Aromatase inhibitors can affect bone mineral density and have been reported to increase the risk of fractures. This article reports on fracture outcomes from a clinical registry of bone mineral density results, identifying results from women undergoing aromatase inhibitor therapy for breast cancer, women with breast cancer not receiving aromatase inhibitor therapy, and women from the general population without breast cancer.
Keywords: Osteoporosis, Breast cancer, Aromatase inhibitors, Bone density, Fracture
Abstract
Background.
Aromatase inhibitors (AIs) used in breast cancer induce loss in bone mineral density (BMD) and are reported to increase fracture risk.
Materials and Methods.
Using a population‐based BMD registry, we identified women aged at least 40 years initiating AIs for breast cancer with at least 12 months of AI exposure (n = 1,775), women with breast cancer not receiving AIs (n = 1,016), and women from the general population (n = 34,205). Fracture outcomes were assessed to March 31, 2017 (mean, 6.2 years for AI users).
Results.
At baseline, AI users had higher body mass index (BMI), higher BMD, lower osteoporosis prevalence, and fewer prior fractures than women from the general population or women with breast cancer without AI use (all p < .001). After adjusting for all covariates, AI users were not at significantly greater risk for major osteoporotic fractures (hazard ratio [HR], 1.15; 95% confidence interval [CI], 0.93–1.42), hip fracture (HR, 0.90; 95% CI, 0.56–1.43), or any fracture (HR, 1.06; 95% CI, 0.88–1.28) compared with the general population.
Conclusion.
Higher baseline BMI, BMD, and lower prevalence of prior fracture at baseline may offset the adverse effects of AI exposure. Although confirmatory data from large cohort studies are required, our findings challenge the view that all women with breast cancer initiating AI therapy should be considered at high risk for fractures.
Implications for Practice.
In a population‐based observational registry that included 1,775 patients initiating long‐term aromatase inhibitor therapy, risk for major osteoporotic fracture, hip fracture, or any fracture was similar to the general population. Higher baseline body mass index, bone mineral density, and lower prevalence of prior fracture at baseline may offset the adverse effects of aromatase inhibitor exposure.
关键词。骨质疏松症 • 乳腺癌 • 芳香化酶抑制剂 • 骨密度 • 骨折
摘要
背景。将芳香化酶抑制剂(AI)用于治疗乳腺癌可导致骨密度(BMD)降低,据报告,可增加骨折 风险。
材料和方法。我们采用基于人群的 BMD 登记,确定了年龄至少为 40 岁的开始接受 AI 治疗乳腺癌的女性,其 AI 暴露时间至少为 12 个月(n = 1 775),患有乳腺癌但未接受 AI 治疗的女性(n = 1 016),以及来自普通人群的女性(n = 34 205)。骨折结果评估至 2017 年 3 月 31 日(AI 使用者平均为 6.2 年)。
结果。基线情况下,与来自普通人群的女性或未使用 AI 的乳腺癌女性患者相比,AI 使用者具有更高的身体质量指数(BMI),更高的 BMD,更低的骨质疏松症患病率和更低的骨折发生率(所有p < 0.001)。在对所有协变量进行校正后,AI 使用者发生严重骨质疏松性骨折 [风险比 (HR),1.15;95% 置信区间 (CI),0.93–1.42]、髋部骨折(HR,0.90;95% CI,0.56–1.43)或任何骨折(HR,1.06;95% CI,0.88–1.28)的风险均不显著高于一般人群。
结论。较高的基线 BMI、BMD 和较低的既往基线骨折发生率可抵消 AI 暴露的不良反应。尽管需要来自大型队列研究的证实性数据,但我们的研究结果对所有实施 AI 治疗的女性乳腺癌患者都应被视为骨折的高危人群这样的观点表示质疑。
实践意义:在一项基于人群的观察性登记中,包括 1 775 名实施长期芳香化酶抑制剂治疗的患者,其出现骨质疏松性骨折、髋部骨折或任何骨折的风险与普通人群相似。较高的基线身体质量指数、骨密度和较低的既往基线骨折发生率可抵消芳香化酶抑制剂暴露的不良反应。
Introduction
According to global estimates for 2018, breast cancer is the most common cancer in women [1]. Breast cancer survivors are known to be at increased risk for osteoporosis and fractures [2]. Aromatase inhibitors are recommended to reduce the risk of cancer recurrence in postmenopausal women with hormone receptor‐positive breast cancer [3]. The use of aromatase inhibitors (AIs) increases bone turnover and induces bone loss at trabecular‐rich bone sites at an average rate of 1% to 3% per year, with reports of up to threefold increased fracture incidence [4], [5]. In contrast, a large nationwide population‐based cohort study using U.S. Medicare data identified minimal excess fracture risk from AI use compared with tamoxifen (11% higher for nonvertebral fractures, not significantly increased for hip fractures) [6].
Observations in the clinical trial setting may differ from routine clinical practice. Therefore, we examined fracture outcomes using a large clinical registry of bone mineral density (BMD) results for the province of Manitoba, Canada, that allowed us to identify women initiating AI therapy for breast cancer, women with breast cancer not receiving AI therapy, and women from the general population without breast cancer.
Materials and Methods
Study Population
We performed a registry‐based cohort study to examine fracture outcomes among women 40 years of age or older who had undergone baseline BMD of the hip between 2005 and 2016 and had at least 1 year of follow‐up, with the date of BMD testing as the index date. In the Canadian province of Manitoba, health services are provided to nearly all residents through a single public health care system [7]. For each health system contact, information is recorded to document the patient's demographics, date and type of service, and diagnosis code(s). Hospital discharge abstracts (diagnoses and procedures) were coded using the International Classification of Diseases (ICD), 9th revision, Clinical Modification (ICD‐9‐CM) prior to 2004 and the 10th revision of ICD, Canadian version (ICD‐10‐CA) and Canadian Classification of Interventions thereafter. Physician billing claims were coded using ICD‐9‐CM as previously described [8], [9]. Information on medication use was obtained from the provincial pharmacy system [10]. BMD testing through the Manitoba Density Program has been managed as an integrated program since 1997 [11]. The Manitoba Density Program maintains a database of all results that can be linked with the other provincial population‐based databases through an anonymous personal identifier. The associated database exceeds 99% in terms of completeness and accuracy [12]. The study was approved by the Research Ethics Board of the University of Manitoba and the Health Information Privacy Committee of Manitoba Health.
Aromatase Inhibitor Use and Breast Cancer Diagnosis
We categorized the women into one of three mutually exclusive subgroups: breast cancer with AI use, breast cancer without AI use, and general population without a breast cancer diagnosis (referent). Breast cancer diagnosis was based upon physician and hospitalization codes for malignant neoplasm of breast (ICD‐9‐CM 174‐175, ICD‐10‐CA C50) during the prior 3 years. This approach identifies breast cancer cases with high sensitivity, specificity and overall accuracy (kappa 0.97) compared with cancer registry data [13]. AI use has been the standard of care for management of estrogen receptor‐positive breast cancer in postmenopausal women since approximately 2005. Information on AI use (Anatomical Therapeutic Chemical classification code L02BG) was obtained from medication dispensation records through the province‐wide retail pharmacy system for up to 5 years before the index date (entire 5 years available in 95%) and 5 years after the index date (entire 5 years available in 61%), was tabulated as yearly total number of medication days, and did not distinguish the specific agent used (anastrozole, letrozole, exemestane) [10]. To classify a patient as an AI user, we required that there be at least 365 days of medication exposure after the index date, with at least 180 days in the first year to exclude delayed treatment initiation. The intensity of exposure was quantified as the medication possession ratio (MPR: medication days dispensed divided by the total number of days). We excluded long‐term AI users (more than 180 days' use prior to the index date) to reflect the common clinical scenario of a women initiating AI therapy when BMD changes are expected to be greatest. Characteristics of the included versus excluded AI users are shown in supplemental online Table 1. Women from the general population (controls) without a breast cancer diagnosis and women with breast cancer but without AI use had no exposure to these medications at any time point.
Assessment of Incident Fractures
Longitudinal health service records (i.e., hospital discharge abstract and physician billing claims) were assessed between April 1, 1987, and March 31, 2017, for the presence of a major osteoporotic fracture (MOF: hip, clinical spine, forearm, and humerus), hip fracture, and any fracture (excluding head/neck, hands/feet, and ankle) not associated with codes indicative of severe trauma (i.e., external injury) using published and validated definitions [8], [14]. Hip and forearm fractures were required to have a site‐specific fracture reduction, fixation, or casting code. To minimize misclassification of prevalent and incident fractures at the same skeletal site, we required that there be no hospitalization or physician visit(s) with the same fracture type in the 12 months preceding an incident fracture. There was no time restriction on prior and incident fractures involving different skeletal sites.
Bone Densitometry and Covariates
All dual‐energy x‐ray absorptiometry (DXA) scans were performed with a commercial fan‐beam device (Prodigy or iDXA; GE Healthcare, Waukesha, WI) and analyzed in accordance with the manufacturer's recommendations. Femoral neck BMD T scores were calculated using the third National Health and Nutrition Examination Survey white female reference values [15]. The DXA instruments were cross‐calibrated using anthropomorphic phantoms, and no clinically significant differences were identified (T score differences <0.1). Short‐term reproducibility (coefficient of variation) for femoral neck BMD from the multiple technologists was 2.3% (over 400 repeat hip DXA scans performed within 28 days).
We also considered multiple covariates that affect fracture risk independent of BMD: age, sex, body mass index (BMI), prior fragility fracture, parental history of hip fracture, current smoking, long‐term oral glucocorticoid use, rheumatoid arthritis diagnosis, and high alcohol consumption [16]. Weight and height were measured at the time of DXA, and BMI was calculated as weight (in kilograms) divided by height (in meters) squared. Other covariates were assessed using a combination of self‐report at the time of DXA and hospital discharge abstracts, physician billing claims, and prescription drug records as previously described [17]. We defined prior fragility fracture as any nontraumatic MOF that occurred before the baseline DXA test, examining medical records back to 1987. Prolonged oral corticosteroid use (>90 days dispensed in the 1 year prior to DXA) was obtained from the provincial pharmacy system [10]. Smoking and parental hip fracture was by self‐report. High alcohol use was directly assessed from 2012 onwards and represented by a proxy variable in earlier years (alcohol substance abuse diagnosis codes). Finally, we also ascertained use of tamoxifen and osteoporosis medications (>180 days dispensed in the 1 year prior and the year following DXA). Osteoporosis medications included oral or parenteral bisphosphonates (∼90% of all osteoporosis medication use), raloxifene, denosumab, calcitonin, teriparatide, or any systemic estrogen product. We categorized osteoporosis medication and tamoxifen use as recent (in the year prior to the index date) and current (in the year after the index date).
Statistical Analysis
Statistical analyses were performed with Statistica (version 13.0, Tibco Software, Palo Alto, CA). Descriptive statistics for demographic and baseline characteristics are presented as means ± SD for continuous variables or number (%) for categorical variables. Analysis of variance and χ2 tests of independence were used to test for between‐subgroup differences. Cumulative incidence of fracture according to breast cancer status and AI use was constructed from Kaplan‐Meier curves to time to first fracture. Curves were compared using the log‐rank test. Cox proportional hazards models were used to test for differences in time to first fracture, with patient subgroup (breast cancer with AI use, breast cancer without AI use, and general population [referent]) as the covariate of interest, controlled for the effect of other covariates and presented as hazard ratio (HR) with 95% confidence intervals (CIs). Models sequentially adjusted for the effects of age alone (Model 1), with addition of clinical risk factors (Model 2), with addition of BMD (Model 3), and with addition of tamoxifen and osteoporosis medication use, prior and current (Model 4). In sensitivity analyses, we also looked at women with minimum 5 years of observation, at women without clinical risk factors at baseline that may trigger BMD testing, at women without osteoporotic BMD, and for an interaction according to age (<65 years vs. ≥65 years). We also looked for evidence of channeling bias among women selected for tamoxifen therapy rather than AIs [18].
Results
The study population included 36,996 women, among whom 1,775 (4.8%) had breast cancer treated with an AI, 1,016 (2.7%) had breast cancer without AI use, and 34,205 (92.5%) were women from the general population (supplemental online Fig. S1). In women with breast cancer initiating AI therapy, median total exposure was 4.2 years (interquartile range 2.9–4.9 years); there was a consistently high level of use: median MPR during the first year was 0.99 (interquartile range 0.90–1.00), and median MPR for up to 5 years was 0.97 (interquartile range 0.84–1.00).
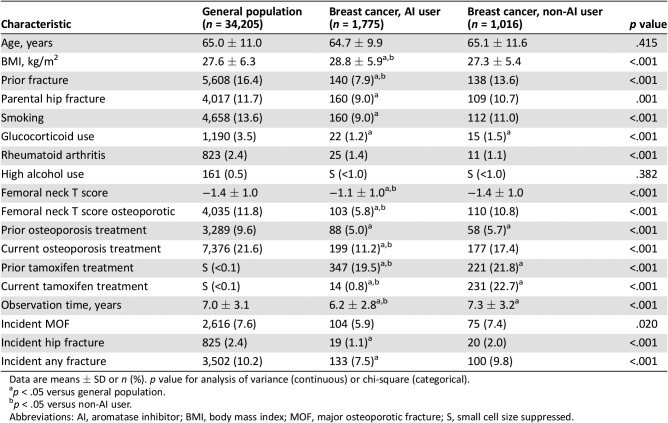
The groups were similar in terms of age at baseline, but there were significant between‐group differences in other characteristics (Table 1). Specifically, AI users had significantly higher BMI than women from the general population and women with breast cancer without AI use (all p < .001). Femoral neck BMD was also greater in AI users, but similar among women from the general population and women with breast cancer not receiving an AI (p < .001), with lower proportions of AI users with BMD T scores in the osteoporotic range or with prior fracture (p < .001). Osteoporosis treatment increased for all subgroups in the year following BMD testing but was significantly less among AI users (p < .001). Furthermore, osteoporosis treatment rates remained lower among AI users even up to 5 years (supplemental online Table 2). Among the subset of women not receiving osteoporosis treatment who underwent a second BMD test, there was significantly greater BMD loss (p < .001) among AI users than the other groups (supplemental online Table 3).
Table 1. Study characteristics stratified by breast cancer status and aromatase inhibitor use.
Data are means ± SD or n (%). p value for analysis of variance (continuous) or chi‐square (categorical).
p < .05 versus general population.
p < .05 versus non‐AI user.
Abbreviations: AI, aromatase inhibitor; BMI, body mass index; MOF, major osteoporotic fracture; S, small cell size suppressed.
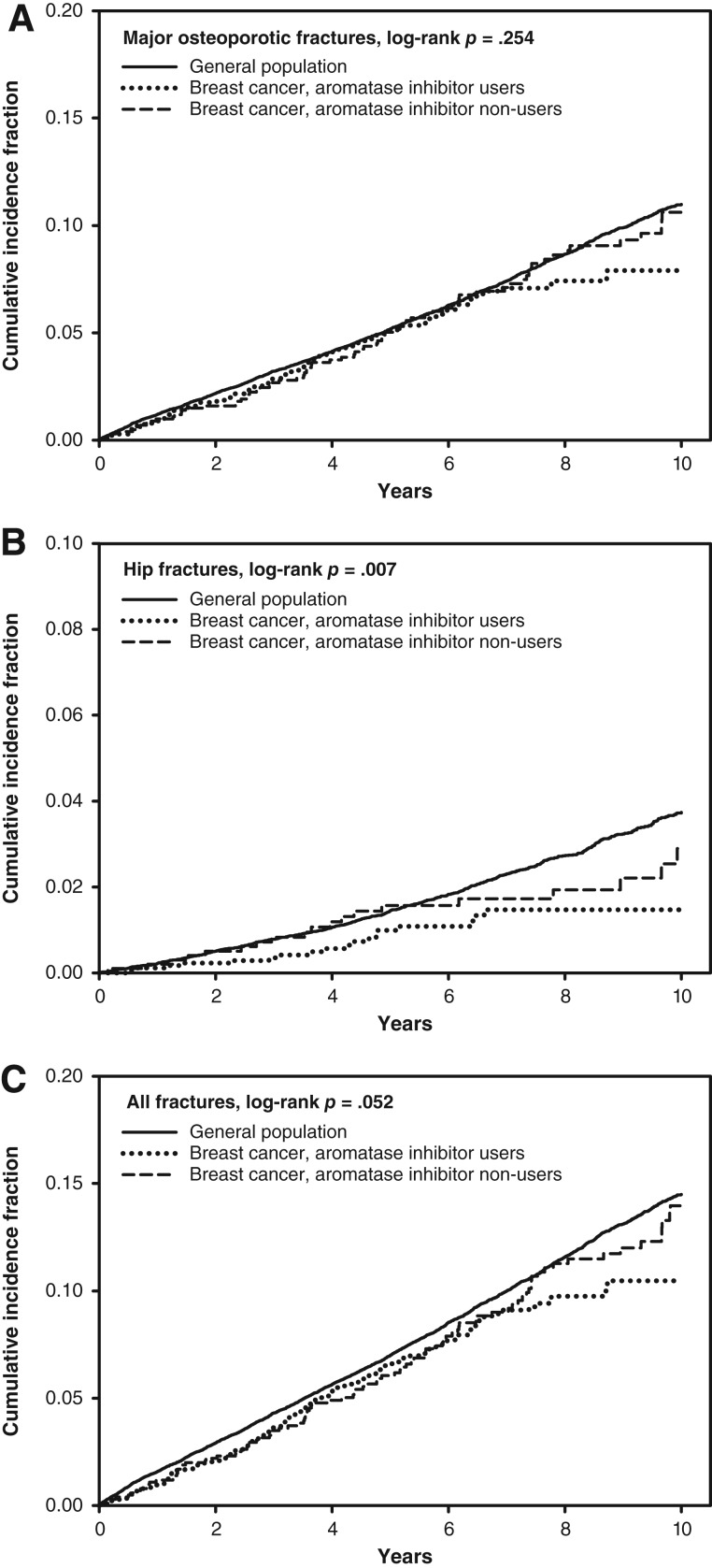
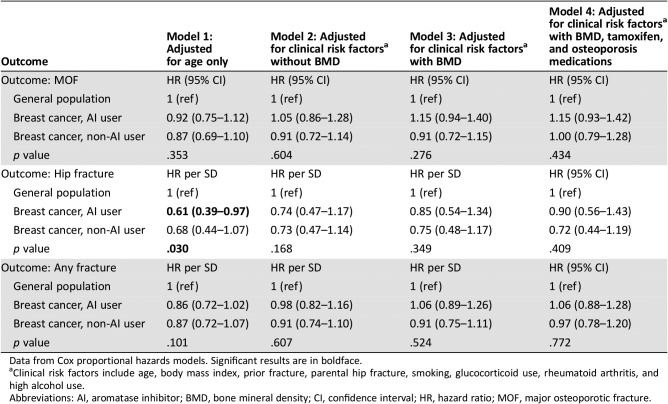
Mean ± SD follow‐up ranged from 6.2 ± 2.8 years in AI users to 7.3 ± 3.2 years for women with breast cancer who were not AI users. During the observation period, incident MOFs were experienced by 2,795 women (104 AI users), incident hip fractures by 864 women (19 AI users), and any clinical fracture by 3,502 women (133 AI users). The crude (unadjusted) cumulative incidence for fracture to 10 years is shown in Figure 1. No significant between‐group differences were seen for incident MOFs. There was a significant difference for incident hip fracture (p = .007), which was lower in AI users than in the other groups, with a similar trend for any fracture (p = .052). Age‐adjusted regression analyses (Table 2) showed similar patterns: no significant difference for incident MOFs (HR, 0.92; 95% CI, 0.75–1.12), lower hip fracture risk among AI users (HR, 0.61; 95% CI, 0.39–0.98), and a nonsignificant lower trend for any fracture among AI users (HR, 0.86; 95% CI, 0.72–1.02). After adjustment for additional covariates (which included the higher BMI and BMD in AI users), hip fracture risk among AI users was similar to the general population.
Figure 1.
Cumulative incidence for fracture according to breast cancer status and aromatase inhibitor use. (A): Major osteoporotic fractures. (B): Hip fractures. (C): All fractures.
Table 2. Hazard ratios with 95% CIs for outcomes of incident fracture according to breast cancer status and aromatase inhibitor use, sequentially adjusted for multiple covariates.
Data from Cox proportional hazards models. Significant results are in boldface.
Clinical risk factors include age, body mass index, prior fracture, parental hip fracture, smoking, glucocorticoid use, rheumatoid arthritis, and high alcohol use.
Abbreviations: AI, aromatase inhibitor; BMD, bone mineral density; CI, confidence interval; HR, hazard ratio; MOF, major osteoporotic fracture.
Sensitivity analyses were performed. Results reported in Table 2 were essentially unchanged when analysis was limited to these women with high adherence to AI therapy and at least 5 years of observation (supplemental online Table 4). Results were also similar when limited to individuals without previous fracture, parental hip fracture, smoking, or glucocorticoid use (supplemental online Table 5). More than half of the women (n = 1,090, 61%) had over 5 years of follow‐up (median MPR for AI use over the 5 years was 0.93; interquartile range, 0.74–0.98). Women with osteoporotic BMD at baseline were overrepresented among non‐AI subgroups. When women with osteoporotic BMD were excluded, there was a nonsignificant trend to higher age‐adjusted MOF risk in AI users (not seen for hip fracture or any fracture), and this completely disappeared with full covariate adjustment (supplemental online Table 6). There was no evidence that AI use affected fracture risk differently in women younger versus older than 65 years (all age‐interaction p > .4). We looked for evidence of channeling bias among women selected for tamoxifen therapy rather than AIs (supplemental online Table 7). Among women with breast cancer who were not AI users, 231 (22.7%) were currently treated with tamoxifen and 77 (7.6%) were new users of tamoxifen. There was no evidence that current or new tamoxifen users had lower femoral neck T scores or were at higher fracture risk than the general population because of preferential channeling of women at high fracture risk from AIs to tamoxifen.
Discussion
This analysis of fracture risk among women with breast cancer initiating AI therapy found unexpected results. Contrary to the suggestion from previous studies that these women would be at increased fracture risk compared with the general population [4], [5], fracture risk was similar to the general population when adjusted for baseline covariates. Higher baseline BMI, BMD, and lower prevalence of prior fracture at baseline among these women compared with the general population and women with breast cancer not receiving AIs may offset the adverse effects of AI exposure.
The fact that hip BMD was relatively better in women initiating AI therapy may at first appear surprising. However, estrogen receptor‐positive breast cancer has been associated with higher baseline BMD, perhaps reflecting circulating estrogen, and this risk is independent of the Gail score [19], [20], [21]. Higher serum estrogen is a risk factor for breast cancer [22] and confers a reduced risk for osteoporotic fracture because of its effect on BMD [23]. Higher BMI is a known risk factor for estrogen receptor‐positive breast cancer and is also correlated with higher BMD and lower fracture risk [24], [25]. Indeed, similarly high BMI has been reported among postmenopausal women with low bone mass and hormone receptor‐positive breast cancer on an aromatase inhibitor [26], [27]. Prior tamoxifen use and/or osteoporosis treatment could have contributed to higher baseline BMD among AI users, but osteoporosis treatment was actually less frequent than for the other subgroups, and our results were unchanged when adjusted for both exposures. Clinical trials in patients with breast cancer receiving adjuvant AIs versus tamoxifen have documented an increased risk of fracture (increase 15%–113%) [4], [28], [29], [30], [31], [32]. Because placebo arms were not included in these trials, the effect of AIs alone on fracture risk is less clear, as tamoxifen has a favorable effect on BMD in postmenopausal women [33], and this appears to translate into a reduced incidence of osteoporotic fracture [34]. Channeling bias, in which women with very low baseline BMD and high fracture risk would not receive AI therapy, cannot be excluded but is unlikely to account for our findings [18]. If this were a frequent occurrence, then mean BMD among women with breast cancer not receiving AIs (and particularly among those selected to receive tamoxifen rather than AIs) should be lower than in the general population, but this was not the case. Regardless of the mechanism, our findings suggest that fracture risk in women encountered in routine clinical practice receiving AI therapy may not be as elevated as has previously been suggested [5] and is consistent with the U.S. Medicare data suggesting little excess risk [6]. A smaller observational real‐life cohort found that 3 years of AI treatment was not associated with a major increase in fracture risk in 267 postmenopausal nonosteoporotic women with breast cancer [35].
Our findings, if confirmed in other large cohort studies and in conjunction with existing data, may help to inform clinical guidelines regarding the role of BMD testing and fracture risk assessment for AI recipients. The National Comprehensive Cancer Network guideline on breast cancer (version 2.2011) recommends BMD monitoring at baseline and periodically in AI recipients. The optimal testing interval is unclear. The American Society of Clinical Oncology (ASCO) has suggested annual DXA assessment of the spine and hip [36]. A U.K. expert group suggested that postmenopausal women with normal BMD at baseline risk are at low risk of developing osteoporosis over a 5‐year treatment and do not require specific intervention or monitoring beyond the usual recommendations for healthy postmenopausal women [4]. Of interest, a systematic review and guideline from Cancer Care Ontario and ASCO concluded that adjuvant bisphosphonates be considered to reduce bone recurrence and improve survival in postmenopausal patients with nonmetastatic breast cancer (effects on fragility fractures in women with low bone mineral density was not addressed in this guideline) [37]. Finally, there is evidence from a large clinical trial (ABCSG‐18) that adjuvant therapy with denosumab substantially reduces fracture risk in women with breast cancer receiving AI therapy, even when BMD is in the normal range [38].
Strengths of our study include the comprehensive population‐based data sources, which allowed us to identify a large cohort of women initiating AI therapy as well as two comparison groups, women with breast cancer who did not receive AI therapy and women from the general population without breast cancer. In addition to BMD results for the population, we were able to assess long‐term medication use and fracture outcomes. Limitations to this study are acknowledged. Lifestyle factors, including diet and exercise, are unavailable through administrative data. Although fractures were ascertained from administrative data, the definitions used have been directly validated against x‐ray confirmed fractures and adopted for national osteoporosis surveillance [8], [9], [14]. Some of the fractures could have been pathologic (related to breast cancer metastases), but this would bias results toward higher fracture rates rather than lower fracture rates in women with breast cancer. Median use of AI therapy was 4.2 years rather than the currently recommended 5 years, although subgroup analysis showed comparable results in women highly adherent to AI therapy during at least 5 years of observation. It is uncertain whether results would differ if recommendations for AI use were to be extended to 10 years [39].
Although confirmatory data from large cohort studies are required, our findings challenge the view that all women with breast cancer initiating AI therapy should be considered at high risk for fractures. In fact, at baseline such women appear to be at slightly lower fracture risk than women from the general population and women with breast cancer not initiating AI therapy because of higher BMI, BMD T score, and lower prevalence of prior fracture. This highlights the importance of identifying those women receiving AI therapy who experience accelerated BMD loss and develop a level of fracture risk at which intervention is warranted.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The authors acknowledge the Manitoba Centre for Health Policy for use of data contained in the Population Health Research Data Repository (HIPC 2016/2017‐29). The results and conclusions are those of the authors, and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, Seniors and Active Living, or other data providers is intended or should be inferred. This article has been reviewed and approved by the members of the Manitoba Bone Density Program Committee. S.N.M. is chercheur‐boursier des Fonds de Recherche du Québec en Santé. L.M.L. is supported by a Tier I Canada Research Chair.
Footnotes
For Further Reading: Jung Il Lee, Jung‐Hwan Yu, Sung Gwe Anh et al. Aromatase Inhibitors and Newly Developed Nonalcoholic Fatty Liver Disease in Postmenopausal Patients with Early Breast Cancer: A Propensity Score‐Matched Cohort Study. The Oncologist 2019;24:e653–e661.
Implications for Practice: Unlike tamoxifen, the role of aromatase inhibitor treatment use in postmenopausal patients with breast cancer in development of fatty liver is not well known. In this propensity‐matched cohort study, postmenopausal patients with breast cancer treated with aromatase inhibitors had increased risk of nonalcoholic fatty liver disease compared with healthy women after menopause, independent of obesity and diabetes mellitus. The results show possible adverse influence of the newly developed fatty liver on breast cancer disease‐free survival and suggest a necessity for further validation. Fatty liver may need to be considered as an adverse event for aromatase inhibitor treatment.
Author Contributions
Conception/design: William D. Leslie
Provision of study material or patients: William D. Leslie
Collection and/or assembly of data: William D. Leslie
Data analysis and interpretation: William D. Leslie, Suzanne N. Morin, Lisa M. Lix, Saroj Niraula, Eugene V. McCloskey, Helena Johansson, Nicholas C. Harvey, John A. Kanis
Manuscript writing: William D. Leslie, Suzanne N. Morin, Lisa M. Lix, Saroj Niraula, Eugene V. McCloskey, Helena Johansson, Nicholas C. Harvey, John A. Kanis
Final approval of manuscript: William D. Leslie, Suzanne N. Morin, Lisa M. Lix, Saroj Niraula, Eugene V. McCloskey, Helena Johansson, Nicholas C. Harvey, John A. Kanis
Disclosures
Suzanne N. Morin: Amgen, Merck (RF); Nicholas C. Harvey: Alliance for Better Bone Health, Amgen, Merck Sharpe & Dohme, Eli Lilly & Co., Servier, Shire, UCB, Radius Health, Consilient Healthcare, Internis Pharma (C/A, H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Bray F, Ferlay J, Soerjomataram I et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Maricic M, Bassford TL et al. Fracture risk among breast cancer survivors: Results from the Women's Health Initiative Observational Study. Arch Intern Med 2005;165:552–558. [DOI] [PubMed] [Google Scholar]
- 3.Burstein HJ, Temin S, Anderson H et al. Adjuvant endocrine therapy for women with hormone receptor‐positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol 2014;32:2255–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid DM, Doughty J, Eastell R et al. Guidance for the management of breast cancer treatment‐induced bone loss: A consensus position statement from a UK Expert Group. Cancer Treat Rev 2008;34(suppl 1):S3–S18. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt N, Jacob L, Coleman R et al. The impact of treatment compliance on fracture risk in women with breast cancer treated with aromatase inhibitors in the United Kingdom. Breast Cancer Res Treat 2016;155:151–157. [DOI] [PubMed] [Google Scholar]
- 6.Neuner JM, Shi Y, Kong AL et al. Fractures in a nationwide population‐based cohort of users of breast cancer hormonal therapy. J Cancer Surviv 2018;12:268–275. [DOI] [PubMed] [Google Scholar]
- 7.Roos NP, Shapiro E. Revisiting the Manitoba Centre for Health Policy and Evaluation and its population‐based health information system. Med Care 1999;37(suppl 6):JS10–JS14. [DOI] [PubMed] [Google Scholar]
- 8.Lix LM, Azimaee M, Osman BA et al. Osteoporosis‐related fracture case definitions for population‐based administrative data. BMC Public Health 2012;12:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Donnell S; Canadian Chronic Disease Surveillance System Osteoporosis Working Group. Use of administrative data for national surveillance of osteoporosis and related fractures in Canada: Results from a feasibility study. Arch Osteoporos 2013;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozyrskyj AL, Mustard CA. Validation of an electronic, population‐based prescription database. Ann Pharmacother 1998;32:1152–1157. [DOI] [PubMed] [Google Scholar]
- 11.Leslie WD, Metge C. Establishing a regional bone density program: Lessons from the Manitoba experience. J Clin Densitom 2003;6:275–282. [DOI] [PubMed] [Google Scholar]
- 12.Leslie WD, Caetano PA, Macwilliam LR et al. Construction and validation of a population‐based bone densitometry database. J Clin Densitom 2005;8:25–30. [DOI] [PubMed] [Google Scholar]
- 13.Lix L, Smith M, Pitz M et al. Cancer Data Linkage in Manitoba: Expanding the Infrastructure for Research. Winnipeg, Canada: Manitoba Centre for Health Policy; 2016. Available at http://mchp‐appserv.cpe.umanitoba.ca/reference//Candata_web_final.pdf. Accessed October 7, 2018.
- 14.Epp R, Alhrbi M, Ward L et al. Radiological validation of fracture definitions from administrative data. J Bone Miner Res 2018;33(supp 1):S275. [Google Scholar]
- 15.Looker AC, Orwoll ES, Johnston CC Jr et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res 1997;12:1761–1768. [DOI] [PubMed] [Google Scholar]
- 16.Kanis JA; World Health Organization Scientific Group . Assessment of Osteoporosis at the Primary Health Care Level. Geneva, Switzerland: World Health Organization; 2007. Available at http://www.shef.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf. Accessed July 2, 2019. [Google Scholar]
- 17.Leslie WD, Lix LM, Johansson H et al. Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res 2010;25:2350–2358. [DOI] [PubMed] [Google Scholar]
- 18.Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med 1991;10:577–581. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Arendell L, Aickin M et al. Hip bone density predicts breast cancer risk independently of Gail score: Results from the Women's Health Initiative. Cancer 2008;113:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grenier D, Cooke AL, Lix L et al. Bone mineral density and risk of postmenopausal breast cancer. Breast Cancer Res Treat 2011;126:679–686. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Kiel DP, Kreger BE et al. Bone mass and the risk of breast cancer among postmenopausal women. N Engl J Med 1997;336:611–617. [DOI] [PubMed] [Google Scholar]
- 22.Cummings SR, Duong T, Kenyon E et al. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA 2002;287:216–220. [DOI] [PubMed] [Google Scholar]
- 23.Cauley JA, Gutai JP, Sandler RB et al. The relationship of endogenous estrogen to bone density and bone area in normal postmenopausal women. Am J Epidemiol 1986;124:752–761. [DOI] [PubMed] [Google Scholar]
- 24.Munsell MF, Sprague BL, Berry DA et al. Body mass index and breast cancer risk according to postmenopausal estrogen‐progestin use and hormone receptor status. Epidemiol Rev 2014;36:114–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson H, Kanis JA, Oden A et al. A meta‐analysis of the association of fracture risk and body mass index in women. J Bone Miner Res 2014;29:223–233. [DOI] [PubMed] [Google Scholar]
- 26.Greenspan SL, Vujevich KT, Brufsky A et al. Prevention of bone loss with risedronate in breast cancer survivors: A randomized, controlled clinical trial. Osteoporos Int 2015;26:1857–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pineda‐Moncusi M, Servitja S, Casamayor G et al. Bone health evaluation one year after aromatase inhibitors completion. Bone 2018;117:54–59. [DOI] [PubMed] [Google Scholar]
- 28.Coates AS, Keshaviah A, Thurlimann B et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine‐responsive early breast cancer: Update of study BIG 1‐98. J Clin Oncol 2007;25:486–492. [DOI] [PubMed] [Google Scholar]
- 29.Coombes RC, Kilburn LS, Snowdon CF et al. Survival and safety of exemestane versus tamoxifen after 2‐3 years' tamoxifen treatment (Intergroup Exemestane Study): A randomised controlled trial. Lancet 2007;369:559–570. [DOI] [PubMed] [Google Scholar]
- 30.Jakesz R, Jonat W, Gnant M et al. Switching of postmenopausal women with endocrine‐responsive early breast cancer to anastrozole after 2 years' adjuvant tamoxifen: Combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 2005;366:455–462. [DOI] [PubMed] [Google Scholar]
- 31.Baum M, Buzdar A, Cuzick J et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early‐stage breast cancer: Results of the ATAC (Arimidex, Tamoxifen Alone or in Combination) trial efficacy and safety update analyses. Cancer 2003;98:1802–1810. [DOI] [PubMed] [Google Scholar]
- 32.Eastell R, Hannon RA, Cuzick J et al. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2‐year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230). J Bone Miner Res 2006;21:1215–1223. [DOI] [PubMed] [Google Scholar]
- 33.Ding H, Field TS. Bone health in postmenopausal women with early breast cancer: How protective is tamoxifen? Cancer Treat Rev 2007;33:506–513. [DOI] [PubMed] [Google Scholar]
- 34.Cooke AL, Metge C, Lix L et al. Tamoxifen use and osteoporotic fracture risk: A population‐based analysis. J Clin Oncol 2008;26:5227–5232. [DOI] [PubMed] [Google Scholar]
- 35.Bouvard B, Soulie P, Hoppe E et al. Fracture incidence after 3 years of aromatase inhibitor therapy. Ann Oncol 2014;25:843–847. [DOI] [PubMed] [Google Scholar]
- 36.Hillner BE, Ingle JN, Chlebowski RT et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol 2003;21:4042–4057. [DOI] [PubMed] [Google Scholar]
- 37.Dhesy‐Thind S, Fletcher GG, Blanchette PS et al. Use of adjuvant bisphosphonates and other bone‐modifying agents in breast cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017;35:2062–2081. [DOI] [PubMed] [Google Scholar]
- 38.Gnant M, Pfeiler G, Dubsky PC et al.; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in breast cancer (ABCSG‐18): A multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2015;386:433–443. [DOI] [PubMed] [Google Scholar]
- 39.Goss PE, Ingle JN, Pritchard KI et al. Extending aromatase‐inhibitor adjuvant therapy to 10 years. N Engl J Med 2016;375:209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]