Abstract
Migratory birds rely on a habitat network along their migration routes by temporarily occupying stopover sites between breeding and non‐breeding grounds. Removal or degradation of stopover sites in a network might impede movement and thereby reduce migration success and survival. The extent to which the breakdown of migration networks, due to changes in land use, impacts the population sizes of migratory birds is poorly understood. We measured the functional connectivity of migration networks of waterfowl species that migrate over the East Asian‐Australasian Flyway from 1992 to 2015. We analysed the relationship between changes in non‐breeding population sizes and changes in functional connectivity, while taking into account other commonly considered species traits, using a phylogenetic linear mixed model. We found that population sizes significantly declined with a reduction in the functional connectivity of migration networks; no other variables were important. We conclude that the current decrease in functional connectivity, due to habitat loss and degradation in migration networks, can negatively and crucially impact population sizes of migratory birds. Our findings provide new insights into the underlying mechanisms that affect population trends of migratory birds under environmental changes. Establishment of international agreements leading to the creation of systematic conservation networks associated with migratory species’ distributions and stopover sites may safeguard migratory bird populations.
Keywords: bird migration, habitat loss, life history, network robustness, population dynamics, species traits, wetland
Introduction
Recent trends in habitat loss and degradation strongly impact the survival and reproduction of wildlife species (Cushman 2006, Studds et al. 2017, Xu et al. 2017). Habitat loss and degradation reduce functional connectivity, i.e., the degree to which landscape elements promote animal movements within and between habitat patches (Taylor et al. 1993, Bélisle 2005, Saura and Rubio 2010). Functional connectivity links landscape features with species’ dispersal traits and is critical for understanding how the spatial distribution of suitable landscapes may influence populations of migratory species. The functional connectivity of migration networks is defined here as the degree to which the habitat configuration facilitates bird movements both within and between the breeding, non‐breeding, and stopover sites.
Many migratory birds rely on a network of habitat patches as they travel between breeding and non‐breeding areas. For example, many birds take advantage of multiple stopover sites during their migration for resting or refueling before migrating further and breeding (Arzel et al. 2006, Newton 2010, Si et al. 2018). Hence, connectivity among sites along migration flyways is essential for their survival and reproduction (Merken et al. 2015) and thereby can play a vital role in the population dynamics of these species. A stable network of stopover sites is an important component for maintaining stable or increasing populations of migratory species (Leito et al. 2015). In contrast, loss of habitat and loss of network connectivity may negatively impact migratory bird populations (Iwamura et al. 2013).
The degree to which losses in the functional connectivity of a migration network, as a consequence of habitat loss and degradation, contribute to population declines in migratory birds remains unknown. So far, the relationships between functional connectivity of migration networks and population trends have not been investigated empirically (Gilroy et al. 2016, Barshep et al. 2017, Studds et al. 2017). One reason for this is the challenge of quantifying the connectivity of habitat networks at a flyway scale over extended periods of time, though such quantification at these relatively large spatial and temporal scales is essential when the goal is to assess links between functional connectivity and population dynamics of migratory species.
We hypothesize that functional connectivity, as well as other previously studied predictors, together drive population trends of migratory birds, so that population sizes of migratory birds decrease with a decreasing functional connectivity of their migration networks. Species, such as those with longer migration distances (Morrison et al. 2013), with a smaller size of their non‐breeding ranges relative to breeding ranges (migratory dispersion; Gilroy et al. 2016), and with smaller breeding ranges (Murray et al. 2014), are more likely to experience population declines. Others, such as species with a relatively small body mass (de Boer et al. 2011), large clutch size (Jiguet et al. 2007), and short generation length (Murray et al. 2014), are less likely to decline.
To test this hypothesis, we measured the functional connectivity of migration networks of eight waterfowl species that winter in the Yangtze River Basin and migrate over the East Asian‐Australasian Flyway. We tested whether a decrease in functional connectivity was correlated with a decrease in non‐breeding population sizes of migratory waterfowl species from 2001 to 2014. Additionally, we included six common predictors for population dynamics: breeding range size, migratory dispersion, migration distance, body mass, generation length, and clutch size. The results can provide new insights into the underlying mechanisms that affect population trends of migratory species, and point out efficient conservation strategies for safeguarding the sustainability of migratory birds under observed land use changes.
Methods
Estimate bird population sizes
We selected eight waterfowl species that have a majority of their East Asian‐Australasian flyway population wintering in the Yangtze River Basin, China (Cao et al. 2010): Tundra Swan (Cygnus columbianus), Swan Goose (Anser cygnoid), Bean Goose (Anser fabalis), Greater White‐fronted Goose (Anser albifrons), Lesser White‐fronted Goose (Anser erythropus), Greylag Goose (Anser anser), Common Teal (Anas crecca), and Northern Pintail (Anas acuta). The flyway population trends for each of these species were estimated by Anatidae non‐breeding counts between 2001 and 2014 in the Yangtze River Basin (Zhang et al. 2015). The non‐breeding population sizes of each year were estimated by the sum of birds counted in all lakes in the Yangtze River Basin, and trends in population sizes were illustrated using a locally weighted scatterplot smoothing (LOWESS) method. Lakes without counting data in certain years were interpolated by means of nearest neighbors. The eight study species in a total of 24 lakes were observed annually during the 14‐yr study period during 2001–2014; thus, we included a total of 2,688 records in the analysis.
Quantify functional connectivity of migration networks
For every year (1992–2015), we constructed migration networks of each of the study species, in which the “nodes” were the connected wetland patches in the suitable sites for each study species in the East Asian‐Australasian Flyway (Fig. 1; Xu et al. 2019). Distances between any two nodes were set at >32.5 km, which is the mean of maximum foraging flight distance of geese and ducks (Johnson et al. 2014). We defined the strength of node‐to‐node connections by the dispersal probability of the study species’ direct movement between two nodes (internode dispersal probabilities). Because a limited number of birds have been ringed or fitted with telemetry equipment, we could not rely on direct calculations of dispersal probabilities between sites. Therefore, we used an indirect method, a decreasing exponential function (Keitt et al. 1997), to quantify internode dispersal probabilities
Figure 1.
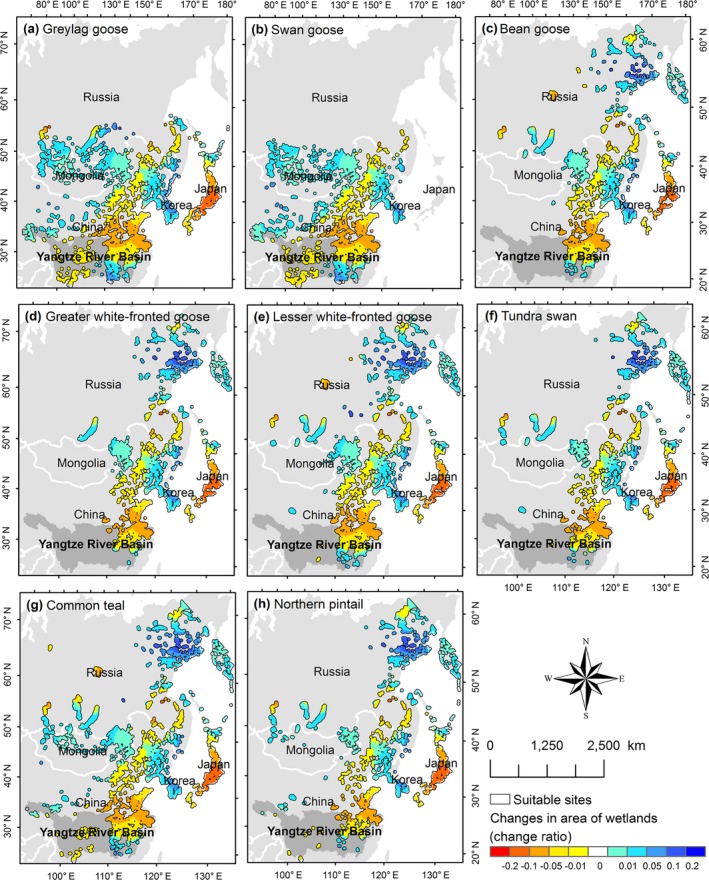
Patterns of habitat loss in the ranges of study species. The suitable sites in the East Asian‐Australasian Flyway for each study species and change ratios of the area of wetlands in the suitable sites during 1992–2012 were both analyzed in a previous study (Xu et al. 2019). Each connected wetland patch (within a distance of 32.5 km) in the suitable sites was defined as a node of the migration networks. Blue indicates the increase of wetland area and red indicates the decrease of it. The map was produced with ArcMap 10.2 (ESRI, Redlands, California, USA) under the cylindrical equal area projection.
| (1) |
P ij is the dispersal probability between node i and j, and d ij is the closest distance between habitat patch i and j, k is a constant defined so that dispersal probability is 50% when d ij equals the median of the migration lags of the study species, i.e., distances between consecutive sites used by migratory birds (Appendix S1: Table S1).
To facilitate comparison of functional connectivity of migration networks between years and species, we measured the functional connectivity of a migration network through an index of “equivalent connected area” (Saura and Pascual‐Hortal 2007, Saura et al. 2014) in square kilometers using a directed graph theory algorithm (Saura et al. 2014). Equivalent connected area is the size of a single patch providing the same level of connectivity as the calculated migration network (Eq. (2)). A larger equivalent connected area means a better‐connected migration network:
| (2) |
where a i and a j represent the area (km2) of habitat sites (i.e., nodes) i and j. P ij * is the maximum product probability, i.e., the “best” paths with one or more steps between nodes i and j. When P ij * = P ij, nodes i and j are close enough for individuals to move directly between them. When P ij * > P ij , the “best” path consists of several steps within the network and involves stepping stones in between nodes i and j. The functional connectivity values were decomposed (contribution, %) into intra, direct, and step fractions, showing the contribution (%) of within‐patch connectivity, direct connections, and use of stepping stones between source and destination patches, respectively (mathematical details are available in Saura et al. 2014). Specifically, intrapatch connectivity is the fraction corresponding to the area of reachable habitat within sites used by migratory birds. Direct connectivity is the amount of intersite connectivity if stepping stones are not used in the movements of birds among sites. Step connectivity is the increase in the amount of connectivity by having higher probabilities of movement between sites due to the use of existing stepping stones. We ran the analyses in R 3.3.1 (R Development Core Team 2018) with Conefor 1.1.6 for directed networks (Saura and Torne 2009).
Additional predictors for bird population changes
We included six species traits as potential predictors in the analyses, i.e., body mass (g), breeding range size (km2), clutch size (the average number of eggs laid; N per female), generation length (yr), migratory dispersion, and migration distance (km). Migratory dispersion is the size of species non‐breeding range relative to that of breeding range (Gilroy et al. 2016). We calculated migratory dispersion, breeding range size, and migration distance on the basis of species’ distribution maps (Birdlife International and NatureServe 2015). We measured both migratory dispersion and breeding range size using a cylindrical equal area projection. Breeding range size was the total area of breeding ranges of a study species. Migratory dispersion was measured by the difference between the log‐transformed area of the non‐breeding area and that of the breeding ranges divided by log‐transformed area of the breeding ranges of a study species (Gilroy et al. 2016). We measured migration distance as the distance between the centroids of breeding and non‐breeding ranges of a study species using an azimuthal equidistant projection. Body mass and clutch size of the study species were obtained from the amniote life‐history database (Myhrvold et al. 2015). Generation lengths were obtained from the IUCN Red List of Threatened Species (Birdlife International 2016).
Statistical analysis
We tested for differences in functional connectivity of migration networks among the different study species using a one‐way ANOVA test followed by Tukey's post hoc tests. Residuals were normally distributed (Kolmogorov‐Smirnov test, P > 0.05).
Phylogenetic non‐independence among species can bias results, and we therefore implemented a phylogenetically corrected model, i.e., a multi‐variable phylogenetic linear mixed model (PLMM; Pearse et al. 2015), to test for the effects of functional connectivity of migration networks and other additional predictors on the population changes of the study species. Data of all study species in each of the survey years (14 yr; 2001–2014) were included in the PLMM. We fitted random effect terms that account for phylogenetic co‐variance (Ives and Helmus 2011), bird species, and year of observation. We acquired a subset tree of the study species based on the Ericson backbone (Ericson et al. 2006) from BirdTree.org (Jetz et al. 2012). The dependent variable was the population change ratio (PCR; Eq. (3)). PCR was calculated as the difference between the population size in a given year i (P i) and the population size of a starting year (P 2001) divided by the population size of the starting year:
| (3) |
Independent variables (described above) included body mass, breeding range size, migratory dispersion, clutch size, generation length, migration distance, and changes in functional connectivity of migration networks of each species. Changes in functional connectivity (CFC) were calculated as the difference in functional connectivity of migration networks between a given year i (FCi) and the starting year (FC2001):
| (4) |
We removed one independent variable with a variance inflation factor larger than 10 (generation length) to reduce the effect of multicollinearity. All factors were scaled to facilitate comparison of the contributions to the prediction. Interaction terms were not fitted in the model due to limited sample sizes. The best model with the smallest Bayesian Information Criterion (BIC) was selected by a backward elimination procedure (Burnham and Anderson 2003). To account for lag effects of changes in functional connectivity of migration networks on changes in population sizes, we fitted the PLMMs with different lag periods (i.e., 1–7 yr; Table 1; Appendix S1: Fig. S1). In these lag models (Table 1), the change in functional connectivity CFC (Lag n) was calculated as the difference in functional connectivity (FC) between a number of years (i.e., the number of lags: n) before the bird survey year (i) and year 1992, the starting year of the functional connectivity measurement:
Table 1.
A test for lag effects of changes in functional connectivity on changes in population sizes for eight waterfowl species, showing the performance of the best models and regression coefficients for predictors included in these models
| Model | Coefficient | Standard error | z | P |
|---|---|---|---|---|
| Lag 1 (N = 112, BIC = 319.9) | ||||
| (Intercept) | 0.175 | 0.253 | 0.691 | 0.490 |
| Changes in functional connectivity | 0.235 | 0.100 | 2.359 | 0.018* |
| Lag 2 (N = 112, BIC = 319.4) | ||||
| (Intercept) | 0.175 | 0.253 | 0.693 | 0.488 |
| Changes in functional connectivity | 0.245 | 0.098 | 2.494 | 0.013* |
| Lag 3 (N = 112, BIC = 318.9) | ||||
| (Intercept) | 0.175 | 0.250 | 0.699 | 0.484 |
| Changes in functional connectivity | 0.254 | 0.095 | 2.674 | 0.007* |
| Lag 4 (N = 112, BIC = 318.8) | ||||
| (Intercept) | 0.175 | 0.249 | 0.703 | 0.482 |
| Changes in functional connectivity | 0.257 | 0.094 | 2.749 | 0.006* |
| Lag 5 (N = 112, BIC = 319.1) | ||||
| (Intercept) | 0.176 | 0.249 | 0.705 | 0.481 |
| Changes in functional connectivity | 0.252 | 0.094 | 2.668 | 0.008* |
| Lag 6 (N = 112, BIC = 319.2) | ||||
| (Intercept) | 0.177 | 0.250 | 0.708 | 0.479 |
| Changes in functional connectivity | 0.249 | 0.093 | 2.677 | 0.007* |
| Lag 7 (N = 112, BIC = 321.9) | ||||
| (Intercept) | 0.173 | 0.252 | 0.688 | 0.492 |
| Changes in functional connectivity | 0.185 | 0.101 | 1.824 | 0.07 |
Notes: The lag periods for measuring changes in functional connectivity are in units of years, i.e., in the model Lag 1, changes in functional connectivity were measured by the change 1 yr before the corresponding population count. The number of lags represents the changes in functional connectivity over the number of years.
The estimated regression coefficient was significant at P ≤ 0.05.
| (5) |
Results
Differences among species
Average functional connectivity of migration networks over the period 1992–2015 significantly differed among the eight study species (Fig. 2a; one‐way ANOVA, F 7, 184 = 23,667, P < 0.001). Among these species, the migration network of the Northern Pintail (Anas acuta) was the least connected with an equivalent connected area of 601,000 km2. The Greylag Goose (Anser anser, equivalent connected area = 908,000 km2), Swan Goose (Anser cygnoid, equivalent connected area = 871,000 km2), and Common Teal (Anas crecca, equivalent connected area = 831,000 km2) had the largest connected migration networks (Fig. 2a).
Figure 2.
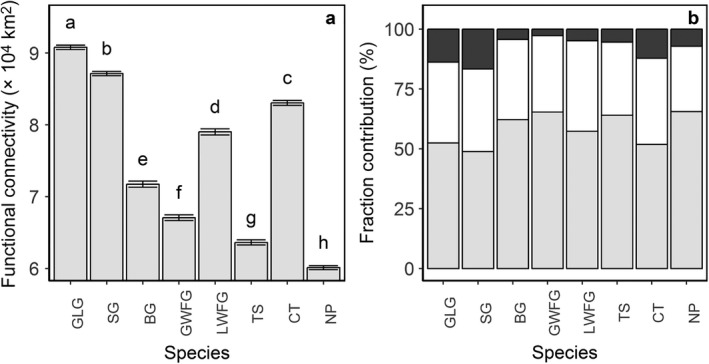
Functional connectivity of migration networks among bird species. (a) The eight study species include the Greylag Goose (GLG) and Swan Goose (SG), Bean Goose (BG), Greater White‐fronted Goose (GWFG), Lesser White‐fronted Goose (LWFG), Tundra Swan (TS), Common Teal (CT), and Northern Pintail (NP). The equivalent connected area (km2 × 10,000) averaged over 1992–2015 is presented per species (mean ± SE). Letters represent group differences as identified by Tukey's multiple comparison test (P ≤ 0.05). (b) Contribution of fractions show intrapatch (light gray), direct (white), and step (dark gray) connections in percentages of functional connectivity.
Intrapatch connectivity contributed more than one‐half the functional connectivity of migration networks for all species. Step connections contributed least, especially for the swan and goose species with major breeding grounds in Russia (i.e., 2% for Greater White‐fronted Goose (Anser albifrons), 4% for Bean Goose (Anser fabalis), 5% for Lesser White‐fronted Goose (Anser erythropus), and 5% for Tundra Swan (Cygnus columbianus), Fig. 2b).
Changes over time
During 2001–2014, the estimated non‐breeding population sizes of the Greylag Goose, Swan Goose, Tundra Swan, Common Teal, and Northern Pintail first increased and then decreased; population sizes of the Bean Goose, Greater White‐fronted Goose, and Lesser White‐fronted Goose first decreased and then either stabilized or slightly increased over the survey period (Fig. 3). Generally, the study species showed a decreasing trend, and at the meantime, the functional connectivity of migration networks of all the eight species consistently and continuously declined since 2001. However, before 2001 (i.e., 1994–1999; periods differ among species), there was an increase in the functional connectivity.
Figure 3.
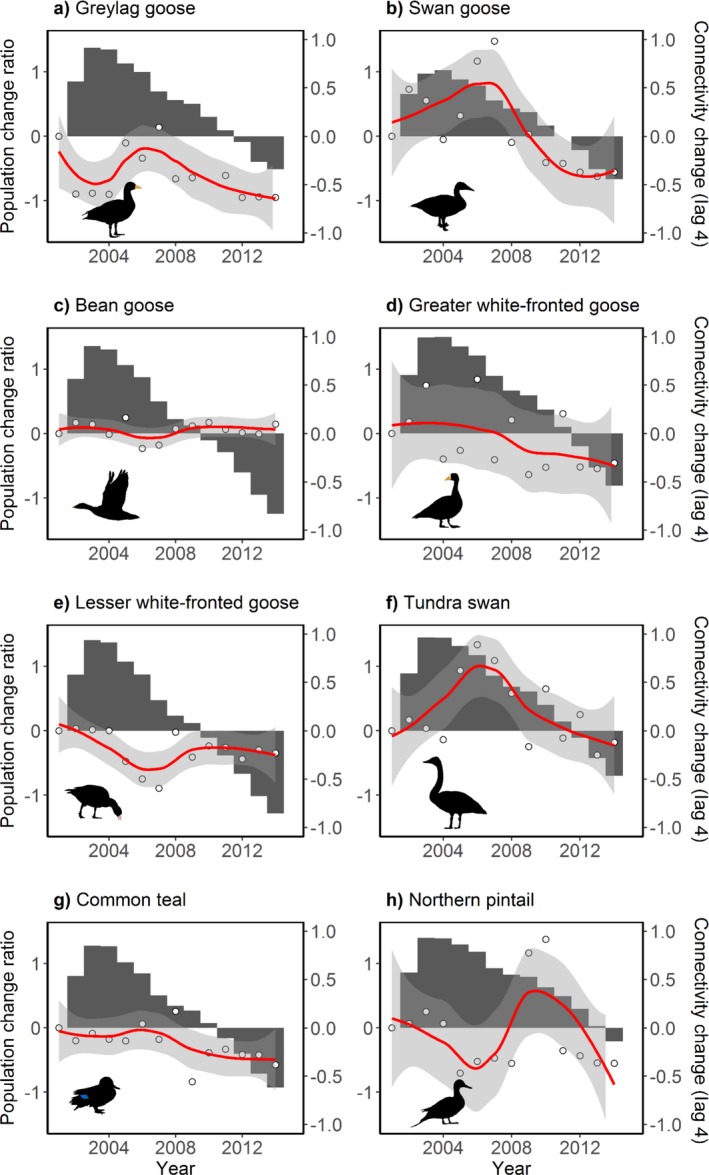
Changes in bird population sizes and in functional connectivity of migration networks. Population change ratio is the difference between the population size in a given year and the population size in 2001 divided by the population size in 2001. Population change ratios are displayed with dots and their trends are represented by smoothed red lines, using a locally weighted scatterplot smoothing method. Connectivity change (gray bars) with a 4‐yr lag is presented as the difference in the equivalent connected area (km2) of the migration network between 4 yr before a given year and year 1992. To facilitate comparison between species, connectivity change (indicated by dark grey bars) is standardized by being divided by the maximum connectivity change. A 4‐yr lag is displayed because the population changes are best explained by the changes in functional connectivity 4 yr before the survey year (Table 1).
Loss of functional connectivity affects bird population dynamics
Among the seven analysed factors, generation length was collinear with other predictors (variance inflation factor >10), and was excluded from the PLMM. The population change ratio was significantly and positively related to changes in functional connectivity of migration networks (N = 112, regression coefficient = 0.24, 95% confidence interval = 0.04–0.44, P = 0.02; Fig. 4a; Table 2). No other variables showed a significant effect, and changes in functional connectivity was the only factor included in the best model (Table 2). When the functional connectivity declined, bird populations declined (Fig. 4b). There was a lag effect of functional connectivity on population changes of migratory birds, with a strongest effect of a 4‐yr lag on the population change ratio (N = 112, regression coefficient = 0.26, 95% confidence interval = 0.07–0.44, P = 0.01; Table 1; Appendix S1: Fig. S1).
Figure 4.
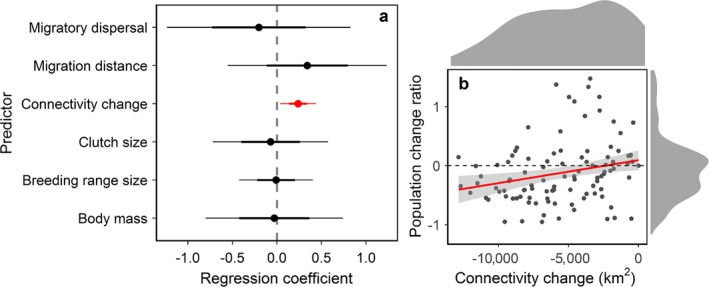
Changes in functional connectivity of migration networks is the only significant predictor for population declines of migratory birds. (a) Estimated coefficients ±95% (thin lines) and 68% confidence interval (thick lines) of predictors for population change ratio using a full model of multivariable phylogenetic linear mixed model. The significant predictor is in red. (b) The effect of changes in functional connectivity on population change ratios of the eight study species. We measured population change ratios using 2001 as the baseline year, the first year of the bird survey. The relationship between population change ratio and changes in functional connectivity were represented by a fitted line (red line) with 95% confidence intervals (gray area). When functional connectivity declines, populations decline. Density plots on top and right show distributions of connectivity change and population change ratio, respectively.
Table 2.
Results of the phylogenetic linear mixed model of species traits on changes in population sizes for eight waterfowl species, showing the performance of the models and regression coefficients for predictors included in these models
| Model | Coefficient | Standard error | z | P |
|---|---|---|---|---|
| Full model (N = 112, BIC = 324.2) | ||||
| (Intercept) | 0.199 | 0.450 | 0.442 | 0.658 |
| Body mass | −0.030 | 0.393 | −0.076 | 0.940 |
| Breeding range size | −0.010 | 0.211 | −0.048 | 0.962 |
| Changes in functional connectivity | 0.238 | 0.103 | 2.308 | 0.021* |
| Clutch size | −0.072 | 0.329 | −0.219 | 0.827 |
| Migration distance | 0.341 | 0.456 | 0.748 | 0.455 |
| Migratory dispersal | −0.203 | 0.525 | −0.386 | 0.699 |
| Best model (N = 112, BIC = 320.7) | ||||
| (Intercept) | 0.169 | 0.256 | 0.661 | 0.508 |
| Changes in functional connectivity | 0.216 | 0.100 | 2.163 | 0.031* |
Note: The full model and the best models with a smallest Bayesian information criterion (BIC) are listed.
The estimated regression coefficient was significant at P ≤ 0.05.
Discussion
We found that loss of functional connectivity in migration networks is a crucial predictor for population declines in migratory birds. Changes in functional connectivity was the only significant factor in the model predicting population changes, outperforming other previously used predictors of population decline and local extinction of migratory birds. Although migratory birds have high flexibility in distribution and migration, their populations declined with a decrease in functional connectivity. Moreover, population sizes responded immediately to connectivity loss, however, when a lag effect was taken into account in the analysis, the impact of changes in functional connectivity on population sizes became even stronger (Table 1; Appendix S1: Fig. S1). The impact of decreasing functional connectivity on population sizes can last long, and was largest using a 4‐yr lag effect.
With the loss of connectivity in their migration network, migratory birds must either adapt to suboptimal resources or accept suboptimal strategies (Weber et al. 1999, Schmaljohann and Both 2017, Si et al. 2018), e.g., longer non‐stop flights and/or suboptimal arrival, departure, and residence times. These adjustments could lead to increasing costs of migration and decreasing efficiency in energy refueling (Goymann et al. 2010); consequently, mortality during migration could increase and breeding success could decline. Carryover effects (Norris et al. 2004) could result in conditions becoming tougher over time for those species that successively lose habitat area along their migration routes. Ultimately, this loss of functional connectivity in migration networks could make it difficult to replenish energy stores during migration and maintain optimal body reserves for reproduction (Norris and Taylor 2006).
A well‐connected network facilitates animal movements and subsequent survival and viability (Crooks and Sanjayan 2006), especially for migratory birds, which pass through long and narrow geographic ranges twice a year. As for the study species, Greylag Goose and Swan Goose with relatively wider and shorter migration extents, have relatively well‐connected migration networks than the other species (e.g., Northern Pintail, Tundra Swan, and Greater White‐fronted Goose). A well‐connected migration network provides sufficient alternative routes for migrants, and not only promotes migratory movements, but also provides more possibilities for range shifts to cope with area‐specific environmental changes. Migratory species that rely on a migration network that is continuously losing connectivity are more likely to experience a population decline. Hence, connectivity of migration networks is an essential element for habitat change analysis for migratory species. Establishment of systematic conservation networks across species migration extents based on international agreements, e.g., the Ramsar Convention on Wetlands of International Importance and the European Union's Biodiversity Strategy 2020, should be comprehensively considered for biodiversity conservation planning.
Stepping stones are essential during seasonal migration. Even during Sahara crossing, migratory birds take short diurnal stopovers in resource‐poor desserts (Schmaljohann et al. 2007) as stepping stones, and birds crossing the Himalayas do the same (Prins and Namgail 2017). However, the step connections in the studied migration networks contributed least to the functional connectivity, which indicated a warning signal that these studied waterfowl species lack stepping stones to facilitate movements between sites. Successive loss of crucial stepping stones in migration routes can lead to the collapse of migration networks, so that migratory movements between breeding and non‐breeding grounds could be completely impeded (Shimazaki et al. 2004). Thus, it is necessary to put an emphasis on protecting critical sites used as stepping stones in migration to enhance the connectivity between isolated sites.
Human‐induced and climate‐driven changes to natural land cover can have large impacts on the connectivity of animal movement networks. Composition and structure of landscape mosaics can explain large‐scale species distribution and richness patterns especially for birds (Xu et al. 2014, Zhang et al. 2018). As indicated by our study, the functional connectivity of migration networks for species in the East Asian‐Australasian Flyway was continuously decreasing from the 2000s. Wetlands are one of the world's mostly threatened habitat types, under influence of climate change and human‐induced habitat destruction (Millennium Ecosystem Assessment 2005, Silva et al. 2007). Wetland loss can isolate and eliminate habitat sites in migration networks, thereby reducing network connectivity. Under high human–bird conflicts of East Asian flyways (Si et al. 2018, 2015), wetlands in this region are widely destroyed by human activities.
Migratory birds that rely the most on degraded stopover sites experienced the largest population decline (Studds et al. 2017) and habitat conditions during migration can influence bird survival (Hewson et al. 2016). Decreasing wetland area and food availability (e.g., via loss of grasslands) can lead to staging sites no longer being utilized by migrants (Verkuil et al. 2012, Zou et al. 2016). The vulnerability of a migratory species that uses a number of wetland sites increases even when only part of the network is negatively affected (e.g., by sea‐level rise or other human‐induced changes (Iwamura et al. 2013). Upon the degradation or loss in individual sites, the chance of a breakdown in both direct and indirect connections between sites increases. It is therefore essential to maintain well‐connected habitat networks by either expanding the area of (protected) sites or by adding new sites to existing networks, to increase the resilience of migrants to environmental change.
Species traits are often associated with population dynamics, as species that vary in traits respond differently to environmental changes (Gaston and Blackburn 1995, Pacifici et al. 2017). Individuals of large‐bodied species might be able to adapt to environmental changes more easily (Pacifici et al. 2017), as they are physiologically more resistant to environmental changes. Thus, species with larger body mass may have an advantage when environmental conditions deteriorate (Pacifici et al. 2017). The connectivity of the studied migration networks decreased continuously over the past 15 yr, under influence of deteriorating environmental conditions, such as habitat loss and fragmentation. However, the expectation that larger species were less affected by the decrease in function connectivity of their migration network could not be confirmed in our study, as the decline in population was similar among species. This is in agreement with, for example, the findings of Jiguet et al. (2007) who also found no relationships between the species body mass and their population trends. The extent to which the species’ life‐history traits affect their population dynamics is dependent on the geographical ranges and orders of species studied (Fritz et al. 2009). We fitted species as a random factor in the PLMM; the signal of the species traits may therefore be weakened. Our results indicate that a loss of functional connectivity of their migration network is a novel and crucial predictor for population declines of migratory birds. Further studies about population declines of migratory birds should take this factor into account. However, other species traits may also be important factors triggering population dynamics of migratory birds. This study included waterfowl species in the East Asian‐Australasian flyway, so future researches investigating whether our findings can be applied to a broader geographical or species range will be valuable.
Supporting information
Acknowledgments
We thank Kevin Matson (Wageningen University, the Netherlands) for his insightful comments on the manuscript, and Henjo de Knegt (Wageningen University, the Netherlands) for help with the spatial analysis. Financial support was provided by the National Natural Science Foundation of China (No. 41471347) and Chinese Scholarship Council (No. 201600090128). We declare no competing interests.
Xu, Y. , Si Y., Wang Y., Zhang Y., Prins H. H. T., Cao L., and de Boer W. F.. 2019. Loss of functional connectivity in migration networks induces population decline in migratory birds. Ecological Applications 29(7):e01960 10.1002/eap.1960
Corresponding Editor: Dianne Brunton.
Data Availability
Data are available on the Dryad Digital Repository: https://doi.org/10.5061/dryad.r901kb6
Literature Cited
- Arzel, C. , Elmberg J., and Guillemain M.. 2006. Ecology of spring‐migrating Anatidae: a review. Journal of Ornithology 147:167–184. [Google Scholar]
- Barshep, Y. , Erni B., Underhill L. G., and Altwegg R.. 2017. Identifying ecological and life‐history drivers of population dynamics of wetland birds in South Africa. Global Ecology and Conservation 12:96–107. [Google Scholar]
- Bélisle, M. 2005. Measuring landscape connectivity: the challenge of behavioral landscape ecology. Ecology 86:1988–1995. [Google Scholar]
- Birdlife International . 2016. The IUCN red list of threatened species. https://www.iucn.org/resources/conservation-tools/iucn-red-list-threatened-species
- Birdlife International and NatureServe . 2015. Bird species distribution maps of the world Version 5.0. BirdLife International, Cambridge, UK: and NatureServe, Arlington, Virginia, USA. [Google Scholar]
- Burnham, K. P. , and Anderson D. R.. 2003. Model selection and multimodel inference: a practical information‐theoretic approach. Springer Science & Business Media, New York, NY. [Google Scholar]
- Cao, L. , Zhang Y., Barter M., and Lei G.. 2010. Anatidae in eastern China during the non‐breeding season: geographical distributions and protection status. Biological Conservation 143:650–659. [Google Scholar]
- Crooks, K. R. , and Sanjayan M.. 2006. Connectivity conservation. Cambridge University Press, Cambridge. [Google Scholar]
- Cushman, S. A. 2006. Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biological Conservation 128:231–240. [Google Scholar]
- de Boer, W. F. , Cao L., Barter M., Wang X., Sun M., van Oeveren H., de Leeuw J., Barzen J., and Prins H. H. T.. 2011. Comparing the community composition of European and Eastern Chinese waterbirds and the influence of human factors on the China waterbird community. Ambio 40:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson, P. G. , Anderson C. L., Britton T., Elzanowski A., Johansson U. S., Källersjö M., Ohlson J. I., Parsons T. J., Zuccon D., and Mayr G.. 2006. Diversification of Neoaves: integration of molecular sequence data and fossils. Biology Letters 2:543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz, S. A. , Bininda‐Emonds O. R., and Purvis A.. 2009. Geographical variation in predictors of mammalian extinction risk: big is bad, but only in the tropics. Ecology Letters 12:538–549. [DOI] [PubMed] [Google Scholar]
- Gaston, K. J. , and Blackburn T. M.. 1995. Birds, body size and the threat of extinction. Philosophical Transactions of the Royal Society B 347:205–212. [Google Scholar]
- Gilroy, J. J. , Gill J. A., Butchart S. H. M., Jones V. R., and Franco A. M. A.. 2016. Migratory diversity predicts population declines in birds. Ecology Letters 19:308–317. [DOI] [PubMed] [Google Scholar]
- Goymann, W. , Spina F., Ferri A., and Fusani L.. 2010. Body fat influences departure from stopover sites in migratory birds: evidence from whole‐island telemetry. Biology Letters 6:478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson, C. M. , Thorup K., Pearce‐Higgins J. W., and Atkinson P. W.. 2016. Population decline is linked to migration route in the Common Cuckoo. Nature Communications 7:12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives, A. R. , and Helmus M. R.. 2011. Generalized linear mixed models for phylogenetic analyses of community structure. Ecological Monographs 81:511–525. [Google Scholar]
- Iwamura, T. , Possingham H. P., Chadès I., Minton C., Murray N. J., Rogers D. I., Treml E. A., and Fuller R. A.. 2013. Migratory connectivity magnifies the consequences of habitat loss from sea‐level rise for shorebird populations. Proceedings of the Royal Society B 280:20130325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetz, W. , Thomas G., Joy J., Hartmann K., and Mooers A.. 2012. The global diversity of birds in space and time. Nature 491:444–448. [DOI] [PubMed] [Google Scholar]
- Jiguet, F. , Gadot A. S., Julliard R., Newson S. E., and Couvet D.. 2007. Climate envelope, life history traits and the resilience of birds facing global change. Global Change Biology 13:1672–1684. [Google Scholar]
- Johnson, W. P. , Schmidt P. M., and Taylor D. P.. 2014. Foraging flight distances of wintering ducks and geese: a review. Avian Conservation and Ecology 9:2. [Google Scholar]
- Keitt, T. H. , Urban D. L., and Milne B. T.. 1997. Detecting critical scales in fragmented landscapes. Conservation Ecology 1:4. [Google Scholar]
- Leito, A. , et al. 2015. The potential impacts of changes in ecological networks, land use and climate on the Eurasian crane population in Estonia. Landscape Ecology 30:887–904. [Google Scholar]
- Merken, R. , Deboelpaep E., Teunen J., Saura S., and Koedam N.. 2015. Wetland suitability and connectivity for trans‐Saharan migratory waterbirds. PLoS ONE 10:e0135445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millennium Ecosystem Assessment . 2005. Ecosystems and human well‐being: wetlands and water. https://www.millenniumassessment.org
- Morrison, C. A. , Robinson R. A., Clark J. A., Risely K., and Gill J. A.. 2013. Recent population declines in Afro‐Palaearctic migratory birds: the influence of breeding and non‐breeding seasons. Diversity and Distributions 19:1051–1058. [Google Scholar]
- Murray, K. A. , Verde Arregoitia L. D., Davidson A., Di Marco M., and Di Fonzo M. M.. 2014. Threat to the point: improving the value of comparative extinction risk analysis for conservation action. Global Change Biology 20:483–494. [DOI] [PubMed] [Google Scholar]
- Myhrvold, N. P. , Baldridge E., Chan B., Sivam D., Freeman D. L., and Ernest S.. 2015. An amniote life‐history database to perform comparative analyses with birds, mammals, and reptiles. Ecology 96:3109. [Google Scholar]
- Newton, I. 2010. The migration ecology of birds. Academic Press, Amsterdam, the Netherlands. [Google Scholar]
- Norris, D. R. , and Taylor C. M.. 2006. Predicting the consequences of carry‐over effects for migratory populations. Biology Letters 2:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, D. R. , Marra P. P., Kyser T. K., Sherry T. W., and Ratcliffe L. M.. 2004. Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proceedings of the Royal Society B 271:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici, M. , Visconti P., Butchart S. H., Watson J. E., Cassola F. M., and Rondinini C.. 2017. Species’ traits influenced their response to recent climate change. Nature Climate Change 7:205–208. [Google Scholar]
- Pearse, W. D. , Cadotte M. W., Cavender‐Bares J., Ives A. R., Tucker C. M., Walker S. C., and Helmus M. R.. 2015. pez: phylogenetics for the environmental sciences. Bioinformatics 31:2888–2890. [DOI] [PubMed] [Google Scholar]
- Prins, H. H. , and Namgail T.. 2017. Bird migration across the Himalayas: wetland functioning amidst mountains and glaciers. Cambridge University Press, Cambridge. [Google Scholar]
- R. Development Core Team , 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Saura, S. , and Pascual‐Hortal L.. 2007. A new habitat availability index to integrate connectivity in landscape conservation planning: comparison with existing indices and application to a case study. Landscape and Urban Planning 83:91–103. [Google Scholar]
- Saura, S. , and Rubio L.. 2010. A common currency for the different ways in which patches and links can contribute to habitat availability and connectivity in the landscape. Ecography 33:523–537. [Google Scholar]
- Saura, S. , and Torne J.. 2009. Conefor Sensinode 2.2: a software package for quantifying the importance of habitat patches for landscape connectivity. Environmental Modelling & Software 24:135–139. [Google Scholar]
- Saura, S. , Bodin Ö., and Fortin M. J.. 2014. Stepping stones are crucial for species’ long‐distance dispersal and range expansion through habitat networks. Journal of Applied Ecology 51:171–182. [Google Scholar]
- Schmaljohann, H. , and Both C.. 2017. The limits of modifying migration speed to adjust to climate change. Nature Climate Change 7:573–576. [Google Scholar]
- Schmaljohann, H. , Liechti F., and Bruderer B.. 2007. Songbird migration across the Sahara: the non‐stop hypothesis rejected! Proceedings of the Royal Society B 274:735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki, H. , Tamura M., Darman Y., Andronov V., Parilov M. P., Nagendran M., and Higuchi H.. 2004. Network analysis of potential migration routes for Oriental White Storks (Ciconia boyciana). Ecological Research 19:683–698. [Google Scholar]
- Si, Y. , Xu Y., Xu F., Li X., Zhang W., Wielstra B., Wei J., Liu G., Luo H., Takekawa J., Balachandran S., Zhang T., de Boer W. F., Prins H. H. T. and, Gong P.. 2018. Spring migration patterns, habitat use, and stopover site protection status for two declining waterfowl species wintering in China as revealed by satellite tracking. Ecology and Evolution 8:6280–6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si, Y. , Xin Q., Prins H. H. T., de Boer W. F., and Gong P.. 2015. Improving the quantification of waterfowl migration with remote sensing and bird tracking. Science Bulletin 60:1984‐1993. [Google Scholar]
- Silva, J. P. , Phillips L., and Jones W.. 2007. LIFE and Europe's wetlands: restoring a vital ecosystem. http://ec.europa.eu
- Studds, C. E. , Kendall B. E., Murray N. J., Wilson H. B., Rogers D. I., Clemens R. S., Gosbell K., Hassell C. J., Jessop R., and Melville D. S.. 2017. Rapid population decline in migratory shorebirds relying on Yellow Sea tidal mudflats as stopover sites. Nature Communications 8:14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, P. D. , Fahrig L., Henein K., and Merriam G. C. F. P. D. D.. 1993. Connectivity is a vital element of landscape structure. Oikos 68:571–573. [Google Scholar]
- Verkuil, Y. I. , Karlionova N., Rakhimberdiev E. N., Jukema J., Wijmenga J. J., Hooijmeijer J., Pinchuk P., Wymenga E., Baker A. J., and Piersma T.. 2012. Losing a staging area: eastward redistribution of Afro‐Eurasian ruffs is associated with deteriorating fuelling conditions along the western flyway. Biological Conservation 149:51–59. [Google Scholar]
- Weber, T. P. , Houston A. I., and Ens B. J.. 1999. Consequences of habitat loss at migratory stopover sites: a theoretical investigation. Journal of Avian Biology 30:416–426. [Google Scholar]
- Xu, C. , Huang Z. Y., Chi T., Chen B. J., Zhang M., and Liu M.. 2014. Can local landscape attributes explain species richness patterns at macroecological scales? Global Ecology and Biogeography 23:436–445. [Google Scholar]
- Xu, W. , Viña A., Kong L., Pimm S. L., Zhang J., Yang W., Xiao Y., Zhang L., Chen X., and Liu J.. 2017. Reassessing the conservation status of the giant panda using remote sensing. Nature Ecology & Evolution 1:1635. [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Si Y., Yin S., Zhang W., Grishchenko M., Prins H. H. T., Gong P., and de Boer W. F.. 2019. Species‐dependent effects of habitat degradation in relation to seasonal distribution of migratory waterfowl in the East Asian‐Australasian Flyway. Landscape Ecology 34:243. [Google Scholar]
- Zhang, Y. , Jia Q., Prins H. H. T., Cao L., and de Boer W. F.. 2015. Effect of conservation efforts and ecological variables on waterbird population sizes in wetlands of the Yangtze River. Scientific Reports 5:17136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Li X., Yu L., and Si Y.. 2018. Multi‐scale habitat selection by two declining East Asian waterfowl species at their core spring stopover area. Ecological Indicators 87:127–135. [Google Scholar]
- Zou, Y. A. , Tang C. D., Niu J. Y., Wang T. H., Xie Y. H., and Guo H.. 2016. Migratory waterbirds response to coastal habitat changes: conservation implications from long‐term detection in the Chongming Dongtan Wetlands, China. Estuaries and Coasts 39:273–286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on the Dryad Digital Repository: https://doi.org/10.5061/dryad.r901kb6


