Characterization of a cellulose‐binding, cellulase‐containing complex in Clostridium thermocellum . J Bacteriol. 1983;156(2):828‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vagin A, Teplyakov A. MOLREP: an automated program for molecular replacement. J Appl Cryst. 1997;30:1022‐1025. [Google Scholar]
- 35. Winn MD, Ballard CC, Cowtan KD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):235‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum‐likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240‐255. [DOI] [PubMed] [Google Scholar]
- 37. Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Perrakis A, Morris R, Lamzin VS. Automated protein model building combined with iterative structure refinement. Nat Struct Biol. 1999;6(5):458‐463. [DOI] [PubMed] [Google Scholar]
- 39. Holm L, Rosenstrom P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545‐W549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870‐1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saitou N, Nei M. The neighbor‐joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406‐425. [DOI] [PubMed] [Google Scholar]
- 43. Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39(4):783‐791. [DOI] [PubMed] [Google Scholar]
- 44. Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor‐joining method. Proc Natl Acad Sci USA. 2004;101(30):11030‐11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44(W1):W242‐W245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mrazek J, Xie S. Pattern locator: a new tool for finding local sequence patterns in genomic DNA sequences. Bioinformatics. 2006;22(24):3099‐3100. [DOI] [PubMed] [Google Scholar]
- 47. Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25(9):1189‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Notredame C, Higgins DG, Heringa J. T‐coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302(1):205‐217. [DOI] [PubMed] [Google Scholar]
- 49. Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14(6):1188‐1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ortiz de Ora L, Lamed R, Liu YJ, et al. Regulation of biomass degradation by alternative sigma factors in cellulolytic clostridia. Sci Rep. 2018;8(1):11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Artzi L, Morag E, Barak Y, Lamed R, Bayer EA. Clostridium clariflavum: key cellulosome players are revealed by proteomic analysis. MBio. 2015;6(3):e00411‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Noach I, Frolow F, Alber O, Lamed R, Shimon LJ, Bayer EA. Intermodular linker flexibility revealed from crystal structures of adjacent cellulosomal cohesins of Acetivibrio cellulolyticus . J Mol Biol. 2009;391(1):86‐97. [DOI] [PubMed] [Google Scholar]
- 53. Goossens K, Willaert R. Flocculation protein structure and cell‐cell adhesion mechanism in Saccharomyces cerevisiae . Biotechnol Lett. 2010;32(11):1571‐1585. [DOI] [PubMed] [Google Scholar]
- 54. Ielasi FS, Goyal P, Sleutel M, Wohlkonig A, Willaert RG. The mannose‐specific lectin domains of Flo1p from Saccharomyces cerevisiae and Lg‐Flo1p from S. pastorianus: crystallization and preliminary X‐ray diffraction analysis of the adhesin‐carbohydrate complexes. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013;69(Pt 7):779‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maestre‐Reyna M, Diderrich R, Veelders MS, et al. Structural basis for promiscuity and specificity during Candida glabrata invasion of host epithelia. Proc Natl Acad Sci USA. 2012;109(42):16864‐16869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gabius HJ, Andre S, Jimenez‐Barbero J, Romero A, Solis D. From lectin structure to functional glycomics: principles of the sugar code. Trends Biochem Sci. 2011;36(6):298‐313. [DOI] [PubMed] [Google Scholar]
- 57. Diderrich R, Kock M, Maestre‐Reyna M, et al. Structural hot spots determine functional diversity of the Candida glabrata epithelial Adhesin family. J Biol Chem. 2015;290(32):19597‐19613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Boraston AB, Bolam DN, Gilbert HJ, Davies GJ. Carbohydrate‐binding modules: fine‐tuning polysaccharide recognition. Biochem J. 2004;382(Pt 3):769‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gilbert HJ, Knox JP, Boraston AB. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate‐binding modules. Curr Opin Struct Biol. 2013;23(5):669‐677. [DOI] [PubMed] [Google Scholar]
- 60. Henshaw J, Horne‐Bitschy A, van Bueren AL, et al. Family 6 carbohydrate binding modules in beta‐agarases display exquisite selectivity for the non‐reducing termini of agarose chains. J Biol Chem. 2006;281(25):17099‐17107. [DOI] [PubMed] [Google Scholar]
- 61. Jamal‐Talabani S, Boraston AB, Turkenburg JP, Tarbouriech N, Ducros VM, Davies GJ. Ab initio structure determination and functional characterization of CBM36; a new family of calcium‐dependent carbohydrate binding modules. Structure. 2004;12(7):1177‐1187. [DOI] [PubMed] [Google Scholar]
- 62. Montanier C, Flint JE, Bolam DN, et al. Circular permutation provides an evolutionary link between two families of calcium‐dependent carbohydrate binding modules. J Biol Chem. 2010;285(41):31742‐31754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Montanier CY, Correia MA, Flint JE, et al. A novel, noncatalytic carbohydrate‐binding module displays specificity for galactose‐containing polysaccharides through calcium‐mediated oligomerization. J Biol Chem. 2011;286(25):22499‐22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. FASTA sequences used during the analysis of the evolutionary relationships of σI factors from cellulolytic clostridia shown in Figure 3.


