Abstract
Objective
To review and summarize the recently developed Canadian Association of Gastroenterology screening recommendations for patients with a family history of colorectal cancer (CRC) or adenoma from a family medicine perspective.
Quality of evidence
A systematic review and meta-analysis was performed to synthesize knowledge regarding family history and CRC. The Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE were searched with the following MeSH terms: colorectal cancers or neoplasms, screen or screening or surveillance, and family or family history. Known hereditary syndromes were excluded. The Grading of Recommendations Assessment, Development and Evaluation methodology was used to establish certainty in reviewed evidence. Most recommendations are conditional recommendations with very low-quality evidence.
Main message
Individuals who have 1 first-degree relative (FDR) with CRC or an advanced adenoma diagnosed at any age are recommended to undergo colonoscopy every 5 to 10 years starting at age 40 to 50 years or 10 years younger than the age at diagnosis of the FDR, although fecal immunochemical testing at an interval of every 1 to 2 years can be used. Individuals with FDRs with non-advanced adenomas or a history of CRC in second-degree relatives should be screened according to average-risk guidelines. Lifestyle modification can statistically significantly decrease risk of CRC and should be considered in all patients.
Conclusion
These guidelines acknowledge the many factors that can increase an individual’s risk of developing CRC and allow for judgment to be employed depending on the clinical scenario. Lifestyle advice already given to patients for weight, blood pressure, and heart disease management will reduce the risk of CRC if implemented, and this combined with more targeted screening for higher-risk individuals will hopefully be successful in decreasing CRC mortality in Canada.
Résumé
Objectif
Passer en revue et résumer les recommandations récemment élaborées par l’Association canadienne de gastroentérologie pour les patients qui ont des antécédents familiaux de cancer colorectal (CCR) ou d’adénomes, et ce, sous l’angle de la médecine familiale.
Qualité des données
Nous avons procédé à une revue systématique et à une méta-analyse pour faire la synthèse des connaissances concernant les antécédents familiaux et le CCR. Une recension a été effectuée dans le registre central des études contrôlées de Cochrane, MEDLINE et EMBASE, à l’aide des expressions MeSH suivantes : colorectal cancers or neoplasms, screen or screening or surveillance et family or family history. Les syndromes héréditaires connus ont été exclus. La méthodologie GRADE (Grading of Recommendations Assessment, Development and Evaluation) a servi à établir la certitude dans l’examen des données probantes. La majorité des recommandations étaient conditionnelles, s’appuyant sur des données de très faible qualité.
Message principal
Il est recommandé que les personnes dont 1 parent du premier degré (PPD) a ou a eu un CCR ou un adénome avancé diagnostiqué à n’importe quel âge subissent une colonoscopie tous les 5 à 10 ans à partir de 40 à 50 ans, ou 10 ans plus tôt que l’âge au moment du diagnostic du PPD, quoiqu’un test immunochimique fécal puisse être utilisé chaque année ou 2. Les personnes qui ont un PPD sans adénome au stade avancé ou celles qui ont des antécédents familiaux de CCR venant de parents du deuxième degré devraient subir un dépistage selon les lignes directrices s’appliquant à un risque moyen. Les modifications au mode de vie peuvent diminuer le risque de CCR de manière statistiquement significative et devraient être envisagées pour tous les patients.
Conclusion
Ces lignes directrices reconnaissent que de nombreux facteurs peuvent augmenter les risques d’une personne de contracter un CCR et laissent place à l’exercice du jugement en fonction du scénario clinique. Les conseils sur le mode de vie déjà donnés aux patients en matière de poids, de pression artérielle et de prise en charge des cardiopathies réduiront le risque de CCR s’ils sont suivis. La mise en pratique de ces conseils, de même qu’un dépistage plus ciblé pour les personnes à risque plus élevé, devraient réussir à réduire la mortalité due au CCR au Canada.
Colorectal cancer (CRC) has the second-highest incidence of all cancers in Canada, with more than 26 000 new cases diagnosed yearly.1 Fortunately, there are robust screening tests available to diagnose CRC, and the pathophysiology of the disease is such that there is a lead time that allows premalignant adenomas to be detected and removed, thereby preventing the occurrence of CRC.
Family physicians are at the forefront of CRC screening and have clear, well-developed guidelines to follow for average-risk populations that have been successfully implemented across Canada.2 However, existing clinical practice guidelines for CRC screening either do not address patients with a family history of CRC or are derived indirectly from studies of average-risk individuals with estimates for those at an increased risk.3 Additionally, there is a high degree of variability among existing guidelines on recommended screening tests, age of initiation, and screening intervals.4,5 Given the heterogeneity of current recommendations and lack of evidence behind these recommendations, it is difficult for family physicians to effectively screen their patients with a family history of CRC. Furthermore, increased use of colonoscopy has resulted in the detection of more polyps and therefore more family members reporting relatives with these lesions. Evidence-based recommendations on screening these individuals are needed. Resources are scarce, and colonoscopy is associated with a statistically significant risk of bleeding and perforation and a small risk of death.6
In 2017, the Canadian Association of Gastroenterology, sponsored in part by an unrestricted grant from the Canadian Partnership Against Cancer, assembled a group of Canadian and American experts, including a family medicine representative, to create screening recommendations for patients with a family history of CRC or adenomas. These guidelines were subsequently endorsed by the American Gastroenterological Association and were published in full in 2018.7 They do not apply to patients with hereditary cancer syndromes such as familial adenomatous polyposis or Lynch syndrome. This article reviews these guidelines from a family medicine perspective, highlighting new CRC screening practices that should be incorporated into family practice and exploring the rationale and evidence behind these recommendations.
Quality of evidence
A systematic review and meta-analysis was performed by the guideline group to synthesize knowledge regarding family history and CRC. The Cochrane Central Register of Controlled Trials, MEDLINE, and EMBASE were searched with the following MeSH terms: colorectal cancers or neoplasms, screen or screening or surveillance, and family or family history. Known hereditary syndromes (eg, Lynch syndrome, familial adenomatous polyposis) were excluded. An a priori decision was made to exclude retrospective studies from the systematic review given resource constraints; however, these articles were included in the meta-analysis. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was used to establish certainty in reviewed evidence.8 A subsequent consensus conference analyzed the GRADE evidence in an Evidence to Decision framework to rank screening strategies based on efficacy, adverse effects, patient values, cost, resource allocation, and equity. In this way, screening recommendations for patients with a family history of CRC or adenomas were developed in an evidence-based fashion, with the strength of recommendation ultimately based on the GRADE analysis (Table 1).
Table 1.
The GRADE assessment of recommendations for screening for CRC in individuals with a family history of nonhereditary CRC or adenomas
| RECOMMENDATION | GRADE CONCLUSION |
|---|---|
| 1 FDR with history of CRC | |
| • Colonoscopy is the preferred screening test | Conditional recommendation, very low-quality evidence |
| • FIT is suggested as a second-line screening option | Conditional recommendation, moderate-quality evidence |
| • Screening should commence at age 40–50 y or 10 y younger than the age of diagnosis of FDR | Conditional recommendation, very low-quality evidence |
| • Screening interval should be 5–10 y for colonoscopy and 1–2 y for FIT | Conditional recommendation, very low-quality evidence |
| ≥ 2 FDRs with CRC | |
| • Colonoscopy is the preferred screening test | Strong recommendation, very low-quality evidence |
| • Colonoscopy should commence at age 40 y or 10 y younger than earliest age of diagnosis of FDR | Conditional recommendation, very low-quality evidence |
| • Colonoscopy screening interval should be 5 y | Conditional recommendation, very low-quality evidence |
| ≥ 1 SDRs with CRC | |
| • Screening should follow average-risk guidelines starting at age 50 y | Conditional recommendation, very low-quality evidence |
| ≥ 1 FDRs with advanced adenomas | |
| • Colonoscopy or FIT are suggested for screening | Conditional recommendation, very low-quality evidence |
| • Screening should commence at age 40–50 y or 10 y younger than age of diagnosis of FDR | Conditional recommendation, very low-quality evidence |
| • Screening interval should be 5–10 y for colonoscopy and 1–2 y for FIT | Conditional recommendation, very low-quality evidence |
| ≥ 1 FDR with non-advanced adenomas or polyp of unknown histology | |
| • Screening should follow average-risk guidelines | Conditional recommendation, very low-quality evidence |
CRC—colorectal cancer; FDR—first-degree relative; FIT—fecal immunochemical testing; GRADE—Grading of Recommendations Assessment, Development and Evaluation; SDR—second-degree relative.
Main message
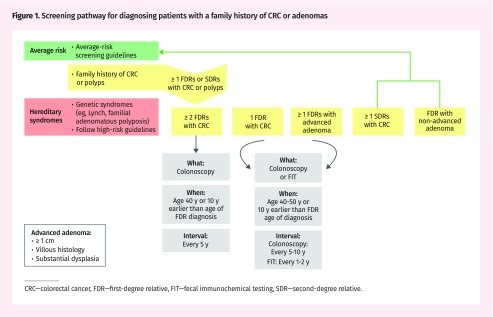
Family physicians should screen patients with a family history of CRC or adenomas as follows (Figure 1).
Figure 1.
Screening pathway for diagnosing patients with a family history of CRC or adenomas
CRC—colorectal cancer, FDR—first-degree relative, FIT—fecal immunochemical testing, SDR—second-degree relative.
First-degree relatives (FDRs) with CRC.
For an individual with 1 FDR with a history of CRC at any age, colonoscopy is suggested as the preferred screening test, and fecal immunochemical testing (FIT) can be considered if colonoscopy is not feasible owing to logistics, patient preference, or patient characteristics. Screening by any method should start at age 40 to 50 years or 10 years younger than the age of the FDR at diagnosis. The screening interval should be every 5 to 10 years for those undergoing colonoscopy and every 1 to 2 years for those being screened with FIT. Colonoscopy frequency after the initial scope will vary depending on the results of the examination.
Given the higher risk of patients with 2 or more FDRs with CRC, more intense screening is warranted. Colonoscopy is recommended for screening, and this should be commenced at age 40 or 10 years younger than the age of the FDR at diagnosis, with ongoing screening every 5 years.
Second-degree relatives with CRC.
In the presence of 1 or more second-degree relatives (SDRs) with CRC, recommendations for screening follow those suggested for the average-risk population, starting at age 50 years.
First-degree relatives with adenomas.
Individuals with any FDRs with advanced adenomas should be screened with colonoscopy or FIT starting at age 40 to 50 years or 10 years younger than the earliest age of diagnosis of adenoma in an FDR. The screening interval should be 5 to 10 years for colonoscopy or 1 to 2 years for FIT. Advanced adenomas are defined as sessile serrated polyps or adenomatous polyps (ie, not hyperplasia) that are 1 cm or larger and have a villous or tubulovillous histology and a high grade of dysplasia. Patients with FDRs with non-advanced adenomas or polyps for which there is no information on size or histology should be screened according to average-risk guidelines.
Family doctors and patients might not know the histology of polyps that were removed. It is important to obtain this information if possible; however, if there is no histology available, polyps might be assumed not to be advanced adenomas.
Background information supporting screening recommendations.
The risk of developing CRC is higher in patients with a family history of CRC. However, the increased risk is small, and it is important to remember that most CRCs are not hereditary. The best estimate of CRC relative risk (RR) in patients with 1 FDR with CRC is likely 2.2 (95% CI 1.7 to 2.7), which was determined in a recent study of CRC incidence in monozygotic twins.9 The RR inferred from a family history of CRC in an SDR is significantly lower than that of CRC in an FDR and, in fact, approaches average risk (pooled risk estimate from meta-analysis of 1.73 [95% CI 1.02 to 2.94]).10 However, a patient’s risk of developing CRC will cumulatively increase with the number of affected FDRs and SDRs, or even third-degree relatives, and the proximity of a patient’s relationship to the index case.11,12
Previous guidelines for CRC screening in patients with a family history stratified patient risk based on age of the family member at diagnosis.13 Patients with relatives who developed cancers at ages younger than 60 were believed to be at higher risk of hereditary as opposed to environmental malignancies, and therefore more intensive screening was suggested.4,14 The evidence does not support this and suggests instead there is a continuum of risk. The new guidelines developed by the Canadian Association of Gastroenterology working group therefore removed the age stratification and grouped all patients with a first-degree family history of CRC in an elevated-risk screening group. The rationale for this change is that, although the RR of developing CRC is certainly higher in patients with an FDR diagnosed at an age younger than 50 years (RR = 3.55; 95% CI 1.84 to 6.83), the RR with an FDR developing CRC at an age older than 60 or 70 is still double that of an individual with no family history.10 Family physicians should treat all patients with a first-degree family history of CRC as higher risk, independent of age of diagnosis. However, clinical judgment should be employed in the investigation of older patients who are more likely to experience colonoscopy complications.
Fuchs et al15 estimated the cumulative incidence of CRC among those with and without a family history, and it is these data that underpin current average-risk screening programs and their recommendations to initiate screening at age 50 years. The cumulative incidence of CRC in patients with a first-degree family history approaches the same threshold as those with no family history 10 years earlier at age 40, hence explaining the earlier initiation of screening recommended in these guidelines.
The optimal interval for screening in patients with a first-degree family history of CRC must balance the risks of unnecessary procedures and the serious implications of the development of “interval cancers.” The recommendation for the interval of 1 to 2 years for FIT testing comes from a single randomized controlled trial that compared screening intervals of 1, 2, and 3 years and found no significant difference in the detection rate of advanced neoplasm, but improved participation rates with testing every 2 years.16 Colonoscopy intervals are rooted in a study that showed that in patients with FDRs with CRC, there was no evidence of advanced adenomas at a 3-year screening interval, but 33% of patients had adenomas present with an interval of 5 years between colonoscopies.17
The inclusion of a range for initiation and interval of screening in the new guidelines allows for clinical judgment to tailor screening to an individual’s risk. Individuals with a single FDR but multiple SDRs with CRC at a young age might benefit from earlier-onset, more intense screening, while those with a single FDR who was more elderly at diagnosis might benefit from less intervention. The range presented is an acknowledgment that guidelines are intended to be interpreted in a clinical context with appropriate judgment and their implementation might be varied based on the nuances of individual cases.
The new guidelines include FIT as an alternative to colonoscopy. Fecal immunochemical testing is a quantitative screening test for occult blood in the stool, with a cutoff point (µg hemoglobin/g) that can be altered to tailor detection rates. Fecal immunochemical testing has been shown to be more accurate than fecal occult blood testing in the detection of CRC, and in 1 interim analysis it had similar detection rates for CRC in average-risk patients compared with colonoscopy.18 Fecal immunochemical testing is generally better accepted by patients, more available in rural areas, and more feasible for patients with multiple medical comorbidities. However, definitive evidence for the equivalence of FIT and colonoscopy for preventing death from CRC is still pending, and FIT has not been tested in higher-risk patients with a family history of CRC. As such, colonoscopy remains the criterion standard for screening patients with a family history of CRC.
Given the new recommendations for more intense screening in patients with a family history of advanced adenomas, family physicians will need to become more aware of the presence of CRC or “polyps” within family members. Patients frequently do not remember colonoscopy results, as shown in a study where only 8% of patients were able to accurately report the size, number, and pathology of polyps removed after colonoscopy.19 It is doubtful whether many patients would be able to accurately distinguish between a polyp and an advanced adenoma in a family member, creating a barrier to effective implementation of these new guidelines. To properly stratify patient risk for CRC screening, family physicians will rely more on high-quality reports of endoscopic findings from other specialists, and there might also need to be public education on accurate awareness of a family history or improved informatics documentation within provincial screening programs.
Currently, anywhere from 3.1% to 12.9% of the population has FDRs with CRC,20,21 meaning that any decisions regarding screening recommendations in this group will have substantial resource use implications. However, given the considerable potential for life-years lost and the substantial costs of the care of patients with metastatic CRC (eg, palliative chemotherapy, diagnostic imaging, hospitalizations), colonoscopy every 5 years starting at age 40 to 50 years was found to be cost effective in screening for CRC in individuals with 1 FDR with CRC.22–25
As family physicians concerned with primary prevention, it is notable that several modifiable risk factors carry almost as much weight as a family history. Inactivity, obesity, and a diet high in processed meats (ie, a non-Mediterranean diet) all increase the risk of developing CRC, with RRs of 1.44 to 1.91 (Table 2).26,27 Alcohol, smoking, and diabetes also statistically significantly increase risk.27 Colorectal cancer screening therefore dovetails with the work that family physicians are already doing advocating for a healthy lifestyle for their patients, and targeted lifestyle advice for those with a strong family history of CRC is warranted.
Table 2.
Effect of lifestyle on RR of developing CRC
| RISK FACTOR | RR |
|---|---|
| BMI > 34 kg/m2 | 1.49 |
| Physical inactivity | 1.44 |
| Smoking, 5 pack-years | 1.06 |
| Non-Mediterranean diet | 1.91 |
Conclusion
The new CRC screening guidelines give family physicians much needed evidence-based recommendations for screening patients with a family history of CRC. There is a new understanding that an individual with an FDR with CRC has an elevated risk of developing CRC beyond that of the general population, independent of the age of onset of the FDR’s CRC. These guidelines acknowledge the many factors that can increase an individual’s risk of developing CRC and allow for judgment to be employed depending on the clinical scenario. There is some flexibility to use FIT if colonoscopy is not accessible or advisable, although families with more than 1 FDR with CRC should always be screened with colonoscopy when possible. Family physicians will need to have a greater awareness of advanced adenomas in families and an appreciation for their differing management from polyps. Lifestyle advice already given to patients for weight, blood pressure, and heart disease management will act to reduce the risk of CRC if implemented, and this combined with more targeted screening for higher-risk individuals will hopefully be successful in decreasing CRC mortality in Canada.
Editor’s key points
▸ These guidelines do not apply to hereditary cancer syndromes (eg, familial adenomatous polyposis, Lynch syndrome).
▸ All patients with a first-degree relative (FDR) with colorectal cancer (CRC) should be considered for high-risk screening, independent of the age at which the FDR developed CRC. Those with 1 or more second-degree relatives with CRC can be treated as average risk.
▸ Individuals with any FDRs with advanced adenomas should be screened as high-risk patients. Individuals with family members with polyps other than documented advanced adenomas can be treated as average risk.
▸ Colonoscopy is the preferred screening test, but fecal immunochemical testing can be considered if colonoscopy is not feasible owing to logistics, patient preference, or patient characteristics.
▸ Lifestyle modifications might be effective in decreasing the risk of developing colon cancer. Several modifiable risk factors carry almost as much weight as a family history: inactivity, obesity, and a diet high in processed meats all increase the risk of developing CRC. Colorectal cancer screening therefore dovetails with the work that family physicians are already doing advocating for a healthy lifestyle for their patients.
Points de repère du rédacteur
▸ Ces lignes directrices ne s’appliquent pas aux syndromes héréditaires de cancer (p. ex. polypose adénomateuse familiale, syndrome de Lynch).
▸ On devrait envisager un dépistage pour risque élevé pour tous les patients dont un parent du premier degré (PPD) a ou a eu un cancer colorectal (CCR), indépendamment de l’âge auquel le PPD du patient a développé un CCR. Les patients dont 1 ou plusieurs parents du deuxième degré a ou a eu un CCR peuvent être considérés comme étant à risque moyen.
▸ Les personnes dont un PPD souffre d’adénomes avancés devraient subir un dépistage à titre de patients à risque élevé. Les personnes dont des membres de la famille ont des polypes autres que des adénomes documentés au stade avancé peuvent être considérées comme à risque moyen.
▸ La colonoscopie est le test de dépistage à privilégier, mais le test immunochimique fécal peut être envisagé si la colonoscopie n’est pas possible pour des motifs logistiques, ou en raison des préférences ou des caractéristiques du patient.
▸ Des modifications au mode de vie peuvent être efficaces pour diminuer le risque de développer un cancer du côlon. Divers facteurs de risque modifiables revêtent autant d’importance que les antécédents familiaux : l’inactivité, l’obésité et un régime riche en viandes transformées augmentent tous le risque de développer un CCR. Le dépistage du CCR concorde donc avec le travail que font déjà les médecins de famille pour préconiser un mode de vie sain chez leurs patients.
Footnotes
Contributors
All authors made substantial contributions to the acquisition, analysis, or interpretation of the literature. The manuscript was initially drafted by Dr Wilkinson, after which it was revised based on input from members of the consensus group and the rest of the authors.
Competing interests
None declared
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian cancer statistics 2017. Toronto, ON: Canadian Cancer Society; 2017. Available from: www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2017-EN.pdf. Accessed 2017 Nov 15. [Google Scholar]
- 2.Canadian Task Force on Preventive Health Care Recommendations on screening for colorectal cancer in primary care. CMAJ. 2016;188(5):340–8. doi: 10.1503/cmaj.151125. Epub 2016 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Care Ontario . ColonCancerCheck screening recommendations summary—2016. Toronto, ON: Cancer Care Ontario; 2016. Available from: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/CCCScreeningRecommendations.pdf. Accessed 2017 Nov 15. [Google Scholar]
- 4.Provenzale D, Jasperson K, Ahnen DJ, Aslanian H, Bray T, Cannon JA, et al. Colorectal cancer screening, version 1.2015. J Natl Compr Canc Netw. 2015;13(8):959–68. doi: 10.6004/jnccn.2015.0116. [DOI] [PubMed] [Google Scholar]
- 5.Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, et al. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112(1):37–53. doi: 10.1038/ajg.2016.492. Epub 2016 Oct 18. [DOI] [PubMed] [Google Scholar]
- 6.Rabeneck L, Paszat LF, Hilsden RJ, Saskin R, Leddin D, Grunfeld E, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135(6):1899–906. doi: 10.1053/j.gastro.2008.08.058. 1906.e1. Epub 2008 Sep 13. [DOI] [PubMed] [Google Scholar]
- 7.Leddin D, Lieberman DA, Tse F, Barkun AN, Abou-Setta AM, Marshall JK, et al. Clinical practice guideline on screening for colorectal cancer in individuals with a family history of non-hereditary colorectal cancer or adenoma: the Canadian Association of Gastroenterology Banff Consensus. Gastroenterology. 2018;155(5):1325–47. doi: 10.1053/j.gastro.2018.08.017. e3. Epub 2018 Aug 16. [DOI] [PubMed] [Google Scholar]
- 8.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graff RE, Möller S, Passarelli MN, Witte JS, Skytthe A, Christensen K, et al. Familial risk and heritability of colorectal cancer in the Nordic Twin Study of Cancer. Clin Gastroenterol Hepatol. 2017;15(8):1256–65. doi: 10.1016/j.cgh.2016.12.041. Epub 2017 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowery JT, Ahnen DJ, Schroy PC, 3rd, Hampel H, Baxter N, Boland CR, et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: a state-of-the-science review. Cancer. 2016;122(17):2633–45. doi: 10.1002/cncr.30080. Epub 2016 Jun 3. Erratum in: Cancer 2017;123(19):3857. Epub 2017 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42(2):216–27. doi: 10.1016/j.ejca.2005.09.023. Epub 2005 Dec 9. [DOI] [PubMed] [Google Scholar]
- 12.Taylor DP, Burt RW, Williams MS, Haug PJ, Cannon-Albright LA. Population-based family history-specific risks for colorectal cancer: a constellation approach. Gastroenterology. 2010;138(3):877–85. doi: 10.1053/j.gastro.2009.11.044. Epub 2009 Dec 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leddin D, Enns R, Hilsden R, Fallone CA, Rabeneck L, Sadowski DC, et al. Colorectal cancer surveillance after index colonoscopy: guidance from the Canadian Association of Gastroenterology. Can J Gastroenterol. 2013;27(4):224–8. doi: 10.1155/2013/232769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tinmouth J, Vella E, Baxter NN, Dubé C, Gould M, Hey A, et al. Colorectal cancer screening in average risk populations: evidence summary. Program in Evidence-Based Care Evidence Summary No. 15-14. Toronto, ON: Cancer Care Ontario; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331(25):1669–74. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 16.Van Roon AH, Goede SL, van Ballegooijen M, van Vuuren AJ, Looman CW, Biermann K, et al. Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut. 2013;62(3):409–15. doi: 10.1136/gutjnl-2011-301583. Epub 2012 Mar 2. [DOI] [PubMed] [Google Scholar]
- 17.Schoenfeld PS, Fincher RK, Military Colorectal Cancer Screening Trials Group Colonoscopic surveillance of patients with a family history of colon cancer and a history of normal colonoscopy: is a five-year interval between colonoscopies appropriate? Clin Gastroenterol Hepatol. 2013;1(4):310–4. doi: 10.1053/s1542-3565(03)00136-8. [DOI] [PubMed] [Google Scholar]
- 18.Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366(8):697–706. doi: 10.1056/NEJMoa1108895. Erratum in: N Engl J Med 2016;374(19):1898. [DOI] [PubMed] [Google Scholar]
- 19.Kumaravel V, Heald B, Lopez R, Hasson H, Schneider K, Burke CA. Patients do not recall important details about polyps, required for colorectal cancer prevention. Clin Gastroenterol Hepatol. 2013;11(5):543–7. doi: 10.1016/j.cgh.2012.12.010. e1–2. Epub 2012 Dec 24. [DOI] [PubMed] [Google Scholar]
- 20.Henrikson NB, Webber EM, Goddard KA, Scrol A, Piper M, Williams MS, et al. Family history and the natural history of colorectal cancer: systematic review. Genet Med. 2015;17(9):702–12. doi: 10.1038/gim.2014.188. Epub 2015 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai FC, Strum WB. Impact of a family history of colorectal cancer on the prevalence of advanced neoplasia at colonoscopy in 4,967 asymptomatic patients. Dig Dis Sci. 2012;57(12):3234–9. doi: 10.1007/s10620-011-2015-1. Epub 2011 Dec 20. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey SD, Wilschut J, Boer R, van Ballegooijen M. A decision-analytic evaluation of the cost-effectiveness of family history-based colorectal cancer screening programs. Am J Gastroenterol. 2010;105(8):1861–9. doi: 10.1038/ajg.2010.185. [DOI] [PubMed] [Google Scholar]
- 23.Wilschut JA, Steyerberg EW, van Leerdam ME, Lansdorp-Vogelaar I, Habbema JD, van Ballegooijen M. How much colonoscopy screening should be recommended to individuals with various degrees of family history of colorectal cancer? Cancer. 2011;117(18):4166–74. doi: 10.1002/cncr.26009. Epub 2011 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouakrim DA, Boussioutas A, Lockett T, Hopper JL, Jenkins MA. Cost-effectiveness of family history-based colorectal cancer screening in Australia. BMC Cancer. 2014;14:261. doi: 10.1186/1471-2407-14-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladabaum U, Ferrandez A, Lanas A. Cost-effectiveness of colorectal cancer screening in high-risk Spanish patients: use of a validated model to inform public policy. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2765–76. doi: 10.1158/1055-9965.EPI-10-0530. Epub 2010 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson CM, Wei C, Ensor JE, Smolenski DJ, Amos CI, Levin B, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control. 2013;24(6):1207–22. doi: 10.1007/s10552-013-0201-5. Epub 2013 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doubeni CA, Fletcher RH. Family history of colorectal cancer: it is time to rethink screening recommendations. Gastroenterology. 2015;149(6):1321–2. doi: 10.1053/j.gastro.2015.09.030. Epub 2015 Sep 28. [DOI] [PubMed] [Google Scholar]