Abstract
Induced pluripotent stem (iPS) cells hold great promise for regenerative medicine and the treatment of various diseases. Before proceeding to clinical trials, it is important to test the efficacy and safety of iPS cell‐based treatments using experimental animals. The common marmoset is a new world monkey widely used in biomedical studies. However, efficient methods that could generate iPS cells from a variety of cells have not been established. Here, we report that marmoset cells are efficiently reprogrammed into iPS cells by combining RNA transfection and chemical compounds. Using this novel combination, we generate transgene integration‐free marmoset iPS cells from a variety of cells that are difficult to reprogram using conventional RNA transfection method. Furthermore, we show this is similarly effective for human and cynomolgus monkey iPS cell generation. Thus, the addition of chemical compounds during RNA transfection greatly facilitates reprogramming and efficient generation of completely integration‐free safe iPS cells in primates, particularly from difficult‐to‐reprogram cells.
Keywords: chemical compounds, ES cell, humans, interferon response, iPS cell, marmosets, miRNA, mRNA, primates, RNA transfection
This study reports a novel method for iPS cell generation by combining RNA transfection and chemical compounds. Our novel method enable highly efficient induction of iPS cells in marmosets, it can be used in other primates as well.
1. INTRODUCTION
An increasing number of clinical trials involving induced pluripotent stem (iPS) cells have been planned since the first transplantation of iPS cells‐derived cells into a patient with age‐related macular degeneration in 2017 (Mandai et al., 2017). Before applying iPS cells into regenerative medicine, it is essential to test their safety and efficacy using experimental animals. Although rats and mice can be used in the first phase of preclinical studies, results obtained using nonhuman primates facilitate a more accurate prediction of the effect of human treatment. Common marmoset (Callithrix jacchus) is a new world monkey that originally inhabited the northeastern coast of Brazil. As common marmoset is relatively easy to handle due to its small body size and mild character, it is extensively used in biomedical research (Okano, Hikishima, Iriki, & Sasaki, 2012). In addition, when compared to other nonhuman primates, only a small number of cells are required for transplantation experiments, which facilitates preclinical studies involving the transplantation of cells that are challenging to prepare. Thus, marmoset is useful for preclinical studies of iPS cell‐based treatment.
The establishment of iPS cells was initially carried out using retorovirus vectors to ectopically express reprogramming factors, such as OCT4 and SOX2 (Park et al., 2008; Takahashi et al., 2007; Takahashi & Yamanaka, 2006; Yu et al., 2007). Retrovirus‐based methods involve the insertion of transgenes into the genome, which potentially causes cancer. Several methods for iPS cell induction without transgene insertion have since been developed, including excisable lentivirus and transposons, episomal plasmids and adenovirus. However, the risks of causing changes in genomic DNA sequences still exist in these DNA‐based methods. Among DNA‐free methods, mRNA transfection methods seem to be superior to others, including protein transfection and Sendai‐virus methods, in terms of efficiency and speed of iPS colony appearance (Gonzalez, Boue, & Izpisua Belmonte, 2011; Schlaeger et al., 2015).
Two types of RNAs are used for RNA transfection‐mediated iPS cell induction: modified RNAs and unmodified RNAs. Transfection of in vitro synthesized RNAs usually causes antiviral interferon responses, which react with dsRNAs (Nallagatla, Toroney, & Bevilacqua, 2011; Sadler & Williams, 2008). This results in global inhibition of cellular translation in transfected cells. To bypass this innate antiviral system, nucleotide bases in mRNAs are slightly altered using pseudouridine for uridine and 5‐methylcytidine for cytidine. Induction of iPS cells has been reported using the modified mRNAs (Mandal & Rossi, 2013; Warren et al., 2010). Furthermore, Poleganov et al. reported successful induction of iPS cells by combining unmodified RNAs and three interferon response suppressor genes (E3, K3 and B18R) from vaccinia virus. This method using unmodified mRNAs can induce iPS cells more efficiently than that using modified mRNAs, possibly due to efficient translation or increased RNA stability (Poleganov et al., 2015).
Several methods have been reported so far for marmoset iPS cell induction. iPS cells have been generated from fetus liver‐, newborn skin‐ and adult bone marrow‐derived cells by introducing reprogramming factors using retrovirus or lentivirus (Tomioka et al., 2010; Wiedemann et al., 2012; Wu, Zhang, Mishra, Tardif, & Hornsby, 2010). More recently, iPS cells were generated without altering the genomic sequence using an excisable transposon vector and episomal vector from postnatal and adult skin cells, respectively (Debowski et al., 2015; Yang et al., 2018). In humans, RNA‐based methods are increasingly applied in the integration‐free generation of iPS cells, as they can lower the risk of introducing mutation (Anokye‐Danso et al., 2011; Mandal & Rossi, 2013; Poleganov et al., 2015; Rohani et al., 2016; Warren et al., 2010; Yoshioka et al., 2013). However, there is no report of generation of marmoset iPS cells using the RNA‐based methods. In the present paper, we report a highly efficient generation of marmoset iPS cells by introducing chemical compounds and a dominant negative form of P53 to a conventional RNA transfection method. Finally, we show that this novel combination is effective for iPS cell induction in other primate species, including humans.
2. RESULTS
2.1. Induction of marmoset iPS cell using RNA transfection method
The efficiency of iPS cell generation varies between cells, and it depends on cellular characteristics, such as epigenetic state and virus transduction efficiency. For experiments testing RNA transfection‐mediated reprogramming in marmosets, we first selected marmoset cells that could be converted into iPS cells using a conventional retrovirus method (Tomioka et al., 2010). Among the five adult marmoset liver‐derived cell lines, iPS cells were generated from only one line (I2965F) using the retrovirus method.
To enable RNA‐based reprogramming in marmosets, we used the RNA transfection method developed by Poleganov et al., which transfects human reprogramming factor mRNAs (OCT4/SOX2/KLF4/C‐MYC/LIN28A/NANOG), human ES cell‐specific miRNAs (302a‐d, 367) and vaccinia virus‐derived interferon response suppressor mRNAs (E3, K3 and B18R). Amino acid sequences of the reprogramming factor mRNAs display 88%‐99% similarity in identity between marmosets and humans (OCT4; 97.5%, SOX2; 98.1%, KLF4; 98.1%, C‐MYC; 96,3%, LIN28A; 99.0%, NANOG; 88.6%), and all the miRNA sequences are identical between the two species. The RNAs were transfected for eight days successively from Days 1 to 8 into four kinds of marmoset cells including the I2965F adult liver‐derived cells selected above (others include newborn skin‐derived cells, adult skin‐derived cells and fetus liver‐derived cells). On Day 5 during the transfection period, cells were passaged onto mouse embryonic fibroblast (MEF) feeder cells. On Day 18, two iPS‐like colonies were observed in I2965F liver‐derived cells (Figure 1a). However, no such colonies were found in the other three cells that were transfected and cultured in parallel. One of the two iPS cell colonies was picked up, and it was cultured in MEF‐conditioned medium using feeder‐free condition. Our unpublished study shows that marmoset ES cells are maintained in an undifferentiated state using this condition (manuscript in preparation). iPS‐like cell line (mRNA_iPS cell) was established from one of the two iPS colonies. The mRNA_iPS cells displayed normal karyotype (Figure S1a).
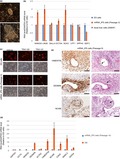
Figure 1.
Generation of marmoset iPS cells by transfection of mRNAs and miRNAs. (a) iPS cell colonies appeared after RNA‐based reprogramming. (b) qPCR analysis of ES cell marker genes in mRNA_iPS cells. Adult liver cells (I2965F), from which mRNA_iPS cells were derived, do not express ES cell marker genes. The number of passages of iPS cells examined is shown in parentheses. Error bars represent SE (N = 3). (c) Immunofluorescence analysis of ES cell surface antigens. ES cell surface antigen‐negative cells are MEF used for feeder cells. (d) qPCR analysis of ES cell marker and differentiation marker genes in EBs. Error bars represent SE (N = 3). (e) Formation of blood vessel‐like structure, muscle‐like structure and neuronal marker‐positive cells in teratoma. They are indicated by arrows. The results of immunostaining using the antibodies indicated and H&E staining are shown. Error bar = 100 μm
2.2. mRNA_iPS cells show characteristics of iPS cells
To confirm that the established cells (mRNA_iPS cells) are iPS cells, expression of pluripotent marker genes was examined. All of the genes examined (NANOG, LIN28A, SALL4, OCT4, SOX2, UTF1, DPPA3, GDF3, SSEA4, TRA1‐60 and TRA1‐81) showed similar expression levels between mRNA_iPS cells and control ES cells established previously (Sasaki, Hanazawa, & Kurita, 2005) (Figure 1b,c, Figure S1b and Table S1).
To examine whether mRNA_iPS cells exhibit the multipotent ability to differentiate into multiple cell lineages, mRNA_iPS cells were differentiated into embryoid bodies (EBs) for 18–21 days. Upon differentiation, OCT4 and NANOG expression dramatically decreased. In contrast, several differentiation markers from all three germ layers increased (Figure 1d and Figure S1c). The teratoma assay was carried out to further examine the differentiation potential. By injection of mRNA_iPS cells under kidney capsule of immunodeficient mice, teratoma was formed. In the teratoma, blood vessel‐like structures containing red blood cells were formed (Figure 1e top). These blood vessel structures were stained with anti‐VIMENTIN antibody that reacts with marmoset VIMENTIN, but not with mouse VIMENTIN. This suggests that they are derived from mRNA_iPS cells. Furthermore, DESMIN‐positive cells forming muscle‐like structures were found in the teratoma (Figure 1e middle), and they were also stained with anti‐VIMENTIN antibody, again suggesting they are derived from marmoset iPS cells. Although we were not able to find neurons based on morphology in the section of H&E staining, we observed populations of neuronal cells by staining with anti‐NCAM antibody (Figure 1e bottom), which specifically react with marmoset antigen. However, we failed to find the evidence of endodermal differentiation. These results are in line with a previous study reporting the difficulty of differentiation into endoderm lineage and frequent differentiation into mesoderm lineage of marmoset ES cells (Sasaki et al., 2005). The results of gene expression and differentiation potential analyses indicate that mRNA_iPS cells are indeed iPS cells. These cells are stably maintained in undifferentiated state for 27 passages (Table S2).
2.3. Chemical compounds promote RNA‐mediated induction
As mentioned above, iPS cells were induced from only one (I2965F adult liver‐derived cells) of the four cell lines tested in parallel using the RNA transfection method. We inferred that increasing reprogramming efficiency would enable the induction of iPS cells from numerous types of cells. Therefore, chemical compounds that have been shown to promote iPS cell induction were added during reprogramming. The following three sets of chemicals were used: (1) Thiazovivin set containing thiazovivin (ROCK inhibitor), SB431542 (TGF‐β/Activin/NODAL inhibitor) and PD0325901 (MEK inhibitor) (Lin et al., 2009), (2) Human iPS reprogramming Boost Supplement II (Boost supplement) containing PS48 (PDK inhibitor), sodium butyrate (Histone deacetylase inhibitor) and TGF‐β inhibitor (Ichida et al., 2009; Zhu et al., 2010) and (3) 3i containing PD0325901 (MEK inhibitor), CHIR99021 (GSK3 inhibitor) and PD173074 (FGFR inhibitor) (Li et al., 2009; Silva et al., 2008).
However, RNA transfection in the presence of any of the three sets of chemicals resulted in massive cell death, and cell numbers decreased considerably after a successive eight‐day transfection (Figure 2a). To alleviate cell death caused by chemicals, the dominant negative form of P53 (P53DD) mRNA was transfected together with other RNAs to inhibit the function of P53, which promotes apoptosis (Bowman et al., 1996; Hafner, Bulyk, Jambhekar, & Lahav, 2019; Hong et al., 2009). As expected, the addition of P53DD markedly increased the cell numbers on Day 9, and they exceeded even the initial cell numbers (Figure 2a).
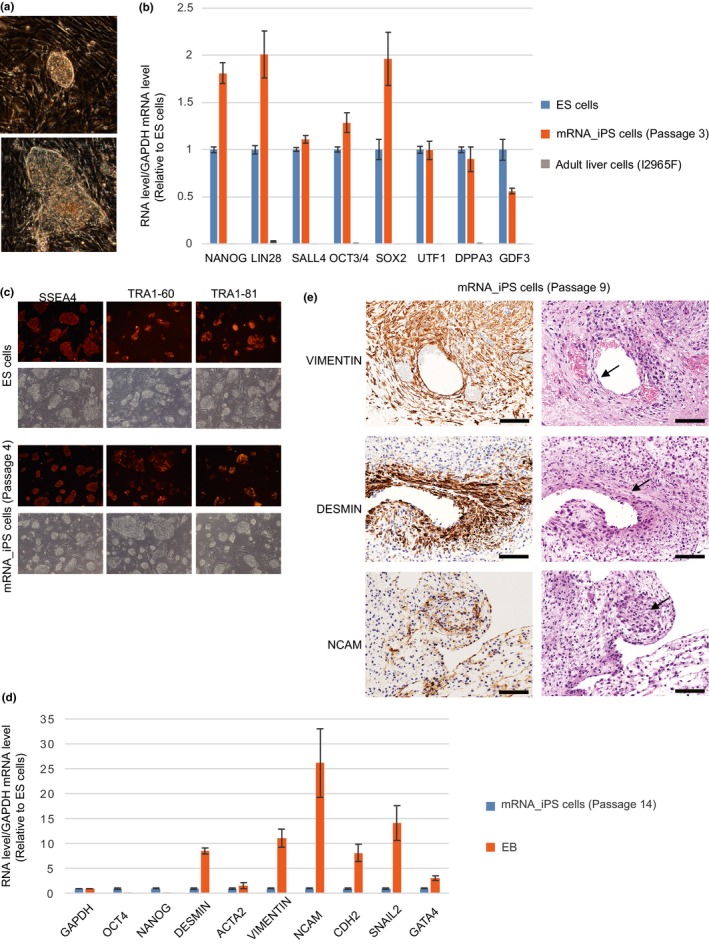
Figure 2.
Addition of chemical compounds promotes RNA‐based reprogramming. (a) Increases in cell numbers following the addition of P53DD. Numbers of cells after successive 8‐day transfection are shown. The arrow and line indicate the initial cell number (8 × 104). (b) Increases in the number of iPS‐like colonies following the addition of chemical compounds. Values above the bars indicate the number of AP‐positive iPS‐like colonies. (c) qPCR analysis of ES cell marker genes in iPS cells generated using chemical compounds. The results of two iPS cell lines are shown for each set of chemical compounds. The number of passages of iPS cells examined is shown in parentheses. Error bars represent SE (N = 3). (d) Normal chromosome numbers of iPS cells generated using chemical compounds. The number of passages of iPS cells examined is shown in parentheses. A representative karyogram of the iPS cells is shown
To examine the effect of chemical compounds on reprogramming efficiency, P53DD mRNA and other RNAs were transfected into I2965F adult liver‐derived cells together with either in the absence or in the presence of one of the three sets of chemicals. Chemicals were added from the start of transfection (day 1) until four days after the passaging (day 13). Addition of chemicals increased the numbers of alkaline phosphatase‐positive iPS‐like colonies 76.2 (Boost supplement), 68.2 (Thiazovivin) and 2.6 (3i)‐fold (Figure 2b). In addition, colonies became discernible earlier by one or two days in the presence of chemicals. The iPS‐like cells induced in the presence of chemicals express ES cell marker genes (NANOG, LIN28A, SALL4, OCT4, SOX2, UTF1, DPPA3, GDF3, DPPA4) at levels similar to those induced without chemicals (Figure 2c). Furthermore, EBs produced from the iPS‐like cells showed the expression of multiple differentiation markers, with accompanying decreases in ES cell markers (Figure S2). In addition, they were stably maintained in undifferentiated state (Table S2). The results indicate that they are indeed iPS cells.
Upon DNA damage, P53 causes arrest of cell cycle to promote DNA repair (Hafner et al., 2019). Although our method only involves transient expression of P53DD, it raises the concern that iPS cells induced using P53DD would frequently display chromosomal abnormality and mutations (Menendez, Camus, & Izpisua Belmonte, 2010; Rasmussen et al., 2014). We therefore examined karyotypes of iPS cells induced in the presence of chemicals and the dominant negative form of P53. For each set of the three chemical sets, two iPS cell lines were examined. All of the six lines examined exhibited normal karyotype (Figure 2d). Thus, we did not find any differences between iPS cells induced in the absence of chemicals and in the presence of chemicals, although the possibility of increased occurrence of DNA mutations in iPS cells induced using P53DD was not examined in this study.
2.4. Induction of iPS cells from various kinds of marmoset cells
All of the iPS cells established above are derived from I2965F adult liver cells. To examine whether iPS cells could be induced from other cell lines using RNA transfection in the presence of chemical compounds and P53DD, six cell lines were tested: three adult (five, six and eight years old) ear (auricle)‐derived, newborn skin‐derived, newborn liver‐derived and fetus skin‐derived cell lines. Each cell was tested under five conditions: (1) P53DD‐, Chemical‐, (2) P53DD+, Chemical‐, (3) P53DD+, Thiazovivin set, (4) P53DD+, Boost supplement and (5) P53DD+, 3i.
When both P53DD and chemicals were absent, none of the iPS‐like colony was formed in three of the six cell lines tested, and only one was formed in two cell lines (Table 1). However, using the same condition, a remarkably large number of iPS‐like colonies was observed in the remaining cell line (fetus skin cell). Remarkably, in the presence of P53DD and any of the three chemical sets, iPS‐like colonies were generated from all of the six cells. Although Thiazovivin set (22–38410 colonies) and Boost supplement (15–11539 colonies) were always more effective than 3i (1–7908 colonies), the effects of the two chemicals varied among cells. For two cell lines (fetus and newborn skin cells), Boost supplement condition produced a higher number of iPS‐like colonies than Thiazovivin set condition. The opposite trend was observed for the other four cell lines (all of the three adult ear‐derived cells and newborn liver‐derived cells). Thus, the optimal chemical condition is different among cells.
Table 1.
Induction of iPS cells from six marmoset cell lines
| Cell line | No. of iPS‐like cells | ||||
|---|---|---|---|---|---|
| No chemical (−P53DD) | No chemical (+P53DD) | Boost supplement (+P53DD) | Thiazovivin (+P53DD) | 3i (+P53DD) | |
| 1. Fetus skin cell | 2,600 | 19,447 | 38,410 | 11,539 | 7,908 |
| 2. Adult ear cell #1 (5‐year) | 1 | 1 | 206 | 320 | 1 |
| 3. Adult ear cell #2 (8‐year) | 0 | 0 | 22 | 44 | 4 |
| 4. Adult ear cell #3 (6‐year) | 0 | 11 | 1600 | 3,400 | 72 |
| 5. Newborn skin cell | 0 | 4 | 800 | 15 | 3 |
| 6. Newborn liver cell | 1 | 0 | 83 | 1,400 | 78 |
To establish iPS cells, we picked up colonies that were formed in the representative condition(s) of each line. iPS cells expressing ES cell markers were successfully established from all of the six lines (Figure S3 and Figure S4). Remarkably, only a subset of the picked colonies retained their original undifferentiated state after passaging four times (Table S3), and, interestingly, proportion of iPS‐like cells that kept undifferentiated state varied among cell types (Table S3), suggesting that the original cell state is important for full reprogramming. Furthermore, both of the two iPS cells once established from eight‐year‐old ear‐derived cells #2 displayed a tendency to differentiate at later passages (Figure S3), consistent with high rate of differentiation of iPS cells from the same cells at earlier passages (Table S3). In contrast, all the iPS cells from the other five cell lines maintained an undifferentiated state (Figure S3), if they were undifferentiated when passaging for P4 culture. Thus, complete reprogramming may be a challenge in some difficult‐to‐reprogram cells.
2.5. Efficient induction of iPS‐like cells from human and cynomolgus monkey fibroblasts
As reprogramming factors used for induction of iPS cells in the present study were derived from humans, other primate iPS‐like cells could be induced using the same set of RNAs and chemical compounds. We first tested human fibroblasts. RNA transfection was carried out under the following three conditions: (1) P53DD‐, Chemical‐, (2) P53DD+, Thiazovivin set and (3) P53DD+, Boost supplement (Table 2). After eight‐day successive transfection (Days 1–8), cells were passaged onto MEF feeder cells (Day 9). Although AP‐positive iPS‐like cell colonies expressing ES cell markers were induced from the human fibroblast even in the absence of chemicals (40 colonies on average), the number of iPS cell colonies formed dramatically increased by adding chemical compounds (Table 2). Boost Supplement (5,160 colonies on average) was more effective than the Thiazovivin set (103 colonies on average) in the induction of iPS‐like cells from human fibroblast. These iPS‐like cell colonies were formed by Day 16 as was the case in marmoset cells (Figure 3a,b). Next, two cynomolgus monkey fibroblast lines were tested using the same conditions. No iPS‐like cell colonies were formed when chemicals were not added (Table 3). Although no colony was formed from one of the two lines even by adding chemical compounds, iPS‐like cell colonies were formed by adding either Boost supplement (189 colonies) or Thiazovivin set (28 colonies) from the other line (Figure 3a,b and Table 3).
Table 2.
Induction of iPS‐like cells from human cells
| Cell line | No. of iPS‐like cells | ||
|---|---|---|---|
| No chemical (−P53DD) | Boost supplement (+P53DD) | Thiazovivin (+P53DD) | |
| Human adult fibroblast rep1 | 66 | 3,806 | 21 |
| Human adult fibroblast rep2 | 14 | 6,514 | 184 |
Figure 3.
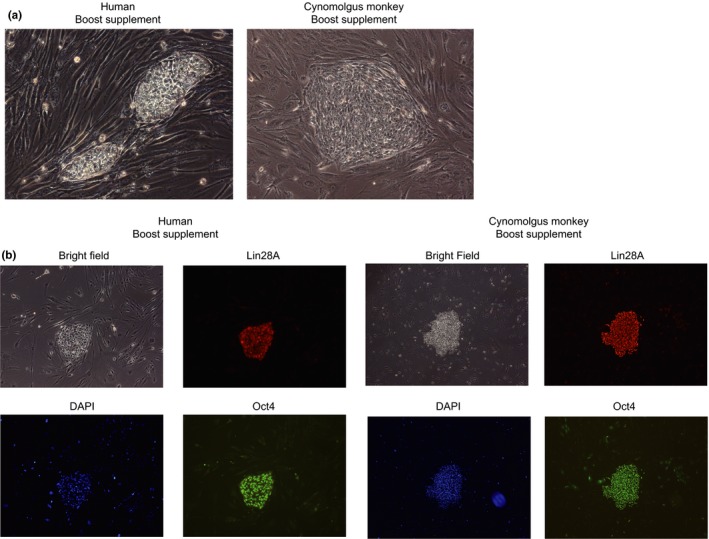
Induction of iPS‐like cells from human and cynomolgus monkey cells. (a) Human and cynomolgus monkey iPS‐like cell colonies observed after the induction. (b) Immunofluorescence analysis of human and cynomolgus iPS‐like cells observed after the induction. The cells were established in the presence of the Boost supplement
Table 3.
Induction of iPS‐like cells from cynomolgus monkey cells
| Cell line | No. of iPS‐like cells | ||
|---|---|---|---|
| No chemical (‐P53DD) | Boost supplement (+P53DD) | Thiazovivin (+P53DD) | |
| Cynomolgus monkey fetus fibroblast #1 | 0 | 189 | 28 |
| Cynomolgus monkey fetus fibroblast #2 | 0 | 0 | 0 |
3. DISCUSSION
The present study demonstrates that marmoset iPS cells can be induced by RNA transfection of reprogramming factors and interferon suppressors. To the best of our knowledge, this is the first report of the generation of marmoset iPS cells using RNA‐based methods. As iPS cells can only be induced from limited cell line using conventional RNA transfection methods, we combined the RNA transfection method with a chemical compound method. This remarkably increases the number of iPS colonies appeared, which makes it possible to generate iPS cells even from difficult‐to‐reprogrammed cells. As this combination has not been reported in any other species, we examined whether this combination is similarly effective in other primate species. As expected, addition of chemical compounds greatly enhanced RNA transfection‐mediated reprogramming in humans and cynomolgus monkeys. Thus, this novel combination is useful for efficient generation of integration‐free safe iPS cells in primates, particularly from cells that are difficult to be reprogrammed.
Dramatic increases in the number of iPS colonies were observed following the addition of chemicals. Among the three chemicals tested, the Thiazovivin set and the Boost supplement were more effective than 3i in all the cells tested. However, the effect of the Thiazovivin set and the Boost supplement varied among cells. We speculate that molecular pathways that need to be modulated for promotion of iPS reprogramming would vary among cell types. We therefore suggest the testing of both the Thiazovivin set and the Boost supplement when inducing iPS cells using the method in this study.
The previous studies used marmosets for the preclinical study of iPS cell‐based treatment for spinal cord injury (Iwai et al., 2015; Kobayashi et al., 2012). The studies demonstrate that the treatment can regenerate neurons in injured spinal cords of marmosets and increase their mobility. Based on the results, transplantation of iPS cell‐derived cells into patients with spinal cord injury is currently planned. However, in the studies, human iPS cell‐ and marmoset ES cell‐derived neuronal stem cells were transplanted by administering immunosuppressive agent, instead of using marmoset iPS cells, which would make more precise evaluation of iPS cell‐based therapy possible. By using the methods developed in this study, marmoset iPS cell can be now efficiently generated from various types of cells. This will promote preclinical studies involving iPS cells in marmosets.
In human, DPPA3 and GDF3 are known to be naïve ES cell marker genes, and they are expressed at low levels in conventional human ES cells (Messmer et al., 2019). Unexpectedly, we observed seemingly moderate levels of DPPA3 and GDF3 gene expression in marmoset ES and iPS cells (Figure 1b and Table S1). Possible underlying bases for the expression of these genes in marmoset ES cells are as follows: (1) species‐specific difference in gene expression and (2) difference in the corresponding developmental stages between marmoset and human ES cells. Further analyses are needed to clarify this issue.
Inhibiting P53 functions is known to promote iPS reprogramming, and P53 inhibitors (siRNAs, chemical compounds and dominant negative forms) are used to increase the reprogramming efficiency (Hong et al., 2009; Kawamura et al., 2009; Marion et al., 2009; Menendez et al., 2010; Rasmussen et al., 2014; Utikal et al., 2009). However, in this study, P53DD mRNA was added for a different purpose: inhibiting cell death caused by stress of chemical compounds during transfection. Thus, the contribution of P53DD to iPS reprogramming in this study is two‐fold: (1) inhibition of cell death and (2) promotion of reprogramming. As P53 is involved in cell cycle arrest and apoptosis when DNA damage occurs (Hafner et al., 2019), cells with chromosome abnormalities or mutations could be reprogrammed to iPS cells without cell death during P53DD expression. There is, therefore, a concern that iPS cells induced using P53 inhibitors have increased chance of gaining mutations (Menendez et al., 2010; Rasmussen et al., 2014). Since sensitivity to chemical stress likely varies among cell types, requirement for P53 inhibitors may be also varying on cell types. In fact, cell death did not become problematic even in the absence of P53DD when inducing iPS cells from a certain cell line using a combination of RNA transfection and chemical compounds (data not shown). Although we did not observe any abnormalities in karyotypes of iPS cells that were generated using P53DD, we cannot rule out the possibility that iPS cells generated using P53DD have increased levels of mutations, and P53DD, therefore, should be omitted when it is not required.
4. EXPERIMENTAL PROCEDURES
4.1. Animals
Animal experiments were carried out after obtaining permission from the Institutional Animal Care and Use Committee at the Central Institution for Experimental Animals (CIEA: 16019A, 17029A and 18031A). Animal tissues were obtained from animals being killed by euthanasia for other experimental purposes. Fetus tissues were acquired from aborted fetuses.
4.2. Cells established from livers, skins and ears
Cultured cells were established by either enzymatic digestion or attaching small pieces of tissues on a culture plate. For enzymatic digestion, tissues were minced, and they were treated with collagenase solution (1 mg/ml Collagenase1 in DMEM) for 30 min at 37°C with shaking. An equal volume of MEF medium (10% FCS/ 1 × Anti‐Anti in DMEM) was added to stop the reaction. After adding 1/1000 volume of 5 mg/ml DNase1 solution, the digested tissue was filtered through 100 μm mesh. Undigested tissue on the mesh was squashed using a syringe gasket. The filtrate was centrifuged to collect cells. The collected cells were cultured in MEF medium.
To attach tissues onto culture plates, tissues cut into small pieces were put onto a culture plate. They were left with the lid open until the surfaces of the tissues dried (~15 min). The pieces were then covered with MEF medium.
Human fibroblasts derived from 75‐year‐old female were purchased from Lonza. Cynomolgus fibroblast cells were established previously from aborted fetuses (Seita et al., 2016).
4.3. RNAs used for transfection
The dominant negative form of P53 (P53DD) mRNA was prepared by in vitro transcription. Multiple cloning site (Not1‐Asc1‐Sal1‐HindIII‐EcoRI) generated by annealing two oligo DNAs was ligated to EcoRI and NotI‐digested pCR4 Blunt‐TOPO‐vector to generate MCS plasmid. To tandemly insert two copies of human β‐globin 3′ UTR, two human β‐globin 3′ UTR DNA fragments were digested using Asc1/Sal1 and Sal1/HindIII. They were then ligated jointly with Asc1 and HindIII‐digested MCS plasmid to generate MCS‐3′ UTR plasmid. The dominant negative form of P53 was amplified from pMXs‐p53DD (Addgene #22729) (Hong et al., 2009). The PCR product was PCR‐amplified again to attach the 5′ UTR of human HBA gene, and the amplified product was ligated to Not1 and Asc1‐digested MCS‐3′ UTR plasmid to generate 5′ UTR‐P53DD‐3′ UTR plasmid. The plasmid was digested with HindIII for in vitro transcription using mMESSAGE mMACHINE T7 Ultra Kit (Ambion). Oligo DNAs and primers used are listed in Table S4.
StemRNA‐NM reprogramming kit (Reprocell) was purchased to obtain other RNAs: human OCT4, SOX2, KLF4, c‐MYC, NANOG, LIN28A (reprogramming factors), Vaccinia virus E3, K3, B18R (interferon suppressors), human miRNA302a, miRNA302b, miRNA302c, miRNA302d and miRNA367 (miRNAs).
4.4. Preparation of conditioned medium
To prepare a conditioned medium, 2 × 106 MEF was plated onto a gelatin‐coated 10‐cm dish using MEF medium. On the following day, the medium was changed to a Primate ES cell medium (ESM, Reprocell) containing 4 ng/ml FGF2 and 1 × Anti‐Anti. On the following day, the MEF‐conditioned medium was collected, and a new Primate ESM was added. The conditioned medium was filtered through a 0.22 μm filter, and FGF2 was again added at a concentration of 4 ng/μl (total concentration is 8 ng/μl). FGF2 was not added to the Primate ESM and conditioned medium when transfection was carried out in the presence of 3i or Thiazovivin set, as they include chemicals (PD173074 and SB431542) that inhibit FGF2 function.
4.5. Induction of iPS cells
The day before transfection (Day 0), a 12‐well plate was coated with iMatrix‐511 silk (Nippi) for at least 1 hr at 37°C. After coating, 8 × 104 cells were plated onto a one well of the 12‐well plate using MEF medium. On the following day (Day 1), the medium was changed to a 0.5 ml conditioned medium. Chemicals were added to the conditioned medium when required. To prepare an RNA–lipid complex, 1.5 μl of RNAiMax (Invitrogen) was added to 62.5 μl of OPTI‐MEM (Invitrogen). In another tube, RNAs (OSKMNL 0.2 μg, EKB 0.075 μg, miRNA 0.1 μg, P53 mRNA 0.05 μg) were added to 62.5 μl of OPTI‐MEM. The solutions in the two tubes were combined and mixed. After incubation for 15 min, the mixed solution was gently dropped onto the medium in the 12‐well plate. The following morning, medium was changed to new conditioned medium, and cells were cultured for 6–8 hr without adding RNA–lipid complex. RNA transfection was daily carried out eight times.
The following day after the last transfection (Day 9), the medium was changed to a new conditioned medium. After culturing for 3–4 hr, cells were dissociated using Accutase and plated onto 10 cm MEF plates (2 × 106 MEF per plate). When a total cell number exceeded 2 × 105, cells were plated onto two or more plates. Cells were cultured in marmoset ESM containing 20% Knockout Serum Replacement (KSR), 100 μM MEM Non‐Essential Amino Acids, 1 × Anti‐Anti, 1 mM L‐Glutamine, 0.2 mM 2‐mercaptoethanol and 4 ng/ml bFGF2. The medium was changed to a new marmoset ESM every day. When chemicals were used for induction, chemicals were added to the medium from Day 1 to Day 13 at the following concentrations: (1) Thiazovivin set, thiazovivin (0.5 μM), SB431542 (2 μM) and PD0325901 (0.5 μM), (2) Human iPS reprogramming Boost Supplement II (Boost supplement) was added at the concentration suggested in manufacture's instruction and (3) 3i containing PD0325901 (0.5 μM), CHIR99021 (3 μM) and PD173074 (0.1 μM). During the entire process of reprogramming, cells were cultured under hypoxic condition (5% O2).
iPS colonies became visible on Days 14–16, and they were picked up on Days 17–20. Picked iPS colonies were then dissociated using 25 μl of Dissociation Buffer (0.25% trypsin/1mM CaCl2/20% KSR/1 mg/ml collagenase IV) for five min at 37°C. The reaction was stopped by adding 200 μl of stop solution (20% KSR in KDMEM) and centrifuged to collect cells. The cells were plated onto 48‐well plates for feeder‐free culture using MEF‐conditioned medium.
4.6. Culture of iPS and ES cells
For feeder‐free culture using MEF‐conditioned medium culture, the conditioned medium was changed every day. For passaging, after dissociation using Accutase (Nacalai), cells were plated at a density of 8 × 104 cells per one well of 6‐well plates using a conditioned medium containing 1.25 ng/μl iMatrix (Nippi) and 10 μM Y27632.
4.7. Embryoid body formation
EB was formed using a protocol as described in the Takara website (http://takara.co.kr/file/manual/pdf/Spin%20Embryoid%20Body%20Formation%20and%20Confirmation%20of%20Pluripotency%20protocol_FL.pdf). Briefly, cells were plated onto an uncoated 96 well plate, and the plate was centrifuged to form cell aggregates. After a seven‐day culture, the formed spin EBs were collected and transferred to a new coated plate (five spin EBs per one well of 24‐well plate). The transferred spin EBs were attached to the plate and then cultured for an additional 11–14 days before analyses.
4.8. RT‐qPCR analysis
RNA was purified from ~5 × 104 cells using NucleoSpin RNA kit (MACHEREY‐NAGEL). cDNA was generated from approximately 200 ng of RNA using SuperScript IV (Thermo Fisher). Oligo dT primer was used for reverse transcription. For qPCR analysis, Power Up SYBR Green Master Mix (ABI) was used. Primers used are listed in Table S4.
4.9. AP staining and immunostaining
AP staining was carried out using the AP Staining Kit (Stemgent) according to the manufacturer's protocol. When many AP‐positive colonies were present on a dish, colonies in a representative area of the dish (e.g., 2 × 2 cm2) were counted to estimate the total number.
For immunofluorescence analyses, culture dishes were washed once with PBS, and cells were fixed using 4% PFA/PBS for 10 min at room temperature. After washing with PBS, blocking was carried out using a blocking solution (1% BSA/5% FBS/0.1% TritonX‐100 in PBS) for 45 min. at room temperature. The blocking solution was removed, and primary antibodies diluted using blocking solution were added. The antibodies used are LIN28A (Cell Signaling #3978, 1:200), OCT4 (Santa Cruz sc‐5279, 1:400) and NANOG (Cell Signaling #4893, 1:400).
4.10. Teratoma formation
Approximately 10 μl of iPS cell suspension (1 × 107 cells/ml) was injected under the kidney capsule of immunodeficient NOD/Shi‐scid, IL‐2Rγ null (NOG) mice. Teratoma was harvested at 7–8 weeks after transplantation, fixed in 10% neutral buffered formalin and embedded in paraffin. Immunostaining was carried out using a Leica Bond‐Max automatic immunostainer (Leica Biosystems, Mount Waverley, VIC, Australia). The antibodies we used for immunostaining were VIMENTIN (Nichirei #422101), DESMIN (Nichirei #422011) and NCAM (Leica NCL‐CD56‐1B6).
4.11. Karyotype analysis
iPS cells were incubated with medium containing 0.1μg/mL colcemid for 3 hr. The cells were then washed in PBS, dissociated using trypsin–EDTA solution and spun down. The pellet was resuspended in hypotonic solution (0.56% KCl). After 20 min incubation at room temperature, they were fixed with fixed solution containing 75% methanol and 25% acetic acid (vol/vol). The fixed cells were spread on slides and air‐dried overnight. The slides were stained with 0.5μg/mL Hoechst 33,258 and 50μg/mL quinacrine mustard for 10 min. At least five metaphase spreads were analyzed for each cell line.
Supporting information
ACKNOWLEDGMENTS
We thank Yuko Yamada and Chia‐Ying Lee for the establishment of marmoset fibroblast cells, Takashi Inoue, Takayuki Mineshige and Terumi Yurimoto for the preparation of marmoset tissues, Andrew Chen for the comment on the manuscript, Masafumi Yamamoto for genotyping of the established iPS cells and Keiko Kishimoto for primer sequences. This research was partially supported by the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from the Japan Agency for Medical Research and development (AMED), Strategic Research Program for Brain Science, “Maintenance of Systems for Creation and Spread of Primate Model Animals” from AMED and Grants‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT/JSPS KAKENHI Grant Number 15H02360).
Watanabe T, Yamazaki S, Yoneda N, et al. Highly efficient induction of primate iPS cells by combining RNA transfection and chemical compounds. Genes Cells. 2019;24:473–484. 10.1111/gtc.12702
Communicated by: Fumio Matsuzaki
REFERENCES
- Anokye‐Danso, F. , Trivedi, C. M. , Juhr, D. , Gupta, M. , Cui, Z. , Tian, Y. , … Morrisey, E. E. (2011). Highly efficient miRNA‐mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell, 8, 376–388. 10.1016/j.stem.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, T. , Symonds, H. , Gu, L. , Yin, C. , Oren, M. , & Van Dyke, T. (1996). Tissue‐specific inactivation of p53 tumor suppression in the mouse. Genes & Development, 10, 826–835. 10.1101/gad.10.7.826 [DOI] [PubMed] [Google Scholar]
- Debowski, K. , Warthemann, R. , Lentes, J. , Salinas‐Riester, G. , Dressel, R. , Langenstroth, D. , … Behr, R. (2015). Non‐viral generation of marmoset monkey iPS cells by a six‐factor‐in‐one‐vector approach. PLoS ONE, 10, e0118424 10.1371/journal.pone.0118424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, F. , Boue, S. , & Izpisua Belmonte, J. C. (2011). Methods for making induced pluripotent stem cells: Reprogramming a la carte. Nature Reviews Genetics, 12, 231–242. [DOI] [PubMed] [Google Scholar]
- Hafner, A. , Bulyk, M. L. , Jambhekar, A. , & Lahav, G. (2019). The multiple mechanisms that regulate p53 activity and cell fate. Nature Reviews Molecular Cell Biology, 20, 199–210. 10.1038/s41580-019-0110-x [DOI] [PubMed] [Google Scholar]
- Hong, H. , Takahashi, K. , Ichisaka, T. , Aoi, T. , Kanagawa, O. , Nakagawa, M. , … Yamanaka, S. (2009). Suppression of induced pluripotent stem cell generation by the p53–p21 pathway. Nature, 460, 1132–1135. 10.1038/nature08235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida, J. K. , Blanchard, J. , Lam, K. , Son, E. Y. , Chung, J. E. , Egli, D. , … Eggan, K. (2009). A small‐molecule inhibitor of tgf‐Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell, 5, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai, H. , Shimada, H. , Nishimura, S. , Kobayashi, Y. , Itakura, G. , Hori, K. , … Okano, H. (2015). Allogeneic Neural Stem/Progenitor Cells Derived From Embryonic Stem Cells Promote Functional Recovery After Transplantation Into Injured Spinal Cord of Nonhuman Primates. Stem Cells Translational Medicine, 4, 708–719. 10.5966/sctm.2014-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura, T. , Suzuki, J. , Wang, Y. V. , Menendez, S. , Morera, L. B. , Raya, A. , … Izpisua Belmonte, J. C. (2009). Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature, 460, 1140–1144. 10.1038/nature08311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, Y. , Okada, Y. , Itakura, G. , Iwai, H. , Nishimura, S. , Yasuda, A. , … Okano, H. (2012). Pre‐evaluated safe human iPSC‐derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS ONE, 7, e52787 10.1371/journal.pone.0052787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Wei, W. , Zhu, S. , Zhu, J. , Shi, Y. , Lin, T. , … Ding, S. (2009). Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell, 4, 16–19. 10.1016/j.stem.2008.11.014 [DOI] [PubMed] [Google Scholar]
- Lin, T. , Ambasudhan, R. , Yuan, X. , Li, W. , Hilcove, S. , Abujarour, R. , … Ding, S. (2009). A chemical platform for improved induction of human iPSCs. Nature Methods, 6, 805–808. 10.1038/nmeth.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai, M. , Watanabe, A. , Kurimoto, Y. , Hirami, Y. , Morinaga, C. , Daimon, T. , … Takahashi, M. (2017). Autologous Induced Stem‐Cell‐Derived Retinal Cells for Macular Degeneration. New England Journal of Medicine, 376, 1038–1046. 10.1056/NEJMoa1608368 [DOI] [PubMed] [Google Scholar]
- Mandal, P. K. , & Rossi, D. J. (2013). Reprogramming human fibroblasts to pluripotency using modified mRNA. Nature Protocols, 8, 568–582. 10.1038/nprot.2013.019 [DOI] [PubMed] [Google Scholar]
- Marion, R. M. , Strati, K. , Li, H. , Murga, M. , Blanco, R. , Ortega, S. , … Blasco, M. A. (2009). A p53‐mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature, 460, 1149–1153. 10.1038/nature08287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez, S. , Camus, S. , & Izpisua Belmonte, J. C. (2010). p53: Guardian of reprogramming. Cell Cycle, 9, 3887–3891. 10.4161/cc.9.19.13301 [DOI] [PubMed] [Google Scholar]
- Messmer, T. , von Meyenn, F. , Savino, A. , Santos, F. , Mohammed, H. , Lun, A. T. L. , … Reik, W. (2019). Transcriptional Heterogeneity in Naive and Primed Human Pluripotent Stem Cells at Single‐Cell Resolution. Cell Reports, 26(815–824), e814 10.1016/j.celrep.2018.12.099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallagatla, S. R. , Toroney, R. , & Bevilacqua, P. C. (2011). Regulation of innate immunity through RNA structure and the protein kinase PKR. Current Opinion in Structural Biology, 21, 119–127. 10.1016/j.sbi.2010.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano, H. , Hikishima, K. , Iriki, A. , & Sasaki, E. (2012). The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Seminars in Fetal and Neonatal Medicine, 17, 336–340. 10.1016/j.siny.2012.07.002 [DOI] [PubMed] [Google Scholar]
- Park, I. H. , Zhao, R. , West, J. A. , Yabuuchi, A. , Huo, H. , Ince, T. A. , … Daley, G. Q. (2008). Reprogramming of human somatic cells to pluripotency with defined factors. Nature, 451, 141–146. 10.1038/nature06534 [DOI] [PubMed] [Google Scholar]
- Poleganov, M. A. , Eminli, S. , Beissert, T. , Herz, S. , Moon, J. I. , Goldmann, J. , … Sahin, U. (2015). Efficient Reprogramming of Human Fibroblasts and Blood‐Derived Endothelial Progenitor Cells Using Nonmodified RNA for Reprogramming and Immune Evasion. Human Gene Therapy, 26, 751–766. 10.1089/hum.2015.045 [DOI] [PubMed] [Google Scholar]
- Rasmussen, M. A. , Holst, B. , Tumer, Z. , Johnsen, M. G. , Zhou, S. , Stummann, T. C. , … Clausen, C. (2014). Transient p53 suppression increases reprogramming of human fibroblasts without affecting apoptosis and DNA damage. Stem Cell Reports, 3, 404–413. 10.1016/j.stemcr.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani, L. , Fabian, C. , Holland, H. , Naaldijk, Y. , Dressel, R. , Loffler‐Wirth, H. , … Stolzing, A. (2016). Generation of human induced pluripotent stem cells using non‐synthetic mRNA. Stem Cell Res, 16, 662–672. 10.1016/j.scr.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Sadler, A. J. , & Williams, B. R. (2008). Interferon‐inducible antiviral effectors. Nature Reviews Immunology, 8, 559–568. 10.1038/nri2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, E. , Hanazawa, K. , Kurita, R. , et al. (2005). Establishment of novel embryonic stem cell lines derived from the common marmoset (Callithrix jacchus). Stem Cells, 23, 1304–1313. [DOI] [PubMed] [Google Scholar]
- Schlaeger, T. M. , Daheron, L. , Brickler, T. R. , Entwisle, S. , Chan, K. , Cianci, A. , … Daley, G. Q. (2015). A comparison of non‐integrating reprogramming methods. Nature Biotechnology, 33, 58–63. 10.1038/nbt.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seita, Y. , Tsukiyama, T. , Iwatani, C. , Tsuchiya, H. , Matsushita, J. , Azami, T. , … Ema, M. (2016). Generation of transgenic cynomolgus monkeys that express green fluorescent protein throughout the whole body. Scientific Reports, 6, 24868 10.1038/srep24868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, J. , Barrandon, O. , Nichols, J. , Kawaguchi, J. , Theunissen, T. W. , & Smith, A. (2008). Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biology, 6, e253 10.1371/journal.pbio.0060253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K. , Tanabe, K. , Ohnuki, M. , Narita, M. , Ichisaka, T. , Tomoda, K. , & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131, 861–872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Takahashi, K. , & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Tomioka, I. , Maeda, T. , Shimada, H. , Kawai, K. , Okada, Y. , Igarashi, H. , … Okano, H. (2010). Generating induced pluripotent stem cells from common marmoset (Callithrix jacchus) fetal liver cells using defined factors, including Lin28. Genes to Cells, 15, 959–969. 10.1111/j.1365-2443.2010.01437.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utikal, J. , Polo, J. M. , Stadtfeld, M. , Maherali, N. , Kulalert, W. , Walsh, R. M. , … Hochedlinger, K. (2009). Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature, 460, 1145–1148. 10.1038/nature08285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, L. , Manos, P. D. , Ahfeldt, T. , Loh, Y.‐H. , Li, H. U. , Lau, F. , … Rossi, D. J. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell, 7, 618–630. 10.1016/j.stem.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann, A. , Hemmer, K. , Bernemann, I. , Gohring, G. , Pogozhykh, O. , Figueiredo, C. , … Muller, T. (2012). Induced pluripotent stem cells generated from adult bone marrow‐derived cells of the nonhuman primate (Callithrix jacchus) using a novel quad‐cistronic and excisable lentiviral vector. Cell Reprogram, 14, 485–496. [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Zhang, Y. , Mishra, A. , Tardif, S. D. , & Hornsby, P. J. (2010). Generation of induced pluripotent stem cells from newborn marmoset skin fibroblasts. Stem Cell Research, 4, 180–188. 10.1016/j.scr.2010.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, G. , Hong, H. , Torres, A. , Malloy, K. E. , Choudhury, G. R. , Kim, J. , & Daadi, M. M. (2018). Standards for deriving nonhuman primate‐induced pluripotent stem cells, neural stem cells and dopaminergic lineage. International Journal of Molecular Sciences, 19(9), 2788. 10.3390/ijms19092788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka, N. , Gros, E. , Li, H. R. , Kumar, S. , Deacon, D. C. , Maron, C. , … Dowdy, S. F. (2013). Efficient generation of human iPSCs by a synthetic self‐replicative RNA. Cell Stem Cell, 13, 246–254. 10.1016/j.stem.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Vodyanik, M. A. , Smuga‐Otto, K. , Antosiewicz‐Bourget, J. , Frane, J. L. , Tian, S. , … Thomson, J. A. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318, 1917–1920. 10.1126/science.1151526 [DOI] [PubMed] [Google Scholar]
- Zhu, S. , Li, W. , Zhou, H. , Wei, W. , Ambasudhan, R. , Lin, T. , … Ding, S. (2010). Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell, 7, 651–655. 10.1016/j.stem.2010.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


