Summary
A systematic review and meta‐analysis were conducted to assess the effectiveness of app‐based mobile interventions for improving nutrition behaviours and nutrition‐related health outcomes, including obesity indices (eg, body mass index [BMI]) and clinical parameters (eg, blood lipids). Seven databases were searched for studies published between 2006 and 2017. Forty‐one of 10 132 identified records were included, comprising 6348 participants and 373 outcomes with sample sizes ranging from 10 to 833, including 27 randomized controlled trials (RCTs). A beneficial effect of app‐based mobile interventions was identified for improving nutrition behaviours (g = 0.19; CI, 0.06‐0.32, P = .004) and nutrition‐related health outcomes (g = 0.23; CI, 0.11‐0.36, P < .001), including positive effects on obesity indices (g = 0.30; CI, 0.15‐0.45, P < .001), blood pressure (g = 0.21; CI, 0.01‐0.42, P = .043), and blood lipids (g = 0.15; CI, 0.03‐0.28, P = .018). Most interventions were composed of four behaviour change technique (BCT) clusters, namely, “goals/planning,” “feedback/monitoring,” “shaping knowledge,” and “social support.” Moderating effects including study design, type of app (commercial/research app), sample characteristics (clinical/non‐clinical sample), and intervention characteristics were not statistically significant. The inclusion of additional treatment components besides the app or the number or type of BCTs implemented did not moderate the observed effectiveness, which underscores the potential of app‐based mobile interventions for implementing effective and feasible interventions operating at scale for fighting the obesity epidemic in a broad spectrum of the population.
Keywords: BCT, diet, intervention, m‐Health, mobile apps, nutrition behaviour, nutritional outcomes, obesity
Abbreviations
- App
application
- BCT
behaviour change technique
- BMI
body mass index
- CONSORT
Consolidated Standards of Reporting Trials
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RCT
randomized controlled trial
1. INTRODUCTION
There were 2.1 billion people1 worldwide classified as overweight or obese in 2013, which equates to 27.5% of all adults. Since being overweight or obese is associated with both physical and mental health consequences2, 3, 4 and huge economic costs,5 it is one of today's most crucial health issues. Since nutrition‐related behaviours are well established as major risk factors in becoming overweight,6, 7 accounting for a considerable percentage of global disability‐adjusted life years,8 preventing obesity, and being overweight are not only of personal matters but also of social, societal, and governmental interest.9
To fight the obesity epidemic, it is important to scale effective, feasible, and affordable interventions that address a broad spectrum of the population. New intervention delivery modes such as e‐Health (web based) and m‐Health (mobile) technologies are booming and have even evoked a “self‐track trend.” The term “m‐Health” refers to the concept of using mobile devices, such as mobile phones, personal digital assistants (PDAs), tablets, wireless devices, and smartphones, in medicine and public health. Early functionalities of these devices were text messaging (SMS), paging, and voice communication.10 However, with the emergence of smartphones, more advanced functionalities including fully automated applications (apps) were developed. App‐based mobile health interventions are a particularly promising method of changing nutrition behaviours and nutrition‐related health outcomes due to the high level of global smartphone penetration and the ease of installing apps in all kinds of mobile devices.10 The advantages of app‐based mobile interventions are numerous, including the possibility of intervening in “real life” and “real time.” while also offering interactivity,11, 12, 13 the ability to tailor interventions to personal needs, and the potential to provide effective and feasible interventions to different target groups.14, 15 So far, however, most users and patients have relied on commercially available app‐based mobile health interventions that have not been empirically evaluated and rarely include evidence‐based strategies for behaviour change.16, 17 The effectiveness of app‐based mobile health interventions must be determined to enable evidence‐based decisions and to evaluate the potential contribution of app‐based mobile interventions as large‐scale health prevention measures.
While an increasing number of systematic reviews have examined technology‐based interventions, a considerable gap exists in the research since most of these reviews have examined combined intervention delivery modes using various e‐Health and m‐Health technologies simultaneously.18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 In addition, they have a specific focus on study selection, target population, and outcome measure. More specifically, most reviews combine different target behaviours (eg, diet and physical activity11, 18, 29, 30, 31, 32, 33); they focus on specific audiences (eg, adults with overweight or obesity34, 35 or with diabetes36, 37 or patients with cancer23) or research designs (eg, randomized controlled trials [RCTs]20, 21, 30, 34, 35, 38, 39, 40) or examine single nutrition‐related health outcomes (eg, weight loss20, 34, 35, 40). The few reviews that specifically look at app‐based mobile interventions are also heterogeneous in scope; some concentrate on single nutrition‐related health outcomes (eg, weight loss,39 diabetes,41, 42 or glycaemic control43) or a specific target group (eg, healthy adults44), while others combine multiple health behaviours (eg, diet and physical acitvity31, 33, 38, 45, 46). Almost all previous reviews analysed the studies narratively, and the number of intervention studies that they included was rather limited, ranging from four44 to 27.31 Not surprisingly, this leads to findings that are divergent, difficult to integrate, and limited in their ability to provide quantified effect sizes on the effectiveness of app‐based mobile health interventions. Only six previous meta‐analyses, which included between seven and 22 studies, revealed that app‐based mobile interventions were associated with significant weight loss39, 40, 47 and improved diabetes indicators.42, 43, 46 On the basis of these findings, app‐based mobile interventions might be a promising approach for combating obesity and nutrition‐related diseases, but to our knowledge, no conclusions about the effects of app‐based mobile interventions on nutrition behaviours and nutrition‐related health outcomes have yet been drawn, and there have been no attempts to quantify the effects. Addressing both nutrition behaviours and nutrition‐related health outcomes provides a more comprehensive picture of the effectiveness of mobile interventions using a fully automated mobile dietary application, as intervention studies often target multiple outcomes within the same study.
Therefore, the aim of this systematic review and meta‐analysis was to address these research gaps by evaluating the effectiveness of mobile interventions using a fully automated mobile dietary application on nutritional outcomes in both healthy and clinical audiences. Nutritional outcomes included (a) nutrition behaviours (primary outcomes) such as nutrition scores and calorie intake and (b) consequent nutrition‐related health outcomes (secondary outcomes) such as obesity indices (eg, body mass index [BMI]) and clinical metabolic parameters (eg, blood lipids). Unlike most previous reviews that have narratively summarized empirical evidence, we add to the literature by conducting both a systematic review and a meta‐analysis using a random effects model for quantifying intervention effects. In addition, we extended the scope of previous reviews by examining relevant moderator effects such as sample characteristics and the duration of the intervention and assessing the “building blocks” of app‐based mobile interventions that target nutrition behaviours and nutrition‐related health outcomes by coding the intervention characteristics according to implemented behaviour change techniques48 (BCTs).
2. METHODS
The systematic review of the literature and quantitative meta‐analysis to evaluate the effectiveness of app‐based mobile interventions on nutrition behaviours and nutrition‐related health outcomes were conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines49 (see Data S1 for the completed PRISMA checklist).
2.1. Search strategy and selection criteria
To investigate the effectiveness of app‐based mobile dietary interventions for both clinical and healthy samples in changing nutrition behaviours and nutrition‐related health outcomes, studies were eligible if (a) they included a mobile intervention using a fully automated mobile application, (b) the app assessed any kind of nutrition behaviour, (c) the measured outcome was nutrition‐related, including either nutrition behaviours or nutrition‐related health outcomes, and (d) they targeted adolescents or adults.
Any intervention study design or study population from clinical and healthy audiences was considered for inclusion. All types and units of measurement for nutrition behaviours and nutrition‐related health outcomes were acceptable (eg, self‐report, objective measures, calories, and kilograms). The app intervention could be a stand‐alone intervention using apps only or a multicomponent intervention where the use of an app was one of several intervention components (eg, face‐to‐face counselling and dietary education). RCTs and pre‐post studies were included if they encompassed an intervention that was delivered through an app that targeted nutrition behaviours or nutrition‐related health outcomes. We considered both between (control‐intervention) and within (pre‐post) comparisons for the quantitative meta‐analyses. We looked at studies between 2006 and June 2017, since smartphones and apps are a recent development over the last 10 years. Studies were excluded if (a) they were not available in English language, (b) they were published before 2006, (c) they were targeting children (12 y of age or younger), (d) they were papers, study protocols, or conference presentations that did not report empirical data, or (e) the app did not assess any kind of nutrition behaviour.
To ensure that the literature search was multidisciplinary and comprehensive, we searched databases from the fields of medicine, nutrition, and sport (MEDLINE, PubMed, PsycInfo, PsycIndex, PsycArticle, SPORTDiscus, and Web of Science) using a predefined systematic database search protocol developed in cooperation with a scientific literature specialist from the university library. The search strategy incorporated both keywords and a controlled vocabulary (eg, Medical Subject Headings and MeSH terms) and free‐text search terms. The complete search terms as well as a specification of the search strategy are provided in Data S2. We also searched reference lists of relevant review papers and retraced study protocols and conference presentations to identify other potentially eligible studies.
After duplicates are removed, articles were selected in a three‐step process (see Figure 1). Firstly, trained reviewers independently screened titles and abstracts according to the four eligibility criteria, with no reviewer screening both the title and abstract of the same paper. The full‐text articles that remained after this first selection were then screened independently by two authors (K.V./D.W.), who also screened a random sample of 25 studies to ensure that the four eligibility criteria were being applied consistently. Disagreements were resolved through discussion by the authors until a consensus was reached.
Figure 1.
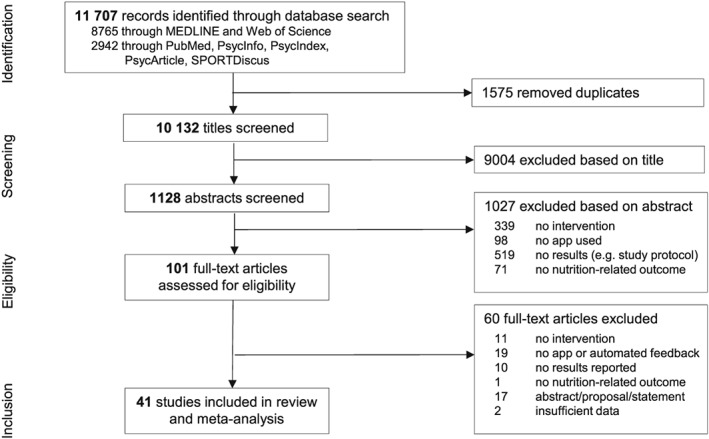
Study selection process
2.2. Data extraction
A standardized manual for data extraction was developed by the authors, and two authors (K.V./D.W.) independently extracted key study and sample characteristics (including study design, number and type of participants, and dropout rate), intervention characteristics (including type of intervention, duration, BCTs,48 and type of app used), nutrition assessment (including quantity and type of food assessed and assessment method), and prespecified outcomes. The information was then entered into a customized Excel database and discussed by the authors. The coders were trained in recognizing BCTs as defined by the v1 taxonomy and reference list.48 The v1 taxonomy includes a total of 93 BCTs such as “goal setting for behaviours” (BCT 1.1), which describes a behaviour goal such as eating five pieces of fruit per day, or “goal setting for outcome” (BCT 1.3), representing a positive outcome of a wanted behaviour, eg, a weight loss goal of 0.5 kg over 1 week as an outcome of a changed eating pattern. Both BCTs belong to the BCT cluster “goals and planning” (BCT 1). In total, the v1 taxonomy includes 93 BCTs, which are grouped into 16 BCT clusters. The coders independently extracted and coded the 93 BCTs and 16 BCT clusters, and disagreements were resolved through discussions between the authors until a consensus was reached. Since missing details in the descriptions of the interventions made it impractical to only analyse BCTs that were implemented in the app, additional treatments were also considered when classifying the implemented intervention strategies. In order to retrieve as much detail and information about the interventions and BCTs as possible, we used three sources: (a) the description and information provided in the retrieved articles, (b) the original apps used in the included studies (which we downloaded for data extraction where possible), and (c) in case of incomplete reports, we contacted the authors and asked for the missing information. Hence, we took a great deal of effort to retrieve as much information as possible, so we could make objective and non‐contentious decisions. Neither authorship, publication journal, nor study results were blinded for data extraction.
2.3. Outcomes
Our primary outcomes were changes in nutrition behaviours, including changes in overall nutrition (eg, healthy eating score), the consumption of specific foods (eg, fruit and vegetables), nutrient intake (eg, vitamin C), and caloric intake. Secondary outcomes were nutrition‐related health outcomes including obesity indices (eg, body weight and BMI) and clinical parameters (eg, blood lipids and blood pressure). See also Data S8 for the identified outcomes included in the present meta‐analysis (three right‐hand columns).
2.4. Study quality assessment
The quality of the included studies was independently evaluated by two authors (K.V./D.W.) according to the 25 criteria outlined by the Consolidated Standards of Reporting Trials (CONSORT) 2010 checklist.50 Criteria and respective items are related to the background and objectives, methods (including participant selection and outcome measures), study analysis and results, and potential selection bias or bias from funding (see Figure 2 and Data S4 for a definition of all 25 criteria). While the CONSORT checklist is intended for controlled trials, most criteria are applicable to other study designs, and the weaker study designs justifiably received a lower score than studies using a controlled trial design. This approach has been used in other reviews.31, 51 Each item of the 25 criteria was rated as 1 (fulfilled), 0.5 (only partially fulfilled), 0 (not fulfilled), or not applicable to the study design. For example, criterion 2, “background and objectives,” entails two items with item 2a, “scientific background and explanation of rationale,” and item 2b, “specific objectives or hypotheses.” Items were averaged per criterion. Adapted from previous reviews,31, 51, 52 study quality was classified as “high,” “fair,” and “low” based on an “overall study quality score” (sum of points). Nonapplicable criteria were discounted from the “overall study quality score,” and as a result, the highest attainable quality score was not 25 for all studies. The study quality score for each study was divided by the highest attainable score and multiplied by 100 to give a percentage of fulfilled criteria, with more than 66.6% = high, 50% to 66.6% = fair, and less than 50% = low study quality (Figure 2). In addition, two authors (K.V./D.W.) independently assessed the risk of bias according to the International Cochrane Collaboration criteria.53 All study criteria were dual coded, and any discrepancies were resolved by consensus.
Figure 2.
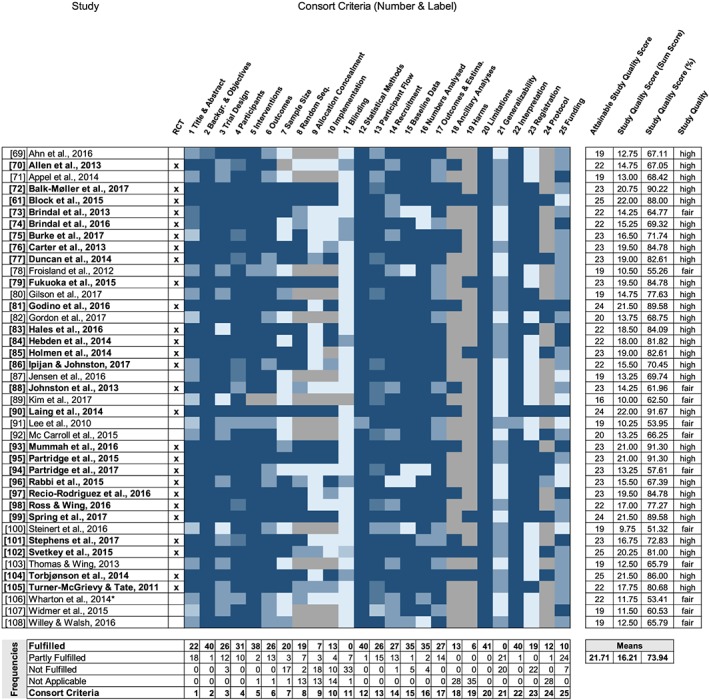
Heat map visualizing the assessment of the 25 Consolidated Standards of Reporting Trials (CONSORT) criteria of study quality for the 41 studies included. Colours range from dark blue (item fulfilled) to light blue (item not fulfilled or unclear) and grey (not applicable). *Wharton et al (2014): semirandomized trial
2.5. Data analysis and synthesis
Standardized effect sizes were calculated to conduct quantitative meta‐analyses. Effect sizes were calculated based on two approaches to account for between group (control‐intervention) and within group (pre‐post) comparisons.54 Effect sizes were calculated separately for each measured primary and secondary outcome. Referring to Higgins and Green,55 outcomes reported for subsamples (eg, gender) and for studies with more than two groups were pooled to create single pairwise comparisons to address the unit‐of‐analysis error due to “double counts.” Specifically, we combined all relevant intervention groups of a study into a single group and combined all relevant control groups into a single control group. Cohen's d was calculated to provide a standardized effect size56 and converted into Hedges' g to correct for the slight upward sample bias.57 Hedges' g is a variation of Cohen's d that corrects for biases because of small sample sizes.57 However, the effect sizes calculated in Cohen's d (see Data S11) or Hedges' g (see Data S12) were highly comparable and did not show marked statistical changes. The magnitude of Hedges' g is interpreted using Cohen's convention as small (0.2), medium (0.5), and large (0.8).56 The standardized mean difference was prioritized for between‐group comparisons, and the standardized mean change was preferred for within‐group comparisons. Reported effect sizes (eg, odds ratios) were transformed into Cohen's d. Furthermore, where possible, effect sizes were calculated from the statistics provided.58, 59 Positive effect sizes refer to intended changes by the intervention, indicating, for example, weight loss or an increase in fruit consumption.
The standardized effect sizes were synthesized using a meta‐analysis model with random effects.60 The choice of a fixed effect or a random effects statistical model affects the method used to calculate the total overall estimate and hence the interpretation of the summary estimates. A fixed‐effect meta‐analysis assumes that all studies are estimating the same (fixed) intervention effect, whereas a random effects meta‐analysis allows for differences in the intervention effect from study to study (see, for example, Riley et al12 and Borenstein et al60). Thus, random effects meta‐analysis models address heterogeneity in the interventions effects caused by differences in study populations, interventions received, follow‐up length, and other factors. However, random effects commonly yield a wider scatter of effect estimates and substantially wider confidence intervals than fixed effect models because each effect size has two components of variation: one due to sampling error and one from the underlying distribution. In one study,61 the within‐group comparison effect sizes for weight (d = 46.57) and BMI (d = 15.00) deviated markedly from other studies, and these two outcomes effects were therefore excluded from the analyses. To address within‐study dependencies, effect sizes of studies measuring multiple outcomes were aggregated using the univariate procedure developed by Borenstein, Hedges, Higgins, and Rothstein60 (BHHR), which is considered as the most precise and least biassed.62 Heterogeneity was investigated by Q‐statistics and Higgins I 2.
The meta‐analysis was conducted in a three‐step process to account for the heterogeneity of study designs and outcome variables: (a) firstly, an all‐encompassing data set was created to calculate an overall effect size across all 373 identified primary and secondary outcomes, including both between‐group and within‐group comparisons. Here, both within‐group and between‐group comparisons within a study were included. Sensitivity analyses were then computed following the recommendation of Viechtbauer and Cheung63 for outlier and influence diagnostic procedures to determine the stability of conclusions. Publication bias was assessed by a visual inspection of the funnel plot and Egger's test.64 Subgroup analyses were conducted by type of comparison (between or within group). (b) Secondly, an adjusted data set was created, which prioritized between‐group comparisons over within‐group comparisons.54, 65 If a primary or secondary outcome was measured as both between‐group and within‐group comparisons, the between‐group comparison was prioritized and included in the data set. Within‐group comparisons were only included when no between‐group comparisons were provided for the outcome. Follow‐up intervals from the baseline were calculated and grouped into short‐term (less than 3 mo), intermediate‐term (3‐6 mo), and long‐term (more than 6 mo) effects.55 In cases where an outcome (eg, BMI) was measured multiple times within a follow‐up interval, the shortest in duration within the respective follow‐up interval was prioritized. Subgroup analyses were performed for the three different follow‐up intervals. Meta‐regressions were also conducted for a priori identified moderators, including study design (RCT vs non‐RCT), sample size and characteristic (clinical vs non‐clinical), study and intervention duration, dropout rate, and the number of included outcomes, as well as intervention characteristics including the type of app (commercial vs research app), the inclusion of treatment components in addition to the app (stand‐alone app [app only] vs app combined with another intervention [app+]), and the number of BCTs implemented as intervention strategies. Furthermore, we conducted meta‐regressions for samples including adolescents vs adults and the quality of the studies according to the 25 CONSORT criteria (high vs fair). We conducted additional moderation analysis to get more insights into the question of whether the presence or absence of each BCT identified in the included intervention studies impacts on the effect size estimates (see Goodwin, Ostuzzi, Khan, Hotopf, and Moss‐Morris,66 Tang, Smith, McSharry, Hann, and French,67 and Williams and French68 for a similar approach). (c) Thirdly, to get more detailed insights into intervention effects, primary and secondary outcomes were analysed separately and further subdivided into short‐term, intermediate‐term, and long‐term follow‐up intervals. In addition, constituent outcomes of nutrition behaviours and nutrition‐related health outcomes were analysed separately (given a sufficient number of studies was available for the respective outcome), including caloric and fruit/vegetable intake as well as obesity indices (including body weight, BMI, body fat, hip, waist, and arm circumference), blood pressure, blood lipids (cholesterol, LDL, HDL, and triglyceride), and blood sugar (including fasting [plasma] glucose, glucose, HbA1c, and glucose/HbA1c).
All analyses were conducted in Statistical Package for the Social Sciences (SPSS) (version 24) and R using the packages compute.es, metafor, MAd, and altmeta.
3. RESULTS
The search identified a total of 11 707 electronic records. After duplicates are removed, 10 132 titles were screened, and the full text of 101 potentially eligible articles was retrieved (see Figure 1). Studies were excluded for multiple reasons, such as not having an intervention component (see Data S3 for a list of the excluded studies). In total, 41 studies61, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108 had investigated the effectiveness of app‐based mobile interventions for improving nutrition behaviours or nutrition‐related health outcomes and met all four eligibility criteria. These 41 studies were then included in the systematic review and quantitative meta‐analysis.
3.1. Study quality
A detailed summary of quality assessments of the studies included according to the CONSORT 2010 checklist50 is presented in Figure 2 and depicted as a heat map. The 25 CONSORT criteria show a considerable variation across the 41 studies (see Data S4 for criteria definitions and details). Overall, study quality ranged from high (29 studies) to fair (12 studies) (see Figure 2 for study details). While the study quality of 89% of the 27 RCT intervention studies was classified as “high,” 43% of the non‐RCT intervention studies were classified as being of “high” quality. On average, the studies included fulfilled 74% of the quality assessment criteria (range: 51%‐92%). Most studies (at least 85%) met the CONSORT criteria requirements of providing a clear scientific rationale and describing their scientific background and objectives (criterion 2), delivered interventions (criterion 5), statistical methods (criterion 12), sample characteristics (criterion 15), and number analysed (criterion 16); they also considered limitations (criterion 20) and provided a consistent and balanced interpretation of their results (criterion 22). A majority of the studies (at least 63%) reported a detailed and complete description of the study design, participants, and outcomes as specified by the CONSORT criteria (criteria 3, 4, 6, 13, 14, and 17). Fewer studies reported sample size calculations (criterion 7) and included randomization (criteria 8‐10). Most of the studies did not include blinding procedures in their study design (criterion 11). Therefore, the results of the evaluation of risk of bias according to the six International Cochrane Collaboration criteria53 indicate a high or unclear risk of bias for the two blinding procedures criteria and the allocation concealment criterion. Risk of bias due to incomplete data or random sequence generation was low (36 studies and 27 studies, respectively). See Data S5 and S6.
3.2. Study and sample characteristics
Overall, the 41 studies contained a total of 6348 participants, yielded 373 primary and secondary outcomes, and were published between 2006 and 2017 (80.5% in 2014 or later). Of these, 27 studies were RCTs with either a two‐arm (19 studies), three‐arm (seven studies), or four‐arm (one study) design. The remaining 14 studies were either single‐arm, pre‐post studies (eight studies), or studies with different control group designs (six studies). Sample size in the studies ranged from 10 to 833 (M = 154.83; SD = 177.80), with an average attrition rate of 18.7% (SD = 16.27; range: 0%‐72%). Sample descriptions were provided for 5701 participants. The mean age was 41.51 years (SD = 13.44; range: 14‐68 y), and 3678 (64.5%) of the sample population were women. Thirty‐eight studies focussed on adults, and three included adolescents aged between 13 to 19 years of age71, 78 and 13 to 17 years87. The average BMI of the sample population was 30.77 kg/m2 (SD = 3.73; range: 22.25‐36.40 kg/m2). Most of the studies focussed on clinical samples, with 16 studies including participants classified as being overweight or obese and eight including patients diagnosed with diabetes or prediabetes. In addition, one study focussed on survivors of endometrial and breast cancer92 and one on smokers.82 The remaining 15 studies focussed on non‐clinical, generally healthy samples. Study durations ranged from 20 days71 to 24 months,81, 102 with an average duration of 24 weeks (SD = 21.71). Intervention duration ranged from 2 weeks96 to 96 weeks81, 102 (M = 21.05; SD = 21.17).
A total of 30 different smartphone apps were used across the studies, mostly running on Android (nine studies), iOS (eight studies), or both (13 studies). To implement the intervention, 17 studies developed their own research app, while 15 used pre‐existing commercial apps. Two studies modified a pre‐existing app to fit their study purposes, and seven did not provide respective information. Eighteen studies included an exclusively app‐based intervention, and 23 studies included additional treatment components, which were delivered to participants either before (13 studies), during (23 studies), and/or after (two studies) the app‐based intervention.
3.3. Classification of implemented BCTs
The classification of implemented intervention strategies for achieving outcome changes according to the BCT taxonomy48 showed that in total, nine of the 16 different BCT clusters with an average of 3.88 (SD = 1.29, range: 1‐6) were employed across the 41 studies. Figure 3 depicts the frequency of BCT clusters implemented across the 41 studies and Table 1 the frequency of inclusion of single BCTs. A detailed summary of BCTs for each study is provided in Data S7.
Figure 3.
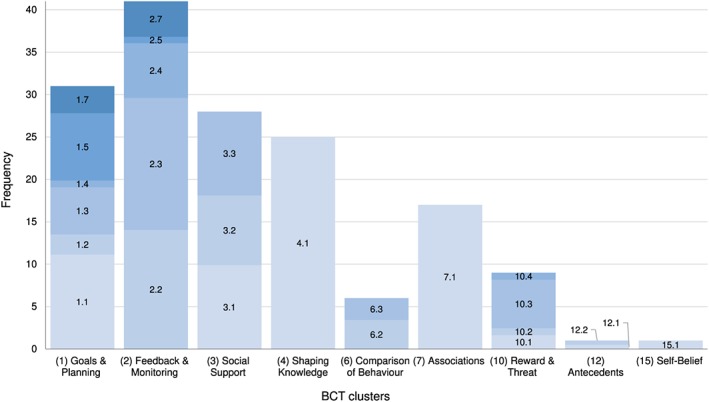
Absolute frequency of behaviour change technique (BCT) clusters implemented across studies (k = 41) and relative proportion of implemented BCTs within each of the BCT clusters, as classified in the BCT taxonomy.48 The number before the decimal point denotes the BCT cluster; decimal and colour denote the specific BCT. Note. Only nine out of the 16 BCT clusters were implemented across the 41 studies. See Table 1 and Data S7 for absolute frequencies of implemented BCTs
Table 1.
Comparisons between effect sizes, according to whether specific BCT clusters and single BCTs were present or absent in the intervention study
| Moderator | Present | Absent | ||||||
|---|---|---|---|---|---|---|---|---|
| k | g | k | g | Estimate | SE | P | CI | |
| BCT 1 | 31 | 0.27 | 10 | 0.21 | 0.06 | 0.13 | .663 | −0.19‐0.30 |
| BCT 1.1 | 28 | 0.26 | 13 | 0.25 | 0.02 | 0.12 | .875 | −0.21‐0.25 |
| BCT 1.2 | 6 | 0.30 | 35 | 0.25 | 0.04 | 0.15 | .803 | −0.26‐0.33 |
| BCT 1.3 | 14 | 0.20 | 27 | 0.29 | −0.10 | 0.11 | .396 | −0.32‐0.13 |
| BCT 1.4 | 2 | 0.30 | 39 | 0.26 | 0.05 | 0.27 | .854 | −0.48‐0.58 |
| BCT 1.5 | 20 | 0.26 | 21 | 0.26 | 0.00 | 0.11 | .973 | −0.21‐0.22 |
| BCT 1.7 | 8 | 0.16 | 33 | 0.28 | −0.11 | 0.13 | .391 | −0.37‐0.15 |
| BCT 2 | 41 | 0 | ||||||
| BCT 2.2 | 37 | 0.26 | 4 | 0.23 | 0.07 | 0.20 | .717 | −0.32‐0.46 |
| BCT 2.4 | 17 | 0.29 | 24 | 0.23 | 0.05 | 0.11 | .623 | −0.16‐0.27 |
| BCT 2.6 | 2 | −0.06 | 39 | 0.27 | −0.29 | 0.29 | .330 | −0.86‐0.29 |
| BCT 2.7 | 11 | 0.25 | 30 | 0.26 | −0.02 | 0.12 | .895 | −0.25‐0.22 |
| BCT 3 | 28 | 0.26 | 13 | 0.24 | 0.03 | 0.12 | .831 | −0.21‐0.27 |
| BCT 3.1 | 12 | 0.32 | 29 | 0.22 | 0.10 | 0.12 | .381 | −0.13‐0.33 |
| BCT 3.2 | 10 | 0.14 | 31 | 0.29 | −0.16 | 0.13 | .209 | −0.40‐0.09 |
| BCT 3.3 | 12 | 0.22 | 29 | 0.28 | −0.06 | 0.11 | .608 | −0.28‐0.17 |
| BCT 4 | 25 | 0.32 | 16 | 0.14 | 0.17 | 0.11 | .116 | −0.04‐0.38 |
| BCT 4.1 | 25 | 0.32 | 16 | 0.14 | 0.17 | 0.11 | .116 | −0.04‐0.38 |
| BCT 6 | 6 | 0.08 | 35 | 0.29 | −0.22 | 0.15 | .142 | −0.51‐0.07 |
| BCT 6.2 | 4 | −0.02 | 37 | 0.28 | −0.29 | 0.19 | .118 | −0.66‐0.08 |
| BCT 6.3 | 3 | 0.11 | 38 | 0.27 | −0.13 | −0.20 | .524 | −0.52‐0.26 |
| BCT 7 | 17 | 0.28 | 24 | 0.24 | 0.05 | 0.11 | .665 | −0.17‐0.26 |
| BCT 7.1 | 17 | 0.28 | 24 | 0.24 | 0.05 | 0.11 | .665 | −0.17‐0.26 |
| BCT 10 | 9 | 0.12 | 32 | 0.29 | −0.17 | 0.13 | .189 | −0.42‐0.08 |
| BCT 10.1 | 2 | −0.19 | 39 | 0.28 | −0.46 | 0.26 | .074 | −0.96‐0.05 |
| BCT 10.3 | 7 | 0.16 | 34 | 0.27 | −0.07 | 0.14 | .647 | −0.35‐0.22 |
Note. BCT cluster and single BCTs as classified in the BCT taxonomy.48 Comparisons were only made if the BCT cluster or single BCT was at least included in two intervention studies; k = number of studies; g = Hedges' g.
Abbreviation: BCT, behaviour change technique.
As Figure 3 illustrates, the BCT intervention strategy clusters “feedback and monitoring” (BCT 2, 41 studies), “goals and planning” (BCT 1, 31 studies), “social support” (BCT 3, 28 studies), and “shaping knowledge” (BCT 4, 25 studies) were implemented in the majority of the 41 studies, while “associations” (BCT 7, 17 studies), “reward and threat” (BCT 10, nine studies), and “comparison of behaviour” (BCT 6, six studies) were less prominent, and the BCT clusters “antecedents” (BCT 12) and “self‐belief” (BCT 15) were only implemented in one study each.
In addition to the 16 main BCT clusters of the BCT taxonomy, intervention strategies can be further classified into 93 BCTs. The number of implemented BCTs across the 41 studies ranged from two to 11, with an average of 6.9 (SD = 2.46). For example, the frequently implemented BCT cluster “goals and planning” (BCT 1) includes nine different BCTs, of which seven were actually implemented across the 41 studies with “goal setting for behaviours” (BCT 1.1, 28 studies), “goal setting for outcome” (BCT 1.3, 14 studies), and “review behaviour goal(s)” (BCT 1.5, 20 studies) being realized the most frequently. The intervention strategies “problem solving” (BCT 1.2, six studies), “action planning” (BCT 1.4, two studies), and “review outcome goal(s)” (BCT 1.7, eight studies) were implemented less often, and strategies such as “discrepancy between current behaviour and goal” (BCT 1.6), “behavioural contract” (BCT 1.8), and “commitment” (BCT 1.9) were implemented in none of the studies. The BCT cluster “feedback and monitoring” includes seven different BCTs, of which five were actually implemented across the 41 studies. The most frequently implemented strategies were “feedback on behaviour” (BCT 2.2, 37 studies), “self‐monitoring of behaviour” (BCT 2.3, 41 studies), “self‐monitoring of outcome(s) of behaviour” (BCT 2.4, 17 studies), and “feedback on outcome(s) of behaviour” (BCT 2.7, 11 studies), while the three remaining strategies were implemented either infrequently or not at all. The third main intervention strategy cluster “social support,” used in 28 studies, encompasses the three BCTs: “unspecified social support” (BCT 3.1, 12 studies), “practical social support” (BCT 3.2, 10 studies), and “emotional social support” (BCT 3.3, 12 studies).
3.4. Assessment of nutrition behaviour
Of the 41 studies, 29 included an app‐based assessment of the total nutrition intake,69, 70, 71, 72, 74, 75, 76, 78, 79, 80, 83, 85, 86, 87, 89, 90, 91, 92, 96, 97, 98, 99, 101, 102, 103, 104, 105, 106, 108 nine an assessment of specific foods61, 77, 82, 84, 93, 94, 95, 100, 107 such as vegetables or specific food consumption patterns (eg, adherence to specific guideline‐based recommendations), one assessed meal replacements,73 and two did not provide further information.81, 88 Twenty one of the 32 studies that provided information about the features implemented for nutrition assessment included a database that participants could search to select the food items and meals they had consumed. Other features included a photo function (11 studies), a barcode scan (13 studies), an open‐format description of food items (10 studies), and the selection of food icons for specific foods (three studies). In addition, 26 studies included a quantitative assessment of the amount of food consumed, with 20 studies providing further details about the assessment. All 20 studies used predefined amounts or quantities specified by the implemented databases, and nine studies also offered users the option to insert their own estimations of the amounts consumed.
3.5. Quantitative synthesis of primary and secondary outcomes
Three hundred seventy‐three outcome effect sizes were reported in the 41 studies, covering a broad range of different primary and secondary outcomes (see Data S8, right‐hand columns, as well as Data S12). The primary outcome, nutrition behaviours, was assessed through both general and specific nutrition scores (eg, Healthy Eating Index [HEI] as a measure of diet quality, which reflects the concordance of dietary pattern with key dietary recommendations from the Dietary Guidelines for Americans109), total caloric intake, the consumption of specific foods (eg, fruits and low‐fat milk), consumption of meal types (eg, take‐away meals), or the intake of specific nutrients (eg, sodium). Secondary outcomes also included a broad range of indicators ranging from obesity indices (eg, body weight, BMI, body fat, and waist circumference) to clinical metabolic parameters (eg, blood pressure, glucose, or triglyceride).
The random effects meta‐analyses based on the all‐encompassing data set including all 373 primary and secondary outcomes effect sizes across the 41 studies showed an overall significant small‐to‐medium positive effect for app‐based mobile interventions, with overall Hedges' g = 0.33 (CI, 0.21‐0.44, P < .001).
To assess the robustness of the effect, we conducted a sensitivity analysis including and excluding outliers.63 One study was identified as an outlier61 (Hedges' g = 1.80). However, removing this study did not appreciably change the overall effect size (Hedges' g = 0.27; CI, 0.20‐0.35). Therefore, we kept the study in the analysis, given our interest in providing a comprehensive assessment of the overall impact of app‐based mobile interventions on nutrition behaviours.
The investigation of publication bias, analysed by a visual examination of the funnel plot with the observed effect size g on the horizontal axis plotted against the standard error, revealed no asymmetry (see Data S9). In addition, Egger's regression coefficient64 did not suggest a publication bias (z = 0.47, P = .636). However, since the Q‐statistics for the overall effect across the 373 effect sizes were significant, indicating considerable heterogeneity between the 41 studies with Q(40) = 312.19, P < .001, and I 2 = 86.79%, the type of comparison (between group or within group) was further examined as a moderator. Analyses indicated a significant moderating effect, Q(1) = 13.13, P < .001, explaining R 2 = 3.63% of the among‐study heterogeneity. Subsequent separate subgroup analyses yielded a significant small effect size of Hedges' g = 0.22 (CI, 0.08‐0.36, P = .002, Q(27) = 200.61, P < .001, I 2 = 83.61%) for between‐group comparisons (k = 28, outcome n = 190) and a significant medium‐to‐large effect size with Hedges' g = 0.47 (CI, 0.29‐0.65, P < .001, Q(33) = 390.52, P < .001, I 2 = 93.61%) for within‐group comparisons (k = 34, outcome n = 183). As the type of comparison (between group or within group) was identified as a significant moderator, further analyses were conducted with the adjusted data set, which prioritized between‐group effects.
The random effects meta‐analyses based on the adjusted data set across the 41 studies including 224 effect sizes revealed an overall significant small but positive effect of Hedges' g = 0.26 (CI, 0.15‐0.36, P < .001). The Q and I 2 statistics indicated considerable heterogeneity across studies with Q(40) = 220.98, P < .001, and I 2 = 80.94% (see Figure 4). Separate meta‐regressions were conducted to identify moderating effects of study design (RCT vs non‐RCT), study quality (high vs fair), sample size and characteristic (non‐clinical vs clinical sample; adolescents vs adults), study and intervention duration, dropout rate, and the number of included outcomes, along with intervention characteristics including the type of the app, the inclusion of treatment components in addition to the app, and the number of BCTs implemented as intervention strategies. The meta‐regressions revealed no significant effects, with .875 ≤ P ≥ .120 (see Data S10). In addition to testing whether or not the number of BCTs implemented had a moderating effect, it was additionally tested whether specific BCTs were a predictor of the pooled effect size (see, for a similar approach, Goodwin et al,66 Tang et al,67 and Williams and French68). The separate meta‐regressions for the implemented BCT clusters and single BCTs (technique present vs not present in the intervention) yielded no significant effect, with .973 ≤ P ≥ .074 (see Table 1).
Figure 4.
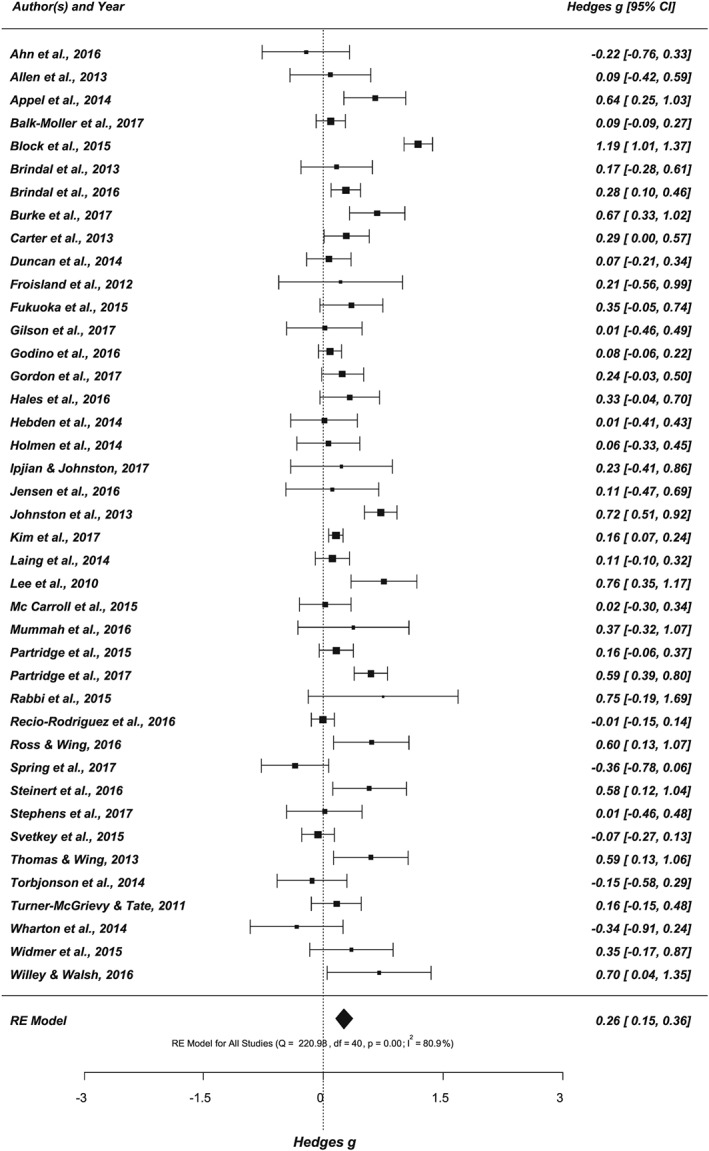
Forest plot showing the effects of app‐based mobile interventions on nutrition behaviours and nutrition‐related health outcomes (k = 41, outcome n = 224; adjusted data set)
3.6. Primary outcomes
Twenty one of the 41 studies assessed at least one primary outcome, addressing the effect of app‐based mobile interventions on nutrition behaviours, resulting in 24 analysed behavioural outcomes (see Figure 5). Analysing the effect of app‐based mobile interventions on behavioural outcomes (k = 21, outcome n = 24) showed a small significant effect size, Hedges' g = 0.19 (CI, 0.06‐0.32, P = .004), with considerable heterogeneity of Q(20) = 57.41, P < .001, and I 2 = 62.96%. On the basis of the number of available studies, behavioural outcomes were further separated into calorie (k = 8, outcome n = 9) and fruit and vegetable intake (k = 8, outcome n = 27). For a detailed summary, see Data S12 and S13. While both outcomes yielded overall positive effects, only the effect for fruit and vegetable intake reached statistical significance with Hedges' g = 0.32 (CI, 0.15‐0.50, P < .001, Q(7) = 11.83, P = .106, I 2 = 24.19%).
Figure 5.
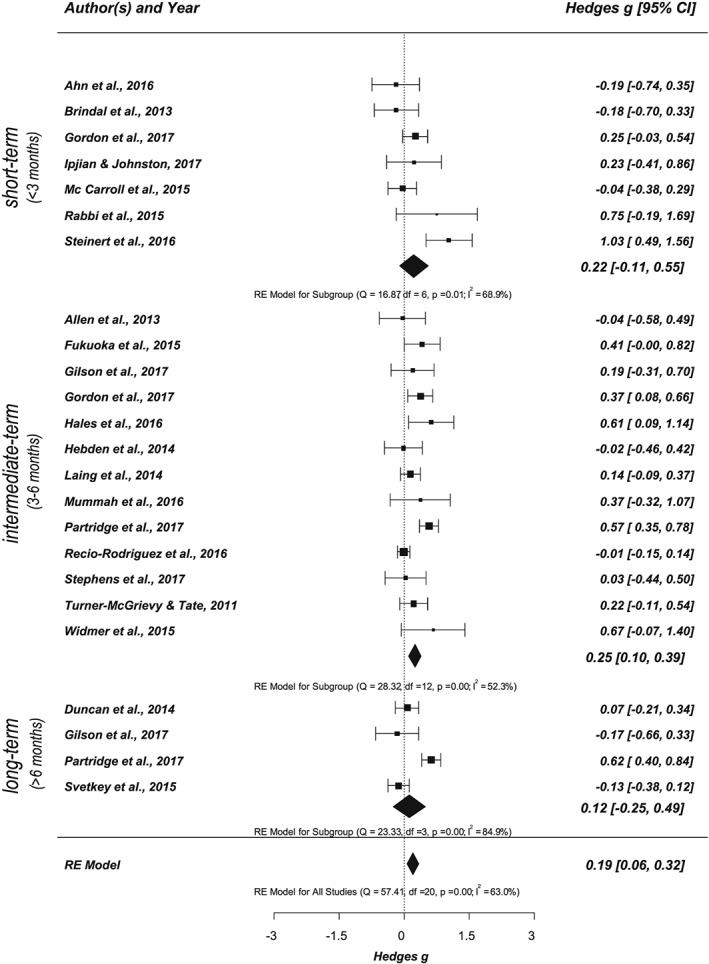
Forest plot showing the effects of app‐based mobile interventions on nutrition behaviours (primary outcome) for short‐term, intermediate‐term, and long‐term follow‐up intervals (k = 21, outcome n = 24; adjusted data set)
3.7. Secondary outcomes
Thirty four of the 41 studies included at least one secondary, nutrition‐related health outcome, resulting in 42 analysed outcomes (see Figure 6). The effect size for nutrition‐related health outcomes was small to medium, with Hedges' g = 0.23 (CI, 0.11‐0.36, P < .001). Effect sizes showed considerable heterogeneity between studies, Q(33) = 202.39, P < .001, and I 2 = 84.15%. Furthermore, based on the number of available studies, assessed nutrition‐related health outcomes were divided into obesity indices (eg, body weight and BMI), blood pressure, blood lipids, and blood sugar (see also Data S13). The strongest effect was found for obesity indices with Hedges' g = 0.30 (CI, 0.15‐0.45, P < .001, Q(31) = 230.49, P < .001, I 2 = 87.88%, k = 32, outcome n = 76). Separate analyses for body weight and BMI also revealed comparable effect sizes with an effect of Hedges' g = 0.27 for body weight (CI, 0.13‐0.41, P < .001, Q(30) = 125.59, P < .001, I 2 = 81.93%, k = 31, outcome n = 39) and an effect of Hedges' g = 0.37 for BMI (CI, 0.18‐0.55, P < .001, Q(16) = 66.35, P < .001, I 2 = 81.55%, k = 17, outcome n = 21). The effects for the other health indicators were also significantly positive but less pronounced, with blood pressure showing an overall effect of Hedges' g = 0.21 (CI, 0.01‐0.42, P = .043, Q(6) = 20.99, P = .002, I 2 = 73.81%, k = 7, outcome n = 19) and blood lipids of Hedges' g = 0.15 (CI, 0.03‐0.28, P = .018, Q(4) = 1.67, P = .797, I 2 = 0.00%, k = 5, outcome n = 22). For cholesterol, a significant positive overall effect was found with Hedges' g = 0.37 (CI, 0.04‐0.71, P = .031, Q(4) = 21.99, P < .001, I 2 = 72.81%, k = 5, outcome n = 7). The effect for blood sugar was also positive but not statistically significant, Hedges' g = 0.18, P = .429 (k = 7, outcome n = 10). We did not conduct separate meta‐analyses for the remaining single health outcomes, since the numbers of studies and outcomes were too small.
Figure 6.
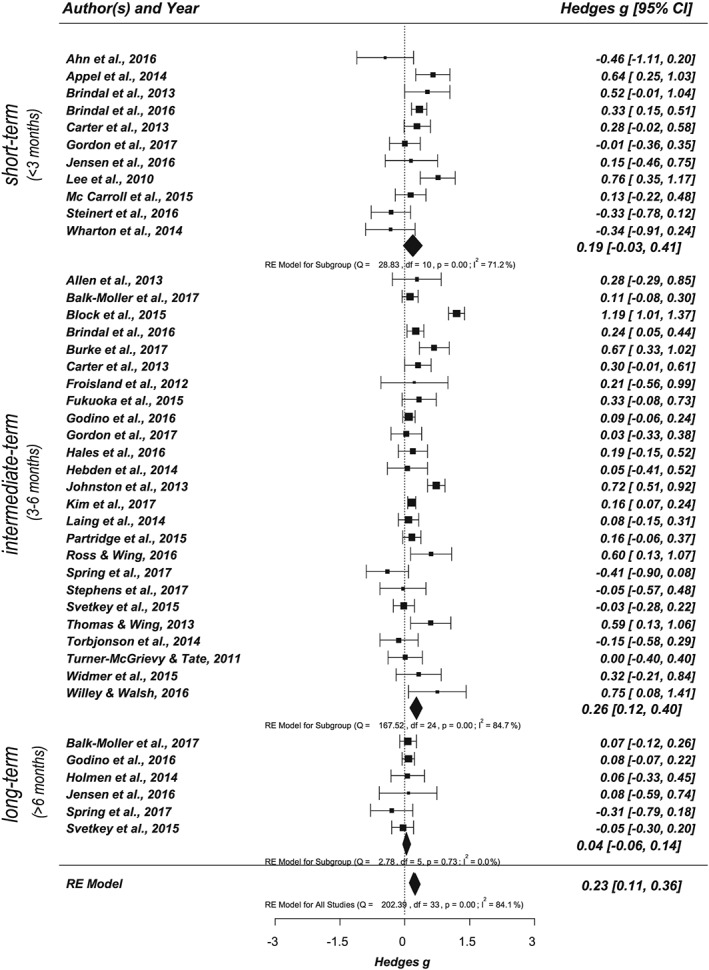
Forest plot showing the effects of app‐based mobile interventions on nutrition‐related health outcomes (secondary outcome) for short‐term, intermediate‐term, and long‐term follow‐up intervals (k = 34, outcome n = 42; adjusted data set)
3.8. Follow‐up intervals: Short‐term, intermediate‐term, and long‐term effects
Effect sizes for different follow‐up intervals were also examined. Assessing short‐term, intermediate‐term, and long‐term effects separately by subgroup analysis revealed positive effects for all follow‐up intervals. However, only studies targeting short‐term and/or intermediate‐term follow‐up intervals yielded significant small effect sizes (short‐term: Hedges' g = 0.27; CI, 0.12‐0.43, P = .001, Q(12) = 22.34, P = .034, I 2 = 48.53%, k = 13, outcome n = 57; intermediate term: Hedges' g = 0.28; CI, 0.15‐0.40, P < .001, Q(28) = 186.20, P < .001, I 2 = 83.85%, k = 29, outcome n = 136). The effects for long‐term intervals did not reach statistical significance (Hedges' g = 0.08; CI, −0.10‐0.39, P = .404, Q(8) = 27.84, P = .001, I 2 = 72.99%, k = 9, outcome n = 31). In a subsequent step, the effects of different follow‐up intervals were examined separately for primary and secondary outcomes (see Figures 5 and 6). For nutrition behaviours (primary outcome), the effect for the intermediate‐term follow‐up interval was statistically significant with Hedges' g = 0.25 (CI, 0.10‐0.39, P < .001, Q(12) = 28.32, P = .005, I 2 = 52.32%, k = 13, outcome n = 49). The effect for nutrition‐related health outcomes (secondary outcome) was also significant and small with Hedges' g = 0.26 (CI, 0.12‐0.40, P < .001, Q(24) = 167.52, P < .001, I 2 = 84.68%, k = 25, outcome n = 86). Effects of short‐term and long‐term intervals did not reach statistical significance for either outcome. An overview of the different levels of analysis and corresponding statistical characteristics is summarized in Data S12.
4. DISCUSSION
This meta‐analysis of data from 41 studies, which included more than 6300 participants, showed that app‐based mobile interventions can be effective for changing nutrition behaviours and their main nutrition‐related health outcomes in a wide range of study settings, with both clinical and generally healthy samples. The study extends previous work by including 373 primary and secondary outcomes addressing nutrition behaviours, obesity indices, and clinical metabolic parameters, revealing small‐to‐moderate positive effect sizes.
To make the results as relevant and robust as possible to inform prevention and clinical practice, we prioritized between group effects, which also resulted in a significant positive effect with Hedges' g = 0.26 (CI, 0.15‐0.36). We found no evidence that the inclusion of additional treatment components besides the app or the number of BCTs implemented moderated the observed effectiveness, which underscores the potential of app‐based mobile interventions for implementing effective and feasible, cost‐effective interventions with a high reach of target groups.
Although the results of studies comparing app‐based mobile intervention versus control and pre‐post comparisons yielded broadly consistent positive results on their relative effectiveness, a substantial diversity was observed in the range of efficacy across measured outcomes. In view of the rapid increase in the prevalence and disease burden of obesity worldwide, it is particularly encouraging that the results of app‐based mobile interventions revealed a positive effect size for changing obesity indices with Hedges' g = 0.30 (CI, 0.15‐0.45) pooled across 32 studies. This confirms previous reviews summarizing the effectiveness of various types of mobile interventions on obesity indices.21, 35, 39, 40, 47 This effectiveness was also observed for clinical metabolic parameters including blood lipids and blood pressure, although with smaller effect sizes, while a positive, non‐significant effect was observed for blood sugar. This differential efficacy across nutrition‐related health outcomes might reflect heterogeneous mechanisms and causes, smaller numbers of studies on clinical metabolic parameters, or different methodological issues affecting studies targeting obesity indices and clinical metabolic parameters. New developments in sensor technology, eg, continuous glucose measurement, seem to offer promising avenues for improving the assessment of the clinical metabolic parameters implicated in body weight regulation.110
4.1. Implications for intervention research and clinical practice
While the pooled effect size indicates a significant overall effect on nutrition behaviours and related health outcomes (Hedges' g = 0.26; CI, 0.15‐0.36), converting this statistical significance into clinical significance is not unequivocally possible. Nakagawa and Cuthill111 and other researchers suggested taking multiple criteria into account besides comparisons with benchmark values (eg, Cohen's classification), such as comparing effect size values of the current work with previous research and practical meaningful measures.
Comparing the effect size values of the current study with previous research shows that the observed effects coincide with m‐Health interventions in the same and other domains. For example, Schippers et al40 reported a pooled body weight reduction of d = −0.23; CI, −0.38 to −0.08 based on 12 studies, which is comparable with the present pooled obesity indices estimate, which includes 32 studies (Hedges' g = 0.30; CI, 0.15‐0.45). The effect size value is also comparable with effect sizes found in previous meta‐analyses of weight loss interventions either using mobile phones21, 112 or not using mobile phones.113 A comparison with other domains leads to similar conclusions. For example, a recent meta‐analysis in the domain of physical activity by Eckerstorfer et al114 found a small overall effect of Hedges' g = 0.29 (CI, 0.20‐0.37), which agrees with another recent meta‐analysis of m‐Health physical activity interventions.115
As pooled overall estimates are based on standardized effect sizes converted from measures with different units (eg, body weight loss in kg, BMI change, etc), translating them into physiological and clinical significance is not directly possible. However, effect sizes in original units, eg, body weight loss in kilograms as a result of the intervention, can be obtained from reported changes in the intervention arms (see also Schippers et al40). Summarizing the 13 intervention studies reporting changes in BMI (kg/m2) in intervention arms (see Data S14, Table S14.1), the average weighted BMI weight loss was −0.90 kg/m2, ranging from 0.2169 to −1.4183 kg/m2. In the 29 studies reporting body weight changes in kilograms (or lbs) in intervention arms (see Data S14, Table S14.2), the reduction in the intervention arm ranged from 0.6469 to −9.65103 kg with an average weight loss of −2.69 kg. This weighted effect size for body weight reduction is similar to those reported in previous meta‐analysis of mobile or e‐Health interventions within adults with chronic diseases (−2.45 kg)47, adults (−3.1 kg)40, and adults with overweight or obesity (−2.70 kg).20 Whether this is or is not enough to result in physiologically relevant effects or clinically meaningful health improvements probably depends on the situation and context.111 For some individuals with overweight or obesity, this weight loss might be insufficient. However, from a public health perspective, small changes might also be considered as relevant (see Fisher et al116 for a discussion).
The overall effectiveness of the interventions did not significantly vary in dependence of whether the interventions were based on apps as stand‐alone or they were combined with additional intervention components. Eighteen of the 41 intervention studies (44%) used an app as a stand‐alone intervention delivery method, while a larger proportion of interventions used an app in combination with other intervention strategies such as groups sessions,75 weekly meetings and online tools,88 coaching calls, text messages, and emails,94 or face‐to‐face contact98, 103 (see Data S8 for a detailed study description). Also, the 11 most successful intervention studies,61, 71, 74, 75, 88, 91, 94, 98, 100, 103, 108 which demonstrated a significant and positive overall pooled effect size, included four intervention studies71, 91, 100, 108 using an app as stand‐alone strategy (36%). Comparing the effect sizes between these “success models” shows an overall comparable result pattern for stand‐alone interventions (range: Hedges' g = 0.58100 to Hedges' g = 0.7691) compared with combined interventions (range: Hedges' g = 0.2874 to Hedges' g = 1.19.61)
A major finding of our analysis is that app‐based mobile interventions are effective in changing nutrition behaviours, which is an essential prerequisite for changes in obesity indices and clinical metabolic parameters.21, 22 However, the number and type of assessed behavioural outcomes varied between studies, which limited the calculation of effect sizes for all specific behavioural outcomes except for caloric and fruit and vegetable intake. A positive effect was found for these two behavioural outcomes, but the pooled effect was only significant for fruit and vegetable intake (Hedges' g = 0.32; CI, 0.15‐0.50), which is one of the most commonly measured dietary change indicators.
Studies showed substantial variations in the nutrition assessment methods, which are often study and/or country specific and are not harmonized.117 This diversity in methods for assessing dietary intake might have contributed to the identified heterogeneity and the smaller effect sizes compared with nutrition‐related health outcomes. It is therefore desirable to harmonize and standardize dietary assessment methods to enable better cross‐study comparisons.
Behaviour assessments also provide a major input for creating tailored feedback implemented in mobile interventions.118 So far, however, the full potential of mobile technology for dietary assessment has barely been realized. The focus is often on conventional food frequency questionnaires relying on retrospective self‐reports, including memory bias.119, 120 Mobile devices utilizing image‐based methods offer in‐the‐moment dietary assessments, and it is likely that automated food identification and portion size estimation will allow more a comprehensive and accurate assessment of the quality and quantity of dietary intake.119
Considering that on average, only four of the 16 different BCT clusters were implemented in the interventions and that mobile devices impose excessive restrictions on message length and interaction duration,121, 122, 123 the generally positive effects we observed across nutrition behaviours and major health outcomes were induced by a highly focussed intervention effort. The BCT clusters that were utilized, which form the central “building blocks” of interventions, mainly encompassed goal setting, feedback and self‐monitoring, information, and social support provision, which coincide with successful conventional individual and group‐based interventions124 and reviews on m‐Health interventions.21, 31, 40, 115, 125, 126, 127, 128 Setting goals, monitoring behaviour, receiving feedback, and reviewing relevant goals in the light of feedback are central to self‐management and behavioural control, as specified by control theories.129, 130 Considering that app‐based mobile interventions can operate at scale and at lower comparable implementation costs than individual and group‐based interventions, they offer the potential to be a cost‐effective method for improving nutrition behaviours and health indicators. A recent systematic review of economic evaluations of m‐Health solutions131 found a consistent overall reporting of positive economic outcomes (eg, increase in life‐years gained, cost savings, and cost‐effectiveness). Of 35 intervention studies using m‐Health as primary intervention component, 26 (74%) reported a positive costing outcome. This supports the notion that mobile intervention might be a viable alternative to more cost‐intensive face‐to‐face intervention formats, offering a potentially more effective alternative to common nonmobile interventions.
Although the number of single BCTs implemented varied from two to 11 between studies, the results did not show the association, which some studies have suggested between greater effectiveness and an increasing number of BCTs.67, 125, 127, 128, 132 We conducted additional moderation analysis to get more insights into the question of whether or not the presence of each of the 24 BCTs identified impacts on the effect size estimates (see Goodwin et al,66 Tang et al,67 and Williams and French68). While we identified 19 BCTs, which were implemented in two or more intervention studies, we did not find evidence that any of the BCTs predicted the pooled effect size. Hence, there does not appear to be a single effective approach to changing nutrition behaviours and their main nutrition‐related health outcomes (see also Goodwin et al66 for similar results but see Olander et al,133 Tang et al,67 and Williams and French68 for significant results). However, the effect of a single BCT may generally be very small.134 For example, according to control theories,129, 130 setting and reviewing goals in the light of feedback are central to self‐management, behavioural control, and ultimately behaviour changes. Accordingly, techniques revolving around goal setting and reviewing of goals are likely to be more effective if implemented conjointly in an intervention. However, “goal setting” (BCT 1.1 and BCT 1.3) was implemented more commonly than “review of goals” (BCT 1.5 and BCT 1.7).
The BCT taxonomy, while already complex with 16 different theory‐based BCT clusters and 93 single BCTs, is mainly focussing on static concepts. However, since mobile interventions distinguish themselves by being interactive, adaptive, time‐sensitive, and intraindividually dynamic,12 more dynamic concepts including the timing of information provision, feedback, and reminders or tailoring tasks and goals to individual progress and capacities as specified in persuasive technology might be essential ingredients of effective focussed mobile interventions.12, 13, 121, 122, 135, 136 Hence, while mobile phones are a promising platform for accessible and cost‐effective interventions, further development and innovation are required to ensure that the medium's possibilities are leveraged to ensure efficacious changes.14, 40
4.2. Study strengths and limitations
To the best of our knowledge, this is one of the most comprehensive and up‐to‐date reviews and meta‐analyses to evaluate the effects of app‐based mobile interventions on both major nutrition‐related health outcomes and intermediate nutrition behaviours across a broad spectrum of the population, combining data from 41 studies, 6348 participants, and 373 outcomes with sample sizes ranging from 10 to 833 participants and including 27 RCTs. The meta‐analyses should therefore provide sufficiently reliable estimates of the intervention effects associated with app‐based mobile interventions targeting nutrition behaviours and related health outcomes. By analysing the currently available evidence for app‐based mobile intervention studies and reporting results separately for study designs and characteristics, we address the observed heterogeneity and provide estimates across the whole range of intervention studies. We report results separately for two outcomes (nutrition behaviours and nutrition‐related health outcomes), each according to short‐term, intermediate‐term, and long‐term follow‐up intervals. Importantly, results showed consistency between study designs, clinical, and generally healthy samples and between the different forms of analysis.
However, our review and meta‐analysis have limitations. Since we strived to cover all the globally available evidence on app‐based mobile interventions targeting nutrition behaviours and nutrition‐related health outcomes, considerable heterogeneity in the results could be due to methodological differences restricting the interpretation. Firstly, the systematic review is based on 30 different apps, with 15 studies using commercial apps. Although meta‐regression did not yield a significant difference in effectiveness between commercial and research apps, considerable heterogeneity within both types of apps might still be of concern. Secondly, estimated intervention effects were smaller in between‐group comparisons than in within‐group comparisons, which are associated with less precise estimations due to within‐subject correlations and potentially confounding variables.54, 65 Thirdly, although the pooled effect size did not vary in dependence of the study or intervention duration (see also Schippers et al40 for similar findings for weight loss interventions), the effect sizes varied between short‐term, intermediate‐term, and long‐term follow‐ups as for the latter, effect sizes were generally smaller and non‐significant. There are several possible explanations for this, as many factors may have caused lower effectiveness for long‐term outcomes. This differential efficacy might indicate a smaller number of studies with long‐term follow‐ups, a decrease in the maintenance of changes,94 or a general lack of effectiveness.72, 81, 99, 102 Specifically, systematic reviews and meta‐analyses have shown that the efficacy of both traditional and technology‐based weight loss interventions is greatest during the first 6 months,20, 137 which might be due to a well‐documented decline in engagement with intervention modalities.138 Another possible explanation could be bias in conducting, analysing, or reporting. In our analysis, neither dropout rate, sample size, study quality, intervention duration, number or type of included BCTs, nor outcomes were associated with differential effectiveness. Also, the funnel plot did not provide consistent evidence for publication bias (see Data S9). However, such plots are known to be insensitive, and it is possible that a small study bias139 might have led to an overestimation of the magnitude of the effectiveness.
Considering the BCTs, we have identified four core clusters including “goals/planning,” “feedback/monitoring,” “shaping knowledge,” and “social support,” but not all interventions using these strategies were more effective. There are various possible reasons for this. The BCTs may be implemented very differently in one study versus another. Since meta‐analyses are reliant on published descriptions of intervention and control conditions, a further “drill down” was limited. We included information from the articles, authors, and apps to decrease the likelihood of missing out on actually implemented BCTs. Since these are different data sources, the surplus of having more detailed information available comes with the downside that the available information differs between studies. As one anonymous reviewer suggested, some apps might, for example, vary the information included depending on how they are used (eg, some interactive apps trigger specific BCTs after repeated and constant use), which might explain why some BCTs are not coded. Methodological limitations may also have contributed. A certain number of studies and a sufficient variation among them in terms of intervention components and effect sizes are needed to have a chance of detecting relevant associations (see also Michie et al134 for a detailed discussion). Specifically, there was insufficient variation between the 41 available studies for the assessment of the relative effectiveness of BCTs or evaluating different BCT combinations.
The potential for sample selection bias is another concern for the quality of mobile intervention studies.40 In this respect, most of the studies scored in the moderate to high range on CONSORT criteria and neither study quality (high vs fair quality) nor study design (RCT vs non‐RCT) moderated the effects. Moreover, the type of study sample did not reveal a significant moderating effect, indicating that the effects were comparable for studies with both clinical and generally healthy samples and across adolescents and adults. Overall, the findings suggest a high potential for app‐based mobile interventions across multiple audiences, offering confidence that app‐based mobile interventions are a promising method for fighting the obesity epidemic in a broad spectrum of the population.
5. CONCLUSION
The present findings represent one of the most comprehensive currently available evidence bases demonstrating that app‐based mobile interventions are effective and highly promising for changing nutrition behaviours and nutrition‐related health outcomes, which is essential for conquering the obesity epidemic. The overall pooled effect size was positive, and in general, the effects were relatively consistent across different outcomes, which provides some confidence in the conclusion that the effects are likely to be small but, to a certain degree, reliable and of practical relevance. Moreover, the present results do not indicate that this generally positive effect is limited to certain populations (healthy vs clinical samples; adolescents vs adults), intervention strategies (app only vs app+; number or type of BCTs), or type of app (commercial vs research). Considering that app‐based mobile interventions can operate at scale and at lower comparable implementation costs than individual and group‐based interventions, they offer the potential to be a cost‐effective method for improving nutrition behaviours and health indicators in a wide range of target groups. However, long‐term follow‐up effect sizes were generally smaller and non‐significant. Hence, increasing engagement with intervention modalities might be a key avenue for future developments. Mobile technologies offer the possibility of realizing more dynamic concepts with interactive, adaptive, time‐sensitive, and intraindividually dynamic strategies. However, all statements comparing the merits of mobile app‐based interventions with other delivery modes must be tempered by the potential limitations of the methodology,140 the complexity of specific populations, and treatment settings. These findings raise important questions around intervention design. Additional research to unpack the effective ingredients of mobile interventions would be helpful to identify which intervention components might be the most likely to be universally effective and which are more contextually dependent.
AUTHORS' CONTRIBUTIONS
Karoline Villinger, Deborah R. Wahl, Harald T. Schupp, and Britta Renner designed the systematic review. Karoline Villinger, Deborah R. Wahl, and Britta Renner did the search. Karoline Villinger and Deborah R. Wahl did the screening and data extraction process, assessed data quality, and analysed data with critical comments from Britta Renner, Harald T. Schupp, and Heiner Boeing. Karoline Villinger, Deborah R. Wahl, and Britta Renner drafted the manuscript with input from Harald T. Schupp and Heiner Boeing. All authors read, commented on, and approved the draft and final manuscripts.
FUNDING INFORMATION
This research was supported by the Federal Ministry of Education and Research and the German Research Foundation within the research projects SmartAct (BMBF grant 01EL1820A) and RiskDynamics (DFG grant FOR 2374) granted to B.R. and H.S. The funding source had no involvement in the study's design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit this article for publication.
DATA SHARING
No additional data available.
Supporting information
Data S1: PRISMA checklist
Data S2: Search strategy
Data S3: List of excluded studies and reasons (k = 60)
Data S4: CONSORT 2010 checklist with definitions
Data S5: Cochrane risk of bias assessment across studies
Data S6: Cochrane risk of bias assessment for each study
Data S7: Behaviour Change Techniques (BCTs) for each study
Data S8: Detailed description of included studies and analysed outcomes
Data S9: Funnel plot
Data S10: Meta‐regression and moderation effect results
Data S11: Effect sizes (Cohen's d) for all analysis levels and outcomes
Data S12: Effect sizes (Hedges’ g) for all analysis levels and outcomes
Data S13: Forest plots for outcomes (I. Nutrition behaviours, Figure 13.1, 13.2; II. Nutrition‐related health outcomes, Figure 13.3 – 13.9)
Data S14: Weighted mean differences for BMI (Table S14.1) and body weight (Table S14.2)
ACKNOWLEDGEMENTS
We thank Alexandra Grimm for supporting the data analyses. We also thank Valentina Boos, Marie‐Luise Diepolder, Timo Dimitriadis, Desiree Katzenberger, Heike Koch, Laura König, Monika May, Sabrina Nöth, Kristian Sabados, Julia Schuhmann, Tobias Volk, Katrin Ziesemer, and Tony Arthur for their valuable support.
CONFLICT OF INTERESTS
No conflict of interest was declared.
Villinger K, Wahl DR, Boeing H, Schupp HT, Renner B. The effectiveness of app‐based mobile interventions on nutrition behaviours and nutrition‐related health outcomes: A systematic review and meta‐analysis. Obesity Reviews. 2019;20:1465–1484. 10.1111/obr.12903
Villinger and Wahl joint first authorship.
REFERENCES
- 1. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766‐781. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity‐related health risk factors, 2001. JAMA. 2003;289(1):76‐79. 10.1001/jama.289.1.76 [DOI] [PubMed] [Google Scholar]
- 3. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523‐1529. 10.1001/jama.282.16.1523 [DOI] [PubMed] [Google Scholar]
- 4. Corica F, Bianchi G, Corsonello A, Mazzella N, Lattanzio F, Marchesini G. Obesity in the context of aging: quality of life considerations. Pharmacoeconomics. 2015;33(7):655‐672. 10.1007/s40273-014-0237-8 [DOI] [PubMed] [Google Scholar]
- 5. Withrow D, Alter D. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. 2011;12(2):131‐141. 10.1111/j.1467-789X.2009.00712.x [DOI] [PubMed] [Google Scholar]
- 6. Thorpe KE. The future costs of obesity: national and state estimates of the impact of obesity on direct health care expenses. A Collaborative Report from United Health Foundation 2009.
- 7. Rodgers RF, Watts AW, Austin SB, Haines J, Neumark‐Sztainer D. Disordered eating in ethnic minority adolescents with overweight. Int J Eat Disord. 2017;50(6):665‐671. 10.1002/eat.22652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224‐2260. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bleich SN, Vercammen KA, Zatz LY, Frelier JM, Ebbeling CB, Peeters A. Interventions to prevent global childhood overweight and obesity: a systematic review. Lancet Diabetes Endocrinol. 2017;6(4):332‐346. 10.1016/S2213-8587(17)30358-3 [DOI] [PubMed] [Google Scholar]
- 10. Ali EE, Chew L, KY‐L Y. Evolution and current status of mHealth research: a systematic review. BMJ Innovat. 2016;2(1):33‐40. 10.1136/bmjinnov-2015-000096 [DOI] [Google Scholar]
- 11. Heron KE, Smyth JM. Ecological momentary interventions: incorporating mobile technology into psychosocial and health behaviour treatments. Br J Health Psychol. 2010;15(1):1‐39. 10.1348/135910709X466063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med. 2011;1(1):53‐71. 10.1007/s13142-011-0021-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nahum‐Shani I, Hekler EB, Spruijt‐Metz D. Building health behavior models to guide the development of just‐in‐time adaptive interventions: a pragmatic framework. Health Psychol. 2015;34(Suppl):1209‐1212. 10.1037/hea0000306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Servick K. Mind the phone. Science. 2015;350(6266):1306‐1309. 10.1126/science.350.6266.1306 [DOI] [PubMed] [Google Scholar]
- 15. Rehg JM, Murphy SA, Kumar S. Mobile Health: Sensors, Analytic Methods, and Applications. Springer: Cham, 2017. 10.1007/978-3-319-51394-2 [DOI] [Google Scholar]
- 16. Bardus M, van Beurden SB, Smith JR, Abraham C. A review and content analysis of engagement, functionality, aesthetics, information quality, and change techniques in the most popular commercial apps for weight management. Int J Behav Nutr Phys Act. 2016;13(1):35 10.1186/s12966-016-0359-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rivera J, McPherson A, Hamilton J, et al. Mobile apps for weight management: a scoping review. JMIR mHealth uHealth. 2016;4(3). 10.2196/mhealth.5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Afshin A, Babalola D, Mclean M, et al. Information technology and lifestyle: a systematic evaluation of internet and mobile interventions for improving diet, physical activity, obesity, tobacco, and alcohol use. J Am Heart Assoc. 2016;5(9):e003058 10.1161/JAHA.115.003058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bardus M, Smith JR, Samaha L, Abraham C. Mobile phone and web 2.0 technologies for weight management: a systematic scoping review. J Med Internet Res. 2015;17(11):e259 10.2196/jmir.5129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hutchesson M, Rollo M, Krukowski R, et al. eHealth interventions for the prevention and treatment of overweight and obesity in adults: a systematic review with meta‐analysis. Obes Rev. 2015;16(5):376‐392. 10.1111/obr.12268 [DOI] [PubMed] [Google Scholar]
- 21. Lyzwinski LN. A systematic review and meta‐analysis of mobile devices and weight loss with an intervention content analysis. J Pers Med. 2014;4(3):311‐385. 10.3390/jpm4030311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olson CM. Behavioral nutrition interventions using e‐ and m‐Health communication technologies: a narrative review. Annu Rev Nutr. 2016;36(1):647‐664. 10.1146/annurev-nutr-071715-050815 [DOI] [PubMed] [Google Scholar]
- 23. Roberts AL, Fisher A, Smith L, Heinrich M, Potts HW. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: a systematic review and meta‐analysis. J Cancer Surviv. 2017;11(6):704‐719. 10.1007/s11764-017-0632-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rose T, Barker M, Jacob CM, et al. A systematic review of digital interventions for improving the diet and physical activity behaviors of adolescents. J Adolesc Health. 2017;61(6):669‐677. 10.1016/j.jadohealth.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Xue H, Huang Y, Huang L, Zhang D. A systematic review of application and effectiveness of mHealth interventions for obesity and diabetes treatment and self‐management. Adv Nutr. 2017;8(3):449‐462. 10.3945/an.116.014100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cotie L, Prince S, Elliott C, et al. The effectiveness of eHealth interventions on physical activity and measures of obesity among working‐age women: a systematic review and meta‐analysis. Obes Rev. 2018;19(10):1340‐1358. 10.1111/obr.12700 [DOI] [PubMed] [Google Scholar]
- 27. Teasdale N, Elhussein A, Butcher F, et al. Systematic review and meta‐analysis of remotely delivered interventions using self‐monitoring or tailored feedback to change dietary behavior. Am J Clin Nutr. 2018;107(2):247‐256. 10.1093/ajcn/nqx048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fedele DA, Cushing CC, Fritz A, Amaro CM, Ortega A. Mobile health interventions for improving health outcomes in youth: a meta‐analysis. JAMA Pediatr. 2017;171(5):461‐469. 10.1001/jamapediatrics.2017.0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Free C, Phillips G, Galli L, et al. The effectiveness of mobile‐health technology‐based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362 10.1371/journal.pmed.1001362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palmer M, Sutherland J, Barnard S, et al. The effectiveness of smoking cessation, physical activity/diet and alcohol reduction interventions delivered by mobile phones for the prevention of non‐communicable diseases: a systematic review of randomised controlled trials. PLoS ONE. 2018;13(1):e0189801 10.1371/journal.pone.0189801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schoeppe S, Alley S, Van Lippevelde W, et al. Efficacy of interventions that use apps to improve diet, physical activity and sedentary behaviour: a systematic review. Int J Behav Nutr Phys Act. 2016;13(1):127 10.1186/s12966-016-0454-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc Nurs. 2013;28(4):320‐329. 10.1097/JCN.0b013e318250a3e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao J, Freeman B, Li M. Can mobile phone apps influence people's health behavior change? An evidence review. J Med Internet Res. 2016;18(11):e287 10.2196/jmir.5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bacigalupo R, Cudd P, Littlewood C, Bissell P, Hawley M, Buckley Woods H. Interventions employing mobile technology for overweight and obesity: an early systematic review of randomized controlled trials. Obes Rev. 2013;14(4):279‐291. 10.1111/obr.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu F, Kong X, Cao J, et al. Mobile phone intervention and weight loss among overweight and obese adults: a meta‐analysis of randomized controlled trials. Am J Epidemiol. 2015;181(5):337‐348. 10.1093/aje/kwu260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta‐analysis. Diabet Med. 2011;28(4):455‐463. 10.1111/j.1464-5491.2010.03180.x [DOI] [PubMed] [Google Scholar]
- 37. Kitsiou S, Paré G, Jaana M, Gerber B. Effectiveness of mHealth interventions for patients with diabetes: an overview of systematic reviews. PLoS ONE. 2017;12(3):e0173160 10.1371/journal.pone.0173160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Covolo L, Ceretti E, Moneda M, Castaldi S, Gelatti U. Does evidence support the use of mobile phone apps as a driver for promoting healthy lifestyles from a public health perspective? A systematic review of randomized control trials. Patient Educ Couns. 2017;100(12):2231‐2243. 10.1016/j.pec.2017.07.032 [DOI] [PubMed] [Google Scholar]
- 39. Mateo GF, Granado‐Font E, Ferré‐Grau C, Montaña‐Carreras X. Mobile phone apps to promote weight loss and increase physical activity: a systematic review and meta‐analysis. J Med Internet Res. 2015;17(11):e253 10.2196/jmir.4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schippers M, Adam P, Smolenski D, Wong H, Wit J. A meta‐analysis of overall effects of weight loss interventions delivered via mobile phones and effect size differences according to delivery mode, personal contact, and intervention intensity and duration. Obes Rev. 2017;18(4):450‐459. 10.1111/obr.12492 [DOI] [PubMed] [Google Scholar]
- 41. Wu Y, Yao X, Vespasiani G, et al. Mobile app‐based interventions to support diabetes self‐management: a systematic review of randomized controlled trials to identify functions associated with glycemic efficacy. JMIR mHealth uHealth. 2017;5(3):e35 10.2196/mhealth.6522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bonoto BC, de Araújo VE, Godói IP, et al. Efficacy of mobile apps to support the care of patients with diabetes mellitus: a systematic review and meta‐analysis of randomized controlled trials. JMIR mHealth uHealth. 2017;5(3):e4 10.2196/mhealth.6309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu I, Kee J, Threapleton D, et al. Effectiveness of smartphone technologies on glycaemic control in patients with type 2 diabetes: systematic review with meta‐analysis of 17 trials. Obes Rev. 2018;19(6):825‐838. 10.1111/obr.1266 [DOI] [PubMed] [Google Scholar]
- 44. DiFilippo KN, Huang W‐H, Andrade JE, Chapman‐Novakofski KM. The use of mobile apps to improve nutrition outcomes: a systematic literature review. J Telemed Telecare. 2015;21(5):243‐253. 10.1177/1357633X15572203 [DOI] [PubMed] [Google Scholar]
- 45. Coughlin SS, Whitehead M, Sheats JQ, Mastromonico J, Hardy D, Smith SA. Smartphone applications for promoting healthy diet and nutrition: a literature review. Jacobs J Food Nutr. 2015;2:021. [PMC free article] [PubMed] [Google Scholar]
- 46. Lunde P, Nilsson BB, Bergland A, Kværner KJ, Bye A. The effectiveness of smartphone apps for lifestyle improvement in noncommunicable diseases: systematic review and meta‐analyses. J Med Internet Res. 2018;20(5):e162 10.2196/jmir.9751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. El Khoury CF, Karavetian M, Halfens RJ, Crutzen R, Khoja L, Schols JM. The effects of dietary mobile apps on nutritional outcomes in adults with chronic diseases: a systematic review. J Acad Nutr Diet. 2019;119(4):626‐651. 10.1016/j.jand.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 48. Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81‐95. 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- 49. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maher CA, Lewis LK, Ferrar K, Marshall S, De Bourdeaudhuij I, Vandelanotte C. Are health behavior change interventions that use online social networks effective? A systematic review. JMIR. 2014;16(2):e40 10.2196/jmir.2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Davies CA, Spence JC, Vandelanotte C, Caperchione CM, Mummery WK. Meta‐analysis of internet‐delivered interventions to increase physical activity levels. Int J Behav Nutr Phys Act. 2012;9(1):52 10.1186/1479-5868-9-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morris SB, DeShon RP. Combining effect size estimates in meta‐analysis with repeated measures and independent‐groups designs. Psychol Methods. 2002;7(1):105‐125. 10.1037/1082-989X.7.1.105 [DOI] [PubMed] [Google Scholar]
- 55. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2. The Cochrane Collaboration, 2005. Available from http://handbook.cochrane.org. [Google Scholar]
- 56. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. ed. Hillsdale: Erlbaum; 1988. [Google Scholar]
- 57. Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107‐128. 10.3102/10769986006002107 [DOI] [Google Scholar]
- 58. Kontopantelis E, Reeves D. MetaEasy: a meta‐analysis add‐in for Microsoft Excel. J Stat Softw. 2009;30(7):1‐25. 10.18637/jss.v030.i07 21666874 [DOI] [Google Scholar]
- 59. Polanin JR, Snilstveit B. Campell methods policy on converting between effect sizes (version 1.1, updated December 2016). The Campbell Collaboration: Oslo, 2016. 10.4073/cmpn.2016.3 [DOI] [Google Scholar]
- 60. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta‐Analysis. John Wiley & Sons: West Sussex; 2011. [Google Scholar]
- 61. Block G, Azar KMJ, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17(10):e240 10.2196/jmir.4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hoyt W, Del Re A. Comparison of methods for aggregating dependent effect sizes in meta‐analysis. Manuscript under review, 2015.
- 63. Viechtbauer W, Cheung MWL. Outlier and influence diagnostics for meta‐analysis. Res Synth Methods. 2010;1(2):112‐125. 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 64. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cuijpers P, Weitz E, Cristea I, Twisk J. Pre‐post effect sizes should be avoided in meta‐analyses. Epidemiol Psychiatr Sci. 2017;26(4):364‐368. 10.1017/S2045796016000809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Goodwin L, Ostuzzi G, Khan N, Hotopf MH, Moss‐Morris R. Can we identify the active ingredients of behaviour change interventions for coronary heart disease patients? A systematic review and meta‐analysis. PLoS ONE. 2016;11(4):e0153271 10.1371/journal.pone.0153271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tang MY, Smith DM, Mc Sharry J, Hann M, French DP. Behavior change techniques associated with changes in postintervention and maintained changes in self‐efficacy for physical activity: a systematic review with meta‐analysis. Ann Behav Med. 2018. 10.1093/abm/kay090 [DOI] [PubMed] [Google Scholar]
- 68. Williams SL, French DP. What are the most effective intervention techniques for changing physical activity self‐efficacy and physical activity behaviour—and are they the same? Health Educ Res. 2011;26(2):308‐322. 10.1093/her/cyr005 [DOI] [PubMed] [Google Scholar]
- 69. Ahn Y, Bae J, Kim HS. The development of a mobile u‐Health program and evaluation for self‐diet management for diabetic patients. Nutr Res Pract. 2016;10(3):342‐351. 10.4162/nrp.2016.10.3.342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Allen JK, Stephens J, Dennison Himmelfarb CR, Stewart KJ, Hauck S. Randomized controlled pilot study testing use of smartphone technology for obesity treatment. J Obes 2013;2013:1‐7 151597 10.1155/2013/151597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Appel HB, Huang B, Cole A, James R, Ai AL. Starting the conversation—a childhood obesity knowledge project using an app. Br J Med Med Res. 2014;4(7):1526‐1538. 10.9734/BJMMR/2014/5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Balk‐Moller NC, Poulsen SK, Larsen TM. Effect of a nine‐month web‐ and app‐based workplace intervention to promote healthy lifestyle and weight loss for employees in the social welfare and health care sector: a randomized controlled trial. J Med Internet Res. 2017;19(4):e108 10.2196/jmir.6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brindal E, Hendrie G, Freyne J, Coombe M, Berkovsky S, Noakes M. Design and pilot results of a mobile phone weight‐loss application for women starting a meal replacement programme. J Telemed Telecare. 2013;19(3):166‐174. 10.1177/1357633X13479702 [DOI] [PubMed] [Google Scholar]
- 74. Brindal E, Hendrie GA, Taylor P, Freyne J, Noakes M. Cohort analysis of a 24‐week randomized controlled trial to assess the efficacy of a novel, partial meal replacement program targeting weight loss and risk factor reduction in overweight/obese adults. Nutrients. 2016;8(5):265 10.3390/nu8050265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Burke LE, Zheng Y, Ma Q, et al. The SMARTER pilot study: testing feasibility of real‐time feedback for dietary self‐monitoring. Prev Med Rep. 2017;6:278‐285. 10.1016/j.pmedr.2017.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Carter MC, Burley VJ, Nykjaer C, Cade JE. Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. J Med Internet Res. 2013;15(4):e32 10.2196/jmir.2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Duncan M, Vandelanotte C, Kolt GS, et al. Effectiveness of a web‐ and mobile phone‐based intervention to promote physical activity and healthy eating in middle‐aged males: randomized controlled trial of the ManUp study. J Med Internet Res. 2014;16(6):40‐60. 10.2196/jmir.3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Froisland DH, Arsand E, Skarderud F. Improving diabetes care for young people with type 1 diabetes through visual learning on mobile phones: mixed‐methods study. J Med Internet Res. 2012;14(4):e111 10.2196/jmir.2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fukuoka Y, Gay CL, Joiner KL, Vittinghoff E. A novel diabetes prevention intervention using a mobile app: a randomized controlled trial with overweight adults at risk. Am J Prev Med. 2015;49(2):223‐237. 10.1016/j.amepre.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gilson ND, Pavey TG, Wright OR, et al. The impact of an m‐Health financial incentives program on the physical activity and diet of Australian truck drivers. BMC Public Health. 2017;17(1):467 10.1186/s12889-017-4380-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Godino JG, Merchant G, Norman GJ, et al. Using social and mobile tools for weight loss in overweight and obese young adults (Project SMART): a 2 year, parallel‐group, randomised, controlled trial. Lancet Diabetes Endocrinol. 2016;4(9):747‐755. 10.1016/S2213-8587(16)30105-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gordon JS, Armin J, Hingle D, et al. Development and evaluation of the see me smoke‐free multi‐behavioral mHealth app for women smokers. Transl Behav Med. 2017;7(2):172‐184. 10.1007/s13142-017-0463-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hales S, Turner‐McGrievy GM, Wilcox S, et al. Social networks for improving healthy weight loss behaviors for overweight and obese adults: a randomized clinical trial of the social pounds off digitally (Social POD) mobile app. Int J Med Inform. 2016;94:81‐90. 10.1016/j.ijmedinf.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 84. Hebden L, Cook A, van der Ploeg HP, King L, Bauman A, Allman‐Farinelli M. A mobile health intervention for weight management among young adults: a pilot randomised controlled trial. J Hum Nutr Diet. 2014;27(4):322‐332. 10.1111/jhn.12155 [DOI] [PubMed] [Google Scholar]
- 85. Holmen H, Torbjornsen A, Wahl AK, et al. A mobile health intervention for self‐management and lifestyle change for persons with type 2 diabetes, part 2: one‐year results from the Norwegian randomized controlled trial RENEWING HEALTH. JMIR mHealth uHealth. 2014;2(4):e57 10.2196/mhealth.3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ipjian ML, Johnston CS. Smartphone technology facilitates dietary change in healthy adults. Nutrition. 2017;33:343‐347. 10.1016/j.nut.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 87. Jensen CD, Duncombe KM, Lott MA, Hunsaker SL, Duraccio KM, Woolford SJ. An evaluation of a smartphone‐assisted behavioral weight control intervention for adolescents: pilot study. JMIR mHealth uHealth. 2016;4(3):e102 10.2196/mhealth.6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Johnston CA, Rost S, Miller‐Kovach K, Moreno JP, Foreyt JP. A randomized controlled trial of a community‐based behavioral counseling program. Am J Med. 2013;126(12):e19‐e24. 10.1016/j.amjmed.2013.04.025 [DOI] [PubMed] [Google Scholar]
- 89. Kim H, Faw M, Michaelides A. Mobile but connected: harnessing the power of self‐efficacy and group support for weight loss success through mHealth intervention. J Health Commun. 2017;22(5):395‐402. 10.1080/10810730.2017.1296510 [DOI] [PubMed] [Google Scholar]
- 90. Laing BY, Mangione CM, Tseng CH, et al. Effectiveness of a smartphone application for weight loss compared with usual care in overweight primary care patients: a randomized, controlled trial. Ann Intern Med. 2014;161(10):5‐12. 10.7326/M13-3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee W, Chae YM, Kim S, Ho SH, Choi I. Evaluation of a mobile phone‐based diet game for weight control. J Telemed Telecare. 2010;16(5):270‐275. 10.1258/jtt.2010.090913 [DOI] [PubMed] [Google Scholar]
- 92. McCarroll ML, Armbruster S, Pohle‐Krauza RJ, et al. Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application. Gynecol Oncol. 2015;137(3):508‐515. 10.1016/j.ygyno.2014.12.025 [DOI] [PubMed] [Google Scholar]
- 93. Mummah SA, Mathur M, King AC, Gardner CD, Sutton S. Mobile technology for vegetable consumption: a randomized controlled pilot study in overweight adults. JMIR mHealth uHealth. 2016;4(2):e51 10.2196/mhealth.5146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Partridge SR, McGeechan K, Bauman A, Phongsavan P, Allman‐Farinelli M. Improved confidence in performing nutrition and physical activity behaviours mediates behavioural change in young adults: mediation results of a randomised controlled mHealth intervention. Appetite. 2017;108:425‐433. 10.1016/j.appet.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 95. Partridge SR, McGeechan K, Hebden L, et al. Effectiveness of a mHealth lifestyle program with telephone support (TXT2BFiT) to prevent unhealthy weight gain in young adults: randomized controlled trial. JMIR mHealth uHealth. 2015;3(2):e66 10.2196/mhealth.4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rabbi M, Pfammatter A, Zhang M, Spring B, Choudhury T. Automated personalized feedback for physical activity and dietary behavior change with mobile phones: a randomized controlled trial on adults. JMIR mHealth uHealth. 2015;3(2):e42 10.2196/mhealth.4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Recio‐Rodriguez JI, Agudo‐Conde C, Martin‐Cantera C, et al. Short‐term effectiveness of a mobile phone app for increasing physical activity and adherence to the Mediterranean diet in primary care: a randomized controlled trial (EVIDENT II Study). J Med Internet Res. 2016;18(12):e331 10.2196/jmir.6814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ross KM, Wing RR. Impact of newer self‐monitoring technology and brief phone‐based intervention on weight loss: a randomized pilot study. Obesity. 2016;24(8):1653‐1659. 10.1002/oby.21536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Spring B, Pellegrini CA, Pfammatter A, et al. Effects of an abbreviated obesity intervention supported by mobile technology: the ENGAGED randomized clinical trial. Obesity. 2017;25(7):1191‐1198. 10.1002/oby.21842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Steinert A, Haesner M, Tetley A, Steinhagen‐Thiessen E. Self‐monitoring of health‐related goals in older adults with use of a smartphone application. Act Adapt Aging. 2016;40(2):81‐92. 10.1080/01924788.2016.1158569 [DOI] [Google Scholar]
- 101. Stephens JD, Yager AM, Allen J. Smartphone technology and text messaging for weight loss in young adults: a randomized controlled trial. J Cardiovasc Nurs. 2017;32(1):39‐46. 10.1097/JCN.0000000000000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Svetkey LP, Batch BC, Lin PH, et al. Cell Phone Intervention for You (CITY): a randomized, controlled trial of behavioral weight loss intervention for young adults using mobile technology. Obesity. 2015;23(11):2133‐2141. 10.1002/oby.21226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Thomas JG, Wing RR. Health‐e‐call, a smartphone‐assisted behavioral obesity treatment: pilot study. JMIR mHealth uHealth. 2013;1(1):e3 10.2196/mhealth.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Torbjornsen A, Jenum AK, Smastuen MC, et al. A low‐intensity mobile health intervention with and without health counseling for persons with type 2 diabetes, part 1: baseline and short‐term results from a randomized controlled trial in the Norwegian part of RENEWING HEALTH. JMIR mHealth uHealth. 2014;2(4):e52 10.2196/mhealth.3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Turner‐McGrievy G, Tate D. Tweets, apps, and pods: results of the 6‐month mobile pounds off digitally (mobile POD) randomized weight‐loss intervention among adults. J Med Internet Res. 2011;13(4):e120 10.2196/jmir.1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wharton CM, Johnston CS, Cunningham BK, Sterner D. Dietary self‐monitoring, but not dietary quality, improves with use of smartphone app technology in an 8‐week weight loss trial. J Nutr Educ Behav. 2014;46(5):440‐444. 10.1016/j.jneb.2014.04.291 [DOI] [PubMed] [Google Scholar]
- 107. Widmer RJ, Allison TG, Lerman LO, Lerman A. Digital health intervention as an adjunct to cardiac rehabilitation reduces cardiovascular risk factors and rehospitalizations. J Cardiovasc Transl Res. 2015;8(5):283‐292. 10.1007/s12265-015-9629-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Willey S, Walsh JK. Outcomes of a mobile health coaching platform: 12‐week results of a single‐arm longitudinal study. JMIR mHealth uHealth. 2016;4(1):131‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95(10):1103‐1108. 10.1016/s0002-8223(95)00300-2 [DOI] [PubMed] [Google Scholar]
- 110. Koydemir HC, Ozcan A. Wearable and implantable sensors for biomedical applications. Annu Rev Anal Chem. 2018;11(1):127‐146. 10.1146/annurev-anchem-061417-125956 [DOI] [PubMed] [Google Scholar]
- 111. Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev. 2007;82(4):591‐605. 10.1111/j.1469-185x.2007.00027.x [DOI] [PubMed] [Google Scholar]
- 112. Khokhar B, Jones J, Ronksley PE, Armstrong MJ, Caird J, Rabi D. Effectiveness of mobile electronic devices in weight loss among overweight and obese populations: a systematic review and meta‐analysis. BMC Obes. 2014;1(1):22 10.1186/s40608-014-0022-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Franz MJ, VanWormer JJ, Crain AL, et al. Weight‐loss outcomes: a systematic review and meta‐analysis of weight‐loss clinical trials with a minimum 1‐year follow‐up. J Am Diet Assoc. 2007;107(10):1755‐1767. 10.1016/j.jada.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 114. Eckerstorfer LV, Tanzer NK, Vogrincic‐Haselbacher C, et al. Key elements of mHealth interventions to successfully increase physical activity: meta‐regression. JMIR mHealth uHealth. 2018;6(11):e10076 10.2196/10076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Direito A, Carraça E, Rawstorn J, Whittaker R, Maddison R. mHealth technologies to influence physical activity and sedentary behaviors: behavior change techniques, systematic review and meta‐analysis of randomized controlled trials. Ann Behav Med. 2016;51(2):226‐239. 10.1007/s12160-016-9846-0 [DOI] [PubMed] [Google Scholar]
- 116. Fisher EB, Fitzgibbon ML, Glasgow RE, et al. Behavior matters. Am J Prev Med. 2011;40(5):e15‐e30. 10.1016/j.amepre.2010.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Brug J, van der Ploeg HP, Loyen A, et al. Determinants of Diet and Physical Activity (DEDIPAC): a summary of findings. Int J Behav Nutr Phys Act. 2017;14(1):150 10.1186/s12966-017-0609-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schembre SM, Liao Y, Robertson MC, et al. Just‐in‐time feedback in diet and physical activity interventions: systematic review and practical design framework. J Med Internet Res. 2018;20(3):e106 10.2196/jmir.8701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Boushey C, Spoden M, Zhu F, Delp E, Kerr D. New mobile methods for dietary assessment: review of image‐assisted and image‐based dietary assessment methods. Proc Nutr Soc. 2017;76(3):283‐294. 10.1017/S0029665116002913 [DOI] [PubMed] [Google Scholar]
- 120. Renner B, Klusmann V, Sproesser G. Assessment of health behaviors In: Wright JD. (eds.) The International Encyclopedia of the Social and Behavioral Sciences. 2nd ed. Elsevier: Oxford, 2015, pp588‐93. 10.1016/B978-0-08-097086-8.14155-8. [DOI] [Google Scholar]
- 121. Fogg BJ. The behavior grid: 35 ways behavior can change. In: Proceedings of the 4th International Conference on Persuasive Technology, 2009: ACM, 2009: 42. 10.1145/1541948.1542001. [DOI]
- 122. Fogg BJ. A behavior model for persuasive design. In: Proceedings of the 4th International Conference on Persuasive Technology, 2009: ACM, 2009: 40. 10.1145/1541948.1541999. [DOI]
- 123. Andrews S, Ellis DA, Shaw H, Piwek L. Beyond self‐report: tools to compare estimated and real‐world smartphone use. PLoS ONE. 2015;10(10):e0139004 10.1371/journal.pone.0139004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta‐regression. Health Psychol. 2009;28(6):690‐701. 10.1037/a0016136 [DOI] [PubMed] [Google Scholar]
- 125. Direito A, Dale LP, Shields E, Dobson R, Whittaker R, Maddison R. Do physical activity and dietary smartphone applications incorporate evidence‐based behaviour change techniques? BMC Public Health. 2014;14(1):646 10.1186/1471-2458-14-646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Matthews J, Win KT, Oinas‐Kukkonen H, Freeman M. Persuasive technology in mobile applications promoting physical activity: a systematic review. J Med Syst. 2016;40(3):72 10.1007/s10916-015-0425-x [DOI] [PubMed] [Google Scholar]
- 127. Middelweerd A, Mollee JS, van der Wal CN, Brug J. Te Velde SJ. Apps to promote physical activity among adults: a review and content analysis. Int J Behav Nutr Phys Act. 2014;11(1):97 10.1186/s12966-014-0097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Samdal GB, Eide GE, Barth T, Williams G, Meland E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults: systematic review and meta‐regression analyses. Int J Behav Nutr Phys Act. 2017;14(1):42 10.1186/s12966-017-0494-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Carver CS, Scheier MF. The self‐attention‐induced feedback loop and social facilitation. J Exp Soc Psychol. 1981;17(6):545‐568. 10.1016/0022-1031(81)90039-1 [DOI] [Google Scholar]
- 130. Gollwitzer P, Oettingen, G . Goal pursuit In: The Oxford Handbook of Human Motivation. Oxford: Oxford University Press; 2012:208‐231. 10.1093/oxfordhb/9780195399820.013.0013 [DOI] [Google Scholar]
- 131. Iribarren SJ, Cato K, Falzon L, Ston PW. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS ONE. 2017;12(2):e0170581 10.1371/journal.pone.0170581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta‐analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4 10.2196/jmir.1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Olander EK, Fletcher H, Williams S, Atkinson L, Turner A, French DP. What are the most effective techniques in changing obese individuals' physical activity self‐efficacy and behaviour: a systematic review and meta‐analysis. Int J Behav Nutr Phys Act. 2013;10(1):29 10.1186/1479-5868-10-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Michie S, West R, Sheals K, Godinho CA. Evaluating the effectiveness of behavior change techniques in health‐related behavior: a scoping review of methods used. Transl Behav Med. 2018;8(2):212‐224. 10.1093/tbm/ibx019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. König LM. Healthy pleasures: integrating food well‐being and simple eating behaviour interventions . Doctoral dissertation, 2018. University of Konstanz, Germany.
- 136. Wahl DR, Villinger K, König LM, Ziesemer K, Schupp HT, Renner B. Healthy food choices are happy food choices: evidence from a real life sample using smartphone based assessments. Sci Rep. 2017;7(1):17069 10.1038/s41598-017-17262-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA. 2014;312(17):1779‐1791. 10.1001/jama.2014.14173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Okorodudu DE, Bosworth HB, Corsino L. Innovative interventions to promote behavioral change in overweight or obese individuals: a review of the literature. Ann Med. 2015;47(3):179‐185. 10.3109/07853890.2014.931102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Libr. 2009. 10.1002/14651858.MR000006.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ioannidis JP. Effectiveness of antidepressants: an evidence myth constructed from a thousand randomized trials? Philos Ethics Humanit Med. 2008;3(1):14 10.1186/1747-5341-3-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: PRISMA checklist
Data S2: Search strategy
Data S3: List of excluded studies and reasons (k = 60)
Data S4: CONSORT 2010 checklist with definitions
Data S5: Cochrane risk of bias assessment across studies
Data S6: Cochrane risk of bias assessment for each study
Data S7: Behaviour Change Techniques (BCTs) for each study
Data S8: Detailed description of included studies and analysed outcomes
Data S9: Funnel plot
Data S10: Meta‐regression and moderation effect results
Data S11: Effect sizes (Cohen's d) for all analysis levels and outcomes
Data S12: Effect sizes (Hedges’ g) for all analysis levels and outcomes
Data S13: Forest plots for outcomes (I. Nutrition behaviours, Figure 13.1, 13.2; II. Nutrition‐related health outcomes, Figure 13.3 – 13.9)
Data S14: Weighted mean differences for BMI (Table S14.1) and body weight (Table S14.2)


