Abstract
Charge detection mass spectrometry (CDMS) of low-level signals is currently limited to the analysis of individual ions that generate a persistent signal during the entire observation period. Ions that disintegrate during the observation period produce reduced frequency domain signal amplitudes, which lead to an underestimation of the ion charge state, and thus the ion mass. The charge assignment can only be corrected through an accurate determination of the time of ion disintegration. The traditional mechanisms for temporal signal analysis have severe limitations for temporal resolution, spectral resolution, and signal-to-noise ratios. Selective Temporal Overview of Resonant Ions (STORI) plots provide a new framework to accurately analyze low-level time domain signals of individual ions. STORI plots allow for complete correction of intermittent signals, the differentiation of single and multiple ions at the same frequency, and the association of signals that spontaneously change frequency.
Keywords: Single ion mass spectrometry, Orbitrap, FT-MS, Ion signal analysis, Charge detection mass spectrometry
Graphical Abstract
Introduction
Conventional mass spectrometers determine the mass-to-charge (m/z) ratio of detected ions by a variety of techniques including the measurement of velocity, frequency, or the stability of ion motion through electrostatic, electrodynamic, and magnetic fields. For multiple-charged ions, after determining m/z, one must then infer the charge (z) of signals based upon the spacing between either adjacent charge states or isotopic peaks to finally determine mass [1, 2]. Specialized charge detection mass spectrometers (CDMS) have been developed which are capable of simultaneously measuring m/z and z for individual ions contained in an electrostatic ion trap from the frequency and amplitude of the resulting signal [3–9]. Due to limited detector sensitivity, this has been applied primarily to large molecules with tens to thousands of charges, but improvements have made this method applicable to ions with less than 10 elementary charges[10, 11]. The signal amplitude determined with this process is dependent not only on the charge of the ion being measured, but also by the duration of signal. A signal that does not persist the entire acquisition period will produce an amplitude less than expected and lead to an underestimation of charge, and subsequently, mass. Intermittent signals must be identified and discarded, which results in longer experiment durations to collect statistically meaningful CDMS distributions.
Since the signal amplitude is directly proportional to the duration of the signal, the ability to accurately determine the time of signal loss would allow for the recovery of these intermittent signals. Temporal analysis of sinusoidal signals has traditionally been performed with short-time Fourier transforms (STFT) [12]. This involves transforms of local sections of the time domain data which provides information about the absence or presence of signal in each section. A distinct weakness of this method is that an increase in temporal resolution results in a decrease in the signal-to-noise ratio and spectral resolution in each section. For example, sectioning a time domain data set into 16 pieces allows for the determination of the time of signal loss with 6% accuracy[12]. However, this results in a fourfold reduction in S/N for each section, conflicting with a single ion signal which may already be on the edge of detectability. The 16-fold reduction in resolution in each section would not be considered problematic for traditional CDMS, where each acquisition includes just one ion, but with the recent push to increase throughput [13, 14], the ability to cleanly resolve adjacent signals becomes critical. Herein, we describe a new method of analyzing individual ion signals termed Selective Temporal Overview of Resonant Ions (STORI). STORI plots provide a way to track the temporal evolution of individual signals without limiting temporal resolution, spectral resolution, or signal levels.
Experimental
A Q Exactive Plus mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) was operated with a reduced HCD pressure (UHV pressure < 3 × 10−11 torr) to minimize the probability of ion/neutral background gas molecule collisions. Electrospray ionization spectra for horse myoglobin (16.9 kDa) were acquired under denaturing conditions using quadrupole isolation to select individual charge states. To reduce the number of ions in each spectrum, samples were diluted to concentrations between 0.05 and 0.1 μM and injection times were limited to under 1 ms. If necessary, instrument ion optics were detuned to further decrease ion transmission [15].
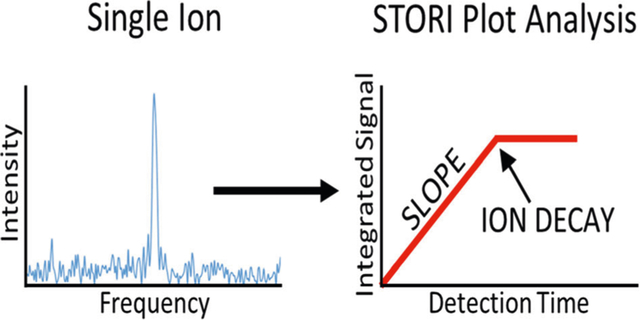
The STORI plot is obtained from the discrete time domain signal S for a frequency of interest ω with the relation:
| (1) |
Equation (1) is closely related to the discrete Fourier transform and can by described as the temporal profile of the summation that yields a specific point in the Fourier transform of the entire signal S, i.e., the temporal evolution of the integrated or “accumulated” signal at frequency ω.
The complex sum (4) for each point in the STORI plot is conveniently calculated as a recursive sum of the real (2) and imaginary (3) components:
| (2) |
| (3) |
The components cannot independently provide quantitative information about the signal amplitude without knowledge of the initial phase and thus, the magnitude is obtained from:
| (4) |
The magnitude STORI is used to determine signal persistence and the rate of signal accumulation.
Results and Discussion
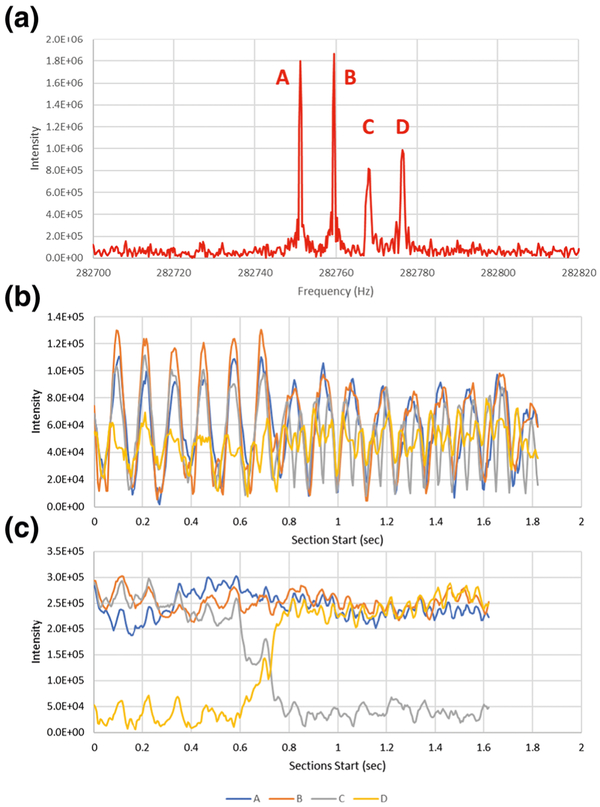
Frequency domain amplitudes are directly dependent on the duration of the signal during data acquisition. Figure 1 a shows the frequency domain spectrum for individual 20+ myoglobin ions that are observed for a period of 1.9 s. In the mass domain, these signals are spaced 0.05 m/z, or 1 Da apart, and are assumed to be ions of different isotopic composition. Based upon intensities and peak widths, the lowest frequency signals labeled A and B appear to persist about twice as long as signals labeled C and D.
Figure 1.
(a) Myoglobin +20 individual ion signals and (b) 50-ms section and (c) 250-ms section STFT temporal intensity plots for each signal in (a)
Figure 1b shows the temporal dependence of the intensities for each signal obtained with a sliding window STFT using 50-ms sections and 5-ms steps [14]. Although short sections could provide useful temporal resolution, there is insufficient spectral resolution to separate adjacent signals. The strong oscillatory nature of the intensities is a result of the time domain beat pattern that is created from the isotopic signals [16]. STFT only becomes informative when the section size is sufficient to clearly resolve adjacent isotopes, as is shown for 250-ms sections in Figure 1c. Based on this STFT, A and B appear to persist the entire acquisition period. The ion which generates signal C disintegrates during the acquisition, with the appearance signal D correlating in time. Interpretation of the graph is non-obvious, but the transition point corresponds to the end of the STFT intensity change, so approximately 800 ms.
Relative to STFT, STORI plots provide a clearer picture of the temporal behavior for each of the signals, without any need to empirically optimize section size as required with STFT. Shown in Figure 2a are STORI plots for A and B, which provide information on two primary characteristics of the signals that both signals persist throughout the acquisition period and that the ions likely have the same charge, since the slopes of the two plots are similar (9.62 × 105 vs. 9.96 × 105). Although these plots appear to be noisy, the wiggles come from mathematical effects of the isotopic beat pattern in the time domain signal [16].
Figure 2.
(a) STORI plots for persistent signals A and B and (b) STORI plots for intermittent signals C and D
It is for signals C and D shown in Figure 2b that STORI plots demonstrate convincing value. Based on the initial slope for C (9.80 × 105), the ion likely has the same charge as A and B. It is also clear that this ion has a time of disintegration (TOD) that occurs at 845 ms. Not coincidentally, this is the same time that signal D appears, indicating it is a product of C. The slope for D (9.58 × 105) matches the other signals, so the 0.05 m/z spacing translates to a 1 Da loss, which is most logically explained as the loss of neutral atomic hydrogen.
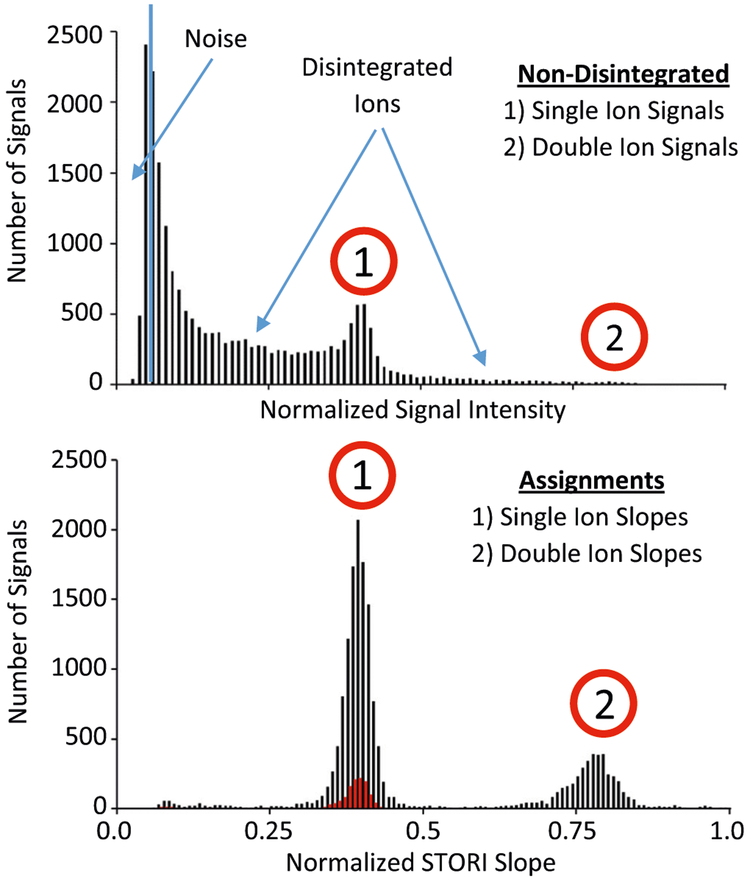
Figure 3 shows histograms of single-ion intensities (top panel) and corresponding STORI slopes (bottom panel) for +20 myoglobin ions collected over 2284 acquisitions. A total of 23,918 signals fall into three categories: low level (2935) from electronic noise that gets past the intensity threshold filters of the embedded processor, mid-level (18,639) that represent individual ions that disintegrate, and finally, the highest level (2344) that represent ions persisting over the full observation period. When STORI analysis is applied, noise signals are discarded and plots that pass strict line fitting filters (R2>0.97) produce distinct slopes for single and double ion signals. Currently, our slope measurement only assigns the first STORI line segment slope. This means that if one ion from a double ion signal disintegrates, only the slope for the double ion signal is recognized. With these limitations, ~ 8000 STORI slopes (black trace; bottom panel) are still rescued from disintegrated ion signals, dramatically increasing the statistical significance of the histograms over just utilizing ion signals that persist the entire observation period (red trace; bottom panel).
Figure 3.
Intensity histogram (top panel) of individual ions collected from the myoglobin +20 charge state. Ion intensities from lowest to highest are shown from left to right and assigned as noise peaks, real ions that disintegrated, and real ions that persist throughout the observation period, respectively. Over-laid STORI slope histograms (bottom panel) of assigned single-and double-ion signals rescuing signals from ions at any TOD (black trace) vs. only ion signals that persisted the entire observation period (red trace)
Proper determination of the STORI slope is dependent on the accuracy of the frequency used in the calculation. To ensure an accurate frequency estimate, the true peak apex is determined by a quadratic fit to the three most intense frequency domain points of the peak [17]. An error in the frequency would result in underestimation of the slope, equivalent to measuring not at the apex of the frequency domain peak but instead down the side of the peak. Fortunately, this type of error is readily apparent in the real and imaginary STORI plots. These components will show curvature instead of the expected straight lines, since the ion signal slowly gets out of phase with the sine and cosine used in the calculation. Supplemental Figures S1 and S2 demonstrate the effect of calculating the STORI plots with frequencies away from the apex of the peak. Reduced STORI slopes mirror the frequency domain peak shape (Figure S1) where real and imaginary components deviate from the expected linear summation pattern (Figure S2).
STORI plots can also be used to characterize signals from less typical acquisition events. For example, two ions at the same frequency can be difficult to distinguish from a single ion of twice the charge, and thus twice the mass, leading to this mass being potentially mistaken for a biologically relevant dimer. However, if one of the pair of ions happens to disintegrate during the observation period, the STORI plot will show a reduction in the slope to about half of the initial value. Figure S3 shows the slope decreases from 2.67 × 106 to 1.27 × 106, clearly indicating a coincidental ion pair. An automated process to distinguish these events and assign double- to single-ion slopes is currently under development.
Conclusion
For CDMS, charge assignment can be impacted when signals do not persist for the entire observation period. Through the use of STORI plots, these signals, instead of being discarded, can be rescued to provide accurate charge assignment and improve the statistical significance of the resulting CDMS spectrum. STORI plots also help to characterize abnormal events, such as multi-ion signals and ion disintegration without compromising between temporal and spectral resolution.
Supplementary Material
Acknowledgements
This work was funded by the Intensifying Innovation program from Thermo Fisher Scientific and was carried out in collaboration with the National Resource for Translational and Developmental Proteomics under Grant P41 GM108569 from the National Institute of General Medical Sciences, National Institutes of Health with additional support from the Sherman Fairchild Foundation.
Footnotes
Electronic supplementary material The online version of this article (http://doi.org/10.1007/s13361-019-02309-0) contains supplementary material, which is available to authorized users.
References
- 1.Mann M, Meng CK, Fenn JB: Interpreting mass spectra of multiply charged ions. Anal. Chem 61, 1702–1708 (1989) [Google Scholar]
- 2.Senko MW, Beu SC, McLafferty FW: Automated assignment of charge states from resolved isotopic peaks for multiply charged ions. J. Am. Soc. Mass Spectrom. 6, 52–56 (1995) [DOI] [PubMed] [Google Scholar]
- 3.Keifer DZ, Pierson EE, Jarrold MF: Charge detection mass spectrometry: weighing heavier things. Analyst. 142, 1654–1671 (2017) [DOI] [PubMed] [Google Scholar]
- 4.Schmidt HT, Cederquist H, Jensen J, Fardi A: Conetrap: a compact electrostatic ion trap. Nucl. Instrum. Methods Phys. Res., Sect. B 173, 523–527 (2001) [Google Scholar]
- 5.Elliott AG, Harper CC, Lin HW, Williams ER: Mass, mobility and MSn measurements of single ions using charge detection mass spectrometry. Analyst. 142, 2760–2769 (2017) [DOI] [PubMed] [Google Scholar]
- 6.Keifer DZ, Shinholt DL, Jarrold MF: Charge detection mass spectrometry with almost perfect charge accuracy. Anal. Chem 87, 10330–10337 (2015) [DOI] [PubMed] [Google Scholar]
- 7.Benner WH: A gated electrostatic ion trap to repetitiously measure the charge and m/z of large electrospray ions. Anal. Chem 69, 4162–4168 (1997) [Google Scholar]
- 8.Halim MA, Clavier C, Dagany X, Kerleroux M, Dugourd P, Dunbar RC, Antoine R: Infrared laser dissociation of single megadalton polymer ions in a gated electrostatic ion trap: the added value of statistical analysis of individual events. Phys. Chem. Chem. Phys 20, 11959–11966 (2018) [DOI] [PubMed] [Google Scholar]
- 9.Elliott AG, Harper CC, Lin HW, Susa AC, Xia Z, Williams ER: Simultaneous measurements of mass and collisional cross-section of single ions with charge detection mass spectrometry. Anal. Chem 89, 7701–7708 (2017) [DOI] [PubMed] [Google Scholar]
- 10.Contino NC, Pierson EE, Keifer DZ, Jarrold MF: Charge detection mass spectrometry with resolved charge states. J. Am. Soc. Mass Spectrom. 24, 101–108 (2013) [DOI] [PubMed] [Google Scholar]
- 11.Contino NC, Jarrold MF: Charge detection mass spectrometry for single ions with a limit of detection of 30 charges. Int. J. Mass Spectrom. 345–347, 153–159 (2013) [Google Scholar]
- 12.Cohen L: Time-frequency distributions-a review. Proc. IEEE 77, 941–981 (1989) [Google Scholar]
- 13.High throughput charge detection mass spectrometry, Botamanenko D, Todd AR, Jarrold MF: MP494, Proceedings of the 67th ASMS Conference on Mass Spectrometry and Allied Topics, Atlanta, Georgia, June 2–6, 2019. [Google Scholar]
- 14.Harper CC, Elliott AG, Oltrogge LM, Savage DF, Williams ER: Multiplexed charge detection mass spectrometry for high-throughput single ion analysis of large molecules. Anal. Chem 91, 7458–7465 (2019) [DOI] [PubMed] [Google Scholar]
- 15.Kafader JO, Melani RD, Senko MW, Makarov AA, Kelleher NL, Compton PD: Measurement of individual ions sharply increases the resolution of orbitrap mass spectra of proteins. Anal. Chem 91, 2776–2783 (2019) [DOI] [PubMed] [Google Scholar]
- 16.Hofstadler SA, Bruce JE Rockwood AL, Anderson GA, Winger BE, Smith RD: Isotopic beat patterns in Fourier transform ion cyclotron resonance mass spectrometry: implications for high resolution mass measurements of large biopolymers. Int. J. Mass Spectrom. Ion Process. 132, 109–127 (1994) [Google Scholar]
- 17.Chen L, Cottrell CE, Marshall AG: Effect of signal-to-noise ratio and number of data points upon precision in measurement of peak amplitude, position and width in fourier transform spectrometry. Chemom. Intell. Lab. Syst 1, 51–58 (1986) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.