Abstract
Background
Irritable bowel syndrome (IBS) is a brain‐gut disorder, of which the natural course varies between patients and is difficult to predict. This study aimed to evaluate symptom evolution over a 5‐year follow‐up period and to identify baseline predictors for symptom severity and quality of life (QoL) at follow‐up.
Methods
Maastricht IBS cohort participants completed questionnaires upon inclusion regarding demographics and lifestyle, gastrointestinal (GI) symptoms, anxiety and depression, and QoL. The same questionnaires, in addition to others, were completed after 5 years. Rome criteria were confirmed face‐to‐face at initial enrollment and through telephonic interviews at follow‐up.
Key Results
At a mean follow‐up of 4.7 years, 379 patients were approached of whom 203 (53.7%) responded. Of these, 161 were reached by telephone and analyzed; 49 (30.4%) did not fulfill the Rome III criteria at follow‐up and had lower levels of GI symptoms and GI‐specific anxiety compared to those remaining Rome III‐positive (P < 0.001). However, Rome III‐negative patients had comparable levels of QoL and life satisfaction, comorbid anxiety and depression, work absenteeism, and impaired productivity. No baseline predictors were found for being Rome III‐positive or Rome III‐negative. However, greater age and lower baseline physical QoL predicted lower physical QoL at follow‐up (P < 0.005 and P < 0.01, respectively), while lower baseline mental QoL predicted lower mental QoL at follow‐up (P = 0.005). Additionally, higher anxiety and depression scores at follow‐up were associated with lower QoL and life satisfaction at follow‐up (P < 0.001).
Conclusions and Inferences
Long‐term QoL and general well‐being might depend on concurrent psychological symptoms, rather than GI symptom improvement.
Keywords: irritable bowel syndrome, natural history, quality of life, Rome criteria, symptom evolution
This 5‐year follow‐up study of the Maastricht IBS cohort showed that 30% of patients did no longer fulfill the Rome III criteria. However, the decrease in GI symptom severity (i.e., being Rome III‐negative at follow‐up) did not impact quality of life nor life satisfaction. Our results indicate that long‐term quality of life and general well‐being might depend on comorbid psychological symptoms, rather than gastrointestinal symptom severity.

Key Points.
Irritable bowel syndrome (IBS) is a chronic disorder that has considerable impact on quality of life (QoL).
After 5‐years follow‐up, 30% of patients with IBS do not fulfill the Rome III criteria.
This does not impact QoL scores, comorbid psychological symptoms, and general life satisfaction. QoL and life satisfaction are associated with concurrent anxiety and depression scores at follow‐up, but not with GI symptom improvement.
1. INTRODUCTION
Irritable bowel syndrome (IBS) is a brain‐gut disorder characterized by a chronic relapsing‐remitting nature of symptoms, including abdominal pain and altered bowel habits. Global prevalence, based predominantly on the Rome III criteria, is estimated at 5%‐20%,1 with varying rates according to geographical area and diagnostic criteria used.2 Recent studies using the more restrictive Rome IV criteria point to lower prevalence rates of 5%‐6%.3, 4
Although the exact pathophysiology of IBS remains incompletely understood, a multifactorial origin is generally recognized, in which dysregulation of the brain‐gut axis has a central role. Other factors include aberrant neuroimmune interactions, visceral hypersensitivity, genetic susceptibility, microbiome alterations, and psychosocial factors.5, 6 As interference of IBS with patients’ everyday lives is extensive7 and treatment results are often unsatisfactory, quality of life is low and comparable to chronic somatic diseases.8
Given the heterogeneous nature of the disorder, symptom patterns vary widely both between and within IBS patients and predicting individual disease courses remains challenging. IBS is known as a chronic, in many patients lifelong, condition with fluctuating gastrointestinal (GI) symptoms. Symptoms such as abdominal pain, constipation, and diarrhea are known to occur in episodes of several days followed by days without symptoms.9 In addition, transitions from one predominant bowel habit type to the other are common and occur in up to 75% of patients.10, 11, 12 Quality of life, on the other hand, has been shown to be relatively stable over a 3‐month period.13 With regard to long‐term symptom variability, several studies have investigated the disease course of IBS and have shown varying results with respect to symptom severity and quality of life. The majority of the prospective studies had a follow‐up period of approximately 1 year, which is relatively short for a condition such as IBS. In addition, there is a lack of follow‐up data for the Dutch population. As implications of IBS on quality of life have shown to vary considerably between different countries,14 it is of added value to expand earlier findings and investigate the natural history of IBS in a Dutch, well‐characterized population.
Finally, as IBS is a symptom‐based diagnosis, it is of interest to assess how symptoms evolve over time and how these symptoms and long‐term quality of life relate to the Rome diagnostic criteria for IBS, in particular when they are confirmed in a telephonic interview rather than a purely survey‐based assessment at follow‐up measurements.
Hence, the current follow‐up study, which is part of a large prospective cohort study on the pheno‐ and genotypical characterization of IBS in the Netherlands, aimed (a) to gain further insight into symptom evolution and long‐term quality of life over a 5‐year follow‐up period; (b) to identify baseline predictors for higher symptom severity and greater quality of life impairment at follow‐up; and (c) to assess how point a and b differ between patients that remain Rome III‐positive and those who are Rome III‐negative at follow‐up.
2. MATERIALS AND METHODS
2.1. Irritable bowel syndrome cohort study
Since 2009, patients with IBS between 18 and 75 years of age who visit the outpatient clinic of the Gastroenterology‐Hepatology division of the Maastricht University Medical Center+ (MUMC+), The Netherlands, are requested to enroll in the Maastricht IBS (MIBS) cohort study. The MUMC + is a university hospital with a combined secondary and tertiary care service in the area of South Limburg, The Netherlands. In addition, patients with IBS are recruited via general practitioners in the area of South Limburg. The MIBS cohort is an extensively phenotyped cohort of IBS patients with a follow‐up measurement 5 years after initial inclusion.15, 16, 17 The research protocol had been approved by the MUMC + Committee of Ethics, and all study procedures were performed in compliance with Good Clinical Practice Guidelines and according to the revised Declaration of Helsinki.18 The study had been registered in the US National Library of Medicine (NCT00775060). All subjects gave a written informed consent prior to participation.
2.2. Subjects
Patients who had been included at least 3 years (5 ± 2 years) before the current study, that is, patients included in the MIBS cohort between September 2009 and September 2014, were eligible for participation in the current follow‐up study. All patients fulfilled the Rome III criteria for IBS at the time of inclusion, which was confirmed by a trained clinical investigator in a face‐to‐face interview. Patients were assigned IBS subtypes based on their reported predominant stool type, that is, diarrhea (IBS‐D), constipation (IBS‐C), a mix of diarrhea and constipation (IBS‐M), or unspecified predominant bowel habit (IBS‐U). Their medical history was taken, and if any alarm symptoms were present or if deemed necessary by the gastroenterologist, GI endoscopy, abdominal imaging, and/or blood, breath and/or fecal analyses were performed to exclude organic diseases. A history of abdominal surgery automatically led to exclusion, except for an uncomplicated appendectomy, hysterectomy, or cholecystectomy.
2.3. Data collection
At the time of enrollment in the MIBS cohort, patients completed several questionnaires, that is, on demographics and lifestyle, GI symptoms, symptoms of comorbid anxiety and depression, and general quality of life (QoL). The data were obtained administering a predefined self‐report questionnaire on demographics and lifestyle, a 14‐day end‐of‐day symptom diary, the gastrointestinal symptom rating scale (GSRS, scale 1‐7, generates symptom scores for the following subdomains: abdominal pain, reflux, diarrhea, constipation, and indigestion),19 the hospital anxiety and depression scale (HADS, scale 0‐3, a screening tool for anxiety and depression),20, 21 and the rand 36‐item short‐form health survey (SF‐36, scale 1‐6, generates a physical and a mental QoL component summary).22, 23
Approximately 5 years (±2 year) after inclusion, patients were invited to complete several follow‐up questionnaires. These included the same questionnaires that were filled out at baseline (with the exception of the end‐of‐day diary), with the addition of other questionnaires to further standardize the phenotyping of our population and to allow valid comparisons to other cohorts (ie, international harmonization). Additional questionnaires included the following: the GSRS specific for IBS (GSRS‐IBS, scale 1‐7, generates symptom scores for the following subdomains: abdominal pain, bloating, constipation, diarrhea, and satiety)24; the Visceral Sensitivity Index (VSI, scale 1‐6, assesses GI‐specific anxiety or, in other words, fear for GI symptoms)25; and the Satisfaction With Life Scale (SWLS, scale 1‐6, assesses individual overall satisfaction with life).26, 27, 28 As it is known that IBS can affect the ability to work,29 a self‐report questionnaire regarding productivity (Productivity Cost Questionnaire (PCQ), validated for the Dutch situation) was included as well.30, 31 Patients could opt for paper or digital web‐based (invitation was sent via email) questionnaires to encourage participation. One reminder was sent when no response had been received within 1 month. A trained clinical investigator contacted all patients who did respond and confirmed the Rome III diagnostic criteria during a telephonic interview with them. Patients were reassigned IBS subtypes based on their predominant bowel habit, during the follow‐up measurement. Reliable information on treatment history was not available for the majority of patients as this was not registered systematically during the follow‐up period. Furthermore, many patients underwent self‐treatment or treatment via their primary care physician, of which data were not available and were therefore not included in the analysis of the current study.
2.4. Statistical analysis
All statistical analyses were carried out using IBM SPSS statistics 23.0 and GraphPad Prism 6.0 for Macintosh. Continuous data are presented as medians and interquartile ranges (IQR) and categorial data as proportions (%). To compare (non‐parametric) continuous data between subgroups, Mann‐Whitney U tests were used. Wilcoxon‐signed‐rank tests were used to evaluate differences within subjects over time. For categorical data, groups were compared using the chi‐square or Fisher’s exact test and differences over time were evaluated using the McNemar’s test. Correlations were assessed according to Spearman. To decrease the false discovery error rate induced by multiple testing, a post hoc correction was applied using the Benjamini‐Hochberg step‐up procedure.32
The patients participating in the follow‐up were divided into two groups for comparison and further analyses, (a) Rome III‐positive: patients who still fulfilled the Rome III criteria for IBS at follow‐up; (b) Rome III‐negative: patients who did not fulfill the Rome III criteria for IBS at follow‐up. Multivariable logistic and linear regression models, respectively, were used to identify independent baseline predictors for (a) the presence of IBS according to the Rome III diagnostic criteria at follow‐up and (b) quality of life at follow‐up. We also used linear regression models to identify characteristics at follow‐up that were associated with quality of life at follow‐up. The β of the linear regression analyses signifies one‐point change in the mental or physical QoL component summary.
3. RESULTS
3.1. Study population for follow‐up of the Rome III Irritable bowel syndrome cohort
At a mean follow‐up time of 4.7 (SD 1.5) years, 379 patients with IBS, Rome III‐positive at inclusion and participating at least 3 (5 ± 2 years) in the Maastricht IBS cohort, were approached for participation in the follow‐up measurement. A total of 203 subjects (53.7%) responded by completing the electronic or paper questionnaires. Of these, 161 could be reached by telephone to confirm the Rome criteria, and these did not differ in demographics nor in symptom scores when compared to the 42 subjects that were not reached. A study flowchart is shown in Figure 1.
Figure 1.
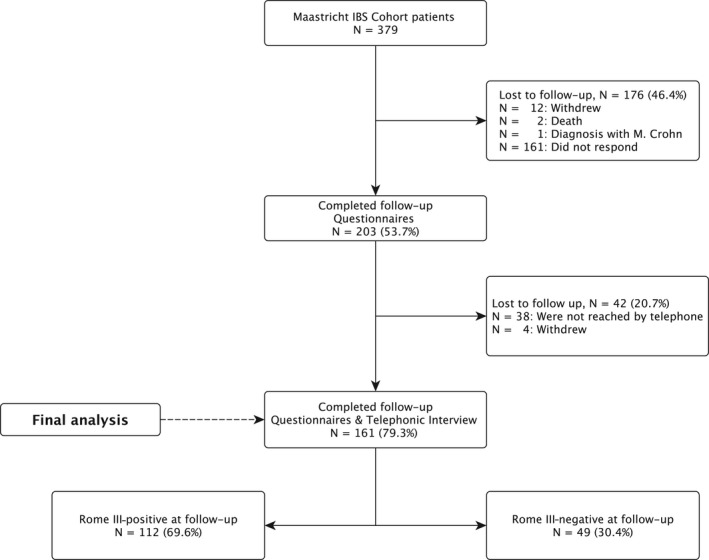
Flowchart of follow‐up of N = 379 IBS patients included in the Maastricht IBS cohort study. IBS, irritable bowel syndrome; N, number
3.1.1. Follow‐up—demographics, clinical characteristics, and symptoms of the Rome III‐positive and Rome III‐negative patients
The 161 patients in whom IBS criteria could be checked by phone were included in the final analysis. The majority of patients was female (74.5%), and median age was 53 years old (IQR = 36‐65).
In total, 49 out of the 161 (30.4%) did no longer fulfill the Rome III criteria for IBS at follow‐up (Figure 1). Demographical and clinical characteristics at follow‐up of the Rome III‐negative and Rome III‐positive patients are shown in Table 1. The groups did not differ significantly in age or gender, although the Rome III‐positive group was slightly younger. As expected, Rome III‐negative patients had significantly lower GI symptom scores compared to Rome III‐positive patients (assessed by both the GSRS and GSRS‐IBS) at follow‐up, which was shown on each of the following subdomains: abdominal pain, bloating, diarrhea, constipation, indigestion (all P < 0.001), and satiety (P < 0.005). Despite having less GI symptoms, they did not have significantly higher quality of life or more satisfaction with life. Although Rome III‐negative patients had significantly less GI‐specific anxiety at follow‐up (P < 0.001), they did not have less symptoms of general anxiety and depression. Moreover, levels of impaired work productivity and absence from work did not differ between both groups.
Table 1.
Demographical and clinical characteristics, at a 5‐y follow‐up period, of patients who still fulfilled the Rome III criteria for IBS at follow‐up (FU Rome III‐positive) and those who did not (FU Rome III‐negative)
| Characteristics at follow‐up | FU Rome III‐positive | FU Rome III‐negative |
|---|---|---|
| N = 112 | N = 49 | |
| Age, median years (IQR) | 52 (34‐65) | 57 (45‐67) |
| Female gender, N (%) | 84 (75.00) | 36 (73.47) |
| BMI, median kg m‐2 (IQR) | 25.15 (21.88‐28.70) | 25.35 (21.62‐28.37) |
| GSRS, median (IQR) | ||
| Abdominal pain | 3.33 (2.67‐4.33) | 2.33 (1.17‐3.00)*** |
| Reflux syndrome | 1.50 (1.00‐3.00) | 1.50 (1.00‐3.00) |
| Diarrhea syndrome | 3.33 (2.00‐4.67) | 1.67 (1.00‐3.00)*** |
| Constipation syndrome | 3.00 (2.00‐4.00) | 2.00 (1.33‐3.00)*** |
| Indigestion syndrome | 3.75 (2.00‐4.67) | 3.00 (2.25‐4.50)*** |
| GSRS‐IBS, median (IQR) | ||
| Abdominal pain | 4.00 (3.5‐5.00) | 2.50 (1.50‐3.50)*** |
| Bloating | 4.33 (3.33‐5.00) | 3.00 (2.00‐4.33)*** |
| Constipation | 2.50 (1.00‐4.00) | 2.00 (1.00‐2.50) |
| Diarrhea | 3.25 (2.25‐4.50) | 2.00 (1.50‐3.00)*** |
| Satiety | 3.00 (2.00‐4.50) | 2.50 (1.25‐3.50)** |
| Comorbid‐FD, N (%) | 61 (55.00) | 20 (41.67) |
| Rome IV‐positive IBS, N (%) | 69 (61.61) | ‐ |
| HADS, score > 8, N (%) | ||
| Depressive symptoms | 26 (23.42) | 8 (16.33) |
| General anxiety symptoms | 33 (29.72) | 8 (16.33) |
| GI‐specific anxiety, median (IQR) | 24.00 (13.00‐37.00) | 8.00 (4.00‐23.00)*** |
| Quality of life, median (IQR) | ||
| PCS | 45.10 (33.01‐48.50) | 48.96 (37.71‐52.99) |
| MCS | 51.27 (37.81‐55.96) | 54.75 (44.61‐57.71) |
| Satisfaction with life, N (%) | ||
| Very unsatisfied | 3 (2.67) | 0 (0.0) |
| Unsatisfied | 7 (6.25) | 2 (4.08) |
| Below average satisfaction | 20 (17.86) | 4 (8.16) |
| Averagely satisfied | 25 (22.32) | 14 (28.57) |
| Satisfied | 37 (33.04) | 20 (40.82) |
| Very satisfied | 20 (17.86) | 9 (18.37) |
| Absence from work, N (%) | 18 (34.62) | 6 (25.00) |
| Impaired productivity at work, N (%) | 30 (61.22) | 15 (62.50) |
Numbers may not add up to total due to missing. Abbreviations: BMI, body mass index (kg m−2); FD, functional dyspepsia; GI, gastrointestinal. GSRS, gastrointestinal symptom rating scale; HADS, hospital anxiety and depression scale; IBS, Irritable bowel syndrome; IQR, interquartile range; MCS, mental quality of life composite summary as assessed by SF‐36; N, number of patients included in the analysis; PCS, physical quality of life composite summary as assessed by SF‐36.
P < 0.001 vs Rome III‐positive;
P < 0.005 vs Rome III‐positive.
3.1.2. Baseline—demographics, clinical characteristics, and symptoms of the Rome III‐positive and Rome III‐negative patients
Table 2 shows baseline demographics and clinical characteristics (at inclusion time into the cohort) of patients who were Rome III‐positive and Rome III‐negative at follow‐up. With regard to baseline levels of GI symptom severity (assessed by the GSRS, as baseline GSRS‐IBS data were not available), it can be noted that Rome III‐negative patients had slightly lower baseline scores for abdominal pain. Nevertheless, after correction for multiple comparisons, this did not reach statistical significance nor did other GI symptoms differ significantly between the groups at baseline. In terms of general anxiety, depression, and general quality of life scores, no baseline differences were observed between Rome III‐positive and Rome III‐negative patients (Table 2). Moreover, the proportion of patients that had been recruited for the cohort from the primary care setting did not differ between Rome III‐positive and Rome III‐negative patients (Table 2). In addition, the distribution in baseline IBS subtypes did not differ between Rome III‐positive and Rome III‐negative patients (Table 2).
Table 2.
Baseline demographical and clinical characteristics of patients still fulfilling the Rome III criteria for IBS at follow‐up (FU Rome III‐positive) and those who did not (FU Rome IV‐negative)
| Characteristics at inclusion | FU Rome III‐positive | FU Rome III‐negative |
|---|---|---|
| N = 112 | N = 49 | |
| Age, median (IQR) | 48 (29‐61) | 51 (39‐61) |
| Gender, N female (%) | 84 (75.0) | 36 (73.47) |
| BMI, median kg m−2 (IQR) | 23.70 (21.50‐28.70) | 24.40 (21.50‐28.05) |
| Secondary/tertiary care, N (%) | 72 (64.29) | 35 (71.43) |
| IBS subtype, N (%) | ||
| IBS‐D | 40 (35.7) | 19 (38.8) |
| IBS‐C | 23 (20.5) | 8 (16.3) |
| IBS‐M | 47 (42.0) | 16 (32.7) |
| IBS‐U | 2 (1.8) | 6 (12.2) |
| GSRS, median (IQR) | ||
| Abdominal pain | 3.67 (2.33‐4.00) | 2.83 (2.00‐3.67) |
| Reflux syndrome | 1.50 (1.00‐2.63) | 1.00 (1.00‐2.88) |
| Diarrhea syndrome | 3.00 (2.33‐4.67) | 2.83 (1.67‐4.25) |
| Constipation syndrome | 3.17 (2.33‐4.00) | 1.49 (2.00‐3.67) |
| Indigestion syndrome | 4.25 (3.38‐5.00) | 3.88 (2.75‐5.00) |
| Diary, median score (IQR) | ||
| Discomfort | 2.29 (2.00‐2.72) | 2.21 (1.64‐2.57) |
| Pain | 2.23 (1.64‐2.79) | 1.93 (1.27‐2.50) |
| Constipation | 1.29 (1.00‐1.79) | 1.14 (1.00‐1.59) |
| Diarrhea | 1.21 (1.07‐1.64) | 1.07 (1.00‐1.38) |
| Bloating | 2.00 (1.52‐2.64) | 2.00 (1.25‐2.43) |
| Flatulence | 2.29 (1.89‐2.89) | 2.23 (1.64‐2.80) |
| Belching | 1.36 (1.00‐2.00) | 1.18 (1.00‐1.92) |
| Nausea | 1.21 (1.00‐1.81) | 1.14 (1.00‐1.57) |
| HADS, score > 8, N (%) | ||
| Depressive symptoms | 20 (19.05) | 6 (5.71) |
| General anxiety symptoms | 41 (38.68) | 17 (38.64) |
| Quality of life, median (IQR) | ||
| PCS | 41.71 (33.90‐48.98) | 45.51 (36.32‐51.74) |
| MCS | 50.20 (41.45‐55.19) | 50.67 (41.38‐57.44) |
Numbers may not add up to total due to missing. Please note that several variables shown in Table 1 derive from questionnaires not administered at baseline and could therefore not be considered in the comparison of baseline variables in Table 2.
Abbreviations: BMI, body mass index (kg m−2); GSRS, gastrointestinal symptom rating scale; HADS, hospital anxiety and depression scale; IBS, Irritable bowel syndrome; IQR, interquartile range; MCS, mental quality of life composite summary as assessed by SF‐36; N, number of patients included in the analysis; PCS, physical quality of life composite summary as assessed by SF‐36; secondary/tertiary care as compared to primary care.
3.1.3. Symptom evolution over time
Table 3 shows the evolution of clinical characteristics and symptoms over time by pairwise comparisons between baseline and follow‐up measurements. Overall, improvements in GI symptom severity (assessed by the GSRS, as baseline GSRS‐IBS data were not available), general quality of life, and general anxiety and depression over a 5‐year period were small and did not reach significance in patients that still met the Rome III diagnostic criteria at follow‐up. Symptom severity for abdominal pain, diarrhea, constipation, and indigestion did improve significantly over time in the Rome III‐negative patients, although they still had “low to moderate” levels of symptoms after 5 years, with a median abdominal pain GSRS score of 2.33 (IQR = 1.33‐3.00), and a median indigestion GSRS score of 2.88 (IQR = 2.25‐3.94). However, similar to the Rome III‐positive patients, the Rome III‐negative patients did not improve significantly in quality of life during the follow‐up period. In addition, their comorbid depression scores were relatively unaltered.
Table 3.
Clinical characteristics, at baseline and at time of follow‐up, of patients who still fulfilled the Rome III criteria for IBS at follow‐up (Rome III‐positive) and patients who did not (Rome III‐negative)
| FU Rome III‐positive IBS patients, N = 112 | Baseline | Follow‐up |
|---|---|---|
| BMI, median kg m−2 (IQR) | 23.70 (21.45‐28.70) | 24.77 (21.92‐28.60)** |
| GSRS, median (IQR) | ||
| Abdominal pain | 3.67 (2.33‐4.00) | 3.33 (2.50‐4.33) |
| Reflux syndrome | 1.50 (1.00‐2.75) | 1.50 (1.00‐3.00) |
| Diarrhea syndrome | 3.00 (2.33‐4.67) | 3.33 (1.83‐4.67) |
| Constipation syndrome | 3.00 (2.33‐4.00) | 3.00 (2.00‐4.00) |
| Indigestion syndrome | 4.00 (3.38‐5.00) | 3.75 (3.00‐4.50) |
| HADS, score > 8, N (%) | ||
| Depressive symptoms | 20 (19.05) | 25 (23.81) |
| General anxiety symptoms | 41 (39.05) | 31 (29.52) |
| Quality of life, median (IQR) | ||
| PCS | 42.19 (34.36‐48.90) | 45.41 (33.44‐48.78) |
| MCS | 49.58 (37.87‐54.96) | 51.07 (37.57‐55.93) |
| FU Rome III‐negative IBS patients, N = 49 | Baseline | Follow‐up |
|---|---|---|
| BMI, median kg m−2 (IQR) | 24.50 (21.30‐28.08) | 25.35 (21.68‐28.73) |
| GSRS, median (IQR) | ||
| Abdominal pain | 2.83 (2.00‐3.67) | 2.33 (1.33‐3.00)* |
| Reflux syndrome | 1.00 (1.00‐2.88) | 1.50 (1.00‐3.00) |
| Diarrhea syndrome | 2.83 (1.67‐4.25) | 1.67 (1.08‐3.00)** |
| Constipation syndrome | 2.50 (2.00‐3.67) | 2.00 (1.33‐3.00)*** |
| Indigestion syndrome | 3.88 (2.75‐5.00) | 2.88 (2.25‐3.94)*** |
| HADS, score > 8, N (%) | ||
| Depressive symptoms | 6 (13.64) | 7 (15.91) |
| General anxiety symptoms | 17 (38.64) | 7 (15.91)∞ |
| Quality of life, median (IQR) | ||
| PCS | 45.51 (36.32‐51.74) | 48.85 (33.14‐52.99) |
| MCS | 50.68 (41.38‐57.44) | 53.38 (43.89‐57.30) |
Numbers may not add up to total due to missing. Abbreviations: BMI, body mass index (kg m−2); GSRS, gastrointestinal symptom rating scale; HADS, hospital anxiety and depression scale; IBS, Irritable bowel syndrome; IQR, interquartile Range; MCS, mental quality of life composite summary as assessed by SF‐36. N, number of patients included in the analysis; PCS, physical quality of life composite summary as assessed by SF‐36.
P < 0.001 vs baseline;
P < 0.005 vs baseline;
P < 0.01 vs baseline;
P < 0.05 vs baseline.
With regard to IBS subtype based on predominant bowel habit, Figure S1 illustrates the proportion of IBS subtypes at baseline and at follow‐up. Sixty‐two (55.4%) out of 112 Rome III‐positive patients had changed IBS subtype during the follow‐up period. In Figure S2, the within‐patient subtype changes from baseline to follow‐up are shown. IBS‐D appeared the most stable subtype (72.5% of Rome III‐positive unchanged), whereas IBS‐M and IBS‐C were least stable (76.6% and 60.9% of Rome III‐positive changed, respectively).
Multivariable regression model
No baseline predictors for fulfilling the Rome III diagnosis for IBS at follow‐up could be identified with regression analyses. When looking for independent baseline predictors concerning quality of life at follow‐up for both groups taken together (N = 161), the combined regression model that included age, gender, baseline GI symptom severity, and baseline anxiety and depression showed that only younger age (β −0.16, 95% CI −0.26; −0.06, P = 0.002) and higher physical quality of life at baseline (β 0.59, 95% CI 0.41; 0.78, P = <0.001) predicted higher physical quality of life at follow‐up. Likewise, only higher mental quality of life at baseline (β 0.46, 95% CI 0.15; 0.77, P = 0.004), but not baseline depression or anxiety scores, predicted higher mental quality of life at follow‐up.
When looking for characteristics at follow‐up that were associated with quality of life at follow‐up, general anxiety (β −1.49, 95% CI −1.90; −1.07, P < 0.001) and depression levels (β −1.00, 95% CI −1.48; −0.53, P < 0.001) at follow‐up were the only two parameters independently associated with mental quality of life at follow‐up after correcting the model for age, gender, IBS subtype, GI symptom severity, GI‐specific anxiety, and general anxiety and depression. No characteristics at follow‐up were found to independently associate with physical quality of life at follow‐up. Baseline IBS subtypes were not included in the models that looked for baseline predictors, as univariate analyses showed no significant differences in subtypes between groups; that is, the distribution in baseline subtypes did not differ significantly between Rome III‐negative and Rome III‐positive patients. Inclusion of these variables into the model would have decreased statistical validity and increased the probability of type I errors.
4. DISCUSSION
In the current prospective study, we evaluated the natural symptom evolution of patients with IBS in the Maastricht IBS cohort over time.33 We demonstrated that 30.4% of patients did no longer fulfill the Rome III criteria after a 5‐year follow‐up period. The most salient finding is that quality of life did not improve significantly in patients who showed a decrease in gastrointestinal symptom severity (ie, being Rome III‐negative at follow‐up), compared to patients who had unaltered symptom severity over time (Rome III‐positive). In addition, general well‐being in terms of comorbid general anxiety and depression, work absenteeism and productivity, and life satisfaction were also comparable in those who still fulfilled the Rome III criteria at follow‐up, when compared to those who did not.
Several studies have investigated IBS symptom evolution in relation to diagnostic criteria in patient cohorts, with varying results. A study by Williams et al using the Rome II criteria reported that 52% had no IBS 2 years after web‐based diagnosis,12 Ford et al found that 28% did not meet the Manning criteria after 10 years,34 Mearin et al reported that abdominal pain frequency decreased in 26% after 1 year,35 and more recently, Card et al reported that 27% of their large study population did not fulfill the Rome III criteria after 1 year.36 Except for the study by Williams et al, the proportion of patients who do not meet the diagnostic criteria after a follow‐up period is thus in line with the findings of this follow‐up study in our Dutch cohort.
Given that clinical treatment decisions often rely on prognostic predictions of the disease course, we sought to identify baseline predictors for a less favorable disease course. This study, however, did not show any baseline characteristics associated with meeting the Rome III criteria at follow‐up. As IBS is a highly heterogeneous disorder, larger study populations might be necessary to investigate what factors influence and thereby predict the natural disease course in (subgroups of) patients with IBS.
Rather unexpectedly, we found that a decrease in GI symptom severity did not lead to an improved quality of life in our study. Clevers et al recently evaluated longitudinal symptom changes over time and, in contrast to our results, found that patients with lower GI symptom severity had significantly higher quality of life scores.37 They also demonstrated that GI‐specific anxiety is associated with an increase in GI symptom severity, which is in agreement with our results as GI‐specific anxiety was significantly lower in the group that did not fulfill the Rome III criteria. The inconsistency between our data in terms of quality of life might be explained in part by the different questionnaires used. Clevers et al used an IBS‐specific questionnaire, the IBS‐QoL,38 which assesses more disease‐specific changes in quality of life in contrast to generic quality of life instruments, such as the SF‐36 that we used. Our data can therefore also be compared with other diseases and with the general population. Both the Rome III‐positive and Rome III‐negative group showed lower mean quality of life than the mean of a Dutch population sample39 and of a US‐based population without a functional GI disorder.40 Additionally, we used the validated SWLS to score overall life satisfaction. This has been used in healthy persons and in patients with Crohn’s disease,41 but, to our knowledge, not in patients with IBS. We found scores comparable to the ones reported by Crohn’s disease patients for both the Rome III‐positive and Rome III‐negative group.
In contrast to GI symptom severity, we found that quality of life did not improve over time in those that were Rome III‐negative at follow‐up. The data reported here suggest that concurrent, but not baseline, psychological comorbidities are more predictive of this impaired long‐term quality of life than GI symptom severity. Several studies in IBS have found similar results, in particular for the mental health‐related quality of life; that is, Naliboff et al reported that psychological distress had a stronger effect on health‐related quality of life than GI symptoms,42 Koloski et al reported that depression was independently associated with mental quality of life,43 and Addante et al reported that perceived stress, and anxiety and depression were significant predictors for mental health‐related quality of life.44 This raises the question whether current treatment goals for IBS in daily clinical practice should be revised. Gaining insight into which symptoms specifically affect a patient’s quality of life can aid in reprioritizing personal treatment goals and increase the chance of a successful individualized treatment trajectory.45 Currently, treatment is merely targeted toward the patient’s predominant GI symptoms, and in many cases, this entails primary treatment of pain, constipation, or diarrhea. Targeting GI symptom improvement without characterizing the full extent of the disorder and possible psychosocial modifiers may therefore contribute to treatment failure concerning quality of life over time. A helpful and pragmatic framework in this regard has been suggested by the Rome expert panel.45 By following their five‐step approach, clinicians can identify if and which psychological factors contribute to the disease burden in the individual patient and consider psychological treatments. In some patients, this might include the use of pharmacological neuromodulators,46 but other therapy options include cognitive behavioral therapy (CBT), gut‐directed hypnotherapy,47 and dynamic psychotherapy.48 Due to the relative lack in therapist availability, clinical implementation of these therapies is still limited. Recent attempts to reduce resource use without compromising effectiveness may assist in making psychological treatment more widely available; for example, group hypnotherapy,49 Internet‐delivered exposure‐based CBT,50 and home‐based CBT.51 In that light, it will be interesting to explore whether these developments can affect long‐term quality of life and well‐being in patients with IBS.
Several limitations of the current study should be noted. Only two time‐points within the 5‐year period were assessed; no further data were available about the period in between the baseline and follow‐up measurement, including data on treatment received in this period. Therefore, we can neither comment about the frequency and duration of IBS flares within these 5 years, nor about the effect of treatment on symptoms, nor on the causal order of the impaired quality of life and psychological comorbidities. Moreover, selection bias cannot be excluded. Follow‐up cohort studies depend on long‐term dedication of their participants, and the proportion of non‐responders (loss to follow) in this study was 46.4%. To assess potential selection bias, we tested for differences in baseline characteristics between responders and non‐responders at follow‐up. The groups did not differ in baseline demographic and lifestyle characteristics, nor in baseline symptom scores and subtypes (data not shown). Hence, there is no clear reason to assume that the non‐responders would have had different outcomes at follow‐up. Finally, a larger sample size would have been desirable. Due to a relatively small Rome III‐negative group, small effects concerning quality of life or other parameters could have been missed, especially since we have corrected for multiple comparisons. Although we argue that this adjustment improves the reproducibility of our results, an unnecessarily high false‐negative rate cannot be ruled out. Because of the small sample size, it is important that our findings are corroborated in other cohorts of patients with IBS. Until then, findings should be considered exploratory and appropriate caution should be taken when interpreting the results of the current study.
Strengths of this prospective follow‐up study include the heterogeneous and well‐characterized Maastricht IBS cohort population; that is, in‐depth phenotyping enables comparison to other large cohorts and future studies should explore possibilities of pooling data from different centers to cluster phenotypes and validate the robustness of findings such as the ones reported here. Moreover, as we have recruited patients from both primary and secondary/tertiary care settings (Table 1), our population is representative for the general Dutch IBS population. Another strength is the evaluation of the Rome criteria in a telephonic follow‐up interview with patients. Due to the current lack of validated biomarkers, IBS remains a symptom‐based diagnosis for which the Rome criteria are used to aid diagnostic accuracy, both in research and clinical settings.52 As the Rome criteria have not been developed nor validated as a self‐administered questionnaire, asking patients to complete the Rome criteria without supervision by a trained researcher may lead to bias and over‐ or under‐estimation of the actual diagnosis, as previously demonstrated by Lovell et al.2 In contrast to earlier studies on follow‐up of IBS, our study method implied a complete reevaluation of the diagnosis in a telephonic interview and, thus, allows for a more valid and less biased interpretation of Rome‐diagnosed IBS prevalence over time.
In conclusion, the current prospective study contributes to existing insight regarding symptom evolution over time in patients with IBS and showed that 30% of patients did no longer fulfill the Rome III criteria after a 5‐year follow‐up period. However, the decrease in GI symptom severity (ie, being Rome III‐negative at follow‐up) did not impact quality of life nor life satisfaction. Our results indicate that long‐term quality of life and general well‐being might depend on comorbid psychological symptoms, that is, affective states, rather than gastrointestinal symptom severity.
CONFLICT OF INTEREST
The authors state that they are no competing interests to declare.
AUTHOR CONTRIBUTIONS
ZW and LV conceived and designed the study, collected the data, analyzed and interpreted the data, and wrote the manuscript; ZM collected the data and constructively reviewed the manuscript; DK interpreted the data and constructively reviewed the manuscript; MH conceived and designed the study, and collected and processed the data; JK, CL, and JM constructively reviewed the manuscript; and DJ and AM conceived and designed the study, interpreted the data, and constructively reviewed the manuscript. All authors approved the final manuscript. Z. Weerts: Guarantor of article.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank Madelon Bosschee and Ayse Bicer for their practical contributions to the study.
Weerts ZZRM, Vork L, Mujagic Z, et al. Reduction in IBS symptom severity is not paralleled by improvement in quality of life in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2019;31:e13629 10.1111/nmo.13629
Zsa Zsa R. M. Weerts and Lisa Vork authors contributed equally to the manuscript.
REFERENCES
- 1. El‐Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151‐5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta‐analysis. Clin Gastroenterol Hepatol. 2012;10(712–721):e714. [DOI] [PubMed] [Google Scholar]
- 3. Van den Houte K, Carbone F, Pannemans J, et al. Prevalence and impact of self‐reported irritable bowel symptoms in the general population. United European Gastroenterol J. 2018;7(2):307‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jossan N, Simren M, Sperber AD, et al. Health care utilization for Rome IV irritable bowel syndrome, a three‐country survey in the general population. Gastroenterology. 2017;152:S68. [Google Scholar]
- 5. Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626‐1635. [DOI] [PubMed] [Google Scholar]
- 6. Holtmann GJ, Ford AC, Talley NJ. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol Hepatol. 2016;1:133‐146. [DOI] [PubMed] [Google Scholar]
- 7. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil. 2017;29(4):e12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health‐related quality of life. Gastroenterology. 2000;119:654‐660. [DOI] [PubMed] [Google Scholar]
- 9. Palsson OS, Baggish J, Whitehead WE. Episodic nature of symptoms in irritable bowel syndrome. Am J Gastroenterol. 2014;109:1450‐1460. [DOI] [PubMed] [Google Scholar]
- 10. Drossman DA, Morris CB, Hu Y, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology. 2005;128:580‐589. [DOI] [PubMed] [Google Scholar]
- 11. Garrigues V, Mearin F, Badia X, et al. Change over time of bowel habit in irritable bowel syndrome: a prospective, observational, 1‐year follow‐up study (RITMO study). Aliment Pharmacol Ther. 2007;25:323‐332. [DOI] [PubMed] [Google Scholar]
- 12. Williams RE, Black CL, Kim HY, et al. Stability of irritable bowel syndrome using a Rome II‐based classification. Aliment Pharmacol Ther. 2006;23:197‐205. [DOI] [PubMed] [Google Scholar]
- 13. Spiegel B, Harris L, Lucak S, et al. Developing valid and reliable health utilities in irritable bowel syndrome: results from the IBS PROOF Cohort. Am J Gastroenterol. 2009;104:1984‐1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corsetti M, Whorwell P. The global impact of IBS: time to think about IBS‐specific models of care? Therap Adv Gastroenterol. 2017;10:727‐736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mujagic Z, Jonkers D, Ludidi S, et al. Biomarkers for visceral hypersensitivity in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29:e13137. [DOI] [PubMed] [Google Scholar]
- 16. Ludidi S, Mujagic Z, Jonkers D, et al. Markers for visceral hypersensitivity in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2014;26:1104‐1111. [DOI] [PubMed] [Google Scholar]
- 17. Thijssen AY, Mujagic Z, Jonkers DM, et al. Alterations in serotonin metabolism in the irritable bowel syndrome. Aliment Pharmacol Ther. 2016;43:272‐282. [DOI] [PubMed] [Google Scholar]
- 18. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191. [DOI] [PubMed] [Google Scholar]
- 19. Svedlund J, Sjodin I, Dotevall G. GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129‐134. [DOI] [PubMed] [Google Scholar]
- 20. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361‐370. [DOI] [PubMed] [Google Scholar]
- 21. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52:69‐77. [DOI] [PubMed] [Google Scholar]
- 22. McHorney CA, Ware JE, Jr. , Raczek AE. The MOS 36‐item short‐form health survey (SF‐36): II. Med Care. 1993;31:247‐263. [DOI] [PubMed] [Google Scholar]
- 23. Farivar SS, Cunningham WE, Hays RD. Correlated physical and mental health summary scores for the SF‐36 and SF‐12 health survey, V.I. Health Qual Life Outcomes. 2007;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wiklund IK, Fullerton S, Hawkey CJ, et al. An irritable bowel syndrome‐specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947‐954. [DOI] [PubMed] [Google Scholar]
- 25. Labus JS, Bolus R, Chang L, et al. The visceral sensitivity index: development and validation of a gastrointestinal symptom‐specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89‐97. [DOI] [PubMed] [Google Scholar]
- 26. Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess. 1985;49:71‐75. [DOI] [PubMed] [Google Scholar]
- 27. Pavot W, Diener E, Colvin CR, Sandvik E. Further validation of the Satisfaction with Life Scale: evidence for the cross‐method convergence of well‐being measures. J Pers Assess. 1991;57:149‐161. [DOI] [PubMed] [Google Scholar]
- 28. Van Beuningen J. Satisfaction with life scale: examining construct validity, Heerlen (The Hague). 2012.
- 29. Frandemark A, Tornblom H, Jakobsson S, Simren M. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem. Am J Gastroenterol. 2018;113(10):1540–1549. [DOI] [PubMed] [Google Scholar]
- 30. Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Hakkaart‐van Roijen L. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health‐related productivity losses. Value Health. 2015;18:753‐758. [DOI] [PubMed] [Google Scholar]
- 31. Bouwmans C, Krol M, Brouwer W, Severens JL, Koopmanschap MA, Hakkaart L. IMTA productivity cost questionnaire (IPCQ). Value Health. 2014;17:A550. [DOI] [PubMed] [Google Scholar]
- 32. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57(1):289–300. [Google Scholar]
- 33. Weerts Z, Vork L, Mujagic Z, et al. The natural history of IBS: a five‐year follow‐up of the Maastricht IBS cohort study, poster sessions, poster 276. Neurogastroenterol Motil. 2018;30:1–186. [Google Scholar]
- 34. Ford AC, Forman D, Bailey AG, Axon AT, Moayyedi P. Fluctuation of gastrointestinal symptoms in the community: a 10‐year longitudinal follow‐up study. Aliment Pharmacol Ther. 2008;28:1013‐1020. doi:10.1111/j.1365-2036.2008.03813.x [DOI] [PubMed] [Google Scholar]
- 35. Mearin F, Badia X, Balboa A, et al. Predictive factors of irritable bowel syndrome improvement: 1‐year prospective evaluation in 400 patients. Aliment Pharmacol Ther. 2006;23:815‐826. [DOI] [PubMed] [Google Scholar]
- 36. Card T, Enck P, Barbara G, et al. Post‐infectious IBS: defining its clinical features and prognosis using an internet‐based survey. United European Gastroenterol J. 2018;6:1245‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clevers E, Tack J, Törnblom H, et al. Development of irritable bowel syndrome features over a 5‐year period. Clin Gastroenterol Hepatol. 2018;16(8):1244–1251.e1 [DOI] [PubMed] [Google Scholar]
- 38. Drossman DA, Patrick DL, Whitehead WE, et al. Further validation of the IBS‐QOL: a disease‐specific quality‐of‐life questionnaire. Am J Gastroenterol. 2000;95:999. [DOI] [PubMed] [Google Scholar]
- 39. Ware JE Jr, Gandek B, Kosinski M, et al. The equivalence of SF‐36 summary health scores estimated using standard and country‐specific algorithms in 10 countries: results from the IQOLA project. International quality of life assessment. J Clin Epidemiol. 1998;51:1167‐1170. [DOI] [PubMed] [Google Scholar]
- 40. Kanuri N, Cassell B, Bruce SE, et al. The impact of abuse and mood on bowel symptoms and health‐related quality of life in irritable bowel syndrome (IBS). Neurogastroenterol Motil. 2016;28:1508‐1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarid O, Slonim‐Nevo V, Pereg A, et al. Coping strategies, satisfaction with life, and quality of life in Crohn’s disease: a gender perspective using structural equation modeling analysis. PLoS ONE. 2017;12:e0172779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naliboff BD, Kim SE, Bolus R, Bernstein CN, Mayer EA, Chang L. Gastrointestinal and psychological mediators of health‐related quality of life in IBS and IBD: a structural equation modeling analysis. Am J Gastroenterol. 2012;107:451‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koloski NA, Boyce PM, Jones MP, Talley NJ. What level of IBS symptoms drives impairment in health‐related quality of life in community subjects with irritable bowel syndrome? Qual Life Res. 2012;21:829‐836. [DOI] [PubMed] [Google Scholar]
- 44. Addante R, Naliboff B, Shih W, et al. Predictors of health‐related quality of life in irritable bowel syndrome patients compared with healthy individuals. J Clin Gastroenterol. 2018;53(4):e142‐e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23:151‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tornblom H, Drossman DA. Psychotropics, antidepressants, and visceral analgesics in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2018;20:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miller V, Carruthers HR, Morris J, Hasan SS, Archbold S, Whorwell PJ. Hypnotherapy for irritable bowel syndrome: an audit of one thousand adult patients. Aliment Pharmacol Ther. 2015;41:844‐855. [DOI] [PubMed] [Google Scholar]
- 48. Guthrie E, Creed F, Dawson D, Tomenson B. A controlled trial of psychological treatment for the irritable bowel syndrome. Gastroenterology. 1991;100:450‐457. [DOI] [PubMed] [Google Scholar]
- 49. Flik CE, Laan W, Zuithoff NPA, et al. Efficacy of individual and group hypnotherapy in irritable bowel syndrome (IMAGINE): a multicentre randomised controlled trial. The Lancet Gastroenterology & Hepatology. 2019; 4(1) 20-31. [DOI] [PubMed] [Google Scholar]
- 50. Ljótsson B, Hedman E, Andersson E, et al. Internet‐delivered exposure‐based treatment vs. stress management for irritable bowel syndrome: a randomized trial. Am J Gastroenterol. 2011;106(8):1481–1491. [DOI] [PubMed] [Google Scholar]
- 51. Lackner JM, Jaccard J, Keefer L, et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology. 2018;155:47‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393‐1407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


